Abstract
Purpose
The extracellular signal-regulated kinase (ERK) 1/2 pathway plays important roles in the regulation of cell proliferation, differentiation and cell survival. The caudal-related homeobox protein CDX2 is essential for the development of the intestine, and is related to gastric and gallbladder cancers with the intestinal phenotype. However, the roles of ERK1/2 phosphorylation (pERK1/2) and CDX2 in cholangiocarcinogenesis remain unknown.
Methods
We investigated the expression of pERK1/2, CDX2 and MUC2 in Thai cholangiocarcinoma (CCA) specimens by means of immunohistochemical staining, and compared the expression of these proteins with clinicopathological factors.
Results
The pERK1/2 protein was expressed in 29 of 59 (49.2%) CCA cases. Interestingly, in tubular-type CCA, the frequency of pERK1/2 expression was associated with a higher grade of differentiation (P = 0.001). CDX2 expression was observed in 22 of the 59 (37.3%) CCA cases, showed a relationship with MUC2 expression (P = 0.001), and was much higher in papillary-type than tubular-type CCA (P = 0.002).
Conclusion
These results imply that pERK1/2 may be important for the differentiation of tubular-type CCA, while CDX2 is related to the intestinal phenotype of papillary-type CCA.
Keywords: pERK1/2, CDX2, MUC2, Cholangiocarcinoma, KRAS
Introduction
Cholangiocarcinoma (CCA) is a primary malignant liver tumor arising from the biliary epithelium with an extremely poor prognosis. Surgical resection remains the only curative treatment and no chemotherapy regimens have yielded satisfactory responsiveness (Olnes and Erlich 2004). Although the etiology of CCA remains unclear, the following factors have been reported to be associated with CCA, that is, primary sclerosing cholangitis in the West, Opisthorchis viverrini (OV) infestation in Thailand, and Clonorchis sinensis infestation and hepatolithiasis in East Asia (Nakanuma et al. 2003; Olnes and Erlich 2004). The highest incidence of CCA has been reported in the Northeastern region of Thailand, where CCA comprises 82% of liver cancer and is strongly associated with OV infestation (Vatanasapt and Sripa 2000).
The extracellular signal-regulated kinase (ERK) 1/2 pathway is a critical signaling pathway that regulates cell proliferation, differentiation and cell survival (Chang et al. 2003). Uncontrolled activation of ERK1/2 leads to malignant transformation both in vitro and in vivo (Webb et al. 1998), and phosphorylated ERK1/2 (pERK1/2) expression has been detected in a variety of human neoplasias (Sebolt-Leopold and Herrera 2004). The mechanisms responsible for this constitutive activation remain unknown, although some has been shown to involve increased expression of growth factors and/or their receptors, and activated mutation of Ras/Raf genes (Chang et al. 2003; Sebolt-Leopold and Herrera 2004). There has been a marked lower frequency of the KRAS codon 12 or 13 mutation in OV-associated Thai CCA cases (8%), compared with Japanese CCA cases without OV infestation (58%) (Kiba et al. 1993) and German cases (45%) (Tannapfel et al. 2003). BRAF mutations have been found in 22% of German CCA cases, the majority being found at codon 599 (Tannapfel et al. 2003).
Caudal-type homeodomain transcription factor 2 (CDX2) regulates axial development and intestinal differentiation, both during the embryonic period and postnatally (Beck et al. 2000). In adults, CDX2 expression is restricted to small and large intestinal epithelial cells (Silberg et al. 2000), where it regulates several gut differentiation-related genes, such as MUC2 (Yamamoto et al. 2003), etc. Ectopic CDX2 expression has been reported in intestinal metaplasia and carcinoma of the stomach, and Barrett’s epithelium of the esophagus (Bai et al. 2002; Yuasa 2003; Eda et al. 2003), and in mucinous intrahepatic CCA and intraductal papillary neoplasia of the liver, where CDX2 expression is closely related to MUC2 expression (Ishikawa et al. 2004).
In this study, we examined the expression of pERK1/2, CDX2 and MUC2 in 59 Thai OV-associated CCA cases by means of immunohistochemical staining, and analyzed the relationship between their expression and clinicopathological factors.
Materials and methods
Clinical samples
Fifty-nine primary CCA specimens were obtained from a hospital affiliated to Khon Kaen University, Khon Kaen, Thailand. The tumors consisted of 36 intrahepatic and 23 extrahepatic CCA. Informed consent was obtained from all patients. Intrahepatic CCA cases were classified using the International Scientific Committee of the International Hepato-Pancreato-Biliary Association classification (Makuuchi et al. 2003). Extrahepatic CCA cases were classified using the fifth edition of the American Joint Commission on Cancer staging system for cancer of the extrahepatic bile duct (Sobin and Wittekind 1997). The histology of CCA was divided into two groups: papillary-type and tubular-type adenocarcinomas, and the tubular group was further classified into well-, moderately and poorly differentiated adenocarcinomas.
Immunohistochemical analyses of pERK1/2, CDX2 and MUC2
Paraffin-embedded primary CCA tissues were cut into 4-μM thick sections. The sections were de-paraffinized and antigen retrieval was performed as described previously (Wu et al. 2005). The sections were incubated overnight at 4°C with anti-phosphorylated-p44/42 MAPK (pERK1/2) (Cell Signaling Technology Inc., Beverly, MA, USA) at a dilution of 1:100, anti-CDX2 (Bio Genex, San Ramon, CA, USA) at a dilution of 1:100 and anti-MUC2 (Ccp58) (Novocastra Laboratories, Newcastle, UK) at a dilution of 1:100. After three washes with PBS, the slides were treated with Envision-labeled polymer peroxidase (Dako Cytomation, Carpinteria, CA, USA) for 1 h at room temperature, and then treated with 0.1% hydrogen peroxide and 0.6 mM 3,3-diaminobenzidine in PBS. Finally, the sections were counterstained with Mayer’s hematoxylin.
The result of pERK1/2 immunostaining was considered to be positive when more than 10% of the tumor cells were stained regardless of whether in the nucleus or the cytoplasm. Concerning CDX2 staining, only nuclear CDX2, i.e., not the cytoplasmic one, was counted. When more than 10% of cancer cells were positive for CDX2 or MUC2, such cases were regarded as positive (Wu et al. 2005).
Mutational analyses of KRAS and BRAF
Genomic DNA from CCA cells was extracted by the standard method. The methods used for KRAS codon 12 mutation analysis involving PCR-restriction fragment length polymorphism (RFLP) were described previously (Yagi et al. 1997). The KRAS codon 13 mutation was analyzed by the RFLP method using mutated primers to introduce a HaeIII site adjacent to codon 13. A PCR product derived from a wild-type sequence can be digested with HaeIII but a product derived from a mutated sequence cannot be digested. The primers used were 5′-TACTGGTGGAGTATTTGATAGTG-3′ (sense) and 5′-TCGTCAAGGCACTCTTGCCTAGG-3′ (antisense). The BRAF mutation at codon 599 was examined by the PCR-single strand conformation polymorphism method. The primers used were 5′-TCATAATGCTTGCTCTGATAGG-3′ (sense) and 5′-CCATCCACAAAATGGATCCAG-3′ (antisense). The PCR products were denatured for 10 min, run through a 17.5% polyacrylamide gel at 15°C, and then detected with a 2D-SILVER STAIN II “DAIICHI” kit (DAIICHI Pure Chemicals, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed with StatView 5.0 software. The results were considered to be significant when the P values were less than 0.05.
Results
Immunohistochemical analysis of pERK1/2
pERK1/2 expression was not detected in the normal bile duct epithelium. The expression of pERK1/2 was detected in the cytoplasm and/or nucleus in 29 (49.2%) of 59 CCA cases (Fig. 1a, b). Many of the positive cases showed pERK1/2 expression preferentially located at the periphery of the tumor. There was no statistical difference between positive pERK1/2 staining and age, sex, tumor size, histological type or distant metastasis (Table 1). However, we found a statistical correlation between pERK1/2 expression and the absence of metastatic lymph node (N0 vs N1-2, P = 0.020) (Table 1), and a higher grade of differentiation in the tubular adenocarcinoma group (P = 0.001) (Table 2).
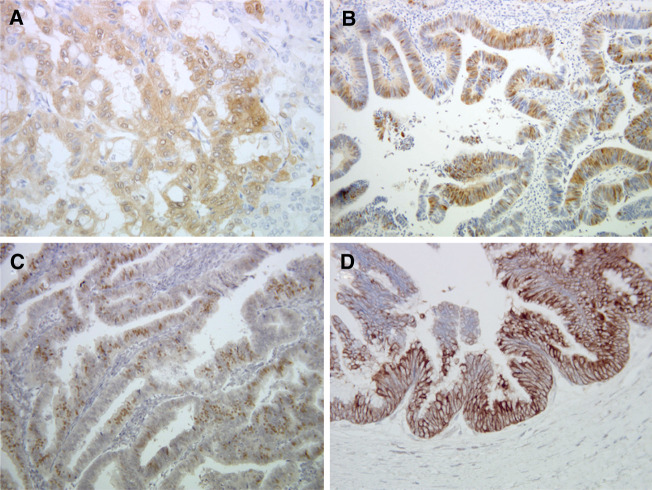
Fig. 1.
Representative examples of immunohistochemical staining of pERK1/2 in a well-differentiated (a) and a papillary-type CCA (b), CDX2 (c), and MUC2 (d) in papillary-type CCA (×200)
Table 1.
Correlation between pERK1/2 expression and clinicopathological factors
| Total | pERK1/2 (+) | pERK1/2 (−) | P value | |
|---|---|---|---|---|
| Age (year ± SD) | 57.4 ± 10.9 | 53.6 ± 9.2 | 0.142a | |
| Sex (F/M) | 14/45 | 7/22 | 7/23 | 0.942b |
| Tumor size (cm ± SD) | 6.1 ± 3.5 | 7.0 ± 3.3 | 0.276a | |
| Location | ||||
| Intrahepatic type | 36 | 19 | 17 | 0.486b |
| Extrahepatic type | 23 | 10 | 13 | |
| Histological type | ||||
| Papillary | 22 | 13 | 9 | 0.239b |
| Tubular | 37 | 16 | 21 | |
| Lymph node metastasisc | ||||
| (+) | 25 | 9 | 16 | 0.020b |
| (−) | 23 | 16 | 7 | |
| Distant metastasisd | ||||
| (+) | 13 | 7 | 6 | 0.752b |
| (−) | 43 | 21 | 22 | |
aStudent’s t test
b χ 2 independence test
cLymph node metastasis data were not available in 11 cases
dDistant metastasis data were not available in three cases
Table 2.
pERK1/2 expression in tubular-type CCA
| Total | pERK1/2 (+) | pERK1/2 (−) | P value | |
|---|---|---|---|---|
| Well | 16 | 11 | 5 | 0.001a |
| Moderately | 11 | 5 | 6 | |
| Poorly | 10 | 0 | 10 |
aMann–Whitney U test
When the CCA cases were divided into intrahepatic and extrahepatic groups, there was no statistical difference in pERK1/2 staining between the two groups (Table 1). In the intrahepatic tubular adenocarcinoma group, pERK1/2 was positive in eight of nine, five of seven, and none of nine cases with well-, moderately and poorly differentiated CCA, respectively, indicating a significant correlation between pERK1/2 expression and a higher grade of differentiation (P = 0.0002). Although the case numbers for the different differentiation grades in the extrahepatic tubular adenocarcinoma group were low (12 cases in total), there was a similar tendency for this correlation (P = 0.113). In addition, in the extrahepatic CCA group, pERK1/2-positive cases were significantly smaller than pERK1/2-negative ones (P = 0.024).
Immunohistochemical analyses of CDX2 and MUC2
Positive staining of CDX2 and MUC2 was detected in 22 (37.3%) and 19 (32.2%) of the 59 CCA cases, respectively (Fig. 1c, d). CDX2-positive CCA cases were significantly more frequent in the papillary-type than the tubular-type CCA (P = 0.002) and were smaller than CDX2-negative ones (P = 0.048) (Table 3). CDX2 expression was highly correlated with MUC2 expression (P = 0.001) (Table 4), but not correlated with the grade of differentiation in the tubular-type CCA cases (Table 5).
Table 3.
Correlation between CDX2 expression and clinicopathological factors
| Total | CDX2 (+) | CDX2 (−) | P value | |
|---|---|---|---|---|
| Age (year ± SD) | 57.1 ± 9.9 | 54.5 ± 10.3 | 0.342a | |
| Sex (F/M) | 14/45 | 5/17 | 9/28 | 0.575b |
| Tumor size (cm ± SD) | 5.5 ± 3.7 | 7.2 ± 3.0 | 0.048a | |
| Location | ||||
| Intrahepatic type | 36 | 12 | 24 | 0.431b |
| Extrahepatic type | 23 | 10 | 13 | |
| Histological type | ||||
| Papillary | 22 | 14 | 8 | 0.002b |
| Tubular | 37 | 8 | 29 | |
| Lymph node metastasisc | ||||
| (+) | 25 | 7 | 18 | 0.087b |
| (−) | 23 | 12 | 11 | |
| Distant metastasisd | ||||
| (+) | 13 | 3 | 10 | 0.228e |
| (−) | 43 | 17 | 26 | |
| pERK1/2 | ||||
| (+) | 29 | 13 | 16 | 0.239b |
| (−) | 30 | 9 | 21 | |
aStudent’s t test
b χ 2 independence test
cLymph node metastasis data were not available in 11 cases
dDistant metastasis data were not available in three cases
eFisher’s exact probability test
Table 4.
Correlation between CDX2 expression and MUC2 expression
| CDX2 (+) | CDX2 (−) | P value | |
|---|---|---|---|
| MUC2 (+) | 13 | 6 | 0.001a |
| MUC2 (−) | 9 | 31 |
a χ 2 independence test
Table 5.
Expression of CDX2 in tubular-type CCA
| Total | CDX2 (+) | CDX2 (−) | P value | |
|---|---|---|---|---|
| Well | 16 | 4 | 12 | 0.441a |
| Moderately | 11 | 3 | 8 | |
| Poorly | 10 | 1 | 9 |
aMann–Whitney U test
When the CCA cases were divided into intrahepatic and extrahepatic groups, we found that CDX2 expression was more frequent at earlier stages (stage II vs. stage III vs. stage IV, P = 0.026) for intrahepatic CCA (Table 6). In extrahepatic CCA, CDX2 expression was positively correlated with a lower T stage (T2 vs. T3, P = 0.023) and a smaller tumor size (P = 0.032), and tended to be more frequent at earlier stages (stage II vs. stage III vs. stage IV, P = 0.053) (Table 6). There was no correlation in the expression patterns between pERK1/2 and CDX2 (Table 3).
Table 6.
Correlation between CDX2 expression, and stages of intrahepatic and extrahepatic CCA
| Total | CDX2 (+) | CDX2 (−) | P value | |
|---|---|---|---|---|
| Intrahepatic | ||||
| Stage II | 5 | 4 | 1 | 0.026a |
| Stage III | 8 | 3 | 5 | |
| Stage IV | 23 | 5 | 18 | |
| Extrahepatic | ||||
| Stage II | 3 | 3 | 0 | 0.053a |
| Stage III | 2 | 1 | 1 | |
| Stage IV | 18 | 6 | 12 | |
aMann–Whitney U test
KRAS and BRAF mutation analysis
By means of PCR-RFLP, we detected a KRAS codon 12 mutation in one case and a KRAS codon 13 mutation in 3 of the 59 CCA cases. All four cases with KRAS mutations were pERK1/2-positive ones. We did not find any BRAF codon 599 mutation in the 59 CCA cases.
Discussion
Increased levels of activated ERK1/2, pERK1/2, have been reported in a variety of human cancers (Sebolt-Leopold and Herrera 2004), and several studies have also provided data on the clinical outcomes of patients with pERK1/2 expression. In non-small cell lung cancers, pERK1/2 expression is correlated with significantly lower survival (Vicent et al. 2004), but is positively correlated with better survival in small cell lung cancers (Blackhall et al. 2003). However, the frequency of pERK1/2 expression in CCA has not been reported. We found that 49.2% of the Thai OV-associated CCA cases were positive for pERK1/2, indicating that activation of the pERK1/2 pathway may be important for CCA formation. Interestingly, the expression of pERK1/2 was significantly correlated with a higher grade of differentiation in the tubular-type CCA. This expression pattern appears to be similar to those of the ERBB2 and COX-2 proteins in CCA cases (Endo et al. 2002). Since activation of ERBB2 up-regulates pERK1/2 (Rescan et al. 2005), and pERK1/2 is a regulator of COX-2 expression (Chun and Surh 2004), this pathway may be important for cholangiocarcinogenesis, particularly for the tubular type.
Several differences in genetic alterations between OV-related Thai CCA and non-OV-related CCA have been reported (Suzuki et al. 2000). Similar to the previous report of a low frequency of KRAS mutations in Thai CCA cases (Kiba et al. 1993), we found a KRAS mutation in 4 (6.8%) of the 59 CCA cases. We also did not find a BRAF mutation in any case, whereas a BRAF mutation has been detected in 15 (21.7%) of 69 German CCA cases (Tannapfel et al. 2003). These data suggest that activated mutation of KRAS/BRAF may not be an important mechanism in OV-related Thai CCA formation.
We found that CDX2 expression was associated with MUC2 expression and more frequently found in papillary-type CCA compared with tubular-type CCA. The result is similar to that for Japanese CCA cases (Ishikawa et al. 2004). Therefore, CDX2 may be important for development of the intestinal phenotype in papillary-type CCA. In contrast to in gastric and gallbladder tubular adenocarcinoma cases, where CDX2 expression is correlated with a higher differentiation grade (Bai et al. 2002; Wu et al. 2005), we did not find such a correlation for tubular-type CCA. Like that of CDX2, MUC2 expression was more frequent in papillary-type than tubular-type CCA (P = 0.006). These data suggest that the papillary type shows a more intestinal phenotype than the tubular type.
CDX2 expression has been shown to be an independent favorable prognosis marker for gastric cancer (Mizoshita et al. 2003) and carcinoma of the ampulla of Vater (Hansel et al. 2005). Although we could not obtain survival data from these CCA cases and the majority of the cases were at late stages (stages III–IV), there may be a tendency for a better prognosis in CDX2-positive CCA cases, as explained below. Firstly, CDX2 expression was significantly more frequent in the papillary-type CCA cases, which have been shown to exhibit better survival after hepatic resection (Tajima et al. 2004). Furthermore, the sizes in CDX2-positive cases tended to be smaller, and intrahepatic and extrahepatic CCA cases with CDX2 expression were associated with an earlier stage.
In conclusion, we found several correlations between the expression of pERK1/2, CDX2 and MUC2, and clinicopathological factors in OV-related Thai CCA cases. In particular, pERK1/2 may be associated with differentiation of tubular-type CCA, while CDX2 may be necessary for the intestinal phenotype of papillary-type CCA. We did not find a correlation between pERK1/2 and CDX2 expression, suggesting that the two proteins are independently associated with different types of CCA. Our data may allow a better understanding of cholangiocarcinogenesis of papillary-type and tubular-type CCA.
Acknowledgment
This work was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Abbreviations
- CCA
Cholangiocarcinoma
- ERK
Extracellular signal-regulated kinase
- OV
Opisthorchis viverrini
References
- Bai YQ, Yamamoto H, Akiyama Y, Tanaka H, Takizawa T, Koike M, Kenji Yagi O, Saitoh K, Takeshita K, Iwai T, Yuasa Y (2002) Ectopic expression of homeodomain protein CDX2 in intestinal metaplasia and carcinomas of the stomach. Cancer Lett 176:47–55 [DOI] [PubMed] [Google Scholar]
- Beck F, Tata F, Chawengsaksophak K (2000) Homeobox genes and gut development. Bioessays 22:431–441 [DOI] [PubMed] [Google Scholar]
- Blackhall FH, Pintilie M, Michael M, Leighl N, Feld R, Tsao MS, Shepherd FA (2003) Expression and prognostic significance of kit, protein kinase B, and mitogen-activated protein kinase in patients with small cell lung cancer. Clin Cancer Res 6:2241–2247 [PubMed] [Google Scholar]
- Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA (2003) Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia 17:1263–1293 [DOI] [PubMed] [Google Scholar]
- Chun KS, Surh YJ (2004) Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem Pharmacol 68:1089–1100 [DOI] [PubMed] [Google Scholar]
- Eda A, Osawa H, Satoh K, Yanaka I, Kihira K, Ishino Y, Mutoh H, Sugano K (2003) Aberrant expression of CDX2 in Barrett’s epithelium and inflammatory esophageal mucosa. J Gastroenterol 38:14–22 [DOI] [PubMed] [Google Scholar]
- Endo K, Yoon BI, Pairojkul C, Demetris AJ, Sirica AE (2002) ERBB-2 overexpression and cyclooxygenase-2 up-regulation in human cholangiocarcinoma and risk conditions. Hepatology 36:439–450 [DOI] [PubMed] [Google Scholar]
- Hansel DE, Maitra A, Lin JW, Goggins M, Argani P, Yeo CJ, Piantadosi S, Leach SD, Biankin AV (2005) Expression of the caudal-type homeodomain transcription factors CDX 1/2 and outcome in carcinomas of the ampulla of Vater. J Clin Oncol 23:1811–1818 [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Sasaki M, Ohira S, Ohta T, Oda K, Nimura Y, Chen MF, Jan YY, Yeh TS, Nakanuma Y (2004) Aberrant expression of CDX2 is closely related to the intestinal metaplasia and MUC2 expression in intraductal papillary neoplasm of the liver in hepatolithiasis. Lab Invest 84:629–638 [DOI] [PubMed] [Google Scholar]
- Kiba T, Tsuda H, Pairojkul C, Inoue S, Sugimura T, Hirohashi S (1993) Mutations of the p53 tumor suppressor gene and the ras gene family in intrahepatic cholangiocellular carcinomas in Japan and Thailand. Mol Carcinog 8:312–318 [DOI] [PubMed] [Google Scholar]
- Makuuchi M, Belghiti J, Belli G, Fan ST, Lau JW, Ringe B, Strasberg SM, Vauthey JN, Yamaoka Y, Yamasaki S, Working Group of the International Scientific Committee of the International Hepato-Pancreato-Biliary Association (2003) IHPBA concordant classification of primary liver cancer: working group report. J Hepatobiliary Pancreat Surg 10:26–30 [DOI] [PubMed] [Google Scholar]
- Mizoshita T, Tsukamoto T, Nakanishi H, Inada K, Ogasawara N, Joh T, Itoh M, Yamamura Y, Tatematsu M (2003) Expression of Cdx2 and the phenotype of advanced gastric cancers: relationship with prognosis. J Cancer Res Clin Oncol 129:727–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanuma Y, Harada K, Ishikawa A, Zen Y, Sasaki M (2003) Anatomic and molecular pathology of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg 10:265–281 [DOI] [PubMed] [Google Scholar]
- Olnes MJ, Erlich R (2004) A review and update on cholangiocarcinoma. Oncology 66:167–179 [DOI] [PubMed] [Google Scholar]
- Rescan C, Le Bras S, Lefebvre VH, Frandsen U, Klein T, Foschi M, Pipeleers DG, Scharfmann R, Madsen OD, Heimberg H (2005) EGF-induced proliferation of adult human pancreatic duct cells is mediated by the MEK/ERK cascade. Lab Invest 85:65–74 [DOI] [PubMed] [Google Scholar]
- Sebolt-Leopold JS, Herrera R (2004) Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer 4:937–947 [DOI] [PubMed] [Google Scholar]
- Silberg DG, Swain GP, Suh ER, Traber PG (2000) Cdx1 and cdx2 expression during intestinal development. Gastroenterology 119:961–971 [DOI] [PubMed] [Google Scholar]
- Sobin LH, Wittekind Ch (1997) In: Sobin LH, Wittekind Ch (eds) International Union Against Cancer (UICC): the TNM classification of malignant tumors, 5th edn. Wiley-Liss, New York
- Suzuki H, Isaji S, Pairojkul C, Uttaravichien T (2000) Comparative clinicopathological study of resected intrahepatic cholangiocarcinoma in northeast Thailand and Japan. J Hepatobiliary Pancreat Surg 7:206–211 [DOI] [PubMed] [Google Scholar]
- Tajima Y, Kuroki T, Fukuda K, Tsuneoka N, Furui J, Kanematsu T (2004) An intraductal papillary component is associated with prolonged survival after hepatic resection for intrahepatic cholangiocarcinoma. Br J Surg 91:99–104 [DOI] [PubMed] [Google Scholar]
- Tannapfel A, Sommerer F, Benicke M, Katalinic A, Uhlmann D, Witzigmann H, Hauss J, Wittekind C (2003) Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut 52:706–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatanasapt V, Sripa B (2000) Liver cancer in Thailand: epidemiology, diagnosis and control. Siriphan Press, Khon Kaen, pp 1–28 [Google Scholar]
- Vicent S, Lopez-Picazo JM, Toledo G, Lozano MD, Torre W, Garcia-Corchon C, Quero C, Soria JC, Martin-Algarra S, Manzano RG, Montuenga LM (2004) ERK1/2 is activated in non-small-cell lung cancer and associated with advanced tumours. Br J Cancer 90:1047–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CP, van Aelst L, Wigler MH, van de Woude GF (1998) Signaling pathways in Ras-mediated tumorigenicity and metastasis. Proc Natl Acad Sci USA 95:8773–8778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, Akiyama Y, Igari T, Kawamura T, Hiranuma S, Shibata T, Tsuruta K, Koike M, Arii S, Yuasa Y (2005) Expression of homeodomain protein CDX2 in gallbladder carcinomas. J Cancer Res Clin Oncol 131:271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi OK, Akiyama Y, Ohkura Y, Ban S, Endo M, Saitoh K, Yuasa Y (1997) Analyses of the APC and TGF-beta type II receptor genes, and microsatellite instability in mucosal colorectal carcinomas. Jpn J Cancer Res 88:718–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Bai YQ, Yuasa Y (2003) Homeodomain protein CDX2 regulates goblet-specific MUC2 gene expression. Biochem Biophys Res Commun 300:813–818 [DOI] [PubMed] [Google Scholar]
- Yuasa Y (2003) Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat Rev Cancer 3:592–600 [DOI] [PubMed] [Google Scholar]