Abstract
Purpose:Adenoid cystic carcinoma (ACC) is one of the most common malignant tumours of the salivary glands characterized by multiple recurrences and distant metastasis resulting in significantly worsening prognosis. Galectin-3, a member of the β-galactoside-binding lectin family, has been implicated in tumour progression, metastasis, and found to have prognostic value. The aim of the study was to determine galectin-3 expression in ACC and correlate it with clinicopathological features and patient survival. Methods: Galectin-3 expression was investigated in paraffin sections of 35 ACC of the head and neck. Patients were divided into two groups based on a threshold of 5% positivity in the tumour cell population. The mean follow-up period for all patients was 90.1 months (range 3–300.1 months). Results: Seventeen (48.6%) tumour specimens were considered galectin-3-positive. Galectin-3 reactivity was significantly associated with regional and distant metastasis (P=0.045 and P<0.001, respectively). There was no statistical significance in the correlation of galectin-3 expression and disease-free survival and overall survival rate (P=0.095 and 0.102, respectively). Conclusion: Galectin-3 may be used as an indicator in the prediction of metastatic spread in ACC.
Keywords: Adenoid cystic carcinoma, Galectin-3, Metastatic spread, Prognosis
Introduction
While adenoid cystic carcinoma (ACC) accounts for less than 1% of all head and neck malignancies and approximately 10% of all salivary gland cancers, this tumour is one of the most common malignancies of the minor salivary glands and the submandibular gland (Pinkston and Cole 1999). Adenoid cystic carcinoma has paradoxical clinical behaviour. It is usually characterized by slow but relentless progression and a prolonged clinical course with multiple local recurrences and distant metastasis. Lymph node metastases from ACC are rare, but its presence has a negative effect on survival (Conley and Dingman 1974). Adenoid cystic carcinoma has a relatively favourable 5- year survival rate of approximately 70%; however, the survival rates are 40% at 10 years and 25% at 15 years (Stell 1986; Spiro and Huvos 1992).
Although optimal therapy for ACC has not been established to date, aggressive local radical excision combined with postoperative radiotherapy appears to decrease the local recurrence rate (Silverman et al. 2004). However, despite locoregional control at the primary site, 35–50% of patients with ACC may still develop distant metastasis even a long time after their initial treatment had resulted in a significantly worse prognosis (Matsuba et al. 1986; Bradley 2004). The incidence of distant metastasis is likely to be more common because over a decade of observation with clinical and radiological evaluations may be required for the detection of mostly asymptomatic distant metastasis. Furthermore, in some patients, once lung metastases are detected, no further metastatic investigations are performed (Spiro 1997). The organs involved with distant metastasis in the order of decreasing frequency are the lung, bone, brain and liver (Spiro et al. 1974). Distant metastasis in the lung is usually slow growing, but when bone metastasis occurs, especially in the spine, the course of disease is usually rapidly fulminant (Bradley 2001).
Galectins are a growing family of β-galactoside-binding lectins identified by characteristic amino acid sequences. These proteins are found in a wide range of tissues and cells and are likely to be involved in a variety of physiological and pathological processes. Galectin-3 formerly known as the Mac-2 antigen, with a molecular weight of 31 kDa, is one of the more extensively studied members of the galectin family. Galectin-3 is involved in various biological phenomena including cell growth, adhesion, differentiation, angiogenesis and apoptosis (Ochieng et al. 2004). Recent research revealed that galectin-3 can be a reliable marker for cancer aggressiveness and metastasis due to its involvement in angiogenesis, cell–matrix interaction, dissemination through blood flow and extravasation (Takenaka et al. 2004). The expression of galectin-3 is associated with tumour invasion and metastatic potential in thyroid and gastric cancers (Xu et al. 1995; Lotan et al. 1994). In contrast, for cancers of breast, prostate and pancreas, the expression of galectin-3 is inversely correlated with metastatic potential (Castronovo et al. 1996; Pacis et al. 2000; Shimamura et al. 2002). The variations in galectin-3 expression in different tumours may depend on tumour-specific factors.
The aim of the present study was to determine the relationship of galectin-3 expression to clinicopathological findings and patient prognosis in ACC of the head and neck.
Materials and methods
Clinical data
Thirty-five previously untreated patients (21 females, 14 males; range 29–81 years; mean age 59.9 years) with ACC of the head and neck for whom adequate follow-up data were obtainable were selected. . The cases were retrieved from the files of the Department of Otolaryngology, Head and Neck Surgery of the University of Marburg, Germany, between July 1974 and August 2004. In most patients (51.4%) ACC originated from the extraoral minor salivary glands. The site distribution of these tumours are summarized in Table 1.
Table 1.
Site distribution of 35 head and neck adenoid cystic carcinomas
| Site | No. (%) | Total % |
|---|---|---|
| Major salivary glands | 11 (100) | 31.4 |
| Parotid gland | 5 (45.5) | 14.3 |
| Submandibular gland | 5 (45.5) | 14.3 |
| Sublingual gland | 1 (9.1) | 2.9 |
| Intraoral minor salivary glands | 4 (100) | 11.4 |
| Hard palate | 1 (25.0) | 2.9 |
| Buccal mucosa | 2 (50.0) | 5.7 |
| Floor of mouth | 1 (25.0) | 2.9 |
| Extraoral minor salivary gland | 18 (100) | 51.4 |
| Maxillary antrum | 2 (11.1) | 5.7 |
| Sphenoid sinus | 2 (11.1) | 5.7 |
| Nasal cavity | 6 (33.3) | 17.1 |
| Hypopharynx | 2 (11.1) | 5.7 |
| Larynx | 3 (16.7) | 8.6 |
| Trachea | 3 (16.7) | 8.6 |
| Lacrimal gland | 1 (2.9) | 2.9 |
| Auricular skin | 1 (2.9) | 2.9 |
The average duration of complaints at diagnosis was 11.8 months (range 1–60 months), and the most commonly reported symptoms were swelling of the tumour area (55%), pain (25%), paresthesia (10%) and bleeding (10%).
Definitively treated groups underwent surgery alone (n=17) or surgery and radiotherapy (n=5) or surgery and chemotherapy (n=6); in two patients surgery was combined with radiotherapy and chemotherapy. Two patients were treated with radiotherapy alone and one patient combined it with chemotherapy because they were considered unresectable, unsuitable for surgery, or refused surgery. Chemotherapy consisted of carboplatin in all cases. Two patients refused any kind of therapy.
About 4 out of 35 patients had enlarged cervical lymph nodes at presentation. The site of the primary was the submandibular gland, larynx, and hypopharynx in two patients. Staging of cervical lymph nodes was defined by sonography and CT scan or MRI of the neck in all cases. The metastatic infiltration of regional lymph nodes could be confirmed by histological examination after neck dissection. The metastatic lymph nodes were at some distance from the primary tumour, so that lymph node involvement by direct extension of the lesion cannot be considered in these cases.
Distant metastasis was observed in 9 out of 35 patients. The site of distant metastasis and the time interval from the primary to diagnosis of metastasis are listed in Table 2. Diagnostic workup for the detection of distant metastasis included conventional chest X-ray, bone scan and CT of the chest and abdomen and/or MRI. In the other 26 patients without distant metastasis, only 13 underwent complete clinical and laboratory evaluations and approximately yearly conventional chest X-rays. The other 13 patients of this group did not have conventional chest X-rays in the course of follow-up. The evidence of distant metastasis should be considered unknown in these patients.
Table 2.
Distant metastasis in adenoid cystic carcinoma patients
| Case no. | Primary site | Site of metastasis | Interval from diagnosis of the primary to diagnosis of metastasis (years) |
|---|---|---|---|
| 6 | Hypopharynx | Lung | 0 |
| 7 | Trachea | Lung | 4.4 |
| 12 | Trachea | Lung/liver | 12.7/16.2a |
| 13 | Maxillary antrum | Lung/bone | 11.7/12a |
| 14 | Lacrimal gland | Bone/liver | 4.9 |
| 18 | Submandibular gland | Lung | 0.9 |
| 21 | Submandibular gland | Lung | 2.1 |
| 29 | Parotid gland | Lung/liver/bone | 1.1 |
| 30 | Hypopharynx | Lung | 4.3 |
a Metachron distant metastasis
The mean follow-up period for all patients was 90.1 months (range, 3–300.1 months), and mean follow-up for the patients who were alive at the end of follow-up was 122.8 months (range, 9–300.1 months). For all patients, the disease-free survival was 49% for 5 years and 44.1% for 10 years of follow-up (n=30). Similarly, overall survival was 54.5% for 5 years and 43.7% for 10 years of follow-up (n=35). Mean survival after distant metastasis was 26.6 months (range, 2.2–93.3, n=9). Of the total patients, 11 patients were alive at the end of follow-up.
Immunohistochemical analysis
Archival, formalin fixed and in paraffin embedded specimens of the untreated primary cancer from all patients were available. All cases were histologically reviewed using haematoxylin and eosin staining to confirm the diagnosis. Perineural invasion and surgical margins of excision were investigated in patients who underwent surgical treatment of the primary tumour. The histological patterns of tumour growth were also studied. The immunohistochemical investigations were performed on deparaffined, 5-μm sections after antigen retrieval using microwave oven heating in 0.01M citrate buffer (pH 6.0). A mouse monoclonal anti-galectin-3 primary antibody (RDI, Flanders, USA) in a dilution of 1:100 and a streptavidin–biotin complex/horseradisch peroxidase kit, with 3,3-diaminobenzidine tetrahydrochloride as the chromogenic substrate (Dako, Hamburg, Germany) was used. After blocking non-specific staining, sections were incubated for 1h with anti-galectin-3 antibody. Haematoxylin was used for counterstaining. Galectin-3 stained sections were reviewed independent of clinical data of the patients. A cutoff point of 5% immunostained tumour cells was chosen, on the basis of an initial overview of the cases, to determine the range of galectin-3-positive cells and as proposed by Penner et al. (2002).
Negative controls in all cases were performed by omitting the primary antibody. Histological samples of anaplastic Ki-1-positive large-cell lymphoma were used as positive control. In addition, tissue specimens of normal salivary glands (six submandibular glands and six parotid glands) were studied.
Statistical analysis
All statistical tests were carried out using SPSS (Version 11.5, SPSS Inc., Chicago, USA). The Fisher’s exact test was used to analyze the galectin-3 dependent reactivity in relation to the different clinicopathological parameters. Overall survival time was calculated from the date of initial diagnosis until death or the last day of follow-up evaluation, whereas disease-free survival was considered to cover the period from the date of therapy to the date of recurrence or metastasis. Survival analysis was computed by means of the Kaplan–Meier method and significant levels were assessed by means of the log-rank test. P-values ≤0.05 were considered to indicate statistical significance.
Results
Galectin-3 expression and clinicopathological features
Normal salivary glands revealed a positive cytoplasmic reaction with the galectin-3 antibody, the positive cells being localized in various zones of the secretory duct system and very weak in some acinar cells. Of the 35 ACC cases immunostained for galectin-3 expression, 17 (48.6%) demonstrated galectin-3 expression in more than 5% of the tumour cells and were considered as galectin-3-positive, whereas in 18 (51.4%) cases the expression was ≤5% of tumour cells and considered as galectin-3-negative. Moderate to strong staining was present in the cytoplasm of galectin-3-positive tumour cells, which was diffuse and often of variable intensity (Fig. 1a). Nuclear localization was also sometimes observed. An intense galectin-3 immunoreactivity was present in nerve fibres (Fig. 1b).
Fig. 1.
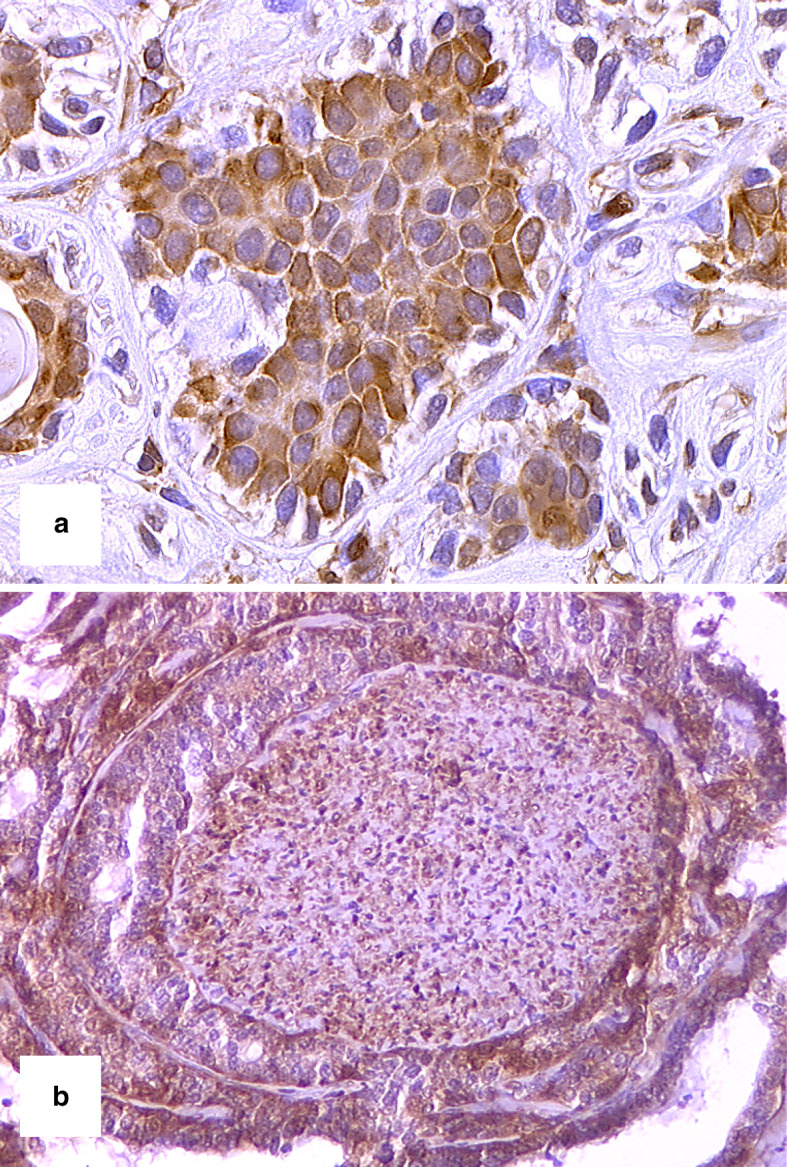
a ACC showing galectin-3 expression in solid units, 400 times, b perineural invasion of galectin-3-positive ACC. The nerve also exhibited galectin-3 immunoreactivity, 200 times
The relationship between galectin-3 expression and clinicopathologic features is summarized in Table 3. Galectin-3 expression was found to be significantly correlated with regional metastasis (P=0.045, n=35) and distant metastasis (P<0.001, n=22). There was no significant difference between the galectin-3-negative and the galectin-3-positive group with respect to age at diagnosis, gender, tumour size, tumour growth pattern, and perineural invasion.
Table 3.
Expression of galectin-3 in 35 adenoid cystic carcinomas and its correlation with clinicopathological parameters
| Galectin-3-negative no. of cases (%) | Galectin-3-positive no. of cases (%) | P-value | |
|---|---|---|---|
| Age at diagnosis | |||
| <60 years | 10 (62.5) | 6 (37.4) | 0.315 |
| ≥60 years | 8 (42.1) | 11 (57.9) | |
| Gender | |||
| Female | 12 (57.1) | 9 (42.9) | 0.500 |
| Male | 6 (42.9) | 8 (57.1) | |
| T classificationa | |||
| 1–2 | 5 (55.6) | 4 (44.4) | 1.000 |
| 3–4 | 13 (56.5) | 10 (43.5) | |
| Regional metastasis | |||
| With | 0 (0) | 4 (100) | 0.045 |
| Without | 18 (58.1) | 13 (41.9) | |
| Distant metastasis | |||
| With | 0 (0) | 9 (100) | <0.001 |
| Without | 13 (100) | 0 (0) | |
| Histologic growth pattern | |||
| Cribriform | 6 (60.0) | 4 (40.0) | 0.836 |
| Solid | 6 (50.0) | 6 (50.0) | |
| Tubular | 6 (46.2) | 7 (53.8) | |
| Perineural invasion | |||
| With | 5 (45.5) | 6 (54.5) | 1.000 |
| Without | 8 (44.4) | 10 (55.6) | |
a In three patients with ACC of the trachea the T classification could not be evaluated due to the loss of TNM classification of tumours of this area
Galectin-3 expression and survival
The disease-free survival and overall survival rate of patients with galectin-3-positive tumours tended to be lower than that of patients with galectin-3-negative tumours, but did not reach statistical significance in the correlation of galectin-3 expression and the disease-free survival, and overall survival rate (P=0.095 vs. 0.102, n=35). Figure 2a and b depict the Kaplan-Meier plot of survival curves of both groups. Median disease-free survival was 5.1 years (95% confidence interval, CI, 2.6–7.6) for the galectin-3-positive group compared with 15.7 years (95% CI, 9.4–22) for the galectin-3-negative group. Similarly, the median overall survival was 7.5 years (95% CI, 4.9–10.1) for the galectin-3-positive group compared with 12.6 years (95% CI, 7.4–17.8) for the galectin-3-negative group.
Fig. 2.
Survival rate according to galectin-3 tumour staining. a Disease-free survival rate (n=30), b overall survival rate (n=35) of patients with ACC of the head and neck
Discussion
Distant metastasis is the main problem in the management of ACC of the head and neck. . The occurrence of distant metastases in patients suffering from ACC is not related to the successful therapy of the primary tumour and the locoregional control rate (Fordice et al. 1999). In a retrospective analysis of 94 patients with ACC, half of the patients showed distant metastases without any hint to locoregional recurrence (Sung et al. 2003). Aggressive therapy of the primary tumour may achieve a high local control rate, however, without improvement of the prognosis due to distant metastases. Kim et al. (1994) investigated the survival rate of patients with ACC after detection of distant metastases and confirmed a survival rate of 41.3% after 3 years and 15.5% after 5 years. A survival rate of 32.3 months after the detection of pulmonary metastases and of 20.6 months after the detection of distant metastases in other organs was evaluated (van der Wal et al. 2002). In the present study, the mean survival after distant metastasis was 26.6 months.
Currently, no therapeutic concept for the treatment of distant metastases of ACC exists. There are controversial opinions on the surgical resection of metastases. The arguments against surgical therapy are based on the expected long survival rates in months in case the presence of pulmonary metastases that frequently occur multiply (Spiro 1997). Liu et al. (1999) showed that pulmonary resection of metastasis may prolong the survival rate. In their studies, patients who had undergone pulmonary metastasectomy had a 5-year survival rate of 84%, which continued to decline until there were no survivors after 14 years. They found no differences in survival rates between those patients with solitary or multiple pulmonary lesions.
The estimated doubling time of pulmonal metastasis in ACC ranges from 86 to 1064 days with an average of 393 days. These findings suggest that metastasis at the cellular level could occur prior (average, 227 months) to clinical presentation of primary cancer (Umeda et al. 1999). Due to the high incidence of occult pulmonary metastases they should be included in the therapy of the primary cancer. To establish an effective therapeutic concept for ACC patients, besides the aggressive therapy of the primary cancer, we think it is important to identify high- risk patients for the development of distant metastases, on the other hand it is essential to introduce an appropriate systemic chemotherapy or immunotherapy for those patient groups.
Currently there are no valid data for both mentioned aspects. The response rates of ACC to conventional cytotoxic chemotherapy such as cisplatin, 5-fluorouracil, epirubicin and antracyclines have been generally modest (Verweij et al. 1996; Hill et al. 1997; Vermorken et al. 1993). There is only little information on the factors promoting distant metastasis. Most investigations published until now describe a significantly higher occurrence of distant metastases in the histologically solid subtype (Perzin et al. 1978). Regarding other factors accompanying a high risk of distant metastasis, such as the size of the primary tumour, there are different and partly controversial statements. So a significantly low metastatic rate is assumed for ACC in the area of the sinunasal tract and palatal region (Sung et al. 2003; Haung et al. 1997). There is a need to explore additional factors for predicting distant metastasis.
In the present study, galectin-3 expression was associated with the increased incidence of regional and distant metastasis in ACC of the head and neck. The immunohistochemical evaluation of galectin-3 might be valuable to identify ACC patients at high risk for the development of metastasis. To our knowledge there are only two studies in the literature about galectin-3 expression in ACC. Xu et al. (2000) showed expression of galectin-3 in 3 out of 14 cases with ACC and concluded that the reduced expression of galectin-3 may be related to their cellular differentiation, since eight out of nine polymorphous low-grade adenocarcinoma, and eight out of nine carcinoma ex-pleomorphic adenoma were galectin-3-positive. In contrast to these findings, in the other study galectin-3 was expressed in eight out of nine cases of ACC and 14 of 14 cases of polymorphous low-grade adenocarcinoma (Penner et al. 2002). In both studies the clinical data of ACC patients are missing.
Further studies involving a larger patient population are required to provide more information regarding galectin-3 expression in ACC and its diagnostic accuracy in patients who have a high risk of distant metastasis. Galectin-3 overexpression has been detected in the serum of patients with metastatic disease compared to sera from patients with localized tumours in various types of cancer (Iurisci et al. 2000). To evaluate the performance of galectin-3 as a diagnostic marker for distant metastasis, a preoperative serological study in ACC patients is also needed.
References
- Bradley PJ (2001) Distant metastases from salivary glands cancer. ORL J Otorhinolaryngol Relat Spec 63:233–242 [DOI] [PubMed] [Google Scholar]
- Bradley PJ (2004) Adenoid cystic carcinoma of the head and neck: a review. Curr Opin Otolaryngol Head Neck Surg 12:127–132 [DOI] [PubMed] [Google Scholar]
- Castronovo V, Van Den Brule FA, Jackers P, Clausse N, Liu FT, Gillet C, Sobel ME (1996) Decreased expression of galectin-3 is associated with progression of human breast cancer. J Pathol 179:43–48 [DOI] [PubMed] [Google Scholar]
- Conley J, Dingman DL (1974) Adenoid cystic carcinoma in the head and neck (cylindroma). Arch Otolaryngol 100:81–90 [DOI] [PubMed] [Google Scholar]
- Fordice J, Kershaw C, El-Naggar A, Goepfert H (1999) Adenoid cystic carcinoma of the head and neck: predictors of morbidity and mortality. Arch Otolaryngol Head Neck Surg 125:149–152 [DOI] [PubMed] [Google Scholar]
- Hill ME, Constenla DO, A’Hern RP, Henk JM, Rhys-Evans P, Breach N, Archer D, Gore ME (1997) Cisplatin and 5-fluorouracil for symptom control in advanced salivary adenoid cystic carcinoma. Oral Oncol 33:275–258 [DOI] [PubMed] [Google Scholar]
- Huang M, Ma D, Sun K, Yu G, Guo C, Gao F (1997) Factors influencing survival rate in adenoid cystic carcinoma of the salivary glands. Int J Oral Maxillofac Surg 26:435–439 [DOI] [PubMed] [Google Scholar]
- Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, Iacobelli S (2000) Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res 6:1389–1393 [PubMed] [Google Scholar]
- Kim KH, Sung MW, Chung PS, Rhee CS, Park CI, Kim WH (1994) Adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg 120:721–726 [DOI] [PubMed] [Google Scholar]
- Liu D, Labow DM, Dang N, Martini N, Bains M, Burt M, Downey R Jr, Rusch V, Shah J, Ginsberg RJ (1999) Pulmonary metastasectomy for head and neck cancers. Ann Surg Oncol 6:572–578 [DOI] [PubMed] [Google Scholar]
- Lotan R, Ito H, Yasui W, Yokozaki H, Lotan D, Tahara E (1994) Expression of a 31-kDa lactoside-binding lectin in normal human gastric mucosa and in primary and metastatic gastric carcinomas. Int J Cancer 56:474–480 [DOI] [PubMed] [Google Scholar]
- Matsuba HM, Spector GJ, Thawley SE, Simpson JR, Mauney M, Pikul FJ (1986) Adenoid cystic salivary gland carcinoma. A histopathologic review of treatment failure patterns. Cancer 57:519–524 [DOI] [PubMed] [Google Scholar]
- Ochieng J, Furtak V, Lukyanov P (2004) Extracellular functions of galectin-3. Glycoconj J 19:527–535 [DOI] [PubMed] [Google Scholar]
- Pacis RA, Pilat MJ, Pienta KJ, Wojno K, Raz A, Hogan V, Cooper CR (2000) Decreased galectin-3 expression in prostate cancer. Prostate 44:118–123 [DOI] [PubMed] [Google Scholar]
- Penner CR, Folpe AL, Budnick SD (2002) C-kit expression distinguishes salivary gland adenoid cystic carcinoma from polymorphous low-grade adenocarcinoma. Mod Pathol 15:687–691 [DOI] [PubMed] [Google Scholar]
- Perzin KH, Gullane P, Clairmont AC (1978) Adenoid cystic carcinomas arising in salivary glands: a correlation of histologic features and clinical course. Cancer 42:265–282 [DOI] [PubMed] [Google Scholar]
- Pinkston JA, Cole P(1999) Incidence rates of salivary gland tumours: results from a population-based study. Otolaryngol Head Neck Surg 120:834–840 [DOI] [PubMed] [Google Scholar]
- Shimamura T, Sakamoto M, Ino Y, Shimada K, Kosuge T, Sato Y, Tanaka K, Sekihara H, Hirohashi S (2002) Clinicopathological significance of galectin-3 expression in ductal adenocarcinoma of the pancreas. Clin Cancer Res 8:2570–2575 [PubMed] [Google Scholar]
- Silverman DA, Carlson TP, Khuntia D, Bergstrom RT, Saxton J, Esclamado RM (2004) Role for postoperative radiation therapy in adenoid cystic carcinoma of the head and neck. Laryngoscope 114:1194–1199 [DOI] [PubMed] [Google Scholar]
- Spiro RH (1997) Distant metastasis in adenoid cystic carcinoma of salivary origin. Am J Surg 174:495–498 [DOI] [PubMed] [Google Scholar]
- Spiro RH, Huvos AG (1992) Stage means more than grade in adenoid cystic carcinoma. Am J Surg 164:623–628 [DOI] [PubMed] [Google Scholar]
- Spiro RH, Huvos AG, Strong EW (1974) Adenoid cystic carcinoma of salivary origin. A clinicopathologic study of 242 cases. Am J Surg 128:512–520 [DOI] [PubMed] [Google Scholar]
- Stell PM (1986) Adenoid cystic carcinoma. Clin Otolaryngol 11:267–291 [DOI] [PubMed] [Google Scholar]
- Sung MW, Kim KH, Kim JW, Min YG, Seong WJ, Roh JL, Lee SJ, Kwon TK, Park SW (2003) Clinicopathologic predictors and impact of distant metastasis from adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg 129:1193–1197 [DOI] [PubMed] [Google Scholar]
- Takenaka Y, Fukumori T, Raz A (2004) Galectin-3 and metastasis. Glycoconj J 19:543–549 [DOI] [PubMed] [Google Scholar]
- Umeda M, Nishimatsu N, Masago H, Ishida Y, Yokoo S, Fujioka M, Shibuya Y, Komori T (1999) Tumour-doubling time and onset of pulmonary metastasis from adenoid cystic carcinoma of the salivary gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 88:473–478 [DOI] [PubMed] [Google Scholar]
- Vermorken JB, Verweij J, de Mulder PH, Cognetti F, Clavel M, Rodenhuis S, Kirkpatrick A, Snow GB (1993) Epirubicin in patients with advanced or recurrent adenoid cystic carcinoma of the head and neck: a phase II study of the EORTC Head and Neck Cancer Cooperative Group. Ann Oncol 4:785–788 [DOI] [PubMed] [Google Scholar]
- Verweij J, de Mulder PH, de Graeff A, Vermorken JB, Wildiers J, Kerger J, Schornagel J, Cognetti F, Kirkpatrick A, Sahmoud T, Lefebvre JL (1996) Phase II study on mitoxantrone in adenoid cystic carcinomas of the head and neck. EORTC Head and Neck Cancer Cooperative Group. Ann Oncol 7:867–869 [DOI] [PubMed] [Google Scholar]
- van der Wal JE, Becking AG, Snow GB, van der Waal I (2002) Distant metastases of adenoid cystic carcinoma of the salivary glands and the value of diagnostic examinations during follow-up. Head Neck 24:779–783 [DOI] [PubMed] [Google Scholar]
- Xu XC, el-Naggar AK, Lotan R (1995) Differential expression of galectin-1 and galectin-3 in thyroid tumours. Potential diagnostic implications. Am J Pathol 147:815–822 [PMC free article] [PubMed] [Google Scholar]
- Xu XC, Sola Gallego JJ, Lotan R, El-Naggar AK (2000) Differential expression of galectin-1 and galectin-3 in benign and malignant salivary gland neoplasms. Int J Oncol 17:271–276 [DOI] [PubMed] [Google Scholar]



