Abstract
Purpose: Several studies indicate that integrin receptors are involved in the regulation of matrix metalloproteinase (MMP) expression. Integrin–ECM ligand interaction leads to phosphorylation of focal adhesion kinase (FAK) and activation of mitogen activated protein kinase pathways. In this present communication, we cultured human cervical cancer cells, SiHa, in the presence of fibronectin to study fibronectin–integrin mediated modulation of MMP activity. Methods: SiHa cells were cultured in serum-free medium (SFCM) in the presence of fibronectin, SFCM was collected and gelatin zymography was performed. Western blot, RT-PCR and immunocytochemistry were performed with SiHa cells cultured in the presence of fibronectin. Results: The culture of SiHa cells in the presence of 50 μg/1.5 ml fibronectin led to expression of pro-MMP-9 and activation of MMP-2 within 2 h. When cells were treated with ERK inhibitor (PD98059) and grown in the presence of fibronectin MMP-2 activation was partially inhibited, but when cells were treated with PI-3K inhibitor (LY294002) and grown in the presence of fibronectin MMP-2 activation was appreciably reduced. Tyrosine phosphorylation of FAK, PI-3K and ERK and nuclear trafficking of ERK were increased in SiHa cells grown in the presence of fibronectin. Increased MT1-MMP mRNA expression and processing of MT1-MMP were also observed in SiHa cells grown in the presence of fibronectin. Conclusions: Our findings indicate that the culture of SiHa cells in SFCM in the presence of fibronectin perhaps generates a signalling cascade which leads to the expression of pro-MMP-9 and the activation of MMP-2 within 2 h. The signalling pathways activated seem to be the FAK/ERK/PI-3K pathway.
Keywords: Fibronectin, Integrin α5β1, MMP-2, MMP-9, FAK, ERK, PI-3K
Introduction
The integrin family of cell surface glycoproteins functions as receptors for extracellular matrix (ECM) proteins such as fibronectin, vitronectin and collagen-IV (Jin and Varner 2004; Hynes 1987). Integrins play important roles in the physiological processes such as neurite outgrowth, gastrulation and in tumour metastasis (Jin and Varner 2004; Hynes 1987; Brakebusch et al. 2002). Binding and clustering of integrins at cell surface leads to the increased tyrosine phosphorylation of 125 kDa focal adhesion kinase (FAK) (Kornberg et al. 1991). Upon integrin–ECM protein interaction, FAK is recruited to focal adhesion sites and autophosphorylated at Y397, creating a binding site for Src Homology 2 (SH2) domain of Src. FAK binds to a number of signalling and structural proteins and also combines with and may activate phosphoinositide-3 kinase (PI-3 kinase) either directly or through Src kinase. This is followed by activation of Ras and activation of the downstream kinase cascade of Raf-1, MEK and extracellular signal related kinase (ERK) (Juliano 2002; Giancotti and Ruoslahti 1999). Thus, integrin mediated adhesion activates various arms of the mitogen activated protein kinase (MAPK) pathway including ERK and c-jun kinase (Jnk) (Giancotti and Ruoslahti 1999 ). It has been reported that the PI-3K and ERK pathways regulate matrix metalloproteinase-2 (MMP-2) expression (Juliano 2002; Zhang et al. 2004). PI-3K regulates membrane type-1 matrix metalloproteinase (MT1-MMP) and MMP-2 activity during melanoma cell vasculogenic mimicry (Hess et al. 2003). Suppression of ERK activation inhibits MMP-2 promoter activity (Pan et al. 2002). FAK is the receptor proximal step in the integrin mediated signal transduction cascade and it has been reported that FAK transfected cells show enhanced MMP-2 and MMP-9 mRNA expression on culture in the presence of fibronectin (Segarra et al. 2005). Thus, integrin mediated signalling initiates cell migration and release of proteolytic enzymes, e.g. MMPs (Seftor et al. 1992; Hofmann et al. 2000; Brakebusch et al. 2002). Integrins also link ECM to cytoskeleton and transmit biochemical signals across the cell membrane helping cell migration (Juliano 2002; Tomasek et al. 1997).
Matrix metalloproteinases (MMPs) are a family of metal dependent endopeptidases, which can degrade a variety of ECM components (Egeblad and Werb 2002; Nelson et al. 2000). High expression and activity of MMPs e.g. MMP-2 (72 kDa gelatinase A) in tumour cells can predict tumour recurrence and is associated with accelerated tumour progression in various carcinomas (Egeblad and Werb 2002; Nelson et al. 2000; Schmalfeldt et al. 2001). The importance of tumour cell surface integrin receptors in the regulation of MMP expression and function has been reported (Hofmann et al. 2000; Lochter et al. 1999). Perturbation of αvβ3 integrin in A375M melanoma cells by specific antibodies or ligands results in enhanced expression of MMP-2 (Seftor et al. 1992). Stimulation of α3β1-tetraspanin complex on breast carcinoma cells upregulates MMP-2 expression (Sugiura and Berditchevski 1999; Kubota et al. 1997). In basal keratinocytes, integrin α2β1 induces MMP-1 expression (Dumin et al. 2001). We have reported the activation of MMP-2 on modulation of α5β1 integrin in B16F10 murine melanoma cells and of α2β1 integrin in human cervical cancer cells, SiHa (Mitra et al. 2003, 2004).
The ECM protein fibronectin is a 440 kDa glycoprotein. The cell adhesive region in the central portion of fibronectin is comprised of at least two minimal amino acid sequences: Arg-Gly-Asp (RGD) and Pro-His-Ser-Arg-Asn (PHSRN) which function in synergy (Ruoslahti 1999; Humphries et al. 1987). α5β1 integrin (fibronectin receptor) binds to -RGD- site (Ruoslahti 1999; Humphries et al. 1987). Ligation of α5β1 integrin with fibronectin results in the accumulation of signalling molecules and cytoskeletal components at focal adhesions, and stimulates tyrosine phosphorylation of specific proteins including FAK, paxillin and p130 CAS (Hocking et al. 1998). The fibronectin–integrin system has provided a valuable model for the study of molecular mechanisms of ligand receptor interactions involved in metastasis. Upregulation of MMP-2 and -9 in fibronectin treated cell lines has been reported (Esparza et al. 1999). In HT-1080 human fibrosarcoma cells, Stanton et al. (1998) observed the activation of MMP-2 after 48 h of culture on fibronectin with increased processing of MT1-MMP. Segarra et al. (2005) reported that FAK transfected cells showed enhanced MMP-2 and MMP-9 expression in SFCM in response to culture on fibronectin even before an increase in MMP-2 and -9 mRNA could be detected by RT-PCR. In this present communication, we report the culture of human cervical cancer cells, SiHa, in serum-free medium in the presence of fibronectin leads to the expression of pro-MMP-9 and the activation of MMP-2 within 2 h. The signalling pathways activated seem to be the FAK/ERK/PI-3K pathways.
Materials and methods
Materials
SiHa human cervical cancer and HT-1080 human fibrosarcoma cell lines were obtained from National Centre for Cell Sciences (NCCS) Pune, India. Anti-α5β1 polyclonal antibody, GRGDSP peptide, Minimal Essential Medium (MEM) and foetal bovine serum (FBS) were purchased from Life Technologies, USA and fibronectin from Roche, Germany. PD98059 (ERK inhibitor) and LY294002 (PI3-K inhibitor) were purchased from Promega, USA. Anti-FAK, anti-phospho FAK, anti-α5 and anti-MMP-2 antibodies were purchased from Becton & Dickinson, USA. Anti-phospho ERK, anti-PI-3K and anti-phospho-tyrosine antibodies were purchased from Santa Cruz, USA. Gelatin Sepharose 4B beads was purchased from Amersham Biosciences, USA. Primers were synthesised by Operon, Germany and RT-PCR kit was purchased from Ambion, USA. FITC-coupled phalloidin, APMA and all other fine chemicals were purchased from Sigma, USA.
Cell culture
SiHa cells were grown and maintained in MEM containing 10% FBS. HT-1080 cells were grown and maintained in MEM containing 20% FBS. Cell lines were cultured in CO2 incubator at 37°C.
Cell adhesion assay
Microtitre plate wells were coated with increasing concentration of fibronectin (in triplicate). The ligand was allowed to bind for 1.5 h at 37°C. Wells were blocked with Buffer A (1% BSA, 1 mM CaCl2, 1 mM MgCl2 in PBS). Cells were washed, suspended in Buffer A, added to wells (50,000 cells/well) and allowed to bind at 37°C for 1.5 h. Wells were washed thrice with Buffer A. The bound cells were trypsinised, counted and expressed as percentage of adhesion. SiHa cells were incubated with α5 monoclonal antibody (1 μg) for 1 h at 37°C with intermittent gentle vortexing and then allowed to bind to fibronectin coated wells (in triplicate). Control SiHa cells were also kept under same conditions (without antibody) for the same period. The bound cells were trypsinised, counted and expressed as percentage of adhesion.
Zymography
SiHa cells (500,000 cells/1.5 ml) were washed thrice with serum-free culture medium (SFCM), grown in SFCM in the presence of increasing concentrations of fibronectin (10 μg, 25 μg and 50 μg/1.5 ml) and SFCM was collected. SiHa cells (500,000 cells/1.5 ml) were grown in SFCM in the presence of 50 μg fibronectin and SFCM was collected at different time points (1 and 2 h). SiHa cells (500,000 cells/1.5 ml) were grown in SFCM in the presence of GRGDSP peptide (100 μg/1.5 ml) and SFCM was collected after 2 h. Gelatinases were separated from 1 ml of SFCM collected at each time point and for each concentration using Gelatin Sepharose 4B beads (25 μl) and shaking for 2 h at 4°C. After washing the beads with TBS-T, gelatinases were eluted with 25 μl 1× sample buffer (0.0625 M Tris, 2% SDS, pH 6.8) for 30 min at 37°C (Toth and Fridman 2001). The eluted samples were run on 10% SDS-PAGE co-polymerised with 0.1% gelatin. Gels were washed in 2.5% Triton-X-100, incubated overnight in reaction buffer (50 mM Tris–HCl, pH 7, 4.5 mM CaCl2, 0.2 M NaCl) and stained with 0.5% Coomassie Blue in 40% methanol and 10% glacial acetic acid. Control cells grown in SFCM without fibronectin for 2 h were processed similarly. SFCM (1.5 ml) was also incubated at 37°C with 50 μg of fibronectin and processed along with experimental samples.
SiHa cells were grown in the presence of ERK inhibitor PD98059 (50 μM) or PI3-K inhibitor LY294002 (50 μM) for 45 min and then with 50 μg fibronectin in SFCM for 2 h at 37°C. Gelatinases were separated from SFCM using gelatin-sepharose beads and were subjected to 10% zymography as described previously. Control cells grown with fibronectin (50 μg/1.5 ml) for 2 h were processed similarly. HT-1080 cells were grown in SFCM for 24 h. Gelatinases (MMP-9, MMP-2) separated by gelatin-sepharose beads from SFCM were used as MMP-9/MMP-2 marker (Toth and Fridman 2001).
Isolation of membrane fraction
SiHa cells (10 × 106) were homogenised in Buffer B (20 mM Tris–Hcl, pH 7.4, 5 mM EDTA, 0.25 M sucrose, 1 mM PMSF) and centrifuged at 1,700g at 4°C for 10 min. The supernatant was centrifuged at 17,000g for 45 min at 4°C. The pellet was homogenised in Buffer B with 30% percoll and centrifuged at 17,000g for 45 min at 4°C. The membrane fraction was collected and washed thrice in Buffer C (20 mM Tris–HCl, pH 7.4, 5 mM EDTA, 1 mM PMSF) (Im and Graham 1990).
Immunoblot analysis
SiHa cell membrane fractions (50 μg) were run on 7.5% SDS-PAGE and transferred onto nitrocellulose membrane. The membrane was blocked using 10% chicken serum in PBS, incubated with anti-α5β1 polyclonal antibody (1:1,000 dilution) for 1.5 h at 37°C, washed with TBS-T, incubated with alkaline phosphatase coupled second antibody (1:1,000 dilution) for 1.5 h at 37°C and washed with TBS-T. Immunoblot was developed using NBT/BCIP (nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indoyl phosphate) as substrate.
Assay of FAK, p-FAK, p-ERK and p-PI-3K
SiHa cells (500,000) were grown with fibronectin (50 μg/1.5 ml) for 30 min, 1 and 2 h and without fibronectin (control). For FAK and p-FAK immunoblots, cells were extracted in Buffer D (1 mM CaCl2, 1 mM MgCl2, 1 mM EDTA, 1 mM PMSF, 1 mM sodium orthovanadate, 1% Triton-X-100 in PBS). For p-ERK immunoblot, cells were extracted in 1X sample buffer. For p-PI-3K immunoblot, cells were extracted in Buffer E (150 mM Nacl, 50 mM Tris, 1% NP-40, pH 8.0). PI-3K was immunoprecipitated from cell lysate using anti-PI-3K antibody (1 μg/ml) and protein G agarose beads. The resultant immune complex and cell lysates (60 μg protein) were run on 7.5% SDS-PAGE and transferred onto nitrocellulose membrane. Immunoblot was blocked with 1% BSA, incubated with anti-FAK, anti-phospho FAK, anti-phospho-ERK or anti-phospho-tyrosine antibodies (1:1,000 dilution) for FAK, p-FAK, p-ERK and p-PI-3K, respectively, for 1.5 h at 37°C, washed with TBS-T, incubated with alkaline phosphatase coupled second antibody (1:1,000 dilution) for 1.5 h at 37°C, washed with TBS-T and developed using NBT/BCIP as substrate.
Assay of MT1-MMP processing
SiHa cells grown with fibronectin (50 μg/1.5 ml) and without fibronectin (control) for 2 h were extracted in 1X sample buffer. Cell lysates (60 μg protein) were run on 7.5% SDS-PAGE and transferred onto nitrocellulose membrane. The membrane was blocked using 1% BSA, incubated with anti-MT1-MMP antibody (1:1,000 dilution) for 1.5 h at 37°C, washed with TBS-T, incubated with alkaline phosphatase coupled second antibody (1:1,000 dilution) for 1.5 h at 37°C, washed with TBS-T and bands were visualised using NBT/BCIP as substrate.
RT-PCR
RNA was extracted from 1 × 106 SiHa cells grown with fibronectin (50 μg/1.5 ml) and without fibronectin (control) for 2 h. Primers used for PCR were: hMMP-2: 5′-GTATTTGATGGCATCGCTCA-3′(forward) and 5′-CATTCCCTGCAAAGAACACA-3′ (reverse); hMMP-9: 5′-CGCTACCACCTCGAACTTTG-3′ (forward) and 5′-GCCATTCACGTCGTCCTTAT-3′ (reverse) (Stygar et al. 2002); hMT1-MMP: 5′-CCCTATGCCTACATCCGTGA-3′ (forward) and 5′-TCCATCCATCACTTGGTTAT-3′ (reverse) and hTIMP-2: 5′-GTTTTGCAATGCAGA TGTAG-3′ (forward) and 5′-ATGTGGAGAAACTCCTGCTT-3′ (reverse) (Kazes et al. 2000). Cells were washed in PBS and RNA was extracted (RNAqueous for PCR, Ambion). Two-step RT-PCR (Retroscript, Ambion) was done with equal amounts of total RNA using specific primers for PCR (MMP-2, MMP-9, TIMP-2 and MT1-MMP). Conditions used for PCR consisted of 24 cycles for MMP-2 or 34 cycles for MMP-9 at 94°C for 30 s, 63°C for 30 s and 72°C for 60 s with a final incubation at 72°C for 3 min (Stygar et al. 2002). For MT1-MMP, PCR consisted of 35 cycles at 94°C for 60 s, 60°C for 60 s and 72°C for 60 s; for TIMP-2, 95°C for 60 s, 52°C for 90 s and 72°C for 60 s (Kazes et al. 2000). GAPDH primers (Bangalore Genei, India) were used as a control to normalise for mRNA integrity and equal loading. Each PCR product (20 μl) was run on a 2.5% agarose gel and bands visualised under UV.
Immunocytochemistry
SiHa cells were grown overnight on coverslips in MEM with 10% FBS, washed and incubated in SFCM with fibronectin (50 μg/1.5 ml) for 1 and 2 h and without fibronectin (control) at 37°C. The coverslips were washed in PBS, fixed with 3.5% formaldehyde, treated with 0.5% Triton-X100 and nonspecific sites were blocked with 1% BSA. Coverslips (fibronectin treated 1 h, 2 h and control) were then treated with anti-ERK antibody (1:500 dilution) at 37°C for 1 h followed by FITC-coupled second antibody (1:1,000 dilution) at 37°C for 1 h in a humidified chamber. Coverslips (fibronectin treated 2 h and control) were also treated with FITC-coupled phalloidin (1:100 dilution) at 37°C for 30 min in a humidified chamber. After washing in PBS, cover slips were mounted on glass slides and observed under fluorescence microscope.
Results
Cell adhesion assay
Cell adhesion assay shows that SiHa cells bind to fibronectin efficiently (Fig. 1a). The incubation of SiHa cells with anti-α5 monoclonal antibody for 1 h at 37°C inhibits the adhesion of cells to fibronectin appreciably confirming the binding of SiHa cells via α5β1 integrin (Fig. 1b). Immunoblot of SiHa cell membrane shows that SiHa cells express both subunits (α5 and β1) of α5β1 integrin (Fig. 1c).
Fig. 1.
Cell adhesion assay and assay of integrin α5β1 in SiHa cells. a Cell adhesion assay. Microtitre plate wells were coated with increasing concentrations of fibronectin (in triplicate). Ligand was allowed to bind for 1.5 h at 37°C. The wells were blocked with Buffer A for 1 h at 37°C. SiHa cells were suspended in Buffer A, added to microtitre plate wells (50,000 cells/well) and allowed to bind for 1.5 h at 37°C. The wells were washed thrice with Buffer A, bound cells were trypsinised, counted on a haemocytometer and percentage of adhesion was calculated. b Cell adhesion assay. SiHa cells were incubated with 1 μg anti-α5 monoclonal antibody for 1 h at 37°C. Control SiHa cells were kept under same conditions (without antibody) for the same period. Both control and anti-α5 antibody treated SiHa cells were added to microtitre plates (50,000 cells/well) and allowed to bind at 37°C for 1.5 h. The wells were washed thrice with Buffer A. The bound cells were trypsinised, counted and expressed as percentage of adhesion. c Immunoblot of integrin α5β1. SiHa cell membrane fraction was run on 7.5% SDS-PAGE and transferred onto nitrocellulose membrane by Western blot. The immunoblot was incubated with anti-α5β1 polyclonal antibody, followed by alkaline phosphatase coupled secondary antibody. Bands were visualised using NBT/BCIP as substrate
Culture of SiHa cells in serum-free medium in the presence of fibronectin activates MMP-2
When 1.5 ml of SFCM was incubated at 37°C with 50 μg of fibronectin and processed along with experimental samples, no MMP activity was detectable (Fig. 2, lane 2).Virtually no MMP activity was detected in SFCM of SiHa cells cultured without fibronectin for 2 h (Fig. 2, lane 3). When SiHa cells were grown in SFCM in the presence of 10 μg/1.5 ml and 25 μg/1.5 ml fibronectin for 2 h, only pro-MMP-2 could be detected (Fig. 2, lanes 4 and 5). When SiHa cells were grown in SFCM in the presence of fibronectin (50 μg/1.5 ml) for 1 h, pro-MMP-9 (92 kDa), pro-MMP-2 (72 kDa) and active MMP-2 (64, 62, 56 and 52 kDa) could be detected (Fig. 2, lane 6). When SiHa cells were grown in SFCM in the presence of fibronectin (50 μg/1.5 ml) for 2 h, activation of MMP-2 was increased. Pro-MMP-9 (92 kDa), pro-MMP-2 (72 kDa) and active MMP-2 (64, 62, 52 and 45 kDa) could be detected (Fig. 2, lane 7). When SiHa cells were treated with GRGDSP peptide (100 μg/1.5 ml) for 2 h, only pro-MMP-9 and pro-MMP-2 could be detected (Fig. 2, lane 8). Figure 2 (lane 1) shows the activation of cervical tissue homogenate (a source of MMP-2) with APMA for 30 min at 37°C. When SiHa cells were grown in the presence of 50 μM ERK inhibitor (PD98059) or 50 μM PI3-K inhibitor (LY294002) and then treated with fibronectin (50 μg/1.5 ml) for 2 h, zymogram shows pro-MMP-9 (92 kDa), pro-MMP-2 (72 kDa) and partial reduction of active MMP-2 in SFCM of ERK inhibitor (PD98059) treated SiHa cells (Fig. 2, lane 9). In SFCM of PI3-K inhibitor (LY294002) treated SiHa cells, pro-MMP-9 (92 kDa), pro-MMP-2 (72 kDa) can be detected but the activation of MMP-2 is appreciably inhibited (Fig. 2, lane 10). Gelatinases (MMP-9 and MMP-2) separated by gelatin-sepharose beads from SFCM of HT-1080 cells (24 h) have been used as MMP-9/MMP-2 marker (Fig. 2, lane M).
Fig. 2.
Culture of SiHa cells in serum-free medium in the presence of fibronectin activates MMP-2. Gelatin zymography of serum-free culture medium (SFCM) incubated at 37°C with 50 μg of fibronectin (lane 2), of culture supernatant of SiHa cells grown in SFCM without fibronectin for 2 h (lane 3), of culture supernatants of SiHa cells grown in SFCM in the presence of 10 μg/1.5 ml and 25 μg/1.5 ml fibronectin for 2 h (lanes 4–5), in the presence of fibronectin (50 μg/1.5 ml) for 1 h (lane 6) and 2 h (lane 7) and in the presence of GRGDSP peptide (100 μg/1.5 ml) for 2 h (lane 8). Gelatin zymography of the culture supernatant of SiHa cells grown in the presence of 50 μM ERK inhibitor (PD98059) and 50 μM PI3-K inhibitor (LY294002) and then treated with fibronectin (50 μg/1.5 ml) for 2 h (lane 9 and lane 10, respectively). Activation of MMP-2 on incubation of cervical tissue homogenate (source of 72 kDa MMP-2) incubation with APMA for 30 min at 37°C (lane 1). Lane M are the gelatinases (MMP-9 and MMP-2) separated by gelatin-sepharose beads from serum-free medium of HT-1080 cells (24 h) which has been used as MMP-9/MMP-2 marker. Gelatinases were separated from SFCM using gelatin-sepharose beads and shaking for 2 h at 4°C. After washing the beads with TBS-T, gelatinases were eluted with 1× sample buffer. The samples were subjected to gelatin zymography on 10% SDS-PAGE co-polymerised with 0.1% gelatin
The role of MT1-MMP in fibronectin mediated activation of MMP-2
Immunoblot shows the processing of MT1-MMP (66 kDa) to 60, 52 and 45 kDa forms in SiHa cells cultured in the presence of fibronectin (50 μg/1.5 ml) for 2 h (Fig. 3, lane 2). Such processing is not seen in SiHa cells grown without fibronectin (Fig. 3, lane 1).
Fig. 3.
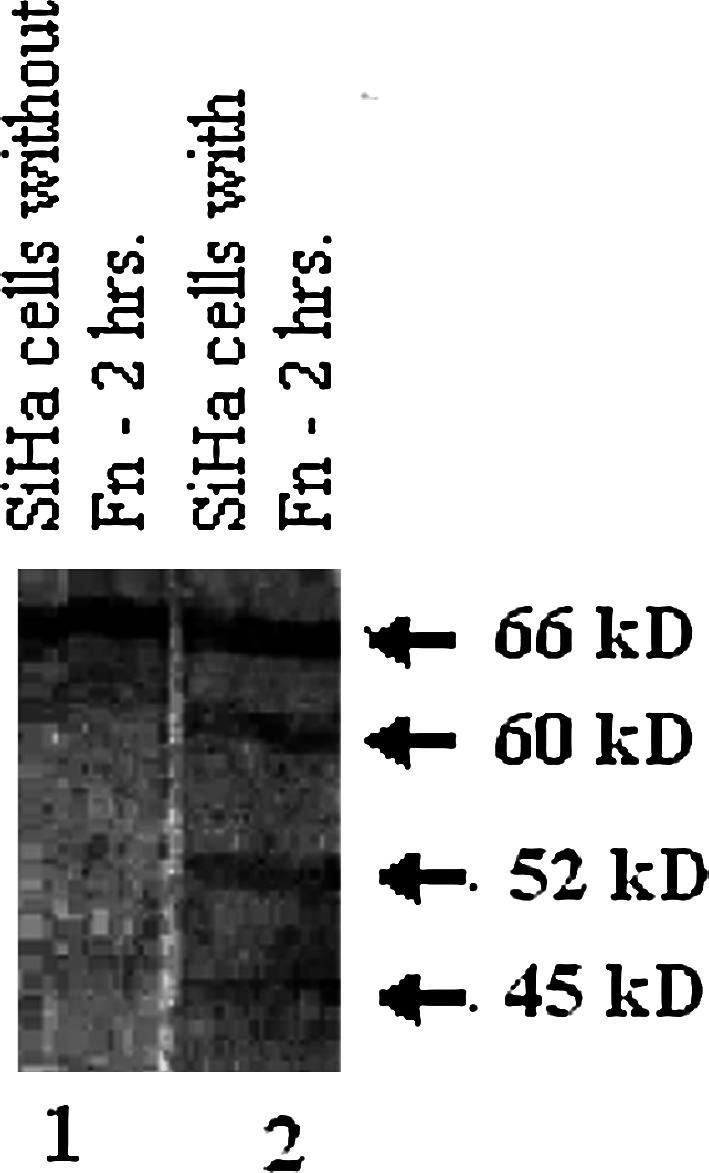
Assay of MT1-MMP processing. SiHa cells were grown in the presence of 50 μg/1.5 ml fibronectin (lane 2) and without fibronectin (lane 1) for 2 h in SFCM. Cell lysates were subjected to electrophoresis on 7.5% SDS-PAGE and proteins were transferred on to nitrocellulose membrane by Western blot. The immunoblot was incubated with anti-MT1-MMP polyclonal antibody for 1.5 h at 37°C followed by incubation with alkaline phosphatase coupled second antibody for 1.5 h at 37°C. Bands were visualised using NBT/BCIP as substrate
RT-PCR
RT-PCR (Fig. 4) shows no appreciable difference in MMP-9 and TIMP-2 mRNA expression in SiHa cells grown in the presence of fibronectin and without fibronectin (control). However, MMP-2 mRNA expression increases few fold and MT1-MMP mRNA expression increases appreciably in SiHa cells grown in the presence of fibronectin (50 μg/1.5 ml) for 2 h (E) compared to control cells (C). GAPDH primers were used as control to normalise for mRNA integrity and equal loading.
Fig. 4.

RT-PCR of MMP-2, MMP-9, TIMP-2 and MT1-MMP. SiHa cells were grown in the presence of 50 μg/1.5 ml fibronectin (lane E) and without fibronectin (lane C) for 2 h in SFCM. Total RNA was extracted from 1 × 106 cells (RNAqueous for PCR, Ambion). Two-step RT-PCR (Retroscript, Ambion) was done with equal amounts of total RNA using specific primers for PCR (MMP 2, MMP 9, TIMP 2 and MT1-MMP) synthesised by Operon, Germany. Twenty microlitres of each PCR product was run on a 2.5% agarose gel and bands visualised under UV. GAPDH primers (Bangalore Genei, India) were used as control to normalise for mRNA integrity and equal loading
The role of FAK, p-FAK, p-ERK and p-PI-3K in fibronectin mediated activation of MMP-2
Figure 5 demonstrates the expression of phospho-ERK (A), phospho-PI-3K (B), phospho-FAK (C) and FAK (D) at different time points of SiHa cell culture in the presence of fibronectin (30 min, 1 and 2 h) and in SiHa cells grown without fibronectin (control). The expression of phospho-ERK and phospho-PI-3K and the phosphorylation of FAK are increased in SiHa cells cultured in the presence of fibronectin for 30 min–2 h compared to control SiHa cells. However, there is no appreciable change in the FAK expression in SiHa cells cultured in the presence of fibronectin and control cells.
Fig. 5.

Immunoblot assay of FAK, p-FAK, p-PI-3K and p-ERK. SiHa cells were grown in the presence of 50 μg/1.5 ml fibronectin for 30 min, 1 and 2 h and without fibronectin in SFCM. Cells were extracted in appropriate buffers. For p-PI-3K Western blot, PI-3K was immunoprecipitated from cell lysate using anti-PI-3K antibody and protein G agarose beads. Samples were subjected to electrophoresis on 7.5% SDS-PAGE and proteins were transferred onto nitrocellulose membrane by Western blot. Immunoblots were incubated with anti-phospho ERK monoclonal antibody (A), anti-phospho tyrosine monoclonal antibody (for phospho PI-3K) (B), anti-phospho FAK monoclonal antibody (C) and anti-FAK polyclonal antibody (D) for 1.5 h at 37°C followed by incubation with alkaline phosphatase coupled second antibodies for 1.5 h at 37°C. Bands were visualised by using NBT/BCIP as substrate
Immunocytochemical localisation of actin and ERK
Figure 6a shows immunocytochemical localisation of actin. SiHa cells grown in the presence of fibronectin show distinct relocalisation of actin in cell adhesion points (2 h). Concentration of actin in focal points is absent in control SiHa cells (C). Figure 6b shows nuclear translocation of ERK. In control SiHa cells (C), ERK is located mainly in the cytosol. When SiHa cells are grown in the presence of fibronectin, ERK translocates to nuclei within 1 h (1, 2 h).
Fig. 6.
Immunocytochemical localization of actin and ERK. a Immunocytochemical localization of actin. SiHa cells grown on coverslips were grown with fibronectin (50 μg/1.5 ml) for 2 h and without fibronectin (control) in serum-free medium. Cells were fixed with 3.5% formaldehyde, treated with 0.5% Triton-X 100 and nonspecific sites were blocked with 1% BSA. Coverslips were treated with FITC-coupled phalloidin for 30 min at 37°C (a: C and 2 h). After extensive washing in PBS, the cover slips were mounted on glass slides and observed under a fluorescence microscope. b Immunocytochemical localization of ERK. SiHa cells grown on coverslips were grown with fibronectin (50 μg/1.5 ml) for 1 and 2 h and without fibronectin (control) in serum-free medium. Cells were fixed with 3.5% formaldehyde, treated with 0.5% Triton-X 100 and nonspecific sites were blocked with 1% BSA. Coverslips were incubated with anti-ERK polyclonal antibody at 37°C for 1 h, washed in PBS and incubated with FITC-coupled second antibody at 37°C for 1 h (b: C, 1 and 2 h). After extensive washing in PBS, the cover slips were mounted on glass slides and observed under a fluorescence microscope
Discussion
In this present communication we report that culture of SiHa cells in SFCM in the presence of fibronectin results in the expression of MMP-9 and the activation of MMP-2 within 1–2 h.
The culture of SiHa cells in SFCM in the presence of fibronectin (50 μg/1.5 ml/500,000 cells) shows expression of MMP-9 and active MMP-2 within 1 h and after 2 h shows much higher MMP-2 activity. When SiHa cells were grown in SFCM in the presence of fibronectin for 2 h, pro-MMP-9 (92 kDa), pro-MMP-2 (72 kDa) and active MMP-2 (64, 62, 52 and 45 kDa) could be detected. SiHa cells grown with lower concentrations of fibronectin (10 and 25 μg/1.5 ml) do not show pro-MMP-9 expression or MMP-2 activation comparable with 50 μg/1.5 ml of fibronectin. In human fibrosarcoma HT-1080 cells, Stanton et al. (1998) reported that soluble fibronectin did not affect MMP-2 expression and activity and MMP-2 activity was induced after 48 h of culture on fibronectin. However, in SiHa cells, we observed the activation of MMP-2 at a much earlier time point. Using molecular motifs for fibronectin binding to integrins α5β1 and α4β1, Jia et al. (2004) reported that ligation of α5β1 with its fibronectin binding motif results in upregulation of MMP-1. However, Werb et al. (1989) reported that binding of fibronectin derived fragments to fibronectin receptor (integrin α5β1) triggers events different from those triggered by binding the native fibronectin ligand. The molecular motif of fibronectin binding to integrin α5β1 is -RGD- (Ruoslahti 1999; Humphries et al 1987). To observe whether this alone can elicit MMP expression and activation, SiHa cells were grown in the presence of GRGDSP peptide in SFCM for 2 h. No such activation of MMP-2 was noticed. This perhaps indicates that a multivalent ligand receptor interaction rather than simple ligand occupancy is required for the induction of MMP-2. Esparza et al. (1999) reported that fibronectin induces coordinated expression of MMP-2 and MMP-9 in human T lymphocyte cell lines and soluble fibronectin results in much higher expression of activated MMPs than solid phase fibronectin. However, in our experiments, SiHa cells grown in the presence of fibronectin show no activation of MMP-9, although pro-MMP-9 expression is appreciably increased. Thus, the culture of SiHa cells in SFCM in the presence of fibronectin probably sends signals via fibronectin receptor integrin α5β1, regulating the activation of MMP-2.
RT-PCR shows that there is no appreciable increase in MMP-9 and TIMP-2 mRNA expression but MMP-2 mRNA expression increases and MT1-MMP mRNA expression is appreciably increased in SiHa cells grown in the presence of fibronectin (50 μg/1.5 ml) for 2 h compared to control SiHa cells. We observed that on culture in the presence of fibronectin, MT1-MMP (66 kDa) is processed to 60, 52 and 45 kDa forms in SiHa cells. Stanton et al. (1998) reported that processing of MT1-MMP to 45 kDa form occurs on culture of HT-1080 cells in the presence of fibronectin. MT1-MMP processing is associated with MMP-2 activation (Stanton et al 1998; Lehti et al 1998). As MT1-MMP has been identified as part of membrane associated MMP-2 activation complex composed of MT1-MMP/TIMP-2/pro-MMP-2/integrin αvβ3 (Hofmann et al. 2000; Strongin et al. 1995), increased MT1-MMP expression and processing may play a significant role in MMP-2 activation. Our observations thus indicate appreciable upregulation of MT1-MMP at the message level and increased MT1-MMP processing which could lead increased MMP-2 activity in fibronectin treated cells. It has been reported that MT1-MMP is involved in the maturation of α3, α5, and αv integrin subunits (Ratnikov et al. 2002). Increased MT1-MMP expression could promote the maturation of integrin receptors. The increased maturation of integrin α5β1 may promote increased attachment to fibronectin, upregulating integrin-mediated signal transduction leading to MMP-2 activation.
Binding of ECM proteins with their respective integrins initiates a signalling cascade that modulates most of the known gene regulatory pathways. Binding of integrins to ECM causes phosphorylation of a nonreceptor tyrosine kinase known as FAK (Kornberg et al 1991). FAK is reported to be in a critical position as a receptor proximal component of both integrin and growth factor receptor signalling pathways (Sieg et al 2000) and plays a critical role in the activation of ERK and MEK (Giancotti and Ruoslahti 1999; Sieg et al 2000). The involvement of FAK in α5β1 mediated signalling was demonstrated by observing tyrosine phosphorylation of FAK. Phosphorylation of FAK is increased in SiHa cells within 30 min–2 h culture in the presence of fibronectin although there is no appreciable change in the FAK expression in the fibronectin treated and control SiHa cells. To study participation of ERK and PI-3K in the integrin mediated signalling for MMP-2 activation, specific inhibitors were used to block respective signalling pathways. In SiHa cells, when ERK inhibitor (PD98059) treated cells were grown in the presence of fibronectin for 2 h, MMP-2 activation was partially inhibited. However, when PI-3K inhibitor (LY294002) treated SiHa cells were grown in the presence of fibronectin for 2 h, MMP-2 activation was inhibited appreciably. Western Blot shows that tyrosine phosphorylation of PI-3K and ERK increases in the SiHa cells grown in the presence of fibronectin compared to control. These results seem to indicate that transduction of α5β1 integrin-mediated signals for modulation of MMP-2 activity in SiHa cells may occur via FAK/ERK/PI-3K pathways.
Integrin-mediated anchorage plays an important role in trafficking of ERK between the cytoplasm and the nucleus (Juliano 2002). Inactive ERK remains in cytoplasm due to its association with MEK. Upon activation, ERK is phosphorylated, dissociates from MEK and enters the nucleus (Adachi et al. 2000). Immunocytochemistry shows that in control SiHa cells, ERK is located in the cytosol. When SiHa cells were grown in the presence of fibronectin, ERK translocates to the nuclei within 1 h. This could strongly indicate the role of ERK in integrin α5β1 mediated signalling. Sato et al. (2005) have reported that activated ERK stimulates MT1-MMP expression and MT1-MMP thus induced plays an important role in sustained activation of ERK. Integrin α5β1 mediated activation of ERK may thus function in a positive feedback loop increasing the MT1-MMP levels and promoting the activation of MMP-2.
Cytoskeletal components play an important role in the regulation of cell motility and migration and can transduce signals from the ECM to nucleus (Tomasek et al. 1997; Rosette and Karin 1995). SiHa cells grown in the presence of fibronectin show distinct relocalisation of actin in adhesion points; such localisation of actin is absent in the control cells. This suggests a change in cytoskeletal organisation in fibronectin treated cells which could lead to enhanced motility and migration.
Our experimental findings in human cervical tumour cells (SiHa) indicate that culture of SiHa cells in the presence of fibronectin generates a α5β1 integrin mediated signalling cascade which leads to early (within 2 h) expression of pro-MMP-9 and activation of MMP-2. These findings perhaps suggest the role of SiHa cell surface α5β1 integrin in the regulation of MMP-2 activity via FAK/ERK/PI-3K pathways. This relationship between integrin and MMP-2 may help in the modulation of invasive potential of tumour cells.
Acknowledgements
The authors acknowledge Director, CNCI for her constant encouragement and financial support and DST (SP/SO/B15/2000) and CSIR (F.NO. 9/30(24)/2001-EMR-1) for financial assistance.
Abbreviations
- APMA
4-Amino phenyl mercuric acetate
- ERK
Extracellular signal related kinase
- ECM
Extracellular matrix
- EDTA
Ethylenediaminetetracetic acid
- FAK
Focal adhesion kinase
- FBS
Foetal bovine serum
- MAPK
Mitogen activated protein kinase
- MMP
Matrix metalloproteinase
- MT1-MMP
Membrane type matrix metalloproteinase
- MEM
Minimal essential medium
- NBT/BCIP
Nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indoyl phosphate
- PMSF
Phenyl methyl sulphonyl fluoride
- PI3-K
Phosphotidylinositide-3 kinase
References
- Adachi M, Fukuda M, Nishida E (2000) Nuclear export of MAP kinase (ERK) involves a MAP kinase kinase (MEK) dependant active transport mechanism. J Cell Biol 148:849–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakebusch C, Bouvard D, Stanchi F, Sakai T, Fassler R (2002) Integrins in invasive growth. J Clin Invest 109:999–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumin JA, Dickeson SK, Stricker TP, Bhattacharyya-Pakrasi M, Roby JD, Santoro SA, Parks WC (2001) Pro-collagenase-1 (matrix metalloproteinase-1) binds the alpha (2) beta(1) integrin upon release from keratinocytes migrating on type I collagen. J Biol Chem 276:29368–29374 [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2:161–174 [DOI] [PubMed] [Google Scholar]
- Esparza J, Vilardell C, Calvo J, Juan M, Vives J, Urbano -Marquez A, Yague J, Cid MC (1999) Fibronectin upregulates gelatinase B (MMP-9) and induces coordinated expression of gelatinase A (MMP-2) and its activator MT1-MMP (MMP-14) by human T lymphocyte cell lines. A process repressed through RAS/MAP kinase signaling pathways. Blood 94:2754–2766 [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E (1999) Integrin signalling. Science 285:1028–1032 [DOI] [PubMed] [Google Scholar]
- Hocking DC, Sottile J, Mckeown-Longo PJ (1998) Activation of distinct α5β1-mediated signalling pathways by fibronectin’s cell adhesion and matrix assembly domains. J Cell Biol 141:241–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann UB, Westphal JR, van Kraats AA, Ruiter DJ, van Muijen GNP (2000) Expression of integrin αvβ3 correlates with activation of membrane-type matrixmetalloproteinase-1 (MT1-MMP) and matrix metalloproteinase 2 (MMP-2) in human melanoma cells in vitro and in vivo. Int J Cancer 87:12–19 [DOI] [PubMed] [Google Scholar]
- Hess AR, Seftor EA, Seftor REB, Hendrix MJC (2003) Phosphoinositide-3 kinase regulates membrane type-1 matix metalloproteinase and MMP-2 activity during melanoma cell vasculogenic mimicry. Cancer Res 63:4757–4762 [PubMed] [Google Scholar]
- Humphries MJ, Komoriya A, Akiyama SK, Olden K, Yamada KM (1987) Identification of two distinct regions of the type III connecting segment of human plasma fibronectin that promote cell type-specific adhesion. J Biol Chem 262:6886–6892 [PubMed] [Google Scholar]
- Hynes RO (1987) Integrins: versatility, modulation and signalling in cell adhesion. Cell 69:11–15 [DOI] [PubMed] [Google Scholar]
- Im MJ, Graham RM (1990) A novel guanine nucleotide binding protein coupled to alpha-1 androgenic receptor by photolabelling on membrane and thereby complex preparation. J Biol Chem 265:18944–18951 [PubMed] [Google Scholar]
- Jia Y, Zeng ZZ, Markwart SM, Rockwood KF, Ignatoski KMW, Ethier SP, Livant DL (2004) Integrin fibronectin receptors in matrixmetalloproteinase-1-dependant invasion by breast cancer and mammary epithelial cells. Cancer Res 64:8674–8681 [DOI] [PubMed] [Google Scholar]
- Jin H, Varner J (2004) Integrins: roles in cancer development and as treatment targets. Brit J Cancer 90:561–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano RL (2002) Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol 42:283–323 [DOI] [PubMed] [Google Scholar]
- Kazes I, Elalamy I, Sraer JD, Hatmi M, Nguyen G (2000) Platelet release of trimolecular complex components MT1-MMP/TIMP-2/MMP-2: involvement in MMP-2 activation and platelet aggregation. Blood 96:3064–3069 [PubMed] [Google Scholar]
- Kornberg L, Earp HS, Parsons JT, Schaller M, Juliano RL (1991) Cell adhesion of integrin clustering increases phosphorylation of a focal adhesion associated tyrosine kinase. J Biol Chem 267:23439–23442 [PubMed] [Google Scholar]
- Kubota S, Ito H, Ishibashi Y, Seyama Y (1997) Anti-α3 integrin antibody induces the activated form of matrixmetalloproteinase 2 (MMP-2) with concomittant stimulaton of invasion through matrigel by human rhabdomyosarcoma cells. Int J Cancer 70:106–111 [DOI] [PubMed] [Google Scholar]
- Lehti K, Lohi J, Valtanen H, Keski-Oja J (1998) Proteolytic processing of membrane-type-1 matrix metalloproteinase is associated with gelatinase A activation at cell surface. Biochem J 334:345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochter A, Navre M, Werb Z, Bissell M (1999) α1 and α2 integrins mediate invasive activity of mouse mammary carcinoma cells through regulation of Stromelysin-1 expression. Mol Biol Cell 10:271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A, Chakrabarti J, Chatterjee A (2003) Binding of α5 monoclonal antibody to cell surface α5β1 integrin modulates MMP-2 and MMP-7 activity in B16F10 melanoma cells. J Environ Pathol Toxicol Oncol 22:167–178 [DOI] [PubMed] [Google Scholar]
- Mitra A, Chakrabarti J, Banerji A, Chatterjee A (2004) Binding of α2 monoclonal antibody to human cervical tumor cell (SiHa) surface α2β1 integrin modulates MMP-2 activity. Gynecol Oncol 94:33–39 [DOI] [PubMed] [Google Scholar]
- Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM (2000) Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol 18:1135–1149 [DOI] [PubMed] [Google Scholar]
- Pan MR, Hung WC (2002) Nonsteroidal anti-inflammatory drugs inhibit matrix metalloproteinase 2 via suppression of the ERK/Sp-1 mediated transcription. J Biol Chem 277:32775–32780 [DOI] [PubMed] [Google Scholar]
- Ratnikov BI, Rozanov DV, Postnova TI, Baciu PG, Zhang H, DiScipio RG, Chestukhina GG, Smith JW, Deryugina EI, Strongin AY (2002) An alternative processing of integrin αv subunit in tumour cells by membrane type-1 matrix metalloproteinase. J Biol Chem 277:7377–7385 [DOI] [PubMed] [Google Scholar]
- Rosette C, Karin M (1995) Cytoskeletal control of gene expression: depolymerization of microtubules activates NF kappa B. J Cell Biol 128:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E (1999) Fibronectin and its integrin receptors in cancer. Adv Cancer Res 76:1–20 [DOI] [PubMed] [Google Scholar]
- Schmalfeldt B, Prechtel D, Harting K, Sparthe K, Rutke S, Konok E, Fridman R, Berger U, Schmitt M, Kuhn W, Lengyl E (2001) Increased expression of matrixmetalloproteinases (MMP-2, MMP-9), and the urokinase-type plasminogen activator is associated with progression from benign to advanced ovarian cancer. Clin Cancer Res 7:2396–2404 [PubMed] [Google Scholar]
- Sato H, Takino T, Miyamori H (2005) Roles of membrane-type matrix metalloproteinase-1 in tumour invasion and metastasis. Cancer Sci 96:212–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seftor REB, Seftor EA, Gehlson KR, Stetler-Stevenson WG, Brown PD, Ruoslathi E, Hendrix MJC (1992) Role of alpha v beta 3 integrin in human melanoma. Proc Natl Acad Sci USA 89:1557–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra M, Vilardell C, Matsumoto K, Esparza J, Lozano E, Serra-Pages C, Urbano-Marquez A, Yamada KM, Maria CC (2005) Dual function of focal adhesion kinase in regulating integrin-induced MMP-2 and MMP-9 release by human T lymphoid cells. Faseb J 19:1875–1877 [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD (2000) FAK integrates growth-factor and integrin signals to promote cell migration. Nature Cell Biol 2:249–256 [DOI] [PubMed] [Google Scholar]
- Stanton H, Gavrilovic J, Atkinson SJ, d’Ortho MP, Yamada KM, Zardi L, Murphy G (1998) The activation of pro-MMP-2 (gelatinase A) by HT-1080 fibrosarcoma cells is promoted by culture on a fibronectin substrate and is concomitant with an increase in processing of MT1-MMP (MMP-14) to a 45 kDa form. J Cell Sci 111:2789–2798 [DOI] [PubMed] [Google Scholar]
- Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI (1995) Mechanism of cell surface activation of 72 kDa Type IV collagenase. J Biol Chem 270:5331–5338 [DOI] [PubMed] [Google Scholar]
- Stygar D, Wang H, Vladic YS, Ekman G, Eriksson H, Sahlin L (2002) Increased level of matrix metalloproteinases 2 and 9 in the ripening process of the human cervix. Biol Reprod 67:889–894 [DOI] [PubMed] [Google Scholar]
- Sugiura T, Berditchevski F (1999) Function of α3β1-tetraspanin protein complexes in tumour cell invasion. Evidence for role of complexes in production of MMP-2. J Cell Biol 146:1375–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Halliday DL, Updike DL, Ahernmoore JS, Vu TK, Liu RW, Howard EW (1997) Gelatinase A activation is regulated by the organization of the polymerized actin cytoskeleton. J Biol Chem 272:7482–7487 [DOI] [PubMed] [Google Scholar]
- Toth M, Fridman R (2001) Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. In: Brooks SA, Schumacher U (eds) Metastasis research protocols, vol I. Humana Press, New Jersey, pp 163–173
- Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH (1989) Signal transduction through the fibronectin receptor induces collagenase and stromeolysin gene expression. J Cell Biol 109:877–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Bar-Elei M, Meloche S, Brodt P (2004) Dual regulation of MMP-2 expression by the type -1 insulin growth factor receptor. J Biol Chem 279:19683–19690 [DOI] [PubMed] [Google Scholar]





