Abstract
Purpose
Among the family of heat shock proteins (HSPs), HSP70 and HSP27 have been implicated in tumorigenesis and chemoresistance, probably via the prevention of apoptosis. HSP27 levels are frequently increased in large populations of tumors of the head and neck, but the mechanism of its chemoresistance is not yet fully understood. In the present study, the role of HSP27 in the resistance to cytotoxic stress was studied in Hep-2 human laryngeal cancer cells.
Method
We established a Hep-2 cell line overexpressing HSP27 and examined whether the expression of HSP27 provides resistance to heat shock and several cytotoxic agents using a MTT colorimetic assay. Cell cycle progression was assessed by flow cytometry and fluorescence staining was performed for F-actin filaments.
Results
HSP27 overexpression induced cellular resistance to heat shock at 45°C for 1 h as well as against several cytotoxic agents, including cisplatin, staurosporin and H2O2. However, no difference in sensitivity to irradiation or serum starvation was found. Moreover, HSP27 overexpressing Hep-2 cells showed a delayed cell growth, compared to control cells. To determine if the decreased cell proliferation in HSP27 overexpressing cells contributed to chemoresistance, control Hep-2 cells were synchronized at the late G1 phase by treatment with mimosine. The synchronized Hep-2 cells were resistant to cisplatin and H2O2, but not to irradiation or serum starvation, correlating the protection effect shown in HSP27 overexpressing cells. These results suggest that the overexpression of HSP27 in Hep-2 cells confers chemoresistance which is associated with the delay in cell growth. We also propose that the stabilization of F-actin observed in Hep-2/hsp27 cells is partly related to the delay in cell cycle progression, by showing that the induction of actin polymerization in Hep-2/neo cells results in the retardation of cell growth as well as a cytoprotective effect as observed in Hep-2/hsp27.
Keywords: HSP27, Laryngeal cancers, Themoresistance, Chemoresistance, Cell cycle delay, Actin polymerization
Introduction
Exposure of cells to elevated temperatures or other environmental stresses induces the synthesis of a specific set of proteins called heat shock proteins (HSPs) (Lindquist 1986; Lindquist and Craig 1988). Their expression renders cells resistant to further stress, resulting in the protection of cells from changes in their environment. Some of the HSPs have been shown to be induced by physiological stress such as infection and ischemia and even under unstressed conditions such as the control of the cell cycle and differentiation, indicating their essential role in normal cellular function (Welch 1993; Walsh et al. 1999; Quintana and Cohen 2005).
Human HSP27 is a member of the family of small heat shock proteins (sHSPs), which represent an abundant and ubiquitous family of stress proteins with a monomeric mass ranging between 15 and 30 kDa (Parcellier et al. 2005). The overexpression of HSP27 or its murine analogue, HSP25, has been shown to protect mammalian cells exposed to a variety of stress stimuli, including heat shock, oxidative stresses, and chemotherapeutic agents (Richards et al. 1996; Fortin et al. 2000) probably by inhibiting the mitochondrial pathway of apoptosis (Mairesse et al. 1998; Bruey et al. 2000). Furthermore, several studies have demonstrated that the expression of HSP27 is increased in various human cancers, suggesting that the cytoprotective activity of HSP27 might affect tumorigenesis and the susceptibility of tumors to cancer treatment (Richards et al. 1996; Vargas-Roig et al. 1998; Fortin et al. 2000). Supporting this postulation, HSP27 expression was reported to be correlated with a poor prognosis after surgery or a resistance to adjuvant therapy in many types of cancers including gastric cancer, breast cancer, osteosarcoma, and prostate carcinoma (Paulus et al. 1993; Nakopoulou et al. 1995; Liu et al. 1996; Uozaki et al. 1997). In head and neck cancers, HSP27 has been frequently shown to be expressed at high levels in squamous cell carcinomas over the oral cavity, the orophyarynx and larynx, as well as in normal upper respiratory tract mucosa (Sugerman et al. 1995; Gandour-Edwards et al. 1998). However, the biological and prognostic significance of its expression in head and neck cancers remains unclear.
Among the therapeutic approaches for the head and neck cancers, induction chemotherapy is an important strategy, especially to cases of the advanced laryngeal cancers, which are the candidates for total laryngectomy. The response to induction chemotherapy determines the subsequent treatments, i.e., whether the target organ is preserved or not (Orus et al. 2000; Leon et al. 2001, 2005). Therefore, the elucidation of the mechanism of chemoresistance of laryngeal cancers may provide the biochemical base for reducing chemoresistance, thereby increasing the survival and organ preservation rates in laryngeal cancers. HSP27-overexpressing Hep-2 laryngeal cancer cells, as one of the candidate proteins for the chemoresistance in laryngeal cancers, were examined by us for their cytoprotective activity. The findings herein show that the overexpression of HSP27 in Hep-2 cells confer chemoresistance but no protective effect against radiation, which is associated with cell growth delay. We also demonstrated that actin polymerization is partly related with the cytoprotective activity of HSP27.
Materials and methods
Cell culture and treatment
Human laryngeal cancer Hep-2 cells and the human cervix epitheloid carcinoma HeLa cells were purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA). Hep-2 cells were grown in Dulbecco’s modified Eagle Medium (DMEM, Gibco-BRL, Grand island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT, USA) and antibiotic solution (100 U/ml of penicillin, 100 μg/ml of streptomycin, Gibco-BRL) in a 5% CO2 humidified incubator at 37°C. For the determination of sensitivity to apoptotic stimuli, cells were plated in 96 well plates at a density of 1 × 104 in 100 μl of medium. After 10–12 h, the attached cells were exposed to various concentrations of selected cytotoxic agents such as cisplatin, staurosporin and H2O2 (Sigma, St. Louis, MO, USA), or serum starved media. For radio resistance and thermo resistance experiments, cells were exposed to dose of radiation from 20 Gy with cobalt-60 or subjected to a heat treatment of 45°C for 2 h, respectively, followed by continued culture at 37°C. To obtain cell populations with synchrony in the G1 phase, cells were treated with 400 μM of mimosine (Sigma), which reversibly blocks the cell cycle at the G1/S-phase boundary, for 20 h before addition of the cytotoxic agents. To examine the effect of actin polymerization on the cytotoxicity, Hep-2/neo cells were treated with 1 μM of Jasplakinolide (Calbiochem, La Jolla, CA, USA) for 3 h, prior to the treatment of various drugs.
Preparation of HSP27-overexpressing Hep-2 cells
The entire coding region of human hsp27 cDNA was generated by polymerase chain reaction (PCR) amplification from cDNA from HeLa cells with primers 5′-gaattcgcgcatgaccgagcgccgcgtc-3′ (forward) and 5′-gaattcttacttggcggcagtctcatc gga-3′ (reverse), provided with the EcoRI site at the 5′ and the 3′ extremity respectively. PCR products were then inserted in the sense orientation into the EcoRI site of a pCXN plasmid containing a β-actin promoter, a CMV enhancer and a neomycin resistant gene (Niwa et al. 1991). Transfection of Hep-2 cells with the HSP27 expressing vector (pCXN-hsp27) was performed using lipofectAMINE (Gibco-BRL). After transfection, the cells were incubated with 1 mg/ml of G418 (Neomycin, Gibco-BRL) for three weeks, and the expression of HSP27 from the pooled population (Hep-2/hsp27) was determined by Western blot analysis. Hep-2 cells transfected with pCXN vector (Hep-2/neo) were used as control cells.
Western blot analysis
Cells were lysed in RIPA buffer (50 mM Tris-HCl, pH7.4, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate), and protein concentrations were determined using BCA reagent (Pierce, Rockford, IL, USA). Aliquots containing 5 μg of protein were subjected to SDS-PAGE using 12.5% gel followed by electro-transfer onto a PVDF membrane (Millipore, Bedford, MA, USA). Immunodetection was performed using monoclonal antibody to HSP27 (1:2,000, Stressgen, Victoria, Canada), HSP70/HSC70 (1:1,000, Stressgen), β-Actin (1:50,000, Sigma), peroxidase-conjugated anti-mouse antibody (1:2,000, Promega, Madison, WI, USA) and ECL system (Amersham Bioscience Corp., Cardiff, UK).
Northern blot analysis
Total RNA was extracted from cells using RNAzol B reagent (TEL-TEST Inc., Friendswood, TX, USA) according to the manufacturer’s instructions. 10 μg of RNAs were separated by 1% agarose electrophoresis, and then transferred onto positively charged nylon membrane (Roche Diagnostic corp., Indianapolis, IN, USA). Before blotting, 28S and 18S ribosomal RNAs were visualized by staining of ethidium bromide to ensure the integrity of the isolated RNA and to quantify RNA loading. The mRNA expression of β-actin was investigated using Digoxigenin (DIG)-labeled specific β-actin DNA probe and DIG system (Roche Diagnostic corp). Dig-labeled β-actin probe was generated by direct PCR labeling using forward primer: 5′-caagagatggccacggctgct-3′, reverse primer: 5′-atactcctgcttgctgatcca-3′ and HeLa cDNA as template.
Analysis of cell viability
Cell viability was measured by the 2-(4,5-dimethyltriazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma) assay, as described previously (Chae et al. 2001). The relative cell survival was calculated as the percentage of absorbance of cells treated with apoptotic stimuli from the absorbance of control cells. For measurement of cell growth, a trypan blue exclusion test was also performed. All assays were repeated at least three times independently, and the data are presented as the average ± SEM value of representative triplicate experiments.
5′-Bromo-2′-deoxy-uridine (BrdU) incorporation assay
To determine cell populations in the S phase, the incorporation of BrdU was monitored as a parameter for DNA synthesis according to the instructions of the manufacturer (Roche Diagnostic corp.). Cells were plated in 96 microtiter plates at a density of 1 × 103 in 100 μl of medium and incubated for the indicated times in the presence or absence of mimosine. 110 μM of BrdU was added to the culture medium for 3 h at 37°C for incorporation into freshly synthesized DNA. Following fixation of the cells, cellular DNA was partially digested by nuclease treatment. A peroxidase-labeled antibody to BrdU and a peroxidase substrate was sequentially added to yield a colored reaction product, which is proportional to the level of BrdU incorporated into cellular DNA.
Morphological counting of apoptotic cells
Propidium iodide (PI, 10 μM, Sigma) and Hoechst 33258 (10 μM, Sigma) were added to the cell culture plate after heat shock, followed by incubation for 10 min at room temperature. The morphology and color of the nuclei were observed by fluorescence microscopy.
Immunofluorescent staining
For staining of actin polymerization, Hep-2/neo and Hep-2/hsp27 cells cultured in a 24-well plate containing a cover slip and were fixed in 3.7% formaldehyde for 5 min, washed with PBS, and then permeabilized with 0.1% Triton X-100 for 15 min. After a wash with PBS, cells were stained with 100 nM of FITC-conjugated phalloidin (Sigma) for 3 h, and finally washed with PBS several times. The F-actin fibers were observed by fluorescence microscopy.
Analysis of cell cycle distribution
1 × 106 cells of Hep-2/neo and Hep-2/hsp27 cells were harvested and washed twice with cold phosphate-buffered saline (PBS). The cells were incubated with 10 μg/ml RNaseA (Sigma) at 37°C for 30 min and then stained with a 50 μg/ml PI solution. Fluorescence emitted from the PI-DNA complex in each cell nucleus was measured after laser excitation at 488 nm with a flow cytometer (FACSorter, Becton Dickinson, San Jose, CA, USA).
Results
Overexpression of HSP27 increases thermotolerance in Hep-2 cells
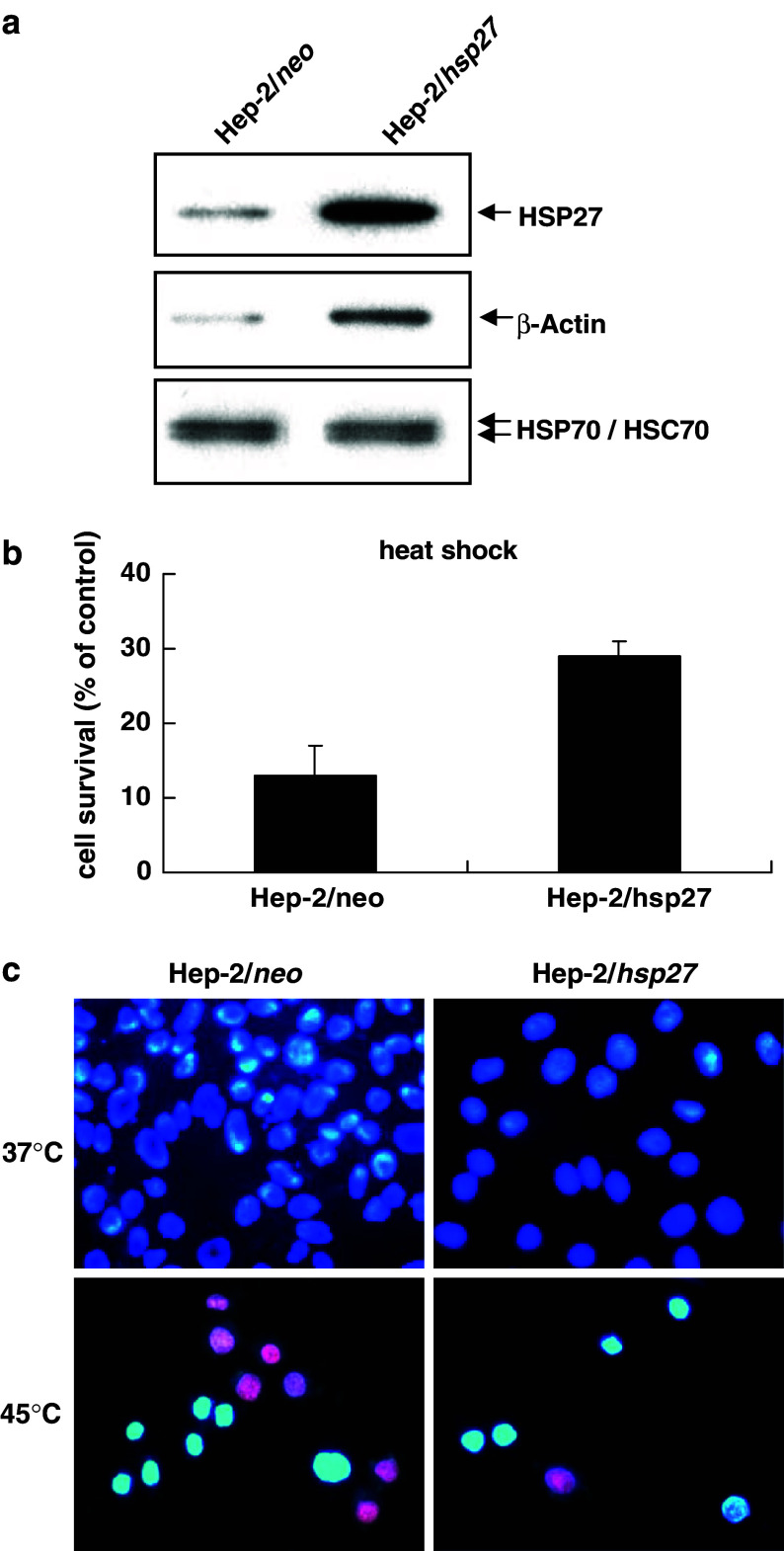
To examine the effect of HSP27 on the susceptibility of Hep-2 laryngeal cancer cells against apoptotic stimuli, we prepared HSP27-overexpressing Hep-2 cells (Hep-2/hsp27). The level of HSP27 expression was examined by Western blot analysis in Hep-2/neo and Hep-2/hsp27 cells. As shown in Fig. 1a, the Hep-2/hsp27 cells showed about eightfold higher expression of HSP27 than the Hep-2/neo cells. Figure 1a also shows that there was no difference in the endogenous level of HSP70/HSC70 between these cells. Therefore, the effect of other HSPs on the responses of the Hep2/neo and Hep2/hsp27 cells against various cytotoxic stimuli can be excluded. In contrast, the level of actin, the polymerization of which is known to be regulated by HSP27 (Lavoie et al. 1993), was increased in Hep-2/hsp27 cells. The stable cells were then tested for resistance to heat shock exposure at 45°C for 60 min. After a 24 h period of recovery, relative cell survival was determined by the MTT method as described in Materials and methods (Fig. 1b). As expected, the survival of Hep-2/hsp27 cells was about twofold higher than that of Hep-2/neo cells, 29% and 13%, respectively. Resistance to heat shock was also confirmed by PI and Hoechst 33258 staining (Fig. 1c). The nuclei of live cells are shown in bright blue, but the nuclei of dead cells are red in color. Most of the Hep-2/hsp27 cells showed bright blue fluorescent condensed nuclei (Fig. 1c right panel), in contrast, Hep-2/neo cells showed about equal amounts of blue and red nuclei (Fig. 1c left panel). These results indicate that the over expression of HSP27 induces thermo resistance and causes an increase in the expression of actin in Hep-2 laryngeal cancer cells.
Fig. 1.
Overexpression of HSP27 increases thermotolerence in Hep-2 cells. a Hep-2 cells were transfected with pCXN or pCXN-hsp27 using the lipofectAMINE reagent, and selected in 1 mg/ml of G418 containing medium for 3 weeks. Protein extracts of Hep-2/hsp27 and Hep-2/neo cells were prepared and assessed by Western blot analysis using Hsp-27, β-Actin and HSP70/HSC70 antibody. b Hep-2/neo and Hep2/hsp27 cells were exposed to 45°C for 60 min. After a 24 h period of recovery, relative cell survival was determined by an MTT assay. Results are mean ± SD of three independent experiments (P < 0.001). c Thermotolerence was also observed by fluorescence microscope after Hoechst 33258 and PI staining
Effect of HSP27 overexpression on the survival of Hep-2 cells to various apoptotic stimuli
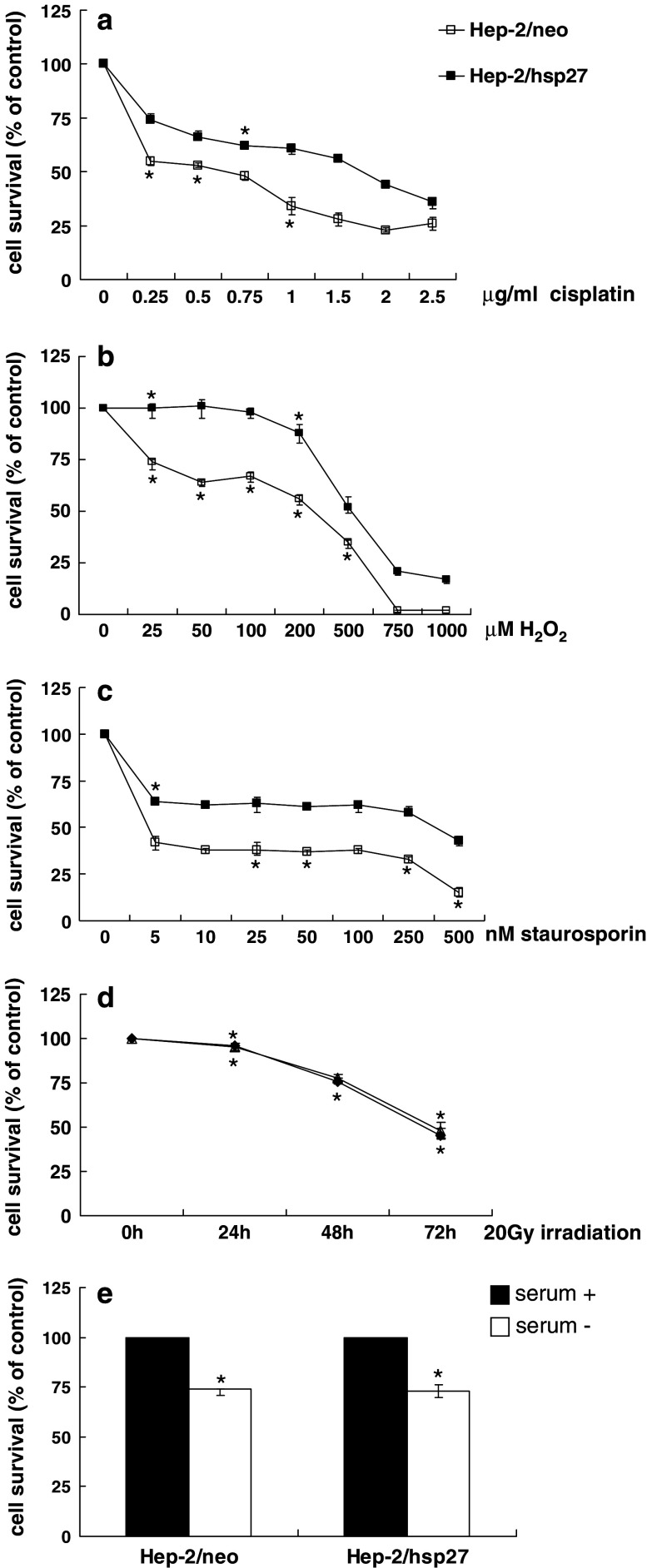
Since small HSPs have been shown to induce resistance against various stresses in many cell types, we determined whether the expression of HSP27 provides chemoresistance in Hep-2 cells where the effect of HSP27 has not yet been determined. Hep-2/neo and Hep-2/hsp27 cells were exposed to various concentrations of cisplatin, H2O2 and staurosporin for 48 h (Fig. 2a–c). HSP27 overexpressing cells demonstrated a significant resistance in response to these treatments compared to control cells. However, no difference in survival was found among control and hsp27 transfected cells after irradiation with 20 Gy (Fig. 2d). Hep-2/neo and Hep-2/hsp27 cells also exhibited a similar survival rate when incubated in the absence of serum in the culture media (Fig. 2e). Collectively, these results indicate that the induction of HSP27 in Hep-2 cells does not provide cellular resistance against all the types of stress in that the overexpression of HSP27 is associated with resistance to heat shock and several cytotoxic agents but not with resistance to irradiation or serum starvation.
Fig. 2.
Effect of HSP27 overexpression on the cytotoxicity of various apoptotic stimuli in Hep-2 cells. For the determination of sensitivity to apoptotic stimuli, Hep-2/neo and Hep-2/hsp27 cells were plated in 96 well plates at a density of 1 × 104 in 100 μl of medium and then treated with the indicated doses of cisplatin (a), H2O2 (b), staurosporin (c) for 48 h. Cells were exposed to 20 Gy of irradiation over 72 h (d) or deprived with serum for 48 h (e). The relative cell survival was evaluated by means of an MTT assay. All assays were performed in triplicate, and the data were presented as average ± SEM value. P < 0.001, *P < 0.01, compared to the value of control
Retarded cell proliferation by HSP27 is associated with chemoresistance
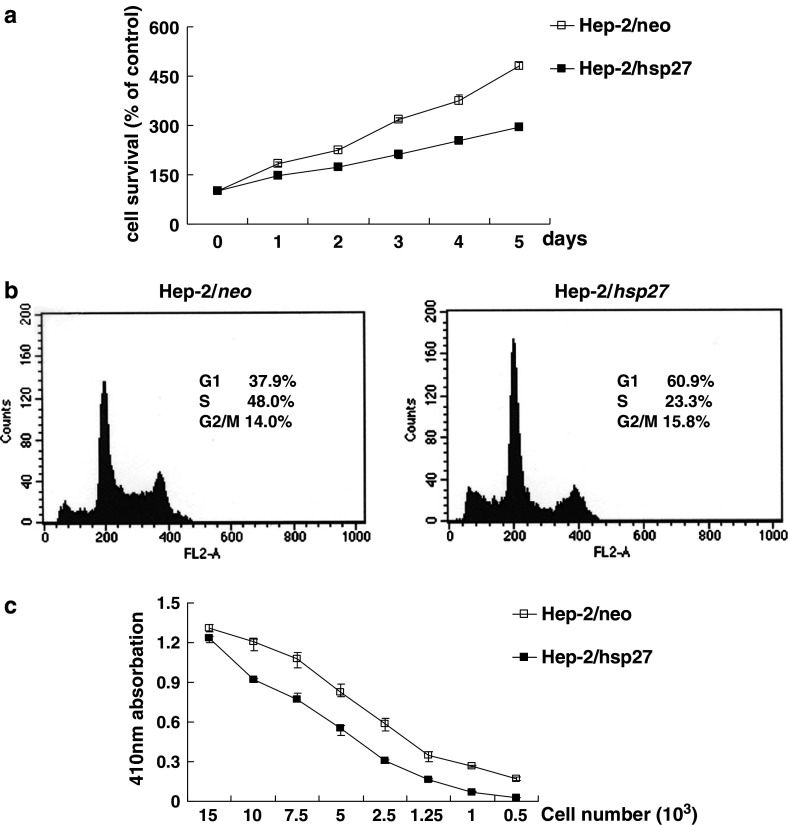
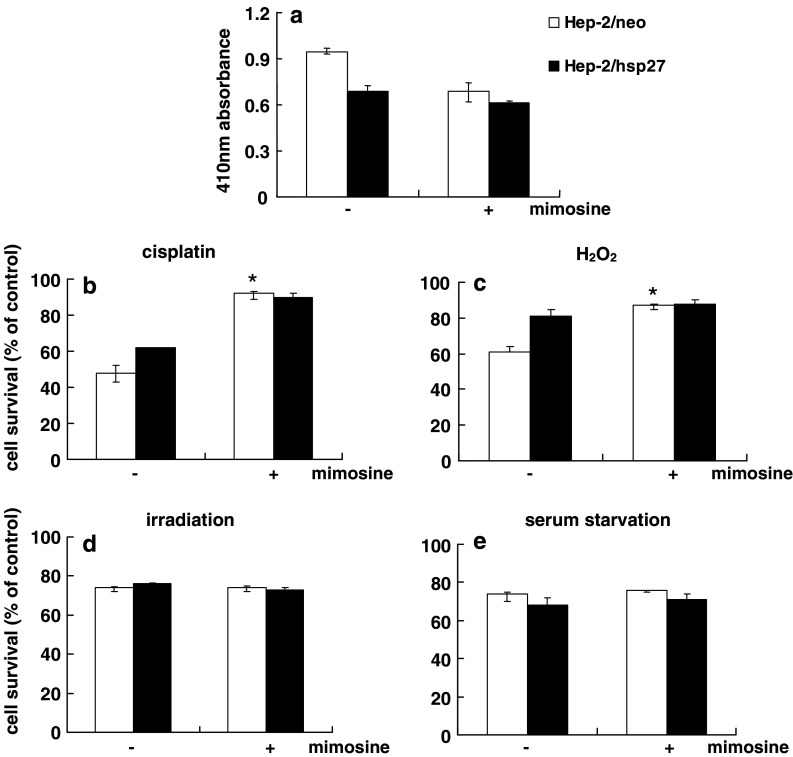
To determine whether the overexpression of HSP27 influences the growth properties of Hep-2 cells, we determined the growth rate of these two types of cells over a 5-day period. From Fig. 3a, it can be seen that Hep-2/hsp27 cells show a significant lower proliferation rate than hep-2/neo cells, as evidenced by a trypan blue exclusion assay, a 2.9 fold and 4.8 fold increase at day-5, respectively, compared to the cell number at day 0. Moreover, Fig. 3b shows that the proportion of Hep-2/hsp27 cells in the G1 phase was higher than that of Hep-2/neo cells, 60.9 and 37.9%, respectively, in the flow cytometric analysis, indicating that the lower proliferation rate in HSP27-overexpressing cells is due to this G1 arrest. A BrdU incorporation assay also demonstrated that the rate of DNA synthesis in Hep-2/hsp27 cells is lower than that of Hep-2/neo cells (Fig. 3c). These results suggest that the delayed cell proliferation might be associated with the chemoresistance of HSP27 in Hep-2 cells. To confirm this assumption, control Hep-2 cells were pretreated with mimosine, which is known to induce cell cycle arrest at the G1/S-phase boundary (Vackova et al. 2003), followed by a determination of cell survival against several cytotoxic agents. As shown in Fig. 4a, treatment of Hep-2/neo cells with mimosine resulted in a decrease in the incorporation of BrdU, by about 35%, reflecting a decreased population of cells in the S phase, comparable with that for mimosine untreated Hep-2/hsp27 cells. When these two cells were further exposed to 1 μg/ml of cisplatin (Fig. 4b), or 200 nM of H2O2 (Fig. 4c), a significant increase in survival was observed in mimosine-treated Hep-2/neo cells compared to the absence of mimosine treatment. However, when these cells were exposed to 20 Gy of irradiation (Fig. 4d) or incubated under serum deprived conditions (Fig. 4e), where no difference was observed in cell survival between control and HSP27-overexpressing Hep-2 cells (Fig. 2), no protective effect was provided by mimosine pretreatment. These results strongly support that the protective effect of HSP27 against several cytotoxic agents is based, at least in part, on the delay in growth arrest in the cell cycle progress.
Fig. 3.
Inhibition of cell proliferation by HSP27. a Hep-2/neo and Hep-2/hsp27 cells were incubated for the indicated times. At each time, viable cells were counted by the trypan blue dye exclusion method (P < 0.001). b Flow cytometric cell cycle analysis. Hep-2/neo and Hep-2/hsp27 cells were stained for DNA by PI, and stained cells were analyzed by flow cytometry using the ModFit DNA analysis program and CellQuest software. c Hep-2/neo and Hep-2/hsp27 cells were plated in 100 μl/well culture medium with the indicated cell numbers. After a 24 h incubation, BrdU was added and its incorporation was determined according to the manufacturer’s instructions. Error bars indicate the mean ± SD from three independent experiments (P < 0.05)
Fig. 4.
Inhibition of cell proliferation by HSP27 is associated with chemoresistance. a Hep-2/neo and Hep-2/hsp27 cells were plated and treated with 400 μM mimosine. After 20 h of incubation, BrdU incorporation assay was performed to confirm the synchronization at G1 phase. Hep-2/neo and Hep-2/hsp27 cells were pretreated with mimosine, which is known to induce cell cycle arrest at the G1/S phase boundary, followed by the determination of cell survival against 1 μg/ml cisplatin (b), 200 nM H2O2 (c), 20 Gy irradiation (d) or serum starvation (e) using an MTT assay. P < 0.005, *P < 0.05, compared to the value of control
Increased polymerization of actin is involved in the protective effect of HSP27 on several chemotherapeutic agents
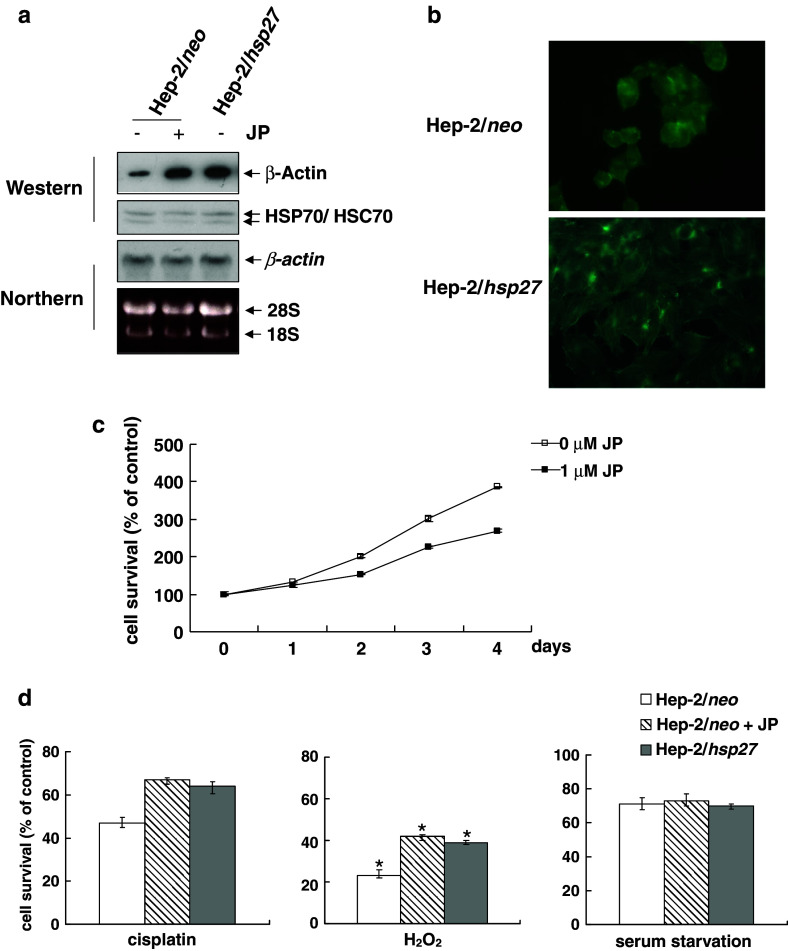
Cytoskeletal integrity is required to progress through G1 to S pass the G1/S in many cell types (Trielli et al. 1996; Bohmer et al. 1996; Reshetnikova et al. 2000). In order to test the possibility that the cell cycle delay in Hep-2/hsp27 cells is related to the increased levels of actin (Fig. 1a), we first examined whether the increased actin protein levels could be attributed to the increased synthesis or the increased polymerization of actin. A Northern blot assay revealed no difference in mRNA levels of actin between Hep-2/neo and Hep-2/hsp27 cells (Fig. 5a), indicating that the transcription rate of actin is not increased in Hep-2/hsp27 cells. When Hep-2/neo cells were treated with Jasplakinolide, an actin polymerizing agent (Bubb et al. 1994; Wei et al. 2001), actin protein levels were significantly enhanced in a Western blot assay, comparable to those in Hep-2/hsp27 cells (Fig. 5a). Immunofluorescent staining for F-actin, polymerized form of actin fillament, also showed a more prominent pattern of F-actin filament at the cortex and of F-actin fiber in the cytoplasm in Hep-2/hsp27 cells than in Hep-2/neo cells (Fig. 5b). Thus, the overexpression of HSP27 appears to increase the stability of actin microfilament, resulting in an increase in overall F-actin levels. To test if the induction of polymerization of actin affects the cell growth, we then treated Hep-2/neo cells with Jasplakinolide, and examined cell growth rate. As shown in Fig. 5c, the growth of Hep-2/neo cells was retarded by the treatment of 1 μM Jasplakinolide, at which actin polymerization was efficiently induced (Fig. 5a). Furthermore, when cisplatin or H2O2 treatment followed the Jasplakinolide pretreatment, the Hep-2/neo cells showed a recovery in cell survival equivalent to the levels of Hep-2/hsp27 cells. However, as in the mimosine-treated case, Jasplakinolide had no effect on the serum starved condition in Hep-2/neo cells (Fig. 5d). Our results, taken together, indicate that the stabilization of F-actin induced by HSP27 affects cell cycle progress, which is related, in part, with the protective ability of HSP27 against apoptosis induced by several agents.
Fig. 5.
Actin polymerization by HSP27 is related with chemoresistance. a Hep-2/neo and Hep-2/hsp27 cells were treated with or without 1 μM of Jasplakinolide (JP) for 3 h. The protein and mRNA expression of β-actin were analyzed by Western (upper panel) and Northern blot analysis (lower panel). b Hep-2/neo and Hep-2/hsp27 cells were fixed and stained using FITC-conjugated phalloidin as described under Materials and methods. The polymerized actin was observed by fluorescence microscope. c Hep-2/neo cells were treated with 1 μM of JP for the 3 h followed by the indicated recovery times in normal medium. The cell viability was determined by the MTT assay. d Hep-2/neo and Hep-2/hsp27 cells were pretreated with 1 μM of JP followed by the determination of cell survival against 1 μg/ml cisplatin (left), 200 nM H2O2(center) or serum starvation (right) using an MTT assay. P < 0.005, *P < 0.05, compared to the value of control (n = 3)
Discussion
In the present study, we showed that in addition to thermoresistance, the overexpression of HSP27 in Hep-2 laryngeal cancer cells confer chemoresistance against cisplatin, staurosporin and H2O2 (Figs. 1, 2). However, the overexpression of HSP27 has no effect on cell death caused by irradiation or serum-deprivation (Fig. 2). Therefore, the mechanisms by which chemotoxic agents induce apoptosis might be different from the mechanisms by which radiation or serum starvation induces cell death, depending on whether they are affected by HSP27 or not. The difference in susceptibility to cisplatin and irradiation in HSP27 overexpressing Hep-2 cells observed in our study could reflect one of the bases for the variable relation of HSP27 expression and the prognosis in laryngeal cancers (Orus et al. 2000; Leon et al. 2001, 2005).
The critical mechanisms by which HSP27 exhibit cytoprotective activity have not yet been clearly elucidated. Recently, it has been demonstrated that HSP27 prevents the activation of procaspase-9 by a specific interaction with cytochrome c and the subsequent inhibition of apoptosome formation (Bruey et al. 2000; Concannon et al. 2001). In addition to the chaperon activity, some post-translational modifications of HSP27 such as phosphorylation or methylglyoxal modification have been also suggested as possible explanations for the ability of HSP27 to inhibit apoptotic pathways (Lavoie et al. 1993; Sakamoto et al. 2002). Our results indicate that HSP27 induced a significant growth retardation in Hep-2 cells as determined by a cell cycle analysis as well as a BrdU incorporation assay (Fig. 3). The treatment of Hep-2/neo cells with mimosine, which induces cell cycle arrest at the G1 phase, confers resistance to cisplatin and H2O2 but not to irradiation or serum-deprivation, correlating the resistance provided by HSP27 (Fig. 4). Furthermore, it has recently been reported that the confluence dependent resistance of Hep-2 cells to drugs is mediated by HSP27 overexpression (Mazurov et al. 2003). Taken together, the delay in cell cycle progress through G1 to S phase appears to be related to the cytoprotective activity of HSP27.
DNA damaging agents such as cisplatin are especially effective for cells in the S phase (Schwartz 1989). Thus, the accumulation of cells in the G1 phase would lead to a decrease in the population in S phase cells that are susceptible to DNA damaging agents. Another possible explanation for the relation between cell cycle delay and a cytoprotecitive effect is that the synthesis of effector molecules, if present, which mediate the apoptotic signal might also be affected by a delay in the cell cycle, leading to an insufficient execution of cell death program. However, the expression of HSP27 in L929 cells resulted in an increased sensitivity to TNF-α, Etoposide, and H2O2 even though HSP27 induces a growth delay in these cells (Mairesse et al. 1998). These findings, along with the results reported herein, imply that growth inhibition induced by HSP27 cannot necessarily be extended to protection against to all the types of cellular stress.
Very little is currently known concerning the mechanism by which HSP27 induces a delay in cell cycle progression. In addition to functioning as a molecular chaperone, HSP27 has also been shown to regulate actin polymerization depending its phosphorylation state (Lavoie et al. 1993; Butt et al. 2001). The cytoprotective effect of HSP27 has also been attributed to its capacity to bind to and stabilize the polymerization of actin (Wang and Spector 1996; Hino et al. 2000). Our results indicate that the increase in the amount of F-actin stress fiber in Hep-2/hsp27 cells compared to Hep-2/neo cells (Fig. 5b). Considering the importance of actin filaments as part of the cytoskeleton for the proliferation and differentiation (Kubler et al. 1991; Launay et al. 2003; Searler et al. 2004), the accumulated F-actin fiber might disturb the balance between polymerized F-actin and soluble G-actin, which can be monitored by the cytokinesis check point (McMillan et al. 1998), slowing the progression through the G1 to the phase. Supporting this assumption, an induction of polymerization of actin by Jasplakinolide resulted in both the retardation of growth of Hep-2/neo cells and an increased survival against cisplatin and H2O2 but not against serum deprivation (Fig. 5c), as observed in Hep-2/hsp27 cells or mimosine treated Hep-2/neo cells (Figs. 2, 4). Therefore, the increased F-actin fibers observed in our studies might, at least partly, explain the delayed cell cycle progress and the cytoprotective activity of HSP27. However, the possibility that an increase in actin polymerization is not the cause of cell cycle retardation, but an accompanying phenomenon of delay in cytokinesis, cannot be excluded.
In conclusion, our findings indicate that the overexpression of HSP27 induces the inhibition of cell growth, which seems to be related with resistance to several cytotoxic compounds not with cytoprotecitve effect against irradiation or serum starvation in Hep-2 cells. Although the stabilization of F-actin filaments is suggested to be one of the possible bases for the delay in cell cycle progression, further studies will be required to define the biochemical properties of HSP27, which is responsible for the regulation of actin dynamics as well as cell cycle progression.
Acknowledgments
This work was supported, in part, by grants from the alumni associated of the Department of Otolaryngology Head and Neck Surgery, The Catholic University of Korea.
Footnotes
Jung-Hee Lee and Dongil Sun have contributed equally to this work.
References
- Bohmer RM, Scharf E, Assoian RK (1996) Cytoskeletal integrity is required throughout the mitogen stimulation phase of the cell cycle and mediates the anchorage-dependent expression of cyclin D1. Mol Biol Cell 7:101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C (2000) Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol 2(9):645–652 [DOI] [PubMed] [Google Scholar]
- Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED (1994) Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem 269(21):14869–14871 [PubMed] [Google Scholar]
- Butt E, Immler D, Meyer HE, Kotlyarov A, Laass K, Gaestel M (2001) Heat shock protein 27 is a substrate of cGMP-dependent protein kinase in intact human platelets: phosphorylation-induced actin polymerization caused by HSP27 mutants. J Biol Chem 276:7108–7113 [DOI] [PubMed] [Google Scholar]
- Chae YJ, Kim HS, Rhim H, Kim BE, Jeong SW, Kim IK (2001) Activation of caspase-8 in 3-deazaadenosine-induced apoptosis of U-937 cells occurs downstream of caspase-3 and caspase-9 without Fas receptor-ligand interaction. Exp Mol Med 33:284–292 [DOI] [PubMed] [Google Scholar]
- Concannon CG, Orrenius S, Samali A (2001) Hsp27 inhibits cytochrome c-mediated caspase activation by sequestering both pro-caspase-3 and cytochrome c. Gene Expr 9:195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin A, Raybaud-Diogene H, Tetu B, Deschenes R, Huot J, Landry J (2000) Overexpression of the 27 KDa heat shock protein is associated with thermoresistance and chemoresistance but not with radioresistance. Int J Radiat Oncol Biol Phys 46:1259–1266 [DOI] [PubMed] [Google Scholar]
- Gandour-Edwards R, Trock BJ, Gumerlock P, Donald PJ (1998) Heat shock protein and p53 expression in head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg 118:610–615 [DOI] [PubMed] [Google Scholar]
- Hino M, Kurogi K, Okubo MA, Murata-Hori M, Hosoya H (2000) Small heat shock protein 27 (HSP27) associates with tubulin/microtubules in HeLa cells. Biochem Biophys Res Commun 271:164–169 [DOI] [PubMed] [Google Scholar]
- Kubler MD, Jordan PW, O’Neill CH, Watt FM (1991) Changes in the abundance and distribution of actin and associated proteins during terminal differentiation of human epidermal keratinocytes. J Cell Sci 100(Pt 1):153–165 [DOI] [PubMed] [Google Scholar]
- Launay S, Brown G, Machesky LM (2003) Expression of WASP and Scar1/WAVE1 actin-associated proteins is differentially modulated during differentiation of HL-60 cells. Cell Motil Cytoskeleton 54(4):274–285 [DOI] [PubMed] [Google Scholar]
- Lavoie JN, Hickey E, Weber LA, Landry J (1993) Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem 268:24210–24214 [PubMed] [Google Scholar]
- Leon X, Quer M, Orus C, Lopez M, Gras JR, Vega M (2001) Results of salvage surgery for local or regional recurrence after larynx preservation with induction chemotherapy and radiotherapy. Head Neck 23:733–738 [DOI] [PubMed] [Google Scholar]
- Leon X, Lopez-Pousa A, de Vega M, Orus C, de Juan M, Quer M (2005) Results of an organ preservation protocol with induction chemotherapy and radiotherapy in patients with locally advanced laryngeal carcinoma. Eur Arch Otorhinolaryngol 262:93–98 [DOI] [PubMed] [Google Scholar]
- Lindquist S (1986) The heat-shock response. Annu Rev Biochem 55:1151–1191 [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22:631–777 [DOI] [PubMed] [Google Scholar]
- Liu FF, Miller N, Levin W, Zanke B,Cooper B, Henry M, Sherar MD, Pintilie M, Hunt JW, Hill RP (1996) The potential role of HSP70 as an indicator of response to radiation and hyperthermia treatments for recurrent breast cancer. Int J Hyperthermia 12:197–208 [DOI] [PubMed] [Google Scholar]
- Mairesse N, Bernaert D, Del Bino G, Horman S, Mosselmans R, Robaye B, Galand P (1998) Expression of HSP27 results in increased sensitivity to tumor necrosis factor, etoposide, and H2O2 in an oxidative stress-resistant cell line. J Cell Physiol 177:606–617 [DOI] [PubMed] [Google Scholar]
- Mazurov VV, Solovieva ME, Leshchenko VV, Kruglov AG, Edelweiss EF, Yakubovskaya RI, Akatov VS (2003) Small heat shock protein hsp27 as a possible mediator of intercellular adhesion-induced drug resistance in human larynx carcinoma HEp-2 cells. Biosci Rep 23:187–197 [DOI] [PubMed] [Google Scholar]
- McMillan JN, Sia RA, Lew DJ (1998) A morphogenesis checkpoint monitors the actin cytoskeleton in yeast. J Cell Biol 142(6):1487–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakopoulou L, Lazaris AC, Baltas D, Giannopoulou I, Kavantzas N, Tzonou A (1995) Prognostic evaluation of oestrogen-regulated protein immunoreactivity in ductal invasive (NOS) breast cancer. Virchows Arch 427:33–40 [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J (1991) Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199 [DOI] [PubMed] [Google Scholar]
- Orus C, Leon X, Vega M, Quer M (2000) Initial treatment of the early stages (I, II) of supraglottic squamous cell carcinoma: partial laryngectomy versus radiotherapy. Eur Arch Otorhinolaryngol 257:512–516 [DOI] [PubMed] [Google Scholar]
- Parcellier A, Schmitt E, Brunet M, Hammann A, Solary E, Garrido C (2005) Small heat shock proteins HSP27 and alphaB-crystallin: cytoprotective and oncogenic functions. Antioxid Redox Signal 7:404–413 [DOI] [PubMed] [Google Scholar]
- Paulus JA, Tucker RD, Flanagan SW, Moseley PL, Loening SA, Park JB (1993) Heat shock protein response in a prostate tumor model to interstitial thermotherapy: implications for clinical treatment. Prostate 23:263–270 [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Cohen IR (2005) Heat shock proteins as endogenous adjuvants in sterile and septic inflammation. J Immunol 175:2777–2782 [DOI] [PubMed] [Google Scholar]
- Reshetnikova G, Barkan R, Popov B, Nikolsky N, Chang LS (2000) Disruption of the actin cytoskeleton leads to inhibition of mitogen-induced cyclin E expression, Cdk2 phosphorylation, and nuclear accumulation of the retinoblastoma protein-related p107 protein. Exp Cell Res 259:35–53 [DOI] [PubMed] [Google Scholar]
- Richards EH, Hickey E, Weber L, Master JR (1996) Effect of overexpression of the small heat shock protein HSP27 on the heat and drug sensitivities of human testis tumor cells. Cancer Res 56:2446–2451 [PubMed] [Google Scholar]
- Sakamoto H, Mashima T, Yamamoto K, Tsuruo T (2002) Modulation of heat-shock protein 27 (Hsp27) anti-apoptotic activity by methylglyoxal modification. J Biol Chem 277(48):45770–45775 [DOI] [PubMed] [Google Scholar]
- Schwartz JL (1989) monofunctional alkylating agent-induced S-phase-dependent DNA damage. Mutat Res 216(2):111–118 [DOI] [PubMed] [Google Scholar]
- Searles CD, Ide L, Davis ME, Cai H, Weber M (2004) Actin cytoskeleton organization and posttranscriptional regulation of endothelial nitric oxide synthase during cell growth. Circ Res 95(5):488–495 [DOI] [PubMed] [Google Scholar]
- Sugerman PB, Savage NW, Xu LJ, Walsh LJ, Seymour GJ (1995) Heat shock protein expression in oral epithelial dysplasia and squamous cell carcinoma. Eur J Cancer B Oral Oncol 31B:63–67 [DOI] [PubMed] [Google Scholar]
- Trielli MO, Andreassen PR, Lacroix FB, Margolis RL (1996) Differential Taxol-dependent arrest of transformed and nontransformed cells in the G1 phase of the cell cycle, and specific-related mortality of transformed cells. J Cell Biol 135:689–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozaki H, Horiuchi H, Ishida T, Iijima T, Imamura T, Machinami R (1997) Overexpression of resistance-related proteins (metallothioneins, glutathione-S-transferase pi, heat shock protein 27, and lung resistance-related protein) in osteosarcoma. Relationship with poor prognosis. Cancer 79:2336–2344 [DOI] [PubMed] [Google Scholar]
- Vackova I, Engelova M, Marinov I, Tomanek M (2003) Cell cycle synchronization of porcine granulosa cells in G1 stage with mimosine. Anim Reprod Sci 77(3–4):235–245 [DOI] [PubMed] [Google Scholar]
- Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR (1998) Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer 79:468–475 [DOI] [PubMed] [Google Scholar]
- Walsh D, Grantham J, Zhu XO, Wei Lin J, van Oosterum M, Taylor R, Edwards M (1999) The role of heat shock proteins in mammalian differentiation and development. Environ Med 43:79–87 [PubMed] [Google Scholar]
- Wang K, Spector A (1996) Alpha-crystallin stabilizes actin filaments and prevents cytochalasin-induced depolymerization in a phosphorylation-dependent manner. Eur J Biochem 242:56–66 [DOI] [PubMed] [Google Scholar]
- Wei L, Wang L, Carson JA, Agan JE, Imanaka-Yoshida K, Schwartz RJ (2001) Beta1 integrin and organized actin filaments facilitate cardiomyocyte-specific RhoA-dependent activation of the skeletal alpha-actin promoter. FASEB J 15(3):785–796 [DOI] [PubMed] [Google Scholar]
- Welch WJ (1993) Heat shock proteins functioning as molecular chaperones: their roles in normal and stressed cells. Philos Trans R Soc Lond B Biol Sci 339:327–333 [DOI] [PubMed] [Google Scholar]