Abstract
Purpose
We examined the growth-inhibitory and apoptosis-inducing effects of vitamin K2 (VK2; menaquinone-4) on various lines of human ovarian cancer cells to study the mechanism of induction of apoptosis by VK2.
Methods
Cell proliferation was determined by XTT method, and apoptotic cells were detected by Hoechst staining. TR3, also known as Nur77 and NGFI-B, was detected by immunoblotting and immunofluorescence analysis. Role of TR3 on induction of apoptosis was examined by a siRNA experiment.
Results and conclusions
We found that PA-1 cells were the most sensitive to VK2 (IC50 = 5.0 ± 0.7 μM), while SK-OV-3 cells were resistant to VK2. Immunoblotting and immunofluorescence analyses indicated that levels of TR3 were elevated in cell lysates 48 h after the start of treatment with 30 μM VK2. In the VK2-treated cells, TR3 accumulated at significant levels in mitochondria, as well as in the nuclei of PA-1 cells. No similar changes were observed in SK-OV-3 cells under the same conditions. Treatment of PA-1 cells with small interfering RNA (siRNA) directed against TR3, and with cycloheximide or SP600125 (an inhibitor of c-jun N-terminal kinase; JNK), separately, inhibited the VK2-induced synthesis of TR3 and apoptosis. From these results, we can conclude that an increase in the synthesis of TR3 and the accumulation of TR3 in mitochondria and in nuclei might be involved in the induction of apoptosis by VK2 and that the synthesis of TR3 might be regulated through a JNK signaling pathway.
Keywords: Apoptosis, Vitamin K2, TR3, JNK, Ovarian cancer cells
Introduction
Vitamin K2 (VK2) induces growth inhibition (Nishikawa et al. 1995; Wang et al. 1995; Hitomi et al. 2005), differentiation (Sakai et al. 1994; Yaguchi et al. 1997; Miyazawa et al. 2001) and apoptosis in various lines of cancer cells (Yoshida et al. 2003; Iwamoto et al. 2004). We reported previously that VK2 induces apoptosis in human ovarian cancer cells (TYK-nu) and pancreatic cancer cells (MIA-Paca-2) (Shibayama-Imazu et al. 2003) and also induces the production of superoxide, with dissipation of the mitochondrial membrane potential (Shibayama-Imazu et al. 2006). Apoptosis induced by VK2 is almost completely inhibited by cycloheximide, which blocks protein synthesis (Shibayama-Imazu et al. 2003). However, the proteins involved in the induction of apoptosis by VK2 remain to be identified.
TR3, also known as Nur77 and neuron growth factor inducible factor I-B (NGFI-B), was originally isolated as the product of an immediate-early gene that is rapidly expressed in response to stimulation by serum or phorbol ester of quiescent fibroblasts (Hazel et al. 1988; Ryseck et al. 1989; Nakai et al. 1990; Herschman 1991). TR3 is also known as a transcription factor that modulates the expression of genes that are linked to the regulation of cell proliferation and apoptosis (John et al. 1994; Weih et al. 1996; Wu et al. 2002). TR3 is strongly expressed in many different lines of cancer cells (Wu et al. 1997; Uemura and Chang 1998), and the constitutive expression of TR3 results in massive cell death (Weih et al. 1996; Xue et al. 1997). These observations suggest that the level of TR3 might be an important factor with respect to the sensitivity of cancer cells to the induction of apoptosis.
Recently, the translocation of TR3 from nuclei to mitochondria was observed upon exposure of various cancer cells to apoptotic stimuli (Li et al. 2000; Wu et al. 2002; Wilson et al. 2003; Lin et al. 2004b), with subsequent depolarization of mitochondrial membranes and the release of cytochrome c from mitochondria into the cytosol (Li et al. 2000). In the present study, we compared the sensitivity to VK2 of five lines of human ovarian cancer cells. We found that PA-1 cells were the most sensitive to VK2 with an IC50 value of 5.0 ± 0.7 μM, while VK2 had almost no effect on SK-OV-3 cells. Using these two cell lines, we investigated the mechanism by which VK2 induces apoptosis. We found marked increases in levels of TR3 in lysates of PA-1 cells during VK2-induced apoptosis, whereas there was practically no change in the level of TR3 in SK-OV-3 cells under the same conditions. Furthermore, both the VK2-induced increase in levels of TR3 and induction of apoptosis in PA-1 cells were inhibited by addition of cycloheximide to the culture medium and by transfection of cells with small interfering RNA (siRNA) directed against TR3, indicating that the increased synthesis of TR3 might be associated with the induction of apoptosis by VK2. Furthermore, cellular localization studies indicated that TR3 accumulated in mitochondria and in nuclei in PA-1 cells during treatment with VK2. The results obtained in the present study suggest that the VK2-induced accumulation of TR3 in both mitochondria and nuclei might be associated with the induction of apoptosis in PA-1 ovarian cancer cells.
Materials and methods
Reagents
Vitamin K2 (VK2; menaquinone 4) was provided by Eisai Chemical Co. Ltd (Ibaraki, Japan). Sodium-3'-[1-[(phenylamino)carbonyl]-3,4-tetrazo-lium-bis(4-methoxy-6-nitro) benzene-sulfonic acid hydrate (XTT), phenazine methosulfate (PMS), cycloheximide (CHX), Hoechst 33342, a cocktail of protease inhibitors, bovine insulin, RPMI 1640 medium, Eagle’s minimum essential medium (MEM) and leptomycin B were obtained from Sigma-Aldrich Co. Ltd (St Louis, MO). Macoy’s 5A medium (modified), fetal bovine serum (FBS) and MEM nonessential amino acids were purchased from Gibco Co. Ltd (Glasgow, UK). SB203580, PD169316 and SP600125 were purchased from Calbiochem (a brand of EMD Biosciences Inc., La Jolla, CA). A Cell-Death Detection ELISAPLUS kit for the detection of released nucleosomes was purchased from Roche Diagnostics GmbH (Mannheim, Germany).
Antibodies
Antibodies against Nur77 (TR3; SC-5569), lamin A/C, was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Monoclonal antibodies against cytochrome oxidase subunit IV (OX) (A-21347) were obtained from Molecular Probes Inc. (Eugene, OR). Antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Chemicon International Co. Ltd (Temecula, CA). Monoclonal antibodies against cytochrome c (6H2.B4) were purchased from BD Pharmingen Co. Ltd (San Diego, CA).
Cell lines and cell culture
All lines of cells originated from human ovarian cancer tissues. Both PA-1 cells and TYK-nu cells were provided by the Japan Health Sciences Foundation (Tokyo, Japan). PA-1 cells were cultured in MEM that contained 10% FBS and 1% MEM nonessential amino acids. TYK-nu cells were maintained in MEM with 10% FBS. SK-OV-3 cells, SW626 cells and OVCAR3 cells were purchased from the American Type Culture Collection (Manassas, VA). SK-OV-3 cells were cultured in Macoy’s 5A medium (modified) supplemented with 10% FBS. SW626 cells were cultured in RPMI 1640 supplemented with 10% FBS. OVCAR3 cells were cultured in RPMI 1640 supplemented with 20% FBS and bovine insulin (1.25 μg/ml; Sigma-Aldrich Co.). All cells were cultured in an atmosphere of 5% CO2 in air at 37°C. Confluent cells were detached from the substratum by treatment with 0.25% trypsin and 0.02% EDTA in phosphate-buffered saline (PBS). Cells were transferred to fresh medium at a concentration of 5 × 104 cells/ml every 3 or 4 days to maintain the logarithmic growth of cells. For assays, cells (5 × 104 cells/ml) were transferred to 10-ml dishes or 24-well plates and incubated overnight at 37°C in fresh medium. Then VK2, dissolved in ethanol, was added (or ethanol was added alone, as a control) to the culture medium.
Quantification of cell proliferation
Cell proliferation was quantified by measuring absorbance at 492 nm after exposure of cells to XTT, which is metabolized by mitochondrial dehydrogenase to yield a formazan dye (Roehm et al. 1991), as described in a previous paper (Shibayama-Imazu et al. 2003). In brief, cells were transferred to 96-well plates at 5 × 103 cells/well and then treated with VK2 at concentrations from 0.39 to 100 μM for 96 h, as indicated. Absorbance was measured after a further 4-h incubation at 37°C with a solution of XTT (0.33 mg/ml) that contained 12.5 μM PMS. Cell growth (as a percentage) was calculated from the absorbance at 492 nm of treated cells relative to the absorbance at 492 nm of nontreated cells.
Analysis of the induction of apoptosis with a cell-death detection kit and Hoechst staining
Cells (1 × 105 cells/ml) were treated with VK2 in 24-well plates. Both the culture medium and cells were collected after brief centrifugation at 200×g for 10 min, and pelleted cells were lysed in lysis buffer supplied with the Cell-Death Detection ELISAPLUS kit. After brief centrifugation at 200×g for 10 min, the supernatant was incubated with histone-specific and DNA-specific antibodies, and released nucleosomes were quantified as described in the instruction manual supplied with the kit. Apoptotic cells were assessed by an examination of nuclear morphology after staining with Hoechst 33342. Cells were stained with 10 μM Hoechst 33342 for 15 min on ice and were examined under a fluorescence microscope.
Preparation of cell lysates and immunoblotting
Cells were collected by centrifugation and washed twice with ice-cold PBS. Then cell lysates were prepared as follows. Cells were suspended in a solution of 50 mM HEPES (pH 7.4), 0.2% Triton X-100, 1 mM EGTA, 1 mM Na2VO4, 100 mM NaCl, 10 mM NaF, 1 mM phenylmethylsulfonylfluoride (PMSF) and a cocktail of protease inhibitors (1/100 volume) and kept on ice for 30 min. The suspension was agitated briefly with a vortex mixer and centrifuged at 4°C for 15 min at 17,400×g. The concentration of protein in the supernatant was determined with a BCA kit (Pierce Co. Ltd, Rockford, IL) or a Bio-Rad protein assay kit (Bio-Rad Co. Ltd, Hercules, CA) with bovine serum albumin (BSA) as the standard, and then an aliquot was supplemented with 1/10 volume of 10× sample buffer [10% SDS, 10% β-mercaptoethanol, 10 mM EDTA, 10% sucrose and 30 mM Tris–HCl (pH 6.8)] for SDS-PAGE, boiled for 5 min and then subjected to SDS-PAGE on a 10% polyacrylamide gel (Laemmli 1970). Bands of proteins were transferred electrophoretically to a nitrocellulose membrane (Schleicher & Schuell, Inc., Keene, NH) in a transfer buffer that contained 192 mM glycine, 20 mM Tris–HCl (pH 8.2) and 20% methanol, and then the membrane was blocked in a blocking solution of 5% nonfat dry milk in TBS [20 mM Tris–HCl (pH 8.0), 0.85% NaCl] for 30 min. Then the membrane was incubated for 18 h at 4°C with monoclonal or polyclonal antibodies (1:1,000; diluted in blocking solution) and washed three times in TBS that contained 0.05% Tween-20. The membrane was incubated for 1 h with horseradish peroxidase-conjugated goat antibodies against mouse IgG or rabbit IgG (Cell Signaling Technology, Inc., Beverly, MA; 1:3,000; diluted in blocking solution). Then it was washed three times with 0.05% Tween 20 in TBS and finally incubated in Western LightningTM Chemiluminescence Reagent (PerkinElmer Life Science Products, Boston, MA). Immune complexes were detected on O-Max X-ray film (Fuji Film Co. Ltd, Kanagawa, Japan).
Preparation of heavy membrane fraction, cytosol and nuclei
The procedure for isolation of the heavy membrane (HM) fraction, which contained mainly mitochondria, was performed as described by Lin et al. (2004a). In brief, cells (1 × 106 cells) were suspended in 0.5 ml of hypotonic buffer [5 mM Tris–HCl (pH 7.5), 5 mM KCl, 1.5 mM MgCl2, 0.1 mM EGTA, 1 mM DTT and a cocktail of protease inhibitors (1/100 volume)] and homogenized 60 strokes with Dounce-homogeneizer on ice. The homogenate was centrifuged at 500×g for 5 min. The resulting supernatant was centrifuged at 10,000×g for 30 min at 4°C to obtain the HM fraction. The supernatant was further centrifuged at 100,000×g for 60 min, and the resulting supernatant was separated as the cytosol. For immunoblotting analysis, HM fraction was suspended in lysis buffer [10 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 5 mM EDTA, 5 mM EGTA, 10 mM sodium pyrophosphate, 5 mM NaF, 2 mM Na2VO4, a cocktail of protease inhibitors (1/100 volume)]. After centrifugation of the mixture at 17,400×g for 15 min, the supernatant was supplemented with 1/10 volume of 10× sample buffer for SDS-PAGE and boiled for 5 min. Nuclei were obtained from the pellet after centrifugation of the homogenate at 500×g for 5 min. The pellet was suspended in nuclear isolation solution (0.2% Triton X-100 in hypotonic buffer), and passed several times through a 22G syringe and centrifuged at 500×g for 10 min. The pellet was dissolved in 2× sample buffer, boiled for 5 min and sonicated for 5 s to break the DNA. The concentration of protein was determined with the Protein Assay kit from Bio-Rad with BSA as the standard.
Immunofluorescence analysis
Cells were cultured overnight on glass coverslips (Matsunami, Tokyo, Japan) and then treated with reagents as indicated. The cells were fixed for 20 min in a solution of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4), permeabilized with 2% Triton X-100 in PBS for 5 min, washed once with PBS and then incubated for 30 min at 37°C in blocking solution (3% normal goat serum and 1% BSA in PBS). For detection of TR3, cells were then incubated overnight at 4°C with TR3-specific rabbit antibodies. After three washes with PBS, cells were incubated for 1 h with fluorescein isothiocyanate-conjugated mouse monoclonal antibodies against rabbit IgG (Molecular Probes, Eugene, OR). Cells were washed three times with PBS and mounted. For detection of mitochondria, we used a mouse monoclonal antibody against cytochrome oxidase subunit IV (OX). As second antibody, we used Cy3-conjugated goat antibodies against mouse IgG (Rockland Inc., Gilbertsville, PA). Fluorescent images were collected and analyzed with a laser-scanning confocal microscope (TCS SP2; LEICA Microsystems Wetzler GmbH, Wetzler, Germany)
Treatment of PA-1 cells with siRNA
Small interfering RNA (siRNA) directed against TR3 (siRNA-TR3) was prepared by Qiagen Inc. (Chatsworth, CA). The sequences of the siRNA were as follows (numbers in parentheses indicate nucleotide positions within the open reading frame of the human gene for TR3): 5'-CGCUUCAUGCCAGCAUUAUd(TT)-3' (929–947) and 5'-AUAAUGCUGGCAUGAAGCGd(TT)-3'. Cells were treated with siRNA according to the instructions provided with the RNAiFectTM transfection reagent (Qiagen K. K., Tokyo, Japan) with slight modifications. In brief, PA-1 cells (5 × 104) were treated with 2.5 μg siRNA-TR3 in MEM medium supplemented with 10% FBS in the presence of the RNAiFectTM transfection reagent. After incubation for 24 h at 37°C, the medium was replaced by fresh MEM medium with 10% FBS, and cells were incubated for a further 24 h. Then, VK2 was added to culture medium and incubation was continued for 72 h. VK2-induced apoptosis was monitored by Hoechst staining after cells had been collected by centrifugation, as described above.
Results
Inhibition of growth of human ovarian cancer cells by vitamin K2
We examined the effects of VK2 on the growth of five lines of ovarian cancer cells. As shown in Fig. 1, VK2 inhibited most effectively the growth of PA-1 cells, whereas the growth of SK-OV-3 cells was practically unaffected. The growth of both OVCAR3 cells and SW626 cells was weakly inhibited. The growth of TYK-nu cells was inhibited by VK2 with an IC50 of 73.0 ± 4.5 μM, which is much higher than the value of IC50, 5.0 ± 0.7 μM, for PA-1 cells. Therefore, we used PA-1 cells and SK-OV-3 cells as VK2-sensitive and VK2-resistant lines, respectively.
Fig. 1.
Effects of vitamin K2 (VK2) on the growth of various lines of human ovarian cancer cells. Cell proliferation was determined by the XTT assay 96 h after the treatment of VK2, as defined and described in “Materials and methods”. Each value is the mean ± SD of the results from three independent experiments. Open square PA-1, filled circle SK-OV-3, open circle OVCAR3, filled square SW626, filled triangle TYK-nu
Induction of apoptosis in PA-1 cells
We monitored the release of fragmented nucleosomes into the cytosolic fraction with a Cell-Death Detection ELISAPLUS kit. The level of nucleosomes in the cytosolic fraction of PA-1 cells began to increase 24 h after the start of incubation with VK2 and continued to increase until at least 72 h after the start of incubation (Fig. 2a). After 72 h, induction of apoptosis was evident in 35% of PA-1 cells, as determined by counting apoptotic cells with condensed and fragmented nuclei after staining with Hoechst 33342 as described in “Materials and methods”. In a previous report (Shibayama-Imazu et al. 2003), we showed that treatment of TYK-nu cells with 150 μM VK2 for 96 h caused the release of cytochrome c. We examined the release of cytochrome c in VK2-treated PA-1 cells. As shown in Fig. 2b, we observed the release of cytochrome c 48 h after the start of incubation with VK2. After 72 h, the level of cytochrome c in the cytosol increased markedly, suggesting that apoptosis had been induced via the same pathway as previously reported for TYK-nu cells (Shibayama-Imazu et al. 2003).
Fig. 2.
Induction of apoptosis in PA-1 cells by vitamin K2 (VK2). PA-1 cells were treated with 30 μM VK2 for up to 72 h. a Apoptosis was monitored, in terms of the release of nucleosomes into the cytosolic fraction, as described in the text. White columns nontreated cells, gray columns VK2-treated cells. b Time-dependent release of cytochrome c in the cytosol prepared from PA-1 cells after treatment with VK2. Cells were treated with 30 μM VK2 and lysed as described in “Materials and methods”. Cytosol obtained from the cells at various time points were subjected to SDS-PAGE on a 15% polyacrylamide gel followed by transferring it to a nitrocellulose membrane. Cytochrome c was detected with specific antibody, as described in the text. Levels of GAPDH confirm the loading of equivalent amounts of protein in each lane
Significant increases in levels of TR3 in PA-1 cells after treatment with VK2
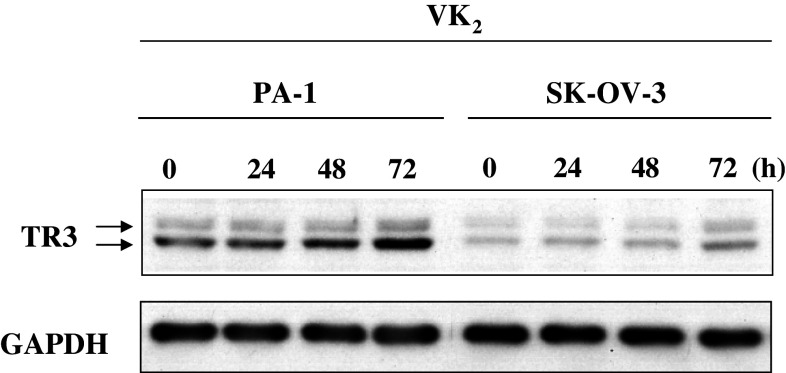
We compared the levels of TR3 in cell lysates of PA-1 cells and SK-OV-3 cells. To our surprise, the level of TR3 in PA-1 cells was four times higher than that in SK-OV-3 cells (compare bands of TR3 at 0 h in Fig. 3). The lysates of both lines of cells also included a small but significant amount of TR3 that generated a band that migrated more slowly than the bulk of TR3 during SDS-PAGE. The more slowly migrating band of TR3 was identified previously as phosphorylated TR3 (Katagiri et al. 2000; Pekarsky et al. 2001).
Fig. 3.
The level of TR3 increased gradually and time-dependently in PA-1 cells upon treatment with vitamin K2 (VK2). Cell lysates were prepared from PA-1 and SK-OV-3 cells 0, 24, 48 and 72 h after the start of treatment with 30 μM VK2. TR3 in the cell lysates (10 μg protein each) was detected by immunoblotting using rabbit TR3-specific polyclonal antibody as described in “Materials and methods”. Levels of GAPDH were confirmed by loading an equal amount of proteins in each lane
When PA-1 cells were treated with 30 μM VK2, the level of TR3 in PA-1 cells increased time-dependently. By contrast, the band of TR3 from SK-OV-3 cells was barely visible and the level of TR3 had increased only slightly 72 h after the start of treatment with VK2, and even then did not reach the level of TR3 in untreated PA-1 cells.
Effects of TR3 synthesis and VK2-induced apoptosis
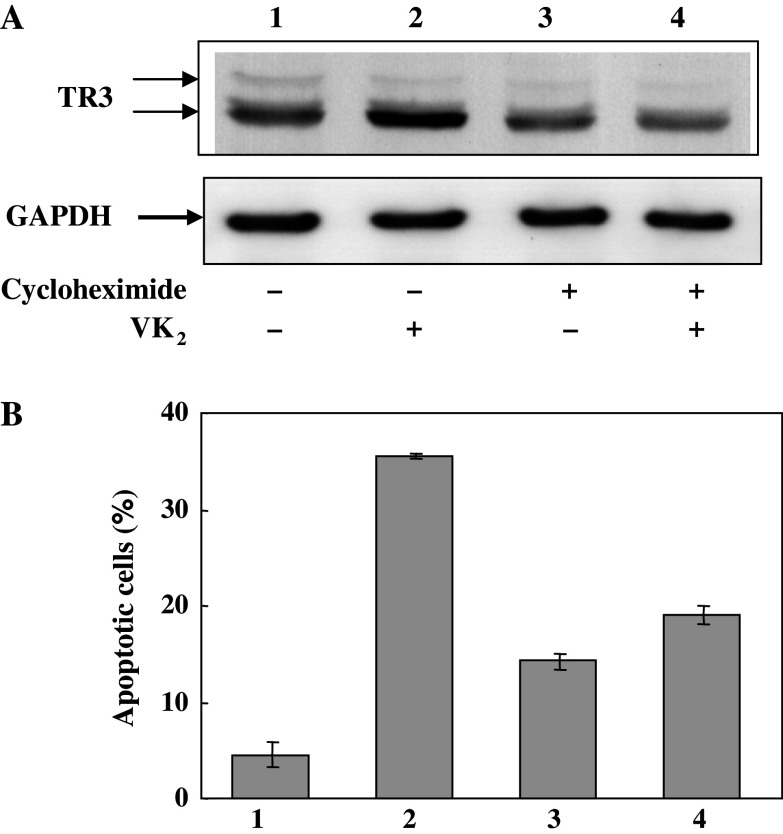
Cycloheximide (CHX) blocks the synthesis of proteins, including those required for signal transduction. When PA-1 cells were treated with VK2 in the presence of 100 nM CHX, subsequent accumulation of TR3 by VK2 was completely inhibited and the level of TR3 was practically unchanged (Fig. 4a). Furthermore, the extent of the induction of apoptosis of PA-1 cells by VK2 was reduced to almost 50% of the control level in the presence of cycloheximide (Fig. 4b). These results suggest that the increase in the TR3 level in PA-1 cells by VK2 treatment is due to the increase in TR3 synthesis and not due to inhibition of its degradation and that TR3 synthesized during treatment with VK2 is required for the induction of apoptosis in PA-1 cells by VK2.
Fig. 4.
Effects of CHX on the synthesis of TR3 and induction of apoptosis upon treatment of PA-1 cells with vitamin K2 (VK2). a After PA-1 cells had been incubated under the conditions indicated for 72 h, cell lysates were prepared, fractionated and immunoblotted to detect TR3 as described in the legend of Fig. 3. b After cells were treated under the same conditions as described in a, apoptotic cells that contained condensed and fragmented chromatin were counted after staining with Hoechst 33342. Lane 1 PA-1 cells incubated without VK2, lane 2 PA-1 cells incubated with 30 μM VK2, lane 3 PA-1 cells incubated with 100 ng/ml CHX, lane 4 PA-1 cells incubated with 100 ng/ml CHX for 30 min before the addition of 30 μM VK2 and subsequent incubation for 72 h in the presence of VK2 and CHX. The results shown are typical of results of two experiments. Values are means ± SD of results from five assays
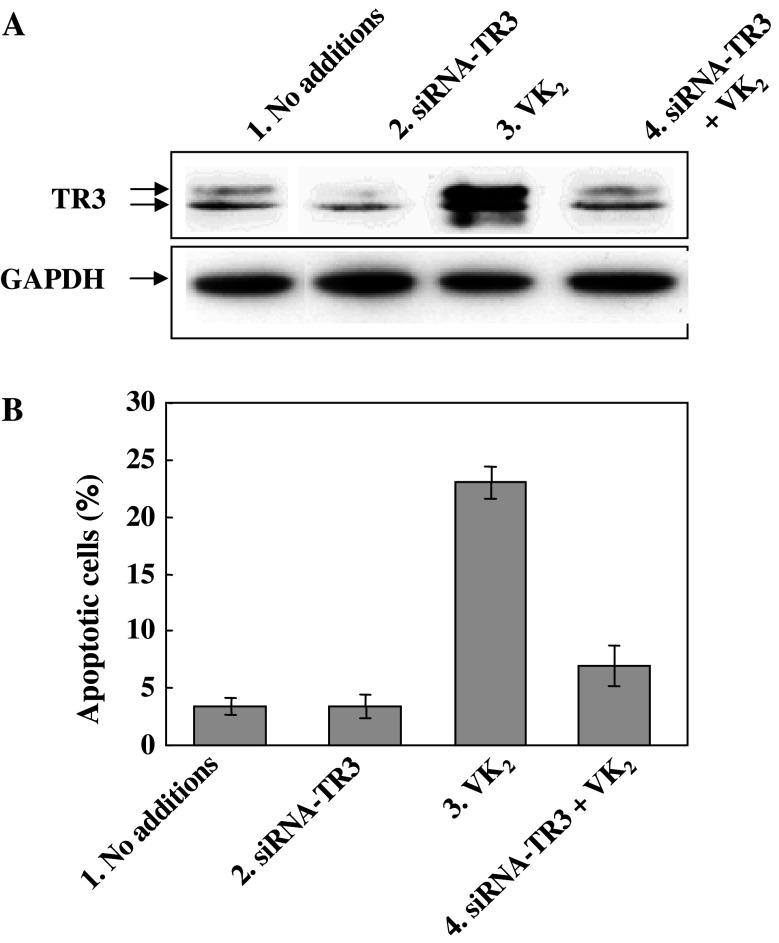
We examined whether siRNA-TR3 treatment might block the induction of apoptosis in PA-1 cells by VK2. When PA-1 cells were transfected with siRNA-TR3, the levels of TR3 in cell lysates fell significantly but small amounts of TR3 were still present (Fig. 5a, lane 2). However, the marked increase in the level of TR3 caused by treatment with VK2 for 72 h was almost completely abolished after transfection of cells with siRNA-TR3 (compare lane 3 with lane 4 in Fig. 5a). Induction of apoptosis by VK2 was also significantly inhibited by siRNA-TR3 (compare columns 3 and 4 in Fig. 5b). Apoptosis was not induced in cells treated with siRNA-TR3 only (column 2 in Fig. 5b). These results suggest that an increase in level of TR3 might be responsible for the induction of apoptosis in PA-1 cells by VK2.
Fig. 5.
Effect of inhibition of the TR3 synthesis on the induction of apoptosis. a PA-1 cells were incubated under the conditions indicated for 72 h, and cell lysates were prepared, fractionated and immunoblotted with TR3-specific antibody. b Apoptotic cells were counted after staining with Hoechst 33342 as described in the legend of Fig. 4. Lane 1 PA-1 cells incubated for 72 h, lane 2 PA-1 cells incubated with siRNA-TR3 (2 μg) for 48 h, lane 3 PA-1 cells treated with 30 μM VK2 for 72 h, lane 4 PA-1 cells incubated with siRNA-TR3 (2 μg) for 48 h and then treated with 30 μM VK2 for 72 h. Values are means ± SD of results from five assays
Increases in levels of TR3 in the mitochondrial fraction upon treatment of cells with VK2
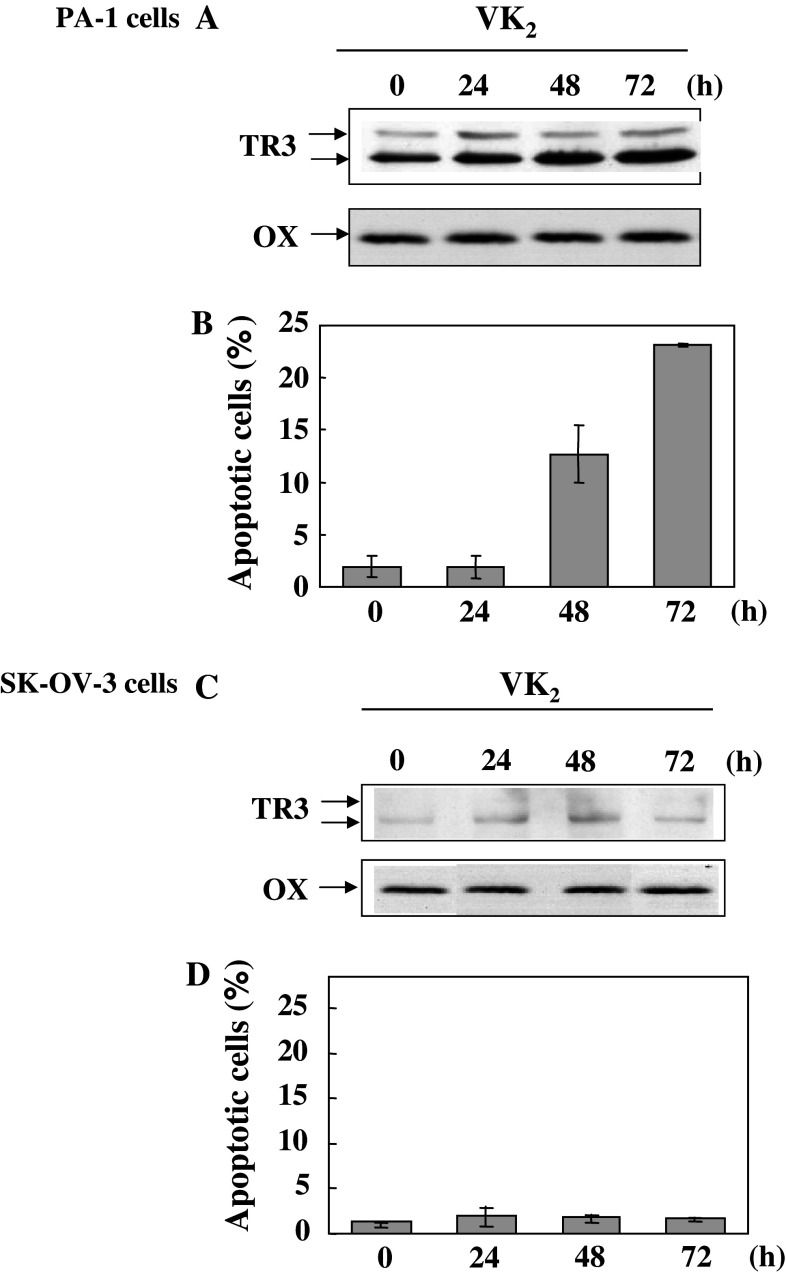
Figure 6a shows that the level of TR3 in the HM fraction increased dramatically 48 and 72 h after the start of the treatment of PA-1 cells with VK2. The level of TR3 in the HM fraction 72 h after the start of treatment of PA-1 cells with VK2 was seven times higher than that of TR3 in the HM fraction of control cells that had not been treated with VK2. In parallel with the increase in the level of TR3, the percentage of apoptotic cells was also increased time-dependently by treatment of PA-1 cells with VK2 (Fig. 6b). By contrast, neither the level of TR3 in the HM fraction nor the percentage of apoptotic cells was practically unchanged by treatment of SK-OV-3 cells with VK2 (Fig. 6c, d).
Fig. 6.
Changes in the levels of TR3 in HM fractions of PA-1 and SK-OV-3 cells after treatment with vitamin K2 (VK2). Both PA-1 and SK-OV-3 cells were treated with VK2 and fractionated to yield an HM fraction after incubation for 0, 24, 48 and 72 h. a and c The HM fractions were immunoblotted with TR3 specific-antibody and OX-specific antibody. b and d Ratio of apoptotic cells was quantitated after staining with Hoechst 33342, as described in the legend of Fig. 4. The intensities of bands of OX shown in each lane confirmed that equal amounts of mitochondria had been loaded in each lane. a,b PA-1 cells; c,d SK-OV-3 cells
Immunofluorescence staining of PA-1 and SK-OV-3 cells during VK2-induced apoptosis
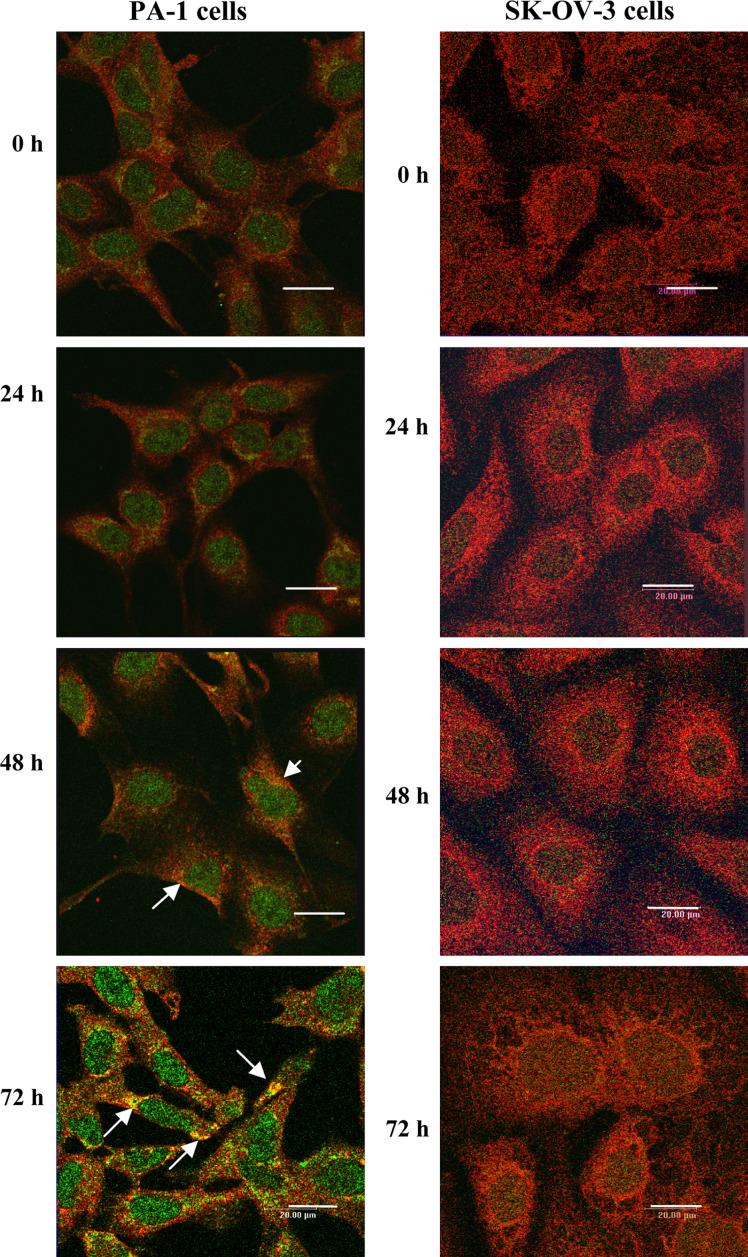
Using an indirect immunofluorescence-staining method, we studied the changes in the distribution of TR3 in both PA-1 and SK-OV-3 cells during treatment with VK2 (Fig. 7). In control PA-1 cells, endogenous TR3 was observed in nuclei. Moreover, no translocation of TR3 from nuclei was evident and instead, the amount of TR3 in nuclei increased 48 and 72 h after the start of treatment with VK2 (Fig. 7). Localization of TR3 on mitochondria, as identified by immunostaining with antibody against OX, was also evident after treatment with VK2 for 48 and 72 h (Fig. 7). By contrast, TR3 was barely detectable in SK-OV-3 cells. Neither an increase in the amount of detectable TR3 nor the aggregation of TR3 was observed at any time after the start of treatment of SK-OV-3 cells with VK2 (Fig. 7).
Fig. 7.
Changes in the distribution of TR3 in PA-1 cells during induction of apoptosis by vitamin K2 (VK2). PA-1 and SK-OV-3 cells were treated with 30 μM VK2 for 0, 24, 48 and 72 h. After fixation of cells as described in the text, cells were subjected to indirect immunofluorescence staining. The TR3-specific polyclonal antibodies reacted with FITC-conjugated antibody against rabbit IgG (green color) and the OX-specific monoclonal antibody (delineating mitochondria) reacted with Cy3-conjugated antibodies against mouse IgG (red color). White arrows indicate typical aggregated mitochondria and TR3. Yellow coloration suggests the localization of TR3 in mitochondria. No changes in the distribution of TR3 were evident in SK-OV-3 cells during treatment with VK2. The images are representative of results from three experiments. The scale bar represents 20 μm
Changes in the distribution of TR3 during VK2-induced apoptosis
As shown in Fig. 8, TR3 in nuclei of PA-1 cells increased 48 and 72 h after the start of treatment of VK2. This result was well coincident with the photographs of the immunofluorescent staining (Fig. 7). The level of TR3 in the cytosol fraction of PA-1 cells was low, but it was also upregulated during induction of apoptosis (Fig. 8). The increase of TR3 in the cytoplasm of PA-1 cells suggests that the synthesis of TR3 in PA-1 cells may be increased by the treatment with VK2.
Fig. 8.
Localization of TR3 in both nuclei and cytosol of PA-1 cells during the induction of apoptosis by treatment of vitamin K2 (VK2). PA-1 cells were treated with VK2 for various times and then fractionated to yield nuclear and cytosolic fractions. Each fraction was analyzed by immunoblotting with TR3-specific antibodies. The intensities of the bands of lamin and GAPDH confirm that equal amounts of the nuclear or cytosolic fractions were loaded
Effects of inhibitors of p38 and JNK on VK2-induced apoptosis
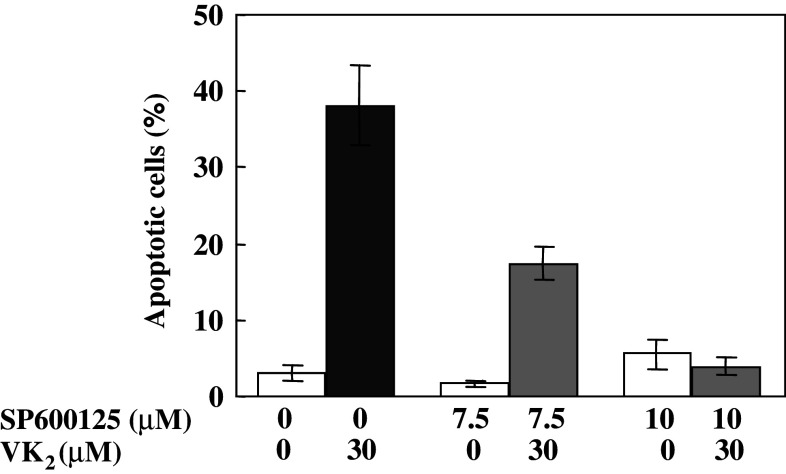
Signaling through the JNK and p38 MAP kinase cascades is frequently associated with the induction of apoptosis (Davis 2000, Tournier et al. 2000; MacFarlane et al. 2000; Holmes et al. 2002, 2003a; Li et al. 1998; Kolluri et al. 2003; Han et al. 2006). Therefore, we examined whether the induction of apoptosis in PA-1 cells by VK2 could be blocked by inhibitors of p38 and JNK. When PA-1 cells were pretreated with SP600125, an inhibitor of JNK, for 60 min, the subsequent induction of apoptosis by VK2 is markedly inhibited in a dose-dependent manner (Fig. 9). In contrast to the effect of JNK inhibitor on VK2-induced apoptosis in PA-1 cells, inhibitors of p38 such as PD169316 or SB203580 had no effect on the induction of apoptosis in the same cell by VK2 (results not shown).
Fig. 9.
Effects of a JNK inhibitor (SP600125) on the induction of apoptosis by vitamin K2 (VK2). PA-1 cells were incubated with 7.5 or 10 μM SP600125 for 1 h before addition of 30 μM VK2. After following incubation of PA-1 cells for 72 h, cells were stained with Hoechst 333452 and apoptotic cells were counted. White columns show percentages of apoptotic cells after treatment without VK2 (0.1% ethanol was added as vehicle). Gray columns show percentages of apoptotic cells after treatment with 30 μM VK2 and SP600125. The black column shows the percentage of apoptotic cells after treatment with 30 μM VK2 alone. Values are means ± SD of results from five assays
Discussion
In the present study, we found that PA-1 ovarian cancer cells were extremely sensitive to the induction of apoptosis by VK2, while ovarian cancer SK-OV-3 cells were resistant. Levels of TR3 in various lines of cells rise upon exposure of cells to apoptotic stimuli, such as 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid (AHPN/CD437) (Li et al. 1998), tetradecanoyl phorbol-1,3-acetate (TPA), VP-16 (Li et al. 2000), and butyrate (Wilson et al. 2003). In harmony with these results, levels of TR3 in PA-1 cells rose significantly upon treatment of cells with VK2, whereas they did not change at all in SK-OV-3 cells under the same conditions. Furthermore, we found that CHX and siRNA-TR3 inhibited the VK2-induced synthesis of TR3 and apoptosis. From our results, we can conclude that an increase in the synthesis of TR3 is associated with the induction of apoptosis in PA-1 cells by VK2.
When the lysate of PA-1 cells was analyzed by immunoblotting with TR3-specific antibodies, two bands were observed (Figs. 3–5). One is the major band and the other is the more slowly migrating minor band. The more slowly migrating band of TR3 was reported to be phosphorylated TR3 (Katagiri et al. 2000; Pekarsky et al. 2001). As shown in Fig. 4, the intensity of the more slowly migrating band of TR3 was practically unchanged by treatment of PA-1 cells with VK2. By contrast, the intensities of the more slowly migrating bands increased evidently by the VK2-treatment as shown in Figs. 3 and 5a, although their degrees of intensification were different between these figures. These results suggest that the degree of phosphorylation of TR3 after its synthesis upon treatment of PA-1 cells with VK2 might be different in spite of the identical experimental conditions. Further studies are required to clarify the mechanism of VK2-induced activation of TR3 phosphorylation.
The level of TR3 in the mitochondrial fraction of PA-1 cells increased markedly 48 h after the start of treatment with VK2. This result is consistent with observations that TR3 accumulates in the HM fraction during the apoptosis that is induced by various inducers of apoptosis, such as TPA and MM11453 (Li et al, 2000). Furthermore, immunofluorecsence studies showed that TR3 accumulated in mitochondria 48 and 72 h after the start of treatment with VK2. It seems, therefore, that TR3 might be one of proteins that disrupt the functions of mitochondria, with the resultant release of cytochrome c. Lin et al. (2004a) demonstrated that elevated levels of TR3 in mitochondria induce the depolarization of mitochondrial membranes by interfering with the antiapoptotic properties of Bcl-2 that are the result of a conformational change. We observed previously that depolarization of the mitochondrial membrane was induced by treatment of TYK-nu cells with VK2 (Shibayama-Imazu et al. 2006). In our present study, we observed the accumulation of TR3 in the HM fraction during VK2-induced apoptosis. Our results suggest that the accumulation of TR3 in mitochondria might be a critical event in the induction of apoptosis.
Li et al. (2000) proposed that TR3 might induce apoptosis via its translocation from the nucleus to the mitochondria, with the resultant release of cytochrome c. In OV-CA-3 cells, TR3 dose move from nuclei and becomes associated with mitochondria upon treatment of cells with the synthetic retinoid, CD437, whereas apoptosis is induced without similar translocation of TR3 upon treatment of the same cells with another synthetic retinoid, N-(4-hydroxyphenyl)retinamide (4-HPR; Holmes et al. 2003b). Thus, the translocation of TR3 from nuclei is thought to be dependent on reagent. In our present study, treatment of PA-1 cells with VK2 did not result in translocation of TR3 from nuclei into the cytoplasm. Indeed, TR3 was found to have accumulated in nuclei 48 and 72 h after the start of treatment with VK2. This is the first report, to our knowledge, regarding treatment of TR3 accumulating in both mitochondria and nuclei during the induction of apoptosis.
In this report, we could not show why TR3 did not translocate from the nuclei to the cytoplasm during the VK2-induced apoptosis of PA-1 cells. Leptomycin B, which is an inhibitor of the chromosomal region maintenance 1 (CRM1)-dependent nuclear export process (Kudo et al. 1999), has been used to block both the translocation of TR3 from nuclei and the induction of apoptosis (Li et al. 2000; Katagiri et al. 2000; Cao et al. 2004). However, the VK2-induced apoptosis of PA-1 cells was not inhibited by 0.05–0.2 nM leptomycin B (results not shown). This observation supports our conclusion that TR3 did not move from the nucleus to the cytosol during the VK2-induced apoptosis of PA-1 cells.
We demonstrated that the VK2-induced apoptosis in ovarian cancer PA-1 cells was inhibited by SP600125, an inhibitor of JNK, but not by inhibitors of p38 such as PD169316 and SB203580 (Fig. 9). JNK is known to regulate the induction of apoptosis (Davis 2000; Tournier et al. 2000). We reported recently that VK2 induces the accumulation of reactive oxygen species (ROS), in particular, superoxide radicals and H2O2, in cells (Shibayama-Imazu et al. 2006). ROS are potent activators of JNK, acting via the oxidative inactivation of endogenous inhibitors of JNK, such as JNK phosphatases and glutathione S-transferase (Chen et al. 2001; Bernardini et al. 2000). Superoxide induces the activation of JNK and cell death in hepatocytes (Lo et al. 1996; Conde de la Rosa et al. 2006). Furthermore, activated JNK-1 efficiently phosphorylates TR3, eliminating its ability to bind to DNA in lung cancer cells (Kolluri et al. 2003). The present study demonstrates a possibility that VK2 might cause accumulation of TR3 in mitochondria directly from ribosomes without translocation from nuclei. Further studies are needed to examine whether VK2 might activate JNK and whether phosphorylation of TR3 by JNK might contribute to the accumulation of TR3 in mitochondria.
In conclusion, we have demonstrated that ovary cancer PA-1 cells are very sensitive to VK2 and that accumulation of TR3 in mitochondria, as well as in nuclei, might be associated in the induction of apoptosis by VK2. This finding suggests that VK2 may have therapeutic efficacy for the clinical treatment of ovary cancer.
Abbreviations
- BSA
Bovine serum albumin
- CHX
Cycloheximide
- FBS
Fetal bovine serum
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- HM
Heavy membrane
- JNK
c-Jun N-terminal kinase
- NGFI-B
Nerve growth factor induced clone B
- OX
Cytochrome oxidase subunit IV
- PBS
Phosphate-buffered saline
- PMSF
Phenylmethylsulfonyl fluoride
- ROS
Reactive oxygen species
- siRNA
Small interfering RNA
- TBS
Tris-buffered saline
- TPA
Tetradecanoylphorbol-1,3-acetate
- VK2
Vitamin K2
- XTT
Sodium-3'-[1-[(phenylamino)carbonyl]-3,4-tetrazolium-bis(4-methoxy-6-nitro)benzene-sulfonic acid hydrate
References
- Bernardini S, Berassola F, Cortese C, Ballerini S, Melino G, Motti C, Bellincampi L, Iori R, Federici G (2000) Modulation of GST P1-1 activity by polymerization during apoptosis. J Cell Biochem 77:645–653 [PubMed] [Google Scholar]
- Cao X, Liu W, Lin F, Li H, Kulluri SK, Lin B, Han Y, Dawson MI, Zhang X (2004) Retinoid receptor regulates Nur77/thyroid hormone receptor 3-dependent apoptosis by modulating its nuclear export and mitochondrial targeting. Mol Cell Biol 24:9705–9725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YR, Shrivastava A, Tan TH (2001) Down-regulation of the c-Jun N-terminal kinase (JNK) phosphatase M3/6 and activation of JNK by hydrogen peroxide and pyrrolidine dithiocarbamate. Oncogene 16:367–374 [DOI] [PubMed] [Google Scholar]
- Conde de la Rosa L, Schoemaker MH, Vrenken TE, Buist-Homan M, Havinga R, Jansen PL, Moshage H (2006) Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: involvement of JNK and ERK MAP kinases. J Hepatol 44:918–929 [DOI] [PubMed] [Google Scholar]
- Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103:239–252 [DOI] [PubMed] [Google Scholar]
- Han YH, Cao X, Lin B, Kolluri SK, Stebbins J, Reed JC, Dawson MI, Zhang XK (2006) Regulation of Nur77 nuclear export by c-Jun N-terminal kinase and Akt. Oncogene 18:2974–2986 [DOI] [PubMed] [Google Scholar]
- Hazel TG, Nathans D, Lau LF (1988) A gene inducible by serum growth factors encodes a member of the steroid and thyroid hormone receptor superfamily. Proc Natl Acad Sci USA 85:8444–8448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman HR (1991) Primary response genes induced by growth factors and tumor promoters. Annu Rev Biochem 60:281–319 [DOI] [PubMed] [Google Scholar]
- Hitomi M, Yokoyama F, Kita Y (2005) Antitumor effects of vitamin K1, K2 and K3 on hepatocellular carcinoma in vitro and in vivo. Int J Oncol 26:713–720 [PubMed] [Google Scholar]
- Holmes WF, Soprano DR, Soprano KJ (2002) Elucidation of molecular events mediating induction of apoptosis by synthetic retinoids using a CD437-resistant ovarian carcinoma cell line. J Biol Chem 277:45408–45419 [DOI] [PubMed] [Google Scholar]
- Holmes WF, Soprano DR, Soprano KJ (2003a) Early events in the induction of apoptosis in ovarian carcinoma cells by CD437: activation of the p38 MAP kinase signal pathway. Oncogene 22:6377–6386 [DOI] [PubMed] [Google Scholar]
- Holmes WF, Soprano DP, Soprano KJ (2003b) Comparison of the mechanism of induction of apoptosis in ovarian carcinoma cells by the conformationally restricted synthetic retinoids CD437 and 4-HPR. J Cell Biochem 89:262–278 [DOI] [PubMed] [Google Scholar]
- Iwamoto J, Takeda T, Sato Y (2004) Effects of vitamin K2 on osteoporosis. Curr Pharm Res 10:2557–2576 [DOI] [PubMed] [Google Scholar]
- John D. Woronics JD, Xallan B, Ngo V, Astar W (1994) Requirement for orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature 367:277–281 [DOI] [PubMed] [Google Scholar]
- Katagiri Y, Takeda K, Yu Z-X, Ferrans VJ, Ozato K, Guroff G (2000) Modulation of retinoid signalling through NGF-induced nuclear export of NGFI-B. Nat Cell Biol 2:435–440 [DOI] [PubMed] [Google Scholar]
- Kolluri SK, Bruey-Sedano N, Cao X, Lin B, Lin F, Han YH, Dawson MI, Zhang XK (2003) Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells. Mol Cell Biol 23:8651–8667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner EP, Wolff B, Yoshida M, Horinouchi S (1999) Leptomycin B inactivates CRM1/exportin by covalent modification at a cysteine residue in the central conserved region. Proc Natl Acad Sci USA 96:9112–9117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, Lin B, Chen G, Lu J, Lin F, Xie Z, Fontana JA, Reed JC, Zhang X, (2000) Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science 289:1159–1164 [DOI] [PubMed] [Google Scholar]
- Li Y, Lin B, Agadir A, Liu R, Dawson MI, Reed JC, Fontana JA, Bost F, Hobbs PD, Zheng Y, Chen GQ, Shroot B, Mercola D, Zhang XK. (1998) Molecular determinants of AHPN (CD437)-induced growth arrest and apoptosis in human lung cancer cell lines. Mol Cell Biol 18:4719–4731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Kolluri SK, Lin F, Liu W, Han Y-H, Cao X, Dawson MI, Reed JC, Zhang X-K (2004a) Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell 116:527–540 [DOI] [PubMed] [Google Scholar]
- Lin X-F, Zhao B-X, Chen H-Z, Ye X-F, Yang C-Y, Zhou H-Y, Zhang M-Q, Lin S-C, Wu Q (2004b) RXRα acts as a carrier for TR3 nuclear export in a 9-cis retinoic acid-dependent manner in gastric cancer cells. J Cell Sci 117:5609–5621 [DOI] [PubMed] [Google Scholar]
- Lo YY, Wong JM, Cruz TF (1996) Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases. J Biol Chem 271:15703–15707 [DOI] [PubMed] [Google Scholar]
- MacFarlane M, Cohen GM, Dickens M (2000) JNK (c-Jun-terminal kinase) and p38 activation in receptor-mediated and chemically induced apoptosis of T-cells: differential requirements for caspase activation. Biochem J 348:93–101 [PMC free article] [PubMed] [Google Scholar]
- Miyazawa K, Yaguchi M, Funato K, Gotoh A, Kawanishi Y, Nishizawa Y, Yuo A, Ohyashiki K (2001) Apoptosis/differentiation-inducing effects of vitamin K2 on HL-60 cells: dichotomous nature of vitamin K2 in leukemia cells. Leukemia 15:1111–1117 [DOI] [PubMed] [Google Scholar]
- Nakai A, Kartha S, Sakurai A, Toback FG, DeGroot LJ (1990) A human early response gene homologous to murine nur77 and rat NGFI-B, and related to the nuclear receptor superfamily. Mol Endocrinol 4:1438–1443 [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Carr BI, Wang M, Kar S, Finn F, Dowd P, Zheng ZB, Kerns J, Naganathan S (1995) Growth inhibition of hepatoma cells induced by vitamin K and its analogs. J Biol Chem 270:28304–28310 [DOI] [PubMed] [Google Scholar]
- Pekarsky Y, Hallas C, Palamarchuk A, Koval A, Bullrich F, Hirata Y, Bichi R, Letofsky J, Croce CM (2001) Akt phosphorylates and regulates the orphan nuclear receptor Nur77. Proc Natl Acad Sci USA 98:3690–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL (1991) An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods 142:257–265 [DOI] [PubMed] [Google Scholar]
- Ryseck RP, Macdonald-Bravo H, Mattei MG, Ruppert S, Bravo R (1989) Structure, mapping and expression of a growth factor inducible gene encoding a putative nuclear hormonal binding receptor. EMBO J 8:3327–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai I, Hashimoto S, Yoda M, Hida T, Ohsawa S, Nakajo S, Nakaya K (1994) Novel role of vitamin K2: a potent inducer of differentiation of various human myeloid leukemia cell lines. Biochem Biophys Res Commun 205:1305–1310 [DOI] [PubMed] [Google Scholar]
- Shibayama-Imazu T, Sakairi S, Watanabe A, Aiuchi T, Nakajo S, Nakaya K (2003) Vitamin K2 selectively induced apoptosis in ovarian TYK-nu and pancreatic MIA Paca-2 cells out of eight solid tumor cell lines through a mechanism different from geranylgeraniol. J Cancer Res Clin Oncol 129:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama-Imazu T, Sonoda I, Sakairi S, Aiuchi T, Wei-wei A, Nakajo S, Itabe H, Nakaya K (2006) Production of superoxide and dissipation of mitochondrial transmembrane potential by vitamin K2 trigger apoptosis in human ovarian cancer TYK-nu cells. Apoptosis 11:1535–1543 [DOI] [PubMed] [Google Scholar]
- Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ (2000) Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathways. Science 288:870–874 [DOI] [PubMed] [Google Scholar]
- Uemura H, Chang C (1998) Antisense TR3 orphan receptor can increase prostate cancer cell viability with etoposide treatment. Endocrinology 139:2329–2334 [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang M, Finn F, Carr BI (1995) The growth-inhibitory effects of vitamin K and their actions on gene expression. Hepatology 22:876–882 [PubMed] [Google Scholar]
- Weih F, Ryseck P, Chen L, Bravo R (1996) Apoptosis of nur77/N10-transgenic thymocytes involves the Fas/Fas ligand pathway. Pro Natl Acad Sci USA 93:5533–5538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AJ, Arango D, Mariadason JM, Heerdt BG, Augenlicht LH (2003) TR3/Nur77 in colon cancer cell apoptosis. Cancer Res 63:5401–5407 [PubMed] [Google Scholar]
- Wu Q, Liu S, Ye X-F, Huang Z-W, Su W-J (2002) Dual roles of Nur77/N10 in selective regulation of apoptosis and cell cycle by TPA and ATRA in gastric cancer cells. Carcinogenesis (Lond) 23:1583–1592 [DOI] [PubMed] [Google Scholar]
- Wu Q, Li Y, Liu R, Agadir M, Lee O, Liu Y, Zhang X (1997) Modulation of retinoic acid sensitivity in lung cancer cells through dynamic balance of orphan receptors nur77 and COUP-TF and their heterodimerization. EMBO J 16:1656–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Chomez E, Castanos-Velez E, Biberfeld P, Perlmann T, Jondal M (1997) Positive and negative thymic selection in T cell receptor-transgenic mice correlate with Nur77 mRNA expression. Eur J Immunol 27:2048–2056 [erratum in Eur J Immunol 27:2748] [DOI] [PubMed] [Google Scholar]
- Yaguchi M, Miyazawa K, Katagiri T, Nishimaki J, Kizaki M, Tohyama K, Toyama K (1997) Vitamin K2 and its derivatives induce apoptosis in leukemia cells and enhance the effect of all-trans retinoic acid. Leukemia 11:779–787 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Miyazawa K, Kasuga I, Yokoyama T, Minemura K, Ustumi K, Aoshima M, Ohyashiki K (2003) Apoptosis induction of vitamin K2 in lung carcinoma cell lines: the possibility of vitamin K2 therapy for lung cancer. Int J Oncol 23:627–632 [PubMed] [Google Scholar]