Abstract
CHD8 mutations cause autism spectrum disorder, cognitive deficits, and macrocephaly. Chd8+/− mouse models exhibit macrocephaly and transcriptional pathology, with inconsistent findings regarding neurogenesis, neuron function, and behavior. Via stereology and single nuclei transcriptomics (snRNA-seq), we found increased Chd8+/− cortical volume was not explained by increase in neuron number. Differential expression (DE) was present across cortical cell types, with excitatory neurons exhibiting high DE burden and shared and subclass-specific DE signatures. Bulk RNA-seq DE of constitutive Chd8+/− and conditional Camk2a-Cre Chd8+/− mice identified shared transcriptional pathology. DE in synaptosomal versus nuclear mRNA identified overlapping DEGs, but also significant differences and exaggerated synaptosomal changes. Building on DE findings implicating glutamatergic neurons, we found Chd8+/− mice exhibited altered excitatory neuron spine density and dynamics, decreased GCaMP activity correlation, and sleep perturbation. Thus, Chd8 haploinsufficiency causes lasting excitatory neuron dysfunction, perturbs RNA regulation beyond transcription, and impacts neuronal properties, cortical microcircuits, and behavior.
Introduction
Mutations in a growing list of genes cause monogenic Neurodevelopmental Disorders (NDDs) with overlapping but complex presentation, including autism spectrum disorder (ASD), intellectual disability (ID), and behavior or psychiatric disorders1,2. CHD8 encodes the chromatin remodeling factor (CRF) Chromodomain Helicase DNA-binding protein 8, and has among the highest rates of protein disrupting rare and de novo mutations in ASD cases1. CHD8-associated NDD (CHD8-NDD) has core but variably expressed features including ASD, ID, and macrocephaly, as well as sleep, gastrointestinal, psychiatric, and neurological issues3–9. CHD8 is ubiquitously expressed and has pleiotropic functions in the developing and mature brain. Clarifying the links between CHD8 haploinsufficiency, macrocephaly, and pathology may offer critical insights into the etiology of the 15% of ASD patients that exhibit more severe cognitive and behavioral phenotypes often with poorer prognosis10. Furthermore, Chd8 has been identified as a central regulator in network studies of ASD-associated loci11–13, and serves as a key example of the many CRF genes implicated in ASD and other NDDs14–18. Defining the pathological consequences associated with CHD8 haploinsufficiency is thus an important step towards understanding both CHD8-specific mechanisms and broader NDD neurobiology.
Chd8 knockout is embryonic lethal in mice19,20, and NDD-relevant phenotypes have been observed across studies of heterozygous Chd8+/− mouse models20–26. Among the consistent findings across constitutive Chd8+/− mouse models is the presence of adult macrocephaly and transcriptional pathology20,22,24,25. Bulk RNA-seq on developing and adult Chd8+/− mouse brain has been performed in a number of studies, finding relatively subtle effects but replicated perturbation of energetics and protein homeostasis and downregulation of synaptic and neuronal genes20,21,24,25,27–30. Recent single cell studies of Chd8+/− mice suggest subtle changes in neurodevelopment and perturbations to neurons31–33, but have lacked resolution to analyze specific subtypes in mature cortex. Studies of Chd8+/− mouse behavior and cognition have been more variable20–26, with evidence that some variability is due to genetic background34,35. Multiple studies of Chd8 haploinsufficiency have identified changes to electrophysiology in the cortex and in in vitro21,24,36,37, indicating impacts on neuron function. In vitro studies in human and non-human primate neurons and human cortical organoids also indicate perturbation to neurogenesis, increases in organoid size paralleling macrocephaly, and neuronal electrophysiological and network level dysfunction31,32,38–41. Finally, conditional Chd8 knockout studies have been conducted targeting specific cell types such as excitatory neurons36,42–46, oligodendrocytes47–49, and astrocytes and microglia50,51. Many of these studies have primarily focused on homozygous conditional mutants, which may not accurately model the heterozygous mutations relevant to NDDs.
Despite extensive study of Chd8 as a representative NDD risk gene, key questions remain regarding how heterozygous mutation leads to pathology at the cellular, molecular, and circuit level. First, no published studies have linked adult macrocephaly to changes in cellular composition or described cell-type specific molecular pathology in adult Chd8+/− mouse brain. Second, it remains unclear whether neuronal dysfunction has arisen from developmental disruption or reflects a continued requirement for full Chd8 dosage in mature neurons, and whether reported RNA expression changes are due to transcriptional or post-transcriptional mechanisms. Third, circuit-level consequences to behavior beyond sociability and cognition remain largely unexplored. To address these gaps, we characterized adult Chd8+/− mice and cerebral cortex across cellular, molecular, functional, and behavioral domains (Figure 1A). Our findings reveal selective vulnerability of cortical glutamatergic neurons to Chd8 haploinsufficiency, implicate disrupted post-transcriptional regulation of synaptosomal RNA homeostasis, and demonstrate associated changes in dendritic spine dynamics, impaired neuronal signaling coherence, and altered sleep architecture in Chd8+/− mice.
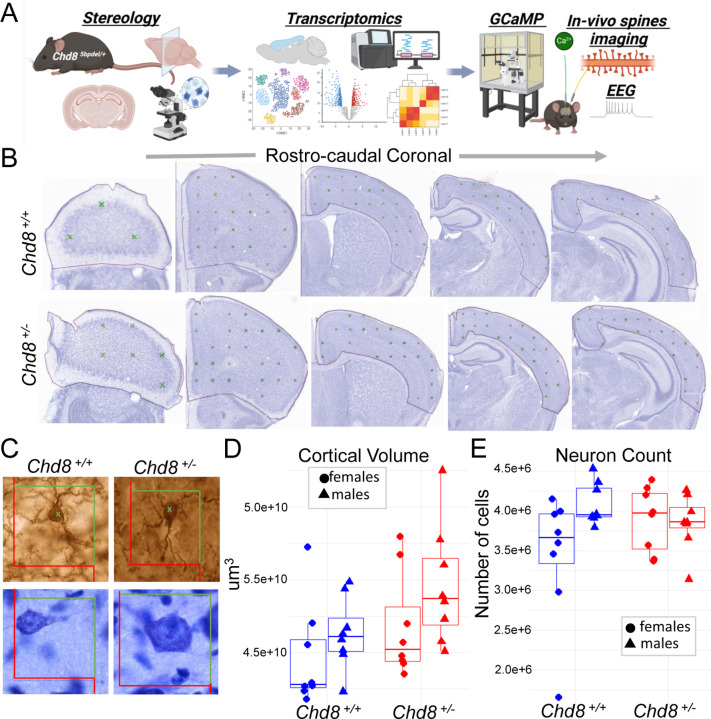
Figure 1. Increased cortical volume in Chd8+/− mice without a corresponding increase in neuron number.
(A) Schematic overview of study investigating the role of Chd8 haploinsufficiency in mature mouse cortex. (B–C) Representative coronal sections across the rostro-caudal extent of the cerebral cortex used for unbiased stereological analysis. (B) Cortical boundaries as determined for volume measurements. (C) Cellular organization visualized using Nissl staining (top) and NeuN immunostaining (bottom) in Chd8+/+ and Chd8+/− mice. (D–E) Stereological profiles across the Z-plane showing (D) cortical volume estimates based on the Cavalieri method and (E) total neuron number as assessed by unbiased stereology in the same rostro-caudal regions. Data are stratified by sex and genotype. Error bars represent SEM. Statistical comparisons were performed using two-way ANOVA (Cortical volume: P=0.032 unstratified, P=0.009 males; P=0.61 females; Neuron Count: P=0.76, unstratified; P=0.37, males; P=0.30, females Student’s t-test need to insert n per group).
Results
Description of Chd8 mutant mouse lines
Constitutive mutant Chd8 mice were generated on the C57BL/6N background via CRISPR/Cas9 targeting exon 5, resulting in a 5 bp deletion that leads to reduced Chd8 mRNA and protein and is early embryonic homozygous lethal. We previously characterized heterozygous mutants from this line (referred to as Chd8+/5bpdel, herein Chd8+/−), reporting differential expression and splicing, altered cortical proliferation, increased brain volume, and phenotypes of altered exploration in open field assay and deficits in fear conditioning and novel object recognition20. We also use a conditional Chd8 loxP mutant on the C57BL/6J background generated by Jackson Laboratory where exon 3 is ablated in the presence of CRE (Chd8em3Lutzy). These were mated to Camk2a-CRE (B6.Cg-Tg(Camk2a-cre)T29–1Stl/J; JAX strain: 005359) to direct conditional heterozygous ablation in post-mitotic forebrain glutamatergic neurons in this line, herein referred to as Chd8+/flox.
Increased adult cortical volume without increase in neuron number or soma size in Chd8+/− mice
We previously identified increased cortical volume and macrocephaly in Chd8+/− mice via structural MRI20. Here, we aimed to replicate this finding and determine whether the volume increase results from a higher number of neurons. We performed unbiased stereological analysis on Nissl-stained brain sections from male and female heterozygous Chd8+/− mice and wild-type littermates at postnatal day 42 (P42) (Figure 1B–C), an age marking the end of adolescence and a mature stage of mouse brain development52. We examined 15–18 representative coronal sections per mouse using established stereological methods to accurately assess cortical volume, neuronal distribution, and soma size. Cortical volume was estimated using the Cavalieri method53,53,54, revealing a significant overall increase in Chd8+/− mice compared to wild-type littermates (Figure 1D, P=0.032, Student’s t-test). When stratified by sex, the increase in cortical volume was more pronounced and independently significant in males (P=0.009 males; P=0.61 females, Student’s t-test) (Supplement). Comparing volume across rostral-caudal sections, Chd8+/− males showed increase in more rostral regions (Supplement).
Next, we investigated whether the cortical volume expansion was associated with an increase in neuronal numbers or soma size. Using the optical fractionator method55, we estimated neuron counts across the cortex. We did not find significant differences in total neuron number in either the full cohort or sex-stratified analyses (P=0.76, unstratified; P=0.37, males; P=0.3, females) (Figure 1E). Finally, we assessed neuronal soma size and found no significant differences between genotype groups in full or sex-stratified analyses (P=0.23, unstratified; P=0.81, males; P=0.19, females) (Supplement). Taken together, these findings replicate the cortical volume increase observed in Chd8+/− mice, with a stronger effect in males, and demonstrate that this expansion is not explained by an increase in neuronal number or soma size. Instead, the increased cortical volume without an increase in numbers results in decreased glutamatergic neuron density in the Chd8+/− cortex.
Cell-type specific cellular and molecular pathology in Chd8+/−l P60 cortex
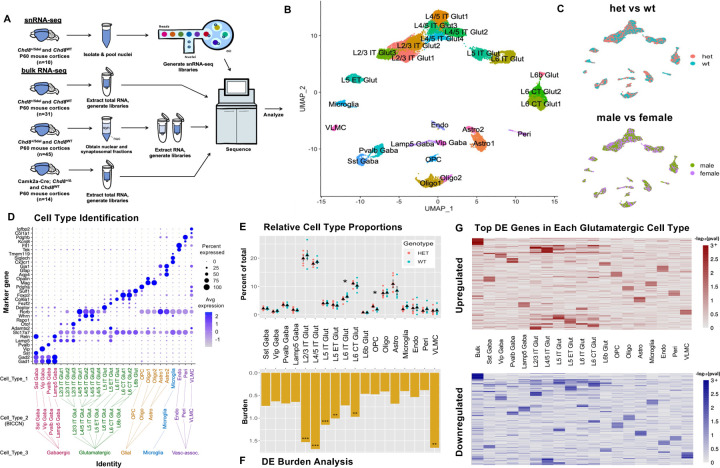
Towards characterizing differences in RNA expression associated with Chd8 haploinsufficiency in adult (P60) mouse cortex, we performed single nucleus RNA sequencing (snRNA-seq) on constitutive Chd8+/− mice, as well as bulk RNA-seq on Chd8+/− and conditional Camk2a-CRE Chd8+/flox mutants, and for nuclear and synaptosomal RNA from Chd8+/− mutants (Figure 2A). For snRNA-seq, we analyzed Chd8+/− (male n = 3, female n= 2) and WT littermates (male n=2, female n=3 WT). Following quality control and ambient RNA correction56, there were 11,751 and 12,169 nuclei from Chd8+/− and WT samples, respectively, which we mapped to 18 cortical cell types based on marker gene expression and integration with the BICCN mouse brain atlas57 (see methods) (Figure 2B and Supplement). There was balanced representation of male and female samples and of Chd8+/− and WT samples (Figure 2C). Chd8 expression was higher in non-neuronal cells, but detectable across all cell types (Figure 2C). There were no significant differences in Chd8 expression between mutant and WT cells, as expected based on a cytosolic nonsense-mediated decay being required for reduced levels of the mutant frameshift transcript. All major cortical cell types were identified, with expected expression of marker genes (Figure 2C). Cell identity was split at three levels: cell type 3 representing general classes (e.g. glutamatergic neurons), cell type 2 associated with specific canonical subtypes (e.g. L4/5 IT), and cell type 1 representing data-driven further sub-clustering of subtypes (e.g. L4/5 IT Glut1). Following UMAP generation and cell type identification, we tested for differences in the relative representation across different cell types, comparing normalized cell proportions between Chd8+/− and WT groups (Figure 2E). At the cell type 2 level, there was significant increase in OPC and decrease in L6 IT, with trending increase in astrocytes and decrease in microglia. These findings are consistent with the results from stereology, demonstrating that macrocephaly and increased cortical volume in Chd8+/− mice is not due to increase in number of glutamatergic neurons and further suggesting that increased cortical volume is not explained by expansion of specific cell types.
Figure 2: Cell type-specific pathology in adult Chd8+/− mouse cortex.
A) Schematic depicting experimental design for RNA-seq and snRNA-seq experiments. B) UMAP of 23,920 cortical nuclei from P60 Chd8+/− and WT littermates with cell types annotated. C) Clustering of Chd8+/− (HET) and WT nuclei (top) and male and female nuclei (bottom) in UMAP space. D) Cell type identification dot plot representing average expression of cell type-specific marker genes in each cell type. Tree (bottom) shows 3 levels of cell type identification (Cell_Type_1–3). E) Cell type proportion comparison between Chd8+/− (HET) and WT nuclei, revealing a significant decrease in mutant L6 IT Glut nuclei (*p = 0.027) and increase in mutant OPCs (*p = 0.048) (Student’s t-test). F) DEG burden across cell types (Burden = DE genes / expressed genes * 100). Significant DE burden was found in L2/3 IT (*p = 0.00001), L4/5 IT (*p = 0.00001), L5 IT (*p = 0.00001), L5 ET (*p = 0.0045), L6 CT Glut (*p = 0.0004), and VLMC (*p = 0.0009) nuclei (permutation test, n= 10,000). G) Heatmap showing –log10(pval) of top 25 unique upregulated (top) and downregulated (bottom) bulk and pseudobulk DEGs in each cell type (p < 0.1).
Next, we performed cell-type specific differential expression (DE) analysis using a cluster-level pseudobulk framework58, where reads are from the relevant cell type are aggregated at the sample level, followed by DE testing using DESeq259 with experimental batch included as a covariate. We performed DE analysis at each level of cell type identity, with a focus on cell type 2 for results here. DE results were stablished using either the full dataset or when down-sampling for equivalent cell/nuclei numbers across cell types and when using both uncorrected and ambient RNA corrected expression values, and similar effects were present when stratifying male and female samples (Supplement). Comparing DE burden (defined as the number of total DE genes divided by genes passing criteria for testing), glutamatergic cell types along with VLMC showed the largest impacts (Figure 3F). Among glutamatergic neurons, L2/3 intra-telencephalic (IT) and L4/5 IT neurons had the highest burden. At the single gene level, the top DE genes (DEGs) varied by cell type, with the exception that glutamatergic neuron subtypes, showed considerable overlap in effects (Figure 3G). We performed gene set enrichment analysis (GSEA) based on DE rank using the clusterProfiler package60 (Supplement). Only glutamatergic neuron subtypes had DE signatures strongly associated with GO terms (FDR < 0.05), with the strongest signatures in L2/3 IT and L4/5 IT. Using a relaxed threshold of uncorrected P < 0.05, upregulated genes in glutamatergic neurons showed shared enrichment for pathways including Synapse organization, Synaptic signaling, and Axon guidance, and Neuron differentiation. Finally, we applied NeuronChat61 to infer cell-cell communication networks comparing receptor-ligand relationships in Chd8+/− and WT cortex based on snRNA-seq data (Supplement). This analysis revealed significantly reduced Neurexin-Neuroligan interaction strength and frequency in Cdh8+/− mice, with the strongest effect in L2/3 IT neurons (Supplement). Conversely, we detected increased communication strength involving glutamate receptors in the Chd8+/− mutants (Supplement). These findings highlight disrupted synaptic signaling in Chd8⁺/⁻ mice and suggest that glutamatergic neuron subtypes, particularly L2/3 IT and L4/5 IT neurons, are disproportionately affected in the adult cortex.
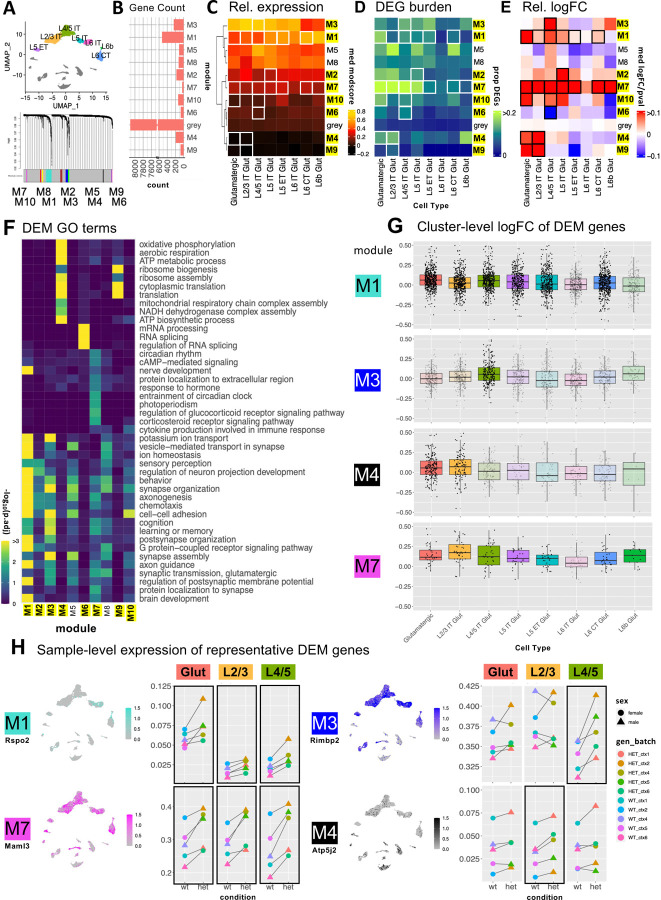
Figure 3: hdWGCNA reveals differentially expressed glutamatergic cell coexpression modules.
A) 10 glutamatergic modules identified via hdWGCNA B) Number of genes per module. C) Relative expression of glutamatergic modules across glutamatergic neuron subtypes (module score = average expression of all module genes compared to expression-matched background genes; DEMs identified via comparison of median pseudo-bulk logFC of module genes to that of the grey module via t-test; DEMs (*p < 0.05) in at least one population are labeled in yellow. D) DEG burden (calculated as in Fig. 2F) of each DEM in each cell type E) Heatmap of relative median pseudo-bulk logFC of module genes for glutamatergic cell types scaled by p-value. White and black boxes in C-E indicate the cell types in which each corresponding module was DE. F) Representative significant GO terms for Biological Processes enriched in glutamatergic modules. (*p < 0.05, **p < 0.01, ***p < 0.001). G) Box plots for 4 representative DEMs showing logFCs of module genes in each glutamatergic subtype. Opaque boxes indicate cell types where the module shown was significantly differentially expressed. H) Feature plots (left) showing relative expression of representative genes from DEMs of interest in UMAP space. Dot plots (right) show median sample-level expression of the same genes. Black boxes indicate cell types where the corresponding module is differentially expressed, or where the direction of change in module expression is consistent across batches.
Shared and cell-type specific DE co-expression modules in Chd8+/− glutamatergic neurons
To capture systems-level expression patterns in glutamatergic neurons, we used hdWGCNA62 to identify gene co-expression modules and then identified differentially expressed modules (DEMs) based on log2-transformed Fold Change (logFC) of module genes (see methods for details). All glutamatergic cell subtypes were used as input to hdWGCNA, which yielded 10 modules accounting for 1,691 (17%) genes, with the remaining 8,115 genes assigned to the grey group (Figure 3A). 8/10 modules were DEMs in at least one glutamatergic subtype, as indicated by highlighted label and box outlines in Figures 2B–E. Two modules (M1 and M7) were DEMs across most glutamatergic subtypes, whereas the other six DEMs (M2, M3, M4, M6, M9, and M10) showed genotype differences in only one or two subtypes. Module level differential expression direction and effect size varied, though all but one DEM (M6 in L4/5 IT) were upregulated in Chd8+/− mutants. The modules varied in gene number (Figure 3B) and relative expression patterns (Figure 3C) across glutamatergic subtypes and cortical layers. The proportion of module genes that were individually DE (Figure 2D) and the module-level logFC (Figure 2E) similarly varied across modules and subtypes.
The glutamatergic co-expression modules showed different association to biological processes based on GO enrichment (Figure 3F). First, 6/10 modules showed overlapping enrichment for a variety of general neuron-associated terms (e.g. Synapse assembly, Axon Guidance, Behavior) without strong module-specific GO terms. These included DEMs M1, M2, and M3, as well as non-DEMs M8 and M5. These modules also showed relative expression differences across glutamatergic subtypes and cortical layers, suggesting that co-expression for these gene sets is primarily driven by expression co-variation associated with cell identity, rather than driven by co-expression associated with biological processes or genotype effects. The remaining four modules (M4, M6, M7, M9) were all DEMs and featured strong module-specific GO enrichment: M4 and M9, associated with Aerobic Respiration and Translation and upregulated in L2/3 IT, M6, associated with mRNA processing and downregulated in L4/5 IT, and M7, which was broadly downregulated and associated with general neuronal terms as well as GO terms represented by Circadian rhythm. In addition to module-specific GO terms, these four modules did not show subtype- or laminar-associated differences in relative expression and had DE signatures limited to individual glutamatergic subtypes. We highlight gene-level differential expression patterns for DEMs of high interest in Figure 3G, focusing on M1 and M7 modules that are broadly DE across glutamatergic subtypes, and M3 and M4 modules that are subtype-specific DEMs with prominent effects in L4/5 IT and L2/3 IT, respectively. Figure 3H shows representative gene expression patterns from these DEMs visualized in UMAPs and pseudobulk-level relative expression plots. See Supplement for extended modules visualizations. M1 (featuring Rspo2) includes a large set of genes that are expressed most highly in deep layer excitatory neurons (L6 CT and L6b), associated with general neuronal and synaptic functions. Although it includes both up- and downregulated genes, M1 shows a significant bias towards upregulation with the strongest effects in L4/5 IT and L5 IT subtypes. In contrast, M7 (featuring Malm3) is a smaller module exhibiting consistent upregulation across all glutamatergic subtypes. It is associated with general neuronal function as well as circadian rhythm and hormone response pathways. M3 (featuring Rimbp2) is selectively upregulated in more superficial glutamatergic subtypes L4/5 IT neurons, with enrichment for neuronal and synaptic processes. In contrast M4 (featuring Atp5j2) shows low expression across glutamatergic subtypes and GABAergic neurons, but relatively higher expression in non-neuronal cell types, with gene ontology terms related to translation and mitochondrial ATP metabolism. The shared dysregulation of M1 and M7 DEMs mirrors overlapping GO enrichment patterns across glutamatergic subtypes observed in GSEA (Supplement), reflecting convergent molecular pathology. The subtype-specific dysregulation of M3 and M4 suggest genotype-dependent perturbations of distinct biological processes particularly affecting L2/3 IT and L4/5 IT neuron populations.
Bulk RNA-seq comparison identifies shared signatures in constitutive versus conditional Chd8 mutants but divergent effects in nuclear versus synaptosomal mRNA fractions
We employed bulk RNA-seq as a complementary approach to snRNA-seq, assaying DE signatures across four contrasts: mRNA from P60 cortex from constitutive Chd8+/− and from conditional Camk2a-Cre x Chd8+/flox mice, and compartment-enriched nuclear and synaptosomal mRNA from P60 cortex from Chd8+/− mice (Figure 4). For all conditions, 4–8 biological replicates for each of male and female mutant Chd8 mice were compared to matched WT littermates, with the primary DESeq2 DE model including sequencing batch and sex as covariates. We also analyzed male and female samples separately, finding generally shared DE signatures (similar as for snRNA-seq) (Supplement). Across the four conditions, the largest number of DEGs at either FDR < 0.05 or P < 0.05 was in the synaptosomal preparation, followed by the nuclear preparation, then full cortex from Chd8+/− and lastly Chd8+/flox (Figure 4A). Chd8 was among the top downregulated DEGs in all but the nuclear mRNA condition, consistent with nonsense-mediated decay of mutant Chd8 transcripts outside the nucleus (Figure 4B). To identify shared and divergent DE signatures across conditions, we compared logFC of DEGs; shared signatures are represented by concordant LogFC direction, condition-specific effects are captured by patterns of change (up or down) in one condition with LogFC around zero in the other condition, and divergent DE signatures are represented by opposite logFC direction. Comparison between Chd8+/− and Chd8+/flox logFC for genes with FDR < 0.2 and P-value < 0.05 in Chd8+/− (Figure 4C, Supplement) captured largely shared signatures, indicating that effects from post-mitotic heterozygous ablation in excitatory neurons accounts for a significant part of DEG transcriptional pathology, DEGs identified in both the nuclear and synaptosomal conditions (FDR < 0.1) showed strong concordance with bulk cortex, however there were striking differences and low concordance between nuclear and synaptosomal enriched mRNA fractions (Figure 4D).
Figure 4: Shared DE in constitutive Chd8+/− and Camk2a-CRE conditional Chd8+/flox mice, with divergent impacts on nuclear and synaptosomal mRNA and protein.
A) Barplot of DEG counts across bulk RNA-seq experiments (constitutive Chd8+/−, Camk2a-CRE conditional Chd8+/flox, and nuclear and synaptosomal preparations from the Chd8+/− line. B) Chd8 is down-regulated in all but the nuclear preparation. C) LogFC concordance between constitutive and conditional datasets for constitutive model DEGs. D) LogFC concordance plots for nuclear and synaptosomal versus whole cortex for DEGs identified in nulear or synaptosomal fractions on left, reduced concordance between nuclear and synapsomal DEG logFC on right. E) Selected enriched GO terms for upregulated (left) and downregulated (right) genes across experiments. F) Downregulation of Dlg2 in synaptosomal RNA. GH) Western blot validating decreased synaptosomal PSD93 protein in Chd8+/− cortex.
To compare pathway-level dysregulation across bulk RNA conditions, we used GSEA to identify Gene Ontology (GO) terms where the annotated gene set exhibited significant shift in LogFC distribution towards up- or downregulation, with shared and condition-specific enrichment for representative terms shown in Figure 4E. Most of the up- and down-regulated terms identified in the constitutive Chd8+/− were shared in the conditional Chd8+/flox, and most terms in the nuclear or synaptosomal enriched conditions were shared in the full cortex preparation, findings that consistent with DEG concordance across contrasts above. We identified shared upregulation of terms including Cell cycle checkpoint, Electron transport chain, Mitochondrial translation, and Chaperone mediated protein folding, showing overlap with terms that were also detected in glutamatergic neuron subtypes in the snRNA-seq data (Supplement and Figure 3). Among upregulated terms that differed between synaptosomal and nuclear conditions were Regulation of gamma-aminobutyric acid secretion and Neuropeptide signaling pathway in the synaptosomal and Regulation of DNA repair and Transport along microtubule in the nuclear fraction. For downregulated terms, Axonogenesis, Synapse organization, Cell adhesion, and Regulation of transcription by RNA Pol II were shared, while Dendrite morphogenesis and Postsynapse organization were example synaptosomal-enriched terms. Overall, bulk RNA-seq results from the full cortex and from the nuclear and synaptosomal conditions showed evidence of concordance with DEG findings from snRNA-seq, though with fairly substantial overall differences in DE signatures (Supplement). The nuclear enriched mRNA showed upregulation of genes associated with the glutamatergic M1 and M7 hdWGCNA modules that were broadly upregulated across glutamatergic subtypes in the snRNA-seq data (Supplement), validating relevance of these broad DEMs. GO terms associated with L2/3 IT enriched upregulated M4 and M9 modules associated with mitochondrial energetics were among the strongest bulk RNA-seq upregulated GSEA hits across conditions. Finally, for the ASD and neuropsychiatric-associated synaptosomal-specific downregulated Dlg2 gene63–66, which encodes the PSD93 excitatory synaptic scaffold protein, we validated reduction at the protein level in synaptosomal preparations (Figure 4F–4H). These results solidify transcriptional pathology in adult cortex due to Chd8 haploinsufficiency and provide insights into cell-autonomous effects in post-mitotic excitatory neurons and suggest differential mRNA levels are driven by both transcriptional and post-transcriptional mechanisms that have heightened impact on synaptosomal mRNA levels.
Altered excitatory neuron spine dynamics and calcium signaling coherence in Chd8+/− mice
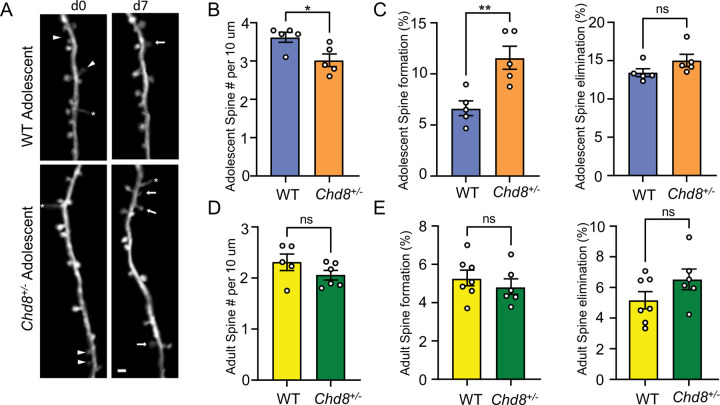
The snRNA-seq and bulk RNA-seq both pointed to cortical excitatory neuron dysfunction and previous studies have identified electrophysiological signatures in vivo and in vitro in glutamatergic neurons21,36,37,40,40,41. To further investigate structural pathology of cortical neurons, we crossed Chd8+/− mice with thy1-YFP mice, which express cytoplasmic yellow fluorescent protein in a sparse subset of layer 5 pyramidal neurons (L5 PyrNs)67, and performed in vivo two-photon (2P) imaging on apical dendrites of L5 PyrNs. We compared the density and dynamics of dendritic spines (postsynaptic structure of majority excitatory synapse) between WT and Chd8+/− littermates at adolescence (P30) and adulthood (P90) mice (Figure 5A). Adolescent Chd8+/− mice had lower spine density on the apical dendritic tufts of L5 PyrNs (Figure 5B). Following the same dendritic segments over seven days, we also found that Chd8+/− mice had increased dendritic spine formation relative to WT mice, but comparable spine elimination (Figure 5C). On the other side, adult Chd8+/− mice had normal spine density and dynamics (Figure 7D–E). These results indicate that Chd8 haploinsufficiency delays spinogenesis of cortical neurons during early development (before P30).
Figure 5: Delayed spinogenesis during Chd8+/− development.
A) Dendritic segment imaged over 7-days in somatosensory cortex of adolescent WT and Chd8+/− mice. Arrowheads: eliminated spines; arrows: formed spines; asterisks: filopodia. Scale bar = 2 μm. B, D) Spine density in adolescent (b) and adult (D) Chd8+/− or WT littermates. C, E) The percentage of spines formed or eliminated over 7d is higher in adolescent (D) and adult (E) Chd8+/−than WT littermates, mean, SEM., unpaired t-test. **p<0.01.
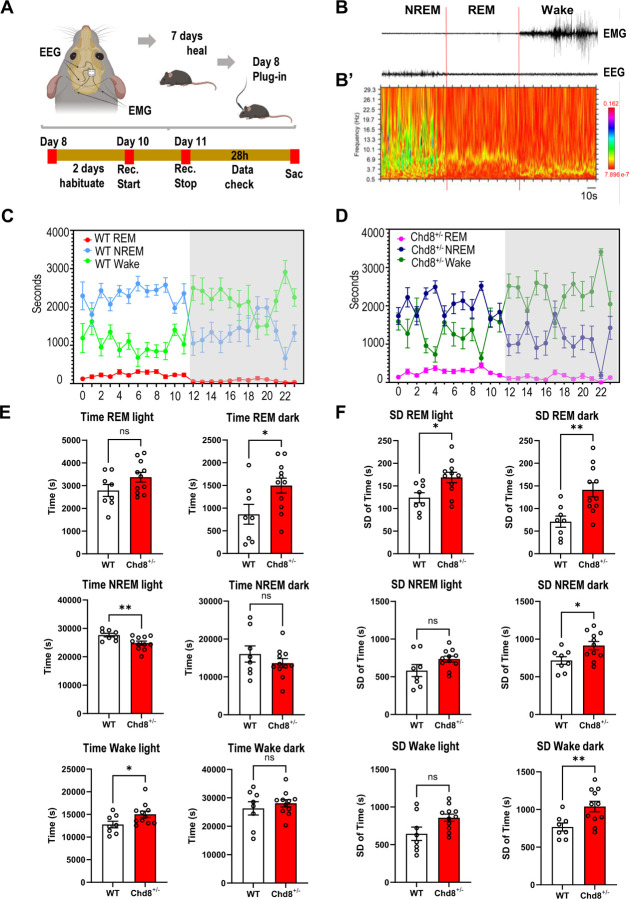
Figure 7: Perturbed sleep patterns in Chd8+/− mice.
A) Schematic of 24hr EEG/EMG sleep analysis. B) Example EEG/EMG traces showing activity during REM, NREM, and Wake state. B’) Spectrogram of the EEG signal in B C) Summary of time spent in each state during each hour of the 24hr monitoring period for wild type (left) and Chd8+/− groups. Mean and SD shown. D) Summary of mean total time spent in each state separated by light and dark stages (left). Summary of SD in time spent in each state separated by light and dark stages. See text for statistical summary.
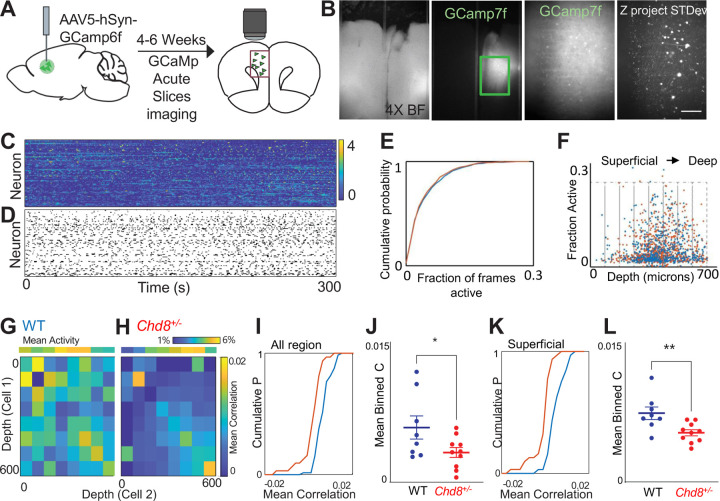
To understand how disruption of Chd8 signaling affects the function of a cortical microcircuit, we performed calcium imaging in acute slices taken from wildtype or Chd8+/− mouse brains expressing the genetically encoded calcium indicator GCamp6f in prefrontal cortex neurons under the control of the human Synapsin promoter (Figure 6A–D). We collected 60-minute recordings from a total of 544 neurons from 8 wildtype slices (68+/−9 neurons/slice) and 818 neurons from 10 Chd8+/− slices (82+/−9 neurons/slice). Overall, the activity of wildtype and Chd8+/−l slices was similar (Figure 6E–F; mean fraction of frames active = 5.2+/−0.2% WT vs 5.0+/−0.2% HET, p = 0.4, t-test). To examine the functional connectivity across the prefrontal microcircuit, we binned neurons into eight 75 μm bins starting at the cortical surface and extending to a depth of 600 μm, and calculated the correlation coefficient between the activity of neurons according to the location of each neurons within the slice, then averaged the correlation coefficient based on the depth of 600 μm, and calculated the correlation coefficient between the activity of neurons according to the location of each neurons within the slice, then averaged the correlation coefficient based on the position of each neuron in the cell pair (Figure 6G–H). Visual examination of the resulting matrix of averaged correlations revealed that neuron pairs from heterozygous Chd8+/−l slices had on average lower correlation coefficients compared to neuron pairs from WT slices, and this was particularly pronounced when at least one neuron was located in the superficial (< 300 μm from the cortical surface) aspect of the slice (Figure 6I–L; mean correlation coefficient WT 0.0057 +/−0.0001 vs 0.0030+/−0.0005, p < 0.05, t-test; n = 8 WT and 10 HET slices; mean correlation coefficient superficial WT 0.0050+/−0.0009 vs HET 0.0021+/−0.0004, p < 0.01, t-test; n = 8 WT and 10 HET slices), suggesting that these neurons are relatively disconnected from deeper layer neurons in the prefrontal microcircuit of mutant Chd8+/−l animals.
Figure 6: Disruption of coordinated activity in Chd8+/− PFC microcircuits.
A. Mice injected with Syn-GCamp6f and activity recorded from acute slices after 4–6 weeks. B, wide field and GFP at 4x (left). 10X of individual neurons and z-projection of standard deviation across frames (right). C, z-scored fluorescence traces from neurons in a single slice. D, Raster of events. E, Cumulative probability function showing overlap in fraction of frames active for neurons from WT (blue) and Chd8+/− (red) slices. F, Activity of each cell as a function of depth from cortical surface. Cells binned by distance from surface (gray boxes) and mean correlations within and between bins calculated. G,H, 8x8 matrix of mean correlation coefficient calculated for cells within each pair of bins, averaged for WT (G) or Chd8+/− (H) slices. I, cumulative distribution function (CDF) of mean correlation across 544 pooled WT (blue) neurons and 818 pooled Chd8+/− (red) neurons for all bins. J, Mean correlation coefficient of WT and Chd8+/− slices (p < 0.05, t-test). K, CDF of the mean value of the subset of bins in which neuron 1 was < 300 microns from cortical surface. L, Mean correlation coefficient of superficial neurons in WT and HET slices (p < 0.01, t-test).
Chd8+/− mice exhibit disrupted sleep
We previously tested Chd8+/− mice across a battery of behavioral assays, finding deficits in novel object recognition and fear conditioning in the learning and memory domain with no differences in sociability using the three-chamber social preference test or in male-female interactions. Sleep issues are a common symptom in humans with CHD8 mutations and the transcriptional dysregulation in Chd8+/− mice featured upregulation of the circadian-associated M7 glutamatergic module. Thus, we used EEG/EMG to continuously record neural activity during a 24-hour period, assigning states of REM, NREM, and Wake, analyzing 11 Chd8+/− (female=7, male=4) and 8 WT littermates (female=4, male=4) (Figure 7A–B’). The total time in seconds that each mouse spent in each state was calculated for each hour (Figure 7C–D). For comparison of sleep patterns, total and standard deviation (SD) were calculated by subject using hourly values and compared for light (Zeitgeber Time ZT0-ZT12) and dark (ZT12-ZT24) phases (Figure 7E–F). Chd8+/− mice had a significant increase in time spent in REM in the dark stage (P = 0.036 [unpaired t-test with Welch’s correction]), and significantly less time spent in NREM (P = 0.008 [unpaired t-test with Welch’s correction]) and increased time spent in wake state (P = 0.040 [unpaired t-test with Welch’s correction]) in the light phase. Comparing SD of the time spent per state across hours, Chd8+/− mice showed higher variability in all states. SD of REM time was significantly higher during both light and dark phases (P = 0.012 and 0.003, respectively), and SD of NREM and Wake time were significantly higher during dark phase (P-values = 0.017 and 0.006, respectively) and marginally increased during the light phase (P = 0.115 and 0.059 respectively). Together results revealed disrupted sleep patterns in Chd8+/− mice, including more time in REM and time spent in Wake in the light stage, and higher variability in time spent in specific sleep/wake states across the light and dark stages.
Discussion
This study extends understanding of how Chd8 haploinsufficiency alters the adult mouse cerebral cortex. Via stereology and snRNA-seq, we show that increased cortical volume in Chd8+/− mice is not due to increase in cortical excitatory neuron numbers, and, in fact, is associated instead with decreased neuron density. While macrocephaly was not due to increased numbers in excitatory neurons, these cells do exhibit sensitivity to Chd8 haploinsufficiency at the level of cell-type specific transcriptional pathology, synaptosomal RNA homeostasis, dendritic spine formation, and signaling coherence. Finally, we expand the phenotypic testing of heterozygous Chd8 mouse models in the domains of sleep and cognitive performance, finding perturbations to sleep state that aligns with high prevalence of sleep disturbance reported in patients carrying CHD8 mutations. Overall, our findings add to the understanding of the effects of heterozygous Chd8 mutation on the mouse cortex, providing new insights into anatomical, cellular, and circuit impacts, dosage sensitivity during brain development and in post-natal postmitotic neurons, and demonstrating presence of sleep disturbance.
Increased brain size during development and increased cortical volume in adult mice has been reported across different mutant Chd8 mouse lines20,25,27,29. Chd8 haploinsufficiency has also been linked to altered neurogenesis in mouse20,25,28 and in vitro in human cellular and organoid brain models32,38. While increased production of cortical excitatory neurons during development leading to increased neurons in the adult cortex seemed a plausible explanation for cortical volume increase, results from both stereology and snRNA-seq show this to not be the case in our Chd8+/− mouse model. In fact, we found the opposite pattern of decreased excitatory neuron density driven by increased volume without increased neuron counts or increased soma size. There was a trend of decrease in proportions of certain glutamatergic types in snRNA-seq data, reaching significance for decreased in L6 IT neurons in the mutants. snRNA-seq indicated significant increase in proportion of OPCs and trend towards increase in astrocytes, raising the possibility that increased proportions of non-neuronal cells may explain some of the increased cortical volume. Whatever the roots of Chd8-associated macrocephaly, it is unclear what the link between this neuroanatomical signature and relevant social and cognitive phenotypes, with a recent study finding little correlation between adult brain weight and behavioral phenotypes for Chd8+/− mice from a series of genetic backgrounds34. It could be that causes of macrocephaly and behavioral phenotypes in Chd8+/− mice are independent, or that the mechanisms for are linked, but with variable anatomical and behavioral outcomes in adult mice. Our results do point to glutamatergic pathology and one intriguing possibility is that changes in neuronal morphology and microstructure organization, for example dendritic arborization, could account for the changes. Further studies will be needed to resolve specific causes of volume increase in Chd8+/− adult cortex.
Via unbiased snRNA-seq, we found that glutamatergic neurons exhibit the strongest transcriptional pathology across cell types in adult mouse Chd8+/− cortex, with the most extensive impacts in L2/3 IT and L4/5 IT subtypes. We identified subtype-specific and shared glutamatergic DEMs, including shared upregulation of M1 and M7 modules, which are associated with general neuron-associated pathways and with specific circadian rhythm GO terms. Transcriptional pathology in bulk RNA-seq analysis of constitutive and conditional Chd8+/− mutants here aligns with CHD8 studies in vitro and using mouse models12,20,22,24,25,28,68, with our study adding substantially via integrating single cell and bulk, constitutive and conditional mouse models, and synaptosomal mRNA fractions. These findings include upregulated aerobic respiration and mitochondrial energetics, which we map to having the strongest signature in L2/3 IT neurons, and downregulation associated with synaptic organization and signaling. Bulk RNA-seq transcriptional changes in excitatory neuron-specific Chd8+/flox mice paralleled changes in constitutive heterozygous Chd8+l- mutants, demonstrating continued requirement for full Chd8 dosage and indicating that excitatory neuron transcriptional phenotypes in mature Chd8+/− mice may be independent of developmental impacts. Our findings of effects in Chd8+/flox mice due to ablation in excitatory neurons are consistent with earlier in vitro studies of both heterozygous and homozygous neuronal ablation40,69, and extend previous studies of adult Chd8-/- conditional mutant mice that did not examine NDD-relevant heterozygous mutants44. We further link transcriptional dysregulation in glutamatergic neurons to functional impacts in these cells, finding altered dendrite spine formation during adolescence which manifests at the circuit level as de-correlated spontaneous prefrontal activity. Finally, though not directly linked to specific cells or circuits, we identified perturbed sleep patterns Chd8+/−. These findings are highly relevant to NDDs, as disrupted synaptic development and altered network activity represent candidate causal mechanisms and sleep disturbances are commonly reported in individuals with ASD and related conditions70 and are one of the more penetrant phenotypes in CHD8-NDD71. Constitutive Chd8+/− mice do not exhibit classic sociability deficits in the three-chamber social preference assay and behavioral and cognitive phenotypes vary across Chd8+/− studies and across mouse genetic backgrounds20–22,24,25,27,34,37. As such, behavioral studies of Chd8 mutant mice have proved challenging to interpret. Our results, along with studies that have identified other phenotypes at cellular, molecular, and anatomical level such as via electrophysiology or structural MRI, highlight molecular endophenotypes that may be more consistent in expression and have high translational relevance. Future studies will be needed to determine which aspects of Chd8+/− mouse phenotypes are driven by developmental versus later requirement for full dosage, which specific neuronal and glial populations are dependent on Chd8, and if molecular, cellular, and neuroanatomical phenotypes are consistent between mutant lines and across mouse and human CHD8+/− models.
One novel finding from our study was the significant differences in DE signatures between nuclear and synaptosomal mRNA preparations. Bulk nuclear RNA-seq recapitulated upregulation observed for the two glutamatergic DEMs with broad dysregulation and had the highest concordance with snRNA-seq DEGs, as well as being strongly concordant with the full bulk cortex RNA-seq. Thus, nuclear RNA changes observed at both single cell/nucleus and bulk level appear robust. Surprisingly, there was relatively weak concordance between synaptosomal and nuclear DEGs, with a stronger overall DE signature in the synaptosomal enriched preparations. These findings indicate disconnect between changes in transcriptional rate, captured by nuclear RNA datasets, and changes in steady-state mRNA levels in Chd8+/− cortex. We and others previously identified altered mRNA splicing in Chd8+/− mouse and in- vitro models20,72, raising the possibility that Chd8 haploinsufficiency directly or indirectly alters RNA processing. ChIP-seq studies of CHD8 have consistently identified enrichment of regulatory targets associated transcription and RNA processing73. Intriguingly, we did observe down-regulation of RNA processing associated with L4/5 IT specific DEM M6. Beyond a better understanding of the molecular function of Chd8, future studies are needed to determine whether the synaptosomal DEG signature is specific to synaptosomal localized mRNA, or a more broadly present in cytosolic mRNA overall, what the cell-type specific impacts are on mRNA processing and localization, whether synaptosomal RNA differences are associated with differences in synaptic protein makeup and synaptic function, and if this finding extends to CHD8+/− human neurons.
There are limitations to this wok that should be considered. The number of replicates (N=5 per genotype) and contrasts (Chd8+/5bpdel versus WT) was restricted for snRNA-seq, somewhat limiting statistical power and scope of these experiments. Additionally, nuclei-based methods may miss changes in cytosolic mRNA levels74,75, which may be relevant based on our bulk analysis. Further, there are challenges with snRNA-seq for DE testing at single gene level using individual cells/nuclei. While Wilcoxon tests are commonly used in single cell differential expression analysis, we found this method yielded high false positive rates (as issue that has been raised previously76. In contrast, pseudobulk and hdWGCNA methods take advantage of aggregating cells or genes to compare expression at the cluster or module level, giving more robust DE results at the cost of reducing resolution for single cell and single gene level effects. Finally, it is unclear how much the transcriptomic pathology extends to protein level or impacts function. With regard to functional studies, we chose three in vivo assays to focus on: spine dynamics, correlated GCaMP signaling, and sleep. Previous studies have not tested these phenotypes, and we believe our results provide new insights into pathology. However, other assays would be valuable and each of the domains we studied could be investigated at higher depth. We did not test conditional Chd8+/flox mice beyond bulk RNA-seq, leaving unanswered whether this model recapitulates other pathology. Finally, Chd8 mutant mouse lines exhibit variation in phenotype penetrance and expressivity, which is at least in part due to the impact of genetic background34. Considering this, our findings may not be conserved in other Chd8 mouse models, though we note overlapping transcriptional signatures described here and in other studies as summarized above. While these limitations hold, our results lay the foundation for future work characterizing constitutive and conditional mutant Chd8 mouse models.
Our study extends understanding of the impacts of Chd8 haploinsufficiency in vivo in mouse brain, with a focus on glutamatergic excitatory neurons. We show that constitutive and conditional heterozygous ablation leads to convergent transcriptional pathology and to impacts on neuronal spinogenesis and circuit function. Our findings illustrate how loss of Chd8, which has received attention for high expression in early development and role as a chromatin remodeler, impacts the cell-type specific makeup and pathology of the adult cortex extending across molecular, cellular, circuit, and organismal phenotypes and providing support for a framework where excitatory neuron dysfunction at the level of synapses and circuits may be an area of casual convergence in monogenic NDDs featuring ASD and ID and raising the possibility that interventions targeting postnatal postmitotic cortical neurons may be effective at addressing at least some of the underlying NDD pathology.
Methods
Animals
Generation of Cas9-mediated 5bp frameshift deletion in exon 5 of Chd8 in mice (here referred to as Chd8+/− mice) was previously described20. For in vivo imaging, this mouse line was crossed with the thy1-YFP-H (JAX strain: 003782) mouse line and maintained in the C57BL6/J (JAX strain: 000664) background. Mutant mice Camk2a-CRE Chd8+/flox were obtained by crossing Chd8 loxP mice on C57BL/6J (Chd8em3Lutzy; JAX strain: 031555) to Camk2a-CRE (B6.Cg-Tg(Camk2a-cre)T29–1Stl/J; JAX strain: 005359) to achieve heterozygous ablation in post-mitotic forebrain glutamatergic neurons. All protocols utilized in the generation of mouse brain samples were approved by the Institutional Animal Care and Use Committees (IACUC) at the University of California Davis and University of California Santa Cruz. Mice were housed in a temperature-controlled vivarium maintained on a 12-h light–dark cycle. We made efforts to minimize pain, distress and the number of animals used in the study.
Histological tissue preparation and Nissl Stain
Thirty-six 42-day-old mice were included in this analysis (16 brains per genotype, 16 per sex). Mice were anesthetized using Isoflurane and perfused transcardially using room temperature saline solution (0.9% NaCl) followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate buffered (PB) saline using a peristaltic pump (Minipuls 3, Gilson) at 20 rpm flow rate. Brains were removed and post-fixed in 4% PFA at 4ºC for 24 h. Fixed brains were cryoprotected in 50 ml of 10% and 20% glycerol solutions with 2% DMSO in 0.1 M phosphate buffer (PB; pH 7.4; for 24 and 72 hours, respectively). Brains were blocked at −2.00mm posterior to the interaural line prior to flash freezing. Sections were cut using a freezing, sliding microtome in 4 series at 60 μm (Microm HM 440E, Microm International, Germany). Every 4th section was collected in 10% formaldehyde in 0.1 M PB (pH 7.4) and post-fixed at 4ºC for 1 week77. Sections were rinsed twice for 1 hour each in 1ml of 0.1M PB, mounted on gelatin-coated slides, and air-dried overnight at 37°C. Defatting was performed in a mixture of chloroform/ethanol (1:1, vol) for 2 x 1 hour. The brains were rehydrated in graded alcohols; 100% ethanol, 100% ethanol, 95% ethanol, 70%, 50% and dipped in deionized water77,78. Sections were stained 40 seconds in a 0.25% thionin solution (Fisher Scientific, Waltham, MA, cat. no. T-409), dehydrated through graded alcohols, differentiated in 95% ethanol and 5 drops of glacial acetic acid, cleared in xylene and cover-slipped with DPX (BDH Laboratories, Poole, UK)77.
Stereology
The inferior boundary of the cortex was defined as the bottom of the agranular insular area (AIA), and the superior boundary was defined as the anterior cingulate area (ACA) or the retrosplenial area (RSA) and the white matter was then followed to the ACA/RSA border79. Neuron number, neuronal soma size, and brain volume were estimated using Stereoinvestigator V10.50 (MBF Biosciences, Williston, VT) attached to a Zeiss Imager.Z2 Vario with a Zeiss AxioCam MRc. An EC PlanNeoFluar 2.5 x objective was used to trace the outline of the cortex and a PlanApoChromat 100x oil objective was used for cell counts and soma size determinations. The volume of the cortex was determined using the Cavalieri method53,54,80. For each mouse, 15–18 sections (480 μm apart) were analyzed with the first section chosen randomly amongst the first through the cortex. Estimates of neuron numbers were obtained using the Optical Fractionator method55,81. Since no lateralization was found in the volumetric estimates (p = 0.65), we sampled the left hemisphere of half of the mice and the right hemisphere of the other half for estimates of neuron numbers. We used the same sections that were used for volumetric estimates. We used a counting frame size of 18x18x10μm with 2 μm guard zones, and a scan grid of 480x480 μm placed at a random angle. Neurons were defined following established protocols53–55,80–82 based on: 1) dark staining, 2) a well-defined nucleolus and 3) a round, non-jagged cell body77,82–85. Section thickness was measured at every counting site. The volume of neuronal somas was determined using the Nucleator method86 during the Optical Fractionator analysis. Comparison of stereological measures (volume, neuron number, and soma size) were performed via ANOVA to examine global effects of sex and genotype and test for sex by genotype interaction, and then via t-test to compare the effect of genotype in males and females. In addition to test results, effect sizes are reported for ANOVA (partial eta squared, ηp2) and t-test (Cohen’s d). Statistical analyses were performed using SPSS (version 26), plots were generated using ggplots2 package in R programming language87.
Rostro-Caudal Volume analysis.
To investigate the effects of genotype and sex on Cavalieri counts, mixed-effects regression models were employed using the `lme4` and `lmerTest` packages in R. The initial model tested the main effect of genotype on Cavalieri counts while accounting for sex, batch effects, and the z-position of the mouse brain sections. Polynomial terms up to the fourth degree for z-position (Zpos) were included to capture potential non-linear relationships observed in exploratory data analysis. Subsequently, a model was fitted to examine the interaction between genotype and sex, including an interaction term for these variables. This model was structured to evaluate whether the effect of genotype on Cavalieri counts varied by sex. The analysis was extended to assess the effects of genotype across different rostral-caudal brain segments. The segments - Rostral, Median, and Caudal - were defined based on changes in direction observed in the z-position plot, aiming to reflect the anatomical structure of the mouse brain. Dummy coding was applied to compare each segment against the reference levels. The final model included these segments as factors, alongside the previously mentioned covariates and polynomial terms for z-position. For data visualization, the `ggplot2` package was utilized to create plots depicting the relationship between z-position and Cavalieri counts. Separate smoothed plot lines were generated for each sex and genotype combination, providing a clear visual representation of the data across different brain regions. All statistical analyses were performed using R. The mixed-effects models allowed for robust estimation of fixed effects while accounting for random effects associated with batch and individual mouse variations.
Adult mouse cortex dissections
Mice were anesthetized with isoflurane and euthanized via cervical dislocation in accordance with institutional animal care protocols. Brains were rapidly extracted, and cerebral cortices were dissected on ice following standard anatomical landmarks. Dissected tissues were snap-frozen in liquid nitrogen or dry ice to preserve molecular integrity and minimize RNA degradation, and stored at −80°C until further use. These samples were subsequently used for RNA extraction, nuclear isolation, or cellular fractionation, as described below.
Cellular Fractionation (synaptosomal preparation)
Brains from Chd8+/5bpdel and wild-type littermates were collected on or around PND60. Following cervical dislocation, brains were sub-dissected to isolate Cortex. Flash frozen cortices were stored at −80C until further processing. On the day of cellular fractionation, samples were treated with Syn-Per reagent (ThermoFisher Scientific Catalog #87793) to obtain cytosol, synaptosome and flowthrough (“total”) fractions. The “total” fraction was then used treated with “NE-PER™ Nuclear and Cytoplasmic Extraction Reagents” (cat #78833) to obtain the nuclear, and a “cleaner cytosol” fractions. Total RNA from these fractions was then extracted using RNA RNAqueous™ Total RNA Isolation Kit (cat# AM1912 ThermoFisher Scientifics). Manufacturer protocols were followed with minor adjustments to increase RNA yield from cellular fractions. Adjustments included combining the cortex samples with 500μl of Syn-Per reagent and 5μl of protease inhibitors during homogenization, based on our starting sample amount, and adding 350μl of RNA extraction lysis buffer immediately after fractionation to the synaptosome and nuclear fractions.
Western blot analysis
Synaptosomal fractions were obtained from cortical extracts from PND 60 micethe using the Syn-Per reagent following manufacturer instructions as described above. Protein concentration was quantified using a BCA protein assay kit (Pierce, 23225) and samples were stored at −80°C until use. For the SDS gel electrophoresis, samples were diluted in 6X Laemmli SDS buffer (375mm Tris-HCl, 9% SDS, 50% glycerol, 0.03% Bromophenol blue) and 5% β-mercaptoethanol, boiled at 70°C for 10 min, and separated on a 4–20% polyacrylamide tris-glycine protein gel (BioRad). Prior loading into the gel, samples were incubated at 70°C for 10 min. After SDS-PAGE, proteins were wet transferred onto a PVDF membrane (Millipore Sigma) overnight at 4°C (13mA, constant current). Membranes were blocked with Intercept PBS blocking buffer (Li-Cor) at room temperature for one hour. Primary antibody PSD93 (1:50, DSHB, Clone N18/28, supernatant fraction) was diluted in 7.5 ml Intercept PBS blocking buffer with 0.1% Tween. Membranes were incubated with the primary antibody solution overnight at 4°C, then washed four times for 10min with PBS with 0.1% Tween (PBST). Fluorescently tagged secondary antibody (Li-Cor) were diluted in 10ml Intercept PBS blocking buffer with 0.1% Tween. After the initial washes, blots were incubated with the secondary antibody solution for one hour at room temperature. Blots were washed an additional four times for 10min with PBST and two times with PBS. Bands were visualized using the Odyssey DLx imaging system (Li-Cor).
RNA Isolation, bulk RNA-Sequencing and bioinformatics analysis
Mouse cortices were collected at around PND60 from the following groups: 16 wild-type (WT) mice and 16 Chd8+/− mice (8 males and 8 females in the WT group; 7 males and 9 females in the Chd8+/− group), as well as 6 WT and 8 Chd8+/flox control mice (4 males and 2 females in the WT group; 5 males and 4 females in the Chd8+/flox group). Samples were snap frozen on dry ice and stored at −80C until RNA preparation. Total RNA was obtained using RNAqueous Total RNA Isolation Kit (cat# AM1912 ThermoFisher Scientifics), and assayed via Agilent RNA 6000 Nano Bioanalyzer kit/instrument. Sample RIN scores ranged from 8 to 9.4. Poly-A-enriched mRNA libraries were prepared at Novogene using Illumina reagents. Libraries were sequenced using Illumina NovaSeq 6000 S4 system, paired-end 150 (PE150) method. Reads were aligned to mouse genome (GRCm38/mm10) using STAR (version 2.5.4b)88, and gene counts were produced using featureCounts89. On average, 50 million paired-end reads (PE) aligned per sample, with a range of 32 to 84 million reads. Data quality was assessed using FastQC90, and principal component analysis (PCA) was used to determine presence of sample outliers. All 24 samples were qualified for the analysis. Raw RNA-seq fastq files and a gene count matrix is available on GEO (accession number TBD). Bioinformatic analysis was performed using R programming language version 4.2.1 (R Development Core Team, 2015) and RStudio integrated development environment version 2023.06.0 (Team R, 2018). Plots were generated using ggplot2 R package version 3.4.0. Heatmaps were generated using pheatmap R package 1.0.12.
Bulk differential expression (DE) analysis
For DE analysis we used edgeR R package91. Genes with a minimum of 1 counts per million (CPM) in at least six samples were included in the analysis. The first surrogate variable from the SVA-batch-correction method was used as a covariate in the edgeR GLM model92. For sex-stratified DE, we used a threshold of CPM > 1 in at least 3 samples, and no batch correction. Reads Per Kilobase per Million mapped reads (RPKM) were used for plotting summary heatmaps and expression data of individual genes.
Bulk gene ontology enrichment analysis
To test for enrichment of GO terms we used the TopGO R package version 2.3471. Mouse Gene Ontology (GO) data was downloaded from Bioconductor (org.Mm.eg.db). For the analysis presented here, we restricted our testing to GO Biological Process annotations and required a minimal node size of 20, and at least 2 significantly DE genes in a GO term. We used the internal ‘weight01’ testing framework and the Fisher exact test, a strategy recommended for gene set analysis that generally accounts for multiple testing comparisons. For GO BP analysis, we reported terms with p-value<0.05. For all enrichment analysis, the test set of DE genes was compared against the background set of genes expressed in our study based on minimum read-count cutoffs described above.
Single Nuclei RNA-seq
Nuclei isolation for snRNA-seq and pooling strategy
Frozen issue from the first 6 samples was processed into nuclei using 2mL glass dounce homogenizers containing 2mL of EZ Prep lysis buffer each. Tissue for 3 samples was dounced over ice, 25 times with pestle A and 35 times with pestle B. Each solution was transferred to a 15 mL conical tube, then the same douncing process was repeated for the second set of 3 samples. Solutions from the latter 3 samples were pooled with those of the first 3 such that each pool contained one male and one female of opposite genotype. An additional 6 mL of EZ Prep lysis buffer was added to each conical tube and the tubes were incubated on ice for 5 minutes. Homogenates were centrifuged at 500xg for 5 minutes at 4°C, the supernatant was discarded, and the pellet was washed twice in NSB (1X PBS with 0.01% BSA and 0.1% RNAse inhibitor). The final pellet was resuspended in 1mL NSB then passed through a 20uM cell strainer. Nuclei were counted using a LUNA-FL cell counter and Acridine Orange/ Propidium Iodide stain. The 10X Genomics nuclei isolation kit, with protocol as described, was used to isolate nuclei for the final 4 samples. These samples were combined into 2 pools, each containing one male and one female of opposite genotype, then nuclei were counted as described previously. Nuclei suspensions had concentrations adjusted to target 10,000 nuclei for sequencing per pool, then used as input for the 10X Genomics 3’ Gene Expression Assay. Libraries were generated according to the v3 protocol by the UC Davis DNA Technologies Core. For the first 3 pools, libraries were sequenced with the Illumina NovaSeq 6000 at 80,000 reads per nucleus. The remaining two pools were sequenced with the Element Biosciences AVITI at 80,000 reads per nucleus.
RNA-seq relevant bioinformatics
Pre-processing of snRNA-seq data and initial QC
De-multiplexed sequencing data were obtained from the UC Davis DNA Technologies Core as FASTQ files. FASTQ files were passed through the Cell Ranger count pipeline for alignment to the mouse mm10 genome, filtering, and creation of feature-barcode matrices. Ambient RNA removal was performed using SoupX (version), and Seurat objects were generated using Seurat (version). These Seurat objects were subjected to initial clustering, and each resulting cluster was filtered based on cluster-specific thresholds for UMI count, feature count, and percentage of mitochondrial reads, which were determined using standard deviation criteria. Mitochondrial genes were then removed from all objects.
Doublet removal
The DoubletFinder package (version) was used to assign nuclei as ‘Singlet’ or ‘Doublet’. Predicted doublets were removed before proceeding with downstream analyses.
Assigning sex and genotype
Nuclei were next assigned as ‘male’ or ‘female’ using the sxreveal package, which uses X- and Y-linked marker gene expression to predict sex. Chd8 genotype was assigned based on the predicted sex and pool-specific metadata. Because each pool consisted of only one male and one female of opposite genotype, all female nuclei in a pool were assumed to be one genotype and all males were assumed to be the other.
Clustering analysis
Seurat objects for each of the 5 samples were individually passed through SCTransform to normalize counts, then all 5 were integrated using IntegrateData. The ‘RNA’ assay of the integrated object was normalized and scaled using NormalizeData and ScaleData. Principal Components Analysis was performed on the ‘Integrated’ assay of the integrated data to identify top variable genes, and FindNeighbors was used to construct a KNN graph based on their euclidean distance in PCA space. The data were then passed through FindClusters to group cells into clusters using the Louvain algorithm. Clusters were visualized with uniform manifold approximation and projection (UMAP).
Cell type annotation
FindAllMarkers was used to identify cluster marker genes. Modules of cell type marker genes described in published scRNA-seq datasets from adult mouse cortex were constructed. Canonical marker genes were added to these modules, then AddModuleScore was used to calculate relative expression of each module in each cluster. The cell type associated with the marker gene module that had the highest expression in each cluster was assigned as that cluster’s identity. All nuclei were assigned identities at 3 levels of classification, with ‘Cell_Type_1’ being the most specific and ‘Cell_Type_3’ the broadest.
Cell type re-clustering
Clusters that were poorly clustered, had a high percentage of mitochondrial reads relative to other clusters, or had ambiguous marker genes (i.e. markers for multiple distinct cell types) were removed before subsequent analysis. The filtered Seurat object was then re-normalized, re-scaled, and re-clustered as described previously.
Differential expression (DE) analysis
A pseudo-bulk approach was used to identify differentially-expressed genes in each cortical cell population. Each cluster was extracted as a subset of the main Seurat object, then raw counts were passed through the DESeq2 pipeline to compare Chd8 mutant and wild-type nuclei within that subset. Batch was included as a covariate in the DESeq2 statistical model. DEGs with an adjusted p-value < 0.05 were deemed significant. This analysis was performed at each of the 3 levels of cell type assignment.
Burden analysis
A minimum expression threshold was determined based on the minimum expression level (‘baseMean’) of DEGs with p-value < 0.05. For each cell type, DE burden was calculated as the percentage of DEGs relative to the total expressed genes that passed the minimum expression threshold. A permutation test with 1,000 iterations was performed to assess the statistical significance of the observed DE burden. This analysis was performed at each of the three levels of cell type classification.
Cell type proportion analysis
For each cell population, nuclei of each genotype were counted, then counts were converted to a proportion of the total cells in each sample. Linear models adjusted for genotype, sex, and batch were used to assess whether or not the proportions of mutant and wild-type nuclei for each cell type were statistically different.
hdWGCNA
High-dimensional weighted gene co-expression network analysis (hdWGCNA) was applied to construct gene co-expression networks for each cell population. Nuclei of specific cell types were grouped by sample and passed through the MetacellsByGroups function to create metacells. Custom parameters for ‘k’, ‘max_shared’, and ‘min_cells’ were chosen for each cell type. We determined the optimal soft power using TestSoftPowers, built the co-expression network with ConstructNetwork, and detected modules via hierarchical clustering. Harmonized module eigengenes (hMEs) were calculated for each module using ModuleEigengenes and GetMEs.
Differential module expression analysis
To test for differences in module gene expression by genotype, we analyzed each module identified in glutamatergic neurons by plotting the log fold change (logFC) of its member genes based on pseudo-bulk differential expression analysis. We then compared the median logFC of each module to that of the corresponding ‘grey’ module using a t-test, applying Bonferroni correction for multiple comparisons. Modules with a Bonferroni-adjusted p-value < 0.05 in at least one glutamatergic subtype were considered significantly differentially expressed.
GO enrichment analysis
GO analysis was performed on significant DEGs and DEMs using enrichGO, with the gene universe being set to all genes expressed in the cell type the DEGs or DEMs were identified in. Genes and modules were annotated with biological process and molecular function terms. Terms were deemed significant if they had a corrected p-value < 0.05.
Gene set enrichment analysis (GSEA)
Gene set enrichment analysis was performed on DEGs from bulk and pseudobulk DE analysis using the clusterProfiler R package60 (version 4.10.0) with the org.Mm.eg.db database (version 3.18.0, Carlson M (2022). _org.Mm.eg.db: Genome wide annotation for Mouse_. R package version 3.15.0.). For pseudobulk DEGs, gene hits from each cell type were filtered to include only genes with adjusted p-value < 0.05 and absolute LogFC > 0.25. Enrichment testing was conducted separately for upregulated and downregulated gene sets using the enrichGO() function, with background genes defined as all genes tested for differential expression in the corresponding analysis. Multiple testing correction was applied using the Benjamini-Hochberg method, and GO terms with adjusted p-values < 0.05 were considered significant.
Calcium Imaging - Stereotactic injections
We utilized adult mice of either sex housed and bred in the UCSF animal facility. To image prefrontal activity in acute slices from wildtype or mutant animals, mice were injected with AAV5.hSyn.GCaMP6f.WPRE.SV40 (Penn Viral Core). Mice were anesthetized with 2% isoflurane and mounted in a stereotactic frame. Craniotomies were made according to stereotaxic coordinates relative to Bregma. Coordinates for injection into mPFC were (in mm relative to Bregma): +1.7 anterior–posterior (AP), –0.3 mediolateral (ML) and –2.75 dorsoventral (DV). Imaging was performed 4–6 weeks later.
Slice preparation and imaging
300 μm coronal slices were prepared as previously described93. Immediately after preparation slices were transferred to an N-Methyl-D-Glucamine (NMDG)-based recovery solution for 10 minutes before being transferred to ACSF for the remainder of their recovery. The NMDG-based solution was maintained at 32° C, and consisted of the following (in mM): 93 N-Methyl-D-Glucamine (NMDG), 93 HCl, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 25 glucose, 20 HEPES, 5 Na-ascorbate, 5 Na-pyruvate, 2 thiourea, 10 magnesium sulfate, 0.5 calcium chloride. ACSF contained the following (in mM): 126 NaCl, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl, and 10 glucose. For imaging slices were perfused with ‘Active’ ACSF which was identical to normal ACSF except containing elevated KCl (3.5 mM vs 2 mM) and reduced CaCl (1.2 mM vs 2 mM). Slice data was acquired at 10 Hz on an Olympus BX51 upright microscope with a 20× 1.0NA water immersion lens, 0.5× reducer (Olympus), and ORCA-ER CCD Camera (Hamamatsu Photonics). Illumination was delivered using a Lambda DG4 arc lamp (Sutter Instruments). Light was delivered through a 472/30 excitation filter, 495nm single band dichroic, and 496nm long pass emission filter (Semrock). All movies consisted of 36000 frames acquired at 10Hz (1 hr) with 4×4 sensor binning yielding a final resolution of 256 × 312 pixels. Light power during imaging was 100 – 500 μW/mm2. The Micro Manager software suite (v1.4, NIH) was used to control all camera parameters and acquire movies. When necessary, drift correction was conducted in MATLAB using custom written code94 modified to utilize individual neurons as fiducials, whose positions were averaged and aligned every 5 seconds. We segmented neuronal signals using a modified PCA/ICA approach95 modified as previously described96 so that each segment was expressed as a binary ROI consisting of pixels representing a single neuron. To deconvolve neuronal signals from background neuropil signals, we subtracted the mean signal from each identified segment from the mean value in pixels surrounding the edge of the segment (we excluded pixels that belonged to another ROI). Signals were subsequently lowpass filtered to remove high frequency noise using the Matlab command: designfilt(‘lowpassfir’, ‘PassbandFrequency’, 0.5, ‘StopbandFrequency’, .65, ‘PassbandRipple’, 1, ‘StopbandAttenuation’, 25). We then detected events corresponding to epochs in which each neuron was active using threshold-based event detection as previously described4. We detected increases in (F-F0)/F0 exceeding 2.5σ over one frame, and then further thresholded these events by keeping only those events which exceeded a 8σ increase over baseline and had an integrated area of 200 σ. σ is the standard deviation of (F-F0)/F0. All further analysis was performed on binary rasters of detected events for each slice.
Cranial Window Implantation Surgery
Cranial window implantation in adult mice (6–8 weeks old) was performed as previously described97. Briefly, the mouse was anaesthetized by gaseous isoflurane (4% for induction, 1.5–2% for maintenance) delivered through a vaporizer system and mounted on a stereotaxic frame. Throughout the surgery the mouse was kept warm with a heating pad. Ophthalmic ointment was applied to prevent eye desiccation and irritation. Carprofen (5 mg/kg, i.p.), buprenorphine (0.1 mg/kg, s.c.), enrofloxacin (5 mg/kg, s.c.), and dexamethasone (2 mg/kg, intramuscular) were administered. The fur over the surgical site was shaved, and the scalp was cleaned with 3 rounds of alternating 70% ethanol and iodine solution (Betadine®). A midline scalp incision was made, and the periosteum was gently scraped off from the skull using a scalpel. A circular piece of the skull (centered at AP = –1 mm, ML = 1.5 mm) was removed with a trephine (diameter = 2.3 mm, Fine Science Tools, Foster City, CA, USA) driven by a high-speed micro-drill (Foredom K1070, Blackstone Industries, LLC, Bethal, CT, USA). The imaging port was made by gluing a circular cover glass (#2, diameter = 2.2 mm) underneath a donut-shaped glass (#1, inner diameter = 2 mm, outer diameter = 3 mm; Potomac Photonics, Inc., Baltimore, MD, USA). The imaging port was mounted so that the bottom cover glass fit snugly into the cranial window and the top glass donut rested above the skull. The imaging port was secured with a UV-cured adhesive (Fusion Flo, Prevest DenPro, Jammu, India) onto the skull. After the solidification of the adhesive, the scalp flaps were closed with suture. After 2 weeks of recovery, the central piece of the scalp was excised, the edge of the scalp flap was secured to the tissue underneath with cyanoacrylate (Vetbond), and a custom-made stainless-steel head-bar was secured over the skull with dental cement (Jet Denture Repair, Lang Dental, Wheeling, IL, USA). The mouse received enrofloxacin, buprenorphine, and carprofen once per day for two extra days post-surgery and was allowed to recover for an additional week prior to imaging.
Thin skull preparation
The thin skull procedure was performed on adolescent (1 month old) mice as previously described98. Briefly, the mouse was anesthetized with a mixture of ketamine (20 mg/ml) and xylazine (2.0 mg/ml) in 0.9% sterile saline administered intraperitoneally (5 ml/kg body weight). Ophthalmic ointment was applied to the eyes to prevent desiccation and irritation, and the fur over the scalp was removed with a blade. A midline incision was made through the scalp and the periosteum was gently scraped off from the skull. A high-speed micro-drill (Foredom K1070, Blackstone Industries, LLC, Bethal, CT, USA) and a microblade were used to thin a small region of the skull to ∼20 μm thickness. A custom-made head-plate with a central opening was attached to the skull by cyanoacrylate glue (Krazy Glue, Elmer’s Products, Westerville, OH, USA), centered over the thinned region. The head-plate was secured onto a custom-made metal baseplate to stabilize the mouse’s head during imaging. Two-photon imaging was performed as described above. After imaging, the head-plate was detached from the skull, the skull was cleaned with sterile saline, and the scalp was sutured. The same region was imaged again 7 days later.
In vivo two-photon (2P) imaging and analysis
2P imaging of dendritic spines was performed on a 2P microscope (Ultima IV, Bruker Co., Middleton, WI) equipped with a 40× NA = 0.8 water immersion objective (LUMPlanFl/IR, Olympus, Japan) and an ultrafast 2P laser (Mai Tai, Spectra-Physics, Santa Clara, CA) operating at 940 nm. The mouse was anaesthetized with an intraperitoneal injection of a mixture of 85 mg/kg ketamine and 8.5 mg/kg xylazine in 0.9% saline and mounted on a custom-made stage for imaging. Stacks of images were acquired with a Z-step size of 1 μm at 4X zoom. Relocation of the same dendrites in subsequent imaging sessions was achieved by reference to blood vessels and the dendritic branching pattern. The same dendrites were imaged again 1 week after the first imaging session.
Data analysis was performed in ImageJ as previously described98. Typically, 150–200 spines were analyzed per animal per session. The percentage of spines formed/eliminated was calculated as the number of spines formed/eliminated divided by the total number of spines counted from the previous imaging session. Dendritic spine density was calculated as the number of dendritic spines per 10 μm of dendritic segment length on the first imaging day.
EEG/EMG recording and analysis
The EEG/EMG implant consists of a mini connector (Samtec Inc., SFMC-102-01-S-D) soldered to 2 EEG and 2 EMG electrodes made from Teflon-coated, multi-filament stainless steel wires (0.002” in diameter, Medwire 316 SS7/44T, Sigmund Cohn Corp.). The mouse was anaesthetized by gaseous isoflurane (4% for induction, 1.5–2% for maintenance) delivered through a vaporizer system and mounted on a stereotaxic frame. Throughout the surgery the mouse was kept warm with a heating pad. Ophthalmic ointment was applied to prevent eye desiccation and irritation. Buprenorphine (0.1 mg/kg, s.c.), carprofen (5 mg/kg, i.p.), and enrofloxacin (5 mg/kg, s.c.) were injected. The fur over the surgical site was shaved, and the scalp was cleaned with 3 rounds of alternating 70% ethanol and iodine solution (Betadine®). A piece of the scalp was excised with scissors to expose the skull. The edge of the scalp flap was secured to the tissue underneath with cyanoacrylate (Vetbond). The exposed skull surface was gently scraped with a scalpel to remove the periosteum. Small holes (one at AP = + 2 mm, ML = 2 mm; the other at AP = −3 mm, ML = 2 mm) were drilled through the skull using a high speed microdrill (Foredom K.1070) with fine drill bits. The EEG electrodes were inserted into the holes, positioned between the skull and the brain surface, and secured with a UV-curable adhesive (Fusion Flo, Prevest DenPro). The EMG electrodes were positioned under the nuchal trapezoid muscles and secured with Vetbond. The exposed skull surface was covered with a thin layer of Vetbond. The EEG/EMG implant connector was then further secured onto the skull by dental cement (Jet Denture Repair, Lang Dental). Enrofloxacin (5 mg/kg, s.c.) and carprofen (5 mg/kg, i.p.) were given at 24 h after surgery. The mouse was allowed to recover for 7 days before EEG/EMG recording starts. A BL-420N biological signal acquisition and processing system (Chengdu Techman, China) was used for EEG/EMG recording. EEG was sampled at 200 Hz and band-pass filtered between 1–100 Hz; EMG was sampled at 200 Hz and band-pass filtered between 1–2K Hz. Recorded data was annotated manually and subsequently processed using custom-written Matlab programs (R2022, MathWorks Inc.).
Acknowledgements
This work was supported by the National Institutes of Health National Institute of Mental Health (NIH NIMH) R01 MH120513 to A.S.N., National Institute of Neurological Disorders and Stroke (K08 NS105938-01A1 to NAF), the Simons Foundation (Award # 604325) and the National Institute of Mental Health (R01MH117961 to VS), as well as the UCSF Dolby Family Center for Mood Disorders. C.P.C was supported by the NIH NIMH Autism Research Training Program fellowship (T32 MH073124-16). A.A.W was supported by NIH NIMH F31 MH119789 and T32 GM007377. We thank Dr. Wenjie Bian and Dr. Luis de Lecea at Stanford University, for their technical advice on EEG/EMG experiments and data analysis.
REFERENCES
- 1.Fu J. M. et al. Rare coding variation provides insight into the genetic architecture and phenotypic context of autism. Nat. Genet. 54, 1320–1331 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satterstrom F. K. et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 180, 568–584.e23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alotaibi M. & Ramzan K. A de novo variant of CHD8 in a patient with autism spectrum disorder. Discov. Craiova Rom. 8, e107 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An Y. et al. De novo variants in the Helicase-C domain of CHD8 are associated with severe phenotypes including autism, language disability and overgrowth. Hum. Genet. 139, 499–512 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Beighley J. S. et al. Clinical Phenotypes of Carriers of Mutations in CHD8 or Its Conserved Target Genes. Biol. Psychiatry 87, 123–131 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernier R. et al. Disruptive CHD8 Mutations Define a Subtype of Autism Early in Development. Cell 158, 263–276 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doummar D. et al. Childhood-onset progressive dystonia associated with pathogenic truncating variants in CHD8. Ann. Clin. Transl. Neurol. 8, 1986–1990 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douzgou S. et al. The clinical presentation caused by truncating CHD8 variants. Clin. Genet. 96, 72–84 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Ostrowski P. J. et al. The CHD8 overgrowth syndrome: A detailed evaluation of an emerging overgrowth phenotype in 27 patients. Am. J. Med. Genet. C Semin. Med. Genet. 181, 557–564 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Amaral D. G. et al. In pursuit of neurophenotypes: The consequences of having autism and a big brain. Autism Res. Off. J. Int. Soc. Autism Res. 10, 711–722 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnard R. A., Pomaville M. B. & O’Roak B. J. Mutations and Modeling of the Chromatin Remodeler CHD8 Define an Emerging Autism Etiology. Front. Neurosci. 9, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotney J. et al. The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nat. Commun. 6, 6404 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willsey H. R., Willsey A. J., Wang B. & State M. W. Genomics, convergent neuroscience and progress in understanding autism spectrum disorder. Nat. Rev. Neurosci. 23, 323–341 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy D. et al. Rare De Novo and Transmitted Copy-Number Variation in Autistic Spectrum Disorders. Neuron 70, 886–897 (2011). [DOI] [PubMed] [Google Scholar]
- 15.O’Roak B. J. et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485, 246–250 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Roak B. J. et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat. Genet. 43, 585–589 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ronemus M., Iossifov I., Levy D. & Wigler M. The role of de novo mutations in the genetics of autism spectrum disorders. Nat. Rev. Genet. 15, 133–141 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Yuen R. K. et al. Genome-wide characteristics of de novo mutations in autism. Npj Genomic Med. 1, 1–10 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishiyama M. et al. Early Embryonic Death in Mice Lacking the β-Catenin-Binding Protein Duplin. Mol. Cell. Biol. 24, 8386–8394 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gompers A. L. et al. Germline Chd8 haploinsufficiency alters brain development in mouse. Nat. Neurosci. 20, 1062–1073 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung H. et al. Sexually dimorphic behavior, neuronal activity, and gene expression in Chd8-mutant mice. Nat. Neurosci. 21, 1218–1228 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Katayama Y. et al. CHD8 haploinsufficiency results in autistic-like phenotypes in mice. Nature 537, 675–679 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Niu X. et al. The Deficiency of the ASD-Related Gene CHD8 Disrupts Behavioral Patterns and Inhibits Hippocampal Neurogenesis in Mice. J. Mol. Neurosci. MN 74, 103 (2024). [DOI] [PubMed] [Google Scholar]
- 24.Platt R. J. et al. Chd8 Mutation Leads to Autistic-like Behaviors and Impaired Striatal Circuits. Cell Rep. 19, 335–350 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suetterlin P. et al. Altered Neocortical Gene Expression, Brain Overgrowth and Functional Over-Connectivity in Chd8 Haploinsufficient Mice. Cereb. Cortex N. Y. N 1991 28, 2192–2206 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiménez J. A. et al. Chd8 haploinsufficiency impairs early brain development and protein homeostasis later in life. Mol. Autism 11, 74 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiménez J. A. et al. Chd8 haploinsufficiency impairs early brain development and protein homeostasis later in life. Mol. Autism 11, 74 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durak O. et al. Chd8 mediates cortical neurogenesis via transcriptional regulation of cell cycle and Wnt signaling. Nat. Neurosci. 19, 1477–1488 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yim K. M. et al. Cell type-specific dysregulation of gene expression due to Chd8 haploinsufficiency during mouse cortical development. bioRxiv 2024.08.14.608000 (2024) doi: 10.1101/2024.08.14.608000. [DOI] [PubMed] [Google Scholar]
- 30.Hurley S. et al. Distinct, dosage-sensitive requirements for the autism-associated factor CHD8 during cortical development. Mol. Autism 12, 16 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Astorkia M., Liu Y., Pedrosa E. M., Lachman H. M. & Zheng D. Molecular and network disruptions in neurodevelopment uncovered by single cell transcriptomics analysis of CHD8 heterozygous cerebral organoids. Preprint at 10.1101/2023.09.27.559752 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulsen B. et al. Autism genes converge on asynchronous development of shared neuron classes. Nature 602, 268–273 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann A. & Spengler D. Single-Cell Transcriptomics Supports a Role of CHD8 in Autism. Int. J. Mol. Sci. 22, 3261 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabbaa M., Knoll A. & Levitt P. Mouse population genetics phenocopies heterogeneity of human Chd8 haploinsufficiency. Neuron 111, 539–556.e5 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabbaa M. & Levitt P. Chd8 haploinsufficiency impacts rearing experience in C57BL/6 mice. Genes Brain Behav. 23, e12892 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellingford R. A. et al. Cell-type-specific synaptic imbalance and disrupted homeostatic plasticity in cortical circuits of ASD-associated Chd8 haploinsufficient mice. Mol. Psychiatry 26, 3614–3624 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellingford R. A., Tojo M., Basson M. A. & Andreae L. C. Male-Dominant Effects of Chd8 Haploinsufficiency on Synaptic Phenotypes during Development in Mouse Prefrontal Cortex. ACS Chem. Neurosci. 15, 1635–1642 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villa C. E. et al. CHD8 haploinsufficiency links autism to transient alterations in excitatory and inhibitory trajectories. Cell Rep. 39, 110615 (2022). [DOI] [PubMed] [Google Scholar]
- 39.Li B. et al. CHD8 mutations increase gliogenesis to enlarge brain size in the nonhuman primate. Cell Discov. 9, 27 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi X. et al. Heterozygous deletion of the autism-associated gene CHD8 impairs synaptic function through widespread changes in gene expression and chromatin compaction. Am. J. Hum. Genet. 110, 1750–1768 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao Y. et al. Multimodal analyses reveal genes driving electrophysiological maturation of neurons in the primate prefrontal cortex. Preprint at 10.1101/2023.06.02.543460 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kweon H. et al. Excitatory neuronal CHD8 in the regulation of neocortical development and sensory-motor behaviors. Cell Rep. 34, 108780 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Haddad Derafshi B. et al. The autism risk factor CHD8 is a chromatin activator in human neurons and functionally dependent on the ERK-MAPK pathway effector ELK1. Sci. Rep. 12, 22425 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawamura A. & Nishiyama M. Deletion of the autism-related gene Chd8 alters activity-dependent transcriptional responses in mouse postmitotic neurons. Commun. Biol. 6, 593 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong C. et al. Conserved and Distinct Functions of the Autism-Related Chromatin Remodeler CHD8 in Embryonic and Adult Forebrain Neurogenesis. J. Neurosci. Off. J. Soc. Neurosci. 42, 8373–8392 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X. et al. Deletion of CHD8 in cerebellar granule neuron progenitors leads to severe cerebellar hypoplasia, ataxia, and psychiatric behavior in mice. J. Genet. Genomics Yi Chuan Xue Bao 49, 859–869 (2022). [DOI] [PubMed] [Google Scholar]
- 47.Kawamura A. et al. Oligodendrocyte dysfunction due to Chd8 mutation gives rise to behavioral deficits in mice. Hum. Mol. Genet. 29, 1274–1291 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Kawamura A. et al. Chd8 mutation in oligodendrocytes alters microstructure and functional connectivity in the mouse brain. Mol. Brain 13, 160 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao C. et al. Dual Requirement of CHD8 for Chromatin Landscape Establishment and Histone Methyltransferase Recruitment to Promote CNS Myelination and Repair. Dev. Cell 45, 753–768.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Megagiannis P. et al. Autism-associated CHD8 controls reactive gliosis and neuroinflammation via remodeling chromatin in astrocytes. Cell Rep. 43, 114637 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takanezawa Y. et al. Microglial ASD-related genes are involved in oligodendrocyte differentiation. Sci. Rep. 11, 17825 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spear L. P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 24, 417–463 (2000). [DOI] [PubMed] [Google Scholar]
- 53.West M. J. & Gundersen H. J. Unbiased stereological estimation of the number of neurons in the human hippocampus. J. Comp. Neurol. 296, 1–22 (1990). [DOI] [PubMed] [Google Scholar]
- 54.Lavenex P. B. Lesions in the budgerigar vocal control nucleus NLc affect production, but not memory, of english words and natural vocalizations. J. Comp. Neurol. 421, 437–460 (2000). [DOI] [PubMed] [Google Scholar]
- 55.West M. J., Slomianka L. & Gundersen H. J. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 231, 482–497 (1991). [DOI] [PubMed] [Google Scholar]
- 56.Young M. D. & Behjati S. SoupX removes ambient RNA contamination from droplet-based single-cell RNA sequencing data. GigaScience 9, giaa151 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao Z. et al. A high-resolution transcriptomic and spatial atlas of cell types in the whole mouse brain. BioRxiv Prepr. Serv. Biol. 2023.03.06.531121 (2023) doi: 10.1101/2023.03.06.531121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murphy A. E. & Skene N. G. A balanced measure shows superior performance of pseudobulk methods in single-cell RNA-sequencing analysis. Nat. Commun. 13, 7851 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Love M. I., Huber W. & Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 1–21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu T. et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innov. Camb. Mass 2, 100141 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao W., Johnston K. G., Ren H., Xu X. & Nie Q. Inferring neuron-neuron communications from single-cell transcriptomics through NeuronChat. Nat. Commun. 14, 1128 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morabito S., Reese F., Rahimzadeh N., Miyoshi E. & Swarup V. hdWGCNA identifies co-expression networks in high-dimensional transcriptomics data. Cell Rep. Methods 3, 100498 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoo T. et al. A DLG2 deficiency in mice leads to reduced sociability and increased repetitive behavior accompanied by aberrant synaptic transmission in the dorsal striatum. Mol. Autism 11, 19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoo T. et al. A Deficiency of the Psychiatric Risk Gene DLG2/PSD-93 Causes Excitatory Synaptic Deficits in the Dorsolateral Striatum. Front. Mol. Neurosci. 15, 938590 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanders B. et al. Transcriptional programs regulating neuronal differentiation are disrupted in DLG2 knockout human embryonic stem cells and enriched for schizophrenia and related disorders risk variants. Nat. Commun. 13, 27 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang X. et al. Common and Rare Genetic Risk Factors Converge in Protein Interaction Networks Underlying Schizophrenia. Front. Genet. 9, 434 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000). [DOI] [PubMed] [Google Scholar]
- 68.Wang P. et al. CRISPR/Cas9-mediated heterozygous knockout of the autism gene CHD8 and characterization of its transcriptional networks in neurodevelopment. Mol. Autism 6, 55 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haddad Derafshi B. et al. The autism risk factor CHD8 is a chromatin activator in human neurons and functionally dependent on the ERK-MAPK pathway effector ELK1. Sci. Rep. 12, 22425 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Earl R. K. et al. Sleep Problems in Children with ASD and Gene Disrupting Mutations. J. Genet. Psychol. 182, 317–334 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.topGO. Bioconductor http://bioconductor.org/packages/topGO/.
- 72.Kerschbamer E. et al. CHD8 suppression impacts on histone H3 lysine 36 trimethylation and alters RNA alternative splicing. Nucleic Acids Res. 50, 12809–12828 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wade A. A., Lim K., Catta-Preta R. & Nord A. S. Common CHD8 Genomic Targets Contrast With Model-Specific Transcriptional Impacts of CHD8 Haploinsufficiency. Front. Mol. Neurosci. 11, 481 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zaghlool A. et al. Characterization of the nuclear and cytosolic transcriptomes in human brain tissue reveals new insights into the subcellular distribution of RNA transcripts. Sci. Rep. 11, 4076 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bakken T. E. et al. Single-nucleus and single-cell transcriptomes compared in matched cortical cell types. PloS One 13, e0209648 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Squair J. W. et al. Confronting false discoveries in single-cell differential expression. Nat. Commun. 12, 5692 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chareyron L. J., Banta Lavenex P., Amaral D. G. & Lavenex P. Stereological analysis of the rat and monkey amygdala. J. Comp. Neurol. 519, 3218–3239 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lavenex P., Lavenex P. B., Bennett J. L. & Amaral D. G. Postmortem changes in the neuroanatomical characteristics of the primate brain: hippocampal formation. J. Comp. Neurol. 512, 27–51 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates. (2019). [Google Scholar]
- 80.Gundersen H. J. & Jensen E. B. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 147, 229–263 (1987). [DOI] [PubMed] [Google Scholar]
- 81.Gundersen H. J. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J. Microsc. 143, 3–45 (1986). [PubMed] [Google Scholar]
- 82.Long J. M. et al. Stereological estimation of total microglia number in mouse hippocampus. J. Neurosci. Methods 84, 101–108 (1998). [DOI] [PubMed] [Google Scholar]
- 83.García-Cabezas M. Á., John Y. J., Barbas H. & Zikopoulos B. Distinction of Neurons, Glia and Endothelial Cells in the Cerebral Cortex: An Algorithm Based on Cytological Features. Front. Neuroanat. 10, 107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haida O. et al. Sex-dependent behavioral deficits and neuropathology in a maternal immune activation model of autism. Transl. Psychiatry 9, 124 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lauber E., Filice F. & Schwaller B. Dysregulation of Parvalbumin Expression in the Cntnap2-/- Mouse Model of Autism Spectrum Disorder. Front. Mol. Neurosci. 11, 262 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gundersen H. J. The nucleator. J. Microsc. 151, 3–21 (1988). [DOI] [PubMed] [Google Scholar]
- 87.Wickham H. Ggplot2: Elegant Graphics for Data Analysis. (Springer, New York, NY, 2009). doi: 10.1007/978-0-387-98141-3. [DOI] [Google Scholar]
- 88.Dobin A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinforma. Oxf. Engl. 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liao Y., Smyth G. K. & Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinforma. Oxf. Engl. 30, 923–930 (2014). [DOI] [PubMed] [Google Scholar]
- 90.Babraham Bioinformatics - FastQC A Quality Control tool for High Throughput Sequence Data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- 91.Robinson M. D., McCarthy D. J. & Smyth G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinforma. Oxf. Engl. 26, 139–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leek J. T., Johnson W. E., Parker H. S., Jaffe A. E. & Storey J. D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinforma. Oxf. Engl. 28, 882–883 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luongo F. J., Horn M. E. & Sohal V. S. Putative Microcircuit-Level Substrates for Attention Are Disrupted in Mouse Models of Autism. Biol. Psychiatry 79, 667–675 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frost N. A., Shroff H., Kong H., Betzig E. & Blanpied T. A. Single-molecule discrimination of discrete perisynaptic and distributed sites of actin filament assembly within dendritic spines. Neuron 67, 86–99 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mukamel E. A., Nimmerjahn A. & Schnitzer M. J. Automated analysis of cellular signals from large-scale calcium imaging data. Neuron 63, 747–760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frost N. A., Haggart A. & Sohal V. S. Dynamic patterns of correlated activity in the prefrontal cortex encode information about social behavior. PLoS Biol. 19, e3001235 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu J. et al. An analog of psychedelics restores functional neural circuits disrupted by unpredictable stress. Mol. Psychiatry 26, 6237–6252 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu T. et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462, 915–919 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]