Abstract
Head and neck cancer is the fifth most common cancer worldwide but the most common malignant disease site in central Asia. The treatment of head and neck cancer is one of the most challenging in clinical oncology because of the high content of hypoxic cells of the cancer which increases resistance to therapy and also because of the high capacity of the cancer to regrow during treatment. For unresectable tumours, radiotherapy and chemotherapy alone or more often in combination is the treatment of choice. The aim of this paper is to review current understanding of carcinogenesis of head and neck cancer in relation to predisposing risk factors in general and for specific sub-sites and how these risk factors interact with the main reported genetic alterations in the progression of the cancer. The implications of these changes in determining choice of therapy are also discussed from a brief historical perspective of the various treatment approaches of head and neck cancer.
Keywords: Head and neck, Risk factors, Genetics, Pathology, Radiotherapy, Cisplatin
Introduction
Head and neck squamous cell carcinoma (HNSCC) is an aggressive malignant tumour type arising from the epithelial lining of mucosal membranes of the upper-aerodigestive tract (i.e. pharynx, hypopharynx and larynx) and the oral cavity. Head and neck cancer is the fifth most common cancer worldwide and the most common tumour site in central Asia. Head and neck cancers include several types of cancer originating from the site. The predominant (95%) type consists of squamous cell carcinomas whilst 4–5% are salivary gland (adeno) carcinomas or melanomas (Vikram et al. 1984).
Surgery, radiation and/or chemotherapy have not improved the 50% overall 5-year survival of this debilitating disease over the past 30 years. Furthermore, because of their anatomical location, treatment can lead to long-term sequelae in survivors, which can have a significant impact on the quality of life. The high mortality rate is likely attributable at least to late presentation with the result that most head and neck cancers are diagnosed at advanced stages of the disease. Earlier diagnosis therefore plays a key role in improving treatment response, and ultimately not only to increasing duration of survival but also the quality of life of the patients.
Predisposing factors and their interaction with epigenetic and genetic alterations in head and neck carcinogenesis
Predisposing factors
The most important predisposing factors in the development of HNSCC, present in 90% of cases, are alcohol and tobacco consumption in developed countries and betel nut chewing in Southeast Asia (Sankaranarayanan et al. 1998; Schantz et al. 2000). Although independent factors individually, alcohol and tobacco consumption when combined, have a synergistic effect on carcinogenic risk (Lewin et al. 1998). Furthermore, while the incidence of HNSCC is much higher in males, more and more females are developing HNSCC as women adopt the male pattern of alcohol and tobacco consumption.
Whereas alcohol consumption and smoking are the predominant risk factors in HNSCC carcinogenesis in general, specific, tumour sub-site related risk factors also have a role in carcinogenesis as discussed in the paragraphs which follow.
Other risk factors that contribute to oral cavity cancers apart from smoking and alcohol consumption include genetic susceptibility, viral infections (human papillomavirus infection) (Herrero et al. 2003) and poor oral hygiene (Rosenquist 2005). In lip cancers, unprotected sun exposure early in life and cumulative sun exposure during outdoor work also contributes to carcinogenesis (Perea-Milla et al. 2003).
Radiotherapy to the head and neck by damaging the surrounding normal organs such as salivary glands is one of the main contributors to salivary gland carcinogenesis. Wood dust inhalation and genetic predisposition have also been implicated as important risk factors. While mobile phone use was also thought to increase the risk of parotid gland tumours, recent studies do not support this hypothesis (Lonn et al. 2006).
Industrial exposures to some materials such as wood, nickel dust chromium, mustard gas, isopropyl alcohol and radium are risk factors associated with the development of cancers in the nasal cavity and paranasal sinuses (Caplan et al. 2000; Feron et al. 2001). Tobacco and alcohol use has less of a role in carcinogenesis at this sub-site.
The development of nasopharyngeal cancers has been attributed to several risk factors including viral infection, diet and occupational exposure to wood dust and genetic susceptibility. In Asia, predominantly in Southern China, the prevalence of nasopharyngeal cancer is particularly high. This has been attributed to the dietary habits of the Chinese population as many cases are associated with the consumption of salt-cured meats and fish (Yuan et al. 2000). It is postulated that cooking such foods results in the release of nitrosamines into the air which is carcinogenic to the mucous membranes of the nasopharynx. Similar carcinogenic effects on the nasopharynx result from the use of certain food preservatives for vegetables, also in Asian countries (Gallicchio et al. 2006). Viral infections with the Epstein–Barr (E–B) virus have been implicated in the development of nasopharyngeal cancers. As the virus has growth transforming potential for human B lymphocyte cells it has the potential for malignant transformation and has been associated with certain human B cell lymphomas and also with the undifferentiated nasopharyngeal carcinoma (Young et al. 1988). The E–B virus is a member of the herpesvirus family and lies dormant in most people. The primary site of E–B virus infection is the oropharyngeal cavity. The infection may be silent or may manifest as infectious mononucleosis.
Similar to oral cancer, oropharyngeal cancers have been associated with poor oral hygiene, mechanical irritation such as from poorly fitting dentures, and lack of teeth as well as tobacco and alcohol consumption (Rosenquist 2005). The use of mouthwash of high alcohol content is also considered a risk factor even though oral hygiene is improved. In addition, viral infections especially with human papillomavirus are major risk factors for the development of oropharyngeal cancers (Herrero et al. 2003). Recent advances in the field of immunology have resulted in the development of a vaccine to prevent most human papillomavirus infections of the cervix. Since the same strains of the virus have been shown to be associated with oropharyngeal cancer, the vaccine could decrease the risk of oral and oropharyngeal squamous cell carcinomas (Closmann 2007).
A large case–control study conducted during 1979–1982 in six centres in South Europe showed that occupational exposure to organic solvents is associated with an increased risk of hypopharyngeal cancer (Berrino et al. 2003). Hypopharyngeal cancer risk was also significantly associated with exposure to mild steel dust, asbestos and iron compounds and fumes (Shangina et al. 2006). Another predisposing factor for cancers of the hypopharynx is Plummer–Vinson (also called Paterson–Kelly) syndrome, a rare disorder that results from iron deficiency (Novacek 2006). This syndrome presents as a triad of dysphagia, iron-deficiency anaemia and oesophageal webs (tissue that grow across the upper part of the oesophagus) resulting in swallowing difficulties.
Occupational exposure, particularly to airborne particles of asbestos has been proposed as a major contributor to the development of laryngeal cancers (Marchand et al. 2000) apart from alcohol consumption and smoking (Menvielle et al. 2004). This is supported by an epidemiological study undertaken among Central and Eastern-European industrial workers which found a significant link between laryngeal cancer and exposure to hard-alloys dust, chlorinated solvents and coal dust. A possible link between high formaldehyde exposure and laryngeal cancer has also been suggested (Shangina et al. 2006).
Epigenetic and genetic alterations and relationship to predisposing factors in head and neck carcinogenesis
Squamous cell carcinomas of the head and neck originate from keratinized epithelial cells of the mucous lining. Differences between normal epithelium and malignant epithelial cells of the head and neck arise as a result of specific alterations in genes controlling DNA repair, proliferation, apoptosis, invasion and angiogenesis. These molecular alterations observed in HNSCC are the consequence of (proto)oncogene activation and tumour suppressor gene inactivation and may arise directly and indirectly. An example of an indirect mechanism in the alteration of gene function is the reversible hypermethylation of the promoter region of the genes reported in many cases of HNSCC (Sanchez-Cespedes et al. 2000; Youssef et al. 2004; Kato et al. 2006). The three main direct mechanisms of changes in gene function occur as a result of (a) inactivation of the p53 tumour suppressor gene, (b) inactivation of the cyclin-dependent kinase (CDK) inhibitor p16 and (c) overexpression of epidermal growth factor receptor (EGFR) (Hardisson 2003). Disturbances in the regulation of a wide variety of intracellular signalling pathways such as those pathways modulating growth and cell proliferation, response to DNA damage, cell cycle progression and programmed cell death ensue downstream of these changes in gene function whether induced directly or indirectly.
The three main direct mechanisms which result in altered gene function and their interaction with known predisposing factors in head and neck carcinogenesis will be briefly discussed below:
Inactivation of the p53 tumour suppressor gene is found in about 50% of HNSCC tumours, being one of the most common events in the development of cancer. This is the result of mutation of the remaining copy of the p53 tumour suppressor gene, the other abnormal copy being inherited by the patient. Head and neck cancers are an ideal tumour model because of well-defined risk factors linked to this malignancy (such as smoking and alcohol consumption). Therefore, characterization of p53 mutations in terms of location and type has been widely performed. The type, frequency and location of p53 mutations have been associated with specific carcinogenic agents. A detailed study on the types and frequencies of p53 mutations has correlated the endogenous and exogenous carcinogens with specific alterations in the DNA (base transitions, typically G → T, deletions and transversions) of the p53 gene in head and neck cancer patients (Olshan et al. 1997).
Cyclins play a crucial role in cell function as these proteins regulate the cell cycle by binding to a kinase the role of which is to transfer phosphate groups from high-energy donor molecules (such as ATP) to specific target molecules (substrates). The purpose of phosphorylation is to energize a molecule so it is able to further participate in biochemical reactions. Therefore, because of their involvement in cell cycle regulation, CDK are key enzymes in the process of cell growth and also cell death. While inhibition of CDK can lead to apoptosis, inactivation of CDK inhibitors can trigger cancer development.
The p16 gene is a tumour suppressor gene involved in cell cycle control. The gene encodes an inhibitor of CDK 4 and 6, which regulate the phosphorylation of retinoblastoma gene (Rb) and the G1 to S phase transition of the cell cycle (Ai et al. 2003). The tumour suppressor function of p16 is attributed to its ability to inhibit the catalytic activity of the CDK 4–6/cyclin D complex that is required for phosphorylation of retinoblastoma protein. This effect blocks the transcription of important cell-cycle regulatory proteins and results in cell-cycle arrest. The expression of p16 is frequently down-regulated in HNSCC, leading to cellular proliferation, and ultimately to the development of locally advanced cancer. Tumours of the larynx have also been associated with a significantly higher frequency of weak p16 expression compared with tumours of the oral cavity and pharynx (Yuen et al. 2002). Furthermore, tobacco and alcohol consumption has been shown to enhance the likelihood of p16 inactivation in all head and neck tumours (Ai et al. 2003).
-
(c)
The epidermal growth factor promotes growth of epidermal cells and regulates (stimulates or inhibits) cell proliferation. EGFR plays a crucial role in head and neck cancer development, growth, metastasis and angiogenesis. Therefore, overexpression of EGFR leads to increased tumour proliferation and other growth-promoting behaviour. Up to 90% of HNSCC exhibit overexpression of EGFR (Kalyankrishna 2006).
Monoclonal antibodies such as cetuximab which has the ability to block EGFR have been investigated as the treatment of HNSCC with promising results (Bonner et al. 2006). Blocking EGFR resulted in inhibition of cell proliferation, enhancement of apoptosis, and reduction in the metastatic and angiogenetic potential of HNSCC.
Mechanisms of progression in the development of head and neck squamous cell carcinoma
Like other cancers, squamous cell carcinomas of the head and neck arise as a result of sequential accumulation of genetic changes. These changes include a combination of inherited and acquired alterations in the DNA sequence, ranging from point mutations to deletions, amplifications and translocations. However, more in-depth clarification of the molecular mechanisms underlying these changes is required to account for the changes. For example, loss of chromosomal region 9p21 is the underlying mechanism for the progression of 70–80% of HNSCC cases (Perez-Ordoňez et al. 2006). As discussed under Sect. ”Epigenetic and genetic alterations and relationship to predisposing factors in head and neck carcinogenesis” above, inactivation of the p16 tumour suppressor gene is one of the earliest detectable genetic change in HNSCC and inactivation of the p53 tumour suppressor gene is present in half of all HNSCC cases. That these changes in gene function may occur as epigenetic phenomenon and not necessarily as a results of somatic mutations are also discussed under Sect. ”Epigenetic and genetic alterations and relationship to predisposing factors in head and neck carcinogenesis.”
Elucidation of the molecular mechanisms leading to the acquisition of an invasive and metastatic phenotype in HNSCC is another area of research needed in the future.
The concept of “field cancerization”, a characteristic of head and neck cancers, was introduced in 1953 (Slaughter and Southwick 1953) based on the hypothesis that prolonged exposure to carcinogens leads to the independent transformation of epithelial cells at multiple sites in the mucosa adjacent to HNSCC. Slaughter’s theory implied that the multiple tumours adjacent to HNSCC arise as a result of independent genetic events. However, current data show that these multiple tumours adjacent to the index HNSCC are clonally related originating from a common preneoplastic progenitor (Bedi et al. 1996; Califano et al. 1996; Perez-Ordoňez et al. 2006). It has been hypothesized that these multiple tumours are actually micrometastases, caused by the migration of transformed cells through the mucosa of the upper aerodigestive tract, either by intraepithelial migration or through the saliva.
Treatment of head and neck cancers
Advanced head and neck cancers are difficult to manage because of (1) their high content of hypoxic cells leading to radio-resistance and (2) their ability to repopulate during treatment. The latter property results from accelerated stem cell division in response to radiation or drug-induced cell kill. The change from conventional radiotherapy (2 Gy/day, 5 days/week over 7 weeks) to altered fractionation schedules (whether hyperfractionated, accelerated or a combination of both regimens) has improved both loco-regional control as well as overall survival in patients with advanced, unresectable tumours (Overgaard et al. 2003). Nevertheless, there is scope for further improvement, as normal tissue toxicity is often a limiting factor when “accelerating” radiotherapy.
The desire for organ preservation in advanced, resectable head and neck cancers has led to the implementation of novel technologies such as 3D-conformal radiotherapy, intensity modulated radiotherapy, and image-guided radiotherapy, all of which allow for better normal tissue protection through more conformal target delivery. The development of prodrugs to overcome tumour hypoxia and of radioprotectors (like amifostine) as an aid in the sparing of normal tissue have brought improved results in the treatment of some head and neck patients. Major interest in immunotherapy, specifically targeted therapies and gene therapy has also gained pace in the past few years.
Applying radiobiological principles to combined modality treatment of head and neck cancer, especially the optimization of the time factor in treatment scheduling, has been shown to enhance tumour response (Peters and Withers 1997). Likewise, explanation of the molecular mechanisms leading to head and neck cancer may result in the identification of new biomarkers of both diagnostic and prognostic value to assist in the clinical management of these patients.
Short historical perspective of therapeutic approaches in treatment of HNSCC
Most probably, the oldest available specimen of a human head and neck cancer is the one found in the skull remains of a female who lived during the Bronze Age (1900–1600 BC).
Hippocrates, considered the father of medicine, is thought to be the first to recognize the differences between benign and malignant tumours. In the fifth century BC Hippocrates described the nasopharyngeal fibroma, a benign tumour that was named as such only in 1940, and he also attributed a common chronic, malignant ulcer at the edge of the tongue to sharp teeth rubbing against the tongue.
Though Greek and Roman medical writings were the foundation of modern medicine, little advancement was made in the management of head and neck cancers until the employment of anaesthesia and surgical excision in the eleventh century. Biopsy studies started to bring some insights into the development of cancer. However, significant success in tumour control took much longer to achieve and did not occur until the twentieth century, when multimodality treatment was introduced and with the improved understanding of the molecular changes underlying the development of cancer.
Surgery was and still remains the treatment of first choice for resectable head and neck cancer. Nevertheless, radiotherapy, chemotherapy and to some extent hyperthermia, either alone or in combination with radiotherapy were used with greater success in the treatment of unresectable and locally advanced carcinomas.
Brachytherapy (using radium surface moulds and plaques) has been successfully applied since 1903 (Mould 1993). For head and neck carcinomas successful treatment reports using surface moulds date back to the 1920 s. For deep seated head and neck cancer interstitial brachytherapy was developed to overcome the side effects of X-ray treatment because much healthy tissue has to be traversed in order to deliver cancericidal doses to the tumour target volume. In 1903, the German physician H. Strebel reported the first interstitial treatment technique with radium (Mould 1993). More sophisticated followed during the second half of the twentieth century with the employment of the afterloading applicators in brachytherapy, and high dose rate treatments now widely available for intracavitary and interstitial brachytherapy.
Treatment of head and neck cancer in the twenty-first century
While surgery or radiation alone is effective in treating most early stage disease, patients with advanced but resectable head and neck tumours are managed by surgery and postoperative radiotherapy or by combined chemo-radiotherapy. Combined modality treatment is also often used for patients with locally advanced, unresectable tumours in recent years since radiotherapy alone leads to a failure-free survival of only 25% (Brizel 1998). To overcome accelerated repopulation during treatment, a typical response of head and neck cancer to the induction of cytotoxic therapy including conventional radiotherapy lead to more rapid proliferation than before the therapy is increasingly replaced by altered fractionation schedules. These strategies involve either giving the radiation treatment in smaller multiple fractions a day (hyperfractionation), or giving the radiation in an accelerated manner to shorten the overall treatment time (accelerated radiotherapy). Head and neck cancer which has a high content of hypoxic cells also benefit from this approach, since the more aggressive treatment results in tumour shrinkage and therefore tumour reoxygenation during treatment. Radiosensitizers, normal tissue protectors, bioreductive drugs and antiangiogenic agents have also been successfully used in addition to radio- and chemotherapy to improve results of treatment.
Tumour site-related treatments
Though surgery (neck dissection) and/or radiotherapy are usually the treatment choice for oral cancers, the occurrence of cumulative morbidity in both has led to other treatment approaches being employed for both cure and palliation of these cancers. A retrospective study (Sturgis et al. 2005) has demonstrated the possible role neoadjuvant chemotherapy with taxane-based regimens might play in the treatment of oral cancers. Pre-clinical studies investigating the effect of induction chemotherapy on treatment outcome have shown an inhibitory effect of the neoadjuvant drug treatment on metastasis of oral squamous cell carcinoma (Kawashiri et al. 2009). These results are indicative of the possible implementation of neoadjuvant chemotherapy as a routine treatment for oral cancers.
Starting as a potential treatment for squamous cell carcinomas of the oral cavity, photodynamic therapy (PDT) has been used in patients with field cancerization of the oral cavity, being an obvious choice over highly mutilating surgery (Grant et al. 1993). PDT also known as photoradiation therapy involves the use of a photosensitizer (photoactive dye which is tumour-specific, most commonly 5-aminolevulinic acid) that is activated by exposure to light of a specific wavelength (usually with 50–100 J/cm2 red or violet laser light) in the presence of oxygen. The light-activated photosensitizer reacts with available oxygen which consequently damages cells and eventually causes local tissue necrosis (Nauta et al. 1996). While the healing process after PDT is smooth, leaving remarkably little scaring, this method is not suitable for deep seated tumours as the PDT effect is too superficial with the currently used photosensitizing agents (Fan et al. 1996). Lately, beside non-thermal lasers, light-emitting diodes have been employed in PDT (Kvaal and Warloe 2007). Due to the damaging effect of PDT upon various cellular components, studies are leading to selective photosensitizers, to ensure long term safety. Consequently, in current oral cancer management, PDT is more often used in the palliative setting than for curative intent.
Salivary gland cancer occurs mostly in the parotid gland, located in front of the ears. However, predisposed individuals are also at risk for development of tumours of the submandibular, sublingual and also the minor salivary glands. For both major and minor resectable salivary gland cancers surgical excision is the most common treatment approach, with or without postoperative radiotherapy. Unresectable tumours are treated with concurrent radiotherapy and chemotherapy using a combination of cytotoxic drugs, including newer cytotoxic agents (e.g. Taxols) and older established cytotoxic drugs (e.g. cisplatin). Irradiation as a single modality treatment, as an alternative to surgery has also been reported to result in significant long-term benefits (Chen et al. 2006). Where surgical excision results in significant morbidity, fast neutron radiotherapy can offer high local-regional control and survival rates and is recommended as initial primary treatment (Douglas et al. 1996).
For the cancer of nasal cavity and paranasal sinuses the primary treatment is the surgical removal of the tumour, though for the early stage disease, radiotherapy yields equivalent results. Furthermore, three-dimensional conformal treatment and, in particular, intensity-modulated radiotherapy, may minimize the occurrence of late complications associated with conventional radiotherapy techniques (Hoppe et al. 2007). For advanced cases, surgery and postoperative radiotherapy is recommended as it has been reported to result in improved local control, absolute survival and less complications when compared with radiation therapy alone (Katz et al. 2002).
The first line treatment employed for nasopharyngeal carcinoma depends on available expertise but may involve surgical resection. Despite its complexity, infratemporal fossa surgery is implemented in several specialized clinical centers to achieve radical removal of the tumour (Fisch 1983). The understanding of the surgical anatomy has made possible the numerous modifications in surgical procedures of skull based neoplasms that were previously regarded as inoperable due to the critical structures involved in the operation. The lateral techniques introduced by Fisch involve an infratemporal fossa approach to tumours of the lateral skull base and temporal bones and they include three different lines of attack. Type A approach is the most commonly used for glomus tumours and cholesteatoma, type B is optimal for the clivus and the superior infratemporal fossa and type C is employed for nasopharynx and paranasal synuses (Fisch et al. 1984).
Among squamous cell carcinomas of the head and neck, nasopharyngeal carcinoma is probably the most radio- and chemo-responsive. Therefore, in centres where the necessary surgical expertise is unavailable, radiotherapy alone or in combination with chemotherapy is the treatment of choice. Loco-regional control of early-stage disease after radiotherapy is very high (Fu 1998). When combined with chemotherapy, radiation treatment achieves better results for late stages of the disease than radiotherapy alone (possibly through the effect of chemotherapy on distant micro-metastatic disease), although, some patients do not respond to drug treatment. Antiangiogenic agents might be beneficial by decreasing tumour microvascular density to reduce the risk of distant metastases.
Multimodality treatment is often employed in the management of both resectable and unresectable oropharyngeal carcinomas. Perioperative chemotherapy, surgery and postoperative chemo-radiotherapy were shown to lead to excellent disease control rates and long-term survival in patients with advanced, resectable cancers (Schuller et al. 2007). The use of pre-treatment PET imaging to determine the primary treatment strategies for patients with aggressive, advanced oropharyngeal tumours is gaining increasing importance because of the prognostic significance of PET imaging. The important role of PET imaging in the management of head and neck cancer was demonstrated in the early 90 s, when independent research groups employed this imaging modality for tumour detection and prediction of treatment outcome (Bailet et al. 1992; Seifert et al. 1992; Greven et al. 1994). It has been demonstrated that pretreatment tumour (18)F-FDG (fluoro-2-deoxy-d-glucose) uptake represents an independent prognostic factor in patients with oropharyngeal squamous cell carcinomas. It was shown that while lack of FDG uptake 4 months after radiotherapy strongly suggests tumour control, high tumour uptake after treatment is associated with an unfavourable outcome. Therefore, tumours having high pretreatment FDG uptake are at greater risk of failure and should be considered for more aggressive multimodality therapy.
Cancer of the hypopharynx is associated with poor prognosis, and the treatment applied should therefore be aggressive. While surgery and radiotherapy remain the first treatment choice for uncommon cases of early stage disease, for the majority which present with advanced stage disease, the choice of treatment depends on various factors such as: the extent of local involvement, particularly the larynx, performance status, the presence or absence of distant metastasis. Chemotherapy administered prior to surgery or radiotherapy helps in organ preservation (Shirinian et al. 1994). Altered fractionation regimens (hyperfractionation) and IMRT used in combination with chemotherapy are also employed to improve treatment results.
Radiotherapy with concurrent cisplatin is the standard treatment for patients with locally advanced laryngeal cancer. A recent study on advanced laryngeal cancers has concluded that patients with complete response to induction chemotherapy (cisplatin) have a high probability of cure after hyperfractionated radiotherapy (Majem et al. 2006). Gene therapy promises to improve the outcome of laryngeal cancer treatment in the near future. Several studies have focused on the p53 tumour suppressor gene, analysing gene and protein status for all HNSCC patients but as yet there is no correlation with patient outcome. However, a significant association between p53 expression and poor patient outcome was found when analysing only patients with laryngeal squamous cell carcinomas (Nylander et al. 2000). It was found that in most laryngeal cancers, the function of p53 gene is down-regulated. To explore the potential use of p53 in gene therapy of laryngeal cancer, Wang et al. (1999) have introduced a wild-type p53 into a laryngeal cancer cell line via a recombinant adenoviral vector. They concluded that the adenovirus-mediated antitumour therapy carrying the p53 gene is an efficient method to inhibit laryngeal cancer growth. The same group has conducted a phase I clinical trial showing the effectiveness of recombinant adenovirus p53 injection in reducing laryngeal cancer progression (Han et al. 2003).
While chemo-radiotherapy is very effective in the management of HNSCC there are situations when this treatment does not succeed. Consequently, surgical therapy for salvage of disease resistance or recurrence after chemoradiation could be the treatment choice. With advances in re-constructive surgery, good functional results can be achieved in the event of failure of radiotherapy ± chemotherapy (Scher and Esclamado 2009). However, earlier results regarding salvage-surgery are inconclusive, as some studies indicate positive outcomes while other studies less favourable results. For example, pharyngeal tumours present with fairly poor postoperative, functional and oncologic results after salvage surgery which makes the decision on primary treatment very important (Julieron and Temam 2004). Also, the limited potential of salvage surgery for previously treated advanced recurrent cancers of the oral cavity, larynx, oropharynx and hypopharynx (Gleich et al. 2004) advises for cautious counselling of those patients contemplating treatment of recurrent cancer. On the other hand, patients with recurrent T1, T2 head and neck tumours are ideal candidates for salvage surgery, achieving a minimum of 2 years disease-free survival after salvage (Kim et al. 2007). Salvage surgery remains a treatment choice for the persistent and recurrent advanced disease. With the large number of patients enrolled in organ preservation protocols, the role of salvage surgery is increasing among the other therapies involved in the management of HNSCC.
Table 1 is a synopsis of the most commonly used treatment modalities as well as other (adjuvant, or single modality) treatment methods in the management of head and neck carcinomas. It is clearly illustrated that for resectable tumours surgery remains the main treatment option with or without adjuvant radiotherapy, while for unresectable cancers, radio- and chemotherapy are employed. Usually, well-established chemotherapeutic agents, like cisplatin, 5-FU, bleomycin, carboplatin and methotrexate are combined with newer chemotherapy drugs, such as paclitaxel, docetaxel, gemcitabine and/or doxorubicin to improve results. Beside radio- and chemotherapy, additional treatment modalities used in the adjuvant setting such as hypoxic cell cytotoxins, normal tissue radioprotectors, antiangiogenic agents which show promise in the clinic.
Table 1.
Summary of tumour site-related treatment options
| Tumour site | First treatment choice | Other treatment options | Comments |
|---|---|---|---|
| Oral cavity | Surgery (neck dissection) | Neoadjuvant chemotherapy | PDT is used for palliation in oral cancers and for superficial treatment in dentistry |
| Radiotherapy | |||
| Salivary glands | Surgery (gland excision) | Neutron beam therapy | Drug combinations with concurrent radiotherapy are employed for the treatment of unresectable tumours |
| Radiotherapy/chemo-therapy | |||
| Nasal cavity and paranasal sinuses | Surgical resection | Radiotherapy/chemotherapy | For early stages surgery and RT yield equivalent results |
| Radiotherapy | |||
| Nasopharynx | Surgery | Antiangiogenic agents | Probably the most radio- and chemo-responsive head and neck cancer |
| Radiotherapy/chemotherapy (low grade squamous carcinoma) | |||
| Oropharynx | Surgery | Pretreatment (18)F-FDG uptake provides guide for the choice of primary treatment modality or combined modality treatment | |
| Radiotherapy | |||
| Chemotherapy | |||
| Hypopharynx | Surgery | Induction chemotherapy | Highly aggressive tumour, needs aggressive treatment approaches |
| Radiotherapy | |||
| Larynx | Surgery | Induction chemotherapy | Induction chemotherapy can preserve the larynx |
| Radiotherapy | Gene therapy | ||
| Chemotherapy |
Cisplatin and radiotherapy in the latest clinical trials
Randomized trials in head and neck cancers have shown that combination treatment (cisplatin–radiotherapy) improves survival compared to radiation treatment alone. This result is due to the cooperation between cisplatin and radiotherapy at the cellular level. Radiosensitization of tumour cells by cisplatin is believed to be mediated through a variety of mechanisms, including inhibition of DNA repair, a radiation-induced increase in cellular platinum uptake, cell-cycle arrest (Lawrence et al. 2003), and inhibition of angiogenesis (Yoshikawa et al. 1997).
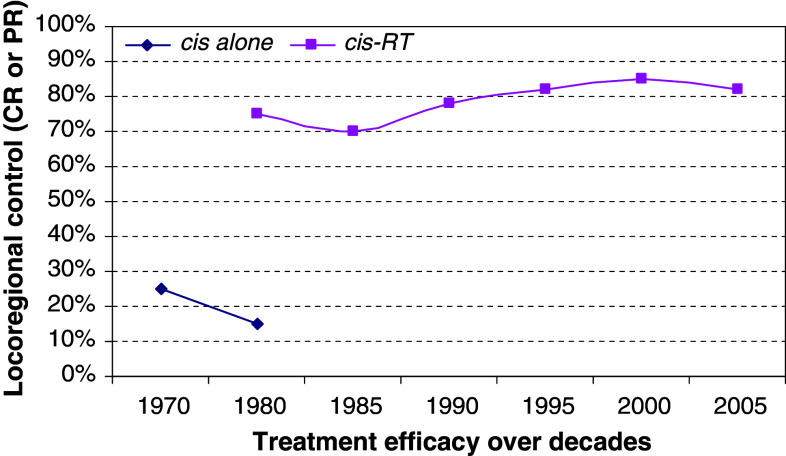
The utilization of cisplatin has progressed from its early use in isolation in the 70 s to a variety of forms of combined chemo-radiotherapy in the subsequent decades. While in the 80 s and early 90 s most of the schedules used high-dose weekly cisplatin administration, in the mid to late 90 s daily low-dose infusions were introduced and outcomes have continuously improved (Marcu et al. 2003). The graph in Fig. 1 presents the treatment efficacy of cisplatin with or without radiotherapy based on clinical trial data over the decades (Jacobs et al. 1978; Sako et al. 1978; Creagan et al. 1983; Leipzig 1983; Al-Sarraf et al. 1987; Gasparini et al. 1991; Fontanesi et al. 1991; Arias et al. 1995; Robbins et al. 1997; Serin et al. 1999; Jeremic et al. 2000; Beckmann et al. 2005; Medina et al. 2006; Vivek et al. 2006). Furthermore, conventional radiotherapy is increasingly replaced by altered fractionation schedules and several trials employing concurrent chemo-irradiation using accelerated concomitant boost radiation therapy for advanced head and neck cancers are in progress or complete (Table 2).
Fig. 1.
Treatment efficacy of cisplatin with or without radiotherapy based on clinical trial data over the decades shown [the cisplatin-alone curve shows partial response (PR) since locoregional control was incomplete and the cisplatin-radiotherapy curve shows complete locoregional response (CR)]
Table 2.
Cisplatin-radiotherapy trials for advanced, unresectable head and neck cancer in the twenty-first century
| Trial | Chemotherapy schedule and total dose | Radiotherapy schedule and total dose | End points | |
|---|---|---|---|---|
| Complete and/or overall response | Toxicity (side effects) | |||
| Vivek et al. 2006 Phase I | 100 mg/m2 infusion on days 1 and 22 | 1.8 Gy during the first 3.5 weeks and two fractions per day, 1.8 and 1.5 Gy boost-separated by > 6 h interval, during the last 2.5 weeks | Complete response 79.1% | 12.5% Patients acute grade 4 toxicity |
| 24 Patients stage III and IV | Total dose: 200 mg/m2 | Total dose: 72 Gy | Overall response 95.8% | 87.5% Patients acute grade 3 toxicity |
| Beckmann et al. 2005 Phase I/II | 40 mg/m2 weekly | 1.8 Gy, days 1–38 and 1.5 Gy boost, days 22–38, twice daily with at least a 6-h interval | 2 year Overall survival 67% | Manageable toxicity |
| 37 Patients stage III and IV oropharynx and hypopharynx | 160 mg/m2 median dose intensity (5.5 weeks) | Total dose: 69.9 Gy | ||
| Medina et al. 2006 Phase II | 40 mg/m2 weekly, for the first 4 weeks | 1.8 Gy on days 1–40 and 1.5 Gy boost on days 25–40 | Complete response 66% overall response 88% estimated overall survival at 4 years was 41% | 85% Patients grade 3 mucositis |
| 94 Patients stage III and IV all sites, no nasopharynx | Total dose: 160 mg/m2 | Total dose: 72 Gy | Manageable toxicity | |
| Garden et al. 2008 Phase II | 100 mg/m2 infusion on days 1 and 22 | 1.8 Gy per fraction, 5 fractions a week, to 54 Gy in 30 fractions over 6 weeks + a second daily dose of 1.5 Gy per fraction (from latter part of week 4) | Complete response 83% | 42% Patients grade 3 and 4 toxicity |
| 84 Patients stage III and IV all sites | Total dose: 200 mg/m2 | Total dose: 72 Gy | Overall survival at 2 years—71.6% | |
| Jeremic et al. 2000 Phase III (prospective randomized trial) | 6 mg/m2 IV bolus 3–4 h after the first RT fraction | 1.1 Gy twice a day, 4.5–6 h inter-fractions over 7 weeks | Complete response 75% | 11% Patients had treatment interruptions because of acute toxicity |
| 65 Patients stage III and IV all sites | Total dose: 210 mg/m2 | Total dose: 77 Gy | Locoregional progression free survival at 5 years—50% | |
The better loco-regional control and the higher percentage of complete survival among patients treated with combined chemo-radiotherapy in the late 90 s and the beginning of the twenty-first century are due to the employment of non-conventionally fractionated radiotherapy and/or chemotherapy. The aggressive treatments lead to more pronounced normal tissue toxicity, which however, can be managed. Nevertheless, the higher tumour control is indicative of effective treatment schedules, maintaining cisplatin as the preferential chemotherapeutical agent for head and neck cancer.
Other chemotherapeutic agents combined with radiotherapy
Although cisplatin has been used successfully in head and neck cancers, it has been avoided by others because of its toxicity, replacing it with analogous anticancer agents, less toxic to the normal tissue. The platinum family has proven efficacy in head and neck carcinomas, therefore, less toxic platinum compounds have been developed and trialed over the years. Carboplatin and oxaliplatin are two representatives of newer agents in the platinum group. Studies comparing the effect of cisplatin and carboplatin on head and neck carcinomas suggest that carboplatin is inferior to cisplatin (Go and Adjei 1999). Although carboplatin has replaced cisplatin in chemotherapy regimens of other neoplasms (such as ovarian cancers), its success in head and neck cancers has not been clinically proven. Similarly, oxaliplatin has been found effective for colorectal cancers (Pandor et al. 2006) but not for squamous cell carcinomas.
Besides platinum compounds, head and neck tumours have been treated with multi-agent chemotherapy, using different classes of drugs. Some of the most commonly used of these drugs are the antimetabolites (5-FU or 5-fluorouracil), antibiotics (mitomycin-C, bleomycin) and taxanes (paclitaxel). Although different classes of drugs have different cytotoxic mechanisms, the interaction of these drugs with radiation enhances the antitumour effect of radiotherapy. While taxanes are able to arrest cells in the radiosensitive phases of their cell cycle (G2 or M), antimetabolites interfere with the DNA synthesis of cells during the S phase of their cell cycle and mitomycin-C has the role of a hypoxic cell cytotoxin, similar to tirapazamine (Milas et al. 2003).
Multi-agent chemotherapy combined with radiation increases treatment toxicity, sometimes not balanced by better tumour control. There has been an ongoing search for novel drugs which would lead to increased therapeutic ratio by making the tumour more radiosensitive and/or preventing critical normal tissue damage. Taxanes, nucleoside analogues (gemcitabine), drugs that are conjugated to polymeric macromolecules (to improve drug delivery) and molecular targeting (EGFR inhibitors) are some of the newer pathways to achieve better tumour control. While some of the drugs have undergone clinical trials and are becoming part of standard care, others are only being subjected to trials, conclusive results of which are not expected for some years to come.
Nowadays, for head and neck tumours the optimal treatment schedule when combining chemotherapy with radiation is the concurrent administration of these two anticancer agents. The trials have shown that induction and/or adjuvant chemotherapy using the currently available drugs are ineffective (Milas et al. 2003). However, the heterogeneity in the design of various concomitant treatment regimens makes it difficult to define the optimal multi-agent chemotherapy for squamous cell carcinomas (Browman et al. 2001). The fact that the most reported chemotherapeutic agent in the head and neck literature is cisplatin, makes this drug, despite its toxicity to the normal tissue, one of the first treatment choices. In the final analysis, the balance between cisplatin’s effectiveness on tumours and its toxicity to the healthy tissue determines whether cisplatin is the right choice of chemotherapeutic agent.
References
- Ai L, Stephenson KK, Ling W, Zuo C, Mukunyadzi P et al (2003) The p16 (CDKN2a/INK4a) tumor-suppressor gene in head and neck squamous cell carcinoma: a promoter methylation and protein expression study in 100 cases. Mod Pathol 16:944–950. doi:10.1097/01.MP.0000085760.74313.DD [DOI] [PubMed] [Google Scholar]
- Al-Sarraf M, Pajak T, Marcial V et al (1987) Concurrent radiotherapy and chemotherapy with cisplatin in inoperable squamous cell carcinoma of the head and neck. An RTOG study. Cancer 59:259–265. doi:10.1002/1097-0142(19870115)59:2%3c259::AID-CNCR2820590214%3e3.0.CO;2-1 [DOI] [PubMed] [Google Scholar]
- Arias F, Dominguez M, Illarramendi J et al (1995) Split hyperfractionated accelerated radiation therapy and concomitant cisplatin for locally advanced head and neck carcinomas: a preliminary report. Int J Radiat Oncol Biol Phys 33:675–682. doi:10.1016/0360-3016(95)00210-P [DOI] [PubMed] [Google Scholar]
- Bailet JW, Abemayor E, Jabour BA, Hawkins RA et al (1992) Positron emission tomography: a new, precise imaging modality for detection of primary head and neck tumors and assessment of cervical adenopathy. Laryngoscope 102:281–288 [DOI] [PubMed] [Google Scholar]
- Beckmann GK, Hoppe F, Pfreundner L, Flentje MP (2005) Hyperfractionated accelerated radiotherapy in combination with weekly cisplatin for locally advanced head and neck cancer. Head Neck 27:36–43. doi:10.1002/hed.20111 [DOI] [PubMed] [Google Scholar]
- Bedi GC, Westra WH, Gabrielson E et al (1996) Multiple head and neck tumors: evidence for a common clonal origin. Cancer Res 56:2484–2487 [PubMed] [Google Scholar]
- Berrino F, Richiardi L, Boffetta P, Esteve J et al (2003) Occupation and larynx and hypopharynx cancer: a job-exposure matrix approach in an international case-control study in France, Italy, Spain and Switzerland. Cancer Causes Control 14:213–223. doi:10.1023/A:1023661206177 [DOI] [PubMed] [Google Scholar]
- Bonner JA, Harari PM, Giralt J, Azarnia N et al (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567–578. doi:10.1056/NEJMoa053422 [DOI] [PubMed] [Google Scholar]
- Brizel DM (1998) Radiotherapy and concurrent chemotherapy for the treatment of locally advanced head and neck squamous cell carcinoma. Semin Radiat Oncol 8:237–246. doi:10.1016/S1053-4296(98)80021-0 [DOI] [PubMed] [Google Scholar]
- Browman G, Hodson I, Mackenzie R, Bestic N, Zuraw L (2001) Choosing a concomitant chemotherapy and radiotherapy regimen for squamous cell head and neck cancer: a systematic review of the published literature with subgroup analysis. Head Neck 20:579–589. doi:10.1002/hed.1081 [DOI] [PubMed] [Google Scholar]
- Califano J, van der RP, Westra W et al (1996) Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res 56:2488–2492 [PubMed]
- Caplan LS, Hall HI, Levine RS, Zhu K et al (2000) Preventable risk factors for nasal cancer. Ann Epidemiol 10:186–191. doi:10.1016/S1047-2797(99)00049-6 [DOI] [PubMed] [Google Scholar]
- Chen AM, Bucci MK, Quivey JM, Garcia J et al (2006) Long-term outcome of patients treated by radiation therapy alone for salivary gland carcinomas. Int J Radiat Oncol Biol Phys 66:1044–1050. doi:10.1016/j.ijrobp.2006.06.050 [DOI] [PubMed] [Google Scholar]
- Closmann JJ (2007) The human papilloma virus, the vaccines, and oral and oropharyngeal squamous cell carcinoma: what every dentist should know. Gen Dent 55(3):252–254 [PubMed] [Google Scholar]
- Creagan E, O’Fallon J, Woods J et al (1983) Cis-diamminedichloroplatinum (II) administered by 24-hour infusion in the treatment of patients with advanced upper aerodigestive cancer. Cancer 51:2020–2023. doi:10.1002/1097-0142(19830601)51:11%3c2020::AID-CNCR2820511110%3e3.0.CO;2-X [DOI] [PubMed] [Google Scholar]
- Douglas JG, Laramore GE, Austin-Seymour M, Koh WJ et al (1996) Neutron radiotherapy for adenoid cystic carcinoma of minor salivary glands. Int J Radiat Oncol Biol Phys 36:87–93. doi:10.1016/S0360-3016(96)00213-1 [DOI] [PubMed] [Google Scholar]
- Fan KF, Hopper C, Speight PM, Buonaccorsi G et al (1996) Photodynamic therapy using 5-aminolevulinic acid for premalignant and malignant lesions of the oral cavity. Cancer 78:1374–1383. doi:10.1002/(SICI)1097-0142(19961001)78:7%3c1374::AID-CNCR2%3e3.0.CO;2-L [DOI] [PubMed] [Google Scholar]
- Feron VJ, Arts JH, Kuper CF, Slootweg PJ et al (2001) Health risks associated with inhaled nasal toxicants. Crit Rev Toxicol 31:313–347. doi:10.1080/20014091111712 [DOI] [PubMed] [Google Scholar]
- Fisch U (1983) The infratemporal fossa approach for nasopharyngeal tumors. Laryngoscope 93:36–44 [DOI] [PubMed] [Google Scholar]
- Fisch U, Fagan P, Valvanis A (1984) The infratemporal fossa approach for the lateral skull base. Otolaryngol Clin North Am 17:513–552 [PubMed] [Google Scholar]
- Fontanesi J, Beckford NS, Lester EP et al (1991) Concomitant cisplatin and hyperfractionated external beam irradiation for advanced malignancy of the head and neck. Am J Surg 162:393–396. doi:10.1016/0002-9610(91)90156-8 [DOI] [PubMed] [Google Scholar]
- Fu KK (1998) Combined radiotherapy and chemotherapy for nasopharyngeal carcinoma. Semin Radiat Oncol 8:247–253. doi:10.1016/S1053-4296(98)80022-2 [DOI] [PubMed] [Google Scholar]
- Gallicchio L, Matanoski G, Tao XG, Chen L et al (2006) Adulthood consumption of preserved and nonpreserved vegetables and the risk of nasopharyngeal carcinoma: a systematic review. Int J Cancer 119:1125–1135. doi:10.1002/ijc.21946 [DOI] [PubMed] [Google Scholar]
- Garden AS, Harris J, Trotti A, Jones CU et al (2008) Long-term results of concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: a phase II trial of the radiation therapy oncology group (RTOG 99–14). Int J Radiat Oncol Biol Phys 71:1351–1355. doi:10.1016/j.ijrobp.2008.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini G, Pozza F, Recher G et al (1991) Simultaneous cis-platinum and radiotherapy in inoperable or locally advanced squamous cell carcinoma of the head and neck. Oncology 48:270–276 [DOI] [PubMed] [Google Scholar]
- Gleich L, Ryzenman J, Gluckman J, Wilson K et al (2004) Recurrent advanced (T3 or T4) head and neck squamous cell carcinoma: is salvage possible? Arch Otolaryngol Head Neck Surg 130:35–38. doi:10.1001/archotol.130.1.35 [DOI] [PubMed] [Google Scholar]
- Go SR, Adjei AA (1999) Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol 17(1):409–422 [DOI] [PubMed] [Google Scholar]
- Grant WE, Hopper C, Speight PM, Macrobert AJ et al (1993) Photodynamic therapy of malignant and premalignant lesions in patients with ‘field cancerization’ of the oral cavity. J Laryngol Otol 107:1140–1145. doi:10.1017/S0022215100125496 [DOI] [PubMed] [Google Scholar]
- Greven KM, Williams DW, Keyes JW Jr, McGuirt WF et al (1994) Positron emission tomography of patients with head and neck carcinoma before and after high dose irradiation. Cancer 74:1355–1359. doi:10.1002/1097-0142(19940815)74:4%3c1355::AID-CNCR2820740428%3e3.0.CO;2-I [DOI] [PubMed] [Google Scholar]
- Han D, Huang ZG, Zhang W, Yu ZK et al (2003) Effectiveness of recombinant adenovirus p53 injection on laryngeal cancer: phase I clinical trial and follow up. Chin J Cancer Res 83:2029–2032 [PubMed] [Google Scholar]
- Hardisson D (2003) Molecular pathogenesis of head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol 260:502–508. doi:10.1007/s00405-003-0581-3 [DOI] [PubMed] [Google Scholar]
- Herrero R, Castellsague X, Plissowska J et al (2003) Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 95:1772–1783 [DOI] [PubMed] [Google Scholar]
- Hoppe BS, Stegman LD, Zelefsky MJ, Rosenzweig KE et al (2007) Treatment of nasal cavity and paranasal sinus cancer with modern radiotherapy techniques in the postoperative setting—the MSKCC experience. Int J Radiat Oncol Biol Phys 67:691–702. doi:10.1016/j.ijrobp.2006.09.023 [DOI] [PubMed] [Google Scholar]
- Jacobs C, Bertino JR, Goffinet DR et al (1978) 24-hour infusion of cis-platinum in head and neck cancers. Cancer 42:2135–2140. doi:10.1002/1097-0142(197811)42:5%3c2135::AID-CNCR2820420508%3e3.0.CO;2-W [DOI] [PubMed] [Google Scholar]
- Jeremic B, Shibamoto Y, Milicic B et al (2000) Hyperfractionated radiation therapy with or without concurrent low-dose daily cisplatin in locally advanced squamous cell carcinoma of the head and neck: a prospective randomized trial. J Clin Oncol 18:1458–1464 [DOI] [PubMed] [Google Scholar]
- Julieron M, Temam S (2004) Locoregional recurrence of ORL cancer: the place of surgery. Bull Cancer 91:863–869 [PubMed] [Google Scholar]
- Kalyankrishna GJ (2006) Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol 17:2666–2672. doi:10.1200/JCO.2005.04.8306 [DOI] [PubMed] [Google Scholar]
- Kato K, Hara A, Kuno T, Mori H et al (2006) Aberrant promoter hypermethylation in oral squamous cell carcinomas and the surrounding normal mucosa. J Cancer Res Clin Oncol 132:735–743. doi:10.1007/s00432-006-0122-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz TS, Mendenhall WM, Morris CG, Amdur RJ et al (2002) Malignant tumors of the nasal cavity and paranasal sinuses. Head Neck 24:821–829. doi:10.1002/hed.10143 [DOI] [PubMed] [Google Scholar]
- Kawashiri S, Noguchi N, Tanaka A, Nakaya H et al (2009) Inhibitory effect of neoadjuvant chemotherapy on metastasis of oral squamous cell carcinoma in a mouse model. Oral Oncol [DOI] [PubMed]
- Kim AJ, Suh JD, Sercarz JA, Abemayor E et al (2007) Salvage surgery with free flap reconstruction: factors affecting outcome after treatment of recurrent head and neck squamous carcinoma. Laryngoscope 117:1019:23 [DOI] [PubMed] [Google Scholar]
- Kvaal SI, Warloe T (2007) Photodynamic treatment of oral lesions. J Environ Pathol Toxicol Oncol 26:127–133 [DOI] [PubMed] [Google Scholar]
- Lawrence TS, Blackstock AW, McGinn C (2003) The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Semin Radiat Oncol 13(1):13–21. doi:10.1053/srao.2003.50002 [DOI] [PubMed] [Google Scholar]
- Leipzig B (1983) Cisplatin sensitization to radiotherapy of squamous cell carcinomas of the head and neck. Am J Surg 146:462–465. doi:10.1016/0002-9610(83)90231-3 [DOI] [PubMed] [Google Scholar]
- Lewin F, Norell SE, Johansson H, Gustavsson P et al (1998) Smoking tobacco, oral snuff, and alcohol in the aetiology of squamous cell carcinoma of the head and neck. Cancer 82:1367–1375. doi:10.1002/(SICI)1097-0142(19980401)82:7%3c1367::AID-CNCR21%3e3.0.CO;2-3 [DOI] [PubMed] [Google Scholar]
- Lonn S, Ahlbom A, Christensen HC, Johansen C et al (2006) Mobile phone use and risk of parotid gland tumor. Am J Epidemiol 164:637–643. doi:10.1093/aje/kwj242 [DOI] [PubMed] [Google Scholar]
- Majem M, Mesia R, Mañós M, Gomez J et al (2006) Does induction chemotherapy still have a role in larynx preservation strategies? The experience of Institut Catala d’Oncologia in stage III larynx carcinoma. Laryngoscope 116:1651–1656. doi:10.1097/01.mlg.0000231736.08477.47 [DOI] [PubMed] [Google Scholar]
- Marchand JL, Luce D, Leclerc A, Goldberg P et al (2000) Laryngeal and hypopharyngeal cancer and occupational exposure to asbestos and man-made vitreous fibers: results of a case-control study. Am J Ind Med 37:581–589. doi:10.1002/(SICI)1097-0274(200006)37:6%3c581::AID-AJIM2%3e3.0.CO;2-D [DOI] [PubMed] [Google Scholar]
- Marcu L, van Doorn T, Olver I (2003) Cisplatin and radiotherapy in the treatment of locally advanced head and neck cancer. Acta Oncol 42(4):315–325. doi:10.1080/02841860310004364 [DOI] [PubMed] [Google Scholar]
- Medina JA, Rueda A, de Pasos AS, Contreras J et al (2006) A phase II study of concomitant boost radiation plus concurrent weekly cisplatin for locally advanced unresectable head and neck carcinomas. Radiother Oncol 79:34–38. doi:10.1016/j.radonc.2006.03.010 [DOI] [PubMed] [Google Scholar]
- Menvielle G, Luce D, Goldberg P, Bugel I et al (2004) Smoking, alcohol drinking and cancer risk for various sites of the larynx and hypopharynx. A case–control study in France. Eur J Cancer Prev 13:165–172. doi:10.1097/01.cej.0000130017.93310.76 [DOI] [PubMed] [Google Scholar]
- Milas L, Mason K, Liao Z, Ang K (2003) Chemoradiotherapy: emerging treatment improvement strategie. Head Neck 25:152–167. doi:10.1002/hed.10232 [DOI] [PubMed] [Google Scholar]
- Mould R (1993) A century of X-rays and radioactivity in medicine. Institute of Physics Publishing, Bristol [Google Scholar]
- Nauta JM, van Leengoed HL, Star WM, Roodenburg JL et al (1996) Photodynamic therapy of oral cancer. A review of basic mechanisms and clinical applications. Eur J Oral Sci 104:69–81. doi:10.1111/j.1600-0722.1996.tb00049.x [DOI] [PubMed] [Google Scholar]
- Novacek G (2006) Plummer–Vinson syndrome. Orphanet J Rare Dis 15:1–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander K, Dabelsteen E, Hall PA (2000) The p53 molecule and its prognostic role in squamous cell carcinomas of the head and neck. J Oral Pathol Med 29:413–425. doi:10.1034/j.1600-0714.2000.290901.x [DOI] [PubMed] [Google Scholar]
- Olshan A, Weissler M, Pei H, Conway K (1997) p53 Mutations in head and neck cancer: new data and evaluation of mutational spectra. Cancer Epidemiol Biomarkers Prev 6:499–504 [PubMed] [Google Scholar]
- Overgaard J, Hansen HS, Specht L, Overgaard M et al (2003) Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet 362:933–940. doi:10.1016/S0140-6736(03)14361-9 [DOI] [PubMed] [Google Scholar]
- Pandor A, Eggington S, Paisley S, Tappenden P, Sutcliffe P (2006) The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation. Health Technol Assess 10:1–204 [DOI] [PubMed] [Google Scholar]
- Perea-Milla L, Minarro-Del Moral RM, Martinez-Garcia C, Zanetti R et al (2003) Lifestyles, environmental and phenotypic factors associated with lip cancer: a case-control study in southern Spain. Br J Cancer 88:1702–1707. doi:10.1038/sj.bjc.6600975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Ordoňez B, Beauchemin M, Jordan RC (2006) Molecular biology of squamous cell carcinoma of the head and neck. J Clin Pathol 59:445–453. doi:10.1136/jcp.2003.007641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters LJ, Withers HR (1997) Applying radiobiological principles to combined modality treatment of head and neck cancer—the time factor. Int J Radiat Oncol Biol Phys 39:831–836. doi:10.1016/S0360-3016(97)00466-5 [DOI] [PubMed] [Google Scholar]
- Robbins KT, Kumar P, Regine W et al (1997) Efficacy of targeted supradose cisplatin and concomitant radiation therapy for advanced head and neck cancer: the Memphis experience. Int J Radiat Oncol Biol Phys 38:263–271. doi:10.1016/S0360-3016(97)00092-8 [DOI] [PubMed] [Google Scholar]
- Rosenquist K (2005) Risk factors in oral and oropharyngeal squamous cell carcinoma: a population-based case-control study in southern Sweden. Swed Dent J Suppl 179:1–66 [PubMed] [Google Scholar]
- Sako K, Razack MS, Kalnins I (1978) Chemotherapy for advanced and recurrent squamous cell carcinoma of the head and neck with high and low dose cis-diamminedichloroplatinum. Am J Surg 136:529–533. doi:10.1016/0002-9610(78)90276-3 [DOI] [PubMed] [Google Scholar]
- Sanchez-Cespedes M, Esteller M, Wu L, Nawroz-Danish H et al (2000) Gene promoter hypermethylation in tumors and serum of head and neck cancer patients. Cancer Res 60:892–895 [PubMed] [Google Scholar]
- Sankaranarayanan R, Masuyer E, Swaminathan R, Ferlay J, Whelan S (1998) Head and neck cancer: a global perspective on epidemiology and prognosis. Anticancer Res 18:4779–4786 [PubMed] [Google Scholar]
- Schantz SP, Huang Q, Shah K, Murty VV et al (2000) Mutagen sensitivity and environmental exposures as contributing causes of chromosome 3p losses in head and neck cancers. Carcinogenesis 21:1239–1246. doi:10.1093/carcin/21.6.1239 [PubMed] [Google Scholar]
- Scher R, Esclamado R (2009) Organ and function preservation: the role of surgery as the optimal primary modality or as salvage after chemoradiation failure. Semin Radiat Oncol 19:17–23. doi:10.1016/j.semradonc.2008.09.004 [DOI] [PubMed] [Google Scholar]
- Schuller DE, Ozer E, Agrawal A, Grecula JC et al (2007) Multimodal intensification regimens for advanced, resectable, previously untreated squamous cell cancer of the oral cavity, oropharynx, or hypopharynx: a 12-year experience. Arch Otolaryngol Head Neck Surg 133:320–326. doi:10.1001/archotol.133.4.320 [DOI] [PubMed] [Google Scholar]
- Seifert E, Schadel A, Haberkorn U, Strauss LG (1992) Evaluating the effectiveness of chemotherapy in patients with head-neck tumors using positron emission tomography (PET scan). Head Neck Oncol 40:90–93 [PubMed] [Google Scholar]
- Serin M, Erkal HS, Cakmak A (1999) Radiation therapy and concurrent cisplatin in management of locoregionally advanced nasopharyngeal carcinomas. Acta Oncol 38:1031–1035. doi:10.1080/028418699432310 [DOI] [PubMed] [Google Scholar]
- Shangina O, Brennan P, Szeszenia-Dabrowska N, Mates D et al (2006) Occupational exposure and laryngeal and hypopharyngeal cancer risk in central and eastern Europe. Am J Epidemiol 164:367–375. doi:10.1093/aje/kwj208 [DOI] [PubMed] [Google Scholar]
- Shirinian MH, Weber RS, Lippman SM, Dimery IW et al (1994) Laryngeal preservation by induction chemotherapy plus radiotherapy in locally advanced head and neck cancer: the M. D. Anderson Cancer Center experience. Head Neck 16:39–44. doi:10.1002/hed.2880160109 [DOI] [PubMed] [Google Scholar]
- Slaughter DP, Southwick HW (1953) “Field cancerization” in oral stratified squamous epithelium. Clinical implications of multicentric origin. Cancer 6:963–968. doi:10.1002/1097-0142(195309)6:5%3c963::AID-CNCR2820060515%3e3.0.CO;2-Q [DOI] [PubMed] [Google Scholar]
- Sturgis EM, Moore BA, Glisson BS, Kies MS et al (2005) Neoadjuvant chemotherapy for squamous cell carcinoma of the oral tongue in young adults: a case series. Head Neck 27:748–756. doi:10.1002/hed.20240 [DOI] [PubMed] [Google Scholar]
- Vikram B, Strong EW, Shah JP et al (1984) Failure at distant sites following multimodality treatment for advanced head and neck cancer. Head Neck Surg 6:730–733. doi:10.1002/hed.2890060305 [DOI] [PubMed] [Google Scholar]
- Vivek RS, Baludavid M, Mohanram R, Chitra et al (2006) Concurrent chemo-irradiation using accelerated concomitant boost radiation therapy in loco-regionally advanced head and neck squamous cell carcinomas. J Cancer Res Ther 2:90–96 [DOI] [PubMed]
- Wang Q, Wu Z, Han D, Zhang W et al (1999) Growth inhibition of human laryngeal cancer cell with the adenovirus-mediated p53 gene. Chin J Cancer Res 11:157–160 [Google Scholar]
- Yoshikawa A, Saura R, Matsubara T (1997) A mechanism of cisplatin action: antineoplastic effect through inhibition of neovascularization. Kobe J Med Sci 43:109–120 [PubMed] [Google Scholar]
- Young LS, Dawson CW, Clark D, Rupani H et al (1988) Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J Gen Virol 69:1051–1065. doi:10.1099/0022-1317-69-5-1051 [DOI] [PubMed] [Google Scholar]
- Youssef E, Lotan D, Issa JP, Wakasa K et al (2004) Hypermethylation of the retinoic acid receptor-ß2 gene in head and neck carcinogenesis. Clin Cancer Res 10:1733–1742. doi:10.1158/1078-0432.CCR-0989-3 [DOI] [PubMed] [Google Scholar]
- Yuan JM, Wang XL, Xiang YB, Gao YT et al (2000) Preserved foods in relation to risk of nasopharyngeal carcinoma in Shanghai, China. Int J Cancer 85:358–363. doi:10.1002/(SICI)1097-0215(20000201)85:3%3c358::AID-IJC11%3e3.0.CO;2-E [DOI] [PubMed] [Google Scholar]
- Yuen W, Man M, Lam K, Kwong Y (2002) Clinicopathological significance of p16 gene expression in the surgical treatment of head and neck squamous cell carcinomas. J Clin Pathol 55:58–60 [DOI] [PMC free article] [PubMed] [Google Scholar]