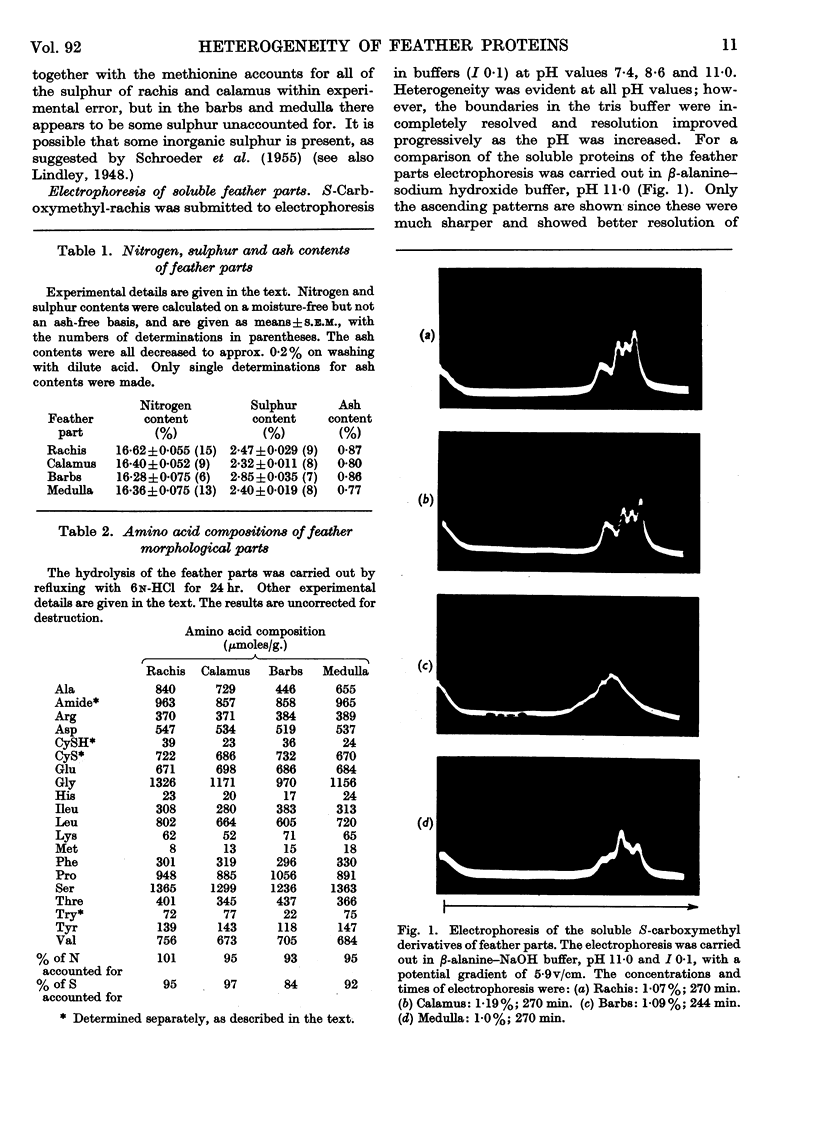
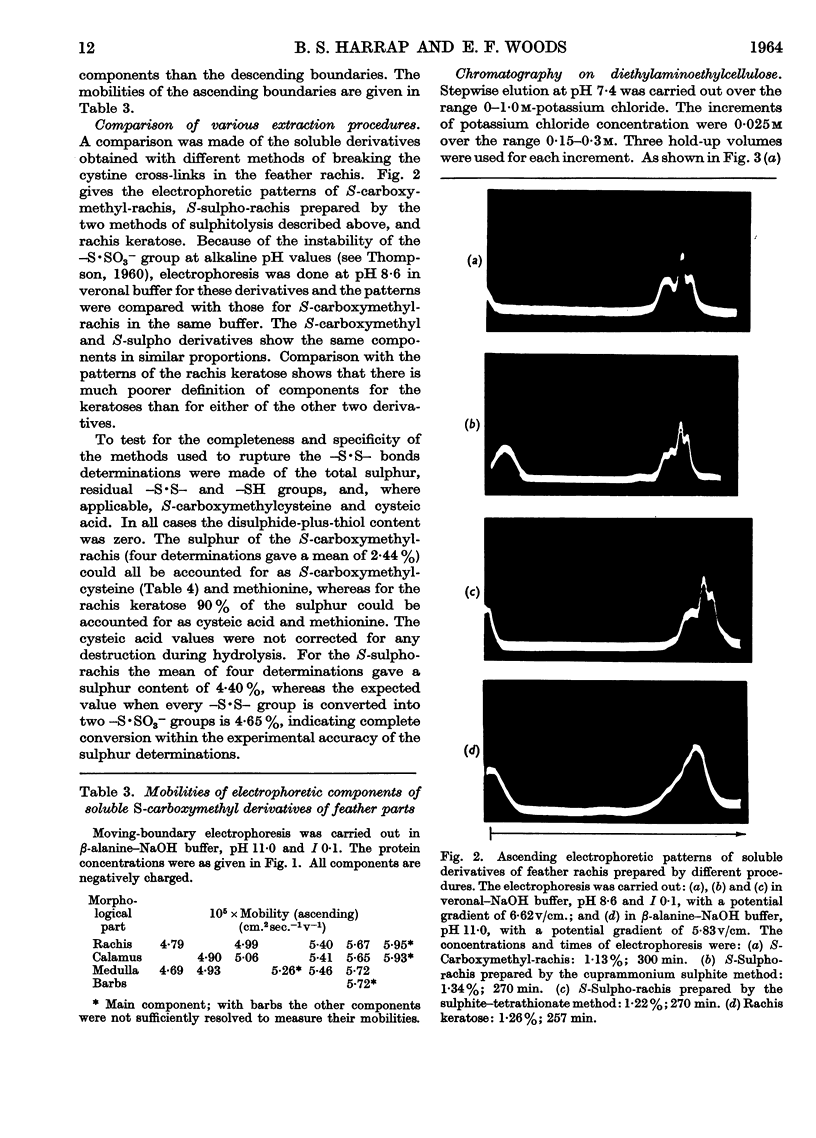
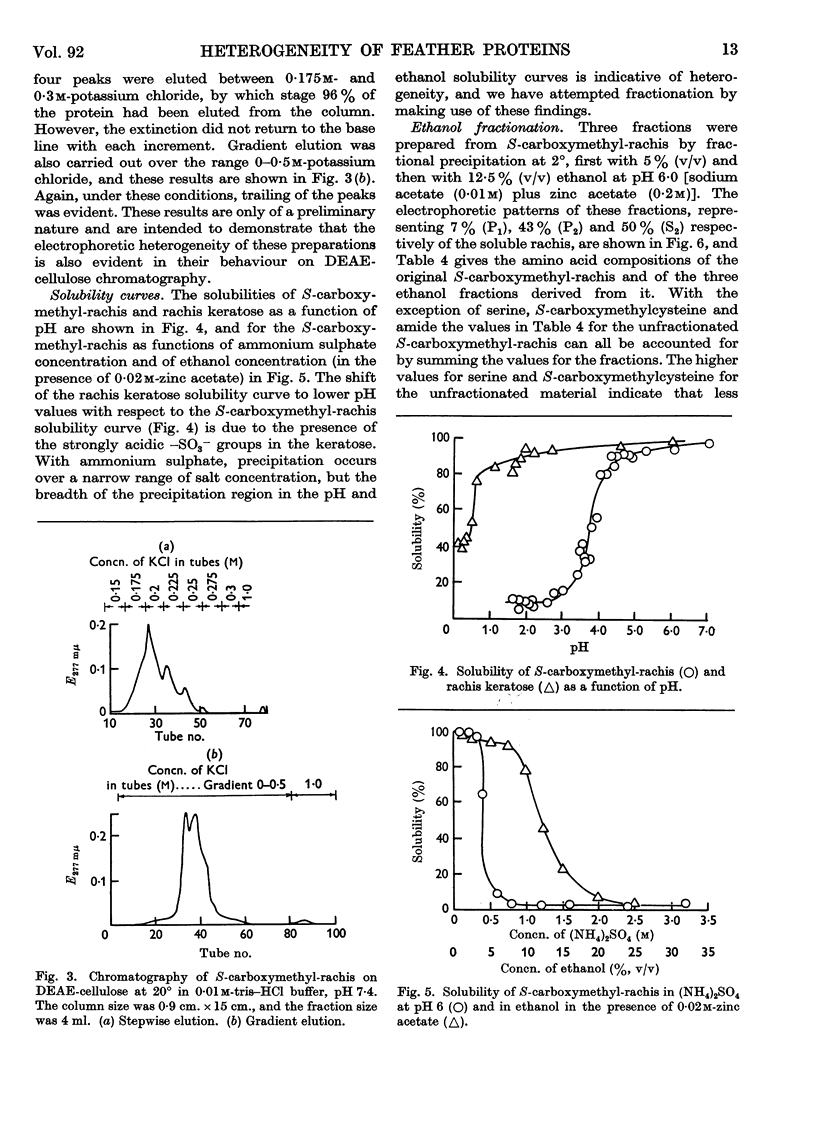
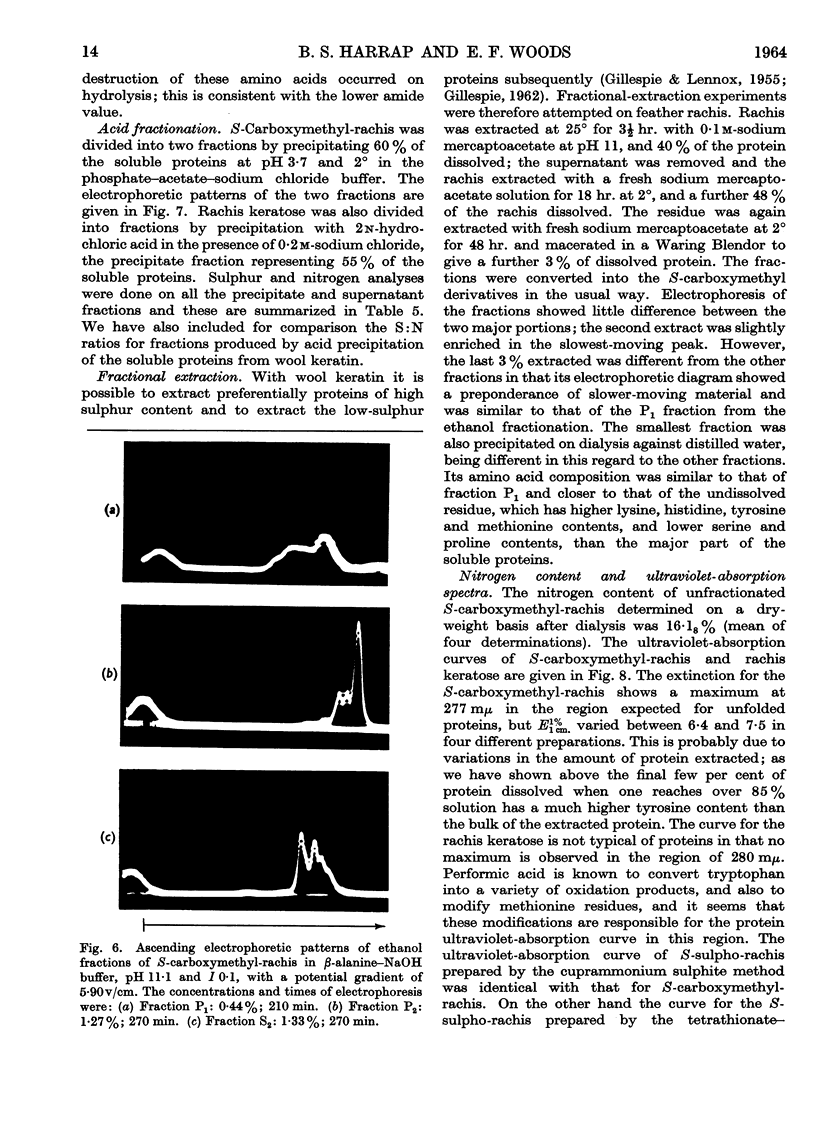
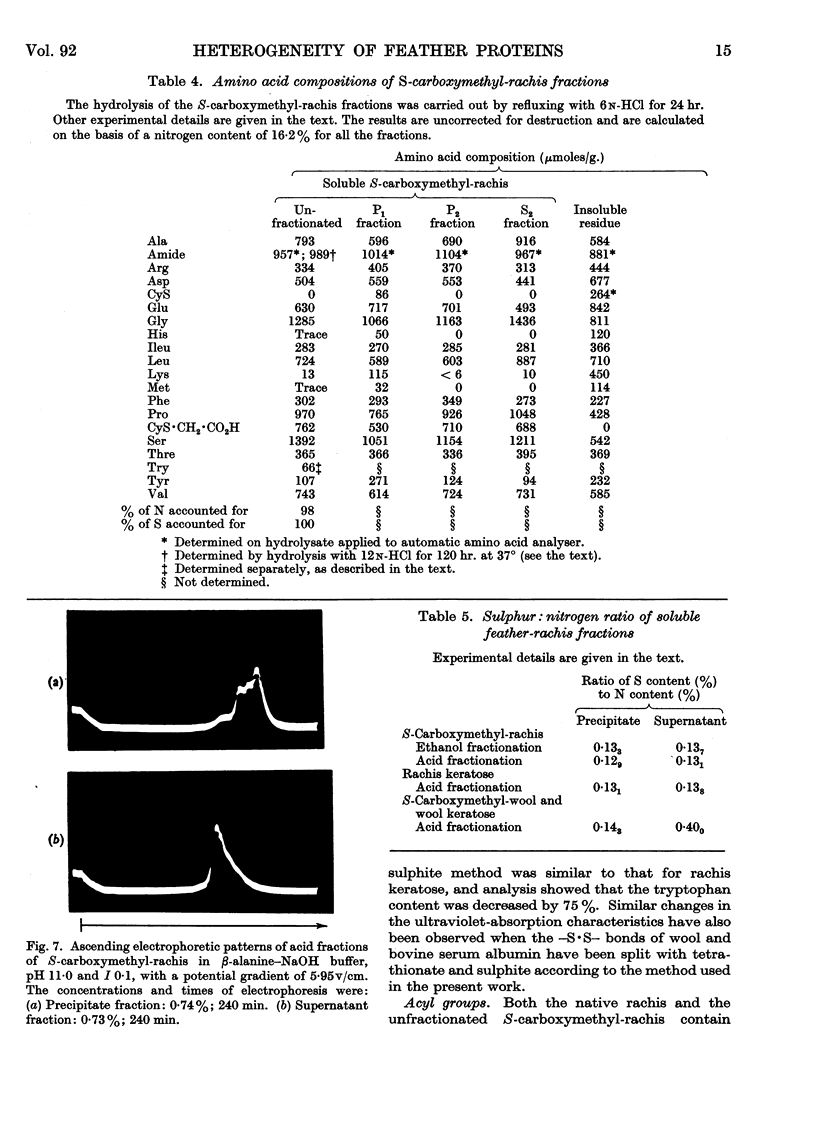
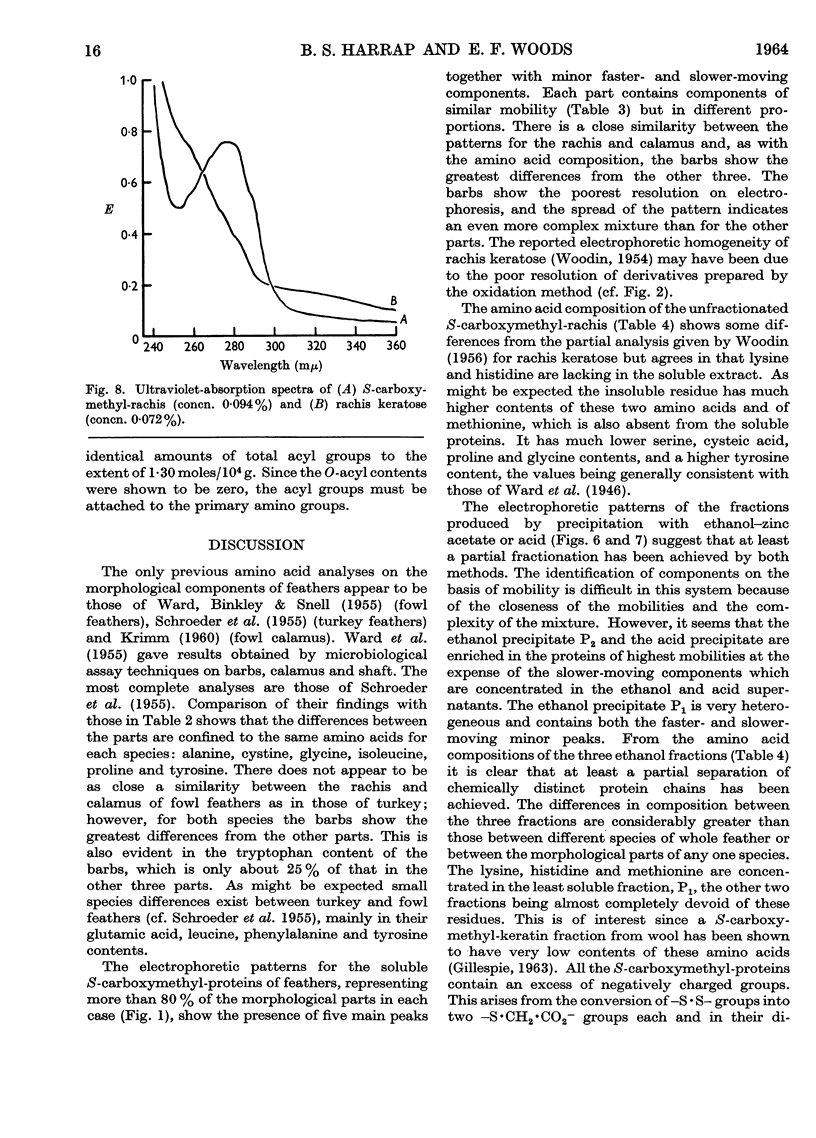
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER P., EARLAND C. Structure of wool fibres; isolation of an alpha and beta-protein in wool. Nature. 1950 Sep 2;166(4218):396–397. doi: 10.1038/166396a0. [DOI] [PubMed] [Google Scholar]
- BAILEY J. L., COLE R. D. Studies on the reaction of sulfite with proteins. J Biol Chem. 1959 Jul;234(7):1733–1739. [PubMed] [Google Scholar]
- BIRBECK M. S., MERCER E. H. The electron microscopy of the human hair follicle. I. Introduction and the hair cortex. J Biophys Biochem Cytol. 1957 Mar 25;3(2):203–214. doi: 10.1083/jcb.3.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRBECK M. S., MERCER E. H. The electron microscopy of the human hair follicle. II. The hair cuticle. J Biophys Biochem Cytol. 1957 Mar 25;3(2):215–222. doi: 10.1083/jcb.3.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRBECK M. S., MERCER E. H. The electron microscopy of the human hair follicle. III. The inner root sheath and trichohyaline. J Biophys Biochem Cytol. 1957 Mar 25;3(2):223–230. doi: 10.1083/jcb.3.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EARLAND C., BLAKEY P. R., STELL J. G. Studies on the structure of keratin. IV. The molecular structure of some morphological components of keratins. Biochim Biophys Acta. 1962 Jan 29;6:268–274. doi: 10.1016/0006-3002(62)90564-4. [DOI] [PubMed] [Google Scholar]
- Filshie B. K., Fraser R. D., Macrae T. P., Rogers G. E. Appendix-X-ray-diffraction and electron-microscope observations on soluble derivatives of feather keratin. Biochem J. 1964 Jul;92(1):18.2–1819. doi: 10.1042/bj0920018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE J. M., SIMMONDS D. H. Amino acid composition of a sulphur-rich protein from wool. Biochim Biophys Acta. 1960 Apr 22;39:538–539. doi: 10.1016/0006-3002(60)90211-0. [DOI] [PubMed] [Google Scholar]
- GUNDLACH H. G., STEIN W. H., MOORE S. The nature of the amino acid residues involved in the inactivation of ribonuclease by iodoacetate. J Biol Chem. 1959 Jul;234(7):1754–1760. [PubMed] [Google Scholar]
- Harrap B. S., Woods E. F. Soluble derivatives of feather keratin. 2. Molecular weight and conformation. Biochem J. 1964 Jul;92(1):19–26. doi: 10.1042/bj0920019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindley H. The reactivity of the combined cystine of proteins other than wool. Biochem J. 1948;42(4):481–485. doi: 10.1042/bj0420481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. Procedures for the chromatographic determination of amino acids on four per cent cross-linked sulfonated polystyrene resins. J Biol Chem. 1954 Dec;211(2):893–906. [PubMed] [Google Scholar]
- ROGERS G. E. Electron microscope studies of hair and wool. Ann N Y Acad Sci. 1959 Nov 20;83:378–399. doi: 10.1111/j.1749-6632.1960.tb40914.x. [DOI] [PubMed] [Google Scholar]
- WHITE F. H., Jr Regeneration of enzymatic activity by airoxidation of reduced ribonuclease with observations on thiolation during reduction with thioglycolate. J Biol Chem. 1960 Feb;235:383–389. [PubMed] [Google Scholar]
- WOODIN A. M. Molecular size, shape and aggregation of soluble feather keratin. Biochem J. 1954 May;57(1):99–109. doi: 10.1042/bj0570099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODIN A. M. Structure and composition of soluble feather keratin. Biochem J. 1956 Aug;63(4):576–581. doi: 10.1042/bj0630576. [DOI] [PMC free article] [PubMed] [Google Scholar]


