Abstract
Suppression of insulin-like growth factor-1 (IGF-1) signaling extends mammalian lifespan and protects against a range of age-related diseases. Surprisingly though, we found that reduced IGF-1 signaling fails to extend the lifespan of mitochondrial mutator mice. Accordingly, most of the longevity pathways that are normally initiated by IGF-1 suppression were either blocked or blunted in the mutator mice. These observations suggest that the pro-longevity effects of IGF-1 suppression critically depend on the integrity of the mitochondrial genome and that mitochondrial mutations may impose a hard limit on mammalian lifespan. Together, these findings deepen our understanding of the interactions between the hallmarks of aging and underscore the need for interventions that preserve the integrity of the mitochondrial genome.
INTRODUCTION
Aging is a complex biological process that is characterized by multiple molecular and cellular hallmarks, including genomic instability, mitochondrial dysfunction and deregulated nutrient sensing1. While significant progress has been made in our understanding of the individual hallmarks of human aging, their interactions remain poorly defined. In addition to obscuring our insight into the basic biology of aging, this gap in our knowledge complicates the development of effective anti-aging interventions, as targeting one hallmark of aging may inadvertently exacerbate others.
One strategy to elucidate the relationships between the hallmarks of aging is to investigate how the disruption of one hallmark affects the trajectory of another. In doing so, it may be possible to assess whether these processes act independently, synergistically, or in opposition of each other as they shape human lifespan. In addition, this strategy may reveal if a hierarchy exists between aging pathways, which could lead to a more integrated and causally ordered model of the aging process. In this study, we apply this strategy to investigate the relationship between two critical drivers of the aging process, mitochondrial mutagenesis2 and IGF-1 signaling3.
A large body of evidence supports the idea that instability of the mitochondrial genome (mtDNA) leads to a progressive decline in mitochondrial function, which accelerates the natural aging process and contributes to a wide variety of age-related diseases, including sarcopenia, neurodegeneration and heart failure2. A similar body of work describes the role of IGF-1 signaling in the aging process. IGF-1 regulates the growth and metabolism of human tissues, and reduced IGF-1 signaling can not only extend mammalian lifespan, but can also confer resistance against various age-related diseases, including neurodegeneration, metabolic decline, and cardiovascular disease3. However, how mitochondrial mutagenesis and IGF-1 signaling interact with each other to shape mammalian lifespan remains unclear.
It is likely though, that mitochondrial mutagenesis and IGF-1 signaling intersect at multiple decisive junctions during the aging process. At a molecular level, mtDNA mutations are a constant source of oxidative stress4, a form of cellular damage that is directly influenced by IGF-1 activity5. At a systemic level, mitochondria serve as central hubs of metabolic activity and respond dynamically to changes in nutrient availability and growth signals6, processes that are tightly regulated by IGF-1 signaling7. And finally, at a macroscopic level, mitochondrial mutations directly contribute to a variety of age-related diseases2,8,9, many of which can be prevented, or slowed down by reduced IGF-1 signaling10–14.
Together, these observations suggest that mitochondrial mutagenesis and IGF-1 signaling do not operate in isolation, but instead converge on overlapping biological pathways that shape the rate and quality of aging. If so, it may be possible to counteract the harmful effects of mtDNA mutations by suppressing IGF-1 signaling, which could improve antioxidant defenses5, enhance mitochondrial turnover15, and delay the onset of age-related diseases16. In support of this idea, we previously found that reduced insulin/IGF-1 signaling can alleviate muscle dysfunction in a C. elegans model of mtDNA disease17. Alternatively, mitochondrial mutagenesis and IGF-1 signaling may act through parallel, but mechanistically distinct pathways, in which case interventions targeting IGF-1 signaling would fail to overcome the progeroid effects of mtDNA instability.
To distinguish between these possibilities, we reduced IGF-1 signaling in mice that carry an error-prone version of DNA Polymerase γ18 (PolgAD257A), the enzyme that replicates the mitochondrial genome. These mice accumulate mtDNA mutations at an accelerated pace19,20, which reduces their lifespan by 40% and leads to a wide variety of age-associated phenotypes, including sarcopenia, cardiomyopathy, anemia, and inflammation18,21. We crossed these mice into a background that is deficient for Pappalysin 1 (Pappa), a metalloproteinase that increases IGF-1 bioavailability by cleaving IGF-binding protein 4 (IGFBP-4)22. In the absence of PAPPA, IGFBP-4 persists and sequesters IGF-1 away from receptors, thereby reducing IGF-1 signaling. When Pappa is deleted in WT mice, they exhibit a 35% increase in lifespan and a reduction in age-related pathologies23–25.
Surprisingly though, we found that Pappa deletion failed to extend the lifespan of PolgAD257A mice, suggesting that the integrity of the mitochondrial genome is required for lifespan extension by reduced IGF-1 signaling. Moreover, many of the pro-longevity programs normally initiated by Pappa deletion, including improved proteostasis, enhanced DNA repair and better telomere maintenance failed to initiate in PolgAD257A; Pappa−/− mice. These observations suggest that mtDNA instability places a hard limit on mammalian lifespan, and that mitochondrial function is required for the successful activation of multiple longevity programs.
RESULTS
Lifespan extension by IGF-1 suppression depends on the integrity of the mitochondrial genome
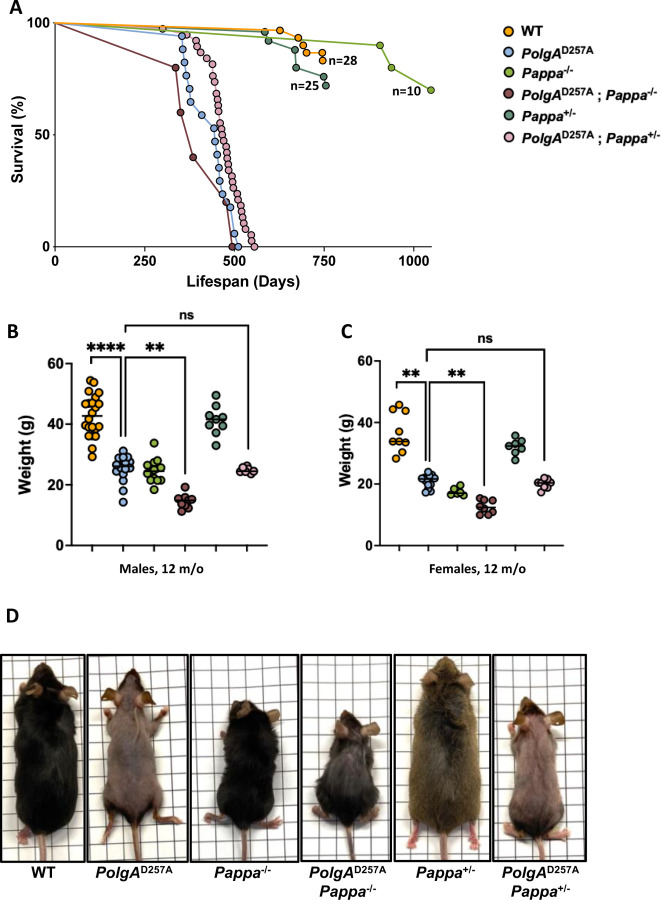
To examine how IGF-1 signaling and mitochondrial mutagenesis interact to shape mammalian lifespan, we crossed PolgAD257A mice into a Pappa−/− background and compared the lifespan of WT mice to PolgAD257A mice, Pappa−/− mice, PolgAD257A; Pappa−/− mice, Pappa+/− mice, and PolgAD257A; Pappa+/− mice. As expected, we found that PolgAD257A mice exhibited a marked reduction in both median and maximum lifespan relative to WT controls, with all PolgAD257A mice expiring before they reached 17 months of age (fig. 1A), a timepoint when all WT mice were still alive. In contrast, the first Pappa−/− mouse did not expire until it reached 28 months of age, consistent with their extended lifespan12. Surprisingly though, a complete deletion of the Pappa gene failed to extend the lifespan of PolgAD257A mice (fig. 1A). Instead, PolgAD257A; Pappa−/− mice seemed to exhibit enhanced frailty, characterized by extensive weight loss as they aged (fig. 1B-D, for a weight distribution of young mice and their progression throughout their lifespan, see fig. S1). A heterozygous deletion of the Pappa gene failed to improve the lifespan of PolgAD257A mice as well (fig. 1A), although this deletion was better tolerated, as indicated by their normalized body weight (fig. 1B,C). Taken together, these results indicate that the pro-longevity effects of IGF-1 suppression are contingent upon the integrity of the mitochondrial genome.
Figure 1|. Lifespan, weight, size and appearance of WT, PolgAD257A and Pappa mutant mice.
A. The lifespan of PolgAD257A is not rescued by deletion of the Pappa gene. B-C. A homozygous deletion of the Pappa gene leads to decreased weight and increased frailty in male and female PolgAD257A mice (male n = 8–22/group, female n = 6–13/group). D. Appearance and size distribution of WT and mutant mice (grid size = 1 cm2).
Deletion of Pappa rescues splenomegaly in PolgAD257A mice
Next, we tested whether loss of Pappa could attenuate the age-related pathology of the mutator mice. Both male and female PolgAD257A mice exhibit pronounced splenomegaly at 12 months of age (fig. 2A-C), and surprisingly, this phenotype was rescued in both sexes by homozygous deletion of the Pappa gene, even after we corrected for the reduced size of Pappa−/− animals (fig. 1D). Interestingly, a heterozygous deletion of Pappa also rescued spleen size in male PolgAD257A mice, although not in female mice, suggesting that male PolgAD257A mice are more sensitive to IGF-1 depletion than females. These findings suggest that depletion of Pappa (loss of one copy of the Pappa gene) can partially recapitulate the impact of a full deletion (loss of both copies of the Pappa gene). These findings indicate that while Pappa deletion is insufficient to extend lifespan in PolgAD257A mice, it is capable of rescuing distinct pathologies that are driven by mtDNA instability.
Figure 2|. The splenomegaly and anemia of PolgAD257A mice are partially rescued by deletion of Pappa.
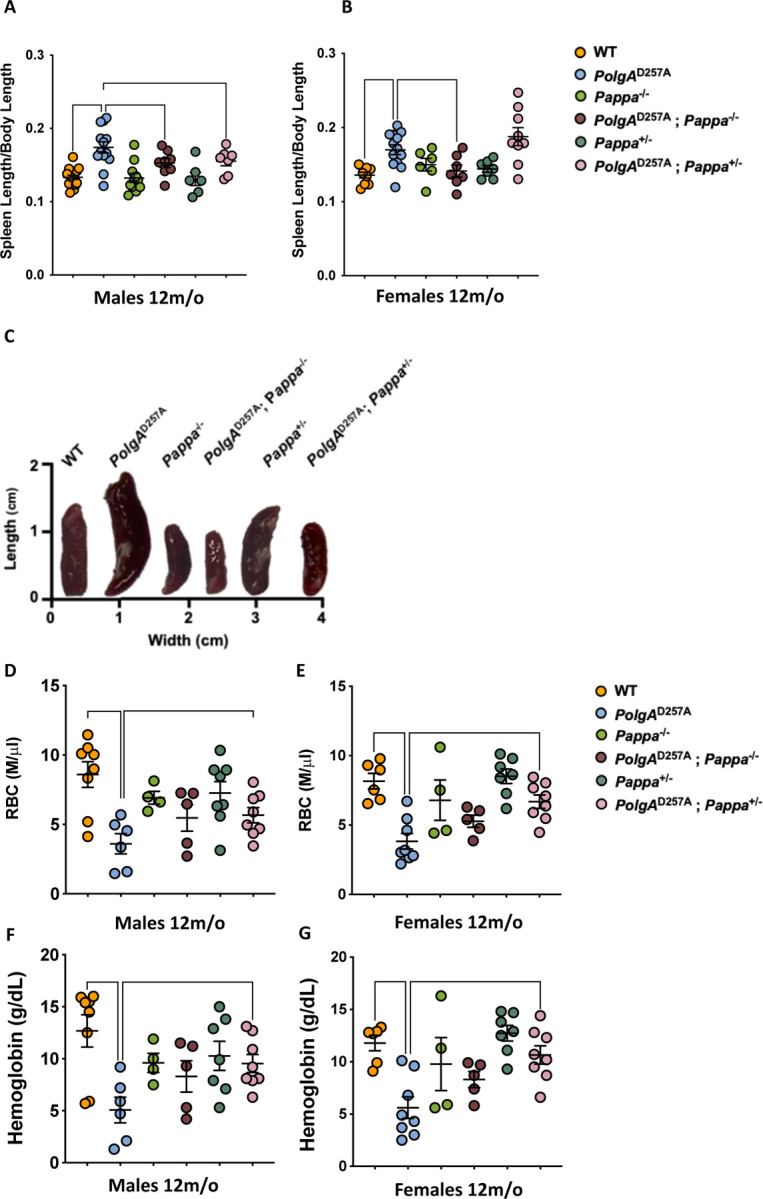
A-C.Male and female PolgAD257A mice display splenomegaly. In both sexes, spleen size is reduced by deletion of two copies of the Pappa gene. In males, deletion of one copy of the Pappa gene is sufficient for spleen reduction as well. Spleen length was normalized to body length (male n = 7–17/group, female n = 6–13/group). D-E. Male and female PolgAD257A mice exhibit reduced RBC counts, which is rescued in both sexes in PolgAD257A; Pappa+/− mice. F-G. Male and female PolgAD257A mice exhibit reduced hemoglobin content, which is rescued in both sexes in PolgAD257A; Pappa+/− mice (male and female n = 4–8/group).
Depletion of Pappa partially rescues anemia in PolgAD257A mice
The reduced spleen size of PolgAD257A; Pappa−/− mice prompted us to investigate whether other phenotypes associated with the PolgAD257A allele were attenuated as well. A second characteristic phenotype of PolgAD257A mice is anemia, which is thought to arise from multiple factors, including splenomegaly, which is an accelerant for the destruction of red blood cells (RBCs), impaired hematopoietic stem cell function, and increased damage to circulating erythrocytes26. Accordingly, we observed that old, but not young PolgAD257A mice displayed a marked reduction in RBCs and hemoglobin content (fig. 2D,E; fig. S2). Excitingly, heterozygous deletion of the Pappa gene partially restored both the RBC levels and hemoglobin content in PolgAD257A Pappa+/− mice (fig. 2F,G). In these experiments, as well as others (see below), PolgAD257A Pappa+/− mice consistently outperformed PolgAD257A Pappa−/− mice, suggesting a dose-dependent reduction in IGF-1 signaling is required for PolgAD257A mice to achieve the greatest benefits.
Depletion of Pappa partially rescues the sex-specific, inflammatory profile of PolgAD257A mice
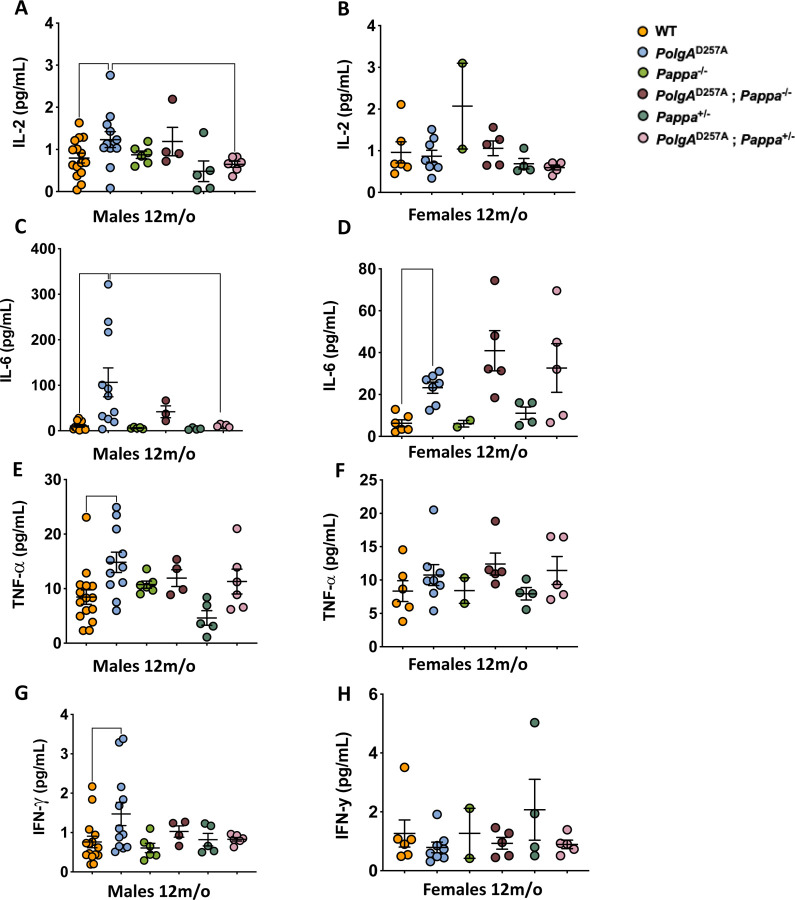
Chronic inflammation is a well-established consequence of mitochondrial dysfunction that is frequently driven by increased reactive oxygen species (ROS) production27, the release of damage-associated molecular patterns28, and activation of innate immune pathways such as the STING pathway29,30. In addition, inflammation is a major cause of anemia in patients with chronic diseases. Accordingly, we examined whether PolgAD257A mice display elevated levels of inflammation as well and found that male PolgAD257A mice exhibit significantly elevated serum levels of IL-2, IL-6, TNF-α, and IFN-γ, cytokines indicative of systemic inflammation and heightened splenic immune activity (fig. 3). Heterozygous deletion of Pappa significantly reduced IL-2 and IL-6 levels in these males, attenuating their inflammatory phenotype. Notably, PolgAD257A; Pappa+/− mice showed greater improvement than PolgAD257A; Pappa−/− mice, reinforcing the importance of IGF-1 dosage in ameliorating mtDNA disease. In addition, we noted that female PolgAD257A mice displayed a muted inflammatory profile compared to male PolgAD257A mice, indicating a sex-specific inflammatory response to mitochondrial mutagenesis. Most likely, this sexual dimorphism is due to the anti-inflammatory31 and anti-oxidant activity32 of estrogen, and other sex-linked factors33. Regardless, the limited inflammatory profile of female PolgAD257A mice remained unchanged upon Pappa deletion (fig. 3h), highlighting the heightened sensitivity of male PolgAD257A mice to IGF-1 modulation.
Figure 3|. Inflammation in PolgAD257A mice is partially rescued by depletion of Pappa.
A, C, E, G. Male PolgAD257A mice display increased levels of serum inflammation markers. IL-2 (A) and IL-6 (C) markers are rescued by deletion of one copy of the Pappa gene. B, D, F, H. Female PolgAD257A mice only display increased levels of IL-6 among the evaluated cytokines, which is not rescued by Pappa deletion (D). (male n = 4–15/group, female n = 2–8/group).
Depletion of Pappa improves muscle function in male PolgAD257A mice
The primary consequence of mitochondrial mutagenesis is impaired energy production, which is particularly detrimental to energy-demanding tissues such as skeletal muscle34. As a result, aged (but not young, fig. S3) PolgAD257A mice exhibit significant reductions in both grip strength and treadmill endurance compared to WT type controls. These assays primarily assess type I (grip strength) and type II (endurance) muscle fiber performance, suggesting functional impairment across both fiber types. Consistent with our previous findings, we observed improved grip strength and endurance in PolgAD257A; Pappa+/− males, but not females and saw no improvement in PolgAD257A; Pappa−/− mice (fig. 4A-D). Instead, PolgAD257A; Pappa−/− mice tended to display reduced, rather than improved grip strength compared to PolgAD257A mice, providing further evidence of their overall frailty.
Figure 4|. Impaired muscle and cardiac function in PolgAD257A mice is partially rescued by deletion of Pappa.

A-D. Male and female PolgAD257A mice display reduced grip strength and endurance. In male mice, these physiological endpoints are improved upon deletion of one Pappa copy in male, but not female mice (male n = 8–32/group, female n = 5–19/group). E-I. Male, but not female, PolgAD257A mice display increased LVID during diastole and systole. Heart size, LVIDd and LVIDs return to WT levels in male mice upon deletion of one or two copies of the Pappa gene (male n = 7–10/group, female n = 4–9/group).
Deletion and depletion of Pappa improve the sex-specific cardiac dysfunction of PolgAD257A mice
Like skeletal muscle, cardiomyocytes rely heavily on continuous energy production, making them particularly vulnerable to mitochondrial dysfunction35,36. In PolgAD257A mice, extensive mitochondrial mutagenesis leads to significant cardiac hypertrophy and enlargement of the left ventricle—a phenotype that mirrors age-related cardiac pathology in humans. To determine whether Pappa deletion could mitigate these effects, we performed echocardiography on 12-month-old mice and found that male PolgAD257A mice exhibited a significant increase in the heart size (fig. 4E-I), as well as the internal diameter of the left ventricle in both diastole (LVIDd) and systole (LVIDs), indicating structural remodeling and reduced cardiac efficiency. Similar to the inflammatory profile of the mice, we did not observe overt cardiomyopathy in female PolgAD257A mice, suggesting a sex-specific impact of mtDNA instability on cardiac health, consistent with previous reports37. Because inflammation can drive cardiomyopathy38, it is possible that the reduced inflammation in female PolgAD257A mice is mechanistically linked to their cardiomyopathy. We found that both PolgAD257A; Pappa+/− and PolgAD257A; Pappa−/− mice showed significant improvement in ventricular dimensions compared to PolgAD257A males. These findings further confirm that depletion of Pappa partially phenocopies a complete deletion. Indeed, out of the 2,950 genes that are significantly altered in the hearts of male Pappa−/− mice vs WT mice, 2,309 move in the same direction in Pappa+/− mice, albeit in a dose dependent manner (Spearman correlation 0.46, p=1.5×10−154, fig. S4). Together, these observations indicate that reduced IGF-1 signaling can partially restore cardiac function even in the context of a high mtDNA mutation burden
The mutation frequency of mtDNA is not affected by deletion of the Pappa gene
To investigate the mechanism by which cardiac function is improved in male PolgAD257A mice following Pappa deletion, we tested whether reduced IGF-1 signaling lowers the mitochondrial mutation burden. Using duplex sequencing39, we generated high-resolution maps of the single base substitutions (fig. 5A), deletions (fig. 5B), and insertions (fig. 5B) that arose across the mitochondrial genome in 12 month old animals. However, the frequency of these mutation classes was unchanged among PolgAD257A, PolgAD257A; Pappa+/−, and PolgAD257A; Pappa−/− mice, as was their mutation spectrum (fig. 5C) and the distribution of mutations across the mitochondrial genome (fig. 5D,E). Digital droplet PCR (fig. 5F) and qPCR (fig. S5) further showed that mtDNA copy number was unchanged among these genotypes. Thus, the improvements in cardiac function are not due to reduced mtDNA mutagenesis or altered mtDNA copy number, but must be mediated by processes that occur after mutation generation.
Figure 5|. Mutagenesis in WT and mutant mice.

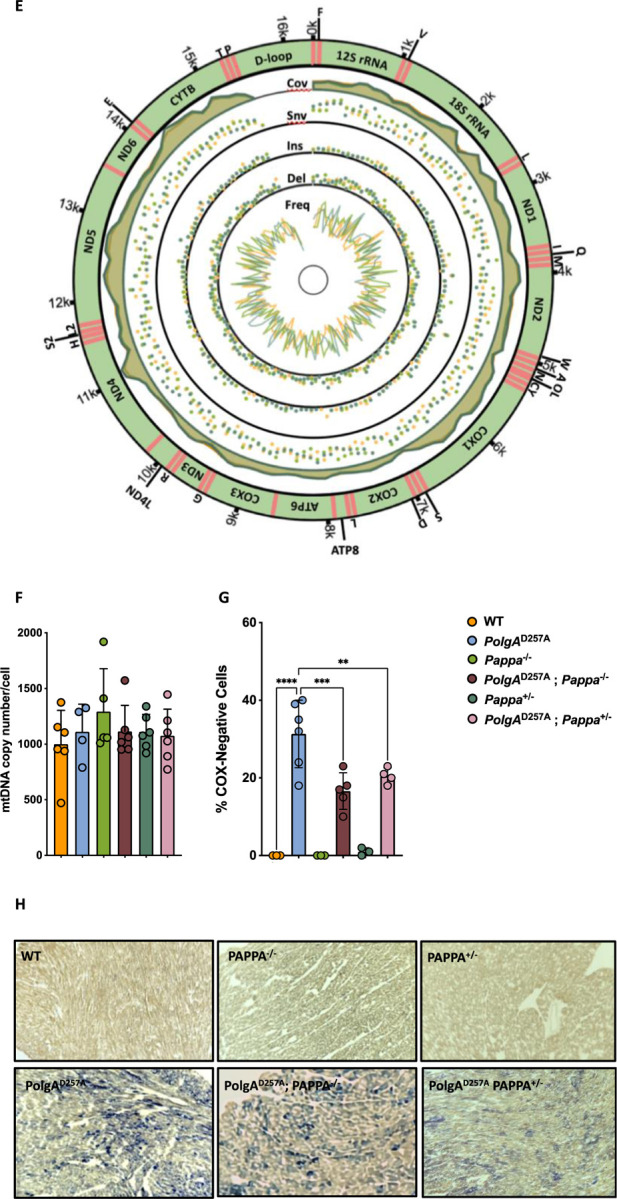
A-C. Male PolgAD257A mice, with or without Pappa deletion display identical mutation rates and spectra for all mutation classes. The same is observed for WT mice with or without Pappa deletion. D,E. Mutations were equally distributed across the mitochondrial genome (n = 5–6/group). First track = coverage. Second track = single nucleotide variants. Third track = insertions. Fourth track = deletions. Fifth track = mutation frequency. F. mtDNA copy number was unchanged by deletion of the Pappa gene (n = 5–6/group). G,H. Deletion of the Pappa gene reduces the number of cardiomyocytes with clonally expanded mtDNA mutations. Blue cells depict cells without WT COX activity (n = 3/group).
Deletion of Pappa slows clonal expansion of mtDNA mutations
While the mutation frequency remained unchanged upon Pappa deletion, the pathogenic consequences of these mutations do not manifest themselves until they clonally expand within individual cells. In both aging humans34 and PolgAD257A mice19, these clonally expanded mutations disrupt cytochrome oxidase activity, impair ATP production and promote cardiomyopathy. To determine whether Pappa deletion influences this expansion process, we stained heart sections from 12-month-old mice for COX/SDH activity (fig. 5H), a technique that can identify cells in which mtDNA mutations have clonally expanded. While COX-negative cells were absent in WT, Pappa+/− and Pappa−/− hearts, PolgAD257A mice exhibited a substantial accumulation of COX-negative cells, consistent with widespread clonal expansion of mtDNA mutations. Remarkably, this phenotype was partially rescued in both PolgAD257A; Pappa+/− (p = 0.0046) and PolgAD257A; Pappa−/− (p = 0.0002) mice (fig. 5G). These findings suggest that although IGF-1 signaling does not affect the generation of mutations, it does affect the rate with which harmful mtDNA mutations clonally expand within cells, thereby preserving mitochondrial function and contributing to improved cardiac health in PolgAD257A mice.
Transcriptomic profiling reveals molecular signatures of cardiomyopathy in male PolgAD257A mice
To investigate the consequences of reduced homoplasmy in PolgAD257A; Pappa−/− mice, we analyzed their heart tissue by RNA-seq. First, we established a baseline, transcriptomic profile of PolgAD257A hearts (fig. S6) by comparing differentially expressed genes between PolgAD257A and WT mice. This analysis confirmed that the hearts of PolgAD257A mice are under substantial metabolic and inflammatory stress. Specifically, PolgAD257A mice exhibited a 3-fold upregulation of Gdf15 (p = 0.0005), a well-established marker of cardiac pathology, inflammation, and metabolic perturbation and a 7-fold increase in Fgf21 (p = 0.03), which encodes a hormone that is released in response to metabolic stress. Consistent with a failing myocardium we also observed a 16-fold upregulation of Myh7 (p < 0.0001) and a 3-fold upregulation of Nppb (p < 0.0001), two canonical markers of the “fetal gene expression program” that can be reactivated during cardiac dysfunction. These transcriptional changes were accompanied by a significant downregulation of genes that encode key components of the contractile apparatus, including Myl1, Myl4, Myl7, and Mybphl, all of which encode myosin light chains essential for sarcomere stability and contraction in striated muscle. Additionally, critical regulators of cardiac pacemaking and excitation-contraction coupling were downregulated, such as voltage-gated calcium (Atp2b2, Cacna2d2) and potassium channels involved in maintaining cardiac electrophysiology. Consistent with their inflammatory profile (fig. 4), we also observed a marked upregulation of chemokines that recruit innate immune cells, including Ccl2 (3-fold), Ccl8 (5-fold), Cxcl5 (3-fold) and Cxcl13 (>20-fold), potent attractants of monocytes, macrophages and neutrophils. Components of the complement system (e.g., C4b, 2.5-fold increase) and pattern-recognition receptors like Tlr7 (2-fold) that are associated with the JAK-STAT signaling pathway were also elevated. Notably, a subset of these inflammatory genes was linked to the activation of the AIM2 inflammasome (enrichment p= 4×10−4), a complex that detects cytosolic double-stranded DNA (including mtDNA that leaks out of dysfunctional mitochondria) and drives innate immune activation40. Together, these findings establish a molecular signature of cardiomyopathy in PolgAD257A mice, characterized by metabolic stress, sarcomeric disorganization, electrophysiological instability, and robust activation of innate immune pathways.
Pappa deletion partially normalizes the transcriptomic profile of male PolgAD257A hearts
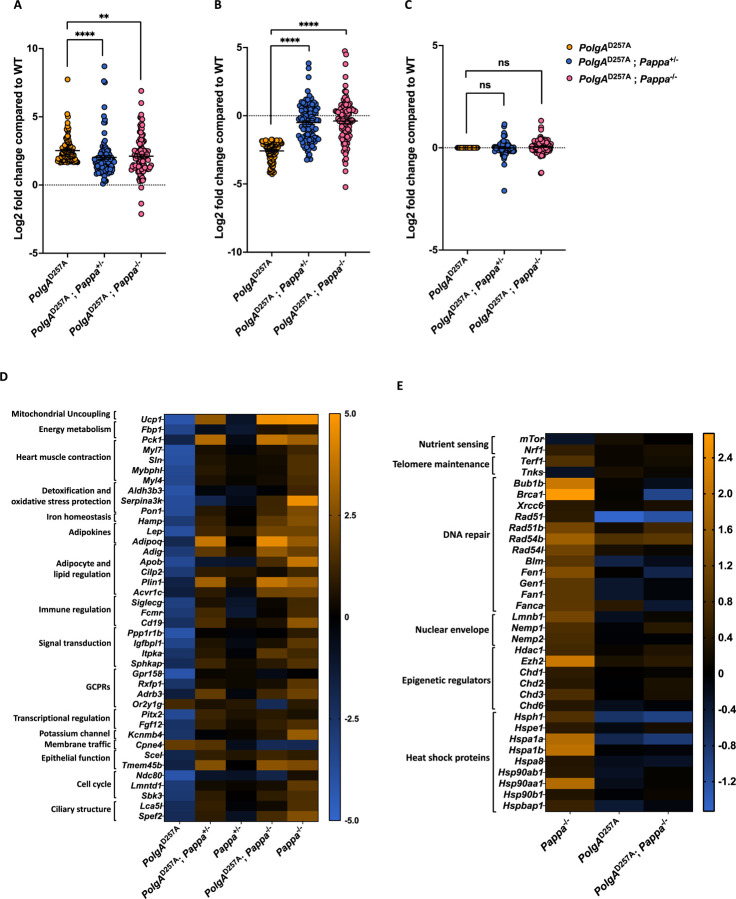
To assess the impact of Pappa deletion on the pathological transcriptomic landscape of PolgAD257A mice, we then compared the changes in expression profile of PolgAD257A vs. WT mice to PolgAD257A; Pappa+/− vs. WT, and PolgAD257A;Pappa−/− vs. WT animals. Remarkably, we found that deletion of one or two copies of the Pappa gene shifted the entire transcriptome of PolgAD257A hearts closer to WT levels (fig. 6A-C). This normalization was most pronounced when we focused on the genes that are most dysregulated in PolgAD257A animals. Out of the 252 genes that were significantly downregulated >2-fold in PolgAD257A hearts, 217 moved closer to WT levels in PolgAD257A; Pappa+/− mice (p < 0.0001) and 203 in PolgAD257A; Pappa−/− mice (p < 0.0001, fig. 6A). A similar trend was observed among upregulated genes. Out of the 244 genes that were significantly upregulated >2-fold in PolgAD257A hearts, 202 moved closer to WT levels in PolgAD257A; Pappa+/− mice (p < 0.0001) and 167 in PolgAD257A; Pappa−/− mice (p < 0.01, fig. 6B). In contrast, genes that were not dysregulated in PolgAD257A mice showed no significant change when Pappa was deleted (fig. 6C).
Figure 6|. Transcriptomic analysis of WT and mutant mice.
A-C. Loss of one or two copies of the Pappa gene normalizes the expression profile of PolgAD257A mice. Genes that are upregulated (A) or downregulated (B) in PolgAD257A mice are normalized upon deletion or depletion of Pappa. Genes that are unchanged in PolgAD257A mice remain unchanged after complete or partial loss of Pappa (C). D. Gene expression patterns suggest that impaired cardiac and metabolic function are rescued by deletion and depletion of the Pappa gene, drastically shifting the expression patterns away from the dysregulation observed in PolgAD257A mice. Interestingly, loss of one copy of Pappa in PolgAD257A mice changes the transcriptional profile in the direction of Pappa−/− compared to Pappa+/−, suggestive of implementation of pathways to rescue the negative consequences of mtDNA mutagenesis. E. Pro-aging related gene programs are dampened by deletion and depletion of Pappa. F. Complete loss of Pappa does not restore expression patterns of mitochondrial transcripts in the PolgAD257A background. G. Lipid metabolism related genes are not upregulated in Pappa+/− mice or PolgAD257A mice, but are upregulated in PolgAD257A; Pappa+/− mice, suggesting context dependent expression patterns (n = 6/group).
Pappa deletion restores metabolic flexibility and cardiac function in male PolgAD257A hearts
To identify the pathways most responsible for the improved cardiac health of PolgAD257A mice, we filtered the transcriptome for genes that change ≥8-fold in expression upon deletion of either one, or two copies of the Pappa gene. This analysis showed that cardiac health was improved at multiple levels of scale. At a functional level, we found that key cardiac contractile genes (Myl7, Myl4, Mybphl, Sln) that were strongly downregulated in PolgAD257A mice, were robustly restored by Pappa deletion, suggesting a rescue of cardiac contractile function (fig. 6D). This functional rescue was accompanied by widespread metabolic reprogramming. Although heart tissue primarily relies on fatty acid oxidation41, it can switch to glucose utilization under stress42. Two genes that enable this metabolic flexibility are PCK1 the rate limiting enzyme for gluconeogenesis, and FBP143, which are significantly downregulated in PolgAD257A hearts, indicating metabolic rigidity. Deletion of Pappa reactivates these genes, indicating enhanced gluconeogenesis and glucose mobilization, which improves the metabolic flexibility of the heart. Systemically, PolgAD257A mice exhibit reduced expression of the adipokines leptin (Lep) and adiponectin (Adipoq), consistent with global metabolic dysfunction. Pappa deletion reversed this trend, restoring expression of both hormones and correcting this endocrine imbalance. Restoration of Adipoq is particularly noteworthy, given its role in insulin sensitivity, mitochondrial biogenesis, and antioxidant defenses44, factors that influence organismal resilience at multiple levels of scale. Further transcriptomic shifts supported improved lipid handling. Pappa deletion upregulated Adipogenin (Adig), which promotes adipocyte differentiation45, and Plin1, which stabilizes lipid droplets and prevents lipotoxicity46. These changes occurred alongside increased expression of Adrb347 and Acvr1c48, regulators of lipid mobilization and adipocyte metabolism. Notably, Adrb3 was co-rescued with Ucp1, suggesting improved thermogenesis and mitochondrial uncoupling and the activation of stress-protective metabolic programs. Together, these changes suggest that Pappa deletion enables PolgAD257A hearts to shift from metabolic exhaustion towards a more flexible, energy and stress-resilient metabolic state.
The PolgAD257A allele blocks anti-aging programs triggered by Pappa deletion
To understand why Pappa deletion can improve cardiac function, but not extend lifespan in PolgAD257A mice, we analyzed the expression of genes that are involved in key anti-aging pathways (fig. 6E). One hallmark of lifespan extension is reduced mTOR signaling49. Accordingly, Pappa−/− mice show reduced mTor expression (p= 0.046), while short-lived PolgAD257A mice exhibit increased expression (p= 0.01) ; however, mTor reduction was partially blocked when Pappa was deleted in PolgAD257A mice, limiting its impact on longevity. Another hallmark of longevity is improved telomere maintenance1. Consistent with their extended lifespan, Pappa−/− mice, show upregulation of Terf1 (p=0.0004) and downregulation of tankyrase (p=0.036), two changes that shield telomeres from damage and recombination and prevent them from triggering cell cycle arrest50. Importantly, neither gene changed expression when Pappa was deleted in a PolgAD257A background. In addition, Pappa−/− mice exhibit overexpression of multiple DNA repair genes that are involved in double strand break repair and telomere maintenance, a key feature of longevity programs51. However, these genes were not overexpressed in PolgAD257A; Pappa−/− mice, demonstrating that the PolgAD257A allele blunts, or blocks this longevity interventions as well. Another feature of long-lived animals is their ability to maintain their epigenetic landscape, which is normally eroded by reduced expression of epigenetic regulators like Hdac1. Accordingly, Pappa−/− mice overexpress multiple chromatin regulators, including Hdac1 (p=0.005), Ezh2 (0.0002), Chd1 (p=0.03), Chd2 (p=0.02), Chd3 (p=0.0007) and Chd6 (p=0.049), none of which were overexpressed in PolgAD257A; Pappa−/− mice. As cells age, the structure of their nuclear envelope tends to deteriorate, a change that is frequently attributed to reduced expression of Lamin B (Lmnb1)52,53. Consistent with their increased longevity, Pappa−/− mice overexpress Lmnb1 (p=0.002) together with Nemp1 (p=0.006) and 2 (p=0.042), two structural proteins of the nuclear envelope; however, we found no overexpression of these genes in PolgAD257A; Pappa−/− mice. As part of a shift from anabolic to catabolic metabolism, deletion of Pappa decreases expression of protein coding genes from the mitochondrial genome, which may reduce mitochondrial dysfunction and ROS production (fig. 6F). This reduction was mediated by reduced expression of the mitochondrial RNA polymerase (Polrmt, p=0.03) and LRPPC (p=0.06), a protein that selectively protects mitochondrial mRNAs, but not tRNAs54,55. Accordingly, the expression of tRNAs was not systemically reduced in Pappa−/− mice. None of these changes were observed in PolgAD257A; Pappa−/− mice. Another hallmark of long-lived cells is improved proteostasis1. Again though, we found that although Pappa deletion significantly upregulated numerous primary heat shock proteins, these genes were not upregulated in PolgAD257A; Pappa−/− mice. Indeed, we found that out of the 32 primary heat shock proteins (HSPs) we detected, 27 were downregulated in PolgAD257A mice (fig. S7A) and none of these genes were rescued in PolgAD257A; Pappa−/− mice. Notably, this pattern was not observed in the DnaJ-family HSP40 of co-chaperones (fig. S7B), which, unlike the primary chaperones, are ATP-independent56. Thus, loss of mtDNA integrity, and reduced ATP production specifically inhibit the expression of ATP-dependent chaperones, contributing to proteostasis failure.
Pappa deletion triggers context-dependent metabolic rewiring
Aging cells frequently exhibit not one, but multiple hallmarks of the aging process. These combinations could lead to complex, context-dependent interactions that result in novel gene expression states that neither hallmark produces alone. To identify such interactions between mitochondrial mutagenesis and IGF-1 signaling, we filtered the transcriptome for genes that remain unchanged upon deletion of Pappa in WT mice (Pappa+/− mice), but were strongly altered (>4-fold) in PolgAD257A; Pappa+/− mice. Of the 13 genes that were identified by this method, 7 were directly involved in lipid metabolism, storage, or mobilization (fig. 6G). These included Adipoq, a protective adipokine in metabolic disease and inflammation; Cidec and Plin1, which regulate lipid droplet formation and adipocyte energy storage46,57, and Apoc1, a lipid- and immune-regulatory apolipoprotein58. Finally, we identified Fasn, an enzyme that is central to fatty acid synthesis59; Elovl6, which catalyzes the elongation of long-chain fatty acids60, and Lrp2, a multifunctional endocytic receptor that internalizes lipoproteins, insulin, leptin, and amyloid-beta61. These results reveal a striking lipid-centric response to a partial deletion of Pappa that only emerges in the presence of mitochondrial dysfunction, underscoring how aging hallmarks can interact with each other to produce context-dependent transcriptional programs. Notably, some of these interactions also occur when both copies of Pappa are deleted, demonstrating that mitochondrial mutations can sensitize cells to modulation by Pappa deletion and offering a plausible explanation for why partial Pappa deletion can improve the phenotype of PolgAD257A mice as well. A similar effect is seen when column 2 and 3 of figure 6D are compared.
DISCUSSION
Aging is a multifactorial process, characterized by the simultaneous emergence of multiple, interconnected molecular and cellular hallmarks. In naturally aging organisms, these hallmarks do arise independently, but instead accumulate and interact within the same tissues and cells over time. As a result, there is a growing recognition that the hallmarks of aging are critically intertwined, and that to fully understand the aging process, it will be essential to tease apart these complex interactions.
In this study, we investigated how mtDNA instability interacts with IGF-1 signaling to shape mammalian lifespan and healthspan. Suppression of IGF-1 signaling through Pappa deletion extends lifespan in WT mice by triggering well-known pro-longevity programs, including enhanced stress resistance, DNA repair, proteostasis, and metabolic reprogramming. However, the same intervention fails to extend lifespan in PolgAD257A mice, which accumulate mtDNA mutations at an accelerated pace. This observation echoes prior studies that showed that calorie restriction62, exercise63–66, or overexpression of a mitochondrially targeted catalase67, did not extend the lifespan of PolgAD257A mice either. Taken together, these studies support that a hierarchy exists among the hallmarks of aging, where mtDNA integrity overrides the impact of longevity interventions, such as IGF-1 signaling, nutrient sensing, exercise and oxidative stress to impose a hard limit on mammalian lifespan.
Although Pappa deletion did not extend the lifespan of PolgAD257A mice, it did attenuate a wide range of age-related phenotypes, including splenomegaly, anemia, chronic inflammation, skeletal muscle decline, and cardiomyopathy. These findings echo those with a mitochondrially targeted catalase, which failed to extend lifespan as well, but improved the cardiomyopathy in PolgAD257A mice35,67. The benefits we observed were especially pronounced in male mice, which seemed to suffer more from mtDNA instability than female mice, possibly due to the anti-oxidant and anti-inflammatory effects of estrogen68. Surprisingly, these improvements were most evident in mice with partial Pappa deletion. This dose-dependent effect suggests that complete loss of Pappa may excessively suppress IGF-1 signaling in the context of high mitochondrial stress, where some degree of anabolic signaling remains necessary to maintain cellular homeostasis. Our transcriptomic analyses demonstrate that the health improvements in PolgAD257A; Pappa+/− mice were mediated, at least in part, by context-dependent interactions between mtDNA instability and IGF-1 signaling. In other words, we observed gene expression states in PolgAD257A; Pappa+/− mice that were not observed in either genotype alone. Such context-dependent effects underscore how important the interactions between the hallmarks of aging are: the outcome is not merely the sum of two interventions, but a new state shaped by their interplay.
This principle was most clearly illustrated by the failure of Pappa deletion to activate canonical longevity pathways in PolgAD257A mice. In WT mice, Pappa deletion upregulates a suite of anti-aging pathways that improve DNA repair, telomere maintenance, proteostasis and chromatin integrity. However, in the presence of mtDNA mutations, most of these pathways are either blocked or blunted, demonstrating that the integrity of the mitochondrial genome is pre-requisite for lifespan extension by reduced IGF-1 signaling. Similarly, previous research has shown that proteostasis69, DNA repair70 and other molecular mechanisms are pre-requisites for lifespan extension by reduced IGF-1 signaling as well. Our observations now suggest that within this framework, mtDNA integrity is not simply one of the many hallmarks of aging, but rather the foundation upon which others are built. And when that platform is broken, downstream hallmarks like proteostasis or DNA repair cannot be engaged by typical means, no matter how favorable the upstream signaling may be.
This insight has major implications for the development of anti-aging therapies. It suggests that interventions that target nutrient-sensing pathways may fail—or even backfire—when applied to organisms or tissues with high levels of mitochondrial damage. As such, the next generation of geroprotective treatments must be tested in diverse models of aging, including those that combine multiple hallmarks, to better understand the scope and boundaries of their efficacy.
Because mtDNA mutations impose a ceiling on mammalian lifespan, it is vital that these treatments also address the stability of the mitochondrial genome. While a reduction in IGF-1 signaling did not alter the frequency of mutations in WT or PolgAD257A mice, it did slow the pace with which they reached homoplasmy. Thus, although it may not be possible today to reduce mitochondrial mutagenesis in human cells, our data shows that it may already be possible to curtail the impact of mtDNA mutations on mammalian healthspan by slowing their clonal expansion.
While the precise mechanism by which Pappa influences clonal expansion of mtDNA mutations remains uncertain, several plausible explanations can be proposed. First, the progression of mtDNA mutations toward homoplasmy is likely influenced by mitochondrial genome replication and segregation dynamics, two processes that can accelerate genetic drift, especially during cell division when mutant genomes may be unequally partitioned between daughter cells. Because Pappa deletion suppresses growth-related pathways these changes may reduce mitochondrial workload and mtDNA replication frequency, thereby slowing genetic drift in post-mitotic cells and limiting mitochondrial segregation via reduced cell turnover. Second, Pappa deletion enhances a wide variety of maintenance processes, which could lead to improved autophagic and mitochondrial quality control mechanisms that selectively eliminates dysfunctional mitochondria and curbs the expansion of mutant clones. Third, emerging evidence suggests that damaged mtDNA is often degraded rather than repaired71. In that context, the enhanced antioxidant capacity observed in Pappa-deficient PolgAD257A mice may lessen oxidative mtDNA damage, decreasing the need for mtDNA turnover and potentially stabilizing the mitochondrial genome by limiting replication-driven amplification of existing mutations.
Regardless, these findings provide a compelling example of how the interplay between distinct hallmarks of the aging process can fundamentally alter the outcome of otherwise beneficial interventions. They reveal that the efficacy of anti-aging strategies like IGF-1 suppression is not absolute, but context-dependent. They are contingent on the integrity of underlying systems, including proteostasis and DNA repair. Without an intact mitochondrial genome though, these pathways cannot be engaged, indicating that mtDNA integrity is upstream from these critical anti-aging pathways. More broadly, our results underscore the need for a more integrated model of aging, one that considers not just individual pathways but their interactions, hierarchies, and points of failure. By mapping these interactions, we can better anticipate the limitations of existing interventions and design next-generation therapies that are robust to the complex biology of aged tissues. In this light, strategies that target the expansion of mtDNA mutations—rather than their origin—may offer a powerful new axis for preserving tissue function and extending healthspan, even when the underlying genomic damage cannot be undone.
METHODS
Transgenic Mice
PolgAD257A mice were purchased from JAX (strain #:017341), while Pappa−/− mice were a generous gift from Dr. Cheryl Conover12. Male PolgAD257A/+; Pappa+/− and female PolgAD257A/+; Pappa+/− were bred to generate mice the genotypes described in this study. To limit mtDNA mutations from being inherited through the germline, separate PolgAD257A/+; Pappa+/− x WT C57Bl6/j crosses were set up to generate “first generation” PolgAD257A/+; Pappa+/− females to be used for breeding. At 21 days, mice were weaned, ear-punched and two PCR reactions were run to genotype each mouse. The first reaction genotyped the PolgAD257A allele, using primers F: 5’-GCCTCGCTTTCTCCGTGACT-3’ and R: 5’-GGATGTGGCCCAGGCTGTAACTCA-3’. To genotype the Pappa allele, primers Fcommon: 5’- TAAGCAGGGGTGGGTCCTTT-3’, Fneo: 5’- TCGCCTTCTATCGCCTTCTTG-3’, and R: 5’- CACTCCTCAGCTTCGGCTTTCA-3’ were used. PCR reactions were run on agarose gels by gel electrophoresis and imaged before analysis. Mice were assayed at 3 months or 12 months of age for experimental studies, while a subset of mice were aged for lifespan experiments and weekly body weight measurements. Animals were scored by our lab and USC’s Department of Animal Resources veterinarians to establish humane endpoints. Mice were euthanized by CO2 exposure.
Grip Strength
Grip strength was assessed using a horizontally mounted bar attached to a sensor (TSE-Systems, 303500-M/E1) designed to measure forelimb strength. Each mouse was allowed to securely grasp the bar before being swiftly and steadily pulled backward. Measurements were recorded only if the mouse let go with both forelimbs at the same time. Ten measurements were taken per animal and averaged to obtain a final value.
Endurance
Mice were acclimated to the treadmill instrument (TSE 303401-M-04/C) 24 hours before testing. During acclimation, animals were placed on the stationary treadmill for five minutes, followed by an increase in speed to 2m/min for five additional minutes. Mice were then tested once each day on the two days following acclimation. The treadmill protocol consisted of 1 m/min for 1 minute, followed by a 1m/minute increase every minute until exhaustion. Time of exhaustion was recorded in seconds. Mice that refused to run would feel a mild shock at the back of the treadmill. The values recorded for each mice over both test days was averaged and reported.
Echocardiography
One week prior to dissection, mice were transported to the molecular imaging center at USC for echocardiography using the VisualSonics Vevo 3100, MX 550 transducer 22–55 MHz. Mice were anesthetized with 2% isoflurane and a depilatory cream (Nair) was used to remove fur prior to echocardiography. Mice were placed in the supine position onto the warmed platform to maintain optimal physiological conditions and their limbs were taped onto the metal EKG leads. Heart rates were monitored and generally maintained at 400–500 beats per minute. Warmed echocardiography gel was placed on the shaved chest and the heart was imaged with a 30 MHz transducer. By placing the transducer along the long-axis of LV and directing it to the right side of the neck of the mouse, two-dimensional LV long-axis can be obtained. The transducer was then rotated clockwise by 90°, and the LV short-axis view was obtained. Transmitral inflow Doppler spectra were recorded in an apical 4-chamber view by placing the sample volume at the tip of the mitral valves. After the scans were concluded, the residual gel was removed, and the mouse was returned to the cage for recovery. Images were subsequently analyzed using the VevoLab software.
Tissue Collection and Analysis
Upon euthanasia, mice were measured, photographed, and the heart was punctured for blood draws for blood composition experiments and serum isolation. Tissues were dissected, placed on a grid sheet for measurements and photography and cut into 4 pieces. One piece was stored in formalin, one piece was frozen in Optimal Cutting Temperature (OCT) compound and two pieces were flash frozen in liquid nitrogen for DNA and RNA extractions. Formalin-fixed tissue was left at room temperature overnight before being washed twice with PBS and stored at 4°C in 70% EtOH. Serum was collected by letting blood clot for 30 min, followed by a 10 minute spin at 1,000g at 4°C. Serum was then collected by aspirating the supernatant and stored at −80°C. IFN-γ, IL2, IL6 and TNF-α levels in serum were measured with commercial immunoassays using a custom V-PLEX Mouse Biomarker Kit (Meso Scale Discovery, Rockville, MD). Blood composition was measured using 40 μl of blood collected in EDTA tubes using the Hemavet 950FS instrument.
Duplex-Sequencing
DNA was extracted from the hearts of experimental mice (n= 5–6/group) using the Qiagen DNeasy Blood and Tissue Kit (cat# 69506), quantified by Qubit 1x dsDNA hs assay kit, and stored at −20C. 500ng of DNA per sample was fragmented using the Biorupter Pico (30 seconds ON, 90 seconds OFF, 6 cycles) and size was measured using the Agilent 4200 TapeStation instrument (fragment size ~300bp). Published protocols39,72 were followed with minor adjustments. Briefly, DNA was end-repaired and adapters (a generous gift from the Kennedy Lab) were ligated to DNA fragments using the Ultra II DNA Library Preparation Kit from NEB (cat# E7103) samples were cleaned up and libraries were quantified by qPCR using SYBR Green iTaq Supermix (Biorad cat#: 1725124) compared to previously generated libraries with target family sizes and on-target efficiencies. This quantification enables us to input the correct number of molecules into the pre-enrichment PCR depending on mtDNA content for each sample to sequence at the target parameters (depth, target raw reads, family size, on-target reads). Libraries were then enriched for mtDNA sequences using the xGen Hybridization Capture Assay (IDT) with a custom Discovery Pool of biotinylated probes (Suppl. File 1), along with their protocol. Final libraries were quantified, pooled and sequenced on a NovaSeq 6000 S2 kit (150PE). The standard duplex-seq pipeline was used to analyze the sequencing data, https://github.com/Kennedy-Lab-UW/Duplex-Seq-Pipeline. Mutation data are attached as supplementary files (Suppl. File 2).
mtDNA Copy Number Measurements
DNA extracted for duplex-sequencing experiments was used for copy number determination. By quantitative PCR, relative mtDNA copy number was measured using 50ng of input DNA and primers targeting mitochondrial ND1 (F: 5’-GCCTGACCCATAGCCATAAT-3’ and R: 5’-TATTCTACGTTAAACCCTGA-3’) and nuclear RPP30 (F: 5’-GCAACCGGAACATAGAGACA-3’ and R: 5’-CTGGCCTTGGAATGGGTAAT-3’). iTaq Universal SYBR Green Supermix was used in these reactions and the protocol was followed accordingly (Biorad cat#: 1725124). By droplet digital PCR (ddPCR), BioRad ddPCR copy number assay primers/probe for Rpp30 (HEX, Assay ID: dMmuCNS822293939) and for ND1 (FAM, Assay ID: dMmuCNS343824284) were used with 2x ddPCR Supermix for Probes (no dUTP) (Biorad cat#: 186–3023).
RNA-seq
Heart tissue from 12 month old, male mice (n = 6/group) was dounced in Trizol/Chloroform and homogenized with with zirconia beads for 20 minutes at 4°C. Samples were then at 10,000g for 5 minutes and supernatant purified according to the RiboPure Yeast RNA Purification Kit (Invitrogen, cat#: AM1926). RNA was DNase treated and mRNA was enriched using the GenElute Direct mRNA miniprep kit (Sigma, cat#: DMN70–1KT) and RNA-seq libraries were generated using the SMARTer Stranded RNA-Seq Kit (Takara Bio, cat#: 634838) and sequenced on a NovaSeq 6000 SP PE150 kit. Sequencing reads were trimmed using fastp73 (version 0.23.2) with the following parameters: “--trim_poly_g --poly_g_min_len 6 --length_required 120”. Trimmed reads were then used to obtain transcripts abundance with Kallisto quant74 (version 0.46) against the Ensembl r96 annotation for Mus musculus. Abundance estimates from Kallisto were then analyzed in R with the package DESeq275 following the standard pipeline to identify genes differentially expressed relative to the WT with FDR < 0.05 (correction for multiple testing). Sequencing data were deposited to SRA under accession PRJNA1271490.
COX-SDH Staining
Frozen OCT blocks containing heart tissue were sectioned onto slides using a Leica CM 1860 Cryostat in 10µm sections and stored at −80°C until they were ready to be stained. COX-SDH staining protocol was adapted from Wanagat et al34. Briefly, heart sections placed on a slide were circled using a PAP pen, fully covered with COX-staining solution and incubated at 37°C for 12 minutes. Slides were then rinsed in 0.1M Tris-HCl and incubated at 37°C with SDH-staining solution for 10 minutes. Afterwards, slides were dehydrated in 70% ethanol, 95% ethanol, 100% ethanol (2x) and xylene before being coverslipped with Cytoseal 60. Slides were imaged on an Echo Revolve microscope at 4x, 10x, and 20x magnifications. Images were analyzed and scored for their % COX-negative cells using QuPath2 software.
Statistics
Group differences means (± SEM) were analyzed by one-way ANOVA with Fisher’s LSD test, Kruskal-Wallis with Uncorrected Dunn’s test for nonparametric distributions, or Welch’s ANOVA when variances were unequal across groups. Significance was defined as p < 0.05. Analyses used GraphPad Prism version 10 (GraphPad Software, San Diego, CA).
Supplementary Material
Acknowledgements
M.V. was supported by NIA awards R01GM124532, R01AG075130, and R01AG083065. S.R.K was supported by NIGMS grant R35GM133428. P.C. was supported by the Hevolution Foundation Award HF-AGE-23-1273964-51. S.J.S. was supported by NIA fellowship F31AG084238.
REFERENCES
- 1.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M. C Kroemer G. The hallmarks of aging. Cell 153, 1194–1217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallace D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39, 359–407 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Junnila R.K., List E.O., Berryman D.E., Murrey J.W. C Kopchick J.J. The GH/IGF-1 axis in ageing and longevity. Nat Rev Endocrinol 9, 366–376 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolesar J.E., et al. Defects in mitochondrial DNA replication and oxidative damage in muscle of mtDNA mutator mice. Free Radic Biol Med 75, 241–251 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Holzenberger M., et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421, 182–187 (2003). [DOI] [PubMed] [Google Scholar]
- 6.McBride H.M., Neuspiel M. C Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol 16, R551–560 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Templeman N.M. C Murphy C.T. Regulation of reproduction and longevity by nutrient-sensing pathways. J Cell Biol 217, 93–106 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bender A., et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 38, 515–517 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa K., et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320, 661–664 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Cohen E., et al. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell 139, 1157–1169 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrington S.C., Simari R.D. C Conover C.A. Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet. Circ Res 100, 1696–1702 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Conover C.A. C Bale L.K. Loss of pregnancy-associated plasma protein A extends lifespan in mice. Aging Cell 6, 727–729 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Vallejo A.N., et al. Resistance to age-dependent thymic atrophy in long-lived mice that are deficient in pregnancy-associated plasma protein A. Proc Natl Acad Sci U S A 106, 11252–11257 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conover C.A., et al. Transgenic overexpression of pregnancy-associated plasma protein-A in murine arterial smooth muscle accelerates atherosclerotic lesion development. Am J Physiol Heart Circ Physiol 299, H284–291 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyons A., et al. Insulin-like growth factor 1 signaling is essential for mitochondrial biogenesis and mitophagy in cancer cells. J Biol Chem 292, 16983–16998 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conover C.A. C Oxvig C. The IGF System and Aging. Endocr Rev 46, 214–223 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haroon S., et al. Multiple Molecular Mechanisms Rescue mtDNA Disease in C. elegans. Cell Rep 22, 3115–3125 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kujoth G.C., et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309, 481–484 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Vermulst M., et al. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat Genet 40, 392–394 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Vermulst M., et al. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat Genet 39, 540–543 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Trifunovic A., et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Laursen L.S., et al. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett 504, 36–40 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Conover C.A., et al. Longevity and age-related pathology of mice deficient in pregnancy-associated plasma protein-A. J Gerontol A Biol Sci Med Sci 65, 590–599 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bale L.K. C Conover C.A. Disruption of insulin-like growth factor-II imprinting during embryonic development rescues the dwarf phenotype of mice null for pregnancy-associated plasma protein-A. J Endocrinol 186, 325–331 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Conover C.A., et al. Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development 131, 1187–1194 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Norddahl G.L., et al. Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell Stem Cell 8, 499–510 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Mittal M., Siddiqui M.R., Tran K., Reddy S.P. C Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20, 1126–1167 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Q., et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman L.E., et al. Mitochondrial DNA replication stress triggers a pro-inflammatory endosomal pathway of nucleoid disposal. Nat Cell Biol 26, 194–206 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Z., et al. Mitochondrial DNA Stress Signalling Protects the Nuclear Genome. Nat Metab 1, 1209–1218 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villa A., Vegeto E., Poletti A. C Maggi A. Estrogens, Neuroinflammation, and Neurodegeneration. Endocr Rev 37, 372–402 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simpkins J.W., Yi K.D., Yang S.H. C Dykens J.A. Mitochondrial mechanisms of estrogen neuroprotection. Biochim Biophys Acta 1800, 1113–1120 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez de Toda I., et al. Sex differences in markers of oxidation and inflammation. Implications for ageing. Mech Ageing Dev 211, 111797 (2023). [DOI] [PubMed] [Google Scholar]
- 34.Wanagat J., Cao Z., Pathare P. C Aiken J.M. Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. Faseb J 15, 322–332 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Dai D.F., et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 119, 2789–2797 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orogo A.M., et al. Accumulation of Mitochondrial DNA Mutations Disrupts Cardiac Progenitor Cell Function and Reduces Survival. J Biol Chem 290, 22061–22075 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golob M.J., et al. Mitochondria DNA mutations cause sex-dependent development of hypertension and alterations in cardiovascular function. J Biomech 48, 405–412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tschope C., et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol 18, 169–193 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy S.R., et al. Detecting ultralow-frequency mutations by Duplex Sequencing. Nat Protoc 9, 2586–2606 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumari P., Russo A.J., Shivcharan S. C Rathinam V.A. AIM2 in health and disease: Inflammasome and beyond. Immunol Rev 297, 83–95 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopaschuk G.D., Ussher J.R., Folmes C.D., Jaswal J.S. C Stanley W.C. Myocardial fatty acid metabolism in health and disease. Physiol Rev 90, 207–258 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Tran D.H. C Wang Z.V. Glucose Metabolism in Cardiac Hypertrophy and Heart Failure. J Am Heart Assoc 8, e012673 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gizak A., Majkowski M., Dus D. C Dzugaj A. Calcium inhibits muscle FBPase and affects its intracellular localization in cardiomyocytes. FEBS Lett 576, 445–448 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Civitarese A.E., et al. Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab 4, 75–87 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alvarez-Guaita A., et al. Phenotypic characterization of Adig null mice suggests roles for adipogenin in the regulation of fat mass accrual and leptin secretion. Cell Rep 34, 108810 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desgrouas C., Thalheim T., Cerino M., Badens C. C Bonello-Palot N. Perilipin 1: a systematic review on its functions on lipid metabolism and atherosclerosis in mice and humans. Cardiovasc Res 120, 237–248 (2024). [DOI] [PubMed] [Google Scholar]
- 47.Collins S. beta-Adrenoceptor Signaling Networks in Adipocytes for Recruiting Stored Fat and Energy Expenditure. Front Endocrinol (Lausanne) 2, 102 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ibanez C.F. Regulation of metabolic homeostasis by the TGF-beta superfamily receptor ALK7. FEBS J 289, 5776–5797 (2022). [DOI] [PubMed] [Google Scholar]
- 49.Johnson S.C., Rabinovitch P.S. C Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature 493, 338–345 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 19, 2100–2110 (2005). [DOI] [PubMed] [Google Scholar]
- 51.Hoeijmakers J.H. DNA damage, aging, and cancer. N Engl J Med 361, 1475–1485 (2009). [DOI] [PubMed] [Google Scholar]
- 52.Freund A., Laberge R.M., Demaria M. C Campisi J. Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell 23, 2066–2075 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bedrosian T.A., et al. Lamin B1 decline underlies age-related loss of adult hippocampal neurogenesis. EMBO J 40, e105819 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruzzenente B., et al. LRPPRC is necessary for polyadenylation and coordination of translation of mitochondrial mRNAs. EMBO J 31, 443–456 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siira S.J., et al. LRPPRC-mediated folding of the mitochondrial transcriptome. Nat Commun 8, 1532 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kampinga H.H. C Craig E.A. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 11, 579–592 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gupta A., et al. Human CIDEC transgene improves lipid metabolism and protects against high-fat dietinduced glucose intolerance in mice. J Biol Chem 298, 102347 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rouland A., et al. Role of apolipoprotein C1 in lipoprotein metabolism, atherosclerosis and diabetes: a systematic review. Cardiovasc Diabetol 21, 272 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jensen-Urstad A.P. C Semenkovich C.F. Fatty acid synthase and liver triglyceride metabolism: housekeeper or messenger? Biochim Biophys Acta 1821, 747–753 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X., Yu H., Gao R., Liu M. C Xie W. A comprehensive review of the family of very-long-chain fatty acid elongases: structure, function, and implications in physiology and pathology. Eur J Med Res 28, 532 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beenken A., et al. Structures of LRP2 reveal a molecular machine for endocytosis. Cell 186, 821–836 e813 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Someya S., et al. Effects of calorie restriction on the lifespan and healthspan of POLG mitochondrial mutator mice. PLoS One 12, e0171159 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ross J.M., et al. Voluntary exercise normalizes the proteomic landscape in muscle and brain and improves the phenotype of progeroid mice. Aging Cell 18, e13029 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clark-Matott J., et al. Metabolomic analysis of exercise effects in the POLG mitochondrial DNA mutator mouse brain. Neurobiol Aging 36, 2972–2983 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Houghton D., et al. Impact of Age-Related Mitochondrial Dysfunction and Exercise on Intestinal Microbiota Composition. J Gerontol A Biol Sci Med Sci 73, 571–578 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maclaine K.D., Stebbings K.A., Llano D.A. C Rhodes J.S. Voluntary wheel running has no impact on brain and liver mitochondrial DNA copy number or mutation measures in the PolG mouse model of aging. PLoS One 15, e0226860 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dai D.F., et al. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell 6, 536–544 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Straub R.H. The complex role of estrogens in inflammation. Endocr Rev 28, 521–574 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Hsu A.L., Murphy C.T. C Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300, 1142–1145 (2003). [DOI] [PubMed] [Google Scholar]
- 70.Hyun M., et al. Longevity and resistance to stress correlate with DNA repair capacity in Caenorhabditis elegans. Nucleic Acids Res 36, 1380–1389 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Valente W.J., et al. Mitochondrial DNA exhibits resistance to induced point and deletion mutations. Nucleic Acids Res 44, 8513–8524 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoekstra J.G., Hipp M.J., Montine T.J. C Kennedy S.R. Mitochondrial DNA mutations increase in early stage Alzheimer disease and are inconsistent with oxidative damage. Ann Neurol 80, 301–306 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen S. Ultrafast one-pass FASTQ data preprocessing, quality control, and deduplication using fastp. SSImeta 2, e107 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bray N.L., Pimentel H., Melsted P. C Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 34, 525–527 (2016). [DOI] [PubMed] [Google Scholar]
- 75.Love M.I., Huber W. C Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.