Abstract
Purpose
Rhabdomyosarcoma (RMS), which is the most common pediatric soft tissue sarcoma, is classified into two major histologic subtypes, embryonal RMS (ERMS) and alveolar RMS (ARMS). RMS is occasionally reported to be the second neoplasm of hereditary retinoblastoma. Osteosarcoma is known as the most common second neoplasm of hereditary retinoblastoma, and tumorigenesis of osteosarcoma has been proven in previous studies to be related to the RB gene (RB1) alteration. Therefore, there might be a correlation between the tumorigenesis of RMS and RB1 alteration.
Methods
We examined the RB protein (pRB) expression and RB1 alteration such as allelic imbalance (gain or loss) and homozygous deletion, using immunohistochemistry, microsatellite makers, and quantitative real-time PCR in 57 sporadic RMS.
Results
Allelic imbalance was more frequently detected in ERMS (13/27), than in ARMS (3/20) (P = 0.04). Homozygous deletion on the protein-binding pocket domain of RB1 was found in 6 of 27 ERMS and in 2 of 20 ARMS (P = 0.24). Furthermore, immunohistochemical pRB labeling indexes (LI) in 31 ERMS (median value, 31%) were significantly reduced in comparison with those observed in 26 ARMS (median value, 85%) (P < 0.0001).
Conclusions
Our results support the assertion that tumorigenesis of RMS may be associated with RB1 alteration especially in ERMS, as previously reported for osteosarcoma. As for the RB pathway, each subtype of RMS may have a different tumorigenesis. In addition, immunohistochemical pRB LI may have the potential to be a useful ancillary tool in the differential diagnosis of RMS subtypes.
Keywords: Rhabdomyosarcoma, Retinoblastoma, Second neoplasm, Immunohistochemistry, Allelic imbalance
Introduction
Rhabdomyosarcoma (RMS) is the most common pediatric soft tissue sarcoma. Two major histologic subtypes of RMS can be identified: embryonal rhabdomyosarcoma (ERMS) and alveolar rhabdomyosarcoma (ARMS). Most ARMS is characterized cytogenetically by t(2;13)(q35;q14) or its variant, t(1;13)(p36;q14). These translocations create the chimeric genes PAX3-FKHR and PAX7-FKHR, respectively. However, no specific fusion gene has been documented in ERMS (Parham and Barr 2002a, b).
Retinoblastoma is hereditary in all patients with bilateral disease and in 10–15% of those with unilateral disease identified by a family history of retinoblastoma. Although the retinoblastoma gene (RB1) was initially cloned as a result of its frequent mutation in retinoblastoma, it is now thought to play a fundamental role in cellular regulation and is the target of tumorigenic mutations in many cell types. Recently, the cure rate of retinoblastoma has been excellent after enucleation or irradiation. Therefore, survivors of hereditary retinoblastoma are at increased risk of developing recurrent tumors or second primary neoplasms. Osteosarcoma is the most common second primary neoplasm after retinoblastoma, while rhabdomyosarcoma is also common as a secondary neoplasm (Aerts et al. 2004; Raymond et al. 2002; Tateishi et al. 2003). The tumorigenesis of these neoplasms may be related to RB1 alteration.
Therefore, we hypothesized that the tumorigenesis of RMS, especially that of ERMS, may be associated with RB1 alteration as well as osteosarcoma. In the current study of a large series of RMS, we analyzed RB1 alteration, including retinoblastoma protein (pRB) expression, allelic imbalance (gain or loss), and homozygous deletion, and compared the frequency of these alterations between ERMS and ARMS.
Materials and methods
Tumor samples
Fifty-seven formalin-fixed, paraffin-embedded RMS specimens (ERMS: 31 cases, ARMS: 26 cases) registered in the Department of Anatomic Pathology, Kyushu University, between 1970 and 2005 were available, and all the samples were from different patients. In addition, frozen tissue samples were available for 20 of the 57 cases (ERMS: 9 cases, ARMS: 11 cases). The diagnosis of all cases was based on light microscopic examination with hematoxylin-eosin (HE) staining according to the most recent WHO classification and, where necessary, immunoperoxidase procedures using the streptavidin-biotin peroxidase (SAB) method were carried out (Parham and Barr 2002a, b). That histological architecture of ERMS is composed of primitive mesenchymal cells in various stages of myogenesis, that is rhabdomyoblasts, with myxoid mesodermal tissues. In addition, ERMS is never arranged in alveolar patterns. ARMS is a primitive round cell neoplasm that cytologically resembles lymphoma and which shows partial skeletal muscle differentiation. That histological architecture produces fibrovascular septa that separate the round cells into discrete nests. All 20 frozen tissue samples were analyzed for PAX3/7-FKHR fusion transcripts as described previously, and these fusion transcripts were expressed only in ARMS (Jin et al. 2003).
Genomic DNA was isolated from paraffin-embedded or frozen material in 47 cases (ERMS: 27 cases, ARMS: 20 cases) using standard proteinase K digestion and phenol/chloroform extraction, and was used for the following molecular analysis.
Allelic imbalance analysis
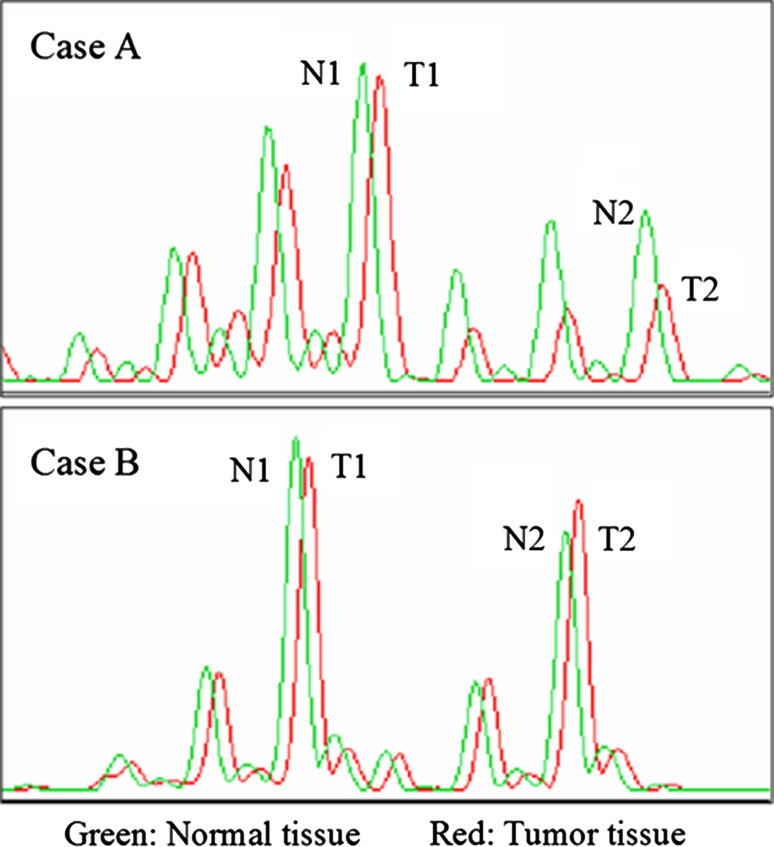
Tumor and normal DNA samples were subjected to PCR using primers for the following five dinucleotide microsatellite markers at 13q12-14: D13S175, D13S153, D13S233, D13S1293, and D13S1312. D13S153 is located within intron 2 of the RB1. The primer sequences were obtained from use of the NCBI UniSTS database (http://www.ncbi.nlm.nih.gov/). The forward primer was end-labeled with 6-carboxyfluorescein (6-FAM) at the 5′ end. The procedure was carried out according to a method described previously (Takahira et al. 2005). The data which were processed using GeneScan software (Applied Biosystems) were compared between the tumor and normal DNA for each patient. Informative cases were defined as cases in which the heterozygous alleles were identified within the normal DNA. Allelic imbalance (gain or loss) was considered to occur when the allelic imbalance index (AI index) was calculated to be less than 0.70 as previously described (Bergthorsson et al. 2006; Nishimura et al. 2006). The AI index was calculated with the allelic ratios of the tumor and normal DNA (select indices <1.0), using the peak areas, with the following formula: AI index = (T1/T2)/(N1/N2) or (T2/T1)/(N2/N1), in which T and N represent “tumor” and “normal”, and 1 and 2 indicate shorter and longer alleles (Fig. 1) (Bergthorsson et al. 2006; Nishimura et al. 2006). Reproducibility was confirmed by 2–4 independent PCR amplifications for each sample.
Fig. 1.
Estimation of allelic imbalance (AI) from the output of the ABI 310 genetic analyzer: chromatograms showing the genotypes of two normal-tumor tissue pairs. AI is evident in case A (2 years old, embryonal rhabdomyosarcoma): AI index = (T2/T1)/(N2/N1) <0.7. The tumor in case B (24 years old, alveolar rhabdomyosarcoma) is unaltered: AI index >0.7
Quantitative real-time PCR for homozygous deletion
The quantitative real-time PCR assay was based on original primers that specifically amplify the protein-binding pocket domain (exons 20–24) of RB1 (GenBank accession no. L11910) and GAPDH (GenBank accession no. AY340484) as a reference gene (Table 1). Using the ABI Prism 7700 Sequence Detection System (PE Applied Biosystems, Foster City, CA, USA), the PCR was performed in a total volume of 50 μl in each well, including 25 μl of SYBR Green PCR Master Mix (PE Applied Biosystems), 50 ng of genomic DNA, and 0.35 μl of 10 μM specific forward and reverse primers, which were determined after the analysis of the optimal concentrations of each primer. Each sample was run in triplicate in separate tubes to permit quantification of the RB1 gene normalized to GAPDH. The PCR condition consisted of an initial denaturation step of 95°C for 10 min, followed by 50 cycles of 95°C for 15 s and 60°C for 1 min (Aveyard and Knowles 2004; Berggren et al. 2003; Labuhn et al. 2001).
Table 1.
Oligonucleotides used for quantitative real-time PCR amplification
| Primer name | Sense 5′–3′ | Antisense 5′–3′ | Annealing (°C) | Size (bp) |
|---|---|---|---|---|
| GAPDH | gggctgctcacatattctgg | gcccaatacgaccaaatctaa | 60 | 80 |
| RB1 exon20 | cactttgtgaacgccttctg | ctggaaaagggtccagatga | 60 | 65 |
| RB1 exon21 | tggcatatgcaaagtgaagaa | cagcatgaggaagatccttg | 60 | 77 |
| RB1 exon22 | gtattctataactcggtcttcatgc | accctggtggaagcatactg | 60 | 68 |
| RB1 exon23 | acccttacggattcctggag | tgttggcagaccttctgaaa | 60 | 79 |
| RB1 exon24 | tggtatttcatcttaacttgacagaat | gaaaatactcaccccgaatga | 60 | 67 |
Data analysis was carried out using ABI Prism 7700 Sequence Detection Software, which calculates threshold cycle numbers (Ct). The Ct value is the cycle at which the fluorescent signal from a sample crosses a threshold as it enters the exponential phase in the PCR reaction. A standard curve is formulated from the starting copy number (x-axis) plotted against the Ct value (y-axis). The Ct values of unknown samples can then be used to determine the copy number from the standard curve. Only standard curves with correlation coefficients of 0.98 and higher were used. The gene dosage ratio was calculated as follows: (average copy number of target gene)/(average copy number of the reference gene). All gene dosage ratios were then normalized against the normal diploid control DNA to give a normalized gene dosage ratio (GDR). This procedure was carried out according to a method described previously (Aveyard and Knowles 2004).
Homozygous deletion was assumed when a paraffin-embedded sample had a GDR less than or equal to 0.36. This allows for the presence of up to 30% contaminating normal cell DNA in the template (Aveyard and Knowles 2004).
Immunohistochemistry
Immunohistochemical study was performed using a streptavidin-biotin-peroxidase method (histofine; Nichirei, Tokyo, Japan). The primary monoclonal antibody for the RB protein used in this study was the antibody for both the hyperphosphorylated and underphosphorylated RB gene product (clone G3-245; BD Pharmingen™, San Diego, CA; 1:800; 15 min microwave). We assessed the immunoreactivity with the labeling index (LI). To determine the RB labeling index (LI), the positively stained nuclei in at least 500 tumor cells were counted by three pathologists (KK, OY, HY) independently for each tumor. Furthermore, the protein expression in tumors was classified into two categories by the LI: heterogeneous (±), less than 50% of LI; homogeneous (+), more than 50% of LI.
Results
Allelic imbalance on chromosome 13q12-14
Allelic imbalance analysis at the 13q12-14 locus was performed in 47 cases. None of the cases showed non-informative findings for all five markers. Allelic imbalance for one or more markers was found in 13 of 27 (48%) cases with ERMS and in 3 of 20 (15%) cases with ARMS (Table 2). Representative examples of allelic imbalance are depicted in Fig. 1. The frequency of allelic imbalance in ERMS was significantly higher than that in ARMS (P = 0.04) (Table 2).
Table 2.
Relationship between RMS subtypes and genealterations
| ERMS | ARMS | P | |
|---|---|---|---|
| Allelic imbalance | |||
| (+) | 13 | 3 | |
| (−) | 14 | 17 | 0.04 |
| Homozygous deleton | |||
| (+) | 6 | 2 | |
| (−) | 21 | 18 | 0.24 |
| Allelic imbalance and/or homozygous deletion | |||
| (+) | 16 | 5 | |
| (−) | 11 | 15 | 0.04 |
Homozygous deletion of protein binding pocket domain of RB1 gene
The results of quantitative real-time PCR for homozygous deletion analysis are summarized in Table 2. None of the cases showed non-informative findings for five exons. Homozygous deletion for one or more exons was more frequently found in ERMS (6/27: 22%) than in ARMS (2/20: 10%), but the difference was not statistically significant (P = 0.24) (Table 2). Furthermore, allelic imbalance and/or homozygous deletion in tumors were found significantly more frequently in ERMS (16/27: 59%) than in ARMS (5/20: 25%) (P = 0.04) (Table 2).
Immunohistochemistry of pRB
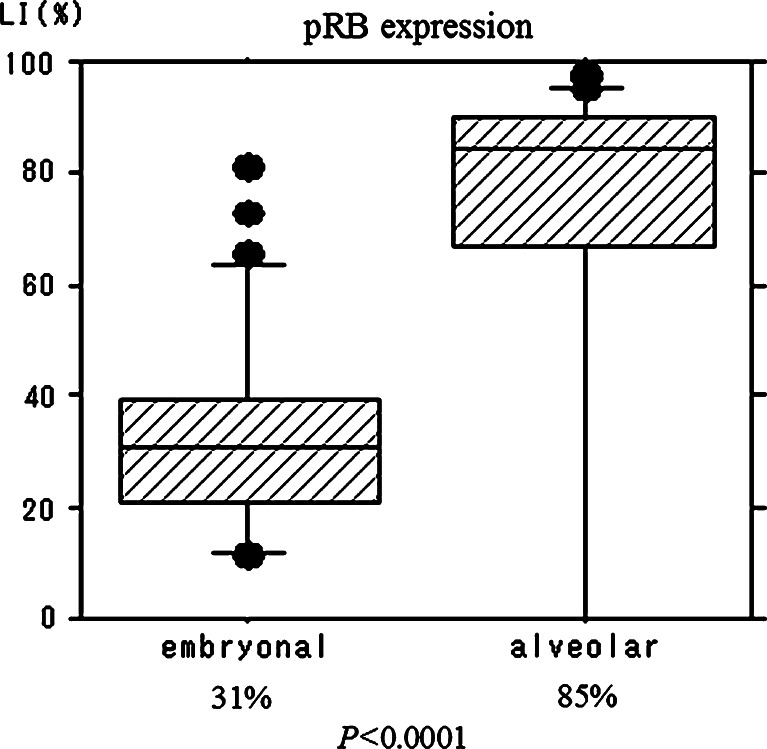
pRB LI was significantly higher in 26 ARMS cases (median value, 85%) than in 31 ERMS cases (median value, 31%) (P < 0.0001; Figs. 2, 3). Moreover, the relationship between allelic imbalance and pRB expression was also evaluated, and is summarized in Table 3. Among allelic-imbalance-positive cases, heterogeneous staining was observed in 11 out of 16 cases (69%), while among allelic-imbalance-negative cases, heterogeneous staining was recognized in 17 out of 31 cases (55%). There was no significant correlation between allelic imbalance and pRB immunohistochemical expression (P = 0.54).
Fig. 2.
Boxplot of retinoblastoma protein (pRB) expression. The median values of the pRB labeling index in embryonal and alveolar rhabdomyosarcoma are 31 and 85%, respectively (P < 0.0001)
Fig. 3.

Immunohistochemical staining of retinoblastoma protein (pRB) expression. a The numbers of tumor cells with a positive nuclear reaction are reduced; the labeling index is 37% (1 year old, embryonal rhabdomyosarcoma). b Most tumor cells show the immunoreaction in the nuclei; the labeling index is 88% (5 years old, alveolar rhabdomyosarcoma)
Table 3.
Relationship between Allelic imbalance (AI) and pRB expression
| Heterogeneous | Homogeneous | Total | |
|---|---|---|---|
| AI (+) | 11 (69%) | 5 (31%) | 16 |
| AI (−) | 17 (55%) | 14 (45%) | 31 |
| P = 0.54 |
Discussion
Hereditary retinoblastoma patients have a high risk of second primary neoplasms such as osteosarcoma, rhabdomyosarcoma, meningioma, and others (Aerts et al. 2004; Tateishi et al. 2003). Generally, such an osteosarcoma is likely to show loss of heterozygosity (LOH) at 13q and alteration of the RB1 tumor suppressor gene (Raymond et al. 2002; Wadayama et al. 1994). According to several previous studies, the frequency of RB1 alterations in sporadic osteosarcoma has been found to vary between 30 and 40% (Raymond et al. 2002). Therefore, tumorigenesis of osteosarcoma was indicated in association with RB1 alteration in part. Rhabdomyosarcoma was also occasionally reported as a second neoplasm of hereditary retinoblastoma (Aerts et al. 2004; Tateishi et al. 2003). Therefore, we analyzed the RB1 alteration of sporadic rhabdomyosarcoma.
In molecular analysis, we identified allelic imbalance at 13q12-14 and/or homozygous deletion of the protein binding pocket domain of RB1 gene in 21 of 47 cases (45%). In particular, in ERMS, the frequency of allelic imbalance at 13q12-14 (48%; 13 of 27 ERMS) was found to be significantly higher than in ARMS (15%; three of 20 ARMS) (P = 0.04). In the previous cytogenetic and comparative genomic hybridization studies, ERMS was found to show a complex structure and numerous chromosomal changes, often including extra copies of chromosome 13 and other changes (Bridge et al. 2002; Gordon et al. 2001; Polito et al. 1999; Xia et al. 2002), and those results supported our results.
At the present time, the common diagnostic factors distinguishing ERMS from ARMS are morphological arrangements of the tumor cells, myogenin immunoreactivity, and specific fusion gene transcripts (Parham and Barr 2002a, b; Hostein et al. 2004). However, when molecular genetic analysis was not available, especially in the solid variant of ARMS, differential diagnosis might be very difficult. In the present study, pRB expression in ARMS subtype (median value of LI, 85%) was almost preserved, whereas, pRB expression in ERMS subtype (median value of LI, 31%) was significantly decreased (P < 0.0001). Therefore, pRB LI has the potential to be a useful ancillary tool in the differential diagnosis of RMS subtypes (Fig. 4).
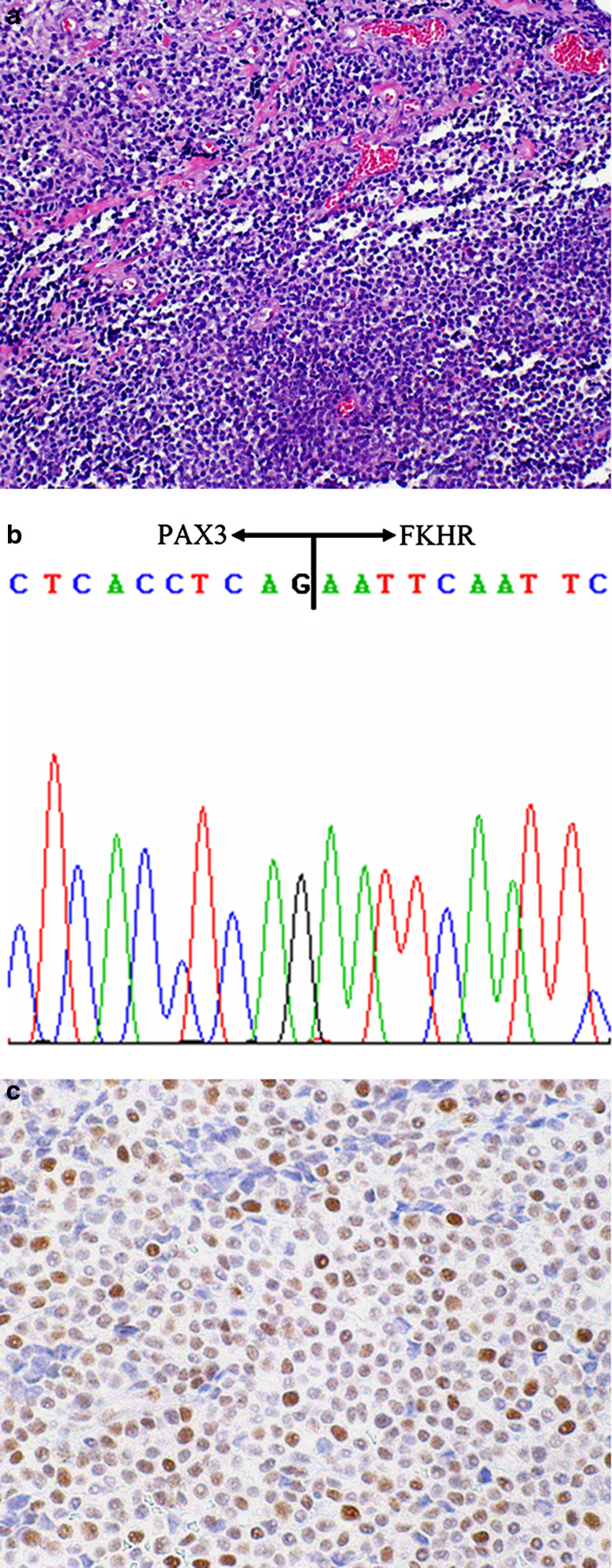
Fig. 4.
Solid variant of alveolar rhabdomyosarcoma (1 month old, orbit). a Although the characteristic alveolar structures are not present, cellular nests are still separated by fibrovascular septa. b PAX3-FKHR fusion gene transcript is detected in this case. c Most tumor cell nuclei show retinoblastoma protein (pRB) immunopositivity
Moreover, our immunohistochemical result suggested that RB1 gene alteration may frequently be present in ERMS, rather than ARMS; may be predominant in second neoplasms of hereditary retinoblastoma. However, the actual subtype of secondary RMS have not been precisely documented in previous studies (Aerts et al. 2004; Tateishi et al. 2003). In addition, no close relationship between pRB expression and allelic imbalance at 13q12-14 was found (P = 0.54). Those results suggested that other alterations such as hypermethylation and mutation may be present.
Although various sarcomas were found to have acquired RB1 mutation in the previous studies (Polito et al. 1999), no such mutations were identified in sporadic cases of rhabdomyosarcoma (Polito et al. 1999). While RB1 mutations were not detected in RMS, genetic changes were found in genes encoding proteins that regulate pRB function (Polito et al. 1999). In particular, RMS cases have been identified with the amplification of the CDK4 gene, and with deletions of the CDKN2A and CDKN2B genes (Polito et al. 1999). However, as pRB expression levels were reduced in our study, the possibility of a non-mutational mechanism causing the reduction of protein expression should also be considered.
Genetically, sarcomas fall into two main categories with regard to tumorigenesis (Helman and Meltzer 2003). One group is characterized by a tumor-specific translocation that seems to be the main pathogenesis of the tumor, and indeed is being incorporated as a diagnostic criterion. Another group is characterized not by a recurring, tumor-specific genetic alteration but by complex karyotypes that are characteristic of severe genetic and chromosomal instability. However, ERMS and ARMS each belong to a different one of those groups despite having the same expression profile of skeletal muscle differentiation; therefore, each subtype of RMS is suggested to have a different tumorigenesis. ARMS is found to have the specific fusion gene transcription of PAX3-FKHR and PAX7-FKHR, and ERMS is found to disclose the complex karyotypes seen in previous studies (Bridge et al. 2002; Gordon et al. 2001; Polito et al. 1999). In particular, in ERMS, allelic loss in chromosomal region 11p15 has also been observed (Xia et al. 2002; Asakura and Rudnicki 2003; Koi et al. 1993; Li et al. 1998; Loh et al. 1992; Scrable et al. 1987). In addition, we found the possibility of a relationship between RB1 gene alteration and tumorigenesis, as is considered to exist in osteosarcoma. However, the possibility that a higher frequency of RB1 alterations is simply related to genetic instability should be considered.
In summary, in each subtype of RMS, we first investigated the RB1 gene alteration, including pRB expression. ERMS significantly showed a higher frequency of RB1 gene and protein alteration than ARMS. Our results support the assertion that the tumorigenesis of rhabdomyosarcoma, especially ERMS, may be associated with RB1 gene alteration, as in osteosarcoma. Each subtype of RMS is suggested to have a different tumorigenesis with respect to the RB pathway. Moreover, pRB LI has the potential to become an ancillary parameter in the differential diagnosis of the RMS subtypes.
Acknowledgments
The English used in this manuscript was revised by KN International (http://www.kninter.com/).
Abbreviations
- RMS
Rhabdomyosarcoma
- ERMS
Embryonal rhabdomyosarcoma
- ARMS
Alveolar rhabdomyosarcoma
- RB1
Retinoblastoma gene
- pRB
Retinoblastoma protein
- LI
Labeling index
- AI
Allelic imbalance
Footnotes
This study was supported by a Grant-in-Aid for Scientific Research (C) (no. 18590332) from the Japan Society for the Promotion of Science, Tokyo, Japan.
References
- Aerts I, Pacquement H, Doz F, Mosseri V, Desjardins L, Sastre X, Michon J, Rodriguez J, Schlienger P, Zucker JM, Quintana E (2004) Outcome of second malignancies after retinoblastoma: a retrospective analysis of 25 patients treated at the Institut Curie. Eur J Cancer 40:1522–1529 [DOI] [PubMed] [Google Scholar]
- Asakura A, Rudnicki MA (2003) Rhabdomyosarcomagenesis-Novel pathway found. Cancer Cell 4:421–422 [DOI] [PubMed] [Google Scholar]
- Aveyard JS, Knowles MA (2004) Measurement of relative copy number of CDKN2A/ARF and CDKN2B in bladder cancer by real-time quantitative PCR and multiplex ligation-dependent probe amplification. J Mol Diagn 6:356–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berggren P, Kumar R, Sakano S, Hemminki L, Wada T, Steineck G, Adolfsson J, Larsson P, Norming U, Wijkstrom H, Hemminki K (2003) Detecting homozygous deletions in the CDKN2A(p16(INK4a))/ARF(p14(ARF)) gene in urinary bladder cancer using real-time quantitative PCR. Clin Cancer Res 9:235–242 [PubMed] [Google Scholar]
- Bergthorsson JT, Agnarsson BA, Gudbjartsson T, Magnusson K, Thoroddsen A, Palsson B, Bjornsson J, Stefansson K, Gulcher J, Einarsson GV, Amundadottir LT, Barkardottir RB (2006) A genome-wide study of allelic imbalance in human testicular germ cell tumors using microsatellite markers. Cancer Genet Cytogenet 164:1–9 [DOI] [PubMed] [Google Scholar]
- Bridge JA, Liu J, Qualman SJ, Suijkerbuijk R, Wenger G, Zhang J, Wan X, Baker KS, Sorensen P, Barr FG (2002) Genomic gains and losses are similar in genetic and histologic subsets of rhabdomyosarcoma, whereas amplification predominates in embryonal with anaplasia and alveolar subtypes. Genes Chromosomes Cancer 33:310–321 [DOI] [PubMed] [Google Scholar]
- Gordon T, McManus A, Anderson J, Min T, Swansbury J, Pritchard-Jones K, Shipley J; United Kingdom Children’s Cancer Study Group; United Kingdom Cancer Cytogenetics Group (2001) Cytogenetic abnormalities in 42 rhabdomyosarcoma: a United Kingdom Cancer Cytogenetics Group Study. Med Pediatr Oncol 36:259–267 [DOI] [PubMed] [Google Scholar]
- Helman LJ, Meltzer P (2003) Mechanisms of sarcoma development. Nat Rev Cancer 3:685–694 [DOI] [PubMed] [Google Scholar]
- Hostein I, Andraud-Fregeville M, Guillou L, Terrier-Lacombe MJ, Deminiere C, Ranchere D, Lussan C, Longavenne E, Bui NB, Delattre O, Coindre JM (2004) Rhabdomyosarcoma: value of myogenin expression analysis and molecular testing in diagnosing the alveolar subtype: an analysis of 109 paraffin-embedded specimens. Cancer 101:2817–2824 [DOI] [PubMed] [Google Scholar]
- Jin L, Majerus J, Oliveira A, Inwards CY, Nascimento AG, Burgart LJ, Lloyd RV (2003) Detection of fusion gene transcripts in fresh-frozen and formalin-fixed paraffin-embedded tissue sections of soft-tissue sarcomas after laser capture microdissection and rt-PCR. Diagn Mol Pathol 12:224–230 [DOI] [PubMed] [Google Scholar]
- Koi M, Johnson LA, Kalikin LM, Little PF, Nakamura Y, Feinberg AP (1993) Tumor cell growth arrest caused by subchromosomal transferable DNA fragments from chromosome 11. Science 260:361–364 [DOI] [PubMed] [Google Scholar]
- Labuhn M, Jones G, Speel EJ, Maier D, Zweifel C, Gratzl O, Van Meir EG, Hegi ME, Merlo A (2001) Quantitative real-time PCR does not show selective targeting of p14(ARF) but concomitant inactivation of both p16(INK4A) and p14(ARF) in 105 human primary gliomas. Oncogene 20:1103–1109 [DOI] [PubMed] [Google Scholar]
- Li M, Squire JA, Weksberg R (1998) Molecular genetics of Wiedemann-Beckwith syndrome. Am J Med Genet 79:253–259 [PubMed] [Google Scholar]
- Loh WE Jr, Scrable HJ, Livanos E, Arboleda MJ, Cavenee WK, Oshimura M, Weissman BE (1992) Human chromosome 11 contains two different growth suppressor genes for embryonal rhabdomyosarcoma. Proc Natl Acad Sci USA 89:1755–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Nishida N, Komeda T, Fukuda Y, Ikai I, Yamaoka Y, Nakao K (2006) Genome-wide semiquantitative microsatellite analysis of human hepatocellular carcinoma: discrete mapping of smallest region of overlap of recurrent chromosomal gains and losses. Cancer Genet Cytogenet 167:57–65 [DOI] [PubMed] [Google Scholar]
- Parham DM, Barr FG (2002a) Alveolar rhabdomyosarcoma. In: Fletcher CDM, Unni KK, Mertens F (eds) WHO classification of tumours, pathology and genetics of tumours of soft tissue and bone. IARG Press, Lyon, pp 150–152 [Google Scholar]
- Parham DM, Barr FG (2002b) Embryonal rhabdomyosarcoma. In: Fletcher CDM, Unni KK, Mertens F (eds) WHO classification of tumours, pathology and genetics of tumours of soft tissue and bone. IARG Press, Lyon, pp 146–149 [Google Scholar]
- Polito P, Dal Cin P, Sciot R, Brock P, Van Eyken P, Van den Berghe H (1999) Embryonal rhabdomyosarcoma with only numerical chromosome changes. Case report and review of the literature. Cancer Genet Cytogenet 109:161–165 [DOI] [PubMed] [Google Scholar]
- Raymond AK, Ayala AG, Knuutila S (2002) Conventional osteosarcoma. In: Fletcher CDM, Unni KK, Mertens F (eds) WHO classification of tumours, pathology and genetics of tumours of soft tissue and bone. IARG Press, Lyon, pp 264–270 [Google Scholar]
- Scrable HJ, Witte DP, Lampkin BC, Cavenee WK (1987) Chromosomal localization of the human rhabdomyosarcoma locus by mitotic recombination mapping. Nature 329:645–647 [DOI] [PubMed] [Google Scholar]
- Takahira T, Oda Y, Tamiya S, Yamamoto H, Kobayashi C, Izumi T, Ito K, Iwamoto Y, Tsuneyoshi M (2005) Alterations of the RB1 gene in dedifferentiated liposarcoma. Mod Pathol 18:1461–1470 [DOI] [PubMed] [Google Scholar]
- Tateishi U, Hasegawa T, Miyakawa K, Sumi M, Moriyama N (2003) CT and MRI features of recurrent tumors and second primary neoplasms in pediatric patients with retinoblastoma. Am J Roentgenol 181:879–884 [DOI] [PubMed] [Google Scholar]
- Wadayama B, Toguchida J, Shimizu T, Ishizaki K, Sasaki MS, Kotoura Y, Yamamuro T (1994) Mutation spectrum of the retinoblastoma gene in osteosarcomas. Cancer Res 54:3042–3048 [PubMed] [Google Scholar]
- Xia SJ, Pressey JG, Barr FG (2002) Molecular pathogenesis of rhabdomyosarcoma. Cancer Biol Ther 1:97–104 [DOI] [PubMed] [Google Scholar]