Abstract
Purpose
Human hepatocellular carcinoma (HCC) is one of the most mortal tumor. In a previous study, we had constructed glycoprotein expression profiles and glycoprotein databases of three human liver cancer cell lines with diverse metastasis potential. In order to discover vital glycoproteins related to pathogenesis and metastasis of HCC, in this study we analyzed previous data with bioinformatic approach.
Methods
We took previous data to draw the protein–protein interaction (PPI) networks of liver cell lines by searching IntACT database and then using Pajeck software. Further more, we compared the differences between the three PPI networks by drawing the PPI networks of differential glycoproteins and by naming differential display PPI networks.
Results
Large numbers of proliferation and apoptosis-relative proteins interact with the differential glycoproteins, and among the differential glycoproteins there are many interactions.
Conclusions
We conclude that neither single nor several proteins cause malignant proliferation of liver cells. “Molecule groups” concept should be introduced into diagnosis and metastasis prediction of the HCC.
Keywords: Protein–protein interaction network, Human hepatocellular carcinoma
Introduction
In the present post-genomics period, understanding the biological significance contained in large biological data has became vital, and thereby bioinformatics plays increasingly important roles. Bioinformatics is a rising subject that synthetically manages mathematics, computer science and biology to elucidate biological significance contained in substantive biological data (Kremer et al. 2005). Proteomic analysis is a failure without bioinformatic tools. At present common bioinformatic tools in proteomic analysis include sequence and structure analyzing software package, molecule biology software, network informatics resource, Protein–protein interaction network software, etc (Englbrecht et al. 2005; Thorgeirsson et al. 2006; Chagoyen et al. 2006; Armano et al. 2005; Fung et al. 2005). Protein–protein interaction network can visually display interactions between proteins, easily making out hub proteins (Deeds et al. 2006; Uetz et al. 2006). In present study, we utilized the searched results from IntACT database and Pajeck software to draw Protein–protein interaction networks. IntACT (http://www.ebi.ac.uk/intact) was constructed by European Bioinfomatics institute in 2003, which provides an open source database and toolkit for storage, presentation and analysis of protein interactions. The web interface provides both textual and graphical representations of protein interactions, and allows exploring interaction networks in the context of the GO and Uniprot-KB annotations of the interacting proteins. IntAct currently contains approximately 2,200 binary and complex interactions imported from the literature and curated in collaboration with the Swiss-Prot team, open to science researchers for free (Hermjakob et al. 2004a, b; Iragne et al. 2005; Hermjakob et al. 2004a, b). Pajeck (http://www.vlado.fmf.uni-lj.si/pub/networks/pajek) is a program for large-scale network analysis, which was tapped by Vladimir Batagelj and Andrej Mrvar and extensively used for dealing with network matter in computer science, sociological and biological research. The program is a powerful and a convenient tool for visually analyzing (Ludemann et al. 2004; Batagelj et al.1998). In a previous study, we had constructed glycoprotein expression profiles and glycoprotein databases of human liver cancer cell lines with diverse metastasis potential (paper has been contributed to Archives of Biochemistry and Biophysics and unpublished till now). In this study, we used bioinformatic tools to analyze these data looking forward to find vital HCC-relative glycoproteins and elucidate the mechanism in pathogenesis and metastasis of HCC.
Methods
IntACT searches
Our constructed glycoprotein databases of three cell lines, namely normal human liver cell line Chang’s liver, non-metastatic human hepatocellular carcinoma (HCC) cell line Hep3B and highly metastatic HCC cell line MHCC97H, are displayed in Table 1. Firstly we converted IPI accession numbers of glycoproteins of our databases to SWISS-PROT accession numbers, and then searched the glycoproteins in IntACT with SP protein names, and saved the web search results as text format.
Table 1.
Identified glycoproteins from human liver cell lines
| Glycoprotein | IPI_human Acc# | Matche-d peptide-s | Protein score | Chang’s -liver | Hep3B | MHC-C -97H |
|---|---|---|---|---|---|---|
| Cell motility | ||||||
| Actin,cytoplasmic2 | IPI00021440 | 13 | 294 | + | + | + |
| Actin, alpha cardiac | IPI00023006 | 10 | 192 | + | + | + |
| Actin, cytoplasmic 1 | IPI00021439 | 13 | 369 | + | + | |
| Actin, alpha skeletal muscle | IPI00021428 | 10 | 204 | + | + | |
| Actin, aortic smooth muscle | IPI00008603 | 9 | 196 | + | + | |
| Actin, gamma-enteric smooth muscle | IPI00025416 | 8 | 188 | + | + | |
| Tubulin beta-5 chain | IPI00142634 | 14 | 235 | + | + | + |
| Tubulin beta-1 chain | IPI00011654 | 13 | 225 | + | + | + |
| Tubulin beta-2 chain | IPI00007752 | 12 | 215 | + | + | + |
| Tubulin beta polypeptide | IPI00031370 | 13 | 175 | + | + | + |
| Tubulin alpha-1 chain | IPI00387144 | 14 | 356 | + | + | |
| Tubulin alpha-6 chain | IPI00218343 | 14 | 354 | + | + | |
| Tubulin alpha 3 | IPI00328163 | 14 | 262 | + | + | |
| Similar to tubulin alpha 2 | IPI00328163 | 10 | 235 | + | + | |
| Cellular organization/biogenesis | ||||||
| keratin 8 | IPI00219205 | 19 | 250 | + | + | |
| Cofilin, non-muscle isoform | IPI00012011 | 5 | 60 | + | + | + |
| Keratin, type I cytoskeletal 18 | IPI00220985 | 17 | 155 | + | + | |
| Vimentin | IPI00216312 | 24 | 289 | + | ||
| Moesin | IPI00219365 | 5 | 94 | + | ||
| Transcription and RNA processing | ||||||
| Heterogeneous nuclear ribonucleoprotein H | IPI00013881 | 10 | 255 | + | + | + |
| Splice isoform A2 of P22626 Heterogeneous nuclear ribonucleoproteins A2/B1 | IPI00414696 | 11 | 142 | + | + | + |
| Splice isoform B1 of P22626 Heterogeneous nuclear ribonucleoproteins A2/B1 | IPI00396378 | 9 | 96 | + | ||
| Paraspeckle protein 1 | IPI00395775 | 11 | 140 | + | ||
| Paraspeckle protein 1 | IPI00395775 | 12 | 90 | + | ||
| Heterogeneous nuclear ribonucleoprotein H | IPI00013881 | 11 | 132 | + | + | |
| Splice isoform A2 of P22626 Heterogeneous nuclear ribonucleoproteins A2/B1 | IPI00414696 | 16 | 397 | + | ||
| JKTBP1delta6 | IPI00045498 | 8 | 120 | + | ||
| Poly (rC) -binding protein 1 | IPI00016610 | 3 | 64 | + | + | + |
| Protein synthesis | ||||||
| Eukaryotic translation initiation factor 3 | IPI00012795 | 8 | 66 | + | ||
| 60S acidic ribosomal protein P0 | IPI00008530 | 11 | 78 | + | + | |
| Energy generation/metabolism | ||||||
| Protein disulfide isomerase precursor | IPI00010796 | 25 | 576 | + | + | + |
| Protein disulfide isomerase A3 precursor | IPI00025252 | 20 | 256 | + | + | + |
| Alpha enolase | IPI00215736 | 12 | 90 | + | + | + |
| Alpha enolase | IPI00215736 | 19 | 271 | + | + | + |
| Beta enolase | IPI00218474 | 7 | 161 | + | + | + |
| Glutamate dehydrogenase 1 | IPI00016801 | 14 | 137 | + | + | + |
| Alpha enolase | IPI00215736 | 15 | 154 | + | + | + |
| Aldolase A | IPI00395757 | 11 | 263 | + | + | |
| Glyceraldehyde-3-phosphate dehydrogenase | IPI00219018 | 6 | 106 | + | + | + |
| Glyceraldehyde-3-phosphate dehydrogenase | IPI00219018 | 11 | 145 | + | + | + |
| Glyceraldehyde-3-phosphate Dehydrogenase,liver (GAPDH) | IPI00247601 | 4 | 115 | + | ||
| Pyruvate kinase, M1 isozyme | IPI00383237 | 24 | 224 | + | + | |
| Phosphoglycerete kinase 1 | IPI00169383 | 18 | 352 | + | + | |
| Phosphoglycerate kinase 1 | IPI00295540 | 18 | 257 | + | + | |
| Phosphoglycerete kinase 1 | IPI00169383 | 12 | 121 | + | + | |
| Bifunctional purine biosynthesis protein | IPI00289499 | 18 | 173 | + | ||
| Aldehyde dehydrogenase 1A1 | IPI00218914 | 10 | 216 | + | + | |
| Guanine deaminase | IPI00032461 | 9 | 87 | + | ||
| Transketolase | IPI00021716 | 10 | 182 | + | ||
| UDP-N-acteylglucosamine pyrophosphorylase 1 | IPI00410113 | 10 | 75 | + | + | + |
| Aldo-keto reductase family 1 member B10 | IPI00105407 | 6 | 115 | + | + | |
| Aldo-keto reductase family 1 member B10 | IPI00105407 | 7 | 180 | + | + | |
| Succinyl-CoA:3-ketoacid-coenzyme A transferase 1, mitochondrial precursor | IPI00026516 | 5 | 168 | + | ||
| Galactokinase | IPI00019383 | 10 | 65 | + | + | + |
| Glucose-6-phosphate dehydrogenase | IPI00289800 | 4 | 87 | + | ||
| Retinal dehydrogenase 1 | IPI00218914 | 3 | 77 | + | ||
| Aldehyde dehydrogenase, mitochondrial precursor | IPI00006663 | 4 | 102 | + | ||
| Redox regulation of the cell | ||||||
| Superoxide dismutase [Mn], mitochondrial precursor | IPI00022314 | 9 | 96 | + | + | + |
| Peroxiredoxin 1 | IPI00000874 | 9 | 134 | + | + | + |
| Peroxiredoxin 2 isoform b | IPI00375400 | 6 | 74 | + | + | + |
| Peroxiredoxin 6 | IPI00220301 | 10 | 429 | + | + | + |
| Signal transduction | ||||||
| 78 kDa glucose-regulated protein | IPI00003362 | 23 | 458 | + | + | |
| Stress-70 protein, mitochondrial precursor | IPI00007765 | 19 | 441 | + | ||
| 14-3-3 protein zeta/delta | IPI00021263 | 9 | 68 | + | + | |
| 150 kDa oxygen-regulated protein precursor | IPI00000877 | 25 | 152 | + | + | + |
| Molecular chaperone | ||||||
| Endoplasmin precursor | IPI00027230 | 24 | 267 | + | + | + |
| Peptidyl-prolyl cis-trans isomerase A | IPI00006664 | 5 | 117 | + | + | + |
| Peptidyl-prolyl cis-trans isomerase A | IPI00006664 | 5 | 182 | + | + | + |
| T-complex protein 1, zeta subunit | IPI00027626 | 24 | 265 | + | + | |
| Intracellular trafficking | ||||||
| Annexin A2 | IPI00414519 | 13 | 230 | + | ||
| Annexin A2 | IPI00414519 | 15 | 111 | + | + | + |
| Annexin A2 | IPI00414519 | 11 | 70 | + | + | |
| Copine 1 | IPI00018452 | 7 | 82 | + | ||
| Proteinase | ||||||
| Cathepsin D precursor | IPI00011229 | 3 | 111 | + | + | + |
| Proteasome subunit beta type 7 precursor | IPI00003217 | 6 | 72 | + | + | + |
| Inhibiting DNA synthesis | ||||||
| Prohibitin | IPI00017334 | 16 | 464 | + | ||
| Ion channel | ||||||
| Chloride intracellular channel protein 1 | IPI00010896 | 2 | 122 | + | + | |
| Unclassified | ||||||
| DnaJ homolog subfamily B member 11 | IPI00008454 | 9 | 119 | + | + | + |
| Similar to RIKEN cDNA 4732481H14 | IPI00401281 | 4 | 112 | + | + | |
| Similar to Kelch motif containing protein | IPI00386640 | 4 | 96 | + | + | + |
Utilizing multiplexed proteomics technique, we had successfully identidfied 80 glycoproteins from three cell lines via peptide mass profiling using MALDI-TOF-MS/MS
Conversion of data format
We used PERL language to compile the program, then utilized the program to read the previously saved text files, gave dots and lines to the image definition using SP protein names as index, and then saved all the results. We converted these saved interaction data into Pajek software format (*.net). The format consists of two parts: vertices containing all protein names and order, and the edges containing information about binary action relation between two dots. Finally protein–protein interaction network figures were created by Pajek software.
Results
Protein–protein interaction (PPI) networks
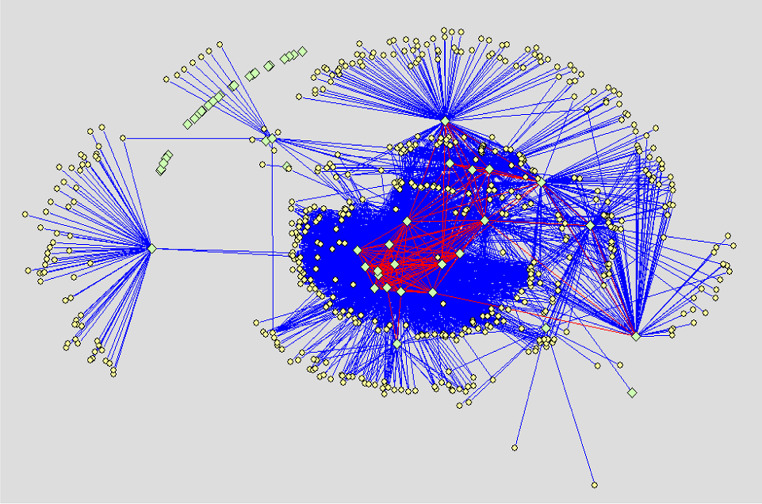
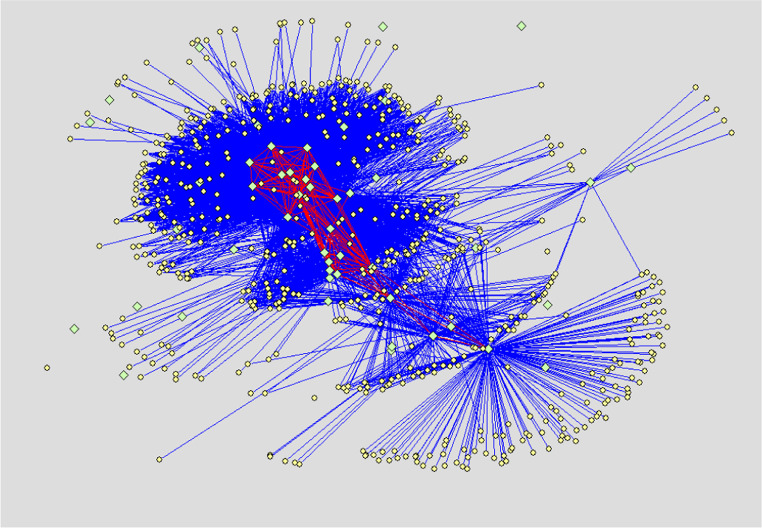
Protein–protein interaction networks of normal human liver cell line Chang’s liver, non-metastatic human hepatocellular carcinoma (HCC) cell line Hep3B and highly metastatic HCC cell line MHCC97H are displayed respectively in Figs. 1, 2 and 3. Results show that the glycoproteins identified in our study extensively interact with proteins reported by literature. Moreover, many interactions also exist between the glycoproteins. The number of proteins interacting with the glycoproteins of Chang’s liver, Hep3B and MHCC97H cell line, respectively, are 571, 645 and 574, and the number of protein binary interactions of three cell lines are 4643, 4373 and 3702, respectively.
Fig. 1.
PPI network of normal human liver cell line Chang’s liver. Ligh green diamonds denote glycoproteins identified in our experiment, Light yellow ellipses denote proteins reported by literature and interacting with the glycoproteins. Blue lines indicate interactions between the glycoproteins identified in our experiment and the proteins reported by literature. Red lines demonstrate interactions between the glycoproteins identified in our experiment
Fig. 2.
PPI network of non-metastatic HCC cell line Hep3B. Light green diamonds denote glycoproteins identified in our experiment, Light yellow ellipses denote proteins reported by literature and interacting with the glycoproteins. Blue lines indicate interactions between the glycoproteins identified in our experiment and the proteins reported by literature. Red lines demonstrate interactions between the glycoproteins themselves identified in our experiment
Fig. 3.
PPI network of high-metastatic HCC cell line MHCC97H. Light green diamonds denote glycoproteins identified in our experiment, Light yellow ellipses denote proteins reported by literature and interacting with the glycoproteins. Blue lines indicate interactions between the glycoproteins identified in our experiment and the proteins reported by literature. Red lines demonstrate interactions between the glycoproteins themselves identified in our experiment
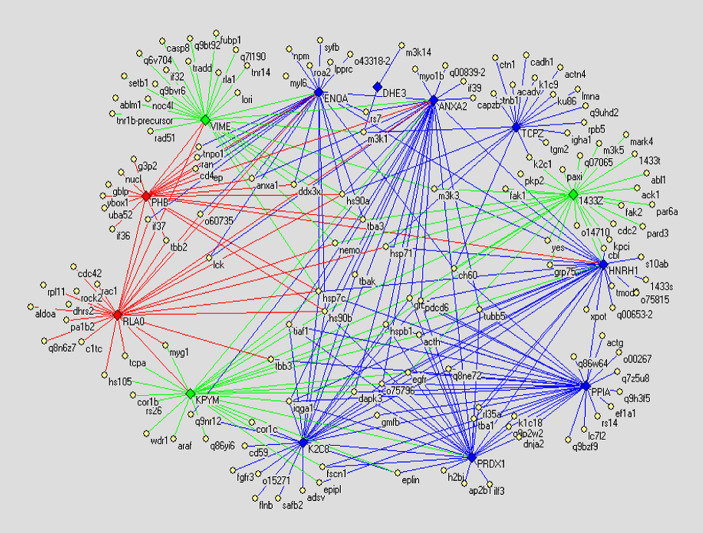
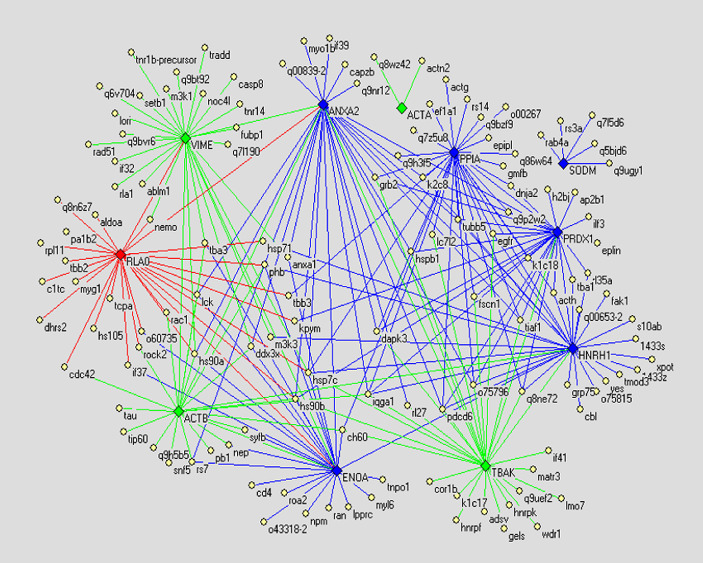
Protein–protein interaction network of different glycoproteins
In order to compare the PPI networks of three cell lines finding vital proteins, we drew the PPI networks of differential glycoproteins. The differential proteins consist of displaying or not displaying glycoproteins and glycoproteins with altered glycosylation. The glycoproteins with altered glycosylation are shown in Tables 2, 3, 4. We take only the differential glycoproteins as center to draw PPI networks. However, since proteins interacting with the differential glycoproteins are so excessive that the interactions cannot be discerned, we filtrated these proteins following the principles given below: reducing function-unclear proteins and redundant proteins. In result, the PPI networks of differential glycoproteins are shown in Figs. 4, 5 and 6.
Table 2.
The identified glycoproteins with alterated glycosylation between Chang’s liver and Hep3B
| No. | Glycoprotein | Theor. Mr | Theor. PI | Chang’s liver | Hep3B |
|---|---|---|---|---|---|
| 15 | Keratin 8 | 53.6711 | 5.52 | + | +↑ |
| 20 | Heterogeneous nuclear ribonucleoprotein H | 49.1984 | 5.89 | + | +↑ |
| 28 | Poly (rC) -binding protein 1 | 37.4739 | 6.66 | + | +↑ |
| 32 | Protein disulfide isomerase A3 precursor | 56.7468 | 5.98 | + | +↑ |
| 34 | Alpha enolase | 47.0083 | 6.99 | + | +↑ |
| 35 | Beta enolase | 46.9733 | 7.59 | + | +↑ |
| 36 | Glutamate dehydrogenase 1 | 61.3592 | 7.66 | + | +↑ |
| 39 | Glyceraldehyde-3-phosphate dehydrogenase | 36.0304 | 8.57 | + | +↓ |
| 50 | UDP-N-acteylglucosamine pyrophosphorylase 1 | 56.9944 | 5.94 | + | +↓ |
| 54 | Galactokinase | 42.2456 | 6.04 | + | +↑ |
| 59 | Peroxiredoxin 1 | 22.0963 | 8.27 | + | +↓ |
| 60 | Peroxiredoxin 2 isoform b | 15.9793 | 6.13 | + | +↓ |
| 65 | 150 kDa oxygen-regulated protein precursor | 111.2662 | 5.16 | + | +↑ |
| 67 | Peptidyl-prolyl cis-trans isomerase A | 17.8698 | 7.82 | + | +↓ |
| 68 | Peptidyl-prolyl cis-trans isomerase A | 17.8698 | 7.82 | + | +↓ |
| 69 | T-complex protein 1, zeta subunit | 57.9876 | 6.23 | + | +↓ |
| 71 | Annexin A2 | 40.3857 | 8.53 | + | +↓ |
| 74 | Cathepsin D precursor | 44.5236 | 6.1 | + | +↑ |
| 78 | DnaJ homolog subfamily B member 11 | 40.4886 | 5.81 | + | +↑ |
↑ and ↓ denote significant up-regulation and down-regulation of glycosylation extent comparing Chang’s liver with Hep3B (P < 0.05)
Table 3.
The identified glycoproteins with alterated glycosylation between Chang’s liver and MHCC97H
| No. | Glycoprotein | Theor. Mr | Theor. PI | Chang’s liver | MHCC -97H |
|---|---|---|---|---|---|
| 20 | Heterogeneous nuclear ribonucleoprotein H | 49.1984 | 5.89 | + | +↑ |
| 21 | Splice isoform A2 of P22626 Heterogeneous nuclear ribonucleoproteins A2/B1 | 35.9839 | 8.67 | + | +↑ |
| 28 | Poly (rC)-binding protein 1 | 37.4739 | 6.66 | + | +↑ |
| 33 | Alpha enolase | 47.0083 | 6.99 | + | +↓ |
| 34 | Alpha enolase | 47.0083 | 6.99 | + | +↓ |
| 35 | Beta enolase | 46.9733 | 7.59 | + | +↓ |
| 50 | UDP-N-acteylglucosamine pyrophosphorylase 1 | 56.9944 | 5.94 | + | +↓ |
| 51 | Aldo–keto reductase family 1 member B10 | 35.9978 | 7.12 | + | +↑ |
| 54 | Galactokinase | 42.2456 | 6.04 | + | +↑ |
| 58 | Superoxide dismutase [Mn], mitochondrial precursor | 24.7066 | 8.35 | + | +↓ |
| 59 | Peroxiredoxin 1 | 22.0963 | 8.27 | + | +↓ |
| 60 | Peroxiredoxin 2 isoform b | 15.9793 | 6.13 | + | +↓ |
| 65 | 150 kDa oxygen-regulated protein precursor | 111.2662 | 5.16 | + | +↑ |
| 68 | Peptidyl-prolyl cis-trans isomerase A | 17.8698 | 7.82 | + | +↓ |
| 71 | Annexin A2 | 40.3857 | 8.53 | + | +↓ |
| 74 | Cathepsin D precursor | 44.5236 | 6.1 | + | +↑ |
| 75 | Proteasome subunit beta type 7 precursor | 30.2883 | 7.57 | + | +↓ |
| 77 | Chloride intracellular channel protein 1 | 26.9058 | 5.09 | + | +↑ |
| 78 | DnaJ homolog subfamily B member 11 | 40.4886 | 5.81 | + | +↑ |
↑ and ↓ denote significant up-regulation and down-regulation of glycosylation extent comparing Chang’s liver with MHCC97H (P < 0.05)
Table 4.
The identified glycoproteins with alterated glycosylation between Hep3B and MHCC97H
| No. | Glycoprotein | Theor. Mr | Theor. PI | Hep3B | MHCC -97H |
|---|---|---|---|---|---|
| 7 | Tubulin beta-5 chain | 49.639 | 4.78 | + | +↓ |
| 8 | Tubulin beta-1 chain | 49.7269 | 4.75 | + | +↓ |
| 9 | Tubulin beta-2 chain | 49.779 | 4.79 | + | +↓ |
| 10 | Tubulin beta polypeptide | 49.9209 | 4.78 | + | +↓ |
| 20 | Heterogeneous nuclear ribonucleoprotein H | 49.1984 | 5.89 | + | +↑ |
| 21 | Splice isoform A2 of P22626 Heterogeneous nuclear ribonucleoproteins A2/B1 | 35.9839 | 8.67 | + | +↑ |
| 28 | Poly (rC) -binding protein 1 | 37.4739 | 6.66 | + | +↑ |
| 32 | Protein disulfide isomerase A3 precursor | 56.7468 | 5.98 | + | +↓ |
| 33 | Alpha enolase | 47.0083 | 6.99 | + | +↓ |
| 34 | Alpha enolase | 47.0083 | 6.99 | + | +↓ |
| 35 | Beta enolase | 46.9733 | 7.59 | + | +↓ |
| 36 | Glutamate dehydrogenase 1 | 61.3592 | 7.66 | + | +↓ |
| 39 | Glyceraldehyde-3-phosphate dehydrogenase | 36.0304 | 8.57 | + | +↑ |
| 40 | Glyceraldehyde-3-phosphate dehydrogenase | 36.0304 | 8.57 | + | +↑ |
| 50 | UDP-N-acteylglucosamine pyrophosphorylase 1 | 56.9944 | 5.94 | + | +↓ |
| 54 | Galactokinase | 42.2456 | 6.04 | + | +↓ |
| 58 | Superoxide dismutase [Mn], mitochondrial precursor | 24.7066 | 8.35 | + | +↓ |
| 65 | 150 kDa oxygen-regulated protein precursor | 111.2662 | 5.16 | + | +↑ |
| 66 | Endoplasmin precursor | 92.4113 | 4.76 | + | +↑ |
| 68 | Peptidyl-prolyl cis-trans isomerase A | 17.8698 | 7.82 | + | +↓ |
| 71 | Annexin A2 | 40.3857 | 8.53 | + | +↑ |
| 74 | Cathepsin D precursor | 44.5236 | 6.1 | + | +↑ |
| 78 | DnaJ homolog subfamily B member 11 | 40.4886 | 5.81 | + | +↓ |
| 80 | Similar to Kelch motif containing protein | 26.0191 | 6.59 | + | +↑ |
↑ and ↓ denote significant up-regulation and down-regulation of glycosylation extent comparing Hep3B with MHCC97H (P < 0.05)
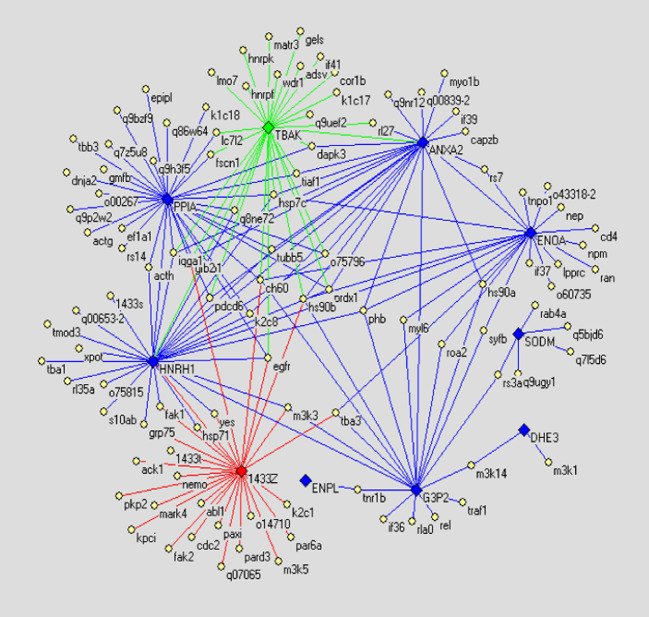
Fig. 4.
The differential display PPI network demonstrating difference between PPI networks of normal human liver cell line Chang’s liver and non-metastatic HCC cell line Hep3B. Diamonds denote differential glycoproteins. Thereinto green ones indicate the glycoproteins only expressed in Chang’s liver; red ones indicate the glycoproteins only expressed in Hep3B; blue ones indicate glycoproteins expressed in both cell lines but their glycosylation altered. Yellow ellipses denote proteins reported by literature. Lines indicate interactions between proteins, in which green lines indicate interactions only existing in Chang’s liver, red lines indicate interactions only existing in Hep3B, and blue lines indicate interactions existing in both cell lines
Fig. 5.
The differential display PPI network demonstrating difference between PPI networks of normal human liver cell line Chang’s liver and highly metastatic HCC cell line MHCC97H. Diamonds denote differential glycoproteins. Thereinto green ones indicate the glycoproteins only expressed in Chang’s liver; red ones indicate the glycoproteins only expressed in MHCC97H; blue ones indicate glycoproteins expressed in both cell lines but their glycosylation alter. Yellow ellipses denote proteins reported by literature. Lines indicate interactions between proteins, in which green lines indicate interactions only existing in Chang’s liver, red lines indicate interactions only existing in MHCC97H, and blue lines indicate interactions existing in both cell lines
Fig. 6.
The differential display PPI network demonstrating difference between PPI networks of non-metastatic HCC cell line Hep3B and highly metastatic HCC cell line MHCC97H. Diamonds denote differential glycoproteins. Thereinto green ones indicate the glycoproteins only expressed in Hep3B; red ones indicate the glycoproteins only expressed in MHCC97H; blue ones indicate glycoproteins expressed in both cell lines but their glycosylation alter. Yellow ellipses denote proteins reported by literature. Lines indicate interactions between proteins, in which green lines indicate interactions only existing in Hep3B, red lines indicate interactions only existing in MHCC97H, and blue lines indicate interactions existing in both cell lines
Discussion
In the present study, to compare PPI networks of three cell lines, we drew the PPI networks of differential glycoproteins based on the PPI networks of total glycoproteins, naming the differential display PPI networks below. In the differential display PPI network (Fig. 4) demonstrating difference between PPI networks of normal human liver cell line and non-metastatic HCC cell line, for instance, glycoprotein VIME (vimentin) is expressed only in normal human liver cell line, and proteins interacting with it include hs90a (Heat shock protein 90-alpha), tba3 (Tubulin alpha-3 chain), m3k1 (Mitogen-activated protein kinase kinase kinase 1), casp8 (Caspase-8 precursor), ran (GTP-binding nuclear protein), etc. Such proteins functionally involve in cell proliferation, motility, apoptosis, etc, according to the GO and uniprot-kb annotations, however, these interactions are void in non-metastatic HCC cell line. Thereby we consider that the lack of the interactions are possibly concerned with the pathogenesis of HCC to a certain extent. On the other hand, Glycoprotein PHB (prohibitin) is expressed only in non-metastatic HCC cell line, and proteins interacting with it include hsp7c (Heat shock cognate 71 kDa protein), ran, tbb2 (Tubulin beta-2 chain), etc. Such proteins are related with cell proliferation and apoptosis similarly, and these interactions are devoid off normal human cell line. Thereby we consider that such interactions are likely to partly precipitate pathogenesis of HCC. In addition, we also investigate glycoproteins with altered glycosylation in Fig. 4, for example, ENOA (Alpha enolase), finding that proteins hsp90b, lck (Proto-oncogene tyrosine-protein kinase), o43318-2 (Mitogen-activated protein kinase kinase kinase 7), tba3, etc. interact with it, Furthermore such proteins functionally involve in proliferation and apoptosis of cell also: so the glycosylation alteration of such glycoproteins might impact on the interactions and consequently promote malignant proliferation. Generally speaking, the proteins interacting with the differential glycoproteins between normal human liver cell line and non-metastatic HCC cell line have haet-shock protein family, cyto-skeletal proteins, mitogen-activated protein kinase kinase kinase family, cell apoptosis-related proteins, tumor necrosis factor receptor superfamily, etc. Further, the differential glycoproteins also interact with each other, hence in the cells the differential glycoproteins, these proliferations and apoptosis-relative proteins form enormous interaction networks, and finally cause malignant proliferation of cells. Analysing the differential display PPI network (Fig. 5) indicating the difference between PPI networks of normal human liver cell line and highly metastatic HCC cell line, we found that Fig. 4 resembles Fig. 5 in the way the differential glycoproteins interact with other proteins, and the proteins interacting with the differential glycoproteins also are mostly proliferative and apoptosis-relative proteins. Similarily we analyzed the differential display PPI network (Fig. 6) indicating the difference between PPI networks of non-metastatic HCC cell line and highly metastatic HCC cell line. As shown in Fig. 6, glycoprotein 1433z (14-3-3zeta) is expressed only in highly metastatic HCC cell line, and the proteins interacting with it, namely tba3, hs90a, m3k1 (Mitogen-activated protein kinase kinase kinase 1), cdc2 (Cell division control protein 2 homolog), Fak1 (Focal adhesion kinase 1) and kpci (Protein kinase C, iota type) are functionally related to cell motility, proliferation, apoptosis and polarization. Hence we suggest that such interactions possibly concern metastasis of the tumor. Regarding other differential glycoproteins in Fig. 6, the proteins interacting with them are also fundamentally heat-shock proteins, cyto-skeletal proteins, cell cycle and apoptosis-relative proteins, cell motility, etc.
In short, from our study we conclude that not single or several proteins promote malignant proliferation of cells, so expecting single or several special proteins as markers for predicting and diagnosing HCC is not feasible, and using one or two proteins as target for treating HCC also is not effectual. “Molecule groups” concept should be introduced into diagnosis and prediction of the tumor metastasis.
Acknowledgments
This research was supported by National Basic 425 Research Priorities Programme (001CB510205), National Nature 426 Science Foundation (30170416) and National Tenth Five-Year Plan Key Scientific Programme (2004BA703B02).
Abbreviations
- HCC
Human hepatocellular carcinoma
- PPI
Protein–protein interaction network
References
- Armano G, Mancosu G, Milanesi L (2005) A hybrid genetic-neural system for predicting protein secondary structure. BMC Bioinformatics 6(Suppl 4):S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batagelj V, Mrvar A (1998) Pajek–program for large network analysis. Connections 21:47–57 [Google Scholar]
- Chagoyen M, Carmona-Saez P, Shatkay H (2006) Discovering semantic features in the literature: a foundation for building functional associations. BMC Bioinformatics 7:41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeds EJ, Ashenberg O, Shakhnovich E (2006) A simple physical model for scaling in protein–protein interaction networks. PNAS 103:311–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englbrecht CC, Facius A (2005) Bioinformatics challenges in proteomics. Comb Chem High Throughput Screen 8:705–715 [DOI] [PubMed] [Google Scholar]
- Fung ET, Weinberger SR, Gavin E (2005) Bioinformatics approaches in clinical proteomics. Expert Rev Proteomics 2:847–862 [DOI] [PubMed] [Google Scholar]
- Hermjakob H, Montecchi-Palazzi L, Bader G, Wojcik J, Salwinski L, Ceol A, Moore S, Orchard S, Sarkans U, Mering CV, Roechert B, Poux S, Jung E, Mersch H, Kersey P, Lappe M, Lix Y, Zeng R, Rana D, Nikolski M, Husi H, Brun C, Shanker K, Grant SGN, Sander C, Bork P, Zhu WM, Pandey A, Brazma A, Jacq B, Vidal M, Sherman D, Legrain P, Cesareni G, Xenarios I, Eisenberg D, Steipe B, Hogue C, Apweiler R (2004a) The HUPO PSI molecular interaction format–a community standard for the representation of protein interaction data. Nat Biotechnol 22:177–183 [DOI] [PubMed] [Google Scholar]
- Hermjakob H, Montecchi-Palazzi L, Lewington C, Mudali S, Kerrien S, Orchard S, Vingron M, Roechert B, Roepstorff P, Valencia A, Margalit H, Armstrong J, Bairoch A, Cesareni G, Sherman D, Apweiler R (2004b) IntAct–an open source molecular interaction database. Nucl Acids Res 32:452–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iragne F, Nikolski M, Mathieu B, Auber D, Sherman D (2005) ProViz is a tool for the visualization of protein–protein interaction networks, developed by the IntAct European project. Bioinformatics 21:272–274 [DOI] [PubMed] [Google Scholar]
- Kremer A, Schneider R, Terstappen GC (2005) A bioinformatics perspective on proteomics: data storage, analysis, and integration. Biosci Rep 25:95–106 [DOI] [PubMed] [Google Scholar]
- Ludemann A, Weicht D, Selbig J, Kopka J (2004) PaVESy: Pathway visualization and editing system. Bioinformatics 20:2841–2844 [DOI] [PubMed] [Google Scholar]
- Thorgeirsson SS, Lee JS, Grisham JW (2006) Functional genomics of hepatocellular carcinoma. Hepatology 43:S145–S150 [DOI] [PubMed] [Google Scholar]
- Uetz P, Dong YA, Zeretzke C, Atzler C, Baiker A, Berger B, Rajagopala SV, Roupelieva M, Rose D, Fossum E, Haas J (2006) Herpesviral protein networks and their Interaction with the human Proteome. Science 311:239–242 [DOI] [PubMed] [Google Scholar]