Abstract
Purpose
CXC chemokine receptor-4 (CXCR4) is closely involved in bone metastasis of prostate cancer, and CXCR4 levels are frequently increased in prostate cancer cells and tissues. In the present study, its biological effects on prostate cancer in vitro and in vivo and feasibility to be a therapy target were investigated using a RNA interfering retrovirus vector targeting CXCR4 gene driven by human prostate-specific antigen promoter (pPSA).
Methods
We established a pPSA-siCXCR4 retrovirus vector and transfected prostate cancer cell PC-3m, LNCaP and breast cancer cell MCF-7, respectively. The expression of CXCR4 mRNA and protein was detected by RT-PCR and western blot, and the ability of adhesion, migration, invasion of prostate cancer cells was assessed using Transwell chamber. A metastasizing model using BALB/cA mice with human bone tissue implantation was established too, and transfected prostate cancer cells were via caudal vein. Survival time of mice suffering bone metastatic tumor as well as the weight and volume of these tumors were recorded and analyzed.
Results
The expression of CXCR4 mRNA and protein in androgen-responsive LNCaP cells was blocked by the pPSA-siCXCR4 vector, but it could not work in non androgen-responsive PC-3m cell and breast cancer cell MCF-7. The results of experiments in vitro also showed that the adhesion, transendothelial migration and invasive ability of transfected LNCaP cells were impaired, while there was no change in PC-3m and MCF-7 cells after transfection. pPSA-siCXCR4 represented a similar inhibitory effect in fluorescent bone metastasis model of LNCaP cells compared with PC-3m cells.
Conclusion
These results suggest that the downstream siRNA controlled by PSA promoter in retrovirus system can express selectively in androgen-responsive prostate cancer in vitro and in vivo, and CXCR4 plays an important role in prostate cancer metastasis. We believe that the pPSA-siCXCR4 retrovirus vector is a potential choice in gene therapy for androgen-responsive prostate cancer.
Keywords: RNA interference, CXCR4, PSA, Prostate cancer, Metastasis
Introduction
Recently, there is an increase in the morbidity of prostate cancer in China, which has ranked second among the morbidity of urologic malignant neoplasms. One of the main causes of death in patients with advanced prostate cancer is pelvic lymph nodes and bone metastasis. It is therefore very important to elucidate the molecular mechanisms underlying prostate cancer metastasis to bones and/or regional lymph nodes and explore pathways preventing such metastasis.
CXC chemokine receptor-4 (CXCR4) is a group of seven-transmembrane, G protein-coupled receptors, which is found to be expressed in many human cancer cells. Stromal-cell-derived factor 1 (SDF-1), the CXCR4 ligand, is expressed by bone stromal cells and microvessel endothelial cell, whose chemotactic and growth-promoting effects are mediated by CXCR4 (Bleul et al. 1996; Ma et al. 1998). Since SDF-1/CXCR4 axis plays a critical role in the process that hematopoietic stem cells home in bone marrow specifically and efficiently (Kollet et al. 2002; Jo et al. 2000; Peled et al. 1999), it is assumed that SDF-1/CXCR4 ligand–receptor system was involved in bone metastasis in the similar way, and intensive investigations have proved this assumption (Payne and Cornelius 2002; Guleng et al. 2005; McGrath et al. 1999; Wang et al. 2006). It has been reported that CXCR4 is highly expressed in many types of cancer such as breast, ovarian, pancreatic, lung and prostate, and the highest SDF-1 expression is detected in those organs that are usually the primary destinations of these cancers metastasis, such as bone marrow, lymph nodes (Mochizuki et al. 2004; Taichman et al. 2002; Muller et al. 2001; Lee et al. 2004; Salvucci et al. 2006; Cabioglu et al. 2005; Scotton et al. 2002; Koshiba et al. 2000; Kijima et al. 2002). And in vivo and in vitro experiments have showed that blockade of the CXCR4/SDF-1 signaling pathway with anti-CXCR4 antibody decreased transendothelial cancer cell migration as well as vascular permeability (Lee et al. 2004; Ottaiano et al. 2005). In prostate cancer cells, high levels of CXCR4 are linked to aggressive phenotypes and positive expression of CXCR4 protein was an independent and superior predictor for bone metastasis (Mochizuki et al. 2004; Taichman et al. 2002). Therefore SDF-1/CXCR4 is considered a promising target for prostate cancer gene therapy.
The success of gene therapy using a RNA interference (RNAi) approach relies on small interfering RNA (siRNA) expression from a highly tissue-specific RNA polymerase II promoter. Song et al. (2004) developed such a prostate-specific vector driven by the human prostate-specific antigen promoter (pPSA), which could be a potential tool for tissue-specific gene therapy. In the present study, we attempted to design and construct a CXCR4-siRNA retrovirus vector driven by PSA promoter, and evaluate its effect on prostate cancer cells PC-3m and LNCaP in vitro and in vivo. Based on the experimental data, we discussed the efficiency of this vector and the feasibility of clinical application.
Materials and methods
CXCR4-siRNA and plasmid preparation
siRNA design and construction of plasmid Pgensil-1-siCXCR4 and PLXSN-G1 were represented in our previous study (Du et al. 2007). PSA promoter was amplified from pDRIVE-pPSA plasmid (Invivogen) with primers carrying BamHI and EcoRI restriction sites (Song et al. 2004). Both PSA promoter and Pgensil-1-siCXCR4 were digested with BamHI and EcoRI, and then linked together to generate Pgensil-1-pPSA-siCXCR4. PLXSN-G1, Pgenesil-1-siCXCR4 and Pgenesil-1-pPSA-siCXCR4 were digested with MluI and EcoRI, and then PLXSN-G1 was linked to Pgenesil-1-siCXCR4 and Pgenesil-1-pPSA-siCXCR4, respectively. The two recombinant products were called PLXSN/EGFP-U6-siCXCR4 and PLXSN/EGFP-pPSA-siCXCR4.
ERFP DsReD2 gene was amplified from plasmid DsReD2 with primers DsReD2-f (5′-AAGGAAAAAAGCGGCCGCGCTACCGGTCGCCAC CATG-3′) and DsReD2-r (5′-GAAGATCTTTCACAGGAACAGGTGGTGGCGGC-3′). This product and PLXSN/EGFP-pPSA-siCXCR4 were both digested with NotI and BglII, and then linked together to generate PLXSN/ DsReD2-pPSA-siCXCR4.
The sequence CGCATGC was used as negative control (HK), and plasmids PLXSN/EGFP-pPSA-siHK and PLXSN/DsReD2-pPSA-siHK were constructed in the same way. All the recombinant plasmids mentioned above were identified by restriction enzymes.
Vector package and transfection
Plasmids PLXSN/EGFP-pPSA-siCXCR4, PLXSN/EGFP-pPSA-siHK, LXSN/DsReD2-pPSA-siCXCR4 and LXSN/ DsReD2-pPSA-siHK were used to transfect PA317 cells with Lipofectamine™ 2000 according to the manufacture’s protocol (Invivogen) and resistant clones were selected by G418. The virus titer was measured using NIH3T3. Prostate cancer cells PC-3m, LNCaP and breast cancer cell MCF-7 were transfected with the virus supernatant of PLXSN/EGFP-pPSA-siCXCR4 and PLXSN/EGFP-pPSA-siHK respectively (MOI 100), while the virus supernatant of LXSN/DsReD2-pPSA-siCXCR4 and LXSN/DsReD2-pPSA-siHK (MOI 100) were used to transfect PC-3m and LNCaP, respectively for experiment in vivo.
Semi-quantitative RT-PCR analysis
Total RNA was extracted from each cancer cell line and reverse-transcribed into cDNA using RNA extraction and MMLV RTase cDNA synthesis Kit (Invivogen). The cDNA was then used to amplify the CXCR4 fragments. Primer sequences for CXCR4 and β-actin and PCR amplification conditions were mentioned in our previous paper (Du et al. 2007). PCR products were run on a 2% agarose gel and scanned with Gel Doc 1000 (Bio-Rad). Graphical analysis was performed with LEICAQ550IW (Germany). Scores of integral absorbance (IA) of each band were calculated, using the formula IA = average absorbance × area. CXCR4 mRNA quantity of each sample was represented in the form of CXCR4 IA/β-actin IA.
Western blotting
PC-3m, LNCaP and MCF7 cells were washed with PBS and lysed in an ice-cold lysis buffer (RIPA-PICT, Roche). Purified lysate (50 µg) of each sample was electrophoresed on 12% SDS-PAGE, transferred to nitrocellulose filters. The filters were blocked overnight at 4°C in TBST containing 8% milk and then incubated for 1 h with 1:2,000 diluted rabbit anti-CXCR4 antibody and 1:2,000 diluted mouse anti-β-actin antibody (Sigma). Goat anti-rabbit or mouse IgG coupled to HRP (1:10,000 or 1:2,000, Sigma) were used as secondary antibodies and the bands were visualized by ECL. Graphical analysis was performed in the same way of semi-quantitative RT-PCR.
In vitro adhesion and transendothelial migration assay
PLXSN/EGFP-pPSA-siCXCR4 or PLXSN/EGFP-pPSA-siHK transfected cells PC-3m and LNCaP were stained with fluorescent dye BCECF-AM (Tojindo). A total of 200 μl cells of each group (1 × 105) were mixed with 100 μl SDF-1 solution with the concentration of 0, 0.2, 20, 200 ng/ml respectively and 100 μl SDF-1 solution (200 ng/ml) which had been boiled for 30 min as well. These cells were incubated 30 min at 37°C, laid on the monolayer formed by human bone marrow endothelial cells (HBMEC) and incubated another 30 min at 37°C. The number of cells adhering to HBMEC monolayer was counted under inverted fluorescent microscope.
For the transendothelial migration assay, HBMEC were put into 12 μm pore size Transwell chamber (GIBCO) to form monolayer. A total of 100 µl prostate cancer cell suspension of each group (5 × 104) was added into the top layer of the chamber, and SDF-1 was added in three different ways: 100 µl 200 ng/ml into both top and bottom layer, 100 µl 200 ng/ml into the top layer, 100 µl 200 ng/ml into the bottom layer, nothing added as blank control. After 48 h, the number of cells in the bottom layer was counted under inverted fluorescent microscope.
In vitro invasion assay
Invasion of prostate cancer and breast cancer cells was assayed in 8-μm pore size Transwell chamber (GIBCO). The bottom of Transwell chamber was coated with 1:8 diluted Matrigel (final concentration 50 mg/l, GIBCO) and air-dried at 4°C. After each well of culture plates 50 µl serum-free medium supplemented with BSA (10 g/l) was added and incubated at 37°C for 30 min, Transwell chambers were put into 24-well culture plates. A total of 400 µl mixture of the supernatant obtained from NIH3T3 cell cultures in serum-free RPMI 1640 and the complete medium (1:1) were added outside chambers, while 50 µl cancer cell suspension containing 2 × 104 cells and RPMI 1640 supplemented with BSA (10 g/l) and fetal bovine serum (1%) were added inside chambers. These chambers were divided into three groups: Group 1, PLXSN/EGFP-pPSA-siCXCR4 transfected prostate cancer cells PC-3m, LNCaP and breast cancer cell MCF-7; Group 2, untreated PC-3m, LNCaP and MCF7 cells added with rabbit anti-CXCR4 antibody (25 µg/l) and kept at 37°C for 30 min; Group 3, PC-3m, LNCaP and MCF7 cells transfected with PLXSN/EGFP-pPSA-siHK. After cultured for 48 h, Transwell chambers were taken out, washed with PBS, fixed in formaldehyde and stained with trypanblue (4 g/l). The number of cells penetrating membrane in every five fields was counted under the inverted microscope and each group was performed six times.
In vivo metastasis assay
Human bone tissues (4 × 3 × 2 mm) were implanted under left and right costal margins of BALB/cA nude mice referred to Yang et al. (2002). After 3 weeks, survived mice were injected using 200 µl transfected prostate cancer cell suspension (1 × 107) through caudal vein. These nude mice were divided into two groups. In one group, PLXSN/DsReD2-pPSA-siCXCR4 transfected LNCaP cells were injected into 24 nude mice and PLXSN/DsReD2-pPSA-siHK transfected LNCaP cells into another 24 nude mice as control. In the other group, PLXSN/DsReD2-pPSA-siCXCR4 and PLXSN/DsReD2-pPSA-siHK transfected PC-3m cells were used.
Location and intensity of RFP fluorescent were observed by a Leica fluorescent stereomicroscope on the 21st, 28th and 35th day after injection. Survival time of each mouse was recorded and autopsy was preformed at once. Weight and volume of bone metastatic tumor of prostate cancer from each mouse were recorded. The rate of tumor inhibition (IR) was calculated using the formula IR = (average tumor weight of control group − average tumor weight of experimental group)/average tumor weight of control group × 100%.
Statistical analysis
SPSS12.0 statistical software was used to analyze the data obtained. The data were represented in the form of  and analysis of variance was applied. P
< 0.05 was taken to demonstrate a statistically significant difference between means.
and analysis of variance was applied. P
< 0.05 was taken to demonstrate a statistically significant difference between means.
Results
Fluorescent detection
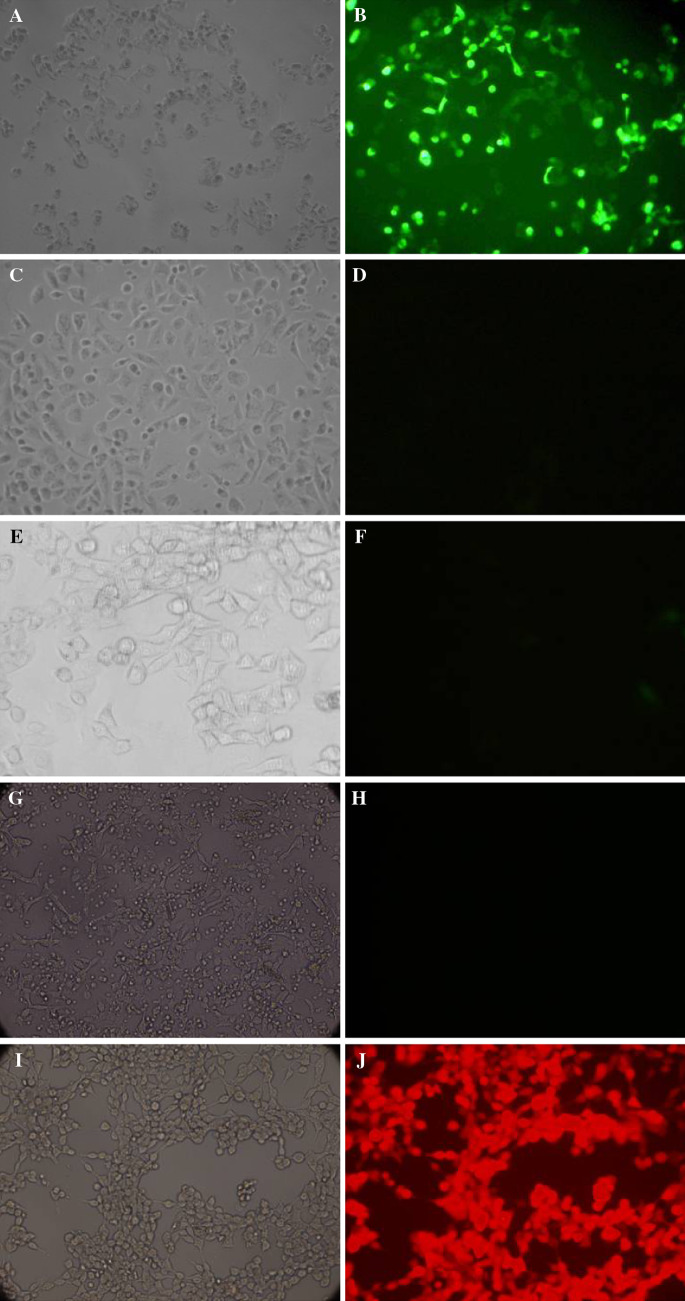
Since PSA promoter was androgen-responsive, GFP and RFP fluorescent were observed in prostate cancer cell LNCaP 1–2 days after transfection and gradually increased, while no fluorescent was detected in prostate cancer cell PC-3m and breast cancer cell MCF7 (Fig. 1).
Fig. 1.
a, c, e, g, i showed the view under light microscope of LNCaP + PLXSN/EGFP-pPSA-siCXCR4, PC-3m + PLXSN/EGFP-pPSA-siCXCR4, MCF-7 + PLXSN/EGFP-pPSA-siCXCR4, PC-3m + LXSN/DsReD2-pPSA-siCXCR4 and LNCaP + LXSN/DsReD2-pPSA-siCXCR4. respectively on the eighth day after transfection. b, d, f, h, j showed the corresponding view under fluorescent microscope. Since LNCaP is androgen-responsive, PSA promoter initiated EGFP and DsReD2 expression. However, in non androgen-responsive cells PC-3m and MCF-7, neither EGFP nor DsReD2 expression was observed after transfected with PLXSN/EGFP-pPSA-siCXCR4 or LXSN/DsReD2-pPSA-siCXCR4
CXCR4 mRNA expression after transfection
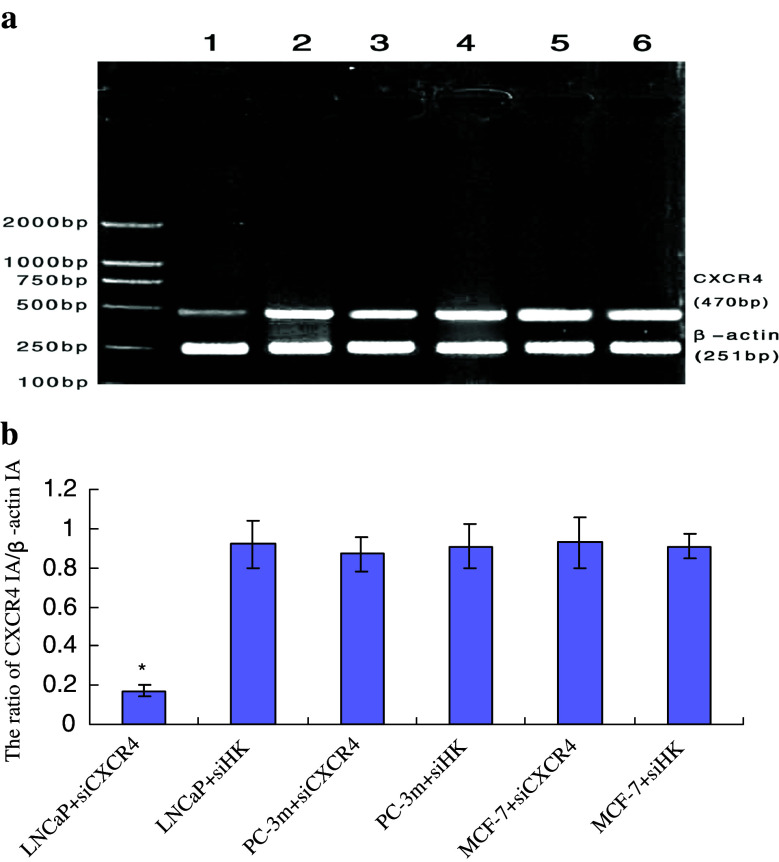
CXCR4 mRNA expression was evaluated by hemi-quantitative RT-PCR. The CXCR4 IA/β-actin IA ratio of each experimental and control group of LNCaP, PC-3m, MCF-7 was 0.17 ± 0.03, 0.92 ± 0.12, 0.87 ± 0.09, 0.91 ± 0.11, 0.93 ± 0.13 and 0.91 ± 0.06, respectively. The average inhibitory rate of siRNA on CXCR4 mRNA for 48 h was (81.53 ± 10.22%). As to androgen-responsive LNCaP cells, CXCR4 expression was greatly depressed in experimental group and this change was statistically significant compared with control (P < 0.05). However, in experimental groups of PC-3m and MCF-7 cells, the change of CXCR4 mRNA expression was not statistically significant compared with control (P > 0.05), which suggested that pPSA-siCXCR4 could not inhibit CXCR4 mRNA expression in these non androgen-responsive cells (Fig. 2).
Fig. 2.
Effect of pPSA-siCXCR4 on CXCR4 mRNA expression in different cells. M marker; 1 LNCaP + PLXSN/EGFP-pPSA-siCXCR4 (siCXCR4); 2 LNCaP + PLXSN/EGFP-pPSA-siCXCR4(siHK); 3 PC-3m + siCXCR4; 4 PC-3m + siHK; 5 MCF-7 + siCXCR4; 6 MCF-7 + siHK. a Agarose electrophoresis of CXCR4 and β-actin amplified from each group, CXCR4 band of LNCaP + siCXCR4 was dimmer than any other band. b The ratio of CXCR4 IA/β-actin IA of six groups were represented in the form of 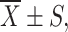 and *
P < 0.05
and *
P < 0.05
CXCR4 protein expression after transfection
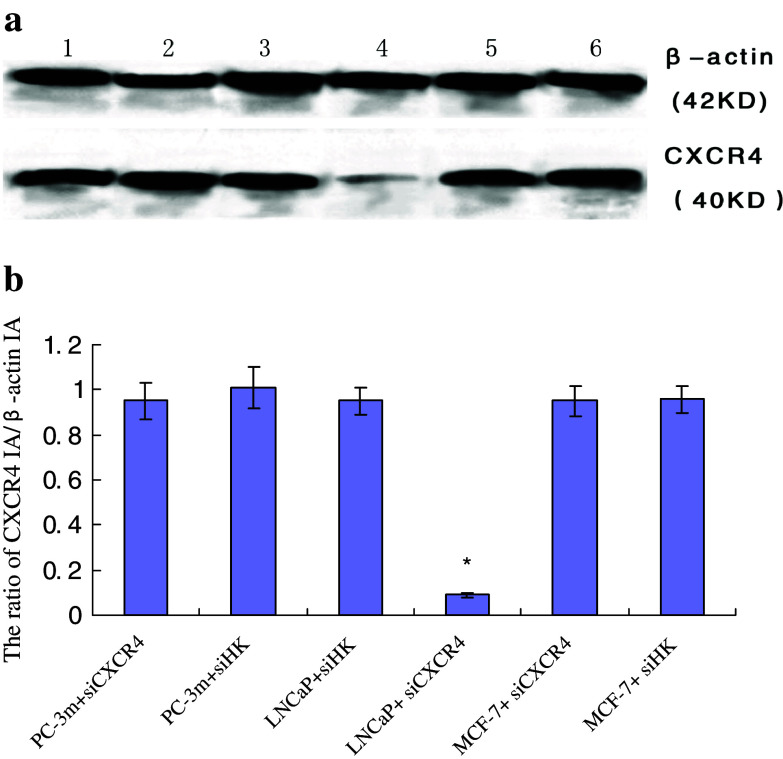
The CXCR4 IA/β-actin IA ratio of each experimental and control group of PC-3m, LNCaP MCF-7was 0.95 ± 0.08, 1.01 ± 0.09, 0.95 ± 0.06, 0.09 ± 0.01, 0.95 ± 0.07 and 0.96 ± 0.06, respectively. The average inhibitory rate of siRNA on CXCR4 mRNA for 48 h was (90.52 ± 9.31%). CXCR4 protein expression was greatly depressed in LNCaP experimental group and this change was statistically significant compared with control (P < 0.05), while in PC-3m and MCF-7 experimental groups, the change of CXCR4 protein expression was not statistically significant compared with control (P > 0.05), and this demonstrated that pPSA-siCXCR4 could not inhibit CXCR4 protein expression in these non androgen-responsive cells as well (Fig. 3).
Fig. 3.
Effect of pPSA-siCXCR4 on CXCR4 protein expression in different cells. 1 PC-3m + siCXCR4; 2 PC-3m + siHK; 3 LNCaP + siHK; 4 LNCaP + siCXCR4; 5 MCF-7 + siCXCR4; 6 MCF-7 + siHK. a Western blot analysis of CXCR4 and β-actin extracted from each group, CXCR4 band of LNCaP + siCXCR4 was dimmer than any other band. b The ratio of CXCR4 IA/β-actin IA of six groups were represented in the form of 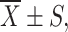 and *
P < 0.05
and *
P < 0.05
Effect of pPSA-siCXCR4 on adhesion and transendothelial migration ability of prostate cancer cells
The number of transfected prostate cancer cells adhering to HBMEC monolayer and migrating into the bottom layer of Transwell chamber was represented in Tables 1 and 2, respectively. In LNCaP group, changes of adhesion and transendothelial migration ability in experimental group was statistically significant compared with control (P < 0.05), while there was no difference between experimental and control group in PC-3m group (P > 0.05).
Table 1.
The number of transfected prostate cancer cells adhering to HBMEC monolayer under the treatment of SDF-1 with different consistency 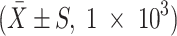
| Group | SDF-1 (ng/ml) | |||||
|---|---|---|---|---|---|---|
| 0 | 0.2 | 2 | 20 | 200 | 200 + boiled | |
| LNCaP + siHK | 1.14 ± 0.01 | 3.33 ± 0.07 | 4.50 ± 0.08 | 4.70 ± 0.17 | 5.93 ± 0.14 | 1.01 ± 0.12 |
| LNCaP + siCXCR4 | 0.27 ± 0.03* | 1.10 ± 0.02* | 1.98 ± 0.03* | 2.24 ± 0.02* | 2.39 ± 0.10* | 0.70 ± 0.27* |
| PC-3m + siHK | 7.64 ± 1.03 | 8.69 ± 0.99 | 11.99 ± 1.77 | 13.77 ± 1.19 | 15.04 ± 1.03 | 7.41 ± 0.60 |
| PC-3m + siCXCR4 | 6.92 ± 0.37 | 8.57 ± 0.94 | 11.25 ± 1.20 | 14.05 ± 0.95 | 15.21 ± 1.03 | 6.97 ± 0.56 |
* P < 0.05 versus LNCaP + siHK group
Table 2.
The number of transfected prostate cancer cells migrating into the bottom layer of Transwell chamber under the treatment of SDF-1 with different consistency 
| Top layer | + | + | − | − |
|---|---|---|---|---|
| Bottom layer | + | − | + | − |
| LNCaP + siHK | 80.71 ± 7.90 | 19.14 ± 2.33 | 176.38 ± 15.67 | 69.28 ± 5.47 |
| LNCaP + siCXCR4 | 51.55 ± 5.87* | 3.07 ± 0.12* | 79.47 ± 6.20* | 30.47 ± 3.04* |
| PC-3m + siHK | 142.40 ± 11.45 | 97.36 ± 11.45 | 322.37 ± 17.46 | 180.72 ± 15.68 |
| PC-3m + siCXCR4 | 127.20 ± 14.82 | 89.91 ± 10.99 | 337.25 ± 12.35 | 156.50 ± 7.50 |
* P < 0.05 versus LNCaP + siHK group
+, 100 µl 200 ng/ml SDF-1 was added; −, nothing was added
Effect of pPSA-siCXCR4 on invasion ability of different cancer cells
For androgen-responsive prostate cancer cell LNCaP, the number of cells penetrating membrane in Group 1 was much less than that of Group 3 and the difference was statistically significant (P < 0.05); but compared with Group 2, there was no statistical difference (P > 0.05). And in PC-3m and MCF-7 cells that are not hormones-responsive, the number of cells penetrating membrane among Groups 1,2 and 3 was not statistically significant (P > 0.05) (Table 3).
Table 3.
Effect of pPSA-siCXCR4 on invasive ability of LNCaP, PC-3m and MCF-7 cells in vitro 
| Group | LNCaP cell | PC-3m cell | MCF-7 cell |
|---|---|---|---|
| siCXCR4 | 139.93 ± 14.25* | 294.74 ± 11.37 | 176.34 ± 12.79 |
| CXCR4 antibody | 152.17 ± 23.44** | 136.30 ± 19.16* | 93.28 ± 14.46* |
| siHK | 348.37 ± 36.40 | 286.90 ± 17.63 | 191.25 ± 9.67 |
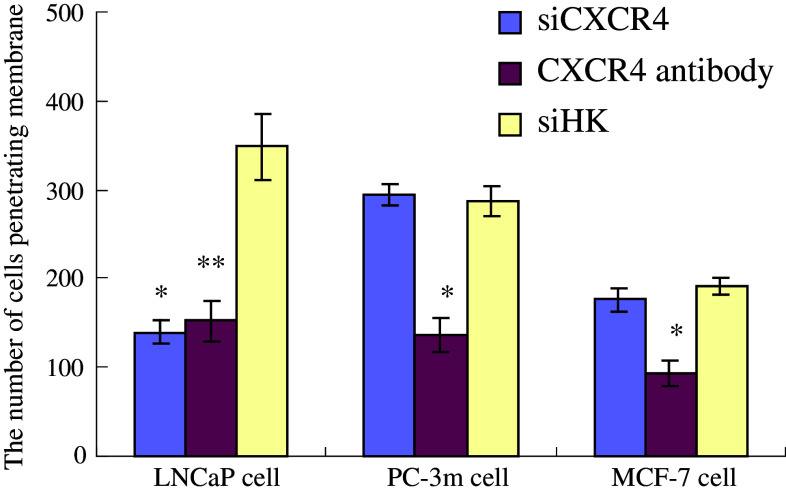 In androgen-responsive prostate cancer cell LNCaP, pPSA-siCXCR4 inhibited cancer cells migration nearly as well as CXCR4 antibody did. But in non androgen-responsive cells PC-3m and MCF-7, only CXCR4 antibody inhibited their migration, which suggested that pPSA-siCXCR4 was highly tissue-specificLNCaP
*
P<0.05 versus siHK group, **
P<0.05 versus siHK group; PC-3m
*
P<0.05 versus siHK group; MCF-7
*
P<0.05 versus siHK group
In androgen-responsive prostate cancer cell LNCaP, pPSA-siCXCR4 inhibited cancer cells migration nearly as well as CXCR4 antibody did. But in non androgen-responsive cells PC-3m and MCF-7, only CXCR4 antibody inhibited their migration, which suggested that pPSA-siCXCR4 was highly tissue-specificLNCaP
*
P<0.05 versus siHK group, **
P<0.05 versus siHK group; PC-3m
*
P<0.05 versus siHK group; MCF-7
*
P<0.05 versus siHK group
Inhibitory effect of siCXCR4 in vivo
It usually took 2 weeks for BALB/cA nude mice to recover after human bone tissue implantation. When the injection of prostate cancer cell suspension was performed, these nude mice became inactive gradually and lost weight. Small lumps could be touched at the implanted location 3 weeks later, and lumps kept growing. From the fourth week after injection, mice died successively. Using the fluorescent stereomicroscope, RFP fluorescent was observed in bone metastatic tumor of prostate cancer and the intensity was strengthened along with the process of tumor enlargement (Fig. 4).
Fig. 4.
a Shows the macrograph of bone metastatic tumor of prostate cancer, b was the corresponding view under fluorescent stereomicroscope, and RFP fluorescent was detected. c, d, e Shows fluorescent images of orthotopic bone metastatic tumor on the second, third, and fourth week after injection, respectively. The intensity of RFP fluorescent was strengthened and volume of tumor enlarged when time went by
The number of nude mice with bone metastatic tumor in each group was shown in Table 4. In PC-3m group, there was no statistical difference between experimental and control group (P > 0.05), while in the LNCaP group, the difference between experimental and control group was statistical (P < 0.05). When every nude mouse died of bone metastatic tumor of prostate cancer, survival time was recorded, tumor was resected immediately to measure weight and volume, and IR was calculated. All the data were represented in Table 5. There was no statistical significance between PC-3m experimental and control group on these parameters mentioned above (P > 0.05), but in LNCaP group, the difference between experimental and control group was significant (P < 0.05), and IR of LNCaP group was higher than that of PC-3m group (P < 0.05). These experimental data demonstrated that pPSA-siCXCR4 could work in vivo as well as in vitro.
Table 4.
The number of mice with bone metastatic tumor in each group
| Group | Number of mice with bone metastatic tumor | |
|---|---|---|
| + | − | |
| PC-3m + DsReD2-siCXCR4 | 10 | 14 |
| PC-3m + DsReD2-siHK | 9 | 15 |
| LNCaP + DsReD2-siCXCR4 | 3 | 21 |
| LNCaP + DsReD2-siHK | 9 | 15 |
DsReD2-siCXCR4: PLXSN/DsReD2-pPSA-siCXCR4, DsReD2-siHK: PLXSN/DsReD2-pPSA-siHK. PC-3m: P > 0.05 versus DsReD2-siHK group, LNCaP: P < 0.05 versus DsReD2-siHK group
Table 5.
Progression of bone metastatic tumor of prostate cancer in different BALB/cA nude mice group 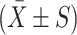
| Group | ST (days) | V (cm3) | W (mg) | IR (%) |
|---|---|---|---|---|
| PC-3m + DsRed2-siiCXCR4 | 30.5 ± 0.7 | 3.54 ± 0.83 | 19 ± 2.1 | 34.5 |
| PC-3m + DsRed2-siHK | 28.2 ± 0.3 | 3.21 ± 0.49 | 20 ± 2.6 | – |
| LNCaP + DsRed2-siiCXCR4 | 41.4 ± 0.4* | 1.73 ± 0.52* | 14 ± 0.7* | 36.3▲ |
| LNCaP + DsRed2-siHK | 32.0 ± 0.6 | 3.13 ± 0.37 | 22 ± 1.8 | – |
ST Survival time, V volume, W weight. *P < 0.05, LNCaP + DsRed2-siiCXCR4 versus LNCaP + DsRed2-siHK, ▲ P < 0.05, LNCaP + DsRed2-siiCXCR4 versus PC-3m + DsRed2-siiCXCR4
Discussion
In the previous study, we constructed a CXCR4-siRNA retrovirus vector which significantly inhibited migration of prostate cancer cells PC-3m and LNCaP (Du et al. 2007), and this time we rebuilt it with a tissue-specific and hormone-dependent promoter, PSA promoter. Results showed that CXCR4 mRNA and protein expression of androgen-responsive prostate cancer cell LNCaP were depressed by this vector, and experiments in vitro demonstrated that adhesion, transendothelial migration and invasion ability of transfected LNCaP cells were greatly impaired as the anti-CXCR4 antibody did. Besides, experiment in vivo showed a similar inhibitory effect of pPSA-siCXCR4 in fluorescent bone metastasis model of LNCaP cells as well. However, a high level of CXCR4 expression and unchanged adhesion, transendothelial migration, invasion and bone metastasis activity were still detected in non androgen-responsive prostate cancer cell PC-3m and breast cancer cell MCF-7 transfected with pPSA-siCXCR4 and the bone metastasis model of PC-3m, which confirmed that this rebuilt vector can inhibit CXCR4 expression in androgen-responsive prostate cancer cell efficiently, stably and tissue-specifically, and decrease the probability of bone metastasis in vitro and in vivo. We believed that our research laid the groundwork for RNAi technology application in clinical therapy of bone metastasis of prostate cancer.
CXCR4 expression has been found to be involved in the malignant progression of tumors (Geminder et al. 2001; Murphy 2001). Higher CXCR4 expression in breast cancer cells was correlated with higher cell motility and metastasis potentiality and down-regulation of CXCR4 inhibited metastasis (Muller et al. 2001; Chen et al. 2003). SDF-1 is the only known ligand for CXCR4 and preferentially expressed in lymph nodes and bone marrow, which are also the most common sites of prostate cancer metastasis. Recent studies (Mochizuki et al. 2004; Wang et al. 2005) proved that prostate cancer cells metastasize to specific sites via cell migration mediated by an interaction between CXCR4 and SDF-1. Furthermore, adhesion-promoting effect of SDF-1 mediated by integrin helps prostate cancer cells adhere to metastasis sites (Taichman et al. 2002), and SDF-1 may also enable cancer cells to thrive and proliferation at metastasis sites by stimulating synergistically with other growth factors (Gupta and Pillarisetti 1999). In all, it is believed that CXCR4/SDF-1 pathway not only mediates tumor metastasis, but also plays a critical role in genesis, development, proliferation and differentiation of cancer cells, which makes CXCR4/SDF-1 axis a new target for cancer gene therapy (Perissinotto et al. 2005; Lee et al. 2005).
RNAi has emerged as one of the most important discoveries of the last years in the fields of molecular biology. RNAi can be induced using virus vectors encoding siRNA allowing a knockdown of specific gene and therefore is a powerful therapeutic agent for cancer. However, off-target, non-specific effects and the relative instability limit RNAi to apply in vivo (Masiero et al. 2007). Particularly, a lack of target specificity of virus vectors would lower the curative effect and cause serious side effects. PSA is a well-characterized biomarker for diagnosis of prostate cancer, whose promoter is androgen-responsive and tissue-specific (Pang et al. 1997; Louie et al. 2003). High-level of androgen introduces the expression of PSA by an enhancer centered at about 4.2 kb on the PSA gene, and RNA polymerase II is directly recruited to the enhancer in an androgen-dependent manner (Louie et al. 2003). Thus PSA promoter is an ideal regulatory element for cancer gene therapy based on tissue-specific siRNA. Song et al (2004) first developed a siRNA expressed from either a vector- or lentiviral-based system using the PSA promoter, which not only specifically reduced the expression of target genes in cells, but also acted in a tissue-specific and hormone-dependent manner. On the basis of their findings, we successfully constructed a pPSA-siCXCR4 retrovirus vector by replacing U6 promoter with human PSA promoter, and experimental data showed that it worked just as we had expected.
To date, the most popular in vivo metastatic animal models for prostate cancer are made by orthotopic inoculation of cancer cells or tissues (Yang et al. 2002; Sato et al. 1997; Yang et al. 1999; Rembrink et al. 1997). In this report, we describe a novel metastasizing model using BALB/cA mice after human bone tissue implantation and injection of prostate cancer cells via caudal vein. Metastatic tumor occurred in implanted bones after PC-3m or LNCaP was used, while there was no evidence that skeleton and other organs of mice were involved in tumor metastasis. The bone is the most common metastatic site for prostate cancer clinically, but in those orthotopic metastasis nude mice models, bone metastasis of human prostate cancer was not so frequent (Sato et al. 1997; Rembrink et al. 1997), which suggested that the microenvironment of murine bone was not fit for the growth of human prostate cancer cells. Therefore the metastatic process in our model is much closer to the natural process of bone metastasis of prostate cancer in human body, and would be more suitable for such researches. In addition, we used not GFP but RFP DsRed2 to construct plasmids for the experiment in vivo, because in the previous attempts, we found that the fluorescent intensity of GFP would be reduced due to the interference of other sequences in the retrovirus vector, while DsRed2 emitted ideal fluorescence with the enhancement of Kozak sequence under the fluorescent stereomicroscope.
In summary, our research strongly demonstrate that this CXCR4-siRNA retrovirus vector driven by PSA promoter is efficient, stable and tissue-specific, which enable it to be a promising therapeutic approach to inhibit bone metastasis of androgen-responsive prostate cancer by blocking CXCR4/SDF-1 axis. And the bone metastatic BALB/cA mice model for prostate cancer described here affords a unique approach to study the mechanism and develop therapy of prostate cancer.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s00432-008-0489-9
References
- Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J (1996) The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382:829–833 [DOI] [PubMed] [Google Scholar]
- Cabioglu N, Sahin A, Doucet M, Yavuz E, Igci A, OYildirim E, Aktas E, Bilgic S, Kiran B, Deniz G, Price JE (2005) Chemokine receptor CXCR4 expression in breast cancer as a potential predictive marker of isolated tumor cells in bone marrow. Clin Exp Metastasis 22:39–46 [DOI] [PubMed] [Google Scholar]
- Chen Y, Stamatoyannopoulos G, Song CZ (2003) Down-regulation of CXCR4 by inducible small interfering RNA inhibits breast cancer cell invasion in vitro. Cancer Res 63:4801–4804 [PubMed] [Google Scholar]
- Du YF, Shi Y, Xing YF, Xiao YJ (2007) Retrovirus-mediated CXCR4-siRNA can inhibit the invasion of prostate carcinoma in vitro. Chin J Cancer Res 19:82–88 [Google Scholar]
- Geminder H, Sagi-Assif O, Goldberg L, Meshel T, Rechavi G, Witz IP (2001) A possible role for CXCR4 and its ligand, the CXC chemokine Stromal cell-derived factor-1, in the development of bone marrow metastasis in neuroblastoma. J Immunol 167:4747–4757 [DOI] [PubMed] [Google Scholar]
- Guleng B, Tateishi K, Ohta M, Kanai F, Jazag A, Ijichi H, Tanaka Y, Washida M, Morikane K, Fukushima Y, Yamori T, Tsuruo T, Kawabe T, Miyagishi M, Taira K, Sata M, Omata M (2005) Blockade of the stromal cell-derived factor-1/CXCR4 axis attenuates in vivo tumor growth by inhibiting angiogenesis in a vascular endothelial growth factor-independent manner. Cancer Res 65:5864–5871 [DOI] [PubMed] [Google Scholar]
- Gupta SK, Pillarisetti K (1999) Cutting edge: CXCR4-Lo: molecular cloning and functional expression of a novel human CXCR4 splice variant. J Immunol 163:2368–2372 [PubMed] [Google Scholar]
- Jo DY, Rafii S, Hamada T, Moore MA (2000) Chemotaxis of primitive hematopoietic cells in response to stromal cell-derived factor-1. J Clin Invest 105:101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijima T, Maulik G, Ma PC, Tibaldi EV, Turner RE, Rollins B, Sattler M, Johnson BE, Salgia R (2002) Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res 62:6304–6311 [PubMed] [Google Scholar]
- Kollet O, Petit I, Kahn J, Samira S, Dar A, Peled A, Deutsch V, Gunetti M, Piacibello W, Nagler A, Lapidot T (2002) Human CD34 (+) CXCR4 (+) sorted cells harbor intracellular CXCR4, which can be functionally expressed and provide NOD/SCID repopulation. Blood 100:2778–2786 [DOI] [PubMed] [Google Scholar]
- Koshiba T, Hosotani R, Miyamoto Y, Ida J, Tsuji S, Nakajima S, Kawaguchi M, Kobayashi H, Doi R, Hori T, Fujii N, Imamura M (2000) Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res 6:3530–3535 [PubMed] [Google Scholar]
- Lee BC, Lee TH, Avraham S, Avraham HK (2004) Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1α in breast cancer cell migration through human brain microvascular endothelial cells. Mol Cancer Res 2:327–228 [PubMed] [Google Scholar]
- Lee BC, Lee TH, Zagozdzon R, Avraham S, Usheva A, Avraham HK (2005) Carboxyl-teminal Src kinase homologous kinase negatively regulates the chemokine receptor CXCR4 through YY1 and impairs CXCR4/CXCL12 (SDF-1alpha)-mediated breast cancer cell migration. Cancer Res 65:2840–2845 [DOI] [PubMed] [Google Scholar]
- Louie MC, Yang HQ, Ma AH, Xu W, Zou JX, Kung HJ, Chen HW (2003) Androgen-induced recruitment of RNA polymerase II to a nuclear receptor-p160 coactivator complex. Proc Natl Acad Sci USA 100:2226–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA (1998) Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA 95:9448–9453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiero M, Nardo G, Indraccolo S, Favaro E (2007) RNA interference: implications for cancer treatment. Mol Aspects Med 28:143–166 [DOI] [PubMed] [Google Scholar]
- McGrath KE, Koniski AD, Maltby KM, Mcgann JK, Palis J (1999) Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol 213:442–456 [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Matsubara A, Teishima J, Mutaquchi K, Yasumoto H, Dahiya R, Usui T, Kamiya K (2004) Interaction of ligand-receptor system between stromal-cell-derived factor-1 and CXC chemokine receptor 4 in human prostate cancer: a possible predictor of metastasis. Biochem Biophys Res Commun 320:656–663 [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wanger SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410:50–56 [DOI] [PubMed] [Google Scholar]
- Murphy PM (2001) Chemokines and the molecular basis of cancer metastasis. N Engl J Med 345:833–835 [DOI] [PubMed] [Google Scholar]
- Ottaiano A, di Palma A, Napolitano M, Pisano C, Pignata S, Tatangelo F, Botti G, Acquaviva AM, Castello G, Ascierto PA, Iaffaioli RV, Scala S (2005) Inhibitory effects of anti-CXCR4 antibodies on human colon cancer cells. Cancer Immunol Immunother 54:781–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S, Dannull J, Kaboo R, Xie Y, Tso CL, Michel K, de Kernion JB, Belldegrun AS (1997) Identification of a positive regulatory element responsible for tissue-specific expression of prostate-specific antigen. Cancer Res 57:495–499 [PubMed] [Google Scholar]
- Payne AS, Cornelius LA (2002) The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol 118:915–922 [DOI] [PubMed] [Google Scholar]
- Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Alon R, Zipori D, Lapidot T (1999) Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 283:845–848 [DOI] [PubMed] [Google Scholar]
- Perissinotto E, Cavalloni G, Leone F, Fonsato V, Mitola S, Grignani G, Surrenti N, Sangiolo D, Bussolino F, Piacibello W, Aqlietta M (2005) Involvement of chemokine receptor 4/stromal cell-derived factor 1 system during osteosarcoma tumor progression. Clin Cancer Res 11:490–497 [PubMed] [Google Scholar]
- Rembrink K, Romijn JC, van der Kwast TH, Rubben H, Schroder FH (1997) Orthotopic implantation of human prostate cancer cell lines: a clinically relevant animal model for metastatic prostate cancer. Prostate 31:168–174 [DOI] [PubMed] [Google Scholar]
- Salvucci O, Bouchard A, Baccarelli A, Deschenes J, Sauter G, Simon R, Bianchi R, Basik M (2006) The role of CXCR4 receptor expression in breast cancer: a large tissue microassay study. Breast Cancer Res Treat 97:275–283 [DOI] [PubMed] [Google Scholar]
- Sato N, Gleava ME, Bruchovsky N, Rennie PS, Beraldi E, Sullivan LD (1997) A metastatic and androgen-sensitive human prostate cancer model using intraprostatic inoculation of LNCaP cells in SCID mice. Cancer Res 57:1584–1589 [PubMed] [Google Scholar]
- Scotton CJ, Wilson JL, Scott K, Stamp G, Wilbanks GD, Fricker S, Bridger G, Balkwill FR (2002) Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res 62:5930–5938 [PubMed] [Google Scholar]
- Song J, Pang S, Lu Y, Yokoyama KK, Zheng JY, Chiu R (2004) Gene silencing in androgen-responsive prostate cancer cells from the tissue-specific prostate-specific antigen promoter. Cancer Res 64:7661–7663 [DOI] [PubMed] [Google Scholar]
- Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK (2002) Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res 15:1832–1837 [PubMed] [Google Scholar]
- Wang J, Wang J, Sun Y, Song W, Nor JE, Wang CY (2005) Diverse signaling pathways through the SDF-1/CXCR4 chemokine axis in prostate cancer cell lines leads to altered patterns of cytokine secretion and angiogenesis. Cell Signal 17:1578–1592 [DOI] [PubMed] [Google Scholar]
- Wang J, Loberg R, Taichman RS (2006) The pivotal role of CXCR12 (SDF-1)/CXCR4 axis in bone metastasis. Cancer Metastasis Rev 25:573–587 [DOI] [PubMed] [Google Scholar]
- Yang M, Jiang P, Sun FX, Hasegawa S, Baranov E, Chishima T, Shimada H, Moossa AR, Hoffman RM (1999) A fluorescent orthotopic bone metastasis model of human prostate cancer. Cancer Res 59:781–786 [PubMed] [Google Scholar]
- Yang M, Baranov E, Wang JW, Jiang P, Wang X, Sun FX, Bouvet M, Moossa AR, Penman S, Hoffman RM (2002) Direct external imaging of nascent cancer, tumor progression, angiogenesis, and metastasis on internal organs in the fluorescent orthotopic model. Proc Natl Acad Sci USA 99:3824–3829 [DOI] [PMC free article] [PubMed] [Google Scholar]