Abstract
Limitations of Poly (ADP‒ribose) Polymerase (PARP) Inhibitors
PARPis have demonstrated efficacy in BRCA-mutated cancers deficient in homologous recombination repair. Furthermore, PARPis have shown efficacy in BRCA-wild-type cancers with a homologous recombination deficiency phenotype known as BRCAness. Current clinically approved PARPis inhibit both PARP1 and PARP2, and their clinical promise is limited by toxicity, resistance, and a lack of combination partners.
Recent Findings
PARP2 inhibition is associated with hematological toxicity, affecting the tolerability and efficacy of monotherapy and combination therapies. Furthermore, synthetic lethality in BRCA-mutated cancers depends mostly on PARP1, whereas PARP2 is not essential. These findings promoted the development of next-generation PARPis with greater selectivity for PARP1 than for PARP2.
Summary
In this review, we discuss the next-generation PARPis that target PARP1 and show promise in terms of improved safety, tolerability, pharmacological profiles, and efficacy compared to existing clinically approved PARPis. These next-generation PARP1-selective inhibitors hold significant promises for improving the survival and outcomes of cancer patients.
Keywords: PARP1, PARP2, DNA damage, Single-strand breaks (SSBs), Double-strand breaks (DSBs), Homologous recombination repair (HRR), Homologous recombination deficiency (HRD)
Introduction
Poly (ADP‒ribose) polymerase (PARP) plays a pivotal role in cellular physiology by catalyzing the transfer of ADP‒ribose from nicotinamide (NAD +) precursors to target proteins, a posttranslational modification called PARylation [1, 2]. This enzymatic process, termed poly (ADP)-ribosylation, regulates vital cellular pathways [2, 3]. PARP1 and PARP2 are the best characterized PARPs (which refers to PARP 1 and 2) and contribute to most PARP activities [4–6]. PARP1 and PARP2 are well characterized for their role in DNA damage repair, ensuring cell survival by modifying proteins involved in chromatin architecture and DNA repair processes [5, 7, 8]. PARP1 has the predominant role in DNA damage repair and PARP2 has a lesser effect [9]. PARP is catalytically activated by single-strand breaks (SSBs) and double-strand breaks (DSBs) [5, 10–13]. Subsequently, PARP promotes the formation of poly (ADP‒ribose) (PAR) chains, which are important for providing a docking platform for DNA repair proteins to damaged sites, facilitating their recruitment [1, 10, 12]. Finally, the PAR chains are removed from substrate proteins by dePARylating enzymes [14, 15]. Recently, a variety of ADP-ribose degrading enzymes have been identified with different substrate specificities. Poly (ADP-ribose) glycohydrolase (PARG) is the major dePARylating enzyme which hydrolyzes the glycosidic linkages between ADP-ribose units of PAR polymers to generate free ADP-ribose monomers [14, 15]. Recently, other dePARylating enzymes such as ADP-ribose hydrolases, phosphodiester ADP-ribose hydrolases, macrodomain-ADP-ribose erasers, have been identified which are also involved in removing PAR chains (reviewed in [16]. Owing to the catalytic function of PARP, PARP can tightly bind to DNA, resulting in “PARP-DNA” trapping, which is stabilized by PARP inhibitors (PARPis), blocking the replication machinery and facilitating the collapse of the replication fork [17]. Disruption of these processes by pharmacological inhibition has emerged as a promising strategy in cancer therapy.
PARP Plays a Role in Base Excision Repair/Single-Strand Break Repair and DNA Replication
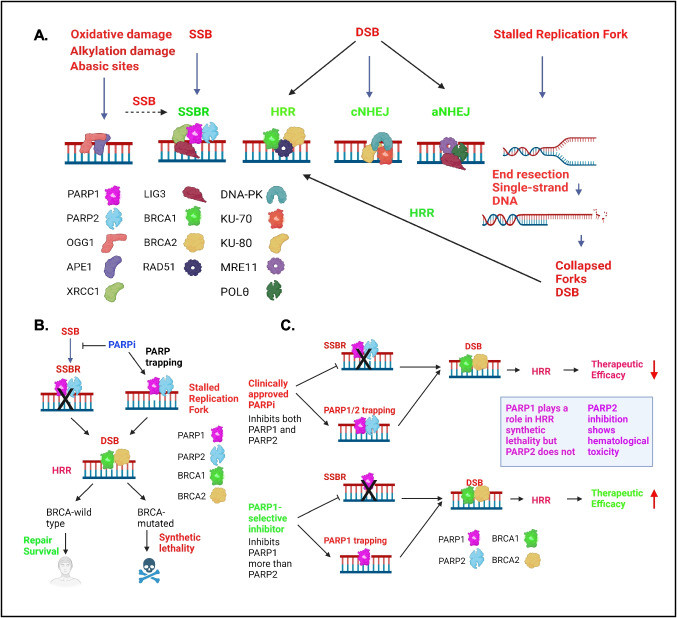
Due to spontaneous DNA damage, oxidative stress, irradiation, and reactive endogenous metabolites, thousands of DNA lesions are introduced in the DNA every day. These DNA modifications are repaired by base excision repair (BER) and single-strand break repair (SSBR). BER is the major pathway for repairing oxidative base damage, alkylation damage, and abasic sites [18]. In BER, damage is detected by OGG1 and APE1, which create SSBs that are repaired by the SSBR pathway [5, 13] (Fig. 1A). PARP1/2 binds to SSBs with high affinity and becomes activated, inducing ADP-ribosylation of histones and other DNA repair proteins and essential for BER/SSBR pathways [5, 19]. The recruitment of the core factor XRCC1 to SSBs is dependent on PARP1 and PARP2 [20]. XRCC1 functions as a scaffold for DNA Polβ, LIG1/3 and PNKP needed for the repair process [5, 18] (Fig. 1). Thus, BER/SSBR restores the damaged DNA to intact DNA [8].
Fig. 1.
Major cellular DNA damage repair pathways related to PARP function and the mechanism of synthetic lethality between PARP inhibitors and HRR deficiency. A. PARP1/2 predominantly play a role in SSBR. Inhibition of SSBR results in DSBs. PARP inhibition and PARP trapping result in stalled replication forks, which are converted to DSBs. In the presence of BRCA1/2, DSBs are predominantly repaired by HRR. In the absence of BRCA1/2, cells repair DSBs via the cNHEJ and aNHEJ pathways. BER (base excision repair), SSBR (single-strand break repair), HRR (homologous recombination repair), cNHEJ (classical nonhomologous end joining), aNHEJ (alternative nonhomologous end joining). B. Schematic showing the synthetic lethality between a PARPi and HRR-deficiency. C. Schematic showing how PARP1-selective inhibitors improve PARP efficacy. PARP1 is needed for the synthetic lethality of HRR and PARP1 selective inhibitors limit toxicity
PARP1 interacts and stimulates the activity of multiple replication proteins and influences DNA replication process [21–24]. Replication stress caused by oncogene activation, dNTP depletion, and unrepaired SSBs. These SSBs encounter the replication machinery resulting in stalled and/or collapsed replication fork with a single-ended DNA DSBs [8]. PARP1 has been implicated in protecting the degradation of stalled DNA replication forks and DSBs by promoting the recruitment of MRE11 and RAD51 [5, 25, 26]. These DSBs are resolved by the homologous recombination repair (HRR) pathway (described further below). PARP1 also plays a role in the prevention of untimely restart of stalled replication forks by binding to RECQ1 and allowing repair of DNA lesions [5]. Recently, PARP1 has been implicated to play a direct role in the Okazaki fragment processing [27, 28]. These studies showed that the unligated Okazaki fragments is one of the major sources of PARP activity and perturbation of the DNA replication proteins LIG1 or FEN1 increases the S phase PARP independent of damaged DNA or replication stress [27, 28].
PARP Inhibitors Inhibit Single-Strand Break Repair, DNA Replication, and induce PARP Trapping Resulting in Double-Strand Breaks which are Repaired by Homologous Recombination Repair Pathway
PARPis catalytically inhibit PARP1/2, and the Inhibition of SSBR by PARPi results in accumulation of SSBs. When these SSBs are unrepaired and enter the S phase, they are converted to more lethal DSBs. Furthermore, upon PARPi treatment, PARP binding to the DNA breaks is stabilized resulting in PARP trapping (Fig. 1A) [17, 29]. These “trapped-PARPs” causes replication fork stalling and SSBs which eventually gets converted to DSBs (Fig. 1A) [17, 30–33] (Fig. 1A). Studies supported that trapped PARPs form a complex with damaged DNA which is more cytotoxic compared with unrepaired SSBs due to PARP inhibition [17]. In cells harboring wild-type BRCA1/2 genes, these DSBs are mainly repaired via the HRR pathway, an error-free repair pathway which predominantly works in S phase and to a lesser extent in G2 phase of cell cycle due to availability of the homologous chromatid for recombination (Fig. 1A) [8, 34–36]. In the canonical HRR, BRCA2 initially binds to RAD51 and localizes to the DSB sites, whereas BRCA1 plays a crucial role in DSB resection and signal transduction [37, 38]. BRCA1 interacts with PALB2 and helps localization of BRCA2 by forming a molecular scaffold of BRCA1-PALB2-BRCA2 needed for HRR [39]. In the absence of functional BRCA1 and BRCA2, DSBs are resolved through the error-prone classical nonhomologous end joining (cNHEJ) pathway, or the alternative nonhomologous end joining (aNHEJ) pathway (Fig. 1A). During cNHEJ process, DNA-PKcs, KU-70, and KU80 bind the DSBs. 53BP1 limits end resection to create an overhang to promote ligation by DNA ligase 4 and XRCC4-XLF [5]. During the aNHEJ, DSBs use microhomologies to align the broken DNA ends. MRE11, POLꝊ and other proteins bind to the microhomology region and LIG3 play a role in the ligation of broken DNA ends (Fig. 1A) [8, 32, 40]. The ligation of broken DNA ends often lead to genomic alterations and increased heterogeneity in the genome [8, 32, 36, 40].
PARP Inhibitors Exhibit Synthetic Lethality in BRCA-Mutated Cancers
As described in the previous section, the unrepaired SSBs, defective replication, and PARP trapping induce replication stress-induced DSBs. BRCA-wild-type cells repair these DSBs via the HRR pathway [37, 38], but the BRCA-mutated cells cannot repair these DSBs. Therefore, inhibition of PARP in BRCA-mutated cells causes synthetic lethality due to the presence of unrepaired DSBs [41–46] (Fig. 1B). As a result, BRCA1/2-mutated cells are hyper reliant on PARP-mediated DNA repair for their survival [31, 41, 42]. This laid the foundation for the development of targeted therapies by exploiting the vulnerability of BRCA1/2-mutated tumors to homologous recombination deficiency (HRD) [47]. Currently, HRD is used as a predictive biomarker for PARP treatment and several methods are being used to determine the level of HRD in cancers [8].
PARP Inhibitors Exhibit Synthetic Lethality in Patients with the Brcaness Phenotype
Numerous investigations have shown that in addition to classical BRCA1 and BRCA2 mutations, a diverse array of molecular alterations can contribute to the development of HRD [40, 48–50]. Defects in these non-BRCA mutated proteins can promote a"BRCAness"phenotype, characterized by biological and clinical features akin to those associated with BRCA1/2 mutations [40, 48–50]. These alterations include mutations in key genes that play a role in HRR, such as RAD50, RAD51, RAD51C, RAD51D, RAD54L, and RAD51C58, as well as the overexpression of the HORMAD1 protein, which suppresses RAD51-dependent HRR [48, 49, 51, 52]. Additionally, defects in DNA damage response genes associated with HRR, such as BARD1, BRIP1, ATM, ATR, CHK1, CHK2, NBS1, PRKDC, RPA1, and DSS1, and mutations in several Fanconi anemia pathway genes, including PALB2, FANCA, FANCC, FANCD2, FANCE, and FANCF, have been implicated in the development of HRD [46, 48, 53]. Moreover, mutations in non-HRR DNA damage response genes such as loss of CDK12 can induce genomic instability resembling BRCAness across various cancer types, indirectly promoting HRR deficiency [54, 55]. CRISPR-Cas9 loss-of-function screens in PARPi-insensitive cell lines has identified most frequently inactivated genes in tumors and showed that XRCC1 is a potential new prognostic biomarker of PARPi sensitivity in prostate cancer [56].
Other mechanisms, including promoter methylation, somatic BRCA1/2 mutation, and gene deletion, can also impair BRCA1/2 function, resulting in a BRCAness phenotype [57–59]. PARPi induce synthetic lethality not only in patients harboring hereditary BRCA1/2 mutations but also in those exhibiting the BRCAness phenotype and HRD [41, 54]. Considering all these factors, HRR gene mutations beyond BRCA1/2 and overall dysregulation of HDR can serve as biomarkers to predict sensitivity to PARPis, thereby guiding personalized therapeutic strategies [48].
First-Generation PARP Inhibitors Targeting both PARP1 and PARP2
Multiple PARPis have undergone successful clinical trials and have been approved by the Food and Drug Administration for ovarian, breast, pancreatic, and prostate cancers, demonstrating the promise of this approach [31, 60, 61]. These PARPis exhibit varying potencies as both PARP catalytic function inhibitors and PARP trapping agents and have been reviewed elsewhere [17, 31, 62, 63]. In general, the first-generation PARPis olaparib, talazoparib, niraparib, rucaparib, veliparib, pamiparib, and fuzuloparib target both PARP1 and PARP2, bind to their active sites and inhibit PARylation. This is because PARP1 and PARP2 share a high degree of sequence homology (69%) at the catalytic domain [64]. Even though all these inhibitors trap PARP at sites of DNA damage they show varied efficacy in PARP trapping [30, 61]. Compared with other agents, talazoparib results in the greatest amount of PARP trapping, in contrast with veliparib, which has weaker PARP trapping activity [30]. Several clinical trials have extensively explored the clinical efficacy of olaparib [65], talazoparib [66, 67], niraparib [68], rucaparib [69], veliparib [70], pamiparib [71], and fuzuloparib [72] in patients harboring BRCA mutations. These first-generation PARPis demonstrate distinct levels of synthetic lethality with HRD tumors and various levels of toxicity due to their differences in PARP selectivity and trapping ability [17, 31, 53, 62, 63, 73]. Specifically, PARPis showed dose limiting toxicities when used in combination with chemosensitizers and radiosensitizers [8, 74]. In addition, while above PARPis have demonstrated promising efficacy, not all patients respond to these treatments, especially BRCA-proficient patients (reviewed in [35, 60, 75]. Furthermore, PARPi efficacy is often diminished by the emergence of drug resistance [60, 76]. Several mechanisms have been demonstrated for PARPi resistance, such as drug efflux [13, 77, 78], decreased PARP trapping to DNA [78, 79], and the restoration of HRR through various mechanisms (reviewed elsewhere), such as BRCA mutation reversion and PARP1 mutation [60, 76, 78, 80–82], increased BRCA and other HRR protein expression [60], the stabilization of the replication fork [13, 76, 78, 83], the inactivation of different NHEJ-promoting factors that inhibit DNA end resection [76], and other processes, such as the loss of POLQ [78], as well as the overexpression of CDK12 and WEE1 [49, 78]. Secondary mutations in BRCA1/2, RAD51 C, RAD51D, and PALB2 are common which restores the functional protein have been reported in many cancer models including breast, ovarian, pancreatic, and prostate cancers (reviewed in [84]. Genome-wide and high-density CRISPER-Cas9 screens identified PARP1 point mutations in the DNA binding site and outside the DNA binding site causing PARPi resistance by lowering PARP trapping [81, 85]. PARP1 mutations p.R591 C and p.848 delY, were identified to cause PARPi resistance. Specifically, the p.R591 C, a clinically relevant PARP mutation found in an olaparib-resistant ovarian cancer patients promotes dissociation of PARP1 from the DNA damage site showing inefficient PARP trapping resulting in PARP resistance [81].
Why the Development of Next-Generation PARP Inhibitors is Important for Improving PARP Inhibitor Efficacy
The first-generation PARPis bind with PARP1 and PARP2 showing similar levels of inhibition [64]. These PARPis generally shows adverse events, including hematological effects, gastrointestinal effects, renal toxicities, fatigue etc. [86–88]. Emerging evidence suggests that the inhibition of PARP2 results in notable hematological toxicity due to the critical role of PARP2 in the survival of hematopoietic stem cells [89, 90]. In preclinical mouse models, the loss of both PARP1 and PARP2 is incompatible with normal embryonic development, whereas the deletion of PARP2, but not PARP1, leads to chronic anemia, supporting a role of PARP2 in erythropoiesis [90]. PARP2 knockout mice exhibit impaired differentiation of erythroid progenitor cells and reduced erythrocytic lifespan, thymopoiesis, adipogenesis, and spermatogenesis, which are correlated with the toxicity of first-generation PARPis [89–93]. The hematologic toxicities limit the use of current clinically approved PARPis as monotherapies, especially in a relapsed setting, and in combination therapy treatments [91, 92]. Another important factor, for developing PARP1 selective inhibitor is that PARP1 plays a major role in 80–90% of PARylation induced by DNA damage, whereas PARP2 accounts for only 5–20% of PARylation [94]. As a result, the synthetic lethality of PARPis in HRR-deficient tumors primarily depends on PARP1 inhibition and trapping, while PARP2 inhibition and trapping are not essential [6, 17]. Therefore, it has been hypothesized that selectively inhibiting PARP1 would reduce PARPi toxicity associated with PARP2 inhibition while improving synthetic lethality in HRD cancers and subsequently improving the efficacy of PARPi treatments. These factors led to the development of next-generation PARPis with greater selectivity for PARP1 than for PARP2.
Next-Generation PARPis Demonstrate Greater PARP1 Selectivity than PARP2 Selectivity and Display Greater PARP Inhibitor Efficacy
The effort to identify PARP1 selective inhibitor led to the first discovery of 2-[1-(4,4-Difluorocyclohexyl)piperidin-4-yl]−6-fluoro-3-oxo-2,3-dihydro-1H-isoindole-4-carboxamide (NMS-P118): A potent, orally available, and highly selective PARP1i with excellent absorption, distribution, metabolism, and excretion (ADME) and pharmacokinetic (PK) profile showing high efficacy in breast cancer and pancreatic cancer xenograft models [95]. Following this discovery, many laboratories tried to develop PARP1 selective inhibitors and some of them are in clinical trial and others are in pre-clinical development.
Next-generation PARP1 Selective PARPis in Clinical Development
Several next-generation PARPis designed with higher PARP1 selectivity than PARP2 selectivity are currently in clinical trials (Table 1). AZD5305 (saruparib), a highly potent and selective PARP1i (reviewed in [93]), exhibited 500-fold selectivity for PARP1 over PARP2 in a PARylation assay (IC50 of 1.55 nM for PARP1 and IC50 of 653 nM for PARP2) [96, 97] and showed excellent PARP1 trapping capacity compared with that of PARP2 [96]. AZD5305 also exhibited greater PARylation (IC50 of 2.3 nM) than talazoparib (IC50 of 5.1 nM) and veliparib (IC50 of 33 nM) did [96]. AZD5305 had a weaker effect on hematopoietic stem/progenitor cells than talazoparib did [97] and had minimal effects on hematological parameters compared to olaparib and niraparib in rat preclinical models [96]. AZD5305 displayed excellent safety and tolerability profiles and favorable pharmacokinetic (PK) and pharmacodynamic (PD) properties in BRCA-mutated tumor models [96]. Mechanistically, AZD5305 induces the accumulation of DNA damage and G2/M cell cycle arrest in HRR-deficient colon, breast, and gastric cancer cell lines [96]. Compared with other PARPis, AZD5305 has better efficacy and improved overall and progression-free survival in HRR-deficient breast, ovarian, gastric, and colon cancer cell lines; breast, ovarian, pancreatic, and colon cancer cell-derived xenograft (CDX) models; and breast cancer patient-derived xenograft (PDX) models [96, 98, 99]. AZD5305 also resulted in significant tumor regression in combination with carboplatin and paclitaxel in BRCA-wild-type breast cancer CDX models and improved tolerability in combination with carboplatin compared with first-generation PARPis in both breast cancer CDX and breast and ovarian cancer PDX models [96, 98]. The phase I/IIa clinical trial PETRA study (NCT04644068,www.clinicaltrial.gov, Table 1) evaluated the safety and early clinical efficacy of AZD5305 as monotherapy or in combination with other anticancer agents (paclitaxel, carboplatin with or without paclitaxel) in advanced solid cancers. Trial results with HRR-deficient (with mutations in one of five HRR genes: BRCA1, BRCA2, PALB2, RAD51C, or RAD51D) breast, ovarian, pancreatic, and prostate cancer patients revealed that, compared with first-generation PARPis, AZD5305 exhibited excellent safety and tolerability profiles and favorable PK and PD properties with a wide therapeutic index [100]. AZD5305 inhibited approximately 90% of PARP activity in tumor tissue collected from biopsies of these patients [100]. Efficacy has been observed in all tumor types across all doses, and mutation types showing great promise compared with clinically approved PARPis [100]. The efficacy and safety of AZD5305 in combination with new hormonal agents (enzalutamide, abiraterone, acetate, and darolutamide) are being assessed in a phase I/IIa PETRANHA study (NCT05367440, www.clinicaltrial.gov, Table 1) of metastatic castration-sensitive and castration-resistant prostate cancers [101]. In addition, the efficacy and safety of AZD5305 in combination with a physician’s choice of new hormonal agents (abiraterone, darolutamide, or orenzalutamide) compared with those of the placebo are being evaluated in a phase III EvoPAR-Prostate01 study with metastatic castration-sensitive prostate cancer (NCT06120491, www.clinicaltrial.gov, Table 1) [102]. Another clinical trial has been initiated in newly diagnosed prostate cancer patients, where AZD5305 will be evaluated alone and in combination with darolutamide, an antiandrogen drug used to treat nonmetastatic castration-resistant prostate cancer in men (NCT05938270, www.clinicaltrial.gov, Table 1). AZD5305 is also in clinical trials in combination with camizestrant, an oral selective estrogen receptor degrader, and will be compared with CDK4/6 inhibitors plus endocrine therapy or with camizestrant in HR-positive, Her2-negative, BRCA1-, BRCA2-, or PALB2-mutated advanced breast cancer (NCT06380751; www.clinicaltrial.gov, Table 1). AZD5305 has also been investigated in combination with datopotamab deruxtecan (Dato-DXd). Dato-DXd is a TROP2-directed antibody drug conjugate composed of a topoisomerase I inhibitor (an exatecan derivative, DXd) conjugated to datopotamab, a humanized anti-TROP2 IgG1 antibody, via a linker. Topoisomerase I inhibitors induce SSBs that require PARPs for their repair. The AZD5305 and Dato-DXd combination enhanced the sensitization of triple-negative breast cancer (TNBC) and non-small cell lung cancer cell lines and resulted in superior tumor growth inhibition in a gastric cancer CDX and a PARPi-resistant ovarian cancer PDX model. This combination is being investigated in clinical trials in patients with advanced/metastatic solid tumors in the TROPION-PanTumor03 study (NCT05489211, www.clinicaltrial.gov, Table 1) [103]. AZD5305 also exhibited promising efficacy with an ATR inhibitor (ATRi) in PARPi- and ATRi-resistant HRR-proficient- and -deficient cell lines, in breast and ovarian cancer CDX, and in TNBC and ER + breast cancer PDX models [104]. Currently, a clinical trial evaluating the AZD5305 and ATRi (ceralasertib) combination (NCT02264678, www.clinicaltrial.gov, Table 1) in advanced solid tumors is ongoing.
Table 1.
Next-generation PARP inhibitors in clinical trials showing increased PARP1 selectivity over PARP2. PARP1 selectivity over PARP2 was measured via an enzymatic/PARylation assay, and DNA trapping was measured via a DNA trapping assay. The clinical trial data are from www.clinicaltrial.gov. mCSPC (metastatic castration-sensitive prostate cancer), mCRPC (metastatic castration-resistant prostate cancer), HRR (homologous recombination repair), HRD (homologous recombination deficiency), IDH (isocitrate dehydrogenase), GBM (glioblastoma)
| PARP inhibitor | PARP selectivity over PARP2 |
PARP1-DNA trapping over PARP2 | Clinical trial | Phase of development (two places) | Cancer type | Key eligibility criteria | Developed by (Company/Institution) |
|---|---|---|---|---|---|---|---|
|
AZD5305 AZD5305 + Paclitaxel AZD5305 + Carboplatin (with or without Paclitaxel) |
500-fold | > PARP2 | NCT04644068 | Phase I/IIa | Solid tumors | Prior PARPi treatment required | AstraZeneca, Cambridge, United Kingdom |
| AZD5305 + new hormonal agents | NCT05367440 | Phase I/IIa |
Prostate (mCSPC and mCRPC) |
no prior PARPi treatment | |||
| AZD5305 + physicians’ choice new hormonal agents | NCT06120491 | Phase III | Prostate (mCSPC) | Confirmed HRRm status | |||
|
AZD5305 AZD5305 + Darolu-tamide |
NCT05938270 | Phase I (to be open) | Prostate | no HRR status requirement published | |||
|
AZD5305 + Camizestrant |
NCT06380751 | Phase III | Breast | BRCA1, BRCA2, or PALB2 Mutations | |||
| AZD5305 + Dato-Dxd | NCT05489211 | Phase II | Solid tumors | no HRR status requirement published | |||
| AZD5305 + Ceralasertib | NCT02264678 | Phase I/Ib | Solid tumors | known or suspected BRCA mutation, PALB2 mutation, RAD51 C/D mutation or HRD positive status | |||
| NMS-03305293 | > 200-fold | No trapping activity | NCT04182516 | Phase I | Solid tumors | BRCA1 and BRCA2 mutation status is not required for enrollment in the dose escalation part, but enrichment with deleterious/pathogenic or likely pathogenic/suspected deleterious BRCA carriers will be attempted | Nerviano Medical Sciences, Italy |
| NMS-03305293 + TMZ | NCT04910022 | Phase I |
Diffused Glioma |
no HRR status requirement published | |||
| NMS-03305293 + TMZ | NCT04910022 | Phase II | IDH wild type GBM |
IDH wild type no HRR status requirement published |
|||
|
AZD9574 AZD9574 + targeted agents |
> 20-fold | > PARP2 | NCT05417594 | Phase I/IIa | Solid tumors | deemed suitable for a Poly ADP-Ribose Polymerase (PARPi) by the Investigator | AstraZeneca United Kingdom |
| HRS-1167 | > PARP2 | Not known | NCT05473624 | Phase I | Solid tumors | HRR gene mutation | Jiangsu HengRui Medicine Co., Ltd., China |
| IMP1734 |
PARP1 selective |
Not known | NCT06253130 | Phase I/II | breast, ovarian, mCRPC | deleterious or suspected deleterious germline or somatic mutations of select HRR genes | Eikon Therapeutics, United States |
NMS-03305293 (NMS-P293) is an oral, PARP1-selective inhibitor showing > 200-fold selectivity for PARP1 over PARP2 in the PARylation assay, but this compound is not a good PARP-trapper [105–107], (Table 1). Owing to its lower inhibitory effect on PARP2, NMS-03305293 spares normal myelocytes [105]. NMS-03305293 exhibited a good PK profile and good ADME properties, including a low efflux ratio and high cross-species metabolic stability in rodents and nonrodent models [105]. NMS-03305293 exhibited good efficacy in BRCA-mutated breast cancer cell lines and CDX models [105, 106]. A phase I dose-escalation and dose expansion study explored the safety, tolerability, and antitumor activity of NMS-03305293 as a single agent in patients with advanced/metastatic, relapsed/refractory solid tumors for whom exhausted standard treatment options or standard therapy are unavailable (NCT04182516, www.clinicaltrial.gov, Table 1). In addition, NMS-03305293 exhibited high blood‒brain barrier (BBB) penetration and represents a novel therapeutic option for glioblastoma (GBM) and localized brain metastasis [106]. On the basis of this property, the PARPA-293–002 study evaluated the safety and efficacy of the NMS-03305293 and temozolomide (TMZ) combination in adult diffuse glioma patients (phase I) and in isocitrate dehydrogenase (IDH) wild-type GBM patients (Phase II) at first relapse (NCT04910022, www.clinicaltrial.gov, Table 1). NMS-03305293 was well tolerated in this clinical trial, with no dose-dependent trend toward myelosuppression and demonstrated encouraging efficacy in combination with TMZ [108].
AZD9547 showed greater PARP1 binding in a fluorescence anisotropy assay, with > 8000-fold greater PARP1 binding than PARP2 binding. AZD9547 is more effective at promoting PARylation in PARP2-/- cells (IC50 of 1.5 nM) than in PARP1-/- cells (IC50 > 30 nM), with ~ 20-fold greater selectivity for PARP1 than for PARP2 in the enzymatic assay. Furthermore, AZD9574 is more potent at PARylation (IC50 of 1.5 nM) than olaparib is (IC50 of 14.7 nM), with ~ 9.8-fold greater PARylation than olaparib in the enzymatic assay [109]. Notably, compared with the first-generation PARPi talazoparib, olaparib, or pamiparib, which trap both PARP1 and PARP2, AZD9547 showed only PARP1 trapping to DNA and no PARP2 trapping. Consequently, compared with olaparib and pamiparib, AZD9547 demonstrated decreased hematologic toxicity in rat preclinical models and displayed favorable PK and PD properties [109]. AZD9574 demonstrated robust anticancer efficacy as a monotherapy in HRR-deficient breast, ovarian, and colon cancer cell lines and in a breast cancer CDX model [109, 110]. Owing to its ability to increase the BBB, AZD9574 showed improved efficacy and extended survival in combination with TMZ in a GBM model [109]. Currently, AZD9574 is in clinical trials as monotherapy and in combination with other anticancer agents for advanced solid tumors (both HRR-proficient and HRR-deficient) that have been used to assess its safety, tolerability, PK, and PD (NCT05417594, www.clinicaltrial.gov; Table 1). This clinical trial will include advanced/relapsed ovarian, breast, pancreatic, and prostate cancer patients who can be treated with PARPis and HER2-negative breast cancer patients with BRCA1/2, PALB2, RAD51C, and RAD51D mutations. This clinical trial will also assess the combination of AZD9574 with trastuzumab deruxtecan in HER2-positive advanced metastatic solid tumors, Dato-DXd in advanced metastatic solid tumors, and TMZ in glioma patients with IDH mutations.
The safety, tolerability, and efficacy of the PARPi HRS-1167 (M9466), a highly selective PARP1i, were assessed in a phase I clinical trial in advanced solid tumors (NCT05473624, www.clinicaltrial.gov, Table 1). Patients with advanced solid tumors that progressed on standard therapies or for whom no standard therapies were available were eligible for the dose escalation study. On the other hand, previously treated patients with germline or somatic BRCA1/2, PALB2, or RAD51 mutations received this drug in the dose expansion cohort. In the clinical trial, no dose-limiting toxicities occurred, and the maximum tolerated dose was not reached. Additionally, HRS-1167 exhibited favorable PK and safety profiles in these patients, indicating promising antitumor efficacy [111].
IMP1734, a selective PARP1i, reduces tumor growth in preclinical models. It is currently being evaluated in clinical trials in patients with advanced, recurrent, and metastatic breast, ovarian, and prostate cancers with mutations in select HRR genes. The primary endpoint is safety and tolerability, and the secondary endpoint is efficacy, progression-free survival, and overall survival. The exploratory endpoints include PK, PD, and patients’ quality of life (NCT06253130, www.clinicaltrial.gov, Table 1) [112].
Preclinical Development of Next-Generation PARP1-Selective PARPis
Several PARPis showing greater selectivity for PARP1 than for PARP2 have shown promising results in preclinical studies.
VB15010 is a novel and potent selective PARP1i (Table 2). VB15010 exhibited greater selectivity for PARP1 than other PARP family members did (30– to 8,000-fold) in enzymatic assays and superior selectivity for PARP1 over PARP2 DNA trapping (> 1700-fold) in DNA trapping assays. VB15010 demonstrated good PK and PD properties and potent antiproliferative activity in both BRCA-mutated and HRD-positive cancer cell lines and demonstrated substantial antitumor activity in BRCA-mutated CDX and HRD-positive PDX models. VB15010 showed excellent bioavailability in animal models and displayed preferable tumor tissue distribution. Overall, higher PARP1 selectivity over PARP2, durable DNA trapping capability, improved PK and PD properties, favorable tumor‒to‒plasma distribution ratios, and potent antitumor activity show promise for enhancing the clinical efficacy of VB15010 and reducing hematological toxicity in patients [113].
Table 2.
Next-generation PARP inhibitors in preclinical stages showing increased PARP1 selectivity over PARP2. PARP1 selectivity over PARP2 was measured via an enzymatic/PARylation assay, and DNA trapping was measured via a DNA trapping assay. TNBC (triple-negative breast cancer)
| PARP inhibitor | PARP1-selectivity over PARP2 | PARP1-DNA trapping over PARP2 | Cell lines/CDX/PDX | Developed by (Company/Institute) |
|---|---|---|---|---|
| VB15010 | 30–8000 fold | 1700-fold | HRR-positive cancers | Shenzhen Yangli Pharmaceutical Technology, China |
| D0112-005 | 2000-fold | 66-fold | Breast | Chengdu Easton Biopharmaceuticals Co., Ltd., Chengdu, China |
| XZP-7797 | > 1000-fold | Not known | Breast, pancreatic | Sihuan Pharmaceutical, China |
| SNV001 | > 500-fold | > PARP2 | Breast | Synnovation Therapeutics, United States |
| ACE86225106 | 72-fold | 131-fold | Solid tumors | Acerand Therapeutics, China and United States |
| DM5167 | 6.88-fold | 348-fold | TNBC | Kainos Medicine, Inc., Republic of Korea |
| HH102007 | > AZD5305 and AZD9574 | > AZD9574 | Breast, pancreatic | Haihe Biopharma, China |
| HSK40495 | > PARP2 | Not known | Breast, colorectal | Haisco Pharmaceutical, China |
| DSB1559 | > AZD5305 | Not known | TNBC | Duke Street Bio Ltd, United Kingdom |
| LAE119 | PARP1 selective | > 1000-fold | Breast, pancreatic, colorectal | Laekna Pharmaceuticals, China |
| DHC-1 | PARP1 selective | Not known | Breast, pancreatic | Qingdao University of Science and Technology, China |
Another PARP1-selective inhibitor, D0112-005, showed 2000-fold selectivity for PARP1 over PARP2 and PARP3 and 66-fold greater PARP1 trapping than PARP2 (Table 2). DO112-005 exhibited a good safety profile in rats and dogs and showed high anti-proliferative activity against BRCA-mutated breast cancer cell lines and CDX models [114].
XZP-7797 is a potent, selective, and CNS-penetrating PARP1-selective inhibitor that shows more than 1000-fold selectivity for PARP1 over PARP2 and other members of the PARP family in PARylation assays and has good ability to trap PARP1 (Table 2). Accordingly, XZP-7797 did not have any adverse hematological effects in a toxicity study using rat model. XZP-7797 also showed good PK and ADME properties and good BBB penetration in a mouse model and demonstrated significant efficacy in breast cancer cell lines, a BRCA1-mutated breast cancer CDX model, and a pancreatic cancer CDX model [115].
SNV001 demonstrated > 500-fold PARP1 selectivity over PARP2 in the PARylation assay and trapped PARP1 on DNA but not PARP2, resulting in less toxicity in mouse models [116] (Table 2). Compared with olaparib, SNV001 dose-dependently inhibited HRD cancer cell lines, including breast and colorectal cancer, and inhibited tumor growth in a BRCA-mutated breast cancer CDX model without any signs of toxicity [116].
ACE86225106, another PARP1-selective inhibitor, showed 72-fold greater PARP1 selectivity over PARP2 in an enzymatic assay and 131-fold greater PARP1 trapping over PARP2 in a DNA-trapping assay (Table 2). ACE86225106 exhibited lower inhibition of hematopoietic stem cells, a good safety profile, and a unique PK profile, with a long half-life and low distribution volume. Consequently, ACE86225106 exhibited robust efficacy in cancer cell lines and CDX models, indicating good clinical utility [117].
DM5167 is a PARP1-selective inhibitor that shows 6.88-fold greater PARP1 selectivity over PARP2 in an enzymatic assay and 348-fold greater PARP trapping activity in a DNA trapping assay (Table 2). Compared with olaparib, DM5167 showed acceptable safety margins and toxicity profiles and demonstrated good anticancer efficacy in TNBC cell lines and CDX models [118].
HH102007, a highly potent PARP1-selective inhibitor, studied extensively and compared with AZ5305 and AZD9574 compounds. HH102007 exhibited greater PARP1 selectivity than did AZD5305 and AZD9574 in enzymatic assays and showed lower hematological toxicity than these compounds in a rat model. However, HH102007 traps more PARP1 than AZD9574 (Table 2). Additionally, HH102007 also exhibited high potency in BRCA-mutated breast cancer cell lines and CDX models alone and in combination with carboplatin, showing greater efficacy than did AZD9574 [119].
HSK40495, a PARP1-selective inhibitor, showed greater PARP1 selectivity than PARP2 in an enzymatic assay and 5000-fold greater selectivity than PARP2 in a protein binding assay (Table 2). Compared with talazoparib, HSK4095 was less cytotoxic to bone marrow progenitor cells and improved the safety profile of the differentiation of myeloid, erythroid, and megakaryocytes. Additionally, HSK40495 has favorable PK properties and potent antitumor activity in BRCA knock-out (KO) colorectal cancer and BRCA-mutated breast cancer cell lines, and BRCA-mutated breast cancer CDX models [120].
DSB1559 demonstrated superior selectivity for PARP1 compared to AZD5305 in cell lines (Table 2). DSB1559 also showed highly desirable PK and ADME properties, indicating its high oral bioavailability in rodent PK studies. Compared with AZD5305, DSB1559 exhibited superior tumor residence time and tumor-to-plasma ratio and significantly reduced tumor growth while enhancing overall survival in a BRCA1-associated immune-competent TNBC model [121].
LAE119, a PARP1-selective inhibitor, demonstrated more than 1000-fold selectivity for PARP1 DNA trapping compared with PARP2, which has good PK profiles and ADME properties (Table 2). In a rat hematologic toxicity assay, LAE1 had minimal effects on hematological parameters and was well tolerated. LAE119 has more efficient anti-proliferative effects on BRCA1/2-mutated breast, colon, and pancreatic cancer cell lines than does AZD5305 and exhibits substantial tumor inhibition with reduced toxicity in BRCA-mutated breast and pancreatic cancer CDX models [122].
DHC-1, a potent and selective PARP1 inhibitor, selectively inhibited PARP1 activity and showed efficacy in BRCA1-deficient breast cancer and BRCA2-deficient pancreatic cancer cell lines (Table 2). DHC1 induced DNA damage and G2/M cell cycle arrest, resulting in enhanced sensitization to oxaliplatin. DHC-1 also exhibited a tumor-inhibitory effect in a CDX model of pancreatic cancer [123].
Conclusions
PARPis altered the treatment paradigm in multiple cancers. PARPis not only improve the treatment outcome of patients with BRCA1/2-mutated cancers but also, as our understanding of HRD and BRCAness has increased, more patients may benefit from these therapies. PAOLA-1, a phase 3 clinical trial, supports potential benefit of HRD in improving the progression-free survival. This trial evaluated the therapy with Olaparib in newly diagnosed advanced ovarian cancer who were receiving chemotherapy and bevacizumab followed by bevacizumab. Prespecified subgroup analysis showed a beneficial progression-free survival in patients with BRCA-mutated and HRD-positive tumors. Furthermore, patients with HRD-positive tumors without BRCA mutation (20%) exhibited substantial clinical benefit compared to Olaparib [124]. Additional biomarkers for response to PARPi are being investigated in other solid tumors. As many cancer therapies may induce DNA damage and target DNA repair pathways, PARPis are potential partners for continually increasing the number of treatments with combination approaches. Even though the currently approved PARPis have shown significant clinical benefits, their toxicities continue to limit their use. Several next-generation PARPis exhibited improved PARP1 selectivity over PARP2, resulting in lower hematological toxicity while demonstrating greater therapeutic efficacy in multiple cancer models (Fig. 1C).
Currently, there is limited data on the resistance patterns for PARP1 inhibitors. Since HRR is most dependent on PARP1 rather than PARP2, we expect similar resistance mechanisms with possible PARP2 compensation. Larger studies will be instrumental in observing the resistance timelines, mechanisms, and patterns to PARP1 specific inhibitors.
Although more work is needed to determine the potential of these new inhibitors in the clinic, to establish drug resistance and effectiveness in combination therapy, the expanding landscape of preclinical research on the development of next-generation PARPis with increased PARP1 selectivity holds great promise for future options for patients. Furthermore, the improved toxicity profile of these new agents will allow for novel combinatorial strategies to further improve patient outcomes. Although there are several strategies for improving PARPi efficacy, increasing PARP1 selectivity is an exciting approach for improving efficacy and tolerability, which will lead to even wider adoption in future clinical practice.
Key References
- Tung NM, Robson ME, Ventz S, Santa-Maria CA, Nanda R, Marcom PK, et al. TBCRC 048: Phase II Study of Olaparib for Metastatic Breast Cancer and Mutations in Homologous Recombination-Related Genes. J Clin Oncol. 2020;38(36):4274–82.
- ○ Key clinical trial establishing the role of PARP inhibitors
- Turner NC, Balmana J, Poncet C, Goulioti T, Tryfonidis K, Honkoop AH, et al. Niraparib for Advanced Breast Cancer with Germline BRCA1 and BRCA2 Mutations: the EORTC 1307-BCG/BIG5-13/TESARO PR-30-50-10-C BRAVO Study. Clin Cancer Res. 2021;27(20):5482–91
- ○ Key clinical trial establishing the role of PARP inhibitors
- Stodtmann S, Eckert D, Joshi R, Nuthalapati S, Ratajczak CK, Menon R, et al. Exposure–Response Model With Time-Varying Predictors to Estimate the Effects of Veliparib in Combination With Carboplatin/Paclitaxel and as Monotherapy: Veliparib Phase 3 Study in BRCA-Mutated Advanced Breast Cancer (BROCADE3) Trial. J Clin Pharmacol. 2022;62(10):1236–46
- ○ Key trial in development of PARP inhibitors and combination strategies
- Dellavedova G, Decio A, Formenti L, Albertella MR, Wilson J, Staniszewska AD, et al. The PARP1 Inhibitor AZD5305 Impairs Ovarian Adenocarcinoma Progression and Visceral Metastases in Patient-derived Xenografts Alone and in Combination with Carboplatin. Cancer Res Commun. 2023;3(3):489–500
- ○ Important work showing the promise of specific PARP1 inhibitors in ovarian cancer models
- Hopkins TA, Shi Y, Rodriguez LE, Solomon LR, Donawho CK, DiGiammarino EL, et al. Mechanistic Dissection of PARP1 Trapping and the Impact on In Vivo Tolerability and Efficacy of PARP Inhibitors. Mol Cancer Res. 2015;13(11):1465–77
- ○ Seminal work defining the mechanisms of PARP inhibitor functions
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21
- ○ Defining BRCA mutation of a therapeutic biomarker
- Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–7
- ○ Defining BRCA mutation of a therapeutic biomarker
- Jamal K, Galbiati A, Armenia J, Illuzzi G, Hall J, Bentouati S, et al. Drug-gene Interaction Screens Coupled to Tumor Data Analyses Identify the Most Clinically Relevant Cancer Vulnerabilities Driving Sensitivity to PARP Inhibition. Cancer Res Commun. 2022;2(10):1244–54
- ○ Establishing genomic vulnerabilities that sensitize cancer cells to PARP inhibitors
Acknowledgements
Figures were created via Biorender.com.
Abbreviations
- PARP
Poly ADP‒ribose polymerase
- SSB
Single-strand break
- DSB
Double-strand break
- BER
Base-excision repair
- HRR
Homologous recombination repair
- HRD
Homologous recombination deficiency
- cNHEJ
Classical nonhomologous end joining
- aNHEJ
Alternative nonhomologous end joining
- I
Inhibitor
- PK
Pharmacokinetics
- PD
Pharmacodynamics
- ADME
Absorption, distribution, metabolism, and excretion
- BBB
Blood–brain barrier
- GBM
Glioblastoma
- TMZ
Temozolamide
- TNBC
Triple-negative breast cancer
- CDX
Cell-derived xenograft
- PDX
Patient-derived xenograft
Author Contributions
Both authors contributed to this manuscript. AR wrote the draft and prepared the figure and tables MO edited the draft and figures.
Funding
The work was supported by: P30 CA082709 Cancer Center Support Grant The Vera Bradley Foundation.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Human and Animal Participants
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of interest
Consulting or Advisory Role Company: AstraZeneca Research Funding Company: Pfizer Recipient: You Company: Bayer Recipient: You Company: Alphageneron Recipient: You Company: Werewolf Therapeutics Recipient: You Company: LaNova Recipient: You Company: Krystal Biotech Recipient: You Company: ImmuneOnco Biopharmaceuticals Recipient: You Company: IDEAYA Biosciences Recipient: You Company: PMV Pharma Recipient: You Company: Monte Rosa Therapeutics Recipient: You Company: Immune-Onc Therapeutics Recipient: You.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alo Ray, Email: aloray@iu.edu.
Mateusz Opyrchal, Email: mopyrch@iu.edu.
References
- 1.Kim MY, Mauro S, Gevry N, Lis JT, Kraus WL. NAD+-dependent modulation of chromatin structure and transcription by nucleosome binding properties of PARP-1. Cell. 2004;119(6):803–14. [DOI] [PubMed] [Google Scholar]
- 2.Alemasova EE, Lavrik OI. Poly(ADP-ribosyl)ation by PARP1: reaction mechanism and regulatory proteins. Nucleic Acids Res. 2019;47(8):3811–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: “PAR-laying” NAD+ into a nuclear signal. Genes Dev. 2005;19(17):1951–67. [DOI] [PubMed] [Google Scholar]
- 4.Szanto M, Brunyanszki A, Kiss B, Nagy L, Gergely P, Virag L, et al. Poly(ADP-ribose) polymerase-2: emerging transcriptional roles of a DNA-repair protein. Cell Mol Life Sci. 2012;69(24):4079–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ray Chaudhuri A, Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol. 2017;18(10):610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronson GE, Piberger AL, Higgs MR, Olsen AL, Stewart GS, McHugh PJ, et al. PARP1 and PARP2 stabilise replication forks at base excision repair intermediates through Fbh1-dependent Rad51 regulation. Nat Commun. 2018;9(1):746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson BA, Kraus WL. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat Rev Mol Cell Biol. 2012;13(7):411–24. [DOI] [PubMed] [Google Scholar]
- 8.Drew Y, Zenke FT, Curtin NJ. DNA damage response inhibitors in cancer therapy: lessons from the past, current status and future implications. Nat Rev Drug Discov. 2024;24:19. [DOI] [PubMed] [Google Scholar]
- 9.Brown JS, O’Carrigan B, Jackson SP, Yap TA. Targeting DNA Repair in Cancer: Beyond PARP Inhibitors. Cancer Discov. 2017;7(1):20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Audebert M, Salles B, Calsou P. Involvement of poly(ADP-ribose) polymerase-1 and XRCC1/DNA ligase III in an alternative route for DNA double-strand breaks rejoining. J Biol Chem. 2004;279(53):55117–26. [DOI] [PubMed] [Google Scholar]
- 11.Shall S, de Murcia G. Poly(ADP-ribose) polymerase-1: what have we learned from the deficient mouse model? Mutat Res. 2000;460(1):1–15. [DOI] [PubMed] [Google Scholar]
- 12.Burkle A. Poly(APD-ribosyl)ation, a DNA damage-driven protein modification and regulator of genomic instability. Cancer Lett. 2001;163(1):1–5. [DOI] [PubMed] [Google Scholar]
- 13.Kanev PB, Atemin A, Stoynov S, Aleksandrov R. PARP1 roles in DNA repair and DNA replication: The basi(c)s of PARP inhibitor efficacy and resistance. Semin Oncol. 2024;51(1–2):2–18. [DOI] [PubMed] [Google Scholar]
- 14.Kamaletdinova T, Fanaei-Kahrani Z, Wang ZQ. The Enigmatic Function of PARP1: From PARylation Activity to PAR Readers. Cells. 2019;8(12):1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrision D, Gravells P, Thompson R, Bryant HE. Poly(ADP-Ribose) Glycohydrolase (PARG) vs. Poly(ADP-Ribose) Polymerase (PARP) - Function in Genome Maintenance and Relevance of Inhibitors for Anti-cancer Therapy. Front Mol Biosci. 2020;7:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Sullivan J, Tedim Ferreira M, Gagne JP, Sharma AK, Hendzel MJ, Masson JY, et al. Emerging roles of eraser enzymes in the dynamic control of protein ADP-ribosylation. Nat Commun. 2019;10(1):1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012;72(21):5588–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krokan HE, Bjoras M. Base excision repair. Cold Spring Harb Perspect Biol. 2013;5(4):a012583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eustermann S, Wu WF, Langelier MF, Yang JC, Easton LE, Riccio AA, et al. Structural Basis of Detection and Signaling of DNA Single-Strand Breaks by Human PARP-1. Mol Cell. 2015;60(5):742–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caldecott KW. Single-strand break repair and genetic disease. Nat Rev Genet. 2008;9(8):619–31. [DOI] [PubMed] [Google Scholar]
- 21.Dantzer F, Nasheuer HP, Vonesch JL, de Murcia G, Menissier-de MJ. Functional association of poly(ADP-ribose) polymerase with DNA polymerase alpha-primase complex: a link between DNA strand break detection and DNA replication. Nucleic Acids Res. 1998;26(8):1891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simbulan-Rosenthal CM, Rosenthal DS, Iyer S, Boulares AH, Smulson ME. Transient poly(ADP-ribosyl)ation of nuclear proteins and role of poly(ADP-ribose) polymerase in the early stages of apoptosis. J Biol Chem. 1998;273(22):13703–12. [DOI] [PubMed] [Google Scholar]
- 23.Smirnova M, Klein HL. Role of the error-free damage bypass postreplication repair pathway in the maintenance of genomic stability. Mutat Res. 2003;532(1–2):117–35. [DOI] [PubMed] [Google Scholar]
- 24.Berti M, Ray Chaudhuri A, Thangavel S, Gomathinayagam S, Kenig S, Vujanovic M, et al. Human RECQ1 promotes restart of replication forks reversed by DNA topoisomerase I inhibition. Nat Struct Mol Biol. 2013;20(3):347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ying S, Hamdy FC, Helleday T. Mre11-dependent degradation of stalled DNA replication forks is prevented by BRCA2 and PARP1. Cancer Res. 2012;72(11):2814–21. [DOI] [PubMed] [Google Scholar]
- 26.Haince JF, McDonald D, Rodrigue A, Dery U, Masson JY, Hendzel MJ, et al. PARP1-dependent kinetics of recruitment of MRE11 and NBS1 proteins to multiple DNA damage sites. J Biol Chem. 2008;283(2):1197–208. [DOI] [PubMed] [Google Scholar]
- 27.Hanzlikova H, Kalasova I, Demin AA, Pennicott LE, Cihlarova Z, Caldecott KW. The Importance of Poly(ADP-Ribose) Polymerase as a Sensor of Unligated Okazaki Fragments during DNA Replication. Mol Cell. 2018;71(2):319–31 e3. [DOI] [PMC free article] [PubMed]
- 28.Vaitsiankova A, Burdova K, Sobol M, Gautam A, Benada O, Hanzlikova H, et al. PARP inhibition impedes the maturation of nascent DNA strands during DNA replication. Nat Struct Mol Biol. 2022;29(4):329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen Y, Aoyagi-Scharber M, Wang B. Trapping Poly(ADP-Ribose) Polymerase. J Pharmacol Exp Ther. 2015;353(3):446–57. [DOI] [PubMed] [Google Scholar]
- 30.Hopkins TA, Shi Y, Rodriguez LE, Solomon LR, Donawho CK, DiGiammarino EL, et al. Mechanistic dissection of PARP1 trapping and the impact on in vivo tolerability and efficacy of PARP inhibitors. Mol Cancer Res. 2015;13(11):1465–77. [DOI] [PubMed] [Google Scholar]
- 31.Scott CL, Swisher EM, Kaufmann SH. Poly (ADP-ribose) polymerase inhibitors: recent advances and future development. J Clin Oncol. 2015;33(12):1397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel AG, Sarkaria JN, Kaufmann SH. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc Natl Acad Sci U S A. 2011;108(8):3406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Lorenzo SB, Patel AG, Hurley RM, Kaufmann SH. The elephant and the blind men: Making Sense of PARP Inhibitors in homologous recombination deficient tumor cells. Front Oncol. 2013;3:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prakash R, Zhang Y, Feng W, Jasin M. Homologous recombination and human health: the roles of BRCA1, BRCA2, and associated proteins. Cold Spring Harb Perspect Biol. 2015;7(4):a016600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol. 2011;5(4):387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461(7267):1071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saleh-Gohari N, Bryant HE, Schultz N, Parker KM, Cassel TN, Helleday T. Spontaneous homologous recombination is induced by collapsed replication forks that are caused by endogenous DNA single-strand breaks. Mol Cell Biol. 2005;25(16):7158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CC, Feng W, Lim PX, Kass EM, Jasin M. Homology-Directed Repair and the Role of BRCA1, BRCA2, and Related Proteins in Genome Integrity and Cancer. Annu Rev Cancer Biol. 2018;2:313–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sy SM, Huen MS, Chen J. PALB2 is an integral component of the BRCA complex required for homologous recombination repair. Proc Natl Acad Sci U S A. 2009;106(17):7155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tutt AN, Lord CJ, McCabe N, Farmer H, Turner N, Martin NM, et al. Exploiting the DNA repair defect in BRCA mutant cells in the design of new therapeutic strategies for cancer. Cold Spring Harb Symp Quant Biol. 2005;70:139–48. [DOI] [PubMed] [Google Scholar]
- 41.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21. [DOI] [PubMed] [Google Scholar]
- 42.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–7. [DOI] [PubMed] [Google Scholar]
- 43.Lord CJ, McDonald S, Swift S, Turner NC, Ashworth A. A high-throughput RNA interference screen for DNA repair determinants of PARP inhibitor sensitivity. DNA Repair (Amst). 2008;7(12):2010–9. [DOI] [PubMed] [Google Scholar]
- 44.Pommier Y, O’Connor MJ, de Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci Transl Med. 2016;8(362):362ps17. [DOI] [PubMed] [Google Scholar]
- 45.Turner NC, Lord CJ, Iorns E, Brough R, Swift S, Elliott R, et al. A synthetic lethal siRNA screen identifying genes mediating sensitivity to a PARP inhibitor. EMBO J. 2008;27(9):1368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66(16):8109–15. [DOI] [PubMed] [Google Scholar]
- 47.Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science. 2017;355(6330):1152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer. 2016;16(2):110–20. [DOI] [PubMed] [Google Scholar]
- 49.Turner N, Tutt A, Ashworth A. Hallmarks of “BRCAness” in sporadic cancers. Nat Rev Cancer. 2004;4(10):814–9. [DOI] [PubMed] [Google Scholar]
- 50.Guo M, Wang SM. The BRCAness Landscape of Cancer. Cells. 2022;11(23). [DOI] [PMC free article] [PubMed]
- 51.Murai J, Pommier Y. BRCAness, Homologous Recombination Deficiencies, and Synthetic Lethality. Cancer Res. 2023;83(8):1173–4. [DOI] [PubMed] [Google Scholar]
- 52.Watkins J, Weekes D, Shah V, Gazinska P, Joshi S, Sidhu B, et al. Genomic Complexity Profiling Reveals That HORMAD1 Overexpression Contributes to Homologous Recombination Deficiency in Triple-Negative Breast Cancers. Cancer Discov. 2015;5(5):488–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abbotts R, Dellomo AJ, Rassool FV. Pharmacologic Induction of BRCAness in BRCA-Proficient Cancers: Expanding PARP Inhibitor Use. Cancers (Basel). 2022;14(11):2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin PH, Chen M, Tsai LW, Lo C, Yen TC, Huang TY, et al. Using next-generation sequencing to redefine BRCAness in triple-negative breast cancer. Cancer Sci. 2020;111(4):1375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bajrami I, Frankum JR, Konde A, Miller RE, Rehman FL, Brough R, et al. Genome-wide profiling of genetic synthetic lethality identifies CDK12 as a novel determinant of PARP1/2 inhibitor sensitivity. Cancer Res. 2014;74(1):287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jamal K, Galbiati A, Armenia J, Illuzzi G, Hall J, Bentouati S, et al. Drug-gene Interaction Screens Coupled to Tumor Data Analyses Identify the Most Clinically Relevant Cancer Vulnerabilities Driving Sensitivity to PARP Inhibition. Cancer Res Commun. 2022;2(10):1244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fugger K, Hewitt G, West SC, Boulton SJ. Tackling PARP inhibitor resistance. Trends Cancer. 2021;7(12):1102–18. [DOI] [PubMed] [Google Scholar]
- 58.Rice JC, Futscher BW. Transcriptional repression of BRCA1 by aberrant cytosine methylation, histone hypoacetylation and chromatin condensation of the BRCA1 promoter. Nucleic Acids Res. 2000;28(17):3233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rice JC, Ozcelik H, Maxeiner P, Andrulis I, Futscher BW. Methylation of the BRCA1 promoter is associated with decreased BRCA1 mRNA levels in clinical breast cancer specimens. Carcinogenesis. 2000;21(9):1761–5. [DOI] [PubMed] [Google Scholar]
- 60.Janysek DC, Kim J, Duijf PHG, Dray E. Clinical use and mechanisms of resistance for PARP inhibitors in homologous recombination-deficient cancers. Transl Oncol. 2021;14(3):101012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murthy P, Muggia F. PARP inhibitors: clinical development, emerging differences, and the current therapeutic issues. Cancer Drug Resist. 2019;2(3):665–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mateo J, Lord CJ, Serra V, Tutt A, Balmana J, Castroviejo-Bermejo M, et al. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol. 2019;30(9):1437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cortesi L, Rugo HS, Jackisch C. An Overview of PARP Inhibitors for the Treatment of Breast Cancer. Target Oncol. 2021;16(3):255–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. BioEssays. 2004;26(8):882–93. [DOI] [PubMed] [Google Scholar]
- 65.Tung NM, Robson ME, Ventz S, Santa-Maria CA, Nanda R, Marcom PK, et al. TBCRC 048: Phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol. 2020;38(36):4274–82. [DOI] [PubMed] [Google Scholar]
- 66.Hurvitz SA, Goncalves A, Rugo HS, Lee KH, Fehrenbacher L, Mina LA, et al. Talazoparib in patients with a germline BRCA-mutated advanced breast cancer: detailed safety analyses from the phase III EMBRACA trial. Oncologist. 2020;25(3):e439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen Y, Rehman FL, Feng Y, Boshuizen J, Bajrami I, Elliott R, et al. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin Cancer Res. 2013;19(18):5003–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turner NC, Balmana J, Poncet C, Goulioti T, Tryfonidis K, Honkoop AH, et al. Niraparib for Advanced Breast Cancer with Germline BRCA1 and BRCA2 Mutations: the EORTC 1307-BCG/BIG5-13/TESARO PR-30-50-10-C BRAVO Study. Clin Cancer Res. 2021;27(20):5482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kristeleit R, Shapiro GI, Burris HA, Oza AM, LoRusso P, Patel MR, et al. A Phase I-II Study of the Oral PARP Inhibitor Rucaparib in Patients with Germline BRCA1/2-Mutated Ovarian Carcinoma or Other Solid Tumors. Clin Cancer Res. 2017;23(15):4095–106. [DOI] [PubMed] [Google Scholar]
- 70.Stodtmann S, Eckert D, Joshi R, Nuthalapati S, Ratajczak CK, Menon R, et al. Exposure-Response Model With Time-Varying Predictors to Estimate the Effects of Veliparib in Combination With Carboplatin/Paclitaxel and as Monotherapy: Veliparib Phase 3 Study in BRCA-Mutated Advanced Breast Cancer (BROCADE3) Trial. J Clin Pharmacol. 2022;62(10):1236–46. [DOI] [PubMed] [Google Scholar]
- 71.Xiong Y, Guo Y, Liu Y, Wang H, Gong W, Liu Y, et al. Pamiparib is a potent and selective PARP inhibitor with unique potential for the treatment of brain tumor. Neoplasia. 2020;22(9):431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee A. Correction to: Fuzuloparib: First Approval. Drugs. 2021;81(13):1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pilie PG, Gay CM, Byers LA, O’Connor MJ, Yap TA. PARP Inhibitors: Extending Benefit Beyond BRCA-Mutant Cancers. Clin Cancer Res. 2019;25(13):3759–71. [DOI] [PubMed] [Google Scholar]
- 74.Lu Y, Liu Y, Pang Y, Pacak K, Yang C. Double-barreled gun: Combination of PARP inhibitor with conventional chemotherapy. Pharmacol Ther. 2018;188:168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D’Andrea AD. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 2015;5(11):1137–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li H, Liu ZY, Wu N, Chen YC, Cheng Q, Wang J. PARP inhibitor resistance: the underlying mechanisms and clinical implications. Mol Cancer. 2020;19(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci U S A. 2008;105(44):17079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giudice E, Gentile M, Salutari V, Ricci C, Musacchio L, Carbone MV, et al. PARP Inhibitors Resistance: Mechanisms and Perspectives. Cancers (Basel). 2022;14(6):1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gogola E, Duarte AA, de Ruiter JR, Wiegant WW, Schmid JA, de Bruijn R, et al. Selective Loss of PARG Restores PARylation and Counteracts PARP Inhibitor-Mediated Synthetic Lethality. Cancer Cell. 2019;35(6):950–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Norquist B, Wurz KA, Pennil CC, Garcia R, Gross J, Sakai W, et al. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J Clin Oncol. 2011;29(22):3008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pettitt SJ, Krastev DB, Brandsma I, Drean A, Song F, Aleksandrov R, et al. Genome-wide and high-density CRISPR-Cas9 screens identify point mutations in PARP1 causing PARP inhibitor resistance. Nat Commun. 2018;9(1):1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Noordermeer SM, van Attikum H. PARP Inhibitor Resistance: A Tug-of-War in BRCA-Mutated Cells. Trends Cell Biol. 2019;29(10):820–34. [DOI] [PubMed] [Google Scholar]
- 83.D’Andrea AD. Mechanisms of PARP inhibitor sensitivity and resistance. DNA Repair (Amst). 2018;71:172–6. [DOI] [PubMed] [Google Scholar]
- 84.Slade D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020;34(5–6):360–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pettitt SJ, Rehman FL, Bajrami I, Brough R, Wallberg F, Kozarewa I, et al. A genetic screen using the PiggyBac transposon in haploid cells identifies Parp1 as a mediator of olaparib toxicity. PLoS ONE. 2013;8(4):e61520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.LaFargue CJ, Dal Molin GZ, Sood AK, Coleman RL. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019;20(1):e15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Madariaga A, Bonilla L, McMullen M, Oza AM, Lheureux S. Efficacy and safety updates of poly ADP-ribose polymerase (PARP) inhibitor maintenance in ovarian cancer from ASCO 2020. Int J Gynecol Cancer. 2020;30(8):1256–7. [DOI] [PubMed] [Google Scholar]
- 88.Oza AM, Matulonis UA, Malander S, Hudgens S, Sehouli J, Del Campo JM, et al. Quality of life in patients with recurrent ovarian cancer treated with niraparib versus placebo (ENGOT-OV16/NOVA): results from a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2018;19(8):1117–25. [DOI] [PubMed] [Google Scholar]
- 89.Farres J, Martin-Caballero J, Martinez C, Lozano JJ, Llacuna L, Ampurdanes C, et al. Parp-2 is required to maintain hematopoiesis following sublethal gamma-irradiation in mice. Blood. 2013;122(1):44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Farres J, Llacuna L, Martin-Caballero J, Martinez C, Lozano JJ, Ampurdanes C, et al. PARP-2 sustains erythropoiesis in mice by limiting replicative stress in erythroid progenitors. Cell Death Differ. 2015;22(7):1144–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381(4):317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hanna C, Kurian KM, Williams K, Watts C, Jackson A, Carruthers R, et al. Pharmacokinetics, safety, and tolerability of olaparib and temozolomide for recurrent glioblastoma: results of the phase I OPARATIC trial. Neuro Oncol. 2020;22(12):1840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheng J, Li Z, Min W. Current status and future promise of next-generation poly (ADP-Ribose) polymerase 1-selective inhibitor AZD5305. Front Pharmacol. 2022;13:979873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matta E, Kiribayeva A, Khassenov B, Matkarimov BT, Ishchenko AA. Insight into DNA substrate specificity of PARP1-catalysed DNA poly(ADP-ribosyl)ation. Sci Rep. 2020;10(1):3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Papeo G, Posteri H, Borghi D, Busel AA, Caprera F, Casale E, et al. Discovery of 2-[1-(4,4-Difluorocyclohexyl)piperidin-4-yl]-6-fluoro-3-oxo-2,3-dihydro-1H-isoindole-4-carboxamide (NMS-P118): A Potent, Orally Available, and Highly Selective PARP-1 Inhibitor for Cancer Therapy. J Med Chem. 2015;58(17):6875–98. [DOI] [PubMed] [Google Scholar]
- 96.Illuzzi G, Staniszewska AD, Gill SJ, Pike A, McWilliams L, Critchlow SE, et al. Preclinical Characterization of AZD5305, A Next-Generation, Highly Selective PARP1 Inhibitor and Trapper. Clin Cancer Res. 2022;28(21):4724–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johannes JW, Balazs A, Barratt D, Bista M, Chuba MD, Cosulich S, et al. Discovery of 5-4-[(7-Ethyl-6-oxo-5,6-dihydro-1,5-naphthyridin-3-yl)methyl]piperazin-1-yl-N-methylpyridine-2-carboxamide (AZD5305): A PARP1-DNA Trapper with High Selectivity for PARP1 over PARP2 and Other PARPs. J Med Chem. 2021;64(19):14498–512. [DOI] [PubMed] [Google Scholar]
- 98.Dellavedova G, Decio A, Formenti L, Albertella MR, Wilson J, Staniszewska AD, et al. The PARP1 Inhibitor AZD5305 Impairs Ovarian Adenocarcinoma Progression and Visceral Metastases in Patient-derived Xenografts Alone and in Combination with Carboplatin. Cancer Res Commun. 2023;3(3):489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moorthy G, Voronova V, Pichardo C, Peskov K, Illuzzi G, Staniszewska A, et al. Abstract 2794: A Quantitative Systems Pharmacology (QSP) model to characterize dose-dependent antitumor activity of AZD5305, PARP1 selective inhibitor, across multiple xenograft models. Cancer Research. 2023;83(7_Supplement):2794. [Google Scholar]
- 100.Yap TA, Im S-A, Schram AM, Sharp A, Balmana J, Baird RD, et al. Abstract CT007: PETRA: First in class, first in human trial of the next generation PARP1-selective inhibitor AZD5305 in patients (pts) with BRCA1/2, PALB2 or RAD51C/D mutations. Cancer Research. 2022;82(12_Supplement):CT007-CT. [Google Scholar]
- 101.Azad A, Voskoboynik M, Joshua AM, Weickhardt AJ, Sankey P, Pacey S, et al. PETRANHA: Phase 1/2 study of AZD5305 + novel hormonal agents in patients with metastatic prostate cancer–Interim safety and pharmacokinetic results. J Clin Oncol. 2024;42(4_suppl):123.38048518 [Google Scholar]
- 102.Chi KN, Agarwal N, Armstrong AJ, Hellmis E, Schlürmann F, Sugimoto M, et al. Phase III, double-blind, placebo-controlled, 2-cohort, randomized study of saruparib (AZD5305) in combination with new hormonal agents in patients with metastatic castration-sensitive prostate cancer with and without homologous recombination repair mutation (EvoPAR-Prostate01). J Clin Oncol. 2024;42(16_suppl):TPS5123-TPS. [Google Scholar]
- 103.Patel G, Owusu Jl, Rane C, Rosen A, Griffin M, Sung M. Abstract 5618: Evaluating the combination of datopotamab deruxtecan (Dato-DXd) with AZD5305, a highly potent, PARP1-selective inhibitor, -in preclinical models. Cancer Res. 2024;84(6_Supplement):5618. [Google Scholar]
- 104.Albertella M, Wijnhoven P, Demin A, Illuzzi G, Ropero AH, Serra V, et al. Abstract B058: Combination of the PARP1-selective inhibitor AZD5305 with the ATR inhibitor ceralasertib for the treatment of PARPi-resistant cancer. Mol Cancer Therapeutics. 2023;22(12_Supplement):058-B. [Google Scholar]
- 105.Ngoi NYL, Leo E, O’Connor MJ, Yap TA. Development of Next-Generation Poly(ADP-Ribose) Polymerase 1-Selective Inhibitors. Cancer J. 2021;27(6):521–8. [DOI] [PubMed] [Google Scholar]
- 106.Montagnoli A, Papeo G, Rainoldi S, Caprera F, Ciomei M, Felder E, et al. Abstract 4843: NMS-P293, a PARP-1 selective inhibitor with no trapping activity and high CNS penetration, possesses potent in vivo efficacy and represents a novel therapeutic option for brain localized metastases and glioblastoma. Cancer Res. 2018;78(13_Supplement):4843. [Google Scholar]
- 107.Montagnoli A, Rainoldi S, Ciavolella A, Ballinari D, Caprera F, Ceriani L, et al. Abstract 1223: NMS-P293, a novel potent and selective PARP-1 inhibitor with high antitumor efficacy and tolerability. Cancer Res. 2016;76(14_Supplement):1223. [Google Scholar]
- 108.Geurts M, Gramatzki D, Merler S, Duca M, Girardi F, Sener UT, et al. Abstract LB_A12: Initial results from 2 Phase I studies of NMS-03305293, a selective PARP1 inhibitor. Molecular Cancer Therapeutics. 2023;22(12_Supplement):LB_A12-LB_A.
- 109.Staniszewska AD, Pilger D, Gill SJ, Jamal K, Bohin N, Guzzetti S, et al. Preclinical Characterization of AZD9574, a Blood-Brain Barrier Penetrant Inhibitor of PARP1. Clin Cancer Res. 2024;30(7):1338–51. [DOI] [PubMed] [Google Scholar]
- 110.Jamal K, Staniszewska A, Gordon J, Wen S, McGrath F, Dowdell G, et al. Abstract 2609: AZD9574 is a novel, brain penetrant PARP-1 selective inhibitor with activity in an orthotopic, intracranial xenograft model with aberrant DNA repair. Cancer Res. 2022;82(12_Supplement):2609. [Google Scholar]
- 111.Wu L, Wang J, Li N, Zhang J, Wang D, Jiang S, et al. A phase 1 study of HRS-1167 (M9466), a highly selective PARP1 inhibitor, in patients (pts) with advanced solid tumors. J Clin Oncol. 2024;42(16_suppl):3154. [Google Scholar]
- 112.Sonpavde GP, Call JA, Falchook GS, Garmezy B, Rasco DW, Liu L, et al. A first-in-human (FIH), phase 1/2, dose-escalation, dose-optimization, and dose-expansion study of PARP1-selective inhibitor IMP1734 in participants with advanced solid tumors. J Clin Oncol. 2024;42(16_suppl):TPS3191-TPS. [Google Scholar]
- 113.Fang DD, Liu Y, Liu Y, Zhang H, Wu J, Wei J, et al. Abstract 4537: VB15010 is a potent PARP1 selective inhibitor and durable trapper with improved pharmacological activities. Cancer Research. 2024;84(6_Supplement):4537. [Google Scholar]
- 114.Li L. Examination of in vitro and in vivo antitumor activity of PARP1 selective inhibitor D0112–005. J Clin Oncol. 2024;42(16_suppl):e15115-e.
- 115.Gao Y, Lu Y, Chen B, Jiang B, Wang F, Zhang G, et al. Abstract LB268: Discovery of a potent, selective and brain penetrating PARP1 inhibitor, XZP-7797. Cancer Res. 2024;84(7_Supplement):LB268-LB.
- 116.Gao M, Li Z, Fan Q, Pan J, Bai Y, Zhang H, et al. Abstract 1648: Discovery of a potent and selective PARP1 inhibitor and trapper with anti-tumor activities in HRD tumors. Cancer Research. 2023;83(7_Supplement):1648. [Google Scholar]
- 117.Wei Y, Li W, Li Z, Li M, Kang K, Wen Z, et al. Abstract 4525: Discovery of a highly potent and selective PARP1 inhibitor with superior PK and safety profiles. Cancer Research. 2024;84(6_Supplement):4525. [Google Scholar]
- 118.Nam S, Chae J, Cho K-O, Koo T-S, Kim J, Jung M, et al. Abstract 509: DM5167, a novel selective PARP1 inhibitor, efficiently reduces growth of triple-negative breast cancers. Cancer Research. 2023;83(7_Supplement):509. [Google Scholar]
- 119.Hou Y, Gao S, Jiang S, Li J, Zhang C, Xu Y, et al. Abstract LB032: HH102007 is a highly selective and potent PARP1 inhibitor and trapper. Cancer Research. 2024;84(7_Supplement):LB032-LB.
- 120.Li F, Gou X, Chen L, Meng Q, Li Y, Dong H, et al. Abstract 4534: HSK40495, a highly selective PARP1 inhibitor with improved hematopoietic safety for the treatment of HRD cancers. Cancer Research. 2024;84(6_Supplement):4534. [Google Scholar]
- 121.Nelson A, Zheng KF, Wanderley C, Michaud DE, Cowley PM, Campbell GM, et al. Abstract 7120: Investigating PARP1 selective inhibitor DSB1559 efficacy in BRCA1-associated triple negative breast cancer. Cancer Research. 2024;84(6_Supplement):7120. [Google Scholar]
- 122.Li M, Chen Y, Chen J, Jiang L, Lin X, Gu J. Abstract 7132: Preclinical characterization of LAE119, a novel PARP1 selective inhibitor and trapper. Cancer Research. 2024;84(6_Supplement):7132. [Google Scholar]
- 123.Wu X, Li Q, Zhang F, Wang L, Wang J, Fan J, et al. Novel poly (ADP-ribose) polymerases inhibitor DHC-1 exhibits in vitro and in vivo anticancer activity on BRCA-deficient pancreatic cancer cells. Food Chem Toxicol. 2021;147:111892. [DOI] [PubMed] [Google Scholar]
- 124.Ray-Coquard I, Pautier P, Pignata S, Perol D, Gonzalez-Martin A, Berger R, et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med. 2019;381(25):2416–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.