Abstract
Tumor immunotherapy has emerged as a formidable strategy, demonstrating substantial achievements in the field of cancer treatment. Despite its remarkable success, intrinsic limitations such as insufficient targeting capabilities, side effects, and resistance to immunotherapy hinder its efficacy. To address these challenges, the utilization of nanomedicines in tumor immunotherapy has been broadly explored, capitalizing on their advantages of targeting delivery capability, loading capacity, modifiability, and biocompatibility. Through rational design approaches, nanomedicines are engineered to meet diverse delivery requirements and synergize with different regimens to maximize therapeutic efficacy while alleviating side effects. This review initially discusses the challenges associated with tumor immunotherapy and underscores the pivotal role played by nanomedicines in overcoming these obstacles. Subsequently, representative types of nanoparticles are systematically introduced based on their structural properties, advantages, potential limitations, and future research directions. Special emphasis is placed on recent advancements in a range of nanomedicines designed for specific tumor immunotherapy strategies. Finally, the clinical applications as well as prospects of nanomedicines are discussed.
Keywords: nanomedicine, nanoparticle, cancer treatment, immunotherapy
Introduction
Tumor immunotherapy represents a promising strategy that aims to stimulate patients’ antitumor immune response for tumor recognition and eradication while also establishing long-term immunological memory to prevent metastasis and recurrence (Kennedy and Salama, 2020). The main categories of immunotherapy encompass immune checkpoint blockades (ICBs), monoclonal antibody (mAb) therapy, tumor vaccines, adoptive cell transfer (ACT), cytokine therapy, oncolytic virus (OV) immunotherapy, and immune combination therapy (Koyande et al., 2022). In comparison to conventional therapeutic modalities for cancer, immunotherapy offers distinct advantages due to its prolonged treatment duration, relatively mild side effects, and favorable efficacy. However, several obstacles still hinder clinical effectiveness. Firstly, inadequate targeting capabilities and limited penetrability through physiological barriers within tumor tissues impede the efficient delivery of immunoregulatory drugs and tumor vaccines (Wang et al., 2022c). Secondly, inflammatory toxicities associated with tumor immunotherapy restrict its clinical applications. For instance, checkpoint blockade toxicity can invade peripheral organs characterized by significant variability and unpredictability due to T-cell hyperreactivity (Khan and Gerber, 2020; Waldman et al., 2020). Finally, primary resistance and acquired resistance to immunotherapy can result in a low immune response clinically (O’Donnell et al., 2019). Notably, primary resistance to anti-programmed cell death-1 (anti-PD1) therapies has been observed in ∼60% of melanoma patients, while acquired resistance has been documented in ∼25% of patients (Topalian et al., 2012; Ribas et al., 2016). Therefore, it is imperative to explore approaches for addressing the aforementioned limitations of tumor immunotherapy.
Nanoparticles (NPs) refer to heterologous small composites typically ranging in size from 1 nm to 100 nm. They can occur naturally such as exosomes, or be artificially synthesized from various materials through nanotechnology. NPs can be categorized into lipid-based, polymer-based, inorganic, biomimetic, and hybrid NPs based on their components. These diverse NPs show promise in optimizing tumor immunotherapy and overcoming existing limitations (Namiot et al., 2023). Owning to the specific scale, NPs can passively traverse through enlarged inter-endothelial gaps of leaky vascular structures and selectively accumulate in tumor tissue (Hobbs et al., 1998; Kalyane et al., 2019). This phenomenon is known as the enhanced permeability and retention (EPR) effect, which allows for passive targeting of antitumor therapeutic cargo-bearing NPs with enhanced intratumoral accumulation, reduced nonspecific uptake, and improved efficacy (Figure 1A). Furthermore, the incorporation of ligand modification such as antibodies, peptides, aptamers, and polysaccharides as well as small molecules confers active targeting capability to NPs for precise action at specific sites (Qian et al., 2019; Yoo et al., 2019; Zhang et al., 2019b; Cho et al., 2020; Yang et al., 2023; Bai et al., 2024), making them suitable for different modalities of immunotherapy such as direct killing of tumor cells, remodelling of tumor microenvironment (TME), and immune activation (Figure 1B; Arranja et al., 2017; Kim et al., 2021; Yang et al., 2021a). In addition to targeted delivery, NPs improve the properties of medicines by improving stability, prolonging circulation time, and promoting specific cellular uptake, which amplifies therapeutic efficacy while minimizing adverse effects (Figure 1C ; Gowd et al., 2022; Katopodi et al., 2022). Moreover, the sophisticated design of NPs is beneficial in orchestrating immune combination therapy in a spatiotemporally controlled manner, thereby demonstrating their potential to overcome the resistance to cancer immunotherapy (Zhu et al., 2021; Figure 1D).
Figure 1.
Schematic diagram of the characteristics of NPs in promoting tumor immunotherapy. (A) EPR effect facilitates passive targeting of NPs to tumor regions, thereby promoting the accumulation of anticancer drugs. (B) Ligand modification broadens the applicability of NPs for delivering drugs to various action sites, enabling direct eradication of tumor cells, TME remodelling, and immune activation. (C) NPs improve drug characteristics by enhancing stability and prolonging circulation time while also facilitating specific cellular uptake to enhance efficacy. (D) Through sophisticated design, NPs mediate a diverse range of therapies such as chemotherapy, radiotherapy, photothermal therapy, and photodynamic therapy in coordination with tumor immunotherapy. The induction of ICD contributes to reversing the immunosuppressive TME and strengthening immune responses synergistically. NIR, near-infrared ray. This figure is created with BioRender.com.
The application of nanotechnology in tumor immunotherapy holds significant promise and potential. Ongoing research endeavors are dedicated to the development of innovative nanomedicines, alongside advancing clinical trials. However, there are some limitations that need to be overcome for their successful implementation. For example, the absence of a comprehensive evaluation regarding potential interactions between nanomedicines and the biological environment, as well as their intricate composition, may give rise to safety concerns (Dormont et al., 2019; Stater et al., 2021). The scale-up manufacturing of nanomedicines poses challenges for ensuring reproducibility of physicochemical characteristics, which is crucial for maintaining their overall performance in vivo (Younis et al., 2022). Therefore, a comprehensive review of the application of nanomedicine in tumor immunotherapy can provide valuable insights for innovative development in preclinical studies and facilitate clinical translation of nanomedicines with enhanced characteristics. This review presents various types of NPs along with their potential limits and discusses the future research directions. Special emphasis is given to the advancements in nanomedicine for tumor immunotherapy. Furthermore, we provide an overview of the current clinical landscape of nanomedicines and outline prospects.
Types of NPs
NPs are heterologous small composites typically ranging in size from 1 nm to 100 nm (Namiot et al., 2023). They can be crafted from diverse materials, including lipids, polymers, and inorganic materials, and may also be derived from natural substances such as self-assembling proteins and exosomes. NPs are commonly categorized into lipid-based NPs, polymer-based NPs, inorganic NPs (iNPs), biomimetic NPs, and hybrid NPs (Figure 2). In this section, we provide an overview of NP classification along with their distinctive properties.
Figure 2.
Schematic diagram of representative types of NPs. Based on the materials utilized, NPs can be categorized into lipid-based, polymer-based, inorganic, biomimetic, and hybrid NPs. Specifically, biomimetic NPs are characterized by their composition of natural macromolecular components. Hybrid NPs are defined as particles composed of at least two constituents. This figure is created with BioRender.com.
Lipid-based NPs
Lipid-based NPs typically present a spherical structure comprising an outer lipid layer and an inner aqueous compartment, while containing various subset structures (Mitchell et al., 2021). Lipid-based NPs offer advantages including robust cellular uptake properties, surface modification flexibility, favorable biocompatibility, and enhanced bioavailability (Kumar et al., 2022). Besides, the lipid formulations are facile to adjust to improve delivery efficiency, increase medication availability, and evade immune system detection (Sheoran et al., 2022). Representative categories of lipid-based NPs include liposomes, micelles, and solid lipid nanoparticles (SLNs).
Liposomes refer to spherical vesicles derived from the self-assembly of amphiphilic lipid molecules in solution, structurally comprising one or more lipid bilayers. They possess stability, high load capacity, biodegradability, and biocompatibility, rendering them available for the delivery of molecules with varying polarities (Guimaraes et al., 2021). The majority of Food and Drug Administration (FDA)-approved nanomedicines for tumor treatment belong to liposomal formulations, such as Doxil, Marqibo, and Onivyde. Liposomes can be subdivided into cationic and anionic types in terms of their surface charge properties. Cationic liposomes can promote cell association, uptake, lysosomal escape, and nucleic acid concentration efficiently, making them widely utilized as optimal carriers for mRNA vaccines (Li et al., 2022c). Conversely, owing to the electrostatic repulsion, anionic liposomes enable reduced nonspecific cell uptake to alleviate cytotoxicity and prolonged circulation in the bloodstream (Large et al., 2021). Based on the aforementioned characteristics, liposomes have extensive applications in tumor immunotherapy.
Phospholipid micelles, self-assembled by amphiphilic molecules above the critical micelle concentration, exhibit distinct structures where the hydrophobic domains aggregate and face away from the aqueous environment, while the hydrophilic domains orient towards water (Feng and Mumper, 2013). They offer the advantages of convenient and reproducible preparation procedures and long-term storage through lyophilization. In terms of drug delivery, they are primarily employed for hydrophobic drug administration while being limited by their small interior hydrophobic space (Banerjee and Onyuksel, 2012). Furthermore, the conjugation of hydrophilic drugs to micelles results in increased lipophilicity and enhanced membrane permeability (Zielinska et al., 2022).
SLNs are spherical vesicles consisting of inner solid lipid and surrounding layers of surfactants as stabilizers, which effectively restrict the mobility of molecules within the lipid matrix and coalescence of NPs (Mirchandani et al., 2021; Zhang et al., 2023d). Surfactants play a pivotal role in polymorphic transitions of lipid core, usually contributing to a more efficient stabilization of SLNs (De Jesus and Zuhorn, 2015). In comparison to other lipid-based NPs, SLNs demonstrate superior physical stability by minimizing drug leakage and burst release, enabling controlled release. Furthermore, the synthesis methods for SLNs without organic solvents not only ensure lower toxicity in the final production but also facilitate convenient large-scale manufacturing (Tenchov et al., 2021).
As one of the most widely utilized types of NPs, there is an urgent necessity for the development of lipid-based NPs in order to establish a more universally applicable method to meet the gradually increasing application requirements, such as organ targeting, payload capacity, safety, dispersive stability, and responsive release. Although additional modifications can enhance the properties of lipid-based NPs, they simultaneously present challenges in terms of toxicity evaluation, scalable production, storage stability, and maintenance of in vivo performance, thereby hindering their widespread clinical application. The adjustment of lipid composition has been increasingly emphasized by recent research due to its feasibility, simplicity, and versatility. Therefore, further investigation into the correlation between lipid structure and biological function is essential for systematically optimizing lipid composition and proportion. Moreover, the introduction of a wider range of artificially synthesized lipid molecules with diverse chemical structures into lipid-based NPs is anticipated, as they have the potential to enhance properties even further.
Polymer-based NPs
Polymer-based NPs are derived from natural or synthetic polymers, encompassing a diverse range of structures, including polymer micelles, polymersomes, dendrimers, and polymeric NPs (Ferrari et al., 2018; Lu et al., 2021; Dong et al., 2024). Natural polymer materials have been widely employed for the synthesis of NPs due to their biocompatibility, biodegradability, non-immunogenicity, and cost-effectiveness (Fazal et al., 2023). Synthetic polymers like poly(ethylene glycol) (PEG), poly(lactic acid) (PLA), and poly(lactic glycolic acid) (PLGA) possess diverse chemical functional groups that offer advantages in terms of easy modification, self-assembling ability, and stimuli-responsiveness based on the presence of sensitive groups and bonds such as tertiary amine groups, acetal bonds, and disulfide bonds (Mukerabigwi et al., 2019; Wang et al., 2022c). Compared to lipid-based NPs, natural and synthetic polymer-based NPs offer the advantages of diverse composition, facile preparation, and functionalization, as well as stability. As a result, polymer-based NPs play a crucial role in intelligent delivery systems, thereby enabling controlled release in response to different pathological stimuli. However, it is noteworthy that stimulus-responsive polymer-based NPs, despite their attractive traits, have been barely tested in clinical trials, which may be attributed to concerns regarding their unstable performance, potential toxicity risks, and challenges associated with scaling up production. Therefore, it is crucial to consider product reproducibility and cost-effectiveness during the preclinical stage design as well as long-term evaluation of safety and stable efficacy in the clinical stage (Javia et al., 2022).
iNPs
iNPs, as a general term, refer to NPs fabricated from a wide array of inorganic materials such as gold, silicon, carbon, iron oxide, and quantum dots. They offer advantages including long-term stability, high surface-to-volume ratio, favorable uniformity, and intrinsic immunogenicity (Hess et al., 2019). In contrast to organic NPs, iNPs possess distinctive optical, electrical, thermal, ultrasonic, and magnetic properties generally, which enable their utilization in cancer diagnosis and imaging. Additionally, they can be used for direct antitumor modalities involving photothermal therapy (PTT) and photodynamic therapy (PDT), as well as combination therapies (Wang et al., 2021a; Zhang et al., 2023a). Representative iNPs incorporate metallic NPs, mesoporous silica NPs (MSNs), and carbon nanotubes (CNTs).
Metallic NPs refer to NPs derived from metals such as gold, silver, iron, and their oxides, exhibiting advantages including high density to promote cell uptake and precise manipulation of size, shape, charge, and surface modification (Evans et al., 2018). Bare gold nanoparticles have been certified to activate immunity and possess intrinsic antitumor capabilities via signaling blockade, rendering them suitable carriers for tumor immunotherapy (Bawage et al., 2016; Saha et al., 2016). Iron NPs are widely utilized as cancer theranostic agents due to their stability, prolonged half-life, and favorable biodistribution (Fernandes, 2023). Additionally, iron NPs exhibit magnetic properties for facilitating magnetic navigation and magnetic hyperthermia, which can enhance immunotherapy (Cheng et al., 2021).
MSNs are distinguished by their large surface areas and porous structures, which confer upon them biodegradability, low toxicity, high loading capacity, and the ability to regulate antigen delivery (García-Fernández et al., 2021). MSNs with larger pore sizes have demonstrated superior efficiency in antigen cross-presentation, reduced production of reactive oxygen species (ROS), faster degradation, and enhanced rates of drug release (Hong et al., 2020). The hydroxyl moieties on the surfaces of MSNs provide numerous sites for facile chemical functionalization to achieve controlled cargo release (Tran et al., 2018; Kankala et al., 2022).
CNTs are synthesized from the rolling of graphene sheets to form tubular and fiber-like structures, which can be further categorized as single-walled and multi-walled varieties based on the arrangement of graphene sheets (Sheikhpour et al., 2020). The exceptional properties of CNTs, such as their high surface area-to-volume ratio, flexible surface chemistry, high drug-loading capacity, and chemical stability, render them highly desirable carriers (Raphey et al., 2019). Moreover, their capability to be internalized through diverse mechanisms, broadly classified into endocytosis and passive diffusion, makes them particularly suitable for application in immunotherapy (Zare et al., 2021). Surface functionalization is usually exploited to enhance cellular uptake of CNTs, and their intrinsic shortcomings, such as poor dispersibility and tendency to aggregate, thereby reducing cytotoxicity (Zhang et al., 2015; Mei et al., 2018).
The safety issue of iNPs remains a major challenge in the field of nanomedicine owing to their complex interaction with the biological environment. Studies have indicated that iNPs, such as Fe2O3 NPs, TiO2 NPs, and Ag NPs, have the potential to induce oxidative stress, biochemical disruption, and genotoxicity (Rizk et al., 2017; Lu et al., 2022; Siddiqui et al., 2023). Moreover, in a study conducted by Peng et al. (2019), iNPs encompassing titanium dioxide, silica, and gold have been proven to cause gaps between endothelial cells, ultimately accelerating the metastasis and promoting the generation of new metastasis sites of breast cancer cells (Peng et al., 2019). The current primary research direction to overcome this challenge involves conducting comprehensive toxicological assessments over the long term, encompassing biodistribution, biological interaction, metabolism, cytotoxicity, and adverse immune stimulation in both in vitro and in vivo settings (Czarnecka et al., 2020; Li et al., 2024b). Additionally, an effective strategy to prevent the detrimental interaction of iNPs with the biological environment and alleviate potential toxicity is by incorporating surface chemistry modulation (Mahamuni-Badiger and Dhanavade, 2023).
Biomimetic NPs
Exogenous NPs are susceptible to the formation of protein corona and undesirable interaction with biological systems (Vijayan et al., 2019). Biomimetic NPs have been engineered to improve the characteristics of synthetic NPs, enhance colloidal stability, prolong circulation time, and mitigate the risk of off-target accumulation and adverse reactions in the complex in vivo environment following administration (Soprano et al., 2022). This section provides an overview of biomimetic NPs, including virus-like particles (VLPs), self-assembling protein NPs, cell membrane-coated NPs, and extracellular vesicles (EVs).
VLPs
VLPs are naturally occurring or artificially designed symmetrical nanostructures formed by self-assembled viral proteins, devoid of viral genetic material. These VLPs serve as a protective and slightly flexible shell for therapeutic cargo, demonstrating biocompatibility, safety, biodegradability, and favorable immunogenicity (He et al., 2022). The structural and functional similarities to viruses confer an inherent affinity of VLPs for specific cellular receptors and host cell entry mechanisms, making them ideal carriers for intracellular delivery (Shan et al., 2023). In addition, the uniform size and defined chemistry of VLPs contribute to the consistent accumulation of cargo and surface modification (Ikwuagwu and Tullman-Ercek, 2022). Leveraging genetic engineering and chemical modification, VLPs present a degree of flexibility in physicochemical properties, which can be tailored for various cargos including chemical drugs, genetic drugs, peptides, and proteins (Chen et al., 2023).
Self-assembling protein NPs
Self-assembling protein NPs are defined as organized aggregated peptidic NPs formed through self-assembly in response to exposure to external stimuli (Pugliese and Gelain, 2017). Ferritin, which plays a crucial role in iron metabolism and homeostasis in organisms, possesses a hollow and spherical nanostructure derived from self-assembled subunits (Mohanty et al., 2022). Due to its H-chain, ferritin exhibits a specific affinity for the transferrin-1 receptor, which is frequently overexpressed on cancer cells, making ferritin an effective carrier for cancer treatment (Obozina et al., 2023). Vault NPs refer to the self-assembling ribonucleoprotein complex comprising 78 copies of major vault protein, demonstrating a symmetrical, barrel-like, hollow structure (Frascotti et al., 2021). These NPs offer several advantages such as favorable size, non-immunogenicity, sufficient interior space, and biodegradability (Rome and Kickhoefer, 2013). Albumin demonstrates an extended half-life and an inherent propensity to accumulate at tumor sites characterized by poor lymphatic drainage, subsequently being preferentially internalized by tumor cells with high metabolic demands (Hoogenboezem and Duvall, 2018). It is noteworthy that, in 2020, an albumin fusion protein was engineered to incorporate Arg–Gly–Asp (RGD) peptide, enzyme digestion sequence, and polyhistidine. This design endowed the protein with the capability to self-assemble into NPs based on the hydrophobicity of polyhistidine (Wang et al., 2020b). These NPs exhibited active targeting capability and facilitated tumor penetration and lysosomal escape by responding to matrix metalloproteinase (MMP)-2 enzyme cleavage and transforming into small histidine micelles.
Cell membrane-coated NPs
The cell membrane coating strategy aims to camouflage NPs with natural cell membranes, allowing these NPs to inherit the natural bio-interfacing properties of the source cells. By leveraging the complex surface architecture and multiple signals of the cell membranes, these cell membrane-coated NPs are capable of concealing exogenous features, evading immune clearance, and traversing diverse biological barriers (Liu et al., 2021). Furthermore, membrane coating can also serve as antigen sources or transfer immunostimulatory signals for immunotherapy, depending on the type of membrane. Additionally, these cell membrane-coated NPs can be further fine-tuned through genetic and metabolic engineering of the source membrane (Fang et al., 2023b). In this section, we will introduce characteristics of several different cell membrane-coated NPs synthesized from those including platelets, cancer cells, red blood cells (RBCs), white blood cells (WBCs), and bacteria.
Platelets confer natural pathophysiological affinity to NPs for tumor cells due to the protein profile expressed on their membrane surface. The efficient interaction between platelet membrane-coated NPs and tumor cells results in prolonged tissue persistence, enhanced efficacy of anticancer drugs, and extended duration of immune memory (Bahmani et al., 2021). In addition, the presence of the cluster of differentiation 47 (CD47) on the platelet membrane can augment the immune evasion capability of NP cores and prolong circulation by interacting with signal regulatory proteins on immune cells (Han et al., 2022).
The membranes of cancer cells possess the capability to facilitate robust homotypic targeting of NPs and prevent the formation of protein coronas due to the presence of receptors on the membranes. Furthermore, antigens and proteins such as CD47 and CD24 can independently contribute to activating antitumor immune responses and promoting the immune evasion of NPs (Lin et al., 2023). Therefore, the coating of cancer cell membranes offers numerous advantages, such as efficient homologous targeting, biocompatibility, prolonged half-life, and enhanced immunogenicity. In a study conducted by Chen et al. (2021a), it was demonstrated that cancer cell membrane-coated polymer NPs displayed an impressive intracellular uptake rate of 61.67% in vitro, along with superior active targeting capabilities and efficient in vivo accumulation, ultimately contributing to enhanced therapeutic efficacy of loaded drugs. Furthermore, it was observed that cancer cell membrane-coated NPs were able to disrupt the migration of cancer cells towards fibroblasts and reduce metastasis (Jin et al., 2019).
The coating of NPs with RBC membranes results in reduced clearance by the reticuloendothelial system, enhanced colloidal stability, extended blood circulation, and improved tumor accumulation (Li et al., 2022b). Additionally, the extraction and purification process of RBC membranes is simplified due to the absence of a nucleus and organelles (Xia et al., 2019). In a groundbreaking approach, modification of RBC membranes with anti-low-density lipoprotein receptor antibodies not only preserves the aforementioned advantages but also confers NPs with potent hypoxic tumor targeting, improved cellular uptake, and significant enrichment in tumor sites (Pan et al., 2022b).
WBCs, functioning as immune cells, possess the capability to detect and respond to pathogens and immune signals, while also naturally homing in on disease sites through cellular surface ligands. Furthermore, the membranes of WBCs enable NP cores to evade phagocytosis by self-recognition with source cells (Wang et al., 2022b). As a result, WBC-coated NPs demonstrate spontaneous tumor targeting, prolonged circulation time, and enhanced accumulation in tumor tissues (Jun et al., 2020).
Bacterial membranes, which display pathogen-associated molecular patterns, can impart NPs with inherent adjuvant properties to stimulate innate immunity and promote adaptive immune responses (Soprano et al., 2022; Zhao et al., 2022b). Additionally, proteins can be readily presented on the bacterial membrane surface through genetic manipulation.
EVs
EVs refer to membrane-enclosed vesicles secreted from cells, which play a crucial role in mediating intracellular communication by packaging a diverse array of signaling molecules. It has been revealed that EVs exhibit significant heterogeneity in their biogenesis, physical characteristics, and content composition, widely involved in the maintenance of cellular homeostasis and regulation of disease progression (Huang et al., 2020a). Importantly, the complex surface proteins and molecular cargo associated with their source cells endow EVs with targeted delivery capacity, enhanced stability during circulation, favorable biocompatibility, and immunoregulatory capability (Tran et al., 2015; Kimiz-Gebologlu and Oncel, 2022; Buzas, 2023). Consequently, EVs have been extensively manipulated for use as delivery systems and therapeutic agents.
According to their biogenesis, EVs are broadly categorized into ectosomes, which originate from the outward budding of the plasma membrane, and exosomes, which are generated by the inward budding of endosomes (Jeppesen et al., 2023). The production of ectosomes has been improved to specifically encapsulate endogenous bioactive molecules leveraging the split green fluorescent protein complementary system, thereby achieving efficient macromolecular delivery and genome modification (Zhang et al., 2020). Exosomes have been extensively engineered for use as tumor vaccines. Tumor-derived exosomes carrying tumor antigens have been demonstrated to elicit potent dendritic cell (DC)-mediated immune responses and reduce the population of regulatory T cells (Tregs) (Wang et al., 2020a). Mature DC-derived exosomes, which retain antigenic peptide–major histocompatibility complex (pMHC) and co-stimulatory molecules from parental DCs, enabling them to directly prime T cells (Lee et al., 2023), are considered promising cell-free DC vaccines with improved penetrating capability, reduced susceptibility to immunosuppressive molecules, enhanced stability during storage, and increased efficiency in activating T and natural killing cells (Luo et al., 2023).
The incorporation of natural biological components, such as proteins and membranes, confers favorable biocompatibility, inherent targeting ability, and unique immunoregulatory capacity to NPs, thereby enhancing tumor immunotherapy. However, this integration simultaneously complicates the interaction mechanism between NPs and the biological environment, thereby exhibiting potential inflammatory risks (Zinger, 2023). In order to address this issue, high-throughput screening methods can be employed to identify biomimetic NPs with optimal characteristics during the research process. The characterization of biomimetic NPs is hindered by their complex structures and compositions, necessitating the establishment of simplified models for separate analysis of performance attributed to specific natural components. Moreover, the synthetic method for biomimetic NPs relies on large-scale cell culture and purification of specific biological components followed by strict storage conditions requirements that impede their clinical translation. Therefore, it is imperative to establish stringent production standards and evaluation criteria to ensure the uniformity of source cells and extracted biological components, as well as the stability in the performance of final biomimetic NP products.
Hybrid NPs
Hybrid NPs, also referred to as multi-component NPs, are defined as nanoscale composites composed of at least two constituents with distinct compositions and properties. They possess the unique capability to integrate the advantages of different materials and overcome the inherent limitations of single materials, while also demonstrating potential for novel properties resulting from hybridization (Rajana et al., 2022; Li et al., 2023c). This section provides a summary of representative categories of hybrid NPs, including metal-organic frameworks (MOFs), mesoporous organosilica nanoparticles (MONs), metallofullerene NPs, and lipid–polymer hybrid NPs (LPHNs).
MOFs
MOFs are crystalline structures with a three-dimensional arrangement, characterized by an organized framework resulting from the self-assembly of metal ions or clusters and organic struts connected by coordination bonds (Luo et al., 2019b). The versatility in coordinating metal ions and organic struts facilitates the synthesis of personalized MOFs tailored to various pharmaceuticals with diverse molecular weights, hydrophilic properties, and sizes (Gao et al., 2021). The significant surface areas, exceptional porosities, and weak coordination bonds of MOFs contribute to their high loading capacity and biodegradability, making them promising candidates for drug delivery systems (Wu and Yang, 2017). The incorporation of specific active metal ions and functional linkers into framework structures can endow the MOFs with diverse functionalities, including stimuli-responsiveness, imaging and biosensing properties, synergistic antitumor effect, and even direct antitumor activity (Gao et al., 2021). For instance, an ultrathin ferrocene-based MOF can function as a photothermal agent and Fenton catalyst to mediate simultaneous photothermal therapy and chemodynamic therapy (CDT) without requiring additional drugs (Deng et al., 2020).
MONs
MONs are engineered to address concerns regarding the potential toxicity associated with the prolonged retention of traditional MSNs, achieved by integrating organic moieties into the Si–O–Si frameworks in a covalent manner to promote degradation under physiological conditions (Cheng et al., 2020). MONs offer numerous advantages including improved biodegradability, biocompatibility, uniform morphology, and large surface areas (Chen and Shi, 2016). Furthermore, the diverse functionalization of organic moieties endows MONs with favorable attributes for controlled release, targeted delivery, and specific interaction with guest molecules through electrostatic forces, chemical bonding, or noncovalent interactions (Yang et al., 2019).
Metallofullerene NPs
Metallofullerene NPs are permanent ionic compounds synthesized by encapsulating metal atoms or metal clusters within a closed fullerene cage, which remain intact until the carbon cage is destroyed (Grebowski and Litwinienko, 2022). In addition to their stability, they offer the advantages of easy functionalization and antitumor activity (Wang and Wang, 2019). Metallofullerene NPs also demonstrate diverse chemical, optical, and magnetic properties due to variations in fullerene cages and metal components (Li et al., 2021). Gd@C82(OH)22 NPs are representative metallofullerene antitumor nanomedicines with biosafety, biocompatibility, and well dispersibility in physiological solutions, which inhibit tumor growth and metastasis via multiple mechanisms (Li et al., 2012, 2019).
LPHNs
LPHNs refer to NPs composed of an internal polymeric core and an outer lipid shell, which may consist of one or multiple layers (Gowsalya et al., 2021). The design of lipid layers aims to enhance the stability of NPs, prevent aggregation, and prolong circulation time following surface engineering, while the polymer core ensures structural integrity to support the lipid layers as well as achieve stimuli-responsive release of diverse cargos (Khalili et al., 2022; Tang et al., 2022). LPHNs possessing high stability, excellent biocompatibility, and low toxicity have the capability to encapsulate a variety of therapeutic agents, regulate cargo release, and achieve synergistic delivery of multiple therapeutic agents (Bose et al., 2017). LPHNs have been validated as an effective delivery system for CRISPR/Cas9 plasmids, ultimately restoring the sensitivity of glioblastoma cells through gene editing facilitated by focused ultrasound and microbubbles (Yang et al., 2021b).
Numerous studies have utilized synergistic combinations of multiple nanomaterials to enhance the performance of nanomedicines and expand the application scope of nanomedicines. However, due to the complex properties exhibited by these hybrid systems, further long-term characterization is necessary to assess their stability, toxicity, drug distribution, metabolism, and immune response in vivo (Dormont et al., 2019; Stater et al., 2021). Additionally, the increasing complexity of nanomedicine design encompassing multiple raw materials and intricate preparation processes complicates scaling up manufacturing as well as subsequent sterilization and storage conditions. Therefore, it is imperative to consider manufacturing constraints for scale-up production of nanomedicines during the initial design phase.
Nanomedicine in tumor immunotherapy
Tumor immunotherapy holds great promise in harnessing the immune system to eliminate tumors, prevent metastasis, and reduce recurrence (Liu et al., 2022d). It encompasses seven main categories, including ICBs, mAb therapy, cytokine therapy, tumor vaccine, ACT, OV immunotherapy, and immune combination therapy (Figure 3). Nevertheless, there remains a pressing need to optimize the delivery efficiency, toxicity profiles, and response rates of tumor immunotherapy agents. Nanomedicine in the form of nanoformulation has the potential to target specific sites, minimize off-target accumulation, control cargo release, and coordinate multiple therapies (Alqosaibi, 2022). Therefore, the application of nanomedicine in tumor immunotherapy aims to address these challenges and maximize efficacy. In the following sections of this review, we will introduce the application of nanomedicines in various modalities of tumor immunotherapy (Table 1).
Figure 3.
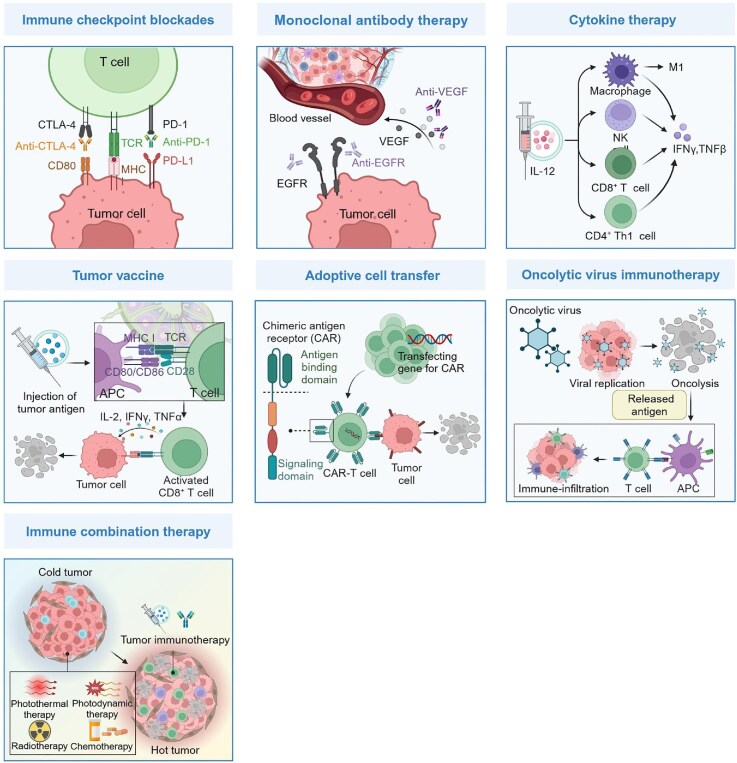
Schematic diagram of the principles of cancer immunotherapy. The fundamental principle of tumor immunotherapy is to stimulate the immune system of patients to recognize and eliminate tumor cells, while also establishing long-term memory. Specifically, ICBs are designed to introduce inhibitors that disrupt the interaction between inhibitory checkpoint molecules and their corresponding ligands, thereby enabling immune cells to function effectively. mAb therapy aims to block signal pathways involved in tumorigenesis or activate the immune response to inhibit tumor growth. Cytokine therapy is intended to supplement various cytokines in order to stimulate multiple immune cells and signal pathways. Tumor vaccines are designed to provide effective tumor antigens for activating antigen-specific responses, ultimately leading to the elimination of tumor cells. ACT, exemplified by CAR-T therapy, aims to harness the potential of natural or genetically modified lymphocytes for the eradication of tumor cells. CAR-T cells are engineered with CAR genes to enhance their capacity in identifying and eliminating cells expressing specific antigens. OV therapy is designed to exploit OVs for the invasion and lysis of tumor cells. Immune combination therapy is intended to integrate immunotherapy with other modalities capable of inducing ICD, thereby remodelling the TME and augmenting the immune response. This figure is created with BioRender.com.
Table 1.
A list of representative NPs applied in immunotherapy across various cancer types.
| Tumor immunotherapy type | Advantage | Major cargo | NP | Cancer type | References |
|---|---|---|---|---|---|
| ICBs | Directly target inhibitory checkpoint molecules and mitigate off-target accumulation | Antibody | • Mesoporous silica NPs • Polymeric NPs • DC-derived nanovesicles |
• Melanoma • Prostate cancer |
Zhao et al. (2021); Jung et al. (2022); Wang et al. (2023a) |
| Small-molecule inhibitor | • Dendrimer-entrapped gold NPs • Lipid–calcium–phosphate NPs • Lipid-based NPs |
• Melanoma • Glioblastoma multiforme • Lung cancer |
Wan et al. (2021); Xue et al. (2021); Hsieh et al. (2022) | ||
| Mediate gene therapy to modulate the expression of multiple checkpoint molecules | siRNA | • ZIF-8 NPs • Polymer-based NPs |
• Osteosarcoma • Colorectal cancer |
Yu et al. (2021); Ge et al. (2022) | |
| miRNA | • Cationic polymer-based NPs | • Colon carcinoma | Nguyen et al. (2021) | ||
| mRNA | • Lipid-based NPs | • Melanoma | Walters et al. (2021) | ||
| mAb therapy | Enhance tumor-specific accumulation, minimize off-target toxicity, and optimize therapeutic efficacy | Bevacizumab | • Polymer-coated human serum albumin NPs | • Colorectal cancer | Luis de Redin et al. (2020) |
| Trastuzumab | • Human ferritin NPs | • Breast cancer | Sevieri et al. (2023) | ||
| Cetuximab | • Silica NPs • Lipid-coated mesoporous silica NPs |
• Hepatocellular carcinoma • Colon carcinoma |
Wang et al. (2017); Chen et al. (2021b) | ||
| Cytokine therapy | Preserve cytokine activity, enhance targeted accumulation, and minimize systemic exposure | Interleukin | • Polymer-based NPs | • Ovarian cancer • Colorectal cancer |
Barberio et al. (2020) |
| Achieve consistent and elevated levels of cytokine expression | mRNA | • Polymer-modified porous silica NPs • Lipid NPs • Cationic liposomes |
• Melanoma • Colon carcinoma |
Lei et al. (2020); Liu et al. (2022b); Shin et al. (2023) | |
| Self-replicating RNA | • Lipid NPs | • Melanoma | Li et al. (2020) | ||
| Tumor vaccine | Facilitate antigen protection, enable efficient delivery, prolong antigen presentation by APCs, and enhance T-cell immune infiltration | Protein/peptide antigen | • Lipid NPs • Polymer-based NPs • Mesoporous silica microrods • Graphene oxide NPs • MnO2 NPs • Ultra-small Fe3O4 NPs • Virus-like gold NPs |
• Melanoma • Colorectal cancer • Colon carcinoma • Lymphoma |
Luo et al. (2019a); Nguyen et al. (2020b); Xu et al. (2022); Zhang et al. (2022a); Gu et al. (2023); Liu et al. (2023b); Su et al. (2022, 2023); Zhao et al. (2022a); Li et al. (2023d); Wang et al. (2023b) |
| mRNA antigen | • Lipid NPs • Ionizable polymeric NPs |
• Melanoma • Glioblastoma multiforme |
Chen et al. (2022) | ||
| Provide abundant antigen epitopes and activate robust immune responses | Natural signal molecules from cells | • Fusion cell membrane-coated NPs • Polymer-based NPs • Tumor cell membrane-coated chitosan-shell PLAG-core NPs • DC-derived exosomes • Tumor cell-derived exosome-like nanovesicles |
• Hepatocellular carcinoma • Colon carcinoma • Melanoma • Lung carcinoma • Breast cancer |
Hu et al. (2021); Zhang et al. (2022b); Zuo et al. (2022); Liu et al. (2023a) | |
| ACT | Promote antigen-specific T-cell proliferation ex vivo | TCR activation and co-stimulation signals | • Iron–dextran NPs • Polymer-based NPs |
/ | Song et al. (2019); Ichikawa et al. (2020). |
| Program T cells directly in situ, eliminating the need for isolating T cells from their physiological environment and reinfusion of CAR-T cells | mRNA | • Polymeric NPs | • Lymphoma • Prostate cancer • Hepatocellular carcinoma |
Parayath et al. (2020) | |
| Plasmid | • Lipid NPs | • Leukemia | Zhou et al. (2022) | ||
| Modulate the immunosuppressive TME to enhance CAR-T cell homing and expansion and sustain their functional persistence | Immunoregulatory agent | • Lipid NPs • Protein nanogels • Multilamellar liposomal vesicles |
• Breast cancer • Melanoma • Ovarian carcinoma |
Siriwon et al. (2018); Tang et al. (2018); Zhang et al. (2018) | |
| OV therapy | Escape from neutralizing antibodies and extend the duration of viral circulation | OV | • Cationic gold NPs | • Prostate cancer | Man et al. (2022) |
| Enable the in situ generation of oncolytic virus and facilitate repeated intravenous administration | Viral RNA genome | • Lipid NPs | • Small cell lung cancer | Kennedy et al. (2022) | |
| Demonstrate inherent selective oncolysis capabilities | No additional cargo | • Repebody-apoptin self-assembled protein NPs | • Breast cancer | Lee et al. (2017) | |
| • Polymer-based NPs | • Pancreatic cancer • Melanoma • Liver cancer |
Liu et al. (2022c); Yu et al. (2023) | |||
| Immune combination therapy | Elicit ICD, remodel the TME, and optimize the effectiveness of combination or sequence treatments | Photothermal agent or photothermal nanomaterial | • Mesoporous silica NPs • Intracellularly generated immunological gold NPs • Ultrasmall FeS-GOx nanodots |
• Melanoma • Breast cancer |
Huang et al. (2021a); Zhang et al. (2019a); Ren et al. (2021) |
| Photosensitizer | • Large-pore mesoporous silica shell • Hyaluronic acid nanomicelles • Polydopamine NPs |
• Metastatic spine tumor • Breast cancer • Colon carcinoma |
Le et al. (2019); Wang et al. (2021c); Wu et al. (2021) | ||
| Chemotherapeutic agent | • Self-assembled polymer micelles • Hollow aluminum hydroxide-modified silica NPs • Metal–phenolic hybrid NPs |
• Breast cancer | Tan et al. (2022); Cheng et al. (2023); Zhang et al. (2023c) | ||
| Radiosensitizing nanomaterial | • Vesicle membrane-coated gold NPs • Lipid-modified manganese diselenide NPs • Hollow MnO2 NPs |
• Colorectal tumor • Esophageal squamous cell carcinoma |
Qin et al. (2021b); Guan et al. (2022); Li et al. (2024a) |
Nanomedicines in ICBs
ICBs were initially developed to disrupt the interaction between inhibitory checkpoint molecules and their corresponding ligands, which have an immunosuppressive effect, thereby enabling immune cells to become activated. Representative inhibitory checkpoints include PD-1, programmed cell death-ligand 1 (PD-L1), indoleamine 2,3-dioxygenase (IDO), and cytotoxic T lymphocyte (CTL)-associated antigen-4 (CTLA-4). Furthermore, an increasing number of stimulatory checkpoint molecules have been harnessed to enhance the immune response through the use of agonistic antibodies in ICB therapy (Marhelava et al., 2019).
Considering the pivotal role of checkpoints in maintaining immune system homeostasis, the unintended off-target accumulation of immune checkpoint inhibitors may trigger immune-related adverse events implicating multiple organs and potentially leading to tumor hyperprogression (Kamada et al., 2019; Okiyama and Tanaka, 2022). Nanomedicine represents a promising approach in the field of immune checkpoint inhibitor delivery, with a diverse array of nanomedicines such as lipid NPs, polymer NPs, biomimetic NPs, MSNs, LPHNs, and others being utilized to modulate checkpoint molecules, demonstrating the capacity to enhance targeting precision and mitigate off-target toxicity (Luo et al., 2018; Zhao et al., 2021; Yu et al., 2022b; Tan et al., 2023). For instance, Wang et al. (2023a) have developed a pH-responsive polymeric nanomedicine that incorporates anti-PD-L1 antibodies and a long-chain PEG coating. The inclusion of the PEG coating serves to mitigate nonspecific cellular uptake and off-tumor accumulation, subsequently facilitating the release of the anti-PD-L1 antibody in response to the acidic TME. In a study conducted by Jung et al. (2022), DC-derived nanovesicles loaded with tumor antigens were employed for the delivery of anti-CTLA-4 antibodies, demonstrating enhanced specific targeting capability towards tumor-specific T cells. As a result, these nanomedicines exhibited enhanced antitumor efficacy without eliciting immune-related adverse events.
Gene therapy provides an alternative approach to traditional antibody-based ICBs, with the potential to either eliminate inhibitory checkpoints or enhance stimulatory checkpoint levels through the introduction of multiple RNAs, thereby contributing to the eradication of cancer. Nanomedicines are increasingly assuming a pivotal role in integrating gene therapy with ICBs (Kiaie et al., 2023). It has been revealed that, in comparison to antibody-based blockades, immune checkpoint silencing based on siRNA-loaded nanomedicines has demonstrated superior efficacy in tumor inhibition (Xue et al., 2021; Won et al., 2022). The observed phenomenon can be attributed to the fundamental suppression of the expression and secretion of checkpoint molecules by siRNA. Moreover, lipid–calcium–phosphate-based NPs have been employed to improve the efficacy of PD-L1 siRNA delivery through the blood–brain barrier (BBB), resulting in the inhibition of glioblastoma multiforme growth (Hsieh et al., 2022). Nanomedicines allow for the simultaneous delivery of multiple RNA molecules to achieve a synergistic effect. An illustrative example is the ROS-responsive polymer nanomedicines co-loaded with siRNAs targeting fibrinogen-like protein 1 (FGL1) and PD-L1, enabling concurrent inhibition of the PD-1/PD-L1 and FGL1/LAG-3 signaling pathways (Wan et al., 2021). It is evident that such dual-pathway blockades have significantly improved the immunosuppressive TME, surpassing single blockade approaches. Lipid nanomedicines co-delivering siRNA and mRNA effectively downregulated PD-L1 expression while concurrently upregulating OX40L levels, resulting in potent inhibition of tumor growth and enhanced immune infiltration with minimal toxicity (Walters et al., 2021).
Another challenge associated with ICBs is the evasion of patients’ immune systems, leading to a low response rate and limited clinical effects. This can be partly attributed to the downregulation of tumor immunogenicity and insufficient infiltration of T lymphocytes (Kim et al., 2018). Therefore, nanomedicines have been developed to harmonize ICBs and multiple immune regulatory reagents to modulate TME as well as alleviate tolerance to ICBs (Wang et al., 2018; Feng et al., 2022). For example, Ge et al. (2022) developed a co-delivery system using modified zeolitic imidazolate framework-8 (ZIF-8) MOFs to encapsulate curcumin (an autophagy activator) and BMS1166 for ICB. This was based on the rationale that autophagy enhances antigen presentation, promotes CTL sensitivity, and induces immunogenic cell death (ICD) (Ge et al., 2022). The results demonstrated that the co-delivery system effectively suppressed tumor growth and elicited strong and durable immune memory against tumor recurrence by distinctly inducing DC maturation and potent ICD. Additionally, polymer nanomedicines were designed to load plasmid DNA encoding immunostimulatory chemokine CCL19 and PD-L1 inhibitor (BMS-1), aiming to stimulate multiple immune regulatory pathways (Yu et al., 2021). Following the administration of these nanomedicines, a significant improvement in TME was observed, characterized by high local concentrations of immunostimulatory cytokines and synergistically enhanced antitumor efficacy. The combination of targeted therapy using BRAF inhibitor and PD-L1 inhibition through miR-200c, delivered via polymer nanomedicines conjugated with CXCR-4 antagonist peptides, resulted in a potent immune response with increased T-lymphocyte infiltration and decreased number of Treg cells (Nguyen et al., 2021).
Overall, the integration of nanomedicines with ICBs holds great promise for mitigating associated adverse effects and improving treatment outcomes by enabling targeted delivery, incorporating gene therapy, and coordinating multiple immunoregulatory agents.
Nanomedicines in mAb therapy
mAbs refer to a class of antibodies that specifically recognize and bind to a particular antigen epitope, and have been extensively investigated in the field of tumor immunotherapy. Structurally, mAbs consist of Fv and Fc regions, which respectively govern the affinity of mAbs and their ability to interact with the immune system (Kimiz-Gebologlu et al., 2018). Taking advantage of their specificity and versatility, mAbs can be engineered to achieve antitumor efficacy through various mechanisms, broadly categorized as either blocking signal pathways involved in tumorigenesis or activating the immune response to inhibit tumor growth (Carvalho et al., 2016). However, mAb therapy is confronted with challenges such as high background expression of the corresponding antigen, undesirable side effects, and development of resistance mechanisms as well as low tumor penetration (Christiansen and Rajasekaran, 2004).
Nanomedicines provide a practical and straightforward approach to enhance mAb therapy. The conjugation of cetuximab with gold NPs improved the inhibition of epidermal growth factor receptor (EGFR) signaling and significantly reduced the receptor recycling process, resulting in enhanced therapeutic effects at lower cetuximab dosages (García-Fernández et al., 2017). A study conducted by Luis de Redín et al. (2020) revealed that bevacizumab-loaded albumin nanomedicines effectively increased intratumor levels of bevacizumab while reducing accumulation in the bloodstream compared to the free formulation, indicating a decreased risk of side effects and improved antitumor efficacy. A study by Truffi et al. (2018) demonstrated that the diversified arrangement of trastuzumab half chains on the surface of magnetic iron oxide NPs contributed to an enhanced antitumor effect and mitigated trastuzumab-resistance in HER2+ breast cancer cells, while also exhibiting favorable targeting properties. Taking into account the ability of ferritin to permeate the trans-BBB via TfR1, Sevieri et al. (2023) synthesized trastuzumab-conjugated ferritin nanomedicines for the treatment of brain metastasis of HER2+ breast cancer. Consequently, these nanomedicines exhibited efficient accumulation in the brain, reduced off-target toxicity, and improved efficacy upon systemic administration. Moreover, mAbs-modified nanomedicines are employed to achieve binary drug delivery in conjunction with additional therapeutic agents, capitalizing on their precise targeting and efficient accumulation in tumors (Wang et al., 2017). An example in point is the development of cetuximab-modified MSN-based nanomedicines by Chen et al. (2021b). These nanomedicines maintained the capability to suppress the EGFR-associated signaling pathway while enhancing the antitumor efficacy of encapsulated chemotherapeutics.
Nanomedicines in cytokine therapy
Cytokines, small soluble proteins secreted by a variety of cells, mediate multiple signaling pathways and directly participate in regulating the immune system. They also play a crucial role in tumor pathogenesis and antitumor immune response. Therefore, cytokines such as interleukin-2 (IL-2), type I interferon (IFN), IL-12, and chemokine (C–C motif) ligand 21 (CCL21) have been employed for antitumor immunotherapy (Qiu et al., 2021). However, frequent and high-dose administration is required for cytokine delivery owing to their short half-life (Dholakia et al., 2022). Additionally, the administration of cytokines often leads to systemic toxicity due to their nonspecific internalization by a wide range of cells with appropriate receptors. In response to these challenges, nanomedicines loaded with cytokines have been developed for cytokine therapy, capitalizing on their ability to achieve sustained drug concentrations and adequate tissue deposition without reaching toxic loading doses associated with systemic administration (Ye et al., 2014; Lacinski et al., 2022). As an example, a liposome conjugated with IL-2 was modified with polyelectrolyte layer-by-layer to construct a nanomedicine (Barberio et al., 2020). The polymer layers served as camouflage to maintain cytokine activity, reduce systemic exposure, and selectively localize on the tumor membrane. This approach resulted in favorable efficacy and reduced toxicity at equivalent doses in multiple cancer models.
The employment of nanomedicines for the transfection of nucleic acid-encoding cytokines offers a preferable alternative to cytokine delivery, due to its advantages in efficient delivery, internalization, and localized and sustained production (Nguyen et al., 2020a). The intratumoral administration of artificially engineered IL-2 encoding mRNA via polyethyleneimine-modified porous silica NPs gave rise to high-level expression of the protein, remodelling of TME, and enhanced antitumor efficacy (Shin et al., 2023). Based on the screening results from a library of diverse ionizable lipids, Xu et al. (2024) synthesized innovative lipid NPs with intrinsic stimulation capability of the nuclear factor-κB (NF-κB)/IRF immune pathway. These NPs significantly enhanced transfection efficiency for IL-12 circular RNA and promoted immune infiltration, leading to tumor regression following a singular intratumoral administration (Xu et al., 2024). Lipid-based NPs encapsulating both IL-12 mRNA and IL-27 mRNA exhibited a significant synergistic effect, leading to the robust infiltration of immune effector cells without inducing systemic toxicity (Liu et al., 2022b). Ionizable lipid nanomedicines containing self-replicating IL-12 RNAs facilitated high-level expression of IL-12, while also eliciting a highly inflamed TME through the intrinsic cytotoxicity of the carriers (Li et al., 2020).
Nanomedicines in the tumor vaccine
The tumor vaccine is a formulation incorporating tumor antigens and adjuvants, aimed at stimulating the antigen-specific response of patients’ immune systems to eliminate tumor cells and establish long-term immunological memory (Gurunathan et al., 2024). The integration of nanomedicines into tumor vaccines as delivery systems enhances the overall vaccine efficacy, providing improved characteristics such as antigen protection, precise targeting, controlled release, prolonged antigen presentation by antigen presenting cells (APC), and enhanced T-cell immune infiltration (Mueller et al., 2015; An et al., 2017; Smith et al., 2022; Zhao et al., 2023). Besides, the nanomedicines possess the capability to optimize vaccine immune phenotype by tailoring physicochemical properties (Qin et al., 2021a). Vaccines formulated at the nanoscale are commonly known as nanovaccines. Various types of nanomaterials have been utilized for the development of tumor nanovaccines, which can be broadly categorized into lipid-based, polymer-based, inorganic-based, and biomimetic tumor nanovaccines based on their respective material compositions.
In the field of nanovaccines, lipid nanomedicines have garnered significant attention, particularly in light of the success achieved with lipid-based COVID-19 mRNA vaccines. The utilization of lipid nanomedicines has been extensive in the development of tumor vaccines and has also been translated into commercially viable products due to their scalable manufacturing processes (Aldosari et al., 2021). Owing to the facile adjustment of lipid composition, a diverse array of lipid-based nanovaccines has been developed. For instance, through screening a library of lipid formulations, lipids with an unsaturated lipid tail, a dihydroimidazole linker, and cyclic amine head groups were identified as optimal candidates for mRNA nanovaccines due to their ability to activate the stimulator of interferon genes (STING) pathway and induce APC maturation, thereby enhancing antitumor efficacy (Miao et al., 2019). Heterocyclic lipid nanomedicines have been utilized for targeted delivery to draining lymph nodes and activation of STING. These nanomedicines were employed to encapsulate ovalbumin (OVA) antigen and signal transducer and activator of transcription 3 (STAT3) siRNA, resulting in a favorable anticancer response (Liu et al., 2023b). Li et al. (2023d) developed minimal-component nanovaccines derived from monophosphoryl lipid A, which is a Toll-like receptor-4 immune agonist. Consequently, these nanovaccines induced a shift in TME to an immunologically ‘hot’ state and successfully increased the ratio of CD8+ T cells/Tregs. Chen et al. (2022) constructed spontaneous lymph node-targeting lipid mRNA nanovaccines without ligand modification as a versatile platform enabling easy production. Consequently, these nanovaccines mitigated undesirable hepatic mRNA expression following subcutaneous administration and elicited enhanced APC infiltration at the tumor sites.
Polymer nanomedicines, characterized by their structural diversity, biocompatibility, biodegradability, and modifiability, have been widely embraced as vaccine platforms. For instance, Xu et al. (2022) designed a mannan-modified polymeric nanovaccine with protein antigens and CpG coupling to the polymeric core via electrostatic interactions. These nanovaccines evoked favorable antitumor effects in several murine tumor models with significantly increased accumulation in CD8+ DCs. Additionally, Zhao et al. (2022a) synthesized simplified polymeric nanovaccines incorporating innate stimulating properties based on polyethyleneimine with azole molecules at the end. These nanovaccines have demonstrated superior performance compared to the traditional ternary vaccine system, offering the advantage of meeting individual requirements through combination with autologous tumor membrane protein antigens. Furthermore, stimuli-responsive polymer nanovaccines exhibit promising potential by enabling intelligent delivery for achieving spatiotemporally controlled release, preventing content inactivation, and enhancing specific uptake of APCs as well as the cytosolic release (Zhou et al., 2020; De Mel et al., 2022; Mao et al., 2023). Su et al. (2023) developed a pH-responsive polymeric nanovaccine from multiple functional blocks, which separately facilitated electrostatic and hydrophobic interactions as well as maintained pharmaceutical stability. As a result, these nanovaccines demonstrated the capability to coordinate bi-adjuvant and peptide neoantigens, leading to the alleviation of tumor immunosuppression and enhanced antitumor responses. A pH-responsive nanovaccine was synthesized leveraging self-assembled polymers, allowing for the simultaneous loading of novel cyclic dinucleotide adjuvants and neoantigens (Su et al., 2022). These nanovaccines facilitated endosomal escape and content release in response to acidic conditions, subsequently eliciting robust antigen-specific T-cell responses with immune memory.
Inorganic nanomedicines with well-defined physicochemical properties, high surface-to-volume ratio, uniformity, and facile preparation are advantageous for the design of nanovaccines (Hess et al., 2019). For instance, a nanovaccine derived from mannose-modified multi-walled CNT exhibited low cytotoxicity, impressive antigen-loading capacity, and specific DC targeting, turning out to evoke an effective immune response (Dong et al., 2019). An antigen-coding DNA-loaded nanovaccine was developed based on mesoporous silica microrods, which have the ability to self-assemble into 3D scaffolds at the injection site (Nguyen et al., 2020b). The macroporous structure of the scaffold facilitated the recruitment and sustained DNA uptake of host DCs, leading to the generation of Th1 humoral responses and CD8+ T-cell responses. In a study conducted by Wang et al. (2023b), Au nanovaccines loading OVA were constructed with virus-like spiky surfaces. These nanovaccines demonstrated efficient delivery and cross-presentation of antigens, along with good biocompatibility and potent adjuvant activity due to their biomimetic structures. The graphene oxide-based nanovaccines synthesized by Zhang et al. (2022a) presented a significant impact on tumor suppression, as they were capable of promoting DC maturation and secretion of proinflammatory cytokines. Several metal nanomedicines, when used as delivery systems, have the capability to directly regulate the immune response through various mechanisms, rendering it feasible to coordinate with nanovaccines (Li et al., 2022a; Yavuz et al., 2023). An illustrative example is the MnO2 nanovaccines loading OVA constructed by Gu et al. (2023), which functioned as a source of Mn2+ to stimulate the STING pathway. As a result, the immunosuppressive TME was reprogrammed through macrophage repolarization and DC maturation. Furthermore, Fe3O4 nanocarriers were identified to be involved in macrophage activation and DC maturation, leading to the inhibition and prevention of subcutaneous or metastatic tumor growth when combined with OVA (Luo et al., 2019a).
Biomimetic nanomedicines have expanded the scope of nanotechnology in the development of nanovaccines, which have been verified to contribute to enhanced biosafety and better antitumor responses (Yang et al., 2020; Johnson et al., 2022). Membrane coated nanovaccines preserve the biological functionality of their source cells and enable the loading of antigens and other therapeutic agents within synthetic cores (Liu et al., 2022a). For instance, the fusion membranes resulting from DCs and tumor cells retained tumor cell membrane antigens as well as MHC and co-stimulatory molecules from mature DCs. As a result, the fusion membrane coating equips NPs with the ability to directly and indirectly initiate specific immune responses (Zhang et al., 2022b). In a study conducted by Liu et al. (2023a), a novel nanovaccine encapsulating tumor-specific antigen (TSA) was camouflaged with cell membranes derived from tumors going through ICD. Leveraging the simultaneous delivery of TSA and a wide range of membrane-associated antigens, this nanovaccine demonstrated the ability to overcome tumor immune suppression and evasion. Exosomes have been extensively employed in the development of tumor nanovaccines, demonstrating superior efficacy compared to liposomal formulations due to the presence of exosomal proteins (Li et al., 2023b). In a study led by Zuo et al. (2022), a personalized nanovaccine with universal applicability was developed using DC-derived exosomes. These exosomes were modified to display an α-fetoprotein epitope, a functional domain of high mobility group nucleosome-binding protein 1, and a hepatocellular carcinoma-targeting peptide. This design enabled the nanovaccine to serve dual functions, (i) as DC vaccines and (ii) to recruit and activate endogenous DCs at tumor sites, thereby eliciting tumor-specific immune responses against neoantigens. Hu et al. (2021) isolated exosomes from tumor cells engineered with the fibroblast activation protein-α gene to develop nanovaccines. It is noteworthy that these exosome-based nanovaccines effectively target both the tumor parenchyma and stroma, thereby mitigating immunosuppression and inhibiting tumor growth in an innovative manner.
In conclusion, a wide range of nanomedicines have been utilized in the development of tumor vaccines, showcasing significant potential in addressing the challenges encountered by traditional vaccine formulations, such as relatively low immunogenicity, limited delivery efficiency, and immunosuppressive TME. The diverse physicochemical properties of nanomaterials and their adaptable modifications contribute to the precise design of nanovaccines tailored for specific antigen-adjuvant combinations, action sites, and types of tumors. This ultimately leads to enhanced immune responses and improved therapeutic efficacy.
Nanomedicines in ACT
ACT is designed to utilize the potential of natural or genetically modified lymphocytes for the identification and eradication of tumors, typically involving original cell extraction, in vitro manipulation, and subsequent back-infusion (Rosenberg et al., 2023). Tumor-infiltrating lymphocytes, engineered T-cell receptor (TCR)-T cells, and chimeric antigen receptor (CAR)-T cells are representative categories of ACT (June et al., 2018). Among them, CAR-T therapy has made remarkable clinical advancements with six products approved by the FDA so far. CAR-T cell therapy entails the modification of T cells to express CARs, enabling them to precisely identify and eliminate cells expressing a specific antigen (Sterner and Sterner, 2021). The current synthesis process of CAR-T cells primarily relies on in vitro gene editing of extracted T cells, which is both costly and intricate (Billingsley et al., 2020). In addition to the complicated in vitro synthesis procedures, the application of CAR-T cell therapy subjects to insufficient T-cell expansion and a short valid period as well (Zheng et al., 2021). To address these issues, the utilization of nanomedicines has been introduced to expedite T-cell expansion, mediate in situ T-cell transfection, and maintain the functional persistence of back-infused CAR-T cells.
The adequate quantity of T cells is a prerequisite for achieving optimal ACT efficacy. Nanomedicines designed to imitate natural APCs by conveying immunostimulatory signals have been broadly employed to promote the activation and proliferation of isolated T cells (Zheng et al., 2021; Figure 4A). These nanomedicines are termed nanoscale artificial APC (nano-aAPC), characterized by providing two signals including TCR activation and co-stimulation (Wang et al., 2021b). As an example, iron–dextran nano-aAPCs carrying anti-CD28 and human MHC-I molecules binding antigenic peptides have possessed the ability to rapidly promote antigen-specific T-cell proliferation up to 1000-fold in vitro (Ichikawa et al., 2020). Additionally, paramagnetic iron–dextran nano-aAPCs have been identified to contribute to the aggregation of TCR clusters in an externally applied magnetic field, thereby further facilitating T-cell activation and proliferation both in vitro and in vivo (Perica et al., 2014). Song et al. (2019) developed a PEGylated PLGA ellipsoidal nano-aAPC coupled with H-2Kb/TRP2180-188-Ig dimers, anti-CD28, and CD47-Fc, achieving enhanced antigen-specific T-cell proliferation while minimizing cellular uptake.
Figure 4.
Schematic diagram of the mechanism of nanomedicines in CAR-T therapy. (A) Through the incorporation of TCR activation signals such as pMHC and co-stimulation signals such as anti-CD28 antibody, nanomedicines are engineered to mimic APCs in order to enhance ex vivo T-cell activation and proliferation. This strategy is referred to as nanoscale artificial APC. (B) The application of DNA/mRNA-bearing nanomedicines enables the direct programming of T cells in situ upon administration, thereby eliminating the need for isolating T cells from their physiological surroundings. (C) Nanomedicines loaded with immunomodulatory reagents, which are coupled to the surface of CAR-T cells, have the ability to infiltrate tumor sites, effectively reshaping the immune microenvironment while simultaneously preserving the viability of CAR-T cells. This figure is created with BioRender.com.
Nanomedicines have the potential to serve as substitutes for conventional transfection methods, such as viral vectors and electroporation. They demonstrate low immunogenicity, improved transfection efficiency, enhanced safety, and reduced cytotoxicity (Zeng et al., 2023). Furthermore, DNA/mRNA-bearing nanomedicines that are modified with targeting ligands provide a straightforward and cost-effective approach to directly program T cells in situ (Figure 4B). This eliminates the necessity of isolating T cells from their physiological environment (Parayath and Stephan, 2021). Importantly, the in situ programming method based on nanomedicines enables precise targeting, reduced nonspecific internalization, and mitigated side effects associated with CAR-T therapy (Sledz et al., 2023). As an example, Parayath et al. (2020) developed injectable polymeric nanomedicines modified with anti-CD8 antibodies for the delivery of CAR-coding mRNA, enabling efficient large-scale manufacture and long-term storage, thus facilitating convenient repeated administration. These nanomedicines transiently reprogramed circulating T cells to activate antitumor responses with transfected T cells expressing CAR for an average of 7 days. Furthermore, lipid nanomedicines modified with the anti-CD3 antibody were employed to encapsulate plasmids containing IL-6 short hairpin RNA (shRNA) and the CD19-CAR gene (Zhou et al., 2022). The resulting IL-6-knockdown CAR-T cells exhibited reduced IL-6 release following transfection, thereby mitigating the risk of cytokine release syndrome while demonstrating antitumor effects comparable to in vitro-prepared CAR-T cells.
The presence of cytokines and inhibitory molecules from tumor-associated immunosuppressive cells and the tumor itself can lead to rapid loss of validity of CAR-T cells (Kong et al., 2023). Therefore, the administration of CAR-T cells necessitates concurrent delivery of carefully selected adjuvants to modulate the immunosuppressive TME and sustain the functional persistence of CAR-T cells. Nanomedicines provide a targeting platform for supporting CAR-T cells while minimizing systemic exposure of adjuvants. The internalizing RGD peptide (iRGD)-modified lipid nanomedicines carrying a combination of phosphatidylinositol 3-kinase (PI3K)-δ inhibitors and α-GalCer agonists have been demonstrated to induce a transition in the TME from suppressive to stimulatory (Zhang et al., 2018). This resulted in the establishment of a therapeutic window lasting 2 weeks following repeated administration, thereby facilitating subsequent CAR-T cell homing, expansion, and successful tumor regression. In addition, surface conjugation with nanomedicines has been demonstrated to synchronize the efficacy of back-infused CAR-T cells and adjuvant treatments preferably, ultimately preserving CAR-T cell activity and enhancing therapeutic efficacy (Stephan et al., 2010; Figure 4C). For example, protein nanogels have been utilized to load the IL-15 superagonist complex and then conjugated in large quantities to the T-cell surface. This backpack strategy allows for higher dosages of cytokine administration tolerance compared to systemic delivery of free cytokines, leading to significant amplification of T cells and ultimately improving CAR-T cell therapy (Tang et al., 2018). Furthermore, coupling nanomedicine with CAR-T cells results in prolonged circulation and reduced liver clearance, taking advantage of the inherent mobility of T cells to penetrate deep inside tumors. This contributes to better performance in restoring the activity of hypofunctional CAR-T cells (Siriwon et al., 2018).
In summary, nanomedicines not only optimize the in vitro manufacturing strategy of CAR-T cells but also offer a versatile platform for in vivo programming and functional maintenance of CAR-T cell-mediated antitumor effects, thereby expanding their potential clinical applications with cost-effectiveness, convenient operation, and personalized design.
Nanomedicines in OV immunotherapy
OVs, comprising protein shells and nucleic acids, present high invasiveness, robust replication, and rapid propagation capabilities within tumor cells. They possess the capability to lyse tumor cells and subsequently trigger an immune response (Fang et al., 2023a). Systemic delivery of OVs is the preferred clinical method owing to its simplicity and extensive coverage of tumor sites. Nevertheless, it faces challenges such as inadequate bioavailability and biodistribution, which hamper its antitumor efficacy (Yokoda et al., 2017). The implementation of nanomedicines can be considered an effective strategy to provide shielding for OVs (Figure 5A). Erythrocyte–liposome hybrid nanovesicles have been demonstrated to fully shield OVs while preserving their structural integrity, thereby preventing neutralization and prolonging circulation following intravenous injection (Huang et al., 2022). Thiolated chitosan nanovesicles functionalized with hyaluronic acid have been adopted to encapsulate the Newcastle disease virus and oral measles vaccine strain (Kousar et al., 2023; Naseer et al., 2023). These nanomedicines sheltered OVs from antibody-mediated clearance and achieved sustained release, active targeting, and receptor-mediated endocytosis. The cationic gold NP coating conferred enhanced internalization and replication capacity to the virus while preventing detrimental blood factor binding (Man et al., 2022).
Figure 5.
Schematic diagram of the mechanism of nanomedicines in OV therapy. (A) Nanomedicines confer protection to the virus against neutralizing antibodies and prolong its circulation time. (B) Nanomedicines loaded with viral RNA genomes function as synthetic OVs, facilitating the virus genome amplification and virus assembly in situ via transfection, thereby inducing oncolysis. (C) The utilization of specific nanomedicines with membranolytic activity allows for the simulation of OVs. These nanomedicines undergo protonation and morphological transformation in response to changes in pH within tumor tissues, thereby activating their membranolytic components to selectively lyse tumor cell membranes. This figure is created with BioRender.com.
The use of nanomedicine to load subunits of OVs presents a promising alternative to using entire OVs, as it helps mitigate the risks associated with excessive immune activation (Diep et al., 2022). As an example, lipid-based NPs have been utilized as synthetic OVs, wherein the viral RNA genome is encapsulated. Following internalization, they subsequently facilitate in situ formation of OVs, leading to oncolysis while evading neutralizing antibodies and allowing for repeated intravenous administration (Kennedy et al., 2022; Figure 5B). Additionally, engineered protein-based nanomedicine derived from the self-assembly of fusion proteins of EGFR-specific repebody and apoptin demonstrates active targeting and selective oncolysis capabilities (Lee et al., 2017). These nanomedicines offer a feasible platform for the systematical delivery of active tumor-selective oncolytic proteins.
Membranolytic nanomedicines with an amphiphilic structure, comprising cationic and hydrophobic moieties, have been utilized to perform a function similar to that of conventional OVs (Park et al., 2018; Figure 5C). As a representative example, Liu et al. (2022c) designed a library of ‘proton transistor’ nanodetergents (pTNTs). In response to subtle pH changes within tumor tissues, pTNTs under went a rapid transition in their protonation state, resulting in a morphological transformation and activation of the membranolytic components. This unique mechanism allowed pTNTs to selectively lyse tumor cell membranes while minimizing toxicity to normal tissues. A pH-sensitive polymer NP composed of poly(ethylene glycol)-poly(2-azepane ethyl methacrylate) was designed analogously (Yu et al., 2023). These polymer NPs maintained their aggregated morphology without exhibiting any cytotoxic effects. Upon protonation in TME, they disassembled into cationic-free chains or smaller cationic NPs, initiating potent membrane-disruptive activity.
Nanomedicines in immune combination therapy
Low immunogenicity of tumors and immunosuppressive TME contribute to the intrinsic resistance of immunotherapy. In the context of combination therapy, there is a growing concentration on integrating ICD with tumor immunotherapy as a universal strategy to address immune resistance and synergistically enhance therapeutic efficacy. ICD refers to a specific process of tumor death followed by the secretion of antigens, proinflammatory factors, and damage-associated molecular patterns, thereby enhancing the immunogenicity and exerting an adjuvant effect (Qi et al., 2021). This mechanism effectively converts the previously ‘cold’ TME into a ‘hot’ one, thereby improving immune infiltration and bolstering tumor immune responses (Ahmed and Tait, 2020).
Nanomedicine offers a versatile platform to harmonize multiple modalities, even in a spatiotemporally controlled manner. Therefore, the use of nanomedicine in combination therapy has the potential to address the limitations of monotherapy and enhance efficacy (Li et al., 2023a). In the subsequent section, we primarily discuss the application of nanomedicines in combining tumor immunotherapy with ICD-inducing therapies, such as PTT, PDT, chemotherapy, and radiotherapy.
Combination of immunotherapy and PTT
PTT involves the use of photothermal agents to induce hyperthermia, leading to destruction of the cell membrane, damage to the cytoskeleton, and inhibition of DNA synthesis, ultimately resulting in apoptosis of cancer cells (Huang et al., 2021b). It has been verified that PTT can improve T-cell infiltration and reduce the number of Tregs, thereby increasing tumor sensitivity to ICBs (Ma et al., 2019). Inorganic photothermal agents such as gold, carbon, sulfide metallic, and black phosphorus can be engineered as nanocarriers for combination therapy (Huang et al., 2021b). As an example, novel immunological gold NPs were synthesized intracellularly and exocytosed as vesicles by murine melanoma cells. Subsequently, these gold NPs, which retained the original tumor antigens, were internalized and secreted by DCs in vitro, thereby functioning similarly to DC vaccines upon injection and mediating PTT as well (Zhang et al., 2019a). Ren et al. (2021) devised self-assembled nanodots to orchestrate cascade reactions, encompassing starvation, chemotherapy, CDT, and PTT. Building on the foundation of anti-CTLA4 checkpoint blockade, the cascade reaction significantly enhanced ICD, facilitated the infiltration of CTLs into distant tumors, and effectively eliminated primary tumors with improved tumor penetration. An MSN-based nanoplatform integrated with a photothermal agent polydopamine and OVA demonstrated remarkable efficacy with a 75% cure rate in B16-OVA melanoma tumors following a single injection and a single round of PTT (Huang et al., 2021a).
Combination of immunotherapy and PDT
PDT involves the activation of photosensitizers through the absorption of excitation light photons. These activated photosensitizers can then interact with endogenous substances or molecular oxygen to generate ROS and singlet oxygen, ultimately resulting in necrosis and apoptosis (Yuan et al., 2021). In addition, the antitumor vascular effect of PDT leads to tumor death by depriving the tumor of oxygen and nutrients (Kong and Chen, 2022). Hypoxia induced by PDT triggers the upregulation of hypoxia-inducible factor 1α, which subsequently increases PD-L1 levels. This elevation in PD-L1 expression enhances the antitumor effects of PDT when combined with ICBs (Yuan et al., 2021). For example, the use of mesoporous NPs to facilitate combination therapy involving IDO-derived peptide nanovaccines, PDT, and a PD-L1 inhibitor resulted in significant accumulation within tumor tissue and a synergistic capacity to induce ICDs. This versatile nanoplatform triggered robust systemic antitumor immunity and effectively delayed the progression of metastatic spinal cancer (Wang et al., 2021c). Furthermore, modification of anti-PD-L1 antibodies on photoresponsive polydopamine NPs enhanced NP binding to cancer cells and subsequently induced a more potent photothermal anticancer effect (Le et al., 2019). Besides, these NPs demonstrated the ability to capture antigens and function as in situ vaccines following PDT-induced ICD, providing long-lasting protection against tumor recurrence. The self-assembled micelles resulting from the photosensitizer Ce6 conjugated to polysaccharide hyaluronic acid functioned as a convenient and light-responsive delivery platform to orchestrate PDT and immunotherapy (Wu et al., 2021). Upon encapsulation of IDO inhibitors, these nanomedicines significantly suppressed triple-negative and poorly immunogenic 4T1 breast cancer.
Combination of immunotherapy and chemotherapy
Chemotherapy has the ability to directly deplete immune-suppressive cells, bolster effector T cells by modulating related cytokine levels, and induce TSA exposure as well as death receptor expression, rendering tumor cells more susceptible (Wu and Waxman, 2018; Yu et al., 2019). Operating as a negative feedback mechanism, PD-L1 undergoes transcriptional upregulation in response to activated NF-κB and various proinflammatory cytokines following chemotherapy. This adaptive response serves to bolster the efficacy of PD-1/PD-L1 inhibitors (Antonangeli et al., 2020; Yamaguchi et al., 2022). A nanomedicine composed of doxorubicin, photosensitizer, and cleavable peptide was engineered to mediate photochemotherapy to effectively deplete immunosuppressive cells such as Tregs and myeloid-derived suppressor cells, as well as remodel TME via ICD (Choi et al., 2021). When coordinating with PD-L1 blockades, these nanomedicines significantly enhanced the immune responses leading to the complete regression of tumors. Through the co-delivery of siCD47, siPD-L1, and camptothecin using nanomedicines developed by Zhang et al. (2023b), TME reversion was facilitated via simultaneous ICD and immune checkpoint molecule downregulation, resulting in improved infiltration of CD68+ macrophages and CD4+ and CD8+ T cells.
Despite the immunoregulatory effect of chemotherapy, which has been widely employed to orchestrate ICBs clinically, chemoresistance remains a major obstacle. A diverse range of NPs have been engineered to reverse chemoresistance by mitigating hypoxia in TME, regulating intracellular homeostasis, and inducing the differentiation of cancer stem cells (Jiang et al., 2022; Wang et al., 2022a; Shen et al., 2023). Jiang et al. (2021) synthesized tumor homing-penetrating peptide-modified nanomedicines loading copper tetrakis(4-carboxyphenyl)porphyrin and paclitaxel. These nanomedicines effectively depleted endogenous glutathione and downregulated P-glycoprotein expression in combination with PD-1/PD-L1 blockades, collectively overcoming chemoresistance and activating an immune response. A metal–phenolic hybrid NP composed of tannic acid and Fe3+ has been employed to deliver paclitaxel (Cheng et al., 2023). Upon internalization by tumors, these NPs depleted adenosine triphosphate (ATP) while disassembling, which suppressed ATP-dependent drug efflux and released paclitaxel. Coordinating with PD-1/PD-L1 blockades and PTT, these NPs alleviated chemoresistance and inhibited tumor progression.
Combination of immunotherapy and radiotherapy
Radiotherapy has the potential to induce DNA damage and facilitate tumor eradication through the use of high-energy ionizing radiation and generated ROS. Additionally, it has been observed that localized treatment of high-dose radiotherapy can trigger systemic immune responses, which contribute to the management of distant metastases, known as the abscopal effect (Herrera et al., 2017). Furthermore, low-dose radiation can promote transient tumor inflammation, recruit DCs, and activate effector CD4+ predominantly to execute a cytolytic transcriptional program, rendering them susceptible to immunotherapy (Herrera et al., 2022). Yu et al. (2022a) developed DC vaccines loaded with whole antigens based on ovalbumin-biomineralized NPs. Consequently, these NP-based DC vaccines, in synergy with radiotherapy, enhanced the infiltration of CTLs, improved OVA-specific immunoglobulin G levels, and reduced the percentage of Treg cells. A glutathione-modified nanomedicine coated with fusing membranes of tumor cell membranes and bacterial outer membranes was constructed to coordinate tumor vaccines and radiotherapy (Pan et al., 2022a). In comparison to the efficacy of sole nanomedicines, the incorporation of radiotherapy significantly increased the ratio of M1 macrophages and CD8+ T cells, while upregulating the IL-6 and IFNγ level, thereby highlighting the pivotal role of radiotherapy in amplifying the immune response.
Furthermore, the nanomedicine-mediated TME remodel contributes to radioresistance reversion. For instance, lipid-modified manganese diselenide NPs have demonstrated the capability of releasing Mn2+ in TME and generating ROS to augment radiosensitivity (Li et al., 2024a). Additionally, the ROS-activated cGAS–STING pathway in conjunction with cytokine secretion improved by Se− contributed to a potent immune response. Analogously, hollow MnO2 NPs loaded with a PI3Kγ inhibitor were utilized to reverse immunosuppressive TME and achieve hypoxia-alleviated radiotherapy through Mn2+-based catalyzing of oxygen synthesis (Guan et al., 2022). When combined with PD-L1 blockade, these NPs significantly inhibited locally residual and distant metastatic tumors. In light of the excessive accumulation of reduced glutathione leading to radioresistance, Song et al. (2024) developed a polymer-based nano-prodrug capable of delivering cinnamaldehyde and all-trans retinoic acid simultaneously in vivo, with the specific aim of depleting glutathione, disrupting redox homeostasis, and enhancing ferroptosis. Subsequently, potent systematic immune responses were elicited by radiotherapy, resulting in tumor suppression when combined with anti-PD-1 therapy.
The utilization of nanomedicine-based immune combination therapy represents a prominent research trend, demonstrating the capacity to reverse immunosuppressive TME and enhance immune responses. The rational design of nanomedicines also exhibits potential in overcoming radio/chemoresistance, thereby further contributing to immune combination therapy. However, a limited understanding of the molecular mechanisms underlying the action of combined drugs presents a significant challenge to rational nanomedicine-based combination therapy. Random combinations of different treatments may exacerbate adverse reactions and induce unexpected cross-resistance, thereby compromising the efficacy of immunotherapy (Haas et al., 2021). Therefore, the identification and mechanistic exploration of tumorigenesis, immune characteristics of TME, treatment response, and resistance development are crucial for informing judicious and precise drug/therapy combinations, thus advancing the development of corresponding nanomedicines (Paudel and Quaranta, 2019; Tutuncuoglu and Krogan, 2019).
Clinical applications of nanomedicines
The field of nanotechnology has significantly advanced the diagnosis, treatment, and prognosis of various diseases, demonstrating a promising potential for widespread clinical application. It is reported that nanomedicines applied in clinical oncology have captured ∼35% of the total market share (Gadekar et al., 2021). The global nanomedicine market is experiencing continuous growth and is projected to reach a value of US  350.8 billion by 2025 (RitaBosetti and Jones, 2019). The FDA has approved multiple antitumor nanomedicines, primarily based on liposomes, polymeric micelles, and albumin NPs for marketing. The majority of ongoing clinical trials are focused on exploring these nanomedicines for new indications and multidrug combination therapy (Huang et al., 2020b).
350.8 billion by 2025 (RitaBosetti and Jones, 2019). The FDA has approved multiple antitumor nanomedicines, primarily based on liposomes, polymeric micelles, and albumin NPs for marketing. The majority of ongoing clinical trials are focused on exploring these nanomedicines for new indications and multidrug combination therapy (Huang et al., 2020b).
The integration of nanomedicine contributes to enhanced safety and favorable efficacy compared to traditional formulations of antitumor agents. A direct comparison between conventional docetaxel and CPC634, an innovative formulation of polymeric micelles cross-linked with docetaxel, revealed that CPC634 exhibited distinct pharmacokinetic profiles with a remarkable 461% increase in intratumoral total docetaxel concentration and reduced risk of neutropenia (Atrafi et al., 2020). Polymeric micellar paclitaxel combined with cisplatin resulted in an objective response rate of 50%, a treatment-related serious adverse event incidence of 9%, and a significant increase in progression-free survival by 1.1 months during the treatment of advanced non-small-cell lung cancer, surpassing solvent-based paclitaxel (Shi et al., 2021). Additionally, several innovative nanomedicines validated in preclinical research have successfully entered the clinical trial stage. AZD2811 refers to a polymeric nanomedicine encapsulating a selective inhibitor of AURKB activity, which has completed a phase I study for advanced solid tumor treatment, demonstrating manageable adverse events and preliminary antitumor efficacy with a stable disease rate of 45.1% (Johnson et al., 2023). A novel formulation utilizing albumin-bound rapamycin NPs, known as nab-sirolimus, completed a phase II clinical trial with an impressive overall response rate of 38.7% and a median duration of response over 3 years and was approved by the FDA for metastatic or locally advanced perivascular epithelioid cell tumors in 2021 (Wagner et al., 2021, 2024).
Despite extensive preclinical research on nanomedicine-mediated tumor immunotherapy and the development of various intelligent delivery systems, only a small fraction of nanomedicine-based immunotherapy progressed to the clinical stage with emphasis on the advancement of cancer nanovaccines and related combination therapies (NCT04573140). A noteworthy illustration is the utilization of nanovaccines loaded with New York esophageal squamous cell carcinoma-1 (NY-ESO-1). NY-ESO-1 exhibits expression in a wide variety of tumor types, while being restrictedly expressed in germ cells and placental cells, thus presenting a promising target for nanovaccines (Thomas et al., 2018). PLAG-based nanovaccines, encapsulating both NY-ESO-1 antigen peptides and threitolceramide-6, are currently undergoing phase I clinical trials (NCT04751786). In a separate phase I clinical study involving advanced or recurrent esophageal cancer, it was reported that NY-ESO-1 nanovaccines, in combination with poly-ICLC, elicited a robust antibody immune response while minimizing toxicities (Ishikawa et al., 2021). Furthermore, the collaboration between cognate antigen-recognizing TCR-T cell therapy and the Pullulan nanovaccine loading long peptide antigens containing the NY-ESO-1 epitope resulted in stable disease duration over 2 years, accompanied by the enduring presence of TCR-T cells in one patient (Ishihara et al., 2023). Personalized vaccines represent a significant area of research that relies on nanomedicines, which have the capability to target multiple neoantigens simultaneously in order to induce a robust immune response (Vormehr et al., 2019). A phase I clinical trial is currently underway for a DOTAP liposome-based autologous total tumor mRNA vaccine, with the aim of addressing early melanoma recurrence following anti-PD-1 therapy (NCT05264974). Another case in point is an mRNA-lipoplex neoantigen vaccine, derived from the autologous resected pancreatic ductal adenocarcinoma tumors, successfully completed a phase I clinical trial (Rojas et al., 2023). This result indicates these autogene nanovaccines elicited potent T-cell responses targeting 24% of vaccine-encoded neoantigens and effectively prolonged the time to tumor recurrence coordinating with a four-drug chemotherapy regimen (mFOLFIRINOX) and atezolizumab.
Ongoing clinical trials have revealed improved characteristics of conventional antitumor agents when formulated as nanomedicines, while also verifying the efficacy of some novel nanomedicines. Within the domain of tumor immunotherapy, utilization of nanomedicines is primarily restricted to their application in developing nanovaccines. Nevertheless, it should be emphasized that, despite extensive preclinical investigations focusing on intricate designs for versatile nanomedicines, it is primarily those with relatively uncomplicated constructs and thorough characterization that have progressed to clinical stages. Moreover, many of these nanomedicines failed to demonstrate satisfactory efficacy or raised safety concerns in clinical settings. The circumstances necessitate further endeavors to investigate the influence of physical and chemical fundamental parameters of nanomedicine on overall behaviors, which play an essential role in the further refined design of nanomedicines with the potential for clinical translation.
Conclusions and perspectives
Nanomedicines have been widely utilized in the field of tumor immunotherapy through rational design and modification, demonstrating the capability to adapt to various delivery requirements and maximize therapeutic efficacy while minimizing side effects. In order to further enhance the rational design of nanomedicines for effective tumor immunotherapy and their clinical translation, it is imperative to conduct in-depth investigations into the correlation between the physicochemical properties of nanomedicines and biological responses. A wider range of accurate models are expected to be established in order to explore and quantify nanomedicine behavior within the biological environment. A comprehensive evaluation of differences and relevance between human and animal species is urgently needed in preclinical research, which facilitates suitable animal model selection to enhance the potential of clinical translation. Additionally, mathematical and computational modelling requires further refinement through the integration of supporting data to accurately simulate human tumor models and predict nanomedicine behavior, thereby facilitating improved adjustment of nanomedicine parameters (Moradi Kashkooli et al., 2021). The development of customizable nanomedicines tailored to individual characteristics, termed as precise healthcare, holds significant promise in addressing the challenges posed by patient heterogeneity and expediting the clinical translation of nanomedicine. However, precise healthcare based on nanomedicine is still in its nascent stages and necessitates further investigation into disease progression, molecular mechanisms, and the development of robust analytical models (Han et al., 2020). The precise manipulation of nanomedicines is somewhat limited by the inherent characteristics of specific nanomaterials and the constraints imposed by synthetic methods (Nakamura and Ohta, 2024). Therefore, it is crucial to further optimize synthetic techniques and develop a universal nanoplatform for future clinical implementation of precision healthcare.
This review initially provides a comprehensive overview of representative types of NPs. Subsequently, a detailed description is presented of the current status of tumor immunotherapy utilizing nanomedicine, along with an introduction to their clinical application status. Finally, the future research direction of nanomedicine is prospectively discussed. It is believed that ongoing researches into the interaction mechanisms between nanomedicines and the biological environment contribute to further refinement in the rational design of nanomedicines. This will facilitate advancements in precise healthcare and ultimately lead to clinical benefits for a broader patient population.
Contributor Information
Zirui Gao, Laboratory of Aging Research and Cancer Drug Target, State Key Laboratory of Biotherapy and Cancer Center, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu 610041, China.
Dandan Wan, Laboratory of Aging Research and Cancer Drug Target, State Key Laboratory of Biotherapy and Cancer Center, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu 610041, China.
Min Luo, Laboratory of Aging Research and Cancer Drug Target, State Key Laboratory of Biotherapy and Cancer Center, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu 610041, China.
Xiawei Wei, Laboratory of Aging Research and Cancer Drug Target, State Key Laboratory of Biotherapy and Cancer Center, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu 610041, China.
Funding
This work was supported by grants from the National Science Foundation for Excellent Young Scholars (32122052), the National Natural Science Foundation Regional Innovation and Development (U19A2003), and the National Natural Science Foundation of China (82273297).
Conflict of interest: none declared.
Author contributions: X.W. and M.L. offered a significant guidance and critically reviewed this manuscript. Z.G. and D.W. drafted the manuscript and made the figures and table for the manuscript, contributing equally to the work. All authors reviewed and agreed on the final manuscript.
References
- Ahmed A., Tait S.W.G. (2020). Targeting immunogenic cell death in cancer. Mol. Oncol. 14, 2994–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldosari B.N., Alfagih I.M., Almurshedi A.S. (2021). Lipid nanoparticles as delivery systems for RNA-based vaccines. Pharmaceutics 13, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqosaibi A.I. (2022). Nanocarriers for anticancer drugs: challenges and perspectives. Saudi J. Biol. Sci. 29, 103298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An M., Li M., Xi J. et al. (2017). Silica nanoparticle as a lymph node targeting platform for vaccine delivery. ACS Appl. Mater. Interfaces 9, 23466–23475. [DOI] [PubMed] [Google Scholar]
- Antonangeli F., Natalini A., Garassino M.C. et al. (2020). Regulation of PD-L1 expression by NF-κB in cancer. Front. Immunol. 11, 584626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arranja A.G., Pathak V., Lammers T. et al. (2017). Tumor-targeted nanomedicines for cancer theranostics. Pharmacol. Res. 115, 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atrafi F., van Eerden R.A.G., van Hylckama Vlieg M.A.M. et al. (2020). Intratumoral comparison of nanoparticle entrapped docetaxel (CPC634) with conventional docetaxel in patients with solid tumors. Clin. Cancer Res. 26, 3537–3545. [DOI] [PubMed] [Google Scholar]
- Bahmani B., Gong H., Luk B.T. et al. (2021). Intratumoral immunotherapy using platelet-cloaked nanoparticles enhances antitumor immunity in solid tumors. Nat. Commun. 12, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Z., Guo L., Huang J. et al. (2024). Biomimetic metal organic frameworks mediated by metformin and 1-MT for enhancing the synergistic treatment of photodynamic therapy, photothermal therapy and immunotherapy. Chem. Eng. J. 479, 147932. [Google Scholar]
- Banerjee A., Onyuksel H. (2012). Peptide delivery using phospholipid micelles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 4, 562–574. [DOI] [PubMed] [Google Scholar]
- Barberio A.E., Smith S.G., Correa S. et al. (2020). Cancer cell coating nanoparticles for optimal tumor-specific cytokine delivery. ACS Nano 14, 11238–11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawage S.S., Tiwari P.M., Singh A. et al. (2016). Gold nanorods inhibit respiratory syncytial virus by stimulating the innate immune response. Nanomedicine 12, 2299–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingsley M.M., Singh N., Ravikumar P. et al. (2020). Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 20, 1578–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose R.J.C., Ravikumar R., Karuppagounder V. et al. (2017). Lipid–polymer hybrid nanoparticle-mediated therapeutics delivery: advances and challenges. Drug Discov. Today 22, 1258–1265. [DOI] [PubMed] [Google Scholar]
- Buzas E.I. (2023). The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 23, 236–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho S., Levi-Schaffer F., Sela M. et al. (2016). Immunotherapy of cancer: from monoclonal to oligoclonal cocktails of anti-cancer antibodies: IUPHAR Review 18. Br. J. Pharmacol. 173, 1407–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Ye Z., Huang C. et al. (2022). Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc. Natl Acad. Sci. USA 119, e2207841119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Zhang L., Li L. et al. (2021a). Cancer cell membrane-coated nanoparticles for bimodal imaging-guided photothermal therapy and docetaxel-enhanced immunotherapy against cancer. J. Nanobiotechnology 19, 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Huang Y., Wang L. et al. (2021b). Cetuximab functionalization strategy for combining active targeting and antimigration capacities of a hybrid composite nanoplatform applied to deliver 5-fluorouracil: toward colorectal cancer treatment. Biomater. Sci. 9, 2279–2294. [DOI] [PubMed] [Google Scholar]
- Chen Y., Shi J.L. (2016). Chemistry of mesoporous organosilica in nanotechnology: molecularly organic–inorganic hybridization into frameworks. Adv. Mater. 28, 3235–3272. [DOI] [PubMed] [Google Scholar]
- Chen Y.L., Bao C.J., Duan J.L. et al. (2023). Overcoming biological barriers by virus-like drug particles for drug delivery. Adv. Drug Deliv. Rev. 203, 115134. [DOI] [PubMed] [Google Scholar]
- Cheng C., Jiang W., Luo Y. et al. (2023). NIR activated multimodal therapeutics based on metal–phenolic networks-functionalized nanoplatform for combating against multidrug resistance and metastasis. Small 19, 2206174. [DOI] [PubMed] [Google Scholar]
- Cheng H.W., Tsao H.Y., Chiang C.S. et al. (2021). Advances in magnetic nanoparticle-mediated cancer immune-theranostics. Adv. Healthc. Mater. 10, e2001451. [DOI] [PubMed] [Google Scholar]
- Cheng Y.Y., Jiao X.Y., Fan W.P. et al. (2020). Controllable synthesis of versatile mesoporous organosilica nanoparticles as precision cancer theranostics. Biomaterials 256, 120191. [DOI] [PubMed] [Google Scholar]
- Cho R., Sakurai Y., Jones H.S. et al. (2020). Silencing of VEGFR2 by RGD-modified lipid nanoparticles enhanced the efficacy of anti-PD-1 antibody by accelerating vascular normalization and infiltration of T cells in tumors. Cancers 12, 3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Shim M.K., Yang S. et al. (2021). Visible-light-triggered prodrug nanoparticles combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. ACS Nano 15, 12086–12098. [DOI] [PubMed] [Google Scholar]
- Christiansen J., Rajasekaran A.K. (2004). Biological impediments to monoclonal antibody-based cancer immunotherapy. Mol. Cancer Ther. 3, 1493–1501. [PubMed] [Google Scholar]
- Czarnecka J., Wiśniewski M., Forbot N. et al. (2020). Cytotoxic or not? Disclosing the toxic nature of carbonaceous nanomaterials through nano–bio interactions. Materials 13, 2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jesus M.B., Zuhorn I.S. (2015). Solid lipid nanoparticles as nucleic acid delivery system: properties and molecular mechanisms. J. Control. Release 201, 1–13. [DOI] [PubMed] [Google Scholar]
- De Mel J., Hossain M., Shofolawe-Bakare O. et al. (2022). Dual-responsive glycopolymers for intracellular codelivery of antigen and lipophilic adjuvants. Mol. Pharm. 19, 4705–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z., Fang C., Ma X. et al. (2020). One stone two birds: Zr-Fc metal-organic framework nanosheet for synergistic photothermal and chemodynamic cancer therapy. ACS Appl. Mater. Interfaces 12, 20321–20330. [DOI] [PubMed] [Google Scholar]
- Dholakia J., Cohen A.C., Leath C. et al. (2022). Development of delivery systems for local administration of cytokines/cytokine gene-directed therapeutics: modern oncologic implications. Curr. Oncol. Rep. 24, 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep Y.N., Kim T.J., Cho H.S. et al. (2022). Nanomedicine for advanced cancer immunotherapy. J. Control. Release 351, 1017–1037. [DOI] [PubMed] [Google Scholar]
- Dong W., Li Z., Hou T. et al. (2024). Multicomponent synthesis of imidazole-based ionizable lipids for highly efficient and spleen-selective messenger RNA delivery. J. Am. Chem. Soc. 146, 15085–15095. [DOI] [PubMed] [Google Scholar]
- Dong Z., Wang Q., Huo M. et al. (2019). Mannose-modified multi-walled carbon nanotubes as a delivery nanovector optimizing the antigen presentation of dendritic cells. ChemistryOpen 8, 915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormont F., Rouquette M., Mahatsekake C. et al. (2019). Translation of nanomedicines from lab to industrial scale synthesis: the case of squalene-adenosine nanoparticles. J. Control. Release 307, 302–314. [DOI] [PubMed] [Google Scholar]
- Evans E.R., Bugga P., Asthana V. et al. (2018). Metallic nanoparticles for cancer immunotherapy. Mater. Today Bio. 21, 673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C., Xiao G.Z., Wang T.X. et al. (2023a). Emerging nano-/biotechnology drives oncolytic virus-activated and combined cancer immunotherapy. Research 6, 0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R.H., Gao W., Zhang L. (2023b). Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat. Rev. Clin. Oncol. 20, 33–48. [DOI] [PubMed] [Google Scholar]
- Fazal T., Murtaza B.N., Shah M. et al. (2023). Recent developments in natural biopolymer based drug delivery systems. RSC Adv. 13, 23087–23121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Mumper R.J. (2013). A critical review of lipid-based nanoparticles for taxane delivery. Cancer Lett. 334, 157–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L.D., Yang L., Li L.J. et al. (2022). Programmed albumin nanoparticles regulate immunosuppressive pivot to potentiate checkpoint blockade cancer immunotherapy. Nano Res. 15, 593–602. [Google Scholar]
- Fernandes D.A. (2023). Review on iron nanoparticles for cancer theranostics: synthesis, modification, characterization and applications. J. Nanopart. Res. 25, 170. [Google Scholar]
- Ferrari R., Sponchioni M., Morbidelli M. et al. (2018). Polymer nanoparticles for the intravenous delivery of anticancer drugs: the checkpoints on the road from the synthesis to clinical translation. Nanoscale 10, 22701–22719. [DOI] [PubMed] [Google Scholar]
- Frascotti G., Galbiati E., Mazzucchelli M. et al. (2021). The vault nanoparticle: a gigantic Ribonucleoprotein assembly involved in diverse physiological and pathological phenomena and an ideal nanovector for drug delivery and therapy. Cancers 13, 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadekar V., Borade Y., Kannaujia S. et al. (2021). Nanomedicines accessible in the market for clinical interventions. J. Control. Release 330, 372–397. [DOI] [PubMed] [Google Scholar]
- Gao P., Chen Y.Y., Pan W. et al. (2021). Antitumor agents based on metal-organic frameworks. Angew. Chem. Int. Ed Engl. 60, 16763–16776. [DOI] [PubMed] [Google Scholar]
- García-Fernández A., Sancenón F., Martínez-Máñez R. (2021). Mesoporous silica nanoparticles for pulmonary drug delivery. Adv. Drug Deliv. Rev. 177, 113953. [DOI] [PubMed] [Google Scholar]
- García-Fernández L., Garcia-Pardo J., Tort O. et al. (2017). Conserved effects and altered trafficking of cetuximab antibodies conjugated to gold nanoparticles with precise control of their number and orientation. Nanoscale 9, 6111–6121. [DOI] [PubMed] [Google Scholar]
- Ge Y.X., Zhang T.W., Zhou L. et al. (2022). Enhancement of anti-PD-1/PD-L1 immunotherapy for osteosarcoma using an intelligent autophagy-controlling metal organic framework. Biomaterials 282, 121407. [DOI] [PubMed] [Google Scholar]
- Gowd V., Ahmad A., Tarique M. et al. (2022). Advancement of cancer immunotherapy using nanoparticles-based nanomedicine. Semin. Cancer Biol. 86, 624–644. [DOI] [PubMed] [Google Scholar]
- Gowsalya K., Yasothamani V., Vivek R. (2021). Emerging indocyanine green-integrated nanocarriers for multimodal cancer therapy: a review. Nanoscale Adv. 3, 3332–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebowski J., Litwinienko G. (2022). Metallofullerenols in biomedical applications. Eur. J. Med. Chem. 238, 114481. [DOI] [PubMed] [Google Scholar]
- Gu Y., Lin S., Wu Y. et al. (2023). Targeting STING activation by antigen-inspired MnO2 nanovaccines optimizes tumor radiotherapy. Adv. Healthc. Mater. 12, 2300028. [DOI] [PubMed] [Google Scholar]
- Guan X., Sun L.P., Shen Y.T. et al. (2022). Nanoparticle-enhanced radiotherapy synergizes with PD-L1 blockade to limit post-surgical cancer recurrence and metastasis. Nat. Commun. 13, 2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes D., Cavaco-Paulo A., Nogueira E. (2021). Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 601, 120571. [DOI] [PubMed] [Google Scholar]
- Gurunathan S., Thangaraj P., Wang L. et al. (2024). Nanovaccines: an effective therapeutic approach for cancer therapy. Biomed. Pharmacother. 170, 115992. [DOI] [PubMed] [Google Scholar]
- Haas L., Elewaut A., Gerard C.L. et al. (2021). Acquired resistance to anti-MAPK targeted therapy confers an immune-evasive tumor microenvironment and cross-resistance to immunotherapy in melanoma. Nat. Cancer 2, 693–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Bartolo R., Li J. et al. (2022). Biomimetic platelet membrane-coated nanoparticles for targeted therapy. Eur. J. Pharm. Biopharm. 172, 1–15. [DOI] [PubMed] [Google Scholar]
- Han S., Shuen W.H., Wang W.-W. et al. (2020). Tailoring precision immunotherapy: coming to a clinic soon? ESMO Open 5, e000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J.Y., Yu L.Y., Lin X.D. et al. (2022). Virus-like particles as nanocarriers for intracellular delivery of biomolecules and compounds. Viruses 14, 1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera F.G., Bourhis J., Coukos G. (2017). Radiotherapy combination opportunities leveraging immunity for the next oncology practice. Cancer J. Clin. 67, 65–85. [DOI] [PubMed] [Google Scholar]
- Herrera F.G., Ronet C., de Olza M.O. et al. (2022). Low-dose radiotherapy reverses tumor immune desertification and resistance to immunotherapy. Cancer Discov. 12, 108–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess K.L., Medintz I.L., Jewell C.M. (2019). Designing inorganic nanomaterials for vaccines and immunotherapies. Nano Today 27, 73–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs S.K., Monsky W.L., Yuan F. et al. (1998). Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc. Natl Acad. Sci. USA 95, 4607–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong X.Y., Zhong X.F., Du G.S. et al. (2020). The pore size of mesoporous silica nanoparticles regulates their antigen delivery efficiency. Sci. Adv. 6, eaaz4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenboezem E.N., Duvall C.L. (2018). Harnessing albumin as a carrier for cancer therapies. Adv. Drug Deliv. Rev. 130, 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H.T., Huang H.C., Chung C.W. et al. (2022). CXCR4-targeted nitric oxide nanoparticles deliver PD-L1 siRNA for immunotherapy against glioblastoma. J. Control. Release 352, 920–930. [DOI] [PubMed] [Google Scholar]
- Hu S., Ma J., Su C. et al. (2021). Engineered exosome-like nanovesicles suppress tumor growth by reprogramming tumor microenvironment and promoting tumor ferroptosis. Acta Biomater. 135, 567–581. [DOI] [PubMed] [Google Scholar]
- Huang C.L., Zhang L., Guo Q. et al. (2021a). Robust nanovaccine based on polydopamine-coated mesoporous silica nanoparticles for effective photothermal–immunotherapy against melanoma. Adv. Funct. Mater. 31, 2010637. [Google Scholar]
- Huang G., Lin G., Zhu Y. et al. (2020a). Emerging technologies for profiling extracellular vesicle heterogeneity. Lab Chip 20, 2423–2437. [DOI] [PubMed] [Google Scholar]
- Huang H., Feng W., Chen Y. et al. (2020b). Inorganic nanoparticles in clinical trials and translations. Nano Today 35, 100972. [Google Scholar]
- Huang H.W., Sun M.C., Liu M.Y. et al. (2022). Full encapsulation of oncolytic virus using hybrid erythroctye–liposome membranes for augmented anti-refractory tumor effectiveness. Nano Today 47, 101671. [Google Scholar]
- Huang X., Lu Y., Guo M. et al. (2021b). Recent strategies for nano-based PTT combined with immunotherapy: from a biomaterial point of view. Theranostics 11, 7546–7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa J., Yoshida T., Isser A. et al. (2020). Rapid expansion of highly functional antigen-specific T cells from patients with melanoma by nanoscale artificial antigen-presenting cells. Clin. Cancer Res. 26, 3384–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikwuagwu B., Tullman-Ercek D. (2022). Virus-like particles for drug delivery: a review of methods and applications. Curr. Opin. Biotechnol. 78, 102785. [DOI] [PubMed] [Google Scholar]
- Ishihara M., Nishida Y., Kitano S. et al. (2023). A phase 1 trial of NY-ESO-1-specific TCR-engineered T-cell therapy combined with a lymph node-targeting nanoparticulate peptide vaccine for the treatment of advanced soft tissue sarcoma. Int. J. Cancer 152, 2554–2566. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Kageyama S., Miyahara Y. et al. (2021). Safety and antibody immune response of CHP-NY-ESO-1 vaccine combined with poly-ICLC in advanced or recurrent esophageal cancer patients. Cancer Immunol. Immunother. 70, 3081–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javia A., Vanza J., Bardoliwala D. et al. (2022). Polymer-drug conjugates: design principles, emerging synthetic strategies and clinical overview. Int. J. Pharm. 623, 121863. [DOI] [PubMed] [Google Scholar]
- Jeppesen D.K., Zhang Q., Franklin J.L. et al. (2023). Extracellular vesicles and nanoparticles: emerging complexities. Trends Cell Biol. 33, 667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., Li X., Zhang F. et al. (2022). Manganese dioxide-based nanocarrier delivers paclitaxel to enhance chemotherapy against orthotopic glioma through hypoxia relief. Small Methods 6, 2101531. [DOI] [PubMed] [Google Scholar]
- Jiang W., Su L., Ao M. et al. (2021). Amplified antitumor efficacy by a targeted drug retention and chemosensitization strategy-based ‘combo’ nanoagent together with PD-L1 blockade in reversing multidrug resistance. J. Nanobiotechnology 19, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Krishnamachary B., Barnett J.D. et al. (2019). Human cancer cell membrane-coated biomimetic nanoparticles reduce fibroblast-mediated invasion and metastasis and induce T-cells. ACS Appl. Mater. Interfaces 11, 7850–7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D.T., Zhou J., Kroll A.V. et al. (2022). Acute myeloid leukemia cell membrane-coated nanoparticles for cancer vaccination immunotherapy. Leukemia 36, 994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.L., Wang J.S., Falchook G. et al. (2023). Safety, tolerability, and pharmacokinetics of Aurora kinase B inhibitor AZD2811: a phase 1 dose-finding study in patients with advanced solid tumours. Br. J. Cancer 128, 1906–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun Y., Tang Z., Luo C. et al. (2020). Leukocyte-mediated combined targeted chemo and gene therapy for esophageal cancer. ACS Appl. Mater. Interfaces 12, 47330–47341. [DOI] [PubMed] [Google Scholar]
- June C.H., O'Connor R.S., Kawalekar O.U. et al. (2018). CAR T cell immunotherapy for human cancer. Science 359, 1361–1365. [DOI] [PubMed] [Google Scholar]
- Jung M., Kang M., Kim B.S. et al. (2022). Nanovesicle-mediated targeted delivery of immune checkpoint blockades to potentiate therapeutic efficacy and prevent side effects. Adv. Mater. 34, e2106516. [DOI] [PubMed] [Google Scholar]
- Kalyane D., Raval N., Maheshwari R. et al. (2019). Employment of enhanced permeability and retention effect (EPR): nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 98, 1252–1276. [DOI] [PubMed] [Google Scholar]
- Kamada T., Togashi Y., Tay C. et al. (2019). PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl Acad. Sci. USA 116, 9999–10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankala R.K., Han Y.H., Xia H.Y. et al. (2022). Nanoarchitectured prototypes of mesoporous silica nanoparticles for innovative biomedical applications. J. Nanobiotechnology 20, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katopodi T., Petanidis S., Tsavlis D. et al. (2022). Engineered multifunctional nanocarriers for controlled drug delivery in tumor immunotherapy. Front. Oncol. 12, 1042125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E.M., Denslow A., Hewett J. et al. (2022). Development of intravenously administered synthetic RNA virus immunotherapy for the treatment of cancer. Nat. Commun. 13, 5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy L.B., Salama A.K.S. (2020). A review of cancer immunotherapy toxicity. Cancer J. Clin. 70, 86–104. [DOI] [PubMed] [Google Scholar]
- Khalili L., Dehghan G., Sheibani N. et al. (2022). Smart active-targeting of lipid–polymer hybrid nanoparticles for therapeutic applications: recent advances and challenges. Int. J. Biol. Macromol. 213, 166–194. [DOI] [PubMed] [Google Scholar]
- Khan S., Gerber D.E. (2020). Autoimmunity, checkpoint inhibitor therapy and immune-related adverse events: a review. Semin. Cancer Biol. 64, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiaie S.H., Salehi-Shadkami H., Sanaei M.J. et al. (2023). Nano-immunotherapy: overcoming delivery challenge of immune checkpoint therapy. J. Nanobiotechnology 21, 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Archer P.A., Thomas S.N. (2021). Innovations in lymph node targeting nanocarriers. Semin. Immunol. 56, 101534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.K., Herbst R.S., Chen L.P. (2018). Defining and understanding adaptive resistance in cancer immunotherapy. Trends Immunol. 39, 624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimiz-Gebologlu I., Gulce-Iz S., Biray-Avci C. (2018). Monoclonal antibodies in cancer immunotherapy. Mol. Biol. Rep. 45, 2935–2940. [DOI] [PubMed] [Google Scholar]
- Kimiz-Gebologlu I., Oncel S.S. (2022). Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Control. Release 347, 533–543. [DOI] [PubMed] [Google Scholar]
- Kong C., Chen X. (2022). Combined photodynamic and photothermal therapy and immunotherapy for cancer treatment: a review. Int. J. Nanomedicine 17, 6427–6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y.J., Tang L., You Y. et al. (2023). Analysis of causes for poor persistence of CAR-T cell therapy. Front. Immunol. 14, 1063454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousar K., Naseer F., Abduh M.S. et al. (2023). CD44 targeted delivery of oncolytic Newcastle disease virus encapsulated in thiolated chitosan for sustained release in cervical cancer: a targeted immunotherapy approach. Front. Immunol. 14, 1175535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyande N.P., Srivastava R., Padmakumar A. et al. (2022). Advances in nanotechnology for cancer immunoprevention and immunotherapy: a review. Vaccines 10, 1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Dkhar D.S., Kumari R. et al. (2022). Ligand conjugated lipid-based nanocarriers for cancer theranostics. Biotechnol. Bioeng. 119, 3022–3043. [DOI] [PubMed] [Google Scholar]
- Lacinski R.A., Markel J.E., Noore J. et al. (2022). Synthesis, characterization, and in vivo cytokinome profile of IL-12-loaded PLGA nanospheres. J. Immunol. Res. 2022, 6993187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large D.E., Abdelmessih R.G., Fink E.A. et al. (2021). Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 176, 113851. [DOI] [PubMed] [Google Scholar]
- Le Q.V., Suh J., Choi J.J. et al. (2019). In Situ nanoadjuvant-assembled tumor vaccine for preventing long-term recurrence. ACS Nano 13, 7442–7462. [DOI] [PubMed] [Google Scholar]
- Lee J.J., Kang J.A., Ryu Y. et al. (2017). Genetically engineered and self-assembled oncolytic protein nanoparticles for targeted cancer therapy. Biomaterials 120, 22–31. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Yam J.W.P., Mao X. (2023). Dendritic cell vaccines: a shift from conventional approach to new generations. Cells 12, 2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S., Zhang X., Men K. et al. (2020). Efficient colorectal cancer gene therapy with IL-15 mRNA nanoformulation. Mol. Pharm. 17, 3378–3391. [DOI] [PubMed] [Google Scholar]
- Li H., Fu Q., Muluh A.T. et al. (2023a). The application of nanotechnology in immunotherapy based combinations for cancer treatment. Recent Pat. Anticancer Drug Discov. 18, 53–65. [DOI] [PubMed] [Google Scholar]
- Li J., Li J., Peng Y. et al. (2023b). Dendritic cell derived exosomes loaded neoantigens for personalized cancer immunotherapies. J. Control. Release 353, 423–433. [DOI] [PubMed] [Google Scholar]
- Li J., Lu W., Yang Y. et al. (2023c). Hybrid nanomaterials for cancer immunotherapy. Adv. Sci. 10, e2204932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Ren H., Zhang Y. (2022a). Metal-based nano-vaccines for cancer immunotherapy. J. Coord. Chem. 455, 214345. [Google Scholar]
- Li J., Wang S.J., Lin X.Y. et al. (2022b). Red blood cell-mimic nanocatalyst triggering radical storm to augment cancer immunotherapy. Nanomicro Lett. 14, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.X., Chen L.L., Su H.R. et al. (2019). The pharmaceutical multi-activity of metallofullerenol invigorates cancer therapy. Nanoscale 11, 14528–14539. [DOI] [PubMed] [Google Scholar]
- Li R., Hao Y., Pan W. et al. (2023d). Monophosphoryl lipid A-assembled nanovaccines enhance tumor immunotherapy. Acta Biomater. 171, 482–494. [DOI] [PubMed] [Google Scholar]
- Li W., Wang C.R., Wang T.S. (2021). Molecular structures and magnetic properties of endohedral metallofullerenes. Chem. Commun. 57, 10317–10326. [DOI] [PubMed] [Google Scholar]
- Li X., Liu H., Gao W. et al. (2024a). Octadecyl gallate and lipid-modified MnSe2 nanoparticles enhance radiosensitivity in esophageal squamous cell carcinoma and promote radioprotection in normal tissues. Adv. Mater. 36, 2311291. [DOI] [PubMed] [Google Scholar]
- Li Y., Fang H., Zhang T. et al. (2022c). Lipid–mRNA nanoparticles landscape for cancer therapy. Front. Bioeng. Biotechnol. 10, 1053197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Su Z., Zhao W. et al. (2020). Multifunctional oncolytic nanoparticles deliver self-replicating IL-12 RNA to eliminate established tumors and prime systemic immunity. Nat. Cancer 1, 882–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.-T., Mei K.-C., Liam-Or R. et al. (2024b). Graphene oxide nanosheets toxicity in mice is dependent on protein corona composition and host immunity. ACS Nano 18, 22572–22585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.Y., Tian Y.H., Nie G.J. (2012). Antineoplastic activities of Gd@C82(OH)22 nanoparticles: tumor microenvironment regulation. Sci. China Life Sci. 55, 884–890. [DOI] [PubMed] [Google Scholar]
- Lin Q., Peng Y., Wen Y. et al. (2023). Recent progress in cancer cell membrane-based nanoparticles for biomedical applications. Beilstein J. Nanotechnol. 14, 262–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Liew S.S., Wang J. et al. (2022a). Bioinspired and biomimetic delivery platforms for cancer vaccines. Adv. Mater. 34, 2103790. [DOI] [PubMed] [Google Scholar]
- Liu J.Q., Zhang C., Zhang X. et al. (2022b). Intratumoral delivery of IL-12 and IL-27 mRNA using lipid nanoparticles for cancer immunotherapy. J. Control. Release 345, 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.D., Huang L.Q., Zhang W.N. et al. (2022c). A transistor-like pH-sensitive nanodetergent for selective cancer therapy (vol 16, pg 795, 2021). Nat. Nanotechnol. 17, 560–560. [DOI] [PubMed] [Google Scholar]
- Liu Q., Hu Y., Zheng P. et al. (2023a). Exploiting immunostimulatory mechanisms of immunogenic cell death to develop membrane-encapsulated nanoparticles as a potent tumor vaccine. J. Nanobiotechnology 21, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Zhang W., Jiao R. et al. (2022d). Rational nanomedicine design enhances clinically physical treatment-inspired or combined immunotherapy. Adv. Sci. 9, e2203921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Sukumar U.K., Kanada M. et al. (2021). Camouflaged hybrid cancer cell–platelet fusion membrane nanovesicles deliver therapeutic microRNAs to presensitize triple-negative breast cancer to doxorubicin. Adv. Funct. Mater. 31, 2103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Wang Q., Feng Y. et al. (2023b). The self-adjuvant heterocyclic lipid nanoparticles encapsulated with vaccine and STAT3 siRNA boost cancer immunotherapy through DLN-targeted and STING pathway. J. Chem. Eng. Data 475, 146474. [Google Scholar]
- Lu C., Lv Y., Kou G. et al. (2022). Silver nanoparticles induce developmental toxicity via oxidative stress and mitochondrial dysfunction in zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 243, 113993. [DOI] [PubMed] [Google Scholar]
- Lu H., Zhang S., Wang J. et al. (2021). A review on polymer and lipid-based nanocarriers and its application to nano-pharmaceutical and food-based systems. Front. Nutr. 8, 783831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis de Redin I., Exposito F., Agueros M. et al. (2020). In vivo efficacy of bevacizumab-loaded albumin nanoparticles in the treatment of colorectal cancer. Drug Deliv. Transl. Res. 10, 635–645. [DOI] [PubMed] [Google Scholar]
- Luo L., Iqbal M.Z., Liu C. et al. (2019a). Engineered nano-immunopotentiators efficiently promote cancer immunotherapy for inhibiting and preventing lung metastasis of melanoma. Biomaterials 223, 119464. [DOI] [PubMed] [Google Scholar]
- Luo L.H., Yang J., Zhu C.Q. et al. (2018). Sustained release of anti-PD-1 peptide for perdurable immunotherapy together with photothermal ablation against primary and distant tumors. J. Control. Release 278, 87–99. [DOI] [PubMed] [Google Scholar]
- Luo S., Chen J., Xu F. et al. (2023). Dendritic cell-derived exosomes in cancer immunotherapy. Pharmaceutics 15, 2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z., Fan S., Gu C. et al. (2019b). Metal-organic framework (MOF)-based nanomaterials for biomedical applications. Curr. Med. Chem. 26, 3341–3369. [DOI] [PubMed] [Google Scholar]
- Ma Y.C., Zhang Y.X., Li X.Q. et al. (2019). Near-infrared II phototherapy induces deep tissue immunogenic cell death and potentiates cancer immunotherapy. ACS Nano 13, 11967–11980. [DOI] [PubMed] [Google Scholar]
- Mahamuni-Badiger P., Dhanavade M.J. (2023). Challenges and toxicity assessment of inorganic nanomaterials in biomedical applications: current status and future roadmaps. J. Drug Deliv. Sci. Technol. 87, 104806. [Google Scholar]
- Man Y.K.S., Aguirre-Hernandez C., Fernandez A. et al. (2022). Complexing the oncolytic adenoviruses Ad∆∆ and Ad-3∆-A20T with cationic nanoparticles enhances viral infection and spread in prostate and pancreatic cancer models. Int. J. Mol. Sci. 23, 8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Ma P., Luo X. et al. (2023). Stimuli-responsive polymeric nanovaccines toward next-generation immunotherapy. ACS Nano 17, 9826–9849. [DOI] [PubMed] [Google Scholar]
- Marhelava K., Pilch Z., Bajor M. et al. (2019). Targeting negative and positive immune checkpoints with monoclonal antibodies in therapy of cancer. Cancers 11, 1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei C.M., Wang N., Zhu X.Q. et al. (2018). Photothermal-controlled nanotubes with surface charge flipping ability for precise synergistic therapy of triple-negative breast cancer. Adv. Funct. Mater. 28, 1805225. [Google Scholar]
- Miao L., Li L., Huang Y. et al. (2019). Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 37, 1174–1185. [DOI] [PubMed] [Google Scholar]
- Mirchandani Y., Patravale V.B., Brijesh S. (2021). Solid lipid nanoparticles for hydrophilic drugs. J. Control. Release 335, 457–464. [DOI] [PubMed] [Google Scholar]
- Mitchell M.J., Billingsley M.M., Haley R.M. et al. (2021). Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A., Parida A., Raut R.K. et al. (2022). Ferritin: a promising nanoreactor and nanocarrier for bionanotechnology. ACS Bio Med Chem Au 2, 258–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi Kashkooli F., Soltani M., Souri M. et al. (2021). Nexus between in silico and in vivo models to enhance clinical translation of nanomedicine. Nano Today 36, 101057. [Google Scholar]
- Mueller S.N., Tian S., DeSimone J.M. (2015). Rapid and persistent delivery of antigen by lymph node targeting PRINT nanoparticle vaccine carrier to promote humoral immunity. Mol. Pharm. 12, 1356–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerabigwi J.F., Yin W., Zha Z. et al. (2019). Polymersome nanoreactors with tumor pH-triggered selective membrane permeability for prodrug delivery, activation, and combined oxidation–chemotherapy. J. Control. Release 303, 209–222. [DOI] [PubMed] [Google Scholar]
- Nakamura N., Ohta S. (2024). Precise control methods of the physicochemical properties of nanoparticles for personalized medicine. Curr. Opin. Biotechnol. 87, 103108. [DOI] [PubMed] [Google Scholar]
- Namiot E.D., Sokolov A.V., Chubarev V.N. et al. (2023). Nanoparticles in clinical trials: analysis of clinical trials, FDA approvals and use for COVID-19 vaccines. Int. J. Mol. Sci. 24, 787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseer F., Ahmad T., Kousar K. et al. (2023). Formulation for the targeted delivery of a vaccine strain of oncolytic measles virus (OMV) in hyaluronic acid coated thiolated chitosan as a green nanoformulation for the treatment of prostate cancer: a viro-immunotherapeutic approach. Inter. J. Nanomed. 18, 185–205. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nguyen H.T., Phung C.D., Tran T.H. et al. (2021). Manipulating immune system using nanoparticles for an effective cancer treatment: combination of targeted therapy and checkpoint blockage miRNA. J. Control. Release 329, 524–537. [DOI] [PubMed] [Google Scholar]
- Nguyen K.G., Vrabel M.R., Mantooth S.M. et al. (2020a). Localized interleukin-12 for cancer immunotherapy. Front. Immunol. 11, 575597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.L., Yin Y., Choi Y. et al. (2020b). Enhanced cancer DNA vaccine via direct transfection to host dendritic cells recruited in injectable scaffolds. ACS Nano 14, 11623–11636. [DOI] [PubMed] [Google Scholar]
- Obozina A.S., Komedchikova E.N., Kolesnikova O.A. et al. (2023). Genetically encoded self-assembling protein nanoparticles for the targeted delivery in vitro and in vivo. Pharmaceutics 15, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell J.S., Teng M.W.L., Smyth M.J. (2019). Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 16, 151–167. [DOI] [PubMed] [Google Scholar]
- Okiyama N., Tanaka R. (2022). Immune-related adverse events in various organs caused by immune checkpoint inhibitors. Allergol. Int. 71, 169–178. [DOI] [PubMed] [Google Scholar]
- Pan P., Dong X., Chen Y. et al. (2022a). A heterogenic membrane-based biomimetic hybrid nanoplatform for combining radiotherapy and immunotherapy against breast cancer. Biomaterials 289, 121810. [DOI] [PubMed] [Google Scholar]
- Pan Y., He Y., Zhao X. et al. (2022b). Engineered red blood cell membrane-coating salidroside/indocyanine green nanovesicles for high-efficiency hypoxic targeting phototherapy of triple-negative breast cancer. Adv. Healthc. Mater. 11, e2200962. [DOI] [PubMed] [Google Scholar]
- Parayath N.N., Stephan M.T. (2021). In situ programming of CAR T cells. Annu. Rev. Biomed. Eng. 23, 385–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parayath N.N., Stephan S.B., Koehne A.L. et al. (2020). In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat. Commun. 11, 6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park N.H., Cheng W., Lai F. et al. (2018). Addressing drug resistance in cancer with macromolecular chemotherapeutic agents. J. Am. Chem. Soc. 140, 4244–4252. [DOI] [PubMed] [Google Scholar]
- Paudel B.B., Quaranta V. (2019). Dynamics of drug response informs rational combination regimens. Sci. Signal. 12, eaax9742. [DOI] [PubMed] [Google Scholar]
- Peng F., Setyawati M.I., Tee J.K. et al. (2019). Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat. Nanotechnol. 14, 279–286. [DOI] [PubMed] [Google Scholar]
- Perica K., Tu A., Richter A. et al. (2014). Magnetic field-induced T cell receptor clustering by nanoparticles enhances T cell activation and stimulates antitumor activity. ACS Nano 8, 2252–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese R., Gelain F. (2017). Peptidic biomaterials: from self-assembling to regenerative medicine. Trends Biotechnol. 35, 145–158. [DOI] [PubMed] [Google Scholar]
- Qi J., Jin F., Xu X. et al. (2021). Combination cancer immunotherapy of nanoparticle-based immunogenic cell death inducers and immune checkpoint inhibitors. Int. J. Nanomedicine 16, 1435–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X.P., Ge L., Yuan K.J. et al. (2019). Targeting and microenvironment-improving of phenylboronic acid-decorated soy protein nanoparticles with different sizes to tumor. Theranostics 9, 7417–7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L., Zhang H.L., Zhou Y. et al. (2021a). Nanovaccine-based strategies to overcome challenges in the whole vaccination cascade for tumor immunotherapy. Small 17, e2006000. [DOI] [PubMed] [Google Scholar]
- Qin X.Y., Yang C.L., Xu H.B. et al. (2021b). Cell-derived biogenetic gold nanoparticles for sensitizing radiotherapy and boosting immune response against cancer. Small 17, e2103984. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Su M., Liu L. et al. (2021). Clinical application of cytokines in cancer immunotherapy. Drug Des. Devel. Ther. 15, 2269–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajana N., Mounika A., Chary P.S. et al. (2022). Multifunctional hybrid nanoparticles in diagnosis and therapy of breast cancer. J. Control. Release 352, 1024–1047. [DOI] [PubMed] [Google Scholar]
- Raphey V.R., Henna T.K., Nivitha K.P. et al. (2019). Advanced biomedical applications of carbon nanotube. Mater. Sci. Eng. C Mater. Biol. Appl. 100, 616–630. [DOI] [PubMed] [Google Scholar]
- Ren H., Yong J., Yang Q. et al. (2021). Self-assembled FeS-based cascade bioreactor with enhanced tumor penetration and synergistic treatments to trigger robust cancer immunotherapy. Acta Pharm. Sin. B 11, 3244–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A., Hamid O., Daud A. et al. (2016). Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. Curr. Med. Res. Opin. 315, 1600–1609. [DOI] [PubMed] [Google Scholar]
- Bosetti Rita, Jones SL. (2019). Cost-effectiveness of nanomedicine: estimating the real size of nano-costs. Nanomedicine 14, 1367–1370. [DOI] [PubMed] [Google Scholar]
- Rizk M.Z., Ali S.A., Hamed M.A. et al. (2017). Toxicity of titanium dioxide nanoparticles: effect of dose and time on biochemical disturbance, oxidative stress and genotoxicity in mice. Biomed. Pharmacother. 90, 466–472. [DOI] [PubMed] [Google Scholar]
- Rojas L.A., Sethna Z., Soares K.C. et al. (2023). Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 618, 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome L.H., Kickhoefer V.A. (2013). Development of the vault particle as a platform technology. ACS Nano 7, 889–902. [DOI] [PubMed] [Google Scholar]
- Rosenberg S.A., Parkhurst M.R., Robbins P.F. (2023). Adoptive cell transfer immunotherapy for patients with solid epithelial cancers. Cancer Cell 41, 646–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Xiong X.H., Chakraborty P.K. et al. (2016). Gold nanoparticle reprograms pancreatic tumor microenvironment and inhibits tumor growth. ACS Nano 10, 10636–10651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevieri M., Mazzucchelli S., Barbieri L. et al. (2023). Ferritin nanoconjugates guide trastuzumab brain delivery to promote an antitumor response in murine HER2+ breast cancer brain metastasis. Pharmacol. Res. 196, 106934. [DOI] [PubMed] [Google Scholar]
- Shan W.J., Wang C.F., Chen H.X. et al. (2023). Rational design of virus-like particles for nanomedicine. ACC Mater. Res. 4, 814–826. [Google Scholar]
- Sheikhpour M., Naghinejad M., Kasaeian A. et al. (2020). The applications of carbon nanotubes in the diagnosis and treatment of lung cancer: a critical review. Int. J. Nanomedicine 15, 7063–7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S., Li T., Fan J. et al. (2023). Lipid–polymer hybrid nanoparticle with cell-distinct drug release for treatment of stemness-derived resistant tumor. Acta Pharm. Sin. B 13, 1262–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheoran S., Arora S., Samsonraj R. et al. (2022). Lipid-based nanoparticles for treatment of cancer. Heliyon 8, e09403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M., Gu A., Tu H. et al. (2021). Comparing nanoparticle polymeric micellar paclitaxel and solvent-based paclitaxel as first-line treatment of advanced non-small-cell lung cancer: an open-label, randomized, multicenter, phase III trial. Ann. Onco. 32, 85–96. [DOI] [PubMed] [Google Scholar]
- Shin H., Kang S., Won C. et al. (2023). Enhanced local delivery of engineered IL-2 mRNA by porous silica nanoparticles to promote effective antitumor immunity. ACS Nano 17, 17554–17567. [DOI] [PubMed] [Google Scholar]
- Siddiqui M.A., Wahab R., Saquib Q. et al. (2023). Iron oxide nanoparticles induced cytotoxicity, oxidative stress, cell cycle arrest, and DNA damage in human umbilical vein endothelial cells. J. Trace Elem. Med. Biol. 80, 127302. [DOI] [PubMed] [Google Scholar]
- Siriwon N., Kim Y.J., Siegler E. et al. (2018). CAR-T cells surface-engineered with drug-encapsulated nanoparticles can ameliorate intratumoral T-cell hypofunction. Cancer Immunol. Res. 6, 812–824. [DOI] [PubMed] [Google Scholar]
- Sledz M., Wojciechowska A., Zagozdzon R. et al. (2023). In situ programming of CAR-T cells: a pressing need in modern immunotherapy. Arch. Immunol. Ther. Exp. 71, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., Wafa E.I., Geary S.M. et al. (2022). Cationic nanoparticles enhance T cell tumor infiltration and antitumor immune responses to a melanoma vaccine. Sci. Adv. 8, eabk3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H., Sun H., He N. et al. (2024). Glutathione depletion-induced versatile nanomedicine for potentiating the ferroptosis to overcome solid tumor radioresistance and enhance immunotherapy. Adv. Healthc. Mater. 13, 2303412. [DOI] [PubMed] [Google Scholar]
- Song S.L., Jin X.X., Zhang L. et al. (2019). PEGylated and CD47-conjugated nanoellipsoidal artificial antigen-presenting cells minimize phagocytosis and augment anti-tumor T-cell responses. Inter. J. Nanomedicine 14, 2465–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soprano E., Polo E., Pelaz B. et al. (2022). Biomimetic cell-derived nanocarriers in cancer research. J. Nanobiotechnology 20, 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stater E.P., Sonay A.Y., Hart C. et al. (2021). The ancillary effects of nanoparticles and their implications for nanomedicine. Nat. Nanotechnol. 16, 1180–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan M.T., Moon J.J., Um S.H. et al. (2010). Therapeutic cell engineering using surface-conjugated synthetic nanoparticles. Nat. Med. 33, 866–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner R.C., Sterner R.M. (2021). CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T., Cheng F.R., Qi J.L. et al. (2022). Responsive multivesicular polymeric nanovaccines that codeliver STING agonists and neoantigens for combination tumor immunotherapy. Adv. Sci. 9, e2201895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T., Liu X., Lin S.B. et al. (2023). Ionizable polymeric nanocarriers for the codelivery of bi-adjuvant and neoantigens in combination tumor immunotherapy. Bioact. Mater. 26, 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J., Ding B.B., Zheng P. et al. (2022). Hollow aluminum hydroxide modified silica nanoadjuvants with amplified immunotherapy effects through immunogenic cell death induction and antigen release. Small 18, e2202462. [DOI] [PubMed] [Google Scholar]
- Tan X., Wang C.H., Zhou H. et al. (2023). Bioactive fatty acid analog-derived hybrid nanoparticles confer antibody-independent chemo-immunotherapy against carcinoma. J. Nanobiotechnology 21, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L., Xiao Q., Yin Y. et al. (2022). An enzyme-responsive and NIR-triggered lipid–polymer hybrid nanoplatform for synergistic photothermal/chemo cancer therapy. Biomater. Sci. 10, 2370–2383. [DOI] [PubMed] [Google Scholar]
- Tang L., Zheng Y.R., Melo M.B. et al. (2018). Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol. 36, 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenchov R., Bird R., Curtze A.E. et al. (2021). Lipid nanoparticles—from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano 15, 16982–17015. [DOI] [PubMed] [Google Scholar]
- Thomas R., Al-Khadairi G., Roelands J. et al. (2018). NY-ESO-1 based immunotherapy of cancer: current perspectives. Front. Immunol. 9, 947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S.L., Hodi F.S., Brahmer J.R. et al. (2012). Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran A.V., Shim K., Vo Thi T.T. et al. (2018). Targeted and controlled drug delivery by multifunctional mesoporous silica nanoparticles with internal fluorescent conjugates and external polydopamine and graphene oxide layers. Acta Biomater. 74, 397–413. [DOI] [PubMed] [Google Scholar]
- Tran T.H., Mattheolabakis G., Aldawsari H. et al. (2015). Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin. Immunol. 160, 46–58. [DOI] [PubMed] [Google Scholar]
- Truffi M., Colombo M., Sorrentino L. et al. (2018). Multivalent exposure of trastuzumab on iron oxide nanoparticles improves antitumor potential and reduces resistance in HER2-positive breast cancer cells. Sci. Rep. 8, 6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutuncuoglu B., Krogan N.J. (2019). Mapping genetic interactions in cancer: a road to rational combination therapies. Genome Med. 11, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan V., Mohapatra A., Uthaman S. et al. (2019). Recent advances in nanovaccines using biomimetic immunomodulatory materials. Pharmaceutics 11, 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vormehr M., Tureci O., Sahin U. (2019). Harnessing tumor mutations for truly individualized cancer vaccines. Annu. Rev. Med. 70, 395–407. [DOI] [PubMed] [Google Scholar]
- Wagner A.J., Ravi V., Riedel R.F. et al. (2021). nab-Sirolimus for patients with malignant perivascular epithelioid cell tumors. J. Clin. Oncol. 39, 3660–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A.J., Ravi V., Riedel R.F. et al. (2024). Phase II trial of nab-sirolimus in patients with advanced malignant perivascular epithelioid cell tumors (AMPECT): long-term efficacy and safety update. J. Clin. Oncol. 42, 1472–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman A.D., Fritz J.M., Lenardo M.J. (2020). A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters A.A., Santacana-Font G., Li J. et al. (2021). Nanoparticle-mediated in situ molecular reprogramming of immune checkpoint interactions for cancer immunotherapy. ACS Nano 15, 17549–17564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan W.J., Huang G., Wang Y. et al. (2021). Coadministration of iRGD peptide with ROS-sensitive nanoparticles co-delivering siFGL1 and siPD-L1 enhanced tumor immunotherapy. Acta Biomater. 136, 473–484. [DOI] [PubMed] [Google Scholar]
- Wang C., Huang X., Wu Y. et al. (2020a). Tumor cell-associated exosomes robustly elicit anti-tumor immune responses through modulating dendritic cell vaccines in lung tumor. Int. J. Biol. Sci. 16, 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Xu X., Xiong S. et al. (2022a). Calcium-chelated nanosystem reversing cancer chemoresistance via replenishing intracellular calcium ions. Chem. Eng. J. 448, 137500. [Google Scholar]
- Wang D., Wang S., Zhou Z. et al. (2022b). White blood cell membrane-coated nanoparticles: recent development and medical applications. Adv. Healthc. Mater. 11, e2101349. [DOI] [PubMed] [Google Scholar]
- Wang J.-K., Zhou Y.-Y., Guo S.-J. et al. (2017). Cetuximab conjugated and doxorubicin loaded silica nanoparticles for tumor-targeting and tumor microenvironment responsive binary drug delivery of liver cancer therapy. Biomater. Adv. 76, 944–950. [DOI] [PubMed] [Google Scholar]
- Wang M.Y., Zhang L., Cai Y.F. et al. (2020b). Bioengineered human serum albumin fusion protein as target/enzyme/pH three-stage propulsive drug vehicle for tumor therapy. ACS Nano 14, 17405–17418. [DOI] [PubMed] [Google Scholar]
- Wang Q., Wang Z., Sun X. et al. (2022c). Lymph node-targeting nanovaccines for cancer immunotherapy. J. Control. Release 351, 102–122. [DOI] [PubMed] [Google Scholar]
- Wang T.S., Wang C.R. (2019). Functional metallofullerene materials and their applications in nanomedicine, magnetics, and electronics. Small 15, e1901522. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhong X., Li J. et al. (2021a). Inorganic nanomaterials with rapid clearance for biomedical applications. Chem. Soc. Rev. 50, 8669–8742. [DOI] [PubMed] [Google Scholar]
- Wang X.Y., Meng F.Y., Yen Y.T. et al. (2021b). Nanotechnology-based CAR-T strategies for improving efficacy and safety of tumor immunotherapy. Adv. Funct. Mater. 31, 2004713. [Google Scholar]
- Wang Y., Lei H., Yan B. et al. (2023a). Tumor acidity-activatable macromolecule autophagy inhibitor and immune checkpoint blockade for robust treatment of prostate cancer. Acta Biomater. 168, 593–605. [DOI] [PubMed] [Google Scholar]
- Wang Y., Song W.T., Hu M.Y. et al. (2018). Nanoparticle-mediated HMGA1 silencing promotes lymphocyte infiltration and boosts checkpoint blockade immunotherapy for cancer. Adv. Funct. Mater. 28, 1802847. [Google Scholar]
- Wang Z., Chen L., Ma Y. et al. (2021c). Peptide vaccine-conjugated mesoporous carriers synergize with immunogenic cell death and PD-L1 blockade for amplified immunotherapy of metastatic spinal. J. Nanobiotechnology 19, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., You T., Cai C. et al. (2023b). Biomimetic gold nanostructure with a virus-like topological surface for enhanced antigen cross-presentation and antitumor immune response. ACS Appl. Mater. Interfaces 15, 13929–13940. [DOI] [PubMed] [Google Scholar]
- Won J.E., Byeon Y., Wi T.I. et al. (2022). Immune checkpoint silencing using RNAi-incorporated nanoparticles enhances antitumor immunity and therapeutic efficacy compared with antibody-based approaches. J. Immunother. Cancer 10, e003928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.H., Xu J.M., Xie Z.X. et al. (2021). Light-responsive hyaluronic acid nanomicelles co-loaded with an IDO inhibitor focus targeted photoimmunotherapy against ‘immune cold’ cancer. Biomater. Sci. 9, 8019–8031. [DOI] [PubMed] [Google Scholar]
- Wu J., Waxman D.J. (2018). Immunogenic chemotherapy: dose and schedule dependence and combination with immunotherapy. Cancer Lett. 419, 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.X., Yang Y.W. (2017). Metal-organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 29, 1606134. [DOI] [PubMed] [Google Scholar]
- Xia Q., Zhang Y., Li Z. et al. (2019). Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta Pharm. Sin. B 9, 675–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Xu Y., Solek N.C. et al. (2024). Tumor-tailored ionizable lipid nanoparticles facilitate IL-12 circular RNA delivery for enhanced lung cancer immunotherapy. Adv. Mater. 36, 2400307. [DOI] [PubMed] [Google Scholar]
- Xu Y., Ma S., Zhao J. et al. (2022). Mannan-decorated pathogen-like polymeric nanoparticles as nanovaccine carriers for eliciting superior anticancer immunity. Biomaterials 284, 121489. [DOI] [PubMed] [Google Scholar]
- Xue X., Li J., Fan Y. et al. (2021). Gene silencing-mediated immune checkpoint blockade for tumor therapy boosted by dendrimer-entrapped gold nanoparticles. Sci. China Mater. 64, 2045–2055. [Google Scholar]
- Yamaguchi H., Hsu J.M., Yang W.H. et al. (2022). Mechanisms regulating PD-L1 expression in cancers and associated opportunities for novel small-molecule therapeutics. Nat. Rev. Clin. Oncol. 19, 287–305. [DOI] [PubMed] [Google Scholar]
- Yang B.W., Chen Y., Shi J.L. (2019). Mesoporous silica/organosilica nanoparticles: synthesis, biological effect and biomedical application. Mater. Sci. Eng. R Rep. 137, 66–105. [Google Scholar]
- Yang M., Li J., Gu P. et al. (2021a). The application of nanoparticles in cancer immunotherapy: targeting tumor microenvironment. Bioact. Mater. 6, 1973–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Zhou Y., Chen J. et al. (2021b). Gene therapy for drug-resistant glioblastoma via lipid–polymer hybrid nanoparticles combined with focused ultrasound. Int. J. Nanomedicine 16, 185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.Q., Yu T., Zeng Y.P. et al. (2020). pH-responsive biomimetic polymeric micelles as lymph node-targeting vaccines for enhanced antitumor immune responses. Biomacromolecules 21, 2818–2828. [DOI] [PubMed] [Google Scholar]
- Yang Z., Li H., Zhang W. et al. (2023). CD163 Monoclonal antibody modified polymer prodrug nanoparticles for targeting tumor-associated macrophages (TAMs) to enhance anti-tumor effects. Pharmaceutics 15, 1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavuz E., Walters A., Chudasama B. et al. (2023). Investigating the potential of silver nanocubes and gold nanocages as protein subunit vaccine carriers. J. Immunol. 210(1_Supplement), 159.14. [Google Scholar]
- Ye H., Tong J., Wu J. et al. (2014). Preclinical evaluation of recombinant human IFNα2b-containing magnetoliposomes for treating hepatocellular carcinoma. Int. J. Nanomedicine 9, 4533–4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoda R., Nagalo B.M., Vernon B. et al. (2017). Oncolytic virus delivery: from nano-pharmacodynamics to enhanced oncolytic effect. Oncolytic Virother. 6, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J., Park C., Yi G. et al. (2019). Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers 11, 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis M.A., Tawfeek H.M., Abdellatif A.A.H. et al. (2022). Clinical translation of nanomedicines: challenges, opportunities, and keys. Adv. Drug Deliv. Rev. 181, 114083. [DOI] [PubMed] [Google Scholar]
- Yu H., Guo H.X., Zu H. et al. (2022a). Therapeutic dendritic cell vaccines engineered with antigen-biomineralized BiS nanoparticles for personalized tumor radioimmunotherapy. Aggregate 3, e194. [Google Scholar]
- Yu R.R., Geng T.T., Wei T.T. et al. (2023). Membrane-disruptive homo-polymethacrylate with both hydrophobicity and pH-sensitive protonation for selective cancer therapy. J. Mater. Chem. B 11, 3364–3372. [DOI] [PubMed] [Google Scholar]
- Yu T., Nie W., Hong Z.H. et al. (2021). Synergy of immunostimulatory genetherapy with immune checkpoint blockade motivates immune response to eliminate cancer. Adv. Funct. Mater. 31, 2100715. [Google Scholar]
- Yu W.D., Sun G., Li J. et al. (2019). Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett. 452, 66–70. [DOI] [PubMed] [Google Scholar]
- Yu Y.J., Li J., Song B.Y. et al. (2022b). Polymeric PD-L1 blockade nanoparticles for cancer photothermal-immunotherapy. Biomaterials 280, 121312. [DOI] [PubMed] [Google Scholar]
- Yuan Z., Fan G., Wu H. et al. (2021). Photodynamic therapy synergizes with PD-L1 checkpoint blockade for immunotherapy of CRC by multifunctional nanoparticles. Mol. Ther. 29, 2931–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare H., Ahmadi S., Ghasemi A. et al. (2021). Carbon nanotubes: smart drug/gene delivery carriers (vol 16, pg 1681, 2021). Inter. J. Nanomedicine 16, 7283–7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q., Liu Z.G., Niu T. et al. (2023). Application of nanotechnology in CAR-T-cell immunotherapy. Chin. Chem. Lett. 34, 107747. [Google Scholar]
- Zhang D., Wu T.T., Qin X.Y. et al. (2019a). Intracellularly generated immunological gold nanoparticles for combinatorial photothermal therapy and immunotherapy against tumor. Nano Lett. 19, 6635–6646. [DOI] [PubMed] [Google Scholar]
- Zhang F., Stephan S.B., Ene C.I. et al. (2018). Nanoparticles that reshape the tumor milieu create a therapeutic window for effective T-cell therapy in solid malignancies. Cancer Res. 78, 3718–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Qi C., Cai K. (2023a). Manganese-based tumor immunotherapy. Adv. Mater. 35, e2205409. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wang J., Cui H. et al. (2023b). Simultaneous knockdown of immune suppressive markers by Tumor microenvironment-responsive multifaceted prodrug nanomedicine. ACS Appl. Mater. Interfaces 15, 12864–12881. [DOI] [PubMed] [Google Scholar]
- Zhang L., Xu L., Wang Y. et al. (2022a). A novel therapeutic vaccine based on graphene oxide nanocomposite for tumor immunotherapy. Chin. Chem. Lett. 33, 4089–4095. [Google Scholar]
- Zhang L., Zhao W., Huang J.K. et al. (2022b). Development of a dendritic cell/tumor cell fusion cell membrane nano-vaccine for the treatment of ovarian cancer. Front. Immunol. 13, 828263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.W., Rong P.F., Chen M.L. et al. (2015). A novel single walled carbon nanotube (SWCNT) functionalization agent facilitating in vivo combined chemo/thermo therapy. Nanoscale 7, 16204–16213. [DOI] [PubMed] [Google Scholar]
- Zhang L.W., Zheng H.A., Cui H.Y. et al. (2023c). Nanomicelle-based redox-responsive dual-prodrug for synergistic breast cancer chemo-immunotherapy. ACS Appl. Nano Mater. 6, 18263–18274. [Google Scholar]
- Zhang W., Jiang Y., He Y. et al. (2023d). Lipid carriers for mRNA delivery. Acta Pharm. Sin. B 13, 4105–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.H., Xu W.H., Lan Y. et al. (2019b). Antitumor effect of hyaluronic-acid-modified chitosan nanoparticles loaded with siRNA for targeted therapy for non-small cell lung cancer. Inter. J. Nanomedicine 14, 5287–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Xu Q., Zi Z. et al. (2020). Programmable extracellular vesicles for macromolecule delivery and genome modifications. Dev. Cell 55, 784–801.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J.Y., Xu Y.D., Ma S. et al. (2022a). A minimalist binary vaccine carrier for personalized postoperative cancer vaccine therapy. Adv. Mater. 34, e2109254. [DOI] [PubMed] [Google Scholar]
- Zhao P.Q., Qiu L.H., Zhou S.Y. et al. (2021). Cancer cell membrane camouflaged mesoporous silica nanoparticles combined with immune checkpoint blockade for regulating tumor microenvironment and enhancing antitumor therapy. Inter. J. Nanomedicine 16, 2107–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R.-M., Hu Y., Chen P. et al. (2023). Zn-dipicolylamine-based ROS-responsive bola-lipid nanoparticles for efficient protein delivery and tumor immunotherapy. ACS Appl. Nano Mater. 6, 21945–21956. [Google Scholar]
- Zhao X., Zhao R., Nie G. (2022b). Nanocarriers based on bacterial membrane materials for cancer vaccine delivery. Nat. Protoc. 17, 2240–2274. [DOI] [PubMed] [Google Scholar]
- Zheng C., Zhang J., Chan H.F. et al. (2021). Engineering nano-therapeutics to boost adoptive cell therapy for cancer treatment. Small Methods 5, e2001191. [DOI] [PubMed] [Google Scholar]
- Zhou J.E., Sun L., Jia Y. et al. (2022). Lipid nanoparticles produce chimeric antigen receptor T cells with interleukin-6 knockdown in vivo. J. Control. Release 350, 298–307. [DOI] [PubMed] [Google Scholar]
- Zhou L., Hou B., Wang D. et al. (2020). Engineering polymeric prodrug nanoplatform for vaccination immunotherapy of cancer. Nano Lett. 20, 4393–4402. [DOI] [PubMed] [Google Scholar]
- Zhu S.M., Zhang T., Zheng L. et al. (2021). Combination strategies to maximize the benefits of cancer immunotherapy. J. Hematol. Oncol. 14, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinska A., Cano A., Andreani T. et al. (2022). Lipid–drug conjugates and nanoparticles for the cutaneous delivery of cannabidiol. Int. J. Mol. Sci. 23, 6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinger A. (2023). Unleashing the potential of cell biomimetic nanoparticles: strategies and challenges in their design and fabrication for therapeutic applications. J. Control. Release 358, 591–600. [DOI] [PubMed] [Google Scholar]
- Zuo B.F., Zhang Y., Zhao K.J. et al. (2022). Universal immunotherapeutic strategy for hepatocellular carcinoma with exosome vaccines that engage adaptive and innate immune responses. J. Hematol. Oncol. 15, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]