Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNHARD S. A. A new method for the determination of the amidase activity of trypsin: kinetics of the hydrolysis of benzoyl-L-arginineamide. Biochem J. 1955 Mar;59(3):506–509. doi: 10.1042/bj0590506. [DOI] [PMC free article] [PubMed] [Google Scholar]
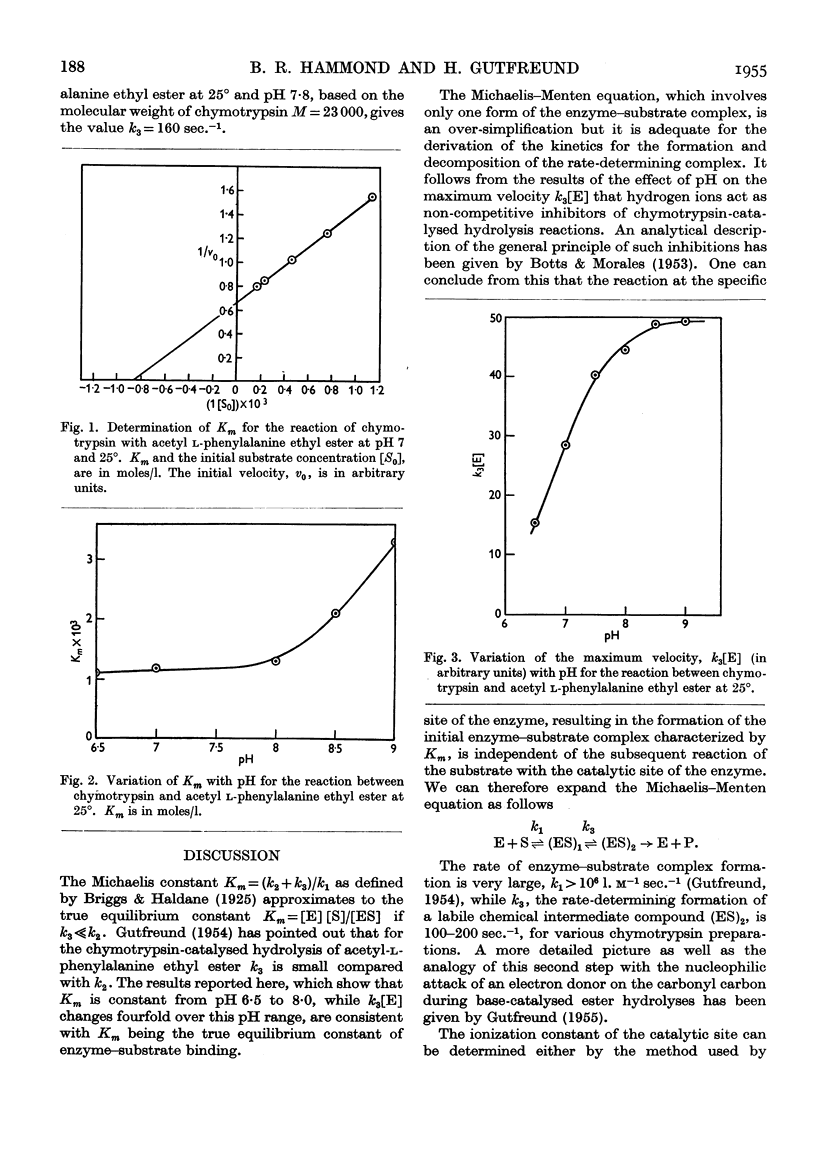
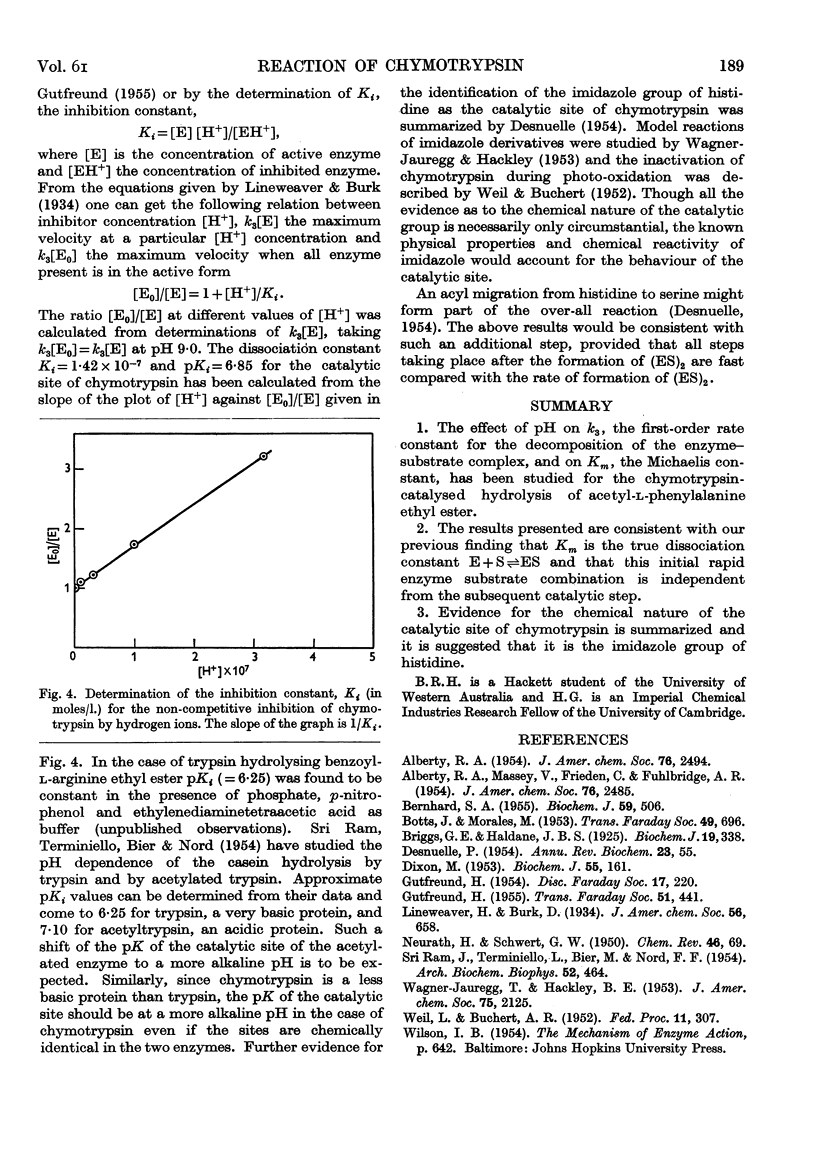
- Briggs G. E., Haldane J. B. A Note on the Kinetics of Enzyme Action. Biochem J. 1925;19(2):338–339. doi: 10.1042/bj0190338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESNUELLE P. Proteolytic enzymes. Annu Rev Biochem. 1954;23:55–78. doi: 10.1146/annurev.bi.23.070154.000415. [DOI] [PubMed] [Google Scholar]
- DIXON M. The effect of pH on the affinities of enzymes for substrates and inhibitors. Biochem J. 1953 Aug;55(1):161–170. doi: 10.1042/bj0550161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAM J. S., TERMINIELLO L., BIER M., NORD F. F. On the mechanism of enzyme action. LVIII. Acetyl trypsin, a stable trypsin derivative. Arch Biochem Biophys. 1954 Oct;52(2):464–477. doi: 10.1016/0003-9861(54)90146-0. [DOI] [PubMed] [Google Scholar]


