Abstract
Objective: To investigate the expression of receptor tyrosine kinase-like orphan receptor 1 (ROR1) in ovarian cancer tissues and its correlation with clinicopathologic features, chemotherapy sensitivity, and prognosis in patients with ovarian cancer. Methods: Paraffin tissue blocks were collected from 227 ovarian cancer patients, and immunohistochemical (IHC) staining was performed to analyze the expression of ROR1. The associations between ROR1 expression and clinicopathologic features, treatment response, and prognosis were evaluated. Results: ROR1 expression and tumor load: higher expression of ROR1 was associated with a heavier tumor load in ovarian cancer patients. ROR1 and chemotherapy: the expression of ROR1 was significantly higher in patients who underwent interval debulking surgery (IDS) compared to primary debulking surgery (PDS) and subsequent chemotherapy (SSC). This suggests that chemotherapy may promote increased ROR1 expression in tumor tissues. Additionally, a trend of higher ROR1 expression was observed in platinum-sensitive patients. Nuclear expression in clear cell ovarian cancer: positive nuclear expression of ROR1 was unexpectedly detected in clear cell ovarian cancer, suggesting a link between ROR1 nuclear translocation and poor prognosis. Conclusion: ROR1 expression may be related to treatment strategy and history of platinum-based chemotherapy in ovarian cancer patients. Targeting ROR1 could represent a promising therapeutic strategy for the treatment of ovarian cancer. Further studies are needed to elucidate the role of ROR1 nuclear translocation in prognosis.
Keywords: ROR1, ovarian cancer, immunohistochemistry
Introduction
Ovarian cancer is one of the main malignancies that threaten women’s health, exhibiting the highest mortality rate among gynecological cancers. Despite some improvement in ovarian cancer prognosis made possible by a combination of surgical intervention and chemotherapeutic agents such as platinum compounds and paclitaxel, the disease poses significant challenges because of delayed clinical diagnosis, chemoresistance, and recurrence [1-3]. Ovarian cancer patients often face significant complications after chemotherapy, including hematological issues, gastrointestinal side effects, mucositis, neurological problems, and other risks such as hearing loss and secondary cancers. To address these challenges, comprehensive preventive measures and supportive care strategies are essential, including pre-treatment assessment, nutritional intervention, psychosocial support, and fertility preservation [1]. While targeted therapies including bevacizumab and poly ADP-ribose polymerase (PARP) inhibitors have demonstrated efficacy in advanced ovarian cancer, there remains a substantial unmet clinical need underscoring the necessity for the development of novel therapeutic targets [1,4].
The ROR1 is a highly conserved type I transmembrane glycoprotein initially identified in human neuroblastoma cells and is a member of the receptor tyrosine kinase (RTK) family [5]. Research has demonstrated that ROR1 is widely expressed during embryonic development and in various human cancers, while its expression in normal tissues is limited [6,7]. In ovarian cancer, data indicate that ROR1 is aberrantly overexpressed in tumor tissues and is associated with a poor prognosis [8]. Targeted therapies against ROR1, including monoclonal antibody, antibody-drug conjugates (ADCs), small molecule inhibitors, and chimeric antigen receptor T (CAR-T) cell therapies, have shown promising preliminary results in the treatment of cancer. For example, monoclonal antibody zilovertamab, when combined with ibrutinib, demonstrated a progression-free survival (PFS) of 35.9 months in a Phase III clinical trial for mantle cell lymphoma (MCL), which is much higher than the 12.8 months observed with ibrutinib alone [9]. Additionally, VelosBio’s ADC VLS-101, which conjugates zilovertamab with the potent cytotoxic agent monomethyl auristatin E (MMAE), has shown encouraging anti-tumor efficacy and safety in clinical trials for lung and breast cancers [10,11]. These findings position ROR1 as a highly promising target for ovarian cancer therapy. However, research on the expression of ROR1 in ovarian cancer tissues and its correlation with chemotherapy sensitivity and patient prognosis remains limited. Given the anticipated increase in the detection of this biomarker with the development and approval of ROR1-targeted therapies, our study aims to analyze a cohort of ovarian cancer tissues subjected to various treatment regimens, thereby supplementing and refining the existing knowledge on ROR1 expression and its clinical significance in ovarian cancer.
Patients and methods
Study populations
This retrospective study retrieved and collected a total of 227 surgical tissue samples from patients with ovarian cancer at West China Second Hospital of Sichuan University in 2023. A cohort of patients with epithelial ovarian cancer was established based on specific inclusion and exclusion criteria: (a) patients with confirmed diagnosis of ovarian cancer through preoperative biopsy or postoperative pathologic examination; (b) participants who consented to participate in the study; (c) availability of surgically obtained histologic formalin fixed paraffin embedded (FFPE) samples; (d) sufficient tissue for IHC staining; (e) complete clinical data. Patients meeting the following criteria were excluded: (a) presence of ovarian tumors other than epithelial ovarian cancer, such as borderline tumors, germ cell tumors, sex cord stromal tumors, etc.; (b) concurrent systemic malignancies; (c) lack of essential clinicopathologic data; (d) insufficient tissue for IHC staining.
Clinical information
The study recorded clinical features and pathologic test results, including patient age, tumor type, tumor grade, tumor site, International Federation of Gynecology and Obstetrics (FIGO) stage, and date of diagnosis. Additionally, information regarding the date of initial surgery, date of recurrence and/or progression, chemotherapy responsiveness, and type of specimen used in the case evaluation process was also collected.
IHC staining
Tissue sections from wax blocks were prepared according to standard procedures, followed by staining of ROR1 antibody (LBP2-ROR1) using the Hyper S9 IHC staining machine from LBP Medical Technology Co., LTD. (Guangzhou, China, 1708001). This machine perfectly mimics human manual operations and the protocol is as follows: (1) antigen retrieval: CC1 for 64 minutes; (2) primary antibody: incubation at 36°C for 28 minutes; (3) secondary antibody: system default addition and incubation conditions; (4) hematoxylin: 8 minutes; (5) bluing: 4 minutes.
Prognostic analysis
The pathologist evaluated ROR1 staining using an H-score, which included evaluation of tumor staining percentage, intensity, and overall positivity or negativity. For patients experiencing relapse, PFS was defined as the time interval between the date of initial surgery and the date of first progression or recurrence. Platinum-free interval (PFI) referred to the duration between the last platinum-containing treatment and the first platinum-containing treatment after relapse.
Statistical analysis
All statistical analyses were conducted using R software (Version 4.2.1). Fleiss’ kappa was used to assess inter-rater agreement among three pathologists. The Kaplan-Meier method was used to evaluate the correlation between variables and survival outcomes, while the log-rank test determined the statistical significance of survival differences between groups. P-value <0.05 was considered significant.
Ethic statement
The Ethics Committee of West China Second Hospital of Sichuan University has granted approval for this retrospective study, which did not involve any intervention in patient treatment or compromise their safety. All patient privacy data were securely protected. The research activities strictly adhere to relevant guidelines and regulations. Prior to surgical procedures, informed consent from all patients was obtained for use of tissue samples and pathologic diagnosis reports exclusively for research purposes.
Results
Patient demographics and ROR1 expression analysis
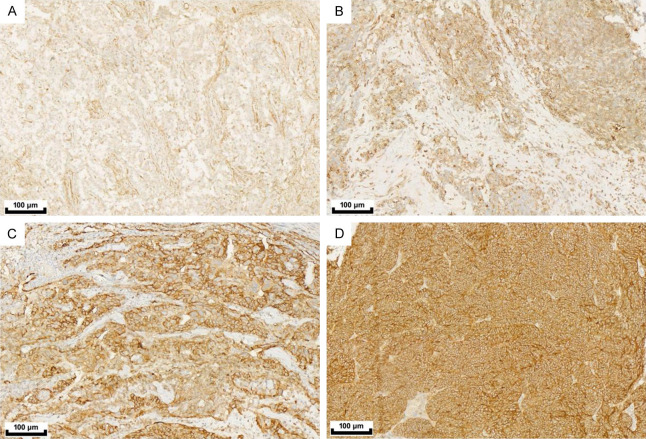
The basic statistical data of the patients are presented in Table 1. The study analyzed a total of 227 ovarian cancer patients who underwent surgery at our hospital in 2023. IHC staining was performed on surgical FFPE specimens to evaluate the expression of ROR1 and investigate its association with clinicopathologic features. The age range of the patients was between 27 and 86 years, with a median age at evaluation being 55.7 years (median: 55 years). Among the tissue types examined, serous epithelial carcinoma accounted for 91.2% (low grade: 202; high grade: 207) while clear cell carcinoma accounted for only 8.8%. According to the FIGO stage classification, the distribution of cases was as follows: Stage I-13 cases (5.7%); Stage II-18 cases (7.9%); Stage III-158 cases (69.6%); and Stage IV-38 cases (16.7%). Of all the IHC samples tested, primary foci contributed to approximately 43.6% (99 cases), whereas metastatic foci contributed about 56.4% (128 cases). Among all tested cases, 70% had a history of chemotherapy, among whom 30.4% were completely sensitive to platinum-based treatment, 25.6% showed partial sensitivity to platinum-based treatment, 7.9% were resistant to platinum-based treatment, and 3.1% were refractory to platinum-based treatment. The H-score method was used as an interpretation standard for assessing ROR1 protein expression through IHC staining intensity (as shown in Figure 1). Since there is currently no recommended cutoff value for ROR1 expression levels, the staining intensity was considered as a continuous variable ranging from low to high (Figure 2).
Table 1.
Patient and case demographics
| Overall (N=227) | |
|---|---|
| Age | |
| Mean (SD) | 55.7 (9.31) |
| Median [Min, Max] | 55.0 [27.0, 86.0] |
| Sample type | |
| Metastases | 99 (43.6%) |
| Primary lesion | 128 (56.4%) |
| FIGO stage | |
| I | 13 (5.7%) |
| II | 18 (7.9%) |
| III | 158 (69.6%) |
| IV | 38 (16.7%) |
| Chemotherapy history | |
| Negative | 41 (18.1%) |
| Positive | 159 (70.0%) |
| Missing | 27 (11.9%) |
| Platinum sensitivity | |
| Platinum-refractory | 7 (3.1%) |
| Platinum-resistant | 18 (7.9%) |
| Potentially platinum-sensitive | 58 (25.6%) |
| Fully platinum-sensitive | 69 (30.4%) |
| Missing | 75 (33.0%) |
| Histologic type | |
| Clear cell adenocarcinoma (CCA) | 20 (8.8%) |
| High-grade serous ovarian cancer (HGSOC) | 5 (2.2%) |
| Low-grade serous ovarian cancer (LGSOC) | 202 (89.0%) |
| Surgical type | |
| PDS | 56 (25%) |
| IDS | 80 (35%) |
| SSC | 91 (40%) |
Figure 1.
The heat map correlation analysis of IHC intensity of ROR1 protein and clinical features of ovarian cancer patients.
Figure 2.
ROR1 IHC interpretation. (A) 0, no staining, (B) Weak (1+) staining, (C) Moderate (2+) staining, (D) Strong (3+) staining. Magnification: 100×.
Correlation analysis of ROR1 expression with disease severity and tissue types
As shown in Figure 3, we conducted a correlation analysis between disease severity and ROR1 expression. Our results suggested that the higher H-score of ROR1, the more serious the FIGO stage of ovarian cancer patients (Figure 3B, P<0.05). We also found that the lesion size was positively correlated with the protein expression of ROR1 but the result was not statistically significant (Figure 3A). In addition, we statistically analyzed the tissue types of the patients, among which 99 cases (43.6%) of IHC samples were from primary sites and 128 cases (56.4%) were from metastatic sites, but there was no significant difference in ROR1 expression between primary and metastatic (Figure 3C).
Figure 3.
Correlation analysis between ROR1 H-score and clinical information. (A) Tumor lesion size (T), (B) FIGO staging, (C) Sample type.
Effect of therapeutic options on ROR1 protein expression in ovarian cancer
We then explored the effect of chemotherapy on the expression of ROR1 protein. As shown in Figure 4A, we first found that the expression of ROR1 in people who had received chemotherapy showed a rising trend. For further discussion, we classified all patients into PDS (no chemotherapy), IDS (neoadjuvant chemotherapy), SSC (recurrence surgery). The study found that the expression of ROR1 in patients with IDS was significantly higher than that of patients with PDS and SSC, possibly because the lesions of patients with IDS were directly affected by neoadjuvant chemotherapy (Figure 4B). Since most chemotherapy regimens for ovarian cancer include platinum, we divided patients into four categories for further study: platinum-refractory, platinum-resistant, platinum-partially sensitive, and platinum-fully sensitive. Unfortunately, we did not find a significant correlation between different platinum sensitivity and ROR1 expression in this manner (Figure 4C). However, the expression of ROR1 appeared to be higher in platinum-sensitive patients.
Figure 4.
Correlation analysis between ROR1 H-score and therapeutic options. (A) Chemotherapy history, (B) Surgery, (C) Platinum sensitivity.
Categorization of ROR1 expression and correlation with clinical characteristics
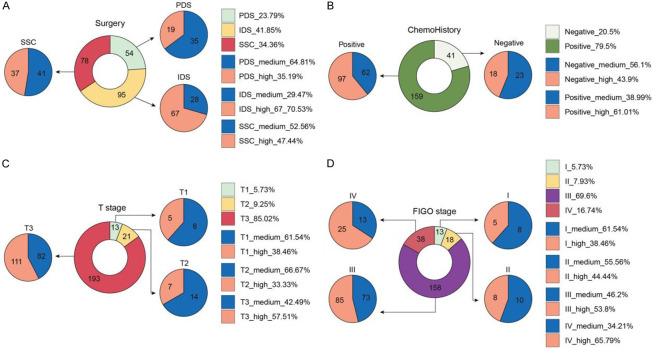
Due to the lack of a recommended cutoff value for ROR1 in relation to clinical prognosis for statistical analysis, all previous results were based on continuous variable analysis of ROR1 protein expression. Therefore, we have used a median H-score score of 200 as the demarcation value and categorized ROR1 expression into high (123 cases) and medium (104 cases), the details are shown in Table 2. We analyzed the correlation between patients’ clinical characteristics using chi-square test. The results indicated that individuals with high expression of ROR1 were significantly older (P<0.05), and those with a history of chemotherapy exhibit higher expression levels (Figure 5). Chi-square analysis did not yield significant results due to substantial differences in sample size across stages; however, there were more cases with pronounced high expression of ROR1 in T3 and IV stage of FIGO.
Table 2.
Demographics of patients and cases with different levels of ROR1 expression intensity
| [ALL] N=227 | Medium N=104 | High N=123 | p.overall | |
|---|---|---|---|---|
| Age | 55.7 (9.31) | 54.3 (10.3) | 56.9 (8.29) | 0.04 |
| Chemotherapy history | 0.048 | |||
| Negative | 41 (20.5%) | 23 (27.1%) | 18 (15.7%) | |
| Positive | 159 (79.5%) | 62 (72.9%) | 97 (84.3%) | |
| Platinum-sensitivity | 0.36 | |||
| Platinum-refractory | 7 (4.61%) | 5 (7.46%) | 2 (2.35%) | |
| Platinum-resistant | 18 (11.8%) | 9 (13.4%) | 9 (10.6%) | |
| Potentially platinum-sensitive | 58 (38.2%) | 22 (32.8%) | 36 (42.4%) | |
| Fully platinum-sensitive | 69 (45.4%) | 31 (46.3%) | 38 (44.7%) | |
| Sample type | 0.266 | |||
| Metastases | 99 (43.6%) | 50 (48.1%) | 49 (39.8%) | |
| Primary lesion | 128 (56.4%) | 54 (51.9%) | 74 (60.2%) | |
| FIGO | 0.256 | |||
| I | 13 (5.73%) | 8 (7.69%) | 5 (4.07%) | |
| II | 18 (7.93%) | 10 (9.62%) | 8 (6.50%) | |
| III | 158 (69.6%) | 73 (70.2%) | 85 (69.1%) | |
| IV | 38 (16.7%) | 13 (12.5%) | 25 (20.3%) | |
| CA125 | 0.798 | |||
| 0 | 1 (0.50%) | 1 (1.15%) | 0 (0.00%) | |
| 1 | 143 (71.1%) | 62 (71.3%) | 81 (71.1%) | |
| 2 | 11 (5.47%) | 4 (4.60%) | 7 (6.14%) | |
| 3 | 46 (22.9%) | 20 (23.0%) | 26 (22.8%) | |
| Histologic type | <0.001 | |||
| CCA | 20 (8.81%) | 18 (17.3%) | 2 (1.63%) | |
| HGSOC | 202 (89.0%) | 86 (82.7%) | 116 (94.3%) | |
| LGSOC | 5 (2.20%) | 0 (0.00%) | 5 (4.07%) | |
| Surgical type | <0.001 | |||
| PDS | 54 (23.8%) | 35 (33.7%) | 19 (15.4%) | |
| IDS | 95 (41.9%) | 28 (26.9%) | 67 (54.5%) | |
| SSC | 78 (34.4%) | 41 (39.4%) | 37 (30.1%) |
Figure 5.
Distribution of cases with high and low ROR1 expression. (A) Surgical types, (B) FIGO stage, (C) T stage, (D) Chemohistory.
Prognostic effect of ROR1 expression
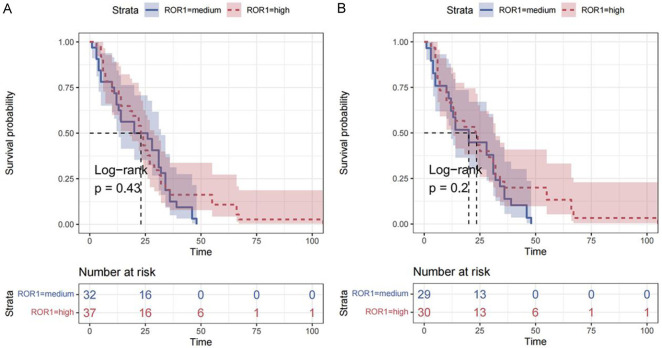
Finally, we conducted univariate Cox regression analysis on all cases according to the high and low expression of ROR1, and observed the PFS and progression-free interval (PFI) respectively. Our results showed that the expression of ROR1 was not an independent risk factor for PFS and PFI according to the data of this batch. However, as shown in Figure 6, there were cases of longer PFS and PFI in the ROR1 high group, which suggested that larger-scale research may be needed for further exploration.
Figure 6.
Cox regression analysis of ROR1 protein expression in ovarian cancer patients. (A) PFS, (B) PFI.
Nuclear translocation of ROR1 in clear-cell ovarian cancer
We unexpectedly found that high-grade serous ovarian cancer had mainly uniform membrane staining, but clear-cell ovarian cancer had ROR1 nuclear staining (Figure 7). It has been reported that the shift of ROR1 protein to the nucleus activates the downstream signaling pathway, which leads to the increased malignancy of tumor cells, and the reduction of membrane receptors may weaken the effect of targeted therapy, which may be one of the factors leading to the poor prognosis of clear cell ovarian cancer [12]. Therefore, it was necessary to quantify the expression level of receptor protein on the membrane before ROR1 targeted therapy, and H-Score was suitable as a method to interpret the IHC strength of cell membrane. In addition, whether the prognosis of other types of tumors is worse due to the nuclear translocation of ROR1 will need further research.
Figure 7.
ROR1 IHC staining of different tissue samples. A. High-grade serous ovarian cancer. B. Clear cell carcinoma of ovary. Magnification: 100×.
Discussion
Although the precise role of ROR1 in malignancies remains incompletely understood, it is crucial for embryonic development, with knockout studies demonstrating that its absence leads to embryonic lethality [5]. Furthermore, ROR1 is absent in most postnatal organs, being present only in select tissues or cells, such as endocrine glands, the gastrointestinal tract, and immature B lymphocytes [7]. Evidence indicates that ROR1 is aberrantly overexpressed in tumor tissues of lung cancer, breast cancer, and chronic lymphocytic leukemia. Under stimulation by Wnt5a, the primary ligand for ROR1 on the cell membrane, transformed cancer cells acquire genomic programs typically active during embryonic development but usually suppressed in adult cells. Specifically, ROR1 can promote tumor cell proliferation, survival, metastasis, and progression through pathways such as PI3K/AKT/mTOR, EGFR, MET, and JAK/STAT, correlating with poor patient prognosis [13-17].
In ovarian cancer, Piki et al [17] discovered significant upregulation of ROR1 expression in clinical samples post-neoadjuvant chemotherapy. Further studies revealed that ROR1 interacts with its major ligand Wnt5a on the cell membrane, activating pro-survival pathways such as AKT/ERK and STAT3 in ovarian cancer cells, suggesting that ROR1 may induce oncogenic signaling by the PI3K/AKT/ERK axis. However, the specific relationship between ROR1 expression and ovarian cancer remains largely unexplored. Previous studies using qPCR and immunohistochemistry have shown that ROR1 expression is significantly higher in ovarian cancer tissues compared to normal ovarian tissues, and ROR1 protein expression is an independent prognostic factor for disease-free survival (DFS) and overall survival (OS). Therefore, ROR1 may serve as a novel prognostic marker for ovarian cancer [8]. However, these studies did not include patients who had undergone any treatment, leaving the changes in ROR1 expression in response to ovarian cancer treatment strategies unexplored.
The current study aimed to refine the data on ROR1 expression in ovarian cancer. Specifically, it aimed to evaluate ROR1 expression in ovarian cancer tissues under different treatment regimens and investigate the correlation between ROR1 expression and clinical case data.
Among the enrolled patients in this study, 89.0% had high-grade serous ovarian cancer, 2.2% had low-grade serous ovarian cancer, and 8.8% had clear cell carcinoma. The distribution of FIGO stages was as follows: 5.7% in stage I, 7.9% in stage II, 69.6% in stage III, and 16.7% in stage IV. Additionally, 56.4% of the samples were from primary ovarian tumors, while 43.6% were from recurrent tumors in the abdominal and pelvic organs. Regarding prior carboplatin treatment, 70.0% had a history of chemotherapy, 18.1% had no chemotherapy history, and 11.9% had missing data. Patients with a history of carboplatin chemotherapy were categorized based on the PFI into four groups: “platinum refractory (PFI ≤ 6 months)”, “platinum resistant (PFI 6-12 months)”, “partially platinum-sensitive (PFI 12-24 months)”, and “fully platinum-sensitive (PFI>24 months)” [18]. Among them, 3.1% were platinum refractory, 7.9% were platinum resistant, 25.6% were partially platinum-sensitive, 30.4% were fully platinum-sensitive, and 33.3% had missing PFI data. According to the type of surgical procedure, 24.7% underwent PDS, 35.2% underwent IDS, and 40.1% underwent SSC [19].
We used the H-score to assess ROR1 expression and found that ROR1 expression was higher in T3 stage tumor tissues compared to T2 and T1 stages. However, this difference was not significant (P=0.065). Interestingly, ROR1 expression was significantly higher in FIGO stage IV tumor tissues compared to stage I (P=0.033). Additionally, we found no significant difference in ROR1 expression between primary and metastatic cancer sites (P=0.20), consistent with previous studies indicating a close relationship between ROR1 and tumor stage [20-22].
We also observed that ROR1 expression was related to the treatment regimen. Patients who underwent IDS had significantly higher ROR1 H-scores compared to those who underwent PDS (P<0.01) and SSC (P<0.01). This may be partially due to the clinical staging of the tumors, and possibly related to the patients’ chemotherapy history. Our results showed a trend of increasing ROR1 expression with the use of and sensitivity to platinum-based chemotherapy, although the differences were not statistically significant. Further analysis using the H-score as a continuous variable revealed no significant enrichment of ROR1 expression with pathological characteristics such as surgical type, chemotherapy history, histologic type, and FIGO stage. However, when categorizing ROR1 expression into high (H-score >200) and medium (H-score ≤ 200) based on the median H-score, we found that patients with IDS treatment, a history of platinum-based chemotherapy, higher tumor diameter (T) and FIGO stage had significantly higher ROR1 expression. This suggests that high ROR1 expression may be associated with more aggressive tumor characteristics and a background of platinum-based chemotherapy. The correlation between high ROR1 expression and chemotherapy is consistent with findings in breast cancer by Zhang et al [23], who showed that chemotherapy can increase ROR1 expression in tumor tissues. Their study also demonstrated that treatment with the humanized anti-ROR1 monoclonal antibody cirmtuzumab inhibited the expression of genes associated with breast cancer stemness and reduced metastasis in mouse models, indicating the potential of ROR1-targeted therapies to improve chemotherapy responses in cancer patients.
Subsequently, we examined the effect of ROR1 expression in ovarian cancer tissues on patient prognosis and PFI. Our data indicated that ROR1 expression levels did not significantly affect PFS or PFI. This contradicts previous studies, such as that by Zhang et al [8], which reported that high ROR1 expression was associated with shorter DFS and OS in ovarian cancer patients. This discrepancy underscores the necessity for further investigation with a larger cohort of ovarian cancer patients to accurately determine the prognostic value of ROR1 expression. Additionally, our analysis revealed that high-grade serous ovarian cancer tissues exhibited uniform cell membrane staining for ROR1, whereas low-grade serous ovarian cancer and clear cell carcinoma tissues showed nuclear staining. This phenomenon warrants further research to understand its implications for the efficacy of ROR1-targeted therapies.
In conclusion, our study indicated that ROR1 expression may be associated with treatment strategy and history of platinum-based chemotherapy in ovarian cancer. The unique observation of ROR1 nuclear translocation in clear cell ovarian cancer highlights the need for further investigation into its biological and clinical significance. While the study provides valuable insight, its limitations, including sample size and lack of longitudinal data, suggest that larger, more diverse cohorts and in-depth molecular analyses are needed to fully understand ROR1’s role and therapeutic potential in ovarian cancer. Targeting ROR1 may offer a promising therapeutic approach, particularly for patients with chemo-resistant tumors. This study supplements existing data on ROR1 expression in ovarian cancer and provides a foundation for the development of personalized treatment.
Disclosure of conflict of interest
None.
References
- 1.Konstantinopoulos PA, Matulonis UA. Clinical and translational advances in ovarian cancer therapy. Nat Cancer. 2023;4:1239–1257. doi: 10.1038/s43018-023-00617-9. [DOI] [PubMed] [Google Scholar]
- 2.Kurnit KC, Fleming GF, Lengyel E. Updates and new options in advanced epithelial ovarian cancer treatment. Obstet Gynecol. 2021;137:108–121. doi: 10.1097/AOG.0000000000004173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69:280–304. doi: 10.3322/caac.21559. [DOI] [PubMed] [Google Scholar]
- 4.Moffat GT, Kong W, Mackay HJ, Mcgee J, Booth CM, Ethier JL. Real-world outcomes associated with bevacizumab combined with chemotherapy in platinum-resistant ovarian Cancer. Gynecol Oncol. 2024;184:51–56. doi: 10.1016/j.ygyno.2024.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Luo D, Qiu X, Zheng Q, Ming Y, Pu W, Ai M, He J, Peng Y. Discovery of novel receptor tyrosine kinase-like orphan receptor 1 (ROR1) inhibitors for cancer treatment. J Med Chem. 2024;67:10655–10686. doi: 10.1021/acs.jmedchem.4c00175. [DOI] [PubMed] [Google Scholar]
- 6.Raivola J, Dini A, Salokas K, Karvonen H, Niininen W, Piki E, Varjosalo M, Ungureanu D. New insights into the molecular mechanisms of ROR1, ROR2, and PTK7 signaling from the proteomics and pharmacological modulation of ROR1 interactome. Cell Mol Life Sci. 2022;79:276. doi: 10.1007/s00018-022-04301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raso MG, Barrientos Toro E, Evans K, Rizvi Y, Lazcano R, Akcakanat A, Sini P, Trapani F, Madlener EJ, Waldmeier L, Lazar A, Meric-Bernstam F. Heterogeneous profile of ROR1 protein expression across tumor types. Cancers (Basel) 2024;16:1874. doi: 10.3390/cancers16101874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Qiu J, Ye C, Yang D, Gao L, Su Y, Tang X, Xu N, Zhang D, Xiong L, Mao Y, Li F, Zhu J. ROR1 expression correlated with poor clinical outcome in human ovarian cancer. Sci Rep. 2014;4:5811. doi: 10.1038/srep05811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HJ, Choi MY, Siddiqi T, Barrientos JC, Wierda WG, Isufi I, Tuscano JM, Lamanna N, Subbiah S, Koff JL. Phase 1/2 study of cirmtuzumab and ibrutinib in mantle cell lymphoma (MCL) or chronic lymphocytic leukemia (CLL) J. Clin. Oncol. 2021;39:7556–7556. [Google Scholar]
- 10.Jiang VC, Liu Y, Mcintosh J, Jordan AA, Wang M. Targeting ROR1 using the antibody drug conjugate Vls-101 in aggressive mantle cell lymphoma. Blood. 2020;136:33. doi: 10.1186/s13045-021-01143-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaisitti T, Arruga F, Vitale N, Lee TT, Ko M, Chadburn A, Braggio E, Napoli AD, Iannello A, Allan JN. ROR1 targeting with the antibody-drug conjugate VLS-101 is effective in Richter syndrome patient-derived xenograft mouse models. Blood. 2021;137:3365–3377. doi: 10.1182/blood.2020008404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng HC, Lyu PC, Lin WC. Nuclear localization of orphan receptor protein kinase (Ror1) is mediated through the juxtamembrane domain. BMC Cell Biol. 2010;11:48. doi: 10.1186/1471-2121-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghia EM, Rassenti LZ, Choi MY, Quijada-Álamo M, Chu E, Widhopf GF 2nd, Kipps TJ. High expression level of ROR1 and ROR1-signaling associates with venetoclax resistance in chronic lymphocytic leukemia. Leukemia. 2022;36:1609–1618. doi: 10.1038/s41375-022-01543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasan MK, Widhopf GF 2nd, Zhang S, Lam SM, Shen Z, Briggs SP, Parker BA, Kipps TJ. Wnt5a induces ROR1 to recruit cortactin to promote breast-cancer migration and metastasis. NPJ Breast Cancer. 2019;5:35. doi: 10.1038/s41523-019-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kipps TJ. ROR1: an orphan becomes apparent. Blood. 2022;140:1583–1591. doi: 10.1182/blood.2021014760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Sun H, Bai C, Hu Y, Tang J, Zhang Y, Chen J, Zhong Z, He Y, Hu K, Yang J. Dihydroartemisinin inhibits tumor progress via blocking ROR1-induced STAT3-activation in non-small cell lung cancer. Int Immunopharmacol. 2024;133:112157. doi: 10.1016/j.intimp.2024.112157. [DOI] [PubMed] [Google Scholar]
- 17.Piki E, Dini A, Raivola J, Salokas K, Zhang K, Varjosalo M, Pellinen T, Välimäki K, Veskimäe KT, Staff S, Hautaniemi S, Murumägi A, Ungureanu D. ROR1-STAT3 signaling contributes to ovarian cancer intra-tumor heterogeneity. Cell Death Discov. 2023;9:222. doi: 10.1038/s41420-023-01527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L, Xie HJ, Li YY, Wang X, Liu XX, Mai J. Molecular mechanisms of platinumbased chemotherapy resistance in ovarian cancer (Review) Oncol Rep. 2022;47:82. doi: 10.3892/or.2022.8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coleridge SL, Bryant A, Kehoe S, Morrison J. Neoadjuvant chemotherapy before surgery versus surgery followed by chemotherapy for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst Rev. 2021;7:CD005343. doi: 10.1002/14651858.CD005343.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heabah NAE, Darwish SA, Eid AM. Evaluation of the prognostic significance of receptor tyrosine kinase-like orphan receptor 1 (ROR1) in lung carcinoma and its relation to lymphangiogenesis and epithelial mesenchymal transition. Pathol Res Pract. 2023;248:154703. doi: 10.1016/j.prp.2023.154703. [DOI] [PubMed] [Google Scholar]
- 21.Mao Y, Xu L, Wang J, Zhang L, Hou N, Xu J, Wang L, Yang S, Chen Y, Xiong L, Zhu J, Fan W, Xu J. ROR1 associates unfavorable prognosis and promotes lymphoma growth in DLBCL by affecting PI3K/Akt/mTOR signaling pathway. Biofactors. 2019;45:416–426. doi: 10.1002/biof.1498. [DOI] [PubMed] [Google Scholar]
- 22.Wei R, Liao X, Li J, Mu X, Ming Y, Peng Y. Novel humanized monoclonal antibodies against ROR1 for cancer therapy. Mol Cancer. 2024;23:165. doi: 10.1186/s12943-024-02075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S, Zhang H, Ghia EM, Huang J, Wu L, Zhang J, Lam S, Lei Y, He J, Cui B, Widhopf GF 2nd, Yu J, Schwab R, Messer K, Jiang W, Parker BA, Carson DA, Kipps TJ. Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody. Proc Natl Acad Sci U S A. 2019;116:1370–1377. doi: 10.1073/pnas.1816262116. [DOI] [PMC free article] [PubMed] [Google Scholar]