Abstract
Background
The diagnosis of pulmonary embolism is difficult because the clinical diagnosis is nonspecific and all of the objective tests have limitations. The assay for plasma d-dimer may be useful as an exclusion test if results are negative. We conducted a prospective cohort study that evaluated the clinical utility (usefulness) of an automated quantitative d-dimer test in the diagnosis of patients with suspected pulmonary embolism.
Methods
Consecutive eligible patients who had clinically suspected PE with nondiagnostic lung scans or negative helical CT scan of the chest results underwent d-dimer testing.
Results
The d-dimer results were negative in 11 of 103 inpatients (10.6%, 95% confidence interval [CI], 5.5 to 18.3%) and 7 of 22 outpatients (31.8%, 95% CI, 13.9 to 54.9%; p = 0.02).
Conclusions
Measurement of plasma d-dimer is of limited clinical utility for inpatients with clinically suspected pulmonary embolism and nondiagnostic lung scans or negative helical CT results at a US academic health center.
Keywords: d-dimer, diagnosis, pulmonary embolism, venous thromboembolism
Abbreviations: CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; VTE, venous thromboembolism
Pulmonary embolism is a major health problem, with an estimated 575,000 persons presenting with clinically suspected pulmonary embolism each year in the United States.1 The diagnosis of pulmonary embolism is difficult because the clinical diagnosis is nonspecific and all of the objective tests have limitations.2,3 The ventilation-perfusion lung scan is nondiagnostic in 60 to 70% of patients.2,3 Combining the clinical assessment with lung scan results or using a clinical algorithm fails to identify 20% of patients with pulmonary embolism.2–4 Pulmonary angiography is the “gold standard,”5 but is invasive, impractical, or unavailable in some clinical settings, and causes cardiopulmonary complications in 3 to 4% of patients.5 Helical (spiral) CT has limited sensitivity (70%) for pulmonary embolism, particularly subsegmental embolism.6,7 Therefore, a negative helical CT scan finding used alone does not exclude pulmonary embolism.6,7 Objective testing for deep-vein thrombosis is useful if results are positive, but negative results do not exclude pulmonary embolism.8 Serial noninvasive testing for proximal deep-vein thrombosis can replace angiography in selected patients with nondiagnostic lung scans or negative helical CT scan findings,9–11 but is not appropriate for the many patients who have inadequate cardiorespiratory reserve.9
The assay for plasma d-dimer, a breakdown product of fibrin, is promising as an exclusion test for pulmonary embolism if results are negative12,13; positive results are highly nonspecific.12,13 Historically, the clinical usefulness (utility) of the d-dimer assay has been hindered by the limited sensitivity and interobserver variation of rapid latex tests, and by the delay and lack of wide availability of sensitive enzyme-linked immunosorbent assay (ELISA) tests. More recently, d-dimer measurement using automated quantitative latex antibody detection and rapid ELISAs with high sensitivity (eg, ≥95%) have become widely available. We conducted a prospective cohort study to evaluate the role of an automated quantitative d-dimer test in the diagnostic management of patients with suspected pulmonary embolism. This article reports the clinical utility of d-dimer for suspected pulmonary embolism in a US academic health center. Clinical utility is defined as the proportion of patients for whom the test provides a definitive management decision. For d-dimer, this is the proportion with a negative result because a positive result is highly nonspecific.12,13
Materials and Methods
The study was conducted at the University of Oklahoma Health Sciences Center teaching hospitals, OU Medical Center, and Veterans Administration Medical Center with beds totaling 842. The estimated number of emergency department visits is 78,500 per year, and the estimated number of admissions are 27,000 per year. The study was approved by the University of Oklahoma Health Sciences Center Institutional Review Board. Consecutive patients, both inpatient and outpatient, who had clinically suspected pulmonary embolism and were referred by their physician for ventilation-perfusion lung scanning or helical CT scanning were eligible for the study. Patients were ineligible if they had one or more of the following: (1) a history of deep-vein thrombosis or pulmonary embolism, (2) documented upper-extremity deep-vein thrombosis, (3) possible pelvic-vein thrombosis due to recent pelvic surgery or pregnancy, (4) compression ultrasound could not be performed due to physical or technical limitations, (5) therapeutic anticoagulation, (6) presence of inferior vena cava filter, (7) indwelling lower extremity venous catheter, or (8) inability to return for follow-up testing. After obtaining informed consent, all eligible patients with nondiagnostic lung scans (low, intermediate, or indeterminate probability) or negative helical CT scan results underwent d-dimer testing and compression ultrasound testing for deep-vein thrombosis of the legs. The d-dimer assay was performed using the quantitative latex method STA-Liatest D-di (Diagnostica Stago; Parsippany, NJ). A negative d-dimer result was defined before the study began as a plasma concentration of <0.47 μg/mL, as recommended by the manufacturer. Patients were managed according to the study design shown in Figure 1 and were assigned to one of four predefined cohorts.
Figure 1.
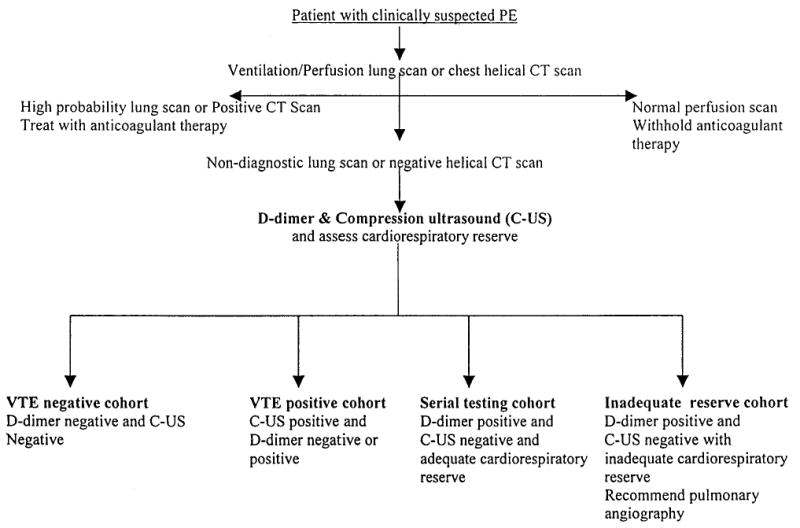
Study design for management of patients with suspected pulmonary embolism (PE).
VTE-Negative Cohort
These patients had negative d-dimer and compression ultrasound results and anticoagulant therapy withheld or withdrawn without further objective diagnostic testing for venous thromboembolism.
VTE-Positive Cohort
These patients had positive compression ultrasound for deep-vein thrombosis and received anticoagulant therapy (regardless of d-dimer results).
Serial Testing Cohort
Patients with a positive d-dimer but negative compression ultrasound findings had a clinical assessment of cardiorespiratory reserve. Cardiorespiratory reserve was defined as inadequate if one or more of the following were present9: (1) pulmonary edema, (2) hypotension, (3) syncope, (4) right ventricular failure, (5) acute tachyarrhythmias, or (6) severe respiratory insufficiency (PO2 < 50 mm Hg or PCO2 > 45 mm Hg). Those with adequate cardiorespiratory reserve underwent repeat testing with compression ultrasound at 5 to 7 days and 10 to 14 days for deep-vein thrombosis. Anticoagulant therapy was withheld if compression ultrasound results remained negative.
Inadequate Reserve Cohort
These patients with positive d-dimer results, negative compression ultrasound results, but inadequate cardiorespiratory reserve had pulmonary angiography recommended.
Perfusion Lung Scanning
Perfusion lung scanning was performed after IV injection of 6 mCi 99mTc macroaggregate albumin using the General Electric Maxxus dual-head gamma camera (GE Medical Systems; Milwaukee, WI) or the Sieman Diacam (Sieman Medical Systems; Iselin, NJ). Ventilation lung scanning was performed after inhalation of 35 mCi 99mTc diethylenetriamine pentaacetic acid aerosol. The criteria for interpreting ventilation-perfusion lung scanning were as defined by the Prospective Investigation of Pulmonary Embolism Diagnosis study.2 Helical CT was performed using one of the following multislice scanners: General Electric Cti, Nxi, Ultra-lite speed, and Hi-speed Advantage scanners (GE Medical Systems) or the Picker 6000 helical CT scanner (Phillips Medical Systems; Milpitas, CA). The criteria for a negative CT result was the absence of any intraluminal filling defects in the pulmonary arteries. Compression ultrasonography was performed using the Acuson 128, Acuson Sequoia scanner (Acuson; Mountainview, CA) or the ATL HDI 5000 (Phillips; Bothell, WA) equipped with a 6-MHz linear-array transuducer. Both the common femoral vein and the popliteal vein were imaged in gray scale as described previously,14,15 and assessed for compressibility. The ultrasonography results were classified as normal if all imaged venous segments were fully compressible, and as abnormal if a noncompressible segment was identified.
Results
Four hundred forty-four consecutive patients were screened. Of these 444 patients, 22 patients had high-probability ventilation-perfusion lung scan results, 43 patients had normal perfusion lung scan findings, and 32 patients had a positive CT scan result for pulmonary embolism. Of the remaining 347 patients with nondiagnostic lung scans or negative helical CT scan results, 222 patients were ineligible (154 inpatients, 53 outpatients, and 15 not recorded). The reasons for ineligibility were as follows: previous venous thromboembolism (n = 39), recent pelvic surgery (n = 12), unable to consent (n = 41), refused consent (n= 37), heparin therapy (n= 10), age <18 years (n = 6), unable to follow-up (n = 18), inmate (n = 3), pregnant or postpartum (n = 17), died prior to consent (n = 4), indwelling lower-extremity venous catheter (n = 3), inferior vena cava filter placement (n = 1), enrollment in another study (n = 7), > 24 h elapsed when screened (n = 12), and not recorded (n = 12).
One hundred twenty-five patients were enrolled (103 inpatients and 22 outpatients). The demographic and clinical characteristics of the enrolled population are given in Table 1.
Table 1.
Demographic and Clinical Characteristics*
| Characteristics | Total (n = 125) | Inpatients (n = 103) | Outpatients (n = 22) |
|---|---|---|---|
| Male gender | 64 (51) | 59 (57) | 5 (23) |
| Female gender | 61 (49) | 44 (43) | 17 (77) |
| Age range, yr | 19–90 | 20–90 | 19–83 |
| CT negative | 72 (58) | 56 (54) | 16 (73) |
| Lung scan nondiagnostic | 53 (42) | 47 (46) | 6 (27) |
| Negative d-dimer | 18 (14) | 11 (11) | 7 (32) |
| Symptoms on presentation | |||
| Dyspnea | 106 (85) | 86 (83) | 20 (91) |
| Chest pain, pleuritic | 24 (19) | 14 (14) | 10 (45) |
| Chest pain, central | 8 (6) | 3 (3) | 5 (23) |
| Syncope | 25 (20) | 22 (21) | 3 (14) |
| Hemoptysis | 17 (14) | 16 (16) | 1 (5) |
| Respiratory rate > 20 breaths/min | 43 (34) | 37 (36) | 6 (27) |
| Chest wall tenderness | 13 (10) | 10 (10) | 3 (14) |
| History | |||
| Surgery in the past 6 mo | 22 (18) | 21 (20) | 1 (5) |
| Myocardial infarction | 23 (18) | 21 (20) | 2 (9) |
| Congestive heart failure | 38 (30) | 33 (32) | 5 (23) |
| COPD | 31 (25) | 26 (25) | 5 (23) |
| Cancer | 27 (22) | 24 (23) | 3 (14) |
Data are presented as No. (%) unless otherwise indicated.
The d-dimer result was negative in 18 of the 125 patients (14.4%; 95% confidence interval [CI], 8.8 to 21.8%). The d-dimer result was negative in 11 of the 103 inpatients (10.6%; 95% CI, 5.5 to 18.3%) and 7 of 22 outpatients (31.8%; 95% CI, 13.9 to 54.9; p = 0.02 for comparison by Fisher exact test).
A total of 18 patients were enrolled in the VTE-negative cohort, 11 patients in the VTE-positive cohort, 46 patients in the serial testing cohort, and 50 patients in the inadequate reserve cohort. The d-dimer result was positive in 11 of 11 patients (100%; 95% CI, 71.5 to 100%) in the VTE-positive cohort (sensitivity 100% for ultrasound-detected deep-vein thrombosis).
Of the total of 444 patients screened for potential participation in the study, 22 patients had high-probability lung scan results, 32 patients had positive helical CT scan results, and 11 patients with nondiagnostic lung scans or negative helical CT scan results had positive findings on compression ultrasonography of the legs. Thus, venous thromboembolism was detected in 65 of the 444 patients (14.6%). This represents a minimum estimate of the prevalence of venous thromboembolism in the screened population because a negative CT finding may fail to detect subsegmental emboli, and not all patients with nondefinitive diagnostic test results underwent pulmonary angiography.
Discussion
Our results indicate that measurement of plasma d-dimer is of limited clinical utility for inpatients with clinically suspected pulmonary embolism and nondiagnostic lung scans or negative helical CT results at an academic health center. The frequency of a negative d-dimer result among such inpatients was only 11%, and is unlikely (p < 0.025), based on the 95% CI, to be > 18%. Thus, for inpatients with nondiagnostic lung scans or negative helical CT results, the plasma d-dimer is of limited utility as an exclusion test for pulmonary embolism, because most patients have positive d-dimer results. The measurement of plasma d-dimer will not obviate the need for further objective testing in most inpatients with nondiagnostic lung scans.
The frequency of a negative plasma d-dimer observed among the inpatients (11%) contrasts with the frequency observed in the outpatients (32%; p = 0.02). This represents a clinically important difference in the utility of the d-dimer assay in these two populations. The reason for this difference is likely the higher prevalence of acute or chronic conditions associated with fibrin generation among the inpatients, such as a history of recent surgery, myocardial infarction, or cancer (Table 1). Most previous studies of d-dimer in patients with suspected pulmonary embolism have included entirely or mostly outpatients,16–22 or failed to report the mix of inpatients and outpatients in the study population. The frequency of a negative d-dimer result among our outpatients is consistent with these prior studies.16–21 Goldstein et al,23 using the SimpliRed d-dimer assay (AGEN Biomedical Limited; Brisbane, Australia), reported a 50% frequency of negative results among inpatients at an academic health center; however, the SimpliRed assay has a lower sensitivity of 84%.12 Further, these investigators used the SimpliRed d-dimer as the first-line screening test, which resulted in both an increase in the number of patients investigated for pulmonary embolism, and increased use of additional objective testing with lung scanning, pulmonary angiography, and helical CT.23 Miron et al, 24 using a rapid ELISA assay, found a negative d-dimer result in only 2 of 55 inpatients (3.6%) with suspected pulmonary embolism, nondiagnostic lung scans, and intermediate clinical prior probability at a university hospital in Switzerland. A recent systematic review22 of strategies for excluding pulmonary embolism noted “whether the diagnostic strategies studied can be used for inpatients with the same results and failure rates requires further testing.” Our findings with a sensitive automated quantitative d-dimer assay indicate important differences in the clinical utility between inpatients and outpatients, and underscore the importance of evaluating new diagnostic approaches in both populations.
Our study has some limitations. First, it was performed in an academic health center, and may not be generalizable to community hospitals with less severely ill inpatients. Second, our focus was on patients with a diagnostic dilemma (ie, those with nondiagnostic lung scans or negative CT results). Our findings do not allow conclusions about the utility of this d-dimer test as a first-line test for inpatients; further studies are needed to evaluate the utility and safety of d-dimer assays with high sensitivity used in this way for inpatients. A rate of negative d-dimer results of even 10% used in this context may still have clinical value. Third, our study is not large enough to make conclusions about the safety of withholding anticoagulant treatment based on negative results by the Sta-Liatest d-dimer alone. These limitations, however, do not change the conclusion that the use of an automated d-dimer with a high sensitivity has a low clinical utility for excluding pulmonary embolism among inpatients with nondiagnostic lung scans or negative CT results at an academic health center.
Acknowledgments
We thank Teresa Beaulieu for help conducting this clinical trial.
Footnotes
From the Department of Medicine (Drs. Rathbun and Whitsett), College of Medicine; and the Department of Biostatistics and Epidemiology (Drs. Vesely and Raskob), College of Public Health, University of Oklahoma Health Sciences Center, Oklahoma City, OK.
Support was provided by a grant from the National Institutes of Health, National Heart Lung and Blood Institute Research Career Training Award (K23) HL04200-03.
STA-Liatest D-dimer reagents were provided by Diagnostica Stago.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (e-mail: permissions@chestnet.org).
References
- 1.Silverstein MD, Holt JA, Mohr DN, et al. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 2.Value of the ventilation/perfusion scan in acute pulmonary embolism: results of the prospective investigation of pulmonary embolism diagnosis (PIOPED); the PIOPED Investigators. JAMA. 1990;263:2753–2759. doi: 10.1001/jama.1990.03440200057023. [DOI] [PubMed] [Google Scholar]
- 3.Hull RD, Hirsh J, Carter CJ, et al. Diagnostic value of ventilation-perfusion lung scanning in patients with suspected pulmonary embolism. Chest. 1985;88:819 –828. doi: 10.1378/chest.88.6.819. [DOI] [PubMed] [Google Scholar]
- 4.Miniati M, Prediletto A, Formichi B, et al. Accuracy of clinical assessment in the diagnosis of pulmonary embolism. Am J Respir Crit Care Med. 1999;159:864 –871. doi: 10.1164/ajrccm.159.3.9806130. [DOI] [PubMed] [Google Scholar]
- 5.Stein PD, Athanasoulis C, Alavi A, et al. Complications and validity of pulmonary angiography in acute pulmonary embolism. Circulation. 1992;85:462–468. doi: 10.1161/01.cir.85.2.462. [DOI] [PubMed] [Google Scholar]
- 6.Rathbun SW, Raskob GE, Whitsett TL. Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: a systematic review. Ann Intern Med. 2000;132:227–232. doi: 10.7326/0003-4819-132-3-200002010-00009. [DOI] [PubMed] [Google Scholar]
- 7.Perrier A, Howarth N, Didier D, et al. Performance of helical computed tomography in unselected outpatients with suspected pulmonary embolism. Ann Intern Med. 2001;135:88–97. doi: 10.7326/0003-4819-135-2-200107170-00008. [DOI] [PubMed] [Google Scholar]
- 8.Turkstra F, Kuijer PM, Van Beek EJ, et al. Diagnostic utility of ultrasonography of leg veins in patients suspected of having pulmonary embolism. Ann Intern Med. 1997;126:776 –781. doi: 10.7326/0003-4819-126-10-199705150-00005. [DOI] [PubMed] [Google Scholar]
- 9.Hull RD, Raskob GE, Ginsberg JS, et al. A non-invasive strategy for the treatment of patients with suspected pulmonary embolism. Arch Intern Med. 1994;154:289 –297. [PubMed] [Google Scholar]
- 10.Kearon C, Ginsberg JS, Hirsh J. The role of venous ultrasonography in the diagnosis of suspected deep venous thrombosis and pulmonary embolism. Ann Intern Med. 1998;129:1044 –1049. doi: 10.7326/0003-4819-129-12-199812150-00009. [DOI] [PubMed] [Google Scholar]
- 11.van Strijen MJ, de Monye W, Schiereck J, et al. Single-detector helical computed tomography as the primary diagnostic test in suspected pulmonary embolism: a multicenter clinical management study of 510 patients. Ann Intern Med. 2003;138:307–314. doi: 10.7326/0003-4819-138-4-200302180-00009. [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg JS, Wells PS, Kearon C, et al. Sensitivity and specificity of a rapid whole-blood assay for d-dimer in the diagnosis of pulmonary embolism. Ann Intern Med. 1998;129:1006 –1011. doi: 10.7326/0003-4819-129-12-199812150-00003. [DOI] [PubMed] [Google Scholar]
- 13.Perrier A, Desmarais S, Miron M, et al. Non-invasive diagnosis of venous thromboembolism in outpatients. Lancet. 1999;353:190 –195. doi: 10.1016/S0140-6736(98)05248-9. [DOI] [PubMed] [Google Scholar]
- 14.Birdwell B, Raskob G, Whitsett T, et al. The clinical validity of normal compression ultrasonography in outpatients suspected of having deep venous thrombosis. Ann Intern Med. 1998;128:1–7. doi: 10.7326/0003-4819-128-1-199801010-00001. [DOI] [PubMed] [Google Scholar]
- 15.Lensing AW, Prandoni P, Brandjes D, et al. Detection of deep-vein thrombosis by real-time B-mode ultrasonography. N Engl J Med. 1989;320:342–345. doi: 10.1056/NEJM198902093200602. [DOI] [PubMed] [Google Scholar]
- 16.De Monye W, Sanson B, Gillavry MR, et al. Embolus location affects the sensitivity of a rapid quantitative d-dimer assay in the diagnosis of pulmonary embolism. Am J Respir Crit Care Med. 2002;165:345–348. doi: 10.1164/ajrccm.165.3.2104099. [DOI] [PubMed] [Google Scholar]
- 17.Burkill GJ, Bell JR, Chinn RF, et al. The use of a d-dimer assay in patients undergoing CT pulmonary angiography for suspected pulmonary embolus. Clin Radiol. 2002;57:41–46. doi: 10.1053/crad.2001.0740. [DOI] [PubMed] [Google Scholar]
- 18.Kovacs MJ, MacKinnon KM, Anderson D, et al. A comparison of three rapid D-dimer methods for the diagnosis of venous thromboembolism. Br J Haematol. 2001;115:140 –144. doi: 10.1046/j.1365-2141.2001.03060.x. [DOI] [PubMed] [Google Scholar]
- 19.Kruip MJ, Slob MJ, Schijen JH, et al. Use of a clinical decision rule in combination with d-dimer concentration in diagnostic workup of patients with suspected pulmonary embolism. Arch Intern Med. 2002;162:1631–1635. doi: 10.1001/archinte.162.14.1631. [DOI] [PubMed] [Google Scholar]
- 20.Perrier A, Desmarais S, Goehring C, et al. D-dimer testing for suspected pulmonary embolism in outpatients. Am J Respir Crit Care Med. 1997;156:492–496. doi: 10.1164/ajrccm.156.2.9702032. [DOI] [PubMed] [Google Scholar]
- 21.Oger E, Leroyer C, Bressollette L, et al. Evaluation of a new, rapid and quantitative d-dimer test in patients with suspected pulmonary embolism. Am J Respir Crit Care Med. 1998;158:65–70. doi: 10.1164/ajrccm.158.1.9710058. [DOI] [PubMed] [Google Scholar]
- 22.Kruip MJ, Leclercq M, van der Heul, et al. Diagnostic strategies for excluding pulmonary embolism in clinical outcome studies: a systematic review. Ann Intern Med. 2003;138:941–951. doi: 10.7326/0003-4819-138-12-200306170-00005. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein NM, Kollef MH, Ward S, et al. The impact of the introduction of a rapid d-dimer assay on the diagnostic evaluation of suspected pulmonary embolism. Arch Intern Med. 2001;161:567–571. doi: 10.1001/archinte.161.4.567. [DOI] [PubMed] [Google Scholar]
- 24.Miron MJ, Perrier A, Bounameaux H, et al. Contribution of noninvasive evaluation to the diagnosis of pulmonary embolism in hospitalized patients. Eur Respir J. 1999;13:1365–1370. doi: 10.1183/09031936.99.13613719. [DOI] [PubMed] [Google Scholar]


