Abstract
Objective
The polymorphism of p53 codon 72 (Arg72Pro) has been suggested to play an important role in many cancers and may influence the response to chemotherapy. Our aim was to investigate the association of p53 Arg72Pro polymorphism with the clinical outcome of gastric cancer patients treated with 5-FU-based adjuvant chemotherapy.
Methods
The p53 codon 72 genotype was determined in blood samples from 110 Chinese patients with gastric cancer treated with 5-fluorouracil (5-FU)-based adjuvant chemotherapy, using polymerase chain reaction-ligation detection reaction (PCR–LDR) method.
Results
Kaplan–Meier survival analysis showed that gastric cancer patients with Pro/Pro genotype had shorter relapse-free survival (χ2 = 10.632, P = 0.005) and overall survival (χ2 = 7.104, P = 0.029) than patients with other genotypes. Cox multivariate analysis showed that Pro/Pro genotype was associated with statistically significant reduced relapse-free survival (adjusted OR = 3.049, 95% CI: 1.363–6.819, P = 0.007) and overall survival (adjusted OR = 2.581, 95% CI: 1.052–6.330, P = 0.038).
Conclusion
These results suggested that p53 codon 72 polymorphism appears to be an independent prognostic factor in gastric cancer patients treated with 5-FU-based adjuvant chemotherapy.
Keywords: Gastric cancer, Chemotherapy, Polymorphism, P53 protein, 5-Fluorouracil, Apoptosis
Introduction
Gastric cancer is the fourth most common cancer and the second most frequent cause of cancer deaths worldwide. Surgery is the primary modality for managing early-stage disease. However, even after radical surgery, the majority of patients with gastric cancer develop local or distant recurrence (Macdonald 2004). Meta-analyses of adjuvant chemotherapy trials have confirmed a survival benefit in favor of the systemic medical treatment (Mari et al. 2000; Carrato et al. 2005; Hejna et al. 2006). Despite the development of new anti-neoplastic agents seen in recent years, 5-fluorouracil (5-FU) remains a cornerstone in the treatment of gastric cancer. However, the response rate is approximately 25%, even if supplemented by leucovorin (CF), which improves the effect of 5-FU. Despite numerous efforts on identifying suitable predictive markers, there is still a lack of accurate markers to discriminate between the patients who are likely to benefit from 5-FU chemotherapy and those who are not (Goekkurt et al. 2006; Ichikawa 2006; Ishida et al. 2002; Ott et al. 2006; Park and Lenz 2006). This means that most of the patients could not get any benefits from the treatment, while suffering from the toxicity of the 5-FU chemotherapy.
The p53 tumor suppressor pathway is well known to be crucial for maintaining genomic integrity and preventing cells from undergoing oncogenic transformation (Vogelstein et al. 2000). Recent studies have highlighted that the p53 codon 72 Arg/Pro polymorphism plays a crucial role in modulating wild-type p53 apoptotic capacity. These two variant protein forms behave differently, as the Arg allele of p53 has been reported to induce apoptosis more effectively than the Pro allele (Dumont et al. 2003; Thomas et al. 1999; Pim and Banks 2004). Although p53 codon 72 polymorphism has been extensively studied for its involvement in cancerogenesis (Storey et al. 1998; Rosenthal et al. 1998; Orsted et al. 2007; Belyavskaya et al. 2006; Koushik et al. 2006; Shen et al. 2004) the predictive value of this important polymorphism for chemotherapy in cancer patients has not been studied thoroughly (Bergamaschi et al. 2003; Matlashewski et al. 1987; Sullivan et al. 2004). A limited number of studies have suggested that the poorer prognosis seems to be associated with the Pro/Pro genotype of p53 codon 72 in breast cancer (Tommiska et al. 2005; Xu et al. 2005), ovarian cancer (Santos et al. 2006; Galic et al. 2007), and lung cancer (Wang et al. 1999), in comparison with the other two genotypes. However, to the best of our knowledge, the predictive value of this polymorphism on chemotherapy for gastric cancer has not been reported.
In this study, we have investigated whether the p53 codon 72 polymorphism could influence the prognosis of the gastric cancer patients receiving 5-FU-based adjuvant treatment.
Materials and methods
Patients
From May 2001 to May 2006, 110 patients with histologically confirmed gastric cancer were enrolled in this study at the 4th Affiliated Hospital of Suzhou University. All those patients received radical surgery and were then treated with 5-FU-based adjuvant treatment (71 received 5-FU/CF/oxaliplatin, 14 were treated with 5-FU/CF/oxaliplatin/taxon, 12 with 5-FU/CF/taxon, 5 with 5-FU/cisplatin, 5 with 5-FU/CF/hydroxycamptothecin, and 3 received 5-FU/CF/oxaliplatin/adriamycin). Follow-up of those patients was performed at 3-month intervals after chemotherapy at outpatient clinics or by routine phone calls. The end of the follow-up period was 16 September 2007. The median follow-up period was 23.8 months (range 5.2–72.1 months). Relapse-free survival (RFS) was defined as the time interval between the date of surgery and the date of relapse of disease or the date of the last clinical evaluation of the patient. Overall survival (OS) was defined as the time between surgery and either death or the time of the last clinical evaluation of the patient. This study was approved by the ethics and research committee of our hospital.
DNA extraction and genotyping
Blood samples were collected in EDTA containing tubes. Genomic DNA was isolated from peripheral blood lymphocytes using Axygene genomic DNA purification Kit (Axygen Biotechnology, China). Genotyping was performed using polymerase chain reaction–ligation detection reaction (PCR–LDR) method. The primers were: forward, 5′-AATGGTTCACTGAAGACCCAGGTCCAGATG-3′ and reverse, 5′-GATACCGTAGCTGCCCTGGTAGGTTTTCTG-3′. The PCRs were carried out on the ABI 9600 (Applied Biosystems, USA) in a total volume of 20 μl including 20 ng genomic DNA, 1× PCR Buffer, 2.5 mM MgCl2, 0.2 mM dNTPs, 0.5μM each primer, and 1 U hot-start Taq DNA polymerase (QIAgen). Cycling parameters were as follows: 95°C for 15 min; 35 cycles of 94°C for 30 s; 60°C for 2 min; 72°C for 60 s; and a final extension step at 72°C for 10 min. The probes for LDR were: common probe, 5′-p-GGGGAGCAGCCTCTGGCATTCTG GGACACTACGGAGGATTA-FAM-3′; G-specific probe, 5′-ACACTTGAACAG GAGGATTAGGAGCTGCTGGTGCAGGGGCCAGGG-3′; C-specific probe, 5′-C TACGGAGGATTATGAGGAGCTGCTGGTGCAGGGGCCAGGC-3′. The common probe was labeled at the 3′-end with 6-carboxyfluorescein (FAM) and was phosphorylated at the 5′-end. The ligation reaction for each PCR product was carried out with a final volume of 20 μl containing 2 μl 10× ligation buffer, 2 μl of PCR product, 1 pmol of each discriminating probe, 20 U Taq DNA ligase (New England Biolabs, USA). The LDR parameters were as follows: 94°C for 2 min, 40 cycles of 94°C for 30 s and 60°C for 2 min. Following the LDR reaction, 1 μl LDR reaction product was mixed with 1 μl ROX and 1 μl loading buffer. The mixture was then analyzed by the ABI Prism 373 DNA Sequencer (Applied Biosystems).
In addition, the representative PCR products were subjected to direct DNA sequencing in an ABI Prism 310 Sequence (Applied Biosystems) to confirm the accuracy of this method.
Statistical analysis
Data analysis was performed using the computer software SPSS13.0. The relationship between the genotype frequencies of p53 codon 72 and clinical characteristics was assessed by χ2 or Fisher’s exact probability tests. Survival curves were generated by the Kaplan–Meier method and verified by the log-rank test. Cox’s proportional hazards regression analysis was used to estimate odds ratios (ORs) and their 95% confidence intervals (CIs), representing the overall relative risk of death associated with p53 condon 72 polymorphism, and to adjust for potential confounding variables. A P value < 0.05 was considered significant.
Results
The allelic discrimination data from PCR–LDR assay were confirmed by direct sequencing of representative PCR products (Fig. 1). In those cases, the genotype determined by the PCR–LDR assay was identical to that determined by DNA sequencing. Of the 110 gastric cancer patients, the frequencies of p53 codon 72 Arg/Arg, Arg/Pro and Pro/Pro genotypes were 21.6% (24/110), 53.6% (59/110) and 24.5% (27/110), respectively. No significant association was found between the age, gender, pathologic stage or tumor differentiation and p53 codon72 genotype (Table 1), suggesting that those patients with different p53 condon genotypes had similar clinical characteristics; thus, the different clinical outcomes to the adjuvant chemotherapy in the three-genotype groups were not due to the potential influence of those clinical characteristics.
Fig. 1.
Genotyping result of p53 codon 72 polymorphism. a Electrophoresis results of PCR–LDR products; b sequencing-verified results of the representative PCR products
Table 1.
The relationship between p53 codon 72 genotypes and clinical characteristics of gastric cancer
| Clinical characteristics | Case (n = 110) | p53 codon 72 | χ2 value | P value | ||
|---|---|---|---|---|---|---|
| Pro/Pro | Arg/Pro | Arg/Arg | ||||
| Age | 0.441 | 0.802 | ||||
| ≥58 | 61 | 16 | 31 | 14 | ||
| <58 | 49 | 11 | 28 | 10 | ||
| Gender | 1.860 | 0.414 | ||||
| Male | 82 | 18 | 44 | 20 | ||
| Female | 28 | 9 | 15 | 4 | ||
| Differentiation | 0.205 | 0.903 | ||||
| Well and moderated | 57 | 15 | 30 | 12 | ||
| Poor | 53 | 12 | 29 | 12 | ||
| TNM stage | 7.802 | 0.099 | ||||
| I–II | 22 | 4 | 9 | 9 | ||
| III | 70 | 17 | 39 | 14 | ||
| IV | 18 | 6 | 11 | 1 | ||
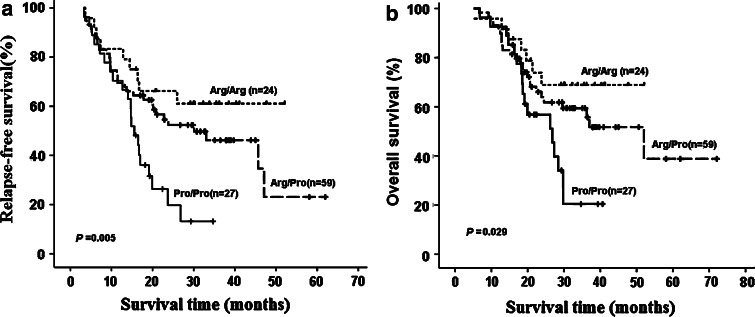
The relapse rate and death rate in patients with Arg/Arg, Arg/Pro and Pro/Pro genotypes were 37.5%(9/24), 52.5%(31/59), 77.8%(21/27) and 29.2% (7/24), 42.4%(25/59), 63.0%(10/27), respectively. Kaplan–Meier survival analysis showed that gastric cancer patients with Pro/Pro genotype had shorter RFS (log-rank χ2 =10.632, P = 0.005) and OS (log-rank χ2 =7.104, P = 0.029) than patients with Arg/Pro or Arg/Arg genotypes (Fig. 2). After adjusting for age, gender, pathologic stage and tumor differentiation, multivariate analysis (Cox’s proportional hazards model) showed that p53 Pro/Pro genotype was a potential risk factor for poor RFS and OS; and no significant difference was found between patients with Arg/Pro or Arg/Arg genotypes on RFS or OS (Table 2). In addition, Cox analysis showed that TNM stage was also a risk factor for OS.
Fig. 2.
Kaplan–Meier curves and log-rank test considering the influence of p53 codon 72 genotypes on relapse-free and overall survival of gastric cancer patients treated with 5-FU-based adjuvant chemotherapy: influence of p53 codon 72 polymorphism on relapse-free survival (a) and overall survival (b)
Table 2.
Results of Cox multivariate analyses for overall and relapse-free survival in patients with gastric cancer
| Variables | Relapse-free survival | Overall survival | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (≥58 vs. <58) | 0.996 (0.971–1.022) | 0.768 | 1.017 (0.998–1.048) | 0.256 |
| Gender (male vs. female) | 0.957 (0.538–1.704) | 0.882 | 1.047 (0.557–1.967) | 0.886 |
| Differentiation (well and moderated vs. poor) | 1.413 (0.840–2.377) | 0.193 | 1.523 (0.842–2.755) | 0.164 |
| TNM stage (I–II vs. III–IV) | 1.726 (0.806–3.693) | 0.806 | 5.935 (1.793–19.646) | 0.004 |
| P53 codon 72 status | ||||
| Arg/Arg | 1.0 (referent) | 1.0 (referent) | ||
| Pro/Pro | 3.049 (1.363–6.819) | 0.007 | 2.581 (1.052–6.330) | 0.038 |
| Arg/Pro | 1.426 (0.671–3.031) | 0.356 | 1.200 (0.514–2.801) | 0.674 |
Discussion
5-FU-based regimens, which showed activity in advanced disease, are one of the most popular modality of choice in clinics in China. Since the response rate of gastrointestinal carcinoma to 5-FU as a single agent is less than 30% (Diasio and Johnson 2000), having an effective assay to predict the patients’ response to a given chemotherapeutic protocol beforehand would greatly enhance the rate of success as well as the life quality of the patients. In this study, we found that p53 codon 72 Pro/Pro genotype was significantly associated with shorter RFS and OS than other genotypes in gastric cancer patients who received 5-FU-based adjuvant chemotherapy, highlighting its potential utility for the rational choosing the 5-FU in the treatment of gastric cancer.
As one of the most important molecules inducing cell apoptosis, the p53 protein was suggested to associate with chemotherapy resistance. Because p53 codon 72 polymorphism modulates the wild-type p53 dependent apoptosis, it is naturally expected that the cancer patients with the different p53 codon 72 genotype respond to the chemotherapy differently. Several reports on breast cancer (Tommiska et al. 2005; Xu et al. 2005), ovarian cancer (Santos et al. 2006), head and neck cancer (Sullivan et al. 2004) have showed that patients with Arg variant had the highest complete response rate, while patients with Pro allele had shorter progression-free survival and overall survival. In addition, a recent investigation showed that p53 Pro/Pro genotype was associated with poorer disease-free survival than other genotypes in breast cancer patients, and the association was especially significant in 137 patients who received adjuvant chemotherapy. Of the 137 patients cohort, 105 patients received 5-FU or 5-FU-based chemotherapy, which suggested that the impact of p53 polymorphism on prognosis of breast cancer patients may be mainly through influencing the efficacy of 5-FU (Toyama et al. 2007). Our results showed that p53 Pro/Pro genotype appears to be an independent prognostic factor in gastric cancer patients treated with 5-FU-based adjuvant chemotherapy, which is consistent with the in vitro study conducted by Sullivan et al. (2004).
Although the influence of p53 codon 72 on chemotherapy in gastric cancer has not been reported, there are studies about the relationship between this polymorphism and the disease risk (Shen et al. 2004) or clinical characteristics. Yi et al. (2006) showed that patients with advanced gastric cancer had a significantly higher frequency of the Arg/Arg allele than early gastric cancer. Another study revealed that Caucasian patients with gastric cardia cancer had a significantly higher frequency of homozygous Arg allele than those with non-cardia tumors, and a significant stepwise increased frequency of codon 72 Arg with age was also observed in patients with gastric cancer (Zhang et al. 2003). A recent meta-analysis also suggested that p53 codon 72 polymorphism may be associated with gastric cancer among Asians and with the location, stage, and histological differentiation of gastric cancer (Zhou et al. 2007). However, Lai et al. (2005) showed that the polymorphism was not associated with age, gender, cell differentiation, lymph node involvement, tumor stage and distant metastasis in patients with gastric cancer. In this study, there was also no significant association observed between p53 codon 72 and age, gender, tumor stage or differentiation. The contradictory results may result from different patients’ population, different specimens (normal or malignant cells) and relative small number of cases in some reports.
Now that the combined chemotherapy is the most common measure to improve the therapy efficiency, a common marker, which could predict the efficacy of several agents may permit a considerably improved survival prediction for the combined chemotherapy of cancers. Most anticancer agents, including 5-FU, platinum, paclitaxel, hydroxycamptothecin, adriamycin, etc., regardless of distinct anti-tumor mechanisms, ultimately kill tumor cells by inducing apoptosis (Kerr et al. 1994). An in vitro study suggested that anticancer agents, including doxorubicin, 5-FU, and cisplatin induced a higher level of apoptosis in human H1299 cells expressing the Arg/Arg genotype of p53 codon 72 than in those expressing the Pro/Pro genotype (Sullivan et al. 2004). Santos et al. (2006) found that p53 Pro allele is associated with a worse prognosis in ovarian cancer treated with cisplatinum/paclitaxel chemotherapy. Xu et al. (2005) showed that breast cancer patients with the Pro/Pro genotype were less sensitive to anthracycline-based neoadjuvant chemotherapy than those with others genotypes. Those reports suggested that p53 codon 72 polymorphism may not only appear to be a valuable factor to predict the efficacy of 5-FU in gastric cancer, but also to act as a common predictor for the efficacy of most anticancer regimens. To validate this, large cases, multi-center perspective trial with multi-group of controls may be necessary to testify the potential utility of p53 codon 72 polymorphism on the prognosis of cancer chemotherapy.
In conclusion, our data shows that the Pro/Pro genotype of p53 codon 72 appears to be an independent prognostic marker in gastric cancer patients who received 5-FU-based adjuvant chemotherapy.
References
- Belyavskaya VA, Vardosanidze VK, Smirnova OY, Karakin EI, Savkin IV, Gervas PA, Cherdyntseva NV, Voevoda MI (2006) Genetic status of p53 in stomach cancer: somatic mutations and polymorphism of codon 72. Bull Exp Biol Med 141:243–246 [DOI] [PubMed] [Google Scholar]
- Bergamaschi D, Gasco M, Hiller L, Sullivan A, Syed N, Trigiante G, Yulug I, Merlano M, Numico G, Comino A, Attard M, Reelfs O, Gusterson B, Bell AK, Heath V, Tavassoli M, Farrell PJ, Smith P, Lu X, Crook T (2003) p53 polymorphism influences response in cancer chemotherapy via modulation of p73-dependent apoptosis. Cancer Cell 3:387–402 [DOI] [PubMed] [Google Scholar]
- Carrato A, Gallego-Plazas J, Guillen-Ponce C (2005) Adjuvant therapy of resected gastric cancer is necessary. Semin Oncol 32:S105–108 [DOI] [PubMed] [Google Scholar]
- Diasio RB, Johnson MR (2000) The role of pharmacogenetics and pharmacogenomics in cancer chemotherapy with 5-fluorouracil. Pharmacology 61:199–203 [DOI] [PubMed] [Google Scholar]
- Dumont P, Leu JI, Della Pietra AC 3rd, George DL, Murphy M (2003) The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat Genet 33:357–365 [DOI] [PubMed] [Google Scholar]
- Galic V, Willner J, Wollan M, Garg R, Garcia R, Goff BA, Gray HJ, Swisher EM (2007) Common polymorphisms in TP53 and MDM2 and the relationship to TP53 mutations and clinical outcomes in women with ovarian and peritoneal carcinomas. Genes Chromosomes Cancer 46:239–247 [DOI] [PubMed] [Google Scholar]
- Goekkurt E, Hoehn S, Wolschke C, Wittmer C, Stueber C, Hossfeld DK, Stoehlmacher J (2006) Polymorphisms of glutathione S-transferases (GST) and thymidylate synthase (TS)–novel predictors for response and survival in gastric cancer patients. Br J Cancer 94:281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald JS (2004) Treatment of localized gastric cancer. Semin Oncol 31:566–573 [DOI] [PubMed] [Google Scholar]
- Hejna M, Wohrer S, Schmidinger M, Raderer M (2006) Postoperative chemotherapy for gastric cancer. Oncologist 11:136–145 [DOI] [PubMed] [Google Scholar]
- Ichikawa W (2006) Prediction of clinical outcome of fluoropyrimidine-based chemotherapy for gastric cancer patients, in terms of the 5-fluorouracil metabolic pathway. Gastric Cancer 9:145–155 [DOI] [PubMed] [Google Scholar]
- Ishida Y, Kawakami K, Tanaka Y, Kanehira E, Omura K, Watanabe G (2002) Association of thymidylate synthase gene polymorphism with its mRNA and protein expression and with prognosis in gastric cancer. Anticancer Res 22:2805–2809 [PubMed] [Google Scholar]
- Kerr JF, Winterford CM, Harmon BV (1994) Apoptosis. Its significance in cancer and cancer therapy. Cancer 73:2013–2026 [DOI] [PubMed] [Google Scholar]
- Koushik A, Tranah GJ, Ma J, Stampfer MJ, Sesso HD, Fuchs CS, Giovannucci EL, Hunter DJ (2006) p53 Arg72Pro polymorphism and risk of colorectal adenoma and cancer. Int J Cancer 119:1863–1868 [DOI] [PubMed] [Google Scholar]
- Lai K, Chen W, Tsai FJ, Li SY, Jeng LB (2005) Arginine and proline alleles of the p53 gene are associated with different locations of gastric cancer. Hepatogastroenterology 52:944–948 [PubMed] [Google Scholar]
- Mari E, Floriani I, Tinazzi A, Buda A, Belfiglio M, Valentini M, Cascinu S, Barni S, Labianca R, Torri V (2000) Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of published randomised trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente). Ann Oncol 11:837–843 [DOI] [PubMed] [Google Scholar]
- Matlashewski GJ, Tuck S, Pim D, Lamb P, Schneider J, Crawford LV (1987) Primary structure polymorphism at amino acid residue 72 of human p53. Mol Cell Biol 7:961–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsted DD, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG (2007) Tumor suppressor p53 Arg72Pro polymorphism and longevity, cancer survival, and risk of cancer in the general population. J Exp Med 204:1295–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott K, Vogelsang H, Marton N, Becker K, Lordick F, Kobl M, Schuhmacher C, Novotny A, Mueller J, Fink U, Ulm K, Siewert JR, Hofler H, Keller G (2006) The thymidylate synthase tandem repeat promoter polymorphism: a predictor for tumor-related survival in neoadjuvant treated locally advanced gastric cancer. Int J Cancer 119:2885–2894 [DOI] [PubMed] [Google Scholar]
- Park DJ, Lenz HJ (2006) Determinants of chemosensitivity in gastric cancer. Curr Opin Pharmacol 6:337–344 [DOI] [PubMed] [Google Scholar]
- Pim D, Banks L (2004) p53 polymorphic variants at codon 72 exert different effects on cell cycle progression. Int J Cancer 108:196–199 [DOI] [PubMed] [Google Scholar]
- Rosenthal AN, Ryan A, Al-Jehani RM, Storey A, Harwood CA, Jacobs IJ (1998) p53 codon 72 polymorphism and risk of cervical cancer in UK. Lancet 352:871–872 [DOI] [PubMed] [Google Scholar]
- Santos AM, Sousa H, Portela C, Pereira D, Pinto D, Catarino R, Rodrigues C, Araujo AP, Lopes C, Medeiros R (2006) TP53 and P21 polymorphisms: response to cisplatinum/paclitaxel-based chemotherapy in ovarian cancer. Biochem Biophys Res Commun 340:256–262 [DOI] [PubMed] [Google Scholar]
- Shen H, Solari A, Wang X, Zhang Z, Xu Y, Wang L, Hu X, Guo J, Wei Q (2004) P53 codon 72 polymorphism and risk of gastric cancer in a Chinese population. Oncol Rep 11:1115–1120 [PubMed] [Google Scholar]
- Storey A, Thomas M, Kalita A, Harwood C, Gardiol D, Mantovani F, Breuer J, Leigh IM, Matlashewski G, Banks L (1998) Role of a p53 polymorphism in the development of human papillomavirus-associated cancer. Nature 393:229–234 [DOI] [PubMed] [Google Scholar]
- Sullivan A, Syed N, Gasco M, Bergamaschi D, Trigiante G, Attard M, Hiller L, Farrell PJ, Smith P, Lu X, Crook T (2004) Polymorphism in wild-type p53 modulates response to chemotherapy in vitro and in vivo. Oncogene 23:3328–3337 [DOI] [PubMed] [Google Scholar]
- Thomas M, Kalita A, Labrecque S, Pim D, Banks L, Matlashewski G (1999) Two polymorphic variants of wild-type p53 differ biochemically and biologically. Mol Cell Biol 19:1092–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommiska J, Eerola H, Heinonen M, Salonen L, Kaare M, Tallila J, Ristimaki A, von Smitten K, Aittomaki K, Heikkila P, Blomqvist C, Nevanlinna H (2005) Breast cancer patients with p53 Pro72 homozygous genotype have a poorer survival. Clin Cancer Res 11:5098–5103 [DOI] [PubMed] [Google Scholar]
- Toyama T, Zhang Z, Nishio M, Hamaguchi M, Kondo N, Iwase H, Iwata H, Takahashi S, Yamashita H, Fujii Y (2007) Association of TP53 codon 72 polymorphism and the outcome of adjuvant therapy in breast cancer patients. Breast Cancer Res 9:R34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ (2000) Surfing the p53 network. Nature 408:307–310 [DOI] [PubMed] [Google Scholar]
- Wang YC, Chen CY, Chen SK, Chang YY, Lin P (1999) p53 codon 72 polymorphism in Taiwanese lung cancer patients: association with lung cancer susceptibility and prognosis. Clin Cancer Res 5:129–134 [PubMed] [Google Scholar]
- Xu Y, Yao L, Ouyang T, Li J, Wang T, Fan Z, Lin B, Lu Y, Xie Y (2005) p53 Codon 72 polymorphism predicts the pathologic response to neoadjuvant chemotherapy in patients with breast cancer. Clin Cancer Res 11:7328–7333 [DOI] [PubMed] [Google Scholar]
- Yi SY, Lee WJ (2006) A p53 genetic polymorphism of gastric cancer: difference between early gastric cancer and advanced gastric cancer. World J Gastroenterol 12:6536–6539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW, Newcomb P, Hollowood A, Feakins R, Moorghen M, Storey A, Farthing MJ, Alderson D, Holly J (2003) Age-associated increase of codon 72 Arginine p53 frequency in gastric cardia and non-cardia adenocarcinoma. Clin Cancer Res 9:2151–2156 [PubMed] [Google Scholar]
- Zhou Y, Li N, Zhuang W, Liu GJ, Wu TX, Yao X, Du L, Wei ML, Wu XT (2007) P53 codon 72 polymorphism and gastric cancer: A meta-analysis of the literature. Int J Cancer 121:1481–1486 [DOI] [PubMed] [Google Scholar]