Abstract
Post-traumatic stress disorder (PTSD) is a psychiatric disorder caused by traumatic past experiences, rooted in the neurocircuits of fear memory formation. Memory processes include encoding, storing, and recalling to forgetting, suggesting the potential to erase fear memories through timely interventions. Conventional strategies such as medications or electroconvulsive therapy often fail to provide permanent relief and come with significant side-effects. This review explores how fear memory may be erased, particularly focusing on the mnemonic phases of reconsolidation and extinction. Reconsolidation strengthens memory, while extinction weakens it. Interfering with memory reconsolidation could diminish the fear response. Alternatively, the extinction of acquired memory could reduce the fear memory response. This review summarizes experimental animal models of PTSD, examines the nature and epidemiology of reconsolidation to extinction, and discusses current behavioral therapy aimed at transforming fear memories to treat PTSD. In sum, understanding how fear memory updates holds significant promise for PTSD treatment.
Keywords: Post-traumatic stress disorder, Fear memory, Reconsolidation, Extinction, Engram
A Synopsis of Post-Traumatic Stress Disorder (PTSD)
PTSD
PTSD is a psychiatric condition characterized by intense fear and memories stemming from past traumatic experiences. It affects ~3.9% of the global population over their lifetime, with rates rising to 5.6% among trauma victims [1] and exceeding 15% among global pandemic survivors [2]. Women are twice as susceptible as men [3]. The coronavirus disease 2019 (COVID-19) pandemic has exacerbated PTSD symptoms worldwide [4, 5], highlighting significant challenges for existing mental health systems [6, 7]. Exposure therapy (ET) is a first-line treatment that facilitates fear memory extinction by safely exposing individuals to traumatic memories [8, 9]. However, this psychotherapy fails to expel fear memories permanently, leading to recurrence and treatment non-completion [10–13]. Given the substantial increase in its prevalence following the COVID-19 pandemic [4, 5], effective management of PTSD, which requires extensive time and professional management, poses significant challenges [11]. Thus, developing more efficient therapeutic strategies urgently requires a deeper understanding of fear memory mechanisms and effective interventions to alleviate the symptoms of PTSD.
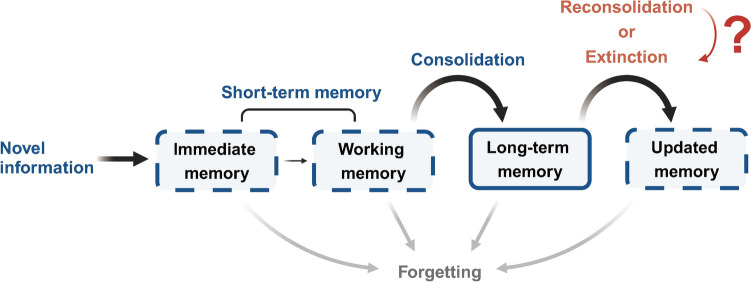
Memory is a dynamic neurophysiological process that retrieves learned information through associative mechanisms [14, 15]. It is widely accepted to involve several stages: encoding, storing, recalling, and forgetting [15] (Fig. 1). Initially, newly-acquired information is encoded rapidly, with immediate memory fading quickly without rehearsal. Reviewing this information helps stabilize memory temporarily against external interference. Short-term memory, crucial for establishing long-term memory, lasts minutes to hours and encompasses immediate and working memory [16]. The process by which newly-encoded memories stabilize over time is termed “consolidation” [17]. As memory consolidates, it becomes resistant to disruption, transforming into long-term memory, which can persist for months [18, 19]. It was broadly agreed that memory consolidation followed a linear progression [20–22] until Prof. Nader introduced the concept of “reconsolidation”, where memories can be reactivated and updated without re-exposure to the original learning environment [23]. Re-exposure to a conditioned stimulus (CS) can either reinforce fear memory retention or facilitate extinction, where a previously unsafe context no longer provokes a fear response [24, 25]. Ideally, memories should be preserved at a level that retains knowledge of threats without negatively affecting mental well-being [26, 27]. Failure to achieve this balance may lead to anxiety and other psychiatric disorders, including PTSD [28, 29]. Current psychotherapies of PTSD often focus on deactivating or impairing strong emotional memories, such as traumatic memories [30, 31]. Recent research on fear memory extinction within the reconsolidation window, known as the “retrieval-extinction protocol”, shows promise in rapidly eliminating fear memories [32, 33]. However, mixed results highlight challenges in achieving consistent outcomes [34–37]. The balance between reconsolidation and extinction processes during memory retrieval is unpredictable [38].
Fig. 1.
A schematic overview of memory simply includes short-term memory (STM), long-term memory (LTM), and updated memory. When new information is learned, it initially forms immediate memory, which lasts for a few minutes and can be extended through review, turning into working memory. Both immediate- and working-memory are termed STM since they are transient. LTM is established through consolidation, making memories more resistant to alterations. Processes like reconsolidation or extinction can update LTM by modifying original memories with new associations. Memories eventually fade if not reinforced. Unstable forms of memories are presented with dashed lines
In this review, we discuss the fundamental aspects of memory reconsolidation and extinction, focusing on the retrieval-extinction protocol and its potential clinical applications. We highlight its relevance for understanding memory modification and its implications for targeting maladaptive memories, particularly those associated with fear and trauma-related disorders [39, 40].
Experimental Models of Fear Conditioning
To understand the mechanisms of memory reconsolidation and extinction, we study them in experimental paradigms. Scientists have developed classical paradigms to study learning and memory, the classical conditioning pioneered by Ivan Pavlov being foundational [16, 41] (Fig. 2). Pavlov paired a neutral stimulus (CS), like a bell, with an unconditioned stimulus (US), such as food, to elicit a conditioned response (CR), like salivation. This laid the groundwork for studying neuronal mechanisms linking two events, predicting CS outcomes based on the US in specific contexts.
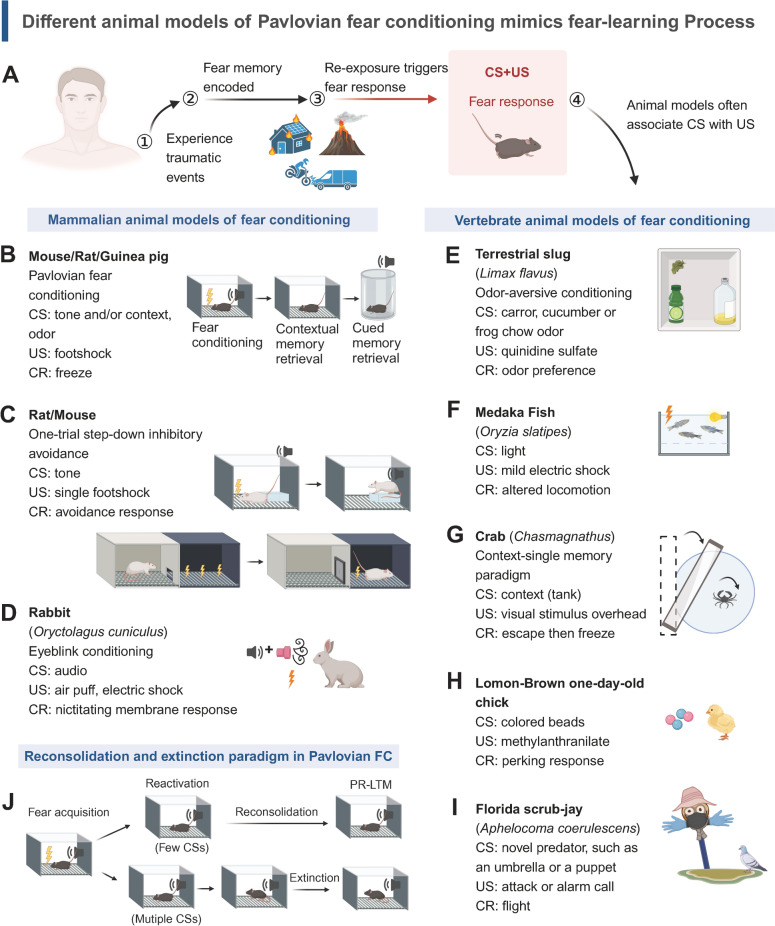
Fig. 2.
Diagrams of experimental animal models for Pavlovian fear conditioning. A Traumatic memories, such as experiencing a fire, a volcano erupting, or a car accident, are stored and retrieved upon re-exposure, forming associative learning processes studied in animal models. These experiments combine CS and US to induce fear responses in various mammalian and vertebrate subjects. B-I Different animal models of fear conditioning. J An overview of memory reconsolidation and extinction paradigms in the Pavlovian FC paradigm. After the fear acquisition, rodents are re-exposed to CSs in the training context to initiate either memory reconsolidation or extinction retrieval. They are then tested for memory retention in post-reactivation long-term tests (often performed 24 h after reactivation)
Classical fear conditioning, also known as Pavlovian fear conditioning (FC), pairs a CS (e.g., tone, odor, context) with a US (e.g., electric shock), to elicit a fear response [42]. In rodents, fear is shown through freezing in response to unexpected shocks, and memory is assessed by freezing duration during conditioning trials (Fig. 2B). Auditory or contextual fear conditioning (CFC) uses tone or context as the CS paired with the US to study different fear memory forms [43–47]. The one-trial step-down inhibitory avoidance (IA) paradigm measures fear-motivated learning by having rodents avoid electric shocks on a grid floor, with step-down latencies indicating IA memory retention [48–50]. Another paradigm, step-through avoidance (Fig. 2C), tests fear memory by measuring the latency to enter a dark box after experiencing shocks [51]. In the context-single memory (CSM) paradigm, crabs are put in a training context and exposed to an overhead visual danger stimulus (VDS), driving them to escape from the visual cue (Fig. 2G). With repeated VDS presentations, crabs develop a strong freezing response instead of escaping [52]. Successful training paradigms enable subjects to associate cues with aversive events, assessed by the degree of freezing, startle reflex, or locomotor activity [53, 54]. Classical fear conditioning has been widely studied across species, from mammals to birds and invertebrates (Fig. 2B-I) [55–61].
In studies of memory reconsolidation and extinction, researchers apply various manipulations during memory retrieval to induce different memory outcomes (Fig. 2J). In Pavlovian FC, the number of CSs presented during retrieval determines whether memory reconsolidation or extinction occurs [62–65]. CFC involves reactivating fear memories in the original or novel context to elicit reconsolidation or extinction, respectively [66, 67]. Some studies reintroduce animals into a novel context for a short duration each day, but this exposure lasts for several consecutive days [68, 69]. Other experiments also vary the number of CSs during retrieval to define reconsolidation or extinction [64–70]. Similarly, in the IA paradigm, subjects are exposed to the training chamber for distinct lengths of time to define two memory forms [71].
Introduction of Reconsolidation and Extinction
This review aims to discuss the retrieval-extinction protocol as an alternative therapy for PTSD treatment. To begin with, we explore the origins and mechanisms underlying memory reconsolidation and extinction, with a focus on their neuronal and molecular profiles. Examining the interplay between these processes provides insights into how memories are modified and regulated over time. Specifically, we explore the neuronal and molecular mechanisms involved in reconsolidation and extinction, integrating experimental evidence from animal models and human fMRI. In this aspect, we seek to contribute to a deeper understanding of memory dynamics and their implications for therapeutic interventions.
Memory Reconsolidation: Origins, Mechanisms, and fMRI Insights
Over a century ago, Müller introduced the term “Konsolidierung” to describe the phase when memories become resistant to interference [17]. They found that interventions following training could impair memory recall, while subsequent recall improved retrieval. Later, Misanin’s research discovered that delivering an electroconvulsive shock (ECS) to fear-conditioned rats after CS exposure induces amnesia [20]. His findings revealed the concept of “reconsolidation”. Reconsolidation, unlike consolidation, is a dynamic process that updates retrieved memories with new protein synthesis. Studies have shown that reconsolidation occurs more quickly and is more susceptible to amnesia than consolidation [72], emphasizing its role in memory modification and maintenance.
Reconsolidation challenges the traditional view of memories as static and unchangeable, suggesting that they are dynamic and can be modified. It occurs during memory retrieval, stabilizing recalled memories in a time-sensitive manner, which is crucial for interventions aimed at reducing fear of memories. Research shows that amnesic agents can disrupt reactivated memories when administered 6 h post-retrieval [73], while treatments before or up to 4 h after retrieval leave fear responses intact [18, 74]. This highlights a reconsolidation window, typically between 0.5 and 6 h post-retrieval, during which memories are more susceptible to modification [75]. Moreover, interventions applied strategically during reconsolidation have been shown to improve retention performance [76, 77]. These findings are key for developing PTSD therapies that target existing memories during this malleable phase.
Understanding how different brain areas interact during memory reconsolidation is crucial for developing therapeutic approaches to disorders like PTSD, where eliminating the fear of memories is key. Several brain regions, particularly the amygdala and hippocampus, play central roles in memory updating and reconsolidation by reorganizing neuronal circuits [78], as shown in Table 1. The amygdala has been extensively studied for its role in the encoding, expression, extinction, and updating of fear memories. Its central region (CeA) plays a key role in encoding salient-specific valence [79]. In addition, the basolateral nucleus of the amygdala (BLA) is responsible not only for processing both positive and negative stimuli [80] but also for storing memory [81]. Despite extensive investigations using lesion and pharmacological approaches, the neuronal mechanisms underlying the amygdala’s involvement in reconsolidation remain inadequately understood. To illustrate, pharmacological inhibition of Thy1-expressing neurons in the BLA [51], or disrupting noradrenergic projections from the locus coeruleus to the BLA can block fear memory reconsolidation [82]. Correspondingly, the hippocampus, crucial for episodic memory and emotional behaviors [83–85], also plays a significant role in reconsolidation [86]. Memory destabilization reduces neuronal activity in regions like CA1, and CA3, and optogenetic inhibition of CA1 during retrieval can cause memory failure [87]. Blocking the projection from the dorsal hippocampus to prelimbic regions of the medial prefrontal cortex (dHPC-mPFC) before retrieval also prevents reconsolidation [88]. Interestingly, identical neuronal populations across distinct brain regions can produce opposing effects. Optogenetic activation of the dorsal dentate gyrus (dDG) during recall blocks memory reconsolidation [66], whereas pharmacological inhibition of the lateral neocortex before re-exposure to the CS achieves a similar result [89]. This discrepancy suggests that diverse populations of neurons, such as CaMKII+ neurons (Ca2+-calmodulin-dependent protein kinase II-positive neurons), play various roles in CFC. However, research on neuronal circuits and the roles of specific neuron types during reconsolidation remains limited (Fig. 3A). Thus, there is a critical need for further studies to prioritize and explore this area.
Table 1.
Molecular mechanisms of memory reconsolidation
| Paradigm | Species | Brain areas | Drug administration | Effect | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Target | Classification | Drug | Time | Within-session recon. | Recon. retention | ||||
| Pavlovian FC | Sprague-Dawley rat | Systemic | Protein synthesis | Inhibitor | ANI | Post-retrieval | ↓ | ↓ | [193] |
| Wistar rat | CXM | ↓ | ↓ | [194] | |||||
| Sprague-Dawley rat | ACC | Protein synthesis | Inhibitor | ANI | Post-retrieval | ↓ | ↓ | [195] | |
| LA | CXM | – | ↓ | [196] | |||||
| Lister rat | Systemic | β-adrenergic receptor | Antagonist | Propranolol | Post-retrieval | – | ↓ | [197] | |
| Amygdala | – | ↓ | |||||||
| Sprague-Dawley rat | Systemic | NMDA-R | Partial agonist | DCS | Post-retrieval | – | ↓ | [198] | |
| Amygdala | – | ↑ | |||||||
| Systemic | Noncompetitive receptor antagonist | MK-801 | Pre-retrieval | – | ↑ | ||||
| Wistar rat | Systemic | Glucocorticoid receptor | Antagonist | RU38486 | Post-retrieval | No effect | ↓ | [199] | |
| Sprague-Dawley rat | BLA | RU486 | – | ↓ | [74] | ||||
| 6 h post-retrieval | – | No effect | |||||||
| LA | AMPA-R | Blocker | NASPM | Post-retrieval | – | ↓ | [200] | ||
| ERK-MARK signaling pathway | Inhibitor | U0126 | Post-retrieval | – | ↓ | [196] | |||
| C57BL/6 mouse | Systemic | MEK signaling pathway | Inhibitor | SL327 | Post-retrieval | – | ↓ | [201] | |
| ERK1 mutant mouse | |||||||||
| BLA | Protein kinase A | Activator | 6-BNZ-cAMP | Post-retrieval | – | ↑ | [202] | ||
| Inhibitor | Rp-cAMPS | – | ↓ | ||||||
| C57BL/6 mouse | Systemic | mTOR | Selective inhibitor of mTOR kinase | Rapamycin | Post-retrieval | ↓ | ↓ | [203] | |
| 12 h post-retrieval | ↓ | – | |||||||
| 24 h post-retrieval | No effect | – | |||||||
| Sprague-Dawley rat | BLA | NF-κB signaling pathway | Direct inhibitor of NF-κB DNA-binding complex | SN50 | Pre-retrieval | No effect | ↓ | [204] | |
| IKK inhibitor | SSZ | Post-retrieval | No effect | ↓ | |||||
| CeA | IKK inhibitor | SSZ | No effect | No effect | |||||
| BLA | Rac | Inhibitor | NSC23766 | Post-retrieval | ↓ | – | [205] | ||
| CeA | No effect | – | |||||||
| CA1 | No effect | – | |||||||
| C57BL/6 mouse | Systemic | HDAC | Inhibitor | VPA | 90 s pre-retrieval | No effect | ↑ | [76] | |
| Sprague-Dawley rat | LA | HDAC | Inhibitor | TSA | 1 h post-retrieval | ↑ | – | [77] | |
| DNMT | Inhibitor | 5-AZA | ↓ | – | |||||
| RG108 | ↓ | – | |||||||
| mRNA synthesis | Inhibitor | α-Amanitin | Post-retrieval | No effect | ↓ | [206] | |||
| Arc/Arg3.1 early gene | Antisense | ODNs | 1 d post-conditioning | No effect | ↓ | [207] | |||
| C57BL/6 mouse | BLA | Actin filament | Arrest | Phalloidin | 0.5 h post-retrieval | ↓ | – | [75] | |
| 6 h post-retrieval | ↓ | – | |||||||
| 24 h post-retrieval | No effect | – | |||||||
| CFC | C57BL/6 mouse | Systemic | Protein synthesis | Inhibitor | ANI | Post-retrieval | ↓ | – | [208] |
| dHPC | ↓ | – | |||||||
| ACC | – | – | |||||||
| Wistar rat | CA1 | – | ↓ | [209] | |||||
| Long-Evans rat | Amygdala | Protein synthesis | Inhibitor | ANI | Post-retrieval | – | ↓ | [210] | |
| Wistar/ST rat | Systemic | NMDA-R | Partial agonist | DCS | Post-retrieval | – | ↑ | [211] | |
| C57BL/6 mouse | Systemic | Noncompetitive receptor antagonist | MK-801 | Post-retrieval | – | ↓ | [212] | ||
| Lister rat | dHPC | Antagonist | D-AP5 | Post-retrieval | ↓ | ↓ | [213] | ||
| C57BL/6 mouse | Systemic | AMPA-R | Potentiator | PEPA | Pre-retrieval | No effect | No effect | [214] | |
| BLA | |||||||||
| Wistar rat | NR | N2A-containing receptor | Antagonist | TCN-201 | Post-retrieval | – | ↓ | [215] | |
| Systemic | CB1/2 receptor | Inhibitor | WIN55,212-2 | Post-retrieval | No effect | – | [209] | ||
| Amygdala | CB1 receptor | Antagonist | AM251 | Post-retrieval | – | ↓ | [216] | ||
| CA1 | ↑ | ↑ | [217] | ||||||
| CB1 receptor | Antagonist | AEA | ↓ | – | |||||
| Systemic | GABAA receptor | Agonist | MDZ | Post-retrieval | – | ↓ | [218] | ||
| Sprague-Dawley rat | Systemic | GABAA receptor | Partial agonist | Flumazenil | Post-retrieval | – | ↓ | [219] | |
| Wistar rat | NR | GABAA receptor | Agonist | Muscimol | Post-retrieval | – | ↓ | [215] | |
| C57BL/6 mouse | Systemic | H3 receptor | Inverse agonist | Thioperamide | Post-retrieval | – | No effect | [212] | |
| Female C57BL/6 mouse | Pitolisant | Post-retrieval | No effect | – | [220] | ||||
| Wistar rat | Amygdala | H3 receptor | Antagonist | Thioperamide | Post-retrieval | – | – | [216] | |
| CA1 | 5-HT5A receptor | Antagonist | SB-699551 | Post-retrieval | – | No effect | [209] | ||
| 3 h post-retrieval | – | ↓ | |||||||
| 5-HT6 receptor | Antagonist | SB-271046 | Post-retrieval | – | No effect | ||||
| 3 h post-retrieval | – | No effect | |||||||
| Agonist | WAY-208466 | Post-retrieval | – | No effect | |||||
| 3 h post-retrieval | – | ↓ | |||||||
| 5-HT7 receptor | Antagonist | SB-269970 | Post-retrieval | – | No effect | ||||
| 3 h post-retrieval | – | ↑ | |||||||
| Agonist | AS-19 | Post-retrieval | – | No effect | |||||
| 3 h post-retrieval | – | No effect | |||||||
| Amygdala | Muscarinic receptor | Antagonist | Scopolamine | Post-retrieval | – | – | [216] | ||
| Sprague-Dawley rat | Systemic | NF-κB signaling pathway | Inhibitor | DDTC | Post-retrieval | ↓ | ↓ | [221] | |
| Lister rat | dHPC | MEK-ERK cascade pathway | Inhibitor | SSZ | Pre-retrieval | ↓ | ↓ | [213] | |
| IKK-NF-κB signaling pathway | Inhibitor | U0126 | No effect | No effect | |||||
| CA1 | IL-1 receptor signaling pathway | Endogenous IL-1 antagonist | IL-1ra | Post-retrieval | ↓ | ↓ | [222] | ||
| Long-Evans rat | Amygdala | CaMKII signaling pathway | Inhibitor | Myr-AIP | Post-retrieval | No effect | ↑ | [95] | |
| C57BL/6 mouse | dHPC | AMPAR endocytosis | Blocker | TAT-GluA23Y | 1 h pre-retrieval | ↑ | - | [223] | |
| 15 min post-retrieval | ↑ | – | |||||||
| 1 d post-retrieval | ↑ | – | |||||||
| Wistar rat | PL | Protein kinase C activity | Estrogen receptor modulator | Tamoxifen | Post-retrieval | ↓ | ↓ | [67] | |
| 6 h post-retrieval | No effect | ↓ | |||||||
| ACC | Post-retrieval | No effect | ↓ | ||||||
| 6 h post-retrieval | No effect | ↓ | |||||||
| PL | PKMζ inhibition | Inhibitor | Chelerythrine | Post-retrieval | ↓ | ↓ | [224] | ||
| ZIP | 1 h post-retrieval | ↓ | ↓ | ||||||
| Sprague-Dawley rat | BLA | Rac | Inhibitor | NSC23766 | Post-retrieval | No effect | – | [205] | |
| CeA | No effect | – | |||||||
| CA1 | ↓ | – | |||||||
| Long-Evans rat | Amygdala | mRNA | Inhibitor | ACT-D | Post-retrieval | No effect | No effect | [210] | |
| C57BL/6 mouse | Systemic | mTOR | Selective inhibitor of mTOR kinase | Rapamycin | Post-retrieval | ↓ | ↓ | [225] | |
| BLA | Actin filament | Arrest | Phalloidin | 0.5 h post-retrieval | ↓ | – | [75] | ||
| 6 h post-retrieval | No effect | – | |||||||
| 24 h post-retrieval | No effect | – | |||||||
| Wistar rat | BLA | Sodium channel | Blocker | TTX | Post-retrieval | – | ↓ | [216] | |
| 96 h post-retrieval | ↓ | – | [226] | ||||||
| NBM | No effect | – | |||||||
| ENT | ↓ | ↓ | [227] | ||||||
| Systemic | Ca2+ signaling pathway | Inhibitor | PD150606 | Post-retrieval | – | ↓ | [228] | ||
| C57BL/6 mouse | dHPC | Inhibitor | ALLN | Post-retrieval | – | ↓ | [229] | ||
| Lister rat | dHPC | Zif268 | ASO | Zif268-ODN | Pre-retrieval | ↓ | ↓ | [222] | |
| BDNF | BDNF-ODN | 1.5 h pre-training | ↓ | ↓ | |||||
| Sequential FC | C57BL/6 mouse | Systemic | β1-adrenergic receptor | Antagonist | Propranolol | Post-US retrieval | No effect | ↓ | [96] |
| Betaxolol | – | ↓ | |||||||
| NMDA | Antagonist | APV | Post-US retrieval | – | ↑ | ||||
| IA | Wistar rat | Systemic | Glucocorticoid receptor | Antagonist | RU38486 | Post-retrieval | ↓ | ↓ | [199] |
| Hippocampus | ↓ | ↓ | |||||||
| Sprague-Dawley rat | Systemic | mTOR | Inhibitor | Rapamycin | Post-retrieval | – | ↓ | [230] | |
| PL | – | ↓ | |||||||
| IL | – | ↑ | |||||||
Recon, reconsolidation; ACC, anterior cingulate cortex; IL, infralimbic region of medial prefrontal cortex; LA, lateral amygdala; CeA, central amygdala; CA1, a subregion of hippocampus; NR, nucleus reuniens; NBM, nucleus basalis magnocellularis; ENT, entorhinal cortex; NMDA-R, N-methyl-D-aspartic acid receptor; AMPA-R, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; BDNF, brain-derived neurotrophic factor; ERK-MAPK signaling pathway, extracellular signal-regulated kinase (ERK)—mitogen-activated protein kinase (MAPK) signaling pathway; MEK signaling pathway, mitogen-activated protein kinase signaling pathway, mTOR, mechanistic target of rapamycin signaling pathway; NF-κB signaling pathway, nuclear factor kappa-B signaling pathway; IKK-NF-κB signaling pathway, IκB kinase-nuclear factor kappa-B signaling pathway; IL-1, interleukin-1; CaMKII, Ca2+-calmodulin (CaM)-dependent protein kinase II; PKMζ, protein kinase M zeta; ASO, antisense oligonucleotides; ACT-D, actinomycin-D; AEA, anandamide; ALLN, N-Acetyl-Leu-Leu-norleucinal; AM251, N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2, 4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; ANI, anisomycin; APV, aminophosphonovaleric acid; CXM, cycloheximide; D-AP5, D-2-Amino-5-phosphovaleric acid; DCS, D-Cycloserine; DDTC, diethyldithiocarbamate; DRB, 5,6-dichloro-1-b-d-ribofuranosylbenzimidazole; MDZ, midazolam; MK-801, dizocilpine; myr-AIP, myristoylated autocamtide-2 related inhibitory peptide; NASPM, 1-naphthylacetylsperimine; ODNs, antisense oligodeoxynucleotides; PEPA, 4-[2-(phenylsulfonylamino)ethylthio]-2,6-difluorophenoxyacetamide; Rac, Ras-related C3 botulinum toxin substrate; Rp-cAMPS, Rp-adenosine 3’,5’-cyclic monophosphorothioate; RG108, N-Phthalyl-L-Tryptophan; SSZ, sulfasalazine; TAT-GluA23Y, HIV TAT-fused GluA2-derived C-terminal peptide; TCN-201, fluoro-N-[4-[[2-(phenylcarbonyl)hydrazino]carbonyl]benzyl]benzenesulphonamide; TSA, trichostatin A; TTX, tetrodotoxin; VPA, valproic acid; ZIP, zeta inhibitory peptide; 5-AZA, 5-AZA-2’-deoxycytidine; 5-HT, serotonin; 6-BNZ-cAMP, N6-benzoyladenosine-3’,5’-cyclic monophosphate
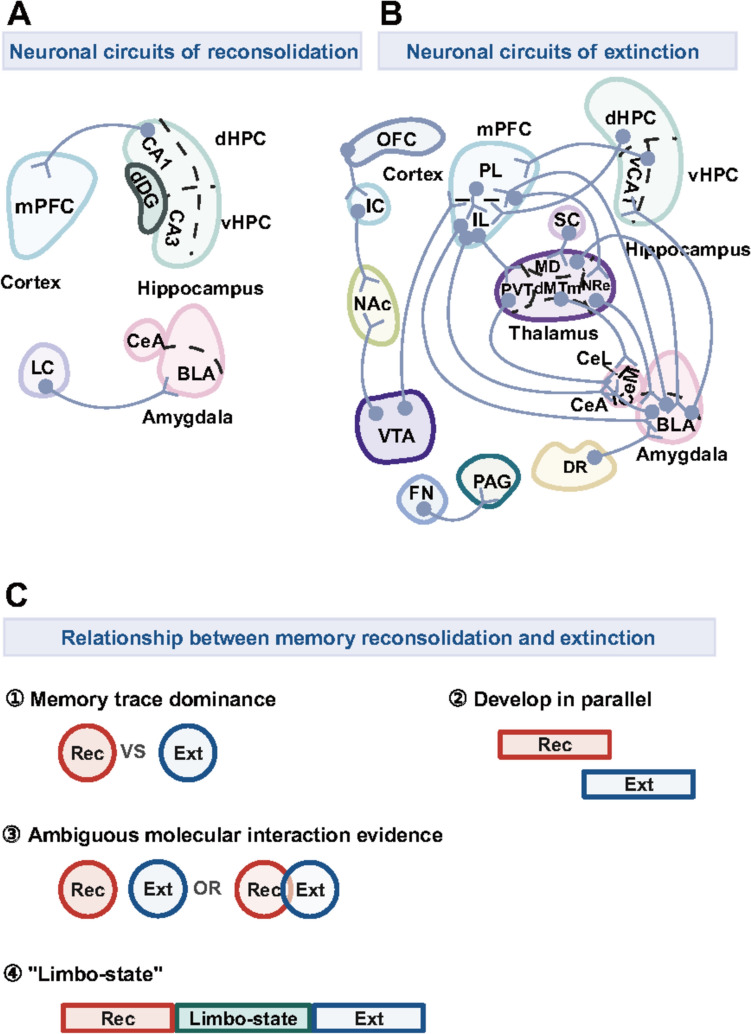
Fig. 3.
Conceptual diagrams of the neuronal circuits of reconsolidation and extinction, as well as their relationship. A Neuronal circuits of reconsolidation mainly involve the cortex, amygdala, and hippocampus. B Neuronal circuits of extinction form a cooperative network across the brain. C (1). Memory trace dominance theory implies a competitive mechanism between reconsolidation and extinction. (2). Nader et al. and Pérez-Cuesta et al. indicate that extinction does not prevent reconsolidation, suggesting dual memory periods may develop in a counterbalanced manner. (3). Molecular evidence regarding reconsolidation and extinction remains unclear from previous studies. (4). The linear memory assumption posits a distinct and resilient period between reconsolidation and extinction of memories. (Rec, Reconsolidation; Ext, Extinction.)
When fear memories are retrieved, increased expression of immediate-early genes like Zif268, Arc, and c-Fos is evident in key brain regions, such as the hippocampus, the amygdala, and the nucleus accumbens (NAc) [90–93]. Genetic studies have shown that serum- and glucocorticoid-induced kinase 3 expression is elevated following retrieval and phosphorylation of GluA1 at Ser831 and Ser845 in the amygdala, hippocampus, and mPFC [94]. Activation markers of proteasome-dependent protein degradation, such as the Lys48-linked poly-ubiquitin chain, also increase following fear memory retrieval [50]. Reactivated fear memories also induce the phosphorylation of CREB and Rpt6-S120, and upregulate proteasome activity in the amygdala and hippocampus [91, 95, 96]. Research on memory reconsolidation often applies pharmacological manipulation targeting diverse receptors or signaling pathways by specific inhibitors or agonists to disrupt the reactivated memory phase. It possesses unique molecular profiles across brain regions, such as CREB-mediated transcription, intracellular signaling pathways, neurotransmitters, or some second messengers (Table 1). For further details, refer to high-profile reviews in the field [97].
Consistent with evidence from rodents, functional magnetic resonance imaging (fMRI) in both healthy subjects and traumatized patients establishes that reconsolidation also requires the cooperation of multiple cerebral areas, notably the amygdala and hippocampus (Table 2). Increasing amygdaloid activity during fear memory retrieval suggests that it predicts fear expression and the activation of fear-related circuits [39]. The hippocampus is responsible for the modification of fear memories during re-exposure, particularly responding to unpredicted reminders associated with the original fear learning context [98]. In declarative tasks, hippocampal activity significantly increases when subjects encounter unpredictable syllable cues, aligning with patterns found during memory reactivation. fMRI studies also highlight a coactivated network involving the midline anterior cingulate cortex (ACC), insular cortex (IC), or amygdala-hippocampus circuit, when addressing dysfunctional fear memories [39]. Enhanced activity in regions like the IC, thalamus, parahippocampal gyrus, and mid-frontal gyri potentially contribute to alleviating the overwhelming stress in traumatized patients following treatments that impair reconsolidation, such as propranolol-induced interventions [99].
Table 2.
Human fMRI evidence of memory reconsolidation
| Paradigm | Subjects | Anatomical structure | Hemisphere | Test memory period | Activity | Function | References |
|---|---|---|---|---|---|---|---|
| Fear conditioning | Healthy subjects | Basolateral amygdala | Bilateral | 1 d post 10 min post-Rec. +Ext. | > | Predict the return of fear | [39] |
| 1 d post 6 h post-Rec. +Ext. | < | ||||||
| dACC | Bilateral | During react. | < | [128] | |||
| vmPFC | < | ||||||
| vmPFC-amygdala | < | Erase fear memory | |||||
| dlPFC | Bilateral | Post-react. | > | Reduce fear | [40] | ||
| Declarative task | Healthy subjects | Hippocampus | Bilateral | Early retrieval post-rec. | < | – | [231] |
| Late retrieval post-rec. | < | ||||||
| Parahippocampus | Early retrieval post-rec. | < | |||||
| Late retrieval post-rec. | < | ||||||
| Prefrontal cortex | Early retrieval post-rec. | < | |||||
| Late retrieval post-rec. | < | ||||||
| Parietal lobe | Early retrieval post-rec. | < | |||||
| Late retrieval post-rec. | < | ||||||
| Temporal lobe | Early retrieval post-rec. | < | |||||
| Late retrieval post-rec. | < | ||||||
| Posterior cingulate cortex | Early retrieval post-rec. | > | |||||
| Late retrieval post-rec. | < | ||||||
| Anterior cingulate cortex | Early retrieval post-rec. | > | |||||
| Late retrieval post-rec. | < | ||||||
| Hippocampus | Left | During React. | < | Detect incongruence and update memory | [98] | ||
| Over faces task | PTSD patients | Thalamus | Right | Propranolol-induced reconsolidation impairment | > | – | [99] |
| ACC | Right | < | Decrease PTSD symptom | ||||
| Mid-frontal gyri | Bilateral | < | |||||
| Mid-cingulate | Bilateral | < | |||||
| Face-location association learning paradigm | Healthy subjects | Hippocampus | Left | After new learning-induced during Rec. | < | Process declarative memories | [232] |
| Amygdala | Right | < | |||||
| Two-day exposure protocol | Arachnophobia subjects | Amygdala | Bilateral | 10 min exposure prior to reactions. | > | Predict fear | [167] |
| Three-day retrieval extinction protocol | Healthy subjects | IT | Bilateral | Post-retrieval extinction | > | Prediction error | [137] |
| dlPFC | > | ||||||
| dlPFC-ACC | > | ||||||
| Autobiographical memory procedure | Healthy subjects | Hippocampus | Bilateral | During react. | < | Positive information update | [233] |
| Ventral striatum | < |
Rec, Reconsolidation; Ext., Extinction; React., Reactivation; <, increased activity; >, decreased activity; ACC, anterior cingulate cortex; dACC, dorsal anterior cingulate; vmPFC, ventromedial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; IT, inferior temporal cortex
Memory Extinction: Origins, Mechanisms, and fMRI Insights
The study of extinction tracks back to Pavlov’s classic experiment where he recorded the gradual disappearance of a dog’s salivary response to food cues when those cues were presented without food [41, 100]. This process, known as “extinction”, refers to the gradual weakening of a CR [101]. Unlike forgetting, extinction involves the formation of a new memory that associates a CS with the absence of a US, requiring new protein synthesis for memory maintenance [102, 103]. Experimental approaches to extinction involve repeated exposure to a CS alone or prolonged exposure in a conditioning context to reduce CRs. The prediction error (PE) between expected and actual outcomes destabilizes the original memory, facilitating the formation of a new CS-no US memory that competes with the original CS-US memory. Extinction encompasses acquisition, retrieval, and retention periods, but over time, vulnerabilities like spontaneous recovery can lead to the return of the original fear memory [104]. Context renewal occurs when a CR reappears outside the extinction context, and reinstatement refers to the return of fear responses if the US unexpectedly reappears, reinstating the CS-US association [105].
The cortex, amygdala, and hippocampus collaborate in fear extinction memory, reflecting an emerging interest in their role in whole-brain networks [28]. Since the neuronal circuits of extinction memory have been well documented [106], in this review we outline neuronal circuits involving diverse subpopulations, summarizing various manipulation techniques such as pharmacological interventions, optogenetics, Tetanus toxin light chain, and optogenetically-induced long-term potentiation across extinction stages (Fig. 3B and Table 3).
Table 3.
Summary of neuronal circuits of extinction memory in fear conditioning
| Paradigm | Extinction period | Neuronal circuits | Neuronal type | Manipulation | Effect on memory | Ref. |
|---|---|---|---|---|---|---|
| Pavlovian FC | Fear conditioning | OFC-IC-NAc | – | Optogenetic activation | ↑ ext. retrieval | [69] |
| Fear conditioning+ Ext. acquisition | IC-NAc | – | TetTox inactivation | ↓ ext. retrieval | ||
| Pre-ext. acquisition | VTA-AcbSh | Dopamine | Pharmacological activation | ↑ext. acquisition | [234] | |
| mPFC-RE | – | Pharmacological inhibition | ↓ext. | [158] | ||
| BLA-mPFC | CaMKII | Optogenetically induced-LTD | ↑ext. | [114] | ||
| Ext. acquisition | PL-IL | Pyramidal | Optogenetic activation | ↑ext. acquisition | [111] | |
| IL-BLA | CaMKII | Optogenetic activation | ↑ext. | [110] | ||
| VTA-NAc mShell | Dopamine | Optogenetic inhibition | ↓ext. consolidation | [235] | ||
| VTA-vmPFC | Dopamine | ↓ext. acquisition | ||||
| DR-BA | 5-HT | Optogenetic activation/inhibition | ↓/↑ ext. | [236] | ||
| FN-vlPAG | Glu→Glu+GABA | Optogenetic activation | ↑ext. | [237] | ||
| SC-MD | GABA→CaMKII | Optogenetic inhibition | ↑ext. | [238] | ||
| MD-BLA | CaMKII-pyramidal | Optogenetic activation | ↓ext. | |||
| dMTm-CeA | CaMKII | Optogenetic inhibition | ↑ext. | [239] | ||
| BLA-vCA1 | Glutamatergic | Optogenetic inhibition | ↓ext. | [119] | ||
| Pre-test | BLA-CeL | Glutamatergic | Pharmacological inhibition | ↓ext. | [68] | |
| Pre-ext. retrieval | IL-PVT | Glutamatergic | Pharmacological inhibition | ↓ext. retrieval | [240] | |
| PVT-CeL | ||||||
| Ext. retrieval | dHPC-IL | CaMKII | Optogenetic activation/inhibition | ↑/↓ext. retrieval | [118] | |
| BLA-mPFC | – | Optogenetic modulation | ↑ext. retrieval | [115] | ||
| vHPC-mPFC | ||||||
| Ext. renewal | vHPC-PL | Glu→GABA | Pharmacological inhibition | ↑ext. | [117] | |
| CFC | Ext. acquisition | PL-amygdala | Pyramidal | Optogenetic activation | ↓ext. | [109] |
| Ext. retrieval | NRe-BLA | – | Optogenetic activation/inhibition | ↑/↓remote ext. | [112] |
Ext., extinction; AcbSh, nucleus accumbens shell; BA, basal amygdala; CeL, central amygdala; dMTm, dorsal midline thalamus; DR, dorsal raphe nucleus; FN, fastigial nucleus; MD, mediodorsal thalamus; NAc mShell, medial shell of nucleus accumbens; NRe, thalamic nucleus reuniens; RE, thalamic nucleus reuniens; OFC, orbitofrontal cortex; PVT, paraventricular nucleus of the thalamus; SC, superior colliculus; vlPAG, ventrolateral periaqueductal gray; vmPFC, ventromedial prefrontal cortex; VTA, ventral tegmental area; GABA, gamma-aminobutyric acid; Glu, glutamate
The PFC plays a key role in the formation and retrieval of extinction memory [107, 108]. Pyramidal projections from the prelimbic cortex (PL) to the amygdala regulate fear expression [109], while infralimbic cortex (IL)-BLA connections facilitate extinction acquisition [110]. The PL also sends excitatory afferents to the IL, which promotes extinction learning, particularly during early acquisition [111]. However, these conclusions lack verification of the tripartite neural circuit projections (PL-IL-BLA) regulating extinction learning. Pharmacological inhibition of the excitatory afferents from the PL to the paraventricular thalamus (PVT) or the PVT to the central lateral amygdala (CeL) before retrieval impairs extinction memory. Monosynaptic retrolabeling can be used to establish the role of the PL-PVT-CeL circuit in fear extinction. Interestingly, the thalamic nucleus reuniens (NRe) works as a hub for IL-BLA projections during remote extinction memory, as indicated by significant c-Fos+ activated cells in the IL-NRe and NRe-BLA projections. The anatomical circuit, verified by the retrograde virus method, suggests that a cortical-thalamic-amygdalar circuit plays a pivotal role in remote memory extinction [112]. Another brain structure that deserves further attention is the IC, a gateway to fear or extinction memory through projections to the CeA or NAc, respectively. The orbitofrontal cortex (OFC) exerts top-down control over this circuit; the OFC-IC-NAc pathway promotes extinction learning and expression [69].
The amygdala plays an essential role in memory extinction. Optogenetic activation of the Thy1-expressing subpopulation of glutamatergic BLA neurons during the extinction acquisition period promotes new learning and consolidation of extinction memory [113]. Similarly, optogenetic modulation of BLA-mPFC afferents improves extinction learning and retrieval both in the Pavlovian and contextual FC paradigms [114, 115]. This effect may be due to optogenetically-induced long-term depression, which reduces presynaptic excitability and fear responses during the extinction retention period. Fear conditioning alters corticotropin-releasing factor-expressing (CRF+) neurons in the CeA to non-CRF+ and somatostatin-expressing neurons in the BLA, while extinction learning reverses this transformation [68]. This dynamic remodeling highlights the inevitable role of excitatory CRF+ neurons in fear activity, as their excitation facilitates extinction learning and their inhibition reinforces fear memory. Intriguingly, inhibitory clusters of intercalated (ITC) neurons located between the basolateral and central region of the amygdala also influence the fear state [64]. The dorsal (ITCdm) and ventral (ITCvm) clusters respond to fear- and extinction-state, respectively. Further, stimulation of ITCvm neurons evokes postsynaptic responses in the medial CeA (CeM), which projects densely to the ventrolateral periaqueductal grey (vlPAG), suggesting that ITCvm neurons reduce the fear response by inhibiting the CeM-vlPAG pathway. Conversely, the ITCdm-BLA-mPFC pathway inhibits extinction learning by suppressing ITCvm neurons. These conclusions require further validation in experimental rodents.
Extinction memory is context-dependent, regulated by hippocampal activity, which is indispensable to spatial representation and fear memory, including extinction learning and fear relapse [116]. Pharmacological inactivation of the ventral hippocampus (vHPC) to the IL pathway facilitates fear extinction [117]. Ex vivo whole-cell recordings have revealed that local parvalbumin-expressing interneurons from the BLA mediate fear renewal by feed-forward inhibition. Likewise, optogenetic activation of the pathway from the dorsal hippocampus (dHPC) to the IL enhances fear extinction memory, but this effect is abolished by conditional deletion of extracellular signal-regulated kinase 2 in the IL [118]. The neighboring hippocampal subregions, dHPC and vHPC, contribute differently to fear extinction memory. Yet, there is a controversy over the role of the BLA in vHPC projections in CS-evoked activity during extinction memory [119]. Optogenetic silencing of this circuit during extinction learning significantly impairs extinction memory by degrading local theta-oscillations and weakening the spatial encoding capacity of the vHPC in a glutamatergic transmission-dependent manner.
Various neurotransmitters are important in fear extinction memory [120, 121]. Classical neurotransmitters (for example, γ-aminobutyric acid, glutamate, dopamine, cannabinoids, glucocorticoids, and norepinephrine) play critical roles in fear extinction memory encoding, recall, and retention. Moreover, intracellular signaling pathways such as L-type voltage-gated Ca2+ channels (LVGCCs) and second messengers are essential for extinction memory storage. These mechanisms are summarized in Table 4.
Table 4.
Molecular mechanisms of extinction memory
| Paradigm | Species | Brain areas | Drug administration | Effect | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Target | Classification | Drug | Time | Within-session ext. | Ext. retention | ||||
| Pavlovian FC | Lister rat | Systemic | NMDA-R | Partial agonist | DCS | Pre-Ext. acquisition | – | – | [198] |
| BLA | – | ↑ | |||||||
| Systemic | NMDA-R | Noncompetitive NMDA-R antagonist | MK-801 | – | ↓ | ||||
| Sprague-Dawley rat | Systemic | Noradrenergic β-receptor | Blocker | Propranolol | Post-conditioning | ↑ | No effect | [241] | |
| C57BL/6 mouse | Systemic | HDAC | Inhibitor | VPA | Pre-Ext. acquisition | ↑ | ↑ | [76] | |
| KCTD8/12 KO mice | Systemic | GABAB | Agonist | Baclofen | Pre-Ext. acquisition | No effect | No effect | [242] | |
| CFC | Lister rat | Systemic | LVGCCs | Blocker | Nimodipine | Pre-Ext. acquisition | ↑ | – | [243] |
| Wistar rat | CA1 | CB1-R | Antagonist | AM251 | Post-Ext. acquisition | No effect | ↓ | [217] | |
| NBM | Sodium channel | Blocker | TTX | 96 h post-conditioning | No effect | – | [244] | ||
| SN | No effect | – | |||||||
| BLA | No effect | – | |||||||
| NBM | 2 h post-Ext. acquisition | – | No effect | ||||||
| SN | – | No effect | |||||||
| BLA | – | ↓ | |||||||
| ENT | Post-Ext. acquisition | ↑ | ↑ | [227] | |||||
| C57BL/6 mouse | Systemic | NMDA-R | Partial agonist | DCS | Pre-Ext. acquisition | ↑ | ↑ | [214] | |
| AMPA-R | Potentiator | PEPA | Pre-ext. acquisition | ||||||
| mTOR | Inhibitor | Rapamycin | Post-Ext. acquisition | ↑ | ↑ | [225] | |||
| dHPC | Ca2+ signaling pathway | Inhibitor | ALLN | Post-Ext. acquisition | – | ↓ | [229] | ||
| IA | Sprague-Dawley rat | Systemic | mTOR | Inhibitor | Rapamycin | Post-Ext. acquisition | ↑ | ↑ | [230] |
| PL | No effect | – | |||||||
| IL | No effect | – | |||||||
| Wistar rat | Systemic | GABA | Topiramate | Ext. acquisition | ↑ | – | [245] | ||
Ext., extinction; IA, inhibitory avoidance; KCTD,K+ channel tetramerization domain; BLA, basolateral nucleus of amygdala; CA1,, a subregion of hippocampus; NBM, nucleus basalis magnocellularis; SN, substantia nigra; ENT, entorhinal cortex; dHPC, dorsal hippocampus; PL, prelimbic region of medial prefrontal cortex; IL, infralimbic region of medial prefrontal cortex; NMDA-R, N-methyl-D-aspartic acid receptor; DCS, D-Cycloserine; MK-801, dizocilpine; myr-AIP, myristoylated autocamtide-2 related inhibitory peptide; HDAC, histone deacetylase; GABAB, gamma-aminobutyric acid type B receptor; LVGCCs, L-type voltage-gated Ca2+ channels; VPA, valproic acid; CB1-R, cannabinoid type-1receptor; AM251, N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2, 4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; TTX, tetrodotoxin; PEPA, 4-[2-(phenylsulfonylamino)ethylthio]-2,6-difluorophenoxyacetamide; mTOR, mechanistic target of rapamycin (mTOR) signaling pathway; ALLN, N-Acetyl-Leu-Leu-norleucinal; GABA, gamma-aminobutyric acid
The temporal dynamics of extinction memory in the human brain are characterized by connectivity between key structures, including the amygdala, hippocampus, and cortex [122–125]. Researchers have focused on hyperactivated or hypoactivated areas during fear acquisition, extinction training, and extinction recall (Table 5). Healthy volunteers exposed to the Pavlovian FC paradigm show strong activation of the amygdala and its connectivity with cortical areas or the anterior hippocampus [126–129]. Even in focal and bilateral vmPFC-lesioned patients, amygdala connectivity remains elevated with aversive stimuli [130], highlighting the vmPFC-amygdala circuit’s role in predicting fear, supported by the finding that proximal threats by visual reality facilitate learned fear memory via suppressing the amygdala-cortical circuit during fear acquisition and extinction [131, 132]. During extinction training, hippocampus-amygdala connectivity increases [129], as the hippocampus distinguishes in the absence of stimuli [133], and the amygdala facilitates novel associations when information changes [126]. The IC and dACC are also active during extinction memory [134, 135], with hyperactivity reported in PTSD patients and healthy participants during extinction conditioning, acquisition, or recall [136]. The dACC activity is governed by the dorsolateral PFC (dlPFC) as seen in studies where healthy volunteers undergoing Pavlovian FC show inactive states in the inferior temporal cortex (IT) and dlPFC, along with suppressed IT-dlPFC and dlPFC-ACC connections during extinction retrieval [137]. As extinction progresses, the auditory association cortex remains active, even when the activity of the vmPFC and amygdala decreases [138]. Unpredicted outcomes in changing contexts activate the posterolateral cerebellum and frontopolar OFC [125, 139]. Fear acquisition inactivates the cerebellum, which shows reduced activity during extinction retrieval [125, 132].
Table 5.
Human fMRI evidence of memory extinction
| Paradigm | Subjects | Anatomical structure | Hemisphere | Test memory period | Activity | Function | Ref. |
|---|---|---|---|---|---|---|---|
| Fear conditioning and extinction paradigm | Healthy subjects | Amygdala | Bilateral | Fear acquisition | < | Fear acquisition and extinction. | [123] |
| Ext. acquisition | < | ||||||
| Healthy right-handed subjects | Amygdala | Right | Ext. acquisition | < | Establish new associations | [126] | |
| Left | > | Encode fear memories | |||||
| Hippocampus | Left | Ext. acquisition | > | ||||
| Fear acquisition | < | – | |||||
| Healthy subjects | vmPFC | Bilateral | Fear acquisition | > | – | [133] | |
| Late ext. | < | Mediate extinction recall | |||||
| Ext. recall | < | ||||||
| Hippocampus | Ext. recall | < | |||||
| Amygdala | Fear acquisition | < | – | ||||
| Late ext. | < | – | |||||
| Healthy subjects | Amygdala | Left | Fear acquisition | < | Involved in the function of the auditory cortex | [138] | |
| Early ext. | < | ||||||
| Late ext. | < | ||||||
| vmPFC | Bilateral | Fear acquisition | > | Recall auditory fear information | |||
| Ext. acquisition | < | ||||||
| dACC | Fear acquisition | < | Maintain fear expression | ||||
| Ext. acquisition | < | – | |||||
| Auditory thalamus | Fear acquisition | < | Maintain durable associations | ||||
| Ext. acquisition | > | ||||||
| Primary auditory cortex | Fear acquisition | < | – | ||||
| Ext. acquisition | < | – | |||||
| Healthy female subjects | Anterior insular | Bilateral | Fear acquisition | < | Predict intrusive memories | [135] | |
| Ext. acquisition | < | ||||||
| dACC | Fear acquisition | < | |||||
| Ext. acquisition | < | ||||||
| Ext. recall | > | ||||||
| PTSD patients | Anterior hippocampus-amygdala regions | Bilateral | Fear acquisition | < | – | [129] | |
| mPFC | – | ||||||
| Anterior hippocampus-amygdala regions | Ext. acquisition | < | Exaggerate threat response | ||||
| Ext. recall | < | ||||||
| Medial prefrontal areas | Ext. recall | < | Regulation deficiency | ||||
| Thalamus | Fear acquisition | > | Regulate arousal and awareness. | ||||
| Ext. acquisition | |||||||
| Ext. recall | |||||||
| PTSD patients | dACC | Bilateral | Fear acquisition | < | Response to cue-elicited fear | [136] | |
| Early ext. acquisition | < | ||||||
| Early ext. recall | < | ||||||
| vmPFC | Late ext. acquisition | < | |||||
| Early ext. recall | < | ||||||
| Two-day Virtual Reality Fear Extinction Experiment | Trauma-exposed children | dACC | Bilateral | Ext. recall | < | Developmental disabilities in extinction neural circuity | [246] |
| Anterior insula | Left | < | |||||
| Fear conditioning-extinction with 3D virtual reality stimuli | Healthy right-handed subjects | Cerebellum lobule VI | Right | Fear acquisition | < | Proximal threats extinction and predict fear reinstatement | [132] |
| Ext. acquisition | |||||||
| Fear reinstatement | |||||||
| Amygdala-mPFC | Late ext. | < | – | ||||
| Fear conditioning-reconsolidation- extinction paradigm | Healthy right-handed subjects | Amygdala | Bilateral | Late ext. | << | Extinction learning | [131] |
| vmPFC-amygdala | Early ext. | > | – | ||||
| Late ext. | < | – | |||||
| Three-day retrieval-extinction protocol | Healthy subjects | IT | Right | Ext. recall | > | – | [137] |
| dlPFC | Right | – | |||||
| dlPFC-ACC | Bilateral | – | |||||
| IT-dlPFC | – | ||||||
| vmPFC | Early ext. | > | – | ||||
| Late ext. | > | – | |||||
| Fear conditioning with cognitive emotion regulation instructions | Healthy right-handed subjects | dlPFC | Left | Ext. acquisition | < | Regulate fear | [127] |
| Middle frontal gyrus | – | ||||||
| vmPFC | – | ||||||
| Posterior insular cortex | > | – | |||||
| Amygdala | – | ||||||
| Cingulate regions | – | ||||||
| dmPFC | – | ||||||
| Cognitive associative learning paradigm | Healthy subjects | Cerebellar cortex lobule VI | Right | Fear acquisition | < | Fear expression | [247] |
| Ext. acquisition | < | ||||||
| Novelty-facilitated extinction paradigm | Healthy subjects | vmPFC | Bilateral | Ext. acquisition | < | Enhance ext. learning | [124] |
| Ext. retention | < | – | |||||
| Thalamus | Ext. acquisition | > | – | ||||
| Ext. retention | – | ||||||
| Insula | Left | Ext. acquisition | > | – | |||
| Ext. retention | – | ||||||
| dACC | Bilateral | Ext. acquisition | < | – | |||
| Superior frontal gyrus | Left | Ext. acquisition | < | – |
<, increased activity; >, decreased activity; Ext, Extinction; vmPFC, ventromedial prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; dACC, dorsal anterior cingulate; IT, inferior temporal cortex
Understanding Their Relationship and Mechanisms
Historical Opinions on Their Relationship
Based on the above evidence, there appear to be competing mechanisms between reconsolidation and extinction [140]. These discrepancies suggest that methodological variables influence fear memory retrieval outcomes. Earlier, Nader et al. found that intra-amygdala infusion of anisomycin (ANI) has an amnesic effect on consolidated memory during the reactivation period in the fear conditioning paradigm [18]. Nevertheless, some findings generated contrasting opinions regarding the effect of ANI on memory retrieval. One group used a one-trial IA task to examine the role of protein synthesis in the hippocampus during extinction memory. As extinction training progresses, the CR of rats gradually diminishes, while a bilateral infusion of ANI into the CA1 region before the extinction retention test completely blocks extinction memory [141]. Furthermore, though infusing ANI into the hippocampus before the first memory retention test impairs memory retrieval at 24 h, it is resistant in the following test sessions, suggesting that the protein inhibitor impairs extinction without affecting consolidated fear memory. The literature contains several inconsistencies, which arise from differences in experimental paradigms, targeted brain areas, and investigators. It is arguable that other studies using similar paradigms in either the amygdala or hippocampus reach the same conclusion as the study of Nader et al. [93], while others suggest that amnesic agents only affect extinction [142].
To reconcile the conflicting findings, several studies have established that reconsolidation and extinction are dissociable on the dominant memory trace (Fig. 3C). The Chasmagnathus model of contextual memory has been used to examine CSM with various contextual exposures, ranging from 5 to 120 min [58]. CSM retention tests showed detectable memory for exposure durations < 60 min, regardless of the contextual environment. For exposure durations > 60 min, CSM is significant in the novel context, but not in the training context, suggesting well-established extinction memory. In addition, they used the protein synthesis inhibitor cycloheximide (CHX) and revealed that after exposure, CHX impaired either reconsolidation or extinction in the 5- or 60-min context exposure groups. These results suggest that reminder duration acts as a switch in determining the outcome of memory retrieval and that reconsolidation and extinction are not mutually exclusive but depend on trace dominance.
Later discoveries refined these findings by showing that non-reinforcement and CS offset are necessary for reconsolidation and extinction [103]. One-trial VDS during re-exposure, with CHX given either 2 h later or immediately after re-exposure, failed to affect reconsolidation. However, re-exposure without one-trial VDS did affect reconsolidation, implying that a US before the CS strengthens reconsolidation, preventing amnesic effects even within the effective time window of CHX. Similar experiments on extinction memory confirmed that non-reinforcement significantly impacts memory retrieval outcomes. These findings contribute to an ineluctable interpretation that it is the non-occurrence of the expected reinforcement, terminated by non-reinforcement, that switches reconsolidation to extinction. Pérez-Cuesta et al.’s research modified the CSM retention paradigm by re-exposing rodents in a training context for 15 mins plus an additional 2 h. They tested reconsolidation before the end of the long exposure and assessed extinction memory afterward. Both reconsolidation and extinction were detectable, indicating that CS-offset initiates extinction even when reconsolidation can be tested during long exposures [143].
Nader et al. pointed out that extinction cannot prevent reconsolidation [144], as inhibiting protein synthesis in the BLA of rodents impairs both recently reactivated and extinguished fear memories. Reconsolidation and extinction memory develop in parallel and can overlap, depending on the number of CS presentations [145]. Pérez-Cuesta et al.’s further experiments [143] showed that pre-exposure administration of CHX and varying re-exposure conditions to CS or CS-US confirmed that reconsolidation initiates earlier than extinction memory, with partial overlap (Fig. 3C). This aligns with Nader et al.’s findings [144], emphasizing that reconsolidation cannot be attenuated to facilitate extinction [146]. There are amnesic effects on context-shift procedures if ANI is given after reactivation. However, the results demonstrated no fear of memory renewal in either the same or a different context, indicating that post-reactivation administration of ANI takes effect on the memory storage trace rather than extinction memory, which does not support this hypothesis that reconsolidation can be attenuated to facilitate extinction.
From a molecular perspective, evidence is mixed regarding the distinctiveness of reconsolidation and extinction pathways. Some studies suggest that these processes have separable biochemical signaling pathways. For instance, pharmacologically inhibiting CB1-R and LVGCCs affects extinction but not reconsolidation in CFC. Conversely, factors such as protein synthesis and N-methyl-D-aspartate receptors (NMDARs), which impact reconsolidation, also impair extinction [147]. Supporting this, the expression of the immediate-early genes Zif268 and Arc is dependent on reconsolidation. Conditional knockdown of these genes combined with protein inhibition results in a blockade of reconsolidation while altering their expression prevents extinction. The above evidence demonstrates that reconsolidation and extinction have distinct molecular signatures.
Some reports have demonstrated that reconsolidation and extinction share predominantly identical molecular characteristics (Fig. 3C). For instance, disruption of CREB-mediated transcription blocks both reconsolidation and extinction in CFC [93]. Immunohistochemical analysis of the transcription factor CREB and the expression of Arc have revealed significant Arc expression in the amygdala and hippocampus during reconsolidation, and in the amygdala and PFC during extinction. Therefore, both processes engage new gene expression in various brain regions, indicating potential interaction between reconsolidation and extinction under certain conditions. Another biomarker in the BLA is calcineurin, which increases during extinction [148]. Its expression pattern remains unchanged even with an NMDA-type glutamate receptor agonist or antagonist, suggesting an insensitive state, termed the “limbo state” where neither process is engaged.
To clarify the concept of the “limbo state” between reconsolidation and extinction, researchers investigated the ERK1/2 signaling pathway in the CFC paradigm. Memory retrieval sessions were divided into four groups based on the number of non-reinforcement sessions (1, 4, 7, and 10) [149]. Minimal CS stimuli (1) represented reconsolidation, and maximal stimuli (10) represented extinction, with intermediate states at 4 and 7 CS stimuli. Intra-BLA administration of the ERK1/2 pathway inhibitor U0126 did not affect memory retrieval during 4/7 CS stimuli, but it did during 1/10 CS stimuli. In addition, manipulating the ERK1/2 pathways with the NMDAR partial agonist D-cycloserine supports the existence of a “limbo state” [62]. This state is a linear progression between reconsolidation and extinction, differing from Pérez-Cuesta et al.’s idea of overlapping reconsolidation and extinction (Fig. 3C). Similar results were found in the IA paradigm, where the ERK signaling pathway was specifically activated during extinction, and CREB expression was anticipated in both reconsolidation and extinction [71]. Since the ERK pathway is upstream of CREB, its activation facilitates extinction while preventing reconsolidated memory, further establishing the “limbo state”. Moreover, a previous study identified “fear neurons” during non-reinforcement reactivation and “extinction neurons” during extinguished memory in the amygdala [150], raising a question about the diversity of neuronal populations involved in fear memory retrieval. Further identification of different neural types responsible for distinct memory stages is necessary.
In summary, reconsolidation and extinction share similar biochemical signatures, but the exact mechanism of how reconsolidation turns into extinction remains largely unknown.
Engram Cells in Memory Reconsolidation and Extinction
Reconsolidation and extinction are both memory-updated processes triggered by the presence of the CS [146], involving new protein synthesis. In addition, these two mnemonic processes share partially-overlapping molecular and neuronal profiles. Questions arise: What proteins are synthesized? Where is the updated memory stored? Are there two phenotypically distinct populations that regulate reconsolidation and extinction separately? Richard Semon’s Engram Theory may help explain these discrepancies. Proposed over a century ago [151], it was not until the turn of the millennium that the “engram cells” theory gained significant attention in the study of memory owing to the technical restrictions of that era [152]. The term “engram” refers to an organic substance that stores memories with three features: (1) activation by a learning experience; (2) modification by an artificial learning experience; and (3) reactivation by subsequent retrieval, often involving non-engram, silent, and active engram cells [151, 153].
The relationship between memory consolidation and engrams remained unexplored until researchers found that protein synthesis inhibitors can induce amnesia, which can be reversed by artificially activating the engram cells labelled during fear training. This phenomenon can also occur when amnesic treatments are administered during the reconsolidation period [154]. During CS retrieval, hippocampal engram cells activated by backward conditioning (US-CS) are labeled, showing that CS retrieval can significantly increase the overlapping proportion of the fear conditioning engram and retrieval-induced engram cells, indicating a reactivation of the fear conditioning engram. This indirect retrieval procedure also underlies the reconsolidation phase, since amnesic treatment into the hippocampus during a post-reactivation period can interfere with the original fear memory [155]. Further, optogenetic activation of c-Fos-tagged dDG ensembles impairs conditioned fear responses during post-reactivation [66], regardless of the valence of the engram. Nevertheless, this freezing decline phenomenon can be replicated if a sufficient proportion of dDG neurons are activated, indicating that the reconsolidation process is not linked to engrams of a specific valence. Furthermore, the reconsolidation process involves different engram cells for fear conditioning and reinstatement, with minimal overlap, implying that reconsolidation re-engagement of fear conditioning engram ensembles may generate new engrams. The verification can be expanded in the amygdala where c-Fos-LacZ transgenic rats in the auditory FC paradigm [156] showed that high neuronal activation leads to the expression of c-Fos and LacZ mRNA, followed by β-galactosidase (β-gal) protein expression. Infusion of Daun02, which disrupts neuronal function when converted to Daunorubicin by β-gal, significantly decreased freezing levels after reactivation, demonstrating that reconsolidation reactivates original fear conditioning engrams rather than creating new engram ensembles. Thus, manipulating reconsolidation engram cells may be less effective, as reconsolidation primarily reactivates existing fear conditioning engrams.
Extensive evidence has shown the widespread existence of extinction engram cells throughout the brain [115, 119, 157–161]. Fear extinction engrams, identified by engram cell labelling technology in transgenic rodents, facilitate the labelling and manipulation of activated neurons across different mnemonic periods. Extinction activates engram ensembles in the dDG, which can be artificially disrupted during extinction retrieval [159]. Moreover, the genetical signature of the amygdaloid extinction engram ensembles has been identified in Ppp1r1b+ BLA neurons [160]. Building upon the “prediction error” theory, it is tempting to speculate that exposing engram cells to a gradually decreasing frequency of non-reinforced stimuli could encode diverse information. Attempts to reverse memory valence can be made by reactivating original engrams while applying conditioning of opposite valence [162], potentially converting related information from negative to positive. Interestingly, in both contextual fear and reward conditioning, engram cells in the dDG encode bidirectional valence with a switch in functional connectivity due to progressively growing synaptic strength [154]. Also, the reward-responsive neurons exhibit functional equivalence with the extinction engrams. This implies that extinction learning represents a positive rewarding experience for fear-conditioned subjects [66]. The engram cell theory provides a cornerstone for explaining memory malleability.
Several studies have revealed that fear acquisition engrams can be suppressed by extinction engram cells, highlighting distinct neuronal ensembles governing fear memory [115, 158, 159]. During the memory updating process initiated by non-reinforced stimulus-initiated fear engrams increasingly overlap with extinction engram cells in the PL [161]. These findings suggest that as extinction learning progresses, some fear engrams may switch into extinction ensembles (Fig. 4). Despite evidence that activated engram cells often inhibit the recruitment of non-activated ones [163, 164], whether a competitive mechanism among engram cells is triggered by the PE remains elusive. Following this question, scientists have demonstrated that it is the “collocation window” between events that establishes competition among engram cells in the lateral amygdala, regulated by parvalbumin interneurons that release GABA [165]. When rodents receive two distinct fear conditioning events within 6 h, engram cells integrate collaborative memories. However, for events >1 day apart, engram cells represent them as independent events. This discovery is consistent with the concept of the “reconsolidation window” [33], which delineates the effective timeframe for rewriting memory. In this context, if the non-reinforcing stimulus is presented during the reactivation period, the already-formed associations in the fear engram cells are overwritten in the absence of the US. If non-reinforcing stimuli are presented during reactivation, the existing associations in fear engram cells may be overwritten in the absence of the US [161]. Fear-related learning mechanisms form strong connections among fear engrams, but concerted efforts are required to degrade these neural associations. Engrams encoding different memories can be reflected in their morphological changes through spine connectivity or vector distance within the population [154, 161]. Caution is warranted regarding the potential of silent engram cells. Emerging evidence supports the hypothesis that it is the silent-state, rather than the latent state, of engram cells in a silent-state, not a latent-state, that makes them inaccessible under natural conditions. However, artificial regulation may facilitate access to these cells, thereby reducing the likelihood of repeated CS exposures activating the dormant engrams [157, 159, 166].
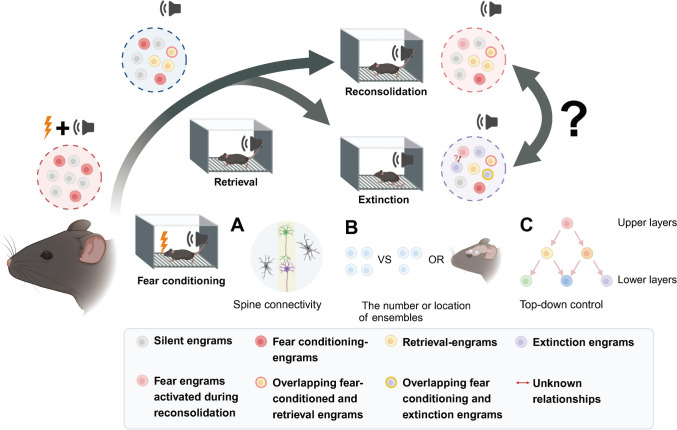
Fig. 4.
A possible role for engram cells pertaining to memory reconsolidation and extinction. This review proposes the existence of distinct engram ensembles during mnemonic stages, such as reconsolidation and extinction. During fear conditioning, a cohort of silent engram cells (grey, silent engrams) encode new information and become conditioning-activated engram cells (scarlet, fear conditioning engrams). Upon memory retrieval, other engram ensembles are activated by the CS (yellow, retrieval engrams). Some conditioned- and retrieval-engrams may overlap (yellow with red border, overlapping fear-conditioned and retrieval engrams). Memory retrieval triggers two opposing stages, namely reconsolidation and extinction. The former process reactivates original fear conditioning engrams (red, fear engrams activated during reconsolidation), and the latter process generates new engram ensembles (violet, extinction engrams). As extinction learning progresses, some fear engrams may switch into extinction ensembles (violet with yellow border, overlapping fear conditioning, and extinction engrams). However, the intrinsic switch mechanism between reconsolidation- and extinction-engrams is not well understood (black question mark). Potential factors govern the competition between reconsolidation and extinction engram cells including spine connectivity (A), the number or location of engram ensembles (B), or projections from upper brain structures (C). The hypothetical mutual interaction between reconsolidation- and extinction-engram cells requires further exploration (tiny red question mark, unknown relationships)
Behavioral Therapy Based on Reconsolidation
Currently, ET aims at weakening traumatic memory associations by inducing reconsolidation and has emerged as a promising alternative to the standard ET strategy. This approach updates memory by decreasing amygdaloid activity [167, 168], thereby reducing the emotional impact of traumatic memories. By presenting unreinforced CSs within this labile, retrieval-induced period, memory is updated so that the CS, once predictive of danger, is now associated with the absence of the US. This shift can potentially lead to the permanent erasure of fear memories without the use of medications [32]. Known as the “retrieval-extinction” the “post-retrieval extinction” (PRE) protocol, or the “reconsolidation of traumatic memories” (RTM) protocol [169–171], this method has advantages over traditional extinction therapy, such as cost-effectiveness and high adherence rates; it is gaining popularity among ET alternative [172, 173]. For limitations of ET, refer to Table 6.
Table 6.
Limitations of exposure therapy among PTSD patients
| Type of exposure therapy | Subjects | Evaluation standard | Course of treatment (weeks/m) | Follow-up sessions | Treatment dropout (%) | Therapeutic outcomes | Limitations | Possible reasons | References |
|---|---|---|---|---|---|---|---|---|---|
| CPT | 94 active service duty members with a diagnosis of PTSD and MDD |
a. MADRS b. CAPS-5 c. PHQ-9 d. PCL-5 |
20 | 3 weeks | 15 | No comparable significant difference. | The use of BA may not be necessary over CPT alone. | Treatment options may be decided by patient preference and shared decision-making | [248] |
| CPT+BA | 21 | ||||||||
| D-CPT | Adolescent PTSD patients (aged 14-21 years, n=38) |
a. Self-developed TAS b. Self-developed TCS c. HAQ d. CAPS-CA |
16-20 | 3 weeks | 13.63 | Therapeutic alliance is positively correlated with reduced PTSD symptom | Therapeutic adherence and competence are negatively correlated to the treatment outcome | Lack of therapist adherence and competence | [249] |
| GBCT |
PTSD veterans (n=98) |
a. CAPS-5 b. BDI-II and BAI c. SCID-IV-TR d. TLEQ e. SF-36 f. CERQ-Short |
16 | – | 38.4 | – | Treatment noncompletion | Reported lifetime trauma exposure, higher depression symptoms. | [250] |
| GPCT |
PTSD veterans (n=100) |
20.2 | 2.389 times more likely to compete for treatment than GBCT groups | – | A group therapy strategy is preferred | ||||
| PE delivered via video teleconferencing | Rural veterans (n=27) |
a. PDS b. BDI c. BAI d. CAPS |
12 | 1 month | 70 in the iPhone group | Decrease PTSD symptom | Significant high attrition | Poor technical support | [251] |
| PE | PTSD male combat veterans (age=65 years, n=87) |
a. CAPS b. SCID-IV-TR c. PCL-S d. PHQ-9 |
12 | 6 weeks | 27 | PTSD symptomology has improved | Hard to maintain therapeutic outcomes | Remote traumatic memories are resistant to interference | [252] |
| RT | |||||||||
| MT |
PTSD veterans (n=370) |
a. PSS-I b. PCL-S |
2 | 6 weeks | 13.6 | No extinguished treatment found | Trauma-focused treatment may not be suggested for improving PTSD symptoms in active-duty military personnel. | Alternative treatments may be needed to investigate better clinical outcomes. | [253] |
| ST | 8 | 24.8 | |||||||
| PCT | 8 | 12.1 | |||||||
| PE with AE (imaginal exposure) | Active duty United States service members (n=125) |
a. PCL-S b. PSS-I c. BDI-II d. BAI e. STAXI-2 f. ADUIT g. SSI |
8–12 | 1 and 6 months | 52.8 | Not as ideal as delivered PET alone | AE intervention fidelity is not systemically assessed post-treatment | Personalized psychotherapy is recommended | [254] |
| 3MDR | Treatment-resistant PTSD veterans (n=43) |
a. CAPS-5 b. PCL-5 c. PABQ |
6 | 12 and 16 weeks | 7 | 45% of patients improved clinically | No statistical variations of PTSD symptom severity between groups | A larger sample size and wider clinical application are needed | [9] |
| VRE | Active duty soldiers with PTSD (n=108) |
a. SUDS b. CAPS |
26 | 3 and 6 months | 44 | A greater decrease in CAPS scores without group difference | Poor emotional engagement | Substantial emotional and stress evaluation should be established | [255] |
| PE | 41 | - | |||||||
| VRET | Active duty soldiers with PTSD (n=153) | a. CAPS | 9 | 3 months | 16.2 | CAPS-score improved 31% | High dropout occurred only in the VRET group | A common phenomenon that is consistent with other reports | [256] |
| CRET | 0 | CAPS-score improved 37% | – | – | |||||
| TMT | Veterans and active duty personnel with PTSD (n=112) |
a. CAPS b. PCL-M c. SCID I and II d. SF-36 e. BRIEF-A f. TRGI g. CGI h. HAMD |
17 | 3 and 6 months | 2 | High-level and satisfactory treatments | Not a randomized controlled trial | Latent variables should be constructed | [257] |
| Web-PE | 40 military personnel with PTSD |
a. CEQ b. CAPS-5 c. PCL-5 d. PHQ-9 e. VR-12 |
8 | 3 and 6 months | 52.6 | Reduced PTSD symptom | High dropout rate | Achieve better therapeutic outcomes in open trial participants. | [258] |
| PCT | 23.8 | No comparable difference, but PCT achieved clinically significant change. | |||||||
| NET | Patients with comorbid BPD and PTSD (n=11) |
a. SCID-I and II b. CTQ |
10 | 12 months | 9.1 | NET is feasible and safe in an inpatient program | Lack of outpatient use | This type of inpatient setting is common in Germany | [259] |
| DET | PTSD patients (n=141) |
a. IDCL b. IES-R c. PDS d. BSI e. PTCI f. IIP-C g. Global Severity Index |
Not mentioned | 6 months | 12.2 | CPT performed significantly better than DET | Unreliable remission rates | Incomplete diagnostic criteria for PTSD symptoms | [260] |
| CPT | 14.9 |
3MDR, virtual reality and motion-assisted exposure therapy; AE, aerobic exercise; AUDIT, Alcohol Use Disorders Identification Test; BA, behavioral activation; BAI, Beck Anxiety Inventory total score; BDI-II and BAI, the Beck Depression Inventory-II and Beck Anxiety Inventory; BPD, Borderline Personality Disorder; BRIEF-A, the Behavior Rating Inventory of Executive Function-Adult Version; BSI, Brief Symptom Inventory; CAPS-5, the Clinician-Administered PTSD Scale for DSM-5; CAPS-CA, the German version of the Clinician-administered PTSD Scale for Children and Adolescents; CEQ, Credibility/Expectancy Questionnaire; CERQ-Short, the Cognitive Emotion Regulation Questionnaire-Short Form CPT, cognitive processing therapy; CGI, Clinical Global Impressions Scale; CHANGE, a coding system designed to capture processes of therapeutic change, including both facilitators and inhibitors of change; CPT, cognitive processing therapy; CRET, control exposure therapy; CTQ, Childhood Trauma Questionnaire; D-CPT, developmentally adapted cognitive processing therapy; DET, dialogical exposure therapy; GBCT, group cognitive behavioral treatment; GPCT, group present-centered treatment; HAQ, the German version of the Helping Alliance Questionnaire; HADS, Hospital Anxiety and Depression Scale; HAMD, Hamilton Rating Scale for Depression; IDCL, the German version of the International Diagnostic Checklist for DSM-IV and ICD; IES-R, Impact of Event Skala-revidierte Version; IIP-C, the Inventory of Interpersonal Problems-Circumplex Version; MADRS, Montgomery-Åsberg Depression Rating Scale; MDD, major depression disorder; MT, massed therapy includes prolonged exposure therapy, cognitive behavioral therapy involving exposure to trauma memories/ reminders are administered altogether; NET, Narrative exposure therapy; PABQ, Posttraumatic Avoidance Behaviour Questionnaire; PCL-5, PTSD Checklist for DSM-5; PCL-M, PTSD Checklist-Military Version; PCL-S, PTSD Checklist-Stressor-Specific Version; PCT, present-centered therapy; PDS, a self-report instrument which can be used for determining PTSD diagnostic status according to DSM-IV criteria and symptom severity; PE/PET, prolonged exposure therapy; PHQ-9, Patient Health Questionnaire-9; PSS-I, PTSD Symptom Scale-Interview; PTCI, Posttraumatic Cognitions Inventory; SCID I and II, the Structured Clinical Interview for DSM-IV to assess other Axis I and II disorders; SCID-IV-TR, the Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition with Psychotic Screen; SF, the Physical Role Functioning subscale of the Medical Outcomes Study Short-Form Health Survey; SF-36, Health-Related Functioning: Medical Outcome Study Short Form-36 Health Survey; SSI, Scale for Suicidal Ideation; ST, spaced prolonged exposure therapy; STAXI-2, State-Trait Anger Expression Inventory; SUDS, subject units of discomfort; TAS, therapeutic adherence scale; TCS: therapeutic competence scale; TRGI, Trauma-Related Guilt Inventory; TLEQ, the Traumatic Life Events Questionnaire; TMT, trauma management therapy; VR-12, the Veterans RAND 12-Item Health Survey; VRE/VRET, visual reality exposure therapy; Web-PE, a web version of Prolonged Exposure Therapy
Several factors contribute to the efficacy of reconsolidation-based protocols. Firstly, it is the timing that matters. The success of this intervention relies heavily on the time window during which memories are temporarily destabilized and susceptible to modification. In humans, reactivating fear memories 10 min, but not 6 h, before extinction produces a durable reduction in fear that can last at least a year [33]. Secondly, a degree of PE is essential for destabilizing memories. Multiple PEs weaken original fear memories more effectively and prevent fear from returning during reinstatement compared to a single-PE strategy [174]. This protocol leverages PE to create a discrepancy between the original learning context and the reactivated context [175]. Moreover, an uncertain number of PEs, rather than a PE alone, may more effectively prevent fear return under a laboratory paradigm [176]. The above evidence suggests that the degree of violation during the memory-updating process facilitates fear attenuation and extinction learning. Thirdly, the unpredictability of interval length is also vital. In a PRE training paradigm, researchers found that distributing random inter-trial intervals (ITIs) during the reactivation, rather than fixed ITIs, leads to a greater erasure of fear memory [177]. Greater variability in ITIs results in a more significant erasure of reconsolidated memory [178]. Finally, PTSD patients harbor persistent fear memories that limit the effectiveness of standard ET. Thus, preventing the recurrence of old fear memories is an important measure of PRE therapy’s robustness. Human studies have shown that PRE can effectively eliminate both recent (1-day-old) and remote (7- and 14-day-old) fear memories [179, 180]. A modified PRE protocol, in which the US is presented before extinction, prevents fear relapse for at least 6 months [179]. Encouragingly, this approach has also shown success in treating naturally-occurring phobias, like arachnophobia [167, 181], and offers promise for those with severe, prolonged trauma exposure [171, 172, 182]. As females are more susceptible than males [183], reconsolidation-based protocols have shown promising results in female patients as well [182]. In sum, these studies provide compelling evidence that reconsolidation-based protocols are not only effective in PTSD, but also offer long-lasting prevention against the return of fear.
Some failures in testing the efficacy of the reconsolidation-based treatments in laboratory-induced fear cannot be overlooked [34, 169, 170, 184], potentially due to the instability of engram cells. Engrams stabilize during consolidation. Stable engrams can be consistently reactivated during both recent and remote memory retrieval, and this persistence is vital for memory maintenance [185]. This rigidity is maintained through mechanisms such as DNA methylation [186] or synaptic strength and spine density augmentation [154]. First, the intensity of extinction training may be insufficient. Experimental fear paradigms applied in healthy participants often span 3 to 4 days, including the extinction training phase, and differ significantly from real-world fear scenarios. Consequently, the artificially-induced extinction memory may fail to establish stable engram cells, leaving it as a fragile, temporary trace unable to counteract the engram connections of the original fear memory because increased ensemble stability is vital for memory permanency [185]. Second, changes in experimental conditions, such as replacing the original aversive stimuli with neutral ones, can contribute to failure. Third, individual differences may cause variability in extinction learning, preventing the elimination of fear memories. Furthermore, the use of medications in some paradigms introduces further uncertainty compared to purely behavioral approaches.
Perspective
PTSD is a mental health disorder inflicted by traumatic experiences, leading to flashbacks, avoidance, and cognition impairments that disrupt daily life [187]. Understanding the pathogenesis of PTSD at the biomolecular and neuronal circuit levels may offer new therapeutic insights. While the role of engram cells in the switch between reconsolidation and extinction remains uncertain, increasing research underscores their importance in both processes, providing a springboard for future work. A key question is what factors govern the competition between reconsolidation and extinction engram cells. Potential influences include spine connectivity, ensemble number, location, and projections from upper brain structures (Fig. 4A-C), though current evidence does not fully explain these assumptions. Achieving these advances requires a detailed mapping of neurocircuitries, supported by technologies such as fMRI and comprehensive electroencephalogram monitoring. In preclinical research, distinct molecular targets involved in memory destabilization are identified by activating encoded engram cells under specific conditions. Techniques such as single-cell sequencing and neural activity-dependent labeling in animal models of PTSD could help uncover biomarkers associated with reconsolidation and extinction engram cells.
For human studies, a standardized protocol should first be developed. Boundary conditions, such as the type of fear memory, memory age, and intensity of stimuli, should closely replicate original memory scenarios to effectively engage engram cells. Engram stability is closely tied to memory context, emphasizing the need to replicate the encoding scenario. Script-driven imagery and music elicit emotional responses to disrupt memory reconsolidation. Interactive tools, like computer games or Virtual Reality (VR) technology, show significant promise as an intervention for PTSD [188]. By generating vivid and immersive artificial environments, VR can effectively incorporate cues associated with individual traumatic experiences, thereby facilitating a more personalized and potentially therapeutic approach to treatment. Multi-center, larger-sample studies are essential to validate the robustness of this methodology. In addition, computer science can predict the outcomes of reconsolidation-based interventions by developing metacognitive frameworks to predict how memory-updating factors may influence the outcomes [189, 190]. Artificial models help further overcome the obstacle of small clinical populations by enabling a better understanding of how individual patient profiles, including etiology, brain circuity, genetic variations, and sex differences modulate the interplay of boundary conditions, identify general patterns, and optimize future treatment strategies.
A key milestone is identifying engram cells in the human brain. Using fMRI data during reactivation may identify brain regions harboring engram cells. It is imperative to develop a behavioral marker of reconsolidation using fMRI at single-cell resolution [191, 192] that can be used in large-scale clinical applications. From brain regions to cellular biomarkers by combining advanced techniques, major efforts should prioritize bridging the gap from the bench to the bedside. Such efforts will not only broaden our understanding of engram cells in PTSD but also lay the groundwork for developing precise and personalized treatments for trauma-related disorders.
Acknowledgments
This review was supported by the National Key Research and Development Project of China (2021ZD0202800) and the National Natural Science Foundation of China (U21A20418, 82003727).
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Jiahui Chen and Zhuowen Fang contributed equally to this review.
Contributor Information
Yanrong Zheng, Email: yanrong_zh@zju.edu.cn.
Zhong Chen, Email: chenzhong@zju.edu.cn.
References
- 1.Koenen KC, Ratanatharathorn A, Ng L, McLaughlin KA, Bromet EJ, Stein DJ. Posttraumatic stress disorder in the world mental health surveys. Psychol Med 2017, 47: 2260–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao W, Liu X, Wang H, Huang Y, Dai Z, Si M, et al. Prevalence and risk for symptoms of PTSD among survivors of a COVID-19 infection. Psychiatry Res 2023, 326: 115304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girgenti MJ, Wang J, Ji D, Cruz DA, Stein MB, et al. Transcriptomic organization of the human brain in post-traumatic stress disorder. Nat Neurosci 2021, 24: 24–33. [DOI] [PubMed] [Google Scholar]
- 4.Chamaa F, Bahmad HF, Darwish B, Kobeissi JM, Hoballah M, Nassif SB, et al. PTSD in the COVID-19 era. Curr Neuropharmacol 2021, 19: 2164–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan K, Gong YM, Liu L, Sun YK, Tian SS, Wang YJ, et al. Prevalence of posttraumatic stress disorder after infectious disease pandemics in the twenty-first century, including COVID-19: A meta-analysis and systematic review. Mol Psychiatry 2021, 26: 4982–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao Y, Sun Y, Meng S, Shi J, Lu L. 2019-nCoV epidemic: Address mental health care to empower society. Lancet 2020, 395: e37–e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khanna A, Jones GB. Envisioning post-pandemic digital neurological, psychiatric and mental health care. Front Digit Health 2021, 3: 803315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guideline Development Panel for the Treatment of Ptsd in Adults AcPA. Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. Am Psychol 2019, 74: 596–607. [DOI] [PubMed]
- 9.van Gelderen MJ, Nijdam MJ, Haagen JFG, Vermetten E. Interactive motion-assisted exposure therapy for veterans with treatment-resistant posttraumatic stress disorder: A randomized controlled trial. Psychother Psychosom 2020, 89: 215–227. [DOI] [PubMed] [Google Scholar]
- 10.Post RM, Kegan R. Prevention of recurrent affective episodes using extinction training in the reconsolidation window: A testable psychotherapeutic strategy. Psychiatry Res 2017, 249: 327–336. [DOI] [PubMed] [Google Scholar]
- 11.Rothbaum BO, McSweeney LB. Patients need to remain in treatment for PTSD to receive the full benefit. J Anxiety Disord 2019, 68: 102156. [DOI] [PubMed] [Google Scholar]
- 12.Wells SY, Morland LA, Hurst S, Jackson GL, Kehle-Forbes SM, Jaime K, et al. Veterans’ reasons for dropping out of prolonged exposure therapy across three delivery modalities: A qualitative examination. Psychol Serv 2023, 20: 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks SK, Greenberg N. Recurrence of post-traumatic stress disorder: Systematic review of definitions, prevalence and predictors. BMC Psychiatry 2024, 24: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okano H, Hirano T, Balaban E. Learning and memory. Proc Natl Acad Sci U S A 2000, 97: 12403–12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zlotnik G, Vansintjan A. Memory: An extended definition. Front Psychol 2019, 10: 2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Squire L, Kandel E. Memory: From mind to molecules. Nat Med, 2003
- 17.Müller GE, Pilzecker A. Experimentelle Beiträge Zur Lehre Vom Gedächtniss [German]. 1 ed: J. A. Barth, Leipzig, 1900.
- 18.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 2000, 406: 722–726. [DOI] [PubMed] [Google Scholar]
- 19.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci 2005, 6: 119–130. [DOI] [PubMed] [Google Scholar]
- 20.Misanin JR, Miller RR, Lewis DJ. Retrograde Amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science 1968, 160: 554–555. [DOI] [PubMed] [Google Scholar]
- 21.Schneider AM, Sherman W. Amnesia: A function of the temporal relation of footshock to electroconvulsive shock. Science 1968, 159: 219–221. [DOI] [PubMed] [Google Scholar]
- 22.Lewis DJ. Sources of experimental Amnesia. Psychol Rev 1969, 76: 461–472. [DOI] [PubMed] [Google Scholar]
- 23.Alberini CM, LeDoux JE. Memory reconsolidation. Curr Biol 2013, 23: R746–R750. [DOI] [PubMed] [Google Scholar]
- 24.Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull 1993, 114: 80–99. [DOI] [PubMed] [Google Scholar]
- 25.Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: Inverse correlation with trace dominance. Science 2003, 301: 1102–1104. [DOI] [PubMed] [Google Scholar]
- 26.Lu Y, Sun XD, Hou FQ, Bi LL, Yin DM, Liu F, et al. Maintenance of GABAergic activity by neuregulin 1-ErbB4 in amygdala for fear memory. Neuron 2014, 84: 835–846. [DOI] [PubMed] [Google Scholar]
- 27.Kintscher M, Kochubey O, Schneggenburger R. A striatal circuit balances learned fear in the presence and absence of sensory cues. Elife 2023, 12: e75703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci 2015, 16: 317–331. [DOI] [PubMed] [Google Scholar]
- 29.Pedraza LK, Sierra RO, de Oliveira Alvares L. Systems consolidation and fear memory generalisation as a potential target for trauma-related disorders. World J Biol Psychiatry 2022, 23: 653–665. [DOI] [PubMed] [Google Scholar]
- 30.Yehuda R, Hoge CW, McFarlane AC, Vermetten E, Lanius RA, Nievergelt CM, et al. Post-traumatic stress disorder. Nat Rev Dis Primers 2015, 1: 15057. [DOI] [PubMed] [Google Scholar]
- 31.Phelps EA, Hofmann SG. Memory editing from science fiction to clinical practice. Nature 2019, 572: 43–50. [DOI] [PubMed] [Google Scholar]
- 32.Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: Key to persistent attenuation of fear memories. Science 2009, 324: 951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 2010, 463: 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gräff J, Joseph NF, Horn ME, Samiei A, Meng J, Seo J, et al. Epigenetic priming of memory updating during reconsolidation to attenuate remote fear memories. Cell 2014, 156: 261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardwicke T, Taqi M, Shanks D. Postretrieval new learning does not reliably induce human memory updating via reconsolidation. Proc Natl Acad Sci U S A 2016, 13: 5206–5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chalkia A, Van Oudenhove L, Beckers T. Preventing the return of fear in humans using reconsolidation update mechanisms: A verification report of Schiller et al. (2010). Cortex 2020, 129: 510–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costanzi M, Cianfanelli B, Santirocchi A, Lasaponara S, Spataro P, Rossi-Arnaud C, et al. Forgetting unwanted memories: Active forgetting and implications for the development of psychological disorders. J Pers Med 2021, 11: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKenzie S, Eichenbaum H. Consolidation and reconsolidation: Two lives of memories? Neuron 2011, 71: 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agren T, Engman J, Frick A, Björkstrand J, Larsson EM, Furmark T, et al. Disruption of reconsolidation erases a fear memory trace in the human amygdala. Science 2012, 337: 1550–1552. [DOI] [PubMed] [Google Scholar]
- 40.Borgomaneri S, Battaglia S, Garofalo S, Tortora F, Avenanti A, di Pellegrino G. State-dependent TMS over prefrontal cortex disrupts fear-memory reconsolidation and prevents the return of fear. Curr Biol 2020, 30: 3672-3679.e4. [DOI] [PubMed] [Google Scholar]
- 41.Pavlov PI. Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Ann Neurosci 2010, 17: 136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang CH, Huang CC, Hsu KS. Generalization of fear inhibition by disrupting hippocampal protein synthesis-dependent reconsolidation process. Neuropsychopharmacology 2011, 36: 1992–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alvarez-Dieppa AC, Griffin K, Cavalier S, McIntyre CK. Vagus nerve stimulation enhances extinction of conditioned fear in rats and modulates arc protein, CaMKII, and GluN2B-containing NMDA receptors in the basolateral amygdala. Neural Plast 2016, 2016: 4273280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tasaka G, Ide Y, Tsukada M, Aihara T. Multimodal cortico-cortical associations induced by fear and sensory conditioning in the guinea pig. Cogn Neurodyn 2022, 16: 283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song C, Stevenson CW, Guimaraes FS, Lee JLC. Bidirectional effects of cannabidiol on contextual fear memory extinction. Front Pharmacol 2016, 7: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alfei JM, De Gruy H, De Bundel D, Luyten L, Beckers T. Apparent reconsolidation interference without generalized Amnesia. Prog Neuropsychopharmacol Biol Psychiatry 2021, 108: 110161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mugnaini M, Alfei JM, Bueno AM, Ferrer Monti RI, Urcelay GP. Fear memory modulation by incentive down and up-shifts. Behav Brain Res 2022, 422: 113766. [DOI] [PubMed] [Google Scholar]
- 48.Valiati FE, Vasconcelos M, Lichtenfels M, Petry FS, de Almeida RMM, Schwartsmann G, et al. Administration of a histone deacetylase inhibitor into the basolateral amygdala enhances memory consolidation, delays extinction, and increases hippocampal BDNF levels. Front Pharmacol 2017, 8: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ganon-Elazar E, Akirav I. Cannabinoid receptor activation in the basolateral amygdala blocks the effects of stress on the conditioning and extinction of inhibitory avoidance. J Neurosci 2009, 29: 11078–11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukushima H, Zhang Y, Archbold G, Ishikawa R, Nader K, Kida S. Enhancement of fear memory by retrieval through reconsolidation. Elife 2014, 3: e02736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilman TL, Dutta S, Adkins JM, Cecil CA, Jasnow AM. Basolateral amygdala Thy1-expressing neurons facilitate the inhibition of contextual fear during consolidation, reconsolidation, and extinction. Neurobiol Learn Mem 2018, 155: 498–507. [DOI] [PubMed] [Google Scholar]
- 52.Pereyra P, Saraco M, Maldonado H. Decreased response or alternative defensive strategies in escape: Two different types of long-term memory in the crab Chasmagnathus. J Comp Physiol A 1999, 184: 301–310. [Google Scholar]
- 53.Pynoos RS, Ritzmann RF, Steinberg AM, Goenjian A, Prisecaru I. A behavioral animal model of posttraumatic stress disorder featuring repeated exposure to situational reminders. Biol Psychiatry 1996, 39: 129–134. [DOI] [PubMed] [Google Scholar]
- 54.Martinho R, Seixas R, Azevedo M, Oliveira A, Serrão P, Moreira-Rodrigues M. Sotalol treatment may interfere with retrieval, expression, and/or reconsolidation processes thus disrupting traumatic memories in a post-traumatic stress disorder mice model. Front Pharmacol 2022, 12: 809271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burhans LB, Smith-Bell CA, Schreurs BG. Propranolol produces short-term facilitation of extinction in a rabbit model of post-traumatic stress disorder. Neuropharmacology 2018, 135: 386–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pinsker HM, Hening WA, Carew TJ, Kandel ER. Long-term sensitization of a defensive withdrawal reflex in Aplysia. Science 1973, 182: 1039–1042. [DOI] [PubMed] [Google Scholar]
- 57.Sekiguchi T, Yamada A, Suzuki H. Reactivation-dependent changes in memory states in the terrestrial slug Limax flavus. Learn Mem 1997, 4: 356–364. [DOI] [PubMed] [Google Scholar]
- 58.Pedreira ME, Maldonado H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron 2003, 38: 863–869. [DOI] [PubMed] [Google Scholar]
- 59.Eisenberg M, Dudai Y. Reconsolidation of fresh, remote, and extinguished fear memory in Medaka: Old fears don’t die. Eur J Neurosci 2004, 20: 3397–3403. [DOI] [PubMed] [Google Scholar]
- 60.Anokhin KV, Tiunova AA, Rose SPR. Reminder effects—reconsolidation or retrieval deficit? Pharmacological dissection with protein synthesis inhibitors following reminder for a passive-avoidance task in young chicks. Eur J Neurosci 2002, 15: 1759–1765. [DOI] [PubMed] [Google Scholar]
- 61.Jones BC, Bebus SE, Ferguson SM, Bateman P, Schoech S. The glucocorticoid response in a free-living bird predicts whether long-lasting memories fade or strengthen with time. Anim Behav 2016, 122: 157–168. [Google Scholar]
- 62.Merlo E, Milton AL, Everitt BJ. A novel retrieval-dependent memory process revealed by the arrest of ERK1/2 activation in the basolateral amygdala. J Neurosci 2018, 38: 3199–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Finnie PSB, Nader K. Amyloid beta secreted during consolidation prevents memory malleability. Curr Biol 2020, 30: 1934-1940.e4. [DOI] [PubMed] [Google Scholar]
- 64.Hagihara KM, Bukalo O, Zeller M, Aksoy-Aksel A, Karalis N, Limoges A, et al. Intercalated amygdala clusters orchestrate a switch in fear state. Nature 2021, 594: 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCullough KM, Chatzinakos C, Hartmann J, Missig G, Neve RL, Fenster RJ, et al. Genome-wide translational profiling of amygdala Crh-expressing neurons reveals role for CREB in fear extinction learning. Nat Commun 2020, 11: 5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grella SL, Fortin AH, Ruesch E, Bladon JH, Reynolds LF, Gross A, et al. Reactivating hippocampal-mediated memories during reconsolidation to disrupt fear. Nat Commun 2022, 13: 4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.da Silva TR, Sohn JMB, Andreatini R, Stern CA. The role of prelimbic and anterior cingulate cortices in fear memory reconsolidation and persistence depends on the memory age. Learn Mem 2020, 27: 292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hartley ND, Gaulden AD, Báldi R, Winters ND, Salimando GJ, Rosas-Vidal LE, et al. Dynamic remodeling of a basolateral-to-central amygdala glutamatergic circuit across fear states. Nat Neurosci 2019, 22: 2000–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Q, Zhu JJ, Wang L, Kan YP, Liu YM, Wu YJ, et al. Insular cortical circuits as an executive gateway to decipher threat or extinction memory via distinct subcortical pathways. Nat Commun 2022, 13: 5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Swiercz AP, Iyer L, Yu Z, Edwards A, Prashant NM, Nguyen BN, et al. Evaluation of an angiotensin Type 1 receptor blocker on the reconsolidation of fear memory. Transl Psychiatry 2020, 10: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fukushima H, Zhang Y, Kida S. Active transition of fear memory phase from reconsolidation to extinction through ERK-mediated prevention of reconsolidation. J Neurosci 2021, 41: 1288–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nader K. Memory traces unbound. Trends Neurosci 2003, 26: 65–72. [DOI] [PubMed] [Google Scholar]
- 73.Pedreira ME, Pérez-Cuesta LM, Maldonado H. Reactivation and reconsolidation of long-term memory in the crab Chasmagnathus: Protein synthesis requirement and mediation by NMDA-type glutamatergic receptors. J Neurosci 2002, 22: 8305–8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jin XC, Lu YF, Yang XF, Ma L, Li BM. Glucocorticoid receptors in the basolateral nucleus of amygdala are required for postreactivation reconsolidation of auditory fear memory. Eur J Neurosci 2007, 25: 3702–3712. [DOI] [PubMed] [Google Scholar]
- 75.Rehberg K, Bergado-Acosta JR, Koch JC, Stork O. Disruption of fear memory consolidation and reconsolidation by actin filament arrest in the basolateral amygdala. Neurobiol Learn Mem 2010, 94: 117–126. [DOI] [PubMed] [Google Scholar]
- 76.Bredy TW, Barad M. The histone deacetylase inhibitor valproic acid enhances acquisition, extinction, and reconsolidation of conditioned fear. Learn Mem 2008, 15: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maddox SA, Schafe GE. Epigenetic alterations in the lateral amygdala are required for reconsolidation of a Pavlovian fear memory. Learn Mem 2011, 18: 579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwapis JL, Jarome TJ, Ferrara NC, Helmstetter FJ. Updating procedures can reorganize the neural circuit supporting a fear memory. Neuropsychopharmacology 2017, 42: 1688–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang T, Yu K, Zhang X, Xiao X, Chen X, Fu Y, et al. Plastic and stimulus-specific coding of salient events in the central amygdala. Nature 2023, 616: 510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim J, Pignatelli M, Xu S, Itohara S, Tonegawa S. Antagonistic negative and positive neurons of the basolateral amygdala. Nat Neurosci 2016, 19: 1636–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu J, Totty MS, Melissari L, Bayer H, Maren S. Convergent coding of recent and remote fear memory in the basolateral amygdala. Biol Psychiatry 2022, 91: 832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haubrich J, Bernabo M, Nader K. Noradrenergic projections from the locus coeruleus to the amygdala constrain fear memory reconsolidation. Elife 2020, 9: e57010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang J, Tambini A, Lapate RC. The Tie that binds: Temporal coding and adaptive emotion. Trends Cogn Sci 2022, 26: 1103–1118. [DOI] [PubMed] [Google Scholar]
- 84.Nyberg N, Duvelle É, Barry C, Spiers HJ. Spatial goal coding in the hippocampal formation. Neuron 2022, 110: 394–422. [DOI] [PubMed] [Google Scholar]
- 85.Yonelinas AP, Ranganath C, Ekstrom AD, Wiltgen BJ. A contextual binding theory of episodic memory: Systems consolidation reconsidered. Nat Rev Neurosci 2019, 20: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zinn R, Leake J, Krasne FB, Corbit LH, Fanselow MS, Vissel B. Maladaptive properties of context-impoverished memories. Curr Biol 2020, 30: 2300-2311.e6. [DOI] [PubMed] [Google Scholar]
- 87.Lux V, Masseck OA, Herlitze S, Sauvage MM. Optogenetic destabilization of the memory trace in CA1: Insights into reconsolidation and retrieval processes. Cereb Cortex 2017, 27: 841–851. [DOI] [PubMed] [Google Scholar]
- 88.Ye X, Kapeller-Libermann D, Travaglia A, Carmen Inda M, Alberini CM. Direct dorsal hippocampal-prelimbic cortex connections strengthen fear memories. Nat Neurosci 2017, 20: 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de la Fuente V, Medina C, Falasco G, Urrutia L, Kravitz AV, Urbano FJ, et al. The lateral neocortex is critical for contextual fear memory reconsolidation. Sci Rep 2019, 9: 12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: Selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci 2001, 21: 2186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hall J, Thomas KL, Everitt BJ. Fear memory retrieval induces CREB phosphorylation and Fos expression within the amygdala. Eur J Neurosci 2001, 13: 1453–1458. [DOI] [PubMed] [Google Scholar]
- 92.Thomas KL, Hall J, Everitt BJ. Cellular imaging with zif268 expression in the rat nucleus accumbens and frontal cortex further dissociates the neural pathways activated following the retrieval of contextual and cued fear memory. Eur J Neurosci 2002, 16: 1789–1796. [DOI] [PubMed] [Google Scholar]
- 93.Mamiya N, Fukushima H, Suzuki A, Matsuyama Z, Homma S, Frankland PW, et al. Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J Neurosci 2009, 29: 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.von Hertzen LSJ, Giese KP. Memory reconsolidation engages only a subset of immediate-early genes induced during consolidation. J Neurosci 2005, 25: 1935–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jarome TJ, Ferrara NC, Kwapis JL, Helmstetter FJ. CaMKII regulates proteasome phosphorylation and activity and promotes memory destabilization following retrieval. Neurobiol Learn Mem 2016, 128: 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang B, Zhu H, Zhou Y, Liu X, Ma L. Unconditioned- and conditioned- stimuli induce differential memory reconsolidation and β-AR-dependent CREB activation. Front Neural Circuits 2017, 11: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bellfy L, Kwapis JL. Molecular mechanisms of reconsolidation-dependent memory updating. Int J Mol Sci 2020, 21: 6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Forcato C, Bavassi L, De Pino G, Fernández RS, Villarreal MF, Pedreira ME. Differential left hippocampal activation during retrieval with different types of reminders: An fMRI study of the reconsolidation process. PLoS One 2016, 11: e0151381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mahabir M, Tucholka A, Shin LM, Etienne P, Brunet A. Emotional face processing in post-traumatic stress disorder after reconsolidation impairment using propranolol: A pilot fMRI study. J Anxiety Disord 2015, 36: 127–133. [DOI] [PubMed] [Google Scholar]
- 100.Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron 2002, 36: 567–584. [DOI] [PubMed] [Google Scholar]
- 101.Pedreira ME, Pérez-Cuesta LM, Maldonado H. Mismatch between what is expected and what actually occurs triggers memory reconsolidation or extinction. Learn Mem 2004, 11: 579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.González-Salinas S, Medina AC, Marín-Vignando V, Ruiz-López CX, Quirarte GL, Prado-Alcalá RA. Protein synthesis is not required for acquisition, consolidation, and extinction of high foot-shock active avoidance training. Behav Brain Res 2015, 287: 8–14. [DOI] [PubMed] [Google Scholar]
- 103.Santini E, Ge H, Ren K, Peña de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci 2004, 24: 5704–5710. [DOI] [PMC free article] [PubMed]
- 104.Popik B, Amorim FE, Amaral OB, De Oliveira Alvares L. Shifting from fear to safety through deconditioning-update. Elife 2020, 9: e51207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dunsmoor JE, Niv Y, Daw N, Phelps EA. Rethinking extinction. Neuron 2015, 88: 47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bouton ME, Maren S, McNally GP. Behavioral and neurobiological mechanisms of Pavlovian and instrumental extinction learning. Physiol Rev 2021, 101: 611–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Szeska C, Pünjer H, Riemann S, Meinzer M, Hamm AO. Stimulation of the ventromedial prefrontal cortex blocks the return of subcortically mediated fear responses. Transl Psychiatry 2022, 12: 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shih CC, Chang YH, Chiou RJ, Chang CH. Analysis of lateral orbitofrontal cortex activation on acquisition of fear extinction and neuronal activities in fear circuit. Brain Struct Funct 2022, 227: 2529–2541. [DOI] [PubMed] [Google Scholar]
- 109.Laricchiuta D, Sciamanna G, Gimenez J, Termine A, Fabrizio C, Caioli S, et al. Optogenetic stimulation of prelimbic pyramidal neurons maintains fear memories and modulates amygdala pyramidal neuron transcriptome. Int J Mol Sci 2021, 22: 810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bukalo O, Nonaka M, Weinholtz CA, Mendez A, Taylor WW, Holmes A. Effects of optogenetic photoexcitation of infralimbic cortex inputs to the basolateral amygdala on conditioned fear and extinction. Behav Brain Res 2021, 396: 112913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marek R, Xu L, Sullivan RKP, Sah P. Excitatory connections between the prelimbic and infralimbic medial prefrontal cortex show a role for the prelimbic cortex in fear extinction. Nat Neurosci 2018, 21: 654–658. [DOI] [PubMed] [Google Scholar]
- 112.Silva BA, Astori S, Burns AM, Heiser H, van den Heuvel L, Santoni G, et al. A thalamo-amygdalar circuit underlying the extinction of remote fear memories. Nat Neurosci 2021, 24: 964–974. [DOI] [PubMed] [Google Scholar]
- 113.Jasnow AM, Ehrlich DE, Choi DC, Dabrowska J, Bowers ME, McCullough KM, et al. Thy1-expressing neurons in the basolateral amygdala may mediate fear inhibition. J Neurosci 2013, 33: 10396–10404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Klavir O, Prigge M, Sarel A, Paz R, Yizhar O. Manipulating fear associations via optogenetic modulation of amygdala inputs to prefrontal cortex. Nat Neurosci 2017, 20: 836–844. [DOI] [PubMed] [Google Scholar]
- 115.Gu X, Wu YJ, Zhang Z, Zhu JJ, Wu XR, Wang Q, et al. Dynamic tripartite construct of interregional engram circuits underlies forgetting of extinction memory. Mol Psychiatry 2022, 27: 4077–4091. [DOI] [PubMed] [Google Scholar]
- 116.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 2008, 33: 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marek R, Jin J, Goode TD, Giustino TF, Wang Q, Acca GM, et al. Hippocampus-driven feed-forward inhibition of the prefrontal cortex mediates relapse of extinguished fear. Nat Neurosci 2018, 21: 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qin C, Bian XL, Wu HY, Xian JY, Cai CY, Lin YH, et al. Dorsal hippocampus to infralimbic cortex circuit is essential for the recall of extinction memory. Cereb Cortex 2021, 31: 1707–1718. [DOI] [PubMed] [Google Scholar]
- 119.Nguyen R, Koukoutselos K, Forro T, Ciocchi S. Fear extinction relies on ventral hippocampal safety codes shaped by the amygdala. Sci Adv 2023, 9: eadg4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cahill EN, Milton AL. Neurochemical and molecular mechanisms underlying the retrieval-extinction effect. Psychopharmacology (Berl) 2019, 236: 111–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry 2007, 12: 120–150. [DOI] [PubMed] [Google Scholar]
- 122.Wen Z, Chen ZS, Milad MR. Fear extinction learning modulates large-scale brain connectivity. Neuroimage 2021, 238: 118261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron 1998, 20: 937–945. [DOI] [PubMed] [Google Scholar]
- 124.Dunsmoor JE, Kroes MCW, Li J, Daw ND, Simpson HB, Phelps EA. Role of human ventromedial prefrontal cortex in learning and recall of enhanced extinction. J Neurosci 2019, 39: 3264–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chang DI, Lissek S, Ernst TM, Thürling M, Uengoer M, Tegenthoff M, et al. Cerebellar contribution to context processing in extinction learning and recall. Cerebellum 2015, 14: 670–676. [DOI] [PubMed] [Google Scholar]
- 126.Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cogn Affect Behav Neurosci 2004, 4: 317–325. [DOI] [PubMed] [Google Scholar]
- 127.Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron 2008, 59: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Feng P, Zheng Y, Feng T. Spontaneous brain activity following fear reminder of fear conditioning by using resting-state functional MRI. Sci Rep 2015, 5: 16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Suarez-Jimenez B, Albajes-Eizagirre A, Lazarov A, Zhu X, Harrison BJ, Radua J, et al. Neural signatures of conditioning, extinction learning, and extinction recall in posttraumatic stress disorder: A meta-analysis of functional magnetic resonance imaging studies. Psychol Med 2020, 50: 1442–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry 2015, 77: 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Feng P, Zheng Y, Feng T. Resting-state functional connectivity between amygdala and the ventromedial prefrontal cortex following fear reminder predicts fear extinction. Soc Cogn Affect Neurosci 2016, 11: 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Faul L, Stjepanović D, Stivers JM, Stewart GW, Graner JL, Morey RA, et al. Proximal threats promote enhanced acquisition and persistence of reactive fear-learning circuits. Proc Natl Acad Sci USA 2020, 117: 16678–16689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 2007, 62: 446–454. [DOI] [PubMed] [Google Scholar]
- 134.Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry 2007, 62: 1191–1194. [DOI] [PubMed] [Google Scholar]
- 135.Miedl SF, Rattel JA, Franke LK, Blechert J, Kronbichler M, Spoormaker VI, et al. Neural processing during fear extinction predicts intrusive memories. Biol Psychiatry Cogn Neurosci Neuroimaging 2020, 5: 403–411. [DOI] [PubMed] [Google Scholar]
- 136.Rougemont-Bücking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, et al. Altered processing of contextual information during fear extinction in PTSD: An fMRI study. CNS Neurosci Ther 2011, 17: 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li J, Chen W, Caoyang J, Hu Y, Yang Y, Xu L, et al. Role of prediction error in destabilizing fear memories in retrieval extinction and its neural mechanisms. Cortex 2019, 121: 292–307. [DOI] [PubMed] [Google Scholar]
- 138.Apergis-Schoute AM, Schiller D, LeDoux JE, Phelps EA. Extinction resistant changes in the human auditory association cortex following threat learning. Neurobiol Learn Mem 2014, 113: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Finger EC, Mitchell DGV, Jones M, Blair RR. Dissociable roles of medial orbitofrontal cortex in human operant extinction learning. Neuroimage 2008, 43: 748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron 2002, 36: 527–538. [DOI] [PubMed] [Google Scholar]
- 141.Vianna MR, Szapiro G, McGaugh JL, Medina JH, Izquierdo I. Retrieval of memory for fear-motivated training initiates extinction requiring protein synthesis in the rat hippocampus. Proc Natl Acad Sci USA 2001, 98: 12251–12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Berman DE, Dudai Y. Memory extinction, learning anew, and learning the new: Dissociations in the molecular machinery of learning in cortex. Science 2001, 291: 2417–2419. [DOI] [PubMed] [Google Scholar]
- 143.Pérez-Cuesta LM, Hepp Y, Pedreira ME, Maldonado H. Memory is not extinguished along with CS presentation but within a few seconds after CS-offset. Learn Mem 2007, 14: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Duvarci S, Mamou CB, Nader K. Extinction is not a sufficient condition to prevent fear memories from undergoing reconsolidation in the basolateral amygdala. Eur J Neurosci 2006, 24: 249–260. [DOI] [PubMed] [Google Scholar]
- 145.Pérez-Cuesta LM, Maldonado H. Memory reconsolidation and extinction in the crab: Mutual exclusion or coexistence? Learn Mem 2009, 16: 714–721. [DOI] [PubMed] [Google Scholar]
- 146.Duvarci S, Nader K. Characterization of fear memory reconsolidation. J Neurosci 2004, 24: 9269–9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci 2004, 24: 4787–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Merlo E, Milton AL, Goozée ZY, Theobald DE, Everitt BJ. Reconsolidation and extinction are dissociable and mutually exclusive processes: Behavioral and molecular evidence. J Neurosci 2014, 34: 2422–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Medina JH, Viola H. ERK1/2: A key cellular component for the formation, retrieval, reconsolidation and persistence of memory. Front Mol Neurosci 2018, 11: 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature 2008, 454: 600–606. [DOI] [PubMed] [Google Scholar]
- 151.Semon RW (1920) Die Mneme als erhaltendes Prinzip im Wechsel des organischen Geschehens. Engelmann, Leipzig. [Google Scholar]
- 152.Josselyn SA, Tonegawa S. Memory engrams: Recalling the past and imagining the future. Science 2020, 367: eaaw4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tonegawa S, Morrissey MD, Kitamura T. The role of engram cells in the systems consolidation of memory. Nat Rev Neurosci 2018, 19: 485–498. [DOI] [PubMed] [Google Scholar]
- 154.Ryan TJ, Roy DS, Pignatelli M, Arons A, Tonegawa S. Engram cells retain memory under retrograde Amnesia. Science 2015, 348: 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ressler RL, Goode TD, Kim S, Ramanathan KR, Maren S. Covert capture and attenuation of a hippocampus-dependent fear memory. Nat Neurosci 2021, 24: 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Luft JG, Popik B, Gonçalves DA, Cruz FC, de Oliveira Alvares L. Distinct engrams control fear and extinction memory. Hippocampus 2024, 34: 230–240. [DOI] [PubMed] [Google Scholar]
- 157.Khalaf O, Resch S, Dixsaut L, Gorden V, Glauser L, Gräff J. Reactivation of recall-induced neurons contributes to remote fear memory attenuation. Science 2018, 360: 1239–1242. [DOI] [PubMed] [Google Scholar]
- 158.Ramanathan KR, Jin J, Giustino TF, Payne MR, Maren S. Prefrontal projections to the thalamic nucleus reuniens mediate fear extinction. Nat Commun 2018, 9: 4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lacagnina AF, Brockway ET, Crovetti CR, Shue F, McCarty MJ, Sattler KP, et al. Distinct hippocampal engrams control extinction and relapse of fear memory. Nat Neurosci 2019, 22: 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang X, Kim J, Tonegawa S. Amygdala reward neurons form and store fear extinction memory. Neuron 2020, 105: 1077-1093.e7. [DOI] [PubMed] [Google Scholar]
- 161.Teng SW, Wang XR, Du BW, Chen XL, Fu GZ, Liu YF, et al. Altered fear engram encoding underlying conditioned versus unconditioned stimulus-initiated memory updating. Sci Adv 2023, 9: eadf0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Redondo RL, Kim J, Arons AL, Ramirez S, Liu X, Tonegawa S. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 2014, 513: 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Morrison DJ, Rashid AJ, Yiu AP, Yan C, Frankland PW, Josselyn SA. Parvalbumin interneurons constrain the size of the lateral amygdala engram. Neurobiol Learn Mem 2016, 135: 91–99. [DOI] [PubMed] [Google Scholar]
- 164.Stefanelli T, Bertollini C, Lüscher C, Muller D, Mendez P. Hippocampal somatostatin interneurons control the size of neuronal memory ensembles. Neuron 2016, 89: 1074–1085. [DOI] [PubMed] [Google Scholar]
- 165.Rashid AJ, Yan C, Mercaldo V, Hsiang HL, Park S, Cole CJ, et al. Competition between engrams influences fear memory formation and recall. Science 2016, 353: 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Abdou K, Shehata M, Choko K, Nishizono H, Matsuo M, Muramatsu SI, et al. Synapse-specific representation of the identity of overlapping memory engrams. Science 2018, 360: 1227–1231. [DOI] [PubMed] [Google Scholar]
- 167.Björkstrand J, Agren T, Åhs F, Frick A, Larsson EM, Hjorth O, et al. Disrupting reconsolidation attenuates long-term fear memory in the human amygdala and facilitates approach behavior. Curr Biol 2016, 26: 2690–2695. [DOI] [PubMed] [Google Scholar]
- 168.Chen W, Li J, Zhang X, Dong Y, Shi P, Luo P, et al. Retrieval-extinction as a reconsolidation-based treatment for emotional disorders: Evidence from an extinction retention test shortly after intervention. Behav Res Ther 2021, 139: 103831. [DOI] [PubMed] [Google Scholar]
- 169.Fricchione J, Greenberg MS, Spring J, Wood N, Mueller-Pfeiffer C, Milad MR, et al. Delayed extinction fails to reduce skin conductance reactivity to fear-conditioned stimuli. Psychophysiology 2016, 53: 1343–1351. [DOI] [PubMed] [Google Scholar]
- 170.Alexandra Kredlow M, Orr SP, Otto MW. Exploring the boundaries of post-retrieval extinction in healthy and anxious individuals. Behav Res Ther 2018, 108: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sturt J, Rogers R, Armour C, Cameron D, De Rijk L, Fiorentino F, et al. Reconsolidation of traumatic memories protocol compared to trauma-focussed cognitive behaviour therapy for post-traumatic stress disorder in UK military veterans: A randomised controlled feasibility trial. Pilot Feasibility Stud 2023, 9: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gray R, Budden-Potts D, Bourke F. Reconsolidation of traumatic memories for PTSD: A randomized controlled trial of 74 male veterans. Psychother Res 2019, 29: 621–639. [DOI] [PubMed] [Google Scholar]
- 173.Tylee DS, Gray R, Glatt SJ, Bourke F. Evaluation of the reconsolidation of traumatic memories protocol for the treatment of PTSD: A randomized, wait-list-controlled trial. J Mil Veteran Fam Health 2017, 3: 21–33. [Google Scholar]
- 174.Chen W, Li J, Xu L, Zhao S, Fan M, Zheng X. Destabilizing different strengths of fear memories requires different degrees of prediction error during retrieval. Front Behav Neurosci 2021, 14: 598924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sevenster D, Beckers T, Kindt M. Prediction error governs pharmacologically induced Amnesia for learned fear. Science 2013, 339: 830–833. [DOI] [PubMed] [Google Scholar]
- 176.Yang Y, Jie J, Li J, Chen W, Zheng X. A novel method to trigger the reconsolidation of fear memory. Behav Res Ther 2019, 122: 103461. [DOI] [PubMed] [Google Scholar]
- 177.Auchter A, Cormack LK, Niv Y, Gonzalez-Lima F, Monfils MH. Reconsolidation-extinction interactions in fear memory attenuation: The role of inter-trial interval variability. Front Behav Neurosci 2017, 11: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Golkar A, Tjaden C, Kindt M. Vicarious extinction learning during reconsolidation neutralizes fear memory. Behav Res Ther 2017, 92: 87–93. [DOI] [PubMed] [Google Scholar]
- 179.Liu J, Zhao L, Xue Y, Shi J, Suo L, Luo Y, et al. An unconditioned stimulus retrieval extinction procedure to prevent the return of fear memory. Biol Psychiatry 2014, 76: 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Steinfurth ECK, Kanen JW, Raio CM, Clem RL, Huganir RL, Phelps EA. Young and old Pavlovian fear memories can be modified with extinction training during reconsolidation in humans. Learn Mem 2014, 21: 338–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lancaster CL, Monfils MH, Telch MJ. Augmenting exposure therapy with pre-extinction fear memory reactivation and deepened extinction: A randomized controlled trial. Behav Res Ther 2020, 135: 103730. [DOI] [PubMed] [Google Scholar]
- 182.Gray RM, Budden-Potts D, Schwall RJ, Bourke FF. An open-label, randomized controlled trial of the reconsolidation of traumatic memories protocol (RTM) in military women. Psychol Trauma 2021, 13: 641–651. [DOI] [PubMed] [Google Scholar]
- 183.Witteveen AB, Young SY, Cuijpers P, Ayuso-Mateos JL, Barbui C, Bertolini F, et al. COVID-19 and common mental health symptoms in the early phase of the pandemic: An umbrella review of the evidence. PLoS Med 2023, 20: e1004206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Stemerding LE, Stibbe D, van Ast VA, Kindt M. Demarcating the boundary conditions of memory reconsolidation: An unsuccessful replication. Sci Rep 2022, 12: 2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Refaeli R, Kreisel T, Groysman M, Adamsky A, Goshen I. Engram stability and maturation during systems consolidation. Curr Biol 2023, 33: 3942-3950.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gulmez Karaca K, Kupke J, Brito DVC, Zeuch B, Thome C, Weichenhan D, et al. Neuronal ensemble-specific DNA methylation strengthens engram stability. Nat Commun 2020, 11: 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vahia VN. Diagnostic and statistical manual of mental disorders 5: A quick glance. Indian J Psychiatry 2013, 55: 220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bisson JI, van Deursen R, Hannigan B, Kitchiner N, Barawi K, Jones K, et al. Randomized controlled trial of multi-modular motion-assisted memory desensitization and reconsolidation (3MDR) for male military veterans with treatment-resistant post-traumatic stress disorder. Acta Psychiatr Scand 2020, 142: 141–151. [DOI] [PubMed] [Google Scholar]
- 189.Santiago RMM, Tort ABL. On the boundary conditions of avoidance memory reconsolidation: An attractor network perspective. Neural Netw 2020, 127: 96–109. [DOI] [PubMed] [Google Scholar]
- 190.Bonsall MB, Holmes EA. Temporal dynamics of trauma memory persistence. J R Soc Interface 2023, 20: 20230108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hao S, Zhu X, Huang Z, Yang Q, Liu H, Wu Y, et al. Cross-species single-cell spatial transcriptomic atlases of the cerebellar cortex. Science 2024, 385: eado3927. [DOI] [PubMed] [Google Scholar]
- 192.He X, Calhoun VD, Du Y. SMART (splitting-merging assisted reliable) independent component analysis for extracting accurate brain functional networks. Neurosci Bull 2024, 40: 905–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Debiec J, Díaz-Mataix L, Bush DEA, Doyère V, Ledoux JE. The amygdala encodes specific sensory features of an aversive reinforcer. Nat Neurosci 2010, 13: 536–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bal NV, Rysakova MP, Vinarskaya AK, Ivanova V, Zuzina AB, Balaban PM. Cued memory reconsolidation in rats requires nitric oxide. Eur J Neurosci 2017, 45: 643–647. [DOI] [PubMed] [Google Scholar]
- 195.Einarsson EÖ, Nader K. Involvement of the anterior cingulate cortex in formation, consolidation, and reconsolidation of recent and remote contextual fear memory. Learn Mem 2012, 19: 449–452. [DOI] [PubMed] [Google Scholar]
- 196.Duvarci S, Nader K, LeDoux JE. Activation of extracellular signal-regulated kinase- mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur J Neurosci 2005, 21: 283–289. [DOI] [PubMed] [Google Scholar]
- 197.Debiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience 2004, 129: 267–272. [DOI] [PubMed] [Google Scholar]
- 198.Lee JLC, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: Inhibition and potentiation. J Neurosci 2006, 26: 10051–10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nikzad S, Vafaei AA, Rashidy-Pour A, Haghighi S. Systemic and intrahippocampal administrations of the glucocorticoid receptor antagonist RU38486 impairs fear memory reconsolidation in rats. Stress 2011, 14: 459–464. [DOI] [PubMed] [Google Scholar]
- 200.Hong I, Kim J, Kim J, Lee S, Ko HG, Nader K, et al. AMPA receptor exchange underlies transient memory destabilization on retrieval. Proc Natl Acad Sci U S A 2013, 110: 8218–8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cestari V, Costanzi M, Castellano C, Rossi-Arnaud C. A role for ERK2 in reconsolidation of fear memories in mice. Neurobiol Learn Mem 2006, 86: 133–143. [DOI] [PubMed] [Google Scholar]
- 202.Tronson NC, Wiseman SL, Olausson P, Taylor JR. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci 2006, 9: 167–169. [DOI] [PubMed] [Google Scholar]
- 203.Mac Callum PE, Hebert M, Adamec RE, Blundell J. Systemic inhibition of mTOR kinase via rapamycin disrupts consolidation and reconsolidation of auditory fear memory. Neurobiol Learn Mem 2014, 112: 176–185. [DOI] [PubMed] [Google Scholar]
- 204.Si J, Yang J, Xue L, Yang C, Luo Y, Shi H, et al. Activation of NF-κB in basolateral amygdala is required for memory reconsolidation in auditory fear conditioning. PLoS One 2012, 7: e43973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wu P, Ding ZB, Meng SQ, Shen HW, Sun SC, Luo YX, et al. Differential role of Rac in the basolateral amygdala and Cornu ammonis 1 in the reconsolidation of auditory and contextual Pavlovian fear memory in rats. Psychopharmacology (Berl) 2014, 231: 2909–2919. [DOI] [PubMed] [Google Scholar]
- 206.Duvarci S, Nader K, LeDoux JE. De novo mRNA synthesis is required for both consolidation and reconsolidation of fear memories in the amygdala. Learn Mem 2008, 15: 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Maddox SA, Schafe GE. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for reconsolidation of a Pavlovian fear memory. J Neurosci 2011, 31: 7073–7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Frankland PW, Ding HK, Takahashi E, Suzuki A, Kida S, Silva AJ. Stability of recent and remote contextual fear memory. Learn Mem 2006, 13: 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Schmidt SD, Furini CG, Zinn CG, Cavalcante LE, Ferreira FF, Behling JK, et al. Modulation of the consolidation and reconsolidation of fear memory by three different serotonin receptors in hippocampus. Neurobiol Learn Mem 2017, 142: 48–54. [DOI] [PubMed] [Google Scholar]
- 210.Parsons RG, Gafford GM, Baruch DE, Riedner BA, Helmstetter FJ. Long-term stability of fear memory depends on the synthesis of protein but not mRNA in the amygdala. Eur J Neurosci 2006, 23: 1853–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Saitoh A, Akagi K, Oka JI, Yamada M. Post-reexposure administration of D-cycloserine facilitates reconsolidation of contextual conditioned fear memory in rats. J Neural Transm (Vienna) 2017, 124: 583–587. [DOI] [PubMed] [Google Scholar]
- 212.Charlier Y, Tirelli E. Differential effects of histamine H3 receptor inverse agonist thioperamide, given alone or in combination with the N-methyl-d-aspartate receptor antagonist dizocilpine, on reconsolidation and consolidation of a contextual fear memory in mice. Neuroscience 2011, 193: 132–142. [DOI] [PubMed] [Google Scholar]
- 213.Lee JLC, Hynds RE. Divergent cellular pathways of hippocampal memory consolidation and reconsolidation. Hippocampus 2013, 23: 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yamada D, Zushida K, Wada K, Sekiguchi M. Pharmacological discrimination of extinction and reconsolidation of contextual fear memory by a potentiator of AMPA receptors. Neuropsychopharmacology 2009, 34: 2574–2584. [DOI] [PubMed] [Google Scholar]
- 215.Troyner F, Bertoglio LJ. Nucleus reuniens of the thalamus controls fear memory reconsolidation. Neurobiol Learn Mem 2021, 177: 107343. [DOI] [PubMed] [Google Scholar]
- 216.Bucherelli C, Baldi E, Mariottini C, Passani MB, Blandina P. Aversive memory reactivation engages in the amygdala only some neurotransmitters involved in consolidation. Learn Mem 2006, 13: 426–430. [DOI] [PubMed] [Google Scholar]
- 217.de Oliveira Alvares L, Pasqualini Genro B, Diehl F, Molina VA, Quillfeldt JA. Opposite action of hippocampal CB1 receptors in memory reconsolidation and extinction. Neuroscience 2008, 154: 1648–1655. [DOI] [PubMed] [Google Scholar]
- 218.Bustos SG, Maldonado H, Molina VA. Midazolam disrupts fear memory reconsolidation. Neuroscience 2006, 139: 831–842. [DOI] [PubMed] [Google Scholar]
- 219.Murkar A, Kent P, Cayer C, James J, Merali Z. Gastrin-releasing peptide attenuates fear memory reconsolidation. Behav Brain Res 2018, 347: 255–262. [DOI] [PubMed] [Google Scholar]
- 220.Brabant C, Charlier Y, Tirelli E. The histamine H-receptor inverse agonist pitolisant improves fear memory in mice. Behav Brain Res 2013, 243: 199–204. [DOI] [PubMed] [Google Scholar]
- 221.Lubin FD, David Sweatt J. The IkappaB kinase regulates chromatin structure during reconsolidation of conditioned fear memories. Neuron 2007, 55: 942–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Barnes P, Kirtley A, Thomas KL. Quantitatively and qualitatively different cellular processes are engaged in CA1 during the consolidation and reconsolidation of contextual fear memory. Hippocampus 2012, 22: 149–171. [DOI] [PubMed] [Google Scholar]
- 223.Rao-Ruiz P, Rotaru DC, van der Loo RJ, Mansvelder HD, Stiedl O, Smit AB, et al. Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nat Neurosci 2011, 14: 1302–1308. [DOI] [PubMed] [Google Scholar]
- 224.da Silva TR, Raymundi AM, Bertoglio LJ, Andreatini R, Stern CA. Role of prelimbic cortex PKC and PKMζ in fear memory reconsolidation and persistence following reactivation. Sci Rep 2020, 10: 4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Blundell J, Kouser M, Powell CM. Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol Learn Mem 2008, 90: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Baldi E, Mariottini C, Bucherelli C. Differential roles of the basolateral amygdala and nucleus basalis magnocellularis during post-reactivation contextual fear conditioning reconsolidation in rats. Neurobiol Learn Mem 2008, 90: 604–609. [DOI] [PubMed] [Google Scholar]
- 227.Baldi E, Bucherelli C. Entorhinal cortex contribution to contextual fear conditioning extinction and reconsolidation in rats. Neurobiol Learn Mem 2014, 110: 64–71. [DOI] [PubMed] [Google Scholar]
- 228.Popik B, Crestani AP, Silva MO, Quillfeldt JA, de Oliveira Alvares L. Calpain modulates fear memory consolidation, retrieval and reconsolidation in the hippocampus. Neurobiol Learn Mem 2018, 151: 53–58. [DOI] [PubMed] [Google Scholar]
- 229.Nagayoshi T, Isoda K, Mamiya N, Kida S. Hippocampal calpain is required for the consolidation and reconsolidation but not extinction of contextual fear memory. Mol Brain 2017, 10: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Levin N, Kritman M, Maroun M, Akirav I. Differential roles of the infralimbic and prelimbic areas of the prefrontal cortex in reconsolidation of a traumatic memory. Eur Neuropsychopharmacol 2017, 27: 900–912. [DOI] [PubMed] [Google Scholar]
- 231.Bavassi L, Forcato C, Fernández RS, De Pino G, Pedreira ME, Villarreal MF. Retrieval of retrained and reconsolidated memories are associated with a distinct neural network. Sci Rep 2019, 9: 784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Shen F, Chen X, Li J, Cao W, Ku Y, Wu J, et al. Mnemonic vulnerability induced by post-activation time-dependent new-learning. Neurobiol Learn Mem 2019, 164: 107047. [DOI] [PubMed] [Google Scholar]
- 233.Speer ME, Ibrahim S, Schiller D, Delgado MR. Finding positive meaning in memories of negative events adaptively updates memory. Nat Commun 2021, 12: 6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kawade HM, Awathale SN, Subhedar NK, Kokare DM. Neuropeptide S facilitates extinction of fear via modulation of mesolimbic dopaminergic circuitry. Neuropharmacology 2022, 221: 109274. [DOI] [PubMed] [Google Scholar]
- 235.Luo R, Uematsu A, Weitemier A, Aquili L, Koivumaa J, McHugh TJ, et al. A dopaminergic switch for fear to safety transitions. Nat Commun 2018, 9: 2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sengupta A, Holmes A. A discrete dorsal raphe to basal amygdala 5-HT circuit calibrates aversive memory. Neuron 2019, 103: 489-505.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Frontera JL, Baba Aissa H, Sala RW, Mailhes-Hamon C, Georgescu IA, Léna C, et al. Bidirectional control of fear memories by cerebellar neurons projecting to the ventrolateral periaqueductal grey. Nat Commun 2020, 11: 5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Baek J, Lee S, Cho T, Kim SW, Kim M, Yoon Y, et al. Neural circuits underlying a psychotherapeutic regimen for fear disorders. Nature 2019, 566: 339–343. [DOI] [PubMed] [Google Scholar]
- 239.Lee JH, Latchoumane CV, Park J, Kim J, Jeong J, Lee KH, et al. The rostroventral part of the thalamic reticular nucleus modulates fear extinction. Nat Commun 2019, 10: 4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tao Y, Cai CY, Xian JY, Kou XL, Lin YH, Qin C, et al. Projections from infralimbic cortex to paraventricular thalamus mediate fear extinction retrieval. Neurosci Bull 2021, 37: 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rodriguez-Romaguera J, Sotres-Bayon F, Mueller D, Quirk GJ. Systemic propranolol acts centrally to reduce conditioned fear in rats without impairing extinction. Biol Psychiatry 2009, 65: 887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ren Y, Liu Y, Zheng S, Luo M. KCTD8 and KCTD12 facilitate axonal expression of GABAB receptors in habenula cholinergic neurons. J Neurosci 2022, 42: 1648–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Flavell CR, Barber DJ, Lee JLC. Behavioural memory reconsolidation of food and fear memories. Nat Commun 2011, 2: 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Baldi E, Bucherelli C. Substantia nigra, nucleus basalis magnocellularis and basolateral amygdala roles in extinction of contextual fear conditioning in the rat. Neurobiol Learn Mem 2010, 94: 199–205. [DOI] [PubMed] [Google Scholar]
- 245.Schmidt do Prado-Lima PA, Perrenoud MF, Kristensen CH, Cammarota M, Izquierdo I. Topiramate diminishes fear memory consolidation and extinguishes conditioned fear in rats. J Psychiatry Neurosci 2011, 36: 250–255. [DOI] [PMC free article] [PubMed]
- 246.Marusak HA, Hehr A, Bhogal A, Peters C, Iadipaolo A, Rabinak CA. Alterations in fear extinction neural circuitry and fear-related behavior linked to trauma exposure in children. Behav Brain Res 2021, 398: 112958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Utz A, Thürling M, Ernst TM, Hermann A, Stark R, Wolf OT, et al. Cerebellar vermis contributes to the extinction of conditioned fear. Neurosci Lett 2015, 604: 173–177. [DOI] [PubMed] [Google Scholar]
- 248.Walter KH, Michael Hunt W, Otis NP, Kline AC, Miggantz EL, Thomsen CJ, et al. Comparison of behavioral activation-enhanced cognitive processing therapy and cognitive processing therapy among U.S. service members: A randomized clinical trial. Psychiatry Res 2023, 326: 115330. [DOI] [PubMed] [Google Scholar]
- 249.Steil R, Weiss J, Renneberg B, Gutermann J, Rosner R. Effect of therapeutic competence, adherence, and alliance on treatment outcome in youth with PTSD treated with developmentally adapted cognitive processing therapy. Child Abuse Negl 2023, 141: 106221. [DOI] [PubMed] [Google Scholar]
- 250.Stoycos SA, Berzenski SR, Gayle Beck J, Unger W, Cappellano JM, Spofford CM, et al. Predictors of treatment completion in group psychotherapy for male veterans with posttraumatic stress disorder. J Trauma Stress 2023, 36: 346–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Franklin CL, Cuccurullo LA, Walton JL, Arseneau JR, Petersen NJ. Face to face but not in the same place: A pilot study of prolonged exposure therapy. J Trauma Dissociation 2017, 18: 116–130. [DOI] [PubMed] [Google Scholar]
- 252.Thorp SR, Glassman LH, Wells SY, Walter KH, Gebhardt H, Twamley E, et al. A randomized controlled trial of prolonged exposure therapy versus relaxation training for older veterans with military-related PTSD. J Anxiety Disord 2019, 64: 45–54. [DOI] [PubMed] [Google Scholar]
- 253.Foa EB, McLean CP, Zang Y, Rosenfield D, Yadin E, Yarvis JS, et al. Effect of prolonged exposure therapy delivered over 2 weeks vs 8 weeks vs present-centered therapy on PTSD symptom severity in military personnel: A randomized clinical trial. JAMA 2018, 319: 354–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Young-McCaughan S, Peterson AL, Mintz J, Hale WJ, Dondanville KA, Borah EV, et al. Testing the role of aerobic exercise in the treatment of posttraumatic stress disorder (PTSD) symptoms in U.S. active duty military personnel: A pilot study. Cogn Behav Ther 2022, 51: 309–325. [DOI] [PubMed] [Google Scholar]
- 255.Reger GM, Smolenski D, Norr A, Katz A, Buck B, Rothbaum BO. Does virtual reality increase emotional engagement during exposure for PTSD? Subjective distress during prolonged and virtual reality exposure therapy. J Anxiety Disord 2019, 61: 75–81. [DOI] [PubMed] [Google Scholar]
- 256.McLay RN, Baird A, Webb-Murphy J, Deal W, Tran L, Anson H, et al. A randomized, head-to-head study of virtual reality exposure therapy for posttraumatic stress disorder. Cyberpsychol Behav Soc Netw 2017, 20: 218–224. [DOI] [PubMed] [Google Scholar]
- 257.Beidel DC, Christopher Frueh B, Neer SM, Lejuez CW. The efficacy of trauma management therapy: A controlled pilot investigation of a three-week intensive outpatient program for combat-related PTSD. J Anxiety Disord 2017, 50: 23–32. [DOI] [PubMed] [Google Scholar]
- 258.McLean CP, Foa EB, Dondanville KA, Haddock CK, Miller ML, Rauch SAM, et al. The effects of web-prolonged exposure among military personnel and veterans with posttraumatic stress disorder. Psychol Trauma 2021, 13: 621–631. [DOI] [PubMed] [Google Scholar]
- 259.Steuwe C, Rullkötter N, Ertl V, Berg M, Neuner F, Beblo T, et al. Effectiveness and feasibility of Narrative Exposure Therapy (NET) in patients with borderline personality disorder and posttraumatic stress disorder - a pilot study. BMC Psychiatry 2016, 16: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Butollo W, Karl R, König J, Rosner R. A randomized controlled clinical trial of dialogical exposure therapy versus cognitive processing therapy for adult outpatients suffering from PTSD after type I trauma in adulthood. Psychother Psychosom 2016, 85: 16–26. [DOI] [PubMed] [Google Scholar]