Abstract
Traditional prognostic determinants in osteosarcoma have included demographics (age, sex), tumour size, site, stage, and the response to chemotherapy. Many of these are determined using varying techniques and units of measurement, which can make comparison between studies difficult. The absence of survival difference between limb sparing surgery and amputation has been repeatedly demonstrated in primary disease, and even in the setting of pathological fracture. On the other hand, there is still some controversy over the existence of increased local recurrence for limb-sparing surgery, and the implications of this. Commonly used prognostic determinants such as metastases, and response to chemotherapy enable a high degree of prognostic accuracy but usually at a late stage in the course of disease. Leading on from this, there is a need to uncover molecular pathways with specific influence over osteosarcoma progression to facilitate earlier treatment changes. Some important pathways are already being defined, for example the association of CXCR4 with metastases on presentation, the likelihood of doxorubicin resistance with positive P-glycoprotein, and the reduced survival prediction of over expressed survivin. It is anticipated that the future of osteosarcoma treatment will involve treatment tailored to the molecular profile of tumours at diagnosis, adjuvant therapy directed towards dysfunctional molecular pathways rather than the use of cytotoxics, and a more standardised approach to the measurement of clinical prognostic factors.
Keywords: Osteosarcoma, Prognosis, Molecular, Staging, Metastasis, Chemotherapy
Introduction
Osteosarcoma is a disease which predominantly targets the adolescent age group (Bielack et al. 2002) and continues to confer a generally poor prognosis in spite of newly devised chemotherapy regimes combined with wide-margin, limb-sparing surgery. The overall relapse-free survival rate over 5 years is approximately 65% (Bielack et al. 2002). There are many studies which have focused on demographic and clinicopathological factors across large sample groups (Bielack et al. 2002; Davis et al. 1994; Pochanugool et al. 1997; Tomer et al. 1999). These have identified response to chemotherapy, tumour size, site and the presence of metastases as being key determinants of prognosis in osteosarcoma.
Molecular markers and pathways contributing to osteosarcoma have been a more recent discovery and their significance as clinically predictive tools is still being defined. Nevertheless, there is already good evidence for a number of these (in particular, P-glycoprotein, CXCR4, uPA/uPAR, and survivin) as being useful both in predicting response to chemotherapy, overall prognosis, the likelihood of metastases at diagnosis, and at the same time providing targets for developing new therapeutic agents. In the last few decades it has become more evident that the way forward in osteosarcoma management, and cancer in general, will be through a focused inter-relation of clinical and molecular information (Papachristou and Papavassiliou 2007; Varmus 2006).
This review will provide a summary of some of the key clinical and molecular prognostic factors.
Clinical factors
The traditional clinical factors influencing prognosis in osteosarcoma have related to the extent of invasion and more specifically, the stage of tumour (Enneking et al. 1980), patient age, the site and size of the tumour, and the response to pre-operative chemotherapy. Other factors influencing prognosis, some of which are more controversial as a result of mixed findings in the literature, include the type of surgery, local recurrence, and the presence and location of metastases. A selection of studies relating to these factors will be discussed in turn.
Tumour site
The two most common sites of osteosarcoma are the distal femur, followed by the proximal tibia. These sites contain large growth plates with high proliferative activity and turnover of bone. Osteosarcoma in either of these bony sites has a relatively favourable prognosis. The proximal tibia is associated with a 5-year survival rate of 77.5%, which is slightly better than the distal femur at 66%. These sites are second (in terms of favoured prognosis) only to the radius at 81.3% (Bielack et al. 2002). An improved prognosis for tumours lying on either side of the knee joint may relate to the invasion barrier (Quan et al. 2005) formed by the high surface area of articular cartilage at the knee, and the large growth plates which are likely to control invasion through the release of high levels of anti-angiogenic factors such as PEDF (Quan et al. 2002).
It is well established that a proximal and axial location of osteosarcoma results in a considerably worse outcome (Bielack et al. 2002). Of this group, pelvic osteosarcomas are the more common, accounting for approximately 7–9% of all osteosarcomas (Ozaki et al. 2003) compared with osteosarcomas of the spine at 0.85–3.0% (Ozaki et al. 2002).
The 5 years survival of osteosarcoma in the pelvis ranges from 27 to 47% (Kawai et al. 1998a; Ozaki et al. 2003). One study reports an overall 5 years survival of only 34% in patients who had undergone excision and although a wide margin of resection was only achieved in 53% of patients, those who had surgery were still better off than those treated with non-surgical therapy (Kawai et al. 1998a). This is quite a significant finding when one considers the relatively high complication rate, including a mortality of 6–9%, associated with the surgical procedure alone (Apffelstaedt et al. 1995, 1996). In terms of overall survival, patients with pelvic osteosarcomas undergoing resection responded more favourably if surgery was combined with radiotherapy (Ozaki et al. 2003).
Osteosarcoma in the spine has also been linked with a very poor prognostic outlook. Studies have reported median survival times of 10–23 months (Shives et al. 1986; Ozaki et al. 2002). Similar to the situation in pelvic osteosarcoma, increased survival has been noted in those undergoing radiotherapy (Ozaki et al. 2002).
One of the highest osteosarcoma survival rates to date has been identified in a study on osteosarcomas of the forearm and hand (Daecke et al. 2005). High-grade tumours of the distal upper limb had a remarkable 86.5% 5-year survival rate, which is consistent with a figure of 81.3% published by Bielack et al. (2002) in tumours arising in the radius (not including the metacarpals and phalanges). Many osteosarcomas of the hand have been associated with the bone surface, and overall, osteosarcomas of the distal upper limb were more likely to be diagnosed as the unconventional histological subtype.
Stage
The clinical stage of osteosarcoma is predictive of the prognosis and following on from this, provides information essential to the selection of surgery. Enneking et al. (1980), who developed the Musculoskeletal Tumour Society Staging System, studied prognosis in relation to this system in 397 cases pooled together from the University of Florida musculoskeletal service and an inter-institutional study formed by the Musculoskeletal Tumour Society. These cases had been treated from 1970 onwards. It should be noted that one of the limiting factors in the study published by Enneking et al. (1980), was the retrospective pooling of data from multiple institutions both in USA and internationally, making a selection of standard treatment measures impossible.
Stage I-A comprises low grade, intracompartmental tumours and confers nearly 100% 5-year survival rate. Unfortunately this stage is much less common than the aggressive types. Stage I-B describes a low grade but extracompartmental lesion. Stage II is the high-grade form of osteosarcoma, and again this is divided into A and B subcategories based on intra- or extracompartmental location. Stage II-B is the most common of all osteosarcoma presentations and at the time of publication carried a significantly worse prognosis, with a 5-year survival rate of around 40%. This figure has improved with the use of pre-operative, multimodal chemotherapy, although not drastically. Foukas et al. (2002) specifically looked at Stage II-B tumours around the knee and found an overall 5-years survival of 47%.
Stage III is defined by the presence of metastases and likewise at the time of publication the 5-years survival was close to 0%. This outcome has changed significantly in the last few decades, most likely through a combination of chemotherapy, helical CT for diagnosing pulmonary metastases, and improved surgical techniques for metastasectomy. In the case of patients with Stage III having only pulmonary metastases at diagnosis, the current 5-years survival may be as high as 68% (Yonemoto et al. 1998).
The American Joint Committee on Cancer (AJCC) Staging System is similar to that just described although it classifies stage III as any tumour with skip metastases, while the extra stage IV is divided up into IV-A, denoting pulmonary metastases, and IV-B denoting ‘other’ metastases. Stages I and II are subdivided into A and B categories depending on tumour size being greater or less than 8 cm in any dimension, rather than intra or extra-compartmental (Greene FL et al. 2002).
Patient age
The pattern of prognosis in different age groups has a tendency towards unfavourable outcomes in patients both younger and older than the adolescent, age-majority. Bacci et al. (2006) showed, on multivariate analysis, a worse prognosis for those patients aged 14 and younger while another study found the 5-years survival in 47 patients greater than 40 years was poor, at 41.65% (Carsi and Rock 2002). Possible reasons for this include greater axial distribution of tumours, more frequent metastases at presentation, and less tolerance of high dose chemotherapy. A higher rate of axial tumours has been reported in those over 40 years (Bielack et al. 2002), and a further study identified an axial site of presentation in 20.5% of those greater than 40 years compared with 4.7% in adolescent patients with a P-value of 0.035 (Jeon et al. 2006). Aksnes et al. (2006) also reported a 40-years cut-off for prognostic significance.
Tumour size
One of the key measures of prognosis in osteosarcoma is tumour size. Although determining tumour size has become more accurate with advances such as magnetic resonance imaging, the actual dimensions correlating best with prognosis are still being defined. The main methods used in previous studies have been absolute tumour length (ATL), relative tumour length (RTL), less frequently absolute tumour plane (ATP), and more recently absolute tumour volume (ATV). Findings of the key studies pertaining to size have been summarised very well in table form (Bieling et al. 1996).
Bieling et al. (1996) also studied 128 patients, all undergoing multi-agent chemotherapy and definitive surgery for primary osteosarcoma, and examined metastasis free survival in relation to absolute and relative length, width, depth, plane, and finally ATV. The often used relative length values of less than 1/3 of the bone of origin, versus greater 1/3 did not reach significance, whereas absolute length values did have a significant prognostic bearing [<10 cm 84% metastasis-free survival (MFS), versus >10 cm 2% MFS]. In addition, absolute width, depth and tumour plane were all significant for predicting prognosis. They found that ATV, calculated with a specific ellipsoid formula based on absolute length, depth and width, had a cut-off value of 150 cm3 giving a 92% 5 year MFS for smaller tumours compared with 58% in tumours with an ATV greater than 150 cm3. It was also noted that the majority of the tumours greater than 150 cm3 were found in the distal femur whereas the smaller tumour group were more often seen in the proximal tibia. This finding may indicate that site and size are linked in determining prognosis. Absolute values were significant on multivariate analysis, while in previous studies on relative values (Bacci et al. 1990; Bieling et al. 1991; Winkler et al. 1984), multivariate analysis was either not performed or not significant.
Other studies have also found the measurement of volume to have prognostic bearing, albeit with variation in the figures. A tumour volume of 200 ml or greater has been associated with a worse prognosis on multivariate analysis (Bacci et al. 2006) while a further study has showed that a volume greater than 400 cm3 has a statistically worse prognosis (Lindner et al. 1999).
Pathological fracture
The fracture of a bone at the site of a malignant tumour is considered by clinicians and patients alike as nothing short of a disaster. In a retrospective study on two groups of approximately 50 patients, one group with pathological fracture, and the other without, the fracture group had a 55% 5 years survival compared with 77% in those without (Scully et al. 2002).
In the clinical setting post-fracture, the key question of limb-sparing surgery versus amputation then arises. One study compared disease-free survival (DFS) in both limb-sparing and amputation patients post-chemotherapy in the context of pathological fracture presentation. No statistical difference was observed in DFS leading the authors to conclude that limb-sparing surgery was equally safe as amputation (Bacci et al. 2003). Similarly, other results (Abudu et al. 1996; Scully et al. 2002) revealed no significant difference in survival between the two options, although Abudu et al. found that a significant increase in local recurrence occurred in the instance of limb sparing surgery. The latter finding, however, is inconsistent with the current understanding of local recurrence as an intrinsically poor prognostic factor. This will be discussed in a later section.
Type of surgery
As mentioned above, the two main surgical options in osteosarcoma are limb-sparing (with wide excision margins and either megaprosthesis or rotationplasty) and amputation. As adjuvant therapies, operative techniques, and diagnostic imaging methods have improved, the choice of amputation has become more infrequent, and is now reserved for tumours involving major neurovascular bundles, extensive tissue invasion, and concomitant infection (Malawer MM 2001). In a comparison of osteosarcoma surgical options, local recurrence rates of 3.5% in amputation, 6.0% in limb-sparing surgery, and 5.7% in rotationplasty have been published, but the differences did not reach significance (Bacci et al. 2006). However, inadequate surgical margins did result in a statistically significant increase in local recurrence. For this reason, the practice of wide-margin excision in limb-sparing surgery must be strictly adhered to.
Functionally superior, limb-sparing surgery (Davis et al. 1999) has been cemented as the default management pathway based on a number of studies (Rougraff et al. 1994; Simon et al. 1986; Sluga et al. 1999; Szendroi et al. 2000) which have all reported no difference in overall survival between amputation and limb-sparing surgery. Grimer et al. (2002) found higher local recurrence (LR) in limb-sparing surgery compared with amputation. This was a study of 202 patients from 3 different centres, where none of the patients undergoing amputation had LR versus 2.5–13% recurrence in limb-sparing surgery depending on the centre. This group still had a better overall 5-years survival than those who had amputation and did not have LR (37 vs. 31%). Another study had very similar findings in that none of those undergoing amputation had LR, whereas 10% of those with limb-sparing surgery had LR (Picci et al. 1994). Eckardt et al. (1985), on the other hand, found a similar rate of LR between the two surgical options (6.4% in sparing surgery vs. 5.8% in amputation).
With regard to surgery on specific pelvic sarcomas (osteosarcoma, chondrosarcoma, and Ewing’s), hemipelvectomy has been compared with limb-sparing surgery and there was no difference in the incidence of LR and no difference in the quality of surgical margins (Kawai et al. 1998b). In spite of this, the hemipelvectomy group had a worse survival than the limb-sparing group with a relative risk of 4. This might be partly attributed to selection of hemipelvectomy in patients with larger tumours, who would do poorly on this basis alone (Ozaki et al. 2003). The morbidity associated with a more extensive operation would also be a significant factor.
Local recurrence
It has previously been discussed that limb-sparing surgery generally results in a slightly higher LR but with better overall survival. There are, however, results which are somewhat contradictory to this pattern. Bacci et al. (1998) reported a higher LR occurring in limb sparing surgery, particularly with inadequate margins, and all patients with LR developed lung metastases at some stage in the course of disease. Regardless of treatment there was a 96.1% mortality in the LR group, compared with 72.1% in those with metastases but no local recurrence. This result suggests that the combination of LR and metastasis is worse than metastasis alone. Also in line with this finding, another study found that the 5-years survival in those with LR and lung metastases was 6% compared with 37% in patients with only metastases (Briccoli et al. 2005).
Grimer et al. (2005) found that in cases of LR the only significant prognostic determinant was the presence of concomitant metastases. In order to tie this in with findings reported by Bacci et al. (1998), it may be that the combination of LR and metastasis has a worse prognosis than metastasis alone, which may be worse than LR alone.
Another different perspective is seen in Nathan et al. (2006) where the strongest correlation with poor prognosis was LR within the first year after resection. Metastasis at the time of recurrence was statistically significant but only with P = 0.04 versus P = 0.001 for LR alone (Nathan et al. 2006).
Metastases
Osteosarcoma metastasises most commonly to the lung (Bacci et al. 2001). Less frequently bone metastases occur and these generally have a much poorer prognosis. Clinical factors predictive of primary metastases include axial tumours, large tumours and a long history of symptoms (Bielack et al. 2002).
Pulmonary metastases which are found at initial diagnosis are generally thought to be associated with a poor outcome (Enneking et al. 1980). Yonemoto et al. (1998) report very different findings, although given a sample size of only nine patients it is unlikely to represent the broad spectrum of osteosarcoma. These investigators found a 5-years survival rate of 64.8% which, surprisingly, was higher than patients who did not have metastases at presentation (62.1%). On the other hand, those that developed pulmonary metastases after chemotherapy were much worse off with a survival rate of 47.5%. One possible explanation may lie in the fact that more patients who were diagnosed with metastases on plain film or CT imaging initially, went on to have metastasectomy via a radical thoracotomy approach. This procedure enabled clinicians to identify and remove approximately four times the number of metastases that were seen on CT. More recent papers have confirmed the superior diagnostic capabilities of transxiphoid lung palpation with video-assisted thorascopic surgery (VATS) compared with helical CT alone (Ambrogi et al. 2000). Likewise, another study found that palpation of the lungs during VATS picked up additional metastases in 22% more cases than helical CT (Parsons et al. 2004).
Even recurrent metastases do not always equate with a sudden downward spiral in the clinical course. After two or more metastasectomies one study population maintained reasonable survival figures of 48% at 5 years (Kandioler et al. 1998). This study also found no survival advantage in the primary histology of the lung metastases (osteosarcoma or epithelial cancer) and no change in survival with variation in size or number of metastases. This was in contrast to a study by Daw et al. (2006), where there was a survival advantage for patients with no more than three lung nodules, and unilateral lung metastases. They also observed significant survival advantages when the interval between the primary tumour resection and first metastectomy was increased (i.e., 20–40 months), and this has been supported by Briccoli et al. (2005). Similarly on a Cox regression model published by Briccoli et al. (2005), the risk of death was slightly less after a second pulmonary metastasectomy (0.974 vs. 0.972) which may be related to the patient’s already prolonged survival on reaching a second metastasectomy. On the basis of reasonable 5-years survival rates, both Briccoli and Kandioler advocate surgery even after multiple recurrences of lung metastases.
Skip lesions in osteosarcoma are those which occur at discontinuous points along the length of a bone. Although found in relatively few patients (1.8–25%) (Sajadi et al. 2004), they carry a particularly bad prognosis even in the modern day treatment era, with a reported survival average of 27.2 months from diagnosis. This is clearly far worse than in patients who only have lung metastases. Sajadi et al. (2004) rightly point out that this does not fit with the current AJCC staging system whereby the presence of lung metastases classifies the patient as stage IV, versus skip lesions without lung metastases which classifies the patient in stage III even though this carries a significantly worse outcome.
Tumour response to chemotherapy
A critical appraisal (Davis et al. 1994) of eight papers concerned with prognostic factors in osteosarcoma, concluded that percentage necrosis of the tumour in response to pre-operative chemotherapy was the most significant factor correlating with disease free survival. The studies reviewed in this appraisal were selected based on strict inclusion standards, including; identifying a clear inception point, a defined outcome, and a minimum follow-up of 36 months. A key point arising from this appraisal was the lack of standard protocols being employed to define percentage necrosis, and even size of the tumour. Establishment and widespread adherence to such protocols would improve the quality of results in any further studies.
Although not usually practical, the most accurate method to determine the percentage necrosis would ideally involve assessment of whole cross-sections of the tumour in the most maximal dimension which could be divided into smaller sections for visualisation. These individual sections should be overlaid with a transparent grid consisting of 2 mm2 and on this, necrotic areas drawn. From this map an accurate percentage necrosis can be determined (Hauben et al. 2002). The most predictive cut off point for favourable or unfavourable response to chemotherapy is greater than 90% or less than 90% necrosis, respectively (Picci et al. 1985).
Response to chemotherapy is also predictive of the need for further resections. Antunes et al. (1999) found that in pulmonary metastectomies, all patients requiring more than one operation had less than 80% necrosis post chemotherapy.
A significant correlation between response to chemotherapy and histological subtype has also been noted (Bacci et al. 2006). Telangiectatic tumours respond best (86.7% good response), followed by osteoblastic (63.9%), fibroblastic (61.7%), chrondroblastic (50.6%), and finally small cell tumours with the worst response (25%). The best chemotherapeutic combination identified in this study was methotrexate, cisplatin, and adriamycin, at a 65.7% good response rate. In this study, multivariate analysis confirmed a significant association between poor response to chemotherapy and a worse prognosis.
Serological markers
A study was performed at the Rizzoli Institute on 560 patients looking at ALP levels in high-grade osteosarcoma (Bacci et al. 2002). High ALP levels correlated with males over 14 years of age, tumour size larger than 150 ml, and osteoblastic subtype. A normal pre-treatment ALP level resulted in a significantly higher 5-years disease free survival (67%) than in patients with high levels (54%). This study also supported the level of chemotherapy-induced necrosis being linked to 5-years survival as an independent predictive factor. A particularly interesting finding was the correlation between high ALP and tumour volume greater than 150 ml (a known prognostic cut-off factor). In future ALP may provide useful prognostic information and potentially a prediction of tumour size when there is difficulty quantifying the size on imaging.
A further study on prognostic factors found that patients with normal serum ALP levels had a significantly longer median time to recurrence (25 months compared with high levels at 18 months) (Bacci et al. 2006). Other studies correlate with these findings (Thorpe et al. 1979; Tomer et al. 1999).
Molecular markers
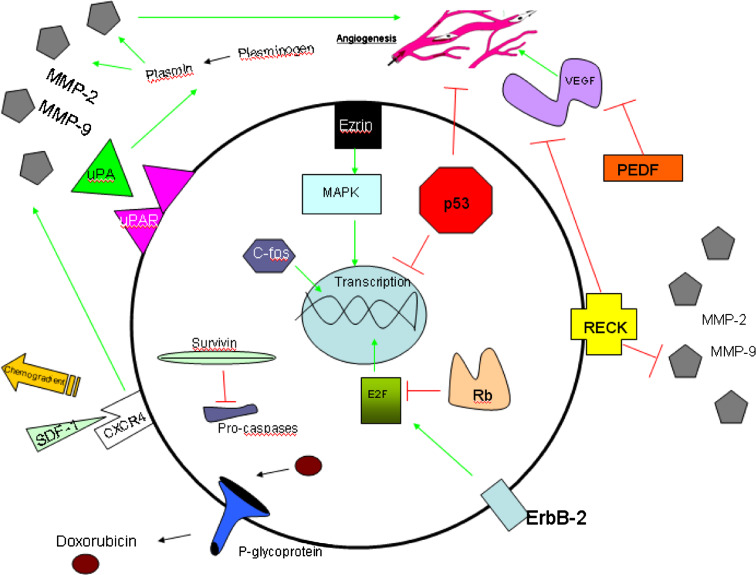
The relative inaccuracy and limitations of standard prognostic markers (tumour size, and percentage necrosis post-chemotherapy), and the fact that many prognostic indicators only become apparent at a late stage in the clinical course, presents a need for more modern quantitative measures to assist in treatment planning. The ultimate aim would be to correlate the up or down-regulation of key molecular pathway components with longer term outcomes (Table 1). This will enable clinicians to more appropriately alter chemotherapy and surgery earlier in the disease process.
Table 1.
Molecular factors associated with prognosis in osteosarcoma
| Factor | General role in cancer | Levels in OS | Prognostic bearing in OS | Potential for therapy in OS |
|---|---|---|---|---|
| VEGF | Angiogenesis | Up | Controversial (Mohammed et al. 2007; Kaya et al. 2000; Hara et al. 2006; Mantadakis et al. 2001) | Yes (Folkman 2004; Tjin Tham Sjin et al. 2006) |
| PEDF | Anti-angiogenesis | Down | Undetermined | Yes, awaiting further preclinical trials (Ek et al. 2007a, b) |
| MMP-2, MMP-9 | Extra-cellular matrix invasion | Up | Correlation (Foukas et al. 2002) | Yes, good results in other cancers (Nemunaitis et al. 1998) |
| uPA/uPAR | Increases plasmin and MMPs. Pro-invasion | Up | Correlation (Choong et al. 1996) | Yes, reduced invasion if down regulated (Dass et al. 2005) |
| RECK | Anti-invasion, anti-angiogenesis | Down | Undetermined | Undetermined |
| P-glycoprotein | Drug resistance. Other unidentified pathways | Up | Correlation, specific to doxorubicin therapy (Baldini et al. 1999; Park et al. 2001) | Undetermined |
| CXCR4 | Chemotaxis, organ-specific metastasis. Pro-invasion | Up | Correlation (Laverdiere et al. 2005) | Yes, good evidence in mice (Perissinotto et al. 2005) |
| p53 | Cell cycle control | Down/mutated | Correlation (Park et al. 2001) | Undetermined |
| ErbB-2 | Cell signalling, proliferation | Mixed results | Controversial (Onda et al. 1996; Somers et al. 2005; Zhou et al. 2003) | Undetermined |
| Survivin | Inhibits apoptosis | Up | Correlation (Osaka et al. 2006) | Undetermined |
| HLA class I | Absence allows immune system evasion | Down | Correlation (Tsukahara et al. 2006) | Undetermined |
| Ezrin | Cell signalling, cell interaction, metastasis | Up | Correlation (Khanna et al. 2004; Park et al. 2006) | Yes, potentially using Rapamycin (Wan et al. 2005) |
| Rb | Tumor suppressor, transcription control | Down/mutated | Correlation (Wadayama et al. 1994; Benassi et al. 1999; Feugeas et al. 1996) | Undetermined |
| PTHrP/PTHR1 | Proliferation, invasion, hypercalcaemia | Up | Undetermined | Undetermined |
| c-Fos | Transcription | Up | Indirect correlation (Gamberi et al. 1998) | Undetermined |
OS osteosarcoma
There is a plethora of molecular pathways involved in tumorigenesis being uncovered, ever increasing the potential options for treatment and diagnosis. For example, these markers may be used to predict specific outcomes such as the likelihood of micrometastases at diagnosis and response to chemotherapy.
The other significant advantage of studying molecular pathways relating to prognosis is that it provides information to assist the development of newer agents which either block or enhance these clinically relevant pathways (Fig. 1). Much attention over the years has been focused on controlling tumour angiogenesis by manipulating the relevant regulatory factors. This research field, pioneered by Judah Folkman (1982; Folkman and Haudenschild 1980), has contributed significantly to the molecular revolution in cancer research.
Fig. 1.
Key molecular factors in osteosarcoma
VEGF and MVD
Vascular endothelial growth factor (VEGF), is a naturally occurring protein known to stimulate the development of microvasculature in tissues generally, and plays a significant role in the progression of many cancers, by increasing their blood supply. There is a concerted effort to utilise anti-angiogenic agents, such as Avastin and Endostatin, which work to inhibit the VEGF signalling pathway (Folkman 2004; Tjin Tham Sjin et al. 2006). In cancer, VEGF expression is stimulated by hypoxic conditions via the up-regulation of hypoxia inducible factor 1a. The prognostic significance of VEGF and microvascular density (MVD) in osteosarcoma, as outlined below, is still controversial and a definitive conclusion has not yet been reached.
Increased VEGF levels in other forms of cancer have already demonstrated a prognostic bearing, for example in a recent study on breast cancer where high VEGF levels correlated with increased MVD, increased frequency of metastases and reduced overall survival (Mohammed et al. 2007). A study on VEGF expression in paraffin-embedded osteosarcoma biopsy samples found VEGF positive samples had a statistically significant correlation with the level of microvascular density (MVD) as measured by CD-34 antibody levels, and also with patient sex, the presence of pulmonary metastases at diagnosis, and finally with the 5-years survival rate (Kaya et al. 2000). Similar findings have been reported in another study (Hara et al. 2006). Contrary to this, Mantadakis et al. (2001), found no relationship between MVD and long-term survival, and a further study found no correlation between VEGF expression or MVD and survival (Ek et al. 2006b). There was an increase in MVD in the samples of patients older than 40 years, and this age group in itself is considered to have a poorer prognosis (Ek et al. 2006b).
One of the more surprising results was published by Kreuter et al. (2004), who in addition to finding no statistical relationship between MVD and age over 40, discovered a prognostic advantage in osteosarcoma with increased MVD. They showed an 89% 5-years survival in high MVD, compared with 49% in low MVD. Furthermore, high MVD was associated with a good response to chemotherapy, which the authors suggest may be due to higher accessibility of chemotherapy to tumour cells.
The results above would naturally bring into question the rational behind anti-angiogenic agents in osteosarcoma. After all, if a higher density of vessels in tumours improves the delivery of chemotherapy and results in significantly better survival, why would one consider inhibiting this process? Other results (Dutour et al. 2005) suggest that anti-angiogenic therapy may still be a very promising therapy in osteosarcoma, however the most appropriate clinical context is yet to be determined. Dutour and fellow researchers infused complexes of liposomes and plasmids expressing endostatin cDNA into rats. Comparing this to a group treated with empty plasmids, they found that endostatin delayed tumour growth and reduced the recurrence of metastasis, confirmed by tomoscintography and PET scanning. In this study, however, chemotherapy was out of the equation and this is likely to be the critical factor in determining whether an increased degree of angiogenesis in a tumour is favourable or unfavourable. It would seem logical that if chemotherapy is not used at all, an increased MVD is unlikely to improve survival. In this case there is probably more benefit in starving the tumour of its blood supply with anti-angiogenic factors, as we see in Dutour et al. (2005). It will be interesting to review studies combining these therapeutic approaches in osteosarcoma using both endostatin and chemotherapy, to see if the effects of chemotherapy are cancelled out to some extent, or conversely if a degree of synergy is present. There are studies already in progress examining the combination of low-dose chemotherapy with celecoxib (a known anti-angiogenic angent) (Stempak et al. 2006).
PEDF
Pigment epithelium derived factor (PEDF) is a protein initially identified by its absence in angiogenic eye disorders, where under normal conditions it has a protective role against excessive angiogenesis (Bouck 2002; Holekamp et al. 2002). PEDF is found to be down regulated in many common cancers (Ek et al. 2006a). It is a significant inhibitor of angiogenesis in osteosarcoma (Ek et al. 2007a) via inhibition of VEGF and induced apoptosis of endothelial cells (Takenaka et al. 2005). PEDF over expression within in vivo models results in suppression of cancer growth, invasion and metastases (Ek et al. 2007b). Thus PEDF has potential in osteosarcoma therapy and staging.
PEDF expression levels have prognostic bearing in both pancreatic adenocarcinoma and non-small cell lung cancer. However, to date, there has been no definitive study correlating PEDF levels and clinical outcomes in osteosarcoma. Given its well-defined anti-cancer effects in osteosarcoma within the laboratory, such a correlation is likely to be present and should be investigated.
MMPs
Matrix metalloproteinases are enzymes normally involved in break down of the extracellular matrix within the context of physiological tissue remodelling and angiogenesis. Excessive production of certain MMPs through dysregulation of transcription (Kido et al. 1999) or lack of MMP inhibitors has been recognised as an important factor in cancer invasion and metastasis. Following on from this, human clinical trials of MMP-inhibitor drugs such as Marimastat have demonstrated an ability to modify tumour progression markers (Nemunaitis et al. 1998).
MMP-9, otherwise known as gelatinase B, is normally associated with bone remodelling, and in dysregulated states such as rheumatoid arthritis and osteosarcoma it appears to have a prominent role. For example, Himelstein et al. (1998) showed positive staining in all paediatric osteosarcomas studied. A linkage with prognosis has also been identified where osteosarcomas staining positively for MMP-9 were associated with an overall 5-years survival of 28% in comparison to 79% for the negative group (Foukas et al. 2002). The importance of MMP-9 in osteosarcoma was also demonstrated by Kido et al. (1999) who showed that over expression of this enzyme in rat osteosarcoma was associated with increased metastatic potential.
The value of this information lies in both the prognostic potential, and the therapeutic relevance. MMP-9 is already known to be inhibited by a number of substances including sulfated glucosamine, histone deacetylases (Vinodhkumar et al. 2007), nitric oxide (Shin et al. 2007), and reversion-inducing cysteine-rich protein with Kazal motifs (RECK) (Kang et al. 2007), with an end result in the lab of reduced invasion and metastasis in cultured cells and animal cancer models.
RECK
RECK is a newly discovered membrane-bound protein which was first identified by Takahashi et al. (1998) by screening a fibroblast cDNA library for genes inducing reversion to a flattened morphology in a Ki-ras transformed fibrosarcoma cell line. In a further study they also discovered RECK’s ability to inhibit MMP-9, MMP-2 and MT1-MMP (Oh et al. 2001). In addition, RECK has an important role in controlling angiogenesis. Oh et al. (2001) found that when fibrosarcoma tumours lacking RECK were implanted into nude mice, they developed extensive, immature and leaky vascular networks. On the other hand, tumours with positive RECK expression had mature vessel walls with less branching formations. These effects have been attributed to RECK’s inhibition of MMPs (which contribute to vascular wall remodelling) and possibly via inhibition of VEGF (Oh et al. 2001).
RECK is down regulated in many common tumours including breast (Span et al. 2003), colorectal (van der Jagt et al. 2006), and lung cancer (Takenaka et al. 2004). In the majority of these studies down regulation is related to a poor outcome. RECK over expression by liposome transfection of SaOS-2 cells (a human osteosarcoma cell line) has been correlated with reduced cell invasion across a matrigel in vitro (Kang et al. 2007). Researchers involved in this initial study also identified down regulation of RECK in a number of osteosarcoma cell lines and human osteosarcoma tissues. Further studies will be required to determine its full potential as a prognostic indicator.
uPA/uPAR
Like RECK, the urokinase plasminogen activator (uPA) system interacts with MMPs, the difference being that uPA upregulates MMPs and promotes invasion whereas RECK does the opposite. The main pathway in this system involves a ligand (uPA) binding to its receptor (uPAR), which subsequently results in uPA becoming active, resulting in cleavage of plasminogen to plasmin (Choong and Nadesapillai 2003). Plasmin breaks down extracellular matrix and activates pro-MMPs, which facilitate cancer invasion (Pillay et al. 2007). The uPA/uPAR system is upregulated in many common tumours, and specifically in colorectal (Ganesh et al. 1994), ovarian (Kuhn et al. 1994), renal (Hofmann et al. 1996) and prostate cancer (McCabe et al. 2000), there is evidence linking an upregulated uPA system with poor survival. In osteosarcoma there is a distinct, inverse relationship between uPA levels and survival time (Choong et al. 1996), and likewise in chondrosarcoma there is increased recurrence and metastases associated with uPA over expression. Down regulating uPAR using antisense clones within an in vivo osteosarcoma model results in reduced primary growth in the tibiae, and inhibition of pulmonary metastases (Dass et al. 2005).
P-glycoprotein
P-glycoprotein (P-gp), a protein responsible for energy-dependent drug efflux and encoded by the multiple durg-resistant-1 (MDR1) gene, has also been found by a number of researchers to be indicative of clinical outcomes. High levels of expressed P-gp in osteosarcoma is associated with a significant reduction in the probability of remaining event-free after diagnosis but does not correlate with the level of post-chemotherapy tumour necrosis (Baldini et al. 1995; Chan et al. 1997). This finding indicates that P-gp may be involved in pathways aside from drug efflux.
In a retrospective study involving immunohistochemical analysis of paraffin-fixed osteosarcoma samples (Baldini et al. 1999) a significant reduction in event-free survival in patients with tumours positive for P-gp was found. A group of 37 patients studied had received doxorubicin as a single agent post-operatively, and the expression of P-gp in this group, along with survival time, was compared with a group who received multi-agent chemotherapy. Not-surprisingly, those who received multi-agent therapy had a longer survival time. Patients solely on doxorubicin with positive P-gp tumours had an even worse survival time but this observation was reversed when P-pg was negative. This made investigators to conclude that P-gp was responsible for tumour cell resistance to doxorubicin, and suggested that clinical testing of P-pg may, in future, influence the choice and dosage of chemotherapy agents.
Other researchers (Park et al. 2001) correlated the co-expression of mutant p53 protein and P-pg with significantly reduced survival time (30% 3-years survival) in comparison to tumours negative for both mutant p53 and P-gp (70% 30-years survival). Enneking stage of the tumour was also statistically related to p53 and P-gp co-expression. Normally the wild-type p53 protein inhibits expression of the MDR1 gene, and the aforementioned researchers (Park et al. 2001) suggest that in the case of the mutant p53, this protein may potentially stimulate MDR1 transcription and therefore P-gp production.
P-gp is a potential marker of increased malignancy as evidenced by increased P-gp levels in lung metastases compared to primary osteosarcoma lesions (Ferrari et al. 2004).
Summarising the results of 14 studies on P-gp and clinical outcomes is a meta-analysis, which also confirms a correlation between positive immunohistochemistry for P-gp and progression of osteosarcoma (Pakos and Ioannidis 2003). In this study, however, P-gp was not found to influence the histologic response to chemotherapy. Such a finding is somewhat conflicting with the understanding of P-gp being involved in chemotherapy resistance by means of actively pumping these agents out of cells. There are likely to be other mechanisms involved in the effects of high P-gp expression, although the authors of this meta-analysis do mention that assessing response to chemotherapy with percentage necrosis is not a very precise measurement.
CXCR4
The chemokine receptor, CXCR4, and its corresponding ligand, stromal cell-derived factor 1 (SDF-1) are understood to play a major role in the metastatic cascade and hence the prognosis for cancer patients. Chemokines released from endothelium in target tissues (i.e., lung and bone) bind to their receptors on tumour cells in the primary lesion and result in both the release of MMPs (Paoletti et al. 2001) and a chemoattractant gradient (Libura et al. 2002), leading to metastasis to the specific target tissue. The CXRR4/SDF-1 system has been found to correlate significantly with the presence of metastases in a number of tumours including prostate, melanoma, breast, and rhabdomyosarcoma (Libura et al. 2002; Murakami et al. 2002; Smith et al. 2004; Taichman et al. 2002). Increased CXCR4 mRNA expression in osteosarcoma tumour samples correlates with reduced overall survival and with the presence of metastases at diagnosis (Laverdiere et al. 2005). CXCR4 is also a potential target for chemotherapy agents. For example, when the T134 peptide was used in a mouse model to inhibit the CXCR4 site it was found that elimination of lung metastases occurred in six out of six mice (Perissinotto et al. 2005). These mice were tested by injecting, both the osteosarcoma cell line, SJSA and T134, concomitantly.
p53
The inactivation of the key tumour-suppressor gene p53 and its protein product plays a prominent role in the development of most tumours, typically through the loss of cell cycle arrest and repair mechanism, and also more recently discovered, a loss of anti-angiogenesis (Teodoro et al. 2007). In many osteosarcoma cell lines, it is noted that rearrangement of the first intron of the p53 gene occurs consistently, and leads to altered protein expression (Chandar et al. 1992). A number of p53 point mutations have also been identified in osteosarcomas (Miller et al. 1996), and a small proportion of osteosarcomas are associated with either germ-line p53 mutations or the Li-Fraumeni syndrome (McIntyre et al. 1994).
In terms of influencing tumour behaviour, one study examining both p53 mutation and MDM2 expression (a p53 binding/inhibiting protein) in osteosarcoma tissue samples, found no significant relationship between p53 and proliferative rate or histological grade of tumours (Lonardo et al. 1997). There was a marginally significant association (P = 0.04) between MDM2 over expression and the presence of recurrence or metastatic disease.
On the other hand, a study mentioned above by Park et al. (2001) looked at prognostic significance of mutant p53 and P-gp co-expression and found a significantly reduced survival time when both these proteins were expressed. Immunohistochemistry was used to detect both proteins and, of note, samples with mutant p53 genes resulted in increased p53 protein because this defective protein has a greater half-life than normal p53 (Finlay et al. 1988).
ErbB-2
The c-erbB-2 gene produces a transmembrane glycoprotein (ErbB-2) which is very similar in structure to the epidermal growth factor receptor. ErbB-2, also known as neu and HER2, plays a significant role in the pathogenesis of breast cancer but its role in osteosarcoma is shrouded in some controversy. Onda et al. (1996) found no evidence of mutation or amplification of the c-erbB-2 gene amongst 26 different osteosarcoma tissues. Rather, they found that in osteosarcoma where the ErbB-2 protein was detected (42%) there was a significant correlation with reduced survival, increased pulmonary metastasis and a poor response to chemotherapy.
Another study, in this case using immunohistochemistry to analyse HER2 in 34 samples of paediatric osteosarcoma, observed predominantly weak staining (Somers et al. 2005). They concluded that a general lack of this protein in the paediatric group indicated a likely poor response to therapeutic targeting of the pathway. Conversely, other researchers found positive cytoplasmic staining in 44% of osteosarcoma samples and positivity was correlated with an increased likelihood of metastases and poor outcome (Zhou et al. 2003). On the basis of these mixed findings, there is some evidence for ErbB-2 as a prognostic indicator, although future studies with larger sample numbers are required to confirm this. Furthermore, drugs blocking ErbB-2 like herceptin, used successfully in breast cancer clinically (Lin and Rugo 2007) and other cancers in vitro (Saeki et al. 2007), may be beneficial if patients with osteosarcoma were found to over express ErbB-2.
Survivin
Survivin is a member of the inhibitor of apoptosis protein family (IAP), and its main action is to bind and inhibit pro-caspases which are an integral part of the apoptosis pathway (Tamm et al. 1998). It is generally known to be expressed in cancer but not in normal tissues (Chiou et al. 2003). Survivin was recently studied in osteosarcoma using RT-PCR for survivin mRNA after extracting RNA from paraffin-embedded osteosarcoma samples (Osaka et al. 2006). No survivin mRNA was identified in normal tissue samples, whereas all 16 osteosarcoma samples demonstrated survivin. Tumours with high levels (any value above the median) were associated with significantly reduced survival time. The converse relationship applied to tumours with low survivin levels. These patterns were consistent in both initial biopsy samples and post-chemotherapy samples.
Another study (Trieb et al. 2003) used immunohistochemistry to detect cytoplasmic and nuclear survivin in 40 osteosarcoma samples (pre-surgery and pre-chemotherapy). The only independent factor correlating with increased survival was the presence of survivin staining in the nucleus. There is no clear explanation for this as yet, but the authors do note that their results are consistent with other survivin studies on gastric (Li et al. 2004) and transitional cell carcinoma (Ku et al. 2004).
Wang et al. (2006) used immunohistochemistry techniques and found a significant correlation between survivin expression and poor differentiation, and also between PCNA (a marker of cell replication) and poor differentiation. They also found a significant correlation between survivin expression and PCNA levels. This further confirms the role of survivin in inhibiting apoptosis and influencing tumour progression.
HLA class I
Immunotherapy for cancer has shown exciting potential and has been a popular focus for research in the last decade. A large number of studies have found HLA class I, usually present on all human cells, to be down-regulated in cancers such as breast, colon, and prostate. The immunological profile of sarcomas has been studied in less detail. Tsukahara et al. (2006) looked at multiple forms of bone and soft tissue sarcoma, including osteosarcoma, chondrosarcoma and malignant fibrous histiocytoma (MFH), and found HLA class I down regulation in 62% overall. In osteosarcomas specifically, a trend towards increased down regulation with an increasing degree of malignancy was noted. HLA class I down regulation was seen in 52% of primary osteosarcomas and 88% of metastatic tumours. This trend also correlated with prognosis on Kaplan Meier plots, where down regulation was associated with significantly less survival (Tsukahara et al. 2006). Mechtersheimer et al. (1990) also studied HLA-I expression and found no down regulation, although sample size was very small at four. The more congruous finding was that these patients all had favourable survival times. This suggests a potential for the use of HLA class I as a prognostic marker, although larger studies are obviously required. As Tsukahara et al. (2006) note, the lack of HLA I in osteosarcoma may weaken the possibility of using immunotherapy, at least with CD8 cell-mediated immunity. Having said this, it may be possible to upregulate HLA I by transfection and perhaps increase immune susceptibility.
Ezrin
The ezrin protein has a role in cell–cell interactions, signal transduction, linkage between actin filaments and the cell membrane, and when upregulated it drives metastasis (Hunter 2004). Increased ezrin expression in paediatric osteosarcoma patients is associated with reduced disease-free intervals, and down regulation of ezrin expression in a mouse model of human osteosarcoma, is associated with pulmonary metastasis inhibition (Khanna et al. 2004). Ezrin appears to mediate its metastatic actions through the MAPK signalling pathway (Khanna et al. 2004). Rapamycin has been found to inhibit ezrin-mediated pathways leading to reduced lung metastases, again in a mouse model of osteosarcoma (Wan et al. 2005).
Park et al. (2006) found a correlation between the presence of ezrin and high-grade osteosarcoma, where 43.7% of these were positive but no low-grade osteosarcomas were positive for ezrin.
Retinoblastoma
The retinoblastoma (Rb) gene is fundamental to the control of the cell cycle. It binds E2F, a nuclear transcription factor, keeping it inactive until the CDK4/CyclinD complex releases E2F via phosphorylation of Rb. Mutations of the Rb gene lead to continuous cell cycling, and this Rb defect is very common in multiple forms of cancer.
Iida et al. (2003) found that in osteosarcoma cell lines lacking an active Rb gene, for example SaOS-2, there was significant resistance to growth suppression with methotrexate, compared with those with the wild-type Rb gene. The loss of the Rb gene may be involved in a familial risk increase for osteosarcoma (Longhi et al. 2001) but the prognostic significance of Rb ‘loss of heterozygosity’ (LOH) has not been fully determined. Heinsohn et al. (2007) found Rb LOH in 39% of 41 osteosarcoma patients but there was no correlation with overall survival or event-free survival in these patients. This was contrasted by Wadayama et al. (1994), who found LOH in 62.9% of osteosarcomas and a significant survival advantage in those patients who were Rb LOH negative. Similar findings have been reported in other studies (Benassi et al. 1999; Feugeas et al. 1996) which are summarised in Table 2.
Table 2.
Summary of finding in four studies examining the prognostic bearing of the retinoblastoma gene LOH in osteosarcoma
| Study | % Rb LOH in OS | Survival advantage with negative LOH |
|---|---|---|
| Heinsohn et al. (2007) | 39% | No |
| Wadayama et al. (1994) | 62.9% | Yes |
| Feugeas et al. (1996) | 70% | Yes |
| Benassi et al. (1999) | 54% negative for functional pRB | Yes |
In three out of four studies there was a significant correlation between absent LOH and improved survival
OS osteosarcoma, LOH loss of heterozygosity
PTHrP/PTHR1
Parathyroid hormone-related peptide and it receptor (PTHR1) are known to be involved in tumour progression, bone metastases and hypercalcaemia due to malignancy. PTHR1 is not entirely dependent on stimulating ligands, showing some autocrine activity. When PTHR1 was ovexpressed in the HOS osteosarcoma cell line by transfection, increased proliferation, motility and invasion through Matrigel was observed suggesting a tumorigenic role (Yang et al. 2007). So far no study has adequately assessed the prognostic bearing of PTHrP/PTHR1 in osteosarcoma, however mixed results have been seen in more common tumours. Patients with glial tumours were observed to have a significantly reduced overall survival with elevated PTHrP expression (Pardo et al. 2004), and in a study of patients with hypercalcaemia of malignancy, PTHrP levels corresponded with a poor prognosis (Truong et al. 2003). On the other hand, over expression of PTHrP in a rat osteoblastic, osteosarcoma cell line was found to reduce cell proliferation by 80% (Pasquini et al. 2002). Similarly in a study of PTHrP immunoreactivity in breast cancer and bony metastases, a favourable prognosis was observed in 79% of positive staining tumours over a median 10-years follow-up (Henderson et al. 2006). These various results suggest that the PTHrP/PTHR1 system has diverging actions on tumour progression, involving progression or inhibition depending perhaps on whether ligand or receptor is upregulated.
c-Fos
The early response gene product, c-Fos protein has an important role in upregulating the transcription proteins, which drive the cell cycle. In many cancers c-Fos levels are abnormally elevated. In osteosarcoma elevated c-Fos levels correspond with higher grades of tumour (Franchi et al. 1998; Wu et al. 1990) and also the propensity to develop metastases (Gamberi et al. 1998). Beedles et al. (1999) were able to show malignant transformation of osteoclasts in transgenic mice when c-Fos was over-expressed.
Discussion
Studying the prognostic factors in osteosarcoma is important for a number of reasons. Detailed studies of prognosis have specifically been performed in osteosarcoma because the prognosis overall is poor and the prognosis has not changed significantly since the introduction of chemotherapy in the 1970s. Further study in this area will firstly allow clinicians to provide a more informed outlook to the patient and their family. Secondly it will determine the combination of treatment and the aggression to which this treatment is implemented from an earlier stage in the disease. Thirdly, the identification of key prognostic factors provides a more vivid target on which to focus anti-cancer research.
In terms of the clinical prognostic factors reviewed, there are clearly a number of discrepancies, but overall the literature supports limb-sparing surgery both in uncomplicated primary disease, and in the setting of pathological fracture (Bacci et al. 2003). Metastasis is clearly unfavourable on the whole, although surprisingly there are some reasonable survival rates for pulmonary metastases (Yonemoto et al. 1998), especially given improved identification and elimination methods using both helical CT (computer tomography) and hand assisted VATS (video-assisted thorascopic surgery). Of the factors which can be consistently assessed on presentation, patient age, absolute tumour volume, stage and site of the tumour remain the most useful because in general they have clear cut-off points identified in the literature between good and bad outcomes, for example age greater than 40 (Aksnes et al. 2006), and tumour volume greater than 150 cm3 (Bieling et al. 1996). The combined value of these factors would be greatly increased, in research and clinical practice, if more uniform measurement techniques were employed across international centres.
Amongst the molecular factors reviewed, many of the dysfunctional pathways are correlated with reduced levels of survival, and in some cases chemotherapy response. Thus, there is a key link between clinical and molecular markers here, with the potential to select higher doses and different combinations of chemotherapy earlier in the disease. In this regard, some particularly exciting findings linking molecular factors with specific disease events include; the association of CXCR4 with micrometastases at diagnosis (Laverdiere et al. 2005), and the association of P-gp with resistance to doxorubicin (Baldini et al. 1999). As further specific relationships are identified, it is likely that molecular markers will assume an increasing influence over future management of osteosarcoma by identifying tumours susceptible to specific molecular therapies.
One of the key drawbacks to the use of molecular markers in prognosis is the variability of expression between tumours of the same histology, and the methods used to measure expression. The same applies to assessment of percentage necrosis in tumours post-chemotherapy, where as previously discussed only by analysing a whole cross-section of tumour will an accurate result be attained (Hauben et al. 2002). This may be at least partially due to heterogeneity of the tumour cell population and its vascular supply. Even in the limited amount of literature reviewed above there are also significant contradictions in results. For example with VEGF and MVD, there are virtually equal numbers of studies supporting or refuting their role as a negative prognostic markers, and Kreuter et al. (2004) finds that these factors in fact convey a prognostic advantage. Conflicting evidence across the literature at this stage makes it very hard therefore, to utilise VEGF and MVD in prognostic protocols clinically. However, there is a good case for improved chemotherapy response and, in some cases survival, in vascular tumours (Kreuter et al. 2004).
As large prognostic studies of the future incorporate an analysis of key molecular factors described above, further relationships with clinical factors will be defined, resulting in earlier and more informed treatment decisions.
Acknowledgments
The authors would like to acknowledge the generous support of the Australian Orthopaedic Association, Cancer Council of Victoria, Faculty of Medicine at the University of Melbourne, and The Royal Australasian College of Surgeons.
Abbreviations
- VEGF
Vascular endothelial growth factor
- MVD
Microvascular density
- PEDF
Pigment epithelium-derived factor
- MMP
Matrix metalloproteinase
- RECK
Reversion-inducing cyteine rich protein with Kazal motifs
- uPA
Urokinase plasminogen activator
- P-gp
P-glycoprotein
- CXCR
Chemokine receptor
References
- Abudu A, Sferopoulos NK, Tillman RM, Carter SR, Grimer RJ (1996) The surgical treatment and outcome of pathological fractures in localised osteosarcoma. J Bone Joint Surg Br 78(5):694–698 [PubMed] [Google Scholar]
- Aksnes LH, Hall KS, Folleraas G, Stenwig AE, Bjerkehagen B, Taksdal I, Winderen M, Bruland OS, Saeter G (2006) Management of high-grade bone sarcomas over two decades: the Norwegian Radium Hospital experience. Acta Oncol 45(1):38–46 [DOI] [PubMed] [Google Scholar]
- Ambrogi V, Paci M, Pompeo E, Mineo TC (2000) Transxiphoid video-assisted pulmonary metastasectomy: relevance of helical computed tomography occult lesions. Ann Thorac Surg 70(6):1847–1852 [DOI] [PubMed] [Google Scholar]
- Antunes M, Bernardo J, Salete M, Prieto D, Eugénio L, Tavares P (1999) Excision of pulmonary metastases of osteogenic sarcoma of the limbs. Eur J Cardiothorac Surg 15(5):592–596 [DOI] [PubMed] [Google Scholar]
- Apffelstaedt JP, Driscoll DL, Karakousis CP (1995) Partial and complete internal hemipelvectomy: complications and long-term follow-up. J Am Coll Surg 181(1):43–48 [PubMed] [Google Scholar]
- Apffelstaedt JP, Driscoll DL, Spellman JE, Velez AF, Gibbs JF, Karakousis CP (1996) Complications and outcome of external hemipelvectomy in the management of pelvic tumors. Ann Surg Oncol 3(3):304–309 [DOI] [PubMed] [Google Scholar]
- Bacci G, Avella M, Picci P, Dallari D, Malaguti C, Biagini R, Ruggieri P, Balladelli A, Ferrari S, Caldora P (1990) Primary chemotherapy and delayed surgery for malignant fibrous histiocytoma of bone in the extremity. Tumori 76(6):537–542 [DOI] [PubMed] [Google Scholar]
- Bacci G, Donati D, Manfrini M, Forni C, Bertoni F, Gherlinzoni F, Biagini R, Campanacci M (1998) Local recurrence after surgical or surgical-chemotherapeutic treatment of osteosarcoma of the limbs. Incidence, risk factors and prognosis. Minerva Chir 53(7–8):619–629 [PubMed] [Google Scholar]
- Bacci G, Ferrari S, Longhi A, Perin S, Forni C, Fabbri N, Salduca N, Versari M, Smith KV (2001) Pattern of relapse in patients with osteosarcoma of the extremities treated with neoadjuvant chemotherapy. Eur J Cancer 37(1):32–38 [DOI] [PubMed] [Google Scholar]
- Bacci G, Longhi A, Ferrari S, Lari S, Manfrini M, Donati D, Forni C, Versari M (2002) Prognostic significance of serum alkaline phosphatase in osteosarcoma of the extremity treated with neoadjuvant chemotherapy: recent experience at Rizzoli Institute. Oncol Rep 9(1):171–175 [PubMed] [Google Scholar]
- Bacci G, Ferrari S, Longhi A, Donati D, Manfrini M, Giacomini S, Briccoli A, Forni C, Galletti S (2003) Nonmetastatic osteosarcoma of the extremity with pathologic fracture at presentation: local and systemic control by amputation or limb salvage after preoperative chemotherapy. Acta Orthop Scand 74(4):449–454 [DOI] [PubMed] [Google Scholar]
- Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P (2006) Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer 106(5):1154–1161 [DOI] [PubMed] [Google Scholar]
- Baldini N, Scotlandi K, Barbanti-Bròdano G, Manara MC, Maurici D, Bacci G, Bertoni F, Picci P, Sottili S, Campanacci M (1995) Expression of P-glycoprotein in high-grade osteosarcomas in relation to clinical outcome. N Engl J Med 333(21):1380–1385 [DOI] [PubMed] [Google Scholar]
- Baldini N, Scotlandi K, Serra M, Picci P, Bacci G, Sottili S, Campanacci M (1999) P-glycoprotein expression in osteosarcoma: a basis for risk-adapted adjuvant chemotherapy. J Orthop Res 17(5):629–632 [DOI] [PubMed] [Google Scholar]
- Beedles KE, Sharpe PT, Wagner EF, Grigoriadis AE (1999) A putative role for c-Fos in the pathophysiology of Paget’s disease. J Bone Miner Res 14(Suppl 2):21–28 [DOI] [PubMed] [Google Scholar]
- Benassi MS, Molendini L, Gamberi G, Ragazzini P, Sollazzo MR, Merli M, Asp J, Magagnoli G, Balladelli A, Bertoni F, Picci P (1999) Alteration of pRb/p16/cdk4 regulation in human osteosarcoma. Int J Cancer 84(5):489–493 [DOI] [PubMed] [Google Scholar]
- Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jürgens H, Winkler K (2002) Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 20(3):776–790 [DOI] [PubMed] [Google Scholar]
- Bieling P, Bielack S, Delling G, Jürgens H, Kotz R, Dose C, Astheimer H, Exner G, Gadner H, Graf N (1991) Neoadjuvant chemotherapy of osteosarcoma. Preliminary results of the cooperative COSS-86 osteosarcoma study. Klin Padiatr 203(4):220–230 [DOI] [PubMed] [Google Scholar]
- Bieling P, Rehan N, Winkler P, Helmke K, Maas R, Fuchs N, Bielack S, Heise U, Jurgens H, Treuner J, Romanowski R, Exner U, Kotz R, Winkler K (1996) Tumor size and prognosis in aggressively treated osteosarcoma. J Clin Oncol 14(3):848–858 [DOI] [PubMed] [Google Scholar]
- Bouck N (2002) PEDF: anti-angiogenic guardian of ocular function. Trends Mol Med 8(7):330–334 [DOI] [PubMed] [Google Scholar]
- Briccoli A, Rocca M, Salone M, Bacci G, Ferrari S, Balladelli A, Mercuri M (2005) Resection of recurrent pulmonary metastases in patients with osteosarcoma. Cancer 104(8):1721–1725 [DOI] [PubMed] [Google Scholar]
- Carsi B, Rock MG (2002) Primary osteosarcoma in adults older than 40 years. Clin Orthop Relat Res 397:53–61 [DOI] [PubMed] [Google Scholar]
- Chan HS, Grogan TM, Haddad G, DeBoer G, Ling V (1997) P-glycoprotein expression: critical determinant in the response to osteosarcoma chemotherapy. J Natl Cancer Inst 89(22):1706–1715 [DOI] [PubMed] [Google Scholar]
- Chandar N, Billig B, McMaster J, Novak J (1992) Inactivation of p53 gene in human and murine osteosarcoma cells. Br J Cancer 65(2):208–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou SK, Jones MK, Tarnawski AS (2003) Survivin—an anti-apoptosis protein: its biological roles and implications for cancer and beyond. Med Sci Monit 9(4):PI25–PI29 [PubMed] [Google Scholar]
- Choong PF, Nadesapillai AP (2003) Urokinase plasminogen activator system: a multifunctional role in tumor progression and metastasis. Clin Orthop Relat Res 415(Suppl):S46–S58 [DOI] [PubMed] [Google Scholar]
- Choong PF, Fernö M, Akerman M, Willén H, Långström E, Gustafson P, Alvegård T, Rydholm A (1996) Urokinase-plasminogen-activator levels and prognosis in 69 soft-tissue sarcomas. Int J Cancer 69(4):268–272 [DOI] [PubMed] [Google Scholar]
- Daecke W, Bielack S, Martini AK, Ewerbeck V, Jürgens H, Kotz R, Winkelmann W, Kabisch H, Kevric M, Bernd L (2005) Osteosarcoma of the hand and forearm: experience of the Cooperative Osteosarcoma Study Group. Ann Surg Oncol 12(4):322–331 [DOI] [PubMed] [Google Scholar]
- Dass CR, Nadesapillai AP, Robin D, Howard ML, Fisher JL, Zhou H, Choong PF (2005) Downregulation of uPAR confirms link in growth and metastasis of osteosarcoma. Clin Exp Metastasis 22(8):643–652 [DOI] [PubMed] [Google Scholar]
- Davis AM, Bell RS, Goodwin PJ (1994) Prognostic factors in osteosarcoma: a critical review. J Clin Oncol 12(2):423–431 [DOI] [PubMed] [Google Scholar]
- Davis AM, Devlin M, Griffin AM, Wunder JS, Bell RS (1999) Functional outcome in amputation versus limb sparing of patients with lower extremity sarcoma: a matched case-control study. Arch Phys Med Rehabil 80(6):615–618 [DOI] [PubMed] [Google Scholar]
- Daw NC, Billups CA, Rodriguez-Galindo C, McCarville MB, Rao BN, Cain AM, Jenkins JJ, Neel MD, Meyer WH (2006) Metastatic osteosarcoma. Cancer 106(2):403–412 [DOI] [PubMed] [Google Scholar]
- Dutour A, Monteil J, Paraf F, Charissoux JL, Kaletta C, Sauer B, Naujoks K, Rigaud M (2005) Endostatin cDNA/cationic liposome complexes as a promising therapy to prevent lung metastases in osteosarcoma: study in a human-like rat orthotopic tumor. Mol Ther 11(2):311–319 [DOI] [PubMed] [Google Scholar]
- Eckardt JJ, Eilber FR, Dorey FJ, Mirra JM (1985) The UCLA experience in limb salvage surgery for malignant tumors. Orthopedics 8(5):612–621 [DOI] [PubMed] [Google Scholar]
- Ek ET, Dass CR, Choong PF (2006a) Pigment epithelium-derived factor: a multimodal tumor inhibitor. Mol Cancer Ther 5(7):1641–1646 [DOI] [PubMed] [Google Scholar]
- Ek ET, Ojaimi J, Kitagawa Y, Choong PF (2006b) Does the degree of intratumoural microvessel density and VEGF expression have prognostic significance in osteosarcoma? Oncol Rep 16(1):17–23 [PubMed] [Google Scholar]
- Ek ET, Dass CR, Contreras KG, Choong PF (2007a) Inhibition of orthotopic osteosarcoma growth and metastasis by multitargeted antitumor activities of pigment epithelium-derived factor. Clin Exp Metastasis 24(2):93–106 [DOI] [PubMed] [Google Scholar]
- Ek ET, Dass CR, Contreras KG, Choong PF (2007b) Pigment epithelium-derived factor overexpression inhibits orthotopic osteosarcoma growth, angiogenesis and metastasis. Cancer Gene Ther 14(7):616–626 [DOI] [PubMed] [Google Scholar]
- Enneking WF, Spanier SS, Goodman MA (1980) A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 153:106–120 [PubMed] [Google Scholar]
- Ferrari S, Bertoni F, Zanella L, Setola E, Bacchini P, Alberghini M, Versari M, Bacci G (2004) Evaluation of P-glycoprotein, HER-2/ErbB-2, p53, and Bcl-2 in primary tumor and metachronous lung metastases in patients with high-grade osteosarcoma. Cancer 100(9):1936–1942 [DOI] [PubMed] [Google Scholar]
- Feugeas O, Guriec N, Babin-Boilletot A, Marcellin L, Simon P, Babin S, Thyss A, Hofman P, Terrier P, Kalifa C, Brunat-Mentigny M, Patricot LM, Oberling F (1996) Loss of heterozygosity of the RB gene is a poor prognostic factor in patients with osteosarcoma. J Clin Oncol 14(2):467–472 [DOI] [PubMed] [Google Scholar]
- Finlay CA, Hinds PW, Tan TH, Eliyahu D, Oren M, Levine AJ (1988) Activating mutations for transformation by p53 produce a gene product that forms an hsc70–p53 complex with an altered half-life. Mol Cell Biol 8(2):531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J (1982) Angiogenesis: initiation and control. Ann N Y Acad Sci 401:212–227 [DOI] [PubMed] [Google Scholar]
- Folkman J (2004) Endogenous angiogenesis inhibitors. APMIS 112(7–8):496–507 [DOI] [PubMed] [Google Scholar]
- Folkman J, Haudenschild C (1980) Angiogenesis in vitro. Nature 288(5791):551–556 [DOI] [PubMed] [Google Scholar]
- Foukas AF, Deshmukh NS, Grimer RJ, Mangham DC, Mangos EG, Taylor S (2002) Stage-IIB osteosarcomas around the knee. A study of MMP-9 in surviving tumour cells. J Bone Joint Surg Br 84(5):706–711 [DOI] [PubMed] [Google Scholar]
- Franchi A, Calzolari A, Zampi G (1998) Immunohistochemical detection of c-fos and c-jun expression in osseous and cartilaginous tumours of the skeleton. Virchows Arch 432(6):515–519 [DOI] [PubMed] [Google Scholar]
- Gamberi G, Benassi MS, Bohling T, Ragazzini P, Molendini L, Sollazzo MR, Pompetti F, Merli M, Magagnoli G, Balladelli A, Picci P (1998) C-myc and c-fos in human osteosarcoma: prognostic value of mRNA and protein expression. Oncology 55(6):556–563 [DOI] [PubMed] [Google Scholar]
- Ganesh S, Sier CF, Heerding MM, Griffioen G, Lamers CB, Verspaget HW (1994) Urokinase receptor and colorectal cancer survival. Lancet 344(8919):401–402 [DOI] [PubMed] [Google Scholar]
- Greene FL, Page DL, Fleming ID et al (eds) (2002) AJCC cancer staging manual, 6th edn. Springer, New York
- Grimer RJ, Taminiau AM, Cannon SR (2002) Surgical outcomes in osteosarcoma. J Bone Joint Surg Br 84(3):395–400 [DOI] [PubMed] [Google Scholar]
- Grimer RJ, Sommerville S, Warnock D, Carter S, Tillman R, Abudu A, Spooner D (2005) Management and outcome after local recurrence of osteosarcoma. Eur J Cancer 41(4):578–583 [DOI] [PubMed] [Google Scholar]
- Hara H, Akisue T, Fujimoto T, Imabori M, Kawamoto T, Kuroda R, Fujioka H, Yamamoto T, Doita M, Kurosaka M (2006) Expression of VEGF and its receptors and angiogenesis in bone and soft tissue tumors. Anticancer Res 26(6B):4307–4311 [PubMed] [Google Scholar]
- Hauben EI, Weeden S, Pringle J, Van Marck EA, Hogendoorn PC (2002) Does the histological subtype of high-grade central osteosarcoma influence the response to treatment with chemotherapy and does it affect overall survival? A study on 570 patients of two consecutive trials of the European Osteosarcoma Intergroup. Eur J Cancer 38(9):1218–1225 [DOI] [PubMed] [Google Scholar]
- Heinsohn S, Evermann U, Zur Stadt U, Bielack S, Kabisch H (2007) Determination of the prognostic value of loss of heterozygosity at the retinoblastoma gene in osteosarcoma. Int J Oncol 30(5):1205–1214 [PubMed] [Google Scholar]
- Henderson MA, Danks JA, Slavin JL, Byrnes GB, Choong PF, Spillane JB, Hopper JL, Martin TJ (2006) Parathyroid hormone-related protein localization in breast cancers predict improved prognosis. Cancer Res 66(4):2250–2256 [DOI] [PubMed] [Google Scholar]
- Himelstein BP, Asada N, Carlton MR, Collins MH (1998) Matrix metalloproteinase-9 (MMP-9) expression in childhood osseous osteosarcoma. Med Pediatr Oncol 31(6):471–474 [DOI] [PubMed] [Google Scholar]
- Hofmann R, Lehmer A, Buresch M, Hartung R, Ulm K (1996) Clinical relevance of urokinase plasminogen activator, its receptor, and its inhibitor in patients with renal cell carcinoma. Cancer 78(3):487–492 [DOI] [PubMed] [Google Scholar]
- Holekamp NM, Bouck N, Volpert O (2002) Pigment epithelium-derived factor is deficient in the vitreous of patients with choroidal neovascularization due to age-related macular degeneration. Am J Ophthalmol 134(2):220–227 [DOI] [PubMed] [Google Scholar]
- Hunter KW (2004) Ezrin, a key component in tumor metastasis. Trends Mol Med 10(5):201–204 [DOI] [PubMed] [Google Scholar]
- Iida K, Nobori T, Matsumine A, Isaka A, Seto M, Shiraishi T, Uchida A (2003) Effect of retinoblastoma tumor suppressor gene expression on chemosensitivity of human osteosarcoma cell lines. Oncol Rep 10(6):1961–1965 [PubMed] [Google Scholar]
- Jeon DG, Lee SY, Cho WH, Song WS, Park JH (2006) Primary osteosarcoma in patients older than 40 years of age. J Korean Med Sci 21(4):715–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandioler D, Krömer E, Tüchler H, End A, Müller MR, Wolner E, Eckersberger F (1998) Long-term results after repeated surgical removal of pulmonary metastases. Ann Thorac Surg 65(4):909–912 [DOI] [PubMed] [Google Scholar]
- Kang HG, Kim HS, Kim KJ, Oh JH, Lee MR, Seol SM, Han I (2007) RECK expression in osteosarcoma: correlation with matrix metalloproteinases activation and tumor invasiveness. J Orthop Res 25:696–702 [DOI] [PubMed] [Google Scholar]
- Kawai A, Healey JH, Boland PJ, Lin PP, Huvos AG, Meyers PA (1998a) Prognostic factors for patients with sarcomas of the pelvic bones. Cancer 82(5):851–859 [PubMed] [Google Scholar]
- Kawai A, Huvos AG, Meyers PA, Healey JH (1998b) Osteosarcoma of the pelvis. Oncologic results of 40 patients. Clin Orthop Relat Res 348:196–207 [PubMed] [Google Scholar]
- Kaya M, Wada T, Akatsuka T, Kawaguchi S, Nagoya S, Shindoh M, Higashino F, Mezawa F, Okada F, Ishii S (2000) Vascular endothelial growth factor expression in untreated osteosarcoma is predictive of pulmonary metastasis and poor prognosis. Clin Cancer Res 6(2):572–577 [PubMed] [Google Scholar]
- Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, Yeung C, Gorlick R, Hewitt SM, Helman LJ (2004) The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med 10(2):182–186 [DOI] [PubMed] [Google Scholar]
- Kido A, Tsutsumi M, Iki K, Takahama M, Tsujiuchi T, Morishita T, Tamai S, Konishi Y (1999) Overexpression of matrix metalloproteinase (MMP)-9 correlates with metastatic potency of spontaneous and 4-hydroxyaminoquinoline 1-oxide (4-HAQO)-induced transplantable osteosarcomas in rats. Cancer Lett 137(2):209–216 [DOI] [PubMed] [Google Scholar]
- Kreuter M, Bieker R, Bielack SS, Auras T, Buerger H, Gosheger G, Jurgens H, Berdel WE, Mesters RM (2004) Prognostic relevance of increased angiogenesis in osteosarcoma. Clin Cancer Res 10(24):8531–8537 [DOI] [PubMed] [Google Scholar]
- Ku JH, Kwak C, Lee HS, Park HK, Lee E, Lee SE (2004) Expression of survivin, a novel inhibitor of apoptosis, in superficial transitional cell carcinoma of the bladder. J Urol 171(2 Pt 1):631–635 [DOI] [PubMed] [Google Scholar]
- Kuhn W, Pache L, Schmalfeldt B, Dettmar P, Schmitt M, Jänicke F, Graeff H (1994) Urokinase (uPA) and PAI-1 predict survival in advanced ovarian cancer patients (FIGO III) after radical surgery and platinum-based chemotherapy. Gynecol Oncol 55(3 Pt 1):401–409 [DOI] [PubMed] [Google Scholar]
- Laverdiere C, Hoang BH, Yang R, Sowers R, Qin J, Meyers PA, Huvos AG, Healey JH, Gorlick R (2005) Messenger RNA expression levels of CXCR4 correlate with metastatic behavior and outcome in patients with osteosarcoma. Clin Cancer Res 11(7):2561–2567 [DOI] [PubMed] [Google Scholar]
- Li YH, Wang C, Meng K, Chen LB, Zhou XJ (2004) Influence of survivin and caspase-3 on cell apoptosis and prognosis in gastric carcinoma. World J Gastroenterol 10(13):1984–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libura J, Drukala J, Majka M, Tomescu O, Navenot JM, Kucia M, Marquez L, Peiper SC, Barr FG, Janowska-Wieczorek A, Ratajczak MZ (2002) CXCR4-SDF-1 signaling is active in rhabdomyosarcoma cells and regulates locomotion, chemotaxis, and adhesion. Blood 100(7):2597–2606 [DOI] [PubMed] [Google Scholar]
- Lin A, Rugo HS (2007) The role of trastuzumab in early stage breast cancer: current data and treatment recommendations. Curr Treat Options Oncol 8:47–60 [DOI] [PubMed] [Google Scholar]
- Lindner NJ, Ramm O, Hillmann A, Roedl R, Gosheger G, Brinkschmidt C, Juergens H, Winkelmann W (1999) Limb salvage and outcome of osteosarcoma. The University of Muenster experience. Clin Orthop Relat Res 358:83–89 [DOI] [PubMed] [Google Scholar]
- Lonardo F, Ueda T, Huvos AG, Healey J, Ladanyi M (1997) p53 and MDM2 alterations in osteosarcomas: correlation with clinicopathologic features and proliferative rate. Cancer 79(8):1541–1547 [PubMed] [Google Scholar]
- Longhi A, Benassi MS, Molendini L, Macchiagodena M, Picci P, Bacci G (2001) Osteosarcoma in blood relatives. Oncol Rep 8(1):131–136 [DOI] [PubMed] [Google Scholar]
- Malawer MM, Sugarbaker PH (2001) Musculoskeletal cancer surgery—treatment of sarcomas and allied diseases, 2nd edn. Kluwer, The Netherlands, p 609 [Google Scholar]
- Mantadakis E, Kim G, Reisch J, McHard K, Maale G, Leavey PJ, Timmons C (2001) Lack of prognostic significance of intratumoral angiogenesis in nonmetastatic osteosarcoma. J Pediatr Hematol Oncol 23(5):286–289 [DOI] [PubMed] [Google Scholar]
- McCabe NP, Angwafo FF III, Zaher A, Selman SH, Kouinche A, Jankun J (2000) Expression of soluble urokinase plasminogen activator receptor may be related to outcome in prostate cancer patients. Oncol Rep 7(4):879–882 [DOI] [PubMed] [Google Scholar]
- McIntyre JF, Smith-Sorensen B, Friend SH, Kassell J, Borresen AL, Yan YX, Russo C, Sato J, Barbier N, Miser J (1994) Germline mutations of the p53 tumor suppressor gene in children with osteosarcoma. J Clin Oncol 12(5):925–930 [DOI] [PubMed] [Google Scholar]
- Mechtersheimer G, Staudter M, Majdic O, Dörken B, Moldenhauer G, Möller P (1990) Expression of HLA-A,B,C, beta 2-microglobulin (beta 2 m), HLA-DR, -DP, -DQ and of HLA-D-associated invariant chain (Ii) in soft-tissue tumors. Int J Cancer 46(5):813–823 [DOI] [PubMed] [Google Scholar]
- Miller CW, Aslo A, Won A, Tan M, Lampkin B, Koeffler HP (1996) Alterations of the p53, Rb and MDM2 genes in osteosarcoma. J Cancer Res Clin Oncol 122(9):559–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed RA, Green A, El-Shikh S, Paish EC, Ellis IO, Martin SG (2007) Prognostic significance of vascular endothelial cell growth factors -A, -C and -D in breast cancer and their relationship with angio- and lymphangiogenesis. Br J Cancer 96(7):1092–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Maki W, Cardones AR, Fang H, Tun Kyi A, Nestle FO, Hwang ST (2002) Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res 62(24):7328–7334 [PubMed] [Google Scholar]
- Nathan SS, Gorlick R, Bukata S, Chou A, Morris CD, Boland PJ, Huvos AG, Meyers PA, Healey JH (2006) Treatment algorithm for locally recurrent osteosarcoma based on local disease-free interval and the presence of lung metastasis. Cancer 107(7):1607–1616 [DOI] [PubMed] [Google Scholar]
- Nemunaitis J, Poole C, Primrose J, Rosemurgy A, Malfetano J, Brown P, Berrington A, Cornish A, Lynch K, Rasmussen H, Kerr D, Cox D, Millar A (1998) Combined analysis of studies of the effects of the matrix metalloproteinase inhibitor marimastat on serum tumor markers in advanced cancer: selection of a biologically active and tolerable dose for longer-term studies. Clin Cancer Res 4(5):1101–1109 [PubMed] [Google Scholar]
- Oh J, Takahashi R, Kondo S, Mizoguchi A, Adachi E, Sasahara RM, Nishimura S, Imamura Y, Kitayama H, Alexander DB, Ide C, Horan TP, Arakawa T, Yoshida H, Nishikawa S, Itoh Y, Seiki M, Itohara S, Takahashi C, Noda M (2001) The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell 107(6):789–800 [DOI] [PubMed] [Google Scholar]
- Onda M, Matsuda S, Higaki S, Iijima T, Fukushima J, Yokokura A, Kojima T, Horiuchi H, Kurokawa T, Yamamoto T (1996) ErbB-2 expression is correlated with poor prognosis for patients with osteosarcoma. Cancer 77(1):71–78 [DOI] [PubMed] [Google Scholar]
- Osaka E, Suzuki T, Osaka S, Yoshida Y, Sugita H, Asami S, Tabata K, Hemmi A, Sugitani M, Nemoto N, Ryu J (2006) Survivin as a prognostic factor for osteosarcoma patients. Acta Histochem Cytochem 39(3):95–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki T, Flege S, Liljenqvist U, Hillmann A, Delling G, Salzer-Kuntschik M, Jürgens H, Kotz R, Winkelmann W, Bielack SS (2002) Osteosarcoma of the spine: experience of the Cooperative Osteosarcoma Study Group. Cancer 94(4):1069–1077 [PubMed] [Google Scholar]
- Ozaki T, Flege S, Kevric M, Lindner N, Maas R, Delling G, Schwarz R, von Hochstetter AR, Salzer-Kuntschik M, Berdel WE, Jürgens H, Exner GU, Reichardt P, Mayer-Steinacker R, Ewerbeck V, Kotz R, Winkelmann W, Bielack SS (2003) Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol 21(2):334–341 [DOI] [PubMed] [Google Scholar]
- Pakos EE, Ioannidis JP (2003) The association of P-glycoprotein with response to chemotherapy and clinical outcome in patients with osteosarcoma. A meta-analysis. Cancer 98(3):581–589 [DOI] [PubMed] [Google Scholar]
- Paoletti S, Borzi RM, Mazzetti I, Magagnoli G, Macor S, Cattini L, Facchini A (2001) Human osteosarcoma cells release matrix degrading enzymes in response to chemokine activation. Int J Oncol 18(1):11–16 [DOI] [PubMed] [Google Scholar]
- Papachristou DJ, Papavassiliou AG (2007) Osteosarcoma and chondrosarcoma: new signaling pathways as targets for novel therapeutic interventions. Int J Biochem Cell Biol 39(5):857–862 [DOI] [PubMed] [Google Scholar]
- Pardo FS, Lien WW, Fox HS, Efird JT, Aguilera JA, Burton DW, Deftos LJ (2004) Parathyroid hormone-related protein expression is correlated with clinical course in patients with glial tumors. Cancer 101(11):2622–2628 [DOI] [PubMed] [Google Scholar]
- Park YB, Kim HS, Oh JH, Lee SH (2001) The co-expression of p53 protein and P-glycoprotein is correlated to a poor prognosis in osteosarcoma. Int Orthop 24(6):307–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HR, Jung WW, Bacchini P, Bertoni F, Kim YW, Park YK (2006) Ezrin in osteosarcoma: comparison between conventional high-grade and central low-grade osteosarcoma. Pathol Res Pract 202(7):509–515 [DOI] [PubMed] [Google Scholar]
- Parsons AM, Detterbeck FC, Parker LA (2004) Accuracy of helical CT in the detection of pulmonary metastases: is intraoperative palpation still necessary? Ann Thorac Surg 78(6):1910–1916. (discussion 1916–1918) [DOI] [PubMed] [Google Scholar]
- Pasquini GM, Davey RA, Ho PW, Michelangeli VP, Grill V, Kaczmarczyk SJ, Zajac JD (2002) Local secretion of parathyroid hormone-related protein by an osteoblastic osteosarcoma (UMR 106–01) cell line results in growth inhibition. Bone 31(5):598–605 [DOI] [PubMed] [Google Scholar]
- Perissinotto E, Cavalloni G, Leone F, Fonsato V, Mitola S, Grignani G, Surrenti N, Sangiolo D, Bussolino F, Piacibello W, Aglietta M (2005) Involvement of chemokine receptor 4/stromal cell-derived factor 1 system during osteosarcoma tumor progression. Clin Cancer Res 11(2 Pt 1):490–497 [PubMed] [Google Scholar]
- Picci P, Bacci G, Campanacci M, Gasparini M, Pilotti S, Cerasoli S, Bertoni F, Guerra A, Capanna R, Albisinni U (1985) Histologic evaluation of necrosis in osteosarcoma induced by chemotherapy. Regional mapping of viable and nonviable tumor. Cancer 56(7):1515–1521 [DOI] [PubMed] [Google Scholar]
- Picci P, Sangiorgi L, Rougraff BT, Neff JR, Casadei R, Campanacci M (1994) Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol 12(12):2699–2705 [DOI] [PubMed] [Google Scholar]
- Pillay V, Dass CR, Choong PF (2007) The urokinase plasminogen activator receptor as a gene therapy target for cancer. Trends Biotechnol 25(1):33–39 [DOI] [PubMed] [Google Scholar]
- Pochanugool L, Subhadharaphandou T, Dhanachai M, Hathirat P, Sangthawan D, Pirabul R, Onsanit S, Pornpipatpong N (1997) Prognostic factors among 130 patients with osteosarcoma. Clin Orthop Relat Res 345:206–214 [PubMed] [Google Scholar]
- Quan GM, Ojaimi J, Nadesapillai AP, Zhou H, Choong PF (2002) Resistance of epiphyseal cartilage to invasion by osteosarcoma is likely to be due to expression of antiangiogenic factors. Pathobiology 70(6):361–367 [DOI] [PubMed] [Google Scholar]
- Quan GM, Slavin JL, Schlicht SM, Smith PJ, Powell GJ, Choong PF (2005) Osteosarcoma near joints: assessment and implications. J Surg Oncol 91(3):159–166 [DOI] [PubMed] [Google Scholar]
- Rougraff BT, Simon MA, Kneisl JS, Greenberg DB, Mankin HJ (1994) Limb salvage compared with amputation for osteosarcoma of the distal end of the femur. A long-term oncological, functional, and quality-of-life study. J Bone Joint Surg Am 76(5):649–656 [DOI] [PubMed] [Google Scholar]
- Saeki H, Yanoma S, Takemiya S, Sugimasa Y, Akaike M, Yukawa N, Rino Y, Imada T (2007) Antitumor activity of a combination of trastuzumab (Herceptin) and oral fluoropyrimidine S-1 on human epidermal growth factor receptor 2-overexpressing pancreatic cancer. Oncol Rep 18(2):433–439 [PubMed] [Google Scholar]
- Sajadi KR, Heck RK, Neel MD, Rao BN, Daw N, Rodriguez-Galindo C, Hoffer FA, Stacy GS, Peabody TD, Simon MA (2004) The incidence and prognosis of osteosarcoma skip metastases. Clin Orthop Relat Res 426:92–96 [DOI] [PubMed] [Google Scholar]
- Scully SP, Ghert MA, Zurakowski D, Thompson RC, Gebhardt MC (2002) Pathologic fracture in osteosarcoma: prognostic importance and treatment implications. J Bone Joint Surg Am 84-A(1):49–57 [PubMed] [Google Scholar]
- Shin CY, Lee WJ, Choi JW, Choi MS, Ryu JR, Oh SJ, Cheong JH, Choi EY, Ko KH (2007) Down-regulation of matrix metalloproteinase-9 expression by nitric oxide in lipopolysaccharide-stimulated rat primary astrocytes. Nitric Oxide 16(4):425–432 [DOI] [PubMed] [Google Scholar]
- Shives TC, Dahlin DC, Sim FH, Pritchard DJ, Earle JD (1986) Osteosarcoma of the spine. J Bone Joint Surg Am 68(5):660–668 [PubMed] [Google Scholar]
- Simon MA, Aschliman MA, Thomas N, Mankin HJ (1986) Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am 68(9):1331–1337 [PubMed] [Google Scholar]
- Sluga M, Windhager R, Lang S, Heinzl H, Bielack S, Kotz R (1999) Local and systemic control after ablative and limb sparing surgery in patients with osteosarcoma. Clin Orthop Relat Res 358:120–127 [PubMed] [Google Scholar]
- Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, Luker GD (2004) CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res 64(23):8604–8612 [DOI] [PubMed] [Google Scholar]
- Somers GR, Ho M, Zielenska M, Squire JA, Thorner PS (2005) HER2 amplification and overexpression is not present in pediatric osteosarcoma: a tissue microarray study. Pediatr Dev Pathol 8(5):525–532 [DOI] [PubMed] [Google Scholar]
- Span PN, Sweep CG, Manders P, Beex LV, Leppert D, Lindberg RL (2003) Matrix metalloproteinase inhibitor reversion-inducing cysteine-rich protein with Kazal motifs: a prognostic marker for good clinical outcome in human breast carcinoma. Cancer 97(11):2710–2715 [DOI] [PubMed] [Google Scholar]
- Stempak D, Gammon J, Halton J, Moghrabi A, Koren G, Baruchel S (2006) A pilot pharmacokinetic and antiangiogenic biomarker study of celecoxib and low-dose metronomic vinblastine or cyclophosphamide in pediatric recurrent solid tumors. J Pediatr Hematol Oncol 28(11):720–728 [DOI] [PubMed] [Google Scholar]
- Szendroi M, Pápai Z, Koós R, Illés T (2000) Limb-saving surgery, survival, and prognostic factors for osteosarcoma: the Hungarian experience. J Surg Oncol 73(2):87–94 [DOI] [PubMed] [Google Scholar]
- Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK (2002) Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res 62(6):1832–1837 [PubMed] [Google Scholar]
- Takahashi C, Sheng Z, Horan TP, Kitayama H, Maki M, Hitomi K, Kitaura Y, Takai S, Sasahara RM, Horimoto A, Ikawa Y, Ratzkin BJ, Arakawa T, Noda M (1998) Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored glycoprotein RECK. Proc Natl Acad Sci USA 95(22):13221–13226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka K, Ishikawa S, Kawano Y, Yanagihara K, Miyahara R, Otake Y, Morioka Y, Takahashi C, Noda M, Wada H, Tanaka F (2004) Expression of a novel matrix metalloproteinase regulator, RECK, and its clinical significance in resected non-small cell lung cancer. Eur J Cancer 40(10):1617–1623 [DOI] [PubMed] [Google Scholar]
- Takenaka K, Yamagishi S, Jinnouchi Y, Nakamura K, Matsui T, Imaizumi T (2005) Pigment epithelium-derived factor (PEDF)-induced apoptosis and inhibition of vascular endothelial growth factor (VEGF) expression in MG63 human osteosarcoma cells. Life Sci 77(25):3231–3241 [DOI] [PubMed] [Google Scholar]
- Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC (1998) IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res 58(23):5315–5320 [PubMed] [Google Scholar]
- Teodoro JG, Evans SK, Green MR (2007) Inhibition of tumor angiogenesis by p53: a new role for the guardian of the genome. J Mol Med. doi:17589818 [DOI] [PubMed]
- Thorpe WP, Reilly JJ, Rosenberg SA (1979) Prognostic significance of alkaline phosphatase measurements in patients with osteogenic sarcoma receiving chemotherapy. Cancer 43(6):2178–2181 [DOI] [PubMed] [Google Scholar]
- Tjin Tham Sjin RM, Naspinski J, Birsner AE, Li C, Chan R, Lo KM, Gillies S, Zurakowski D, Folkman J, Samulski J, Javaherian K (2006) Endostatin therapy reveals a U-shaped curve for antitumor activity. Cancer Gene Ther 13(6):619–627 [DOI] [PubMed] [Google Scholar]
- Tomer G, Cohen IJ, Kidron D, Katz K, Yosipovitch Z, Meller I, Zaizov R (1999) Prognostic factors in non-metastatic limb osteosarcoma: a 20-year experience of one center. Int J Oncol 15(1):179–185 [DOI] [PubMed] [Google Scholar]
- Trieb K, Lehner R, Stulnig T, Sulzbacher I, Shroyer KR (2003) Survivin expression in human osteosarcoma is a marker for survival. Eur J Surg Oncol 29(4):379–382 [DOI] [PubMed] [Google Scholar]
- Truong NU, deB Edwardes MD, Papavasiliou V, Goltzman D, Kremer R (2003) Parathyroid hormone-related peptide and survival of patients with cancer and hypercalcemia. Am J Med 115(2):115–121 [DOI] [PubMed] [Google Scholar]
- Tsukahara T, Kawaguchi S, Torigoe T, Asanuma H, Nakazawa E, Shimozawa K, Nabeta Y, Kimura S, Kaya M, Nagoya S, Wada T, Yamashita T, Sato N (2006) Prognostic significance of HLA class I expression in osteosarcoma defined by anti-pan HLA class I monoclonal antibody, EMR8–5. Cancer Sci 97(12):1374–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Jagt MF, Sweep FC, Waas ET, Hendriks T, Ruers TJ, Merry AH, Wobbes T, Span PN (2006) Correlation of reversion-inducing cysteine-rich protein with kazal motifs (RECK) and extracellular matrix metalloproteinase inducer (EMMPRIN), with MMP-2, MMP-9, and survival in colorectal cancer. Cancer Lett 237(2):289–297 [DOI] [PubMed] [Google Scholar]
- Varmus H (2006) The new era in cancer research. Science 312(5777):1162–1165 [DOI] [PubMed] [Google Scholar]
- Vinodhkumar R, Song YS, Ravikumar V, Ramakrishnan G, Devaki T (2007) Depsipeptide a histone deacetlyase inhibitor down regulates levels of matrix metalloproteinases 2 and 9 mRNA and protein expressions in lung cancer cells (A549). Chem Biol Interact 165(3):220–229 [DOI] [PubMed] [Google Scholar]
- Wadayama B, Toguchida J, Shimizu T, Ishizaki K, Sasaki MS, Kotoura Y, Yamamuro T (1994) Mutation spectrum of the retinoblastoma gene in osteosarcomas. Cancer Res 54(11):3042–3048 [PubMed] [Google Scholar]
- Wan X, Mendoza A, Khanna C, Helman LJ (2005) Rapamycin inhibits ezrin-mediated metastatic behavior in a murine model of osteosarcoma. Cancer Res 65(6):2406–2411 [DOI] [PubMed] [Google Scholar]
- Wang W, Luo H, Wang A (2006) Expression of survivin and correlation with PCNA in osteosarcoma. J Surg Oncol 93(7):578–584 [DOI] [PubMed] [Google Scholar]
- Winkler K, Beron G, Kotz R, Salzer-Kuntschik M, Beck J, Beck W, Brandeis W, Ebell W, Erttmann R, Göbel U (1984) Neoadjuvant chemotherapy for osteogenic sarcoma: results of a Cooperative German/Austrian study. J Clin Oncol 2(6):617–624 [DOI] [PubMed] [Google Scholar]
- Wu JX, Carpenter PM, Gresens C, Keh R, Niman H, Morris JW, Mercola D (1990) The proto-oncogene c-fos is over-expressed in the majority of human osteosarcomas. Oncogene 5(7):989–1000 [PubMed] [Google Scholar]
- Yang R, Hoang BH, Kubo T, Kawano H, Chou A, Sowers R, Huvos AG, Meyers PA, Healey JH, Gorlick R (2007) Over-expression of parathyroid hormone Type 1 receptor confers an aggressive phenotype in osteosarcoma. Int J Cancer 121(5):943–954 [DOI] [PubMed] [Google Scholar]
- Yonemoto T, Tatezaki S, Ishii T, Satoh T, Kimura H, Iwai N (1998) Prognosis of osteosarcoma with pulmonary metastases at initial presentation is not dismal. Clin Orthop Relat Res 349:194–199 [DOI] [PubMed] [Google Scholar]
- Zhou H, Randall RL, Brothman AR, Maxwell T, Coffin CM, Goldsby RE (2003) Her-2/neu expression in osteosarcoma increases risk of lung metastasis and can be associated with gene amplification. J Pediatr Hematol Oncol 25(1):27–32 [DOI] [PubMed] [Google Scholar]