Abstract
Obesity and aging share comorbidities, phenotypes, and deleterious effects on health that are associated with chronic diseases. However, distinct features set them apart, with underlying biology that should be explored and exploited, especially given the demographic shifts and the obesity epidemic that the world is facing.
INTRODUCTION
The World Health Organization (WHO) maintains a strategic vision that “all people can live long and healthy lives” (1). However, with a rapid increase in aging population and daunting demography shifts, the challenge that all countries will face soon requires global and collaborative actions to minimize health, social, and economic repercussions (2). Regardless of education, socioeconomic variables, and geographical location, women live longer than men despite enduring poorer health throughout life. However, except for Alzheimer’s disease, the mortality rates associated with chronic conditions are lower for women than men. The underlying biology of these mortality and morbidity differences remains poorly understood and studied. Aging, defined as an intrinsic and progressive decline in structural and physiological function, is recognized as the major risk factor for all chronic diseases and as a determinant for multimorbidity late in life and, ultimately, mortality. Thus, as the geroscience hypothesis posits because the physiology of aging plays a major role in most chronic diseases, therapeutically addressing aging directly should delay the onset or reduce the severity of multiple chronic diseases. In model organisms, interventions targeting aging have led to consistent increases in lifespan and preservation of health until late in life, often in a sex-specific manner.
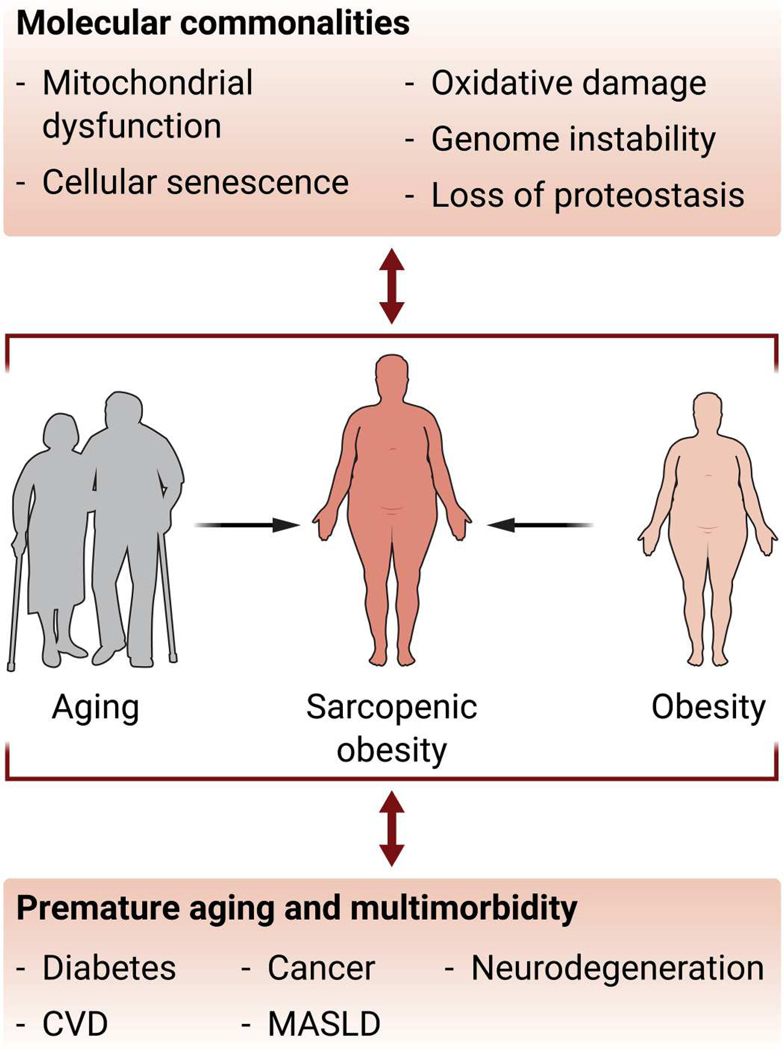
Achieving and maintaining a healthy life in the elderly are affected by a confluence of metabolic risks, including obesity. In 2017–2018, the prevalence of obesity reached ~40 to 45% at any age range (3), and by 2030, one of two adults will have obesity (4). The rapid pace of population aging, together with the growing rate of obesity, represents a serious threat to global health, particularly with obesity influencing the severityof noncommunicable conditions such as cardiovascular disease, diabetes, and cancer. Nine hallmarks of aging were defined more than a decade ago [recently revisited in (5)], which led to the notion that interventions aimed at interfering with biological aging might be more attainable. Thereafter, several efforts were made to identify commonalities between molecular aging and chronic diseases, including obesity (6–8). Accumulation of oxidative damage, increased genome instability, inflammation, and apoptosis, as well as reduction of mitochondrial function, autophagy, and cellular proteostasis, have all been documented to occur in both aging and obesity (Fig. 1). Consistent with these shared features of aging, obesity has been shown to shorten lifespan and promote a premature aging phenotype (8–10). However, the biology underlying the association between obesity and increased mortality remains poorly understood, except in the case of sarcopenic obesity, where obesity is accompanied by decreased skeletal muscle mass. At a population level, the prevalence of obesity increases with age until relatively late in life, at which point body weight loss is often observed in both humans and laboratory animals. Nonetheless, even in genetically identical laboratory animals housed and fed under the same conditions, there is extensive variability in how body weight and body fat change across life in individual animals, and this is even more pronounced in human populations where environmental factors and lifestyle choices play a prominent role. The number of studies directly comparing how age affects the development of obesity is limited, with studies in animal models showing conflicting results on whether increases in body weight in response to a high-fat diet are more pronounced and whether many of the negative metabolic effects associated with obesity are enhanced in aged animals (11, 12). Because obesity and aging are complex processes sharing molecular commonalities, dissecting how aging exacerbates obesity and obesity-associated symptoms is complex and challenging, especially because most research on the relationship between aging and obesity has been cross-sectional. To address this, longitudinal studies of aging in individuals with obesity are needed to properly evaluate the direct relationship between obesity and increased mortality, as shown for sarcopenic obesity, as well as the consequences of any such long-term association.
Fig. 1. Molecular and physiological similarities between obesity and aging.
Shown is a schematic depiction of how aging and obesity are associated with similar molecular changes, increase the severity of common diseases, and together promote the development of sarcopenic obesity. CVD, cardiovascular disease; MASLD, metabolic dysfunction–associated steatotic liver disease.
Body fat distribution, resting energy expenditure, and aging
Many antiaging interventions can lead to weight loss, and, conversely, treatments for obesity have the potential to extend health-span and lifespan. Indeed, obesity and aging are both complex and highly heterogeneous conditions, as is the relationship between the two. Obesity is defined by a body mass index (BMI) higher than 30 kg/m2; however, BMI is not sufficient to indicate the degree to which adiposity may contribute to metabolic dysfunction or other phenotypic changes associated with aging. The two primary forms of adipose tissue, visceral (VAT) and subcutaneous (SAT), have different impacts on many aspects of physiology, with VAT playing a more prominent role in promoting insulin resistance and inflammation. Consistent with this, numerous studies have shown that the associations between adiposity and mortality risk differ for SAT and VAT, including work showing that increased visceral adiposity is a stronger indicator of mortality risk than BMI, whereas increased SAT tissue is associated with lower mortality risk (13). Similarly, SAT was found to be negatively associated with mortality in overweight women (14). The proportion of VAT to SAT varies considerably between men and women and generally increases with age, together with changes in adipokine signaling and higher amounts of circulating inflammatory factors. Although many circulating proinflammatory adipokines increase in abundance as a function of age and obesity, adiponectin, one of the most important anti-inflammatory adipokines, increases with age but declines in individuals with obesity (15). Thus, an adequate balance between pro- and anti-inflammatory markers appears essential for delaying disease onset, as shown for centenarians (16). The remodeling of adipose tissue and changes in cellular composition that occur with both aging and obesity are also associated with an accumulation of immune cells, although important differences in immune cell types present in adipose tissue of aged individuals versus individuals living with obesity have been identified (17). Intermuscular and intramuscular adipose tissue accumulation also increases with age (18), with thigh intermuscular fat associated with an increased risk of mortality in men (14). An increase in visceral obesity and total amount of adipose tissue aggravates sarcopenia (19), an age-related loss of muscle mass and strength naturally occurring with age. In this scenario, obesity combined with sarcopenia, known as sarcopenic obesity, has synergistic impact on (long-term) disability, metabolic diseases, comorbidities, and mortality and is thus a potentially life-threatening scenario for older adults (20).
A reduction in metabolic rate has long been considered one of the likely drivers of increased adiposity with age. A recent study in humans, however, reported that energy expenditure adjusted by body mass does not decline until after the age of 60 and is therefore unlikely to drive the increased body weight in middle-aged individuals, nor is it associated with an increase in either total or percentage of body fat (21). Longitudinal studies have reported a decline in resting metabolic rate with age, whereas an increase in metabolic rate late in life has been associated with mortality, multimorbidity, and some age-related diseases (22). Moreover, unintentional body weight loss in older individuals is accompanied by increased mortality risk (23). Retention of fat mass late in life is predictive of increased survival both under conditions of normal aging and in response to antiaging interventions in laboratory animals (24, 25). Young individuals living with obesity experience years of life lost from diabetes and cardiovascular disease to a greater extent compared with their older counterparts (10). Together, adiposity can accelerate aging in younger individuals, but the relationship between fat mass and mortality changes as we get older. Obesity (BMI > 30) is associated with greater mortality risk; however, overweight individuals (BMI = 25 to 30) have similar or, in some cases, lower mortality risk than individuals in the healthy (BMI = 18.5 to 24) or underweight (BMI < 18.5) range (26, 27), indicating that there is not a simple and linear relationship between body mass and aging/mortality. The potential benefits of being above what is considered a “healthy weight” appear to be even more pronounced in elderly populations (23). Indeed, interventions to reduce body weight in elderly populations can sometimes confer health and longevity benefits, although these approaches have also been reported to be less beneficial or even detrimental (28). Going forward, longitudinal assessments in animal models and human studies should explore in greater depth how changes in body weight, fat mass, and type of adipose tissue (VAT versus SAT) affect age-related phenotypic changes and mortality risk at different life stages. In turn, insights gained from these studies will further elucidate the complex relationship that exists between adiposity and aging and provide more personalized treatment recommendations at different ages.
Will targeting adipose tissue restore longevity?
Because obesity and aging share molecular hallmarks, it is reasonable to hypothesize that therapies targeting adipose tissue for the treatment of obesity might also affect the aging process or influence longevity. Nutritional interventions reducing energy intake are frequently used for first-line management of obesity and related metabolic disorders. Daily calorie restriction without malnutrition reduces body weight, enhances health, and extends lifespan, likely by postponing and lowering the incidence of age-associated chronic diseases, including obesity. Interestingly, molecular pathways linked to weight loss and longevity are reshaped in human adipose tissue in response to caloric restriction (29). Beyond calories, strategies to modulate food intake such as meal size, time between meals, the length of fasting, and the time of calorie loading have the capacity to affect organismal metabolism and survival, with notable outcomes in both dietary-induced and genetically modified preclinical models of obesity (30–32). In addition, the alignment of feeding patterns with circadian components of appetite and hunger appears to achieve the greatest benefits (30, 33, 34). Strikingly, favorable effects on health and survival have been found for energy restriction–based interventions in the absence of body weight loss or despite the presence of weight-regain cycles (35, 36). Nevertheless, other studies indicate that the potential benefits of energy restriction–based interventions are weight loss dependent (37, 38). In addition, whereas many interventions improve metabolic function and reduce mortality under both healthy and obese conditions (39, 40), others only lead to lifespan increases under conditions of obesity while still conferring robust physiologic health benefits in otherwise healthy mice (41, 42). Isocaloric amounts of a ketogenic diet also extend longevity and health in adult mice in the absence of body weight change (43). Moreover, recent work from the U.S. National Institute on Aging intervention testing program reported lifespan extension for some of the most effective antiaging compounds (rapamycin, acarbose, and 17α-estradiol) usually without reductions in body weight and often in a sex-specific manner (44). In fact, rapamycin led to a substantial increase in body weight. This study also observed higher numbers of inflammatory macrophages in VAT of rapamycin-treated animals, which is contrary to what would be expected from an antiaging intervention, because inflammatory macrophages have been shown to accumulate with age in adipose tissues (44). Together, although obesity may promote some aspects of the aging process, the underlying factors that drive aging and obesity are in many ways distinct. Overall, emerging insights into the difference between healthspan and lifespan should be taken into consideration when interpreting the effects of antiobesity and antiaging interventions.
Jointly with changes in dietary habits, promotion of physical activity is included within the first-line choices for obesity treatment. The health benefits of therapeutic exercise in obesity are unquestionable and include increases in energy expenditure, reduction of adipose tissue, and reduction of chronic inflammation, among others. By restoring organismal health, moderate-intensity exercise has been shown to sustain health and to attenuate the major hallmarks of aging, thus lowering all-cause mortality risk (45, 46). Interestingly, intermittent but vigorous physical activity in overweight sedentary individuals appears to elicit similar effects to vigorous physical activity in exercisers, reducing all-cause cardiovascular disease and cancer mortality (47). Overall, regular physical activity in humans seems to be associated with an increase in life expectancy by 0.4 to 6.9 years (48). In animal models, different exercise paradigms have been found to have beneficial effects on parameters associated with healthspan, but published works have failed to demonstrate a substantial improvement in maximal lifespan extension (49, 50). Physical activity has been shown to increase fat-free mass and improve bone mass, thus partially preventing sarcopenia and fractures late in life (46). Aging and obesity also cause myokine dysregulation, and in this scenario, exercise may also counteract sarcopenic obesity (51).
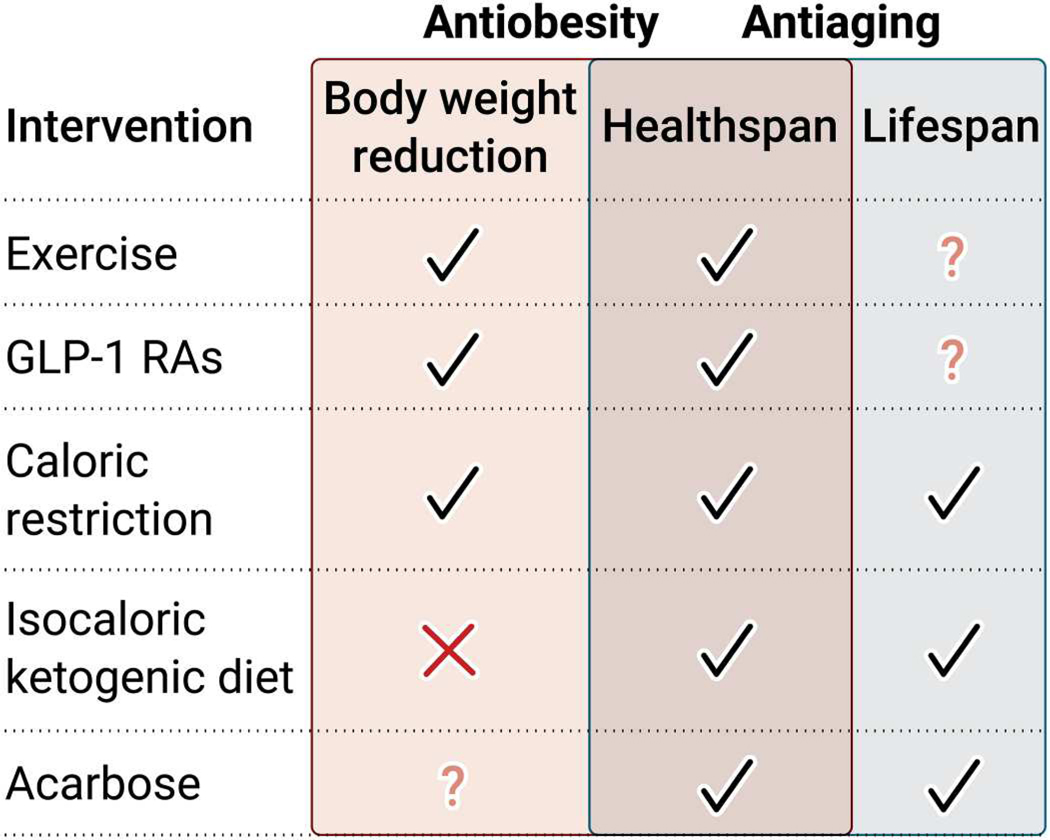
Nonetheless, despite being considered the real polypill, exercise and other lifestyle changes are generally associated with poor adherence, and weight regain is routinely observed shortly after the intervention ends. Nine U.S. Food and Drug Administration (FDA)–approved antiobesity medications are available for adjunct use with long-term weight loss strategies. Among them, the glucagon-like peptide-1 receptor agonists (GLP-1 RAs) liraglutide and semaglutide have been shown to reduce body weight, decrease appetite, and increase feelings of fullness and satiety and have recently been considered by some as a potential gerotherapeutic drug. Weekly administration of the GLP-1 RA tirzepatide also provided substantial and sustained reductions in body weight (52). Interestingly, the effects of GLP-1 RAs on adipose tissue are depot specific, enhancing browning or promoting healthy adipose tissue expansion in some cases (53). Molecularly, GLP-1 RAs protect from major aging-related risk factors, including increased oxidative stress, cellular senescence, mitochondrial dysfunction, and chronic inflammation (54). There have been recent reports on the protective roles of GLP-1 RAs against multiple age-related diseases, including reversal of brain aging and prevention against sarcopenic obesity (54, 55). Moreover, the longevity-extending effects of acarbose may be partially mediated by GLP-1 production (56). Therefore, although lifespan extension mediated by GLP-1 is not yet reported, these studies expose GLP-1 RAs as promising therapeutic strategies to slow the aging process. However, although some antiaging interventions reduce body weight, and some antiobesity treatments have the potential to extend lifespan, other interventions have been shown to be selective to just one of these conditions (Fig. 2), and it remains unclear whether treatments designed to reduce adiposity will extend lifespan, especially in healthy individuals.
Fig. 2. Ways in which different antiobesity and antiaging therapies affect body weight, healthspan, and lifespan.
Schematic representation of pharmacological (GLP-1 RAs and acarbose), nutritional (caloric restriction and isocaloric ketogenic diet), and lifestyle (exercise) interventions targeting adiposity and longevity.
CONCLUSION AND FUTURE DIRECTIONS
Translational longitudinal studies of aging are needed to define the normal trajectories of change in aging phenotypes in the absence and presence of chronic diseases and the impact that interventions known to promote health and survival in model organisms have on those trajectories. Such studies will aid in our understanding of the underlying biological mechanisms of aging, thereby enabling the development of interventions and therapies to extend the disease-free period of life and promote healthy aging.
Acknowledgments:
We thank M. Raley for figure design and M. Bernier for feedback and editing the manuscript.
Funding:
The work was funded by the Intramural Research Program of the National Institutes of Health/NIA, the Talento Grant from the Comunidad de Madrid (2018-T1/BMD-11966 to A.D.-R.), a Ramon y Cajal Award (RYC2021-033751-I to A.D.-R.) from the Spanish Ministry of Science and Innovation (MCIN/AEI/10.13039/501100011033) and the European Union NextGenerationEU/PRTR, RETOS projects program (PID2019-106893RA-100 to A.D.-R.) of MCIN/AEI, Ramón Areces Foundation, (CIVP21S13338 to A.D.-R.), and Asociación Española contra el Cáncer AECC (IDEAS222846DIAZ to A.D.-R.).
Footnotes
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.World Health Organization, “Global strategy and action plan on ageing and health”; www.who.int/publications/i/item/9789241513500. [Google Scholar]
- 2.United Nations, “Decade for healthy aging”; www.decadeofhealthyageing.org/. [Google Scholar]
- 3.Centers for Disease Control and Prevention, National Center for Health Statistics; www.cdc.gov/nchs/data/hestat/obesity-adult-17-18/obesity-adult.htm#table1. [Google Scholar]
- 4.Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW, Gortmaker SL, Projected US state-level prevalence of adult obesity and severe obesity. N. Engl. J. Med 381, 2440–2450 (2019). [DOI] [PubMed] [Google Scholar]
- 5.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G, Hallmarks of aging: An expanding universe. Cell 186, 243–278 (2023). [DOI] [PubMed] [Google Scholar]
- 6.Santos AL, Sinha S, Obesity and aging: Molecular mechanisms and therapeutic approaches. Ageing Res. Rev 67, 101268 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Tam BT, Morais JA, Santosa S, Obesity and ageing: Two sides of the same coin. Obes. Rev 21, e12991 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Ahima RS, Connecting obesity, aging and diabetes. Nat. Med 15, 996–997 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Nunan E, Wright CL, Semola OA, Subramanian M, Balasubramanian P, Lovern PC, Fancher IS, Butcher JT, Obesity as a premature aging phenotype - Implications for sarcopenic obesity. Geroscience 44, 1393–1405 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grover SA, Kaouache M, Rempel P, Joseph L, Dawes M, Lau DCW, Lowensteyn I, Years of life lost and healthy life-years lost from diabetes and cardiovascular disease in overweight and obese people: A modelling study. Lancet Diabetes Endocrinol. 3, 114–122 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Liu C-Y, Chang C-W, Lee H-C, Chen Y-J, Tsai T-H, Chiau J-SC, Wang T-E, Tsai M-C, Yeung C-Y, Shih S-C, Metabolic damage presents differently in young and early-aged C57BL/6 mice fed a high-fat diet. Int. J. Gerontol 10, 105–111 (2016). [Google Scholar]
- 12.Korou LMA, Doulamis IP, Tzanetakou IP, Mikhailidis DP, Perrea DN, The effect of biological age on the metabolic responsiveness of mice fed a high-fat diet. Lab. Anim 47, 241–244 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Lee SW, Son JY, Kim JM, Hwang S-S, Han JS, Heo NJ, Body fat distribution is more predictive of all-cause mortality than overall adiposity. Diabetes Obes. Metab 20, 141–147 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Koster A, Murphy RA, Eiriksdottir G, Aspelund T, Sigurdsson S, Lang TF, Gudnason V, Launer LJ, Harris TB, Fat distribution and mortality: The AGES-Reykjavik study. Obesity (Silver Spring) 23, 893–897 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mancuso P, Bouchard B, The impact of aging on adipose function and adipokine synthesis. Front Endocrinol. (Lausanne) 10, 137 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerli R, Monti D, Bistoni O, Mazzone AM, Peri G, Cossarizza A, di Gioacchino M, Cesarotti MEF, Doni A, Mantovani A, Franceschi C, Paganelli R, Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mech. Ageing Dev 121, 37–46 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Frasca D, Blomberg BB, Adipose tissue, immune aging, and cellular senescence. Semin. Immunopathol 42, 573–587 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Figueiredo P, Marques EA, Gudnason V, Lang T, Sigurdsson S, Jonsson PV, Aspelund T, Siggeirsdottir K, Launer L, Eiriksdottir G, Harris TB, Computed tomography-based skeletal muscle and adipose tissue attenuation: Variations by age, sex, and muscle. Exp. Gerontol 149, 111306 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim TN, Park MS, Ryu JY, Choi HY, Hong HC, Yoo HJ, Kang HJ, Song W, Park SW, Baik SH, Newman AB, Choi KM, Impact of visceral fat on skeletal muscle mass and vice versa in a prospective cohort study: The Korean Sarcopenic Obesity Study (KSOS). PLOS ONE 9, e115407 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roh E, Choi KM, Health consequences of sarcopenic obesity: A narrative review. Front Endocrinol. (Lausanne) 11, 332 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pontzer H, Yamada Y, Sagayama H, Ainslie PN, Andersen LF, Anderson LJ, Arab L, Baddou I, BeduAddo K, Blaak EE, Blanc S, Bonomi AG, Bouten CVC, Bovet P, Buchowski MS, Butte NF, Camps SG, Close GL, Cooper JA, Cooper R, Das SK, Dugas LR, Ekelund U, Entringer S, Forrester T, Fudge BW, Goris AH, Gurven M, Hambly C, Hamdouchi AE, Hoos MB, Hu S, Joonas N, Joosen AM, Katzmarzyk P, Kempen KP, Kimura M, Kraus WE, Kushner RF, Lambert EV, Leonard WR, Lessan N, Martin C, Medin AC, Meijer EP, Morehen JC, Morton JP, Neuhouser ML, Nicklas TA, Ojiambo RM, IAEA DLW Database Consortium, Daily energy expenditure through the human life course. Science 373, 808–812 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zampino M, Alghatrif M, Kuo P-L, Simonsick EM, Ferrucci L, Longitudinal changes in resting metabolic rates with aging are accelerated by diseases. Nutrients 12, 3061 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy RA, Patel KV, Kritchevsky SB, Houston DK, Newman AB, Koster A, Simonsick EM, Tylvasky FA, Cawthon PM, Harris TB, Weight change, body composition, and risk of mobility disability and mortality in older adults: A population-based cohort study. J. Am. Geriatr. Soc 62, 1476–1483 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, González-Reyes JA, Cortassa S, Kaushik S, Gonzalez-Freire M, Patel B, Wahl D, Ali A, Calvo-Rubio M, Burón MI, Guiterrez V, Ward TM, Palacios HH, Cai H, Frederick DW, Hine C, Broeskamp F, Habering L, Dawson J, Beasley TM, Wan J, Ikeno Y, Hubbard G, Becker KG, Zhang Y, Bohr VA, Longo DL, Navas P, Ferrucci L, Sinclair DA, Cohen P, Egan JM, Mitchell JR, Baur JA, Allison DB, Anson RM, Villalba JM, Madeo F, Cuervo AM, Pearson KJ, Ingram DK, Bernier M, de Cabo R, Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab. 23, 1093–1112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palliyaguru DL, Shiroma EJ, Nam JK, Duregon E, Teixeira CVL, Price NL, Bernier M, Camandola S, Vaughan KL, Colman RJ, Deighan A, Korstanje R, Peters LL, Dickinson SL, Ejima K, Simonsick EM, Launer LJ, Chia CW, Egan J, Allison DB, Churchill GA, Anderson RM, Ferrucci L, Mattison JA, de Cabo R, Fasting blood glucose as a predictor of mortality: Lost in translation. Cell Metab. 33, 2189–2200. e3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowman K, Atkins JL, Delgado J, Kos K, Kuchel GA, Ble A, Ferrucci L, Melzer D, Central adiposity and the overweight risk paradox in aging: Follow-up of 130,473 UK biobank participants. Am. J. Clin. Nutr 106, 130–135 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pavela G, Yi N, Mestre L, Lartey S, Xun P, Allison DB, The associations between relative and absolute body mass index with mortality rate based on predictions from stigma theory. SSM Popul. Health 19, 101200 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eglseer D, Traxler M, Embacher S, Reiter L, Schoufour JD, Weijs PJM, Voortman T, Boirie Y, Cruz-Jentoft A, Bauer S, Nutrition and exercise interventions to improve body composition for persons with overweight or obesity near retirement age: A systematic review and network meta-analysis of randomized controlled trials. Adv. Nutr 14, 516–538 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spadaro O, Youm Y, Shchukina I, Ryu S, Sidorov S, Ravussin A, Nguyen K, Aladyeva E, Predeus AN, Smith SR, Ravussin E, Galban C, Artyomov MN, Dixit VD, Caloric restriction in humans reveals immunometabolic regulators of health span. Science 375, 671–677 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruddick-Collins LC, Morgan PJ, Fyfe CL, Filipe JAN, Horgan GW, Westerterp KR, Johnston JD, Johnstone AM, Timing of daily calorie loading affects appetite and hunger responses without changes in energy metabolism in healthy subjects with obesity. Cell Metab. 34, 1472–1485.e6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pak HH, Haws SA, Green CL, Koller M, Lavarias MT, Richardson NE, Yang SE, Dumas SN, Sonsalla M, Bray L, Johnson M, Barnes S, Darley-Usmar V, Zhang J, Yen C-LE, Denu JM, Lamming DW, Fasting drives the metabolic, molecular and geroprotective effects of a calorie-restricted diet in mice. Nat. Metab 3, 1327–1341 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishra A, Longo VD, Fasting and fasting mimicking diets in obesity and cardiometabolic disease prevention and treatment. Phys. Med. Rehabil. Clin. N. Am 33, 699–717 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Acosta-Rodríguez V, Rijo-Ferreira F, Izumo M, Xu P, Wight-Carter M, Green CB, Takahashi JS, Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice. Science 376, 1192–1202 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vujović N, Piron MJ, Qian J, Chellappa SL, Nedeltcheva A, Barr D, Heng SW, Kerlin K, Srivastav S, Wang W, Shoji B, Garaulet M, Brady MJ, Scheer FAJL, Late isocaloric eating increases hunger, decreases energy expenditure, and modifies metabolic pathways in adults with overweight and obesity. Cell Metab. 34, 1486–1498.e7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith DL, Yang Y, Nagy TR, Patki A, Vasselli JR, Zhang Y, Dickinson SL, Allison DB, Weight cycling increases longevity compared with sustained obesity in mice. Obesity (Silver Spring) 26, 1733–1739 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM, Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 27, 1212–1221.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, Strycula P, Najjar SS, Ferrucci L, Ingram DK, Longo DL, Mattson MP, A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am. J. Clin. Nutr 85, 981–988 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, Philip E, Vittinghoff E, Heymsfield SB, Olgin JE, Shepherd JA, Weiss EJ, Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: The TREAT randomized clinical trial. JAMA Intern. Med 180, 1491–1499 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell SJ, Martin-Montalvo A, Mercken EM, Palacios HH, Ward TM, Abulwerdi G, Minor RK, Vlasuk GP, Ellis JL, Sinclair DA, Dawson J, Allison DB, Zhang Y, Becker KG, Bernier M, de Cabo R, The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep. 6, 836–843 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin Y-K, Canto C, Scheibye-Knudsen M, Krawczyk M, Irusta PM, Martín-Montalvo A, Hubbard BP, Zhang Y, Lehrmann E, White AA, Price NL, Swindell WR, Pearson KJ, Becker KG, Bohr VA, Gorospe M, Egan JM, Talan MI, Auwerx J, Westphal CH, Ellis JL, Ungvari Z, Vlasuk GP, Elliott PJ, Sinclair DA, de Caboa R, SRT1720 improves survival and healthspan of obese mice. Sci. Rep 1, 70 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R, Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 8, 157–168 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Couteur DL, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA, Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444, 337–342 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, Perez G, Gutierrez-Casado E, Koike S, Knotts TA, Imai DM, Griffey SM, Kim K, Hagopian K, McMackin MZ, Haj FG, Baar K, Cortopassi GA, Ramsey JJ, Lopez-Dominguez JA, A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab. 27, 1156 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mau T, O’Brien M, Ghosh AK, Miller RA, Yung R, Lifespan extension drug interventions affect adipose tissue inflammation in aging. J. Gerontol. A Biol. Sci. Med. Sci 75, 89–98 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu Y, Fernández-García B, Lehmann HI, Li G, Kroemer G, López-Otín C, Xiao J, Exercise sustains the hallmarks of health. J. Sport Health Sci 12, 8–35 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garatachea N, Pareja-Galeano H, Sanchis-Gomar F, Santos-Lozano A, Fiuza-Luces C, Morán M, Emanuele E, Joyner MJ, Lucia A, Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 18, 57–89 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stamatakis E, Ahmadi MN, Gill JMR, Thøgersen-Ntoumani C, Gibala MJ, Doherty A, Hamer M, Association of wearable device-measured vigorous intermittent lifestyle physical activity with mortality. Nat. Med 28, 2521–2529 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reimers CD, Knapp G, Reimers AK, Does physical activity increase life expectancy? A review of the literature. J. Aging Res 2012, 243958 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaanholt LM, Daan S, Garland T, Visser GH, Exercising for life? Energy metabolism, body composition, and longevity in mice exercising at different intensities. Physiol. Biochem. Zool 83, 239–251 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Samorajski T, Delaney C, Durham L, Ordy JM, Johnson JA, Dunlap WP, Effect of exercise on longevity, body weight, locomotor performance, and passive-avoidance memory of C57BL/6J mice. Neurobiol. Aging 6, 17–24 (1985). [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, Lv J, Wang C, Ren Y, Yong M, Myokine, a key cytokine for physical exercise to alleviate sarcopenic obesity. Mol. Biol. Rep 50, 2723–2734 (2023). [DOI] [PubMed] [Google Scholar]
- 52.Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, Kiyosue A, Zhang S, Liu B, Bunck MC, Stefanski A; SURMOUNT-1 Investigators, Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med 387, 205–216 (2022). [DOI] [PubMed] [Google Scholar]
- 53.Bakkar NMZ, AlZaim I, El-Yazbi AF, Depot-specific adipose tissue modulation by SGLT2 inhibitors and GLP1 agonists mediates their cardioprotective effects in metabolic disease. Clin. Sci. (Lond.) 136, 1631–1651 (2022). [DOI] [PubMed] [Google Scholar]
- 54.Peng W, Zhou R, Sun ZF, Long JW, Gong YQ, Novel insights into the roles and mechanisms of GLP-1 receptor agonists against aging-related diseases. Aging Dis. 13, 468–490 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Z, Chen X, Vong JSL, Zhao L, Huang J, Yan LYC, Ip B, Wing YK, Lai H-M, Mok VCT, Ko H, Systemic GLP-1R agonist treatment reverses mouse glial and neurovascular cell transcriptomic aging signatures in a genome-wide manner. Commun. Biol 4, 656 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCarty MF, DiNicolantonio JJ, Acarbose, lente carbohydrate, and prebiotics promote metabolic health and longevity by stimulating intestinal production of GLP-1. Open Heart 2, e000205 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]