Abstract
Purpose
Preclinical models showed TAC-101 (4-[3,5-bis(trimethylsilyl) benzamide] benzoic acid), an oral synthetic retinoid, has anti-tumor activity in hepatocellular carcinoma (HCC). A phase I/II study was performed in advanced HCC patients (pts).
Patients and methods
Thirty-three patients were enrolled. During Phase I, pts received 40 mg daily for 14 days q3 weeks; 2 of 5 patients developed DLT so dose was reduced to 20 mg/day. Twenty-eight patients received 20 mg/day.
Results
No pt had a CR or PR, but 12 of 21 (57%) had SD. Two pts (9.5%) had late PR after discontinuing TAC-101. Median survival (MS) for all 28 pts treated with 20 mg/day was 12.7 months (95% CI 8.8–22.7); MS for 21 evaluable pts was 19.2 months (95% CI 10.4–27.6).
Conclusions
20 mg of TAC- was well tolerated. Significant disease stabilization (12/21 pts, 57%), 2 late PRs, and prolonged MS (19.2 months) suggest that TAC-101 provides meaningful patient benefit.
Keywords: Hepatocellular carcinoma, Clinical trial, Retinoids, TAC-101, Survival
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common solid tumor and the third leading cause of cancer death worldwide(Parkin et al. 1999a, b) Although infrequent in the USA, its incidence in this country has risen steadily since 1975 (El-Serag and Mason 1999; Kim et al. 2002; El-Serag et al. 2003). HCC prevalence in the USA and Western countries is expected to continue to increase over the next several decades largely as a consequence of chronic hepatitis C (HCV) viral infection, which is the leading underlying HCC etiology in these regions.(El-Serag et al. 2003) More than 75% of HCC patients present with advanced disease and are not candidates for liver transplantation, surgical resection, regional or ablative therapies. Median survival for patients with unresectable HCC ranges from 4 to 8 months although some HCC display a more indolent natural history (Abrams et al. 1998; Leung et al. 2002a, b). HCC is resistant to most cytotoxic chemotherapies; even transient disease stabilization is uncommon in patients with advanced disease. Numerous chemotherapy regimens have been studied in HCC; however, cytotoxic chemotherapy has not improved patient survival and can have significant toxicity. Clearly improved systemic therapies are needed for advanced HCC patients.
TAC-101 (4-[3,5-bis(trimethylsilyl) benzamido] benzoic acid) is an orally bioavailable synthetic retinoid, an analogue of vitamin A (retinol) that binds to nuclear retinoic acid receptor-alpha (RAR-α), activates RAR-α transcriptional activity, and has demonstrated antitumor activity in primary and metastatic liver cancer preclinical models (Murakami et al. 1998a, 1998b). TAC-101 inhibits tumor growth in the liver, exhibits low toxicity, and markedly increases survival in both the primary HCC and metastatic colon cancer models (Minagawa et al. 2004). One possible mechanism of action of TAC-101 in HCC is regulation of transcription factors such as activated protein-1 (AP-1). TAC-101 inhibits both the interaction of AP-1 with consensus DNA sequence and the transcriptional activity of AP-1 possibly via the retinoid signal (Murakami et al. 1999; Lin et al. 2000). AP-1 plays a pivotal role in the progression of cancer by inducing transcription of several factors that have a consensus binding site on its promoter region (Matthews et al. 2007). Among the components of the AP-1 proto-oncogene family, c-Jun is over expressed in HCC tissue (Yuen et al. 2001; Guo et al. 2005). Transition of the G0 phase to G1 in the cell cycle is accelerated by c-Jun and is reflected in cell cycle progression and proliferation of tumor cells in HCC tissue (Mechta-Grigoriou et al. 2001; Yuen et al. 2001). Also essential for development of liver tumor, c-Jun acts as an antagonist for the proapoptotic activity of p53 (Eferl et al. 2003). Thus, interference of AP-1 activity by TAC-101 may possibly contribute to the delay of disease progression in HCC.
Methods
Patient selection
This was an open-label, phase I/II trial of oral TAC-101 in patients with advanced (ineligible for surgery or loco-regional therapies) HCC. Eligibility criteria included the following age 18–80 years with a pathologic diagnosis of HCC; at least one non-irradiated, bidimensionally measurable lesion; up to two prior systemic treatments; clear evidence of progressive disease (PD) after the most recent treatment regimen; cirrhosis of Childs-Pugh classification no greater than B; baseline ECOG performance status 0, 1 or 2; life expectancy of at least 12 weeks. Liver-directed therapy, including chemoembolization or radio-frequency ablation, was allowed. Any prior radiation or systemic therapy was to have been completed and all treatment-related toxicity resolved more than 21 days before study entry. Patients who were pregnant, or breast feeding were excluded and women taking oral contraceptives or hormone replacement were required to discontinue such treatment. Organ function requirements included creatinine <1.5× upper limit of normal (ULN), transaminases <2.5× ULN, albumin ≥2.8 g/dL, INR <1.5× ULN, total bilirubin ≤2.0 mg/dL, hemoglobin ≥10.0 g/dL, total WBC count ≥2.0 × 109/L, absolute neutrophil count (ANC) ≥1.0 × 109, platelet count ≥40 × 109/L, and fasting triglyceride concentrations of ≤400 mg/dL for men and ≤325 mg/dL for women. Patients were also excluded if they had any personal history of or first degree relative with abnormal bleeding or clotting; lower extremity Doppler duplex ultrasounds were performed in all patients at baseline. Patients receiving therapeutic regimens of anticoagulants were not eligible. Patients were also excluded if they were taking azoles, or tetracyclines (because of the potential for drug interactions) or any medications known or suspected to predispose to increased risk of venous thromboembolism. The study was approved by the Institutional Review Board at the University of Texas M.D. Anderson Cancer Center (Houston, TX, USA). Written informed consent was obtained from all patients before study enrollment according to federal and institutional guidelines. The study was conducted in accordance with the Declaration of Helsinki.
Study design and treatment plan
The phase I component evaluated the pharmacokinetics of TAC-101 and established the maximum tolerated dose (MTD) over two courses of treatment; the Phase II component evaluated the anti-tumor response to treatment at the MTD. Patients were treated with TAC-101 once daily for 14 consecutive days, followed by 7 drug-free days (a 21-day treatment course).
The initial patient cohort was treated at 40 mg/day. If dose-limiting toxicity (DLT) occurred during the first two courses of treatment in more than one of six patients at the initial dose level, six additional patients were to be enrolled at 20 mg/day. If DLT did not occur during the first two0 courses of treatment in more than one of six patients at a dose level, six additional patients were to be enrolled at 60 mg/day. The MTD was defined as the dose level immediately below the dose at which DLT was experienced in two or more of six or fewer patients.
The Phase 2 component began immediately after the MTD (20 mg/day) for TAC-101 was established. Patients were treated with 20 mg per day of TAC-101 in 21-day courses and evaluated for responses every two courses. Time to disease progression (TTP), time to treatment failure (TTF), safety, tolerability, and overall survival were secondary endpoints. All patients treated at the 20 mg/day dose in the Phase I and/or Phase II portions of the study were included in the analysis of the Phase 2 portion of the trial.
Dose limiting toxicity was defined as any Grade 3 or 4 toxicity according to National Cancer Institute-Common Toxicity Criteria (NCI-CTC) Version 2.0 that was considered to be possibly, probably, or definitely related to the study drug. For this study, additional DLTs were specified and included platelet count <20 × 109/L, total WBC <1.0 × 109/L or ANC <0.5 × 109/L of more than 3 days duration, or bilirubin >10 × ULN. DLT was also defined to include adverse events that resulted in a decision by either the patient or the investigator to request the patient’s removal from the study.
Assessments
Pharmacokinetics
During Phase I, the following pharmacokinetic parameters were estimated on Day 1: maximum plasma concentration (C max), time to maximum concentration (t max), area under the concentration–time curve (AUC) calculated from 0 to 24 h (AUC0−24) and from 0 to infinity (AUC0–∞), and elimination half-life (t 1/2). Blood samples were collected on Day 1 at the following time points: pretreatment, and postdose at 1, 2, 4, 6, 8, and 24 h. Pharmacokinetic assessments were conducted using a standard two-stage approach using noncompartmental techniques for the patient plasma TAC-101 data set.
Efficacy
The WHO criteria were used to evaluate unblinded radiographic tumor response at the study site. MRI or CT was used to identify measurable disease, and the same imaging modality used at baseline was used during follow-up. Baseline evaluations were performed within 4 weeks before beginning treatment and radiographic tumor assessments were obtained at the end of every two treatment courses.
A retrospective reanalysis of survival and of tumor response was performed for this study and included additional data collected retrospectively from medical records at MD Anderson Cancer Center. The radiologic tumor responses were reassessed by an independent radiologist in a blinded manner using Response Evaluation Criteria in Solid Tumors (RECIST).
Safety
To assess the safety of TAC-101 in Phase I and Phase II patients underwent periodic physical examination, review of adverse events and concomitant medication use, and routine hematologic and biochemical testing, including a thrombosis panel.
Statistical considerations
The number of patients in the Phase 2 component was determined by the “optimal” 2-stage design (Simon 1989). The lowest acceptable anti-tumor response frequency, p 0, was set at 5% and the anti-tumor response that was of definite interest, p 1, was set at 20%. At p 0 = 0.05 and p 1 = 0.20, the Type 1 and Type 2 error probabilities, α and β, are set at 0.05 and 0.10, respectively. In the original analysis, patients who participated in the Phase 1 study at the MTD, received 21 or more doses of TAC-101, and had at least one tumor assessment 38 days or more after the initial dose, were included in the evaluable patient population. In the retrospective reanalysis, one additional patient who was noted retrospectively to have had a mixed diagnosis at baseline was excluded from the evaluable patient population. All data were summarized using descriptive statistics for continuous variables and frequency and percentage for discrete variables. All statistical analyses were performed using SAS. Median times to event were estimated using the Kaplan–Meier method. For the original study, best response by WHO criteria, duration of stable disease (defined as time from day of first dose of TAC-101 until PD was observed), and overall survival (OS; defined as time from day of first dose of TAC-101 until death from any cause) were summarized. For the retrospective analysis, best response by RECIST criteria, progression-free survival (time from first dose of TAC-101 to date of PD or death due to any cause), and OS (defined as time from day of first dose of TAC-101 until death from any cause) were summarized.
Results
Patient characteristics
Between May 2001 and September 2004, 33 patients were enrolled and participated in the study. Twelve patients (eight men and four women, 30–72 years of age) participated in the Phase I component of the trial, and received TAC-101 at dose levels of 40 mg/day (5) or 20 mg/day (7). Twenty-one more patients participated in Phase II and each received 20 mg/day of TAC-101. Data from the 12 patients who participated in the Phase I portion of the study were used to determine the MTD; data from all 28 patients who received 20 mg/day (7 from the “Phase I” portion, 21 from the “Phase II” portion) were used for efficacy analyses as well as for safety analyses for that dose level. Table 1 summarizes patient characteristics and Table 2 summarizes baseline HCC characteristics. A majority of the patients had cirrhosis (21/28, 75%) and 21 of 28 (75%) had received some kind of prior treatment, including 17 patients (61%) who had received prior systemic treatment.
Table 1.
Patient characteristics: all treated patients
| Category | Phase I | Phase II |
|---|---|---|
| (n = 12) | (n = 28) | |
| Gender, n (%) | ||
| Male | 8 (66.7) | 18 (64.3) |
| Female | 4 (33.3) | 10 (35.7) |
| Age (years) | ||
| Mean | 55.9 | 62.8 |
| Median | 61.0 | 66.5 |
| Standard deviation | 12.96 | 14.41 |
| Range | 30–72 | 30–85 |
| Race, n (%) | ||
| White | 8 (66.7) | 17 (60.7) |
| Black | 0 (0.0) | 2 (7.1) |
| Asian | 2 (16.7) | 2 (7.1) |
| Hispanic | 2 (16.7) | 7 (25.0) |
| Child-Pugh Classification, n (%) | ||
| A | 11 (91.7) | 25 (89.3) |
| B | 1 (8.3) | 3 (10.7) |
| CLIP score, n (%) | ||
| 0–2 | 6 (50.0) | 23 (82.1) |
| 3 | 2 (16.7) | 1 (3.6) |
| 4 | 3 (25.0) | 3 (10.7) |
| 5–6 | 1 (8.3) | 1 (3.6) |
| ECOG performance statusa, n (%) | ||
| 0 | 4 (33.3) | 7 (25.0) |
| 1 | 8 (66.7) | 21 (75.0) |
| Type of prior treatment, n (%) | ||
| Chemotherapy | 3 (25.0) | 17 (60.7) |
| Surgery (other than diagnostic) | 2 (16.7) | 9 (32.1) |
| Radiotherapy | 1 (8.3) | 4 (14.3) |
| Immunotherapy-interferon | 1 (8.3) | 1 (3.6) |
| Chemoembolization | 0 (0.0) | 1 (3.6) |
| None | 9 (75.0) | 7 (25.0) |
| Number of prior systemic treatment regimens, n (%) | ||
| 0 | 9 (75.0) | 11 (39.3) |
| 1 | 2 (16.7) | 15 (53.6) |
| 2 | 1 (8.3) | 2 (7.1) |
aOne patient in the Phase II portion did not have an assigned ECOG PS
Table 2.
Baseline HCC characteristics: all treated patients
| Characteristic | Phase I | Phase II |
|---|---|---|
| (n = 12) | (n = 28) | |
| HCC Etiology (evaluable patients) n = 28, n (%) | ||
| Cirrhosis | 21 (75%) | |
| Metabolic syndromea | 5 (15%) | |
| Alcohol use | 5 (18%) | |
| Otherb | 4 (14%) | |
| Positive hepatitis testing, n (%) | ||
| Antibody to hepatitis B core antigen | 5 (41.7) | 5 (17.9) |
| Antibody to hepatitis B surface antigen | 1 (8.3) | 1 (3.6) |
| Hepatitis B surface antigen | 1 (8.3) | 2 (7.1) |
| Antibody to hepatitis C Virus | 6 (50.0) | 9 (32.1) |
| Lesion sites | ||
| Site(s) of measurable lesionsc, n (%) | ||
| Liver | 12 (100.0) | 23 (82.1) |
| Lung | 2 (16.7) | 4 (14.3) |
| Lymph Node | 1 (8.3) | 3 (10.7) |
| Adrenal | 0 | 2 (7.1) |
| Other | 0 | 2 (7.1) |
| Site(s) of nonmeasurable lesionsc, n (%) | ||
| Liver | 7 (58.3) | 21 (75.0) |
| Lung | 4 (33.3) | 6 (21.4) |
| Lymph Node | 5 (41.7) | 9 (32.1) |
| Adrenal | 0 | 1 (3.6) |
| Other | 1 (8.3) | 2 (7.1) |
| Tumor morphologyd, n (%) | ||
| Massive or extension >50% | 5 (41.7) | 6 (21.4) |
| Multinodular and extension ≤50% | 7 (58.3) | 20 (71.4) |
| Uninodular and extension ≤50% | 0 | 2 (7.1) |
| Baseline AFP, n (%) | ||
| <400 ng/mL | 5 (41.7) | 19 (67.9) |
| ≥400 ng/mL | 7 (58.3) | 9 (32.1) |
aDefined as documented history of diabetes mellitus, hypertension, abnormal lipid profile, with or without obesity
bOther includes 1 hemachromatosis, 1 autoimmune, 2 hormone replacement
cPatients may have had more than one site of disease under measurable or nonmeasureable lesions
dPatients are only counted once within a category for tumor morphology
One patient who had received 20 mg TAC-101 was found retrospectively to have had a mixed histologic diagnosis at baseline and was excluded from all of the retrospective analyses. This patient was a 63-year-old white male who did not have cirrhosis, tested negative for hepatitis, and had an ECOG status of 1 and a Child-Pugh score of A at baseline. He discontinued from the study due to PD, received treatment with several additional chemotherapy agents post-TAC-101 treatment and survived 839 days from the first dose of TAC-101.
Determination of maximum tolerated dose (MTD)
The MTD for TAC-101 was 20 mg/day, that is, one de-escalation from the Phase I starting dose. During Phase I, two of five patients (40.0%) in the 40-mg/day group experienced DLTs. One patient developed a deep venous thrombosis, grade 3 dermatitis, fatigue, arthralgia, and myalgia. The second patient experienced Grade 3 vena cava embolism, dermatitis, and fatigue. Therefore, the dose of TAC-101 was de-escalated to 20 mg/day. At the 20 mg/day dose, one of the first six patients entered discontinued treatment after one cycle due to progressive disease; therefore, a seventh patient was added to the 20 mg/day cohort. Of the seven patients in the 20-mg/day group in Phase I, one patient experienced a drug-related Grade 3 AE, increased serum AST level. One patient experienced a drug-related Grade 4 AE (pulmonary embolism, PE). The PE in this patient was asymptomatic and was detected on routine restaging evaluation at the end of Course 2. The MTD of TAC-101 in this study was, therefore, determined to be 20 mg/day for the dosing regimen 14 days on/7 days off.
Pharmacokinetics
Table 3 presents a summary of the pharmacokinetics of TAC-101. Area under the concentration–time curve and C max values increased with dose, and no evidence of nonlinearity was found in apparent drug clearance across the two dose levels. No association was evident between apparent drug clearance and either patient body surface area or weight. For the 20 mg dose, T max was 4.3 hours (range 2–6), C max was 242 ng/ml (131–351), AUC0–24 and AUC0–∞ (ng h/mL) were 3067.6 (1575.2–4325.1) and 4241.1 (2998.4–4995.1), respectively.
Table 3.
Summary of TAC-101 pharmacokinetics
| Parameter | N | Mean | Range | % CV |
|---|---|---|---|---|
| 20 mg dose level | ||||
| AUC0–24 (ng h/mL) | 4 | 3067.6 | 1575.2–4325.1 | 38 |
| AUC0–∞ (ng/h/mL) | 3 | 4241.2 | 2998.4–4995.1 | 26 |
| C max (ng/mL) | 5 | 242.0 | 131–351 | 33 |
| T 1/2 (mins) | 3 | 483.1 | 272.1–788 | 56 |
| 40 mg dose level | ||||
| AUC0–24 (ng h/mL) | 5 | 6871.8 | 4248.4–9278.5 | 35 |
| AUC0–∞ (ng/h/mL) | 2 | 6841.8 | 4570.1–9113.5 | 47 |
| C max (ng/mL) | 5 | 542.0 | 376–718 | 23 |
| T 1/2 (mins) | 2 | 340.5 | 323.2–357.7 | 7.17 |
AUC0–24 area under the TAC-101 time, concentration curve from time 0 to 24 h (i.e., dosing interval), AUC0-∞ area under the TAC-101 time, concentration curve from time 0 extrapolated to infinity; t1/2 half-life; Cmax: maximal concentration of TAC-101
Note: Only patients in Phase I underwent PK analysis (N = 11). One 20 mg patient did not have sufficient samples for any PK analysis; one 20-mg patient had no 24-h sample collected; two 40-mg patients had no 8-h sample collected; one 40-mg and one 20-mg patient had relatively delayed absorption which disallowed accurate estimation of the terminal elimination rate
Clinical efficacy
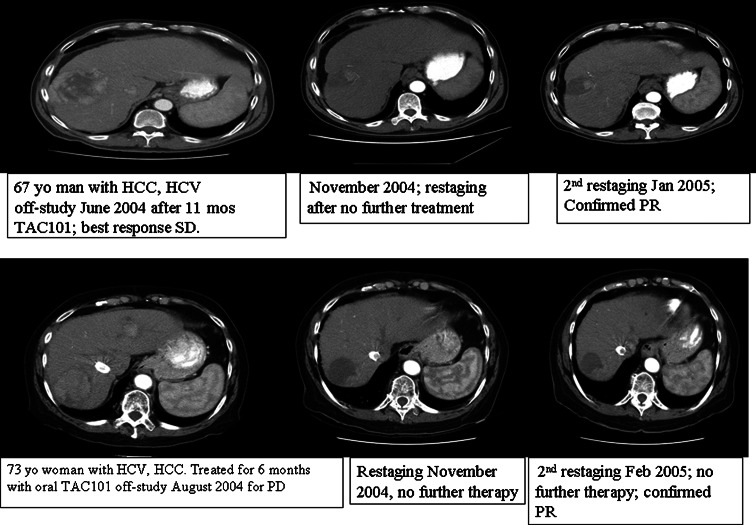
The primary outcome objective of this study was anti-tumor response rate based on unblinded image readings and according to WHO criteria. A retrospective re-analysis of all radiographic images was performed by an independent radiologist using modified RECIST criteria. The best tumor responses according to both criteria are summarized for the evaluable patients and for all patients in Table 4. No patients experienced complete, partial or minor responses (CR, PR, or MR) during the study according to WHO criteria. The study was terminated after completion of the first stage of the Phase II portion since the primary objective RR of 20% was not achieved. Among patients treated with 20 mg/day, 12 of 21 evaluable (Phase II) patients (57.1%) experienced SD; the remainder had PD. In the 12 patients who had SD in the evaluable population, the mean duration of SD was 5.2 months (range 2.8–12 months). Interestingly, two patients had evidence of tumor shrinkage, decreased vascularization and necrosis confirmed as a PR by WHO criteria on restaging CT scans 3 and 6 months after discontinuing TAC-101 (Fig. 1).
Table 4.
Efficacy results: evaluable and all treated patients
| Category | Evaluable patientsa treated with 20 mg/day | All patients treated with 20 mg/day |
|---|---|---|
| Original study | (n = 21) | (n = 28) |
| Best response by WHO Criteria n (%) | ||
| CR | – | – |
| PR | – | – |
| MR | – | – |
| SD (n) | 12 (57.1) | 12 (42.9) |
| PD (n) | 9 (42.9) | 11 (39.3) |
| Duration of stable disease for patients with SD (months) | ||
| Mean | 5.2 | NA |
| Median | 4.5 | NA |
| Standard deviation | 2.9 | NA |
| Range | 2.8–12 | NA |
| Overall survival (months) | ||
| Median survival | 19.2 | 12.7 |
| 95% CI | 10.4–27.6 | 8.8–22.7 |
| Retrospective reanalysis | (n = 20)b | (n = 27)b |
| Best response by modified RECIST Criteria, n (%) | ||
| CR | – | – |
| PR | 1 (5.0) | 1 (3.7) |
| MR | NA | NA |
| SD | 16 (80.0) | 17 (63.0) |
| PD | 3 (15.0) | 7 (25.9) |
| No post-baseline Assessments | – | 2 (7.4) |
| Progression free survival (months) | ||
| Median survival | 3.4 | 3.2 |
| 95% CI | 3.0–5.8 | 3.0–5.8 |
| Overall survival (months) | ||
| Median survival | 16.7 | 12.6 |
| 95% CI | 10.0–30.1 | 7.9–22.0 |
CR complete response, PR partial response, MR minor response, SD stable disease, PD progressive disease, NA data not available, CI confidence intervals
aEvaluable patients were defined as patients who participated in the Phase 1 study at the MTD, who received 21 or more doses of TAC-101, and had at least 1 tumor assessment 38 days or more after the initial dose
bOne patient with a mixed diagnosis at baseline was excluded from all retrospective efficacy analyses
Fig. 1.
Post-TAC-101 tumor responses seen in two patients
Median survival (MS) for all 28 patients treated with 20 mg/day was 12.78 months (95% CI 8.8–22.7), and 19.2 months for the 21 evaluable patients (95% CI 10–27.6), both figures substantially longer than MS achieved for patients treated in numerous clinical trials of systemic agents in patients with advanced, unresectable HCC (Table 4).
When retrospectively evaluated by modified RECIST criteria, among the 20 evaluable patients, one (5.0%) had PR, 16 (80.0%) had SD, and 3 (15.0%) had PD. Reanalysis of MS gave results similar to those observed in the original study for both patient populations. Median progression free survival was 3.2 months for all patients and 3.4 months for evaluable patients in the reanalysis (see Figs. 2, 3). This relatively brief PFS appears discordant with the encouraging MS figures of 12.78 and 19.2 months. Consideration of the TAC-101 mechanism of action may help explain this difference. TAC-101 is not cytotoxic to tumors, rather the agonistic action of TAC-101 on the RARα receptor results in induction of various DNA changes, the end result of which is to inhibit tumor growth and angiogenesis. These processes require time compared to the direct action of the cytotoxic tumor “inhibitors”. Thus it can be inferred that the effect of TAC-101 will occur slowly.
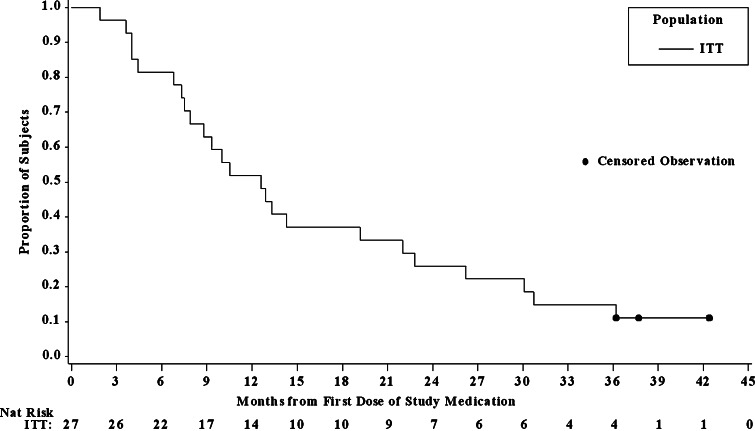
Fig. 2.
Kaplan–Meier overall survival curve for 27 patients with advanced HCC included in the ITT population of the reanalysis. All patients were treated with 20 mg/day TAC-101
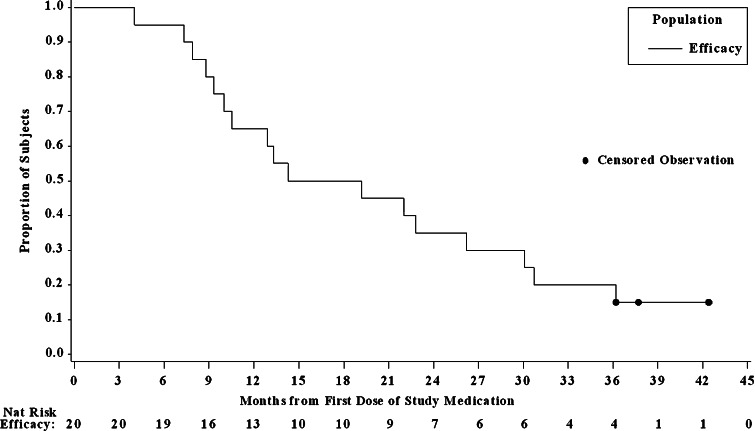
Fig. 3.
Kaplan–Meier overall survival curve for 20 patients with advanced HCC included in the evaluable population of the reanalysis. All patients were treated with 20 mg/day TAC-101
Adverse events
Table 5 summarizes the drug-related AEs during all treatment courses (in order of decreasing overall frequency in the 20 mg group). At a dose of 20 mg/day for 14 days followed by 7-day rest periods, TAC-101 was generally well tolerated. The most frequently occurring drug-related AEs in patients who received 20 mg/day included increased blood triglyceride levels (60.7%); fatigue (57.1%); dermatitis (57.1%); pruritus (50.0%); nausea (46.4%); dry skin (39.3%); myalgias (39.3%); dry mouth (28.6%); arthralgias (25.0%); anorexia (21.4%), diarrhea (21.4%), and headache (21.4%).
Table 5.
Drug related adverse events during all treatment courses all treated patients
| Grade | 40 mg | 20 mg | ||
|---|---|---|---|---|
| n = 5 | n = 28 | |||
| 1/2 | 3/4 | 1/2 | 3/4 | |
| Elevated triglycerides | 1 | 0 | 17 | 0 |
| Fatigue | 3 | 2 | 15 | 1 |
| Dermatitis | 3 | 2 | 15 | 1 |
| Pruritus | 3 | 0 | 13 | 1 |
| Nausea | 2 | 0 | 13 | 0 |
| Dry Skin | 1 | 0 | 11 | 0 |
| Myalgias | 2 | 1 | 11 | 0 |
| Dry Mouth | 0 | 0 | 8 | 0 |
| Arthralgia | 3 | 1 | 7 | 0 |
| Anorexia | 1 | 0 | 6 | 0 |
| Diarrhea | 1 | 0 | 6 | 0 |
| Headache | 4 | 0 | 6 | 0 |
| AST increase | 2 | 0 | 3 | 2 |
| Vomiting | 1 | 0 | 4 | 0 |
| Back pain | 0 | 0 | 4 | 0 |
| Taste disturbance | 0 | 0 | 4 | 0 |
| ALT increase | 0 | 0 | 3 | 0 |
| Weakness | 0 | 0 | 3 | 0 |
| Alopecia | 3 | 0 | 2 | 0 |
| Thrombosis/PE | 0 | 2a | 0 | 2b |
| Dyspepsia | 0 | 0 | 2 | 0 |
| Pain in limb | 0 | 0 | 2 | 0 |
| Pancreatitis | 0 | 0 | 0 | 1 |
| Leukopenia | 1 | 0 | 1 | 0 |
| Constipation | 2 | 0 | 0 | 0 |
| Rigors | 2 | 0 | 0 | 0 |
aOne patient experienced vena caval embolism and the other developed DVT
bOne patient developed PE in Phase 1 and the other DVT in Phase 2
Five of 28 patients (17.9%) treated with 20 mg/day experienced ≥1 of the following drug-related Grade 3 or 4 AEs: increased serum AST level, dermatitis, fatigue, pancreatitis which resolved with conservative management, pruritus, DVT and PE. Five of the 28 (17.9%) experienced AEs leading to discontinuation of TAC-101 therapy. The following AEs resulted in discontinuation of TAC-101 therapy: fatigue, pulmonary embolism, pathologic fracture, increased serum AST levels, and dermatitis. Upon discontinuation of TAC-101, fatigue, dermatitis, and AST elevation resolved during the 3-week off-study evaluation period. With the exception of pathologic fracture, the AEs leading to discontinuation were considered to be drug related. The patient who developed a PE was successfully treated with anticoagulation without clinical sequelae.
Discussion
Significant advances have been made in treatment of patients with small, localized HCC through improved surgical and liver transplantation candidate selection, and demonstration of modest survival benefit from loco-regional treatments such as ablation and trans-arterial chemoembolization. However, a majority of all patients diagnosed with HCC have advanced disease and are not candidates for these therapies. Developing effective systemic therapies for patients with advanced HCC is an imperative in oncology.
In the absence of a randomized trial, deriving conclusions as to the relative benefits of any systemic therapy is always challenging. The results of this single-arm study of TAC-101 can only be evaluated in the context of trial results in similar patient populations. Such comparisons to historical controls are particularly difficult in HCC due to the significant heterogeneity inherent in this patient population. However, the intent of traditional Phase II single-arm trials is to identify a reasonable “biological signal” of potential patient benefit, and thus provide a sound rationale for further drug development.
In this study, while no radiographic responses were seen on treatment, the median survival of the 21 evaluable patients was 19.2 months, and of all 28 patients treated with 20 mg/day TAC-101 was 12.7 months, both of which are substantially longer than the median survival in numerous published chemotherapy trials in HCC. Advanced HCC patients who are not eligible for surgical or loco-regional therapies are well-documented to have median survival in the 6–8 month range (Table 6) with only the exception of sorafenib 400 mg BID, which extended median survival to 9.2 months in a Phase II study (N = 137) and to 10.7 months (299 patients) compared with 7.9 months (303 patients) for placebo in a Phase III study.(Abou-Alfa et al. 2006; Llovet et al. 2007) The patients treated in this study of TAC-101 generally were a moderate to poor-prognostic group: a majority had cirrhosis (21/28, 75%); 75% (21/28) were ECOG PS 1, 32% had HCV, and 5 pts were CLIP score 3 or higher, which is predictive of very poor prognosis.(Hasumi et al. 2000; Llovet and Bruix 2000) We believe this study of TAC-101 provides suggestion of disease stabilization and prolonged survival in a representative advanced HCC patient population. In addition, 2 patients experienced PR and decreased tumor vascularization several months after stopping TAC-101 and without further treatment. The late responses seen in these two patients are difficult to explain by chance alone, although “spontaneous” tumor shrinkage in the absence of anti-cancer therapy has been documented (Kato et al. 2004; Ventegodt et al. 2004; Ohtani et al. 2005). Potential mechanisms for these late responses remain speculative.
Table 6.
Summary of selected clinical trials of systemic therapy in advanced HCC patients
| Study | Regimen | Study type | N | RR% | SD% | MS (months) |
|---|---|---|---|---|---|---|
| Current study | TAC-101 | Phase II | 21/28 | 0% | 57.1%/42.9% | 19.2/12.7a |
| Abou-Alfa et al. (2006) | Sorafenib | Phase II | 137 | 8 | 33.6 | 9.2 |
| Llovet et al. (2007) | Sorafenib vs. placebo | Phase III | 602 (299/303) | 2.3% | 10.7 vs. 7.9 (p = 0.00058) | |
| Zhu et al. (2006) | GEMOX-bevacizumab | Phase II | 33 | 20 | 27 | 9.6 |
| Gish (Gish et al. 2007) | Nolatrexed vs. doxorubicin | Phase III | 446 | 1.4 vs. 4.0 | NA | 4.8 vs. 7.1 |
| Boige et al. (2006) | Irinotecan | Phase II | 25 | 0% | 52 | 7.4 |
| Yeo et al. (2005) | Doxorubicin vs. PIAF | Phase III | 86/91 | 10.5 vs. 20.9 | 43 vs. 38 | 6.8 vs. 8.7 (p = 0.83) |
| Posey et al. (2005) | T138067 vs. doxorubicin | Phase II/III | 169/170 | 2 vs. 4 | 43 vs. 43 | 5.7 vs. 5.6 |
| Ikeda et al. (2005) | 5FU, mitoxantrone, cisplatin | Phase II | 50 | 27 | 53 | 11.6 |
| Barbare et al. (2005) | Tamoxifen vs. BSC | Phase II | 210/210 | NA | NA | 4.8 vs. 4.0 |
| Philip et al. (2005) | Erlotinib | Phase II | 38 | 9 | 50 | 13 |
| Patt et al. (2005) | Thalidomide | Phase II | 37 | 6 | 31 | 6.8 |
| Lee et al. (2004) | Doxorubicin and cisplatin | Phase II | 37 | 18.9 | 16.2 | 7.3 |
| Guan et al. (2003) | Gemcitabine, std. vs. fixed-dose | Phase II, | 25/23 | 4 vs. 0 | NA | 3.2 vs. 3.2 |
| Fuchs et al. (2002) | Gemcitabine | Phase I | 30 | 0 | 30 | 6.9 |
| Leung et al. (2002a, b) | PIAF | Phase II | 149 | 16.8 | NA | 7.1 |
| Mok et al. (1999) | Nolatrexed vs. doxorubicin | Phase II | 32/12 | 0 | 21.8 vs. 16.7 | 4.6 vs. 3.5 |
| Meyskens et al. (1998) | B-all-trans-retinoic acid | Phase II | 18 | 0 | NA | 4.0 |
GEMOX gemcitabine, oxaliplatin; PIAF cisplatin, interferon, Adriamycin, 5FU; NA not available
aMS 19.2 months for 21 evaluable pts; 12.7 months for all pts treated at MTD
Retinoids such as TAC-101 represent a class of agents that has not been extensively studied in HCC. In this trial, TAC-101 was generally well tolerated by patients with the principal adverse events consisting of fatigue, skin changes and venous thrombotic events (VTEs). The initial dose-escalation study of TAC-101 in patients with advanced cancer showed a similar toxicity profile.(Rizvi et al. 2002) Retinoids are capable of inhibiting growth and inducing differentiation in tumor models, including HCC (Yasuda et al. 2002; Matsushima-Nishiwaki et al. 2003). In clinical studies, retinoids have demonstrated potential benefit in both the prevention and treatment of cancer. Treatment of patients with 13-cis-retinoic acid reverses oral leukoplakia and reduces the incidence of second primary tumors in head and neck cancers (Lippman et al. 1989, 1993a, b; Sankaranarayanan and Mathew 1996; Dimery et al. 1997; Lippman 1997; Lotan 1997; Papadimitrakopoulou and Hong 1997) In the treatment of acute promyelocytic leukemia (APL), all-trans-retinoic acid (ATRA) induces complete, albeit temporary, remissions in a majority of patients.(Wang et al. 1990; Bruserud and Gjertsen 2000; Zhang et al. 2000) In a seminal trial by Muto and colleagues, polyprenoic acid, an oral acyclic retinoid (AR) administered for 1 year in a randomized, placebo-controlled study of HCC patients who underwent surgical resection or percutaneous injection of ethanol (PEI), decreased the incidence of second primary tumors and prolonged survival in the patients who received AR (Muto et al. 1996; 1999; Takai et al. 2005).
TAC-101 has very specific binding affinity to RARα but not to RARβ, γ or to RXRs. Through its binding to RARα, TAC-101 interferes with the binding of AP-1 to DNA, and inhibits production of urokinase plasminogen activator (uPA) and matrix metalloproteinase 9 (MMP-9). In several studies, TAC-101 has shown inhibition of tumor growth and hepatic metastases in several cancers including colorectal, gastric, pancreatic, and lung cancer and inhibits angiogenesis via down regulation of vascular endothelial growth factor (VEGF) mRNA and protein in a colon cancer model (Hashimoto et al. 1996; Murakami et al. 1998a, b; Oikawa 1998; Fujimoto et al. 1999; Miyaguchi et al. 2001; Oikawa et al. 2001; Rizvi et al. 2002; Lee et al. 2003; Miyagawa et al. 2003; Sano et al. 2003; Satake et al. 2003; Yoshimura et al. 2003; Minagawa et al. 2004; Shudo et al. 2004; Suzuki et al. 2004; Sako et al. 2005). In a gastric cancer animal model, TAC-101 was shown to decrease cell-induced angiogenesis (decreased microvessel density and new blood vessel formation). TAC-101 was investigated in four human pancreatic cancer cell lines and results suggested that TAC-101 inhibits pancreatic cancer cell growth by G1 phase cell-cycle arrest resulting from the reduction of RB gene phosphorylation and the up-regulation of p21 and p27 as well as induction of apoptosis.(Fujimoto et al. 1999) The mechanisms of action of TAC-101 and other retinoids are considered cytostatic as opposed to cytotoxic, and this may account for the improved survival, rather than tumor responses, seen in HCC patients treated with TAC-101.
This trial terminated after the first stage based on apparent lack of efficacy, and it was only with continued observation of the patients that the biologic activity of TAC-101 became evident. The results described here suggest that outcome measures other than traditional radiographic tumor shrinkage are needed to accurately assess non-cytotoxic drug activity and potential patient benefit. TAC-101 merits further study in randomized trials with correlative studies designed to further explore the mechanism of action of this novel agent in patients with HCC.
References
- Abou-Alfa GK, Schwartz L, Ricci S et al (2006) Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 24(26):4293–4300 [DOI] [PubMed] [Google Scholar]
- Abrams RA, Pajak TF, Haulk TL, Flam M, Asbell SO (1998) Survival results among patients with alpha-fetoprotein-positive, unresectable hepatocellular carcinoma: analysis of three sequential treatments of the RTOG and Johns Hopkins Oncology Center. Cancer J Sci Am 4(3):178–184 [PubMed] [Google Scholar]
- Barbare JC, Bouche O, Bonnetain F et al (2005) Randomized controlled trial of tamoxifen in advanced hepatocellular carcinoma. J Clin Oncol 23(19):4338–4346 [DOI] [PubMed] [Google Scholar]
- Boige V, Taieb J, Hebbar M et al (2006) Irinotecan as first-line chemotherapy in patients with advanced hepatocellular carcinoma: a multicenter phase II study with dose adjustment according to baseline serum bilirubin level. Eur J Cancer 42(4):456–459 [DOI] [PubMed] [Google Scholar]
- Bruserud O, Gjertsen BT (2000) New strategies for the treatment of acute myelogenous leukemia: differentiation induction-present use and future possibilities. Stem Cells 18:157–165 [DOI] [PubMed] [Google Scholar]
- Dimery IW, Hong WK, Lee JJ et al (1997) Phase I trial of alpha-tocopherol effects on 13-cis-retinoic acid toxicity. Ann Oncol 8(1):85–89 [DOI] [PubMed] [Google Scholar]
- Eferl R, Ricci R, Kenner L et al (2003) Liver tumor development. c-Jun antagonizes the proapoptotic activity of p53. Cell 112(2):181–192 [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Davila JA, Petersen NJ, McGlynn KA (2003) The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med 139(10):817–823 [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Mason AC (1999) Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 340(310):745–750 [DOI] [PubMed] [Google Scholar]
- Fuchs CS, Clark JW, Ryan DP et al (2002) A phase II trial of gemcitabine in patients with advanced hepatocellular carcinoma. Cancer 94(12):3186–3191 [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Hosotani R, Doi R et al (1999) Induction of cell-cycle arrest and apoptosis by a novel retinobenzoic-acid derivative, TAC-101, in human pancreatic-cancer cells. Int J Cancer 81(4):637–644 [DOI] [PubMed] [Google Scholar]
- Gish RG, Porta C, Lazar L et al (2007) Phase III randomized controlled trial comparing the survival of patients with unresectable hepatocellular carcinoma treated with nolatrexed or doxorubicin. J Clin Oncol 25(21):3069–3075 [DOI] [PubMed] [Google Scholar]
- Guan Z, Wang Y, Maoleekoonpairoj S et al (2003) Prospective randomised phase II study of gemcitabine at standard or fixed dose rate schedule in unresectable hepatocellular carcinoma. Br J Cancer 89(10):1865–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Guo Y, Xiao S, Shi X (2005) Protein kinase p-JNK is correlated with the activation of AP-1 and its associated Jun family proteins in hepatocellular carcinoma. Life Sci 77(15):1869–1878 [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Kagechika H, Kawachi E et al (1996) Evaluation of differentiation-inducing activity of retinoids on human leukemia cell lines HL-60 and NB4. Biol Pharm Bull 19:1322–1328 [DOI] [PubMed] [Google Scholar]
- Hasumi A, Matsui H, Sugioka A et al (2000) Precancerous conditions of biliary tract cancer in patients with pancreaticobiliary maljunction: reappraisal of nationwide survey in Japan. J Hepatobiliary Pancreat Surg 7(6):551–555 [DOI] [PubMed] [Google Scholar]
- Ikeda M, Okusaka T, Ueno H, Takezako Y, Morizane C (2005) A phase II trial of continuous infusion of 5-fluorouracil, mitoxantrone, and cisplatin for metastatic hepatocellular carcinoma. Cancer 103(4):756–762 [DOI] [PubMed] [Google Scholar]
- Kato H, Nakamura M, Muramatsu M, Orito E, Ueda R, Mizokami M (2004) Spontaneous regression of hepatocellular carcinoma: two case reports and a literature review. Hepatol Res 29(3):180–190 [DOI] [PubMed] [Google Scholar]
- Kim WR, Brown RS Jr, Terrault NA, El-Serag H (2002) Burden of liver disease in the United States: summary of a workshop. Hepatology 36(1):227–242 [DOI] [PubMed] [Google Scholar]
- Lee J, Park JO, Kim WS et al (2004) Phase II study of doxorubicin and cisplatin in patients with metastatic hepatocellular carcinoma. Cancer Chemother Pharmacol 54(5):385–390 [DOI] [PubMed] [Google Scholar]
- Lee SJ, Ohashi Y, Sakurai H, Saiki I (2003) TAC-101 inhibits intrahepatic metastasis of orthotopically implanted murine hepatocellular carcinoma. Cancer Lett 198(2):169–177 [DOI] [PubMed] [Google Scholar]
- Leung CS, Tang CN, Fung KH, Li MK (2002a) A retrospective review of transcatheter hepatic arterial embolisation for ruptured hepatocellular carcinoma. J R Coll Surg Edinb 47(5):685–688 [PubMed] [Google Scholar]
- Leung TW, Tang AM, Zee B et al (2002b) Factors predicting response and survival in 149 patients with unresectable hepatocellular carcinoma treated by combination cisplatin, interferon-alpha, doxorubicin and 5-fluorouracil chemotherapy. Cancer 94(2):421–427 [DOI] [PubMed] [Google Scholar]
- Lin F, Xiao D, Kolluri SK, Zhang X (2000) Unique anti-activator protein-1 activity of retinoic acid receptor beta. Cancer Res 60(12):3271–3280 [PubMed] [Google Scholar]
- Lippman SM (1997) Head and neck chemoprevention: recent advances. Cancer Control 4(2):128–135 [DOI] [PubMed] [Google Scholar]
- Lippman SM, Benner SE, Hong WK (1993a) Chemoprevention strategies in lung carcinogenesis. Chest 103(1 Suppl):15S–19S [DOI] [PubMed] [Google Scholar]
- Lippman SM, Benner SE, Hong WK (1993b) Retinoids in chemoprevention of head and neck carcinogenesis. Prev Med 22(5):693–700 [DOI] [PubMed] [Google Scholar]
- Lippman SM, Garewal H, Meyskens FL Jr (1989) Retinoids as potential chemopreventive agents in squamous cell carcinoma of the head and neck. Prev Med 18:740–48 [DOI] [PubMed] [Google Scholar]
- Llovet JM, Bruix J (2000) Prospective validation of the Cancer of the Liver Italian Program (CLIP) score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology 32(3):679–680 [DOI] [PubMed] [Google Scholar]
- Llovet JM, Ricci S, Mazzaferro V et al (2007) Sorafenib improves survival in advanced Hepatocellular Carcinoma (HCC): results of a Phase III randomized placebo-controlled trials (SHARP trial). American Society of Clinical Oncology Annual Meeting, Chicago [Google Scholar]
- Lotan R (1997) Retinoids and chemoprevention of aerodigestive tract cancers. Cancer Metastasis Rev 16(3–4):349–356 [DOI] [PubMed] [Google Scholar]
- Matsushima-Nishiwaki R, Okuno M, Takano Y, Kojima S, Friedman SL, Moriwaki H (2003) Molecular mechanism for growth suppression of human hepatocellular carcinoma cells by acyclic retinoid. Carcinogenesis 24(8):1353–1359 [DOI] [PubMed] [Google Scholar]
- Matthews CP, Colburn NH, Young MR (2007) AP-1 a target for cancer prevention. Curr Cancer Drug Targets 7(4):317–324 [DOI] [PubMed] [Google Scholar]
- Mechta-Grigoriou F, Gerald D, Yaniv M (2001) The mammalian Jun proteins: redundancy and specificity. Oncogene 20(19):2378–2389 [DOI] [PubMed] [Google Scholar]
- Meyskens FL Jr, Jacobson J, Nguyen B, Weiss GR, Gandara DR, MacDonald JS (1998) Phase II trial of oral beta-all trans-retinoic acid in hepatocellular carcinoma (SWOG 9157). Invest New Drugs 16(2):171–173 [DOI] [PubMed] [Google Scholar]
- Minagawa N, Nakayama Y, Inoue Y et al (2004) 4-[3, 5-Bis(trimethylsilyl) benzamido] benzoic acid inhibits angiogenesis in colon cancer through reduced expression of vascular endothelial growth factor. Oncol Res 14(9):407–414 [DOI] [PubMed] [Google Scholar]
- Miyagawa N, Homma T, Kagechika H, Shudo K, Nagai H (2003) Effect of synthetic retinoid, TAC-101, on experimental autoimmune disease. Pharmacology 67(1):21–31 [DOI] [PubMed] [Google Scholar]
- Miyaguchi T, Nomata K, Noguchi M et al (2001) TAC-101, a novel retinobenzoic-acid derivative, enhances gap junctional intercellular communication among renal epithelial cells treated with renal carcinogens. Anticancer Res 21:4025–4030 [PubMed] [Google Scholar]
- Mok TS, Leung TW, Lee SD et al (1999) A multi-centre randomized phase II study of nolatrexed versus doxorubicin in treatment of Chinese patients with advanced hepatocellular carcinoma. Cancer Chemother Pharmacol 44(4):307–311 [DOI] [PubMed] [Google Scholar]
- Murakami K, Wierzba K, Sani M et al (1998a) TAC-101, a benzoic acid derivative, inhibits liver metastasis of human gastrointestinal cancer and prolongs the life-span. Clin Exp Metastasis 16:323–331 [DOI] [PubMed] [Google Scholar]
- Murakami K, Matsuura T, Sano M et al (1998b) 4-[3, 5-Bis(trimethylsilyl) benzamido] benzoic acid (TAC-101) inhibits the intrahepatic spread of hepatocellular carcinoma and prolongs the life-span of tumor-bearing animals. Clin Exp Metastasis 16(7):633–643 [DOI] [PubMed] [Google Scholar]
- Murakami K, Yamaura T, Suda K et al (1999) TAC-101 (4-[3, 5-bis(trimethylsilyl) benzamido]benzoic acid) inhibits spontaneous mediastinal lymph node metastasis produced by orthotopic implantation of Lewis lung carcinoma. Jpn J Cancer Res 90(11):1254–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto Y, Moriwaki H, Ninomiya M et al (1996) Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N Engl J Med 334(24):1561–1567 [DOI] [PubMed] [Google Scholar]
- Muto Y, Moriwaki H, Saito A (1999) Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma. N Engl J Med 340(13):1046–1047 [DOI] [PubMed] [Google Scholar]
- Ohtani H, Yamazaki O, Matsuyama M et al (2005) Spontaneous regression of hepatocellular carcinoma: report of a case. Surg Today 35(12):1081–1086 [DOI] [PubMed] [Google Scholar]
- Oikawa T (1998) Control of tumor-related angiogenesis. Hum Cell 11:201–206 [PubMed] [Google Scholar]
- Oikawa T, Murakami K, Sano M, Shibata J, Wierzba K, Yamada Y (2001) A potential use of a synthetic retinoid TAC-101 as an orally active agent that blocks angiogenesis in liver metastases of human stomach cancer cells. Jpn J Cancer Res 92(11):1225–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitrakopoulou VA, Hong WK (1997) Retinoids in head and neck chemoprevention. Proc Soc Exp Biol Med 216(2):283–290 [DOI] [PubMed] [Google Scholar]
- Parkin DM, Pisani P, Ferlay J (1999a) Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer 80(6):827–841 [DOI] [PubMed] [Google Scholar]
- Parkin DM, Pisani P, Ferlay J (1999b) Global cancer statistics. CA Cancer J Clin 49(1):33–64 [DOI] [PubMed] [Google Scholar]
- Patt YZ, Hassan MM, Lozano RD et al (2005) Thalidomide in the treatment of patients with hepatocellular carcinoma: a phase II trial. Cancer 103(4):749–755 [DOI] [PubMed] [Google Scholar]
- Philip PA, Mahoney MR, Allmer C et al (2005) Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular cancer. J Clin Oncol 23(27):6657–6663 [DOI] [PubMed] [Google Scholar]
- Posey J, Johnson P, Mok T, Hirmand M, Dahlberg S, Kwei L, Leung T (2005) Results of a phase 2/3 open-label, randomized trial of T138067 versus doxorubicin (DOX) in chemotherapy-naive, unresectable hepatocelular carcinoma (HC). 2005 ASCO Annual Meeting, Orlando, FL, USA
- Rizvi NA, Marshall JL, Ness E et al (2002) Initial clinical trial of oral TAC-101, a novel retinoic acid receptor-alpha selective retinoid, in patients with advanced cancer. J Clin Oncol 20(16):3522–3532 [DOI] [PubMed] [Google Scholar]
- Sako T, Nakayama Y, Minagawa N et al (2005) 4-[3, 5-Bis(trimethylsily) benzamido] benzoic acid (TAC-101) induces apoptosis in colon cancer partially through the induction of Fas expression. In Vivo 19:125–132 [PubMed] [Google Scholar]
- Sankaranarayanan, Mathew RB (1996) Retinoids as cancer-preventive agents. IARC Sci Publ (139):47–59 [PubMed]
- Sano K, Takayama T, Murakami K, Saiki I, Makuuchi M (2003) Overexpression of retinoic acid receptor alpha in hepatocellular carcinoma. Clin Cancer Res 9(10 Pt 1):3679–3683 [PubMed] [Google Scholar]
- Satake K, Takagi E, Ishii A et al (2003) Anti-tumor effect of vitamin A and D on head and neck squamous cell carcinoma. Auris Nasus Larynx 30(4):403–412 [DOI] [PubMed] [Google Scholar]
- Shudo K, Kagechika H, Yamazaki N et al (2004) A synthetic retinoid Am80 (tamibarotene) rescues the memory deficit caused by scopolamine in a passive avoidance paradigm. Biol Pharm Bull 27:1887–1889 [DOI] [PubMed] [Google Scholar]
- Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10(1):1–10 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Aoki D, Oie S et al (2004) A novel retinoid, 4-[3, 5-bis (trimethylsilyl) venzamido] benzoic acid (TAC-101), induces apoptosis of human ocarian carcinoma cells and shows potential as a new antitumor agent for clear cell adneocarcinoma. Gynecol Oncol 94:643–649 [DOI] [PubMed] [Google Scholar]
- Takai K, Okuno M, Yasuda I et al (2005) Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma. Updated analysis of the long-term follow-up data. Intervirology 48(1):39–45 [DOI] [PubMed] [Google Scholar]
- Ventegodt S, Morad M, Hyam E, Merrick J (2004) Clinical holistic medicine: induction of spontaneous remission of cancer by recovery of the human character and the purpose of life (the life mission). Sci World J 4:362–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Sun GL, Lu JX, Gu LJ, Huang ME, Chen SR (1990) Treatment of acute promyelocytic leukemia with all-trans retinoic acid in China. Nouv Rev Fr Hematol 32(1):34–36 [PubMed] [Google Scholar]
- Yasuda I, Shiratori Y, Adachi S et al (2002) Acyclic retinoid induces partial differentiation, down-regulates telomerase reverse transcriptase mRNA expression and telomerase activity, and induces apoptosis in human hepatoma-derived cell lines. J Hepatol 36(5):660–671 [DOI] [PubMed] [Google Scholar]
- Yeo W, Mok TS, Zee B et al (2005) A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst 97(20):1532–1538 [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Uchida G, Okazaki M et al (2003) Differential expression of heparin-binding EGF-like growth factor (HB-EGF) mRNA in normal human keratinocytes induced by a variety of natural and synthetic retinoids. Exp Cell Res 12(S2):28–34 [DOI] [PubMed] [Google Scholar]
- Yuen MF, Wu PC, Lai VC, Lau JY, Lai CL (2001) Expression of c-Myc, c-Fos, and c-jun in hepatocellular carcinoma. Cancer 91(1):106–112 [DOI] [PubMed] [Google Scholar]
- Zhang JW, Wang JY, Chen SJ, Chen Z (2000) Mechanisms of all-trans retinoic acid-induced differentiation of acute promyelocytic leukemia cells. J Biosci 25(3):275–284 [DOI] [PubMed] [Google Scholar]
- Zhu AX, Blaszkowsky LS, Ryan DP et al (2006) Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol 24(12):1898–1903 [DOI] [PubMed] [Google Scholar]