Abstract
Purpose
To compare the efficacy and safety of a biweekly CHOP regimen consisting of cyclophosphamide (CPA), doxorubicin (DOX), vincristine (VCR), and prednisolone (PSL) and those of a biweekly THP-COP regimen containing pirarubicin (THP), an anthracyclin with less cardiotoxicity than DOX.
Methods
A prospective, randomized phase II study with 80 patients (40 receiving CHOP or THP-COP) less than 70 years of age with previously untreated aggressive non-Hodgkin’s lymphoma (NHL). The regimens consisted of DOX or THP 50 mg/m2, CPA 750 mg/m2, VCR 1.4 mg/m2, and PSL 100 mg/body administered for 5 days every 2 weeks for eight cycles.
Results
No significant differences in known prognostic factors were found between the two groups. Complete remission rate was 72.5% (72.5% for CHOP, 72.5% for THP-COP). The 5-year overall survival rate was 49.2% (43.7% for CHOP, 54.0% for THP-COP). When the patients were divided into groups with favorable or poor prognostic factors according to the International Prognostic Index, survival of the former group (L/LI) was superior to that of the later group (HI/H), regardless of chemotherapy regimen (P<0.001). Although grade 3 cardiotoxicity occurred in one patient in the CHOP group, no fatal toxic reactions occurred in either group. The THP-COP produced results equivalent to those of CHOP regarding efficacy and safety in aggressive NHL patients less than 70 years of age.
Conclusions
Although both regimens effectively treated those patients with favorable prognostic factors, neither was satisfactory for treating those with poor prognostic factors.
Keywords: Biweekly CHOP, Aggressive non-Hodgkin’s lymphoma, Pirarubicin, Doxorubicin
Introduction
The conventional treatment of patients with intermediate grade (aggressive) non-Hodgkin’s lymphoma (NHL) is a doxorubicin-containing combination chemotherapy, particularly the CHOP regimen consisting of cyclophosphamide (CPA), doxorubicin (DOX), vincristine (VCR), and prednisolone (PSL), which was developed in the last 20 years. Complete remission (CR) rates following treatment with the CHOP regimen ranges from 45% to 55%; consequently, less than 40% of the patients may be cured. To improve upon these results, intensive combination chemotherapy known as second- or third-generation regimens were introduced in the 1980s. These intensive regimens have been shown to result in a CR rate greater than 60% with long-term survival in more than 50% of the patients. However, the superiority of these new regimens over the CHOP regimen has not been demonstrated in trials designed for that purpose (Fisher et al. 1993). Since 1993, CHOP therapy has been considered to be the gold standard chemotherapy regimen. However, when patients treated with the CHOP regimen were classified into prognostic groups in accordance with the International Prognostic Index (IPI), the 5-year survival rates were 73% for the low risk group, 51% for the low-intermediate risk group, 43% for the high-intermediate risk group, and 26% for the high-risk group (The International Non-Hodgkin’s Lymphoma Prognostic Factors Project 1993). These observations indicate that the CHOP regimen fails to achieve a satisfactory outcome in most cases of aggressive NHL. To improve upon the efficacy of chemotherapy for treating aggressive NHL in patients with poor prognostic factors, recent studies have investigated whether dose intensification could be achieved by combining granulocyte colony-stimulating factor (G-CSF) into a modified escalating-dose CHOP regimen or a short-interval CHOP regimen (Tanosaki et al. 1994; Santoro et al. 1999). Pirarubicin (tetrahydropyranyl-adriamycin: THP), a derivative of DOX, is reportedly an anthracyclin with less cardiotoxicity than DOX (Umezawa et al. 1979; Miller and Salewski 1994; Takagi and Oguro 1987) and a THP-COP regimen consisting of THP, CPA, VCR, and PSL has been administered for the treatment of NHL in elderly patients (Kitamura and Takaku 1990; Aoki et al. 1998). However, the efficacy of the THP-COP regimen for the treatment of NHL in younger patients is still unknown. We have compared the efficacy and safety of a biweekly CHOP regimen with G-CSF and those of a biweekly THP-COP regimen in a prospective, randomized phase II study in previously untreated aggressive NHL patients less than 70 years of age.
Materials and methods
Patient selection
For this study, we consecutively enrolled patients less than 70 years of age with intermediate-grade NHL (Working-Formulation groups D through G) (The non-Hodgkin’s lymphoma pathologic classification project 1982), confirmed by biopsy but previously untreated. Additionally, these patients were not infected with human immunodeficiency virus or human T-cell lymphotropic virus type I. All patients had NHL in either stage II, III, or IV of the disease according to the Ann Arbor classification (Carbone et al. 1971). Clinical staging (CS) evaluation consisted of: a physical examination, chest radiology, computed tomography, and magnetic resonance imaging of the brain, neck, chest, abdomen, and pelvis; bone marrow aspiration and biopsy; gallium scintigraphy; and laboratory measurements of serum aspartate aminotransferase, total bilirubin, alkaline phosphatase, creatinine, lactate dehydrogenase (LDH), C-reactive protein (CRP), soluble interleukin-2 receptors (sIL-2R), and peripheral blood counts. All patients had normal cardiac function (left ventricular ejection fraction of >50%), a serum creatinine concentration lower than 1.6 mg/dl, no severe complications, no multiple malignancies, performance status (PS) ranging from 0 to 3, and evaluable lesions. All patients gave written informed consent according to institutional guidelines and the Declaration of Helsinki.
Treatment
Patients were randomly assigned to receive eight cycles of CHOP or THP-COP therapy. Each regimen consisted of CPA (750 mg/m2, given as a 2-h intravenous drip infusion on day 1, DOX or THP (50 mg/m2, given as a 30-min intravenous drip infusion on day 1, VCR [1.4 mg/m2 (maximal dose, 2.0 mg]), given intravenously in a bolus over 5 min on day 1, and PSL (100 mg daily, given orally on days 1–5). G-CSF was administered subcutaneously at 2 µg/kg on days 6–12. The chemotherapy cycles were repeated at 14-day intervals if the absolute neutrophil count was more than 1×109/l and the platelet count was more than 50×109/l. Patients with a bulky mass received radiotherapy ranging from 30 Gy to 40 Gy after chemotherapy. Patients who relapsed or had disease progression after CHOP or THP-COP, and patients who were resistant to CHOP or THP-COP received the P-IMVP-16/CBDCA regimen (Sawada et al. 2002), which consisted of methylprednisolone, ifosfamide, methotrexate, etoposide, and carboplatin, as a second-line regimen. Some of the patients with refractory or relapsed NHL who responded to the P-IMVP-16/CBDCA received high-dose chemotherapy followed by autologous hematopoietic stem cell transplantation (autoHSCT).
Response criteria
Tumor response for chemotherapies was evaluated after the second, fourth, sixth, and eighth (final) course; however, patients with an aggravation tendency after each course of chemotherapy were considered as failures at those points. Response to treatment was categorized according to the response criteria defined by the International Workshop (Cheson et al. 1999). The disappearance of all evidence of disease for at least 1 month qualified as complete remission (CR). Patients with residual masses were classified as uncertain CR (CRu), which denotes the disappearance of all disease apart from residual radiological abnormalities (less than 25% of the initial volume) of uncertain significance. Partial response (PR) was defined as a >50% decrease in the sum of the products of the greatest perpendicular diameters (SPD) of all measurable lesions, lasting for at least 28 days. When the criteria for CR, CRu, or PR were not met, the case was considered to be a treatment failure. Progressive disease (PD) was defined as either a >50% increase from the nadir in the SPD of the measurable lesions of CRu, PR, or non-responders, or the appearance of a new lesion. Overall survival (OS) was measured from the time of randomization until death from any cause. Progression-free survival (PFS) was defined for all eligible patients, including non-responders, as the interval from the day of initiation of chemotherapy to the day on which progression or death due to any cause was observed. Time to progression (TTP) was defined for all responders (CR, CRu, PR) as the interval from the day of initiation of chemotherapy to the day on which progression was observed.
Statistical analysis
The end points were response rate, survival period, and life-threatening or fatal toxic effects. Data from all patients were analyzed on an intention-to-treat basis. Estimates of survival were determined by the Kaplan-Meier method. Patients’ characteristics and adverse effects were compared by chi-square test. Univariate analyses of several pretreatment features for their effect on attaining CR or PR were performed using chi-square test. Analyses of several pretreatment features for their effect on survival were performed using the log-rank test based on the Kaplan-Meier method (Kaplan and Meier 1958). Multivariate analysis was performed using the Cox regression technique to define the prognostic significance of selected covariates (Cox 1972).
Results
Patients characteristics
Although 85 patients were enrolled in the study between April 1995 and December 2000, five patients were excluded either because they declined to participate or because they were transferred to other hospitals. Thus, 80 consecutive patients (52 men and 28 women, median age 55 years; range 19–69 years) were eligible. We randomly assigned 40 patients to receive the CHOP regimen or 40 patients to receive the THP-COP regimen. Their NHL subtypes included: follicular large cell, five patients (three in CHOP group, two in THP-COP group); diffuse small-cleaved cell, two patients (1, 1); diffuse mixed small and large cell, six patients (3, 3); and diffuse large cell, 67 patients (34, 33). The histology was also reclassified by the new WHO lymphoma classification (Jaffe et al. 2001) as follows: follicular lymphoma, five patients (three in CHOP group, two in THP-COP group); mantle cell lymphoma, six patients (3, 3); diffuse large B-cell lymphoma, 55 patients (28, 27); and peripheral T-cell lymphoma, 14 patients (6, 8). No significant differences were found between the CHOP regimen group and the THP-COP regimen group according to patient characteristics.
Response to therapy
Of the 80 patients who were evaluated for response to treatment, 67 (83.8%) reached CR, CRu, or PR. The CHOP group had a CR/CRu rate of 72.5% (29/40) and a PR rate of 12.5% (5/40); the THP-COP group had a CR/CRu rate of 72.5% (29/40) and a PR rate of 10.0% (4/40). No significant differences in response rates were found between the CHOP and THP-COP groups (Table 1).
Table 1.
Therapeutic effects (response rates) CR+CRu/Total(%)
| All patients | CHOP | THP-COP | ||
|---|---|---|---|---|
| Overall patients | 58/80 (72.5) | 29/40 (72.5) | 29/40 (72.5) | |
| Sex | Male | 38/52 (73.1) | 18/23 (78.3) | 20/29 (69.0) |
| Female | 20/28 (71.4) | 11/17 (64.7) | 9/11 (81.8) | |
| Age | 60> | 30/37 (81.1) | 19/22 (86.4) | 11/15 (73.3) |
| 60≦ | 28/43 (65.1) | 10/18 (55.6) | 18/25 (72.0) | |
| LDH | Normal | 31/36 (86.1) | 15/16 (93.8) | 16/20 (80.0) |
| Elevated | 27/44 (61.4) | 14/24 (58.3) | 13/20 (65.0) | |
| Clinical Stage | II | 20/24 (83.3) | 7/10 (70.0) | 13/14 (92.3) |
| III, IV | 38/56 (67.9) | 22/30 (73.3) | 16/26 (61.5) | |
| Performance status | 0,1 | 52/64 (81.3) | 27/32 (84.4) | 25/32 (78.1) |
| 2,3 | 6/16 (37.5) | 2/8 (25.0) | 4/8 (50.0) | |
| Extranodal involvement | ||||
| 0,1 | 40/54 (74.1) | 22/29 (75.9) | 18/25 (72.0) | |
| 2≦ | 18/26 (69.2) | 7/11 (63.6) | 11/15 (73.3) | |
| International prognostic index | ||||
| L | 19/20 (95.0) | 8/9 (88.9) | 11/11 (100) | |
| LI | 12/15 (80.0) | 6/8 (75.0) | 6/7 (85.7) | |
| HI | 16/20 (80.0) | 9/10 (90.0) | 7/10 (70.0) | |
| H | 11/25 (44.0) | 6/13 (46.2) | 5/12 (41.7) | |
| sIL-2R | ||||
| 1,000> | 29/31 (93.5) | 14/14 (100) | 15/17 (88.2) | |
| 1,000≦ | 29/49 (59.2) | 15/26 (57.7) | 14/23 (60.9) | |
| Histology (phenotype) | ||||
| T | 9/14 (64.3) | 3/6 (50.0) | 6/8 (75.0) | |
| B | 49/66 (74.2) | 26/34 (76.5) | 23/32 (71.2) | |
Survival
The median follow-up time was 42 months. The OS rate was 74.3% after 2 years and 49.2% after 5 years (Fig. 1a). The PFS rate was 60.5% after 2 years and 46.8% after 5 years, with a median PFS of 50 months. The Kaplan-Meier curve of TTP in all responders was 72.0% after 2 years and 58.4% after 5 years, with a median TTP of 62 months (Fig. 1a). When the 80 patients in this study were separated into either a group with favorable (low or low-intermediate) prognostic factors or one with poor (high-intermediate or high) prognostic factors according to the IPI risk groups, the OS of the group with favorable prognostic factors (93.5% after 2 years and 77.6% after 5 years) was superior to that of the group with poor prognostic factors (60.0% after 2 years and 30.7% after 5 years) (P<0.001) (Fig. 1b).
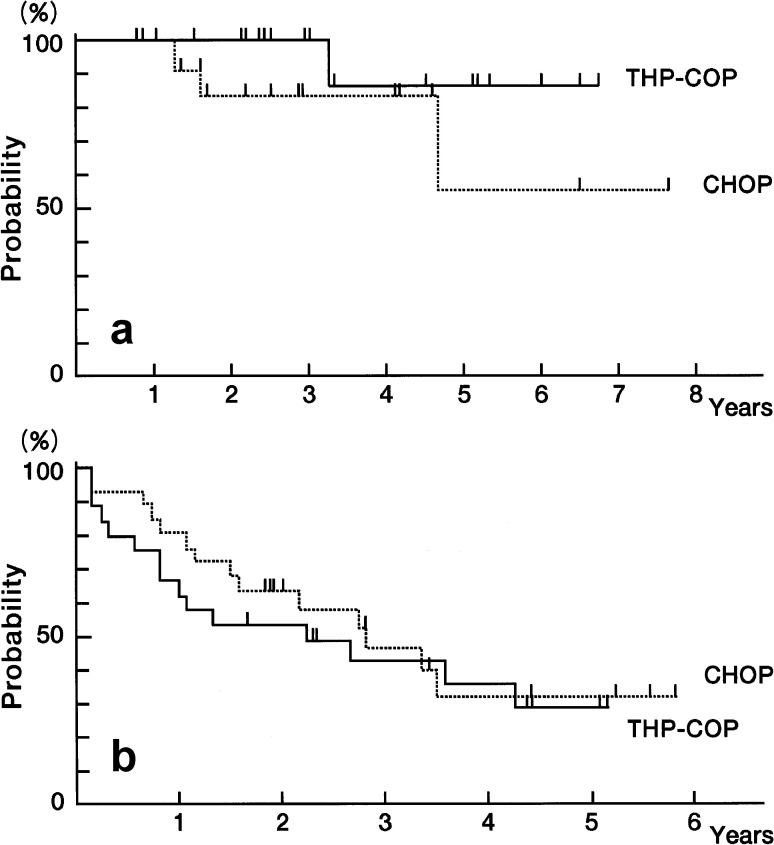
Fig. 1.
a Survival curves of aggressive non-Hodgkin’s lymphoma patients treated with biweekly CHOP or THP-COP (OS overall survival, TTP time to progression); b Comparative overall survival curves of aggressive non-Hodgkin’s lymphoma patients treated with biweekly CHOP or THP-COP according to the International Prognostic Index risk groups
In the CHOP regimen group, the OS rate was 73.8% after 2 years and 43.7% after 5 years, with a median survival time of 54 months (Fig. 2a). The PFS rate was 59.2% after 2 years and 41.6% after 5 years, with a median PFS of 49 months. The Kaplan-Meier curve of TTP in responders with the CHOP regimen was 69.2% after 2 years and 48.4% after 5 years, with a median TTP of 56 months (Fig. 2b). In the THP-COP regimen group, the OS rate was 74.8% after 2 years and 54.0% after 5 years, with a median survival time of 55 months (Fig. 2a). The PFS rate was 61.8% after 2 years and 51.9% after 5 years, with a median PFS of 49 months. The Kaplan-Meier curve of TTP in responders with the THP-COP regimen was 74.9% after 2 years and 67.4% after 5 years, with a median TTP of 64 months (Fig. 2b). No significant differences were found in OS, PFS, and TTP between the CHOP and THP-COP groups. Neither were significant differences found in the survival of the NHL patients, according to the IPI risk groups, in OS between the CHOP and THP-COP groups (Fig. 3a, Fig. 3b).
Fig. 2a, b.
Comparative survival curves of aggressive non-Hodgkin’s lymphoma patients treated with biweekly CHOP and THP-COP. a overall survival; b time to progression
Fig. 3a, b.
Comparative overall survival curves of aggressive non-Hodgkin’s lymphoma patients treated with biweekly CHOP and THP-COP according to the International Prognostic Index risk groups. a overall survival of the patients with favorable prognostic factors (low, low-intermediate); b overall survival of the patients with poor prognostic factors (high, high-intermediate)
Prognostic factors
Univariate analysis demonstrated that PS, CS, LDH, sIL-2R, CRP, and the number of extranodal involvement were prognostic factors for OS. Multivariate analysis of these factors demonstrated that PS was a single independent prognostic factor for OS (P<0.01, risk ratio 3.3, 95% confidence interval 1.56–6.81).
Toxicity and reasons for stopping therapy
Adverse effects were coded according to the National Cancer Institute-Common Toxicity criteria version 2.0. Although biweekly CHOP and THP-COP regimens commonly caused neutropenia, G-CSF treatment overcame it. Pneumonia due to neutropenia developed in one patient in the CHOP group and in two patients in the THP-COP group; the three cases were resolved with antibiotics, and completed the chemotherapy. Nonhematologic adverse effects such as gastrointestinal, hepatic, cardiac, renal, or neurologic damages were generally mild and tolerable (Table 2). In one patient given the biweekly CHOP regimen, we found grade 3 cardiac damage that was reversible. However, the patient completed the chemotherapy. Regimen-related death was not observed in either of the treatment groups. Apart from treatment failure or toxicity, two patients discontinued the treatment after the fifth course of CHOP or the sixth course of THP-COP, respectively, because of rejection by the patients.
Table 2.
Adverse effects (>grade 2)
| CHOP n=40 (%) | THP-COP n=40 (%) | |
|---|---|---|
| Hematological toxicity | ||
| Anemia | 2 (5.0) | 1 (2.5) |
| Neutropenia | 12 (30.0) | 13 (32.5) |
| Thrombocytopenia | 1 (2.5) | 2 (5.0) |
| Non-hematological toxicity | ||
| Nausea/vomiting | 2 (5.0) | 1 (2.5) |
| Stomatitis | 2 (5.0) | 2 (5.0) |
| Diarrhea | 0 | 0 |
| Constipation | 1 (2.5) | 1 (2.5) |
| Liver dysfunction | 1 (2.5) | 2 (5.0) |
| Renal dysfunction | 1 (2.5) | 0 |
| Hematuria | 1 (2.5) | 0 |
| Arrythmia | 1 (5.5) | 0 |
| Heart failure | 1 | 0 |
| Respiratory failure | 0 | 1 (2.5) |
| Neuropathy | 1 (2.5) | 2 (5.0) |
| Infection | 1 (2.5) | 2 (5.0) |
Discussion
The CHOP regimen was clearly not sufficient for treating aggressive NHL, because it resulted in a CR rate of less than 35% in patients with advanced-stage aggressive NHL (Fisher et al. 1993). Because trials of the CHOP regimen were carried out when G-CSF was not available for clinical use, dose intensity was reduced due to myelosuppression, primarily neutropenia, leading to a decrease in the efficacy of treatment. A close association between dose intensity and survival has been reported (DeVita et al. 1987). Kwak et al. reported a significant correlation between the dose intensity of CPA and DOX and the survival time in patients with diffuse large-cell lymphoma (Kwak et al. 1990). Therefore, chemotherapeutic approaches at high-dose intensities performed in combination with G-CSF have recently been carried out (Shipp et al. 1995; Niitsu and Umeda 1998; Witzig et al. 1998). In the present study, we designed biweekly CHOP and THP-COP regimens of high-dose intensity administered in combination with G-CSF. Unfortunately, although the efficacy of the regimens for NHL patients with favorable (low- and low-intermediate risk) prognostic factors was relatively satisfactory, that for patients with poor (high-intermediate and high-risk) prognostic factors was not. Our data suggest that biweekly CHOP and THP-COP regimens with G-CSF fails to achieve a satisfactory outcome for patients with aggressive NHL, especially in patients with poor prognostic factors.
To improve on the prognosis for young NHL patients with poor prognostic factors, high-dose chemotherapy combined with autoHSCT as a front-line treatment has recently been employed. Some studies have demonstrated the superiority of high-dose chemotherapy followed by autoHSCT (Gianni et al. 1997; Haioun et al. 1997). The efficacy of high-dose chemotherapy as a front-line chemotherapy for NHL patients with poor prognostic factors has not yet been defined (Haioun et al. 2000). Further clinical investigations and randomized studies of NHL patients with poor prognostic factors will clarify the usefulness and indication of high-dose chemotherapy followed by autoHSCT. Meanwhile, Niitsu et al. conducted trials with a CyclOBEAP regimen consisting of CPA, VCR, bleomycin, etoposide, DOX, and PSL followed by G-CSF, in which the dose intensity was higher than that of MACOP-B, and achieved a CR rate of 87% and a 2-year survival rate of 91.3% (Niitsu et al. 2000). New generation chemotherapy at high-dose intensities using G-CSF chemotherapy followed by autoHSCT should be considered for treating NHL patients with poor prognostic factors as well as high-dose chemotherapy followed by autoHSCT.
THP is a derivative of DOX developed in Japan, and its cardiotoxicity is lower than that of DOX (Umezawa et al. 1979; Miller and Salewski 1994; Takagi and Oguro 1987). Elderly patients often have cardiac dysfunction and treatment with anthracyclines often has to be discontinued in elderly patients because of cardiac toxicity. Adverse drug reactions should be taken into consideration when a chemotherapeutic regimen is chosen. Because THP has a lower cardiotoxicity than DOX, clinical trials in the treatment of elderly patients with NHL have been performed (Kitamura and Takaku 1990). Niitsu et al. showed that the THP-COPBLM regimen containing THP was highly effective and suggested that THP could be used at a dose of 50–100 mg/m2 higher than that of DOX (Niitsu and Umeda 1997; Niitsu and Umeda 1999). Aoki et al. showed that chemotherapy regimens containing THP are useful and safe for elderly patients with NHL and that THP showed better results than DOX (Aoki et al. 1998). Few reports on the efficacy of THP for treating NHL in young patients are available (Niitsu and Umeda 1998; Takagi et al. 1990). In the present study, efficacy and safety were prospectively compared between biweekly THP-COP and CHOP regimens in the treatment of aggressive NHL in patients less than 70 years of age. No statistically significant differences were found and we concluded that biweekly THP-COP therapy was not inferior to biweekly CHOP therapy in effectively treating aggressive NHL in patients less than 70 years of age. In this study, only one patient, in the CHOP group, showed cardiotoxicity. If THP has less cardiotoxicity than DOX and the efficacy of THP-COP is similar to that of CHOP, THP might be an alternative anthracyclin to DOX in the treatment of NHL.
Biweekly CHOP and THP-COP regimens were effective and safe in patients less than 70 years of age with aggressive NHL, and with favorable prognostic factors. To reduce cardiotoxicity, the THP-COP regimen may be preferable to the CHOP regimen. However, in the treatment of aggressive NHL patients with poor prognostic factors, further therapeutic strategies such as new generation chemotherapy, myeloablative high-dose chemotherapy with HSCT, non-myeloablative HSCT, monoclonal antibody therapy, or a combination of therapies should be considered.
References
- Aoki S, Tsukada N, Nomoto N, Maruyama S, Takahashi M, Moriyama Y, Shibata A, Aizawa Y (1998) Effect of pirarubicin for elderly patients with malignant lymphoma. J Exp Clin Cancer Res 17:465–470 [PubMed] [Google Scholar]
- Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M (1971) Report of the committee on Hodgkin’s disease staging classification. Cancer Res 31:1860–1861 [PubMed] [Google Scholar]
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Kippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP (1999) Report of an International Workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol 17:1244–1253 [DOI] [PubMed] [Google Scholar]
- Cox DR (1972) Regression models and life-tables (with discussions). J Roy Stat Soc (Series B) 34:187–220 [Google Scholar]
- DeVita VT Jr, Hubbard SM, Longo DL (1987) The chemotherapy of lymphomas: looking back, moving forward—The Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res 47:5810–5824 [PubMed] [Google Scholar]
- Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, Glick JH, Coltman CA, Miller TP (1993) Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med 328:1002–1006 [DOI] [PubMed] [Google Scholar]
- Gianni AS, Bregni M, Siena S, Brambilla C, Nicola MD, Lombardi F, Gandola L, Tarella C, Pileri A, Ravagnani F, Valagussa P, Bonadonna G (1997) High-dose chemotherapy and autologous bone marrow transplantation compared with MACOP-B in aggressive B-cell lymphoma. N Engl J Med 336:1290–1297 [DOI] [PubMed] [Google Scholar]
- Haioun C, Lepage E, Gisselbrecht C, Bastion Y, Coiffier B, Brice P, Bosly A, Dupriez B, Nouvel C, Tilly H, Lederlin P, Biron P, Briere J, Gaulard P, Reyes F for the Group d’Etude des Lymphomes de I’Adulte (1997) Benefit of autologous bone marrow transplantation over sequential chemotherapy in poor-risk aggressive non-Hodgkin’s lymphoma: updated results of the prospective study LNH87–2. J Clin Oncol 15:1131–1137 [DOI] [PubMed] [Google Scholar]
- Haioun C, Lepage E, Gisselbrecht C, Salles G, Coiffier B, Brice P, Bosly A, Morel P, Nouvel C, Tilly H, Lederlin P, Sebban C, Briere J, Gaulard P, Reyes F (2000) Survival benefit of high-dose therapy in poor-risk aggressive non-Hodgkin’s lymphoma: final analysis of the prospective LNH87–2 protocol—a Group d’Etude des Lymphomes de I’Adulte Study. J Clin Oncol 18:3025–3030 [DOI] [PubMed] [Google Scholar]
- Jaffe ES, Harris NL, Stein H, Vardiman JW (eds) (2001) World Health Organization classification of tumors, pathology and genetics. Tumors of haematopoietic and lymphoid tissues. IARC Lyon, pp 109–236
- Kaplan EL, Meier P (1958) Non-parametric estimation from incomplete observations. J Am Stat Assoc 53:457–481 [Google Scholar]
- Kitamura K, Takaku F (1990) Pirarubicin, a novel derivative of doxorubicin, in THP-COP therapy for non-Hodgkin’s lymphoma in the elderly. Am J Clin Oncol 13[Suppl]:S15-S19 [DOI] [PubMed]
- Kwak LW, Halpern J, Olshen RA, Horning SJ (1990) Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: results of a tree-structured survival analysis. J Clin Oncol 8:963–977 [DOI] [PubMed] [Google Scholar]
- Miller AA, Salewski E (1994) Prospects for pirarubicin. Med Pediatr Oncol 22:261–268 [DOI] [PubMed] [Google Scholar]
- Niitsu N, Umeda M (1997) THP-COPBLM (pirarubicin, cyclophosphamide, vincristine, prednisone, bleomycin and procarbazine) regimen combined with granulocyte colony-stimulating factor (G-CSF) for non-Hodgkin’s lymphoma in elderly patients: a prospective study. Leukemia 11:1817–1820 [DOI] [PubMed] [Google Scholar]
- Niitsu N, Umeda M (1998) Biweekly THP-COPBLM (pirarubicin, cyclophosphamide, vincristine, prednisone, bleomycin, and procarbazine) regimen combined with granulocyte colony-stimulating factor (G-CSF) for intermediate and high grade non-Hodgkin’s lymphoma. Leukemia 12:1457–1460 [DOI] [PubMed] [Google Scholar]
- Niitsu N, Umeda M (1999) Response and adverse drug reactions to combination chemotherapy in elderly patients with aggressive non-Hodgkin’s lymphoma: comparison of CHOP, COP-BLAM, COP-BLAM III, and THP-COPBLM. Eur J Haematol 63:337–344 [DOI] [PubMed] [Google Scholar]
- Niitsu N, Okamoto M, Kuraishi Y, Nakamura S, Kodama F, Hirano M, The Adult Lymphoma Treatment Study Group (2000) CyclOBEAP (cyclophosphamide, vincristine, bleomycin, etoposide, doxorubicin, prednisolone) regimen with granulocyte colony-stimulating factor (G-CSF) for patients with aggressive non-Hodgkin’s lymphoma: a pilot study. Eur J Haematol 65:188–194 [DOI] [PubMed] [Google Scholar]
- Santoro A, Balzarotti M, Tondini C, Zanini M, Giardini R, Latteri F, Rampinelli I, Bufalino R (1999) Dose-escalation of CHOP in non-Hodgkin’s lymphoma. Ann Oncol 10:519–525 [DOI] [PubMed] [Google Scholar]
- Sawada M, Tsurumi H, Yamada T, Hara T, Fukuno K, Goto H, Shimizu M, Kasahara S, Yoshikawa T, Kanemura N, Oyama M, Takami T, Moriwaki H (2002) A prospective study of P-IMVP-16/CBDCA: a novel salvage chemotherapy for patients with aggressive non-Hodgkin’s lymphoma who had previously received CHOP therapy as first-line chemotherapy. Eur J Haematol 68:354–361 [DOI] [PubMed] [Google Scholar]
- Shipp MA, Neuberg D, Janicek M, Canellos GP, Shulman LN (1995) High-dose CHOP as initial therapy for patients with poor-prognosis aggressive non-Hodgkin’s lymphoma: a dose-finding pilot study. J Clin Oncol 13:2916–2923 [DOI] [PubMed] [Google Scholar]
- Takagi T, Oguro M (1987) (2”-R)-4’-o-Tetrahydropyranyladriamycin, a new anthracyclin derivative; its effectiveness in lymphoid malignancies. Cancer Chemother Pharmacol 20:151–154 [DOI] [PubMed] [Google Scholar]
- Takagi T, Sakai C, Oguro M (1990) Combination chemotherapy with pirarubicin (THP), cyclophosphamide, vincristine, and prednisolone (VEP-THP therapy) in the treatment of non-Hodgkin’s lymphoma. Oncology 47:25–28 [DOI] [PubMed] [Google Scholar]
- Tanosaki R, Okamoto S, Akatsuka N, Ishida A, Michikawa N, Masuda Y, Uchida H, Murata M, Kizaki M, Ikeda Y (1994) Dose escalation of biweekly cyclophosphamide, doxorubicin, vincristine, and prednisolone using recombinant human granulocyte colony stimulating factor in non-Hodgkin’s lymphoma. Cancer 74:1939–1944 [DOI] [PubMed] [Google Scholar]
- The International Non-Hodgkin’s Lymphoma Prognostic Factors Project (1993) A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med 329:987–994 [DOI] [PubMed] [Google Scholar]
- The non-Hodgkin’s lymphoma pathologic classification project (1982) National Cancer Institute-sponsored study of classifications of non-Hodgkin’s lymphomas. Summary and description of a working formulation for clinical usage. Cancer 49:2112–2135 [DOI] [PubMed] [Google Scholar]
- Umezawa H, Takahashi Y, Kinoshita M, Naganawa H, Masuda T, Ishizuka M, Tatsuta K, Takeuchi T (1979) Tetrahydropyranyl derivatives of daunomycin and adriamycin. J Antibiot 32:1082–1084 [DOI] [PubMed] [Google Scholar]
- Witzig TE, Camoriano JK, Schroeder G, Kurtin PJ, Habermann TM (1998) A phase I trial of high-dose ProMACE-CytaBOM with granulocyte colony stimulating factor for patients with non-Hodgkin’s lymphoma. Leukemia Lymphoma 28:307–314 [DOI] [PubMed] [Google Scholar]