Abstract
Purpose
Renal cell carcinoma (RCC) is characterized by a variable and unpredictable clinical course. Thus, accurate prediction of the prognosis is important in clinical settings. We conducted microarray-based study to identify a novel prognostic marker in conventional RCC.
Patients and methods
The present study included the patients surgically treated at Kyoto University Hospital. Gene expression profiling of 39 samples was carried out to select candidate prognostic markers. Quantitative real-time PCR of 65 samples confirmed the microarray experiment results. Finally, we evaluated the significance of potential markers at their protein expression level by immunohistochemically analyzing 230 conventional RCC patients.
Results
Using expression profiling analysis, we identified 14 candidate genes whose expression levels predicted unfavorable disease-specific survival. Next, we examined the expression levels of nine candidate genes by quantitative real-time PCR and selected CUB-domain containing protein 1 (CDCP1) for further immunohistochemical analysis. Positive staining for CDCP1 inversely correlated with disease-specific and recurrence-free survivals. In multivariate analysis including clinical/pathological factors, CDCP1 staining was a significant predictor of disease-specific and recurrence-free survivals.
Conclusions
We identified CDCP1 as a potential prognostic marker for conventional RCC. Further studies might be required to confirm the prognostic value of CDCP1 and to understand its function in RCC progression.
Electronic supplementary material
The online version of this article (doi:10.1007/s00432-008-0412-4) contains supplementary material, which is available to authorized users.
Keywords: CDCP1, Microarray, Prognosis, Renal cell carcinoma
Introduction
Renal cell carcinoma (RCC) has a wide variety of clinical presentations. Approximately 30% patients present with metastatic disease, and up to 40% patients with localized RCC have recurrences after undergoing nephrectomy (Lam et al. 2005). Although most patients with metastatic disease have an unfavorable prognosis, frequently resulting in death within a year of diagnosis, some patients survive for years while the disease slowly progresses. At present, the histological stage, grade, and performance status are considered to be the most reliable prognostic factors (Shimazui et al. 1996; Mejean et al. 2003). Recently, to enhance the prediction of RCC prognosis, several groups have developed comprehensive integrated staging systems based on clinical and histological factors (Lam et al. 2005).
Treatment of metastatic RCC remains challenging because it is highly resistant to chemotherapy and radiation. Although immunotherapy with interleukin-2 and/or interferons has been the mainstay for almost 20 years, the response rates are generally poor, ranging from 4 to 25% (Gleave et al. 1998; Motzer and Russo 2000). Recently, new therapeutic approaches based on molecular targeting have been reported, and several agents have shown encouraging results in clinical trials (Stadler 2005). In the near future, as various options for the treatment of metastatic RCC become available, better understanding of the prognostic factors would enable better selection of patients who might benefit from either conventional immunotherapy or new therapeutics. Therefore, it is important to identify reliable molecular markers for RCC prognosis because only clinical/histological factors are inadequate for the prognostication of RCC patients (Mejean et al. 2003; Maruyama et al. 2006).
High-throughput transcriptional profiling has emerged as a powerful approach for simultaneously screening a large number of genes. Recently, several studies have reported a set of genes that is associated with RCC prognosis (Takahashi et al. 2001; Vasselli et al. 2003; Jones et al. 2005; Kosari et al. 2005; Sultmann et al. 2005). However, the routine use of microarrays for predicting the RCC outcome is impractical because microarray facilities are expensive and are not commonly available. As an alternative method, quantitative real-time PCR assay or immunohistochemistry are particularly feasible in clinical settings. In addition, the scope of microarrays is limited in terms of gene expression quantification, and their results need to be validated using more sensitive experimental procedures including the above-mentioned methods (Chuaqui et al. 2002). In the present study, we analyzed the expression profile of 39 RCC samples to select candidate prognostic markers. Next, we used quantitative real-time PCR assay to confirm whether the expression levels of nine candidate genes are associated with the prognosis of 65 RCC patients. Thus, we identified CUB-domain containing protein-1 (CDCP1) as a potential prognostic marker. Finally, we examined the prognostic significance of CDCP1 protein expression by immunohistochemically studying 230 patients.
Materials and methods
RCC patients and samples
From January 1991 to December 2002, 240 patients underwent surgery and were histologically diagnosed with conventional RCC at Kyoto University Hospital. Of these 240 patients, 230 were enrolled in the present study. Ten patients were excluded because three patients had bilateral RCC, and formalin-fixed paraffin blocks were not available for the remaining seven patients. Of the 230 patients, 39 and 65 patients were enrolled in the microarray and quantitative real-time PCR experiments, respectively. There was an overlap of 25 patients, who were included in both experiments. Immunohistochemical analysis was conducted in all 230 patients. Mean age was 61.0 years (range: 29–90 years), and the male-to-female ratio was 2.8:1. The overall median follow-up was 41.4 months. A total of 31 patients died and median follow-up in surviving patients was 45.0 months. Tumors were staged and graded according to the International TNM classification system and the Fuhrman grading system (Goldstein 1999). Each tissue specimen was snap-frozen at −80°C immediately after surgical resection. Table 1 shows the characteristics of the patients with conventional RCC included in the microarray study (n = 39), quantitative real-time PCR (n = 65), and immnohistochemical analysis (n = 230). Informed consent was obtained from each patient. The institutional review board of the Kyoto University Graduate School of Medicine approved the present study.
Table 1.
Characteristics of RCC patients included in microarray (n = 39), quantitative real-time PCR (n = 65), and immunohistochemical (n = 230) studies
| No. of patients (%) | |||
|---|---|---|---|
| Microarray study | Real-time PCR | Immunohistochemical study | |
| Age (years) | |||
| <60 | 11 (28.2) | 19 (29.2) | 88 (38.3) |
| 60 or older | 28 (71.8) | 46 (70.8) | 142 (68.7) |
| Gender | |||
| Male | 27 (69.2) | 45 (69.2) | 169 (73.5) |
| Female | 12 (31.8) | 20 (30.8) | 61 (26.5) |
| Stage | |||
| T1a | 11 (28.2) | 25 (38.5) | 101 (43.9) |
| T1b&T2 | 16 (40.0) | 23 (35.4) | 80 (34.8) |
| >T2 | 12 (31.8) | 17 (26.2) | 49 (21.3) |
| N0M0 | 30 (76.9) | 51 (78.5) | 195 (84.8) |
| >N0 or M1 | 9 (23.1) | 14 (21.5) | 35 (15.2) |
| Grade | |||
| G1&G2 | 31 (79.5) | 45 (69.2) | 179 (77.8) |
| G3&G4 | 8 (20.5) | 20 (30.8) | 51 (22.2) |
| Dead of RCC | 9 (23.1) | 13(20.0) | 31 (13.5) |
RNA extraction and microarray experiment
Total RNA was isolated using RNeasy Kit and RNase-Free DNase (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. RNA integrity was verified by using the Agilent Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). Gene expression experiments were carried out using oligonucleotide-DNA microarrays. Detailed experimental procedures are described in Supplementary materials and methods.
Quantitative real-time PCR
cDNA was synthesized using First-Strand cDNA Synthesis kit (Amersham Biosciences, Piscataway, NJ, USA) as described in the manufacturer’s protocol. The experimental procedures for real-time PCR are described in Supplementary materials and methods. The primer sequences are shown in Supplementary Table 1S.
Immunohistochemistry
Formalin-fixed paraffin sections (4 μm thick) were deparaffinized in xylene and rehydrated with graded ethanol. Endogenous peroxidase was inactivated by incubating the sections in 3% hydrogen peroxide for 30 min at room temperature. Antigen retrieval involved heat treatment for CDCP1 staining. Subsequently, the sections were incubated overnight at 4°C with polyclonal goat antibody against CDCP1 (Abcam, Cambridge, UK; 100:1 dilution), which was used in the previous study (Ikeda et al. 2006). After the primary antibody was washed, the sections were stained with Dako LSAB+ system (Dako, Glostrup, Denmark) and counterstained with Meyer’s hematoxylin. Biotin Blocking System (Dako) was used to examine CDCP1 expression in the normal kidney. The primary antibody was not used in the staining procedure for the control experiments and no specific staining was confirmed. CDCP1 staining was scored as follows: negative, <10% carcinoma cells stained and positive, ≥10% carcinoma cells stained. The percentage of stained cells was estimated in several fields (200×). Immunohistochemical scoring was performed in a single laboratory and was interpreted without any knowledge of the clinical data.
Statistics
Disease-specific survival was defined as the interval between surgery and death from RCC. Recurrence-free survival was defined as the interval between surgery and the subsequent appearance of local recurrence or metastatic disease.
To identify candidate genes whose expression levels correlated with disease-specific survival, the Cox proportional hazard model was fitted for each gene in the microarray experiment, as described previously (Neben et al. 2004). The cut-off level was defined as a hazard ratio (HR) of >2.5 and P < 0.01 in order to identify a set of genes associated with unfavorable survival.
The relationship between CDCP1 staining and clinical/pathological parameters was evaluated using Fisher’s exact test in an immunohistochemical study. Survival curves were generated by the Kaplan–Meier method. The log-rank test was used to compare the survival curves and for univariate analysis. Multivariate analysis was conducted using the Cox proportional hazards model with a forward stepwise selection procedure. Statistical significance was set at P < 0.05. Statistical analyses were performed using the software package R (Ihaka and Gentlaman 1996) or Dr. SPSS II (SPSS, Chicago, IL, USA).
Results
Identification of candidate genes related to RCC survival by using microarray and quantitative real-time PCR
By analyzing the expression profile of 39 RCC samples, we identified 14 genes whose expression levels predicted unfavorable disease-specific survival (HR > 2.5, P < 0.01; Supplementary Table 2S). Of the 14 genes, we selected 9 genes, which are known to be involved in cell proliferation, apoptosis, or cancer development, and evaluated their expression in 65 samples by quantitative real-time PCR. According to the expression levels of the target gene, 65 patients were divided into two groups, and the disease-specific survival rate was compared (Supplementary Fig. 1S). Of the 9 genes analyzed, only CDCP1 was significantly associated with prognosis (P = 0.039). Thus, we performed immunohistochemical analysis on CDCP1 in the 230 patients, which was described as follows.
Immunohistochemical analysis of CDCP1
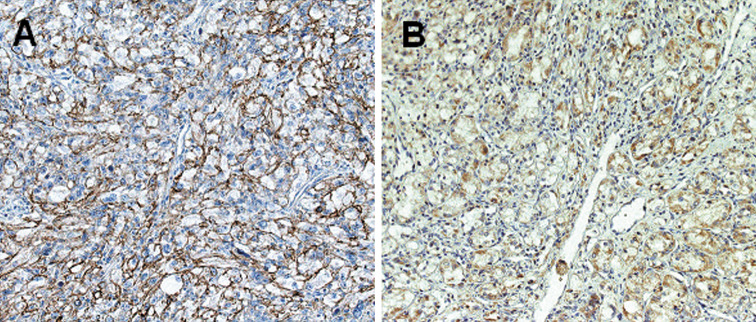
The patterns of CDCP1 expression in the carcinoma cells were observed as membranous and/or cytoplasmic staining (Fig. 1). The heterogeneous distribution of CDCP1 in both plasma membrane and cytoplasm has also been observed in colon cancers (Scherl-Mostageer et al. 2001). In the present study, CDCP1 staining was not detected in the glomerular and tubular cells of normal kidney. CDCP1 staining was positive in 77 cases (33.5%) and was significantly associated with the T stage, the presence of metastasis and the histological grade (P = 0.028, P = 0.002, and P < 0.001, respectively; Table 2).
Fig. 1.
Membranous (a) or cytoplasmic (b) staining of CDCP1 in the carcinoma cells
Table 2.
Relationship between CDCP1 staining and clinicopathological factors
| CDCP1 staining | P value | ||
|---|---|---|---|
| Negative | Positive | ||
| Age (years) | |||
| <60 | 58 | 30 | 0.887 |
| 60 or older | 95 | 47 | |
| Gender | |||
| Male | 108 | 61 | 0.205 |
| Female | 45 | 16 | |
| Stage | |||
| T1&T2 | 127 | 54 | 0.028 |
| >T2 | 26 | 23 | |
| N0M0 | 138 | 57 | 0.002 |
| >N0 or M1 | 15 | 70 | |
| Grade | |||
| G1&G2 | 130 | 49 | <0.001 |
| G3&G4 | 23 | 28 | |
Disease-specific survival
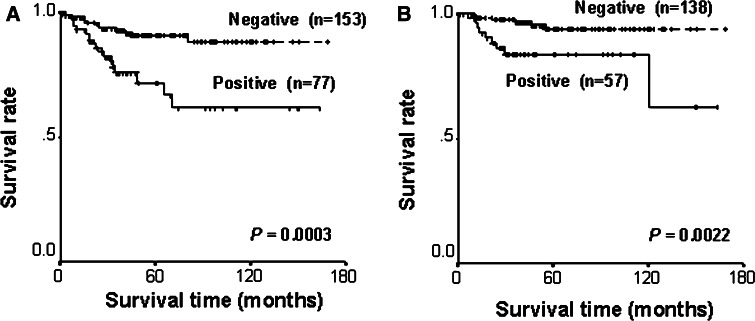
Univariate analysis of potential prognostic impact of clinical, pathological, and immunohistochemical parameters identified the presence of symptoms, T stage >2, the presence of nodal or distant metastasis, histological grade ≥3, and CDCP1 positive staining as being significantly associated with shorter disease-specific survival (P < 0.0001, P < 0.0001, P < 0.0001, P < 0.0001, and P = 0.0003, respectively; Table 3). Disease-specific survival rates at 5 years for patients with CDCP1 positive and negative tumors were 71.7, and 90.8%, respectively (Fig. 2a). In the multivariate analysis including clinical/histological factors and the status of CDCP1 staining, the presence of metastasis and CDCP1 positive staining were significant predictors of shorter disease-specific survival (P < 0.001, P = 0.042, respectively; Table 3).
Table 3.
Univariate and mutivariate analyses of factors related to disease-specific survival
| Variable | No. of patients (n = 230) | Univariate | Mutivariate | ||
|---|---|---|---|---|---|
| P value | Hazard ratio | 95% CI | P value | ||
| Age (years) | |||||
| <60/60 or older | 88/142 | 0.79 | – | – | – |
| Gender | |||||
| Male/Female | 169/61 | 0.48 | – | – | – |
| Presentating symptoms | |||||
| Incidental/Symptomatic | 144/86 | <0.0001 | – | – | – |
| Stage | |||||
| T1–2/>T2 | 181/49 | <0.0001 | – | – | – |
| N0M0/Other | 195/35 | <0.0001 | 34.92 | 14.58–83.63 | <0.001 |
| Grade | |||||
| G1–2/G3–4 | 179/51 | <0.0001 | – | – | – |
| CDCP1 expression | |||||
| Negative/Positive | 153/77 | 0.0003 | 2.2 | 1.53–4.69 | 0.042 |
Fig. 2.
Disease-specific (a) and recurrence-free (b) survivals according to CDCP1 staining
Recurrence-free survival
Recurrence-free survival was analyzed in 195 patients with localized RCC. Univariate analysis identified the presence of symptoms, T stage > 2, and CDCP1 positive staining as being significantly associated with shorter disease-specific survival (P = 0.023, P = 0.0063, and P = 0.0022, respectively; Table 4). Recurrence-free survival rates at 5 years for patients with CDCP1 positive and negative tumors were 83.6, and 93.9%, respectively (Fig. 2b). In the multivariate analysis, T stage > 2 and CDCP1 positive staining were significant predictors of shorter recurrence-free survival in the multivariate analysis (P = 0.042 and P = 0.015, respectively; Table 4).
Table 4.
Univariate and mutivariate analyses of factors related to recurrence-free survival
| Variable | No. of patients (n = 195) | Univariate | Mutivariate | ||
|---|---|---|---|---|---|
| P value | Hazard ratio | 95% CI | P value | ||
| Age (years) | |||||
| <60/60 or older | 79/116 | 0.77 | – | – | – |
| Gender | |||||
| Male/Female | 139/56 | 0.72 | – | – | – |
| Presentating symptoms | |||||
| Incidental/Symptomatic | 139/56 | 0.023 | – | – | – |
| Stage | |||||
| T1–2/>T2 | 165/30 | 0.0063 | 2.98 | 1.03–10.69 | 0.042 |
| Grade | |||||
| G1–2/G3–4 | 166/29 | 0.23 | – | – | – |
| CDCP1 expression | |||||
| Negative/Positive | 138/57 | 0.0022 | 3.71 | 1.29–10.69 | 0.015 |
Discussion
RCC comprises a heterogeneous group of tumors and is classified into various subtypes according to not only the morphological features but also the commonly identified genetic abnormalities (Bodmer et al. 2002). Conventional RCC is the major subtype of RCC, accounting for up to 80% of kidney cancers, and it is characterized by chromosome 3p deletion and frequent mutation of the von Hippel-Lindau gene. In the present study, we performed microarray-based screening of prognostic markers for conventional RCC. To our knowledge, five groups have previously reported gene signatures that defined RCC outcome based on transcriptional gene expression profiling (Takahashi et al. 2001; Vasselli et al. 2003; Jones et al. 2005; Kosari et al. 2005; Sultmann et al. 2005). Strikingly, there is almost no overlap among each set of significant genes identified in these studies. This is probably due to the difference in the design, methods and case subjects of each study. Of these studies, that by Kosari et al. identified 34 candidate biomarkers in a microarray experiment, and by immunohistochemistry in an independent group of patients, they confirmed that one of the candidate biomarkers (survivin) is inversely associated with prognosis (Kosari et al. 2005).
We demonstrated that CDCP1 is a potential prognostic marker for conventional RCC. In 2001, Scherl-Mostageer et al. first identified CDCP1 as the novel gene that is overexpressed in lung and colon carcinomas by using representational difference analysis and cDNA microarray (Scherl-Mostageer et al. 2001). Thereafter, Hooper et al. independently isolated CDCP1 as the protein that is preferentially expressed in metastatic variants of Hep3, an epidermoid carcinoma cell line, over non-metastatic variants (Hooper et al. 2003). CDCP1 is a transmembrane protein containing three extracellular CUB domains and intracellular tyrosine residues phosphorylated by the src family. CUB domains are structurally related to immunoglobulins and are thought to play important roles in adhesion processes (Nentwich et al. 2002). CDCP1 reportedly interacts with other proteins involved in cell adhesion and cell-matrix association such as N-cadherin and matriptase (Bhatt et al. 2005), whose expression has been reported to correspond to RCC progression (Shimazui et al. 2006; Jin et al. 2006). In addition, the src family has been shown to not only form a complex with phosphorylated CDCP1 but also regulate the expression of CDCP1 protein in a cell cycle-dependent manner (Bhatt et al. 2005). Src is known to promote the motility and invasiveness of cancer cells (Yeatman 2004). Thus, CDCP1 might be implicated in cancer invasion and metastasis through src activation, although its precise function remains unclear. The activation of src has been reported in RCC cell lines (Yonezawa et al. 2005) but it has not been examined in primary tissues. Further analysis detailing the relationship between src activation and CDCP1 expression in RCC might become relevant to clinical practice because several src kinase inhibitors have been developed and have undergone clinical trials in other types of cancers (Yeatman 2004).
We recognize some limitations of the present study. First, among the nine candidate prognostic markers identified by using microarray experiment, only CDCP1 was linked to prognosis in qantitative real-time PCR assay. Thus, the quality control in microarray experiments may have been insufficient in the present study. Second, the population of immunohistochemical study was small and limited to patients referred to a single hospital. In addition, the median follow-up-time of 45.0 months for surviving patients was short. Thus, the present results need to be corroborated in independent patient populations. Recently, Kim et al. conducted a tissue array-based immunohistochemical study to investigate the prognostic value of several molecular markers including carbonic anhydrase 9, PTEN, and p53 (Kim et al. 2005). The combination of gene expression profiling and tissue-array technology might be a powerful approach for the identification of potential prognostic markers in RCC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1S. Survival curves for patients with conventional RCC in relation to the expression of 9 candidate genes. CDCP1 expression was significantly associated with prognosis (shown in the rounded rectangle) (TIFF 55 kb)
Table 1S. The sequences of primers used in the present study (XLS 15 kb)
Table 2S. Genes whose expression levels correlate with unfavorable prognosis in microarray experiment (XLS 17 kb)
Supplementary materials and methods (DOC 23 kb)
Acknowledgments
This work was supported in part by NEDO (New Energy and Industrial Technology Development Organization) through its “Project for Developing Biotechnology IT Integration Equipment (P03013).”
References
- Bhatt AS, Erdjument-Bromage H, Tempst P, Craik CS, Moasser MM (2005) Adhesion signaling by a novel mitotic substrate of src kinases. Oncogene 24:5333–5343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer D, van den Hurk W, van Groningen JJ, Eleveld MJ, Martens GJ, Weterman MA, van Kessel AG (2002) Understanding familial and non-familial renal cell cancer. Hum Mol Genet 11:2489–2498 [DOI] [PubMed] [Google Scholar]
- Chuaqui RF, Bonner RF, Best CJ, Gillespie JW, Flaig MJ, Hewitt SM, Phillips JL, Krizman DB, Tangrea MA, Ahram M, Linehan WM, Knezevic V, Emmert-Buck MR (2002) Post-analysis follow-up and validation of microarray experiments. Nat Genet 32(Suppl):509–514 [DOI] [PubMed] [Google Scholar]
- Gleave ME, Elhilali M, Fradet Y, Davis I, Venner P, Saad F, Klotz LH, Moore MJ, Paton V, Bajamonde A (1998) Interferon gamma-1b compared with placebo in metastatic renal-cell carcinoma. Canadian Urologic Oncology Group. N Engl J Med 338:1265–1271 [DOI] [PubMed] [Google Scholar]
- Goldstein NS (1999) Grading of renal cell carcinoma. Urol Clin North Am 26:637–642 [DOI] [PubMed] [Google Scholar]
- Hooper JD, Zijlstra A, Aimes RT, Liang H, Claassen GF, Tarin D, Testa JE, Quigley JP (2003) Subtractive immunization using highly metastatic human tumor cells identifies SIMA135/CDCP1, a 135 kDa cell surface phosphorylated glycoprotein antigen. Oncogene 22:1783–1794 [DOI] [PubMed] [Google Scholar]
- Ihaka R, Gentlaman R (1996) R: a language for data analysis and graphics. J Comput Graph Stat 5:299–314 [Google Scholar]
- Ikeda JI, Morii E, Kimura H, Tomita Y, Takakuwa T, Hasegawa JI, Kim YK, Miyoshi Y, Noguchi S, Nishida T, Aozasa K (2006) Epigenetic regulation of the expression of the novel stem cell marker CDCP1 in cancer cells. J Pathol 210:75–84 [DOI] [PubMed] [Google Scholar]
- Jin JS, Chen A, Hsieh DS, Yao CW, Cheng MF, Lin YF (2006) Expression of serine protease matriptase in renal cell carcinoma: correlation of tissue microarray immunohistochemical expression analysis results with clinicopathological parameters. Int J Surg Pathol 14:65–72 [DOI] [PubMed] [Google Scholar]
- Jones J, Otu H, Spentzos D, Kolia S, Inan M, Beecken WD, Fellbaum C, Gu X, Joseph M, Pantuck AJ, Jonas D, Libermann TA (2005) Gene signatures of progression and metastasis in renal cell cancer. Clin Cancer Res 11:5730–5739 [DOI] [PubMed] [Google Scholar]
- Kim HL, Seligson D, Liu X, Janzen N, Bui MH, Yu H, Shi T, Belldegrun AS, Horvath S, Figlin RA (2005) Using tumor markers to predict the survival of patients with metastatic renal cell carcinoma. J Urol 173:1496–1501 [DOI] [PubMed] [Google Scholar]
- Kosari F, Parker AS, Kube DM, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Vasmatzis G (2005) Clear cell renal cell carcinoma: gene expression analyses identify a potential signature for tumor aggressiveness. Clin Cancer Res 11:5128–5139 [DOI] [PubMed] [Google Scholar]
- Lam JS, Shvarts O, Leppert JT, Figlin RA, Belldegrun AS (2005) Renal cell carcinoma 2005: new frontiers in staging, prognostication and targeted molecular therapy. J Urol 173:1853–1862 [DOI] [PubMed] [Google Scholar]
- Maruyama R, Yamana K, Itoi T, Hara N, Bilim V, Nishiyama T, Takahashi K, Tomita Y (2006) Absence of Bcl-2 and Fas/CD95/APO-1 predicts the response to immunotherapy in metastatic renal cell carcinoma. Br J Cancer 95:1244–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejean A, Oudard S, Thiounn N (2003) Prognostic factors of renal cell carcinoma. J Urol 169:821–827 [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Russo P (2000) Systemic therapy for renal cell carcinoma. J Urol 163:408–417 [PubMed] [Google Scholar]
- Neben K, Korshunov A, Benner A, Wrobel G, Hahn M, Kokocinski F, Golanov A, Joos S, Lichter P (2004) Microarray-based screening for molecular markers in medulloblastoma revealed STK15 as independent predictor for survival. Cancer Res 64:3103–3111 [DOI] [PubMed] [Google Scholar]
- Nentwich HA, Mustafa Z, Rugg MS, Marsden BD, Cordell MR, Mahoney DJ, Jenkins SC, Dowling B, Fries E, Milner CM, Loughlin J, Day AJ (2002) A novel allelic variant of the human TSG-6 gene encoding an amino acid difference in the CUB module. Chromosomal localization, frequency analysis, modeling, and expression. J Biol Chem 277:15354–15362 [DOI] [PubMed] [Google Scholar]
- Scherl-Mostageer M, Sommergruber W, Abseher R, Hauptmann R, Ambros P, Schweifer N (2001) Identification of a novel gene, CDCP1, overexpressed in human colorectal cancer. Oncogene 20:4402–4408 [DOI] [PubMed] [Google Scholar]
- Shimazui T, Oosterwijk E, Debruyne FM, Schalken JA (1996) Molecular prognostic factor in renal cell carcinoma. Semin Urol Oncol 14:250–255 [PubMed] [Google Scholar]
- Shimazui T, Kojima T, Onozawa M, Suzuki M, Asano T, Akaza H (2006) Expression profile of N-cadherin differs from other classical cadherins as a prognostic marker in renal cell carcinoma. Oncol Rep 15:1181–1184 [PubMed] [Google Scholar]
- Stadler WM (2005) Targeted agents for the treatment of advanced renal cell carcinoma. Cancer 104:2323–2333 [DOI] [PubMed] [Google Scholar]
- Sultmann H, von Heydebreck A, Huber W, Kuner R, Buness A, Vogt M, Gunawan B, Vingron M, Fuzesi L, Poustka A (2005) Gene expression in kidney cancer is associated with cytogenetic abnormalities, metastasis formation, and patient survival. Clin Cancer Res 11:646–655 [PubMed] [Google Scholar]
- Takahashi M, Rhodes DR, Furge KA, Kanayama H, Kagawa S, Haab BB, Teh BT (2001) Gene expression profiling of clear cell renal cell carcinoma: gene identification and prognostic classification. Proc Natl Acad Sci USA 98:9754–9759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasselli JR, Shih JH, Iyengar SR, Maranchie J, Riss J, Worrell R, Torres-Cabala C, Tabios R, Mariotti A, Stearman R, Merino M, Walther MM, Simon R, Klausner RD, Linehan WM (2003) Predicting survival in patients with metastatic kidney cancer by gene-expression profiling in the primary tumor. Proc Natl Acad Sci USA 100:6958–6963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman TJ (2004) A renaissance for SRC. Nat Rev Cancer 4:470–480 [DOI] [PubMed] [Google Scholar]
- Yonezawa Y, Nagashima Y, Sato H, Virgona N, Fukumoto K, Shirai S, Hagiwara H, Seki T, Ariga T, Senba H, Suzuki K, Asano R, Hagiwara K, Yano T (2005) Contribution of the Src family of kinases to the appearance of malignant phenotypes in renal cancer cells. Mol Carcinog 43:188–197 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 1S. Survival curves for patients with conventional RCC in relation to the expression of 9 candidate genes. CDCP1 expression was significantly associated with prognosis (shown in the rounded rectangle) (TIFF 55 kb)
Table 1S. The sequences of primers used in the present study (XLS 15 kb)
Table 2S. Genes whose expression levels correlate with unfavorable prognosis in microarray experiment (XLS 17 kb)
Supplementary materials and methods (DOC 23 kb)