Abstract
Ursolic acid (UA) is a pentacyclic triterpenoid, with anti-cancer and anti-inflammatory properties. Sphingomyelin (SM) hydrolysis generates lipid messengers regulating cell survival. Earlier studies showed that UA has anti-proliferative and apoptotic effects on HT29 cells, accompanied by a rapid increase in alkaline sphingomyelinase (Alk-SMase) activity. This study examines the effect of orally administered UA on the formation of aberrant crypt foci (ACF) and intestinal SMase activity in azoxymethane (AOM)-treated rats. Sprague-Dawley rats were divided into eight groups, receiving AOM or vehicle, and fed normal diet or pellets containing 0.11% UA in the initiation or promotion/progression phase. The formation of ACF in the colon and the activities of three types of mucosal SMase were examined. UA significantly reduced the incidence of ACF containing three or more crypts in the initiation group, but had no significant effect in the promotion/progression group. AOM reduced mucosal Alk-SMase activity, and the inhibitory effects could not be prevented by UA. However, in both AOM-treated and normal rats, UA increased the activity of colonic neutral SMase markedly and that of acid SMase activity mildly. These results indicate that UA has chemopreventive effects in the initiation phase of colon cancer associated with changes in SM metabolism.
Keywords: Ursolic acid, Colon cancer, Sphingomyelinase, Azoxymethane, Aberrant crypt foci, Rat
Introduction
Cancer of the colon is one of the leading causes of cancer morbidity and mortality in the Western world (Kochanek et al., 2004). Even if the exact mechanisms are still unknown, it is well established that dietary factors are of huge importance in colon carcinogenesis. In the search for links between diet and colon neoplasia, sphingolipid metabolism in the gut is a subject that has raised a great deal of interest among researchers in recent years.
Sphingomyelin (SM) is a sphingolipid present in eukaryotic cell membranes, and a constituent of many foods, e.g. meat, eggs and dairy products. The hydrolysis of SM generates ceramide, sphingosine and sphingosine-1-phosphate, which are all known to act as regulators of cellular proliferation, differentiation and apoptosis (Spiegel and Merrill 1996). Dietary supplementation of SM has been found to inhibit chemically induced colon tumorigenesis in animal models (Lemonnier et al. 2003). There are at least five known types of sphingomyelinase (SMase) among them, neutral, acid and alkaline SMase, which are named after their respective pH optimum (Goni and Alonso 2002). All of these three enzymes are present in the intestinal tract. The acid and alkaline SMases can be identified in both intestinal content and mucosal cells, whereas the neutral SMase activity is mainly identified in the mucosal cells (Duan et al. 1995). The reduction of alkaline SMase has been found in colon cancer tissues as well as in precancer conditions (i.e. ulcerative colitis and adenoma) (Hertervig et al. 1997, 1999; Sjoqvist et al. 2002). The reduction is further enhanced and followed by reduction of both acid and neutral SMase activities under the transformation from adenomas to carcinomas. The development of colon cancer is therefore associated with a defective SM metabolism, resulting in a reduction of ceramide levels (Selzner et al. 2001). Purified intestinal alkaline SMase has also been shown to specifically inhibit the proliferation and DNA synthesis of HT-29 human colon carcinoma cells in culture accompanied by an increase in SM hydrolysis and ceramide production (Hertervig et al. 2003).
Ursolic acid (UA) is a pentacyclic triterpenoid, which can be found in seaweed, the wax-like coatings of fruits, and in herbs such as thyme, lavender and oregano, among others. It has been used in cosmetics and food preparations, and was long considered to be biologically inactive (Liu 1995). In recent years, however, UA has been found to possess anti-inflammatory properties, mediated via selective inhibition of cyclooxygenase-2 and possibly 5-lipooxygenase, as well as having anti-cancer effects on a variety of cell lines (Ringbom et al. 1998; Subbaramaiah et al. 2000; Novotny et al. 2001).
In a previous study, we found that UA has anti-proliferative and apoptotic effects in HT-29 human colon adenocarcinoma cells. We also showed that UA significantly increased alkaline SMase (Alk-SMase) activity in HT29 cells, which occurred prior to caspase activation (Andersson et al. 2003). This rapid increase in Alk-SMase activity might be caused by a direct interaction of the compound with the enzyme, because we have found that UA specifically enhances the activity of purified rat intestinal Alk-SMase in test tubes (Andersson et al. 2006).
The aim of this study was to investigate the effects of orally administered UA on the formation of aberrant crypt foci (ACF), which are considered to be the earliest morphological markers of dysplasia (Bird and Good 2000) in rats treated with the colon-specific carcinogen azoxymethane (AOM), and also on the activities of the enzymes responsible for intestinal sphingolipid metabolism in the rats. Our results shed further light on the potential of UA as a chemopreventive compound against colon cancer.
Methods
Materials
Bovine milk SM was provided by Dr. Nyberg at Skane Dairy Co. (Malmö, Sweden) and labelled with [N-14C-CH3] choline by Dr. Peters at Astra Zeneca as described (Stoffel 1975). UA (88.1% purity) was purified at Sami Labs Limited (Bangalore, India). AOM was purchased from Sigma-Aldrich (Stockholm, Sweden). Female Sprague Dawley rats (∼100 g) were obtained from Mollegaard, Denmark.
Preparation of the diet containing UA
The dietary chow containing UA was prepared based on the method of Lillienau et al. (1993) with minor modifications. Briefly, UA was dissolved in 99.5% ethanol in a concentration of 1%. The standard chow R34 (Lactamin AB, Sweden) was soaked in the solution for 5 min to be saturated with the solution. The reduction of the solution volume was measured, and the incorporated UA to the pellets were calculated. The pellets were removed and air-dried at room temperature overnight. Using this procedure, a chow containing 0.11% (w/w) UA was obtained. The air-dried diet was stored at 4°C and given to the animals daily.
Experimental animal protocol
Female Sprague-Dawley rats, initially ∼7 weeks old and weighing about 100 grams, were kept under normal housing conditions, and had ad libitum access to drinking water. The rats were divided into eight experimental groups (see Fig. 1). To examine the effects of UA on initiation of ACF (protocol A), the rats in groups 2 (n = 12) and 3 (n = 15) were injected subcutaneously with AOM (15 mg/kg bodyweight) once weekly for two consecutive weeks, whereas those in group 1 (n = 6) served as blank and received no AOM. The rats in groups 1 and 2 were fed standard food pellets, and those in group 3 were fed pellets containing 0.11% UA, beginning on the same day as the first AOM injection. The rats in groups 1–3 were anesthetized 4 weeks after the first AOM injection with an intramuscular injection containing ketamine (60 mg/kg bodyweight) and xylazine (8 mg/kg bodyweight). The colons were excised, whereafter the animals were killed by puncture of the aorta.
Fig. 1.
Experimental design. The arrows mark the time for the AOM injections; the parts underlined mark the duration of the UA feeding. For each Protocol (A and B), there was also a blank group, receiving neither AOM nor UA, not included in the figure
To investigate the effects of UA on progression and outgrowth of ACF (protocol B), rats in groups 5 (n = 15) and 6 (n = 18) were injected subcutaneously with AOM (15 mg/kg bodyweight) once weekly for two consecutive weeks, whereas group 4 (n = 6) served as blank and received no AOM. The rats in groups 4 and 5 were fed standard food pellets, and those in group 6 were fed pellets containing 0.11 % UA, beginning 4 weeks after the first AOM injection. Eight weeks after the first AOM injection, the rats in groups 4–6 were operated and examined as described above. To study the effects of UA alone on SMase activity, rats in group 7 were fed standard diet and those in group 8 were fed 0.11% UA for 4 weeks.
Throughout the study, all of the rats were given 20 g food/rat/day, and the remaining food in each cage was weighed daily as a measure of the appetite. Apart from the food intake, the animals were examined daily for signs of disease as well as changes in stool consistency or signs of macroscopical rectal bleeding.
This study was approved by the local animal research ethics committee of Malmö/Lund, Sweden.
ACF counting
The ACF were visualized according to Bird (1987). In brief, colons were cut open longitudinally, rinsed in saline and then fixed flatly between two filter papers and put in 10% buffered formalin for a minimum of 24 h. They were then stained for about 30 s in 10% buffered formalin containing 0.3% methylene blue, quickly washed in saline and examined under a dissecting microscope by an examiner unaware of which group each colon belonged to.
SMase assay
The colons were cut open longitudinally, rinsed with saline and the mucosa was scraped and homogenized in a homogenisation buffer containing 50 mM Tris, 2 mM EDTA, 1 mM PMSF, 1 mM benzamidine, 0.5 mM DTT and bile salts (2.5 mg/ml), pH 7.4. The samples were sonicated and centrifuged at 13,000 rpm for 10 min, and the supernatant was saved for SMase assays. The activities of three types of SMase (alkaline, acid and neutral) were determined according to Duan and Nilsson (2000). Briefly, the supernatant was incubated at 37°C using [14C-SM] (0.8 μM, 8,000 dpm) as a substrate in different pH optimal buffers (for Alk-SMase, the buffer contained 6 mM taurocholate, pH 9.0, for A-SMase 0.12% Triton X100, pH 5.0 and for N-SMase 0.12% Triton X100, pH 7.0). The reaction was stopped by adding chloroform/methanol; the samples were thereafter centrifuged, and the production of 14C-phosphocholine in the upper phase was determined by liquid scintillation and the activity was expressed as pmole/h/mg sample proteins.
Statistical analysis
The results are presented as mean ± standard error. The data were analysed by non-paired Student’s t test and P < 0.05 was considered statistically significant.
Results
Animal condition
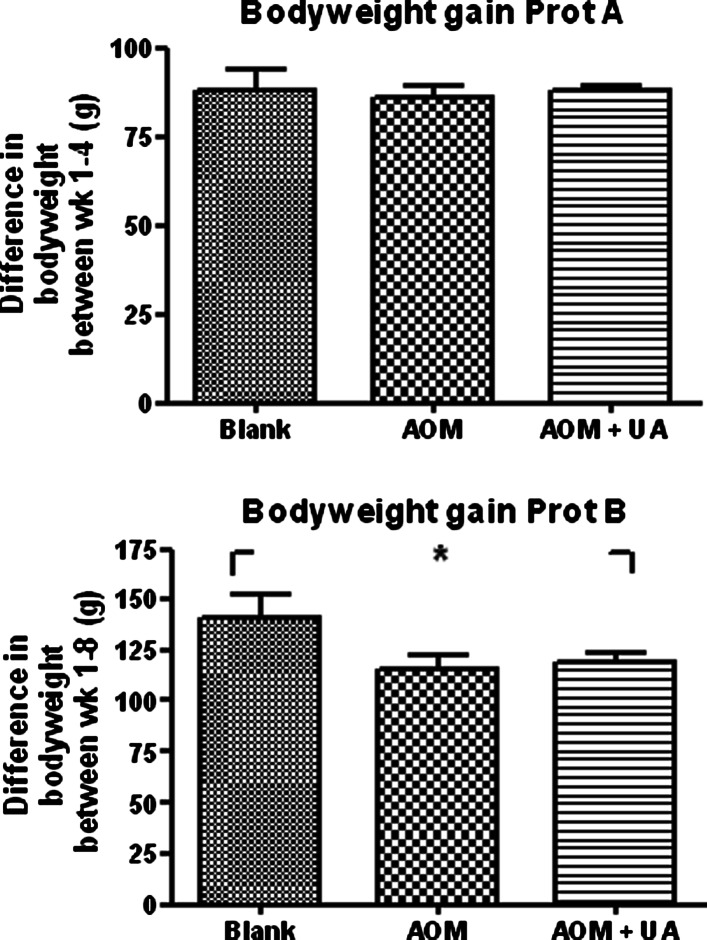
As can be seen in Fig. 2, there were no differences in bodyweight gain between the rats receiving UA and AOM and those receiving AOM only during the first 4 weeks. In Protocol B, there was a 15% decrease in bodyweight gain in the UA + AOM group compared to the Blank group and the AOM group had an 18% decrease compared to the blank group. There were no differences between the groups in neither Protocol A or B regarding stool consistency, appetite, or overall well-being (data not shown). Neither were there any differences in body weight and stool consistency in the normal rats fed with standard diet or the diet containing UA.
Fig. 2.
Upper panel Average gain of bodyweight in Protocol A from the first AOM injection until the rats were killed after 4 weeks. Lower panel Average gain of bodyweight in Protocol B from the first AOM injection until the rats were killed after 8 weeks. * = P < 0.05
Effects of UA on ACF
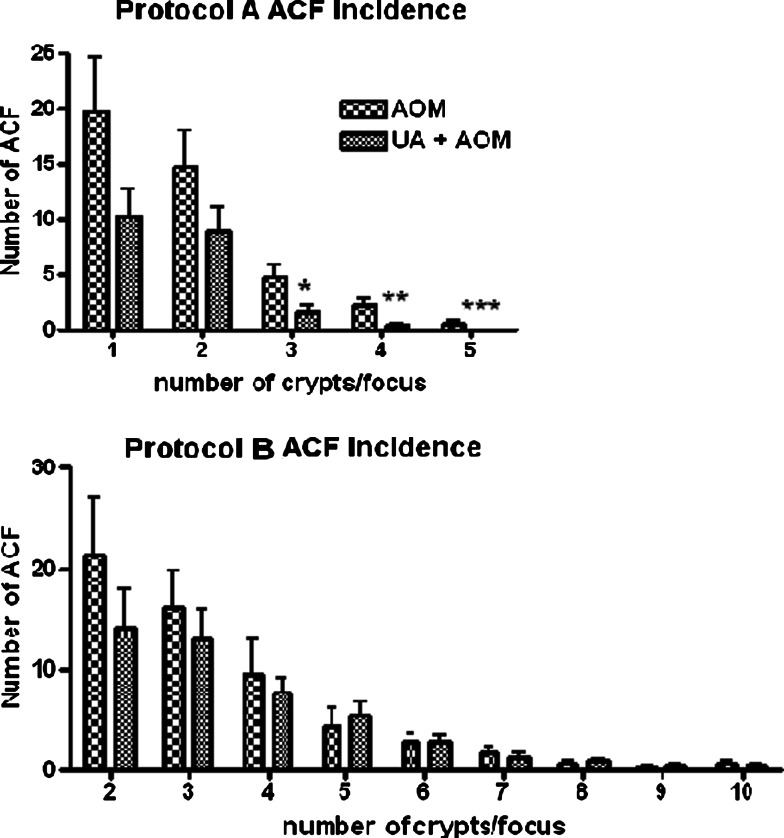
The formations of ACF in both initiation phase and progression phase are shown in Fig. 3. Administration of UA during the initiation phase (Protocol A) reduced the number of ACF of all sizes, but the reduction was specifically significant for those containing three crypts or more. UA administered during the progression/outgrowth phase (Protocol B) had no significant effect on ACF incidence, but as also can be seen in the figure, there was consistent tendency towards fewer ACF in all the UA-treated rats, regardless of ACF size.
Fig. 3.
Upper panel Comparison of the number of ACF between the UA + AOM and AOM groups in protocol A (initiation phase), sorted after number of crypts per ACF. The rats were killed 4 weeks after the first AOM injection, their colons excised, fixed in formalin and stained with methylene blue; whereafter the number of ACF was counted under a dissecting microscope. Lower panel Comparison of the number of ACF between the UA + AOM and AOM groups in protocol B (progression/outgrowth phase), sorted after number of crypts per ACF. The rats were killed 8 weeks after the first AOM injection. The ACFs were examined similarly as described above. * = P < 0.05, ** = P < 0.01, *** = P < 0.001
Colonic mucosal SMase activity
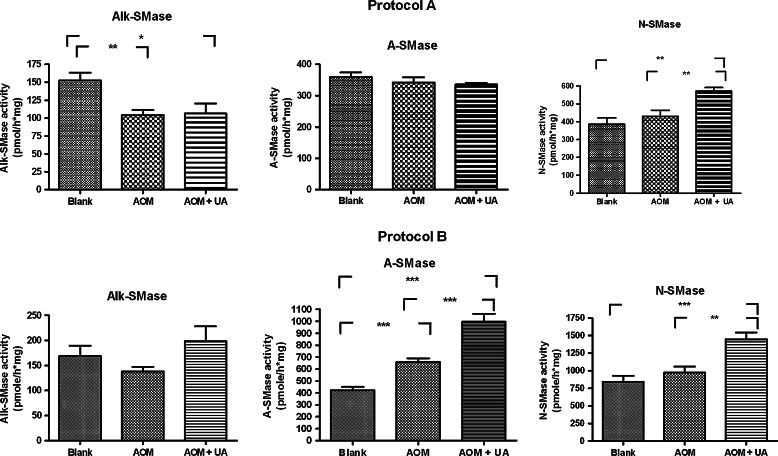
The changes of three types of SMase induced by AOM and UA in protocols A and B are shown in Fig. 4. As can be seen in the upper left panel, after 4 weeks AOM had significantly reduced the colonic mucosal Alk-SMase activity by 32%, an effect that could not be reversed by UA. In the progression/outgrowth protocol B, there were no significant differences in Alk-SMase activity between the groups, although the activity in the UA treated group was slightly higher than the others.
Fig. 4.
Activites of Alk-, A- and N-SMase in colonic mucosa in Protocol A (initiation phase, upper panel) and B (progression/outgrowth phase, lower panel). The rats were killed and the colonic mucosa was scraped and homogenized. The activities of the three SMases were measured using 14C-SM as a substrate. * = P < 0.05, ** = P < 0.01, *** = P < 0.001
As for neutral SMase (N-SMase), AOM administration did not show any significant effect on this enzyme in both initiation and progression phases. However, UA significantly increased N-SMase activity by 50% in the initiation phase and by 77% in the progression phase.
The colonic acid SMase (A-SMase) activity was not changed in the initiation phase. However, in the progression phase (protocol B), AOM itself significantly increased the A-SMase activity by 57%, indicating that increase of A-SMase is a late event after AOM injection. The increase was further enhanced by UA.
How administration of UA alone affected colonic mucosal SMase activity is shown in Fig. 5. Four-week administration of UA in normal rats significantly increased N-SMase activity by 190% and that of A-SMase by 65%. No changes of Alk-SMase activity could be identified.
Fig. 5.
Activites of Alk-, A- and N-SMase in colonic mucosa from rats treated with UA for 4 weeks. The rats were killed and the colonic mucosa was scraped and homogenized. The activities of the three SMases were measured using 14C-SM as a substrate. * = P < 0.05
Discussion
The aim of this study was to investigate the effects of UA as a chemopreventive agent in a rat model treated with AOM. Although UA and other plant-derived pentacyclic triterpenoids have attracted a lot of interest among cancer researchers in recent years, there has, to our knowledge, not yet been any study published where UA has been tested in vivo as a diet supplementation for chemopreventive purposes.
In this study, we show that UA administration to rats during the cancer initiation phase significantly reduces the incidence of ACF containing three or more crypts. This finding indicates that UA has chemopreventive effects against colon cancer, because it has been shown previously that the development of larger ACF (>3 crypts) is predictive of the tumor incidence (Pretlow et al. 1992). However, when we started the UA supplementation 4 weeks after the first AOM injection, i.e. during the progression/outgrowth phase, the inhibitory effects were not statistically significant, even if there still was a consistent tendency towards inhibition in the UA group. This might indicate that once the first mutation in AOM-induced carcinogenesis has occurred (Takahashi and Wakabayashi 2004), the preventive effects of UA are significantly reduced. Whether one can regain the inhibitory effects by increasing the dose of UA might be worth testing, especially since our earlier studies showed that UA has proapoptotic and antiproliferative effects on the poorly differentiated and highly malignant human colon adenocarcinoma cell line HT29 (Andersson et al. 2003). Because of the limited number of other studies in vivo, there is little knowledge about which doses have the most therapeutic efficacy. Since UA is considered to have low toxicity (Liu 1995) and because we did not find any side effects in this study, increasing the UA intake appears feasible in any future studies.
The other main part of this study focuses on the effects of UA and AOM on SMase activity in the rat intestine. Previous studies have shown that Alk-SMase is the major SMase in the gut, and that the activity of alk-SMase is significantly decreased in both cancerous and precancerous tissues (Hertervig et al. 1997, 1999; Sjoqvist et al. 2002). In earlier studies, we have also found that UA rapidly increases the Alk-SMase activity in human colon cancer cells (Andersson et al. 2003). In this study, we did not find any significant increase of Alk-SMase by UA neither in AOM treated nor normal rats. These results might indicate that UA does not affect the expression of Alk-SMase in the mucosa, and that the previous positive finding in the in vitro experiments were caused by a direct interaction of UA with the enzyme, which is located on the surface of the plasma as an ectoenzyme (Duan et al. 2003). In support of the hypothesis, we have shown that in test tubes UA increased the activity of purified Alk-SMase (Andersson et al. 2006).
Although Alk-SMase was not affected by UA in the present study, it was significantly decreased by AOM. As expected, the AOM-induced reduction was not reversed by UA. This finding further strengthens the hypothesis that reduction of Alk-SMase activity is an early event in colon carcinogenesis, and there is a possibility that this reduction facilitates or even initiates ACF formation in the colon. This might also explain why UA had no significant effect on ACF incidence when it was administered with a starting point 4 weeks after the first AOM injection.
Another novel finding in this study is that UA significantly increased the colonic neutral SMase activity in normal and AOM treated rats during the initiation and progression/outgrowth phases. N-SMase is an enzyme which exists in membrane-bound and cytosolic forms, both of which have been proposed to have an integral role in cellular apoptosis (Chatterjee 1999). N-SMase can be activated by many factors such as NSAID, alcohol, and oxidative stress and also some antioxidants as recently reviewed (Marchesini and Hannun 2004). Inhibition of neutral SMase induces cell proliferation, whereas overexpression of neutral SMase results in growth arrest (Marchesini et al. 2004). A recent study by Wu et al. (2005b) also showed that feeding nude mice with fish oil inhibited the growth of breast cancer xenografts accompanied by an activation of N-SMase in the tumors.
Although we do not know the mechanism behind the activity increase in our study, one possibility might be an increase of arachidonic acid induced by UA, because UA has been reported to inhibit the activities of both cyclooxygenase and lipoxygenase (Najid et al. 1992), and arachidonic acid has been shown to increase N-SMase activity in HL-60 cells (Jayadev et al. 1994) and colon cancer cells (Chan et al. 1998). This increase of N-SMase activity by UA might compensate for the reduced SM hydrolytic capacity caused by the reduced Alk-SMase activity by AOM, and perhaps antagonize the formation of ACF.
As shown in the study, the basal N-SMase activity is higher in protocol B than in protocol A. Since the rats in protocol B is older than those in protocol A, it may indicate an increase of the enzyme with age. In cultured cells it was found that N-SMase increased with the confluence (Marchesini et al. 2004). In addition, the A and N-SMase are not as stable as Alk-SMase, and freezing the sample will reduce the activities. In this experiment, as the samples in control and the treated groups were analysed under the same conditions, it does not affect the reliability of the results.
Besides N-SMase, in the present study we also found an increase in colonic mucosal A-SMase activity by AOM in the progression/outgrowth protocol B, which was further increased by UA. The increase in A-SMase by AOM may be stress-related, as A-SMase is sensitive to stress stimulation (Gulbins and Li 2006). The enhancement by UA could be an additive effect, as UA alone in the diet increased acid SMase activity. The significance of this finding is unclear. A-SMase has been proposed to play signalling roles in apoptosis especially under stress conditions (Gulbins and Li 2006), but previous studies from our lab has not shown evidence for an initiating role of A-SMase in colon cancer cell apoptosis (Wu et al. 2005a; Liu et al. 2006). However, the possibility that the increased A-SMase by UA also counteracts the reduced SM hydrolysis due to the reduced Alk-SMase activity induced by AOM cannot be excluded.
To summarize, in this study we have shown that oral administration of UA has inhibitory effects on the formation of ACF, particularly on those with three or more crypts when administered during the initiation phase. The finding that AOM significantly reduced the activity of Alk-SMase, the major SMase in the gut, further supports the importance of Alk-SMase in preventing colon carcinogenesis. UA may counteract the effect of AOM mainly by increasing N-SMase activity, and thus holds promise as a possible chemopreventive agent against colon cancer.
Acknowledgments
Dr. Sankaran Natarajan at Sami Labs Limited, Bangalore, India, is gratefully acknowledged for providing UA. This work was supported by the grants from Swedish Cancer Society, Albert Påhlsson Foundation, Swedish Nutrition Foundation, and the research foundation of University Hospital of Lund, Sweden.
References
- Andersson D, Liu JJ, Nilsson A, Duan RD (2003) Ursolic acid inhibits proliferation and stimulates apoptosis in HT29 cells following activation of alkaline sphingomyelinase. Anticancer Res 4:3317–3322 [PubMed] [Google Scholar]
- Andersson D, Nilsson Å, Duan R-D (2006) Ursolic acid and other pentacyclic triterpenoids stimulate intestinal alkaline sphingomyelinase in vitro. Eur J Lipid Sci Technol 2:103–108 [Google Scholar]
- Bird RP (1987) Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett 2:147–151 [DOI] [PubMed] [Google Scholar]
- Bird RP, Good CK (2000) The significance of aberrant crypt foci in understanding the pathogenesis of colon cancer. Toxicol Lett 112–113:395–402 [DOI] [PubMed] [Google Scholar]
- Chan TA, Morin PJ, Vogelstein B, Kinzler KW (1998) Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci USA 2:681–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S (1999) Neutral sphingomyelinase: past, present and future. Chem Phys Lipids 1–2:79–96 [DOI] [PubMed] [Google Scholar]
- Duan RD, Nilsson A (2000) Sphingolipid hydrolyzing enzymes in the gastrointestinal tract. Methods Enzymol 311:276–286 [DOI] [PubMed] [Google Scholar]
- Duan RD, Nyberg L, Nilsson A (1995) Alkaline sphingomyelinase activity in rat gastrointestinal tract: distribution and characteristics. Biochim Biophys Acta 1:49–55 [DOI] [PubMed] [Google Scholar]
- Duan RD, Bergman T, Xu N, Wu J, Cheng Y, Duan J et al (2003) Identification of human intestinal alkaline sphingomyelinase as a novel ecto-enzyme related to the nucleotide phosphodiesterase family. J Biol Chem 40:38528–38536 [DOI] [PubMed] [Google Scholar]
- Goni FM, Alonso A (2002) Sphingomyelinases: enzymology and membrane activity. FEBS Lett 1:38–46 [DOI] [PubMed] [Google Scholar]
- Gulbins E, Li PL (2006) Physiological and pathophysiological aspects of ceramide. Am J Physiol Regul Integr Comp Physiol 1:R11–R26 [DOI] [PubMed] [Google Scholar]
- Hertervig E, Nilsson A, Nyberg L, Duan RD (1997) Alkaline sphingomyelinase activity is decreased in human colorectal carcinoma. Cancer 3:448–453 [PubMed] [Google Scholar]
- Hertervig E, Nilsson A, Bjork J, Hultkrantz R, Duan RD (1999) Familial adenomatous polyposis is associated with a marked decrease in alkaline sphingomyelinase activity: a key factor to the unrestrained cell proliferation? Br J Cancer 2:232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertervig E, Nilsson A, Cheng Y, Duan RD (2003) Purified intestinal alkaline sphingomyelinase inhibits proliferation without inducing apoptosis in HT-29 colon carcinoma cells. J Cancer Res Clin Oncol 10:577–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayadev S, Linardic CM, Hannun YA (1994) Identification of arachidonic acid as a mediator of sphingomyelin hydrolysis in response to tumor necrosis factor alpha. J Biol Chem 8:5757–5763 [PubMed] [Google Scholar]
- Kochanek KD, Murphy SL, Anderson RN, Scott C (2004) Deaths: final data for 2002. Natl Vital Stat Rep 5:1–115 [PubMed] [Google Scholar]
- Lemonnier LA, Dillehay DL, Vespremi MJ, Abrams J, Brody E, Schmelz EM (2003) Sphingomyelin in the suppression of colon tumors: prevention versus intervention. Arch Biochem Biophys 2:129–138 [DOI] [PubMed] [Google Scholar]
- Lillienau J, Crombie DL, Munoz J, Longmire-Cook SJ, Hagey LR, Hofmann AF (1993) Negative feedback regulation of the ileal bile acid transport system in rodents. Gastroenterology 1:38–46 [DOI] [PubMed] [Google Scholar]
- Liu J (1995) Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol 2:57–68 [DOI] [PubMed] [Google Scholar]
- Liu F, Cheng Y, Wu J, Tauschel HD, Duan RD (2006) Ursodeoxycholic acid differentially affects three types of sphingomyelinase in human colon cancer Caco 2 cells. Cancer Lett 1:141–146 [DOI] [PubMed] [Google Scholar]
- Marchesini N, Hannun YA (2004) Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol 1:27–44 [DOI] [PubMed] [Google Scholar]
- Marchesini N, Osta W, Bielawski J, Luberto C, Obeid LM, Hannun YA (2004) Role for mammalian neutral sphingomyelinase 2 in confluence-induced growth arrest of MCF7 cells. J Biol Chem 24:25101–25111 [DOI] [PubMed] [Google Scholar]
- Najid A, Simon A, Cook J, Chable-Rabinovitch H, Delage C, Chulia AJ et al (1992) Characterization of ursolic acid as a lipoxygenase and cyclooxygenase inhibitor using macrophages, platelets and differentiated HL60 leukemic cells. FEBS Lett 3:213–217 [DOI] [PubMed] [Google Scholar]
- Novotny L, Vachalkova A, Biggs D (2001) Ursolic acid: an anti-tumorigenic and chemopreventive activity. Minireview. Neoplasma 4:241–246 [PubMed] [Google Scholar]
- Pretlow TP, O’Riordan MA, Somich GA, Amini SB, Pretlow TG (1992) Aberrant crypts correlate with tumor incidence in F344 rats treated with azoxymethane and phytate. Carcinogenesis 9:1509–1512 [DOI] [PubMed] [Google Scholar]
- Ringbom T, Segura L, Noreen Y, Perera P, Bohlin L (1998) Ursolic acid from Plantago major, a selective inhibitor of cyclooxygenase-2 catalyzed prostaglandin biosynthesis. J Nat Prod 10:1212–1215 [DOI] [PubMed] [Google Scholar]
- Selzner M, Bielawska A, Morse MA, Rudiger HA, Sindram D, Hannun YA et al (2001) Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res 3:1233–1240 [PubMed] [Google Scholar]
- Sjoqvist U, Hertervig E, Nilsson A, Duan RD, Ost A, Tribukait B et al (2002) Chronic colitis is associated with a reduction of mucosal alkaline sphingomyelinase activity. Inflamm Bowel Dis 4:258–263 [DOI] [PubMed] [Google Scholar]
- Spiegel S, Merrill AH Jr (1996) Sphingolipid metabolism and cell growth regulation. Faseb J 12:1388–1397 [DOI] [PubMed] [Google Scholar]
- Stoffel W (1975) Chemical synthesis of choline-labeled lecithins and sphingomyelins. Methods Enzymol 35:533–541 [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Michaluart P, Sporn MB, Dannenberg AJ (2000) Ursolic acid inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Cancer Res 9:2399–2404 [PubMed] [Google Scholar]
- Takahashi M, Wakabayashi K (2004) Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci 6:475–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Cheng Y, Jonsson BA, Nilsson A, Duan RD (2005) Acid sphingomyelinase is induced by butyrate but does not initiate the anticancer effect of butyrate in HT29 and HepG2 cells. J Lipid Res 9:1944–1952 [DOI] [PubMed] [Google Scholar]
- Wu M, Harvey KA, Ruzmetov N, Welch ZR, Sech L, Jackson K et al (2005) Omega-3 polyunsaturated fatty acids attenuate breast cancer growth through activation of a neutral sphingomyelinase-mediated pathway. Int J Cancer 3:340–348 [DOI] [PubMed] [Google Scholar]