Abstract
Parkinson's disease (PD) is a prevalent neurodegenerative disorder without a definitive cure. Oxidative stress is significantly implicated in its pathogenesis, prompting interest in N-acetylcysteine (NAC), a strong antioxidant and cysteine precursor, as a potential therapeutic agent. We conducted a bibliometric analysis of 421 Scopus articles to assess current trend and future potential of research on the use of NAC in Parkinson's disease. The number of publications related to this topic reached the peaked in 2010 and gradually decreased afterward. We identified 4 main clusters of research theme related to the potential mechanism of NAC effects on Parkinson's disease progression. These include research focusing on NAC effects on oxidative stress, dysfunction of the mitochondria, aberrant protein accumulation and clearance and inflammation. Investigating NAC effect for Parkinson's disease in human is a potential research area.
Keywords: Bibliometric analysis, N-acetylcysteine, Network analysis, Parkinson's disease
الملخص
مرض باركنسون هو اضطراب عصبي تنكسي شائع ليس له علاج شاف. يلعب الإجهاد التأكسدي دورا هاما في تطور المرض، مما أثار الاهتمام بـمركب ن-أسيتيل-سيستين، وهو مضاد أكسدة قوي ومادة أولية للسيستين، كعامل علاجي محتمل. قمنا بتحليل 4٢١ مقالة منشورة في قاعدة بيانات سكوبس لتقييم التوجه الحالي والإمكانات المستقبلية للبحوث المتعلقة باستخدام ن-أسيتيل-سيستين في مرض باركنسون. بلغ عدد المنشورات المتعلقة بهذا الموضوع ذروته في عام ٢٠١٠، ثم انخفض تدريجيا بعد ذلك. حددنا أربع مجموعات بحثية رئيسية تتعلق بالآلية المحتملة لتأثيرات ن-أسيتيل-سيستين على تطور مرض باركنسون. وتشمل هذه الأبحاث التي تركز على تأثيرات ن-أسيتيل-سيستين على الإجهاد التأكسدي، وخلل الميتوكوندريا، وتراكم البروتينات غير الطبيعي، وتصفية البروتينات، والالتهاب. ويُعد البحث في تأثير ن-أسيتيل-سيستين على مرض باركنسون لدى البشر مجالا بحثيا محتملا.
الكلمات المفتاحية: مرض باركنسون, ن-أسيتيل سيستين, التحليل الببليومتري, تحليل الشبكة
Introduction
N-acetylcysteine (NAC) serves as a precursor of cysteine, the sulphur-containing amino acid; this amino acid is essential for glutathione synthesis, one of the major antioxidants in our body.1 In 1985, NAC was approved for treating paracetamol poisoning by FDA. Now, intravenous NAC administration becomes the standard protocol for the treatment of paracetamol poisoning across the globe.2 NAC has also been used for indications beyond its original role as an antidote of paracetamol poisoning. For decades, it has been widely used as a mucolytic agent in chronic obstructive pulmonary disease.3 Owing to the antioxidant and anti-inflammation activities, NAC has potential efficacy for the treatment of health conditions related to oxidative stress, infection, inflammation, and toxic damage.1 For example, NAC has been used as a renal protective agent in contrast-induced nephropathy,4 as an adjuvant therapy for polycystic ovary syndrome,5 and SARS-CoV-2 infection.6
The interest in using NAC to treat neurological disorders has been growing over the past years as evidence of the involvement of inflammation and oxidative stress in these conditions is accumulating.7 Of note, emerging studies suggest that NAC may be implicated in other processes involved in various psychiatric and neurological disorders such as dysregulation of dopamine system, apoptosis, neurogenesis, mitochondrial dysfunction and neuroadaptation and plasticity.8,9 Furthermore, NAC has been demonstrated to correct glutamate dysregulation and modify intracellular and extracellular glutamate, the most significant excitatory neurotransmitter.10 Parkinson's disease has been shown to disrupt these pathways, and NAC's ability to modify these pathways offers justification for investigating NAC's potential as a treatment for Parkinson's disease.11
Studies of NAC for Parkinson's disease have been a topic of interest in the last decades. A quick search in Pubmed conducted on May 2024 for papers published before the year 2000 using N-acetylcysteine as the keyword resulted in 4340 papers. The number skyrocketed to 22.097 papers in 2024 suggesting the substantial increase in research productivity of NAC use in the last two decades. However, there has been a lack of scientific data regarding evaluation of research productivity as well as current trend on the use of NAC for Parkinson's disease. To the best of our knowledge, there is only one bibliometric analysis on NAC that focuses on its use for paracetamol overdose, published nearly a decade ago.2 Bibliometric analysis uses quantitative data and statistical analysis to provide information on current state of scientific research and to identify novelty and future perspective in a particular area of research.12,13 Bibliometric analysis allows us to assess research productivity, to identify active research as well as potential collaborators, to sort trending topics and to track the dynamic trends over time which can help researcher determine new lines of research.14
This study aimed to assess the global research trends on NAC in the context of PD, using bibliometric tools to analyze publication trends, identify core research themes, and suggest future research directions, clarifying the current research landscape with the aim of guiding ongoing and future studies on the potential of NAC as a therapeutic option for PD.
Materials and Methods
Search strategy
We retrieved bibliometric data from Scopus, one of the largest electronic databases, on 12th of August 2024 using the following search criteria: TITLE-ABS-KEY (n-acetylcysteine OR acetylcysteine) AND TITLE-ABS-KEY (“Parkinson disease” OR "Parkinson's disease”)) AND (LIMIT-TO (DOCTYPE, “ar”)) AND (LIMIT-TO (LANGUAGE, “English”)) AND (LIMIT-TO (PUBSTAGE, “final”)).
Data analysis
To analyze the data, we used three software including VOSviewer, RStudio, and Biblioshiny. We used R package's bibliometrics which were developed for quantifying informetric and scient metrics. Following installation and loading of R package for Bibliometrix into R Studio, launching of the Biblioshiny application was started and bibliometric data from Scopus was processed. For visualization, bibliometric data were exported to VOSviewer version 1.6.14 and Biblioshiny for further analysis. To create term maps, we used co-occurrence analysis using author keywords as the unit of analysis. A thesaurus was created and then appended such that VOSviewer would recognize terms such as N-Acetylcysteine, n-acetyl-l-cysteine and n-acetylcysteine as the same term. The co-occurrence of keywords measures the relatedness of the terms.
Results
A total of 421 articles published from 1985 to 12th of August 2024 were collected. This covers 192 sources, 2008 authors, 955 keywords and 22.551 references. Details of the search result are described in Table 1.
Table 1.
Main information related to publication data on the use of NAC for Parkinson's disease.
| Description | Results | |
|---|---|---|
| Main information of data | ||
| Period included | 1985:2024 | |
| Sources (Journals, Books, etc.) | 192 | |
| Documents | 421 | |
| % Of growth rate per year | 6.58 | |
| Average of document age | 12.5 | |
| Average of citations per document | 54.99 | |
| References | 22551 | |
| Contents of document | ||
| Keywords plus/ID | 4911 | |
| Author's keywords/DE | 955 | |
| Authors | ||
| Authors | 2008 | |
| Authors of single-authored documents | 9 | |
| Authors collaboration | ||
| Single-authored documents | 10 | |
| Co-authors/Document | 5.98 | |
| % Of international co-authorships | 22.33 | |
| Document types | ||
| Article | 421 |
Global trends of NAC research on Parkinson's disease based on the total number of publications each year
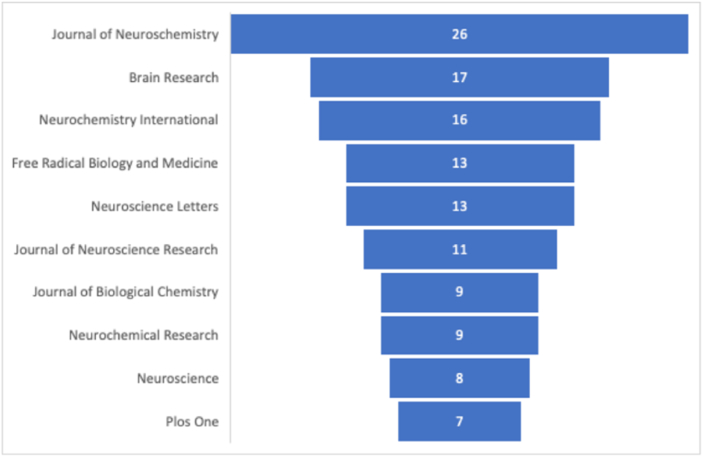
The first work on NAC and Parkinson's disease was introduced in 1985 when Perry and colleagues15 reported the protective effects of four antioxidants including NAC on dopaminergic cells of the substantia nigra following administration of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxin. However, no publications related to this topic could be found until 10 years later. In 1995, Colton and colleagues16 showed that NAC treatment enhanced the tyrosine hydroxylase-containing (TH+) neurons survival cultured from embryonic rat mesencephalon. Since then, the number of publications reporting the work on NAC and Parkinson's disease showed an upward trend reaching its peak in 2010 with 26 articles published in this year. However, after this point, the trend was reversed with decreased growth in publication continuing until August 2024. In 2023, publication quantity was half the quantity of 2010 data (see Figure 1). Based on the number of publications, 10 journals were identified to be the core journals that published articles related to NAC and Parkinson's disease (see Figure 2).
Figure 1.
Trend of NAC research on Parkinson's disease based on annual number of publications.
Figure 2.
Top 10 journals contributing to research related to NAC and Parkinson's disease.
Keywords analysis and network visualization of co-occurrence
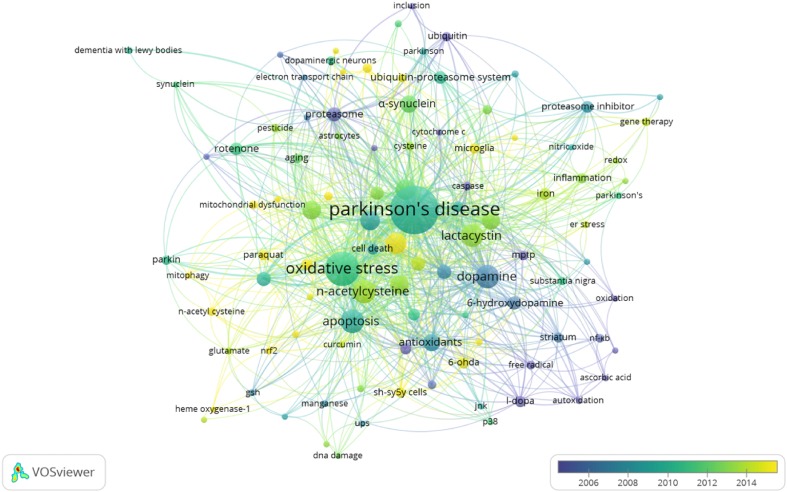
Bibliometric analysis identified four clusters of research theme based on the frequency of co-occurrence of keywords and the level of agreement between keywords in the mapping structure. These clusters are depicted in different colors as shown in Figure 3. The frequency of keyword occurrences represents the research focus of earlier studies. Details of keywords in each cluster are presented in Table 2.
Figure 3.
Network visualization of keyword co-occurrence. Different colors represent different clusters; the frequency of keyword occurrence corresponds to the size of the circle, the bigger the circle size, the more frequent the keyword occurs; the distance between circles represents the strength of relationship between keywords with closer distance suggesting stronger relationship.
Table 2.
Cluster analysis of keywords.
| Clusters | Keywords |
|---|---|
| Cluster 1 (red) - 35 items | 6-Hydroxydopamine, 6-ohda, antioxidants, apoptosis, reactive oxygen species, ros, ascorbic acid, autophagy, autoxidation, calcium, catecholamine, DNA damage, dopamine, erk, free radical, free radicals, gsh, heme oxygenase-1, jnk, l-dopa, lactacystin, manganese, neurotoxicity, nf-κb, nrf2, oxidation, p38, Parkinson disease, pc12 cell, pc12 cells, reactive oxygen species (ros), sh-sy5y cells, striatum, ubiquitin proteasome system, ups |
| Cluster 2 (green) - 32 items | Aggresome, aging, alpha-synuclein, alzheimer's disease, astrocytes, caspase, cysteine, cytochrome c, dementia with lewy bodies, dopaminergic neuron, dopaminergic neurons, electron transport chain, glutathione, inclusion, mpp+, n-acetyl-l-cysteine, neuroinflammation, Parkinson, Parkinson's disease, parkinsonism, pesticide, proteasome, proteasome inhibition, rotenone, sh-sy5y, sh-sy5y cell, stem cells, synuclein, thiol, ubiquitin, ubiquitin-proteasome system |
| Cluster 3 (blue) - 19 items | Anti-inflammatory, antioxidant, cell death, curcumin, cytotoxicity, deferoxamine, dj-1, glutamate, mitochondria, mitochondrial dysfunction, mitophagy, mptp, n-acetyl cysteine, n-acetylcysteine, neurodegenerative diseases, oxidative stress, paraquat, parkin, Parkinson's disease |
| Cluster 4 (yellow) - 19 items | Copper, er stress, ferroptosis, gene therapy, inflammation, iron, microglia, neurodegeneration, neuroprotection, nitric oxide, Parkinson's, proteasome inhibitor, redox, substantia nigra, unfolded protein response, glial cell line-derived neurotrophic factor |
Note: bold words indicate keywords with the highest frequency of co-occurrence.
The most frequent keywords for the first cluster are apoptosis, reactive oxygen species, dopamine, and antioxidant which could signify studies related to the apoptotic death of dopaminergic neurons in Parkinson's disease resulting from oxidative stress and how antioxidant such as NAC could be used to treat this condition. In the second cluster, the most frequent keywords are α-synuclein, glutathione, proteasome, proteasome inhibition, and ubiquitin-proteasome system suggesting the role of aberrant protein aggregate such as α-synuclein aggregate in Parkinson's disease pathogenesis and how antioxidant such as glutathione and NAC may target the system involved in the disposal of abnormal proteins including the ubiquitin-proteasome system. The third cluster is composed of keywords such as antioxidant, mitochondria, mitochondrial dysfunction, and oxidative stress. This reflects research on how NAC may slow the progression of Parkinson's disease by maintaining mitochondrial function, which shields dopaminergic neurons from cell death brought on by oxidative stress. Lastly, the most frequent keywords in cluster 4 are neurodegeneration, neuroprotection, iron, inflammation, and microglia which underline the role of NAC in reducing inflammation induced neurodegeneration in Parkinson's disease.
Figure 4 provides a comprehensive overview of the evolving research topics overtime. From this historical perspective, we can see that the focus of research in the field is changing. Keywords used in earlier studies include proteasome, free radical, antioxidants, GSH (glutathione), oxidation, and l-Dopa while newer studies emphasis on keywords such as mitochondrial dysfunction, microglia, astrocytes, glutamate, NRF-2, ER stress, and iron suggesting the change in research interest and priorities.
Figure 4.
Overlay visualizations of NAC for Parkinson's disease-related publications.
Discussion
One of the most prevalent neurodegenerative diseases, Parkinson's disease is typified by the death or degeneration of dopaminergic neurons that connect to the striatum from the midbrain's substantia nigra.17 The pathological hallmark of Parkinson's disease includes the formation of Lewy bodies composed of abnormal protein aggregates such as α-synuclein and ubiquitin inside these neurons. It is still unclear what precise process causes neuronal death in Parkinson's disease. However, a number of mechanisms, such as oxidative stress, mitochondrial malfunction, apoptosis, inflammation, and excitotoxicity, have been linked to neurodegeneration and cell death.18 In addition, defects in systems required for the breakdown of abnormal protein aggregation such as the ubiquitin-proteasome system has been linked with the progression of Parkinson's disease.19 Here, we conducted a network analysis with VOSviewer and identified clusters of research theme related to possible mechanism of action of NAC on Parkinson's disease.
Firstly, studies have examined the connection between oxidative stress and dopamine cell death, as well as the potential of antioxidants like NAC to stop neuronal loss, particularly that caused by apoptosis. It is commonly recognized that Parkinson's disease causes dopaminergic cell death due to the buildup of free radicals, such as reactive oxygen species (ROS), which intensify oxidative stress.20,21 Studies using in vitro model of Parkinson's disease16,22,23 and in vivo model15,24, 25, 26, 27 demonstrated the positive benefit of NAC in protecting neurons from oxidative stress. Using MN9D cell, a murine mesencephalon – derived dopaminergic neuronal cell line, Choi and colleagues (1999) showed that administration of 6-hydroxydopamine (6-OHDA) generated ROS which in turn activated the c-Jun N-terminal kinase (JNK) pathway leading to apoptotic cell death. Of interest, NAC treatment attenuated the ROS-mediated JNK activation and rescued cell death.27 Similarly, NAC prevented apoptosis of Parkinson's disease cybrids cells22 and TH + cells in the striatum following MPTP injection in mice by suppressing JNK activation26). Other study showed that NAC decreased apoptosis by inhibiting caspase-3 activity, poly (ADP-ribose) polymerase cleavage, and ROS production.23
Secondly, research in Parkinson's disease has looked at abnormal protein aggregation and protein clearance system such as the ubiquitin-proteasome system and the autophagy system. One of the main features of Parkinson's disease is the buildup of α-synuclein aggregates in the substantia nigra in the form of Lewy bodies.28 This aberrant protein buildup induces cytochrome c production and malfunction of the mitochondria29 and initiates endoplasmic reticulum (ER) stress which leads to apoptotic cell death.30 Ubiquitin-proteasome system is the main protein clearance route in neurons. Studies showed that Parkinson's disease compromises this system.31 Proteasome inhibition has been shown to enhance free radical formation which in turn increases protein oxidation that further compromises the degradative capacity of proteasome.32 NAC therapy prevented the apoptosis caused by proteasome inhibitor MG132 applied in SH-SY5Y cells. NAC treatment also inhibited proteasome-induced autophagy, reduced ER stress, blocked glutathione depletion and ROS formation which finally increases cell viability.32 In addition, increasing glutathione level in substantia nigra by NAC administration was able to protect mice from α-synuclein toxicity.33
Thirdly, there has been interest in studying mitochondrial malfunction in Parkinson's disease. One of the pathological processes in Parkinson's disease is believed to be abnormal mitochondrial architecture and functioning.34 Impaired mitochondrial function reduces cellular capacity to generate energy which then leads to increased formation of ROS. Oxidative damage and programmed cell death will occur as a result.35 Early studies have shown that defect in mitochondrial complex I activity was found in the substantia nigra of Parkinson's disease patients.36,37 Next, it was found that cytoplasmic hybrid (cybrid) cell lines from Parkinson's disease patients were more susceptible to toxin-induced apoptosis with 20 % reduction in mitochondrial complex 1 activity.38 The defects in mitochondrial complex 1 activity causes a reduction in ATP synthesis which promotes neuronal degeneration.34 NAC has been shown to increase complex 1 activity in synaptic mitochondria of aged mice which raised the potential benefit for Parkinson's disease treatment.39 In vitro, NAC treatment ameliorates the increased level of cell-free mitochondrial DNA, preserves the function of complex 1 and prevents mitochondrial dysfunction caused by rotenone exposure.40 In addition, NAC treatment restored the reduction of parkin level, an important protein in mitochondrial dynamic.41 Parkin is required for maintaining normal function and morphology of mitochondria34 to protect dopaminergic neurons in the substantia nigra and striatum from rotenone toxicity.41
Lastly, the fourth cluster mainly consists of keywords such as neurodegeneration, neuroprotection, iron, inflammation, microglia, and ferroptosis suggesting the research focusing on the involvement of inflammation pathway in Parkinson's disease. Misfolded α-synuclein aggregates leading to dysfunctional mitochondria and neuroinflammation could be the underlying mechanism of Parkinson's disease.42 Tumor necrosis factor alpha (TNF-α), interleukin 1β, interleukin 2, and interleukin 6 are inflammatory cytokines that promote neuroinflammation and cell death when α-synuclein builds up.43 Using cellular model of Parkinson's disease, Kaya and colleagues (2023) showed that NAC treatment prevented cell death by blocking inflammatory cascade induced by TNF-α and overexpression of α-synuclein.43 NAC effect was mediated by a reduction in the expression of Toll-like receptor 2 (TLR2), which plays an important role in neuroinflammation. Being highly expressed in neurons and microglia, TLR2 would recognize α-synuclein protein and trigger inflammation.44 People with Parkinson's disease have higher TLR2 expression, which may indicate that the inflammatory pathway plays a role in the illness.45
A large body of preclinical studies demonstrates the potential utility of NAC in the treatment of Parkinson's disease, among other conditions.41,46, 47, 48 Whether this effect translates to human will require further investigation. A preliminary clinical data suggested the beneficial effects of NAC in people with Parkinson's disease.49 This finding was followed up with a pilot study by Monti and colleagues (2019) who showed that NAC treatment improved clinical outcomes and increased dopamine transporter binding in the striatum of people with Parkinson's disease.50 However, clinical study investigating NAC impact in Parkinson's disease is very limited. We believe that investigating the efficacy of NAC for Parkinson's disease in human could be a potential new line of research.
Current pharmacotherapies for Parkinson's disease only address the symptoms and decelerate disease progression but none can fully cure Parkinson's disease. Thus, there is an urgent need to explore new therapeutic options; one way is by repurposing currently available drug. NAC is readily available in the market and is not under any patent restriction. NAC also has good safety profile.51,52 NAC has been shown to improve health conditions related to inflammation and oxidative stress.1 It was postulated that NAC effect in improving the symptoms of people with Parkinson's disease was mediated by its direct action as an antioxidant and by restoring glutathione level.50 Intravenous administration of NAC at 150 mg/kg was able to boost the level of glutathione in both the blood and the brain53 while 4 weeks of oral NAC (6000 mg/day) significantly increased systemic antioxidant parameters in people with Parkinson's disease.54 Given the role of oxidative stress in the pathophysiology of Parkinson's disease, these findings raise the possibility of using NAC for the treatment of Parkinson's disease. More recently, NAC increases dopamine release and prevents degeneration of dopaminergic neurons55 to improve motor deficits in animal models of Parkinson.56 Together, this suggests the potential benefit of NAC for the treatment of Parkinson's disease. To sum up, considering NAC safety51 and potential benefit, further studies to look at NAC clinical efficacy for Parkinson's disease are warranted.
We acknowledge that this study has a limitation. We performed literature search only in one database which was Scopus. Thus, literature published in non-Scopus journals may not be covered in our study despite their contribution to research productivity. Future studies could include more databases (e.g., MEDLINE, Embase, Google Scholar) to expand the coverage of the literature search and data analysis.
Conclusion
A bibliometric analysis of 421 articles published in Scopus to assess research theme, trend, and future perspective of research on the use of NAC for Parkinson's disease was conducted. Four major research themes were identified. While previous research has concentrated on how NAC affects oxidative stress and cell death in Parkinson's disease, other studies have explored other approach to see the effects of NAC including on mitochondrial function and inflammation. While many studies were conducted in vitro and in vivo animal model, studies in human are lacking suggesting the potential exploration of NAC utility for Parkinson's disease in human.
Ethics approval
Not required.
Authors contributions
MM: conceptualization, methodology, original draft preparation; NW: Data curation and analysis, FKS: manuscript writing - review and editing, data analysis; AP: review and manuscript editing; RDY: methodology, review and manuscript editing: BW: conceptualization, review and manuscript editing. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Source of funding
No specific grant from a public, private, or nonprofit funding organization was obtained for this study.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgment
We'd like to thank Universitas Sebelas Maret (UNS) Surakarta which provide support for publishing this manuscript (PKGR UNS Grant 371/UN27.22/PT.01.03/2025).
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Dodd S., Dean O., Copolov D.L., Malhi G.S., Berk M. N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility. Expet Opin Biol Ther. 2008;8:1955–1962. doi: 10.1517/14728220802517901. [DOI] [PubMed] [Google Scholar]
- 2.Zyoud S.H., Al-Jabi S.W., Sweileh W.M., Awang R., Waring W.S. Global research productivity of N-acetylcysteine use in paracetamol overdose: a bibliometric analysis (1976–2012) Hum Exp Toxicol. 2015;34:1006–1016. doi: 10.1177/0960327114565494. [DOI] [PubMed] [Google Scholar]
- 3.Dekhuijzen P.N.R., Van Beurden W.J.C. The role for N-acetylcysteine in the management of COPD. Int J Chronic Obstr Pulm Dis. 2006;1:99–106. doi: 10.2147/copd.2006.1.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quintavalle C., Donnarumma E., Fiore D., Briguori C., Condorelli G. Therapeutic strategies to prevent contrast-induced acute kidney injury. Curr Opin Cardiol. 2013;28:676–682. doi: 10.1097/HCO.0b013e3283653f41. [DOI] [PubMed] [Google Scholar]
- 5.Rizk A.Y., Bedaiwy M.A., Al-Inany H.G. N-acetyl-cysteine is a novel adjuvant to clomiphene citrate in clomiphene citrate–resistant patients with polycystic ovary syndrome. Fertil Steril. 2005;83:367–370. doi: 10.1016/j.fertnstert.2004.07.960. [DOI] [PubMed] [Google Scholar]
- 6.Gamarra-Morales Y., Herrera-Quintana L., Molina-López J., Vázquez-Lorente H., Machado-Casas J.F., Castaño-Pérez J., et al. Response to intravenous N-acetylcysteine supplementation in critically Ill patients with COVID-19. Nutrients. 2023;15:2235. doi: 10.3390/nu15092235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hald A., Lotharius J. Oxidative stress and inflammation in Parkinson's disease: is there a causal link? Exp Neurol. 2005;193:279–290. doi: 10.1016/j.expneurol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Dean O., Giorlando F., Berk M. N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiatr Neurosci. 2011;36:78–86. doi: 10.1503/jpn.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samuni Y., Goldstein S., Dean O.M., Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta (BBA)-Gen Subj. 2013;1830:4117–4129. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Bradlow R.C.J., Berk M., Kalivas P.W., Back S.E., Kanaan R.A. The potential of N-acetyl-L-cysteine (NAC) in the treatment of psychiatric disorders. CNS Drugs. 2022;36:451–482. doi: 10.1007/s40263-022-00907-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mustapha M., Taib C.N.M. MPTP-induced mouse model of Parkinson's disease: a promising direction for therapeutic strategies. Bosn J Basic Med Sci. 2021;21:422. doi: 10.17305/bjbms.2020.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamali M., Jahaninafard D., Mostafaie A., Davarazar M., Gomes A.P.D., Tarelho L.A.C., et al. Scientometric analysis and scientific trends on biochar application as soil amendment. Chem Eng J. 2020;395 [Google Scholar]
- 13.Li Y., Jiang S., Wang T., Lin Y., Mao H. Research on biochar via a comprehensive scientometric approach. RSC Adv. 2018;8:28700–28709. doi: 10.1039/c8ra05689g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Battisti F., Salini S. Robust analysis of bibliometric data. Stat Methods Appt. 2013;22:269–283. [Google Scholar]
- 15.Perry T.L., Yong V.W., Clavier R.M., Jones K., Wright J.M., Foulks J.G., et al. Partial protection from the dopaminergic neurotoxin N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine by four different antioxidants in the mouse. Neurosci Lett. 1985;60:109–114. doi: 10.1016/0304-3940(85)90229-0. [DOI] [PubMed] [Google Scholar]
- 16.Colton C.A., Pagan F., Snell J., Colton J.S., Cummins A., Gilbert D.L. Protection from oxidation enhances the survival of cultured mesencephalic neurons. Exp Neurol. 1995;132:54–61. doi: 10.1016/0014-4886(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 17.Samii A., Nutt J.G., Ransom B.R. Parkinson's disease. Lancet. 2004;363:1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- 18.Samii A., Calne D.B., Oeriel W., LeWitt P. Research into the etiology of Parkinson's disease. Parkinson Disease, London, Martin Dunitz. 1999:229–236. [Google Scholar]
- 19.Beitz J.M. Parkinson's disease: a review. Front Biosci (Schol Ed) 2014;6:65–74. doi: 10.2741/s415. [DOI] [PubMed] [Google Scholar]
- 20.Guo J., Zhao X., Li Y., Li G., Liu X. Damage to dopaminergic neurons by oxidative stress in Parkinson's disease. Int J Mol Med. 2018;41:1817–1825. doi: 10.3892/ijmm.2018.3406. [DOI] [PubMed] [Google Scholar]
- 21.Prasad K.N. Oxidative stress, pro-inflammatory cytokines, and antioxidants regulate expression levels of microRNAs in Parkinson's disease. Curr Aging Sci. 2017;10:177–184. doi: 10.2174/1874609810666170102144233. [DOI] [PubMed] [Google Scholar]
- 22.Onyango I.G., Tuttle J.B., Bennett Jr JP. Activation of p38 and N-acetylcysteine-sensitive c-Jun NH2-terminal kinase signaling cascades is required for induction of apoptosis in Parkinson's disease cybrids. Mol Cell Neurosci. 2005;28:452–461. doi: 10.1016/j.mcn.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Molina-Jimenez M.F., Sanchez-Reus M.I., Benedi J. Effect of fraxetin and myricetin on rotenone-induced cytotoxicity in SH-SY5Y cells: comparison with N-acetylcysteine. Eur J Pharmacol. 2003;472:81–87. doi: 10.1016/s0014-2999(03)01902-2. [DOI] [PubMed] [Google Scholar]
- 24.Offen D., Ziv I., Sternin H., Melamed E., Hochman A. Prevention of dopamine-induced cell death by thiol antioxidants: possible implications for treatment of Parkinson's disease. Exp Neurol. 1996;141:32–39. doi: 10.1006/exnr.1996.0136. [DOI] [PubMed] [Google Scholar]
- 25.Soto-Otero R., Méndez-Álvarez E., Hermida-Ameijeiras Á., Muñoz-Patiño A.M., Labandeira-Garcia J.L. Autoxidation and neurotoxicity of 6-hydroxydopamine in the presence of some antioxidants: potential implication in relation to the pathogenesis of Parkinson's disease. J Neurochem. 2000;74:1605–1612. doi: 10.1046/j.1471-4159.2000.0741605.x. [DOI] [PubMed] [Google Scholar]
- 26.Park S.-W., Kim S.-H., Park K.-H., Kim S.-D., Kim J.-Y., Baek S.-Y., et al. Preventive effect of antioxidants in MPTP-induced mouse model of Parkinson's disease. Neurosci Lett. 2004;363:243–246. doi: 10.1016/j.neulet.2004.03.072. [DOI] [PubMed] [Google Scholar]
- 27.Choi W., Yoon S., Oh T.H., Choi E., O'Malley K.L., Oh Y.J. Two distinct mechanisms are involved in 6-hydroxydopamine-and MPP+-induced dopaminergic neuronal cell death: role of caspases, ROS, and JNK. J Neurosci Res. 1999;57:86–94. doi: 10.1002/(SICI)1097-4547(19990701)57:1<86::AID-JNR9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 28.Gómez-Benito M., Granado N., García-Sanz P., Michel A., Dumoulin M., Moratalla R. Modeling Parkinson's disease with the alpha-synuclein protein. Front Pharmacol. 2020;11:356. doi: 10.3389/fphar.2020.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitamura M., Kato H., Saito Y., Nakajima S., Takahashi S., Johno H., et al. Aberrant, differential and bidirectional regulation of the unfolded protein response towards cell survival by 3′-deoxyadenosine. Cell Death Differ. 2011;18:1876–1888. doi: 10.1038/cdd.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verfaillie T., Jäger R., Samali A., Agostinis P. Endoplasmic reticulum stress in health and disease. 2012. ER stress signaling pathways in cell survival and death; pp. 41–73. [Google Scholar]
- 31.Cook C., Stetler C., Petrucelli L. Disruption of protein quality control in Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a009423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng B., Anand P., Kuang A., Akhtar F., Scofield V.L. vol. 2016. Parkinsons Dis; 2016. N-Acetylcysteine in combination with IGF-1 enhances neuroprotection against proteasome dysfunction-induced neurotoxicity. (SH-SY5Y cells). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark J., Clore E.L., Zheng K., Adame A., Masliah E., Simon D.K. Oral N-acetyl-cysteine attenuates loss of dopaminergic terminals in α-synuclein overexpressing mice. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moon H.E., Paek S.H. Mitochondrial dysfunction in Parkinson's disease. Exp Neurobiol. 2015;24:103. doi: 10.5607/en.2015.24.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banaclocha M.M. Therapeutic potential of N-acetylcysteine in age-related mitochondrial neurodegenerative diseases. Med Hypotheses. 2001;56:472–477. doi: 10.1054/mehy.2000.1194. [DOI] [PubMed] [Google Scholar]
- 36.Schapira A.H.V., Cooper J.M., Dexter D., Clark J.B., Jenner P., Marsden C.D. Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 37.Schapira A.H.V., Cooper J.M., Dexter D., Jenner P., Clark J.B., Marsden C.D. 1989. Mitochondrial complex I defi ciency in Parkinson's disease. [DOI] [PubMed] [Google Scholar]
- 38.Swerdlow R.H., Parks J.K., Miller S.W., Davis R.E., Tuttle J.B., Trimmer P.A., et al. Origin and functional consequences of the complex I defect in Parkinson's disease. Ann Neurol: Off J Am Neurol Assoc Child Neurol Society. 1996;40:663–671. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- 39.Banaclocha M.M., Martínez N. N-acetylcysteine elicited increase in cytochrome c oxidase activity in mice synaptic mitochondria. Brain Res. 1999;842:249–251. doi: 10.1016/s0006-8993(99)01819-3. [DOI] [PubMed] [Google Scholar]
- 40.Kwok W.T.-H., Kwak H.A., Andreazza A.C. N-acetylcysteine modulates rotenone-induced mitochondrial Complex I dysfunction in THP-1 cells. Mitochondrion. 2023;72:1–10. doi: 10.1016/j.mito.2023.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Rahimmi A., Khosrobakhsh F., Izadpanah E., Moloudi M.R., Hassanzadeh K. N-acetylcysteine prevents rotenone-induced Parkinson's disease in rat: an investigation into the interaction of parkin and Drp1 proteins. Brain Res Bull. 2015;113:34–40. doi: 10.1016/j.brainresbull.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Kaur R., Mehan S., Singh S. Understanding multifactorial architecture of Parkinson's disease: pathophysiology to management. Neurol Sci. 2019;40:13–23. doi: 10.1007/s10072-018-3585-x. [DOI] [PubMed] [Google Scholar]
- 43.Kaya Z.B., Karakoc E., McLean P.J., Saka E., Atilla P. Post-inflammatory administration of N-acetylcysteine reduces inflammation and alters receptor levels in a cellular model of Parkinson's disease. FASEB Bioadv. 2023;5:263. doi: 10.1096/fba.2022-00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kouli A., Horne C.B., Williams-Gray C.H. Toll-like receptors and their therapeutic potential in Parkinson's disease and α-synucleinopathies. Brain Behav Immun. 2019;81:41–51. doi: 10.1016/j.bbi.2019.06.042. [DOI] [PubMed] [Google Scholar]
- 45.Drouin-Ouellet J., St-Amour I., Saint-Pierre M., Lamontagne-Proulx J., Kriz J., Barker R.A., et al. Toll-like receptor expression in the blood and brain of patients and a mouse model of Parkinson's disease. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berman A.E., Chan W.Y., Brennan A.M., Reyes R.C., Adler B.L., Suh S.W., et al. N-acetylcysteine prevents loss of dopaminergic neurons in the EAAC1−/− mouse. Ann Neurol. 2011;69:509–520. doi: 10.1002/ana.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodríguez-Pallares J., Rey P., Soto-Otero R., Labandeira-Garcia J.L. N-acetylcysteine enhances production of dopaminergic neurons from mesencephalic-derived precursor cells. Neuroreport. 2001;12:3935–3938. doi: 10.1097/00001756-200112210-00016. [DOI] [PubMed] [Google Scholar]
- 48.Muñoz A.M., Rey P., Soto-Otero R., Guerra M.J., Labandeira-Garcia J.L. Systemic administration of N-acetylcysteine protects dopaminergic neurons against 6-hydroxydopamine-induced degeneration. J Neurosci Res. 2004;76:551–562. doi: 10.1002/jnr.20107. [DOI] [PubMed] [Google Scholar]
- 49.Monti D.A., Zabrecky G., Kremens D., Liang T.W., Wintering N.A., Cai J., et al. N-Acetyl cysteine may support dopamine neurons in Parkinson's disease: preliminary clinical and cell line data. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monti D.A., Zabrecky G., Kremens D., Liang T., Wintering N.A., Bazzan A.J., et al. N-acetyl cysteine is associated with dopaminergic improvement in Parkinson's disease. Clin Pharmacol Ther. 2019;106:884–890. doi: 10.1002/cpt.1548. [DOI] [PubMed] [Google Scholar]
- 51.Slattery J., Kumar N., Delhey L., Berk M., Dean O., Spielholz C., et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. 2015;55:294–321. doi: 10.1016/j.neubiorev.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 52.Muthmainah M., Sketriene D., Anversa R.G., Harris E., Griffiths S., Gogos A., et al. Exploring the utility of N-acetylcysteine for loss of control eating: protocol of an open-label single-arm pilot study. Pilot Feasibility Stud. 2025;11 doi: 10.1186/s40814-025-01598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holmay M.J., Terpstra M., Coles L.D., Mishra U., Ahlskog M., Öz G., et al. N-acetylcysteine boosts brain and blood glutathione in gaucher and Parkinson diseases. Clin Neuropharmacol. 2013;36:103–106. doi: 10.1097/WNF.0b013e31829ae713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coles L.D., Tuite P.J., Öz G., Mishra U.R., Kartha R.V., Sullivan K.M., et al. Repeated-dose oral N-acetylcysteine in Parkinson's disease: pharmacokinetics and effect on brain glutathione and oxidative stress. J Clin Pharmacol. 2018;58:158–167. doi: 10.1002/jcph.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.El-Habta R., af Bjerkén S., Virel A. N-acetylcysteine increases dopamine release and prevents the deleterious effects of 6-OHDA on the expression of VMAT2, α-synuclein, and tyrosine hydroxylase. Neurol Res. 2024;46:406–415. doi: 10.1080/01616412.2024.2325312. [DOI] [PubMed] [Google Scholar]
- 56.Caridade-Silva R., Araújo B., Martins-Macedo J., Teixeira F.G. N-acetylcysteine treatment may compensate motor impairments through dopaminergic transmission modulation in a striatal 6-hydroxydopamine Parkinson's disease rat model. Antioxidants. 2023;12 doi: 10.3390/antiox12061257. [DOI] [PMC free article] [PubMed] [Google Scholar]