Abstract
This Perspective covers discovery and mechanistic aspects as well as initial applications of novel ionization processes for use in mass spectrometry that guided us in a series of subsequent discoveries, instrument developments, and commercialization. Vacuum matrix-assisted ionization on an intermediate pressure matrix-assisted laser desorption/ionization source without the use of a laser, high voltages, or any other added energy was simply unbelievable, at first. Individually and as a whole, the various discoveries and inventions started to paint, inter alia, an exciting new picture and outlook in mass spectrometry from which key developments grew that were at the time unimaginable, and continue to surprise us in its simplistic preeminence. We, and others, have demonstrated exceptional analytical utility. Our current research is focused on how best to understand, improve, and use these novel ionization processes through dedicated platforms and source developments. These ionization processes convert volatile and nonvolatile compounds from solid or liquid matrixes into gas-phase ions for analysis by mass spectrometry using e.g., mass-selected fragmentation and ion mobility spectrometry to provide accurate, and sometimes improved mass and drift time resolution. The combination of research and discoveries demonstrated multiple advantages of the new ionization processes and established the basis of the successes that lead to the Biemann Medal and this Perspective. How the new ionization processes relate to traditional ionization is also presented, as well as how these technologies can be utilized in tandem through instrument modification and implementation to increase coverage of complex materials through complementary strengths.
Introduction
The accumulation of discoveries, developments, and implementations of new ionization processes for use in biological MS was honored with the Biemann Medal to Prof. Sarah Trimpin by The American Society for Mass Spectrometry (ASMS). Together with collaborators from academia, government, and industry, key limitations of materials characterization have been identified for which current analytical technology are insufficient. Material characterization is an essential component of materials developments, clinical precision and timeliness, and safety, and, consequently, commercialization. The objective of our work is, inter alia, to improve the utility through simplicity, speed, and sensitivity of the new ionization processes in mass spectrometry (MS), with some emphasis on vacuum matrix-assisted ionization (vMAI),1,2 in order to broaden the scope of materials for which molecular surface and chemical defect analyses can be applied. Recent summaries include research on fundamentals and applications and, in some cases, are supported by movies showcasing the operation of different methods.3–9 In this Perspective we present work delivered in the Biemann lecture10 and other ASMS presentations from us and other researchers to demonstrate scientific and broader societal impact. We first start with the current state-of-the-art of the new technologies. Subsequently, we include a synopsis of discoveries going from the solvent-free and voltage-free experiments to the dedicated sources presenting vMAI as the newest, simplest, and a robust alternative within these new ionization processes, as well as the lessons learned toward taking informed steps through hypothesis-driven fundamental research questions guiding and enabling the discoveries to advance the new technologies and applications. To assist the reader, Scheme 1 summarizes the path of the discoveries and inventions, how they are interconnected fundamentally and how they have been used by us and others, as well as the homebuilt modifications to commercially available manual and automated platforms (inlet ionization) and sources (vacuum ionization). The discoveries led to 11 issued patents and two additional patents at various stages of intellectual protection. In the interest of the academic character of the Biemann Special Issue and perceived conflict of interest, we refrain from referring to any of the patents that resulted from this research.
Scheme 1. Timeline for the Path of Discoveries of New Ionization Processes Developed into Ionization Methods with Applicability Ranging from Imaging to Liquid Chromatography and Spanning Pressure Regimes from Above Atmospheric Pressure (AP) to Low Pressure (High Vacuum).a.

a Inlet ionization (in red color) is referred to when the ionization is assisted by an inlet tube, preferably heated, that connects a higher pressure with a lower pressure region by default available with AP ionization mass spectrometers. Depending on the vendor, mass spectrometer specific ion source configurations can be used “as is” or with inlet modifications. In MAI, a laser is not used to initiate ionization of the matrix:analyte sample. An inlet tube is also used with SAI, but the matrix is a liquid, typically the solvent used to dissolve the analyte. Vacuum ionization (in blue) is referred to when the matrix:analyte is introduced directly into the sub-AP of the mass spectrometer, typically on a substrate without use of an inlet tube. vLSI proved that a heated inlet tube was not necessary; instead, multiply charged ions are obtained from a thermal process assisted by the laser. Without the use of a laser, using the matrix 3-nitrobenzonitrile (3-NBN) under vacuum (vMAI) conditions, the matrix:analyte sample produces ESI-like charge states. Using vMAI, the changes in the instrument can be as minimal as with inlet ionization, or substantial, by replacing the source and inlet to the mass spectrometer. The purple color combines substantially the aspects of inlet and vacuum ionization. MSTM products include manual and automated Ionique platforms and sources developed through the National Science Foundation (NSF) STTR and SBIR funding.
Experimental
Details are included in the SI of this contribution.
Results
1. State-of-the-Art Technologies for Fast, Sensitive, and Robust Analyses Without Mass Spectrometry Expertise
We start this Perspective at the current state of development and then describe the important progression from its inception, bringing us full circle from the first observation of vMAI spontaneously producing gas-phase ions simply by exposing the matrix:analyte sample to the vacuum of the mass spectrometer. Briefly, this was accomplished on Waters SYNAPT G2 intermediate pressure matrix-assisted laser desorption/ionization (MALDI) sources using the vMAI matrix 3-nitrobenzonitrile (3-NBN) and required 2 minutes to insert the sample into vacuum and positioned in front of the ion transfer region. Because 3-NBN sublimes, especially when exposed to vacuum conditions, we needed a faster means of delivering the sample. This was accomplished in the Trimpin academic laboratory at Wayne State University (WSU) with a probe inserted through a ball valve providing ca. 10-second introduction.11 The results, as described below, were so enticing that MSTM, a university spin-off company between WSU and the University of the Sciences, founded to commercialize the new ionization technologies, built a probe device to test on a Thermo Orbitrap mass spectrometer.12 The results, especially from the second-generation probe device were astonishing in sensitivity, robustness to carryover and instrument contamination as described in more detail later on. While the Trimpin group focused on fundamentals of the ionization process and discovery of new matrixes which had been too volatile to survive the time constraints of the vacuum (v)MALDI source introduction (described later), MSTM focused on achieving automated high throughput, while maintaining the advantages of a vacuum-probe.11,12 In order to give the reader a feel for the analytical power of the new ionization technologies described herein, we begin with the developments relative to high throughput vMAI.
The performance of the manual vacuum-probe on the Q-Exactive Focus12 convinced MSTM to develop a high throughput vMAI source using a novel sample introduction approach. The success of the probe sources,11,12 even though limited to single samples, inspired a new plate source (Figure 1) concept for sample introduction to vacuum with the potential for sensitive, robust, and high throughput analysis using vMAI. In spite of the promise of the plate source concept, the version shown in Figure 1 was limited in that great care had to be exercised to prevent venting the instrument, cross contamination and carryover, and additionally, reproducibility was generally poor, and the limitation to alternating two samples. However, the approach was promising, and because this vMAI concept, in theory, can be applied to any atmospheric pressure ionization (API) mass spectrometer (vendor neutral) providing capabilities of high mass resolution, accurate mass measurement, MS/MS, and ion mobility spectrometry (IMS)-MS technology for structural characterization studies, MSTM applied for and was awarded NSF SBIR Phase I grant. The roadblocks were successfully addressed during the NSF SBIR Phase I award period (Figure 2).
Figure 1.
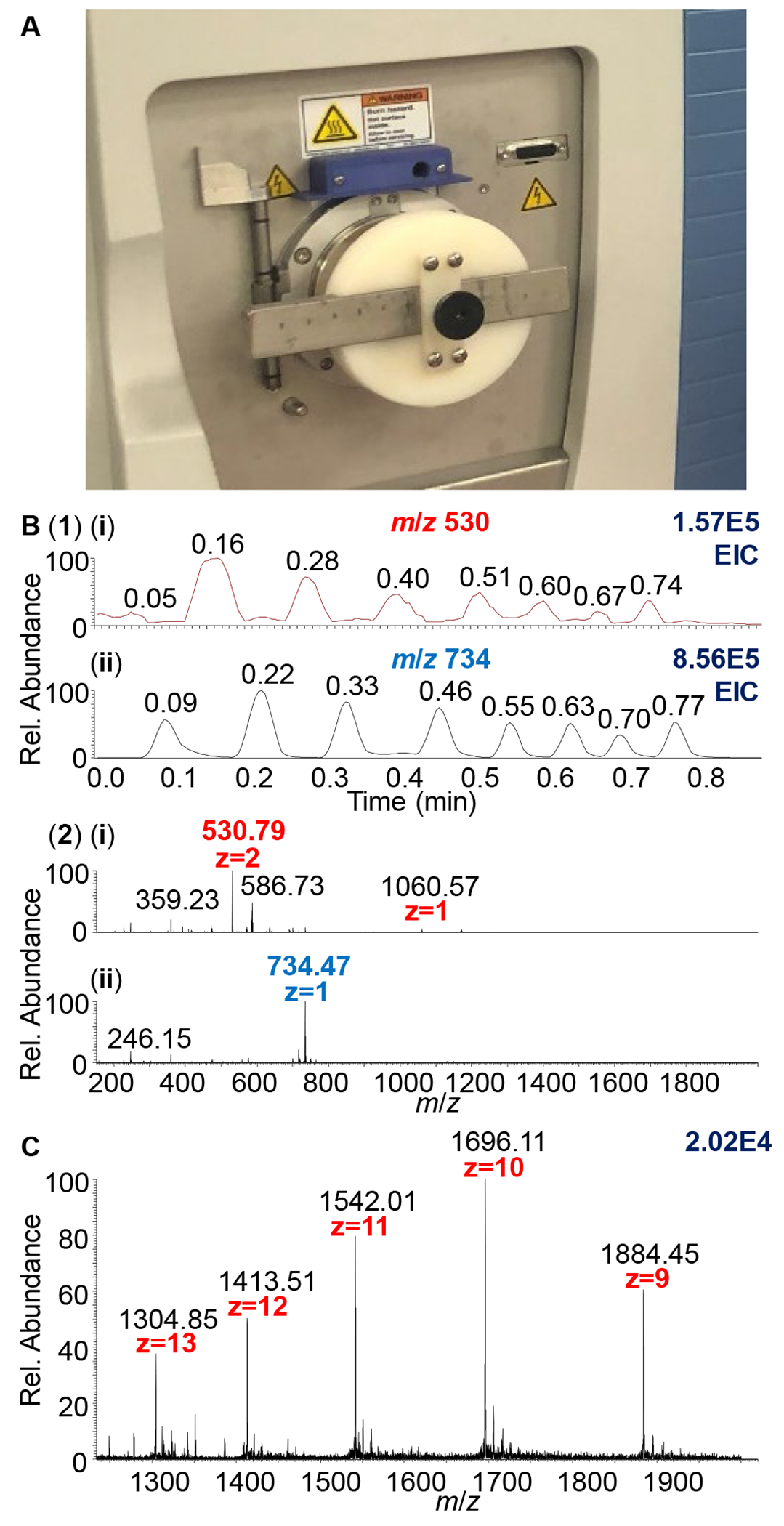
Sampling by vMAI-MS directly from AP into sub-AP of the mass spectrometer. (A) Photograph of the source setup using plate introduction in which each matrix:analyte sample is exposed to the sub-AP in successions; (B1) Chronograms displaying 2 samples acquired in less than 4 s per sample and (B2) mass spectra of the two compounds: (i) bradykinin and (ii) azithromycin. (C) Mass spectrum of the multiply charged ions of myoglobin, molecular weight ~ 16.9 kDa.
Figure 2.

(A) MSTM vMAI/vMALDI prototype manual rapid analysis vacuum source; (1) valve plate, (2) sample plate (glass slide required for TG vMALDI), (3) spacer plate with channels therethrough, (4) channel positions where samples applied to sample plate align, (5) laser beam (represented by pointed blue arrow) aligned with channel in flange (not shown) to vacuum for TG vMALDI. Ionization in vMAI occurs when a spacer plate channel aligned with a vMAI sample aligns with the flange channel and thus exposed to vacuum. Multisample plates are exchanged in ca. 2 sec. (B) vMAI mass spectrum of 2 picomoles of ubiquitin in detergent using 3-NBN as matrix applied to a glass slide. Instrument Q-Exactive Focus. Movie-S01 is included to demonstrate the acquisition of myoglobin in detergent using the plate vMAI/vMALDI source.
A number of modifications were made to address issues of inconsistency, carryover, and ease of instrument venting. Two important findings were (1) airflow used to influence the rate of ionization and sweep ions, charged clusters, and clusters toward the mass analyzer was inconsistent and chiefly responsible for inconsistent results, and (2) incorporation of a spacer plate between the sample and entrance to the vacuum of the mass analyzer essentially eliminated carryover and instrument contamination. The successful development and implementation of the plate source concept (Figure 2A) has allowed vMAI analysis speeds of 4 sec/sample including the time required to load samples using 8-well sample plates.13 Future applications of the plate source may be enhanced by the finding that almost all compounds which sublime at an appreciable rate when placed in sub-atmospheric pressure (sub-AP) conditions produce gas-phase ions opening the potential for selective ionization.14 Two contributions in this Biemann Special issue provide detailed information toward innovative instrumentation, new measurement capabilities, and fundamentals that can be now studied.13,14
Although a laser adds complexity and costs, MSTM later expanded the plate vacuum ionization source to include vMALDI (Figure 2A).13 The new plate vacuum ionization source with the two modes of operation (vMAI/vMALDI) operates directly from AP while only the sample being ionized is at the vacuum of the first vacuum stage of the mass spectrometer. The combined vMAI and vMALDI plate source gives either singly (vMALDI) or multiply charged (vMAI) ions with the same mass spectrometer and no waiting time for the sample to be introduced to vacuum through a vacuum lock. Samples can be loaded on a glass slide and affixed to a spacer plate with channels therethrough so that each sample fits within a channel. This sample plate assembly (sample and spacer plates) can displace another sample assembly plate, or a valve plate, by sliding over the flat flange face. Samples are sequentially be exposed to vacuum when the channel it resides within is over the opening in the mass spectrometer inlet flange. The spacer plate eliminates cross contamination caused by samples in wells of the sample plate having direct contact with the flange surface. A key concept is that the sample plate assembly remains at atmospheric pressure (AP) while samples isolated by the spacer plate channels are individually exposed to vacuum. Spacer plates can be rapidly replaced without venting the instrument if contaminated, which is not possible if the flange surface were to become contaminated.
The plate multi-mode vacuum ionization source of vMAI and vMALDI uses a flange component that is rather simple in concept as it consists of the flange having a flat surface with an opening (ca. ¼”) to the instrument vacuum. Details are included in ref. 13. Vacuum is maintained by having flat plates slide over the flat flange surface. Ionization commences with vMAI when a matrix:analyte sample experiences vacuum and with vMALDI when the matrix:analyte sample in vacuum is ablated by the laser beam. With vMALDI, analysis speeds of one sample per second with full mass range acquisitions have been achieved.13,15 In an interesting example of the complementary nature of the two ionization methods, polyethylene glycol (PEG)-2000 and ubiquitin were mixed and applied on adjacent positions on a glass slide, one sample using 2,5-dihydroxyacetophenone (2,5-DHAP) as matrix, and the other 3-NBN.13 A nitrogen laser was used to ablate the 2,5-DHAP prepared sample (vMALDI), and the 3-NBN sample spontaneously produced gas-phase ions when exposed to sub-AP (vMAI). With 3-NBN, multiply charged ions of ubiquitin were observed, but no PEG ions, and with 2,5-DHAP, singly charged ions of the PEG sample were observed but no ions from ubiquitin, possibly because with MALDI, they are out of the instruments’ mass range. The acquisitions were obtained just a few seconds apart. This new vMAI plate source was also used to investigate the applicability of analyzing e.g., proteins in the presence of detergents in collaboration with Dr. Vladislav Lobodin (BASF Corporation). Highly charged ubiquitin ions (Figure 2B) and even out of a variety of different detergents, without dilution, were ionized in a straightforward manner. Movie S-01 shows ubiquitin in detergent solution, acquired in overall just under 10 seconds, is included to visualize this point, as it is nothing short of astonishing! Future applications of the plate source may be enhanced by the finding that almost all compounds which sublime when placed in sub-AP conditions produce gas-phase ions. At present, the ions observed from most subliming matrices, at least when no analyte is present, are unknown, but this may suggest the potential for finding matrices for selective ionization.14 Two contributions in this Biemann Special issue provide detailed information toward innovative instrumentation, new measurement capabilities, and fundamentals that can be now studied.13,14
MSTM’s current aim is to build an ultimately simple, highly robust, high throughput, automated source based on vMAI and vMALDI capitalizing on high speed complementary analyses. Meanwhile, work in progress in the Trimpin group (Figure S1) is to implement the vacuum source to optionally include vMAI, vMALDI, and vacuum laserspray ionization (vLSI), including laser alignments both in transmission geometry (TG), and reflection geometry (RG) (the default laser configuration with commercial instruments), based on the plate source concept while offering inlet ionization and ESI capabilities. This next generation multi-mode ionization source, ideally available on any vendor’s mass spectrometers, has the potential to be transformative in how direct ionization is achieved with high speed, sensitivity, and robustness with the full scope of capabilities of state-of-the-art mass analyzers, including tandem mass analyzers, IMS interfaced with MS, and with collision induced dissociation (CID), electron transfer dissociation (ETD) and other fragmentation technologies. Such developments could not have been imagined just a few years ago. In the next sections are described how we systematically developed the new technologies based on the new ionization processes.
Lesson 1:
A novel ion source makes vacuum ionization as simple to use as ambient ionization, but with unprecedented speed and robustness ref. 13
Multi-mode ionization sources are cost-effective and analytically exceptional in information coverage, as shown in more detail in ref. 13
Exciting new fundamental research can now be performed to decipher the basics associated with the new ionization processes because the same mass spectrometers can be used under well-functioning conditions for the specific method of interest. Initial progress is detailed in ref. 14
Chemical, clinical and biological applications for sensitive analyses can rapidly be achieved with new and established ionization methods
2. Implementation of Imaging MS using Transmission Geometry Laser Ablation and Unexpected Observation of Multiply Charged Ions
Our research broadly relates to developing technology for molecular characterization of surfaces to provide spatially resolved chemical information of biological and synthetic materials using MS. While surface chemistry is economically extremely important, molecular methods of surface characterization, including the molecular structures of organic and inorganic compounds in surface layers, healthy versus diseased tissue, etc. lag behind materials characterization in solution. Lack of methods with high mass and spatial resolution, as well as sensitivity capable of characterizing complex surfaces and surface reactions are limiting developments in surface characterization, clinical chemistry, and ultra-fast analyses, as examples.
Looking back, the start of the exciting ionization inventions was a relatively trivial idea to improve tissue imaging.16,17 The hypothesis was that high resolution and fast imaging MS directly from AP could be improved using TG-MALDI. Solvent-free sample preparation was used to minimize alteration to the tissue and maximize the chemical information as well as minimize the time of analysis to better study and understand complex organs such as brain function, in collaboration with Michael Walker (Indiana University, Psychology and Brain Sciences), and precise and human error independent assessment of cancer boundaries. TG using LD and MALDI had been performed previously at vacuum and AP.18,19 A “field-free TG-AP-MALDI” proposal by Trimpin eliminated the voltages applied to extract the ions to overcome so-called “rim losses”20,21 as well as offering simplicity to the set-up and experimental execution (Figure S2).16,17 Unlike previous TG-MALDI results, initial voltage-free experiments produced reasonably abundant analyte ions almost immediately from small molecules such as drugs and lipids using the MALDI matrix 2,5-dihydroxybenzoic acid (2,5-DHB).16,17 Elimination of voltage was achieved by placing the point of ion production close (1-5 mm) to the inlet of the mass spectrometer in which the ablated material is flow entrapped into the sub-AP of the mass spectrometer vacuum through the restricted inlet. Unexpectedly for a MALDI-like setup, multiply charged ions of peptides and even proteins were obtained.16,22–25 Only in the presence of high voltages were singly charged ions observed, additionally to the multiply charged peptide ions (Figure S3). Dr. John Callahan (Navel Research Labs) and collaborators used voltage with AP-MALDI and observed only singly charged ions, as expected.19 In our experiments, undesired metal cation and matrix attachments to the singly charged ions, and an increase in chemical background, were observed using higher voltages (Figure S3). The use of solvent-free matrix preparation led to ease in sample preparation and potential improvements in imaging experiments16,17 but also the observation of only singly charged ions.22 This was the first indication of the importance of the solvent, especially the protic solution component, and possibly many other ionization processes relative to the formation of multiply charged ions.
The implementation of MS imaging was a success26–28 for singly and even for the surprising case of multiply charged ions using Thermo mass spectrometers.29 Fast single-shot-one-mass spectrum output16,17 along with multiply charged ions22,23 nearly identical to electrospray ionization (ESI) from a MALDI-like experiment were initially observed using the MALDI matrix, 2,5-DHB, and a ~250 °C Thermo inlet tube. Spatial resolution of ca. 20 μm was achieved.3,17,26,27,30 In brief, an automated X,Y,Z-stage was used to manipulate tissue sections3,28,29 on a glass slide relative to the inlet aperture of the mass spectrometer and laser beam to obtain a series of mass spectra from which an image can be constructed for any mass-to-charge (m/z) value. Different sizes (including lengths, diameters, and shapes) and materials (various metals and glass) inlet apertures, commercial or homebuilt, were studied relative to ion abundance in collaboration with Drs. Jeff Brown (Waters), David Clemmer (Indiana University), Donald Hunt (University of Virginia), Barbara Larsen (DuPont), Alexander Makarov (Thermo), Alan Marshall (Florida State University), Charles McEwen (University of the Sciences, now Saint Joseph’s University, SJU), Paul Stemmer (WSU Institute of Environmental Health Sciences), Steffen Weidner (Bundesanstalt für Materialforschung und –prüfung, BAM, Germany). Even a 1-m long extended stainless steel inlet tube provided gaseous ions of the analytes.4 The quality of imaging results, and the spatial resolution, are typically limited by sensitivity. An increase in sensitivity is known to be associated with vacuum MALDI,31,32 although the ionization efficiency of MALDI is substantially lower than with ESI.33,34
Initial fundamental research with a laser on an API mass spectrometer demonstrated the importance of inlet temperature,22,23,35,36 as well as collisions with surfaces such as gases and obstructions in the production of ions,36–40 and subsequently to the term “laser (as in MALDI) spray (as in ESI) ionization” or LSI.23 Because of the production of multiply charged ions originating from a solid surface, both CID and ETD from mass-selected gaseous analyte ions were demonstrated.23,30,40,41 As one example, multiply charged myelin basic protein (MBP) peptide ions from mouse brain tissue were gas-phase sequenced for the first time by a LSI-ETD approach.30
Lesson 2:
Fast imaging from AP with good spatial resolution using MS was achieved, but this outcome while useful is not transformative
The breakthrough was that ESI-like ions are observed from MALDI-like experiments
The mechanism is an important unknown
“If at first the idea is not absurd, then there is no hope for it.” Albert Einstein42
3. Discovery of a New ‘Ion Source’: The Heated Inlet Tube
Mass spectrometers and IMS-MS instruments that operate without a heated inlet tube were initially difficult to use with the new ionization processes providing only poor analyte ion abundance, even with retrofitted homebuilt inlet tubes.24–26,28,38,40,43–46 An improvement in the ion abundance was accomplished using a 90° bent inlet tube, possibly forcing the material to collide with the surface,43 however, the restricted airflow prohibited long term use for, e.g., imaging applications (Figure S4). Key factors in obtaining analytically useful results early on were a heated inlet tube, collisions (Figure S5), and the matrix used.23–30,35–40,43–47 For example, using the MALDI matrix 2,5-DHB, and especially with the less volatile α-cyano-4-hydroxycinnamic acid (CHCA)48 and sinapinic acid (SA) matrixes, a matrix dependent inlet tube temperature between 200 and >400 °C was necessary to initiate efficient ionization.40,45 Switching from 2,5-DHB,22,23,43 a MALDI matrix,49 to the more volatile 2,5-DHAP, also a MALDI matrix50 (Scheme S1), allowed ionization with lower inlet tube temperature including observation of ESI-like multiply charged ions.51 Nevertheless, reliable ion formation, especially for imaging applications, remained a difficult task on certain mass spectrometers equipped with skimmer cone inlets, even with the 2,5-DHAP matrix.44
At the time, we assumed the laser was responsible for the ionization,35 and thus a number of experiments did not make sense. The undergraduate student, Christopher Lietz (WSU), for example, reported that when he accidentally touched the inlet tube with the matrix:analyte applied to a glass plate, better analyte ion abundance was obtained as compared to using solely the laser. The graduate student, Beixi Wang (WSU), accumulated results with a neodymium-doped yttrium aluminium garnet (Nd:YAG) laser provided by Dr. Arthur Suits (WSU). The laser used at 1064 nm, did not work well with the known MALDI conditions e.g., glycerol, but when she used 2,5-DHAP (Figure S6) matrix instead, which absorbs in the ultraviolet (UV), significantly better results were obtained. Essentially the same ion abundance, charge states, and drift times were observed at 1064, 532, and 355 nm using the same UV matrix with standards and mouse brain tissues provided by Dr. Ken Mackie’s laboratory (Indiana University, Psychology and Brain Sciences).40,47
Directly after a conversation between Trimpin and McEwen discussing these and other seemingly “odd” results from the McEwen lab, such as the ionization continuing after the laser was off, it seemed that the laser may not be responsible for ionization to occur. McEwen then went to his laboratory and tapped a glass plate, with the matrix 2,5-DHB and a peptide analyte sample applied, against the heated inlet tube of a Thermo Exactive mass spectrometer without the use of a laser. He immediately called back with the news that the laser was, in fact, not necessary. A flurry of experiments were initiated by both laboratories to introduce the analyte in a variety of new ways to the inlet of the mass spectrometer (through space by vibrations, acoustic, a punch device, as well as directly by tapping, etc.)10,52–54 and to better define mechanistic aspects, some of which have been covered in previous papers.3–9,40,55–58 In one example, the graduate student, Vincent Pagnotti (SJU), substituted the laser and glass microscope slide with a ‘BB gun’ and metal sample plate as a visual demonstration that almost identical mass spectra were obtained by either the BB or laser approaches.52,55 The successful outcome of forming highly abundant multiply charged analyte ions, unequivocally demonstrated that the laser wavelength has no influence on the experimental results other than delivering the matrix:analyte sample into the inlet tube of the mass spectrometer, and thus, explaining the previously “odd” results. Other examples of experiments to better define this new ionization method include gas pressure (above and below AP) and gas type (Figure S7) with and without the laser, in the positive and negative modes as a means to improve the experimental outcome.38,40,45 These experiments in sum proved successful in increasing the analyte ion abundance, but none provided the desired breakthrough for analytical utility with mass spectrometers not already equipped with a heated inlet tube ‘source’.40,43–46 Consequently, a heated inlet tube used with some API mass spectrometers is an effective ion source, but the path forward,24–27,41,53–59 has not always been straightforward. It was our understanding at that time that mass spectrometers with skimmer cone inlets were not as analytically useful using inlet ionization as mass spectrometers with heated inlet tubes.
The evolvement of the TG alignment for being possibly associated or responsible for the unusual observations of multiply charged ions was eliminated by using a MassTech AP-MALDI source in collaboration with Larsen, which uses the laser alignment in RG.51 Other experimental designs were tested to improve our understanding and the utility of the formation of multiply charged ions directly from surfaces. In 2013 McEwen’s group demonstrated the use of LSI to simultaneously image lipids, peptides, small proteins and drugs.60 The same year Dr. Rainer Cramer (University of Reading, UK) and Dr. Klaus Dreisewerd (University of Münster, Germany) reported the use of liquid matrixes for AP-MALDI using a heated ion transfer tube on a AB SCIEX Q-Star Pulsar instrument and obtaining multiply charged ions of peptides and proteins.61 In 2018, a miniature ion trap mass spectrometer was coupled with LSI by Dr. Wei Xu’s group (Beijing Institute of Technology) for the direct detection of peptides, proteins, and drugs in complex samples such as blood and urine, and lipids from tissues.62 The same group, then adapted this setup to a 2-dimensional (2D) moving platform for high throughput analysis. Drugs, vitamin B, and peptides were analyzed in diluted blood demonstrating a speed of 10 seconds per sample.63 Direct bacterial analysis was demonstrated with this system; 21 bacteria were differentiated at both the genus and species classification utilizing orthogonal partial least squares (OPLS).64
Lesson 3:
Confirmation that the laser is not responsible for analyte ionization (therefore not “MALDI” but MAI)
LSI is MAI with use of a laser to transfer matrix:analyte into the ionization region with high spatial resolution
Confirmation that gas flow, collisions, and heat matter
The research goal is to make LSI and MAI analytically useful and mass spectrometer vendor independent
4. Expanding our Fundamental Knowledge of the New Ionization Processes Leading to Unique New Ionization Methods With and Without a Laser from Solids and Liquids
Studies that form key pillars in the path of these innovative technology developments were made essentially at the same time. Both developments grew, at least in part, out of the success of the more volatile matrix 2,5-DHAP, relative to 2,5-DHB (for more detailed information about matrixes see Scheme S1), that gave good results on the AP-MALDI source used under LSI conditions.51 First, we describe developments of vLSI in Trimpin’s laboratory,65 and second, solvent-assisted ionization (SAI) in McEwen’s laboratory,56 and from each, we briefly expand on some of the current uses and further developments by the community.
Using the commercial intermediate pressure (vacuum) MALDI source of a Waters SYNAPT G2 mass spectrometer and 2,5-DHAP as matrix gave exceptionally clean and highly abundant multiply charged ions upon laser ablation.40,65–67 Hence, no inlet tube is necessary when using the ‘right’ matrix in what again seems like a MALDI experiment. Because of the formation of multiply charged ions, the mass range is extended and because of the fundamentals that were already established from AP, we used the term vLSI.65 Our hypothesis is that an important difference between LSI and MALDI in forming ESI-like multiply charged ions is the volatility of the matrix. This was shown by us, and others.40,51–54,58,68–70 During these studies an even better performing matrix, 2-nitrophloroglucinol (2-NPG), was discovered66 and successfully used for the first imaging applications of several multiply charged MBP peptide ions from a mouse brain tissue using an intermediate pressure MALDI source and the laser in RG alignment.71 The applied laser fluences and voltages were kept low to increase the relative ion abundance of the multiply charged ions.40,45,51,65,68,71 Because of the relatively high volatility of 2-NPG, but the good ion abundance of multiply charged ions, we decided to use a mixture of matrixes, adding 90% of 2,5-DHAP and 10% of 2-NPG, thereby reducing the volatility of the matrix composition and allowing molecular imaging of tissue samples that intrinsically require longer acquisition times.71 IMS-MS was fundamental to enhance the separation of lipids, peptides, and proteins directly from the tissue.65,67,71 These results were encouraging, but still with the limitation of the unintentional matrix sublimation of 2-NPG in a vacuum source.
We, and others, have shown the utility of the new ionization processes for surface analyses and imaging in the positive and negative mode, in RG and TG modes, and from AP and intermediate pressure for lipids, polymers, peptides, and small proteins. As an example, using vLSI, we developed methods to detect and image gadolinium-containing complexes synthesized in the laboratory of our collaborator, Dr. Matthew Allen (WSU). These complexes are developed for potential use as contrast agents for magnetic resonance imaging (MRI).72,73 Traditional MALDI-time of flight (TOF) mass spectrometers were limited in analyzing these compounds. Using the commercial intermediate pressure MALDI-SYNAPT G2 with MALDI matrixes either failed, or produced significant background, fragment ions, and less resolved ions as compared to the 2-NPG matrix in terms of mass resolution (e.g., MALDI 25,312 and LSI 35,711, Figure S8), stressing the importance and broader applicability of 2-NPG as a new matrix. A variety of complexes with different side chain functionalities were analyzed. In some cases, gadolinium complexes associated with matrix ions. The interpretation of the formation of an ion consisting of matrix adduction to analyte was verified by MS/MS (Figure S9). It is hypothesized that the nitro group of the matrix interacts as an additional ligand to the metal center.74 Further studies will be required to understand the fundamentals as it may reveal important mechanistic information of the synthesized structures as well as the ionization mechanism using this and other matrixes in MS.
Using an MS imaging approach, we analyzed mouse brain tissue sections in which a synthesized gadolinium(II) texaphyrin was applied to the tissue surface after sacrificing the animal. According to previous studies in which a multiple imaging approaches were used to visualize the location where the compound resides/accumulates.75 The 2-NPG/2,5-DHAP binary matrix was spray-coated71 onto the tissue section and the image acquired using laserspray conditions. The obtained MS images reflect the isotopic distribution of gadolinium with m/z’s ranging from 917 to 925 (Figure S10) by both the relative ion abundances of the isotopic distributions and the ion intensities of the images. This is contrary to e.g., the typical lipids composition, in a similar m/z range. This relationship between isotopic versus image intensities serve as additional verification that this is indeed the synthesized gadolinium-containing product signal without the necessity of performing MS/MS for confirmation. No other gadolinium-containing isotopic distributions were obvious suggesting initial biological stability of the potential MRI contrast agent in biological applications. The location of this chemical within the mouse brain tissue section matched well with that of the myelin areas of the brain obtained using other imaging approaches.75
The matrix 2-NPG is, to date, one of the most valuable vacuum ionization matrixes in conjunction with the use of a laser. With this matrix, high vacuum MALDI-TOF mass spectrometers were used to ionize and detect the highest charge states so far reported under high vacuum conditions, and even in the reflectron detection mode.45,58,66,68 Such results were highly sought after since the 1990s.76–78 In 2014, Dr. Lingjun Li’s group (University of Wisconsin-Madison) reported on the use of LSI using 2-NPG on an LTQ-Orbitrap XL mass spectrometer with an intermediate pressure MALDI source and CID as well as high-energy collision dissociation (HCD) for in-situ protein identification, visualization and characterization.79 The 2-NPG matrix reinforced our hypothesis that multiply charged ions require more volatile matrixes being capable of rapidly subliming or evaporating from multiply charged gas-phase matrix particles containing analyte as discussed in a 2012 mechanism paper.40
The need for volatility to obtain multiply charged ions and the need for stability for long imaging experiments are counter to each other. Binary matrix combinations have somewhat been able to improve the stability without interfering too much with abundances of multiply charged ions. However, the key to successful imaging with more volatile matrixes is to align the laser in TG for shortening the overall acquisition time (single-shot-one-mass spectrum to give a pixel). Initial imaging successes have been reported by several groups using 2,5-DHAP and 2-NPG, respectively, with vacuum MALDI instruments (Orbitrap; TOF).79–81 As examples, Dr. Richard Caprioli’s group (Vanderbilt University) demonstrated the utility of the use of TG-MALDI and 2,5-DHAP matrix achieving protein images with 1 μm resolution.82 Dr. Kermit Murray’s group (Louisiana State University) included the use of two component matrix using 2-NPG and silica nanoparticles, producing multiply charged ions from tissue on a high vacuum MALDI-TOF.83 In a more recent study by Dr. Jeongkwon Kim’s group (Chungnam National University),84 2-NPG compared with other MALDI matrixes, demonstrated the ability to generate multiply charged ions of proteins such as bovine serum albumin (BSA +4 charge states) and immunoglobulin G (IgG +6 charge states). The addition of hydrochloric acid (HCl) enhanced the absolute protein peak intensities producing more abundant charge states,84 as previously reported for new matrixes in MS.40,45,59,85 In the IgG study using linear detection mode, the typical wide unresolved molecular ion peaks (width ca. 10000 in m/z) associated with metastable ions and matrix and metal cation adductions, typical for a MALDI process,86–89 were observed. In contrast, intact protein ions, e.g. BSA, were also detected highly charged (+14) in the reflectron mode, and the peak width (+9 charge state) had a more analytically useful signal width of ca. 600 in m/z.68 The success is attributed to the softness associated with these new matrixes leading to intact and not metastable molecular ions or fragment ions typically observed with MALDI. MALDI is therefore a harder ionization method than LSI. These matrices, especially those without hydroxyl functionalities, show a general trend of not attaching matrix and metal cations.1,10,14,29,40,45,66,68,71,90
Another key pillar was the discovery of SAI in McEwen’s laboratory.56 Vincent Pagnotti employed solvents used to dissolve analyte(s) as the matrix. Considering how often ESI is used in conjunction with a heated inlet tube, it is amazing this was the first reported example in which a solvent was introduced into an inlet tube to a mass spectrometer, preferably heated, to cause ionization of the analyte contained in the solvent without application of voltage (as in ESI91) or high gas flow (as in sonic spray92).52,56,93 In retrospect, this is a variation of MAI, in that a matrix is used but is in the form of a solvent instead of a solid phase compound. In an early example, the solvent with the sample was introduced using a silica capillary into the inlet tube showing ESI-like multiply charged ions.52,53,56,93–97 It has been shown that it is unnecessary to have e.g., a fused silica tube “inside” of the inlet tube (the source). Flush or “through space” configurations of sample delivery is sufficient, although typically less sensitive.59 There is also no need to add a solid or liquid matrix as was shown by ionizing small and large molecules “neat”.40 Adding matrix, however, greatly increases the sensitivity of the experiment. A variety of methods to introduce the samples follow, and a few are summarized with photos (Figure S4 and S11) including, e.g., lasers and liquid junctions.3,4,7,9,46,52,53,94,96 Coupling SAI to liquid chromatography (LC)/MS was demonstrated, separating and ionizing components from a tryptic digestion of BSA, as well as analysis of steroids with improved sensitivity relative to ESI.94–100 High (ca. 100 μL) to low (ca. 0.2 μL) flow rates were shown to be applicable.52,56,95,98–104 An alternative to voltage-free SAI is application of voltage to the solution being introduced into to the heated inlet tube through fused silica tubing. The sensitivity and breadth of compounds ionized/analyzed was improved relative to SAI or ESI by the voltage (V)SAI method.52,93,95,101–104 VSAI (or ESII) appears to operate through both the SAI and ESI mechanisms.102 Surface analysis without the need of a matrix was demonstrated by capturing material laser ablated from a surface in a solvent flowing directly into a heated inlet tube. The laser provides spatial resolution and without addition of a matrix, solid or solvent, directly to, e.g., a tissue surface, maintaining the spatial location of compounds;3,9,94,103 Dr. Shubhashis Chakrabarty (USDA Bioscience Research Center) in this Biemann Special Issue reported on the use of electrospray ionization inlet, termed VSAI in this perspective, for the quantitative analysis of veterinary drugs (LOQ for zilpaterol, 100 pg/g) and PFOS/PFHxS (LOQ 0.4 – 50 ng/mL) in animal tissue and fluids without chromatographic separation using MS/MS. The quantitative results for LC/ESI-MS and ESII-MS/MS for the drugs ranged from 0.993 – 0.999 for the tested tissues and urine.104
An automated X,Y,Z-stage was used not only in imaging tissue sections,3,28,29 but also, e.g., in the automated analysis of well plates using the SAI approach.59,94 A motion-controlled arm was used to sequentially move an array of pipet tips containing the respective samples which were obtained from a well plate just prior to MS analysis. This approach readily detected the different sample solutions, and their relative concentrations were displayed using an ion intensity map (image of an m/z value of interest in a targeted or nontargeted approach). The gas flow and sub-AP draws the liquid from the pipet tips into the inlet through space. This mist typically leads to ‘splashing’ and therefore undesired deposition on the outside of the inlet leading to carryover, unless, e.g., the use of pipet tips containing wash solvent are applied between acquisitions.
The use of solvents as matrixes is the basis of ESI and SAI. Solvents expand the range of matrixes available to inlet ionization. McEwen’s laboratory early on demonstrated a variety of unique introduction methods e.g., the eye of a sewing needle was used to sample an analyte solution,52,53 as is demonstrated using the liquid matrix glycerol without voltages or a laser on an LTQ Velos with an inlet tube at relatively high temperature (Figure S12A and Table S1). Likely the easiest manner to ionize single samples has been use of pipet tips, as is shown for the date rape drug gamma-hydroxybutyric acid (GHB) in collaboration with Samantha Leach (Department of Forensic Sciences, Washington, DC). Data was acquired in the negative mode on an LTQ Velos at 50 °C inlet temperature using the solution as supplied (Figure S12B). In most of the cases, though not surprising, the diverse variations of SAI have been accomplished using Thermo mass spectrometers that are already equipped with a heated inlet tube. At the ASMS Conference in 2011, McEwen’s group introduced a liquid bridge SAI-surface sampling methods to analyze two and three-dimensional objects using a flow of solvent delivered to a sample under ambient conditions and then introducing the solvent/sample directly to the mass spectrometer inlet (Figure S13).52,53 The following year at ASMS, Wang presented this concept analyzing a drug-treated mouse brain tissue using continuous and discontinuous (discreet) flow.94,105 An improved system using the manual Ionique platform, a syringe pump, and a gutted ballpoint pen was presented by our group in collaboration with MSTM in Trimpin’s ASMS talk from 2016 (Figure 3).106 The pen, constructed by Sara Madarshahian (SJU), held two fused silica capillaries together so that one delivered solvent and the other transferred the solvent to the inlet of the mass spectrometer where ionization occurs. Simply touching a surface of a sample (e.g., tissue) and then collecting dissolved compounds using a liquid junction approach,9 or by sequential transfer of defined extraction droplets to the inlet of the mass spectrometer allowed surface analysis of extracted compounds,3,9,106 similar to earlier work, just more stable and therefore easier and effective. For instruments without a heated inlet tube, it was effective to construct an inlet tube to ionize nonvolatile compounds even from 100% aqueous solution.37,46,52,53,56,59,93,94,98,101 Eventually we learned to analyze samples by SAI on Waters mass spectrometers, e.g., a Waters SYNAPT and Xevo mass spectrometers.7,9,46,93,98,95,107,108 Inlet tubes (not extensions) varied in length from ca. 1 mm to over 1 m with materials ranging from stainless steel, copper, and glass.3,4,9,41,43,93,98,107,109 In one example, a homebuilt heated inlet capillary (3” x 20 gauge) coupled to a Waters Xevo-TQ-S was used, and with this modification peptides and proteins were acquired,9 and with high ion abundance for 50 fmol of lysozyme using 0.1% formic acid (charge states +7 to +9) and 1% proprionic acid, (charge states +7 to +14), respectively (Figure S14). Addition of super-saturated gases into the sample solution,110 increased ion abundances. The addition of solid co-matrixes (e.g., 3-NBN) to the solvent matrix have been shown to lower the temperature requirements and, in turn, improve the quality of the mass spectral results. Likewise, volatile organic solvents reduce the inlet temperature required with SAI. McEwen’s group studied the softness of the new ionization processes relative to ESI on the same mass spectrometer; SAI was softer than ESI and MAI when the inlet tube was heated.94
Figure 3.

Analysis of surfaces with solvent-assisted ionization using the MSTM Pen. The mass spectrum shows in red the pesticide thiabendazole m/z 202.04; the highest abundance of the pesticide was found in the stem area of the apple. Inset: photograph of the setup: (1) manual MSTM platform with (2) fused silica capillary from the (3) MSTM Pen sampling of the (4) surface of an apple. Data were acquired on Thermo Orbitrap Exactive mass spectrometer. See a more detailed description of the MSTM Pen in Figure S1C inset, Movie-S04 and compare with Figure S13.
Different means to ionize and introduce liquid samples into the inlet of the mass spectrometer have been described by others. Interested in the chemistry in aerosol droplets in the atmosphere, Dr. Murray Johnston’s group (University of Delaware) demonstrated the influence of an inlet tube heated to 950 °C by customizing the inlet cone of a SYNAPT G2S.111 Their system uses an atomizer to generate droplets that later are introduced into the modified heated inlet tube where the ionization process occurs; this method is called droplet-assisted ionization inlet (DAII).111 Fundamental details were also provided.112 Another approach, a voltage-free ionization method presented by Drs. Stephen Valentine and Peng Li (West Virginia University), called “vibrating sharp-edge spray ionization” (VSSI), uses a piezoelectric transducer to generate an aerosol from the sample placed on a glass slide and placed close to the inlet tube of the mass spectrometer.113 The transducer is operated at low voltage input (12.7 to 30.6 V, peak to peak) and low power consumption (~150 mW) compared with other ultrasonic114 and surface nebulization methods.115 In the VSSI approach, the high-frequency vibration at the sharp-edge is proposed to cause the detachment of small liquid droplets from the bulk fluid, resulting in a continuous spray.113 This group optimized VSSI to be used as an interface to LC or other continuous flow-based applications by attaching a piece of fused silica capillary on the VSSI, called cVSSI,116 resulting in the ionization of metabolites, peptides, and proteins comparable with LC-ESI-MS. cVSSI was later improved to be a useful method for native MS in negative mode, delivering the liquid sample and providing a good signal with a minimal amount of sample at flow rates of 0.2 to 1 μL min−1.117 The group is working to elucidate the ionization mechanisms involved and obtain a direct comparison with ESI using water and methanol as solvents, as is described in this Biemann Special issue.118 The surface acoustic waves (SAW) processes have been used in the electronic industry119 and more recently have been adopted in MS.120–122 Kostiainen’s group (University of Helsinki) presented the ambient method solvent jet desorption capillary photoionization (DCPI) which combines two processes to analyze polar and non-polar compounds.123 A solvent jet is directed onto a sample surface, and the extracted ions are ejected and directed into a heated extended inlet tube on an Agilent 6330 ion trap mass spectrometer where photoionization by vacuum UV (VUV) photons takes place. Testing different molecular weight compounds, the group concluded that there are two ionization processes occurring, for small molecules via photoionization and for larger molecules ionization is by SAI.
Other groups are now working with inlet ionization methods for use as, e.g., surface analyses tools, specifically with the help of a heated inlet tube. The advantage of this approach is the easy manipulation of samples (liquid or solid) outside the mass spectrometer. One example with important clinical applications is the MasSpec Pen by Dr. Livia Eberlin’s group (The University of Texas at Austin).124,125 A liquid junction was used in combination with SAI to detect cancerous tissue on a Q-Exactive Hybrid Quadrupole-Orbitrap mass spectrometer. Using a stop flow of water to sample the surface of the tissue, then taking advantage of the pressure differential, the sample/solvent is drawn into the inlet tube of the mass spectrometer.124 A modified MasSpec Pen has been integrated into the da Vinci surgical system125 and is sold to Genio Technologies, Inc.126 Dr. Akira Motoyama’s laboratory (Shiseido Co., Ltd.) attributed the ionization mechanism of zero volt paper spray to SAI.127
The methods developed for introducing samples directly into the AP inlet aperture are attractive for their simplicity, but in practice, there could be issues of instrument contamination and carryover between successive samples, similar to other solvent-based API methods used in MS.
Lesson 4:
A matrix can be a solid or solvent or combinations
Volatility of the matrix is important, especially for producing multiply charged ions, even from vacuum
The new ionization processes allow truly different ion sources such as simply an inlet tube which can be extremely short or surprisingly long
SAI developments have seen the greatest expansion, but progress in MAI may change that
5. Fundamentally-Driven Arguments Leading to the Surprising Discovery of Ionization Seemingly Not Possible
In this section we first describe the discovery of the “unthinkable”, at the time, using intermediate pressure (vacuum ionization conditions) on an intermediate pressure MALDI source.1,2 The concept was later transferred to AP (inlet ionization) using an API source.128 We follow briefly with simplifications and showcase initial applications. These applied examples were critical in our path forward.
The discovery of LSI was based on considerations of ESI and MALDI mechanistic aspects because of similarities to both: one in terms of charge states (ESI), and the other, in terms of the matrix (MALDI). The use of a laser in TG without voltages gives good results, minimizing the background noise and providing little or no metal cation or matrix adductions, and working from AP decreases fragmentation. The problem-solving principle described by “Occam’s razor” or “law of parsimony” offers the insight that the right answer will be found if one pursues the path that “entities should not be multiplied without necessity”.129 In other words, limit your assumptions to a bare minimum. Following this guiding strategy, we looked at what is truly known about ESI and MALDI and used that to explain LSI, and, with subsequent inventions, MAI, and SAI with the least assumptions.40 An important difference between MALDI and ESI is the production of singly charged ions by MALDI which limits the analysis of larger molecules with most mass analyzers, versus ESI that provides multiply charged ions. Because we were observing ESI-like charge states with MALDI-like “tools”, the most straightforward explanation is that they are made from charged droplet (molten matrix) or particles and like ESI requires removal of the matrix, which in part was published in the Special Issue honoring John Fenn.35 The heated inlet tube and collisions with a surface would be means to aid desolvation of the charged gas-phase matrix particles. The use of collision surfaces or obstructions are well-known to enhance desolvation of charged droplets in ESI.130–135 We noted that higher melting and presumably less volatile matrixes required higher inlet temperature.35
Trimpin hypothesized that if some matrixes require high inlet tube temperature to produce ions (e.g., CHCA >300 °C) and others lower temperature (e.g., 2,5-DHAP <50 °C)3,4,8,10,40,45,51 that more volatile matrix compounds might further lower the temperature requirement (Scheme S1). This hypothesis guided us in pursuing studies on potential new solid matrixes with increased volatility, initially leading us to the discovery of vLSI (note, on the contrary, MALDI matrixes were optimized for decades for stability under vacuum). A screening study discovered new matrixes with improved analytical utility, expansion to different mass spectrometers, and importantly, to obtain a better understanding of the mechanism.
• Vacuum ionization conditions
Nearly at the end of this lengthy “matrix” study, in which it was found that most compounds when introduced into a hot enough inlet tube produce ions but few at a low inlet temperature, a backordered chemical was finally tested for its potential as a matrix for MAI on a Thermo LTQ Velos and vacuum LSI on a Waters SYNAPT G2. Trimpin and the graduate student, Ellen Inutan (WSU), discovered the pinnacle of the astonishing ionization processes. That is, with the matrix 3-NBN, analyte ions were observed in high abundance and multiply charged at room temperature without initiating the laser on an intermediate pressure MALDI source of a SYNAPT G2 (Figure S15)!1,2 Proteins were spontaneously converted to gas-phase ions and readily detected, from less than pmol amounts, from uncleaned samples (e.g., whole blood),136 and, astonishingly, even the BSA protein (66 kDa) produced mass spectra with high charge states.2 Because of the multiply charging, the mass range of the intermediate pressure Q-TOF (SYNAPT G2) was extended. Many other matrixes followed this initial discovery (Scheme S1), especially after we learned from the literature that the 3-NBN compound has strong triboluminescence behavior137 and began also targeting molecules known to have this feature. With this breakthrough discovery, analytical useful ionization and a broad range of applications followed and finally vendor independent. A few examples are briefly detailed below.
Using the 3-NBN matrix, reaction products were monitored in positive and negative modes. One of these studies, in collaboration with Dr. Mary Kay Pflum (WSU), provided evidence of the utility of vMAI (Figure S16), where ESI or MALDI were not successful, at least not in such a simple straightforward manner.10 One strength is that no acid is required in vMAI70 avoiding undesired hydrolysis hampering some ESI and MALDI applications when standard operation of acid addition is used to improve ionization.138 Other labile post-translational modifications (PTM’s) have been successfully analyzed because no laser is necessary nor is the addition of acids.40,108 Another example is the differentiation of bacterial samples. The ability to differentiate between strains of the bacteria Escherichia coli,9,10,139,140 simply by placing the samples containing the 3-NBN matrix in the intermediate pressure MALDI source without engaging the laser was demonstrated (Figure S17) and provided important proof-of-principle results for subsequent ion source developments by MSTM, detailed below. vMAI using the commercial intermediate pressure MALDI source identified for the first time the unknown antimicrobial peptide (16-mer oncocin) in collaboration with Dr. Christine Chow’s laboratory (WSU).141 Several other types of samples and surfaces have been directly analyzed, including tissues, without the use of a laser,2,6–10,105,136,142 demonstrating that vMAI is not only simple but also highly sensitive and applicable to a variety of analytical challenges.
A simple method to characterize a specific spatially resolved area of a surface is to place a matrix solution on the area of interest, briefly dry, and insert the sample into the vacuum of the mass spectrometer. Only the area where the matrix is applied produces ions.2,8,10,105,136 The ion duration can be several minutes depending on various criteria (pressure, temperature, and source geometry, matrix, sample preparation, and importantly how much matrix is used in the analysis).1,2,10,70 Because of the sensitivity of this approach, we expect that even smaller depositions of the matrix solution will improve spatial resolution measurements from surfaces without the use of a laser.
Because 3-NBN sublimes10,70,143 when in an intermediate pressure source, typically for several minutes, in principle, one has this time to acquire several samples on a plate with a single plate introduction to vacuum (ca. 2 min with the Waters intermediate pressure MALDI source). No carryover between two samples was observed for thin layer chromatography plates because only if the specific sample is located directly in front of the entrance aperture to the hexapole ion guide do the gaseous ions transmit to the mass analyzer and become detected (Figure S18) as long as the two spots were sufficiently separated. Using improved sample preparation protocols, 24 samples applied to a metal plate as 6 different analytes in 4 repeats produced excellent mass spectra of all components in 4 min, not including the 2 min sample loading.8,10,144 Introducing a sample directly into vacuum, similar to vacuum MALDI but without the use of a laser,1,2 provides sensitivity and robustness to carryover and instrument contamination at least comparable to MALDI.145 The typical mass spectrometer capabilities (IMS, MS/MS) can be readily used without employing the laser.1,2,136 Initial experiments demonstrated that the generation of MS images using this ionization method is possible using the vacuum MALDI plate and interface (Figure S19). A very limited number of mass spectrometer vendors currently provide such vacuum MALDI capabilities on API mass spectrometers, but they still exist.
Dr. Malcolm Clench’s group (Sheffield Hallam University) in 2019 implemented vMAI on a modified Q-TOF mass spectrometer fitted with an orthogonal intermediate pressure MALDI source and generated initial results for the generation of images of lipids on brain tissue adding 5% chloroform to the 3-NBN matrix solution.146 Others have also used the vacuum source without engaging the laser. In 2014, Dr. Charles Wilkins’ group (University of Arkansas) studied vMAI on a Bruker Fourier transform ion cyclotron resonance (FTICR) mass spectrometer. This was the first time that 3-NBN was used in relatively high vacuum (hence, colder conditions caused by evaporative cooling), and the ionization event took about 30 min.147 Using the laser (355 nm) to ablate the 3-NBN matrix with absorption at around 266 nm,68 produced essentially the same mass spectrometric results with the exception of speeding up the sample consumption to ca. 2 min. This group showed the utility of vMAI and 3-NBN to ionize lipids, demonstrating the potential of vMAI-FTMS to differentiate edible oils by their profile of non-polar and polar lipids and its utility for taxonomic identification of bacteria using crude bacteria extracts.148 Meanwhile, Li’s group used vMAI149 to analyze peptides with PTM’s, as well as the characterization by CID and HCD on an adapted intermediate pressure MALDI-LTQ-Orbitrap XL.
• Inlet ionization conditions
vMAI was first discovered using the intermediate pressure MALDI source on a SYNAPT G2 mass spectrometer. With volatile matrixes which sublime when exposed to sub-AP conditions, the 2 minutes to load the sample can be problematic, thus, a faster and more convenient sample introduction to vacuum was sought. The easiest means was the introduction into the inlet aperture of an API mass spectrometer using e.g., glass plates or pipet tips as sample substrates (Figure S20).128,145,150 This approach provided exceptional simplicity and good sensitivity, without any optimization or application of significant heat (ca. 30 °C). Analytically useful results were obtained, e.g., (Figure S21 to S25).128,145,150–152 Multiply charged ions are produced similar to ESI so that any mass spectrometer designed for ESI can be used with MAI because the only requirement is the access to the sub-AP of the mass spectrometer and an inexpensive matrix such as 3-NBN. With this matrix, an inlet tube is unnecessary and, in fact, added heat typically hampers analyte ion abundance (note, with room temperature liquid matrices, cooling to solid state made them perform as a MAI matrix).70,150 The condition required for ionization to occur is that the matrix must sublime/evaporate.1,4,40 The vMAI matrixes are so volatile that many of them disappear from a glass plate held at room temperature over a 3 to 24 h period, as was monitored by microscopy.70
The 3-NBN matrix is selective in that many common contaminants observed with ESI are not efficiently ionized with 3-NBN, partly because 3-NBN discriminates strongly against ionization by metal cation addition.1,2,4,7,70,107,136,145 Of course, this is often an advantage, specifically for biological MS as is shown in Figure S21 of proteins in collaboration with Mackie and Dr. Young-Hoon Ahn (WSU). The N-lysine methyltransferase (SMYD2) protein, with an expected molecular weight of 49,668 Da (experimental determined 49,844 Da) and EBOV VP40, a recombinant Ebola virus VP40 matrix protein, with a molecular weight of “ca. 35 kDa” (experimentally determined 35127 Da), were readily ionized from buffer solutions diluted 4:1 with water and mixed with 3-NBN. The cone temperature of the SYNAPT G2 was set to 30 °C. For the deconvolution we used MagTran,153 a Freeware deconvolution software for ESI mass spectral data. The protein:matrix (3-NBN) samples were crystallized preferably on the outside of a pipet tip so that the entire sample enters the sub-AP of the mass spectrometer inlet. Using this approach most proteins (Table S2) were readily detected up to ~50 kDa,145,150 while, previously, for BSA and bacteriorhodopsin (~27 kDa), a membrane protein, optimization was required using less volatile matrices or the intermediate pressure source and the 3-NBN matrix.2,107,136 However, Ebola EBOV VP40 matrix protein was readily detected in buffer without cleanup.154 Using the pipet tip approach,9,145,150 there is some of a concern for contamination just like with any other direct injection method used in MS.18,19,31–34 While 3-NBN discriminates against metal adduction, other MAI matrixes ionize analyte by metal cation adduction, in particularly with less basic compounds.155,156 The selectivity as well as ionization is matrix dependent.6–10,14,40,45,70,155,156
Sensitivity equivalent to nanoESI is achieved using the 3-NBN matrix and the MSTM Ionique manual platform to insert a few nanoliters of the matrix:analyte sample dried on the tip of a syringe needle directly into the inlet of an API mass spectrometer.157 As an example, in collaboration with Chow, a synthesized heptapeptide on TentaGel® resin beads of 75 μm size was photo-cleaved and promptly analyzed using MAI-MS and MS/MS and considerations such as charge states as well as CID and ETD on two different mass spectrometers. The collected peptide material was used for the measurements shown in Figure S22. A slight preference for peptide sequencing using ETD and ProSight Lite158 for data interpretation is observed.
The most commonly used matrixes in MS to date are depicted in Scheme S1. Binary matrixes have been discovered which provide increased sensitivity and may provide tunable selectivity and desirable synergistic properties.7,9,14,15,40,45,159 This area of research is in its infancy but holds considerable promise for further improving the MAI and vMAI processes. A large number of compounds are found to act as matrixes, including solvents.1,6,14,23,45,51,55,56,70,155 These solid and liquid matrixes and matrix combinations can be used with or without employing a laser, high or low voltages, at various temperatures (above and below room temperature), and gas flow, but they are typically not necessary and can hamper the production of ions.4,6,7,9,10,40,70,150 While a laser is not required for ionization,1,2,41,52–59,94,105,107,128,139–142,144–157 lasers have been used to achieve high spatial resolution, as in LSI and laser ablation (LA) SAI/MAI.3,9,16,17,26–30,69,94,103,160
A variety of different matrixes showed improvements for different analyte types. Synthetic carbohydrate-lipid conjugates were successfully ionized in the negative ion mode using 2-bromo-2-nitropropane-1,3-diol (bronopol) matrix (Scheme S1). The ionization occurs in the negative ion mode through deprotonation (Figure S23).155 Importantly, using traditional ESI or MALDI analyses failed. Dr. Zhongwu Guo’s group (WSU), for the first time, was able to obtain molecular ions of what they synthesized,161 and seized the opportunity for improving their synthetic and clean up procedures.
The MAI matrix 1,2-dicyanobenzene (1,2-DCB) (Scheme S1) improves the negative mode detection of gangliosides and cardiolipins. The analysis of gangliosides directly from tissue sections in the negative ion mode was developed by undergraduate Corinne Lutomski (WSU) on the Z-spray source of a Waters SYNAPT G2 using a glass plate containing 10 μm thick mouse brain tissue, provided by Mackie’s group and coated with 1,2-DCB. IMS separation was used to help further separate and identify gangliosides and cardiolipins by inclusion of drift times and the concept of nested dataset (Figure S24).162 Cardiolipins are abundant in mitochondria.163 The acquisition conditions were also applicable for the analysis of mitochondrial membrane extracts (refer to data in SI). The experimental setup consisted of the direct extraction of these compounds from the liver, brain, and heart of rats. The MAI analyses were performed with a pipet tip by bringing the tip holding the matrix:analyte sample close enough to the inlet, or touching against the inlet of an ESI source, typically held at 50 °C, on a Waters SYNAPT G2 instrument. These studies were conducted by a visiting graduate student from the WSU school of medicine laboratory of Drs. Thomas H. Sanderson and Karin Przyklenk, and suggests potential use for medical applications (Figure S25). The developed conditions were subsequently used by the Greenberg laboratory (WSU Biology) for the analyses of lipids. Halverson’s group (University of Oslo, Norway), expert in blood spot analyses, demonstrated an initial, but promising utility of MAI-MS of proteomic applications using 3-NBN as matrix.164 In this Special Biemann issue this group employs vMAI-IMS-MS of intact proteins as a potential means to differentiate protein isoforms.165 The capability of analyzing small sample volumes is important across all disciplines. Other positive attributes of the new ionization processes are the simplicity and ease of use as well as their usefulness in many disciplines including human health settings, e.g., clinical use in the Detroit Medical Center (DMC) hospital laboratory, headed by Dr. Srinivas Narayan.142,142,152,150,166,167
In order to automate MAI analyses, sequential samples held at AP using an array of pipet tips were demonstrated on two different mass spectrometers; a linear ion trap LTQ Velos and on a Waters SYNAPT G2. A programmable X,Y,Z-stage was used to align and move samples on pipet tips at AP to the vacuum orifice entrance. Eight samples consisting of small and large compounds were analyzed in 1.22 min.150 The same two instruments and MAI were subjected to a reproducibility study, using six drugs, two matrixes, and on different days. The reproducibility is improved with improvements in matrix:analyte sample introduction into sub-AP of the mass spectrometer and quantification was demonstrated to be comparable with ESI and MALDI using internal standards.152 In collaboration with Narayan’s DMC hospital laboratory, the urine of a newborn was identified to have been exposed to drugs from the mother during pregnancy at levels similar to those determined by ESI.152 With MAI, one can manipulate the cross-contamination issues using different approaches such as choosing a more volatile matrix, taking advantage of the airflow between samples, or by increasing the inlet temperature.6,70,150,168,169
The AP approach of MAI using one of the self-ionizing matrices appears applicable on a wide variety of instruments, as has been shown in proof-of-principle applications through the years, e.g., various Thermo, Waters, Advion, Bruker, SCIEX, Agilent, and homebuilt IMS-MS instruments (Figure S26).10,13,15,17,28,41,43,46,55,145,147,164,166,170 In Movie S-02 we show that the Advion Nanomate can be retrofitted to perfrom MAI eliminating the expensive ESI plate and pipet tips used with the Nanomate. Early work also showed the applicability of “dissolved MAI” (depending on the viewers eye, a “solid containing SAI” or “matrix enhanced SAI” or “liquid enhanced MAI” etc. approach) where a solvent is introduced to the inlet containing a MAI matrix (Figure S27).45 This led to another approach that we used in the Trimpin laboratory in which a premixed matrix:analyte solution is continuously infused into the inlet of the mass spectrometer prolonging the ion abundance and decreasing the inlet temperature requirements as well as cleaning up mass spectra.45,46,107,108 This idea originated from undergraduate students Alicia Richards and Chris Lietz (WSU) who worked on tissue analyses, including improving spatial resolution in imaging. The first laser and matrix enhanced SAI studies were also developed in which the laser ablated tissue plume was captured by the solvent (Figure S11) and subsequently solid matrices added to the solvent and not to the tissue (refer to data in SI).3,9,69,94 The rational was that the matrices would assist in ionization that is more specific and/or in higher ion abundance which we knew at that time to occur inside the inlet tube of the mass spectrometer and the laser will provide ablation from pristine surfaces that stay pristine. With the various surface approaches, other than LSI where even proteins were detected and peptides gas-phase sequenced,23,24,26,30,40 we noticed that preferably small molecules and intrinsically singly charged ions were detected,52,53,94,96,105 however very effectively! The new ionization methods, of course, are limited by the extraction method used, which with a regular solvent matrix is quite ineffective. The good news is that SAI works from 100% water. A relative comprehensive study on surface analyses details the different surface analyses approaches and provides comparisons to the different matrixes used including the 3-NBN matrix.94,96,105 For the direct analysis of tissue sections (Figure S28) provided by Mackie’s laboratory we demonstrated considerable improvement using a laser and a binary matrix combination consisting of 97% 3-NBN and 3% 2,5-DHAP that allows enough stability on the tissue, enough absorption at the laser wavelength (337 nm, N2 laser) and sufficient volatility in the gas phase, at least in parts associated with sufficient airflow using a slightly modified skimmer inlet160 on a SYNAPT G2. A protein with an average molecular weight of 14,135 Da was detected, along with lipids. Operating from AP, 3-NBN, which has absorption at 266 nm,68 can be used with a laser and a variety of different laser wavelengths, as is the case with other MAI matrixes.147,160 However, the volatile matrix (or matrix component) will sublime spontaneously and continuously, as has been observed at vacuum.1,2,71,136
The idea of mixing solution with the best performing matrix (3-NBN) was also shown by, e.g., Drs. Marcos Eberlin and Damilla de Morais (University of Campinas) in 2015 who reported 3-NBN as a solvent dopant to increase the sensitivity in the method called easy ambient sonic-spray ionization (EASI-MS) and desorption ESI (DESI),171 as did others using liquid/solid binary matrixes.172 Other research groups have modified the MAI approach including the composition of the matrix. Murray’s laboratory developed a variation of MAI using an electrically actuated pulse valve to deliver the matrix:sample into the inlet of the mass spectrometer. This group discussed possible applications for imaging using the pulse valve to provide spatial resolution.173 This group also presented another modification of MAI174 using a piezoelectric cantilever with a needle tip attached that was used to strike the surface of 25-μm thick aluminum foil holding the matrix:analyte. The use of silica nanoparticles and the 2-NPG matrix with laser ablation was also demonstrated by this group83 This binary matrix was added on the top of frozen mouse brain tissue and resulted in enhanced detection of multiply charged proteins. In a study by Li’s group, three ionization methods, MALDI, LSI, and MAI,175 were compared using an AP-MALDI source (MassTech) with the laser aligned in RG coupled to a Q-Exactive HF Hybrid Quadrupole-Orbitrap mass spectrometer and compared to an intermediate pressure MALDI-LTQ-Orbitrap XL. This study concluded that LSI and MAI expand the m/z range and improve the intentional fragmentation efficiency using ETD because it provides the beneficial multiply charged ions in comparison with the singly charged ions from MALDI. In another recent report the tip of a wire containing crystallized matrix:analyte was introduced through the interface capillary of the mass spectrometer into the sub-AP of an ion funnel.176 In this vacuum region, the matrix containing the analyte is removed by sublimation from the wire and the formed gaseous charged clusters and ions are collected by the ion funnel and transferred to the mass analyzer. The objective of this work was to study the photophysical properties and photochemical activity of fullerene-dyad to generate reactive oxygen species (ROS).
IMS-MS has now become ‘mainstream’ technology for gas-phase separation and structural characterization offering unique opportunities to enhance molecular analyses and imaging where LC cannot be applied.177 Combining vMAI surface analysis with high resolution MS, IMS, and MS/MS provides exceptionally powerful tools common with API but not high vacuum TOF mass spectrometers. Clinical and medical relevant studies were discussed above. Here, we discuss different compound classes making up, e.g., industrially important materials and/or increased molecular weight. Together with Dr. Barbara Larsen (DuPont), we demonstrated the utility of the ‘pictorial snapshot’178 using vMAI-IMS-MS as a means of observing small changes in complex polymer systems that only differ in shape, as described in this Biemann Special issue.179 We hypothesize that this approach can also be valuable in detecting small differences in the chemical composition of surfaces. Polymers (e.g., fiber from a new car interior) and additives were differentiated by an IMS-MS approach developed by undergraduate Tarick El-Baba (WSU).180 In another example, the ability of vMAI in combination with IMS-MS to detect even small changes in the conformations of the protein lysozyme under reducing conditions was demonstrated (Figure S29).181 More unfolding studies were performed using a MAI-IMS-MS approach. For example, using 2-dimensional IMS-MS plots, unfolding processes can be rapidly monitored (Figure S30A) and, unlike ESI, without concerns for capillary clogging.182,183 We used the Orbitrap Fusion in Stemmer’s laboratory to decipher the differences of two mass units as a result of cleavage of 4 disulfide bridges in lysozyme (Figure S30B). Structural similarities and differences have been reported using the well-studied model system of ubiquitin on commercial and homebuilt IMS-MS mass spectrometers based on MAI, SAI, and ESI methods.46 As an interesting note, on the homebuilt IMS-MS instrument in Clemmer’s laboratory,46 the ion abundance was noticeably improved by adding the matrix containing protein solution directly into the inlet of the mass spectrometer instead of introducing the dried matrix:analyte crystals. A burst of analyte ions was recorded after ca. 15 to 20 seconds after introduction to the vacuum. We hypothesize that the sample solution ‘collects’ on the ‘jet disrupter’ and that, after the solvent evaporates from the matrix and analyte and conditions become “right”, the ubiquitin ions are “explosively” produced under sub-AP conditions. Such an explosive process can sometimes be observed when removing 3-NBN crystals from the original bottle on a spatula. More details on aspects of “sublimation boiling” are discussed in ref. 14. The drift times of the different charge states were the same and others different using the well-established IMS-MS instrument in collaboration with Clemmer’s group.46 For some drift time distribution, sharper forms were observed suggesting improvements in IMS resolution. In the area of IMS using the new ionization processes there is much work to be accomplished in the future.
Thus, for a breadth of materials characterization, an increase in ionization sensitivity, selectivity, and range of compounds detected, a direct analysis approach not only enhances monolayer surface analyses,184 but also bulk analyses where chemical complexity is combined with the need for direct analyses without use of liquid separation technologies. These include targeted high throughput analyses and the need for rapid answers, common challenges in the industry and in the clinical area. The use of a “vacuum MALDI source without employing a laser”, was demonstrated as proof-of-principle, but in reality is not a practical or economical approach. MALDI, which is most commonly used with MALDI-TOF,87–89,185–189 suffers from several disadvantages relative to vMAI-MS including higher low-mass chemical background, frequently harsher ionization resulting in significantly more fragmentation, and lower mass resolution and mass accuracy on MALDI-TOF versus many ESI-based mass spectrometers (e.g., Orbitrap).138 Distinguishing fragment ions from molecular ions can be difficult when they occur during ionization (in-source fragmentation) or during sample preparation (hydrolysis). Further, fewer laboratories have MALDI-TOF than ESI mass spectrometers, for which only the latter can be readily retrofitted with more than one ionization source. As depicted in Scheme 1, API mass spectrometers can be retrofitted for use with the new ionization processes with relatively minor instrument modifications, or more effectively through source replacements. An advantage of LDI, MALDI, LSI, LA-SAI/MAI is the inherent spatial resolution afforded by laser ablation, especially with the laser aligned in TG,3,16–19,23,28–30,82,190,191 but RG has advantages such as the laser beam not having to penetrate the sample and substrate.3,10,51,65–69,71 Employing any one of the discovered ionization methods, the acquisition conditions are more gentle leading to lower chemical background and undesired fragmentation making the ionization processes more similar to that of ESI in analytical utility than that of MALDI,3,9,16,17,22,25,40,52,53,69,94,107,192 as well as the fundamental relationships.193
General advantages of the new ionization processes for molecular chemical surface analyses include the simplicity of the methods which translates into lower cost, its ability to operate on common mass spectrometers used with ESI or AP chemical ionization (APCI) including ultra-high resolution and mass accuracy instruments, the ability to produce multiply charged ions for improved mass-selected fragmentation and IMS separations, and the softness of the ionization process. However, analyte ionization after introduction to the inlet aperture of the ESI mass spectrometer, if not done correctly, can persist for several seconds using the traditional ESI source inlet as an inlet ionization source. Thus, the disadvantage of MAI using the inlet of an ESI mass spectrometer as an ion source is associated with fast analyses, and frequently carryover between samples, especially with high concentration of analyte.150 One way to obtain some meaningful control over the ion duration is with increased inlet temperature4,6–10,70,128,145,150 as was also briefly described above. Further, the ionization process has been shown to require matrix sublimation which, intrinsically, also helps eliminate matrix-related instrument contamination.6,54,69 In other words, sample introduction through the AP inlet was insufficiently robust and too slow for some applications involving the desire, or need, for ultra-high speed analyses. Thus, not only is the commercial vacuum MALDI source not ideally suited, but also the commercial ESI and APCI sources are limited in their utility, despite their successful initial applications using the new ionization processes developed into new methods for use in MS. To be clear, the same issues, and sometimes worse, are observed using ESI.
In order to get to a stage of advanced applications, dedicated sources and platforms need to be developed. Before we describe these instrument efforts, we dedicate a section to mechanistic arguments relative to the exceptionally interesting and important function of a “matrix” that empowers large nonvolatile compounds such as fragile proteins to be analyzed using either a solid, solvent, or combinations matrix without the need of any of the known tools in MS such as high voltages, laser(s), or even heat being applied so long as the source geometry is “correct” for the new ionization processes.
Lesson 5:
“Life is infinitely stranger than anything which the mind of man could invent”, by Sir Arthur Conan Doyle194
The ion source can be as simple as a hole to the vacuum of the mass spectrometer, but there is room for improvement
With vacuum ionization it is more difficult to create meaningful improvements, but it is potentially more important because of robustness and sensitivity
Intriguing new applications possible (when the technology is developed properly)
6. Mechanistic Arguments Relative to Matrixes, Pressure Regions, and Charge States
The previous sections some fundamentals and mechanistic arguments were presented obtained from studying these new ionization processes over the last decade. Our research has been guided through successive fundamentally driven-research questions for which we have published a number of papers.1,4,6–10,14,35,40,45,47,54,69,94,106 It should be noted that the most recent of these fundamental papers is included in this Biemann Special Issue and the interested reader is referred to that work which is only summarized herein.14 The following section is devoted to the fundamentals related to “the matrix”, and characteristics of a ‘good’ matrix, as well as the charge states obtained under different pressure conditions. More details can be found in the Supplemental Information of this Perspective.
Common solvents and even MALDI matrixes (Scheme S1) produce analyte ions with charge states similar to ESI when traversing an inlet tube from a higher to a lower pressure.3–10,16,17,22–26,28–30,40,41,43–47,51,52,55,56,59,60,66,93–95,98,106–108 Lower inlet temperature is applicable with volatile matrices, at least in SAI and for certain cases MAI and LSI.14,40,45,56,59,107,108 An important development was the discovery of matrixes which produce ions simply by exposure to sub-AP conditions at room temperature without need of an inlet tube. Thus, the first of these matrix compounds, 3-NBN, produces abundant analyte ions when introduced into a room temperature inlet tube, a skimmer separating AP from vacuum, or directly into vacuum on a substrate.1,2,4–15,58,69,70,90,96,103,105–109,128,135,136,139–142,144,145,147–152,154,157,159,164–176,179–184 Because 3-NBN sublimes under vacuum (as is observed with the bare eye, see Movies 3), it is pumped from lens elements it might collect on and is thus self-cleaning. In the case where the matrix:analyte sample is delivered into vacuum on a removable substrate, and remaining matrix and nonvolatile analyte is removed with the substrate greatly reducing the potential for contamination10–15).6 However, the importance of a subliming matrix goes beyond self-cleaning in that the sublimation process is hypothesized to drive ionization by expelling matrix particles into the gas phase in a process which may be akin to boiling in liquids.14 The sublimation process under vacuum is seen in Figure S31 and Movie S-03.70 The expelled particles need to acquire a charge, and while statistical charging will occur, a process such as that responsible for charge separation in triboluminescence seems to be necessary to achieve the high charge states and ion abundances observed. Indeed, several of the more successful MAI matrixes (Scheme S1)1,2,4,8–10,14,70,136 discovered are known to be triboluminescent, a process that has been known to be involved with charge separation: the light emitted due to a discharge between oppositely charged faces.195,196 Coumarin,1,70,197,198 bronopol,70,155 and ice57,199 are examples of matrixes with especially low health concerns and might be used directly, e.g., living tissue, without the use of a laser. It is not good enough to have an effective charge separation. Sufficient volatility is also important to allow loss of matrix from the charged gas-phase particles to produce the bare ions, as well as the proper technology (described in Section 1) to make and improve use of these ‘self ionizing’ matrices.13,14
The use of matrixes, of course, is not novel. In field desorption (FD) the use of benzonitrile is fundamental for the preparation of carbon dendrites in the emitters,200,201 and even though plasma desorption (PD),202 by itself does not need a matrix, it was improved with the use of nitrocellulose and glutathione matrixes.203,204 Fast atom bombardment (FAB) introduced the use of glycerol as a liquid matrix.205 Later, with the development of MALDI, the use of glycerol as a binary mixture with cobalt powder was successful for ionizing small proteins.206 The use of nicotinic acid, a small molecular weight solid phase compound as matrix was used to ionize larger proteins.207 Matrix enhanced SIMS is yet another technology that is improved for increasing the assessable mass range.208 In the case of ESI, the analyte needs to be dissolved into a liquid matrix (solvent) to be dispersed in a fine aerosol.91 The matrix plays a critical role in the ionization processes used in MS for nonvolatile compounds (Scheme S2).
Experiments suggest that, similar to desolvation in ESI, the charged matrix particles in MAI, which includes LSI and SAI, must ‘desolvate’ by a sublimation or evaporation process. We find evidence that for some less volatile matrixes or low energy conditions, loss of matrix from the charged particles/clusters is not complete even by the time they reach TriWave IMS region of the SYNAPT G2(S) mass spectrometers. It was suggested as far back as 2011 that the helium gas associated with the IMS region may be of critical importance in providing sufficient energy in removing matrix from the charged matrix:analyte particles.4,6–8,65 This suggests that improved ‘desolvation’ conditions, and/or more volatile matrixes could potentially improve sensitivity. Our laboratory has discovered a number of ‘self ionizing’ matrixes (501,10,14,55,70 versus >200 MAI matrixes at relatively high heat,3,22–30,35,40,41,43–45,47,51–56,58–60 Scheme S3) that spontaneously produce analyte ions when associated with analyte, dried, and exposed to sub-AP conditions without the need of a laser, ion bombardment, voltage, or heat.1,2,4–15 Similar to MALDI, each matrix has different compound selectivity as well as preferences relative to positive or negative ions but also relative to pressure and temperature conditions. For the 3-NBN matrix using the intermediate pressure source on a Waters Q-TOF SYNAPT G2, BSA ions were detected with up to +37 charges (vMAI),2 and from AP using a modified skimmer cone orifice on the same instrument produced +39 charges (MAI) with the inlet temperature set at 30 °C.8 In comparison, using the matrix 2-NPG under high vacuum on a Bruker MALDI-TOF with the use of a laser, +14 charges are detected.8,68 On the other hand, using the 2-NPG matrix with the use of a heated (200 °C) inlet tube of an API Thermo LTQ Velos instrument, +67 charges are observed for BSA (MAI).85 A modified SYNAPT G2S in Johnston’s lab produced the same high charge states as ESI, on the order of 67, using a homebuilt heated inlet tube.111 Vacuum ionization produces lower charge states than inlet ionization, and for the largest pressure differences between two instruments (TOF vs. linear ion trap), the charge state differences are most significant.
In MALDI, clusters have been discussed40,77,209–214 as an alternative to the photoionization mechanism.215–219 We, therefore, hypothesize that the successes of MAI matrix compounds, which also represent LSI and SAI matrixes, are associated with three key steps for ionization to occur: (1) sublimation/evaporation which assists in expelling small matrix particles from the surface into the gas phase,1,4,6–10,14,69,70 (2) a triboluminescence-like process of charge separation which results in the expelled particles being positively and negatively charged,1,2,4,6–10,14,70 and (3) sublimation/evaporation of the matrix from the charged gas-phase particles to release the bare analyte ions.4,6,8,10,14,35,40,55,56 Directly related to this argument, but separate from the ionization process itself, may be the necessity for analyte molecules to be solvated by residual solvent, or the matrix, in order to be able to produce gas-phase analyte ions. Attempting to remove the role solvent plays by drying the matrix and analyte is not an easy task made more difficult by the propensity of the matrix to sublime, but protein in powdered form, where the residual water is likely minute, produces gaseous analyte ions by traversing through a heated inlet tube.6,7,40 Recent laserless results using a binary matrix combination of 3-NBN/CHCA demonstrated improvements in ionization of lysozyme,14 which may be explained by CHCA, a typical MALDI matrix,48 aiding in keeping the protein solvated rather than as aggregates.
The ionization parameters are more restrictive under vacuum relative to the high gas flow with inlet introduction.65–68,220 However, the rapid introduction of the sample into the sub-AP (see probe and plate source descriptions in this Perspective) offers opportunity in terms of more volatiles matrices. As is summarized in Table 1, the new matrixes, selected for their known ability to triboluminesce,137 and hypothesized volatility, were subjected to a study including AP, intermediate pressure and high vacuum mass spectrometers, with and without the use of a laser. Triboluminescence and volatility together appear to be of key importance for the better matrixes used in these new ionization processes, similar to what was suggested in previously reported work.1,2,4,6–10,14,15,70,220 In other words, fewer matrixes are providing matrix assistance for either forming multiply or singly charged ions of the analytes studied when decreasing the pressure from atmospheric, to intermediate, to high vacuum. Matrices that produced multiply charged ions from AP (inlet ionization), may produce no ions or singly charged ions at intermediate pressure (vacuum ionization).
Table 1.
Potential MAI Matrix Compounds Studied under Different Conditionsa
| Matrix | MAI | vMAI probe | vMAI | TBL | m.p. [°C] | MW [Da] | UV [nm] |
|---|---|---|---|---|---|---|---|
| 2H-Chromen-2-one (coumarin) | √ √ √ | √ √ √ | √ √ √ | y | 68-76 | 146.14 | 266 |
| Isopropyl phenylcarbamate (propham) | √ √ √ | √ √ √ | √ √ √ | y | 90 | 179.22 | 266 |
| 1:1 Binary 1,3-dinitrobenzene and 1,3-dicyanobenzene | √ √ √ | √ √ √ | √ √ √ | u | u | u | u |
| 1,3-Dinitrobenzene | √ √ | √ √ | √ √ | u | 84-86 | 168.11 | 266 |
| 1,8-Ethylenenaphthalene (acenaphthene) | √ √ | √ √ | √ √ | y | 90-94 | 154.21 | 266 |
| Benzene-1,3-diol (resorcinol) | √ | √ √ | √ √ | y | 109-112 | 110.11 | 266 |
| Isobenzofuran-1,3-dione (phthalic anhydride) | √ | √ √ | √ √ | y | 131-134 | 148.12 | 266 |
| 6-Methylcoumarin | √ √ | √ | x | u | 73-76 | 160.17 | 266 |
| 9,10-Dihydroanthracene | √ √ | x | √ | y | 103-107 | 180.25 | 266 |
| 1-(4-Tert-butylphenyl)-3-(4-methoxyphenyl)-propane-1,3-dione (avobenzone) | √ √ | x | x laser355 | u | 83.5 | 310.39 | 355 |
| 1,3-Bis-(3,5-dimethylphenyl)-propane-1,3-dione | √ √ | x | x laser355 | u | 96 | 280.37 | 355 |
| 1,3-Diphenyl-1,3-propanedione | √ | x | x laser355 | y | 77-79 | 224.25 | 355 |
The following symbols and abbreviations denote: TBL, triboluminescence210 (y, yes, n, no, u, unknown), m.p., melting point (obtained from publicly available commercial sources). MW, molecular weight. UV, ultraviolet (absorption measurements, Figure S33). The averaged abundances of a mixture of analytes composed of peptides as large as insulin: √, low, √√, medium, and √√√, high abundance of multiply charged analyte ions without the use of a laser. x, no analyte ions without a laser; x laser355, 355 nm wavelength used at low laser fluence. MAI refers to introducing the matrix:analyte mixture to the AP inlet into the mass spectrometer and vMAI introducing the sample using the intermediate pressure MALDI sample introduction of the Waters SYNAPT G2S. For results obtained on high vacuum TOF mass spectrometer at various wavelengths, refer to ref. 68 and Figure S32 – S37.
Previous measurements on high vacuum TOF instruments studied peptides, small proteins and proteins up to a molecular weight of BSA (67 kDa) using the matrix 2-NPG and commercial MALDI-TOF mass spectrometers at facilities of University of Toledo, DuPont (Larsen), BAM (Weidner), and WSU (Lumigen Instrument Center).66,68 Results showed that on high vacuum TOF, only larger proteins provided high charge states, but not as high as on intermediate pressure sources of API mass spectrometers. Larger proteins also showed a biomodal distribution of charge states potentially indicating two ionization paths under intermediate pressure vacuum conditions which seemed to correlate with the AP distribution superimposed over the high vacuum distribution.68 More systematic studies under high vacuum TOF conditions are needed to elucidate and more completely understand the capabilities of matrixes, especially as a function of the pressure (vacuum) as well as considering temperature and literature known triboluminesence characteristics. Most of high vacuum TOF measurements described in the following studies were performed in our collaborator’s laboratory, Dr. Chi-Kung Ni (Academia Sinica, Taiwan), which has reconstructed a commercial high vacuum MALDI-TOF with flexible use of laser wavelengths and cooling capabilities.221 For a more detailed description of the fundamental studies in collaboration with Ni, refer to the discussion in SI.
To appreciate the changes observed based on vacuum conditions, we first describe general trends that are summarized in Scheme S3 relative to matrixes used without and with a laser and the more challenging formation of ions, especially multiply charged, of bovine insulin and peptides at colder vacuum conditions. An interesting observation is that the abundances of multiply charged ions from AP may vary,8–10,23,40,45,46,51,55,70 however, the formation of only singly charged ions under voltage-free conditions, with or without a laser, has only been observed for smaller molecules under sample preparation methods that avoided to use of a solvent matrix.22 From intermediate pressure, a limited number of matrixes under specific acquisition conditions allowed the observation of either solely singly versus multiply charged ions from the same matrix:analyte sample which was influenced by added energy.9,40 As examples, the MAI and LSI matrixes (2,5-DHAP and 2-NPG)4,8,65–68,70,71 produced multiply charged ions from AP and intermediate pressure. In the case of 2,5-DHAP, solely singly charged ions of angiotensin I (1295 Da) can be formed at will from the same matrix:analyte spot when high energy is imparted (laser, voltages, gases) and at low energy conditions almost exclusively multiply charged ions.40 More recently, the matrix 3-NBN was shown to produce singly charged ions of the MBP peptide (MW 1833) without using a laser, while lower voltage conditions and gas flow allowed “switching” to the multiply charged ions of this peptide when operating from intermediate pressure (note, this clean charge state switch with 3-NBN matrix and no laser was not possible with the smaller peptide angiotensin I)9 a switch that so far was not possible from AP conditions. Under high vacuum, only singly charged ions of bovine insulin are observed with 2,5-DHB, and ions are only observed when there is a “match” between the matrix absorption and the laser wavelength used.68 The charge state observed for insulin with the matrix 2,5-DHAP were singly with some low abundant doubly charged ions. Contrary, using a laser at the wavelength of absorption of the 3-NBN matrix, which is 266 nm, initiates analyte ionization where the ions observed are multiply charged even on a high vacuum TOF mass spectrometer. This could suggest that the laser adds the additional ‘heat’ (thermal energy) necessary for desolvation under these high vacuum, cold conditions. High vacuum studies applying well-developed methods used to understand the MALDI mechanism showed that with increasing laser fluence added to the MAI matrix 3-NBN, an increasing degree of singly charged ions are formed.58,68 AP measurements that did not show this charge state dependence. These studies confirmed previous results from us and others, including those with less volatility.1–10,40,45,70 Ni’s group used theoretical calculations (molecular geometry and reaction barrier [ab initio calculations58]) and concluded that the mechanism of ionization using 3-NBN as a matrix and the 266 nm laser which forms gaseous highly charged ions of bovine insulin under high vacuum conditions,68 is different than that of MALDI as compared to known MALDI matrix calculations.58 An example of bovine insulin using 3-NBN at high vacuum is shown in Figure S32.
Absorption measurements of MAI matrixes were performed by Weidner (Figure S33) and the knowledge used for laser dependent studies with MAI and LSI from AP (N2 at 337 nm; Nd:YAG at 355 nm), intermediate pressure (Nd:YAG at 355 nm), and high vacuum (Nd:YAG at various wavelengths). The most interesting matrixes that were shipped to our collaborator, Ni, for high vacuum, laser wavelength, fluence (Figure S34), and temperature dependent studies. We asked, ‘is it sufficient to have matrixes with high volatility and/or triboluminescence to observe multiply charged ions of relatively small compounds at high vacuum?’. The answers were clear: matrixes that perform well from higher pressure (AP) no longer work well at intermediate pressure and either fail at high vacuum or only produce singly charged ions. In more detail, ionization of bovine insulin at high vacuum involved use of a laser, and using various wavelengths depending on the matrix absorption. The matrixes studied at their absorption showed that even relatively volatile MAI matrixes with known triboluminescence no longer formed highly charged analyte ions from high vacuum (Table S3, Figure S35). Also, a study of different coumarin derivatives showed that the nonfunctionalized coumarin requires the least laser fluence and provides the highest ion abundance (Figure S36). Overall, two matrix results that stand out are methyl-5-nitro-2-furoate and coumarin. Methyl-5-nitro-2-furoate,70 one of the most volatile matrixes, no longer produced multiply charged analyte ions, but only singly charged ions from bovine insulin (Figure S37), similar to other MAI matrixes studied at high vacuum (e.g., 2,5-DHAP and 2-NPG).68,220 Coumarin is also a rather volatile matrix, and is also a known triboluminescent compound.1,70,197,198 The coumarin matrix enabled the formation of the doubly charged bovine insulin ions but not to the degree of the 3-NBN matrix under the same conditions or to the degree that coumarin forms multiply charged ions at higher pressure.1,70 These studies suggest that at high vacuum, matrix volatility and degree of multiple charging do not correlate. High vacuum conditions appear to ‘freeze out’ multiple charging while singly charged ions became a viable alternative ionization pathway. Evaporative cooling under high vacuum is a potential answer to the observed results that prompted a series of matrix studies under cold conditions.1,55,70,220
Initial studies with a SYNAPT G2 in which the plate was cooled to −80 °C and promptly introduced to the intermediate pressure of the mass spectrometer extended the ion duration from a few minutes to several minutes. The effect of temperature on initiating the sublimation process was also examined, as seen in the total ion chronograms (TIC’s) (Figure S38). In a subsequent studies angiotensin I without and with the use of a laser and different matrices showed curious results (Figure S39). Using the matrixes 2-NBN and 3-NBN, doubly charged ions were produced when the laser was fired immediately and shortly after insertion into vacuum, but two minutes after loading 2-NBN, without firing the laser, singly charged ions became dominant. Preliminary data on a ca. −80 °C cooling system on a high vacuum TOF mass spectrometer, similar to previous work,221 in Ni’s laboratory, produced singly charged ions for insulin using laser ablation (266 nm) with the 3-NBN matrix,220 however, at room temperature multiply charged ions were observed at high vacuum.68 The triboluminescence compound (3-NBN) ‘lost’ its ability to ‘supercharge’ under low temperature high vacuum conditions (note, during freezing the sample, water content became ice which is also a triboluminescence material,199 for which the effects are unknown). Scheme S3 summarizes connections between temperature, pressure, and the number of matrixes which are operational at various pressures and their propensity to form singly or multiply charged ions. These are preliminary results, and many more dedicated studies will be needed to understand the underlying fundamental reasons for the vacuum and temperature dependence for the respective matrix molecules. In other words, just like one can enhance the new processes to improve solvation, charging and desolvation, it seems one can freeze out these processes. Much more work will need to accomplished in this area before this can be understood and more specifically as it relates to charge states in MS, e.g., ESI- vs. MALDI-like.
We used RAMAN spectroscopy to study the potential presence of molecular interactions between the matrix:analyte.7 In one study, we analyzed the matrix 3-NBN, the analyte angiotensin II, and the matrix:analyte combination. One of the challenges in using what is considered the ‘normal’ sample preparation and extract meaningful data is that the analyte is in low abundance relative to the presence of the matrix (ca. 1:100,000 is common). Preliminary results, however, suggest there is an interaction between the matrix and analyte, even though other studies have indicated analyte incorporation into the matrix is not necessary.1,7,58 A more systematic study needs to be performed in order to conclude what the implications of these interactions in terms of sublimation and ion generation might be. Single crystal X-ray results show that the 2-NPG matrix contains water molecules in its crystal structure,7,166 whereas this was not the case for 3-NBN.222 We hypothesize that obtaining a better understanding of the process that leads to improved results with some binary matrixes7,14,15,40,45,60,71,159 will allow us to better tailor the solvation and ionization processes to achieve improved results.
Relative to the molecular composition of the matrix, we more recently published a fundamental study that focused on the importance of water (and other protic solvents) being present during vMAI sample preparation, especially for ionization of peptides and proteins.7,222 Matrix combinations have been found which produce different selectivity than the individual compounds used as matrixes and some provide as much as two orders of magnitude improved sensitivity versus the individual matrixes. With relatively little research effort in binary matrix combinations, we produced a number of matrix combinations that the students called ‘super matrixes’ (e.g., 1:1, v/v of 1,3-DCB and 1,3-DNB, and 2:1 v/v 3-NBN and CHCA) (Table 1). The combination of matrix compounds produces this synergy.7,14,15,159 We hypothesize that by gaining an understanding of how this synergy works and combining that with our basic understanding of matrix selectivity, we will be able to tune matrix combinations and improve selectivity. Such an outcome will be especially useful for surface analysis such as tissues, films, and monolayers.2,10,15,71,105,136,160,170,184
One constant from the beginning of MAI was the recognition that sample preparation matters. However, to date, no study of sample preparation has yielded a specific protocol to achieve the best possible results, although several papers have dealt with specific opportunities related to sample preparation.1,2,45,70,71,223 In one example, screening through ca. two dozen MAI matrixes using solutions with different pH values showed no improvements relative to the pH after the compounds are dissolved, and, instead, typically, a noticeable decrease in analyte ion abundance.70 Analyte in basic solutions were frequently detected in the positive mode and with charge states similar to ESI.45,70 Several students have suggested that crystallization and ionization are affected by laboratory conditions, especially humidity, in addition to the solvent composition (with student comments such as ‘films are bad’, ‘crystals are good’, small crystals for AP and larger crystals for vacuum conditions are observational reports and not supported by everyone). A systematic study of sample preparation protocols, especially with additives223 and under stable atmospheric conditions, will likely lead to increased sensitivity and ionization stability. It is likely that crystallization as a purification process is important relative to the results observed.
For several of our collaborators, the main interest is the identification of materials directly from surfaces. Successful molecular analysis by MS requires removal of molecules from the surface as gas-phase ions. Ideally, this occurs under mild conditions that do not alter the molecular structures as they exist on the surface. With vMAI, as with MALDI, the solvent used to dissolve the matrix, and likely the matrix itself, are primarily responsible for dissolution (solvation) of the adsorbed surface layer. Thus, both the solvent and the matrix are potentially important in enhancing the sensitivity of surface analysis simply by the efficient dissolution of the surface layer. We, therefore, instituted a brief search for more effective solvents for analysis directly from surfaces, with the realization that dissolution is likely compound specific. Both water (ε 80.4) and methanol (ε 35) have significant dielectric constants at room temperature, and these solvents seem necessary for high sensitivity MAI. We, therefore, looked at formamide (ε 111).224 It will be of interest to determine if formamide or similar dielectric constant material plays a large role in surface analysis. Recent studies identified formamide as an alternative solvent for vMAI15 (Figure S40) suggesting its potential use in surface analysis of polar and ionic compounds. Formamide may enhance the analysis not only through improved solvation of compounds distributed on a surface but also for its charge separation properties.224,225
Molecular modeling can provide insights into chemical processes once initial research has been accomplished. Using the density functional theory (DFT) calculations, in collaboration with Dr. Bernhard Schlegel (WSU), for water as a matrix in SAI, the ionization of small molecules containing electron-donating groups followed closely the pKa, but not perfectly.226 Similar studies for MAI were less conclusive relative to the performance dependence to the analyte basicity and the MAI matrix 3-NBN. The analyses of larger molecules using SAI or MAI cannot be modelled by DFT. However, the experimental results for the simple case of MAI with the 3-NBN matrix dissolved in water and acetonitrile or acetone support the relationship of basicity (isoelectric point) of the proteins having critical influence (Table S2). The only protein not detected so far was monoacylglycerol lipase (MAGL), membrane-associated member of the serine hydrolase familie with a ca. 33 kDa molecular weight, and having an isoelectric point of ca. 6.5.154 Considering the isoelectric points, the results do not indicate a correlation with how easy one can observe a protein. Much more work will need to be obtained before this can be further generalized. Mechanistic studies concerning vLSI, MAI, and MALDI were first presented in 2012.40 The importance of increasingly volatile matrix compounds in the production of multiply charged ions and the ability the obtain analyte ions using 2,5-DHAP matrix with the inlet at 40 °C were early hints that vMAI might be possible.40 The discovery of the new ionization processes, in particularly the startling case of vMAI, has greatly exceeded our expectations and that of the community as evidenced by honoring Trimpin with the Biemann Medal.
Lesson 6:
How matrixes function is complex, but important to understand
There are significant differences between AP inlet, intermediate pressure, and low pressure ionization using the same matrix and sometimes the same mass spectrometer. Fewer compounds act as MAI matrixes as the pressure is decreased, and is especially pronounced at high vacuum
Matrix absorption and volatility are a necessary but not sufficient condition for forming the multiply charged ions at high vacuum by laser ablation. A process like triboluminescence may also be helpful
Less volatile matrixes require higher energy input, and higher energy is associated with singly charged ions.
7. Advanced Technologies and Enhanced Applications using Inlet and Vacuum Ionization
Evidence of exciting applications and therefore the need for a focused approach to instrumentation dedicated to the new ionization processes has been presented. For a meaningful path forward it became necessary to found the university spin off company, MSTM, LLC. The fundamental studies depicted throughout this text guided and enabled successful platform and source designs with applications being key to demonstrating the utility of the new ionization technologies.
• Inlet ionization – Ionique platforms and other automated approaches
The development of the manual152 and the automated227 Ionique platforms are making the new ionization methods more reliable than using the initial approaches of, e.g., pipet tips, eye of a needle, or paper.52,53,59,128,150 The manual and automated Ionique platforms developed by MSTM, LLC through NSF STTR funding support multiple ionization methods, including MAI, SAI, VSAI , and ESI.7–10,52,101 Initial applications to the clinical area were tested by analyzing raw urine samples obtained from the DMC hospital (Figure S41). Forensics MS applications have also been demonstrated.90,142,170
Samples in microtiter wells were analyzed using MAI. The manual Ionique platform allows MAI by inserting solid matrix:analyte sample on a 1 μL zero dead volume syringe needle into the inlet tube of a mass spectrometer. Typically, a few tenths of a microliter of analyte solution are drawn into the syringe needle and expelled back into the analyte solution leaving only sample that adheres to the syringe needle walls (ca. 50 nL).157 This is followed by drawing in a similar volume of matrix solution and expelling to dry on the tip of the syringe needle. SAI can be performed by inserting a few tenths of a microliter of an analyte solution into the heated inlet tube using such a syringe. With ESI, 1 μL is typically slowly expelled from the syringe capillary, but about 1-3 cm in front of the inlet and with a voltage applied to the metal syringe needle. Each method has advantages relative to certain compound classes which are clearly seen with complex mixtures. Interestingly, with biological samples, ESI and SAI are prone to ionize by protonation and metal cation adduction. The advantages are that compounds which ionize only by metal cation adduction are observed, but typically not with MAI using 3-NBN. However, the downside is that some compounds are ionized by proton, sodium, and potassium cation adduction in ESI and SAI, while in MAI only by protonation, greatly simplifying the mass spectra. With bacterial samples acquired MAI, protein multiply charged ions were frequently some of the most abundant ions observed in the mass spectrum and only protonated, whereas with ESI, without addition of formic acid, these ions had multiple cation adduction and were frequently of low abundance relative to low-mass ions making them easily overlooked. Initial results with the manual platform using MAI showed that with a reproducible sample introduction, quantification with the use of internal standards was equivalent to ESI, but faster. Finally, the manual Ionique platform can be interfaced with LC using ESI, SAI, or VSAI.102 Other approaches using continuous or discreet flow have been established.106 In one example, an undergraduate student rapidly analyzed 25 standards of carbohydrates from our collaborator, Dr. Crich (University of Georgia). The ionization method used was SAI and the analyses were performed on the SYNAPT G2S with the modified heated inlet at 250 °C. Figure S42 shows a comparison of results between SAI and ESI. The applicability to lanthanide analyses is shown using the heated MSTM inlet tube and the manual Ionique platform on the SYNAPT G2S (Figure S43, Table S4(a) and S4(b)). The approach is applicable to observing inorganic molecular species using low sample volume.166,228 This was also demonstrated using uranyl nitrate and 3-NBN matrix in the negative detection mode by Dr. Matthew Wellon’s group at Savannah River National Laboratory (see also contribution in this Special Biemann issue using a pipet tip approach) and by MSTM. These measurement capabilities may be important for nuclear safe guards.229
The MSTM automated Ionique platform operates with 96- or 384-well plates, vials, and surface sampling has been demonstrated (Movie S-04). Different wells are analyzed with a washing step in between leading to acquisition speeds of ca. 1 minute per sample but allows elimination of expendables such as pipet tips and special emitter plates. Using this robotic platform MSTM, with help from Kevin Rackers (Automation Techniques) and Drs. Ana Djuric (WSU College of Engineering), Tamara Hendrickson (WSU), and Vladimir Shulaev (University of North Texas), differentiated among different strains of Escherichia coli. A comparison using ESI and MAI on the automated Ionique platform retrofitted to the Q-Exactive Focus Orbitrap is shown in Figure 4A. Three-dimensional principal component analysis (3D-PCA) was used to evaluate and contrast the performance of the methods with 25 BSL-1 bacteria, including the E. coli (Figure 4B). A comparison of ESI (consuming 1 μL), SAI (0.2 μL), and MAI (0.1 μL) of bacterial extract solution is provided in Figure S44. ESI and SAI detected similar ions in the low mass range, whereas MAI (3-NBN matrix) favors the higher mass ions. Importantly, MAI, like ESI, is able to ionize small to large molecules giving more complete microorganism fingerprints.9,10,15,107,139–141 The success of MAI is related to the ionization of peptides and small proteins out of these “dirty” samples.2,136 Clearly, for best results, a combination of ESI and MAI is desirable from the same API (high resolution) instrument. Overall, over 5000 samples were acquired using the automated Ionique without indication of instrument contamination.
Figure 4.

Bacterial strain differentiation using two ionization methods, MAI and ESI, on the same platform and mass spectrometer. (A) Photograph of the automated Ionique multi-ionization platform mounted on a Thermo Q-Exactive Focus Orbitrap without any further modifications (source override included in platform) and Movie-S04 shows the operation. (B) Differentiation of bacteria using 3D-PCA: (1) under standard MAI conditions, 4 out of 5 E. coli strains were correctly discriminated using 3D-PCA analyses. As a comparison, using (2) typical ESI conditions, only 2 of 5 were clearly separated.
The Ionique platforms, automated and manual, are able to retrofit to instruments using a MSTM inlet tube that readily replaces the skimmer cone of commercial sources as does other automated approaches (Figure S45). One of the advantages of using the Waters SYNAPT mass spectrometers for automation experiments is the availability to perform IMS which adds a dimension of separation in addition to m/z. As one example, the well-defined mixture of β-amyloid (1-42) and (42-1) prepared in the ratio of 1:1 (v:v) was analyzed using MAI-IMS-MS (Figure 5). Both β-amyloid (1-42) and reverse peptide (42-1) showed the expected23 multiply charged ions and are indistinguishable from each other as shown in the obtained mass spectrum (Figure 5A). Of course, MS alone cannot distinguish one isomer from the other. However, two different IMS drift time distributions were observed for each of the respective multiply charged ions indicating the difference in the collisional cross section of the isomers, present in the mixture, leading to effective gas-phase separation (Figure 5B), as was previously established for LSI.44 This is accomplished using the Ionique automated platform in ca. 1 minute plus software interpretation time. While this is sufficient speed for an academic laboratory, it is not for numerous industrial applications of importance. For this reason, we focused efforts on enhancing the speed of analyses based on MAI and SAI (Figure S46–48) with and without the use of a laser.
Figure 5.

Ionique automated MAI-IMS-MS of an isomeric mixture of β-amyloid (1–42) and reverse peptide (42–1): (A) total mass spectrum and (B) 2-dimensional plot of IMS-MS with the color code indicating the ion abundances of the respective charge states detected from +3 to +7. Insets: extracted drift time distributions. Data were acquired on a Waters SYNAPT G2S mass spectrometer using the MSTM automated multimode Ionique platform 225 replacing the ESI source using the retrofitted MSTM inlet tube modification and the matrix 3-NBN (100 mg of 3-NBN was prepared in 3 mL of 3:1 (v:v) ACN:water). The drift time results compare well with those obtained by LSI-IMS-MS on a SYNAPT G2.44
In one approach, we studied the use of a laser to ablate solutions in well plates. This approach uses an X,Y,Z-stage in combination with an in-house 3D printed well plate holder, developed by undergraduate Frank Yenchick, which moves a well to briefly touch the MSTM bent inlet tube to withdraw a solution containg analyte (Figure S46), as is shown in Movie S-05. In an alternative approach, the path of the laser beam from an Nd:YAG laser at various wavelengths was used to generate a mist from each well plate that, at least in part, was captured in the extended inlet tube for ionization by SAI. The laser can be arranged in TG (Figure 6A and Figure S47) and RG (Figure S48). Using TG, special well plates with sufficient transmission at the laser wavelength were required. The TIC of different repetition rates using a Nd:YAG laser at 532 nm and firing the laser at 1 Hz is provided in Figure 6B. Only low analyte ion abundance was observed at the lowest acquisition times (Figure 6C(2)) but was augmented with increased laser repetition rates (Figure 6C(3)). There was a challenge with sample-to-sample carryover which frequently is also observed with direct ionization approaches including ESI and their newer developments, typically referred to as ‘ambient ionization’.230,231 We hypothesized that vacuum ionization methods should not suffer these same issues. For this reason, we increasingly turned our attention to vacuum ionization, which continued to proceed in parallel to the inlet ionization developments but were technically more difficult.
Figure 6.

Fast analysis using TG laser ablation-SAI-MS on an IMS-MS instrument. (A) Photographs of the source setup with inset: green arrow indicates the path of the laser beam through the bottom of the optically transparent well plate. (1) Nd:YAG laser beam locally heats the solvent (methanol) containing the analyte (erythromycin) in the well plate, (2) focusing lens, (3) mirror, (4) automated XYZ-stage equipped with the custom built plate holder to secure the well plate, (5) well plate in close proximity to a customized inlet entrance, (6) inlet entrance of bent MSTM inlet tube. (B) (1) TIC at 2 sec acquisition of laser firing at 1 Hz, (2) TIC of 1 sec acquisition 1 Hz, (3) EIC of 1 sec acquisition of m/z 734 with laser firing at 1Hz, and (C) mass spectra from (1) from B(1), (2) from (B2), and (3) from (B2) at higher laser repetition rate. The Nd:YAG laser was operated at 532 nm wavelength, Movie-S05 shows the operation of the entire system. Multiply charged ions are shown in Figure S46. Data acquired on a commercial Z-spray source of a Waters SYNAPT G2S; for specific details and additional results, refer to Figures S45–S48.
• Vacuum ionization sources – probe based and similar conventional valve based approaches
Because of carryover issues limiting speed of analysis with AP sample introduction approaches, we spent additional effort on sample introduction directly into vacuum. The matrix 3-NBN is exceptionally sensitive for analyses of drugs, peptides, and proteins.1,2,4–15,142,152,150,166,232 However, vMAI using the commercial intermediate pressure sample introduction of a MALDI source into the vacuum of the mass spectrometer initially was not very useful because sample loading requires nearly two minutes, and initially only a single sample could be loaded at a time.1,2,136 To circumvent the slow loading, and to provide a means to better study this ionization process and eliminate the long sample introduction, a vacuum-probe device was designed for Waters (Figure 7A) mass spectrometers replacing the commercial API sources.11 Single sample introduction via a probe containing the matrix:analyte sample on the tip and introduced to the vacuum of a mass spectrometer through a ball valve by hand takes less than 10 seconds without need of any additional pumping.6–12,46,165,169,179 Abundant analyte ions with little background were achieved from low femtomoles of sample loaded without use of a laser, high voltage, or applied heat. Importantly, biological fluids, analyte in buffers, and high concentration samples were acquired without any carryover or any indication of instrument contamination as long as the probe tip was well cleaned.6–12,165,169
Figure 7.

Use of a vacuum probe source for vMAI-MS: (A) Photographs: (1) the source housing open and the approximate position of the probe relative to the entrance to the mass spectrometer, (2) source housing closed as used for analyses. (B) (1) (i) swine liver tissue to be analyzed being touched with the end of probe and (ii) with matrix solution applied to end of probe and dried. (2) (i) Tissue in solvent solution used for extraction, (ii) probe with extract solution and matrix dried on the tip. (C) Mass spectra: (1) from B1 and (2) from B2. The extracted solution was mixed with 3-NBN in acetonitrile (3:1 v/v), and 0.1 μL was applied to the end of the probe for the acquisition. Data acquired on a SYNAPT G2 mass spectrometer. Tentative assignments are based on m/z and published work (Ref. 237–240): TG: triacylglycerol; PC: phosphatidylcholine; SM: sphingomyelin. Assignments in blue are only observed by direct, in red by extraction, and in purple by both approaches. The ion at m/z 663 is a commonly observed using 3-NBN as matrix.14
These studies suggested that the vacuum ionization approach virtually eliminates instrument contamination even with dirty samples because almost all of the nonvolatile materials remain on the probe surface to be removed from the instrument. Likewise, carryover is eliminated even with highly concentrated samples, and importantly, the method is suitable for surface analysis of small (<1 mm2) sample areas as was previously shown on commercial intermediate pressure sources.1,2,136 Because there is no physical contact, and no additional energy is added to ablate the material from a surface, contrary to, e.g., MALDI, only materials volatile enough to sublime or evaporate leave the probe surface, except for the minute amount emitted as charged matrix:analyte particles. Another key advantage of fast sample introduction is that volatile matrixes are applicable such as coumarin and methyl 2-methyl-3-nitrobenzoate which sublime before reaching the ionization region using the commercial mechanical MALDI sample introduction to the vacuum of the same mass spectrometer.1,70 This is expected to enhance the sensitivity by (1) not ‘losing’ the sample due to improper source introduction and (2) improving desolvation of the matrix from the charged clusters. Because the ionization is now also robust one can analyze without concerns materials directly from biological matrixes, buffers, and synthetic starting materials (Figure S49)8–10,142,145 etc. Even proteins that are deposited on a surface together with a matrix are detected, and charge states can be extracted to give an additional value of confirmation using the drift time values associated with their respective cross sections previously determined by an ESI approach from solutions.233–236
For more meaningful research related to vMAI in the MSTM laboratory and seeing the promise of the vacuum-probe method on the Waters SYNAPT G2, MSTM subsequently built a second generation vacuum-probe device for a Thermo Q-Exactive Focus Orbitrap mass spectrometer and demonstrated low attomole detection limits for the peptide angiotensin I and no carryover when loading 1011 times more of this analyte, so long as the probe tip was replaced or thoroughly cleaned. These results were acquired with 3-NBN as matrix using ion extraction voltage to direct ions into the ‘S’ lens of the Q-Exactive Focus. Additional airflow with low voltages can be employed to enhance certain analyses. The drug fexofenadine was detected with S/N >10/1 applying only 7 attomoles, and from 100 attomoles applied directly to a metal surface as a demonstration of analysis of a microscopic deposit.9
Similar to MALDI, with certain compounds such as peptides and drugs, cleaning the surface of the sample plate after analysis so that the analyte is not observed in subsequent analyses can be difficult. For example, with erythromycin placed on a metal probe at 1 μM concentration and dried, multiple washing steps with several common solvents failed to remove the sample sufficiently so that it is not observed in significant abundance in a subsequent vMAI analysis where only matrix solution is added. However, using the solvent used with vMAI, but without the matrix added, and applying it to the surface followed by nanoESI did not detect the drug. These and other results reiterate not only the sensitivity, but that the matrix may play a key role in dissolution and solvation of the analyte. Adding the matrix on top of the tissue as is shown in Figure 7B (as would be the case of e.g., a biopsy sample) results in a lower abundance of ions but additional signals relative to a typical Folch extract.237,238 The respective mass spectral data is shown in Figure 7C1 and C2 with tentative assignements obtained using refs. 239–242. The use of the vMAI probe demonstrated its potential for rapid analyses.12 This is possible because there is no physical contact and ionization ceases when not in close proximity to the ion transmission region. In this experiment, nothing can be learned about true speed and potential carryover issues from one sample to the next because the same sample was acquired multiple times by moving the probe in and out of the ion transmission window.
Ionization is rather limited for some materials using MS such as less basic compounds, including carbohydrates and synthetic polymers requiring the addition of salts to obtain good results, and in some cases the use of binary matrixes to enhance detection. Using the vMAI probe on the SYNAPT G2S, we analyzed and confirmed in a “walk-up” manner synthesized carbohydrates from our collaborator Dr. Nguyen’s group (WSU) (Figure S50). Yet another specific example is shown in Figure S51 in collaboration with Dr. Scott Grayson’s laboratory (Tulane University) in which the graduate student, McKenna J. Redding, travelled to WSU. Using the vMAI probe on the SYNAPT G2S, a combination of tetrabenzylidene G1 and octabenzylidene G2 2,2-bismethylolproprionic acid (bis-MPA) dendrimers243,244 were readily analyzed with the addition of barium salt solution to 3-NBN matrix solution. In collaboration with Larsen, branched polymers were readily differentiated using the vacuum-probe vMAI-IMS-MS approach without the time consuming clean up between samples,179 contrary to using ESI-IMS-MS.178 While highly charged polymer ions, important for distinguishing differences in polymer architectures using MS in combination IMS “fingerprint”245 analysis have been difficult with the matrix 3-NBN, other MAI matrixes have been successful.156,179,180,184 Another improvement was the introduction of use of divalent cations.179,246 Binary matrixes and salts may open up many more research opportunities in the future. Therefore, with vacuum ionization using a simple probe device we gained the freedom to improve our understanding of materials of all sorts because of the high ion abundance and without worry of source and instrument contamination. Using a vacuum-probe we are finally comfortable to measure any sample because we do now know that we can’t harm the instrument or cause extra work because of a dirty inlet.
Peptides, proteins, drugs, pesticides, and consumer products have been analyzed on a mass range limited, portable mass spectrometer (QDa from Waters) with surprisingly little chemical background by simply removing and overriding the API source and using a sample introduction of choice, e.g., the round end of glass capillary.166 For proteins, in-source decay conditions (ca. 20-80 V) typically improved protein analyses by likely aiding shedding of the matrix from the charged clusters more effectively. A miniature vacuum-probe was recently designed, constructed, and applied with this portable mass spectrometer by Dr. I-Chung Lu’s group (National Chung Hsing University),247 which just like the larger mass spectrometer, makes the measurements more robust and reduces even further the pumping load. A minimalistic (‘mini’) vacuum/inlet-source was previously designed, enabling the analysis of samples using a simple on/off valve controlling airflow from the AP to the vacuum. This device was light-weight providing reasonably fast analyses with simplicity and high sensitivity, and as a bonus offered insights into a flexible valve design.109 Drug tablets, e.g., propranolol was detected simply by adding the matrix 3-NBN and moving the tablet close enough to the ‘mini’ source inlet166 on a Waters SYNAPT G2S (Figure S52). The drawback of the ‘mini’ design was carryover from valve contamination. The accumulated vacuum ionization results led to the bold and successful research described in Section 1 of this Perspective. For those only interested in analyzing samples by vMAI and single sample analyses are sufficient, the vacuum-probe is an excellent choice.
Lesson 7:
Information extractable from dedicated platforms is more fundamentally and analytically useful
Unprecedented simplicity, speed, and robustness is achievable with dedicated inlet and vacuum ionization sources
Clinical applications are within reach
Conclusion and Outlook
As a personal note, the success I’ve (ST) had that lead to the Biemann Medal belongs, in a great measure, to a long list of students and collaborators, a number of whom are co-authors on this Perspective. It has been my privilege to work with talented undergraduate and graduate students and postdocs of different genders and ethnicities from around the globe. There are too many talented students to list them all here, but I’m especially indebted to Prof. Ellen Inutan, my first graduate student, and a gifted researcher who first observed vMAI. I like the saying attributed to Henry Ford; “Whether you think you can, or you think you can’t--you’re right”,194 because my group accomplished the most when we all believed. I am amazed at how far we have come since the first observation of multiply charged ions from what appeared to be a MALDI method. Happily, a number of groups are using, and further developing, the ionization discoveries. The new vMAI developments performed on a portable instrument166 have been included in a popular undergraduate textbook.248 Area where progress has been made through these discoveries include laser and matrix assisted (enhanced) ionization, in situ, in vivo, intraoperative, rapid ID, RealTime, monitoring, molecular guidance in healthcare, environmental, nuclear, and forensics settings and safeguards.
Because the new ionization methods are based on heretofore unknown ionization processes, and having received almost no development, relative to well-optimized ionization methods of ESI and MALDI over the past 30 years,206,207,249,250 we can expect that the new methods lie at the very beginning of their technology life cycle curve. Thus, improvements are far more likely than with mature ionization technologies. Therefore, sensitivity increases, which can be expected from dedicated studies, will allow simultaneous ionization of multiple components in complex surface chemistries. Our expectation is that in the immediate future we can improve the detection limit of vMAI by physical source constructions best suited for the new ionization processes making it a low-cost and rapid method for robust, laser-free, low-resolution, imaging with high sensitivity. Further, understanding the role the matrix plays in nearly all ionization methods such as ESI, MALDI, DESI, as well as the novel ionization processes described here, in displacing compounds from a surface should further improve molecular (surface) analysis. We hypothesize that monolayer molecular and bulk surface analysis can be made into a rapid routine technology using readily available mass spectrometers through application of the exceptionally unique vMAI technology combined with the latest vacuum source technology. Defects caused by chemical contaminants can be analyzed in seconds without a laser, high voltages, desolvation gases or any of the traditional means, an important point for ionizing valuable samples. Examples include bacteria extracts,7,9,10,139–141,227 fungi,13,15 drugs,10,13,90,109,142,152,150,166,232 starting materials9,10,142 and synthetic products,75,156,179,180,184 consumer products,15,166,246 proteins,1,2,10,13–15,46,136,165 tissue and other biological materials including whole blood and urine,8–10,136,152 environmental contaminants,104 and nuclear safety,229 to name a few studies by us and collaborators. More broadly, the new processes permit simple and inexpensive analyses through dedicated developments of robust instruments producing quality data in a wide range of applications. Relevant to Dr. Klaus Biemann’s legacy,251–257 we will put our best efforts forward in making MS analysis of even large proteins, as well as other demanding molecules, fast and easy to achieve, even by a novice.
Overall Lesson:
Optimization of ESI and MALDI, led to broad applicability that was unimaginable at the beginning
Similar advances in science is not unexpected for the new ionization processes
Supplementary Material
Acknowledgment
Trimpin has been gifted by a wonderful body of all sorts of teachers from parents, family, school teachers, professors, colleagues, students, and postdocs. The authors are grateful for the generous support from NSF CAREER 0955975, NSF CHE-1411376 (to ST), NSF Phase I 1417124 and Phase II 1556043 including TECP and NSF SBIR Phase I 1913787 (to CNM and ST), CHE-1112289 (to CNM), CHE-1500201 and CHE-1904584 (CNV), CHE-1904670 (MKP), CHE-1807358 (SMG), as well as Eli Lilly Young Investigator Award in Analytical Chemistry, DuPont Young Professor Award, and BASF financial support (to ST), WSU Schaap Faculty Scholar, OVPR Grants Plus (to ST) and CVRI ISIS (to THS and ST), WSU Rumble and Schaap graduate (to EDI and BW) and undergraduate fellowships (to ALR, CBL, and FSY). NIH R01CA095142 and NIH R01GM090270 (to ZG), NIH R01 DA011322 and DA021696 (to KM), NIH R01 GM098285 (HMN), NIH R01 HL131740 (to Y.-H. A). We also thank Dr. David Crich (UGA) for providing standards. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
The Supplemental PDF file contains additional details to the experimental information, movies, and Figures supporting the description of this text.
Conflict of Interest
The corresponding author is a shareholder of MSTM, LLC.
References
- 1.Trimpin S; Inutan ED Matrix Assisted Ionization in Vacuum, a Sensitive and Widely Applicable Ionization Method for Mass Spectrometry. J. Am. Soc. Mass Spectrom 2013, 24(5), 722–732. [DOI] [PubMed] [Google Scholar]
- 2.Inutan ED; Trimpin S Matrix Assisted Ionization Vacuum (MAIV), a New Ionization Method for Biological Materials Analysis using Mass Spectrometry. Mol. Cell Proteomics 2013, 12(3), 792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trimpin S; Wang B; Lietz CB; Marshall DD; Richards AL; Inutan ED New Ionization Processes and Applications for Use in Mass Spectrometry. Crit. Rev. Biochem. Mol 2013, 4(5), 409–429. [DOI] [PubMed] [Google Scholar]
- 4.Trimpin S “Magic” Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom 2016, 27(1), 4–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peacock PM; Zhang W-J; Trimpin S Advances in Ionization for Mass Spectrometry. Anal. Chem 2017, 89(1), 372–388. [DOI] [PubMed] [Google Scholar]
- 6.Trimpin S; Lu I-C; Rauschenbach S; Hoang K; Wang B; Chubatyi ND; Zhang W-J; Inutan ED; Pophristic M; Sidorenko A; McEwen CN Spontaneous Charge Separation and Sublimation Processes Are Ubiquitous in Nature and in Ionization Processes in Mass Spectrometry. J. Am. Soc. Mass Spectrom 2018, 29(2), 304–315. [DOI] [PubMed] [Google Scholar]
- 7.Trimpin S; Inutan ED; Karki S; Elia EA; Zhang W-J; Weidner SM; Marshall DD; Hoang K; Lee C; Davis ETJ; Smith V; Meher AK; Cornejo MA; Auner GW; McEwen CN Fundamental Studies of New Ionization Technologies and Insights from IMS-MS. J. Am. Soc. Mass Spectrom 2019, 30(6), 1133–1147. [DOI] [PubMed] [Google Scholar]
- 8.Trimpin S Novel Ionization Processes for Use in Mass Spectrometry: ‘Squeezing’ Nonvolatile Analyte Ions from Crystals and Droplets. Rapid Commun. Mass Spectrom 2019, 33 (S3), 96–120. [DOI] [PubMed] [Google Scholar]
- 9.Trimpin S; Marshall DD; Karki S; Madarshahian S; Hoang K; Meher AK; Pophristic M; Richards AL; Lietz CB; Fischer JL; Elia EA; Wang B; Pagnotti VS; Lutomski CA; El-Baba TJ; Lu I; Wager-Miller J; Mackie K; McEwen CN; Inutan ED An Overview of Biological Applications and Fundamentals of New Inlet and Vacuum Ionization Technologies. Rapid Commun. Mass Spectrom 2021, 35 Suppl 1:e8829. [DOI] [PubMed] [Google Scholar]
- 10.Trimpin S Biemann Medal Plenary Lecture. 67th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Atlanta, GA, June 2–6, 2019. [Google Scholar]
- 11.Lu I-C; Pophristic M; Inutan ED; McKay RG; McEwen CN; Trimpin S Simplifying the Ion Source for Mass Spectrometry. Rapid Commun. Mass Spectrom 2016, 30(23), 2568–2572. [DOI] [PubMed] [Google Scholar]
- 12.Trimpin S; Pophristic M; Adenijii-Adele A; Tomsho JW; McEwen CN Vacuum Matrix-Assisted Ionization Source Offering Simplicity, Sensitivity, and Exceptional Robustness in Mass Spectrometry. Anal. Chem 2018, 90(19), 11188–11192. [DOI] [PubMed] [Google Scholar]
- 13.Hoang K; Trimpin S; McEwen CN; Pophristic M A Combination MAI and MALDI Vacuum Source Operational from AP for Fast, Robust, and Sensitive Analyses. J. Am. Soc. Mass Spectrom 2021, 32(1), 124–132. [DOI] [PubMed] [Google Scholar]
- 14.McEwen CN; Inutan ED; Moreno-Pedraza A; Lu IC; Hoang K; Pophistic M; Trimpin S Sublimation Driven Ionization for Use in Mass Spectrometry: Mechanistic Implications. J. Am. Soc. Mass Spectrom 2021, 32(1), 114–123. [DOI] [PubMed] [Google Scholar]
- 15.Trimpin S; Moreno-Pedraza A; Hoang K; Pophristic M; Inutan ED; Marshall DD; Karki S; Meher A; Yenchick FS; Lee C; El-Baba TJ; Wang B; Mokahal H; Jarois DR; Redding MJ; Kish M; Davis ETJ; Austin CA; Sohizad R; Musavi A; Simich M; Pflum M-K; Verani CN; Halvorsen TG; Grayson SM; Guo Z; McEwen CN Multi-Functional Vacuum Ionization Source for MAI, LSI, and MALDI: Operational from AP for Comprehensive, Low-Cost Data-Mining in Mass Spectrometry, 68th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Online Conference, May 31–June 4, 2020. (oral presentation) [Google Scholar]
- 16.Trimpin S; Inutan ED; Herath TN; Weidner SM; Kowalski P; Claude E; Major H; McEwen CN; Mackie K; Walker JM Total Solvent-Free Analysis using Mass Spectrometry. 57th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Philadelphia, PA, May 31–June 4, 2009. (oral presentation) [Google Scholar]
- 17.Trimpin S; Herath TN; Inutan ED; Cernat SA; Miller JB; Mackie K; Walker JM Field-Free Transmission Geometry Atmospheric Pressure Matrix-Assisted Laser Desorption/Ionization for Rapid Analysis of Unadulterated Tissue Samples. Rapid Commun. Mass Spectrom 2009, 23(18), 3023–3027. [DOI] [PubMed] [Google Scholar]
- 18.Pérez J; Petzold CJ; Watkins MA; Vaughn WE; Kenttämaa HI Laser Desorption in Transmission Geometry inside a Fourier-Transform Ion Cyclotron Resonance Mass Spectrometer. J. Am. Soc. Mass Spectrom 1999, 10(11), 1105–1110. [DOI] [PubMed] [Google Scholar]
- 19.Galicia MC; Vertes A; Callahan JH Atmospheric Pressure Matrix-Assisted Laser Desorption/Ionization in Transmission Geometry. Anal. Chem 2002, 74(8), 1891–1895. [DOI] [PubMed] [Google Scholar]
- 20.Willoughby RC; Sheehan EW Apparatus and Method for Focusing and Selecting Ions and Charged Particles at or near Atmospheric Pressure, 2004, US Patent 6,744,041.
- 21.Willoughby RC How Do We Eliminate Ion Losses at AP? Keeping the Barn Doors Open. LCMS Chem-Space Associates. 2006, 1(1), 1–3. [Google Scholar]
- 22.Trimpin S; Inutan ED; Herath TN; McEwen CN Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Method for Selectively Producing Either Singly or Multiply Charged Molecular Ions. Anal. Chem 2010, 82(1), 11–15. [DOI] [PubMed] [Google Scholar]
- 23.Trimpin S; Inutan ED; Herath TN; McEwen CN Laserspray Ionization, a New Atmospheric Pressure MALDI Method for Producing Highly Charged Gas-Phase Ions of Peptides and Proteins Directly from Solid Solutions. Mol. Cell. Proteomics 2010, 9(2), 362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trimpin S; Inutan ED; Wang B; Marshall DD; Cudry F; Lietz CB; Leach S; Richards A Alternatives in IMS-MS - Total Solvent-Free Analysis and Structures of Highly Charged Laserspray Ions. 58th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Salt Lake City, UT, May 23–27, 2010. (oral presentation) [Google Scholar]
- 25.Inutan ED; Trimpin S Laserspray Ionization (LSI) Ion Mobility Spectrometry (IMS) Mass Spectrometry (MS) of Proteins. 58th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Salt Lake City, UT, May 23–27, 2010. (poster presentation) [DOI] [PubMed] [Google Scholar]
- 26.Trimpin S Imaging Mass Spectrometry (MS) at Atmospheric and Intermediate Pressure using Laserspray Ionization (LSI). 59th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Denver, CO, June 5–9, 2011. (oral presentation) [Google Scholar]
- 27.Richards AL; Lietz CB; Ren Y; Wager-Miller J; Mackie K; Trimpin S Laserspray Ionization MSn and High Spatial Resolution Tissue Imaging of Labile Gangliosides and Other Lipids. 59th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Denver, CO, June 4–9, 2011. [Google Scholar]
- 28.Richards AL; Lietz CB; Wager-Miller JB; Mackie K; Trimpin S Imaging Mass Spectrometry in Transmission Geometry. Rapid Commun. Mass Spectrom 2011, 25(6), 815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richards AL; Lietz CB; Wager-Miller J; Mackie K; Trimpin S Localization and Imaging of Gangliosides in Mouse Brain Tissue Sections by Laserspray Ionization Inlet. J. Lipid Res 2012, 53(7), 1390–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inutan ED; Richards AL; Wager-Miller J; Mackie K; McEwen CN; Trimpin S Laserspray Ionization, a New Method for Protein Analysis Directly from Tissue at Atmospheric Pressure with Ultrahigh Mass Resolution and Electron Transfer Dissociation. Mol. Cell. Proteomics 2011, 10(2), M110.000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCombie G; Knochenmuss R Enhanced MALDI Ionization Efficiency at the Metal-Matrix Interface: Practical and Mechanistic Consequences of Sample Thickness and Preparation Method. J. Am. Soc. Mass Spectrom 2006, 17(5), 737–745. [DOI] [PubMed] [Google Scholar]
- 32.Lu I-C; Lee C; Lee Y-T; Ni C-K Ionization Mechanism of Matrix-Assisted Laser Desorption/Ionization. Annual Rev. Anal. Chem 2015, 8(1), 21–39. [DOI] [PubMed] [Google Scholar]
- 33.Page JS; Kelly RT; Tang K; Smith RD Ionization and Transmission Efficiency in an Electrospray Ionization—Mass Spectrometry Interface. J. Am. Soc. Mass Spectrom 2007, 18(9), 1582–1590. [DOI] [PubMed] [Google Scholar]
- 34.Marginean I; Page JS; Tolmachev AV; Tang K; Smith RD Achieving 50% Ionization Efficiency in Subambient Pressure Ionization with Nanoelectrospray. Anal. Chem 2010, 82(22), 9344–9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McEwen CN; Trimpin S An Alternative Ionization Paradigm for Atmospheric Pressure Mass Spectrometry: Flying Elephants from Trojan Horses. Int. J. Mass Spectrom 2011, 300(2–3), 167–172. [Google Scholar]
- 36.Wang T; Zydel F; Harron A; Chubatyi N; McEwen CN Improving Ion Efficiency in Laserspray (LSI) and Sonic Spray Ionization (SSI) Mass Spectrometry. 58th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Salt Lake City, UT, May 23–27, 2010. (poster presentation) [Google Scholar]
- 37.Chubatyi N; Pagnotti V; McEwen C An Analysis of Voltage Free Mass Spectrometry Techniques: Sonicspray Ionization (SSI) and Solvent Assisted Inlet Ionization (SAII). 59th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Denver, CO, June 5–9, 2011. (poster presentation) [Google Scholar]
- 38.Lietz CB; Richards AL; Ren Y; Trimpin S Highs and Lows: Small to Large Protein Analysis with Matrix-Assisted Inlet Ionization (MAII) Techniques in Positive and Negative Ion Mode. 59th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Denver, CO, June 5–9, 2011. (poster presentation) [Google Scholar]
- 39.Chubatyi ND; Wang T; McEwen CN More Inclusive or Selective Ionization for Mass Spectrometry using Obstructive Sonic Spray Ionization and Voltage Polarity Switching. Rapid Commun. Mass Spectrom 2012, 26(23), 2763–2769. [DOI] [PubMed] [Google Scholar]
- 40.Trimpin S; Wang B; Inutan ED; Li J; Lietz CB; Harron A; Pagnotti VS; Sardelis D; McEwen CN A Mechanism for Ionization of Nonvolatile Compounds in Mass Spectrometry: Considerations from MALDI and Inlet Ionization. J. Am. Soc. Mass Spectrom 2012, 23(10), 1644–1660. [DOI] [PubMed] [Google Scholar]
- 41.Nyadong L; Inutan ED; Wang X; Hendrickson CL; Trimpin S; Marshall AG Laserspray and Matrix-Assisted Ionization Inlet Coupled to High-Field FT-ICR Mass Spectrometry for Peptide and Protein Analysis. J. Am. Soc. Mass Spectrom 2013, 24(3), 320–328. [DOI] [PubMed] [Google Scholar]
- 42.The Ultimate Quotable Einstein; Calaprice A, Ed.; Princeton University Press: Princeton, NJ, 2011. [Google Scholar]
- 43.Inutan E; Trimpin S Laserspray Ionization (LSI) Ion Mobility Spectrometry (IMS) Mass Spectrometry. J. Am. Soc. Mass Spectrom 2010, 21(7), 1260–1264. [DOI] [PubMed] [Google Scholar]
- 44.Inutan ED; Trimpin S Laserspray Ionization-Ion Mobility Spectrometry–Mass Spectrometry: Baseline Separation of Isomeric Amyloids without the Use of Solvents Desorbed and Ionized Directly from a Surface. J. Proteome Res 2010, 9(11), 6077–6081. [DOI] [PubMed] [Google Scholar]
- 45.Li J; Inutan ED; Wang B; Lietz CB; Green DR; Manly CD; Richards AL; Marshall DD; Lingenfelter S; Ren Y; Trimpin S Matrix Assisted Ionization: New Aromatic and Nonaromatic Matrix Compounds Producing Multiply Charged Lipid, Peptide, and Protein Ions in the Positive and Negative Mode Observed Directly from Surfaces. J. Am. Soc. Mass Spectrom 2012, 23(10), 1625–1643. [DOI] [PubMed] [Google Scholar]
- 46.Inutan ED; Jarois DR; Lietz CB; El-Baba TJ; Elia EA; Karki S; Sampat AAS; Foley CD; Clemmer DE; Trimpin S Comparison of Gaseous Ubiquitin Ion Structures Obtained from a Solid and Solution Matrix using Ion Mobility Spectrometry-Mass Spectrometry. Rapid Commun. Mass Spectrom 2021, 35 Suppl 1:e8793. [DOI] [PubMed] [Google Scholar]
- 47.Wang B; Inutan ED; Wager-Miller J; Mackie K; Suits AG; Trimpin S Highly Charged Protein Ions Generated by an UV-Matrix using Various Laser Wavelengths Ablation. 59th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Denver, CO, June 5–9, 2011. (poster presentation) [Google Scholar]
- 48.Beavis RC; Chaudhary T; Chait BT α-Cyano-4-Hydroxycinnamic Acid as a Matrix for Matrix-assisted Laser Desorption Mass Spectromtry. Org. Mass Spectrom 1992, 27(2), 156–158. [Google Scholar]
- 49.Strupat K; Karas M; Hillenkamp F 2,5-Dihydroxybenzoic Acid: A New Matrix for Laser Desorption—Ionization Mass Spectrometry. Int. J. Mass Spectrom 1991, 111, 89–102. [Google Scholar]
- 50.Wenzel T; Sparbier K; Mieruch T; Kostrzewa M 2,5-Dihydroxyacetophenone: A Matrix for Highly Sensitive Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometric Analysis of Proteins using Manual and Automated Preparation Techniques. Rapid Commun. Mass Spectrom 2006, 20(5), 785–789. [DOI] [PubMed] [Google Scholar]
- 51.McEwen CN; Larsen BS; Trimpin S Laserspray Ionization on a Commercial Atmospheric Pressure-MALDI Mass Spectrometer Ion Source: Selecting Singly or Multiply Charged Ions. Anal. Chem 2010, 82(12), 4998–5001. [DOI] [PubMed] [Google Scholar]
- 52.McEwen CN; Pagnotti VS; Sardelis D; Chubaty ND; Harron A Fundamentals and Applications of Inlet Ionization: Ionization Methods for Small and Large Molecules. 59th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Denver, CO, June 5–9, 2011. (oral presentation) [Google Scholar]
- 53.Pagnotti VS; McEwen CN Inlet Ionization: An Investigation into the Method That Produces Ions from an Instrument Inlet Absent of Lasers, or Voltages. 59th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Denver, CO, June 5–9, 2011. (oral presentation) [Google Scholar]
- 54.Trimpin S; Lutomski C; El-Baba TJ; Wang B; Imperial L; Woodall D; Kumar R; Harless B; Foley C; Liu CW; Inutan ED Surprising New Ionization Methods for Mass Spectrometry, Mechanistic Insights and Potential Practical Utility. 62nd Annual ASMS Conference on Mass Spectrometry and Allied Topics, Baltimore, MD, June 15–19, 2014. (oral presentation) [Google Scholar]
- 55.McEwen CN; Pagnotti VS; Inutan ED; Trimpin S New Paradigm in Ionization: Multiply Charged Ion Formation from a Solid Matrix without a Laser or Voltage. Anal. Chem 2010, 82(22), 9164–9168. [DOI] [PubMed] [Google Scholar]
- 56.Pagnotti VS; Chubatyi ND; McEwen CN Solvent Assisted Inlet Ionization: An Ultrasensitive New Liquid Introduction Ionization Method for Mass Spectrometry. Anal. Chem 2011, 83(11), 3981–3985. [DOI] [PubMed] [Google Scholar]
- 57.Pagnotti VS; Chakrabarty S; Wang B; Trimpin S; McEwen CN Gas-Phase Ions Produced by Freezing Water or Methanol for Analysis using Mass Spectrometry. Anal. Chem 2014, 86(15), 7343–7350. [DOI] [PubMed] [Google Scholar]
- 58.Lee C; Inutan ED; Chen JL; Mukeku MM; Weidner SM; Trimpin S; Ni C Toward Understanding the Ionization Mechanism of Matrix-assisted Ionization using Mass Spectrometry Experiment and Theory. Rapid Commun. Mass Spectrom 2021, 35 Suppl 1:e8382. [DOI] [PubMed] [Google Scholar]
- 59.Wang B; Trimpin S High-Throughput Solvent Assisted Ionization Inlet for Use in Mass Spectrometry. Anal. Chem 2014, 86(2), 1000–1006. [DOI] [PubMed] [Google Scholar]
- 60.Harron AF; Hoang K; McEwen CN High Mass Resolution Tissue Imaging at Atmospheric Pressure using Laserspray Ionization Mass Spectrometry. Int. J. Mass Spectrom 2013, 352, 65–69. [Google Scholar]
- 61.Cramer R; Pirkl A; Hillenkamp F; Dreisewerd K Liquid AP-UV-MALDI Enables Stable Ion Yields of Multiply Charged Peptide and Protein Ions for Sensitive Analysis by Mass Spectrometry. Angew. Chem. Int. Ed. Engl 2013, 52(8), 2364–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhai Y; Liu S; Gao L; Hu L; Xu W Direct Biological Sample Analyses by Laserspray Ionization Miniature Mass Spectrometry. Anal. Chem 2018, 90(9), 5696–5702. [DOI] [PubMed] [Google Scholar]
- 63.Zhou C; Wu H; Zhang X; Zhang Y; Xie W; Xu W High-Throughput and Direct Sample Screening using a Laser Spray Ionization Miniature Mass Spectrometer. Anal. Chem 2019, 91(14), 8808–8813. [DOI] [PubMed] [Google Scholar]
- 64.Liu S; Zuo J; Lu Y; Gao L; Zhai Y; Xu W Direct Bacteria Analysis using Laserspray Ionization Miniature Mass Spectrometry. Anal. Bioanal. Chem 2019, 411(18), 4031–4040. [DOI] [PubMed] [Google Scholar]
- 65.Inutan ED; Wang B; Trimpin S Commercial Intermediate Pressure MALDI Ion Mobility Spectrometry Mass Spectrometer Capable of Producing Highly Charged Laserspray Ionization Ions. Anal. Chem 2011, 83(3), 678–684. [DOI] [PubMed] [Google Scholar]
- 66.Trimpin S; Ren Y; Wang B; Lietz CB; Richards AL; Marshall DD; Inutan ED Extending the Laserspray Ionization Concept to Produce Highly Charged Ions at High Vacuum on a Time-of-Flight Mass Analyzer. Anal. Chem 2011, 83(14), 5469–5475. [DOI] [PubMed] [Google Scholar]
- 67.Inutan ED; Wang B; Wager-Miller J; Mackie K; Trimpin S A Commercial Intermediate Pressure MALDI-IMS-MS Producing Multiply Charged Laserspray Ionization Vacuum Ions (LSIV). 59th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Denver, CO, June 5–9, 2011. (poster presentation) [Google Scholar]
- 68.Trimpin S; Lee C; Weidner SM; El-Baba TJ; Lutomski CA; Inutan ED; Foley CD; Ni C-K; McEwen CN Unprecedented Ionization Processes in Mass Spectrometry Provide Missing Link between ESI and MALDI. ChemPhysChem 2018, 19(5), 581–589. [DOI] [PubMed] [Google Scholar]
- 69.El-Baba T; Imperial L; Inutan ED; Trimpin S Fundamental Studies on Matrix Assisted Ionization Vacuum (MAIV), a New Ionization Method Using New Matrices. 61st Annual ASMS Conference on Mass Spectrometry and Allied Topics, Minneapolis, MN, June 9–13, 2013. (poster presentation) [Google Scholar]
- 70.Trimpin S; Lutomski CA; El-Baba TJ; Woodall DW; Foley CD; Manly CD; Wang B; Liu C-W; Harless BM; Kumar R; Imperial LF; Inutan ED Magic Matrices for Ionization in Mass Spectrometry. Int. J. Mass Spectrom 2015, 377(SI), 532–545. [Google Scholar]
- 71.Inutan ED; Wager-Miller J; Mackie K; Trimpin S Laserspray Ionization Imaging of Multiply Charged Ions using a Commercial Vacuum MALDI Ion Source. Anal. Chem 2012, 84(21), 9079–9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pierre VC; Allen MJ; Caravan P Contrast Agents for MRI: 30+ Years and Where Are We Going? J. Biol. Inorg. Chem 2014, 19(2), 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Runge VM Safety of the Gadolinium-Based Contrast Agents for Magnetic Resonance Imaging, Focusing in Part on Their Accumulation in the Brain and Especially the Dentate Nucleus: Invest. Radiol 2016, 51(5), 273–279. [DOI] [PubMed] [Google Scholar]
- 74.Zhao SN; Song ZZ; Zhu M; Meng X; Wu LL; Song SY; Wang C; Zhang HJ Highly Thermostable Lanthanide Metal–Organic Frameworks Exhibiting Unique Selectivity for Nitro Explosives. RSC Adv. 2015, 5, 93–98. [Google Scholar]
- 75.Vithanarachchi SM; Foley CD; Trimpin S; Ewing JR; Ali MM; Allen MJ Myelin-Targeted, Texaphyrin-Based Multimodal Imaging Agent for Magnetic Resonance and Optical Imaging: Myelin-Targeted Multimodal Texaphyrins. Contrast Media Mol. Imaging 2016, 11(6), 492–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karas M; Bahr U Laser Desorption Ionization Mass Spectrometry of Large Biomolecules. TrAC Trends in Anal. Chem 1990, 9(10), 321–325. [Google Scholar]
- 77.Karas M; Glueckmann M; Schaefer J Ionization in Matrix-Assisted Laser Desorption/Ionization: Singly Charged Molecular Ions are the Lucky Survivors. J. Mass Spectrom 2000, 35(1), 1–12. [DOI] [PubMed] [Google Scholar]
- 78.Niu S; Zhang W; Chait BT Direct Comparison of Infrared and Ultraviolet Wavelength Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry of Proteins. J. Am. Soc. Mass Spectrom 1998, 9(1), 1–7. [DOI] [PubMed] [Google Scholar]
- 79.Chen B; Lietz CB; Li L In Situ Characterization of Proteins using Laserspray Ionization on a High-Performance MALDI-LTQ-Orbitrap Mass Spectrometer. J. Am. Soc. Mass Spectrom 2014, 25(12), 2177–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen S; Leibly D; Loo JA Highly Multiple Charging with 2-Nitrophloroglucinol for MALDI Time-of-Flight Mass Spectrometry. 60th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Vancouver, Canada, May 20–24, 2012. (poster presentation) [Google Scholar]
- 81.Rizzo DG; Spraggins JM; Rose KL; Caprioli RM Imaging and Accurate Mass Identifications of Intact Proteins above 10 kDa using Multiply Charged Ions and High Resolution MS. 61st Annual ASMS Conference on Mass Spectrometry and Allied Topics, Minneapolis, MN, June 9–13, 2013. (poster presentation) [Google Scholar]
- 82.Zavalin A; Junhai Y; Hayden K; Vestal M; Caprioli RM Tissue Protein Imaging at 1 μm Laser Spot Diameter for High Spatial Resolution and High Imaging Speed using Transmission Geometry MALDI TOF MS. Anal. Bioanal. Chem 2015, 407(8), 2337–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Banstola B; Murray KK A Nanoparticle Co-matrix for Multiple Charging in Matrix-assisted Laser Desorption Ionization Imaging of Tissue. Rapid Commun. Mass Spectrom 2021, 35 Suppl 1:e8424. [DOI] [PubMed] [Google Scholar]
- 84.Choi H; Lee D; Kim Y; Nguyen H-Q; Han S; Kim J Effects of Matrices and Additives on Multiple Charge Formation of Proteins in MALDI–MS Analysis. J. Am. Soc. Mass Spectrom 2019, 30(7), 1174–1178. [DOI] [PubMed] [Google Scholar]
- 85.Lietz CB; Richards AL; Ren Y; Trimpin S Inlet Ionization: Protein Analyses from the Solid State without the Use of a Voltage or a Laser Producing up to 67 Charges on the 66 kDa BSA Protein: Letter to the Editor. Rapid Commun. Mass Spectrom 2011, 25(22), 3453–3456. [DOI] [PubMed] [Google Scholar]
- 86.IgG Calibration Standard Kit for AB SCIEX MALDI-TOF Instruments. https://sciex.com/content/dam/SCIEX/pdf/customer-docs/user-guide/mass-spectrometry-4317093E.pdf
- 87.Signor L; Erba EB Matrix-assisted Laser Desorption/ionization Time of Flight (MALDI-TOF) Mass Spectrometric Analysis of Intact Proteins Larger than 100 kDa. J. Vis. Exp 2013, 79(e50635), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koomen JM; Russell WK; Hettick JM; Russell DH Improvement of Resolution, Mass Accuracy, and Reproducibility in Reflected Mode DE-MALDI-TOF Analysis of DNA using Fast Evaporation–Overlayer Sample Preparations. Anal. Chem 2000, 72(16), 3860–3866. [DOI] [PubMed] [Google Scholar]
- 89.Chen W-Y; Chen Y-C Reducing the Alkali Cation Adductions of Oligonucleotides using Sol–Gel-Assisted Laser Desorption/Ionization Mass Spectrometry. Anal. Chem 2003, 75(16), 4223–4228. [DOI] [PubMed] [Google Scholar]
- 90.Elia E; Leach S; Lu IC; Pophistic M; Trimpin S Development of a High Sensitive Method for Direct Characterization of Forensic Samples. 65th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Indianapolis, Indiana, June 4–8, 2017. (oral presentation) [Google Scholar]
- 91.Fenn J; Mann M; Meng C; Wong S; Whitehouse C Electrospray Ionization for Mass Spectrometry of Large Biomolecules. Science 1989, 246(4926), 64–71. [DOI] [PubMed] [Google Scholar]
- 92.Hirabayashi A; Sakairi M; Koizumi H Sonic Spray Mass Spectrometry. Anal. Chem 1995, 67(17), 2878–82. [DOI] [PubMed] [Google Scholar]
- 93.Pagnotti VS; Chakrabarty S; McEwen CN Solvent Assisted Inlet Ionization (SAII): Perspectives on Source Design, Application, and Ionization Mechanism. 61st Annual ASMS Conference on Mass Spectrometry and Allied Topics, Minneapolis, MN, June 9–13, 2013. (poster presentation) [Google Scholar]
- 94.Wang B; Li J; Trimpin S A New Approach using Solvent Assisted Inlet Ionization for High Throughput Analysis, Surface Analysis, and Liquid Chromatography-Mass Spectrometry. 60th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Vancouver, BC, Canada, May 20–24, 2012. (poster presentation) [Google Scholar]
- 95.Pagnotti V; Chakrabarty S; Harron A; McEwen CN Enhancing Solvent Assisted Inlet Ionization: The use of Carbonation and Other Super Saturated Gases as Solution Modifiers. 60th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Vancouver, BC, Canada, May 20–24, 2012. (poster presentation) [Google Scholar]
- 96.Wang B; Trimpin S Simply Applying Heat and/or Vacuum for Ionization of Small and Large Nonvolatile Molecules for Use in MS and its Applications. 61st Annual ASMS Conference on Mass Spectrometry and Allied Topics, Minneapolis, Minnesota, June 9–13, 2013. (poster presentation) [Google Scholar]
- 97.Fenner MA; McEwen CN Survival Yield Comparison between ESI and SAII: Mechanistic Implications. Int. J. Mass Spectrom 2015, 378, 107–112. [Google Scholar]
- 98.Pagnotti VS; Inutan ED; Marshall DD; McEwen CN; Trimpin S Inlet Ionization: A New Highly Sensitive Approach for Liquid Chromatography/Mass Spectrometry of Small and Large Molecules. Anal. Chem 2011, 83(20), 7591–7594. [DOI] [PubMed] [Google Scholar]
- 99.Wang B; Inutan ED; Trimpin S A New Approach to High Sensitivity Liquid Chromatography-Mass Spectrometry of Peptides using Nanoflow Solvent Assisted Inlet Ionization. J. Am. Soc. Mass Spectrom 2012, 23(3), 442–445. [DOI] [PubMed] [Google Scholar]
- 100.Chubatyi ND; Pagnotti VS; Bentzley CM; McEwen CN High Sensitivity Steroid Analysis using Liquid Chromatography/Solvent-assisted Inlet Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom 2012, 26(8), 887–92. [DOI] [PubMed] [Google Scholar]
- 101.Pagnotti VS; Chakrabarty S; Harron AF; McEwen CN Increasing the Sensitivity of Liquid Introduction Mass Spectrometry by Combining Electrospray Ionization and Solvent Assisted Inlet Ionization. Anal. Chem 2012, 84(15), 6828–6832. [DOI] [PubMed] [Google Scholar]
- 102.Fenner MA; Chakrabarty S, Wang B, Pagnotti VS; Hoang K; Trimpin S; McEwen CN A New LC/MS Method Providing Improved Sensitivity: Electrospray Ionization Inlet. Anal. Chem 2017, 89(9), 4798–4802. [DOI] [PubMed] [Google Scholar]
- 103.El-Baba TJ; Lutomski CA; Wang B; Inutan ED; Trimpin S Toward High Spatial Resolution Sampling and Characterization of Biological Tissue Surfaces using Mass Spectrometry. Anal. Bioanal. Chem 2014, 406(17), 4053–4061. [DOI] [PubMed] [Google Scholar]
- 104.Chakrabarty S; Shelver WL; Smith DJ Electrospray Ionization Inlet Tandem Mass Spectrometry: A Hyphenated Method for the Sensitive Determination of Chemicals in Animal Tissues and Body Fluids. J. Am. Soc. Mass Spectrom 2021, 32(1), 14–20. [DOI] [PubMed] [Google Scholar]
- 105.Wang B; Dearring CL; Wager-Miller J; Mackie K; Trimpin S Drug Detection and Quantification Directly from Tissue using Novel Ionization Methods for Mass Spectrometry. Eur. J. Mass Spectrom 2015, 21(3), 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Trimpin S, Rauschenbach S, Kern K, Lu IC, Inutan ED, Fischer JL, Thawoos S Devereaux ZJ, Zhang WJ, Zhang W, Dillenburger M, Rӓder HJ, Mülslen K, Hoang K, Sidorenko A, Pophristic M, McEwen CN, Fundamental Studies of Inlet Ionization at Atmospheric Pressure. 64th Annual ASMS Conference on Mass Spectrometry and Allied Topics, San Antonio, TX, June 5–9, 2016. (oral presentation) [Google Scholar]
- 107.Trimpin S; Wang B Inlet and Vacuum Ionization from Ambient Conditions, in Ambient Ionization Mass Spectrometry by Marek Domin and Robert Cody, 2014, 423–444. [Google Scholar]
- 108.Marshall DD; Inutan ED; Wang B; Liu C-W; Thawoos S; Wager-Miller J; Mackie K; Trimpin S A Broad-Based Study on Hyphenating New Ionization Technologies with MS/MS for PTMs and Tissue Characterization. Proteomics 2016, 16(11–12), 1695–1706. [DOI] [PubMed] [Google Scholar]
- 109.Lu I-C; Elia EA; Zhang W-J; Pophristic M; Inutan ED; McEwen CN; Trimpin S Development of an Easily Adaptable, High Sensitivity Source for Inlet Ionization. Anal. Methods 2017, 9(34), 4971–4978. [Google Scholar]
- 110.Pagnotti VS; Chakrabarty S; McEwen CN Carbonation and Other Super Saturated Gases as Solution Modifiers for Improved Sensitivity in Solvent Assisted Ionization Inlet (SAII) and ESI. J. Am. Soc. Mass Spectrom 2013, 24(2), 186–192. [DOI] [PubMed] [Google Scholar]
- 111.Horan AJ; Apsokardu MJ; Johnston MV Droplet Assisted Inlet Ionization for Online Analysis of Airborne Nanoparticles. Anal. Chem 2017, 89(2), 1059–1062. [DOI] [PubMed] [Google Scholar]
- 112.Apsokardu MJ; Kerecman DE; Johnston MV Ion Formation in Droplet-assisted Ionization. Rapid Commun. Mass Spectrom 2021, 35 Suppl 1:e8227. [DOI] [PubMed] [Google Scholar]
- 113.Li X; Attanayake K; Valentine SJ; Li P Vibrating Sharp-edge Spray Ionization (VSSI) for Voltage-free Direct Analysis of Samples using Mass Spectrometry. Rapid Commun. Mass Spectrom 2021, 35 Suppl 1:e8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu C-I; Wang Y-S; Chen NG; Wu C-Y; Chen C-H Ultrasound Ionization of Biomolecules: Ultrasound Ionization of Biomolecules. Rapid Commun. Mass Spectrom 2010, 24(17), 2569–2574. [DOI] [PubMed] [Google Scholar]
- 115.Huang Y; Yoon SH; Heron SR; Masselon CD; Edgar JS; Tureček F; Goodlett DR Surface Acoustic Wave Nebulization Produces Ions with Lower Internal Energy than Electrospray Ionization. J. Am. Soc. Mass Spectrom 2012, 23(6), 1062–1070. [DOI] [PubMed] [Google Scholar]
- 116.Ranganathan N; Li C; Suder T; Karanji AK; Li X; He Z; Valentine SJ; Li P Capillary Vibrating Sharp-Edge Spray Ionization (cVSSI) for Voltage-Free Liquid Chromatography-Mass Spectrometry. J. Am. Soc. Mass Spectrom 2019, 30(5), 824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li C; Attanayake K; Valentine SJ; Li P Facile Improvement of Negative Ion Mode Electrospray Ionization using Capillary Vibrating Sharp-Edge Spray Ionization. Anal. Chem 2020, 92(3), 2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jayasundara K; Li C; DeBastiani A; Sharif D; Li P; Valentine SJ Physicochemical Property Correlations with Ionization Efficiency in Capillary Vibrating Sharp-Edge Spray Ionization (cVSSI). J. Am. Soc. Mass Spectrom 2021, 32(1), 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Delsing P; Cleland AN; Schuetz MJA; Knörzer J; Giedke G; Cirac JI; Srinivasan K; Wu M; Balram KC; Bäuerle C; Meunier T; Ford CJB; Santos PV; Cerda-Méndez E; Wang H; Krenner HJ; Nysten EDS; Weiß M; Nash GR; Thevenard L; Gourdon C; Rovillain P; Marangolo M; Duquesne J-Y; Fischerauer G; Ruile W; Reiner A; Paschke B; Denysenko D; Volkmer D; Wixforth A; Bruus H; Wiklund M; Reboud J; Cooper JM; Fu Y; Brugger MS; Rehfeldt F; Westerhausen C The 2019 Surface Acoustic Waves Roadmap. J. Phys. D: Appl. Phys 2019, 52(35), 353001. [PMC free article] [PubMed] [Google Scholar]
- 120.Heron SR; Wilson R; Shaffer SA; Goodlett DR; Cooper JM Surface Acoustic Wave Nebulization of Peptides as a Microfluidic Interface for Mass Spectrometry. Anal. Chem 2010, 82(10), 3985–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ho J; Tan MK; Go DB; Yeo LY; Friend JR; Chang H-C Paper-Based Microfluidic Surface Acoustic Wave Sample Delivery and Ionization Source for Rapid and Sensitive Ambient Mass Spectrometry. Anal. Chem 2011, 83(9), 3260–3266. [DOI] [PubMed] [Google Scholar]
- 122.Sun D; Böhringer KF; Sorensen M; Nilsson E; Edgar JS; Goodlett DR Droplet Delivery and Nebulization System using Surface Acoustic Wave for Mass Spectrometry. Lab Chip 2020, 20(17), 3269–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haapala M; Teppo J; Ollikainen E; Kiiski I; Vaikkinen A; Kauppila TJ; Kostiainen R Solvent Jet Desorption Capillary Photoionization-Mass Spectrometry. Anal. Chem 2015, 87(6), 3280–3285. [DOI] [PubMed] [Google Scholar]
- 124.Zhang J; Rector J; Lin JQ; Young JH; Sans M; Katta N; Giese N; Yu W; Nagi C; Suliburk J; Liu J; Bensussan A; DeHoog RJ; Garza KY; Ludolph B; Sorace AG; Syed A; Zahedivash A; Milner TE; Eberlin LS Nondestructive Tissue Analysis for Ex Vivo and in Vivo Cancer Diagnosis using a Handheld Mass Spectrometry System. Sci. Transl. Med 2017, 9(406), eaan3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Keating MF; Zhang J; Feider CL; Retailleau S; Reid R; Antaris AL; Hart B; Tan G; Milner TE; Miller K; Eberlin LS Integrating the MasSpec Pen to the Da Vinci Surgical System for in Vivo Tissue Analysis during a Robotic Assisted Porcine Surgery. Anal. Chem 2020, 92(17), 11535–11542. [DOI] [PubMed] [Google Scholar]
- 126.Innovation to Enterprise. I2E, Inc., viewed 22 November 2020, https://I2e.Org/I2e-Management-Co-Inc-Leads-1-25-Million-Investment-in-Tulsa-Based-Ms-Pen-Technologies-Inc/.
- 127.Motoyama A; Kihara K Zero Volt Paper Spray Ionization Mass Spectrometry for Direct Analysis of Samples on Filter Paper Substrate. Rapid Commun. Mass Spectrom 2012, 29(20), 1905–1916. [DOI] [PubMed] [Google Scholar]
- 128.Trimpin S; Inutan ED New Ionization Method for Analysis on Atmospheric Pressure Ionization Mass Spectrometers Requiring Only Vacuum and Matrix Assistance. Anal. Chem 2013, 85(4), 2005–2009. [DOI] [PubMed] [Google Scholar]
- 129.Schaffer J What Not to Multiply Without Necessity. Australas. J. Philos 2015, 93(4), 644–664. [Google Scholar]
- 130.Krutchinsky AN; Chernushevich IV; Spicer VL; Ens W; Standing KG Collisional Damping Interface for an Electrospray Ionization Time-of-Flight Mass Spectrometer. J. Am. Soc. Mass Spectrom 1998, 9(6), 569–579. [Google Scholar]
- 131.Page JS; Bogdanov B; Vilkov AN; Prior DC; Buschbach MA; Tang K; Smith RD Automatic Gain Control in Mass Spectrometry using a Jet Disrupter Electrode in an Electrodynamic Ion Funnel. J. Am. Soc. Mass Spectrom 2005, 16(2), 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Page JS; Tolmachev AV; Tang K; Smith RD Theoretical and Experimental Evaluation of the Low m/z Transmission of an Electrodynamic Ion Funnel. J. Am. Soc. Mass Spectrom 2006, 17(4), 586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Page JS; Tang K; Kelly RT; Smith RD Subambient Pressure Ionization with Nanoelectrospray Source and Interface for Improved Sensitivity in Mass Spectrometry. Anal. Chem 2008, 80(5), 1800–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kurulugama RT; Valentine SJ; Sowell RA; Clemmer DE Development of a High-Throughput IMS-IMS-MS Approach for Analyzing Mixtures of Biomolecules. J Proteomics 2008, 71(3), 318–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Trimpin S; McEwen CN Redefining the Ion Source for MS and IMS-MS. 32nd ASMS Asilomar Conference: Novel Instrumentation in Mass Spectrometry and Mobility Spectrometry, Pacific Grove, CA, 2016. (oral presentation) [Google Scholar]
- 136.Inutan ED; Wager-Miller J; Narayan SB; Mackie K; Trimpin S The Potential for Clinical Applications using a New Ionization Method Combined with Ion Mobility Spectrometry-Mass Spectrometry. Int. J. Ion Mobil. Spec 2013, 16(2), 145–159. [Google Scholar]
- 137.Sweeting LM Triboluminescence with and without Air. Chem. Mater 2001, 13(3), 854–870. [Google Scholar]
- 138.Nadler WM; Waidelich D; Kerner A; Hanke S; Berg R; Trumpp A; Rösli C MALDI versus ESI: The Impact of the Ion Source on Peptide Identification. J. Proteome Res 2017, 16(3), 1207–1215. [DOI] [PubMed] [Google Scholar]
- 139.McEwen CN; Marshall DD; Karki S; Pophristic M; Hoang K; Trimpin S; Adenijii-Adele A; Tomsho JW More Inclusive Ionization Demonstrated for Direct Bacteria Differentiation by Combining Automated ESI, MAI, and SAI Methods. 67th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Atlanta, GA, June 2–6, 2019. (oral presentation) [Google Scholar]
- 140.Marshall DD; Karki S; Hoang K; Pophistic M; Lee C; Inutan ED; Leach S; McEwen CN; Trimpin S Examining the Discrimination Power of MAI for Identification of Microorganism. 67th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Atlanta, GA, June 2–6, 2019. (poster presentation) [Google Scholar]
- 141.Muthunayake NS; Islam R; Inutan ED; Colangelo W; Trimpin S; Cunningham PR; Chow CS Expression and In Vivo Characterization of the Antimicrobial Peptide Oncocin and Variants Binding to Ribosomes. Biochemistry 2020, 59(36), 3380–3391. [DOI] [PubMed] [Google Scholar]
- 142.Inutan ED; Meher AK; Marshall DD; Verani C; Narayan SB; Trimpin S Matrix Assisted Ionization Vacuum for Fast Analysis Directly from Biological or Synthetic Environments. 66th Annual ASMS Conference on Mass Spectrometry and Allied Topics, San Diego, CA, June 3–7, 2018. (poster presentation) [Google Scholar]
- 143.Mitchell J Jr.; Deveraux HD Determination of Traces of Organic Compounds in the Atmosphere-Role of Detectors in Gas-Chromatography. Anal. Chim. Acta 1978, 100, 45–52. [Google Scholar]
- 144.Trimpin S; Karki S; Marshall DD; Inutan ED; Meher AK; Madarshahian S; Fenner M; McEwen CN Combining Novel and Traditional Ionization Methods for Mass Spectrometry for More Comprehensive Analyses. LCGC North America. 2018, 16(1), 12–17, 27. [Google Scholar]
- 145.Trimpin S; Thawoos S; Foley CD; Woodall DW; Li J; Inutan ED; Stemmer PM Rapid High Mass Resolution Mass Spectrometry using Matrix-Assisted Ionization. Methods 2016, 104, 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Harding A; Hough J; Curtis C; Kinsman D; Clench M Matrix-Assisted Ionisation in Vacuum Mass Spectrometry and Imaging on a Modified Quadrupole-Quadrupole-Time-of-Flight Mass Spectrometer. J. Spectral Imaging 2019, 8(1), a12. [Google Scholar]
- 147.Wang B; Tisdale E; Trimpin S; Wilkins CL Matrix-Assisted Ionization Vacuum for High-Resolution Fourier Transform Ion Cyclotron Resonance Mass Spectrometers. Anal. Chem 2014, 86(14), 6792–6796. [DOI] [PubMed] [Google Scholar]
- 148.Liyanage R; Gidden J; Wilkins CL; Lay JO Matrix-assisted Ionization Fourier Transform Mass Spectrometry for the Analysis of Lipids. Rapid Commun. Mass Spectrom 2021, 35 Suppl 1:e8349. [DOI] [PubMed] [Google Scholar]
- 149.Chen B; Lietz CB; OuYang C; Zhong X; Xu M; Li L Matrix-Assisted Ionization Vacuum for Protein Detection, Fragmentation and PTM Analysis on a High Resolution Linear Ion Trap-Orbitrap Platform. Anal. Chim. Acta 2016, 916, 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Woodall DW; Wang B; Inutan ED; Narayan SB; Trimpin S High-Throughput Characterization of Small and Large Molecules using Only a Matrix and the Vacuum of a Mass Spectrometer. Anal. Chem 2015, 87(9), 4667–4674. [DOI] [PubMed] [Google Scholar]
- 151.Chakrabarty S; Pagnotti VS; Inutan ED; Trimpin S; McEwen CN A New Matrix Assisted Ionization Method for the Analysis of Volatile and Nonvolatile Compounds by Atmospheric Probe Mass Spectrometry. J. Am. Soc. Mass Spectrom 2013, 24(7), 1102–1107. [DOI] [PubMed] [Google Scholar]
- 152.Chakrabarty S; DeLeeuw JL; Woodall DW; Jooss K; Narayan SB; Trimpin S Reproducibility and Quantification of Illicit Drugs using Matrix-Assisted Ionization (MAI) Mass Spectrometry. Anal. Chem 2015, 87(16), 8301–8306. [DOI] [PubMed] [Google Scholar]
- 153.Zhang Z; Marshall AG A Universal Algorithm for Fast and Automated Charge State Deconvolution of Electrospray Mass-to-Charge Ratio Spectra. J. Am. Soc. Mass Spectrom 1998, 9(3), 225–233. [DOI] [PubMed] [Google Scholar]
- 154.Thawoos S; Wager-Miller J; Inutan ED; Devereaux ZJ; Foley CD; Ahn YH; Mackie K; Stemmer PM; Trimpin S Rapid Analysis of Proteins on High-Resolution Mass Spectrometers using Matrix-Assisted Ionization. 64th Annual ASMS Conference on Mass Spectrometry and Allied Topics, San Antonio, TX, June 5–9, 2016. (poster presentation) [Google Scholar]
- 155.Wang B; Liao G; Guo Z; Trimpin S Characterization of Carbohydrate-Monophosphoryl Lipid Conjugate Cancer Vaccine Candidates using Matrix Assisted Ionization Vacuum Mass Spectrometry. 62nd Annual ASMS Conference on Mass Spectrometry and Allied Topics, Baltimore, MD, June 15–19, 2014. (poster presentation) [Google Scholar]
- 156.Fischer JL; Lutomski CA; El-Baba TJ; Siriwardena-Mahanama BN; Weidner SM; Falkenhagen J; Allen MJ; Trimpin S Matrix-Assisted Ionization-Ion Mobility Spectrometry-Mass Spectrometry: Selective Analysis of a Europium-PEG Complex in a Crude Mixture. J. Am. Soc. Mass Spectrom 2015, 26(12), 2086–2095. [DOI] [PubMed] [Google Scholar]
- 157.Hoang K; Pophristic M; Horan AJ; Johnston MV; McEwen CN High Sensitivity Analysis of Nanoliter Volumes of Volatile and Nonvolatile Compounds using Matrix Assisted Ionization (MAI) Mass Spectrometry. J. Am. Soc. Mass Spectrom 2016, 27(10), 1590–1596. [DOI] [PubMed] [Google Scholar]
- 158.DeHart CJ; Fellers RT; Fornelli L; Kelleher NL; Thomas PM Bioinformatics Analysis of Top-Down Mass Spectrometry Data with ProSight Lite. In: Wu C, Arighi C, Ross K (eds) Protein Bioinformatics. Methods in Molecular Biology, Vol 1558. Humana Press, New York, NY., 2017, pp. 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McEwen CN; Pophristic M; Hoang K New Insights Relative to Matrices in Mass Spectrometry. 68th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Online Conference, May 31–June 4, 2020. (poster presentation) [Google Scholar]
- 160.Lutomski CA; El-Baba TJ; Inutan ED; Manly CD; Wager-Miller J; Mackie K; Trimpin S Transmission Geometry Laserspray Ionization Vacuum using an Atmospheric Pressure Inlet. Anal. Chem 2014, 86(13), 6208–6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang Q; Zhou Z; Tang S; Guo Z Carbohydrate-Monophosphoryl Lipid A Conjugates Are Fully Synthetic Self-Adjuvanting Cancer Vaccines Eliciting Robust Immune Responses in the Mouse. ACS Chem. Biol 2012, 7(1), 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lutomski CA; El-Baba TJ; Wager-Miller J; Mackie K; Trimpin S Ion Mobility Spectrometry and Tandem Mass Spectrometry for the Characterization of Gangliosides from Mouse Brain Tissue using Vacuum Ionization. 62nd Annual ASMS Conference on Mass Spectrometry and Allied Topics, Baltimore, MD, June 15–19, 2014. (poster presentation) [Google Scholar]
- 163.Oemer G; Lackner K; Muigg K; Krumschnabel G; Watschinger K; Sailer S; Lindner H; Gnaiger E; Wortmann SB; Werner ER; Zschocke J; Keller MA Molecular Structural Diversity of Mitochondrial Cardiolipins. Proc. Natl. Acad. Sci. USA 2018, 115(16), 4158–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Skjærvø Ø; Trimpin S; Halvorsen TG Matrix-assisted Ionization Mass Spectrometry in Targeted Protein Analysis – An Initial Evaluation. Rapid Commun. Mass Spectrom 2021, 35 Suppl 1:e8437. [DOI] [PubMed] [Google Scholar]
- 165.Halvorsen TG; Levernæs MCS; Rosting C Matrix-Assisted Ionization and Tandem Mass Spectrometry Capabilities in Protein Biomarker Characterization-An Initial Study Using the Small Cell Lung Cancer Biomarker Progastrin Releasing Peptide as a Model Compound. J. Am. Soc. Mass Spectrom 2021, 32(2), 611–614. [DOI] [PubMed] [Google Scholar]
- 166.Devereaux ZJ; Reynolds CA; Fischer JL; Foley CD; DeLeeuw JL; Wager-Miller J; Narayan SB; Mackie K; Trimpin S Matrix-Assisted Ionization on a Portable Mass Spectrometer: Analysis Directly from Biological and Synthetic Materials. Anal. Chem 2016, 88(22), 10831–10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Inutan ED; Halvorsen TG; Zhang WJ; Lu IC; Elia EA; Gibson S; Wang B; Wager-Miller J; Narayan SB; Allen MJ; Mackie K; Trimpin S Matrix-Assisted Ionization - Ion Moblility Spectrometry - Mass Spectrometry for the Characterization of Drugs Directly from Biological or Synthetic Environments. 65th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Indianapolis, IN, June 4–8, 2017. (oral presentation) [Google Scholar]
- 168.Trimpin S A New Ionization Method for Volatile and Nonvolatile Compounds Requiring Only Vacuum and Matrix Assistance. 61st Annual ASMS Conference on Mass Spectrometry and Allied Topics, Minneapolis, MN, June 9–13, 2013. (oral presentation) [Google Scholar]
- 169.Trimpin S; Thawoos S; Lu IC; DeLeeuw JL; Hoang K; Devereaux Z; Pophristic M; Chakrabarty S; Caruso JA; Stemmer P; McEwen NC Simplifying Mass Spectrometry Through New Ionization Technology: Application to Drugs and Clinical Analyses. Spectrosc. 2016, 14(2), 8–19. [Google Scholar]
- 170.Trimpin S; Reynolds C; Foley CD; Chakrabarty S; Woodall DA; DeLeeuw JL; Fischer JL; Thawoos S; Harless BM; Verani C; Allen MJ; Sanderson T; Przyklenk K; Stemmer P Matrix Assisted Ionization: Enhancing Mass Spectrometry through Proper Sampling Conditions on Small Portable to High Performance Mass Spectrometers. 63rd Annual ASMS Conference on Mass Spectrometry and Allied Topics, St. Louis, MO, May 31–June 4, 2015. (oral presentation) [Google Scholar]
- 171.Santos JM; Vendramini PH; Schwab NV; Eberlin MN; de Morais DR A Dopant for Improved Sensitivity in Easy Ambient Sonic-Spray Ionization Mass Spectrometry: A Dopant for Improved Sensitivity in EASI-MS. J. Mass Spectrom 2016, 51(1), 53–61. [DOI] [PubMed] [Google Scholar]
- 172.Kanaki K; Pergantis SA Use of 3-Nitrobenzonitrile as an Additive for Improved Sensitivity in Sonic-spray Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom 2014, 28(23), 2661–2669. [DOI] [PubMed] [Google Scholar]
- 173.Banstola B; Murray KK Pulsed Valve Matrix-Assisted Ionization. Analyst 2017, 142(10), 1672–1675. [DOI] [PubMed] [Google Scholar]
- 174.Banstola B; Szot CW; Deenamulla Kankanamalage AP; Murray KK Piezoelectric Matrix-Assisted Ionization. Eur. J. Mass Spectrom 2019, 25(2), 202–207. [DOI] [PubMed] [Google Scholar]
- 175.Chen B; OuYang C; Tian Z; Xu M; Li L A High Resolution Atmospheric Pressure Matrix-Assisted Laser Desorption/Ionization-Quadrupole-Orbitrap MS Platform Enables in Situ Analysis of Biomolecules by Multi-Mode Ionization and Acquisition. Anal. Chim. Acta 2018, 1007, 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rybkin A. Yu.; Belik A. Yu.; Tarakanov PA; Taziev KR; Kozlov AV; Goryachev NS; Sulimenkov IV; Kozlovskiy VI; Romanenko YV; Koifman OI; Kotelnikov AI Pyropheophorbide-Fullerene Dyad: Synthesis and Photochemical Properties. Macroheterocycles 2019, 12(2), 181–186. [Google Scholar]
- 177.Wilkins CL; Trimpin S Ion Mobility Spectrometry: Theory and Applications; CRC Press, 2010. [Google Scholar]
- 178.Trimpin S; Clemmer DE Ion Mobility Spectrometry/Mass Spectrometry Snapshots for Assessing the Molecular Compositions of Complex Polymeric Systems. Anal. Chem 2008, 80(23), 9073–9083. [DOI] [PubMed] [Google Scholar]
- 179.Austin CA; Inutan ED; Bohrer BC; Li J; Fischer JL; Wijerathne K; Foley CD; Lietz CB; Woodall DW; Imperial LF; Clemmer DE; Trimpin S; Larsen BS Resolving Isomers of Star-Branched Poly(Ethylene Glycols) by IMS-MS using Multiply Charged Ions. J. Am. Soc. Mass Spectrom 2021, 32(1), 21–32. [DOI] [PubMed] [Google Scholar]
- 180.El-Baba TJ; Lutomski CA; Wang B, Trimpin S Characterizing Synthetic Polymers and Additives using New Ionization Methods for Mass Spectrometry. Rapid Commun. Mass Spectrom 2014, 28(11), 1175–1184. [DOI] [PubMed] [Google Scholar]
- 181.Lutomski C; Wang B; Inutan ED; Trimpin S Production of Multiply Charged Ions from the Solid State by Matrix Assisted Ionization Vacuum: Does pH Matter? 61st Annual ASMS Conference on Mass Spectrometry and Allied Topics, Minneapolis, MN, June 9–13, 2013. (poster presentation) [Google Scholar]
- 182.Woodall DW; Lutomski CA; Trimpin S Insights into Gas-Phase Protein Conformations from Matrix-Assisted Ionization (MAI) using Ion Mobility Spectrometry-Mass Spectrometry. 63rd Annual ASMS Conference on Mass Spectrometry and Allied Topics, St. Louis, MO, May 31–June 4, 2015. (poster presentation) [Google Scholar]
- 183.Inutan ED; El-Baba TJ; Walker A; Woodall DA; Stemmer PM; Cisneros GA; Clemmer DE; Trimpin S Ubiquitin Ion Structures from the Solid State using Nothing More than a Small Molecule and Vacuum of an IMS-MS Instrument. 64th Annual ASMS Conference on Mass Spectrometry and Allied Topics, San Antonio, TX, June 5–9, 2016. (oral presentation) [Google Scholar]
- 184.El-Baba TJ; Wickramasinghe LD; Siriwardena-Mahanama BN; Allen MJ; Verani C; Trimpin S Characterization of Monolayer Films of Asymmetric Metallosurfactants Other Coordination Compounds by Matrix Assisted Ionization Vacuum and Mass Spectrometry. 62nd Annual ASMS Conference on Mass Spectrometry and Allied Topics, Baltimore, MD, June 15–19, 2014. (poster presentation) [Google Scholar]
- 185.Singhal N; Kumar M; Kanaujia PK; Virdi JS MALDI-TOF Mass Spectrometry: An Emerging Technology for Microbial Identification and Diagnosis. Front. Microbiol 2015, 6, 791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vestal ML Evolution of Quantitative MALDI-TOF Mass Spectrometry for Clinical Applications. Clinical Chemistry 2016, 62(1), 20–23. [DOI] [PubMed] [Google Scholar]
- 187.Spraggins JM; Rizzo DG; Moore JL; Noto MJ; Skaar EP; Caprioli RM Next-Generation Technologies for Spatial Proteomics: Integrating Ultra-High Speed MALDI-TOF and High Mass Resolution MALDI FTICR Imaging Mass Spectrometry for Protein Analysis. Proteomics 2016, 16(11–12), 1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wu T; Zhang C; Ren H; Xi Y; Du Y; Peng Y Solvent Effect in Polymer Analysis by MALDI-TOF Mass Spectrometry. Int. J. Polym. Anal. Charact 2017, 22(2), 160–168. [Google Scholar]
- 189.Vestal M; Li L; Dobrinskikh E; Shi Y; Wang B; Shi X; Li S; Vestal C; Parker K Rapid MALDI-TOF Molecular Imaging: Instrument Enhancements and Their Practical Consequences. J. Mass Spectrom 2020, 55(8), e4423. [DOI] [PubMed] [Google Scholar]
- 190.Moskovets E; Jackson SN; Muller L; Barbacci D; Doroshenko V; Schultz A; Woods AS High-Resolution Tissue Imaging using Subatmospheric and Atmospheric MALDI Sources. 65th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Indianapolis, IN, June 4–8, 2017. (poster presentation) [Google Scholar]
- 191.Bowman AP; Bogie JFJ; Hendriks JJA; Haidar M; Belov M; Heeren RMA; Ellis SR Evaluation of Lipid Coverage and High Spatial Resolution MALDI-Imaging Capabilities of Oversampling Combined with Laser Post-Ionisation. Anal. Bioanal. Chem 2020, 412(10), 2277–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kumar R; Liu C-W; Lutomski C; Inutan E; Trimpin S Tandem Mass Spectrometry Sequencing of Post-Translationally Modified Peptides using Matrix Assisted Ionization Vacuum on an ESI Source. 62nd Annual ASMS Conference on Mass Spectrometry and Allied Topics, Baltimore, MD, June 15–19, 2014. (poster presentation) [Google Scholar]
- 193.Inutan ED; Trimpin S Mass Spectrometer Vacuum Enables Dislodging Matrix/Analyte Clusters and Forming Gas-Phase Ions from Proteins and Protein Complexes from Surfaces including Tissue. 60th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Vancouver, Canada, May 20–24, 2012. (poster presentation) [Google Scholar]
- 194.Goodreads Site. viewed 22 November 2020, https://Www.Goodreads.Com/Quotes/978-Whether-You-Think-You-Can-or-You-Think-You-Can-t--You-Re. Goodreads, Inc, 2020. [Google Scholar]
- 195.Olawale DO, Okoli OOI, Fontenot RS, Hollerman WA, Triboluminescence: Theory, Synthesis, and Application; Eds.; Springer International Publishing: Switzerland, 2016. [Google Scholar]
- 196.Xie Y; Li Z Triboluminescence: Recalling Interest and New Aspects. Chem 2018, 4(5), 943–971. [Google Scholar]
- 197.Zink JI; Klimt W Triboluminescence of Coumarin. Fluorescence and Dynamic Spectral Features Excited by Mechanical Stress. J. Am. Chem. Soc 1974, 96(14), 4690–4692. [Google Scholar]
- 198.Hardy GE; Baldwin JC; Zink JI; Kaska WC; Liu P-H; Dubois L Triboluminescence Spectroscopy of Aromatic Compounds. J. Am. Chem. Soc 1977, 99(11), 3552–3558. [Google Scholar]
- 199.Quickenden TI; Selby BJ; Freeman CG Ice Triboluminescence. J. Phys. Chem. A 1998, 102(34), 6713–6715. [Google Scholar]
- 200.Beckey HD Field Desorption Mass Spectrometry: A Technique for the Study of Thermally Unstable Substances of Low Volatility. Int. J. Mass Spectrom. Ion Processes 1969, 2(6), 500–503. [Google Scholar]
- 201.Beckey HD Experimental Techniques in Field Ionisation and Field Desorption Mass Spectrometry. J. Phys. E: Sci. Instrum 1979, 12(2), 72–83. [Google Scholar]
- 202.Torgerson DF; Skowronski RP; Macfarlane RD New Approach to the Mass Spectroscopy of Non-Volatile Compounds. Biochemical and Biophysical Research Communications 1974, 60(2), 616–621. [DOI] [PubMed] [Google Scholar]
- 203.Jonsson GP; Hedin AB; Hakansson PL; Sundqvist BUR; Saeve BGS; Nielsen PF; Roepstorff Peter.; Johansson K. Erik; Kamensky Ivan.; Lindberg MSL Plasma Desorption Mass Spectrometry of Peptides and Proteins Adsorbed on Nitrocellulose. Anal. Chem 1986, 58(6), 1084–1087. [Google Scholar]
- 204.Cotter RJ Plasma Desorption Mass Spectrometry: Coming of Age. Anal. Chem 1988, 60(13), 781A–793A. [DOI] [PubMed] [Google Scholar]
- 205.Barber M; Bordoli RS; Sedgwick RD; Tyler AN Fast Atom Bombardment of Solids as an Ion Source in Mass Spectrometry. Nature 1981, 293, 270–275. [Google Scholar]
- 206.Tanaka K; Waki H; Ido Y; Akita S; Yoshida Y; Yoshida T; Matsuo T Protein and Polymer Analyses up to m/z 100 000 by Laser Ionization Time-of-Flight Mass Spectrometry. Rapid Commun. Mass Spectrom 1988, 2(8), 151–153. [Google Scholar]
- 207.Karas Michael.; Hillenkamp Franz. Laser Desorption Ionization of Proteins with Molecular Masses Exceeding 10,000 Daltons. Anal. Chem 1988, 60(20), 2299–2301. [DOI] [PubMed] [Google Scholar]
- 208.Lerach JO; Keskin S; Winograd N Investigations Into the Interactions of a MALDI Matrix with Organic Thin Films Using C60+ SIMS Depth Profiling. Surf. Interface Anal 2014, 46(Suppl 1), 67–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kinsel GR; Edmondson RD; Russell DH Profile and Flight Time Analysis of Bovine Insulin Clusters as a Probe of Matrix-Assisted Laser Desorption/Ionization Ion Formation Dynamics. J. Mass Spectrom 1997, 32, 714–722. [DOI] [PubMed] [Google Scholar]
- 210.Kinsel GR; Gimon-Kinsel ME; Gillig KJ; Russell DH Investigation of the Dynamics of Matrix-Assisted Laser Desorption/Ionization Ion Formation using an Electrostatic Analyzer/Time-of-Flight Mass Spectrometer. J. Mass Spectrom 1999, 34, 684–690. [Google Scholar]
- 211.Fournier I; Brunot A; Tabet JC; Bolbach G Delayed Extraction Experiments using a Repulsive Potential before Ion Extraction: Evidence of Clusters as Ion Precursors in UV-MALDI. Part I: Dynamical Effects with the Matrix 2,5-Dihydroxybenzoic Acid. Int. J. Mass Spectrom 2002, 213(2–3), 203–215. [Google Scholar]
- 212.Krutchinsky AN; Chait BT On the Nature of the Chemical Noise in MALDI Mass Spectra. J. Am. Soc. Mass Spectrom 2002, 13, 129–134. [DOI] [PubMed] [Google Scholar]
- 213.Karas M; Krüger R Ion Formation in MALDI: The Cluster Ionization Mechanism. Chem. Rev 2003, 103(2), 427–440. [DOI] [PubMed] [Google Scholar]
- 214.Jaskolla TW; Karas M Compelling Evidence for Lucky Survivor and Gas Phase Protonation: The Unified MALDI Analyte Protonation Mechanism. J. Am. Soc. Mass Spectrom 2011, 22(6), 976–988. [DOI] [PubMed] [Google Scholar]
- 215.Knochenmuss R; Vertes A Time-Delayed 2-Pulse Studies of MALDI Matrix Ionization Mechanisms. J. Phys. Chem. B 2000, 104(23), 5406–5410. [Google Scholar]
- 216.Knochenmuss R; Zenobi R MALDI Ionization: The Role of In-Plume Processes. Chem. Rev 2003, 103(2), 441–452. [DOI] [PubMed] [Google Scholar]
- 217.Lu I-C; Lee C; Lee YT; Ni CK Ionization Mechanism of Matrix-Assisted Laser Desorption/Ionization. Ann. Rev. Anal. Chem 2015, 8(1), 21–39. [DOI] [PubMed] [Google Scholar]
- 218.Lu IC; Chu KY; Lin CY; Wu SY; Dyakov YA; Chen JL; Gray-Weale A; Lee YT; Ni CK Ion-to-Neutral Ratios and Thermal Proton Transfer in Matrix-Assisted Laser Desorption Ionization. J. Am. Soc. Mass Spectrom 2015, 26(7), 1242–1251. [DOI] [PubMed] [Google Scholar]
- 219.Gray-Weale A; Ni CK Comment on: “Energetics and Kinetics of Thermal Ionization Models of MALDI” by Richard Knochenmuss. J. Am. Soc. Mass Spectrom 2015, 26(12), 2162–2166. [DOI] [PubMed] [Google Scholar]
- 220.Trimpin S; Inutan ED; Lee C; Elia EA; Pophristic M; Marshall DD; Karki S; I-Chung L; Auner G; Wager-Miller J; Mackie K; Shulaev V; Weidner SM; Ni CK; McEwen CN Sub-Atmospheric Pressure Matrix-Assisted Ionization (MAI) Provides Simplicity, Sensitivity, and Robustness. 66th Annual ASMS Conference on Mass Spectrometry and Allied Topics, San Diego, CA, June 3–7, 2018. (oral presentation) [Google Scholar]
- 221.Liang C-W; Chang P-J; Lin Y-J; Lee Y-T; Ni C-K High Ion Yields of Carbohydrates from Frozen Solution by UV-MALDI. Anal. Chem 2012, 84(8), 3493–3499. [DOI] [PubMed] [Google Scholar]
- 222.McEwen CN; Hoang K; Fenner MA; Pophristic M; Trimpin S; Elia EA; Meher AK; Inutan ED Mechanistic Insights Gained from Spontaneous Ionization using Solid or Solvent Matrices. 65th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Indianapolis, IN, June 4–8, 2017. (oral presentation) [Google Scholar]
- 223.Chubatyi ND; McEwen CN Improving the Sensitivity of Matrix-Assisted Ionization (MAI) Mass Spectrometry using Ammonium Salts. J. Am. Soc. Mass Spectrom 2015, 26(10), 1649–1656. [DOI] [PubMed] [Google Scholar]
- 224.Leader GR The Dielectric Constant of Formamide. J. Am. Chem. Soc 1951, 73(2), 856–857. [Google Scholar]
- 225.Geiser L; Cherkaoui S; Veuthey J-L Potential of Formamide and N-Methylformamide in Nonaqueous Capillary Electrophoresis Coupled to Electrospray Ionization Mass Spectrometry. J. Chromatogr. A 2002, 979 (1–2), 389–398. [DOI] [PubMed] [Google Scholar]
- 226.Zhang WJ; Thapa B; Schlegel B; Trimpin S Developing a Fundamental Understanding of Inlet Ionization through Density Functional Theory (DFT). 65th Annual ASMS Conference on Mass Spectrometry and Allied Topics, Indianapolis, IN, June 4–8, 2017. (poster presentation) [Google Scholar]
- 227.Karki S; Meher AK; Inutan ED; Pophristic M; Marshall DD; Rackers K; Trimpin S; McEwen CN Development of a Robotics Platform for Automated Multi-ionization Mass Spectrometry. Rapid Commun. Mass Spectrom 2021, 35 Suppl 1:e8449. [DOI] [PubMed] [Google Scholar]
- 228.Basal LA; Bailey MD; Romero J; Ali MM; Kurenbekova L; Yustein J; Pautler RG; Allen MJ Fluorinated Eu II -Based Multimodal Contrast Agent for Temperature- and Redox-Responsive Magnetic Resonance Imaging. Chem. Sci 2017, 8(12), 8345–8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Mannion DR; Mannion JM; Kuhne WW; Wellons MS Matrix-Assisted Ionization of Molecular Uranium Species. J. Am. Soc. Mass Spectrom 2021, 32(1), 8–13. [DOI] [PubMed] [Google Scholar]
- 230.Cooks RG; Ouyang Z; Takats Z; Wiseman JM Ambient Mass Spectrometry. Science 2006, 311(5767), 1566–1570. [DOI] [PubMed] [Google Scholar]
- 231.Feider CL; Krieger A; DeHoog RJ; Eberlin LS Ambient Ionization Mass Spectrometry: Recent Developments and Applications. Anal. Chem 2019, 91(7), 4266–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.McLaughlin G; Morris N; Kavanagh PV; Power JD; O’Brien J; Talbot B; Elliott SP; Wallach J; Hoang K; Morris H; Brandt SD Test Purchase, Synthesis, and Characterization of 2-Methoxydiphenidine (MXP) and Differentiation from its Meta- and Para-Substituted Isomers: Characterization of 2-, 3- and 4-Methoxydiphenidine Isomers. Drug Test. Anal 2016, 8(1), 98–109. [DOI] [PubMed] [Google Scholar]
- 233.Dugourd Ph.; Hudgins RR; Clemmer DE; Jarrold MF High-Resolution Ion Mobility Measurements. Rev. Sci. Instrum 1997, 68(2), 1122–1129. [Google Scholar]
- 234.McLean JA; Ruotolo BT; Gillig KJ; Russell DH Ion Mobility–Mass Spectrometry: A New Paradigm for Proteomics. Int. J. Mass Spectrom 2005, 240(3), 301–315. [Google Scholar]
- 235.Bohrer BC; Merenbloom SI; Koeniger SL; Hilderbrand AE; Clemmer DE Biomolecule Analysis by Ion Mobility Spectrometry. Annual Rev. Anal. Chem 2008, 1(1), 293–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.El-Baba TJ; Fuller DR; Hales DA; Russell DH; Clemmer DE Solvent Mediation of Peptide Conformations: Polyproline Structures in Water, Methanol, Ethanol, and 1-Propanol as Determined by Ion Mobility Spectrometry-Mass Spectrometry. J. Am. Soc. Mass Spectrom 2019, 30(1), 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Folch J; Lees M; Stanley S A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem 1957, 226(1), 497–509. [PubMed] [Google Scholar]
- 238.Iverson SJ; Lang SLC; Cooper MH Comparison of the Bligh and Dyer and Folch Methods for Total Lipid Determination in a Broad Range of Marine Tissue. Lipids 2001, 36(11), 1283–1287. [DOI] [PubMed] [Google Scholar]
- 239.Kim Y; Shanta SR; Zhou L-H; Kim KP Mass Spectrometry Based Cellular Phosphoinositides Profiling and Phospholipid Analysis: A Brief Review. Exp. Mol. Med 2010, 42(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sud M; Fahy E; Cotter D; Brown A; Dennis EA; Glass CK; Merrill AH; Murphy RC; Raetz CRH; Russell DW; Subramaniam S LMSD: LIPID MAPS Structure Database. Nucleic Acids Res 2007, 35(Supplemental Issue_1), D527–D532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Liebisch G; Vizcaíno JA; Köfeler H; Trötzmüller M; Griffiths WJ; Schmitz G; Spener F; Wakelam MJO Shorthand Notation for Lipid Structures Derived from Mass Spectrometry. J. Lipid Res 2013, 54(6), 1523–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fahy E; Subramaniam S; Brown HA; Glass CK; Merrill AH; Murphy RC; Raetz CRH; Russell DW; Seyama Y; Shaw W; Shimizu T; Spener F; van Meer G; VanNieuwenhze MS; White SH; Witztum JL; Dennis EA A Comprehensive Classification System for Lipids. J. Lipid Res 2005, 46(5), 839–862. [DOI] [PubMed] [Google Scholar]
- 243.Grayson SM; Myers BK; Bengtsson J; Malkoch M Advantages of Monodisperse and Chemically Robust “SpheriCal” Polyester Dendrimers as a “Universal” MS Calibrant. J. Am. Soc. Mass Spectrom 2014, 25(3), 303–309. [DOI] [PubMed] [Google Scholar]
- 244.Giesen JA; Diament BJ; Grayson SM Iodine-Containing Mass-Defect-Tuned Dendrimers for Use as Internal Mass Spectrometry Calibrants. J. Am. Soc. Mass Spectrom 2018, 29(3), 490–500. [DOI] [PubMed] [Google Scholar]
- 245.Trimpin S; Clemmer DE; Larsen BS Snapshots, Conformation, and Bulk Fragmentation Analyses for the Characterization of Polymeric Architectures using ESI-IMS-MS. In Ion Mobility Spectrometry: Theory and Applications; CRC Press: US, 2010; pp 215–236. [Google Scholar]
- 246.Inutan ED; Jarois DR; Meher AK; Karki S; Fischer JL; Imperial LF; Foley CD; El-Baba TJ; Lutomski CL; Trimpin S New MS Concepts for Synthetic Polymers and Additives Characterization. Rapid Commun. Mass Spectrom 2020, 34 Suppl 2:e8768. [DOI] [PubMed] [Google Scholar]
- 247.Chen Y; Lu I Novel Ion Source for a Portable Mass Spectrometer. Rapid Commun. Mass Spectrom 2021, 35 Suppl 1:e8503. [DOI] [PubMed] [Google Scholar]
- 248.Harris DC; Charles AL Quantitative Chemical Analysis, 10th ed.; W. H. Freeman: US, 2020. [Google Scholar]
- 249.Yamashita M; Fenn JB Electrospray Ion Source. Another Variation on the Free-Jet Theme. J. Phys. Chem 1984, 88(20), 4451–4459. [Google Scholar]
- 250.Whitehouse CM; Dreyer RN; Yamashita Masamichi.; Fenn JB Electrospray Interface for Liquid Chromatographs and Mass Spectrometers. Anal. Chem 1985, 57(3), 675–679. [DOI] [PubMed] [Google Scholar]
- 251.Khorana HG; Gerber GE; Herlihy WC; Gray CP; Anderegg RJ; Nihei K; Biemann K Amino Acid Sequence of Bacteriorhodopsin. Proc. Natl. Acad. Sci. USA 1979, 76(10), 5046–5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Biemann K; Scoble H Characterization by Tandem Mass Spectrometry of Structural Modifications in Proteins. Science 1987, 237(4818), 992–998. [DOI] [PubMed] [Google Scholar]
- 253.Johnson RS; Martin SA; Biemann K Collision-Induced Fragmentation of (M + H)+ Ions of Peptides. Side Chain Specific Sequence Ions. Int. J. Mass Spectrom. Ion Processes 1988, 86, 137–154. [Google Scholar]
- 254.Biemann K Contributions of Mass Spectrometry to Peptide and Protein Structure. Biol. Mass Spectrom 1988, 16(1–12), 99–111. [DOI] [PubMed] [Google Scholar]
- 255.Biemann K Sequencing of Peptides by Tandem Mass Spectrometry and High-Energy Collision-Induced Dissociation. In Methods in Enzymology; Elsevier, 1990, 193, 455–479. [DOI] [PubMed] [Google Scholar]
- 256.Biemann K Appendix 5. Nomenclature for Peptide Fragment Ions (Positive Ions). In Methods in Enzymology; Elsevier, 1990, 193, 886–887. [DOI] [PubMed] [Google Scholar]
- 257.Rhomberg AJ; Biemann K Mass Spectrometric Analysis of Highly Acidic Polysaccharides. In: Jackson P, Gallagher JT (eds) A Laboratory Guide to Glycoconjugate Analysis. BioMethods. Birkhäuser; Basel, 1997. 10.1007/978-3-0348-7388-8_5 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


