Abstract
Currently, progression of prostate cancer to androgen independence remains the primary obstacle to improved survival. In order to improve overall survival, novel treatment strategies that are based upon specific molecular mechanisms that prolong the androgen-dependent state and that are useful for androgen-independent disease need to be identified. Both epidemiological as well as pre-clinical data suggest that omega-3 fatty acids are effective primary tumor prevention agents, however their efficacy at preventing and treating refractory prostate cancer has not been as thoroughly investigated. We used an in vitro model of androgen ablation to determine the effect of treatment with omega-3 fatty acids on the progression to an androgen-independent state. The omega-3 fatty acids DHA and EPA were able to prevent progression of LNCaP cells while the omega-6 fatty acid AA actually promoted cell growth under conditions of hormone depletion. These results correlated with a decrease in the expression of the androgen receptor as well as suppression of the Akt/mTOR signaling pathway. Connecting the mechanisms by which omega-3 fatty acids affect phenotypic outcome is important for effective exploitation of these nutrient agents as a therapeutic approach. Understanding these processes is critical for the development of effective dietary intervention strategies that improve overall survival.
Keywords: omega-3 fatty acids, prostate cancer, hormone independence
Background
One of the prime reasons of prostate cancer-related deaths today is progression of the disease to a hormone-refractory state. Thus, understanding the mechanisms underlying this progression will be beneficial in preventing the disease to become hormone-independent. Epidemiological studies suggest that a large portion of prostate cancer development can be associated with lifestyle practices. Nutrition appears to be one of the major risk factors responsible for the differences in the global distribution of prostate cancer (1). The role of dietary fat in development and progression of prostate cancer has gained considerable importance in the last several years. Examples of dietary fat that impact PCa include omega-3 and omega-6 polyunsaturated fatty acids (PUFAs), both of which play important roles in many normal human biological processes (2). As humans cannot synthesize sufficient quantities of omega-6 and omega-3 PUFAs, they are considered essential fatty acids. In animals, including the man, the PUFAs of both the families are biosynthesized from one precursor, linoleic acid (18:2omega-6) and α-linoleic acid (18:3omega-3) respectively. The omega-6 PUFAs (e.g. arachidonic acid) are primarily obtained from vegetable oils such as corn oils and the omega-3 PUFAs are obtained primarily from cold-water fish (eicosapentaenoic acid, EPA and docosahexaenoic acid, DHA) and some plants such as canola and flax (α-linolenic acid, which can be converted to EPA and DHA).
Several reports indicate that poly-unsaturated fatty acids (PUFA) play a major role in promoting or inhibiting several types of tumors including the hormone-responsive breast and prostate tumors. Both in vitro as well as in vivo studies have demonstrated that diets rich in omega-3 fatty acids inhibit tumor growth, whereas omega-6 fatty acids promote tumor growth (3–6). Growing epidemiological studies suggest that there is an inverse correlation between diets rich in omega-3 fatty acids, and the incidence of breast and prostate cancer (7, 8 ). However, the connection between omega-3 fatty acids and hormone progression, as well as the mechanism(s) by which they may be mediating their effects on androgen dependence remains unclear.
The tumor-suppressing effects of the omega-3 fatty acids is thought to be in part due to the modulation of gene expression and signal transduction pathways eventually leading to apoptotic cell death (9). Increasing evidence suggests that fatty acid signaling is, at least in part, mediated through the PI3K/Akt pathway (10, 11). We have previously shown that eicosapentaenoic acid (EPA), an omega-3 fatty acid, effectively inhibits Akt phosphorylation and activity in breast cancer cells (12). Other reports have indicated that fatty acids that promote tumor cell proliferation stimulate the Akt pathway and those that inhibit tumor cell growth down-regulates Akt activity (13, 14). Thus, omega-3 fatty acids are surprisingly potent and efficacious broad-spectrum protein kinase inhibitors, suggesting that PI-3 kinase and Akt may also be targets of action for omega-3 fatty acids in vivo in prostate cancer cells.
In addition to reactivation of the androgen receptor (15), growing evidence suggests a significant role for Akt in the development of hormone-independent prostate disease (16–19). We and others have demonstrated that overexpression of Akt in tamoxifen-responsive breast cancer cells results in inhibition of tamoxifen-induced apoptotic regression (16). Others have demonstrated that inhibition of Akt in prostate cells abrogates HER-2/neu-induced androgen receptor (AR) signaling and cell survival/growth effects in the absence or presence of androgen (17), and that successful progression to an androgen-independent state requires intact PI3K signaling (19). Thus, inhibition of the Akt pathway is emerging as an attractive clinical objective for the prevention of hormone-refractory disease.
In the current study, we demonstrate that treatment of hormone-responsive prostate cancer cells with omega-3 fatty acids impedes the progression from a hormone-responsive to hormone-refractory phenotype. Additionally this inhibitory effect of omega-3 fatty acids is associated with changes in pathways such as modulation of the androgen receptor as well as signaling pathways such as the Akt pathway. These studies indicate a novel mechanism by which the omega-3 fatty acid may mediate their anti-tumor effects and suggest that these dietary factors, currently available in supplemental form, may be effective in preventing progression to androgen-independent disease.
Materials and Methods
Cell lines
LNCaP prostate cancer cells were obtained from the American Type Culture Collection and maintained in RPMI 1640 (Gibco/BRL, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS) (Sigma, St Louis, MO) and 6 ng/ml of bovine insulin (Sigma).
Generation of Clones and Selection of Hormone-Independent LNCaP clones
Forty (40) single-cell suspension subclones were isolated from a population of hormone-sensitive heterogeneous LNCaP cells that were maintained in RPMI 1640 with 10% FBS and 6 ng/ml insulin until confluency. Each of the 40 subclones was then separated into eight treatment groups: complete medium with 10% Fetal bovine serum (FBS), complete medium with 10% charcoal stripped serum (CS)[hormone-ablation], CS with 1nM synthetic androgen, R1881 ((17β-hydroxy-17α-methyl-19-norandrost-4,9,11-trieomega-3-one; Perkin Elmer, Boston, MA) (R1881), CS with 10uM Akt inhibitor (Akt Inhibitor II (SH-5), Calbiochem, San Diego, CA) CS plus the simple fatty acids docosahexaenoic acid (DHA, C22:6, (all cis-4,7,10,13,16,19)), eicosapentaenoic (EPA, C20:5 (all cis-5,8,11,14,17)) or arachidonic acid (AA, C20:4 (all cis-5,8,11,14)) dissolved in ethanol (Matreya, LLC, Pleasant Gap, PA). The Calbiochem Akt inhibitor I and the PI3 kinase inhibitor LY294002 were used for a comparison. The clones were maintained in these growth conditions for 10 weeks and then analyzed. Fixed numbers of cells were seeded for each passage and the total cell number and the number of viable cells were determined by Trypan blue dye exclusion assay with every passage. Cells were classified as arrested or proliferating based on the cell counts obtained every 2 weeks until the end of week 10. Proliferating cells demonstrated continual doubling, whereas arrested cells were defined as those that did not demonstrate doubling but were still viable in a week’s time.
Western Blot Analysis
Cells were harvested followed by lysis with 1x lysis buffer [50 mmol/L Tris-HCl (pH 7.5), 120 mmol/L NaCl, 1% NP40, 1 mmol/L EDTA (pH 8.0), 5 mmol/L EGTA (pH 7.5), 50 mmol/L NaF, 40 mmol/L ß-glycerolphosphate, 100 µmol/L Na orthovanadate, 1 mmol/L benzamidine, and protease inhibitor mixture] , vortexed and centrifuged at 13000g for 20 mins to obtain supernatents as whole cell lysates. Total protein in the extracts was estimated by the Bradford Assay (Bio-Rad, CA). Fixed amount of protein was loaded on a polyacrylamide gel followed by transfer to a nitrocellulose membrane. Transferred proteins were then subjected to immunodetection by antibodies against phospho-Akt (Ser-473), total Akt, phospho-S6, total S6, tuberin (TSC-2), phospho-Erk1/2, total Erk1/2 (all from Millipore, Billerica, MA), androgen receptor and actin (Santa Cruz Biotechnologies, Santa Cruz, CA). Signal was detected using the enhanced chemiluminescence (ECL) system (Amersham, Arlington Heights, IL).
Kinase Assay
Kinase activity assays were used to measure the effects of treatment on Akt activity, as has been described previously (20, 21). Briefly, after appropriate treatment, cells were lysed in 1x lysis buffer and total Akt immunoprecipitated with 1 µg of total Akt antibody. Immunocomplexes were incubated with 5 µg of Akt substrate, a RPRAATF sequence peptide ( Diagnostic International, San Antonio, TX) and 32ATP (PerkinElmer, Boston, MA). ATP incorporation was measured by scintillation counting. Activity is presented relative to that obtained with CS + I (100%) and is the combination of three independent experiments.
Cell Proliferation Assay
Cell growth was assessed by MTT (3, 4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazoliumbromide) (Sigma) dye conversion at 570 nm following manufacture's instructions. Briefly, cells were seeded 5 × 103 per well in a 96-well flat bottom plate. Cells were grown in the following treatment conditions: FBS, CS, CS with R1881, CS with Akt I, CS with AA and CS with DHA. Cell growth was then assessed after 96 hours of continuous treatment. The results are an average of three independent experiments and are presented relative to those results obtained with FBS.
Statistics
All statistics were done using one-way Analysis of Variance (ANOVA).
Results
Omega-3 Fatty Acid Inhibition of Akt Activity and Proliferation in Prostate Cancer Cells
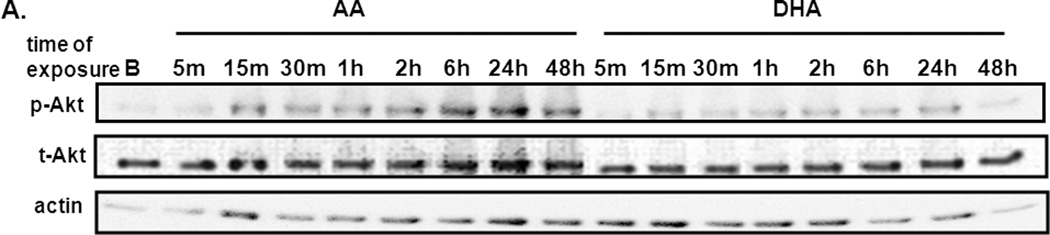
Recent studies have demonstrated that fatty acid regulation of tumor cell growth is mediated, at least in part, via activation of PI-3 kinase and Akt (14). To study the effect of omega-3 and omega-6 fatty acids on Akt activity in prostate cancer cells, we determined Akt phosphorylation and kinase activity after exposure of the omega-6 arachidonic acid (AA) and the omega-3 docosahexaenoic acid (DHA) in LNCaP cells grown in the absence of hormones (Fig. 1). We found that as in the breast cancer cells, insulin-induced Akt phosphorylation in LNCaP prostate cancer cells increased over time when cells are exposed to 40 uM AA, but only minimally increased when exposed to DHA, suggesting that omega-3 fatty acids block growth factor activation of Akt in prostate cancer cells (Fig 1A). We also assessed Akt kinase activity in control LNCaP prostate cancer cells exposed for 24 hours to charcoal-stripped serum supplemented with 100 nM insulin (CS, black bar), insulin-supplemented CS media with 40μM AA (white bars) or DHA (striped bars) (Fig. 1B). Treatment with AA increased Akt activity levels by 80% whereas treatment with the omega-3 DHA fatty acid decreased Akt1 activity by almost 40% compared to that observed in the CS + I treatment. These data indicate that similar to what we found in breast cancer cells, omega-3 fatty acids are able to suppress the activation and activity of Akt1 in prostate cancer cells in the absence of hormones.
Figure 1. Effects of PUFAs on Akt phosphorylation and kinase activity in LNCaP prostate cancer cells.
A., phospho-473 and total Akt expression levels were measured by Western blot analysis in LNCaP cells exposed to charcoal-stripped serum supplemented 100 nM insulin and 40μM of the omega-6 arachidonic acid (AA) or the omega-3 docosahexaenoic acid (DHA) from 5 min to 48 hr. Actin was used as a loading control. B., Akt kinase activity assays were performed on LNCaP cells exposed for 24 hours to charcoal-stripped serum supplemented with 100 nM insulin (CS, black bar), insulin-supplemented CS media with 40μM AA (white bar) or DHA (striped bar). Results are presented as percentage of activity obtained from CS + I treated cells and are a combination of 3 independent experiments. * p ≤ .005 compared to CS + I, ** p ≤ .005 between AA and DHA treatments
DHA Suppresses Growth of Hormone-Independent LNCaP Clones
Since DHA was able to suppress Akt activity in LNCaP cells, and Akt activity has been shown to at least in part mediate hormone-independent prostate cancer growth, we next assessed the efficacy of DHA to suppress proliferation in AR-positive, hormone independent subclones of LNCaP cells, under conditions of hormone ablation, similar to conditions in patients undergoing hormone therapy for metastatic disease. As seen in Figure 2, relative to growth in complete FBS, hormone-independent LNCaP cells were able to proliferate at similar levels whether androgen was present or not (R1881 v. CS). Treatment with the Akt inhibitor (vertical stripes bar) decreased proliferation by 50%. Treatment with 20 μM AA (white bar) maintained proliferation at levels similar to those observed with androgen supplementation. Significantly, treatment with 20 μM DHA (slanted stripes bar) suppressed proliferation by 65%, indicating that at the concentrations employed DHA appears to be a better growth suppressant than the Akt inhibitor.
Figure 2. DHA Suppresses Hormone-independent LNCaP Proliferation.
Hormone-independent LNCaP cells were assessed by MTT analysis for proliferation after exposure for 96 hr to media containing charcoal-stripped serum (CS, black), CS media supplemented with 1 nm of the synthetic androgen R1881 (diamonds), CS with 10 μM Akt inhibitor (vertical stripes), CS with 40 μM AA (white) or CS with 40 μM DHA (slanted stripe). Data is presented relative to growth in FBS and is the combination of three independent experiments done in triplicate. * p ≤ .05
Continual Exposure to Omega-3 Fatty Acids Inhibits Prostate Cancer Cell Progression to Androgen Independence
We used an in vitro model that mimics many of the features of prostate cancer progression and gives rise to androgen-independent cell sublines to investigatethe effects of exposure to different PUFAs on prostate cancer progression in a model of hormone ablation.. The salient result from these studies was that both the omega-3 fatty acids, EPA and DHA, were able to prevent the progression of LNCaP cells to a state of androgen-independence, while the omega-6 fatty acid, AA, actually promoted cell growth under conditions of hormone depletion. The results with EPA suggest that this is not specific to DHA, but is more broadly applicable to other omega-3 fatty acids as well.
As seen in Figure 3, within the androgen-depleted media only group (CS), 35% of the clones that initially demonstrated growth arrest in response to hormone ablation eventually progressed to hormone independence (Arrested, then Recovered) while 42.5% of the clones that were initially arrested remained so through week 10 (continually arrested). Within the group of clones that were grown in charcoal-stripped serum supplemented with synthetic androgen, R1881 (marble bars), 27.5% arrested and never recovered, 45% arrested but eventually recovered, and 25% never arrested (continuously proliferating). Interestingly, only 17.5% of the group grown in charcoal-stripped serum supplemented with 20 µM of AA remained arrested throughout the study. 30% of the clones arrested but then recovered, while over 52% of the clones never arrested. In fact, the AA group had as many clones recover from androgen-depletion as was found in the charcoal-stripped alone group (CS, black bars), and had more clones that never arrested compared to the CS group, suggesting that AA actually protects prostate cancer cells from androgen-deprivation induced growth arrest. We found that importantly for our study, only one of the clones progressed when exposed to 20 µM of the omega-3 fatty acid EPA, and none of clones progressed when continually exposed to 20 µM DHA. Continual exposure to 10 µM of the Akt I resulted in sustained arrest for all of the clones, while continual exposure to 20 µM LY294002 resulted in cell death for all of the clones by week 5. The data obtained from the Arrested groups (continually arrested and arrested the recovered) suggest that omega-3 fatty acids might have benefit in preventing the progression of prostate cancer. The data that was obtained from the continuously proliferating group, in which the omega-3 fatty acids prevented cell proliferation under conditions of hormone-depletion, while the omega-6 fatty acid actually promoted androgen-independent proliferation is in agreement with the many studies, including our own, that have demonstrated that a fish oil diet, that is enriched for omega-3 fatty acids, inhibits tumor cell growth by itself (22–24).
Figure 3. Exposure of LNCaP prostate cancer cells to omega-3 fatty acids prevents progression.
40 individual single cell LNCAP prostate cancer cell clones were exposed continuously to media containing charcoal-stripped media (CS, black), or CS media with 1 nM R1881 (marble), 10 μM of an Akt inhibitor (Akt I, vertical stripes), CS with 20 μM of the omega-6 fatty acid AA (white), CS with 20 μM of the omega-3 fatty acid DHA (slanted stripes) or 20 μM of the omega-3 fatty acid EPA (horizontal stripes). Presented are the % of clones that were continuously arrested, were arrested at week 5 but had recovered by week 10 (acquired resistance), never arrested (de novo resistance), or were proliferating at week 5 but eventually arrested (slow responders).
mTOR Activity is Blunted by Omega-3 Fatty Acids
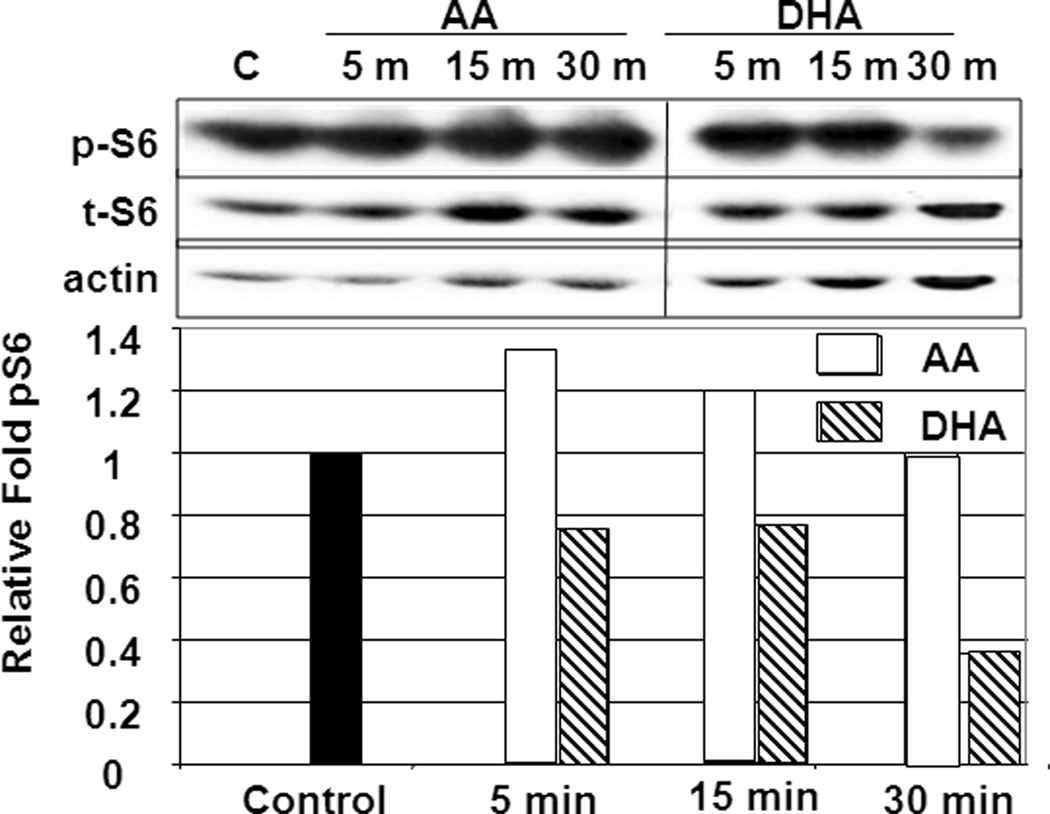
Previous studies from our laboratory (21), as well as from Scott Lowe’s laboratory (25) have indicated that in models of Akt-induced resistance, inhibition of mTOR signaling restores therapeutic response. In addition to being a mediator of growth factor signaling, mTOR is also a nutrient sensor. To determine what effect short-term exposure of omega-3 fatty acids have on mTOR activity in prostate cancer cells, we exposed LNCaP prostate cancer cells from 5 to 30 minutes to serum-free media supplemented with 100 nM insulin and either 40 μM AA or 40 μM DHA (Fig. 4A). mTOR activity was assessed by Western blot analysis based upon the phosphorylation status of the S6 ribosome, a downstream target of mTOR signaling. Exposure to AA increased phospho S6 levels in the first 5 minutes of exposure while DHA decreased phospho-S6 levels. After 30 minutes of exposure to AA, phospho-S6 levels were still above control, while the DHA treated cells demonstrated more than 50% reduction in phospho-S6 levels. These data indicate that mTOR activity is responsive to fatty modulation, either directly or indirectly through Akt.
Figure 4. PUFA modulation of mTOR activity.
A. Short-Term treatment. Protein lysates from LNCaP cells exposed to serum-free media containing 100 nM insulin (Control, black) or SF media with 100 nM insulin with 40 μM AA (white) or 40 μM DHA (slanted stripes) for 5, 15 and 30 minutes were examined by Western blot analysis for expression of phosphorylated S6 (pS6) and total S6 (t-S6). Actin was used as a loading control. Relative levels of pS6 expression were determined by densitometry analysis and are standardized to total S6 and then Control levels. Presented is a representation of at least three independent assays all with similar results.
B. Long-Term treatment. Protein lysates from LNCaP subclones exposed for 10 weeks to 10% complete media (C), charcoal-stripped serum (CS), CS with AA (AA), CS with DHA (DHA) were analyzed by Western blot analysis for expression of phospho-Akt, ribosomal phospho-S6, total S6, phosphor Tuberin (TSC2),phosphor-Erk1/2 and total Erk1/2. Presented are two representative clones of a total of 11 tested. Actin was used as a loading control.
To determine what effect long-term exposure to PUFAs have on mTOR activity, we selected two of the clones 2 and 6 that progressed to hormone-independence, generated as described in figure 3. At the end of 10 weeks levels of phospho-Akt, phospho-S6, total S6 and phospho-tuberin were determined. As seen in Figure 4B, phospho-Akt and phospho-tuberin levels were also significantly reduced with treatment with DHA and increased with treatment with AA. Phosphorylation of tuberin indicates activation of the mTOR pathway. To further confirm the effect on the mTOR pathway we observed that phosphorylated levels of S6 was significantly decreased in DHA-treated cells, compared to the AA cells. Hence our results show that the mTOR pathway is blunted in DHA treated cells as compared to CS treated while the pathway is upregulated in AA treated cells. Phospho-Erk1/2 and total Erk1/2 had no effect on any of the treatments suggesting that the MAP kinase pathway is not affected by the omega-3 fatty acids in hormone-independent prostate cancer cells.
Suppression of Progression is Associated with Suppression of Androgen Receptor Expression
Several recent studies have demonstrated that AR over-expression is one of the characteristics of prostate cancer that progresses to hormone independence (26). In addition to modulating Akt-regulated pathways, we also observed that clones that progressed to hormone-independence showed increased levels of AR protein levels as compared to their hormone-dependent syngenic clones. Exposure to the omega-6 fatty acid AA had a minimal effect on suppressing androgen deprivation-induced expression of AR. Intriguingly, long-term treatment with the omega-3 fatty acids resulted in loss of androgen receptor expression (Fig. 5).
Figure 5. AR expression is suppressed in LNCaP cells treated with omega-3 fatty acids.
Lysates from LNCaP cells exposed for 10 weeks to media with complete FBS (C), media with charcoal-stripped FBS (CS), CS with the omega-6 fatty acid AA (AA), and CS with the omega-3 fatty acid DHA (DHA) were examined by Western blot analysis for expression of the androgen receptor protein (AR). Actin was used as a loading control.
Discussion
Several reports have suggested that nutrition may play a major role in the incidence, progression and clinical outcome of prostate cancer (1, 27). Typically, specific foods such as dairy products and red meat have shown to increase the risk of prostate cancer (27, 28) whereas fiber-containing foods, lycopene and cruciferous vegetables have shown to be protective against the disease (29, 30). In addition to these, dietary fat has shown to play an important role in promoting or inhibiting prostate cancer growth. Epidemiological studies have indicated that omega-3 fatty acids inhibit growth of prostate cancer cells and omega-6 fatty acids promote the disease (2). Based on the evidences it has been speculated that the omega-3 fatty acids may reduce the risk of prostate cancer and also inhibit growth of developing prostate tumors. However, the most important concern of prostate cancer treatment today is that the disease progresses to a hormone-refractory state which is when conventional treatments become unsuccessful. Hence it is crucial in identify compounds that may have the potential to prevent the progression of the disease to hormone-independence. Our study shows that in an in vitro model, long-term treatment with the omega-3 fatty acids such as DHA and EPA may prevent hormone-dependent prostate cancer cells from progressing to hormone-independent. We observed that clones from a heterogeneous population of LNCaP cells that are dependent on androgens for their growth can have the capability of becoming hormone-independent when grown in the absence of androgens long-term. This mimics many of the features of prostate cancer progression where in the initial stages of the disease, a hormone-sensitive prostate tumor regresses after hormone-ablation but subsequently adapts to the stress of hormonal deficiency and begins to grow aggressively. More importantly we observed that treatment with DHA and EPA prevented those clones of LNCaP cells from growing as aggressively in the absence of androgens, as the untreated clones. This is consistent with recent reports using animal models, in which diets enriched for omega-3 fatty acids enhance response to androgen deprivation (31). To elucidate the mechanism of action of DHA, we studied several different molecular pathways. Prior studies have established that PI3K/Akt signaling is one pathway that is frequently upregulated in a large portion of hormone-refractory metastatic prostate cancer patients and that there is a clear association between PI3K/Akt signaling and hormone independence (32). Murillo et al demonstrated that intact PI-3K/Akt pathway is required for LNCaP cells to progress to hormone-refractory state and that active Akt activity increases as the cells transform from hormone-dependent to hormone-independent (19). The addition of exogenous arachidonic acid has shown to stimulate the activity of class Ia PI3K in human myeloid and endothelial cells resulting in the phosphorylation of Akt on Thr-308 and Ser-473, while treatment with omega-3 fatty acids reduced the activity of protein kinase C, cAMP-dependent protein kinase A, mitogen-activated protein kinase, and Ca2+/calmodulin-dependent protein kinase in the CNS (33). Our results showed that acute and chronic treatment with an Akt specific inhibitor impeded the growth of LNCaP cells in a similar manner as treatment with DHA. We also observed that treatment of LNCaP cells short-term and long-term with the omega-3 fatty acids blunted active Akt levels in the cells as well as the phosphorylated levels of its downstream targets tuberin and the ribosomal protein S6. Tuberin is a negative regulator of the mTOR pathway and phosphorylation of tuberin by Akt activated the mTOR pathway. S6 is a downstream target of the mTOR pathway and is activated by phosphorylation by active Akt (34). Hence, our results suggest that DHA could be acting by down-regulating the Akt/mTOR pathway.
Perhaps most importantly, we determined changes in the AR levels during progression to hormone-independence. It is well known that one of the mechanisms by which prostate cancer cells become hormone-independent is by increasing levels and activity of the androgen receptor, thereby sensitizing the receptor to low levels of circulating androgens (15). Intriguingly, a recent report by Locke, et al, (35) indicate that AA is able to upregulate steroidgenesis in prostate cells, thereby promoting androgen receptor activity. Consistent with the previous reports, our results showed that the hormone-independent LNCaP clones showed a significant increase in AR levels as compared to their hormone-dependent clones. However, treatment with DHA inhibited the upregulation of AR indicating that DHA could possibly play a role in modulating and regulating AR pathway.
Previous studies provide strong evidence that the poly-unsaturated fatty acids affect the growth of cancer cells by competing with the enzymes involved in the prostaglandin pathway such as cyclooxygenase-2, and 15-lipoxygenase-1 (36). In our study, we show for the first time that the PUFAs can prevent progression to hormone-independence of prostate cancer cells by other mechanisms such as modifying signal transduction pathways like the Akt/mTOR pathway or altering nuclear receptor expression. Further molecular analysis is required to identify which of these mechanisms is most important in preventing progression to hormone-independence by the omega-3 fatty acids so as to develop potential therapeutic strategies for treatment regimens in a manner that will kill cancer cells and cause least toxicity to normal cells.
Acknowledgements
These studies were supported by National Cancer Institute grant CA099115 (deGraffenried).
References
- 1.Cheung E, Wadhera P, Dorff T, Pinski J. Diet and prostate cancer risk reduction. Expert Rev Anticancer Ther. 2008;8:43–50. doi: 10.1586/14737140.8.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Astorg P. Dietary N-6 and N-3 polyunsaturated fatty acids and prostate cancer risk: a review of epidemiological and experimental evidence. Cancer Causes Control. 2004;15:367–386. doi: 10.1023/B:CACO.0000027498.94238.a3. [DOI] [PubMed] [Google Scholar]
- 3.O'Shea M, Devery R, Lawless F, Murphy J, Stanton C. Milk fat conjugated linoleic acid (CLA) inhibits growth of human mammary MCF-7 cancer cells. Anticancer Res. 2000;20:3591–3601. [PubMed] [Google Scholar]
- 4.Park Y, Allen KG, Shultz TD. Modulation of MCF-7 breast cancer cell signal transduction by linoleic acid and conjugated linoleic acid in culture. Anticancer Res. 2000;20:669–676. [PubMed] [Google Scholar]
- 5.Cave WT., Jr Omega-3 polyunsaturated fatty acids in rodent models of breast cancer. Breast Cancer Res Treat. 1997;46:239–246. doi: 10.1023/a:1005923418886. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes G, Venkatraman JT. Modulation of breast cancer growth in nude mice by omega 3 lipids. World Rev Nutr Diet. 1991;66:488–503. doi: 10.1159/000419316. [DOI] [PubMed] [Google Scholar]
- 7.Wu AH. Diet and breast carcinoma in multiethnic populations. Cancer. 2000;88:1239–1244. doi: 10.1002/(sici)1097-0142(20000301)88:5+<1239::aid-cncr10>3.3.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Terry P, Wolk A, Persson I, Magnusson C. Brassica vegetables and breast cancer risk. Jama. 2001;285:2975–2977. doi: 10.1001/jama.285.23.2975. [DOI] [PubMed] [Google Scholar]
- 9.Gutt CN, Brinkmann L, Mehrabi A, Fonouni H, Muller-Stich BP, Vetter G, Stein JM, Schemmer P, Buchler MW. Dietary omega-3-polyunsaturated fatty acids prevent the development of metastases of colon carcinoma in rat liver. Eur J Nutr. 2007;46:279–285. doi: 10.1007/s00394-007-0662-y. [DOI] [PubMed] [Google Scholar]
- 10.Couplan E, Le Cann M, Le Foll C, Corporeau C, Blondel M, Delarue J. Polyunsaturated fatty acids inhibit PI3K activity in a yeast-based model system. Biotechnol J. 2009;4:1190–1197. doi: 10.1002/biot.200800229. [DOI] [PubMed] [Google Scholar]
- 11.Wan M, Li Y, Xue H, Li Q, Li J. Eicosapentaenoic acid inhibits TNF-alpha-induced Lnk expression in human umbilical vein endothelial cells: involvement of the PI3K/Akt pathway. J Nutr Biochem. 2007;18:17–22. doi: 10.1016/j.jnutbio.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Friedrichs WE, Fernandes G, Macias R, Silva J, Li X, deGraffenried LA. A Diet Rich in Omega-3 Fatty Acids Enhances Tamoxifen Response in Akt-Induced Resistant Breast Cancer. 27th San Antonio Breast Cancer Symposium; San Antonio, TX. 2004. [Google Scholar]
- 13.Hardy S, Langelier Y, Prentki M. Oleate activates phosphatidylinositol 3-kinase and promotes proliferation and reduces apoptosis of MDA-MB-231 breast cancer cells, whereas palmitate has opposite effects. Cancer Res. 2000;60:6353–6358. [PubMed] [Google Scholar]
- 14.Hii CST, Moghadammi N, Dunbar A, Ferrante A. Activation of the Phosphatidylinositol 3-Kinase-Akt/Protein Kinase B Signaling Pathway in Arachidonic Acid-stimulated Human Myeloid and Endothelial Cells. INVOLVEMENT OF THE ErbB RECEPTOR FAMILY. J. Biol. Chem. 2001;276:27246–27255. doi: 10.1074/jbc.M103250200. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol. 2008;8:440–448. doi: 10.1016/j.coph.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 17.Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz G, Yan DH, Hung MC. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000;60:6841–6845. [PubMed] [Google Scholar]
- 18.Li P, Nicosia SV, Bai W. Antagonism between PTEN/MMAC1/TEP-1 and androgen receptor in growth and apoptosis of prostatic cancer cells. J Biol Chem. 2001;16:16. doi: 10.1074/jbc.M010226200. [DOI] [PubMed] [Google Scholar]
- 19.Murillo H, Huang H, Schmidt LJ, Smith DI, Tindall DJ. Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology. 2001;142:4795–4805. doi: 10.1210/endo.142.11.8467. [DOI] [PubMed] [Google Scholar]
- 20.Franke TF. Assays for Akt. Methods Enzymol. 2000;322:400–410. doi: 10.1016/s0076-6879(00)22039-9. [DOI] [PubMed] [Google Scholar]
- 21.deGraffenried LA, E.Friedrichs W, Russell DH, Donzis EJ, Middleton AK, Silva JM, Roth RA, Hidalgo M. Inhibition of mTOR Activity Restores Tamoxifen Response in Breast Cancer Cells with Aberrant Akt Activity. Clin Cancer Res. 2004;10:8059–8067. doi: 10.1158/1078-0432.CCR-04-0035. [DOI] [PubMed] [Google Scholar]
- 22.Rose DP, Connolly JM. Effects of dietary omega-3 fatty acids on human breast cancer growth and metastases in nude mice. J Natl Cancer Inst. 1993;85:1743–1747. doi: 10.1093/jnci/85.21.1743. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes G, Chandrasekar B, Troyer DA, Venkatraman JT, Good RA. Dietary lipids and calorie restriction affect mammary tumor incidence and gene expression in mouse mammary tumor virus/v-Ha-ras transgenic mice. Proc Natl Acad Sci U S A. 1995;92:6494–6498. doi: 10.1073/pnas.92.14.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rose DP, Cohen LA. Effects of dietary menhaden oil and retinyl acetate on the growth of DU 145 human prostatic adenocarcinoma cells transplanted into athymic nude mice. Carcinogenesis. 1988;9:603–605. doi: 10.1093/carcin/9.4.603. [DOI] [PubMed] [Google Scholar]
- 25.Wendel H-G, Stanchina Ed, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 26.Taplin ME, Balk SP. Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem. 2004;91:483–490. doi: 10.1002/jcb.10653. [DOI] [PubMed] [Google Scholar]
- 27.Berkow SE, Barnard ND, Saxe GA, Ankerberg-Nobis T. Diet and survival after prostate cancer diagnosis. Nutr Rev. 2007;65:391–403. doi: 10.1111/j.1753-4887.2007.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 28.Shirai T, Asamoto M, Takahashi S, Imaida K. Diet and prostate cancer. Toxicology. 2002;181–182:89–94. doi: 10.1016/s0300-483x(02)00260-3. [DOI] [PubMed] [Google Scholar]
- 29.Hebert JR, Hurley TG, Olendzki BC, Teas J, Ma Y, Hampl JS. Nutritional and socioeconomic factors in relation to prostate cancer mortality: a cross-national study. J Natl Cancer Inst. 1998;90:1637–1647. doi: 10.1093/jnci/90.21.1637. [DOI] [PubMed] [Google Scholar]
- 30.Giovannucci E, Clinton SK. Tomatoes, lycopene, and prostate cancer. Proc Soc Exp Biol Med. 1998;218:129–139. doi: 10.3181/00379727-218-44277. [DOI] [PubMed] [Google Scholar]
- 31.McEntee MF, Ziegler C, Reel D, Tomer K, Shoieb A, Ray M, Li X, Neilsen N, Lih FB, O'Rourke D, Whelan J. Dietary n-3 polyunsaturated fatty acids enhance hormone ablation therapy in androgen-dependent prostate cancer. Am J Pathol. 2008;173:229–241. doi: 10.2353/ajpath.2008.070989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Kreisberg JI, Ghosh PM. Cross-talk between the androgen receptor and the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer. Curr Cancer Drug Targets. 2007;7:591–604. doi: 10.2174/156800907781662248. [DOI] [PubMed] [Google Scholar]
- 33.Mirnikjoo B, Brown SE, Kim HF, Marangell LB, Sweatt JD, Weeber EJ. Protein kinase inhibition by omega-3 fatty acids. J Biol Chem. 2001;276:10888–10896. doi: 10.1074/jbc.M008150200. [DOI] [PubMed] [Google Scholar]
- 34.Cai SL, Tee AR, Short JD, Bergeron JM, Kim J, Shen J, Guo R, Johnson CL, Kiguchi K, Walker CL. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol. 2006;173:279–289. doi: 10.1083/jcb.200507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Locke JA, Guns ES, Lehman ML, Ettinger S, Zoubeidi A, Lubik A, Margiotti K, Fazli L, Adomat H, Wasan KM, Gleave ME, Nelson CC. Arachidonic acid activation of intratumoral steroid synthesis during prostate cancer progression to castration resistance. Prostate. 2010;70:239–251. doi: 10.1002/pros.21057. [DOI] [PubMed] [Google Scholar]
- 36.Kelavkar UP, Hutzley J, Dhir R, Kim P, Allen KG, McHugh K. Prostate Tumor Growth and Recurrence Can Be Modulated by the omega-6:omega-3 Ratio in Diet: Athymic Mouse Xenograft Model Simulating Radical Prostatectomy. Neoplasia. 2006;8:112–124. doi: 10.1593/neo.05637. [DOI] [PMC free article] [PubMed] [Google Scholar]