Abstract
Purpose
This trial is the first to compare directly the clinical response to and safety of oral and intravenous (IV) ibandronic acid for metastatic bone disease.
Methods
Patients ≥18 years with breast, prostate, lung, urogenital or colon cancer received IV ibandronic acid 6 mg infused over 15 min every 28 days or oral ibandronic acid 50 mg/day. Clinical response was determined using bone scintigraphy, radiography and serum C-terminal telopeptide of type I collagen (S-CTX) at months 3–6. Adverse events and biochemical safety measures were recorded.
Results
A total of 84.6 and 88.5% of patients had a complete/partial response to IV and oral ibandronic acid, respectively. Median percentage decreases in S-CTX were −39 and −35%, respectively. Bone pain scores decreased and analgesic use increased from month 0–3 and were stable from months 3–6. Both formulations improved physical and functioning scores.
Conclusion
Oral and IV ibandronic acid for bone metastases have similar efficacy and tolerability.
Keywords: Bone markers, Bone metastases, Efficacy, Ibandronic acid, Intravenous, Oral, Safety
Introduction
In many advanced types of cancer, primary tumours have a tendency to metastasize to the skeleton, causing osteolysis as well as abnormal new bone formation (Roodman 2004). The mechanisms, which govern tumour growth in bone, are complex: tumours release various factors that affect the function of osteoblasts and osteoclasts; in turn, the bone matrix produces growth factors that allow the tumour to thrive.
Metastatic bone disease (MBD) is a significant feature of many advanced cancers, occurring in 75% of breast and prostate cancer patients and in 15–40% of patients with lung, colon, or kidney cancers (Coleman 2001). MBD is associated with skeletal-related events (SREs), such as bone pain, spinal cord compression (associated with paralysis) (Coleman 2006) and hypercalcaemia of malignancy (Diel et al. 2000). SREs and bone pain limit patient mobility and have a considerable negative impact on a patient’s quality of life (QoL). For breast cancer patients with MBD the median survival time is 2.5 years after MBD diagnosis (Diel et al. 2000; Domchek et al. 2000); therefore, it is important to find an effective long-term therapy for the management of SREs and associated bone pain. While surgery, radiotherapy, and opioids can alleviate bone pain, >20% of patients are resistant to these treatments (Yau et al. 2004; McPartland and Grosjean 2004).
Bisphosphonates are the standard of care for treating MBD (Coleman 2001; Diel et al. 2000; Mystakidou et al. 2005), and several are used in the clinical setting (zoledronic acid, ibandronic acid, pamidronate and clodronate). Ibandronic acid is the only bisphosphonate available in both oral and intravenous (IV) formulations. Results from individual phase 3 trials suggest similar efficacy of oral and IV ibandronic acid for the prevention of SREs in women with breast cancer and MBD (Diel et al. 2000; Body et al. 2003, 2004). However, the efficacy and safety of oral and IV formulations of ibandronic acid have not been evaluated previously in a head-to-head study. The primary objective of this study was to evaluate the efficacy (clinical response) and safety of oral and IV ibandronic acid in patients with various tumour types. The secondary study objective was to determine the influence of ibandronic acid on analgesic use, metastatic bone pain, and QoL.
Materials and methods
Study design
The study was a 6-month, prospective, randomized, open-label, two-arm, single centre, efficacy and safety trial conducted at the Pain Relief & Palliative Care Unit, Areteion Hospital, Department of Radiology of the University of Athens School of Medicine. Patients received oral ibandronic acid (50 mg tablet/day, before breakfast) or 6 mg IV ibandronic acid infused over 15 min every 28 days. Two treatment groups of 26 patients were matched for age, gender, weight, height, blood pressure, site of primary tumour, site and number of bone metastases, and number of irradiated sites. This study was designed and conducted in accordance with the Helsinki Declaration and the protocol was approved by the Institutional Review Board at each study site. All patients gave appropriate written informed consent before any study procedures were initiated.
Patients were ≥18 years of age and had solid malignancies with at least one bone lesion, normal renal function (creatinine ≥1.5 mg/dL) and hepatic function, World Health Organization performance status 0, Ι, ΙΙ, life expectancy ≥6 months, and normocalcaemia or asymptomatic hypercalcaemia. Patients were excluded if they were pregnant or lactating, currently being treated with nephrotoxic drugs (e.g. diuretics, cisplatin) or osteoclast activity modulators (e.g. mithramycin, calcitonin etc.), had renal or hepatic insufficiency, or had an active infection.
Clinical response assessment
Clinical response to IV and oral ibandronic acid treatment was determined by changes in bone scintigraphy and radiography after 3 and 6 months of treatment (Yahara et al. 2003). A response was defined as complete if a resolution and complete recalcification of all osteolytic metastases were observed. A partial response was defined as the resolution of some, but not all, existing osteoblastic metastases, or a ≥50% decrease in the size of measurable osteoblastic metastases and a ≥30% decrease in the size of evaluable osteoblastic metastases, or at least partial recalcification of ≥1 osteolytic metastases and no new bone metastases. Stable disease was defined as no change in metastases in size or number, and worsening disease was defined as new bone metastases or growth in existing bone metastases. Serum C-terminal telopeptide of type I collagen (S-CTX), a biochemical marker of bone turnover, was assessed at 3 and 6 months.
Pain/QoL assessments
At 3 and 6 months the percentage of patients that had an increased, decreased, or fixed dose of analgesic, the median percentage change in pain, and the median percentage change in QoL were measured. The Greek Brief Pain Inventory (G-BPI) was used to measure metastatic bone pain. The selected G-BPI items measured severity of pain by worst pain (item 3) and average pain (item 5), as well as interference due to pain in the following activities: general activity (item 9i), walking (item 9iii), working (item 9iv), and enjoyment of life (item 9vii). Scoring was based on a 10-point scale (0 = no pain/does not interfere with activity; 10 = unbearable pain/completely interferes with activity). QoL was determined using the functional assessment of cancer therapy-general (FACT-G; total physical and total function well-being scales). The physical and functional QoL subscales both consisted of seven questions; each question was measured on a 4-point scale (0 = no QoL; 4 = very high QoL). The sum of these seven questions in each subscale determined the total physical and functional QoL score. Question 8 of each subscale (e.g. physical 8 and function 8) measured how much the physical and functional well-being of the patient affects his/her QoL on a 10-point scale (0 = not at all; 10 = very much).
Safety
Safety was evaluated through the recording of adverse events during the treatment period. A complete blood count monitored levels of haemoglobin, platelets and white blood cells. Levels of urea, serum creatinine, serum γ-glutamyl transferase, alkaline phosphatase, haematocrit (Ht), bilirubin, serum microalbumin and serum calcium (Ca) were measured at 3 and 6 months.
Statistical method
The patients’ characteristics at baseline were compared using Chi-square and Fisher’s exact tests. Comparisons of absolute values of variables between groups at baseline were performed using the independent samples t test or the Welch test (in the case of unequal variance). Comparisons of variables during the observation period (baseline vs. each time point) were performed using paired samples t test.
Pain, QoL, and markers of bone turnover were assessed as median percentage changes from baseline at 3 and 6 months. Analgesic use was assessed in terms of the percentage of patients requiring a change in dose. Clinical response to ibandronic acid after 3 and 6 months was evaluated with the bone metastasis-free interval analysis using the Kaplan–Meier method. Comparison of percentage change from baseline of all parameters during the observation period between the two groups was analyzed using the Mann–Whitney test. All tests were two-sided with a significance level of 0.05. Statistical data processing was carried out with SPSS software version 10.00 (SPSS Inc., Chicago, Illinois).
Results
Patient demographics
A total of 52 patients were recruited into the study, and all were randomized to treatment. Demographic characteristics were similar in both groups (Table 1). Of the 52 patients, 17 (32%) had concomitant diseases. Primary tumour sites were breast, lung, urogenital, colon and prostate cancer (Table 2). Skeletal metastases were found in spine (n = 24), flat bones (n = 17), upper (n = 5) and lower (n = 7) extremities, skull (n = 3) and sacro-iliac joint (n = 5). A total of 22 patients had more than 3 foci of bone metastasis. Forty-two percent of patients had undergone surgery and 85% had previously received radiation therapy (Table 3).
Table 1.
Patient demographics and baseline characteristics
| Ibandronic acid treatment arm | ||
|---|---|---|
| Intravenous (n = 26) | Oral (n = 26) | |
| Age in years, mean (SD) | 66.9 (10.7) | 65.8 (10.7) |
| Gender, n (male:female) | 12:14 | 12:14 |
| Weight in kg, mean (SD) | 71.6 (9.5) | 70.6 (8.0) |
| Height in m, mean (SD) | 1.66 (0.08) | 1.65 (0.08) |
| Pain indices, mean (SD) | ||
| Worst pain | 6.35 (1.4) | 6.38 (1.4) |
| Average pain | 5.15 (1.7) | 5.15 (1.4) |
| Interference of pain in general activity | 6.08 (1.8) | 6.27 (1.3) |
| Interference of pain in walking | 4.77 (2.6) | 6.00 (2.6) |
| Interference of pain in work | 5.19 (2.2) | 6.08 (1.7) |
| Interference of pain in enjoyment of life | 5.84 (2.2) | 5.96 (1.9) |
| Quality-of-life indices, mean (SD) | ||
| Total physical | 16.42 (3.9) | 15.04 (3.4) |
| Total function | 10.69 (3.9) | 9.81 (2.6) |
| Physical 8 | 5.64 (1.6) | 6.08 (1.3) |
| Function 8 | 5.65 (1.7) | 6.19 (1.4) |
| Analgesic use, n (%) | ||
| Paracetamol + codeine tabletsa | 4 (15) | 2 (8) |
| Transdermal therapeutic system—fentanylb | 19 (73) | 23 (88) |
| Slow-release morphine capsulesc | 3 (11) | 1 (4) |
a1,500–2,000 mg Paracetamol tablets + 90–120 mg codeine tablets
b25–200 μg/72 h
c60–240 mg Twice daily
Table 2.
Metastatic bone disease history
| Ibandronic acid treatment arm | ||
|---|---|---|
| Intravenous (n = 26) | Oral (n = 26) | |
| Sites of disease, n | ||
| Breast | 7 | 7 |
| Lung | 6 | 6 |
| Urogenital | 3 | 4 |
| Colon | 3 | 4 |
| Prostate | 4 | 1 |
| Other | 3 | 4 |
| Sites of bone metastases, n | ||
| Upper extremities | 5 | 1 |
| Lower extremities | 7 | 7 |
| Flat bones | 17 | 17 |
| Skull | 3 | 2 |
| Cervical spine | 1 | 3 |
| Thoracic spine | 13 | 13 |
| Lumbar spine | 6 | 11 |
| Sacral spine | 4 | 2 |
| Sacro-iliac joint | 5 | 4 |
| Total number of metastasesa | 61 | 60 |
| Bone metastases per patient, n | ||
| <3 foci | 15 | 15 |
| ≥3 foci | 11 | 11 |
aTotal number of metastases exceeds number of patients because some patients had >1 site of metastasis
Table 3.
Prior therapy for metastatic bone disease
| Ibandronic acid treatment arm | ||
|---|---|---|
| Intravenous (n = 26) | Oral (n = 26) | |
| Surgery, n | ||
| Yes | 9 | 13 |
| No | 17 | 13 |
| Radiotherapy, n | ||
| Yes | 23 | 21 |
| No | 3 | 5 |
| Number of radiated sites, n | ||
| =1 | 20 | 22 |
| >2 | 6 | 4 |
Mean pain scores, analgesic use and QoL indices at baseline were similar for both treatment groups (Table 1). Biochemical measures at baseline were also comparable.
Efficacy
A total of 26 patients received oral ibandronic acid (50 mg tablet/day) and 26 received 6 mg IV ibandronic acid infused over 15 min every 28 days. At 6 months, IV and oral ibandronic acid therapies produced similar effects on bone metastases; 84.6 and 88.5% of patients had a complete or partial response in IV and oral ibandronic acid treatment groups, respectively (Fig. 1a). In addition, the median percentage decreases in S-CTX levels after 6 months of treatment were also similar in the IV and oral ibandronic acid groups −39 and −35%, respectively (Fig. 1b).
Fig. 1.
Clinical response to intravenous (IV) and oral ibandronic acid therapy. a The size and number of bone metastases were measured by bone scintigraphy and radiography after 6 months of ibandronic acid treatment and the percentage of patients who showed a complete or partial response, stable disease, or worsening disease were determined. b Serum C-terminal telopeptide of type I collagen (S-CTX) levels after 3 and 6 months of IV or oral ibandronic acid treatment (median percentage change from baseline)
Pain indices
After 3 months of ibandronic acid treatment, about 70% of patients increased their analgesic use (Fig. 2a). The proportion of patients increasing analgesic use was slightly higher with oral ibandronic acid than with IV ibandronic acid. There was no change in analgesic use in approximately 80% of patients who were receiving ibandronic acid (as either IV or oral therapy) from 3 to 6 months (Fig. 2b).
Fig. 2.
Percentage of patients with a change in analgesic use during intravenous (IV) and oral ibandronic acid treatment. The quantity of analgesia was measured at baseline, and month 3 and 6. The median percentage change in analgesic use was determined a from baseline to 3 months and b from 3 to 6 months
Bone pain scores decreased by a similar amount in both treatment arms (Fig. 3a). The largest decreases in bone pain were observed between baseline and 3 months, with a 57% decrease in the worst pain category and a 70% decrease in the average pain category. After 3 months of both IV and oral ibandronic acid therapy, the interference of pain in general activity was decreased by 66 and 65% and the interference of pain in enjoyment of life was decreased by 80 and 75% in the IV and oral treatment arms, respectively. These effects were maintained from 3 to 6 months of therapy (Fig. 3b).
Fig. 3.
Change in bone pain indices during intravenous (IV) and oral ibandronic acid treatment. Severity of bone pain was measured at baseline, and month 3 and 6 by the Greek bone pain inventory (G-BPI) questionnaire. Median percentage change in bone pain score from baseline was determined for the following items: a worst and average pain and b interference of pain in general activity, walking, work, and enjoyment of life
QoL
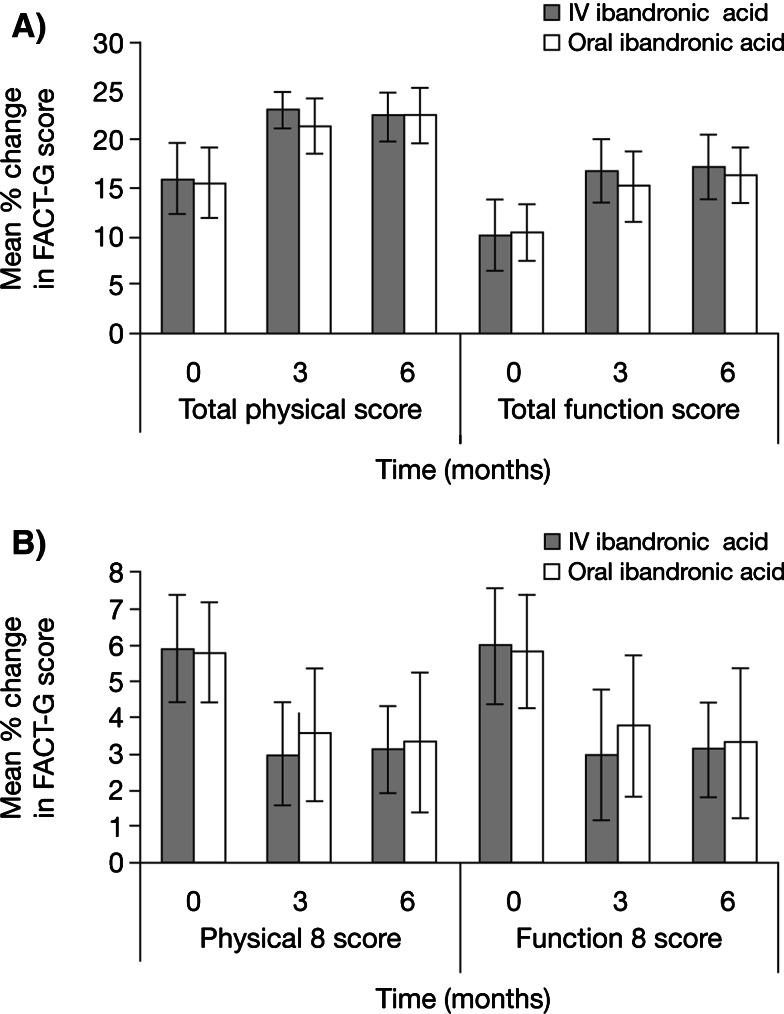
Mean total physical score increased from approximately 16 points at baseline to 22.4 and 22.5 at 6 months in the IV and oral ibandronic acid treatment arms, respectively (Fig. 4a). After 6 months of ibandronic acid, mean total functional score increased from approximately 10 points to 17.2 in the IV and 16.3 in the oral ibandronic acid treatment groups (Fig. 4a). The physical 8 and function 8 scores decreased after 3 months of IV or oral ibandronic acid treatment and this effect was sustained at 6 months (Fig. 4b). The physical 8 and function 8 scores also decreased with IV or oral ibandronic acid, indicating improved functioning.
Fig. 4.
Change in quality of life (QoL) indices during intravenous (IV) and oral ibandronic acid treatment. QoL was determined at baseline, and month 3 and 6 using the functional assessment of cancer therapy-general (FACT-G) questionnaire. Mean values a for total physical and functional indices as well as b for the response to the question 8 of the physical and functional question sets were determined
Safety
Of the 52 patients who entered the study, 34 patients completed the study (18 patients receiving IV treatment and 16 patients in the oral group). A total of 9 patients withdrew from the study, 6 patients treated with IV ibandronic acid and 3 patients treated with oral ibandronic acid. No drug-related adverse events were reported. Seven patients died in the oral ibandronic acid arm and two patients died in the IV ibandronic acid arm; however, these deaths were not related to the study drug.
The median percentage change in Ht, haemoglobin, platelets, white blood count, alkaline phosphatase, γ-glutamyl transferase, urea, and serum creatinine levels did not significantly deviate from baseline after 3 and 6 months of ibandronic acid treatment (Table 4). Ca levels decreased to a similar extent at 3 and 6 months in both treatment arms, with significance only in the IV arm (6.9% decrease at 6 months). Bilirubin levels increased at 3 and 6 months in both treatment arms, but significantly in the oral ibandronic acid treatment arm (9.2% increase at 6 months, P < 0.05). Serum microalbumin decreased significantly at 3 and 6 months in both treatment arms (4.6 and 10.2% decrease after 6 months in IV and oral treatment arms, respectively, P < 0.05).
Table 4.
Changes in biochemical safety indices from baseline after 3 and 6 months of ibandronic acid treatment
| Median change from baseline (%) | ||||
|---|---|---|---|---|
| 3 months | 6 months | |||
| IV (n = 26) | Oral (n = 26) | IV (n = 26) | Oral (n = 26) | |
| Biochemical indices | ||||
| Haematocrit | 0.01 | −2.52 | −0.44 | −2.47 |
| Haemoglobin | 0.02 | −3.91 | −0.79 | −1.56 |
| Platelets | −8.08 | −10.62 | −0.99 | −10.33 |
| White blood cells | −9.09 | −2.07 | 3.12 | −8.64 |
| Alkaline phosphatase | 14.29 | 19.35 | 8.25 | 22.97 |
| γ-glutamyl transferase | 7.58 | 0.00 | 5.90 | −7.69 |
| Microalbumin | −1.86a | −4.31a | −4.58a | 10.21a |
| Calcium | −4.58a | −5.14 | −6.91 | −2.12 |
| Bilirubin | 8.73 | 11.25a | 21.67 | 9.19a |
| Urea | −7.14 | 4.00 | −6.67 | 10.86 |
| Serum creatine | 0.00 | −4.17 | 0.00 | 0.00 |
IV intravenous
aP ≤ 0.05
Discussion
In this first direct comparative trial of oral and IV ibandronic acid, these two formulations provided similar clinical responses in patients with MBD arising from a variety of primary tumour types. The relationship between cancer type and ibandronic acid was non-significant; in general, all patients responded equally to therapy. It should be noted, however, that ibandronic acid is only currently indicated for MBD. The majority of patients in each group achieved a complete or partial response as defined in the protocol, indicating the resolution of bone metastases and the prevention of new metastases within 6 months. In phase III trials with ibandronic acid (IV or oral formulations), changes in levels of bone marker turnover or bone pain were reported by 3 months (Body et al. 2003; Body et al. 2004). In the two-year phase III trial with intravenous ibandronic acid, 49% of patients did not experience a new bone event (Body et al. 2003). This earlier results suggests bone stabilization occurred within months of the onset of therapy and, furthermore, this was maintained for up to two years. The median time to first bone event was 50–91 weeks in these earlier trials. Overall, the data presented here are consistent with earlier findings with ibandronic acid, suggest onset of bone stabilization occurs within months of beginning treatment, and can be sustained for years. Direct comparisons of both formulations of ibandronic acid in longer clinical trials are warranted and would benefit from the direct measurement of skeletal events as a primary endpoint.
These clinical efficacy measures were accompanied by a reduction in serum levels of the marker of bone turnover S-CTX in patients receiving either oral or IV ibandronic acid (as shown by median change from baseline at 3 and 6 months). Biochemical bone markers are often used in the research setting but are not yet validated for use in the clinic. Bone markers appear to act as surrogates of disease progression and as predictors of fracture incidence and survival in patients with MBD (Brown et al. 2005; Coleman 2002). Several studies have demonstrated their value in evaluating clinical response to bisphosphonate therapy, as shown by the relationship between suppressed bone marker levels and a reduction in SREs, including bone pain (Coleman et al. 2005; Garnero 2001; Brown et al. 2003; Lipton et al. 1998, 2005; Berenson et al. 2001; Berruti et al. 1999). In addition to the assessment of bone resorption via serum and urinary CTX, serum levels of bone formation markers, such as bone-specific alkaline phosphatase, amino-terminal procollagen propeptide of type I collagen or osteocalcin have also been investigated in previous trials. In a comparative study of oral ibandronic acid and IV zoledronic acid in patients with breast cancer and MBD, levels of bone formation and resorption markers were reduced from baseline to a similar extent in both treatment groups, suggesting that oral ibandronic acid is non-inferior to zoledronic acid on these measures of efficacy (Body et al. 2007). It would be interesting to directly compare the effect of oral and IV ibandronic acid on bone formation markers (not conducted in the present study), and to evaluate their effects on bone resorption/formation within a shorter time period than 3 or 6 months, as there is some evidence that levels of bone markers may fall within a few days of the patient receiving bisphosphonate therapy (Body et al. 2007).
The secondary efficacy endpoints assessed in the present study were bone pain, analgesic use and QoL. The results showed a reduction in bone pain with oral and IV ibandronic acid that were comparable, and maintained over 6 months of treatment. However, it is not possible to separate the effect of ibandronic acid on bone pain from analgesic medications, particularly during the first 3 months when patients in both arms increased their use of analgesics. From 3 to 6 months, however, analgesic use remained constant and bone pain continued to be reduced, suggesting that an actual pain-relieving effect of ibandronic acid was present and improved physical functioning was observed. The effect of ibandronic acid on bone pain in patients with breast cancer and MBD is supported by large-scale, randomized, controlled, phase 3 clinical trials of oral and IV ibandronic acid. Significant reductions in bone pain below baseline occurred within weeks of starting therapy and were maintained for up to 2 years, accompanied by lower analgesic consumption and improved QoL (Yahara et al. 2003; Coleman et al. 2005). While 14 patients in the current study had breast cancer (the approved indication for ibandronic acid), the remaining 38 patients had other tumour types (lung, urogenital, colon and prostate cancer). The promising effects of ibandronic acid on bone pain have been previously reported for patients with prostate, bladder and urologic cancer (6 mg IV every day for 3 days, followed by standard 4-weekly maintenance dosing) and in multiple myeloma (but with a non-standard 2 mg IV dose) (Heidenreich et al. 2003; Ohlmann and Heidenreich 2003; Menssen et al. 2002). Complete pain resolution has also been achieved with IV ibandronic acid 6 mg combined with radiotherapy in patients with lytic, sclerotic and mixed bone metastases from various tumours (Vassiliou et al. 2007).
Both oral and IV ibandronic acid were well tolerated in the current study, with few significant changes in biochemical markers of safety over 6 months of treatment (they remained within the normal range), and no drug-related adverse events led to withdrawal from the study. Serum creatinine levels (a marker of renal function) were unchanged. Unlike some other IV bisphosphonates (Markowitz et al. 2002; Chang et al. 2003), ibandronic acid appears to have a negligible effect on renal function over up to 4 years of treatment (effect similar to placebo) (Pecherstorfer et al. 2006); this is an important consideration for patients receiving concomitant potentially nephrotoxic anticancer therapies or those patients with pre-existing renal insufficiency. Other clinical advantages of this renal profile are reductions in physician/nurse time for managing renal adverse events, and lack of mandatory monitoring of kidney function prior to each infusion (Jackson 2005).
The limitations of the current study are the relatively small patient population and short study duration (6 months). However, the efficacy and safety results obtained support those of the three phase 3 trials of oral and IV ibandronic acid that led to approval of ibandronic acid for MBD from breast cancer. The availability of two efficacious formulations of ibandronic acid allows physicians and patients a choice of treatment according to individual circumstance and preference. It also offers the option of consistency of care between the inpatient and outpatient setting. IV ibandronic acid administered in the hospital by 15-min infusion once a month offers patients a regular opportunity to interact with their support network. This may then be followed by administration of oral ibandronic acid independently at home. The oral formulation is a convenient treatment for long-term administration and because it does not require hospital visits patients do not experience problems with travel, waiting, and scheduling to receive their treatment. This also lowers the burden of bisphosphonate care on the health care system and increases the availability of resources (i.e. staff and infusion chairs). The efficacy and safety results of our study support the interchangeability of oral and IV ibandronic acid within the clinicians’ therapeutic options for patients with MBD. In addition to efficacy, the pharmacokinetic equivalence of the two formulations is well supported by preclinical data (Leyland-Jones 2004).
Acknowledgment
The authors received assistance from a medical communications agency in drafting the manuscript.
Conflict of interest
None of the authors has a conflict of interest.
Funding source
There is no sponsor or funding source to declare.
References
- Berenson JR, Rosen LS, Howell A et al (2001) Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer 91:1191–1200. doi:10.1002/1097-0142(20010401)91:7<1191::AID-CNCR1119>3.0.CO;2-0 [DOI] [PubMed] [Google Scholar]
- Berruti A, Dogliotti L, Gorzegno G et al (1999) Differential patterns of bone turnover in relation to bone pain and disease extent in bone in cancer patients with skeletal metastases. Clin Chem 45:1240–1247 [PubMed] [Google Scholar]
- Body JJ, Diel IJ, Lichinitser MR, for the MF 4265 Study Group et al (2003) Intravenous ibandronate reduces the incidence of skeletal complications in patients with breast cancer and bone metastases. Ann Oncol 14:1399–1405. doi:10.1093/annonc/mdg367 [DOI] [PubMed] [Google Scholar]
- Body JJ, Diel IJ, Lichinitzer M et al (2004) Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: results from two randomised, placebo-controlled phase III studies. Br J Cancer 90:1133–1137. doi:10.1038/sj.bjc.6601663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Body JJ, Lichinitser M, Tjulandin S, Garnero P, Bergstrom B (2007) Oral ibandronate is as active as intravenous zoledronic acid for reducing bone turnover markers in women with breast cancer and bone metastases. Ann Oncol 18:1165–1171. doi:10.1093/annonc/mdm119 [DOI] [PubMed] [Google Scholar]
- Brown JE, Thomson CS, Ellis SP, Gutcher SA, Purohit OP, Coleman RE (2003) Bone resorption predicts for skeletal complications in metastatic bone disease. Br J Cancer 89:2031–2037. doi:10.1038/sj.bjc.6601437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JE, Cook RJ, Major P et al (2005) Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumours. J Natl Cancer Inst 97:59–69 [DOI] [PubMed] [Google Scholar]
- Chang JT, Green L, Beitz J (2003) Renal failure with the use of zoledronic acid. N Engl J Med 349:1676–1679. doi:10.1056/NEJM200310233491721 [DOI] [PubMed] [Google Scholar]
- Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27:165–176. doi:10.1053/ctrv.2000.0210 [DOI] [PubMed] [Google Scholar]
- Coleman RE (2002) The clinical use of bone resorption markers in patients with malignant bone disease. Cancer 94:2521–2533. doi:10.1002/cncr.10522 [DOI] [PubMed] [Google Scholar]
- Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 12(20 suppl):6243s–6249s. doi:10.1158/1078-0432.CCR-06-0931 [DOI] [PubMed] [Google Scholar]
- Coleman RE, Major P, Lipton A et al (2005) Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol 23:4925–4935. doi:10.1200/JCO.2005.06.091 [DOI] [PubMed] [Google Scholar]
- Diel IJ, Solomayer EF, Bastert G (2000) Treatment of metastatic bone disease in breast cancer: bisphosphonated. Clin Breast Cancer 1:43–51 [DOI] [PubMed] [Google Scholar]
- Domchek SM, Younger J, Finkelstein DM, Seiden MV (2000) Predictors of skeletal complications in patients with metastatic breast carcinoma. Cancer 89:363–368. doi:10.1002/1097-0142(20000715)89:2<363::AID-CNCR22>3.0.CO;2-3 [DOI] [PubMed] [Google Scholar]
- Garnero P (2001) Markers of bone turnover in prostate cancer. Cancer Treat Rev 27:187–192. doi:10.1053/ctrv.2000.0213 [DOI] [PubMed] [Google Scholar]
- Heidenreich A, Ohlmann C, Olbert P, Hegele A (2003) High-dose ibandronate is effective and well tolerated in the treatment of pain and hypercalcaemia due to metastatic urologic cancer. Eur J Cancer 1(Suppl 5):S270 [Google Scholar]
- Jackson GH (2005) Renal safety of ibandronate. Oncologist 10(Suppl 1):14–18. doi:10.1634/theoncologist.10-90001-14 [DOI] [PubMed] [Google Scholar]
- Leyland-Jones B (2004) Pharmacokinetic and clinical equivalence of oral and intravenous ibandronate for metastatic bone disease. Eur J Cancer suppl 2:9–12 [Google Scholar]
- Lipton A, Demers L, Curley E et al (1998) Markers of bone resorption in patients treated with pamidronate. Eur J Cancer 34:2021–2026. doi:10.1016/S0959-8049(98)00277-9 [DOI] [PubMed] [Google Scholar]
- Lipton A, Hei Y, Coleman R, Major P, Cook R (2005) Suppression of bone turnover markers by zoledronic acid and correlation with clinical outcome. J Clin Oncol 23(Suppl 16 S):115 Abstract 532 [Google Scholar]
- Markowitz GS, Fine PL, D’agati VD (2002) Nephrotic syndrome after treatment with pamidronate. Am J Kidney Dis 39:1118–1122. doi:10.1053/ajkd.2002.32797 [DOI] [PubMed] [Google Scholar]
- McPartland C, Grosjean L (2004) Treatment of painful bone metastases in Europe and Canada: the role of bisphosphonates. Ann Oncol 15(Suppl 3):iii50 abstract [Google Scholar]
- Menssen HD, Sakalová A, Fontana A et al (2002) Effects of long-term intravenous ibandronate therapy on skeletal-related events, survival, and bone resorption markers in patients with advanced multiple myeloma. J Clin Oncol 20:2353–2359. doi:10.1200/JCO.2002.02.032 [DOI] [PubMed] [Google Scholar]
- Mystakidou K, Katsouda E, Stathopoulou E, Vlahos L (2005) Approaches to managing bone metastases from breast cancer: the role of bisphosphonates. Cancer Treat Rev 31:303–311. doi:10.1016/j.ctrv.2005.03.005 [DOI] [PubMed] [Google Scholar]
- Ohlmann C, Heidenreich A (2003) Efficacy of ibandronate in the management of painful osseous metastases due to hormone refractory prostate cancer. Support Care Cancer 11:396 Abstract A–38 [Google Scholar]
- Pecherstorfer M, Rivkin S, Body JJ, Diel I, Bergström B (2006) Long-term safety of intravenous ibandronic acid for up to 4 years in metastatic breast cancer: an open-label trial. Clin Drug Investig 26:315–322. doi:10.2165/00044011-200626060-00002 [DOI] [PubMed] [Google Scholar]
- Roodman GD (2004) Mechanisms of bone metastasis. N Engl J Med 350:1655–1664. doi:10.1056/NEJMra030831 [DOI] [PubMed] [Google Scholar]
- Vassiliou V, Kalogeropoulou C, Giannopoulou E, Leotsinidis M, Tsota I, Kardamakis D (2007) A novel study investigating the therapeutic outcome of patients with lytic, mixed and sclerotic bone metastases treated with combined radiotherapy and ibandronate. Clin Exp Metastasis 24:169–178. doi:10.1007/s10585-007-9066-x [DOI] [PubMed] [Google Scholar]
- Yahara J, Noguchi M, Noda S (2003) Quantitative evaluation of bone metastasis in patients with advanced prostate cancer during systemic treatment. BJU Int 92:379–384. doi:10.1046/j.1464-410X.2003.04362.x [DOI] [PubMed] [Google Scholar]
- Yau V, Chow E, Davis L, Holden L, Schueller T, Danjoux C (2004) Pain management in cancer patients with bone metastases remains a challenge. J Pain Symptom Manage 27:1–3. doi:D:10.1016/j.jpainsymman.2003.10.003 [DOI] [PubMed] [Google Scholar]