Abstract
Purpose
Ansaplastic thyroid cancer (ATC) is one of the most lethal malignancies, but the carcinogenic mechanism of ATC has not been clarified. Recently, we performed a cDNA microarray analysis and identified transmembrane protein 34 (TMEM34) that down-regulated in anaplastic thyroid cancer cell lines (ACL)s as compared to normal thyroid tissues.
Methods
To investigate the role of TMEM34 in ATC carcinogenesis, we examined expression levels of TMEM34 in ACLs as well as differentiated thyroid cancers (DTC)s and normal human tissues. To explore the effect of TMEM34 in ATC development, cell-growth assays with KTA2 cells were performed.
Results
Expression of TMEM34 was down-regulated in all 11 ACLs, as compared to either normal thyroid tissues or cell lines derived from papillary or follicular thyroid cancers. TMEM34 was expressed ubiquitously in normal human tissues tested. Transfection of TMEM34 into KTA2 cells led to inhibition of cell growth.
Conclusions
Our findings suggest that TMEM34 might be a tumor suppressor gene, associated with the development of ATC from DTC.
Keywords: Anaplastic thyroid cancer, cDNA microarray, Gene expression, Molecular marker, TMEM34
Introductions
Thyroid cancers can be classified into papillary (PTC), follicular (FTC), anaplastic [Ansaplastic thyroid cancer (ATC)], or medullary (MTC) types. On the basis of clinicopathological observations, most ATCs appear to arise from transformation of differentiated thyroid cancers (DTC)s of papillary or follicular type (Venkatesh et al. 1990; Demeter et al. 1991; Passler et al. 1999; Wiseman et al. 2003). However, the genetic mechanism of anaplastic transformation of DTC to ATC remains unclear. We have been attempting to identify prognostic markers and drug-target molecules in ATC by cDNA microarray technology, and lately have discovered specific changes in expression of several genes in ATCs and in cell lines derived from anaplastic thyroid cancers (ACL)s (Onda et al. 2004).
In this paper, we describe a novel member of the group identified by expression profiling, the gene encoding transmembrane protein 34 (TMEM34), by focusing on anonymous transcribed elements like hypothetical genes and ESTs. The official symbol is TMEM34; aliases are HGNC 25587 and FLJ10846, with NCBI accession number NM_018241. Its chromosomal location is 4q31.21. The coding sequence is 1,317 bp and mRNA consists of 2,913 bp according to the NCBI “Gene” database. A full-length cDNA of this gene was cloned in 2001 and sequenced from human fetal-liver tissue at 22 weeks of gestation (Yu et al. 2001). However, the function of TMEM34 has not been described nor has any association between this gene and human cancers been reported previously. In this study, we investigated the mechanism of down-regulation of TMEM34 in thyroid-cancer cells and performed functional analysis.
Materials and methods
Cell lines
Eleven cell lines derived from human anaplastic thyroid cancers were used for this study: 8305c, 8505c, ARO, FRO, TTA1, TTA2, TTA3, KTA1, KTA2, KTA3, and KTA4. Lines 8305c and 8505c were maintained in Dulbecco’s Modified Eagle Medium (Invitrogen, Carlsbad, CA, USA) and ARO and FRO were maintained in minimum essential medium (MEM). The other seven lines were maintained in RPMI 1640. All media contained 10% fetal bovine serum (FBS) without antibiotics. Follicular thyroid cancer cells (WRO) and papillary thyroid cancer cells (NPA) were also cultured in RPMI 1640 with 10% FBS, in a 37°C incubator under 5% CO2 atmosphere.
Specimens for normal control
Normal thyroid-gland tissues were obtained from patients undergoing surgery for papillary thyroid cancer. All samples came from the Ito Hospital, Tokyo, after informed consent was obtained from each patient.
Semiquantitative RT-PCR (SQ-PCR)
cDNA was reverse-transcribed from 10 μg of each total RNA in the usual manner. To adjust the amount of transcribed cDNA, GAPDH was selected as an internal control and SQ-PCR was performed as previously described (Onda et al. 2005); the primer sequences for GAPDH were 5′-ggaaggtgaaggtcggagt-3′ (forward) and 5′-tgggtggaatcatattggaa-3′ (reverse). After adjustment of concentrations, SQ-PCR experiments for TMEM34 were performed using primers 5′-ggcttggtttattgctggaa-3′ (forward) and 5′-gtgtgggcccaatattcatc-3′ (reverse); all primers were designed with primer 3 (http://www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi) after sequence information was obtained from NCBI GenBank (http://www.ncbi.nlm.nih.gov/). Each SQ-PCR experiment was performed with 1 ul of cDNA for template, 5 U of Takara EX Taq (Takara, Otsu, Japan), 1× PCR buffer (10 mM Tris-HCl, 50 mM KCl, 1.5 mM MgCl2), 10 nM dNTPs, and 10 pmol each of the forward and reverse primer in 30 ul of total reaction mixture. PCR conditions were 94°C for 2 min as the denaturing step, 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s for 30 cycles in a Thermal Cycler PTC-200 (MJ Research Inc., Waltham, MA).
For comparison of gene expression between ACLs and normal thyroid-gland tissues, a 2.0% agarose gel was used to separate 10 ul of each SQ-PCR product, which was visualized by ethidium bromide staining. The band intensity for each sample was measured by AlphaImager 3300 (AlphaInonotech, San Leandro, CA) after subtraction of background. A 16-bit imaging score was acquired from each sample. All SQ-PCR experiments were done in duplicate.
Quantitative RT-PCR (Q-PCR)
Q-PCR experiments were performed with an ABI PRISM 7700 Sequence Detector (Applied Biosystems, Foster City, CA, USA) according to the comparative-threshold (Ct) cycle method, as previously described (Onda et al. 2005). All quantitative RT-PCR products were visualized on a 2% agarose gel to ensure a single product. A difference in expression between normal thyroid tissue and sample X of an ACL is defined as follows:
 |
(Ct-TMEM34, GAPDH are threshold cycles for amplification of TMEM34 and GAPDH, respectively)
 |
(Average of Ct of five normal thyroid tissues)
 |
Expression ratio of TMEM34 to normal thyroid tissue (sample X/average of five normal thyroid tissues) = 2(-Ct X)
Expression of TMEM34 in normal human tissues
To confirm the expression status of TMEM34 in human organs containing thyroid tissue, we performed SQ-PCR experiments with Human MTC panels 1 and 2 (BD Bioscience, Palo Alto, CA, USA), using the SQ-PCR conditions described above.
Transformation of plasmid TMEM34
A TMEM34 expression vector, pcDNA TMEM34, was constructed using a pcDNA3.1 directional TOPO expression kit (Invitrogen, Carlsbad, CA, USA) as described previously (Onda et al. 2005). To generate a full-length coding sequence of TMEM34, PCR was performed using the cloning forward primer: 5′-caccatgccttgcacttgtacctgga-3′ and the reverse primer: 5′-ggaatccacggatttatct-3′. The PCR product of 1,318 bp contained a CACC sequence in front of the ATG start codon, and lacked the TAG stop codon present in the original TMEM34 sequence. After chemical transformation, the proper insert was confirmed by colony PCR with a combination of T7 forward primer (5′-taatacgactcactataggg-3′) and a sequence that would bind within the insert as the reverse primer. After identifying clones with the correct sequence, colonies were cultivated in LB medium at 37°C, overnight and plasmid was extracted from them.
Transfection of TMEM34 to the KTA2 cell line
At first, we confirmed transfection of efficiency in KTA2 cell by lipofectamine assays. The day before transfection, 3,000 KTA2 cells were plated in a 24-well plate and cultured under the conditions described previously; the cells reached 60% confluence after 24 h. The medium was replaced prior to transfection. Then, 400 ng of lacZ were mixed up to 50 ul of Opti-MEM I medium (Invitrogen, Carlsbad, CA) and cultured with the same conditions for 48 h. After staining cells β-gal, we estimated transfection efficiency of KTA2 cells.
Next, to achieve a pcDNA TMEM34/XP-1 complex, 200 ng of pcDNA TMEM34 was mixed in 50 μl of Opti-MEM I medium and siPORT XP-1 (Ambion, Austin, TX). The complex was transfected into a prepared KTA2 cell line. Cultured transfectants were collected and RNA was extracted with TRIzol (Invitrogen). Expression of TMEM34 was evaluated by SQ-PCR in the manner described above.
Cell-growth assay
To determine the effect of TMEM34 in ACLs, cell-growth assays (Smith et al. 2003) were performed. About 3,000 KTA2 cells were plated on 24-well plates and after 24 h, 200 ng of pcDNA TMEM34 was transfected. Cells were fixed with 10% formalin for 10 min at determined time points (days 1, 3, and 6 after transfection). After two washes with PBS, the fixed cells were stained with 0.1% crystal violet for 10 min, then washed three times with water to remove excess dye. Crystal violet was eluted from the stained cells with 200 μl of 10% acetic acid, and absorbance at 590 nm was measured with a spectrophotometer. Differences in cell growth were calculated with student’s t-tests, using Statview version 5.0. (SAS Institute Inc., Cary, NC, USA).
Screening for mutations in the TMEM34 gene by single-strand conformational polymorphism (SSCP)
To search for genomic alterations of the TMEM34 gene in thyroid cancers, we used SSCP to detect mutations in 11ACLs. PCR analysis of each exon of TMEM34 was performed as previously described (Onda et al. 2005), using the primers shown in Table 1. PCR products were denatured for 10 min at 95°C and placed immediately on ice, to prevent annealing of single-stranded products. Electrophoresis was carried out on a 6% Lone Ranger gel (Cambrex, East Rutherford, NJ, USA) with 5% glycerol, at 5W for 4 h. DNA fragments were stained with PlusOne DNA Silver Staining Kits (Pharmacia Biotech, Tokyo, Japan).
Table 1.
Primers for SSCP analysis of TMEM34 gene
| Name | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| TMEM34-Exon1 | acgaatcacctgcctcagac | gtacaggctgatggtgcaga |
| TMEM34-Exon2 | tggccaagctcttttgtttt | agccaacacaacagtagtggaa |
| TMEM34-Exon3 | aaatttctgttttccttttccaa | tgactccttgtcaataacatactttca |
| TMEM34-Exon4 | tgcaactatctttaacatctgtgct | tgcttaaatgaaaaaccaaaaca |
| TMEM34-Exon5 | ctttggggaaaatctgatgc | ttcccacataagatcacaagga |
| TMEM34-Exon6 | tgctgtattttgaagtacgattttg | aaaaagaagcaagccttatcct |
| TMEM34-Exon7 | gcctagaaaagtttgcctgaaa | caacggtttttactgccctta |
| TMEM34-Exon8 | ggtacacaggttcactgga | ccccataccctgtgtgttgt |
| TMEM34-Exon9 | tttaaatccaaaggtggctgtt | ttgccctagtaggagaaattcaa |
| TMEM34-Exon10 | taccacagtgcaggctcatc | tatggtctcaagcaca |
Results
Down-regulation of TMEM34 in ACLs
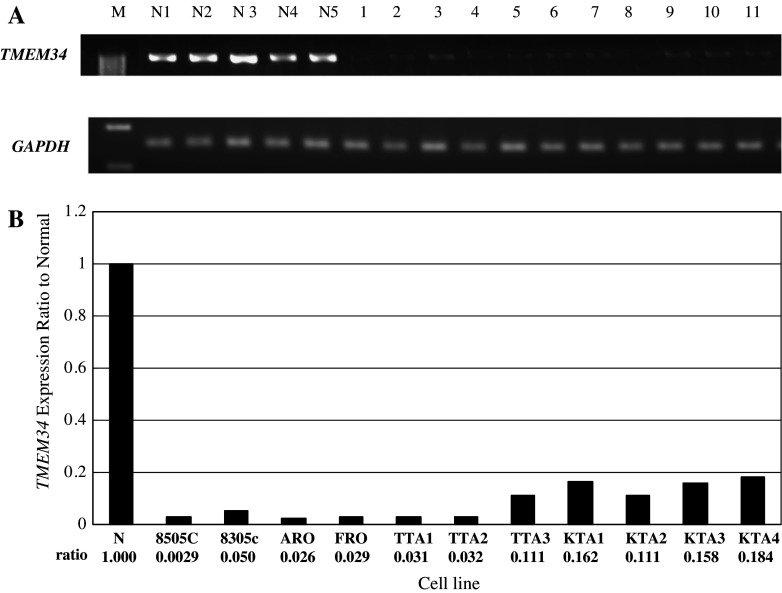
Expression of TMEM34 was significantly down regulated in ACLs as compared to normal thyroid (Fig. 1a). In addition, we performed quantitative RT-PCR, to assess the transcriptional differences in TMEM34 between the ACLs and normal thyroid, using the same panels of cells (Fig. 1b). Average expression ratios of GAPDH and TMEM34 revealed that TMEM34 was significant under-expressed in ACLs.
Fig. 1.
a Down-regulation of TMEM34 in ACLs as compared to normal thyroid tissues as shown by SQ-PCR. The lane designations are follows: M, size markers; N1-5, normal thyroid samples; 1, 8305c; 2, 8505c; 3, ARO; 4, FRO; 5, TTA1; 6, TTA2; 7, TTA3; 8, KTA1; 9, KTA2; 10, KTA3; 11, KTA4. b Results of quantitative RT-PCR. The average expression level of TMEM34 among five normal thyroid tissues was set at 1.00, then relative expression ratios were calculated between normal and cancerous tissue. TMEM34 expression ratios were below 20% of normal thyroid in all ACLs examined
We also examined expression of TMEM34 in cell lines derived from follicular thyroid cancers (WRO) and papillary thyroid cancers (NPA), to assess whether the loss of expression of TMEM34 was specific to ACLs. TMEM34 expression was significantly reduced in ACLs as compared to the differentiated thyroid-cancer cell lines (Fig. 2).
Fig. 2.
Expression of TMEM34 in cell lines derived from differentiated thyroid (follicular and papillary) cancers, WRO and NPA, respectively, as shown by SQ-PCR. The following are the lane designations: M, size marker; 1, WRO; 2, NPA; 3, ARO; 4, FRO; 5, 8505c; 6, 8305c
TMEM34 was expressed in normal human tissues tested: lung, kidney, spleen, pancreas, thymus, prostate, testis, ovary, small intestine, and thyroid (Fig. 3).
Fig. 3.
Expression of TMEM34 in normal human tissues. The following are the designations: M, size marker; 1, heart; 2, brain; 3, placenta; 4, lung; 5, liver; 6, skeletal muscle; 7, kidney; 8, spleen; 9, pancreas; 10, thymus; 11, prostate; 12, testis; 13, ovary; 14, small intestine; 15, colon; 16, leukocyte; 17, thyroid
Exogenous expression of pcDNA TMEM34 in the KTA2 cell line
To confirm expression of pcDNA TMEM34 prior to undertaking cell-growth assays, we evaluated expression of this gene by SQ-PCR, 72 h after transfection of pcDNA TMEM34 vector to KTA2 cells. TMEM34 was over-expressed significantly compared to the control, pcDNA empty vector (Fig. 4).
Fig. 4.
Exogenous expression of pcDNA TMEM34 in KTA2 cells, measured by SQ-PCR. Compared to control (pc DNA empty vector), cells containing the expression vector strongly over-expressed the gene 72 h after transfection. The following are the designations: M, size marker; 1–3, control; 4–6, pc-DNA TMEM34
To clarify the effect of TMEM34 on ACLs, we observed cell growth on days 1, 3 and 6 after transfection. The assays indicated statistically significant suppression of growth by day 6, compared to the control (P=0.0016, t-test, Fig. 5).
Fig. 5.
Cell-growth assay, showing absorbances of the cultures at 590 nm on days 1, 3, and 6 after transfection of 200 ng of pcDNA TMEM34 into KTA2 cells. Transfected cells revealed a significant reduction of growth compared to the control (P=0.0016 at day 6, t-test)
Mutation screening by single-strand conformational polymorphism (SSCP)
We used SSCP to examine exons 1–10 of TMEM34 for possible genomic alterations in 11 ACLs. The results for exons 5, 8 and 9 are shown in Fig. 6. No altered migration patterns were observed in any exons of the gene among the 11 ACL cell lines.
Fig. 6.
SSCP evaluation of exons 5, 8 and 9 of the TMEM34 gene in 3 normal thyroid tissues and 11 ACLs. No mutations were evident in any exon. The following are the designations: 1–3, normal thyroid samples; 4, 8305c; 5, 8505c; 6, ARO; 7, FRO; 8, TTA1; 9, TTA2; 10, TTA3; 11, KTA1; 12, KTA2; 13, KTA3; 14, KTA4
Discussion
Anaplastic thyroid cancer is one of the most aggressive human malignancies. ATC appears to be derived from follicular epithelial cells, arising mainly by transformation of pre-existing differentiated thyroid cancers (Venkatesh et al. 1990; Demeter et al. 1991; Passler et al. 1999; Wiseman et al. 2003). Molecular studies suggest that RAS oncogenes are frequently mutated in ATC (Lemoine et al. 1989; Stringer et al. 1989; Suarez 1998); mutations of p53 (Nakamura et al. 1992; Moretti et al. 1997) and beta-catenin (Garcia-Rostan et al. 1999) are also common in ATC and may be associated with anaplastic transformation. Furthermore, BRAF mutations are found in PTCs and in ATCs arising from PTC (Nikiforova et al. 2003; Quiros et al. 2005).
In this study, we analyzed 11 ACLs on a cDNA microarray and discovered several specific changes in gene expression, related to ATC carcinogenesis (Onda et al. 2004). TMEM34 was one of the genes down regulated in these cancer lines. TMEM34 had been cloned and sequenced from human fetal liver obtained at 22 weeks of gestation (Yu et al. 2001). The protein contains at least five transmembrane domains and one ER-membrane domain. The TMEM34 gene locates at 4q31, where loss of heterozygosity (LOH) has been detected in glioblastomas (Hu et al. 2003) and nasopharyngeal carcinomas (Shao et al. 2000). However, in a previous study, we were unable to detect LOH at 4q in primary ATCs (Kitamura et al. 2000).
We have shown here that TMEM34 expression is down regulated in ACLs as compared either to normal thyroid tissues or papillary or follicular thyroid-cancer cell lines, while the gene was expressed ubiquitously in normal human tissues from various organs. In view of these results, we suggest that loss of TMEM34 might be associated with the development of ATC from DTC or normal thyroid gland.
To confirm whether the activity of the TMEM34 gene product can suppress progression of ATC, we analyzed cell growth assays. At first, we confirmed transfection of efficiency in KTA2 cell by lipofectamine assays. The transfection efficiency of KTA2 cell was about 40% (data was not shown). Exogenous expression of TMEM34 suppressed KTA2 cell growth significantly compared to the control by day 6 (P=0.0016, t-test). In addition, we performed cell growth assay with KTA3 (anaplastic thyroid cancer) cell. Exogenous expression of TMEM34 tended to suppress KTA3 cell growth compared to the control, however, there were not statistically significant differences (data was not shown). The reasons were thought that differences of the transfection efficiency, characteristics and the rate of cell growth between KTA2 and KTA3. Judging from the microscopic observation, no morphological change of apoptosis was confirmed. So it is speculated that cell growth suppression was caused by cell cycle delay. We suggest that TMEM34 possesses tumor-suppressor activity.
In this study we were not able to examine LOH because normal paired DNA samples were not available. However, we did search for mutations within the gene by SSCP and found no altered migration patterns in any exons among 11 ACLs. Therefore the mechanism leading to down regulation of TMEM34 in ATCs requires further examination.
In conclusion, to our knowledge this is the first report of altered TMEM34 expression in human cancer. Our findings suggest that the product of TMEM34 might be a tumor suppressor whose loss is linked to development of ATC from DTC.
Acknowledgment
The authors thank to Drs. Mitsuji Nagahama and Kouichi Ito, Ito Hospital, Tokyo, Japan for providing thyroid materials.
References
- Demeter JG, De Jong SA, Lawrence AM, Paloyan E (1991) Anaplastic thyroid carcinoma: risk factors and outcome. Surgery 110:956–961 [PubMed] [Google Scholar]
- Garcia-Rostan G, Tallini G, Herrero A, D’Aquila TG, Carcangiu ML, Rimm DL (1999) Frequent mutation and nuclear localization of beta-catenin in anaplastic thyroid carcinoma. Cancer Res 59:1811–1815 [PubMed] [Google Scholar]
- Hu J, Jiang C, Ng HK, Pang JC, Tong CY, Chen S (2003) Genome-wide allelotype study of primary glioblastoma multiforme. Chin Med J (Engl) 116:577–583 [PubMed] [Google Scholar]
- Kitamura Y, Shimizu K, Tanaka S, Ito K, Emi M (2000) Allelotyping of anaplastic thyroid carcinoma: frequent allelic losses on 1q, 9p, 11, 17, 19p, and 22q. Genes Chromosomes Cancer 27:244–251 [PubMed] [Google Scholar]
- Lemoine NR, Mayall ES, Wyllie FS, Williams ED, Goyns M, Stringer B, Wynford-Thomas D (1989) High frequency of ras oncogene activation in all stages of human thyroid tumorigenesis. Oncogene 4:159–164 [PubMed] [Google Scholar]
- Moretti F, Farsetti A, Soddu S, Misiti S, Crescenzi M, Filetti S, Andreoli M, Sacchi A, Pontecorvi A (1997) p53 re-expression inhibits proliferation and restores differentiation of human thyroid anaplastic carcinoma cells. Oncogene 14:729–740 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Yana I, Kobayashi T, Shin E, Karakawa K, Fujita S, Miya A, Mori T, Nishisho I, Takai S (1992) p53 gene mutations associated with anaplastic transformation of human thyroid carcinomas. Jpn J Cancer Res 83:1293–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforova MN, Kimura ET, Gandhi M, Biddinger PW, Knauf JA, Basolo F, Zhu Z, Giannini R, Salvatore G, Fusco A, Santoro M, Fagin JA, Nikiforov YE (2003) BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab 88:5399–5404 [DOI] [PubMed] [Google Scholar]
- Onda M, Emi M, Yoshida A, Miyamoto S, Akaishi J, Asaka S, Mizutani K, Shimizu K, Nagahama M, Ito K, Tanaka T, Tsunoda T (2004) Comprehensive gene-expression profiling of anaplastic thyroid cancers with cDNA microarray of 25,344 genes. Endocr Relat Cancer 11:843–854 [DOI] [PubMed] [Google Scholar]
- Onda M, Akaishi J, Asaka S, Okamoto J, Miyamoto S, Mizutani K, Yoshida A, Ito K, Emi M (2005) Decreased expression of haemoglobin beta (HBB) gene in anaplastic thyroid cancer and recovery of its expression inhibits cell growth. Br J Cancer 92:2216–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passler C, Scheuba C, Prager G, Kaserer K, Flores JA, Vierhapper H, Niederle B (1999) Anaplastic (undifferentiated) thyroid carcinoma (ATC). A retrospective analysis. Langenbecks Arch Surg 384:284–293 [DOI] [PubMed] [Google Scholar]
- Quiros RM, Ding HG, Gattuso P, Prinz RA, Xu X (2005) Evidence that one subset of anaplastic thyroid carcinomas are derived from papillary carcinomas due to BRAF and p53 mutations. Cancer 103:2261–2268 [DOI] [PubMed] [Google Scholar]
- Shao JY, Wang HY, Huang XM, Feng QS, Huang P, Feng BJ, Huang LX, Yu XJ, Li JT, Hu LF, Ernberg I, Zeng YX (2000) Genome-wide allelotype analysis of sporadic primary nasopharyngeal carcinoma from southern China. Int J Oncol 17:1267–1275 [DOI] [PubMed] [Google Scholar]
- Smith LL, Coller HA, Roberts JM (2003) Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nat Cell Biol 5:474–479 [DOI] [PubMed] [Google Scholar]
- Stringer BM, Rowson JM, Parkar MH, Seid JM, Hearn PR, Wynford-Thomas D, Ingemansson S, Woodhouse N, Goyns MH (1989) Detection of the H-RAS oncogene in human thyroid anaplastic carcinomas. Experientia 15:372–376 [DOI] [PubMed] [Google Scholar]
- Suarez HG (1998) Genetic alterations in human epithelial thyroid tumours. Clin Endocrinol (Oxf) 48:531–546 [DOI] [PubMed] [Google Scholar]
- Venkatesh YS, Ordonez NG, Schultz PN, Hickey RC, Goepfert H, Samaan NA (1990) Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer 66:321–330 [DOI] [PubMed] [Google Scholar]
- Wiseman SM, Loree TR, Rigual NR, Hicks WL Jr, Douglas WG, Anderson GR, Stoler DL (2003) Anaplastic transformation of thyroid cancer: review of clinical, pathologic, and molecular evidence provides new insights into disease biology and future therapy. Head Neck 25:662–670 [DOI] [PubMed] [Google Scholar]
- Yu Y, Zhang C, Zhou G, Wu S, Qu X, Wei H, Xing G, Dong C, Zhai Y, Wan J, Ouyang S, Li L, Zhang S, Zhou K, Zhang Y, Wu C, He F (2001) Gene expression profiling in human fetal liver and identification of tissue- and developmental-stage-specific genes through compiled expression profiles and efficient cloning of full-length cDNAs. Genome Res 11:1392–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]