Abstract
Purpose
Several reports indicate a complexity in glycosyltransferase activities which lead to several tumor associated carbohydrate structures in gastric carcinoma. The present study was aimed to identify the carbohydrate associated transferases which exhibit the most marked and consistent change of activity in gastric tumorigenesis.
Methods
We examined the levels of fucosyl, β-galactosyl-, β-N-acetylgalactosaminyl, sialyl- and glycan:sulfotransferase activities, which generate the outer ends of oligosaccharide chains in tumorous and adjacent normal gastric tissues of the same patient in ten gastric carcinoma cases by using well defined specific synthetic acceptors utilized in our several earlier published studies as referenced in the text (e.g. Chandrasekaran et al. in J Biol Chem 279:10032–10041, 2004; Biochemistry 44:15619–15635, 2005; Carbohydr Res 341:983–994, 2006).
Results
Among glycosyltransferases only α1,2-fucosyltransferase (FT) was unique in showing a remarkable 40–90% decrease of activity in seven cases. Uniquely several fold elevation of Gal3Sulfo-T2 (1.9 → 156.7 fold) and Gal3Sulfo-T4 (2.4 → 149.0 fold) activities in all ten cases and moderate elevation of GlcNAc6Sulfo-T (1.3 → 37.5 fold) activities in nine cases were identified. Poorly differentiated Signet ring cell carcinoma expresses mainly Gal3Sulfo-T2 activity whereas poorly differentiated adenocarcinoma express predominantly Gal3Sulfo-T4 activity and also GlcNAc6Sulfo-T activity. But, very low level of these sulfotransferase activities were identified in moderately differentiated gastric carcinomas as well as non-epithelial gastric stromal sarcoma.
Conclusion
Up regulation of glycan:sulfotransferase activities and down regulation of α1,2-fucosyltransferase activity are apparently associated with human gastric tumorigenesis.
Keywords: Carbohydrate, Enzyme activities, Gastric tumor, Glycosyltransferases, Sulfotransferases
Introduction
Biosynthesis of carbohydrate structures is tissue-specific and developmentally regulated by glycosyltransferases (Paulson et al. 1989; O’Hanlon et al. 1989). During carcinogenesis, aberrant glycosylation leads to the development of tumor subpopulations with different adhesion properties (Taniguchi et al. 1996; Hiraiwa et al. 1996). Alterations of mucins during the pathogenesis of cancer have been well documented (Hakomori et al. 2002). The under-glycosylation of mucins results in the creation of tumor associated cryptic carbohydrate core structures Tn, sialyl Tn and T (Taylor-Papadimitriou et al. 1999). Werther et al. (1996) examined the frequency of sialyl Tn expression and its prognostic value in gastric cancer by immunohistochemical analysis of 340 gastric tumors and found that sialyl Tn expression is a marker of gastric cancer progression suggesting that cancer associated mucins play a role in the malignant behavior of the tumor. Santos-Silva et al. (2005) have reported that polymorphism in the MUC1 tandem repeat exerts influence on the expression of cryptic carbohydrate core structures in gastric cancer cells and the aggressive gastric tumors tend to express T antigen. Immunohistochemical study of Amado et al. (1998) for the expression of dimeric sialyl Lewisx in 97 gastric carcinomas revealed a correlation between high expression of dimeric sialyl Lewisx and venous invasion and poor outcome in gastric cancer patients. Petretti et al. (1999) studied RNA expression of several glycosyltransferases in surgical specimens of gastric carcinomas and found significant enhancement in the expression of ST3Gal IV and FucT IV.
A comprehensive study by Carvalho et al. (1999) on mucin expression in a panel of gastric carcinoma cell lines found no apparent relationship between the mucin core proteins and the simple mucin type or Lewisx carbohydrate antigens that are expressed in each cell line. Thus there exists a complexity in glycosyltransferase activities which lead to several tumor associated carbohydrate structures in gastric carcinoma. A recent comparative study of gene-expression profiles of adenocarcinoma metastases and primary adenocarcinomas (Ramaswamy et al. 2003) indicated that a subset of primary tumors resembled metastatic tumors with respect to a gene-expression signature and that solid tumors carrying this gene-expression signature were most likely to be associated with metastasis and poor clinical outcome. These results further suggested that the metastatic potential of human tumors is encoded in the bulk of a primary tumor and thus challenged the notion that metastases arise from rare cells within a primary tumor that have the ability to metastasize (Poste and Fidler 1980). Thus, it became obvious that a very consistent change of some biological events in a primary tumor as opposed to the normal tissue has the possibility of being related to the malignant potential of the tumor. Recently, we examined the patterns of glycosyl- and glycan-sulfotransferase activities in several cancer cell lines and the resulting data strongly suggested an association of unique carbohydrate structures with signature potential in individual cancers (Chandrasekaran et al. 2006). The present study was aimed to identify the glycosyltransferase candidate which exhibits the most marked and consistent change of activity in gastric tumorigenesis, by comparing the pattern of various glycosyl and glycan:sulfotransferase activities in tumorous and normal gastric tissues of each patient in ten gastric carcinoma cases.
Materials and methods
Tissue specimens
Human gastric tumor specimens were obtained from pathology after surgical procedures at Roswell Park Cancer Institute and stored frozen within 1 h at −70°C.
We studied the gastric tumor as well as non-tumor stomach tissue specimens from the same patient in ten gastric carcinoma cases and, in addition, two gastric tumor specimens in which case normal stomach tissue specimens were unavailable. The pathology report on the tumors is presented in Table 1. When the samples were collected from pathology, a portion of the sample adjacent to that used for enzyme assay was fixed in formalin and embedded in paraffin. Slides were prepared from the paraffin blocks, and stained with hematoxylin and eosin by standard procedures. A board-certified pathologist (KT) studied the slides to determine the distribution of cell types within the tumor tissue compared to the control tissue from the same case (although in three cases non-tumor tissue was not available). Due to the invasiveness of the gastric cancer in these cases, both the tumor and the non-tumor tissue samples generally contained smooth muscle. The smooth muscle component existed at roughly equivalent percentage in the tumor sample and non-tumor control on a case by case basis sometimes being a little larger in either tumor or non-tumor sample as shown in Table 2. The major difference between the normal and tumor sample in this study was that the tumor contained malignant epithelial cells, and the normal never did. With the exception of case 7323, the amount of protein solubilized from the tumor and the corresponding non-tumor specimen by Triton X-100 did not vary much as evident from the values reported in Table 2. Thus, it becomes evident that a comparison of each glycosyltransferase and glycan:sulfotransferase activity per mg protein of the tissue extract between tumor and the corresponding non-tumor specimen is quite meaningful in understanding the quantitative change of each enzyme activity in tumorigenesis.
Table 1.
Cancer diagnostic details of the patients
| Case | Site | Histology | Differentiation | STG | Date Last Cont. | Patient status | Cancer status |
|---|---|---|---|---|---|---|---|
| 7323 | Cardia, NOS | Mucin producing adenocarcinoma | Poor | 3A | 6/8/2005 | Alive | Unknown/ indeterminate |
| 7741 | Overlapping lesion of stomach | Signet ring cell CA | Poor | 9/19/1994 | Dead | Evidence of this cancer | |
| 7405 | Lesser curvature of stomach, NOS | Signet ring cell CA | Poor | 3A | 4/9/1995 | Dead | Evidence of this cancer |
| 7570 | Gastric antrum | Adenocarcinoma nos | Poor | 3A | 12/1/2000 | Dead | Unknown/ indeterminate |
| 9529 | Gastric antrum | Adenocarcinoma nos | Poor | 1B | 7/31/1997 | Dead | No evidence of this cancer |
| 11681 | Body of stomach | Adenocarcinoma nos | Moderate | 3A | 10/26/2005 | Alive | No evidence of this cancer |
| 11868 | Lesser curvature of stomach, NOS | Signet ring cell CA | Poor | 3A | 3/4/2003 | Dead | No evidence of this cancer |
| 11954 | Lesser curvature of stomach, NOS | Signet ring cell CA | Moderate | 2 | 1/29/2002 | Dead | Evidence of this cancer |
| 11847 | Gastric antrum | Mucin producing adenocarcinoma | Poor | 2 | 4/5/2006 | Alive | No evidence of this cancer |
| 12015 | Body of stomach | Adenocarcinoma nos | Moderate | 1B | 7/20/2006 | Alive | No evidence of this cancer |
| 7532 | Stomach, NOS | Litinis plastica | Poor | 4 | 2/28/1995 | Dead | Evidence of this cancer |
| 11577 | Lesser curvature of stomach, NOS | Gastrointestinal stromal sarcoma | Non-epithelial poor | 6/12/2005 | Dead | Unknown/ indeterminate |
Table 2.
Details of the gastric tumor and non-tumor specimens from the 12 patients
| Case | Smooth muscle (%) | Specimen weight gram | aProtein (mg) in Triton X-100 | |||
|---|---|---|---|---|---|---|
| Solubilized extract per gram tissue | ||||||
| Tumor | Non-tumor | Tumor | Non-tumor | Tumor | Non-tumor | |
| 7323 | 30 | 40 | 2.3 | 1.9 | 23.5 | 112.0 |
| 7741 | 40 | 30 | 1.9 | 3.5 | 67.4 | 52.8 |
| 7405 | 50 | 30 | 2.5 | 1.9 | 53.6 | 58.5 |
| 7570 | 20 | 20 | 0.8 | 0.8 | 80.0 | 80.0 |
| 9529 | 30 | 20 | 0.6 | 0.8 | 67.5 | 60.4 |
| 11681 | 10 | 10 | 2.1 | 0.6 | 80.2 | 68.9 |
| 11868 | 30 | 20 | 1.9 | 4.2 | 42.2 | 54.6 |
| 11954 | 70 | N/A | 1.0 | 6.0 | 41.0 | 60.0 |
| 11847 | 0 | 10 | 0.5 | 1.7 | 82.8 | 52.4 |
| 12015 | 10 | 20 | 2.0 | 0.6 | 86.9 | 52.9 |
| 7532 | 60 | N/Ab | 3.6 | N/A | 58.3 | |
| 11577 | 0 | N/Ac | 3.5 | N/A | 76.8 | |
N/A Not available
a Protein was measured by the BCA method (Pierce Chemicals, Rockford, IL, USA ) with bovine serum albumin as the standard
b Tumor infiltrating the muscle
c Gist–100% tumor
Acceptor compounds
The synthetic compounds used as acceptors in this study have already been used in our earlier studies (Chandrasekaran et al. 1995, 1996, 2001, 2004, 2005, 2006) and, thus, are well-documented acceptors for measuring the reported enzyme activities.
Processing of tissue specimens
The tissues were homogenized at 4°C with four volumes of 0.1 M Tris Maleate pH 7.2, 0.1% NaN3 using kinematica. After adjusting the concentration of TritonX-100 to 2%, these homogenates were mixed in the cold room for 1 h using Speci-Mix (Thermolyne) and then centrifuged at 20,000g for 1 h at 4°C. The clear fat-free supernatant was stored frozen at −20°C until use. Aliquots of 10 μL from this extract were used in assays run in duplicates. Glycosyltransferase activity in cell lysate was determined by mixing the lysates with acceptor and radiolabeled monosaccharide donor under the reaction conditions detailed below, followed by separation of unreacted donor from the radioactive product using anionic or hydrophobic chromatography. In all cases, the radioactive content of isolated products was determined by using 3a70 scintillation cocktail (research Products International, Mount Prospect, IL, USA) and a Beckman LS9000 scintillation counter. Controls for each assay contained the reaction mixture with everything except the acceptor. Radioactivity of product was subtracted from that of control to obtain the results presented in the Tables. All assays were run in duplicate. Results from duplicate runs did not vary by more than 5%. The following are the conditions for individual enzymatic assays. Reaction temperature in all cases was 37°C. α2,3- and α2,6 Sialyltransferase (ST) assay reactions proceeded for 2 h in a mixture containing 100 mM sodium cacodylate buffer (pH 6.0), 7.5 mM acceptor, CMP-[9-3H] NeuAc (typically 0.2 μCi) and 10 μl cell extract in a total volume of 20 μl (Chandrasekaran et al. 1995, 2005).
βGlcNAc:β1,4Gal-T and αGalNAc:β1,3Gal-T assay mixtures in duplicate contained 0.1 M Hepes–NaOH pH 7.0, 7 mM ATP, 20 mM Mn acetate, 1 mM UDP-Gal. UDP [14C]Gal (0.05 μCi; 327 mCi/mmol; Amersham), 0.5 mM acceptor (unless otherwise stated) and the enzyme in a total volume of 20 μL (Chandrasekaran et al. 2001). It was incubated for 4 h βGlcNAc:β1,4GalNAc-T assay mixtures in duplicate contained 0.1 M Hepes–NaOH ph7.0, 7 mM ATP, 20 mM Mn acetate. UDP [3H] GalNAc (0.20 μCi; 7.8 Ci/mmol: New England Nuclear Corp.) 7.5 mM acceptor and incubated for 4 h (Chandrasekaran et al. 2001). α1,2, α1,6-, α1,3- and α1,4-Fucosyltransferase (FT) assay reactions were carried out for 2 h in a reaction mixture containing 50 mM Hepes buffer (pH 7.5), 5 mM MnC12, 7 mM ATP, 3 mM NaN3, 3 mM synthetic acceptor or 40 μg of fetuin-based acceptor, 0.05 μCi GDP-[14C]Fuc (290 mCi/mmol) and 10 μl cell extract in a total volume of 20 μl (Chandrasekaran et al. 1996). Sulfotransferase (Sulfo-T) assay reactions took 2 h and required a mixture containing 100 mM Tris–Maleate (pH 7.2), 5 mM Mg Acetate, 5 mM ATP, 10 mM NaF, 10 mM BAL, 7.5 mM acceptor, 0.5 μCi of [35S]PAPS (specific activity 2.4 Ci/mmol) and 10 μl of cell extract in a total volume of 30 μl (Chandrasekaran et al. 2004).
Dowex-1-Cl or Sep-Pak C18 cartridges were used to isolate radiolabeled product from the reaction mixture. For Gal-T, GalNAc-T and FT assays, the incubation mixture was diluted with 1 ml water and passed through a 1 ml bed volume of Dowex-1-Cl column (Chandrasekaran et al. 1996, 2001). The column was washed twice with 1 ml water. The breakthrough and the water wash contained the [14C]-galactosylated or [14C]-fucosylated products formed with neutral acceptors. About 3 ml of 0.1 M NaCl was used to obtain [14C]-fucosylated products from sialylated acceptors after water elution. For sialyltransferase assays, the radioactive products from benzylglycosides were separated by hydrophobic chromatography on Sep-Pak C18 cartridge (Water, Milford, MA, USA), and elution of the product was done with 3 ml methanol (Chandrasekaran et al. 2005; Palcic et al. 1988). For sulfotransferase assays, elution of the [35S]-sulfated compound from Dowex-1-Cl column could be achieved by 3 ml of 0.2 M NaCl (Chandrasekaran et al. 2001).
Effect of divalent cations on gastric tumor Gal:3-O-sulfotransferase activity
For seeing the effect of divalent cations on Gal:3-O-sulfotransferase activity the incubation mixture contained varying concentration (1–50 mM) of Mg acetate, Mn acetate or Ca acetate under the standard incubation conditions. Gal3Sulfo-T-A activity present in GC11847 was assayed using 3-O-Me Galβ1,4GlcNAcβ1,6(Galβ1,3)GalNAcα-O-Bn as the acceptor. Gal3Sulfo-T-B activity expressed by GC11868 was measured with the acceptor Galβ1,4GlcNAcβ1,6(3-O-MeGalβ1,3)GalNAcα-O-Bn.
Results
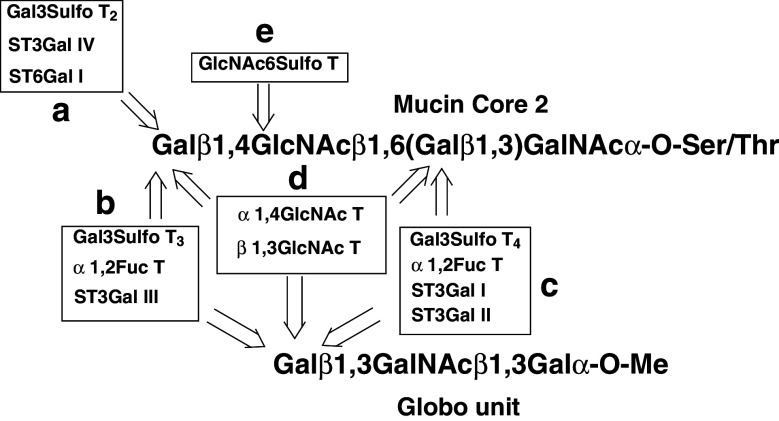
Several glycosyltransferases and glycan-sulfotransferases act on the non-reducing terminal of mucin Core2 tetrasaccharide and Globo backbone unit, leading to a complexity of cancer-associated terminal glycan structures as shown in Fig. 1.
Fig. 1.
Carbohydrate chain terminal modifying glycosyl- and sulfo-transferases. a Sulfation or sialylation of Gal at C-3 OH, sialylation of Gal at C-6 OH, b, c sulfation or sialylation of Gal at C-3 OH, fucosylation of Gal at C-2 OH, d N-acetylglucosaminylation of Gal C-3 or C-4 OH, e sulfation of GlcNAc at C-6 OH
Hence, we examined the levels of many of these enzyme activities in gastric tumor tissue as well as non-tumor gastric tissue of the same patient in ten gastric cancer cases for identifying any significant consistent change in any of these enzyme activities in gastric cancer.
Fucosyltransferase activities in gastric carcinoma
α1,3-FT activity was the major FT activity in both normal and tumor specimens in all cases. In nine cases, the tumor specimens as compared to normal contained lower α1,3-FT activity in the range of 40–90%. On the other hand, α1,4-FT activity was found to be significantly increased in five cancer cases (GC7323; GC7741; GC11868; GC11954 and GC11847). There was a marked decrease (40–90%) of α1,2-FT activity in seven cancer cases (GC7405, GC7570, GC9529, GC11681, GC11868, GC11954 and GC12015). FTVI activity was present in all specimens. It is interesting to note that four tumor specimens (GC11847, GC11954, GC7741 and GC7323) exhibited, respectively, 8.9, 10.5, 51.4 and 199.5 fold of this activity as compared to the corresponding normal specimen. Further, a highly noteworthy finding is that the gastrointestinal stromal tumor specimen #11577 was distinct in having a low level of α1,3-FT activity and negligible amounts of α1,4-FT, α1,2-FT and FTVI activities as compared to other tumor specimens (see Table 3).
Table 3.
The levels of fucosyltransferase activities in human gastric carcinoma
| Case | Tissue specimen | Fucosyltransferase activity: incorporation of [14C] Fuc (CPM × 10−3) into the acceptor catalyzed by 1 mg protein of the tissue extract | |||
|---|---|---|---|---|---|
| α1,3-FT activity [2-O-MeGalβ1,4 GlcNAc] | α1,4-FT activity [2-O-MeGalβ1,3 GlcNAc] | α1,2-FT activity [Galβ-O-Bn] | FT VI activity [GlcNAc β1,4 GlcNAcβ-O-Bn] | ||
| 51 | NS7323 | 166.4 | 18.4 | 19.9 | 0.8 |
| GC7323 | 655.0 ↑(3.9) | 671.6 ↑(36.5) | 131.9 ↑(6.6) | 159.6 ↑(199.5) | |
| 2 | NS7741 | 449.6 | 68.3 | 127.2 | 3.1 |
| GC7741 | 390.5 ↓(0.9) | 193.9 ↑(2.8) | 111.2 ↓(0.9) | 159.2 ↑(51.4) | |
| 3 | NS7405 | 494.1 | 9.1 | 146.8 | 138.6 |
| GC7405 | 382.6 ↓(0.8) | 2.3 ↓(0.3) | 83.3 ↓(0.6) | 60.6 ↓(0.4) | |
| 4 | NS7570 | 448.8 | 12.3 | 197.2 | 16.7 |
| GC7570 | 180.2 ↓(0.4) | 0 ↓(0) | 16.5 ↓(0.1) | 1.2 ↓(0.1) | |
| 5 | NS9529 | 428.4 | 405.2 | 109.4 | 137.9 |
| GC9529 | 198.2 ↓(0.5) | 211.3 ↓(0.5) | 22.0 ↓(0.2) | 2.7 ↓(0.02) | |
| 6 | NS11681 | 367.8 | 173.6 | 102.7 | 5.4 |
| GC11681 | 278.9 ↓(0.8) | 91.2 ↓(0.5) | 34.4 ↓(0.3) | 3.8 ↓(0.7) | |
| 7 | NS11868 | 533.2 | 268.3 | 76.8 | 42.7 |
| GC11868 | 448.3 ↓(0.8) | 322.6 ↑(1.2) | 49.0 ↓(0.6) | 51.7 ↑(1.2) | |
| 8 | NS11954 | 422.2 | 71.5 | 62.9 | 1.5 |
| GC11954 | 184.0 ↓(0.4) | 201.3 ↑(2.8) | 35.0 ↓(0.6) | 15.8 ↑(0.5) | |
| 9 | NS11847 | 526.3 | 189.5 | 70.9 | 13.6 |
| GC11847 | 352.8 ↓(0.7) | 325.6 ↑(1.7) | 132.1 ↑(1.9) | 121.0 ↑(8.9) | |
| 10 | NS12015 | 483.5 | 417.0 | 108.9 | 227.0 |
| GC12015 | 328.7 ↓(0.7) | 294.5 ↓(0.7) | 62.5 ↓(0.6) | 181.7 ↓(0.8) | |
| 11 | GC7532 | 452.2 | 193.4 | 88.5 | 90.5 |
| 12 | GC11577 | 107.0 | 0.9 | 2.5 | 0.8 |
Values in parentheses indicate the fold of enzyme activity in tumor specimen as compared to that of corresponding normal tissue of the patient
NS Normal stomach, GC gastric carcinoma
↑ Increase, ↓ decrease
When compared to Signet ring cell carcinoma specimens (7741, 7405, 11868, with the exception of 11954), all the adenocarcinoma specimens (7570, 9529, 11681, 11847 and 12015) showed a greater decrease of α1,3-L-FT activity. On the other hand, three out of four specimens from Signet ring cell carcinoma (7147, 11868 and 11954) showed an increase in α1,4-FT and FTVI activities whereas three adenocarcinoma specimens (9529, 11681 and 12051) contained a decreased level of these activities. All the FT activities examined in Cardia site tumor (7323) exhibited a high level of these activities as compared to the non-tumor specimen and the reason for this may be due to the fact that the amount of protein extracted from this tumor specimen was far less than that of the non-tumor specimen (see Table 2).
Sialyltransferase activities in gastric carcinoma
ST3(O) activity, which forms 3′-sialyl T hapten (NeuAcα2,3Galβ1,3GalNAcα) was the predominant sialyltransferase activity in all gastric tissue specimens. This activity was increased (1.7–172.0 fold) in six (GC7323, GC7405, GC9529, GC11868, GC11954 and GC12015) and decreased in four (GC7741, GC7570, GC11681 and GC11847) gastric tissue specimens. ST6(N) activity synthesizing 6′-LacNAc type 2 unit (NeuAcα2,6Galβ1,4GlcNAcβ-) ranged from 3–60% of the ST3(O) activity in the tissue specimens studied and ST3(N) activity forming 3′-sialyl LacNAc was even far less than ST6(N) activity. Three out of four specimens from Signet ring cell carcinoma (7405, 11868 and 11954) but only two out of five specimens from adenocarcinoma with the exception of GC7323 containing less extractable protein (see Table 2) as compared to NS7323 showed an increased level of ST3 (O) activity, which is the most predominant sialyltransferase activity in all specimens (see Table 4).
Table 4.
The levels of sialyltransferase activities in human gastric carcinoma
| Tissue specimen | Sialyltransferase activity: incorporation of [9−3H]NeuAc(CPM × 10−3) catalyzed by 1 mg protein of the tissue extract | ||
|---|---|---|---|
| ST3(O) | ST3(N) | ST6(N) | |
| NS7323 | 1.9 | 0.4 | 0 |
| GC7323 | 326.8 ↑(172.0) | 7.1 ↑(4.3) | 0 |
| NS7741 | 184.5 | 2.1 | 0.4 |
| GC7741 | 53.8 ↓ (0.3) | 3.1 ↑(1.5) | 32.9 ↑(82.2) |
| NS7405 | 54.4 | 1.9 | 15.4 |
| GC7405 | 145.1 ↑(2.7) | 4.0 ↑(2.1) | 8.3 ↓(0.5) |
| NS7570 | 234.2 | 1.1 | 16.2 |
| GC7570 | 155.2 ↓(0.7) | 1.6 ↑(1.5) | 6.4 ↓(0.4) |
| NS9529 | 104.2 | 1.7 | 48.3 |
| GC9529 | 240.3 ↑(2.3) | 2.3 ↑(1.4) | 11.9 ↓(0.2) |
| NS11681 | 306.5 | 2.6 | 8.3 |
| GC11681 | 245.1 ↓(0.8) | 2.2 ↓(0.8) | 38.4 ↑(4.6) |
| NS11868 | 180.4 | 0.7 | 24.5 |
| GC11868 | 309.0 ↑(1.7) | 2.5 ↑(3.6) | 25.2 ↑(1.0) |
| NS11954 | 15.6 | 0 | 0 |
| GC11954 | 122.7 ↑(7.9) | 0 | 7.3 ↑ |
| NS11847 | 379.7 | 0.6 | 40.5 |
| GC11847 | 83.3 ↓(0.5) | 4.2 ↑(7.0) | 65.0 ↑(1.6) |
| NS12015 | 148.6 | 3.6 | 50.1 |
| GC12015 | 308.2 ↑(2.1) | 3.8 ↑(1.1) | 41.5 ↓(0.8) |
| GC7532 | 114.0 | 1.1 | 8.7 |
| GC11577 | 91.9 | 2.7 | 8.2 |
Values in parentheses indicate the fold of enzyme activity in tumor specimen as compared to that of corresponding normal tissue of the patient
Acceptors used: ST3(O): 3-O-MeGalβ1,4GlcNAcβ1,6(Galβ1,3)GalNAcα-O-Bn; ST3(N): 2-O-MeGalβ1,3GlcNAcβ-O-Bn; ST6(N): the values obtained by using the acceptor Galβ1,4GlcNAcβ1,6(3-O-MeGalβ1,3)GalNAcα-O-Bn were corrected by subtracting the values obtained by using Galβ1,3GlcNAcβ-O-Bn, which is an acceptor for ST3(N) and also to some extent active with ST3(O)
NS Normal stomach, GC gastric carcinoma
↑ Increase, ↓ decrease
Glycan:sulfotransferase activities in gastric carcinoma
Both Gal3Sulfo-T4 activity specific for Galβ1,3GalNAcα- and Gal3Sulfo-T2 activity utilizing mainly Galβ1,4GlcNAcβ- were found, respectively, at 2.4 → 61.7 fold and 1.7 → 156.7 fold elevated level in all the gastric tumor specimens studied. On the other hand, an increased level of GlcNAc6-Sulfo-T activity was also evident but to a lesser extent in nine gastric tumor specimens. Five tumor specimens, namely GC7323, GC7570, GC9529, GC11847 and GC12015, contained predominantly Gal3Sulfo-T4 while Gal3Sulfo-T2 dominated in three specimens, namely GC7405, Gc7741 and GC11868 (see Table 5).
Table 5.
The levels of glycan:sulfotransferase activities in human gastric carcinoma
| Tissue specimen | Glycan:sulfotransferase activity: incorporation of [35S]sulfate(CPM × 10−3) catalyzed by 1 mg protein of the tissue extract | ||
|---|---|---|---|
| Gal3Sulfo-T4 | Gal3Sulfo-T2 | GlcNAc6Sulfo-T | |
| NS7323 | 0.2 | 0 | 2.3 |
| GC7323 | 12.3 ↑(61.5) | 1.4 ↑ | 86.3 ↑(37.5) |
| NS7741 | 3.4 | 0.8 | 33.8 |
| GC7741 | 49.1 ↑(14.4) | 60.7 ↑(75.9) | 43.8 ↑(1.3) |
| NS7405 | 12.9 | 2.1 | 19.5 |
| GC7405 | 91.4 ↑(7.1) | 329.0 ↑(156.7) | 53.7 ↑(2.8) |
| NS7570 | 27.8 | 1.1 | 31.1 |
| GC7570 | 75.8 ↑(2.7) | 11.7 ↑(10.6) | 49.8 ↑(1.6) |
| NS9529 | 6.7 | 1.4 | 6.0 |
| GC9529 | 34.3 ↑(5.1) | 2.6 ↑(1.9) | 3.3 ↓(0.6) |
| NS11681 | 0.7 | 1.3 | 2.9 |
| GC11681 | 2.3 ↑(3.3) | 2.7 ↑(2.1) | 4.1 ↑(1.4) |
| NS11868 | 1.8 | 10.1 | 1.8 |
| GC11868 | 111.0 ↑(61.7) | 208.1 ↑(20.6) | 13.6 ↑(7.6) |
| NS11954 | 0.1 | 0.1 | 0.1 |
| GC11954 | 0.5 ↑(5.0) | 2.6 ↑(26.0) | 0.7 ↑(7.0) |
| NS11847 | 0.4 | 0.3 | 1.9 |
| GC11847 | 59.6 ↑(149.0) | 2.1 ↑(7.0) | 5.2 ↑(2.7) |
| NS12015 | 7.1 | 0.4 | 1.9 |
| GC12015 | 16.8 ↑(2.4) | 3.3 ↑(8.3) | 15.3 ↑(8.1) |
| GC7532 | 56.2 | 2.3 | 25.7 |
| GC11577 | 4.6 | 1.1 | 1.3 |
Values in parentheses indicate the fold of enzyme activity in tumor specimen as compared to that of corresponding normal tissue of the patient
Acceptors used: Gal3Sulfo-T4: 3-O-MeGalβ1,4GlcNAcβ1,6(Galβ1,3)GalNAcα-O-Bn; Gal3Sulfo-T2: Galβ1,4GlcNAcβ1,6(3-O-MeGalβ1,3)GalNAcα-O-Bn; GlcNAc6Sulfo-Tf: GlcNAcβ1,3Galβ1,4Glc
NS Normal stomach, GC gastric carcinoma
↑ Increase, ↓ decrease
β-GlcNAc:β1,4-Gal/GalNAc and α-GalNAc:β1,3-Gal transferase activities in gastric carcinoma
Except for three tumor specimens, namely GC7323, GC11868 and GC11954, exhibiting an increase in β1,4Gal-T activity, other tumor specimens contained lower level of this activity than the corresponding normal specimens. But, in addition to the three tumor specimens (GC7323, GC11868 and GC11954), three other tumor specimens (GC7570, GC9529 and GC11681) also showed an increased level of β1,4GalNAc-T activity. It is remarkable that two tumor specimens (GC7570 and GC9529) from adenocarcinoma contained only 30 and 40% level of α-GalNAc:β1,3Gal-T activity as compared to the normal specimens. On the contrary, GC7323 as compared to other tumor specimens had a high level of this activity, which exceeded even the levels of its β1,4Gal/GalNAc-T activities. The level of αGalNAc:β1,3Gal-T activity was higher than β1,4Gal/GalNAc-T activity in Litinis plastica specimen GC7532 (see Table 6).
Table 6.
The levels of β-GlcNAc: β1,4Gal/GalNAc and α-GalNAc: β1,3Gal transferase activities in human gastric carcinoma
| Tissue specimen | β1,4Gal/GalNAc-T activities: incorporation of [14C]Gal or [3H]GalNAc(CPM × 10−3) into 3-O-MeGalβ13(GlcNAcβ16)GalNAcα-O-βn catalyzed by 1 mg protein of the tissue extract | α-GalNAc: β1,3GalT activity: incorporation of [14C]Gal(CPM x 10−3) into 4-fluoro GlcNAcβ1,6GalNAcα-O-βn catalyzed by 1 mg protein of the tissue extract | |
|---|---|---|---|
| β1,4Gal-T | β1,4GalNAc-T | β1,3Gal-T | |
| NS7323 | 26.7 | 15.3 | 39.7 |
| GC7323 | 161.9 ↑(6.1) | 61.0 ↑(4.0) | 244.9 ↑(6.2) |
| NS7741 | 172.2 | 51.7 | 36.6 |
| GC7741 | 111.6 ↓(0.6) | 27.2 ↓(0.5) | 43.3 ↑(1.2) |
| NS7405 | 167.8 | 44.3 | 34.8 |
| GC7405 | 151.9 ↓(0.9) | 38.1 ↓(0.9) | 44.1 ↑(1.3) |
| NS7570 | 232.2 | 36.8 | 70.2 |
| GC7570 | 199.0 ↓(0.9) | 44.0 ↑(1.2) | 19.4 ↓(0.3) |
| NS9529 | 275.0 | 38.5 | 102.0 |
| GC9529 | 258.4 ↓(0.9) | 43.9 ↑(1.1) | 36.3 ↓(0.4) |
| NS11681 | 229.8 | 37.9 | 98.9 |
| GC11681 | 205.7 ↓(0.9) | 40.3 ↑(1.1) | 83.0 ↓(0.8) |
| NS11868 | 255.3 | 36.0 | 67.2 |
| GC11868 | 403.9 ↑(1.6) | 50.5 ↑(1.4) | 65.9 ↓(1.0) |
| NS11954 | 113.2 | 12.2 | 25.6 |
| GC11954 | 124.1 ↑(1.1) | 25.8 ↑(2.1) | 31.6 ↑(1.2) |
| NS11847 | 362.5 | 39.7 | 88.1 |
| GC11847 | 211.4 ↓(0.6) | 32.4 ↓(0.8) | 138.0 ↑(1.6) |
| NS12015 | 264.5 | 74.8 | 110.6 |
| GC12015 | 207.0 ↓(0.8) | 28.7 ↓(0.4) | 166.4 ↑(1.5) |
| GC7532 | 92.9 | 28.9 | 103.3 |
| GC11577 | 62.3 | 10.1 | 23.8 |
Values in parentheses indicate the fold of enzyme activity in tumor specimen as compared to that of corresponding normal tissue of the patient
NS Normal stomach, GC gastric carcinoma
↑ Increase, ↓ decrease
A comparison of the levels of glycosyltransferase activities and glycan:sulfotransferase activities between normal and tumor gastric tissue specimens from the same patient
Figure 2 depicts the levels of these activities in tumor specimens as fold of the activities present in the corresponding normal tissue specimens. The arrow on the Y-axis indicates onefold, which is the activity in normal specimens. Anything below the arrow signifies a decrease and above the arrow, an increase of activity which is found in tumor specimens Fig. 3
Fig. 2.
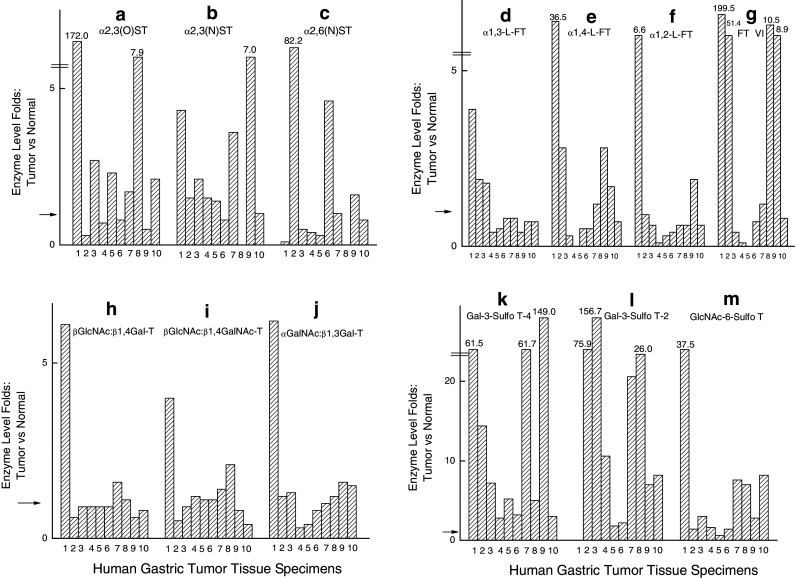
The levels of the activities (in fold) of glycosyltransferases and glycan:sulfo-transferases in human gastric tumor specimens as compared to that of the corresponding normal gastric tissues. a α2,3(O)ST activity, b α2,3(N)ST activity, c α2,6(N)ST activity, d α1,3-FT activity, e α1,4-FT activity, f α1,2-FT activity, g FTVI activity, h β1,4Gal-T activity, i β1,4GalNAc-T activity, j αGalNAc:β1,3Gal-T activity, k Gal3Sulfo-T-A activity, l. Gal3Sulfo-T-B activity, m GlcNAc6 Sulfo-T activity. The arrow on the Y-axis is the level of enzyme activity which is onefold in the normal gastric tissue specimen as compared to the corresponding gastric tumor specimen
Fig. 3.
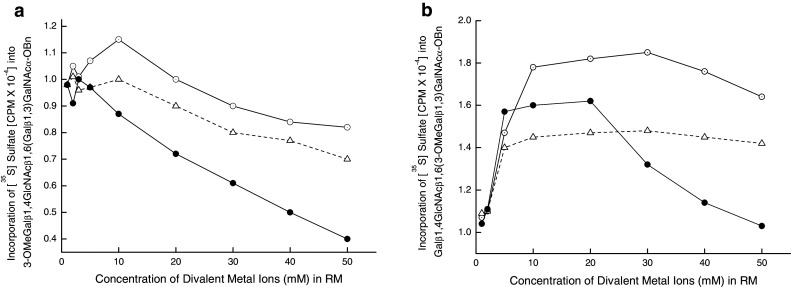
Influence of the divalent metal ions on Gal3Sulfo-T activities of gastric tumor specimens. a GC11847, b GC11868, filled circle Mn, open circle Ca, open triangle Mg
From Fig. 2a, it is evident that α2,3(O)ST activity is over-expressed in six gastric carcinoma cases which include two cases of poorly and one case of moderately differentiated Signet ring cell carcinoma as well as adenocarcinoma. The next predominant activity, namely α2,6(N)ST activity (Fig. 2c) is elevated in three carcinoma cases. The minor activity, namely α2,3(N)ST activity is apparently elevated in seven carcinoma cases (Fig. 2b). The α1,3-FT activity (Fig. 2d) shows a decrease in nine carcinoma cases except for the Cardia site tumor, whereas α1,4FT activity ( Fig. 2e) is definitely at increased level in five carcinoma cases. A marked depression of α1,2-FT activity is quite evident in seven carcinoma cases (Fig. 2f), whereas an augmentation of FTVI activity is a striking observation in four carcinoma cases (Fig. 2g). Further, it is interesting to note that both α1,4FT and FTVI activities showed the same trend of either increase or decrease in all tumor specimens. Figure. 2h shows an increase of β1,4Gal-T activity in two carcinoma cases and a remarkable 40% decrease of this activity in two carcinoma cases. Similarly, β1,4GalNAc-T activity (Fig. 2i) showed a significant increase in two carcinoma cases and a meaningful decrease in another two carcinoma cases. From Fig. 2j, a decrease of 60–70% of αGalNAc:β1,3Gal-T activity is evident in two carcinoma cases and a sixfold elevation of this activity in one carcinoma case.
In contrast to the fluctuation in the levels of glycosyl transferase activities in tumor specimens when compared to that of the corresponding normal specimens, the gastric carcinoma tumor specimen studied here expressed an elevated level of Gal3Sulfo-T-A and Gal3Sulfo-T-B with respect to that of the corresponding normal specimens (Fig. 2k, l). GlcNAc6Sulfo-T (Fig. 2m) also exhibited an increased level in nine carcinoma tumor specimens. But the elevation of activity was more pronounced in the case of Gal3Sulfo-T-A and Gal3Sulfo-T-B than in the case of GlcNAc6Sulfo-T.
One notable exception is the tumor specimen 7323 (tumor site Cardia) which was unique in exhibiting high level (several fold elevation) of all the enzymes examined in this study when compared to the level of these enzymes in the corresponding non-tumor gastric tissue from Cardia site.
The identity of Gal:3-O-Sulfo transferases in human gastric tumor specimens
A consistent marked elevated Gal:3-O-Sulfotransferase activities in gastric tumors prompted us to establish further the identity of these enzymes with the known cloned Gal3Sulfotransferases (Chandrasekaran et al. 2004).
Acceptor-specificities (see Table 7)
Table 7.
Establishing the identity of Gal: 3-O-Sulfotransferases in human gastric tumor specimens
| Acceptor | Gal: 3-O-Sulfotransferase activity % | |||
|---|---|---|---|---|
| GC9529 | GC11847 | GC7405 | GC11868 | |
| Galβ1,4GlcNAc | 3.2 | 8.8 | 100.0 (2438) | 100.0 (17550) |
| Galβ1,3GalNAcα-O-Al | 100.0 (2679) | 100.0 (3409) | 22.7 | 16.5 |
| 3-O-MeGalβ1,4GlcNAcβ1,6(Galβ1,3)GalNAcα-O-Bn | 230.2 | 326.8 | 48.4 | 42.9 |
| Galβ1,4GlcNAcβ1,6(3-O-MeGalβ1,3)GalNAcα-O-Bn | 11.1 | 16.7 | 80.1 | 73.2 |
| Galβ1,3(GlcNAcβ1,6)GalNAcα-O-Al | 489.4 | 657.3 | 112.8 | 90.2 |
| Galβ1,3GalNAcβ1,3Galα-O-Me | 222.8 | 304.2 | 210.7 | 178.0 |
| Fetuin triantennary asialo glycopeptide | 21.3 | 15.9 | 44.0 | 29.7 |
The values in parentheses are the actual CPM designated as 100% for the acceptor Galβ1,3GalNAcα-O-Al in the case of GC9529 and GC11847 and for the acceptor Galβ1,4GlcNAc in the case of GC7405 and GC11868
We studied two specimens, GC9529 and GC11847, containing mostly Gal3SulfoT-A activity and another two specimens, GC7405 and GC11868, expressing mainly Gal3SulfoT-B activity. The Gal3Sulfo-T of GC9529 and GC11847 utilized Galβ1,3GalNAcα-O-Al; 3-O-MeGalβ1,4GlcNAcβ1,6(Galβ1,3)GalNAcα-O-Bn; Galβ1,3(GlcNAcβ1,6)GalNAcα-O-Al; and Galβ1,3GalNAcβ1,3Galα-O-Me. The activities were GC9529: 100.0, 230.2, 489.4 and 222.8%; and GC11847: 100.0, 326.8, 657.3 and 304.2%, respectively. On the other hand, Gal3Sulfo-Ts of GC7405 and GC11868 were very active with Galβ1,4GlcNAc; Galβ1,4GlcNAcβ1,6(3-O-MeGalβ1,3)GalNAcα-O-Bn; Galβ1,3(GlcNAcβ1,6)GalNAcα-O-Al; and Galβ1,3GalNAcβ1,3Galα-O-Me (GC7405 ; 100.0, 80.1, 112,8 and 210.7%; and GC11868: 100.0, 73.2, 90.2 and 178.0%, respectively). Gal3Sulfo-Ts of GC9529 and GC11847 were thus identified as Gal3Sulfo-T4 (Chandrasekaran et al. 2004). Gal3Sulfo-Ts of GC7405 and GC11868 were found to be less active towards Fetuin triantennary asialo glycopeptides, which was established as the best acceptor in our earlier studies for Gal3Sulfo-T3 (Chandrasekaran et al. 2006). Further, the above enzymes were very active towards Galβ1,3GalNAcβ1,3Galα-O-Me whereas Gal3Sulfo-T3 showed very low activity towards this acceptor. Based on the data obtained for acceptor-specificities, Gal3Sulfo-Ts of GC7405 and GC11868 were found to have the identity of cloned Gal3Sulfo-T2 (Chandrasekaran et al. 2004).
Effect of divalent metal ions on Gal3Sulfo-T activities (see Fig. 3)
The influence of Ca2+, Mn2+ and Mg2+ ions on the Gal3Sulfo-T activities of GC11847 containing Gal3Sulfo-T-A and GC11868 having Gal3Sulfo-T-B was studied. None of these divalent metal ions had any stimulating effect on Gal3Sulfo-T of GC11847. In fact, a gradual decline in enzyme activity was noticed upon increasing the concentration of Mn2+ in the reaction mixture. Thus, this enzyme resembled Gal3Sulfotransferas from breast tumor (Chandrasekaran et al. 1997) as well as the cloned Gal3Sulfo-T4 (Chandrasekaran et al. 2004). On the other hand, the metal ions Ca2+, Mn2+ and Mg2+ stimulated the activity of GC11868. Mn2+ stimulated the activity between 5 and 20 mM and then the activity decreased reaching the initial level at 50 mM. This pattern of influence by Mn2+ on the activity was quite similar to that of Gal3Sulfotransferase from colon tissue (Chandrasekaran et al. 1997) and cloned Gal3Sulfo-T2 (Chandrasekaran et al. 2004). On the contrary, the activity of cloned Gal3Sulfo-T3 was stimulated by Mn2+ reaching the maximum at 40–50 mM (Chandrasekaran et al. 2004). Thus, Gal3Sulfo-T of GC11847 was similar to GalSuflo-T4 and that of GC11868 closely resembled Gal3Sulfo-T2.
Correlation between glycan:sulfotransferase activities and the gastric tumor types
From the data organized in Table 8, it is evident that poorly differentiated Signet ring cell carcinoma specimens (7741, 7405 and 11868) in contrast to the other gastric tumor specimens express an elevated level of Gal3Sulfo-T2 whereas poorly differentiated adenocarcinoma specimens (7323, 7570, 9529 and 11847) as well as Litinis Plastica (7532) contain predominantly Gal3Sulfo-T-4 and in three of these cases (7323, 7570 and 7532) a high level of GlcNAc6Sulfo-T could also be noticed. Further, it becomes evident from the data that a very low level of these sulfotransferase activities is expressed by moderately differentiated gastric carcinoma (11954, 11687 and 12015) and also by the stromal tumor (11577). Interestingly, two poorly differentiated gastric carcinoma patients whose tumor specimens (7323 and 11847) showed an increase in α1,2FT activity are still alive, one free of this cancer and the cancer status of the other is unknown to us. Furthermore, two moderately differentiated gastric adenocarcinoma patients (11681 and 12015) are still alive and there is no evidence of this cancer in both cases.
Table 8.
Correlating the glycan:sulfotransferase activities with the gastric tumor types
| Gastric tumor | Specimen | Stage of cancer | Patient status | α1,2-FT Activity decrease (%) | Gal3Sulfo-T2 | Gal3Sulto-T4 | GlcNAc6SulfoT |
|---|---|---|---|---|---|---|---|
| Signet ring cell carcinoma–Krukenberg tumor | |||||||
| Poorly differentiated | 7741 | D (E) | 10 | 60.7 (75.9) | 49.1 (14.4) | 43.8 (1.3) | |
| 7405 | 3A | D (E) | 40 | 329.0 (156.7) | 91.4 (7.1) | 53.7 (2.8) | |
| 11868 | 3A | D (NE) | 40 | 208.1 (20.6) | 111.0 (61.7) | 13.6 (7.6) | |
| Moderately differentiated | 11954 | 2 | D (E) | 40 | 2.6 (26.0) | 0.5 (5.0) | 0.7 (7.0) |
| Adenocarcinoma | |||||||
| Poorly differentiated | 7323 | 3A | A (UN) | (Increase 5.6 fold) | 1.4 (14.0) | 12.3 (61.5) | 86.3 (37.5) |
| 7570 | 3A | D (UN) | 90 | 11.7 (10.6) | 75.8 (2.7) | 49.8 (1.6) | |
| 9529 | 1B | D (NE) | 80 | 2.6 (1.9) | 34.3 (5.1) | 3.3 (0.6) | |
| 11847 | 2 | A (NE) | (Increase 0.9 fold) | 2.1 (7.0 | 59.6 (149.0) | 5.2 (2.7) | |
| Moderately differentiated | 11681 | 3A | A (NE) | 70 | 2.7 (2.1) | 2.3 (3.3) | 4.1 (1.4) |
| 12015 | 1B | A (NE) | 40 | 3.3 (8.3) | 16.8 (2.4) | 15.3 (8.1) | |
| Litinis Plastica | |||||||
| Poorly differentiated | 7532 | 4 | D (E) | 2.3 | 56.2 | 25.7 | |
| Non-epithelial Stromal Sarcoma | |||||||
| Poorly differentiated: | 11577 | D (UN) | 1.1 | 4.6 | 1.3 | ||
The values in parentheses are the fold of activity in tumor as compared to that of non-tumor specimen from the same patient
D Dead, E evidence of cancer; NE no evidence of cancer; UN unknown
Discussion
Although the overall incidence of gastric cancer has steadily declined in the United States, more than 12,000 persons died from gastric cancer in 2003 (Layke and Lopez 2004). The incidence of distal stomach tumors has greatly declined, but reported cases of proximal gastric carcinomas, including tumors at the gastroesophageal junction, have increased (Layke and Lopez 2004). Early diagnosis of gastric cancer is difficult because most patients are asymptomatic in the early stage (Layke and Lopez 2004). The incidence of gastric cancer in developing countries is much higher and is second only to lung cancer in rates of mortality (Layke and Lopez 2004). Ninety-five percent of all malignant gastric tumors are adenocarcinomas, the remaining 5% include lymphomas, stromal tumors and other rare tumors (Layke and Lopez 2004).
Many risk factors have been associated with the development of gastric cancer, and the pathogenesis is most likely multifactorial. One postulation on the development of this disease involves a succession of histologic changes that commence with atrophic gastritis, advance to mucosal metaplasia, and eventually result in a malignancy (Layke and Lopez 2004). Genetic abnormalities (such as DNA aneuploidy, oncogene amplification or mutation, and allelic loss of tumor suppressor genes) are not understood well enough to allow formulation of a sequence of progression to the development of gastric carcinoma (Natomi et al. 1993).
Tumor development and growth can be viewed as uncontrolled tissue growth. As normal organ development and growth relies on the blood supply, tumor growth relies on blood supply by the blood vessels (Kobayashi et al. 1999). It has been known that a growing tumor secretes factors that induce blood vessel growth (i.e., neovascularization) to support its own growth and survival (Folkman 1995). A primary cell type of the blood vessels, especially microvessels in a tumor, is an endothelial cell. Therefore, focus has been to discover factors that specifically control endothelial cell proliferation (Davis et al. 2003). It has been recognized for some time that cancer is largely a disease of genes, in which cumulative mutation in a spectrum of proto-oncogenes and tumor suppressor genes lead to the initiation and progression of cancer. However, it is also very clear that mutations of these genes alone do not determine the disease (Sogn et al. 2005). The roles of other factors are just beginning to be understood (Sogn et al. 2005). The tumor microenvironment needs to be completely characterized for understanding its role in tumor progression and metastasis (Sogn et al. 2005). Glycoproteins play a role in pathological processes due to immunological response to the altered oligosaccharides (Fukuda 1996). They have roles in cell adhesion during inflammation and metastasis (Fukuda 1996). Hence it is anticipated that glycoproteins may have a definite role in tumor microenvironment.
Recently an important role for galectins in cancer micrometastasis became evident from the report of Khaldoyandidi et al. (2003) that interactions between T-antigen of breast cancer cells and galectin-3 mediate both homotypic and heterotypic intercellular adhesion of metastatic breast cancer cells under conditions of flow in vitro and in vivo. A complex pattern of various galectins in several human tumor cell lines but a preferential expression of galectin-4 in colon tumor cell lines was identified by Lahn et al. (2001) by RT-PCR analysis. A substitution of sulfate at C-3 position of β-galactosyl residue in T-hapten enhanced its binding efficiency about 15-fold towards galectin-4 and threefold towards galectin-3 (Ideo et al. 2002). Similarly, the studies of our laboratory on galectin specificities showed that 3-O-Sulfo Galβ1,4GlcNAc as compared to Galβ1,4GlcNAc was threefold more efficient in binding to galectin-1 (Allen et al. 1998). Thus, the enhancement of the binding ability to galectins by 3-O-Sulfation of β-galactosyl residues appears to be a common characteristic of galectin family. In addition, 3′-sialylated Core1 in contrast to 3′-sulfated Core1 had very weak affinity for galectin-4 (Ideo et al. 2002). Hippo et al. (2001) analyzed six gastric cancer cell lines by Northern blot and observed an up-regulation of galectin-4. Sulfatide was found as a major acidic glycolipid in human gastric mucosa (Natomi et al. 1993) and the expression of cerebroside sulfotransferase mRNA in endoscopic bioptic specimens of eleven gastric cancer cases was reported by Kobayashi et al. (1999). The present study finds several fold consistent increase in Gal3Sulfotransferase activity in gastric tumor. The resulting 3-O-Sulfogalactosyl residues in gastric tumor cell glycoproteins and glycolipids may facilitate the interaction of gastric tumor cells with galectin-4, resulting in intercellular homotypic and heterotypic adhesion of gastric cancer cells.
In summary, it becomes evident from the present study that among the various transferases, which can modify the terminal Gal residues in carbohydrate chains as shown in Fig. 1, only Gal3Sulfotransferases show a consistent several fold elevated activity in all gastric tumor specimens: Gal3-sulfo-T4 (Gal3-sulfortransferase specific for Galβ1,3GalNAcα-O-Ser/Thr) and GlcNAc6-Sulfo-T are apparently associated with poorly differentiated gastric adenocarcinoma whereas poorly differentiated Signet ring cell gastric carcinoma expresses a high level of Gal3Sulfo-T2, a Gal3Sulfotransferase acting on Galβ1,4GlcNAcβ- terminal unit. The most consistent change in glycosyltransferase activity could be found only with α1,2-FT. A significant decrease in this activity was seen in the range 40–90% in seven gastric tumor specimens. Thus, the present study was able to show that down regulation of α1,2-FT activity accompanied by induction of Gal3-O-Sulfotransferase activities acting on Gal terminals could be involved in the facile sulfation of carbohydrate chains, which may contribute to the microenvironment suitable for interaction with galectin4 in promoting tumor growth and metastasis. Furthermore, the sulfation of Gal terminal as well as GlcNAc residues would render the carbohydrate chains strong anionic charge, resistance to degradation by sialidases, galactosidases and hexosaminidases and this would increase their half-life in receptor-mediated glycoprotein clearance. A recent investigation employing microarray approach for studying functional glycomics underscores the importance of glycosyltransferase activity data by pointing out that the significance of the results derived from microarray and other gene expression data, from a general perspective, would be most powerful when used in conjunction with other biochemical data (Comelli et al. 2006). A very recent study employing carbohydrate microarrays shows that sulfate groups modulate siglec binding to sialyl-Lewis x sequence (Campanero-Rhodes et al. 2006).
The present study finds a definite relationship between enzyme activities modifying carbohydrate chain terminal structures and various clinically-relevant aspects of human gastric cancer, by examining gastric tissue specimens from a small number (12) of gastric cancer patients. The present results need further validation from a study of larger number of gastric cancer patients. We are now in the process of initiating such a study.
Acknowledgments
This work was supported by the NIH (USA) Grant CA35329 and Comprehensive Cancer Center Support Grant CA160561. We thank Ms. Charlene Romanello for her excellent secretarial assistance, and Ms. Nancy Reska for her invaluable technical assistance in the Tissue Procurement Resource.
References
- Allen HJ, Ahmed H, Matta KL (1998) Binding of synthetic sulfated ligands by human splenic galectin 1, a β-galactoside-binding lectin. Glycoconj J 15:691–695 [DOI] [PubMed] [Google Scholar]
- Amado M, Carneiro F, Seixas M, Clausen H, Sobrinho-Simoes M (1998) Dimeric sialyl-Le(x) expression in gastric carcinoma correlates with venous invasion and poor outcome. Gastroenterology 114:462–470 [DOI] [PubMed] [Google Scholar]
- Campanero-Rhodes MA, Childs RA, Kiso M, Komba S, Narvor CL, Warren J, Otto D, Crocker PR, Feizi T (2006) Carbohydrate microarrays reveal sulphation as a modulator of siglec binding. Biochem Biophys Res Commun 292:1141–1146 [DOI] [PubMed] [Google Scholar]
- Carvalho F, David L, Aubert JP, Lopez-Ferrer A, De Bolos C, Reis CA, Gartner F, Peixoto A, Alves P, Sobrinho-Simoes M (1999) Mucins and mucin-associated carbohydrate antigens expression in gastric carcinoma cell lines. Virchows Arch 435:479–485 [DOI] [PubMed] [Google Scholar]
- Chandrasekaran EV, Jain RK, Larsen RD, Wlasichuk K, Matta KL (1995) Selectin-ligands and tumor associated carbohydrate structures: specificities of α2,3-sialyltransferases in the assembly of 3′-sialyl, 6-sulfo/sialyl Lewis a and x, 3′-sialyl, 6′-sulfo Lewis x and 3′-sialyl, 6-sialyl/sulfo blood group T-hapten. Biochemistry 34:2925–2936 [DOI] [PubMed] [Google Scholar]
- Chandrasekaran EV, Jain RK, Larsen RD, Wlasichuk K, DiCioccio RA, Matta KL (1996) Specificity analysis of three clonal and five non-clonal α1,3-l-fucosyltransferases with sulfated, sialylated, or fucosylated synthetic carbohydrates as acceptors in relation to the assembly of 3′-sialyl-6′-sulfo Lewis x (the l-selectin ligand) and related complex structures. Biochemistry 35:8925–8933 [DOI] [PubMed] [Google Scholar]
- Chandrasekaran EV, Jain RK, Vig R, Matta KL (1997) The enzymatic sulfation of glycoprotein carbohydrate units: blood group T-hapten specific and two other distinct Gal:3-O-sulfotransferases as evident from specificities and kinetics and the influence of sulfate and fucose residues occurring in the carbohydrate chain on C-3 sulfation of terminal Gal. Glycobiology 7:753–768 [DOI] [PubMed] [Google Scholar]
- Chandrasekaran EV, Chawda R, Piskorz C, Locke RD, Ta A, Sharad G, Odunsi K, Lele S, Matta KL (2001) Human ovarian cancer, lymphoma spleen, and bovine milk GlcNAc:β1,4Gal/GalNAc transferases: two molecular species in ovarian tumor and induction of GalNAcβ1,4Glc synthesis by α-lactalbumin. Carbohydr Res 334:105–118 [DOI] [PubMed] [Google Scholar]
- Chandrasekaran EV, Lakhaman SS, Chawda R, Piskorz CF, Neelamegham S, Matta KL (2004) Identification of physiologically relevant substrates for cloned Gal:3-O-sulfotransferases (Gal3STs): distinct high affinity of Gal3ST-2 and LS180 sulfotransferase for the Globo H backbone, Gal3ST-3 for N-glycan multiterminal Galβ1, 4GlcNAcβ- units and 6-sulfoGalβ1, 4GlcNAcβ-, and Gal3ST-4 for the mucin core-2 trisaccharide. J Biol Chem 279:10032–10041 [DOI] [PubMed] [Google Scholar]
- Chandrasekaran EV, Xue J, Xia J, Chawda R, Piskorz C, Locke RD, Neelamegham S, Matta KL (2005) Analysis of the specificity of sialyltransferases toward mucin Core2, Globo, and related structures. Identification of the sialylation sequence and the effects of sulfate, fucose, methyl and fluoro substituents of the carbohydrate chain in the biosynthesis of selectin and siglec ligands and novel sialylation by cloned α2,3(O)sialyltransferase. Biochemistry 44:15619–15635 [DOI] [PubMed] [Google Scholar]
- Chandrasekaran EV, Xue J, Neelamegham S, Matta KL (2006) The pattern of glycosyl- and sulfotransferase activities in cancer cell lines: a predictor of individual cancer-associated distinct carbohydrate structures for the structural identification of signature glycans. Carbohydr Res 341:983–994 [DOI] [PubMed] [Google Scholar]
- Comelli EM, Head SR, Gilmartin T, Whisenant T, Haslam SM, North SJ, Wong N, Kudo T, Narimatsu H, Esko JD, Drickamer K, Dell A, Paulson JC (2006) A focused microarray approach to functional glycomics: transcriptional regulation of the glycome. Glycobiology 16:117–131 [DOI] [PubMed] [Google Scholar]
- Davis DW, McConkey DJ, Zhang W, Herbst RS (2003) Antiangiogenic tumor therapy. BioTechniques 34:1048–1063 [DOI] [PubMed] [Google Scholar]
- Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1:27–31 [DOI] [PubMed] [Google Scholar]
- Fuchs CS, Mayer RJ (1995) Gastric carcinoma. N Engl J Med 333:32–41 [DOI] [PubMed] [Google Scholar]
- Fukuda M (1996) Possible roles of tumor-associated carbohydrate antigens. Cancer Res 56:2237–2244 [PubMed] [Google Scholar]
- Hakomori S (2002) Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci USA 99:10231–10233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippo Y, Yashiro M, Ishii M, Taniguchi H, Tsutsumi S, Hirakawa K, Kodama T, Aburatani H (2001) Differential gene expression profiles of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Res 61:889–895 [PubMed] [Google Scholar]
- Hiraiwa N, Dohi T, KawakamiKimura N, Yumen M, Ohmori K, Maeda M, Kannagi R (1996) Suppression of sialyl Lewis X expression and E-selectin-mediated cell adhesion in cultured human lymphoid cells by transfection of antisense cDNA of an alpha1 → 3 fucosyltransferase (Fuc-T VII). J Biol Chem 271:31556–31561 [DOI] [PubMed] [Google Scholar]
- Ideo H, Seko A, Ohkura T, Matta KL, Yamashita K (2002) High-affinity binding of recombinant human galectin-4 to SO3- → 3Galβ1 → 3GalNAc pyranoside. Glycobiology 12:199–208 [DOI] [PubMed] [Google Scholar]
- Khaldoyanidi SK, Glinsky VV, Sikora L, Glinskii AB, Mossine VV, Quinn TP, Glinsky GV, Sriramarao P (2003) MDA-MB-435 human breast carcinoma cell homo- and heterotypic adhesion under flow conditions is mediated in part by Thomsen–Friedenreich antigen–galecin-3 interactions. J Biol Chem 278:27–4134 [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Honke K, Tsunematsu I, Kagaya H, Nishikawa S, Hokari K, Kato M, Takeda H, Sugiyama T, Higuchi A, Asaka M (1999) Detection of cerebroside sulfotransferase mRNA in human gastric mucosa and adenocarcinoma. Cancer Lett 138:45–51 [DOI] [PubMed] [Google Scholar]
- Lahm H, André S, Hoeflich A, Fischer JR, Sordat B, Kaltner H, Wolf E, Gabius HJ (2001) Comprehensive galectin fingerprinting in a panel of 61 human tumor cell lines by RT-PCR and its implications for diagnostic and therapeutic procedures. J Cancer Res Clin Oncol 127:375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layke JC, Lopez PP (2004) Gastric cancer: diagnosis and treatment options. Am Fam Physician 69:1133–1140 [PubMed] [Google Scholar]
- Natomi H, Saitoh T, Sugano K, Iwamori M, Fukayama M, Nagai Y (1993) Systemic analysis of glycosphingolipids in the human gastrointestinal tract: enrichment of sulfatides with hydroxylated longer-chain fatty acids in the gastric and duodenal mucosa. Lipids 28:737–742 [DOI] [PubMed] [Google Scholar]
- O’Hanlon TP, Lau KM, Wang XC, Lau JT (1989) Tissue-specific expression of beta-galactoside alpha 2,6-sialyltransferase. J Biol Chem 264:17289–17394 [PubMed] [Google Scholar]
- Palcic MM, Heerze LD, Pierce M, Hindsgaul O (1988) The use of hydrophobic synthetic glycosides as acceptors in glycosyltransferase assays. Glycoconj J 5:49–63 [Google Scholar]
- Paulson JC, Weinstein J, Schauer A (1989) Tissue-specific expression in of sialyltransferases. J Biol Chem 264:10931–10934 [PubMed] [Google Scholar]
- Petretti T, Schulze B, Schlag PM, Kemmner W (1999) Altered mRNA expression of glycosyltransferases in human gastric carcinomas. Biochim Biophys Acta 1428:209–218 [DOI] [PubMed] [Google Scholar]
- Poste G, Fidler IJ (1980) The pathogenesis of cancer metastasis. Nature 283:139–146 [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Ross KN, Lander ES, Golub TR (2003) A molecular signature of metastasis in primary solid tumors. Nat Genet 33:49–54 [DOI] [PubMed] [Google Scholar]
- Santos-Silva F, Fonseca A, Caffrey T, Carvalho F, Mesquita P, Reis C, Almeida R, David L, Hollingsworth MA (2005) Thomsen–Friedenreich antigen expression in gastric carcinomas is associated with MUC1 mucin VNTR polymorphism. Glycobiology 15:511–517 [DOI] [PubMed] [Google Scholar]
- Sogn JA, Anton-Culver H, Singer DS (2005) Meeting reports: NCI think tanks in cancer biology. Cancer Res 65:9117–9120 [DOI] [PubMed] [Google Scholar]
- Taniguchi N, Yoshimura M, Miyoshi E, Ihara Y, Nishikawa A, Fuji S (1996) Remodeling of cell surface glycoproteins by N-acetylglucosaminyltransferase III gene transfection: modulation of metastatic potentials and down regulation of hepatitis B virus replication. Glycobiology 6:691–694 [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J, Burchell J, Miles DW, Dalziel M (1999) MUC1 and cancer. Biochim Biophys Acta 1455:301–313 [DOI] [PubMed] [Google Scholar]
- Werther JL, Tatematsu M, Klein R, Kurihara M, Kumagai K, Llorens P, Neto JG, Bodian C, Pertsemlidis D, Yamachika T, Kitou T, Zkowitz S (1996) Sialosyl-Tn antigen as a marker of gastric cancer progression: an international study. Int J Cancer 69:193–199 [DOI] [PubMed] [Google Scholar]