SUMMARY
Background
Carbapenem-resistant Acinetobacter baumannii (CRAB) poses a critical global health threat, particularly among hospitalized and critically ill patients, due to its association with severe, difficult-to-treat infections. Its high resistance stems from genomic plasticity, biofilm formation, and environmental persistence, leading to limited treatment options and high mortality. Existing treatments often rely on sulbactam-based combinations, yet resistance continues to rise and new agents remain limited.
Aims
This review aims to highlight the therapeutic potential of zosurabalpin, a first-in-class lipopolysaccharide (LPS) transport inhibitor with selective activity against A. baumannii, by summarizing preclinical and early clinical data on its efficacy, mechanism of action, pharmacokinetics, and safety.
Sources
Peer-reviewed publications from PubMed and Scopus, clinical data from ClinicalTrials.gov, and relevant conference materials ECCMID and IDWeek.
Content
Zosurabalpin inhibits the LptB-FGC complex, blocking LPS transport and causing lethal intracellular accumulation. Preclinical data demonstrate potent in vitro and in vivo activity against CRAB, with high selectivity and favorable pharmacokinetics. Phase 1 studies report good tolerability and a promising safety profile.
Its novel mechanism makes zosurabalpin a strong candidate for treating CRAB.
Implications
With rising resistance and limited effective treatments, zosurabalpin offers a new, targeted therapeutic approach. Continued clinical development could help close a critical gap in the management of multidrug-resistant Acinetobacter baumannii.
Keywords: Carbapenem-resistant Acinetobacter baumannii (CRAB), lipopolysaccharide (LPS) transport system, LPS transport inhibitors, zosurabalpin
INTRODUCTION
Carbapenem-resistant Acinetobacter baumannii (CRAB) is considered a life-threatening pathogen associated with invasive infections in hospitalized and critically ill patients. Over the past decades, CRAB infections have become a major concern due to healthcare challenges they pose, including difficult-to-treat infections that lead to substantial morbidity, mortality and increased healthcare costs due to prolonged hospitalization and treatment [1, 2]. Several factors contribute to the difficulty in managing Acinetobacter baumannii infections, primarily stemming from the bacterium’s unique biological characteristics [3]. First, Acinetobacter baumannii can persist in hospital environments and infect patients who are often already critically ill [4]. Second, it has a growing capacity to develop antibiotic resistance, due to its highly adaptable and plastic genome [5]. Third, Acinetobacter baumannii can form biofilms, which offer a protective niche that enhances its survival and resistance under harsh conditions [6]. Treating CRAB infections is particularly challenging due to the gradual loss of efficacy of most available antimicrobial drugs [1, 2]. This results in limited therapeutic options and often necessitates the use of combination therapies or older antibiotics such as polymyxins, which raise significant safety concerns. These issues are compounded by the prolonged stagnation in antibiotic development - no new class of antibiotics has been introduced into clinical practice in the past five decades [7]. Perhaps the most significant challenge in the therapeutic management of Acinetobacter baumannii infections is the difficulty distinguishing colonization from true infection. This is especially problematic in critically ill patients with multiple comorbidities, often raising the dilemma of whether poor outcomes are due to inadequate antibiotic therapy or underlying host factors [8].
The aim of this study - following a brief overview of the clinical challenges posed by CRAB infections, including high antibiotic resistance and limited therapeutic options - is to highlight the potential of zosurabalpin, a first-in-class inhibitor of the LptB-FGC complex, with selective activity against Acinetobacter baumannii. This review summarizes preclinical and early clinical data demonstrating zosurabalpin’s antibacterial efficacy, mechanism of action, pharmacokinetics, and tolerability. The data presented are based on a comprehensive literature search of peer-reviewed articles and reviews in PubMed and Scopus, along with completed and ongoing studies registered in ClinicalTrials.gov. Relevant abstracts and presentations from recent ECCMID and IDWeek meetings were also reviewed. Keywords used in the search included “CRAB”, “zosurabalpin”, “RG6006”, “LPS transporter” and “LPS transport inhibitor”.
CURRENT THERAPEUTIC OPTIONS AND INVESTIGATIONAL ANTIMICROBIALS AGAINST CRAB
The most recent guidelines for treatment of CRAB infections recommend using at least two antibiotics, preferably including an agent containing sulbactam. The recommended first-line regimen is a combination of sulbactam-durlobactam with a carbapenem. If sulbactam-durlobactam is unavailable, alternatives include high-dose ampicillin-sulbactam combined with at least one additional agent, such as polymyxin B, minocycline, tigecycline, or cefiderocol. According to current recommendations, while emerging evidence supports the efficacy of fosfomycin when used in combination with other agents, and the drug may be useful in regions where it is available, neither fosfomycin nor rifampicin is recommended as part of combination therapy, nor is the use of inhaled antibiotics [7, 9]. Recognizing the urgency of the situation, the World Health Organization classified Acinetobacter baumannii as a critical priority pathogen on its global priority list of antibiotic-resistant bacteria, emphasizing the need for the development of new antimicrobials [10]. Among the most promising alternatives under investigation (Table 1) are newer beta-lactamase inhibitors and their combinations with older beta-lactam antibiotics (e.g., cefepime/zidebactam, imipenem/funobactam, xeruborbactam), novel polymyxins (MRX-8, QPX9003, SPR206, SPR741), and the potential of phage therapy [11–14]. A particularly noteworthy development is the emergence of a new antibiotic class - LPS transport inhibitors. Among them, zosurabalpin has gained special attention due to its specificity for Acinetobacter baumannii. In contrast to β-lactamase inhibitors (e.g., durlobactam), which restore β-lactam efficacy by inhibiting class A, C, and D β-lactamases, or cefiderocol, a siderophore cephalosporin that hijacks bacterial iron uptake systems to enter the cell and inhibit cell wall synthesis, zosurabalpin - a first-in-class LptB2FGC inhibitor - disrupts lipopolysaccharide (LPS) transport. This leads to impaired outer membrane integrity and bacterial death. It offers a novel mechanism that does not rely on β-lactam activity and may circumvent conventional resistance pathways.
Table 1.
Antibiotic classes of drugs used in current therapy or under investigation against infections caused by resistant Acinetobacter baumannii.
| Antibiotic class | In current use | Under investigation |
|---|---|---|
| β-lactamase inhibitors | Durlobactam, Sulbactam | Xeruborbactam, Funobactam, Zidebactam, Pralurbactam (FL058), Nacubactam, Taniborbactam, ETX0282, Ledaborbactam (VNRX-5236), ANT3310 |
| Polymyxins | Polymyxin B, Colistin | MRX-8, QPX9003, SPR206, SPR741 |
| Tetracycline derivatives | Minocycline, Tigecycline, Eravacycline | Zifanocycline, Omadacycline |
| Siderophore cephalosporins | Cefiderocol | – |
| Carbapenems | Imipenem-cilastatin, Meropenem | – |
| Aminoglycosides | Amikacin | Apramycin (Phase 1) |
| Phosphonic antibiotics | Fosfomycin | |
| Rifamycins | Rifampin, Rifabutin, Rifapentine | Rifabutin for infusion (BV100) |
| Bacterial topoisomerase inhibitor | – | BWC0977 (Oxazolidinone containing NBTI) |
| LPS transport inhibitors | – | Zosurabalpin |
LIPOPOLYSACCHARIDE TRANSPORT SYSTEM AS A NEW THERAPEUTIC TARGET
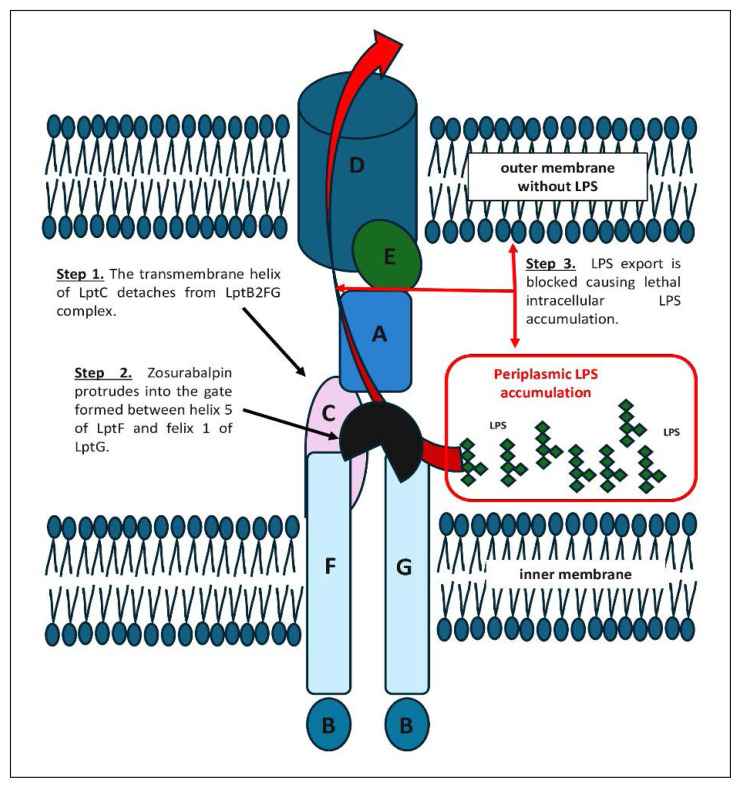
The outer membrane of Gram-negative bacteria functions as a barrier to many antibiotics, making these organisms particularly difficult to eradicate. A central component of this membrane is LPS, an amphipathic glycolipid essential for the outer membrane integrity and bacterial resistance [15]. LPS is transported from the inner membrane to the cell surface via the Lpt multiprotein complex (Figure 1) [15, 16]. This transport system comprises three main components: the inner membrane complex LptB2FGC, the periplasmic bridge protein LptA, and the outer membrane complex LptDE. LPS is extracted from the inner membrane by LptB2FG, passed through the periplasm via a bridge formed by LptC and LptA, and ultimately delivered to the outer membrane by LptDE. This transport process is essential for the viability of nearly all gram-negative bacteria. If LPS fails to reach the outer membrane, precursor molecules accumulate in the inner membrane, compromising outer membrane integrity and leading to bacterial death. Because of its indispensable role, the LPS transport system represents an attractive target for novel antibiotics. Inhibiting this pathway offers several advantages, including the potential for targeting outer membrane or periplasmic components, thereby reducing the need for extensive intracellular drug accumulation. [17, 18]. The Lpt transport machinery is highly conserved in structure and function across gram-negative bacteria [18]. Interestingly, in species that can survive without LPS, such as Acinetobacter baumannii, disruption of the LPS biogenesis reduces growth and virulence while increasing susceptibility to other antibiotics [18]. In recent years, compounds targeting key components of the Lpt system - LptB, LptA, and LptD – have shown antimicrobial potential (Table 2) [17–20]. However, none have succeeded clinically due to issues like host toxicity, poor solubility, and limited intracellular penetration [20]. Among these, zosurabalpin stands out by targeting the inner membrane LptB2FGC complex, a validated and promising site for antibiotic intervention.
Figure 1.
Mechanism of action of zosurabalpin through inhibition of the LptB2FGC transporter complex in Acinetobacter baumannii (based on concepts and structures described in references [18], [21], [23–24]).
Table 2.
Antimicrobial compounds targeting different sites of the LPS transporter multiprotein complex.
| Target Site | Antimicrobial Compounds |
|---|---|
| LptB | Compound 1 and 2, 4-phenylpyrrolocarbazole derivative 1b, novobiocin |
| LptA | Thanatin, IMB-881 |
| LptD | L27-11, Murepavadin (POL7080) |
| LptB 2 FGC | Zosurabalpin |
ZOSURABALPIN: A UNIQUE LPTB2FGC INHIBITOR
Discovery
Zosurabalpin is an experimental antibiotic classified as a tethered macrocyclic peptide (MPC), with its chemical structure and related data available in PubChem (https://pubchem.ncbi.nlm.nih.gov/compound/Zosurabalpin) [18, 21, 22]. Several well-known antibiotics – such as polymyxins, bacitracin and daptomycin – belong to this class, which includes compounds with narrow-spectrum but potent antibacterial activity [21]. To identify novel agents against CRAB, Bradley’s team screened 44,985 MCPs from Tranzyme Pharma in a whole-cell phenotypic assay [23]. This effort led to the identification of RO7036668, featuring a 17-membered macrocyclic ring composed of an L-Orn-L-Orn-L-N(Me)-Trp motif and a di-o-tolylsulfane moiety [21, 23]. It showed activity against Acinetobacter baumannii with a minimum inhibitory concentration (MIC) of 4 μg/ml, but little to no effect against other bacteria. Subsequent hit-to-lead optimization produced RO7075573 by modifying the macrocycle - replacing L-Orm with LLys, adding a pyridine ring, and introducing dichlorobenzene substitutions. This compound exhibited potent activity against a broad panel of Acinetobacter baumannii strains, including multidrug-resistant isolates (MIC: ≤0.06 – 0.5 μg/ml). However, its intravenous use in rats caused significant toxicity. Further structural optimization led to zosurabalpin, an amphoteric benzoic acid derivative. It retained strong activity (MIC: 0.25 mg/L) against Acinetobacter baumannii while improving tolerability, making it a promising clinical candidate.
Mechanism of action
Zosurabalpin targets the LptB2FGC complex on the inner membrane surface, inhibiting LPS extraction and preventing its transport to the outer surface (Figure 1) [23, 24]. This disruption leads to the toxic accumulation of LPS biosynthesis intermediates within the bacterial cell, as the transport process is initiated but cannot be completed - ultimately resulting in bacterial cell death. The drug appears to act by recognizing a composite binding site formed by both the Lpt transporter and its substrate, LPS. Within the LptB2FGC complex, LptC plays a crucial role in transferring LPS from LptF to LptA [25]. When the transmembrane helix of LptC detaches from the LptB2FG, allowing LptC to move away from LptFG, LPS binds to the LptB2FG complex. Zosurabalpin protrudes into the gate formed between helix 5 of LptF and helix 1 of LptG, forming hydrogen bonds with Thr321 on LptF and electrostatic interactions with Glu58 and Glu249 on LptF via the central L-Lys residue [21, 24]. This interference disrupts LptB2FGC function, blocks LPS export, and causes lethal intracellular accumulation. This unique mechanism makes zosurabalpin a promising antibiotic against Acinetobacter baumannii.
The potential molecular targets of zosurabalpin were identified by inducing spontaneous resistance in Acinetobacter baumannii strains exposed to gradually increasing concentrations of the antibiotic [23]. Gene sequencing analysis revealed 28 different mutations in the gene encoding LptF and two unique mutations in LptG, both components of the LptB2FGC complex, a central part of the LPS transport system. These findings strongly suggest that zosurabalpin targets the LptB2FGC complex. In another effort to elucidate the antibacterial mechanism of tethered MCP antibiotics, Kahne’s group used cryo-electron microscopy (cryo-EM) to study RO7196472 [24]. Their analysis showed that the molecule binds to pockets formed by the arrangement of side chains of various amino acids within the transmembrane helices of LptF and LptG [18, 24]. It appears to trap an intermediate form of the LptB2FG-LPS complex, while the overall conformation of the complex remains essentially stable with or without RO7196472. This suggests that the cyclic peptide binds to a pre-existing structural state. By analyzing the cryo-EM structures of zosurabalpin, RO7196472, and RO7075573 bound to the LptB2FG-LPS complex, Kahne’s team observed dissociation of the MCP-targeted LptC transmembrane helix from the complex. Finally, supporting evidence that MCPs exert their antibacterial effect by causing toxic intracellular LPS accumulation – rather than by depleting outer membrane LPS – was derived from the observation that Acinetobacter baylyi strains lacking LPS in their outer membrane (with ~ 85% LptB2FG sequence identity to Acinetobacter baumannii) were able to grow in vitro even in the presence of high concentrations of RO7196472 [18, 24].
Activity against Acinetobacter baumannii – In vitro and in vivo studies
In both in vitro and in vivo studies to date, zosurabalpin has demonstrated excellent antibacterial activity against highly resistant CRAB pathogens. Notably, zosurabalpin appears to act selectively against Acinetobacter baumannii, showing similar efficacy in both susceptible and multidrug-resistant strains, while exhibiting no activity against other gram-negative bacteria [23, 24]. Structural comparisons of the LptB2FG:LPS:ZAB complex in Acinetobacter baylyi with the LptB2FG:LPS structure in Escherichia coli revealed differences in the LptF helices and LPS binding sites. These structural variations may explain zosurabalpin’s narrow spectrum, which is primarily restricted to Acinetobacter baumannii [24, 26]. Although current studies remain limited and variations in virulence and drug responsiveness have been observed - with some mutants exhibiting reduced fitness or treatment response - available data suggest that zosurabalpin resistance is mediated by mutations in three key targets: LptF/G, affecting drug binding and target engagement; LpxM involved in LPS synthesis and potentially altering drug interaction or inhibition; and AdeRS, a two-component system that modulates efflux, reducing intracellular zosurabalpin concentrations [27].
In vitro studies
Bradley et al. confirmed the potent in vitro efficacy of zosurabalpin against severe invasive Acinetobacter baumannii infections, testing 129 resistant and multidrug-resistant clinical isolates from diverse infection sites [23]. The MIC required to inhibit 90% of the isolates (MIC90) was 1 mg/L – substantially lower than tigecycline (8 mg/L), colistin (>16 mg/L), and meropenem (>16 mg/L). In another study, Hawser et al. evaluated zosurabalpin against 150 randomly selected Acinetobacter spp. isolates (100 Acinetobacter baumannii, 50 non-Acinetobacter baumannii), 65% of which were multidrug-resistant, collected from China in 2021 [28]. Zosurabalpin demonstrated activity against all Acinetobacter spp., with an MIC50/90 values of 0.12/0.5 μg/mL and 0.25/1 μg/mL in Mueller Hinton broth supplemented with 20% horse serum and human serum, respectively (MIC range 0.015/0.03 to 8 μg/mL). Among 133 isolates belonging to the Acinetobacter baumannii-calcoaceticus complex, the MIC50/90 values were 0.12/0.25 μg/mL (horse serum) and 0.25/0.5 μg/mL (human serum), with similar potency against carbapenem-resistant isolates. Regarding its bactericidal activity, Erbetti et al. demonstrated that zosurabalpin achieved ≥99.9% reduction (or ≥3 log10 CFU) in all eight CRAB isolates tested (MIC range: 0.12 to 8 mg/L), though it exhibited relatively slow killing kinetics (≥12 hours), with no bacterial regrowth observed at ≥8x or 16x MIC, depending on the strain [29].
In vivo studies
Zosurabalpin exhibited potent efficacy in a neutropenic mouse pneumonia model using a pan-drug-resistant Acinetobacter isolate, as well as in models of sepsis and femur/lung infections caused by CRAB strains. In these models, zosurabalpin achieved dose-independent bacterial load reductions, with >5-log10 CFU decreases observed at the highest daily dose (360 mg/kg/day) [23]. Pharmacokinetic profiling revealed high clearance (51 mL/min/kg), low volume of distribution (0.7 L/kg), short half-life (0.3 hours) and moderate protein binding (37% unbound fraction). Similarly, Erbetti et al. confirmed zosurabalpin’s in vivo bactericidal activity in neutropenic murine thigh and lung models, demonstrating net bacterial reductions after 24 hours and sustained suppression over a 48-hour at various dosing levels [29]. Zosurabalpin has progressed into Phase I clinical trials, with early results confirming its safety, tolerability and favorable pharmacokinetic. In the first-in-human study reported by Guenther et al., 64 healthy volunteers received single intravenous doses ranging from 10 mg to 2000 mg. The drug was generally well tolerated, with mild, dose-dependent, and fully reversible infusion-related reactions as the primary adverse effect. Plasma exposure (Cmax and AUCinf) increased approximately proportionally with the dose up to 1000 mg. Radioactive labeling ([14C]-zosurabalpin) showed that the drug was eliminated in roughly equal proportions via urine and feces [30].
CONCLUSIONS
This review highlights zosurabalpin as a first-in-class LptB2FGC inhibitor, introducing a novel antimicrobial mechanism that disrupts LPS transport and selectively targets Acinetobacter baumannii, a pathogen of critical global health concern. It consolidates preclinical and early clinical evidence and provides an in-depth examination of zosurabalpin’s discovery, mechanism of action, structure-activity optimization, and unique selectivity profile. By offering an up-to-date synthesis of mechanistic insights and experimental data, this review addresses a gap in the literature on LPS transport inhibitors and presents zosurabalpin as a promising therapeutic candidate for combating CRAB infections.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to report.
Funding: No funding was received for conducting this manuscript.
REFERENCES
- 1.O’Donnell JN, Putra V, Lodise TP. Treatment of patients with serious infections due to carbapenem-resistant Acinetobacter baumannii: how viable are the current options? Pharmacotherapy. 2021;41(9):762–780. doi: 10.1002/phar.2607. [DOI] [PubMed] [Google Scholar]
- 2.Butler DA, Biagi M, Tan X, et al. Multidrug resistant Acinetobacter baumannii: resistance by any other name would still be hard to treat. Curr Infect Dis Rep. 2019;21(12):46. doi: 10.1007/s11908-019-0706-5. [DOI] [PubMed] [Google Scholar]
- 3.Shan W, Kan J, Cai X, et al. Insights into mucoid Acinetobacter baumannii: a review of microbiological characteristics, virulence, and pathogenic mechanisms in a threatening nosocomial pathogen. Microbiol Res. 2022;261:127057. doi: 10.1016/j.micres.2022.127057. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-López F, Martínez-Meléndez A, Villareal-Treviño L, et al. Contamination of healthcare environment by carbapenem-resistant Acinetobacter baumannii. Am J Med Sc. 2022;364(6):685–694. doi: 10.1016/j.amjms.2022.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Karampatakis T, Tsergouli K, Behzadi P. Pan-genome plasticity and virulence factors: a natural treasure trove for Acinetobacter baumannii. Antibiotics (Basel) 2024;13(3):257. doi: 10.3390/antibiotics13030257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong CSC, Lau YY, Michels PAM, et al. Insights into biofilm-mediated mechanisms driving last-resort antibiotic resistance in clinical ESKAPE pathogens. Crit Rev Microbiol . 2025:1–26. doi: 10.1080/1040841X.2025.2473332. [DOI] [PubMed] [Google Scholar]
- 7.Tamma PD, Heil EL, Justo JA, et al. Infectious Disease Society of America 2024 guidance on the treatment of antimicrobial-resistant gram-negative infections. Clin Infect Dis. 2024:ciae403. doi: 10.1093/cid/ciae403. [DOI] [PubMed] [Google Scholar]
- 8.Richards GA, Perovic O, Brink AJ. The challenges of difficult-to treat Acinetobacter infections. Clin Microbiol Rev. 2024;37(4):e0009324. doi: 10.1128/cmr.00093-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paul M, Carrara E, Retamar P, et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant gram-negative bacilli (endorsed by European Society of Intensive Care Medicine) Clin Microbiol Infect. 2022;28:521–47. doi: 10.1016/j.cmi.2021.11.025. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. WHO bacterial priority pathogens list. 2024. [Accessed May 8, 2024]. Available at: https://www.who.int/publications/i/item/9789240093461.
- 11.Arshad N, Azzam W, Zilberberg M, et al. Acinetobacter baumannii complex infections: new treatment options in the antibiotic pipeline. Microorganisms. 2025;13(2):356. doi: 10.3390/microorganisms13020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heimann D, Kohnhäuser D, Kohnhäuser A, et al. Antibacterials with novel chemical scaffolds in clinical development. Drugs. 2025;85(3):293–323. doi: 10.1007/s40265-024-02137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marino A, Augello E, Stracquadanio S, et al. Unveiling the secrets of Acinetobacter baumannii: resistance, current treatments, and future innovations. Int J Mol Sci. 2024;25(13):6814. doi: 10.3390/ijms25136814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eslami M, Safaripour A, Banihashemian SZ, et al. Innovative antibiotic therapies for carbapenem-resistant gram-negative bacterial infections: clinical efficacy, safety, and comparative studies. Microorganisms. 2025;13(2):295. doi: 10.3390/microorganisms13020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitfield C, Trent MS. Biosynthesis and export of bacterial lipopolysaccharides. Annu Rev Biochem. 2014;83:99–128. doi: 10.1146/annurev-biochem-060713-035600. [DOI] [PubMed] [Google Scholar]
- 16.Di Lorenzo F, Duda KA, Lanzetta R, et al. A journey from structure to function of bacterial lipopolysaccharides. Chem Rev. 2022;122:15767–15821. doi: 10.1021/acs.chemrev.0c01321. [DOI] [PubMed] [Google Scholar]
- 17.Owens TW, Taylor RJ, Pahil KS, et al. Structural basis of unidirectional export of lipopolysaccharide to the cell surface. Nature. 2019;567:550–553. doi: 10.1038/s41586-019-1039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weng Q, Zhang F, Zheng Q. Zosurabalpin: a novel tethered macrocyclic peptide antibiotic that kills carbapenem-resistant Acinetobacter baumannii. MedComm(2020) 2024;5(8):e696. doi: 10.1002/mco2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabnis A, Edwards A. Lipopolysaccharide as an antibiotic target. Biochim Biophys Acta Mol Cell Res. 2023;1870(7):119507. doi: 10.1016/j.bbamcr.2023.119507. [DOI] [PubMed] [Google Scholar]
- 20.Romano KP, Hung DT. Targeting LPS biosynthesis and transport in gram-negative bacteria in the era of multi-drug resistance. Biochim Biophys Acta Mol Cell Res. 2023;1870(3):119407. doi: 10.1016/j.bbamcr.2022.119407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang WJ, Dong XM, Li GB. Macrocyclic peptides: up-and-coming weapons to combat antimicrobial resistance. Signal Transduct Target Ther. 2024;9(1):81. doi: 10.1038/s41392-024-01813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luther A, Bisang C, Obrecht D. Advances in macrocyclic peptide-based antibiotics. Bioorg Med Chem. 2018;26(10):2850–2858. doi: 10.1016/j.bmc.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Zampaloni C, Mattei P, Bleicher K, et al. A novel antibiotic class targeting the lipopolysaccharide transporter. Nature. 2024;625:566–571. doi: 10.1038/s41586-023-06873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pahil KS, Gilman MSA, Baidin V, et al. A new antibiotic traps lipopolysaccharide in its intermembrane transporter. Nature. 2024;625:572–577. doi: 10.1038/s41586-023-06799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson A, Ruiz N. The transmembrane α-helix of LptC participates in LPS extraction by the LptB2 FGC transporter. Mol Microbiol. 2022;118(1–2):61–76. doi: 10.1111/mmi.14952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Orlando BJ, Liao M. Structural basis of lipopolysaccharide extraction by the LptB2FGC complex. Nature. 2019;567(7749):486–490. doi: 10.1038/s41586-019-1025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ortombina A, Erbetti I, Dylus D, et al. Mechanism of in vitro resistance to zosurabalpin (RG6006) in Acinetobacter baumannii. Program and Abstracts of the 34th Congress of the European Society of Clinical Microbiology and Infectious Diseases (ECCMID); Barcelona, Spain. 2024; European Society of Clinical Microbiology and Infectious Diseases.; p. 2650. Poster 2379. [Google Scholar]
- 28.Hawser S, Kothari N, Valmont T, et al. Activity of the novel antibiotic zosurabalpin (RG6006) against clinical Acinetobacter isolates from China. Open Forum Infect Dis. 2023;10(2):ofad500.1754. [Google Scholar]
- 29.Erbetti I, Ferrari L, Ortombina A, et al. 2019 In vitro and in vivo killing kinetics of zosurabalpin (RG6006) against Acinetobacter baumannii. Open Forum Infect Dis. 2023;10(2):οfad500.1733. [Google Scholar]
- 30.Guenther A, Millar L, Messer A, et al. 2126. Safety, tolerability, and pharmacokinetics (PK) in healthy participants following single dose administration of zosurabalpin, a novel pathogen-specific antibiotic for the treatment of serious Acinetobacter infections. Open Forum Infect Dis. 2023;10(2):ofad500.1749. [Google Scholar]