Abstract
Purpose: Bone morphogenetic proteins (BMPs) are members of the transforming growth factor-beta family and play an important role in the regulation of embryonic vasculogenesis but their role in postnatal angiogenesis remains to be clarified. In this study we investigated a possible role of BMP-2 in the promotion of tumor angiogenesis. Methods: We studied the effect of BMP-2 on human dermal microvascular endothelial cells (HDMECs) and examined a possible angiogenic activity of BMP-2 with the mouse sponge assay. The effect of BMP-2 overexpression on tumor vascularization was also analyzed in xenografts of human BMP-2 transfected MCF-7 breast cancer cells (MCF-7/BMP2) in mice. Results: BMP receptor activation selectively induced the phosphorylation of p38 mitogen-activated protein kinase (MAPK) in contrast to the ERK1/2 MAP kinases. In keeping with this finding, BMP-2 had no significant effect on endothelial cell proliferation but promoted HDMEC tube formation in the matrigel assay. The transcription factor inhibitor of differentiation 1 (Id1), which is known to play an important role in neovascularization of tumors, was confirmed as a BMP target in HDMECs. Immunohistochemical analysis of sponge sections revealed that BMP-2 induced vascularization and showed an additive enhancement of angiogenesis with VEGF. In the murine breast cancer xenograft model, human MCF-7 cells with stable overexpression of BMP-2 developed vascularized tumors while empty vector control MCF-7 cells failed to form tumors. Conclusions: We conclude that activation of the BMP pathway by BMP-2 can promote vascularization and might be involved in tumor angiogenesis possibly by stimulating the Id1 and p38 MAPK pathway.
Keywords: Tumor angiogenesis, Breast cancer, Vascular endothelial growth factor (VEGF), Bone morphogenetic protein 2 (BMP-2)
Introduction
In the adult human organism, angiogenesis is essential for the female reproductive cycle and for the repair and remodeling of tissues. Neovascularization plays also a crucial role in pathological processes such as tumor growth and metastasis (Carmeliet 2000).
Bone morphogenetic proteins (BMPs) are members of a large family of TGF-beta-related growth factors that use similar signal transduction pathways that involve transmembrane serine threonine kinase receptors and Smad proteins. They are involved in the regulation of physiologic angiogenesis during embryonic development and bone formation (Hogan 1996). Both endothelial cells and vascular smooth muscle cells have been found to express BMPs, including BMP-2 (Willette et al. 1999; Glienke et al. 2000). Knockout studies have confirmed that BMPs regulate vasculogenesis during embryonic development (Moser 2003). Functional deletion of BMP-4 and the BMP I receptor in mice leads to impaired mesoderm precursors required for vascular development (Mishina et al. 1995; Winnier et al. 1995). Mice with a functional knockout of the BMP transcription factors Smad 1 or Smad 5 died at approximately 9.5–10.5 weeks and had defects in angiogenesis (Lechleider et al. 2001; Yang et al. 1999). Smad 5 mutant embryos had enlarged blood vessels, a decrease in smooth muscle cells and contained mesenchymal cells, which were unable to direct angiogenesis (Yang et al. 1999). Mice lacking TGF-beta receptors also died in midgestation with defects in angiogenesis (Goumans and Mummery 2000). Interestingly, both familial primary pulmonary hypertension (FPPH) and sporadic primary pulmonary hypertension (PPH) have been associated with germline mutations in the BMP-receptor (BMPR) type II gene, suggesting that BMPs also play an important role in the maintenance of normal pulmonary vascular physiology (de Caestecker and Meyrick 2001).
The expression of several BMPs has been described as altered in solid tumors in comparison to unaffected tissue, e.g., BMP-6, BMP-7 and BMP-2 (Bentley et al. 1992; Clement et al. 1999; Schwalbe et al. 2003). Previously, we and others presented data that breast carcinomas showed in part elevated levels of BMP-2 on the mRNA as well as the protein level (Arnold et al. 1999; Clement et al. 2000). The Id basic-helix-loop-helix transcription factors that play a vital role in angiogenesis have been identified as a target of the BMP signaling pathway. During embryogenesis and tumorigenesis, Id1 and Id3 are abundantly expressed during blood vessel formation (Benezra et al. 2001). Id1/Id3 double-null mice show abnormal vasculogenesis, forming enlarged, dilated blood vessels, and Id+/−1 /Id3−/−3 double-mutant mice failed to support tumor growth and metastasis from xenografts due to poor vascularization (Lyden et al. 1999).
Because of this background we investigated the role of BMP-2 in the promotion of tumor angiogenesis. We studied the effect of BMP-2 on HDMECs and examined a possible angiogenic activity of BMP-2 with in vitro and in vivo angiogenesis assays and in a murine breast cancer xenograft model with stable overexpression of BMP-2.
Methods
Materials and cells
The rabbit polyclonal antibody to Id1 was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz Biotechnology, INC, Calne, UK). The mouse monoclonal antibodies to phospho-Erk1 and 2 as well as the polyclonal goat antibody to phospho-p38 MAPK were obtained from Transduction Laboratories (BD Biosciences, Cowley, UK), mouse monoclonal antibody to BMP-2 (clone 65529.111) was purchased from R & Dsystems (Abingdon, UK), mouse monoclonal antibody to CD31 (clone JC/70A) was obtained from Signet Laboratories (Dedham, USA). All other reagents were supplied by Sigma (Poole, UK), unless stated otherwise. Recombinant BMP-2 (rhBMP-2) was a gift from Peter Hortschansky, Jena, Germany. RhBMP-2 was used at a concentration which is established to give maximal specific biological activity (200 ng/ml). Human dermal microvascular endothelial cells (HDMEC) were purchased from Clonetics BioWhittaker (Wokingham, Berkshire, UK) and were cultured in MCDB131 medium (Invitrogen Paisley, UK) containing 20% fetal calf serum (Sigma, Dorset, UK), 100 u/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine, 5 IU/ml heparin, and 50 μg/ml endothelial cell growth supplement (Sigma, Dorset, UK). Cells were routinely split 1 in 3 and were used up to the eighth passage. The full-length human BMP-2 cDNA constructs were subcloned into the pcDNA3.1 expression vector and used for stable transfections (MCF-7/BMP-2). For control, pcDNA3.1 alone was transfected into MCF7 cells and the established cell line was designed as MCF7/3.1. Transfection of the respective plasmids into the MCF-7 cells was carried out by the lipofectamine method according to standard protocols. BMP-2 protein expression was confirmed by western blot analysis. Cells were routinely cultured at 37°C and 5% CO2 in Dulbeccos’s modified Eagle’s medium (DMEM, with 4.5 mg/ml glucose) supplemented with 10% (v/v) fetal calf serum, with the antibiotics such as penicillin (100 U/ml) and streptomycin (100 mg/ml), and 4 mM L-glutamine (Invitrogen Ltd, Paisley, UK).
Immunoblotting
All cell extracts were prepared at 4°C. The cell lysis buffer used for preparing total cell extracts was a urea denaturing buffer (6.7 M urea, 10 mM Tris–HCl (pH 6.8), 5 mM DTT, 1% SDS, and 10% glycerol) supplemented with complete mini protease inhibitor cocktail tablets (Roche Diagnostics, Sussex, UK). Cultured cells were washed once with ice-cold PBS, scraped off and centrifuged at 13,000 rpm for 20 s, and 250 ml of the urea buffer added directly to the cell pellet. This was rapidly homogenized on ice for 15 s by using a Ultra-Turrax homogenizer at full speed (IKA, Düsseldorf, Germany).
The detergent compatible (DC) Protein Assay (Bio-Rad, Hertfordshire, UK) was used to estimate the protein concentration of extracts according to the manufacturer’s protocol. Cell extracts (50–60 mg/lane) were subjected to reduction of SDS polyacrylamide (10–12%) gel electrophoresis. The resolved proteins were then electroblotted (semi-dry) onto a Millipore Immobilon-P transfer membrane (polyvinylidene fluoride microporous membrane). Bound antibodies were detected using the chemiluminescent substrate ECL+Plus (Amersham Biosciences, Buckinghamshire, UK).
Endothelial cell proliferation
5×104 HDMECs were seeded in each well of a gelatine-coated 6 well plate and cultivated in 20% FCS overnight. Media were changed and HDMECs were treated with 200 ng/ml rhBMP-2 or vehicle control for additional 48 h either in 20% or in 2% FCS, trypsinized and the cell number analysed with a Coulter counter.
Matrigel endothelial cell tube formation assay
Growth factor-reduced Matrigel (BD Biosciences Discovery Labware, Bedford, MA, USA) was added to 24-well plates and allowed to gelatenize for 30 min at 37 °C. HDMEC were seeded at a density of 4×104 cells/well in 1 ml of MCDB131 medium containing 2% FCS. Cells were then treated with vehicle control, 100 ng/ml rhBMP-2, 200 ng/ml rhBMP-2, 20 ng/ml rhVEGF, or 200 ng/ml rhBMP-2 combined with 20 ng/ml rhVEGF. After 24 h, tube formation was assessed by phase contrast microscopy and recorded with a digital camera (Nikon E950, Tokyo, Japan). Tube formation was analyzed by Scion imaging software for Windows (Scion corporation, Frederick, USA).
Mouse subcutaneous sponge angiogenesis assay
All animal experiments were carried out in accordance with the British Home Office Animals (Scientific Procedures) Act 1986 under license number PPL 701/4949. C57 black mice were anesthetized using fluothane, and each received a subcutaneous sterile polyether sponge disc (8×8 mm) (Caligen Foam Ltd. Accrington, UK) placed underneath the dorsal skin. Twelve C57 black mice were divided into four groups (A, B, C, D, n=3 for each group). All mice received a subcutaneous sterile polyether sponge (type 611–7) disc (8 mm diameter) under the dorsal skin (day 0). Administration of rhVEGF 165 and/or rhBMP-2 diluted in PBSA through the skin (day 1–7) into the sponges was performed daily for 7 days as follows:
Group A: PBSA
Group B: 100 ng rhVEGF165
Group C: 100 ng rhBMP-2
Group D: 100 ng rhVEGF165 and 100 ng rhBMP-2
Animals were sacrificed by cervical dislocation after 7 days and the sponges were rapidly excised. The sponges were fixed in 10% formalin at 4°C for 1 h, immersed in 75% ethanol for 30 min, and then kept in 90% ethanol. For analysis of vessel growth, samples were embedded in paraffin wax and 10 μm-thick sections evenly distributed through each sponge were stained with von Willebrand factor antibody (Dako, Cambridgeshire, UK).
Tumorigenicity assay
The tumorigenicity assays are also covered by the license number PPL 701/4949. 2×107 MCF-7/BMP2 or MCF-7/3.1 were injected s.c. into athymic mice (female NuNu mice), five mice in each individual experiment as described earlier (Clement et al. 2005). The mice were monitored twice a week for tumor formation at the injection sites and tumor sizes were determined with a caliper. The mice were killed when tumors reached 1.44 cm2 . Tumors were removed and fixed in formalin for further analysis.
Immunohistochemistry
Sections (6 μm) of paraffin-embedded sponges were stained with von Willebrand factor Abs (Dako), by using Dako Envision System and Vector Laboratories ABC Vectastain kits. Antigen retrieval by proteolytic digestion was performed. The slides were dewaxed and rehydrated and incubated in a trough of distilled water at 37°C for 10 min and in 200 ml of phosphate-buffered saline (PBSA: 0.13 mol/l NaCl, 0.002 mol/l KCl, 0.01 mol/l NazHP04, 0.002 mol/l KH2 PO4) containing 25 μg of protease type 24 (Sigma, Poole, Dorset, UK) at 37°C for a further 10 min. Slides were then incubated in 0.1% trypsin for 20 min at 37°C and washed twice in PBS.
The sections were immmunostained with an antibody against von Willebrand factor (VWf), diluted 1:400 for 1 h at room temperature. The sections were rinsed in PBSA and incubated with a biotinylated goat anti-rabbit antibody (Dako) diluted 1:100 for 30 min at room temperature. The vessels were visualized with 3,3′-diaminobenzidine (DAB) (Dako) and counterstained with hematoxylin.
Staining of breast cancer xenograft tumors was performed on paraffin-embedded and formalin-fixed 5-μm sections. After dewaxing and rehydration, endogenous peroxidase was blocked in a 0.5% hydrogen peroxide in water solution for 30 min. Antigen retrieval involved pressure cooking in 50 mmol Tris/0.2 mmol EDTA buffer for 180 s or incubation at 60°C for 16 h in the same buffer. Following antigen retrieval, slides were pretreated with peroxidase blocking reagent for 5 min (part of DAKO Envision-Plus kit, DakoCytomation, K4010). The primary mouse monoclonal anti-human CD31 antibody (Signet Laboratories, USA) at a dilution of 15 μg/ml was incubated on sections for 90 min at room temperature in a humid chamber. This was followed by incubation with a polyclonal rabbit anti-mouse immunoglobulins link antibody diluted to 1:50 (DAKO Z0454). After washing in PBS, staining was visualized using a DAKO Envision-Plus Rabbit HRP System (DakoCytomation, K4010) and diamino-benzidine yielding a dark brown reaction product. Slides were counterstained with hematoxylin and coverslipped using Glycergel aqueous mounting medium (DakoCytomation, C0563). Vessels in the breast cancer xenograft tumors were also identified using an antibody against VWf as described above.
Vascular grade index
Chalkley vascular count (CVC) was quantitatively determined in the mouse subcutaneous sponge angiogenesis assay using Chalkley point array counting methodologies described by us and others previously (Fox et al. 1995; Leek et al. 1996). Vessels are immunohistochemically stained using an antibody against VWf as described above. Using this system, CVC was determined microscopically by enumerating the numbers of vessels in three areas of the sponge section at high power (×25 objective), which showed the most dense staining, the so-called ‘hotspot’ areas.
Statistical analysis
Comparisons between groups were made applying the Wilcoxon-matched pairs test
Results
BMP-2 activates Id1 expression and p38 MAPK phosphorylation
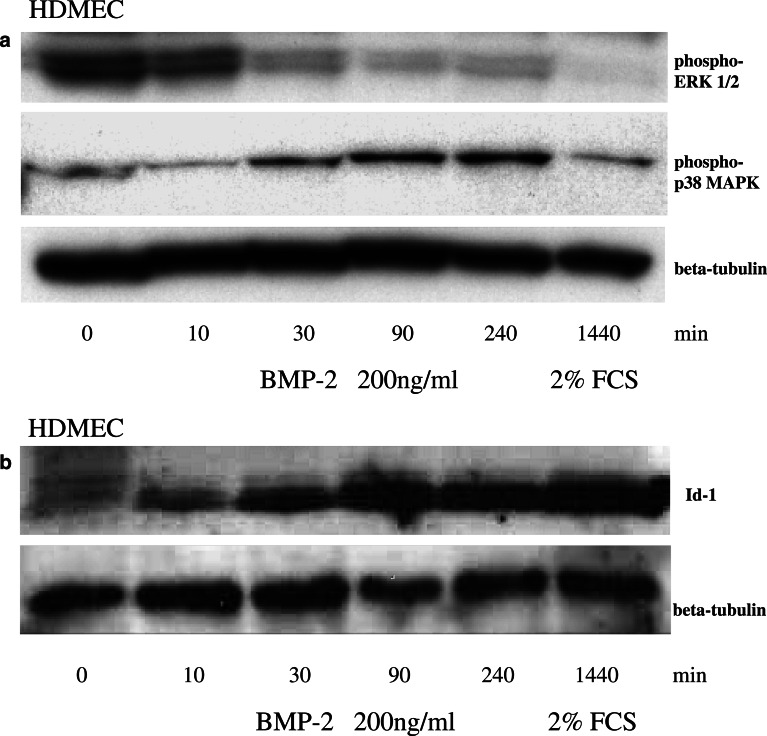
Bone morphogenetic protein-2 has recently been reported to increase Id1 expression in bovine aortic endothelial cells (BAEC) and human umbilical vein endothelial cells (HUVEC) (Langenfeld and Langenfeld 2004). We could confirm that BMP-2 stimulates the expression of this crucial transcription factor for neoangiogenesis in HDMECs. Immunoblotting of cell extracts isolated from the HDMECs incubated under serum-free conditions with 200 ng/ml rhBMP-2 showed a time-dependent upregulation of Id1 reaching a maximum effect after 90 min (Fig. 1b).
Fig. 1.
Time-dependent downregulation of ERK1/2 phosporylation and upregulation of p38 MAPK phosphorylation and Id1 protein expression by BMP-2. HDMEC cells were incubated with 200 ng/ml rhBMP-2. ERK1/2 and p38 MAPK protein phosphorylation (a) and Id1 (b) and β-tubulin (a and b) protein expression were visualized by immunoblotting of cell extracts isolated from the HDMEC cells at the indicated time points
ERK1/2 phosphorylation represents a signal transduction pathway that is known to mediate a mitogenic cellular response in endothelial cells to the proangiogenic proteins VEGF and fibroblast growth factor (FGF). Activation of p38 is involved in endothelial actin cytoskeletal reorganization and induction of adhesion molecule expression, leading to alterations of endothelial cell function (Landry and Huot 1995; Rousseau et al. 1997). In contrast to the data reported in BAEC and HUVEC, we were unable to find an activation of the ERK1/2 phosphorylation in HDMECs that were incubated under serum-free conditions with 200 ng/ml rhBMP-2. However, we could demonstrate a time-dependant upregulation of p38 MAPK phosphorylation reaching a maximum effect after 240 min of rhBMP-2 incubation (Fig. 1a).
BMP-2 stimulates tube formation without affecting endothelial cell proliferation
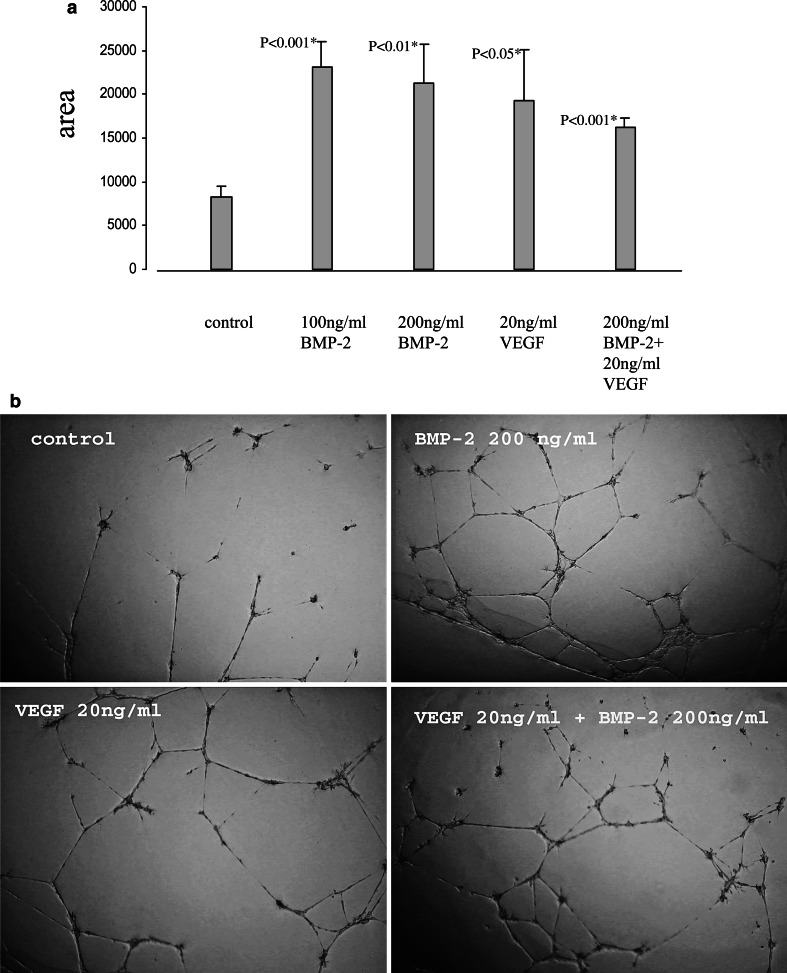
The differential regulation of the MAPK pathways by rhBMP-2 suggested a stimulation of endothelial cell function rather then proliferation. As expected, incubation with 200 ng/ml BMP-2 in 20% or 2% FCS revealed no significant effect on HDMEC proliferation compared to controls (Fig. 2). To study whether rhBMP-2 induced tube formation, HDMEC were plated in growth factor-reduced Matrigel-coated wells and treated with vehicle control, 100 ng/ml rhBMP-2, 200 ng/ml rhBMP-2, 20 ng/ml rhVEGF, or 200 ng/ml rhBMP-2 combined with 20 ng/ml rhVEGF. Detection of tube formation after 24 h showed that rhBMP-2, rhVEGF and combined treatment increased the formation of tube-like structures in HDMEC compared to vehicle control (Fig. 3a, b). Interestingly, combined treatment with rhBMP-2 and rhVEGF had no synergistic effect on tube formation.
Fig. 2.
Bone morphogenetic proteins-2 shows no significant effect on HDMEC proliferation. 5×104 HDMECs were seeded in each well of a gelatine-coated 6-well plate and cultivated either in serum-reduced conditions with 2% FCS (a) or with 20% FCS (b) overnight. Media were changed and HDMECs were incubated for an additional 48 h with the indicated conditions, trypsinized and the cell number analyzed with a Coulter counter
Fig. 3.
Bone morphogenetic proteins-2 and VEGF-dependant stimulation of tube formation. Growth factor-reduced Matrigel was added to 24-well plates and allowed to gel for 30 min at 37°C. HDMEC were seeded at a density of 4×104 cells/well in 1 ml of MCDB131 medium containing 2% FCS. Cells were then treated with vehicle control, 100 ng/ml BMP-2, 200 ng/ml BMP-2, 20 ng/ml VEGF, or 200 ng/ml BMP-2 combined with 20 ng/ml VEGF. After 24 h, tube formation was assessed by phase contrast microscopy and recorded with a digital camera (b). Total tube length was calculated by Scion imaging software for Windows (a)
BMP-2 promotes angiogenesis in the mouse subcutaneous sponge assay
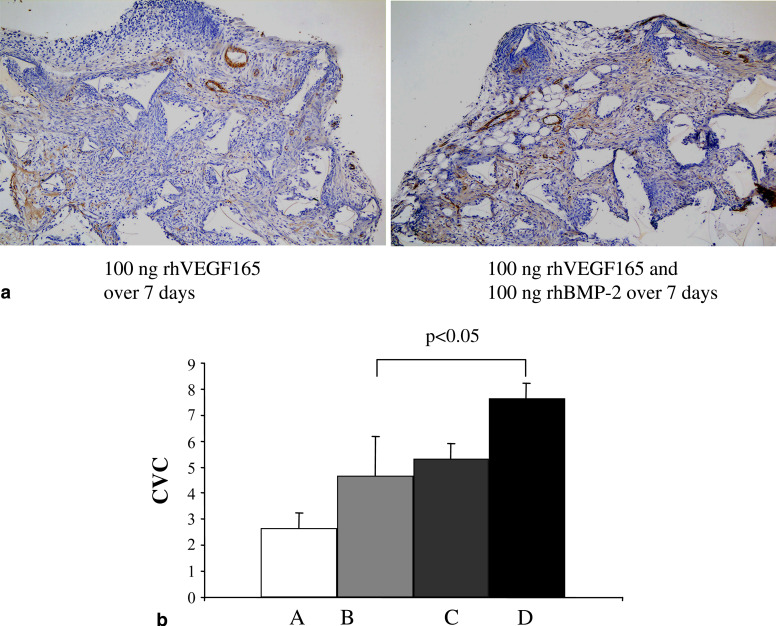
To assess the effect of BMP-2 on blood vessel formation in vivo, C57 black mice received a subcutaneous polyether sponge under the dorsal skin, and rhBMP-2 and/or rhVEGF was administered through the skin for 7 days into the sponges. Immunohistochemical analysis of neovascularization revealed that rhBMP-2 as well as rhVEGF increased angiogenic activity with significant additive enhancement of sponge vascularization in the combined treatment group (P<0.05) (Fig. 4).
Fig. 4.
Bone morphogenetic proteins-2 stimulates angiogenesis in the murine sponge assay. Twelve C57 black mice were divided into four groups (A, B, C, D, n=3 for each group). All mice received a subcutaneous sterile polyether sponge (type 611–7) disc (8 mm diameter) under the dorsal skin (day 0). Administration of rhVEGF 165 and/or rhBMP-2 diluted in PBSA through the skin (day 1–7) into the sponges was performed daily for 7 days as follows: A PBSA, B 100 ng rhVEGF165, C 100 ng rhBMP-2 and D 100 ng rhVEGF165 and 100 ng rhBMP-2. Animals were sacrificed after 7 days and the sponges were fixed in formalin, paraffin embedded and immunohistochemically analyzed with anti-mouse VWf-antibody for neovascularization (CVC)
Stably BMP-2 transfected MCF-7 breast cancer cells induce vascularized xenograft tumors in nude mice
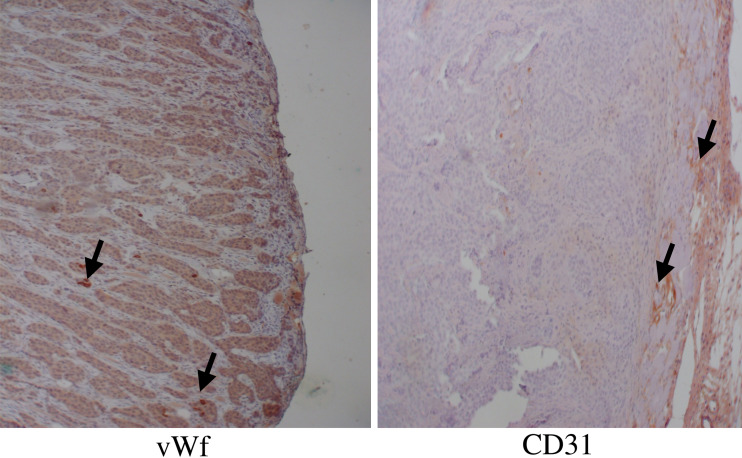
To further evaluate the effect of endogenous BMP-2 expression on tumour angiogenesis, we also analyzed vascularization in xenografts of the stably BMP-2 transfected MCF-7 human breast cancer cells and the empty vector control cells MCF-7/3.1 in nude athymic mice. Within 4–6 weeks all five mice injected with BMP-2 transfected MCF-7 cells developed tumors with 1–1.5 cm2 in size, while the injection of the empty vector control cells MCF-7/3.1 cells failed to form tumors. Immunohistochemical analysis of CD31 and vWf expression as well as macroscopic inspection of the BMP-2 transfected MCF-7 tumors revealed that these tumors were characterized by vascularization from the murine tumor stroma (Fig. 5). Detailed results concerning the growth of the xenograft tumors are given in a separate paper that has been published previously by the authors (Clement et al. 2005).
Fig. 5.
Bone morphogenetic proteins-2 induces tumor formation and neovascularization in a human xenograft model. For tumorigenicity assays, 2×107 MCF-7/BMP2 or MCF-7/3.1 were injected s.c. into athymic mice (female NuNu mice), five mice in each individual experiment. The mice were monitored twice a week for tumor formation at the injection sites and tumor sizes were determined with a caliper. The mice were killed when tumors reached 1.44 cm2. Tumors were removed, fixed in formalin, paraffin embedded and immunohistochemically analyzed with anti-mouse CD31-antibody and anti-mouse vWf-antibody for neovascularization (arrows)
Discussion
Bone morphogenetic proteins are involved in the regulation of physiologic vasculogenesis during embryonic development. Targeted disruption of the BMP 2/4 transcription factor Smad 5 led to the disorganization of yolk sac vasculature and heart development (Chang et al. 1999). However, a role of BMP-2 on postnatal vascular development has not been established.
From the literature, it is known that MCF7 breast cancer cells fail to induce the formation of tumors after subcutaneous inoculation into athymic nude mice (Noel et al. 1993). Using two in vivo models, we can show that stable BMP-2 transfection of MCF-7 cells leads to the formation of vascularized tumors formed in nude mice and that recombinant BMP-2 increases angiogenic activity in the murine sponge vascularization assay. Previously, we have demonstrated that BMP-2 is expressed in the majority of breast cancers when compared to normal mammary tissue (Raida et al. 2005). BMP-2 is secreted from murine osteoblastic cells and is stored in the extracellular matrix (Suzawa et al. 1999). Therefore, endogenous BMP-2 may enhance the development of a blood supply in these tumors.
The demonstration that BMP-2 promotes tube formation, induces phosphorylation of p38, and increases Id1 expression shows that BMP-2 does activate endothelial cells. BMP-2/4 has been shown to activate Erk-l/2 in osteoblasts (Lou et al. 2000) and recently to induce phosphorylation of Erk-l/2 in human aortic endothelial cells (HAEC) but not in human umbilical vein endothelial cells (HUVEC) (Langenfeld and Langenfeld 2004). Our data confirm that different endothelial cells respond differently to BMP-2. VEGF has been shown to mediate a cellular response in endothelial cells through the activation of Erk-1/2 (Wu et al. 2000). Erk-l/2 regulates several critical cellular functions in endothelial cells including proliferation (Tanaka et al. 1999; Liu et al. 2001). In keeping with this mitogenic activity in endothelial cells, treatment with VEGF in addition to BMP-2 led to a additive enhancement of angiogenesis in the sponge assay while combined treatment had no additive effect on tube formation. The differences in proliferation and activation of tube formation may indicate that BMP-2 is required more for the functional maturation of the blood vessels rather than as a sole initiator of angiogenesis.
Prior studies have demonstrated that Id plays an important role in the regulation of angiogenesis during embryonic development and on postnatal endothelial cells (Benezra et al. 2001). Mice with a double knockout of Idl–ld3 show vascular abnormalities in the forebrain and do not support the growth of xenograft tumors, probably due to the inability to form a neovasculature (Lyden et al. 1999). Sustained expression of Id in cells delays the onset of replicative senescence in human endothelial cells (Tang et al. 2002) and induces tube fomation and migration of endothelial cells (Zebedee and Hara 2001). Consistent with our findings, BMP-2 was recently shown to directly stimulate Id1 and phosphorylation of Smad l/5 and/or 8 in HAEC and HUVEC (Langenfeld and Langenfeld 2004).
Bone morphogenetic protein 2 may promote angiogenesis by mechanisms other than directly stimulating endothelial cells. BMP-2 is chemotactic for human monocytes and stimulates the expression of the angiogenic cytokine transforming growth factor beta 1 (Cunningham et al. 1992). Circulating monocytes that leave the blood stream and enter tissues, where they differentiate into macrophages, are known to be present in breast tumors, where they can secrete cytokines that promote an angiogenic response (Leek and Harris 2002). The recruitment of VEGF-responsive bone marrow-derived precursors is necessary and sufficient for tumor angiogenesis (Lyden et al. 2001). It has been suggested that BMPs form part of the complement of cytokines regulating the development of hematopoietic progenitors (Detmer et al. 2002). Recently, Marrony et al. (2003) demonstrated that BMP-2 specifically stimulated the release of placenta growth factor (PlGF) in human mesenchymal stem cells (MSC). PlGF has been shown to play an important role in the recruitment of endothelial and hematopoietic stem cells (HSC) in pathological conditions such as angiogenesis during ischemia, inflammation, wound healing, and cancer (Carmeliet et al. 2001; Hattori et al. 2002). Multipotent adult progenitor cells (MAPCs) have been generated from a nonendothelial bone marrow stem cell, which engraft in vivo and contribute to neoangiogenesis (Reyes et al. 2002). MAPCs could differentiate into MSC in the tumor stroma and might be stimulated by paracrine BMP-2 expression of cancer cells to produce PlGF which then recruits and expands endothelial progenitors to support tumor vascularization.
The autocrine effects of BMP-2 on tumor growth are still not fully understood. Studies have shown that recombinant BMP-2/4 inhibits the growth of tumors cells in vitro (Ghosh-Choudhury et al. 2000; Tada et al. 1998). However, transfection of a dominant-negative BMP-2 type receptor inhibited the growth of breast cancer cell lines, suggesting that BMP-2 may actually stimulate proliferation of tumor cells (Pouliot et al. 2003). In prostate cancer cells, BMP-2 inhibited cell proliferation in the presence of androgen, while in the absence of androgen, BMP-2 stimulated cell growth (Ide et al. 1997). The autocrine effects of BMP-2 on the proliferation of tumor cells may therefore be dependent on the presence of specific cytokines in the tumor environment.
This study demonstrates that BMP-2 enhances angiogenesis in an in vivo tumor model. BMP-2 increases Id1 expression and p38 phosphorylation in endothelial cells with a corresponding stimulation of tube formation. Our data suggest that BMP-2 induces angiogenesis in part by activating endothelial cell function. This study provides further evidence that BMP-2 expression in breast cancer plays an important role in the process of tumorigenesis and suggests that inhibition of this pathway may be of value therapeutically.
Acknowledgements
This work was supported in part by Grants of Cancer Research, UK and Dr. Mildred Scheel Foundation, Germany. We thank Christine Günther for her technical expertise and help in matrigel endothelial cell tube formation assay.
References
- Arnold SF, Tims E, McGrath BE. (1999) Identification of bone morphogenetic proteins and their receptors in human breast cancer cell lines: importance of BMP-2. Cytokine 11:1031–1037 [DOI] [PubMed] [Google Scholar]
- Benezra R, Rafii S, Lyden D (2001) The Id proteins and angiogenesis. Oncogene 20:8334–8341 [DOI] [PubMed] [Google Scholar]
- Bentley H, Hamdy FC, Hart KA, Seid JM, Williams JL, Johnstone D, Russell RG (1992) Expression of bone morphogenetic proteins in human prostatic adenocarcinoma and benign prostatic hyperplasia. Br J Cancer 66:1159–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P (2000) Mechanisms of angiogenesis and arteriogenesis. Nat Med 6:389–395 [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, Vandendriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG (2001) Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med 7:575–583 [DOI] [PubMed] [Google Scholar]
- Chang H, Huylebroeck D, Verschueren K, Guo Q, Matzuk MM, Zwijsen A (1999) Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development 126:1631–1642 [DOI] [PubMed] [Google Scholar]
- Clement JH, Sänger J, Höffken K (1999) Expression of bone morphogenetic protein 6 in normal mammary tissue and breast cancer cell lines and its regulation by epidermal growth factor. Int J Cancer 80:250–256 [DOI] [PubMed] [Google Scholar]
- Clement JH, Marr N, Meissner A, Schwalbe M, Sebald W, Kliche KO, Höffken K, Wölfl S (2000) Bone morphogenetic protein 2 (BMP-2) induces sequential changes of Id gene expression in the breast cancer cell line MCF-7. J Cancer Res Clin Oncol 126:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement JH, Raida M, Sänger J, Bicknell R, Liu J, Naumann A, Geyer A, Waldau A, Hortschansky P, Schmidt A, Höffken K, Wölfl S, Harris AL (2005) Bone morphogenetic protein 2 (BMP-2) induces in vitro invasion and in vivo hormone independent growth of breast carcinoma cells. Int J Oncol (in press) [PubMed]
- Cunningham NS, Paralkar V, Reddi AH (1992) Osteogenin and recombinant bone morphogenetic protein 2B are chemotactic for human monocytes and stimulate transforming growth factor beta 1 mRNA expression. Proc Natl Acad Sci USA 89:11740–11744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Caestecker M, Meyrick B (2001) Bone morphogenetic proteins, genetics and the pathophysiology of primary pulmonary hypertension. Respir Res 2:193–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmer K, Walker AN (2002) Bone morphogenetic proteins act synergistically with haematopoietic cytokines in the differentiation of haematopoietic progenitors. Cytokine 17:36–42 [DOI] [PubMed] [Google Scholar]
- Fox SB, Leek RD, Weekes MP, Whitehouse RM, Gatter KC, Harris AL (1995) Quantitation and prognostic value of breast cancer angiogenesis: comparison of microvessel density, Chalkley count, and computer image analysis. J Pathol 177:275–283 [DOI] [PubMed] [Google Scholar]
- Ghosh-Choudhury N, Woodruff K, Qi W, Celeste A, Abboud SL, Ghosh Choudhury G (2000) Bone morphogenetic protein-2 blocks MDA MB 231 human breast cancer cell proliferation by inhibiting cyclin-dependent kinase-mediated retinoblastoma protein phosphorylation. Biochem Biophys Res Commun 272:705–711 [DOI] [PubMed] [Google Scholar]
- Glienke J, Schmitt AO, Pilarsky C, Hinzmann B, Weiss B, Rosenthal A, Thierauch KH (2000) Differential gene expression by endothelial cells in distinct angiogenic states. Eur J Biochem 267:2820–2830 [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Mummery C (2000) Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol 44:253–265 [PubMed] [Google Scholar]
- Hattori K, Heissig B, Wu Y, Dias S, Tejada R, Ferris B, Hicklin DJ, Zhu Z, Bohlen P, Witte L, Hendrikx J, Hackett NR, Crystal RG, Moore MA, Werb Z, Lyden D, Rafii S (2002) Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat Med 8:841–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BL (1996) Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev 10:1580–1594 [DOI] [PubMed] [Google Scholar]
- Ide H, Yoshida T, Matsumoto N, Aoki K, Osada Y, Sugimura T, Terada M (1997) Growth regulation of human prostate cancer cells by bone morphogenetic protein-2. Cancer Res 57:5022–5027 [PubMed] [Google Scholar]
- Landry J, Huot J (1995) Modulation of actin dynamics during stress and physiological stimulation by a signaling pathway involving p38 MAP kinase and heat-shock protein 27. Biochem Cell Biol 73:703–707 [DOI] [PubMed] [Google Scholar]
- Langenfeld EM, Langenfeld J (2004) Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Mol Cancer Res 2:141–149 [PubMed] [Google Scholar]
- Lechleider RJ, Ryan JL, Garrett L, Eng C, Deng C, Wynshaw-Boris A, Roberts AB (2001) Targeted mutagenesis of Smad1 reveals an essential role in chorioallantoic fusion. Dev Biol 240:157–167 [DOI] [PubMed] [Google Scholar]
- Leek RD, Harris AL (2002) Tumor-associated macrophages in breast cancer. J Mammary Gland Biol Neoplasia 7:177–189 [DOI] [PubMed] [Google Scholar]
- Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL (1996) Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 56:4625–4629 [PubMed] [Google Scholar]
- Liu S, Yu D, Xu ZP, Riordan JF, Hu GF (2001) Angiogenin activates Erk1/2 in human umbilical vein endothelial cells. Biochem Biophys Res Commun 287:305–310 [DOI] [PubMed] [Google Scholar]
- Lou J, Tu Y, Li S, Manske PR (2000) Involvement of ERK in BMP-2 induced osteoblastic differentiation of mesenchymal progenitor cell line C3H10T1/2. Biochem Biophys Res Commun 268:757–762 [DOI] [PubMed] [Google Scholar]
- Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O’Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, Benezra R (1999) Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 401:670–677 [DOI] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S (2001) Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 7:1194–1201 [DOI] [PubMed] [Google Scholar]
- Marrony S, Bassilana F, Seuwen K, Keller H (2003) Bone morphogenetic protein 2 induces placental growth factor in mesenchymal stem cells. Bone 33:426–433 [DOI] [PubMed] [Google Scholar]
- Mishina Y, Suzuki A, Ueno N, Behringer RR (1995) Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev 9:3027–3037 [DOI] [PubMed] [Google Scholar]
- Moser M, Binder O, Wu Y, Aitsebaomo J, Ren R, Bode C, Bautch VL, Conlon FL, Patterson C (2003) BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol Cell Biol 23:5664–5679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel A, De Pauw-Gillet MC, Purnell G, Nusgens B, Lapiere CM and Foidart JM (1993) Enhancement of tumorigenicity of human breast adenocarcinoma cells in nude mice by matrigel and fibroblasts. Br J Cancer 68:909–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot F, Blais A, Labrie C (2003) Overexpression of a dominant negative type II bone morphogenetic protein receptor inhibits the growth of human breast cancer cells. Cancer Res 63:277–281 [PubMed] [Google Scholar]
- Raida M, Clement JH, Ameri K, Han C, Leek RD, Harris AL (2005) Expression of bone morphogenetic protein 2 (BMP-2) in breast cancer inhibits hypoxic cell death. Int J Oncol 26:1465–1470 [PubMed] [Google Scholar]
- Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM (2002) Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest 109:337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau S, Houle F, Landry J, Huot J (1997) p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene 15:2169–2177 [DOI] [PubMed] [Google Scholar]
- Schwalbe M, Sanger J, Eggers R, Naumann A, Schmidt A, Hoffken K, Clement JH (2003) Differential expression and regulation of bone morphogenetic protein 7 in breast cancer. Int J Oncol 23:89–95 [PubMed] [Google Scholar]
- Suzawa M, Takeuchi Y, Fukumoto S, Kato S, Ueno N, Miyazono K, Matsumoto T, Fujita T (1999) Extracellular matrix-associated bone morphogenetic proteins are essential for differentiation of murine osteoblastic cells in vitro. Endocrinology 140:2125–2133 [DOI] [PubMed] [Google Scholar]
- Tada A, Nishihara T, Kato H (1998) Bone morphogenetic protein 2 suppresses the transformed phenotype and restores actin microfilaments of human lung carcinoma A549 cells. Oncol Rep 5:1137–1140 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Abe M, Sato Y (1999) Roles of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase in the signal transduction of basic fibroblast growth factor in endothelial cells during angiogenesis. Jpn J Cancer Res 90:647–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Gordon GM, Nickoloff BJ, Foreman KE (2002) The helix-loop-helix protein id-1 delays onset of replicative senescence in human endothelial cells. Lab Invest 82:1073–1079 [DOI] [PubMed] [Google Scholar]
- Willette RN, Gu JL, Lysko PG, Anderson KM, Minehart H, Yue T (1999) BMP-2 gene expression and effects on human vascular smooth muscle cells. J Vasc Res 36:120–125 [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL (1995) Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev 9:2105–2116 [DOI] [PubMed] [Google Scholar]
- Wu LW, Mayo LD, Dunbar JD, Kessler KM, Baerwald MR, Jaffe EA, Wang D, Warren RS, Donner DB (2000)Utilization of distinct signaling pathways by receptors for vascular endothelial cell growth factor and other mitogens in the induction of endothelial cell proliferation. J Biol Chem 275:5096–5103 [DOI] [PubMed] [Google Scholar]
- Yang X, Castilla LH, Xu X, Li C, Gotay J, Weinstein M, Liu PP, Deng CX (1999) Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development 126:1571–1580 [DOI] [PubMed] [Google Scholar]
- Zebedee Z, Hara E (2001) Id proteins in cell cycle control and cellular senescence. Oncogene 20:8317–8325 [DOI] [PubMed] [Google Scholar]