Abstract
Since the mid-20th century, the interaction between light and inorganic semiconductors plays not only a key role in numerous fascinating phenomena but also provides the physical foundations for the development of many modern technologies focused on health, environmental, and energy solutions. Among these materials, silver-based bimetallic semiconductors have garnered attention due to their enhanced functional properties, which are controlled by the presence and distribution of structural and electronic defects. These defects directly impact key physicochemical properties, making them essential for the development of materials with improved functionalities. Modifying synthetic and postsynthetic parameters is crucial for controlling the type, density, and distribution of these defects in materials. However, achieving precise control of these defects remains a challenge and requires a deeper understanding of the relationship between synthetic conditions and defect formation. This work provides a comprehensive review of how modifications in synthesis methods influence material properties, with a particular focus on understanding their impact on material defects. Specifically, this study examines silver-based bimetallic semiconductors, including Ag2WO4, Ag2MoO4, and Ag2CrO4. Additionally, strategies involving advanced defect characterization techniques such as photoluminescence spectroscopy (PL), X-ray photoelectron spectroscopy (XPS), electron paramagnetic resonance (EPR), and positron annihilation lifetime spectroscopy (PALS) are discussed, as these methods are gaining prominence in defect analysis. By exploring the interplay between synthetic control and its impact on defects in these materials, this study highlights the critical role of defect engineering in advancing the application potential of silver-based bimetallic semiconductors.
Introduction
In recent years, inorganic semiconductors have gained increased prominence in academia and industry by their potential to overcome the challenges of availability, high-cost processing, and toxicity, which have been insurmountable for the development of new technologies that enhance quality of life on a global scale. According to the Semiconductor Industry Association (SIA), global semiconductor sales reached 527 billion dollars in the second half of 2023, and future projections indicate that this figure could triple within the next decade. This growing demand is expected to stimulate investments in this field, not only for the development of traditional semiconductors used in electronic devices but also for the advancement of cutting-edge technologies that can leverage the unique properties of these materials. Historically, the center of semiconductor investments has centered around conventional electronic applications, such as components in everyday devices. However, as science and societal needs continue to evolve, emerging fields - such as catalysts and biomaterials - are gaining increased prominence. This shift reflects the inherent versatility of these materials to be an indispensable component in a wide range of sectors, from healthcare to environmental protection, and enabling them to address increasingly complex technological and social challenges.
Classically, a semiconductor is defined as a material whose electronic structure features a moderate band gap between the valence band (VB), comprising orbitals filled with electrons strongly bound to the atoms, and the conduction band (CB), made up of empty or partially filled orbitals where electrons (e–) can move freely, thus enabling electrical conductivity. Unlike conductors, which have overlapping VB and CB, and insulators, which have very wide band gaps, semiconductors allow some electrons to be excited from the VB to the CB through thermal, optical, or electrical stimulation for example. This process generates charge carriers that are highly reactive, paving the way for numerous technological applications. However, the classical definition of semiconductors, typically based on bulk materials, becomes more complex when particle size is reduced and quantum effects come into play. Under these conditions, the semiconductor’s surface takes on a crucial role. Each crystallographic plane may exhibit slightly different band gaps due to specific atomic arrangements at the surface and the presence of under-coordinated atomic sites, called clusters. They generate regions of varying electron density, both positive and negative, which function similarly to charge carriers in conventional bulk semiconductors. As a result, the morphology and particle size play a critical role in determining the electronic behavior and the overall activity of the semiconductor. These clusters are closely linked to atomic vacancies present on different crystal surfaces, which exhibit distinct local metal coordination environments and unsaturated bonds. Consequently, they differ in several aspects, including their local electric field effects and electron confinement capabilities.
Defects are general perturbations of the periodic atomic arrangements, which inevitably exist in semiconductor materials. i.e. they are ubiquitous and have a pronounced effect on their physical and chemical properties and thus open new opportunities for obtaining efficient defected materials As it was noted: “Even if there is one atom vacancy in one hundred million host atoms, the electronic structure of the material will change intensely”. Oxygen vacancies being intrinsic defects commonly found on the surface of real semiconductors, even in infinitesimal concentration, are found to play a more decisive role in determining the kinetics, energetics, and reactive mechanisms. Specifically, introducing defects into these materials induces the formation of intermediate electronic states within the forbidden region of the band gap. Such defects affect electronic transitions between the atomic clusters that form the material, allowing for fine-tuning of its electronic structure and charge distribution. Therefore, defect engineering is recognized as an effective route to obtaining highly active materials by tuning the atom coordination number and electronic structure, optical, charge separation, and surface properties, and therefore to improve their activity, making them suitable for a wide range of functional applications.
It is important to note that processes such as the formation of surface clusters with different electronic densities and the creation of prepopulated intermediate electronic states occur in all semiconductors, including established examples like TiO2, ZnO, WO3, CeO2, Fe2O3, and Fe3O4. Ag-based bimetallic semiconductors, such as Ag2WO4, Ag2CrO4, and Ag2MoO4, are particularly interesting because they introduce additional complexity to the electronic structure by combining the unique properties of Ag and metal cations with oxygen-based lattices. This combination not only enhances their electronic and optical properties but also promotes superior performance in applications like photocatalysis, antimicrobial activity, and energy conversion, making them ideal candidates for advanced technological solutions.
In this context, Ag2WO4, Ag2CrO4, and Ag2MoO4 semiconductors have attracted growing interest due to their well-defined crystal structures and their capacity for extensive physicochemical tailoring. Among them, Ag2WO4 stands out not only for its polymorphic diversity but also for its remarkable responsiveness to external excitation, including electron beams and laser irradiation, which can induce the formation of metallic Ag nanoparticles directly on the material’s surface, as highlighted by Gouveia et al. This phenomenon, also observed in Ag2CrO4 and Ag2MoO4, reflects the intrinsic photo- and electro-sensitivity of these Ag-based semiconductors, allowing their surface reactivity to be tuned without altering the bulk crystalline framework. − Thanks to its broad-spectrum activity and high chemical reactivity, Ag2WO4 has been successfully employed in a range of applications, including photocatalytic degradation of organic pollutants, antimicrobial coatings, and as a component in heterojunction systems for environmental and energy-related technologies. Ag2MoO4 has demonstrated strong potential in redox-based catalysis, including Fenton-like and ozonation processes, owing to its efficient electron transfer behavior and chemical stability. Meanwhile, Ag2CrO4 stands out for its high absorption in the visible spectrum and pronounced photoactivity, making it particularly effective for solar-driven degradation of pollutants. Together, these materials offer tunable band structures, controlled morphologies, and dynamic surface states, key attributes that support efficient charge separation, robust reactive oxygen species (ROS) generation, and high operational stability. As such, their combined structural, electronic, and functional advantages position them as compelling platforms for innovations in photocatalysis, environmental remediation, renewable energy, and biomedical applications.
To further enhance their functional properties, defect engineering has emerged as a powerful strategy to manipulate the electronic and surface characteristics of these materials. Introducing and controlling defects not only improves catalytic activity but also plays a key role in modifying surface reactivity, particularly by facilitating the adsorption of reactants and the generation of active sites. In this regard, the incorporation of different metal cations within a single crystalline lattice promotes distinctive electronic interactions with oxygen anions, leading to the formation of intermediate states within the band gap. , As a result, it becomes possible to finely tune energy levels and improve charge carrier mobility. Additionally, the intrinsic structural complexity of these materials, expressed through their diverse atomic clusters and crystallographic terminations, can significantly influence their surface dynamics, even under dark conditions. This variety of clusters types is not merely a structural feature, but a key factor in enabling the formation of a wide variety of structural defects, such as vacancies, distortions, and nonstoichiometric sites. These defects, in turn, directly modulate the material’s reactivity by influencing parameters like charge carrier separation, adsorption capacity, and the generation of reactive oxygen species. As a result, Ag-based bimetallic semiconductors become highly effective in processes that rely on efficient charge transport, reactive species production, or selective interaction with electromagnetic radiation, reinforcing their growing relevance in catalysis, sensing technologies, energy conversion systems, and functional biomaterials.
Building upon this foundation, the present study investigates how different synthetic strategies for obtaining Ag-based bimetallic semiconductors, Ag2WO4, Ag2CrO4, and Ag2MoO4, can modulate defect density, chemical composition, and spatial distribution within the material matrix. A range of complementary characterization techniques are explored to analyze these defect structures and their impact on functionality. By correlating synthetic parameters with defect-related behavior, this work provides valuable insights for the rational design of efficient and multifunctional Ag-based semiconductors, offering a roadmap for future developments in this rapidly evolving field. Unlike previous works, this review provides a comprehensive overview of pristine Ag-based materials, highlighting their structural and functional versatility, current synthetic advancements, and unresolved challenges, thereby extending the existing knowledge in the field.
Structural and Electronic Features
At first glance the primary difference among Ag2WO4, Ag2CrO4, and Ag2MoO4 materials lies in the choice of lattice formers, while Ag consistently serves as the lattice modifier across all compounds (see Figure A).
1.

(A) Structure and (B) electronic positions of VB and CB of silver-based bimetallic semiconductors.
Among these materials, Ag2WO4 has attracted significant attention due to its advanced antimicrobial properties, which often outperform conventional Ag nanoparticles. This material exhibits three polymorphs: the thermodynamically stable α-Ag2WO4, the metastable β-Ag2WO4, and the least stable γ-Ag2WO4. The α-Ag2WO4 polymorph has an orthorhombic structure with the space group Pn2n and a remarkably complex arrangement at the cluster level. It is composed of three types of distorted tetrahedral [WO6] clusters and six distinct Ag clusters, including two [AgO7], one [AgO6], two [AgO4], and one [AgO2]. This structural complexity allows for a wide variety of defects to form in both the bulk and surface of the material. In contrast, the β-Ag2WO4 polymorph has a hexagonal structure with the space group P63/m, featuring two types of W clusters ([WO4] and [WO5]) and two types of Ag clusters ([AgO5] and [AgO6]). Lastly, the γ-Ag2WO4 polymorph, which is the least stable, adopts a cubic spinel structure with the space group Fd3̅m. Its simpler architecture consists of [WO4] and [AgO6] clusters.
Similarly, Ag2MoO4 exists in two polymorphs: the thermodynamically stable β-Ag2MoO4 and the metastable α-Ag2MoO4. The β-Ag2MoO4 polymorph has a cubic spinel structure, identical to γ-Ag2MoO4, with the space group Fd3̅m. It is composed of [MoO4] and [AgO6] clusters. The α-Ag2MoO4 polymorph, on the other hand, adopts an inverse tetragonal spinel structure with the space group P4122. This structure is more complex, owing to its different coordination number, and consists of [MoO6] and [AgO6] clusters. In contrast to the aforementioned materials, Ag2CrO4 do not exhibit polymorphism. The structure of Ag2CrO4 is orthorhombic, with the space group Pnma, and is composed of [AgO4], [AgO6], and [CrO4] clusters. These structural differences reflect their suitability for specific applications, particularly in photocatalysis.
From an electronic perspective, most of these materials are classified as p-type semiconductors, meaning that holes are the predominant charge carriers. The exception is α- Ag2MoO4, which is an n-type semiconductor characterized by electrons as the primary charge carriers. This distinction arises from the higher density of states in the CB of α-Ag2MoO4. The VB in these materials is primarily formed by contributions from Ag and O atoms, while the lattice formers play a more significant role in shaping the CB. The band gap (E gap) of these materials varies widely, reflecting their diverse electronic properties. For Ag2WO4 polymorphs, the E gap ranges from 3.0 to 3.3 eV. In the case of Ag2MoO4, the stable β-phase has the highest E gap, between 3.4 and 3.6 eV, while the metastable α-phase has the lowest E gap, ranging from 1.2 to 1.4 eV, making it particularly suitable for visible light applications. Ag2CrO4 has band gap of 1.8 eV, which are ideal for photocatalytic processes as they absorb light in the visible region of the electromagnetic spectrum. The positions of the VB and CB are critical for predicting the photocatalytic efficiency of these materials, especially their ability to generate ROS (Figure B). Theoretical predictions indicate that Ag2CrO4 has CB levels below the redox potential of the O2/•O2 – pair (0.05 eV), while α-Ag2MoO4 uniquely exhibits both a CB below the O2/•O2 – redox potential and a VB above the H2O/•OH redox potential (2.30 eV). These insights guide the development of advanced photocatalytic systems for environmental remediation, highlighting the importance of managing ROS production for efficient pollutant removal.
Defects in Semiconductors
In a classical description of a semiconductor, one might ideally expect a perfectly ordered crystalline lattice, with every atom occupying its intended site. However, structural and electronic imperfections - commonly referred to as defects - inevitably exist in semiconductor materials along the crystal growth process. , As mentioned above, these defects can profoundly influence a semiconductor’s electrical, optical, mechanical, and chemical properties. − While the traditional understanding of semiconductors often focuses on bulk-scale behavior, advances in nanotechnology and surface science have unveiled a complex variety of surface defects that open pathways to innovative applications and tailored functionalities (Figure ).
2.
(A) Classical and (B) quantum surface defects.
When considering a bulk semiconductor in its most straightforward form, defects typically manifest as point defects (vacancies, interstitials, or substitutional atoms), line defects (dislocations), or surface/interface defects (grain boundaries and phase boundaries). Point defects often emerge when an atom is missing, occupying an incorrect location, or substituted by a different species, and each of these scenarios can introduce localized states in the band structure. Dislocations arise along lines in the crystal where the periodic atomic arrangement is disrupted, creating areas of stress that can scatter charge carriers. Surface or interface defects become relevant when grains of different orientations meet, or when a secondary phase merges with the primary phase.
These defects can profoundly influence a semiconductor’s electrical, optical, mechanical, and chemical properties. − While the traditional understanding of semiconductors often focuses on bulk-scale behavior, advances in nanotechnology and surface science have unveiled a complex variety of quantum and surface defects and vacancies which are responsible of a plethora of quantum effects, making them suitable for multidomain applications and innovative functionalities (Figure ). Different research groups have published works aimed at elucidating the role of defects and vacancies in modulating the electronic structure, optical, and surface-active sites. ,
As the focus shifts from the bulk structure to the material’s surfaces - especially at the nanoscale -the concept of defects broadens to include what can be termed “quantum” or “surface” defects. , In semiconductors with a wide array of structural clusters, the notion of quantum clusters as a type of quantum defect becomes critically important. These quantum clusters emerge from local alterations in electronic density and may have multiple origins. They can be intrinsic - such as undercoordinated surface clusters - or induced through synthetic modifications that increase the prevalence of these undercoordinated sites. Additionally, the incorporation of foreign elements into the semiconductor’s structure can trigger a cascade effect, adjusting local electronic density to compensate for electron deficits or surpluses. This expanded concept of quantum clusters also helps clarify how charge-carrier pairs (electrons and holes) are formed and behave within a semiconductor. By creating discrete electronic states in the band gap that are mirrored in different surface electronic densities, these clusters can influence the dynamics of electron–hole generation, trapping, and recombination. Whether they bolster beneficial interactions (e.g., enhanced adsorption of reactants) or promote unfavorable electron–hole recombination, quantum clusters strongly affect the material’s overall functionality and performance.
Vacancies, whether metallic or oxygen-related, play a crucial role in determining the structural, electronic, and functional properties of semiconductors. , Metallic vacancies, often associated with cationic sites, can disrupt lattice symmetry and create localized electronic states, altering charge distribution and mobility. Similarly, oxygen vacancies, among the most studied, introduce intermediate energy levels within the bandgap that directly influence optical and electronic behaviors, such as photoluminescence, conductivity, and catalytic activity. , These defects, whether present in bulk or concentrated at the surface within quantum clusters, serve as key sites for chemical reactions, adsorption, or charge trapping, making them integral to the performance of semiconductor-based technologies. Oxygen vacancies, in particular, are critical for tailoring semiconductor functionality. They often determine the relationship between the degree of structural order/disorder and electronic dynamics, acting as centers for electron trapping and recombination. In quantum clusters, undercoordinated clusters with different oxygen vacancies modify the local surface electronic density, leading to changes in bandgap energies and directly impacting the surface properties of the material. This alteration can be observed in various applications, such as the generation of ROS, where oxygen vacancies can enhance their production, or in the surface interactions of these materials with other molecules, as they can amplify surface reactivity.
To rationally exploit defect production in materials, such as vacancies, it is crucial to use appropriate characterization techniques, in addition to maintaining rigorous synthetic control, as even small modifications can be critical to defect formation. Strict synthetic control directly influences the degree of order/disorder in these materials and, consequently, their defects, enabling fine-tuning of material performance. Synthetic and postsynthetic approaches that can effectively modify nature and distribution of these material defects will be highlighted and analyzed in detail in the next section. Equally important to synthetic control is the use of advanced characterization techniques to analyze and, where possible, quantify these defects, as structure–property correlations are essential for the rational development of materials. Techniques such as photoluminescence spectroscopy (PL), X-ray photoelectron spectroscopy (XPS), electron paramagnetic resonance (EPR), and positron annihilation lifetime spectroscopy (PALS) have gained prominence for identifying the types, densities, and spatial distributions of surface defects, particularly those related to vacancies. Like synthetic strategies, these techniques will also be discussed in subsequent sections.
From Defects to Applications
Rather than being seen merely as imperfections, structural defects in Ag-based semiconductors are now recognized as critical elements for tuning the activity of advanced materials. When rationally introduced, these defects act as functional levers that modulate surface chemistry, charge transport, and overall reactivity, parameters essential for applications such as photocatalysis, gas sensing, and antimicrobial action. Importantly, the presence of defects induces short-range structural distortions, which are directly reflected in the local electronic behavior, both in the bulk and at the surface. These alterations modify the dynamics of charge carrier recombination, typically by introducing new defect states within the band gap (between VB and CB) that act to slow down recombination processes (Figure ). Depending on their energetic position, these defect states can be classified as shallow, located near the band edges, or deep, positioned closer to the center of the band gap. In either case, they fundamentally reshape the local electronic structure, thereby influencing the material’s overall functional performance.
3.
Schematic representation of how structural defects induce short-range distortions that impact local electronic properties in Ag-based semiconductors.
In photocatalytic systems, controlled introduction of oxygen vacancies can reduce the bandgap of the semiconductor, extending their photoresponse into the visible region. , These vacancies not only facilitate light harvesting but also promote the separation of photogenerated charge carriers, lowering recombination losses. More importantly, they create active sites where redox reactions can occur more efficiently, fundamental for pollutant degradation. Similar principles apply to gas sensors and antimicrobial systems, where surface activation and reactive species generation are equally important, albeit through different mechanisms.
In antimicrobial contexts, the presence of surface defects, particularly those that involve under-coordinated Ag atoms, supports the release of Ag+ ions and enhances the production of ROS, expanding their full potential and enhancing their antimicrobial performance. Unlike conventional photocatalysis, where light is essential to initiate charge separation, defect-rich Ag-based semiconductors can generate ROS even in the absence of ligth. This occurs because certain defect states host pre-excited or loosely bound electrons capable of engaging in redox processes at the surface. Although the ROS generation is less intense in the dark, exposure to light significantly amplifies this activity by promoting additional charge excitation and boosting overall reactivity. In gas sensors, similar surface defects act as molecular recognition sites, where adsorbed analytes modulate local electronic properties and conductivity through specific interactions.
Doping strategies further enhance the utility of defect engineering. Incorporating heteroatoms can lead to lattice distortions that favor defect formation, such as the substitution of oxygen by nonmetals or the generation of cationic vacancies to balance charge. This approach simultaneously tunes light absorption, electronic conductivity, and surface polarity, yielding improved performance across different applications. Co-doping using two or more dopants can offer synergistic effects by balancing defect formation energy with band structure optimization.
The key mechanisms behind the performance of Ag-based semiconductors, such as ROS generation, surface charge modulation, and molecular interactions, are fundamentally linked and span their main applications. Whether employed in pollutant degradation, gas sensing, or antimicrobial activity, their functionality is inherently driven by surface-mediated processes. By precisely tailoring surface defect chemistry, it becomes possible to exploit a unifying operational principle across these diverse fields. As scientific insight advances, the development of defect-engineered materials evolves toward a defect-by-design approach, in which reactivity, selectivity, and long-term stability are deliberately integrated into the crystal structure from the very beginning.
Synthetic Strategies for Defect Control: Defect Engineering
Synthetic Parameters
Controlling and even inducing defects intentionally has become a key strategy for optimizing semiconductor functionality. Several synthetic and postsynthetic approaches facilitate this level of control. For instance, doping (the purposeful introduction of foreign atoms at low concentrations) can alter the density of point defects and shift the Fermi level, enhancing conductivity or manipulating light absorption. Thermal treatments in specific atmospheres (oxidizing, reducing, or inert) can trigger the formation or healing of vacancies. Reducing the particle size from bulk to nano increases surface area, thus magnifying the importance of surface and quantum defects. Adjusting the stoichiometry–such as subtly changing the ratio of metal ions to oxygen–can foster oxygen vacancies, which in turn strongly affect the electronic behavior. Physical processes like electron/light irradiation or plasma treatments can also create or repair defects in targeted regions, offering further avenues for fine-tuning the material’s properties. Understanding the nature and control of these defects is vital for designing semiconductors that meet the specific demands of emerging technologies (see Figure ).
4.
Synthetic parameters for the synthesis of silver-based bimetallic semiconductors.
These conditions profoundly impact material defects and, consequently, surface engineering outcomes. Structural surface defects, modulated by factors such as agitation, microwave power, or ultrasound intensity, create surfaces with tailored chemical and physical properties. For instance, stabilizing surfaces with quantum clusters offers distinct active sites for chemical reactions, modifying material behavior. Additionally, controlling the degree of order and disorder–reflected in the type and density of defects–affects electronic properties such as charge mobility and optical response, enabling precise tuning of the functional characteristics of semiconductors.
Ag-based bimetallic semiconductors are commonly synthesized through bottom-up processes, offering remarkable flexibility in adjusting reaction parameters to fine-tune material properties (see Table ). Techniques such as coprecipitation, solvothermal, microwave-assisted, and sonochemical methods are widely utilized, each providing unique advantages for controlling defect density and morphology. For example, in coprecipitation methods, the mode of reagent addition (e.g., dripping, pouring, or injection) significantly influences the nucleation rate and crystal growth, thereby modulating the structural order or disorder of the synthesized materials. Our research group successfully altered the typical hexagonal rod-like morphology of α-Ag2WO4 into parallelepipeds by modifying the coprecipitation process through the injection of AgNO3 in a solution of DMSO containing the former lattice. Similarly, Liu et al. synthesized one-dimensional α-Ag2WO4 nanowires by introducing AgNO3 during a coprecipitation process, followed by microwave-assisted hydrothermal treatment and in situ nucleation of Ag nanoparticles. The resulting Ag/Ag2WO4 composites exhibited enhanced photocatalytic activity, which was attributed to a combination of factors: the surface plasmon resonance (SPR) effect of Ag, bandgap narrowing, and the formation of a Schottky barrier at the Ag/semiconductor interface. Together, these mechanisms contribute to improved light absorption across a broader spectral range and more efficient generation and separation of photogenerated charge carriers. We demonstrated that the morphology of Ag2CrO4 could be manipulated by adjusting the rate of lattice modifier addition during coprecipitation, transitioning from rapid direct addition (25 mL/s) to a controlled dripping process (10 mL/min). This modulation not only influenced particle size and surface structure but also played a decisive role in the type and distribution of structural defects, particularly oxygen vacancies and shallow trap states, which in turn directly impacted the material’s PL behavior and electronic properties.
1. Summary of Different Synthesis Methods for Ag-Based Semiconductors .
| material | synthetic method | solvent | temperature (°C) | additive | morphology | defect analysis | application | ref |
|---|---|---|---|---|---|---|---|---|
| β-Ag2MoO4 | coprecipitation | water | 90 | polyhedral-like | photocatalysis of RhB | |||
| sonochemical | water | 45 | potatoes-like | |||||
| hot solution ion injection with fast cooling | water | 90 | irregular shape | |||||
| conventional hydrothermal | water | 90 | irregular polyhedrons | |||||
| α-Ag2WO4 | coprecipitation with fast injection | water and DMSO | 120 | hexagonal rod-like | ||||
| α-Ag2WO4 | conventional hydrothermal | water | 150 | nanowires | XPS | photocatalysis of MB | ||
| Ag2CrO4 | coprecipitation | water | 90 | polyhedral-like | PL | |||
| α-Ag2WO4 | sonochemical followed by conventional hydrothermal | water | 140 | hexagonal rod-like | antimicrobial | |||
| Ag2CrO4 | microemulsion | cyclohexane/n-hexanol | RT | Triton X-100 | spheres | photocatalysis of MB | ||
| coprecipitation | water | RT | irregular shape | |||||
| conventional hydrothermal | water | 160 | irregular shape | |||||
| α-Ag2WO4 | coprecipitation | water | 10–90 | PVP | hexagonal rod-like and parallelepiped-like | XPS | photocatalysis of MB and MO | |
| α-Ag2WO4 | coprecipitation | water | 30–70 | PVP | hexagonal rod-like and parallelepiped-like | XPS | antimicrobial | |
| α-Ag2WO4 | microwave hydrothermal | water | 100–160 | SDS | hexagonal rod-like | antimicrobial and photocatalysis of RhB and Rh6G | ||
| β-Ag2MoO4 | conventional hydrothermal | water | 100–160 | potatoes-like and coral-like | photocatalysis of RhB | |||
| β-Ag2MoO4 | coprecipitation | water | 450–525 | NaOH | potatoes-like | XPS | NH3 gas sensor | |
| α-Ag2WO4 | microwave hydrothermal | water | 140 | hexagonal rod-like | PL | antimicrobial and photocatalysis of RhB | ||
| Ag2CrO4 | conventional hydrothermal | water | 350 | NaOH | irregular shape | photocatalysis of MB and MO | ||
| β-Ag2MoO4 | coprecipitation | DEG or DMSO/H2O | 0–80 | spheres, truncated octahedra, cubes, cuboctahedra | photocatalysis of RhB | |||
| α-, β-, and γ-Ag2WO4 | coprecipitation | water | 25 | hexagonal rod-like, rod-like, and Polyhedral-like | PL and XPS | antimicrobial and photocatalysis of RhB | ||
| α-Ag2WO4 | coprecipitation | water | 90 | SDS | hexagonal rod-like and cubes | PL | photocatalysis of RhB | |
| α-Ag2WO4 | sonochemical | water | 25 | citric acid, tartaric acid, and benzoic acid | rod-like | PL and XPS | photocatalysis of RhB | |
| β-Ag2MoO4 | coprecipitation | PMAA | 80 | hollow spheres | photocatalysis of MO | |||
| γ-Ag2WO4 | coprecipitation | water | RT | PVP | rod-like and Polyhedral-like | photocatalysis of mb | ||
| β-Ag2MoO4 | microwave hydrothermal | water | 180 | PVP | cube | PL and XPS | photocatalysis of RhB | |
| α- and β-Ag2MoO4 | coprecipitation | water | PVP | butterfly like | photocatalysis of MB | |||
| β-Ag2MoO4 | microwave hydrothermal | water | 120–150 | NH4OH | polyhedral-like and octahedral rod | PL | ||
| Ag2CrO4 | coprecipitation | water | 30–90 | NH4OH | polyhedral-like | PL | antimicrobial and Photocatalysis of RhB | |
| β-Ag2MoO4 | microwave hydrothermal | water | 150 | PVP | coral-like | antimicrobial and Photocatalysis of RhB | ||
| microwave hydrothermal | ethanol | cube | ||||||
| conventional hydrothermal | water | KOH | coral-like | |||||
| α-Ag2WO4 | coprecipitation | water or ethanol | 90 | NH4OH | hexagonal rod-like | PL | antimicrobial | |
| β-Ag2MoO4 | coprecipitation | water or ethanol | 90 | NH4OH | polyhedral-like | PL | antimicrobial | |
| β-Ag2MoO4 | coprecipitation | water or ethanol | 90 | NH4OH | polyhedral-like | XPS | antimicrobial | |
| β-Ag2MoO4 | coprecipitation | water, methanol, ethanol, propanol, or 1-butanol | 60 | irregular shape | ||||
| α- and β-Ag2WO4 | coprecipitation | water | 60 | rod-like | PL | photocatalysis of reactive orange 86 | ||
| β-Ag2MoO4 | coprecipitation | water | RT | PVP | cubes, octahedral, and rods | |||
| Ag2CrO4 | microwave hydrothermal | water | 180 | irregular shape | photocatalysis of PCP-Na | |||
| α-Ag2WO4 | sonochemical | water | 70 | citric acid | nano rod | antimicrobial | ||
| α-Ag2WO4 | coprecipitation | water | 70 | hexagonal rod-like | cytotoxicity | |||
| coprecipitation | SDS | cube | ||||||
| sonochemical | citric acid | nano rod |
RT = room temperature; RhB = rhodamine B; MO = methyl orange; MB = methylene blue.
In solvothermal and microwave-assisted synthesis, parameters such as applied power, agitation, and reaction time significantly impact defect density and distribution. Microwave-assisted methods, for instance, provide rapid and uniform heating, promoting homogeneous structures through repeated dissolution and recrystallization processes. Lower microwave power conditions, however, may result in higher defect densities. Sonochemical methods also offer precise defect engineering capabilities, as the intensity of ultrasonic power determines the cavitation effects that create specific microstructural defects under localized high-energy conditions. Nobre et al. synthesized α-Ag2WO4 using the sonochemical method alone and in combination with a conventional hydrothermal process. They observed that combining these methods induced morphological changes, enhancing the material’s antimicrobial efficiency. MIC values confirmed the higher susceptibility of E. coli, with values as low as 31.25 μg/mL, and 250 μg/mL for MRSA, both obtained from the sample synthesized by the sonochemical method followed by 12 h of conventional hydrothermal treatment. Similarly, Lopes et al. studied the synthesis of β-Ag2MoO4 using coprecipitation, sonochemical, hot-solution ion injection with rapid cooling, and conventional hydrothermal methods. They found that each synthesis route led to distinct morphologies and stabilized surfaces, affecting the types of surface quantum clusters formed. Notably, the β-Ag2MoO4 microcrystals synthesized by the coprecipitation method achieved the highest photocatalytic efficiency, reaching a discoloration rate of 85.12% under UV–C irradiation after 240 min. This enhanced performance was strongly associated with the stabilization of high-energy (112) facets, the formation of [AgO5·xVO] clusters, and the resulting increase in active sites for radical generation. Particle size was also significantly influenced by the choice of synthesis method. For instance, Xu et al. explored the synthesis of Ag2CrO4 using microemulsion, coprecipitation, and conventional hydrothermal processes. The microemulsion method yielded the smallest particles (∼30 nm), which increased the material’s surface area and enhanced photocatalytic efficiency for methylene blue degradation under visible light, reaching a kinetic rate constant (k) of 0.033 min–1. These findings underscore how different synthetic strategies can modulate defect density, morphology, and surface properties, ultimately tailoring the performance of Ag-based bimetallic semiconductors for specific applications.
When discussing the synthesis of Ag-based bimetallic semiconductors, techniques such as coprecipitation, solvothermal, microwave-assisted, and sonochemical methods are employed as previously observed. Coprecipitation is widely recognized for its simplicity, low cost, and ease of scale-up, although it may offer limited control over particle size and morphology. Solvothermal synthesis, on the other hand, allows for better crystallinity and morphological control, but often requires high temperatures, long reaction times, and organic solvents, which may raise environmental concerns. , Microwave-assisted and sonochemical methods have attracted attention due to their ability to significantly reduce reaction time and improve energy efficiency. − The sonochemical route, in particular, benefits from the generation of localized high temperatures and pressures through acoustic cavitation, leading to the formation of nanostructured materials with unique properties. However, both microwave and sonochemical approaches still face challenges regarding scalability and uniformity in large-scale production. In practical terms, cost, efficiency, and environmental impact are strongly dependent on the degree of morphological control required for a specific application, making it difficult to define a single ″best″ method. Instead, the selection of the synthesis route should consider not only the targeted physicochemical features of the material but also the feasibility of scaling up the process while maintaining performance and sustainability.
Beyond particle formation and morphological control, these synthesis methods also play a critical role in the modulation of defect density and distribution, which are central to surface and defect engineering strategies in Ag-based semiconductors. The ability to tailor defects, such as vacancies, dislocations, and local distortions, through controlled variations in synthesis parameters has a direct impact on the material’s physicochemical behavior. Different synthetic methods can be tailored to influence structural order, defect distribution, and chemical composition. Moreover, even within a single synthesis route, parameters such as reaction time, temperature, pH, and the presence of surfactants or reductive/oxidative atmospheres can lead to markedly different outcomes in terms of defect formation, crystallinity, and morphology, ultimately modulating the material’s physicochemical behavior. As a consequence, it becomes possible to strategically introduce energy states associated with these defects, tuning the electronic structure and enhancing functional properties relevant to catalytic, electronic, and optical applications.
For example, varying the coprecipitation temperature for synthesizing α-Ag2WO4 with polyvinylpyrrolidone (PVP) as a surfactant directly impacts the material’s morphology. , Higher temperatures promote the longitudinal growth of nanorods, reduce their cross-sectional areas, and introduce surface irregularities, thereby increasing the material’s surface area. The sample synthesized at 30 °C was more effective for methylene blue degradation, whereas the one obtained at 90 °C showed superior photocatalytic performance for methyl orange degradation. These changes influence the ROS generation, leading to modified photocatalytic responses. Similarly, increasing the temperature in the microwave-assisted hydrothermal synthesis of α-Ag2WO4 reduces particle size, enhancing photocatalytic efficiency for rhodamine B and rhodamine 6G degradation, and the antibacterial activity of the samples against E. coli. Our research group demonstrated that synthesizing β-Ag2MoO4 at different temperatures (100–160 °C) via microwave-assisted hydrothermal methods alters the morphology of particles from potato-like to coral-like structures, significantly improving photocatalytic activity against RhB, with the sample synthesized at 120 °C being the most effective, reaching a k of 0.0257 min–1, likely due to the participation of crystallite defects, deformations in [AgO6] and [MoO4] clusters, and the presence of oxygen vacancies throughout the crystal lattice. Furthermore, calcination temperature also plays a pivotal role, as evidenced by Wang et al., who observed that β-Ag2MoO4 calcined at 500 °C exhibited superior ammonia detection performance compared to samples calcined at other temperatures.
In addition to temperature, reaction time and pressure also influence defect density and material properties. Prolonged reaction times often yield more ordered materials, while shorter durations can introduce disorder and heterogeneity. High-pressure conditions suppress bulk defect formation, whereas ambient or subatmospheric pressures favor the creation of less dense structures rich in vacancies. For instance, our research group demonstrated that longer irradiation times during microwave-assisted synthesis α-Ag2WO4 increase the dimensions of hexagonal rods and reduce defect densities, as indicated by the decrease of the PL emission. These subtle differences among the (001), (010), and (101) surfaces illustrate the strong influence of surface type on material reactivity (see Figure ). By controlling the combination of these surface terminations within the overall morphology, it is possible to finely tune the distribution and accessibility of active sites, an effect reflected in the enhanced photocatalytic degradation of rhodamine B and antimicrobial performance, particularly in samples synthesized within just 4 min, which exhibited the most effective behavior. In addition, we investigated β-Ag2MoO4:Eu materials and observed that longer microwave irradiation times led to a gradual reduction in defect density, as evidenced by decreased PL intensity and supported by PALS, which revealed a systematic decrease in positron lifetimes (τ1 and τ2) and an increase in I2, indicating a reduction in defect size and a redistribution in defect types at the nanoscale. Similarly, Miclau et al. synthesized Ag2CrO4 under pressures ranging from 400 to 800 bar, observing a morphological transition from irregular polyhedral to rod-like structures at higher pressures. This transformation directly improved photocatalytic activity for the degradation of methyl orange and methylene blue dyes.
5.

Polyhedron energy profiles and the morphologies for the α-Ag2WO4 samples synthesized by the CP method followed by microwave irradiation for different times. Reprinted with permission from ref . Copyright (2020) Springer Nature.
Beyond physical parameters, chemical factors also play a critical role in controlling defect density in materials. Chemical variables in the synthesis process, such as the use of surfactants (anionic or cationic), reagent ratios (stoichiometric or nonstoichiometric), solvent selection (altering polarity), pH control, and doping with heteroatoms, offer numerous possibilities for modifications during synthesis, enabling precise adjustments to the structural and consequently electronic properties of these materials. These chemical synthesis parameters complement the physical conditions of synthesis, such as temperature, pressure, and reaction time, resulting in a multifaceted approach to defect engineering. For instance, Warmuth et al. demonstrated that simultaneous variation of temperature (0–80 °C) and solvent composition (ethylene glycol and DMSO–water mixtures) can significantly influence the morphology of β-Ag2MoO4, yielding structures such as spheres, cubes, octahedra, and truncated octahedra depending on the specific conditions applied. Among these, the truncated octahedra β-Ag2MoO4 particles exhibited the highest photocatalytic activity under simulated sunlight, likely due to the predominance of (111) crystal facets, which were shown to be more catalytically active than the (100) facets.
Surfactants play a crucial role in modifying surface and bulk defects by selectively interacting with specific crystal facets during the synthesis process. Acting as capping agents, they stabilize high-energy surfaces, influencing nucleation and growth processes. This interaction often results in the formation of surface vacancies, steps, and other defects that are beneficial for catalytic and adsorption applications. The type of surfactant, cationic, anionic, or nonionic, further modulates electrostatic and steric interactions, thereby affecting the density, nature, and distribution of defects.
Our research group synthesized α-Ag2WO4 via coprecipitation with and without the anionic surfactant sodium dodecyl sulfate (SDS). The presence of the surfactant induced a morphological transformation from the typical hexagonal rod shape to a more defined cubic morphology, a change attributed to the preferential stabilization of specific crystallographic facets. SDS promotes the formation of anisotropic nanostructures by selectively adsorbing onto certain crystal surfaces, thereby directing facet-dependent growth during synthesis. This restructuring was accompanied by the emergence of less defect-rich quantum clusters, reducing the material’s photocatalytic activity. These findings reinforce that the presence of SDS not only guides the formation of the cuboid-like α-Ag2WO4 morphology through selective surface stabilization, particularly of the (100) and (001) facets, but also plays a critical role in reducing the density of under-coordinated clusters, which, although favorable for structural order, ultimately results in lower photocatalytic performance when compared to the defect-rich rod-like counterpart.
Similarly, we used carboxylic acids as surfactants to reduce particle size of α-Ag2WO4, enhancing RhB photodegradation efficiency. Carboxylic acids can act as both surfactants and ligands, introducing oxygen-rich functional groups that affect the chemical environment around defect sites. This effect is closely linked to the dynamic interplay between the formation of the aqueous Ag complex and the chelation process with the carboxylate groups. The competition between Ag–O (from hydration) and Ag–carboxylic acid bonds determine the availability and controlled release of Ag+ ions during synthesis. As a result, carboxylic acids act as templating agents, directing particle nucleation and growth. The stability of the chelate complex formed during synthesis plays a key role: lower chelate stability leads to faster Ag+ release and larger particles, while more stable chelation results in smaller, well-defined nanostructures. These molecular-level interactions directly influence the final size and morphology of the as-synthesized α-Ag2WO4 samples.
Surfactants also stabilize metastable phases, as shown by Wang et al. with β-Ag2WO4 using poly(methacrylic acid) (PMAA), and Andrade Neto et al., who synthesized γ-Ag2WO4 using PVP. The use of PVP has been shown to induce significant morphological changes in β-Ag2MoO4 synthesized via microwave-assisted methods. Specifically, it transformed the morphology from potato-like to cubic, which was associated with improved photocatalytic activity, as confirmed by PL analyses. When PVP was employed in a coprecipitation method using dropwise addition, the resulting β-Ag2MoO4 particles exhibited a distinctive butterfly like morphology. PVP is a nonionic surfactant widely used to control particle size and shape due to its strong affinity for metal ions while PMAA is another anionic polymer capable of tuning surface charge and porosity through pH-responsive behavior. ,
In another study, our research group synthesized β-Ag2MoO4 at varying temperatures (120–150 °C) using a microwave-assisted hydrothermal method, with and without the complexation of Ag+ ions by NH3. NH3 serves a dual role, not only as a pH modifier but also as a ligand that complexes Ag+ ions, forming [Ag(NH3)2]+ species that influence the nucleation rate and final crystalline structure. While temperature alone did not significantly alter the materials, the inclusion of NH3 as a complexing agent had a profound effect on stabilizing the (111) surface. In contrast, when NH3 was not used, the (001) and (011) surfaces were stabilized. Additionally, the use of NH3 proved essential in modulating the density of oxygen vacancies within the material. Similar findings were reported by Assis et al., who explored the synthesis of Ag2CrO4 with and without Ag+ complexation by NH3. Their results underscored the pivotal role of both the complexing agent and temperature in controlling the material’s morphology (Figure ). This, in turn, enabled fine-tuning of its photocatalytic, antimicrobial, and cytotoxic activities. Notably, the samples synthesized with NH3 showed a preferential exposure of surfaces rich in undercoordinated Ag atoms and coordinated Cr clusters, such as the (011), (001), and (111) planes. These facets, characterized by lower Ag oxidation states and planar [AgO6]-derived geometries with minimal distortion, were strongly correlated with enhanced photocatalytic activity, highlighting the surface-dependent nature of the material’s performance.
6.
Theoretical and experimental morphologies obtained by tuning the values of E surf for Ag2CrO4. The FE-SEM images for each sample are inserted for comparison purposes. Surface energy value is in J/m2. Reprinted with permission from ref . Copyright (2021) Springer Nature.
Solvent selection significantly impacts defect formation by affecting reaction kinetics, diffusion rates, and crystal growth. Polar solvents typically enhance ion dissociation, promoting uniform crystal growth and reducing defect density, whereas nonpolar solvents often slow reactions, leading to disordered or amorphous structures with higher defect densities. Our research group synthesized α-Ag2WO4 using water, ethanol, and ammonia as solvents via coprecipitation. Materials synthesized in water and ammonia exhibited flower-like morphologies composed of clustered hexagonal rods, while ethanol yielded dispersed rods with increased oxygen vacancy density. This morphological evolution, confirmed by FE-SEM imaging, is consistent with an oriented aggregation mechanism, where the nature of the solvent influences the self-organization and crystallographic alignment of growing particles. In water, the rapid reaction kinetics favor disordered nucleation, leading to more defective surfaces, while in ammonia, the formation of the [Ag(NH3)2]+ complex slows Ag+ release, allowing more controlled growth and surface definition. In contrast, the use of ethanol restricts particle interaction, promoting the formation of isolated nanorods. This increase in the oxygen vacancies were verified by red-shifted PL emissions, that can enhance the antimicrobial efficiency of the ethanol-synthesized sample.
Similar observations were made for β-Ag2MoO4, where ethanol stabilized the (001) surface, facilitating ROS production and improving photocatalytic efficiency. The use of different alcohols in the synthesis of β-Ag2MoO4 was also evaluated, revealing that increasing the chain length of the alcohol led to noticeable changes in particle size and morphology. Specifically, as the alcohol chain length increased, the morphology shifted from a complex polyhedral structure to the formation of rod-shaped particles. First-principles calculations combined with experimental analyses showed that this morphological evolution is closely linked to surface exposure, with a higher proportion of the (001) surface correlating with enhanced antibacterial activity, highlighting the critical role of crystal facet engineering in tuning the functional properties of β-Ag2MoO4.
Reagent ratios also play a critical role in defect engineering, directly impacting vacancy formation. For instance, the excess of metal precursors can enhance the formation of anion vacancies, while deficiencies may lead to cation vacancies. For metastable phases of Ag2WO4 (β and γ), the molar ratios of reagents are crucial. Specifically, the stoichiometric ratio of AgNO3 to Na2WO4 governs the formation of distinct cluster species, such as [AgO6] and [WO4] for γ-Ag2WO4 at higher concentrations, and [AgO x ] (x = 2, 4, 6, 7) and [WO y ] (y = 4, 5) for α- and β-Ag2WO4 at lower concentrations. These variations directly influence the symmetry, order, and morphology of the resulting polymorphs, illustrating the complex interplay between precursor concentration, cluster formation, and crystallization dynamics. Chen and Xu achieved α and β phases through coprecipitation, using stoichiometric amounts for the α phase and a 1:5 Ag/W ratio for the β phase.
The pH of the reaction medium is also a fundamental variable in the synthetic chemical environment and, consequently, in the formation of defects in these materials. Under more acidic conditions, the formation of these materials can be hindered, leading to an increase in defect density and the generation of vacancies. Conversely, in basic conditions, the formation of these materials is often favored, resulting in the development of crystalline phases with a higher degree of order. Thus, even minimal pH adjustments can modify these materials, and this level of control can be valuable for designing materials tailored for specific applications. Della Rocca et al. conducted a statistical factorial design study to synthesize Ag2MoO4, exploring the effects of surfactant (PVP) addition combined with pH variation in the reaction medium. Their results demonstrated that pH plays a crucial role in stabilizing the α phase over the β phase of Ag2MoO4. Furthermore, these pH adjustments influence the formation of other phases, leading to the production of Ag2O under basic conditions and MoO3 in acidic environments. Similarly, pH is a decisive factor in the synthesis of Ag2CrO4. When the pH deviates significantly from 9.5 - whether more acidic or basic - it stabilizes alternative phases of Ag chromates (e.g., AgCrO2) or Ag oxides, demonstrating the profound impact of pH on phase stability and material properties.
Doping
Doping is one of the most precise and targeted strategies for defect engineering, enabling atomic-level modifications at the crystal lattice. By introducing foreign ions into the structure, substitutional or interstitial defects are created, along with localized distortions that significantly alter the material’s electronic and optical properties (see Table ). For example, doping these materials with transition metals can introduce new electronic states within the bandgap, enhancing properties such as conductivity, photoluminescence, and photocatalytic activity. However, the choice of dopant and its concentration must be carefully optimized to avoid clustering or phase segregation, which can diminish the desired effects.
2. Summary of Different Dopings in Ag-Based Semiconductors .
| material | synthetic method | dopant | concentration (%) | morphology | defect analysis | application | ref |
|---|---|---|---|---|---|---|---|
| α-Ag2WO4 | microwave hydrothermal | Ni2+ | 0–8 | hexagonal rod-like and hexagons | PL | ||
| α-Ag2WO4 | microwave hydrothermal | Mo6+ | 25 | hexagonal rod-like | PL | photocatalysis of RhB | |
| α-Ag2WO4 | coprecipitation | Cu2+ | 0–16 | hexagonal rod-like | PL and XPS | antimicrobial | |
| α-Ag2WO4 | coprecipitation | Zn2+ | 0–25 | hexagonal rod-like | PL | ||
| α-Ag2WO4 | coprecipitation | V4+/5+ | 0–4 | hexagonal rod-like and parallelepiped-like | PL, XPS, and EPR | sulfide Oxidation | |
| β- and α-Ag2WO4 | coprecipitation | Eu3+ | 2 | PL | |||
| α-Ag2WO4 | coprecipitation | Eu3+/Li+ | 1–8 | hexagonal rod-like | PL | ||
| α-Ag2WO4 | coprecipitation | Pr3+, Sm3+, Eu3+, Tb3+, Dy3+ and Tm3+ | 1 | hexagonal rod-like | PL | ||
| β-Ag2WO4 | coprecipitation | S2+, P3+, and B3+ | 20 | hexagonal rod-like and irregular shape | XPS | photocatalysis of carmine dye | |
| β-Ag2MoO4 | coprecipitation | Eu3+ | 0–1 | potato-like and cubes | PL | ||
| β-Ag2MoO4 | microwave hydrothermal | Eu3+ | 1 | cubes | PL and PALS | ||
| β-Ag2MoO4 | coprecipitation | Dy3+ | 0–2.5 | PL and PALS | |||
| Ag2CrO4 | coprecipitation | Eu3+ | 0–1 | irregular shape | PL and XPS | photocatalysis of 4-NP and ciprofloxacin | |
| Ag2CrO4 | coprecipitation | Zn2+ | 1–4 | irregular shape | PL and XPS | photocatalysis of RhB and antimicrobial |
RhB = rhodamine B; 4-NP = 4-nitrophenol.
The doping process involving transition metals like Mo, Ni, Zn, and Cu has been shown to drastically influence the oxygen vacancy density in α-Ag2WO4, while also inducing morphology changes that depend on dopant concentration. − Increased defect density correlates strongly with the addition of dopant clusters, which modify the electronic densities in both the bulk and the surface of the material. This delicate balance between order and disorder introduced by doping is a key factor in altering the photocatalytic responses of these materials. Pereira et al. doped α-Ag2WO4 with up to 8% Ni2+ and observed that increasing Ni2+ concentration led to a reduction in the size of the hexagonal rod-like morphology. This morphological change was attributed to the stabilization of the surface energy of the (001) facet, which initially suppresses crystal growth along the (110) direction, followed by destabilization of the (010) surface. In this context, Ni2+ plays a dual role by decreasing the concentration of oxygen vacancies and reducing the overall defect density of the material. When Mo6+ is used as a dopant in α-Ag2WO4, the microwave-assisted hydrothermal synthesis time plays a key role in determining the type of defects formed in the material. Shorter synthesis times are associated with a higher concentration of deep-level defects, while longer durations tend to promote the formation of shallow defects. These defect profiles directly influence the photocatalytic performance, with deep-level defects, often linked to electronic states near the conduction and valence bands, enhancing the generation of ROS. On the other hand, doping α-Ag2WO4 with Cu2+ increases the material’s defect density, which results in a loss of morphological definition. However, this structural disorder contributes to enhanced antimicrobial activity, significantly reducing the minimum inhibitory concentration (MIC) from 500 to 31.25 μg/mL for MRSA and from 125 to 15.62 μg/mL for C. albicans. A similar result was observed for Zn2+ doping, where the loss of morphological definition was accompanied by an increase in oxygen vacancy density (Figure ).
7.

Schematic illustration of the proposed growth mechanism leading to the formation of α-Ag2–2x Zn x WO4 (0 ≤ x ≤ 0.25) microcrystals. Reprinted with permission from ref . Copyright (2017) American Chemical Society.
For instance, Gupta et al. observed that high concentrations of Eu3+ ions could restructure the crystal lattice of α-Ag2WO4, leading to the formation of the β phase when synthesized via coprecipitation. This aliovalent doping strategy not only induced an orthorhombic-to-hexagonal phase transition at room temperature, but also significantly influenced the optical properties of the resulting material. These findings demonstrate that defect engineering through rare-earth doping can not only modulate the structural phase but also tailor the electronic and optical responses of Ag-based semiconductors, potentially extending their functionality to applications. On the other hand, our research group reported that lower concentrations of Eu3+ and Li+ ions increased the defect density in α-Ag2WO4, enhancing its optical properties. The presence of Li+ as a codopant facilitated a more homogeneous incorporation of Eu3+ into the lattice with minimal structural distortion, relieving internal strain and enabling more efficient red-light emission from Eu3+ centers. Rare-earth ions (Pr3+, Sm3+, Eu3+, Tb3+, Dy3+ and Tm3+) at concentrations below 1% have also been effective in altering the PL emission characteristics of α-Ag2WO4, owing to their unique emission signatures.
Nonmetallic dopants such as S, P, and B have also proven highly effective in tuning the crystallinity, phase composition, and defect density of β-Ag2WO4, leading to significant enhancements in photocatalytic activity. Using a CTAB-assisted precipitation method, S-, P-, and B-doped Ag2WO4 materials were synthesized with controlled nanostructures and increased surface hydroxylation. Among these, S-doped Ag2WO4 exhibited a notably high defect density and amorphous character, particularly in the β-phase, which was linked to superior photocatalytic and adsorption performance. The presence of mesoporosity and N–S bonds, combined with an optimized β-/α-phase ratio, enabled rapid dye adsorption and efficient charge separation. These results reinforce that nonmetallic doping, when combined with surface-active agents, not only modulates defect structures but also enhances the functional performance of Ag-based semiconductors.
We prepared β-Ag2WO4 samples doped with Eu3+ in concentrations ranging from 0.25% to 1.00% and observed a progressive morphological transformation from irregular, potato-like structures to well-defined cubic particles. This shift is associated with the incorporation of [EuO6] distorted octahedra, which locally disrupt the lattice symmetry and induce rearrangements in electronic density. Morphological analyses further revealed that increasing Eu3+ content promoted the formation of surface defects, while simultaneously leading to more geometrically defined particles at higher doping levels, highlighting the dual effect of Eu3+ in both defect modulation and morphology control.
For Ag2CrO4, Eu3+ doping proved to be a highly effective strategy for enhancing photocatalytic performance, particularly in the degradation of antibiotics and pesticides. Even at low concentrations (0.25%), Eu3+ incorporation modified the oxygen vacancy density and introduced localized structural distortions, as confirmed by XPS and PL analyses. These changes disrupted the short-range symmetry of the lattice, leading to alterations in particle size, morphology, and electronic configuration. Notably, the doped material exhibited reduced charge recombination and improved catalytic efficiency, underscoring the pivotal role of Eu3+ in tailoring both structural and functional properties. Zn2+ doping in Ag2CrO4 led to notable improvements in photocatalytic, antibacterial, and antifungal activities, primarily through structural reorganization rather than modification of oxygen vacancy-related defects. The introduction of Zn2+ ions reduced intrinsic disorder and induced changes in particle size and morphology, resulting in enhanced RhB degradation under visible light. These effects became more pronounced with increasing Zn2+ content, reaching optimal performance at 2% doping. Both experimental data and DFT-based theoretical calculations revealed that Zn2+ doping modulates the electronic and structural order–disorder balance and alters surface energies, highlighting it as an effective strategy to tune material functionality.
The doping process can significantly influence the morphology, stability, and surface reactivity of synthesized materials by promoting the formation of exposed surfaces composed by mor active sites. When combined with key physical parameters, such as temperature, pressure, and reaction time, and chemical variables including pH, precursor concentration, and the presence of surfactants or complexing agents, doping becomes a powerful tool for tailoring defect structures. Despite its potential, this multifactorial approach is often underexplored. A deeper understanding of the interplay between doping and synthesis conditions enables more rational material design, where the degree of order/disorder and defect distribution can be precisely modulated to enhance the functional performance of the final material.
Postsynthesis Modifications
Postsynthesis modifications can also be useful in altering the structural and electronic properties of these materials, changing their defect densities and electronic characteristics. Techniques such as electron beam irradiation (EBI) and light beam irradiation have proven effective in modulating these properties (see Table ). EBI can induce drastic changes in the electronic structure of the material, which are not intrinsic to the irradiation technique itself but are particularly dependent on the nature of the modified material , Meanwhile, laser or light irradiation can promote atomic migration, surface modifications, or even phase transitions. , These methods open up a wide range of possibilities for structural and electronic alterations in these materials, enabling the creation of new functional materials with enhanced performance in various applications.
3. Summary of Different Post-synthesis Modifications in Ag-Based Semiconductors .
| material | synthetic method | postsynthesis modification | parameters | defect analysis | application | ref |
|---|---|---|---|---|---|---|
| α-Ag2WO4 | coprecipitation | EBI | 200 kV | XPS | ||
| α-Ag2WO4 | microwave hydrothermal | EBI | 30 kV/30 min | antimicrobial | ||
| α-Ag2WO4 | coprecipitation | EBI | 20 kV/20 min | |||
| β-Ag2MoO4 | coprecipitation | EBI | 5 kV/5 min | |||
| β-Ag2MoO4 | coprecipitation | EBI | 5–20 kV/5 min | PL | ||
| β-Ag2MoO4 | coprecipitation | EBI | 30 kV/6 min | |||
| α-Ag2WO4 | coprecipitation | EBI | 15 kV/5 min | antimicrobial and antitumor | ||
| femtosecond laser irradiation | 800 nm/30 fs/1 kHz/200 mW | |||||
| α-Ag2WO4 | microwave hydrothermal | EBI | 30 kV/2 min | PL and XPS | ||
| femtosecond laser irradiation | 800 nm/30 fs/1 kHz/200 mW | |||||
| α-Ag2WO4 | coprecipitation | EBI | 15 kV/5 min | PL, XPS and Magnetic analyses | ||
| femtosecond laser irradiation | 780 nm/150 fs/1 kHz/200 mW | |||||
| α-Ag2WO4 | coprecipitation | EBI | 30 kV/30 min | antimicrobial | ||
| femtosecond laser irradiation | 800 nm/30 fs/1 kHz/200 mW | |||||
| α-Ag2WO4 | coprecipitation | femtosecond laser irradiation | 800 nm/30 fs/1 kHz/200 mW | antimicrobial | ||
| α-Ag2WO4 | commercial | laser irradiation | 532 nm/10 ns/50 kHz/150 mJ | PL and XPS | photocatalysis of MO |
MO = methyl orange.
According to results reported in the literature, particularly by our research group, Ag clusters in Ag-based bimetallic semiconductors are particularly susceptible to the EBI process. This process induces an increase in Ag–O bond distances within the clusters, resulting in the migration of Ag atoms from the internal structure to the surface of the material. Our research group demonstrated this effect by modifying α-Ag2WO4, synthesized via a microwave method using PVP as a surfactant, through EBI using a scanning electron microscope operating at 30 kV for 30 min. This modification led to the formation of Ag nanoparticles decorating the surface of the α- Ag2WO4 particles. The process involved the internal reduction of Ag ions within the material’s framework, causing an electronic restructuring that significantly enhanced its antimicrobial efficiency. Specifically, it reduced the minimum inhibitory concentration against methicillin-resistant S. aureus (MRSA) by a factor of 5. Similar results regarding electron irradiation-induced modifications were reported for the metastable β-Ag2WO4 phase, complemented by theoretical calculations and modeling. Comparable findings were also observed both experimentally and theoretically for Ag2CrO4, further demonstrating the transformative potential of electron irradiation in tailoring the properties of Ag-based materials.
The modification of Ag-based bimetallic semiconductors using light or laser irradiation can significantly alter their structural, electronic, and optical properties by inducing localized energy absorption and defect generation. These effects are primarily due to the interaction of high-energy photons with the material, which can trigger different processes. For instance, laser irradiation can create or heal defects by rearranging atoms within the crystal lattice, leading to changes in oxygen vacancy density or the formation of metallic nanoparticles on the surface due to localized reduction. This is particularly relevant in Ag-based bimetallic semiconductors, where Ag clusters are highly responsive to photon-induced energy. Lin et al. modified commercial α-Ag2WO4 using a continuous 532 nm laser in solution, successfully reducing its bandgap from 3.22 to 2.78 eV. This electronic restructuring was attributed to distortions in the [WO6] clusters, leading to enhanced photocatalytic activity for methyl orange degradation and improved hydrogen generation efficiency.
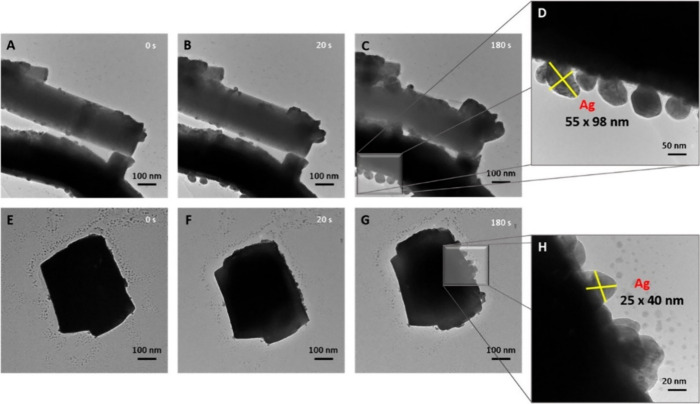
In a previous work, it was observed that femtosecond pulsed laser modification was more effective than electron irradiation for producing highly efficient antimicrobial materials. The localized yet highly energetic effects of femtosecond pulsed lasers (800 nm) caused significant electronic and structural disruptions, making the material up to 64 times more effective against fungi (Candida albicans) compared to the unmodified material, and four times more effective than the material modified with electron irradiation. Similar results were reported by Macedo et al., who used these two techniques to modify different morphologies of α-Ag2WO4, achieving antimicrobial efficiencies against methicillin-resistant S. aureus that were 64 times higher than those of unmodified materials (Figure ). Moreover, these modifications have shown potential for selective interactions with cancer cells (MB49) over healthy cells (BALB3T3), offering a promising strategy for the development of novel materials with targeted biomedical applications. In summary, electron and/or laser irradiation provide a facile in situ synthesis of Ag nanoclusters for promoting highly reactive based bimetallic semiconductors with multifunctional properties and then applications.
8.
TEM images of Ag NPs/α-Ag2WO4 composites within a controlled time of exposure to a 200 kV electron beam. (A–D) Ag NPs/α-Ag2WO4-RE and (E–H) Ag NPs/α-Ag2WO4–CE samples. (A, E) t = 0 s; (B, F) t = 20 s; (C, G) t = 180 s; (D, H) higher magnifications of the nanoparticles after 180 s. Reprinted with permission from ref . Copyright (2019) American Chemical Society.
Analysis of Defects
The analysis of defects in these materials is crucial, as they significantly influence their electronic, optical, and catalytic properties. Various methods are available to study these defects, with PL spectroscopy being one of the most fundamental techniques. This technique can provide insights into the electronic structure of materials through intracluster interactions, particularly by analyzing the emission spectra they produce. , Briefly, when a semiconductor material is excited with a light source (light or laser), electrons are promoted from the VB to the CB with energy above the bandgap value. Through decay processes, these electrons can emit radiation at specific wavelengths. The broad-band theory offers an interpretation of PL emission spectra in terms of defect energy levels within the forbidden region, assessing the medium-range structural order/disorder. According to this theory, as proposed in the works of Longo et al., higher-intensity emissions in the higher-energy regions of the electromagnetic spectrum (toward the left) correspond to shallow defects. These shallow defects are characterized by energy levels located near the VB and CB and are often associated with the intrinsic structural defects of the material. Conversely, emissions in lower-energy regions (toward the right) correspond to deep defects, which are located further within the forbidden region of the bandgap. Deep defects are frequently associated with oxygen vacancies, and many of these defects may already be occupied by pre-excited electrons, making them distinct from shallow structural defects. , In semiconductors, differentiating between shallow and deep defects is critical for understanding medium-range order, as shallow defects can directly influence charge transport, while deep defects can affect electron recombination processes in these materials. Additionally, another important aspect is analyzing the emission intensity of these spectra. Reductions in emission intensity are directly associated with a higher degree of structural organization in the semiconductor, while an increase in intensity is linked to a higher defect density. This makes PL spectroscopy an essential tool for analyzing defects in advanced semiconductor materials. It is also worth noting that PL spectroscopy analyzes the material as a whole, without separating the bulk from the surface, whereas other techniques may have a greater surface-specific character compared to PL.
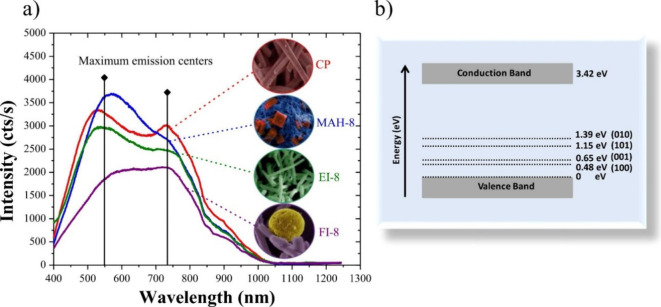
Our research group has been studying, through PL analysis, how synthetic modifications can alter the defect density of these semiconductors. In a previous study, it was observed that α-Ag2WO4 synthesized at lower temperatures (100 °C) exhibited a reduction in PL intensity, whereas samples synthesized at higher temperatures (160 °C) showed an increase in PL intensity. This result demonstrates that temperature can regulate defect density during the microwave-assisted hydrothermal synthesis process. In another work, Lin et al. demonstrated that commercial α-Ag2WO4 modified with a laser (λ = 532 nm) underwent drastic electronic changes, including a reduction in the bandgap (from 3.22 to 2.78 eV) accompanied by an increase in PL emission intensity, indicating an increase in defect density, particularly in the forbidden region of this material. On the other hand, the use of the surfactant SDS in the synthesis of α-Ag2WO4 drastically reduced the PL intensity, suggesting that SDS induces a significant reduction in defect density, accompanied by morphological and surface changes that are critical to the material’s activity. Teixeira et al. demonstrated that both EBI modification and femtosecond pulsed laser modification increased the density of oxygen vacancies and, consequently, the defect density in α-Ag2WO4, as shown by PL analysis (see Figure ). These findings highlight that the synthesis route is a critical factor in modulating the defect density of these materials, providing a valuable tool for tailoring their properties for specific applications.
9.
(a) PL spectra and maximum emission centers of the CP, MAH-8, EI-8, and FI-8 samples. (b) Comparative diagram of the band gap value of the optimized structure (3.42 eV) and band gap values for (100), (010), (001), and (101) surfaces. Reprinted with permission from ref . Copyright (2022) Elsevier.
Magnetic analyses as a function of applied field and temperature can also play a pivotal role in characterizing defects, particularly the vacancies created in these materials. These measurements are especially valuable because certain vacancies, such as oxygen vacancies, can exhibit magnetic properties, providing deeper insights into the electronic and structural changes within the material. Previous studies have shown that variations in local vacancy density are the primary factors influencing the induction of magnetism in α-Ag2WO4 modified bay electrons and laser. These findings align with temperature-dependent PL analyses, which offer complementary information about the electronic transitions and defect-related emissions. Magnetic measurements can therefore provide an additional layer of understanding by directly probing the magnetic contributions of defects and correlating them with structural and electronic characteristics. Despite these promising results, further studies are required to establish consistent trends and meaningful associations between vacancy density and magnetic behavior in these materials. By integrating magnetic analysis with PL spectroscopy and other characterization techniques, researchers can develop a more comprehensive understanding of defect dynamics and their impact on the functional properties of Ag-based bimetallic semiconductors.
X-ray photoelectron spectroscopy (XPS) can be a valuable tool for characterizing defects, particularly those related to surface oxygen vacancies, as its analysis typically penetrates between 1–10 nm, equivalent to a few atomic layers of the material. High-resolution spectra for the O 1s atom can be particularly useful for this analysis. The signal associated with O 1s can be deconvoluted into different components in these materials, primarily corresponding to three binding energy (BE) ranges. These components are associated with lattice oxygen (oxygen atoms constituting the clusters in these materials), oxygen vacancies, and adsorbed oxygen species on the surface (such as hydroxyl groups, adsorbed water, or chemisorbed oxygen). The asymmetry of the O 1s signal in these spectra arises from the combined presence of these different oxygen species occurring in varying proportions. This makes XPS a powerful technique for detecting and quantifying oxygen vacancies, as well as for understanding their contribution to the electronic structure and surface chemistry of the material. In a previous study by our group, small changes in oxygen vacancies were observed in α-Ag2WO4 when modified by EBI or laser irradiation. Ribeiro et al. also demonstrated in their work that the use of carboxylic acids in the synthesis of α-Ag2WO4 not only alters particle size but also significantly changes the density of oxygen vacancies in these materials, initially reducing them from 49.63% to as low as 28.39% when citric acid was used as a surfactant (Figure ). However, it is important to note that inconsistencies exist in the literature regarding the precise identification and quantification of oxygen vacancies using XPS in other semiconductors. Factors such as experimental conditions, data analysis approaches, and specific material properties can lead to misinterpretations of these spectra. Thus, it is of utmost importance to develop a solid and standardized set of methodologies to be applied to the analysis of oxygen vacancies in these and other semiconductors.
10.
High-resolution XPS spectra of the O 1s orbital of α-Ag2WO4 (A), α-Ag2WO4-tartaric acid (B), α-Ag2WO4-benzoic acid (C), and α-Ag2WO4-citric acid (D) samples. Reprinted with permission from ref . Copyright (2022) MDPI.
Electron paramagnetic resonance (EPR) is a highly effective technique for studying paramagnetic species, such as unpaired electrons associated with defects in semiconductor materials. Like PL spectroscopy, EPR analyzes the material as a whole, without differentiating between surface and bulk. By detecting distinct magnetic environments, EPR works by measuring the interaction of unpaired electrons with an external magnetic field, providing detailed insights into the electronic structure and defect dynamics of the material. What sets EPR apart from other methods is its unique ability to differentiate between different types of oxygen vacancies when a magnetic field is applied. Since oxygen vacancies can exist in neutral (VO), cationic (VO·), or doubly ionized (VO··) states, cationic vacancies, in particular, can be detected by EPR due to their unpaired electron. , Cationic oxygen vacancies produce characteristic signals in the range of g = 2.003–2.280, which are widely documented in studies of other semiconductor metal oxides. , This precise capability allows EPR to go beyond the mere detection of defects, enabling researchers to identify and characterize their specific types, offering a resolution that other techniques, such as PL and XPS, cannot achieve. In a study by Patrocínio et al., EPR was used to explore the impact of vanadium doping on α-Ag2WO4. In addition to clearly identifying the oxidation states of the constituent metals, the results revealed significant changes in the density of cationic oxygen vacancies (g = 2.260), with a higher density of these species found in the material doped with 1% vanadium (Figure ). These findings were consistent with PL and XPS data, confirming the complementary nature of the three methods.
11.

EPR spectra of the undoped (AW) and V-doped (AW1 V, AW2 V, and AW4 V) samples recorded at 120 K. (a) Magnetic field swept from 225 to 435 mT. (b) Magnetic field swept from 320 to 365 mT. The background spectrum (BKGD) was obtained by measuring an empty sample tube. Reprinted with permission from ref . Copyright (2023) Royal Society of Chemistry.
Positron annihilation lifetime spectroscopy (PALS) is one of the most sensitive and precise techniques for analyzing surface defects in materials. PALS works by detecting the interactions of positrons with the material’s structure. These positrons are annihilated based on the size, concentration, and type of defects in the material, making it an extremely useful technique not only for analyzing oxygen vacancies but also for measuring any voids within the material. This analysis is particularly useful for identifying subtle changes in defect characteristics, such as complex vacancy clusters and their surrounding chemical environments, which other techniques may not detect. PALS has a greater penetration depth than XPS, typically reaching up to several tens of nanometers, making it highly effective for surface analysis. The penetration depth depends on the positron energy and the density/porosity of the material being analyzed. Our group has demonstrated in several studies the use of PALS to analyze surface defects in Ag-based bimetallic materials (Figure ). In the case of α-Ag2WO4, the use of different solvents during synthesis (such as water and ethanol) resulted in a drastic alteration in the nature and size of the surface defects observed. Samples synthesized in water contained smaller defects, specifically Ag–O vacancy complexes, with an average size equivalent to a trivacancy, formed by two oxygen vacancies surrounding one Ag vacancy. On the other hand, samples synthesized in ethanol exhibited larger and more complex defects, primarily involving Ag vacancies. For this material, it was found that after the EBI process, these defects could be further modified, leading to an increase in the total defect concentration. This indicates that EBI promotes significant changes in the defect environment, increasing both the size and number of these defects. Macchi et al. also employed PALS to investigate how microwave irradiation time influences the size and type of defects in β-Ag2MoO4:Eu with a fixed doping concentration of 1%. Their results showed that as the microwave irradiation time increased, a reduction in the positron annihilation lifetime was observed, corresponding to a decrease in the size of these complex defects. This suggests that extended microwave irradiation promotes the stabilization of smaller defect structures, further highlighting the sensitivity of PALS in monitoring subtle defect transformations. Similarly, Sudarshan et al. analyzed the impact of Dy3+ doping on β-Ag2MoO4 obtained via the coprecipitation method at room temperature. Their results showed that the incorporation of Dy3+ ions generates cation vacancies and surface defects, as confirmed by PALS, which also provided evidence of Dy3+ stabilization at Ag+ sites.
12.
Positron wave function isodensities calculated for monovacancy states: (A) V O created between one [WO6] and one [AgO4] cluster; (B) V W created inside a [WO6] cluster; (C) V Ag formed inside a [AgO4] cluster; and (D) V Ag created within a [AgO2] cluster. The positron isodensities are represented by three surface contours, which correspond to 70, 50, and 30% of the maximum positron density. For the sake of clarity, only the participating clusters are shown in the figures. W atoms are represented in red, Ag atoms in blue, and O atoms in dark gray. Missing atoms related to the different monovacancies are labeled. Positron wave function isodensities calculated for vacancy complexes: (E) V Ag + V O divacancy created inside a [AgO2] cluster; (F) V Ag + 2V O trivacancy corresponding to the whole missing [AgO2] cluster; (G) V Ag + 2V O trivacancy formed inside a [AgO4] cluster; and (H) 2V Ag + 4V O hexavacancy created between a [AgO2] cluster and a neighboring [AgO7] cluster. The positron isodensities are represented by three surface contours, which correspond to 70, 50, and 30% of the maximum positron density within the contour. For the sake of clarity, only the participating clusters are shown in the figures. Ag atoms are shown in blue and O atoms in dark gray. Missing atoms related to the different monovacancies forming the vacancy complexes are labeled. Reprinted with permission from ref . Copyright (2021) American Chemical Society.
Trends and Perspectives
The future of Ag-based semiconductors relies increasingly on refined defect engineering strategies to fully exploit their potential across diverse fields such as photocatalysis, antimicrobial treatment, and gas sensing. ,, These materials, particularly in their bimetallic and oxide forms (Ag2WO4, Ag2MoO4, and Ag2CrO4), benefit from intrinsic and extrinsic defect structures, including oxygen vacancies, cation deficiencies, and lattice distortions, which can modulate their electronic structure, surface reactivity, and light absorption characteristics. However, unlocking these advantages comes with significant challenges. A major hurdle lies in the reproducible control of defect type, concentration, and spatial distribution during synthesis. Small deviations in conditions such as pH, precursor ratio, or reaction temperature can dramatically alter the defect landscape. As a result, material properties may vary unpredictably, limiting scalability and industrial adoption. Moreover, precise defect tuning at the laboratory scale often proves difficult to replicate in large-scale processes, leading to inconsistencies in performance.
In addition, Ag-based semiconductors suffer from several material-specific limitations that can be addressed through tailored defect engineering. One of the most critical issues is photocorrosion, particularly under prolonged illumination, which leads to structural instability. Introducing stable defect structures, such as deeply trapped oxygen vacancies or substitutional dopants, can help redistribute local charge density, reducing the oxidation of Ag sites and improving photostability. Similarly, the use of aliovalent doping can stabilize Ag in its lower oxidation state, minimizing Ag+ release and thus mitigating ionic leaching, especially relevant in biological or aqueous environments.
A further challenge is that while surface defects enhance charge separation and ROS formation, an excess of uncontrolled defects often results in increased carrier recombination. To counter this, defect profiles must be tuned precisely in terms of both concentration and spatial distribution. This can be achieved by combining bottom-up synthesis with in situ annealing, redox-controlled atmospheres, or defect-selective surfactants that direct defect formation to targeted facets. In antimicrobial systems, defects are key for ROS-mediated membrane disruption, yet maintaining their activity over time is challenging. Here, dynamic defect structures, such as reversible vacancies or redox-active defect clusters, offer a route to regenerate surface reactivity during use. Moreover, codoping strategies can introduce synergistic defect interactions, where one dopant creates active sites while another stabilizes the structure, enhancing both performance and durability. Ultimately, the success of Ag-based semiconductors under real operating conditions depends on developing defect architectures that are not only active but also thermodynamically and kinetically stable, enabling long-term function without structural degradation.
In complex architectures like doped or multicomponent systems, defect interactions become more nuanced. While not directly addressed in this study, heterojunctions offer a promising route to improve performance by integrating materials with complementary band alignments. When combined with controlled defect engineering, such systems can potentially boost charge separation efficiency. However, interfacial defects must be carefully managed to avoid new recombination pathways that could compromise the intended synergistic effects.
To navigate these complex interdependencies, theoretical tools like density functional theory (DFT) and molecular dynamics simulations are increasingly indispensable. ,,, These models allow for the prediction of defect energetics, charge distribution, and band alignment, enabling rational design before experimental synthesis. When integrated with machine learning (ML), these tools can process large data sets to uncover nonobvious correlations between synthesis parameters and material performance. Artificial intelligence further enhances this framework by enabling accelerated material discovery through data-driven prediction of defect-property relationships. This approach reduces reliance on trial-and-error experimentation and helps prioritize promising candidates for synthesis and testing.
Ultimately, addressing these challenges requires interdisciplinary collaboration among chemists, physicists, engineers, and data scientists. From materials design to device integration, a unified approach is needed to ensure that defect-engineered Ag-based semiconductors retain their targeted functionalities in real-world applications. With continued innovation in synthesis, modeling, and characterization, these materials are poised to play a transformative role in next-generation technologies for energy conversion, environmental purification, and healthcare.
Acknowledgments
This study was financed, in part, by the São Paulo Research Foundation (FAPESP), Brazil, process numbers #2023/08525-7 and #2013/07296-2. J.A. would like to thank Universitat Jaume I (#UJI-B2022-56), Generalitat Valenciana (Conselleria de Innovacion, Universidades, Ciencia y Sociedad Digital - #CIAICO/2021/122), and Ministerio de Ciencia, Innovación y Universidades (#PID2022-141089NB-I00) for financially supporting this research. The authors would also like to thank M.A. San-Miguel, C.C. Foggi, A.F. Gouveia, I. Pinatti, N.G. Macedo, G. Minguez-Vega, and R. Llusar for their contributions to the development of some of the works discussed here, which greatly enhanced the understanding of this class of materials.
Glossary
ABBREVIATIONS
- PL
photoluminescence spectroscopy
- XPS
X-ray photoelectron spectroscopy
- EPR
electron paramagnetic resonance
- PALS
positron annihilation lifetime spectroscopy
- SIA
semiconductor industry association
- VB
valence band
- CB
conduction band
- E gap
band gap
- ROS
reactive oxygen species
- PVP
polyvinylpyrrolidone
- EBI
electron beam irradiation
- SDS
sodium dodecyl sulfate
- PMAA
poly(methacrylic acid)
- MRSA
methicillin-resistant S. aureus
- DFT
density functional theory
- ML
machine learning
- AI
artificial intelligence
M.A.: Conceptualization, methodology, investigation, data curation, formal validation, resources, writing–original draft, review, and editing, visualization, and project administration. A.C.M.R., J.A., and E.L.: Writing–review and editing.
This study was financed, in part, by the São Paulo Research Foundation (FAPESP), Brazil, process numbers #2023/08525-7 and #2013/07296-2. The Article Processing Charge for the publication of this research was funded by the Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior (CAPES), Brazil (ROR identifier: 00x0ma614).
The authors declare no competing financial interest.
References
- https://www.semiconductors.org/2024-state-of-the-u-s-semiconductor-industry/.
- Gupta, K. M. ; Gupta, N. . Semiconductor Materials: Their Properties, Applications, and Recent Advances BT - Advanced Semiconducting Materials and Devices; Gupta, K. M. ; Gupta, N. Eds.; Springer International Publishing: Cham, 2016; pp 3–40. 10.1007/978-3-319-19758-6_1. [DOI] [Google Scholar]
- Zhang Z., Yates J. T. J.. Band Bending in Semiconductors: Chemical and Physical Consequences at Surfaces and Interfaces. Chem. Rev. 2012;112(10):5520–5551. doi: 10.1021/cr3000626. [DOI] [PubMed] [Google Scholar]
- Jasieniak J., Califano M., Watkins S. E.. Size-Dependent Valence and Conduction Band-Edge Energies of Semiconductor Nanocrystals. ACS Nano. 2011;5(7):5888–5902. doi: 10.1021/nn201681s. [DOI] [PubMed] [Google Scholar]
- Gouveia A. F., Lipsky F., San-Miguel M. A., Longo E., Andrés J.. What’s behind Their Various Activities? Discerning the Importance of Surface Terminations in Ag3PO4 Semiconductor from DFT Calculations. Computational Materials Today. 2024;1:100001. doi: 10.1016/j.commt.2024.100001. [DOI] [Google Scholar]
- Morandi S., Fioravanti A., Cerrato G., Lettieri S., Sacerdoti M., Carotta M. C.. Facile Synthesis of ZnO Nano-Structures: Morphology Influence on Electronic Properties. Sens. Actuators, B. 2017;249:581–589. doi: 10.1016/j.snb.2017.03.114. [DOI] [Google Scholar]
- Maarisetty D., Baral S. S.. Defect Engineering in Photocatalysis: Formation, Chemistry, Optoelectronics, and Interface Studies. J. Mater. Chem. A. 2020;8(36):18560–18604. doi: 10.1039/D0TA04297H. [DOI] [Google Scholar]
- Ma X., Tang X., Hu Z., Zhen M., Shen B., Guo S.-Q., Dong F.. Oxygen Vacancies Assist a Facet Effect to Modulate the Microstructure of TiO2 for Efficient Photocatalytic O2 Activation. Nanoscale. 2023;15(2):768–778. doi: 10.1039/D2NR05849A. [DOI] [PubMed] [Google Scholar]
- Li H., Li J., Ai Z., Jia F., Zhang L.. Oxygen Vacancy-Mediated Photocatalysis of BiOCl: Reactivity, Selectivity, and Perspectives. Angew. Chem., Int. Ed. 2018;57(1):122–138. doi: 10.1002/anie.201705628. [DOI] [PubMed] [Google Scholar]
- Queisser H. J., Haller E. E.. Defects in Semiconductors: Some Fatal, Some Vital. Science (New York, N.Y.) 1998;281(5379):945–950. doi: 10.1126/science.281.5379.945. [DOI] [PubMed] [Google Scholar]
- Braga, A. R. C. ; Trindade, L. G. ; Ramos, S. P. ; Bürck, M. ; Nakamoto, M. M. ; Bernardo, L. R. ; Libero, L. O. ; Gouveia, A. F. ; Assis, M. . Design Ag-Based Semiconductors for Antimicrobial Technologies: Challenges and Future Trends BT - Nanomaterials for Biomedical and Bioengineering Applications; Javed, R. ; Chen, J.-T. ; Khalil, A. T. Eds.; Springer Nature Singapore: Singapore, 2024; pp 277–300. 10.1007/978-981-97-0221-3_11. [DOI] [Google Scholar]
- Gouveia A. F., Roca R. A., Macedo N. G., Cavalcante L. S., Longo E., San-Miguel M. A., Altomare A., da Silva G. S., Andrés J.. Ag2WO4 as a Multifunctional Material: Fundamentals and Progress of an Extraordinarily Versatile Semiconductor. Journal of Materials Research and Technology. 2022;21:4023–4051. doi: 10.1016/j.jmrt.2022.11.011. [DOI] [Google Scholar]
- Lempos P. S., Silva G. S., Roca R. A., Assis M., Torres-Mendieta R., Béltran-Mir H., Mínguez-Vega G., Cordoncillo E., Andrés J., Longo E.. Laser and Electron Beam-Induced Formation of Ag/Cr Structures on Ag2CrO4. Phys. Chem. Chem. Phys. 2019;21(11):6101–6111. doi: 10.1039/C8CP07263A. [DOI] [PubMed] [Google Scholar]
- Andrés J., Ferrer M. M., Gracia L., Beltran A., Longo V. M., Cruvinel G. H., Tranquilin R. L., Longo E.. A Combined Experimental and Theoretical Study on the Formation of Ag Filaments on β-Ag 2 MoO 4 Induced by Electron Irradiation. Part. Part. Syst. Charact. 2015;32:646–651. doi: 10.1002/ppsc.201400162. [DOI] [Google Scholar]
- Rodrigues-Pinheiro A., Longo E., Andrés J., San-Miguel M. A.. Electron Irradiation-Induced Structural and Electronic Transformations in β-Ag2MoO4: Atomistic Insights into the Formation of Ag Nanoparticles. J. Phys. Chem. C. 2025;129(15):7402–7412. doi: 10.1021/acs.jpcc.4c08139. [DOI] [Google Scholar]
- Chauhan A., Kumar R., Sharma S., Raizada P., Sonu, Selvasembian R., Hussain C. M., Thakur S., Singh P., Sudhaik A.. Recent Updates on Ag2WO4-Based S-Scheme Heterojunction Photocatalytic Materials for Environmental Remediation. Inorg. Chem. Commun. 2024;170:113370. doi: 10.1016/j.inoche.2024.113370. [DOI] [Google Scholar]
- Della Rocca D. G., Peralta R. M., Peralta R. A., Peralta Muniz Moreira R. de F.. Recent Development on Ag2MoO4-Based Advanced Oxidation Processes: A Review. Reaction Kinetics, Mechanisms and Catalysis. 2021;132(1):1–35. doi: 10.1007/s11144-021-01934-9. [DOI] [Google Scholar]
- Wang H., Wang T., Zhu Z., Ren S., Huang Y., Qian S., Tang W., Yin X., Niu H., Wang X., Wu T., Gao S.. Construction of Multifunctional Photothermal/Photocatalytic Materials Based on the Principle of Three Primary Colors: A Case Study of g-C3N4/Ag2CrO4. Carbon Energy. 2025:e711. doi: 10.1002/cey2.711. [DOI] [Google Scholar]
- Wang H., Zhang K. H. L., Hofmann J. P., de la Peña O’Shea V. A., Oropeza F. E.. The Electronic Structure of Transition Metal Oxides for Oxygen Evolution Reaction. J. Mater. Chem. A. 2021;9(35):19465–19488. doi: 10.1039/D1TA03732C. [DOI] [Google Scholar]
- Goodenough J. B.. Perspective on Engineering Transition-Metal Oxides. Chem. Mater. 2014;26(1):820–829. doi: 10.1021/cm402063u. [DOI] [Google Scholar]
- Dorini T. T., Lipsky F., Rodrigues-Pinheiro A., Andrés J., Longo E., San-Miguel M. A.. Mapping and Characterization of the Surface Structure and Electronic Properties of β-Ag2MoO4 from DFT Thermodynamic Calculations. Surfaces and Interfaces. 2025;56:105530. doi: 10.1016/j.surfin.2024.105530. [DOI] [Google Scholar]
- Lacerda L. H. da S., Longo E., Andrés J., San-Miguel M. A.. A Diagnosis Approach for Semiconductor Properties Evaluation from Ab Initio Calculations: Ag-Based Materials Investigation. J. Solid State Chem. 2022;305:122670. doi: 10.1016/j.jssc.2021.122670. [DOI] [Google Scholar]
- Alvarez-Roca R., Gouveia A. F., De Foggi C. C., Lemos P. S., Gracia L., Da Silva L. F., Vergani C. E., San-Miguel M., Longo E., Andrés J.. Selective Synthesis of α-, β-, and γ-Ag2WO4Polymorphs: Promising Platforms for Photocatalytic and Antibacterial Materials. Inorg. Chem. 2021;60(2):1062–1079. doi: 10.1021/acs.inorgchem.0c03186. [DOI] [PubMed] [Google Scholar]
- Assis M., Cordoncillo E., Torres-Mendieta R., Beltrán-Mir H., Mínguez-Vega G., Oliveira R., Leite E. R., Foggi C. C., Vergani C. E., Longo E., Andrés J.. Towards the Scale-up of the Formation of Nanoparticles on α-Ag 2 WO 4 with Bactericidal Properties by Femtosecond Laser Irradiation. Sci. Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-19270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos P. S., Altomare A., Gouveia A. F., Nogueira I. C., Gracia L., Llusar R., Andrés J., Longo E., Cavalcante L. S.. Synthesis and Characterization of Metastable β-Ag2WO4: An Experimental and Theoretical Approach. Dalton Trans. 2016;45(3):1185–1191. doi: 10.1039/C5DT03754A. [DOI] [PubMed] [Google Scholar]
- Roca R. A., Lemos P. S., Gracia L., Andrés J., Longo E.. Uncovering the Metastable γ-Ag2WO4 Phase: A Joint Experimental and Theoretical Study. RSC Adv. 2017;7(10):5610–5620. doi: 10.1039/C6RA24692C. [DOI] [Google Scholar]
- Gouveia A. F., Sczancoski J. C., Ferrer M. M., Lima A. S., Santos M. R. M. C., Li M. S., Santos R. S., Longo E., Cavalcante L. S.. Experimental and Theoretical Investigations of Electronic Structure and Photoluminescence Properties of β-Ag2MoO4Microcrystals. Inorg. Chem. 2014;53(11):5589–5599. doi: 10.1021/ic500335x. [DOI] [PubMed] [Google Scholar]
- Beltrán A., Gracia L., Longo E., Andrés J.. First-Principles Study of Pressure-Induced Phase Transitions and Electronic Properties of Ag 2 MoO 4. J. Phys. Chem. C. 2014;118(7):3724–3732. doi: 10.1021/jp4118024. [DOI] [Google Scholar]
- Lopes F. H. P., Noleto L. F. G., Vieira V. E. M., de Sousa P. B., Jucá A. C. S., Oliveira Y. L., Costa K. R. B. S., Almeida M. A. P., Gouveia A. F., Cavalcante L. S.. Experimental and Theoretical Correlation of Modulated Architectures of β-Ag2MoO4Microcrystals: Effect of Different Synthesis Routes on the Morphology, Optical, Colorimetric, and Photocatalytic Properties. Journal of Inorganic and Organometallic Polymers and Materials. 2023;33(2):424–450. doi: 10.1007/s10904-022-02509-4. [DOI] [Google Scholar]
- Pinatti I. M., Tello A. C. M., Trench A. B., de Foggi C. C., Pereira P. F. S., Teixeira M. M., Jacomaci N., Andrés J., Longo E.. Zinc-Substituted Ag2CrO4: A Material with Enhanced Photocatalytic and Biological Activity. J. Alloys Compd. 2020;835:155315. doi: 10.1016/j.jallcom.2020.155315. [DOI] [Google Scholar]
- Wang J., Guo R., Bi Z., Chen X., Hu X., Pan W.. A Review on TiO2–x-Based Materials for Photocatalytic CO2 Reduction. Nanoscale. 2022;14(32):11512–11528. doi: 10.1039/D2NR02527B. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Osetsky Y. N., Weber W. J.. Tunable Chemical Disorder in Concentrated Alloys: Defect Physics and Radiation Performance. Chem. Rev. 2022;122(1):789–829. doi: 10.1021/acs.chemrev.1c00387. [DOI] [PubMed] [Google Scholar]
- Huang M. H., Naresh G., Chen H.-S.. Facet-Dependent Electrical, Photocatalytic, and Optical Properties of Semiconductor Crystals and Their Implications for Applications. ACS Appl. Mater. Interfaces. 2018;10(1):4–15. doi: 10.1021/acsami.7b15828. [DOI] [PubMed] [Google Scholar]
- Pastor E., Sachs M., Selim S., Durrant J. R., Bakulin A. A., Walsh A.. Electronic Defects in Metal Oxide Photocatalysts. Nature Reviews Materials. 2022;7(7):503–521. doi: 10.1038/s41578-022-00433-0. [DOI] [Google Scholar]
- Mishra R. K., Verma K., Singh D. S.. Defect Engineering in Nanomaterials: Impact, Challenges, and Applications. Smart Mater. Manuf. 2024;2:100052. doi: 10.1016/j.smmf.2024.100052. [DOI] [Google Scholar]
- Shivaleela, B. ; Hanagodimaht, S. M. . Defects in Electroceramics BT - Defects Engineering in Electroceramics for Energy Applications; Kumar, U. , Ed.; Springer Nature Singapore: Singapore, 2024; pp 31–51. 10.1007/978-981-97-9018-0_2. [DOI] [Google Scholar]
- Li Y.-F., Selloni A.. Pathway of Photocatalytic Oxygen Evolution on Aqueous TiO2 Anatase and Insights into the Different Activities of Anatase and Rutile. ACS Catal. 2016;6(7):4769–4774. doi: 10.1021/acscatal.6b01138. [DOI] [Google Scholar]
- Zhang S., Zhang Z., Si Y., Li B., Deng F., Yang L., Liu X., Dai W., Luo S.. Gradient Hydrogen Migration Modulated with Self-Adapting S Vacancy in Copper-Doped ZnIn2S4 Nanosheet for Photocatalytic Hydrogen Evolution. ACS Nano. 2021;15(9):15238–15248. doi: 10.1021/acsnano.1c05834. [DOI] [PubMed] [Google Scholar]
- Muhammad P., Zada A., Rashid J., Hanif S., Gao Y., Li C., Li Y., Fan K., Wang Y.. Defect Engineering in Nanocatalysts: From Design and Synthesis to Applications. Adv. Funct. Mater. 2024;34(29):2314686. doi: 10.1002/adfm.202314686. [DOI] [Google Scholar]
- Bassett L. C., Alkauskas A., Exarhos A. L., Fu K.-M. C.. Quantum Defects by Design. Nanophotonics. 2019;8:1867. doi: 10.1515/nanoph-2019-0211. [DOI] [Google Scholar]
- Assis M., Castro M. S., Aldao C. M., Buono C., Ortega P. P., Teodoro M. D., Andrés J., Gouveia A. F., Simões A. Z., Longo E., Macchi C. E., Somoza A., Moura F., Ponce M. A.. Disclosing the Nature of Vacancy Defects in α-Ag2WO4. Mater. Res. Bull. 2023;164(5):112252. doi: 10.1016/j.materresbull.2023.112252. [DOI] [Google Scholar]
- Ai M., Zhang J.-W., Wu Y.-W., Pan L., Shi C., Zou J.-J.. Role of Vacancies in Photocatalysis: A Review of Recent Progress. Chem. – Asian J. 2020;15(22):3599–3619. doi: 10.1002/asia.202000889. [DOI] [PubMed] [Google Scholar]
- Li G., Blake G. R., Palstra T. T. M.. Vacancies in Functional Materials for Clean Energy Storage and Harvesting: The Perfect Imperfection. Chem. Soc. Rev. 2017;46(6):1693–1706. doi: 10.1039/C6CS00571C. [DOI] [PubMed] [Google Scholar]
- Ye K., Li K., Lu Y., Guo Z., Ni N., Liu H., Huang Y., Ji H., Wang P.. An Overview of Advanced Methods for the Characterization of Oxygen Vacancies in Materials. TrAC Trends in Analytical Chemistry. 2019;116:102–108. doi: 10.1016/j.trac.2019.05.002. [DOI] [Google Scholar]
- Huang Y., Yu Y., Yu Y., Zhang B.. Oxygen Vacancy Engineering in Photocatalysis. Solar RRL. 2020;4(8):2000037. doi: 10.1002/solr.202000037. [DOI] [Google Scholar]
- Pacchioni G.. Oxygen Vacancy: The Invisible Agent on Oxide Surfaces. ChemPhysChem. 2003;4(10):1041–1047. doi: 10.1002/cphc.200300835. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Xie G., Duan M., Liu Y., Cai Y., Xu M., Zhao K., Tai H., Jiang Y., Su Y.. Zinc Oxide Nanorods for Light-Activated Gas Sensing and Photocatalytic Applications. ACS Applied Nano Materials. 2023;6(19):17445–17456. doi: 10.1021/acsanm.3c02403. [DOI] [Google Scholar]
- Raizada P., Soni V., Kumar A., Singh P., Parwaz Khan A. A., Asiri A. M., Thakur V. K., Nguyen V.-H.. Surface Defect Engineering of Metal Oxides Photocatalyst for Energy Application and Water Treatment. Journal of Materiomics. 2021;7(2):388–418. doi: 10.1016/j.jmat.2020.10.009. [DOI] [Google Scholar]
- Assis M., Ribeiro L. K., Gonçalves M. O., Staffa L. H., Paiva R. S., Lima L. R., Coelho D., Almeida L. F., Moraes L. N., Rosa I. L. V, Mascaro L. H., Grotto R. M. T., Sousa C. P., Andrés J., Longo E., Cruz S. A.. Polypropylene Modified with Ag-Based Semiconductors as a Potential Material against SARS-CoV-2 and Other Pathogens. ACS Applied Polymer Materials. 2022;4(10):7102–7114. doi: 10.1021/acsapm.2c00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assis M., Breitenbach G. L., Martí M., Sánchez-Safont E., Alfaro-Peyró A., Cabedo L., Garcia-Verdugo E., Andrés J., Serrano-Aroca Á.. Synergistic Integration of α-Ag2WO4 into PLA/PBAT for the Development of Electrospun Membranes: Advancing Structural Integrity and Antimicrobial Efficacy. ACS Appl. Mater. Interfaces. 2024;16(46):63404–63418. doi: 10.1021/acsami.4c16618. [DOI] [PubMed] [Google Scholar]
- Sharma M., Sajwan D., Gouda A., Sharma A., Krishnan V.. Recent Progress in Defect-Engineered Metal Oxides for Photocatalytic Environmental Remediation. Photochem. Photobiol. 2024;100(4):830–896. doi: 10.1111/php.13959. [DOI] [PubMed] [Google Scholar]
- Reddy P., Hoffmann M. P., Kaess F., Bryan Z., Bryan I., Bobea M., Klump A., Tweedie J., Kirste R., Mita S., Gerhold M., Collazo R., Sitar Z.. Point Defect Reduction in Wide Bandgap Semiconductors by Defect Quasi Fermi Level Control. J. Appl. Phys. 2016;120(18):185704. doi: 10.1063/1.4967397. [DOI] [Google Scholar]
- Pan X., Yang M.-Q., Fu X., Zhang N., Xu Y.-J.. Defective TiO2 with Oxygen Vacancies: Synthesis, Properties and Photocatalytic Applications. Nanoscale. 2013;5(9):3601–3614. doi: 10.1039/c3nr00476g. [DOI] [PubMed] [Google Scholar]
- Mallick H. K., Zhang Y., Pradhan J., Sahoo M. P. K., Pattanaik A. K.. Influence of Particle Size and Defects on the Optical, Magnetic and Electronic Properties of Al Doped SnO2 Nanoparticles. J. Alloys Compd. 2021;854:156067. doi: 10.1016/j.jallcom.2020.156067. [DOI] [Google Scholar]
- Lee S. A., Jeong H., Woo S., Hwang J.-Y., Choi S.-Y., Kim S.-D., Choi M., Roh S., Yu H., Hwang J., Kim S. W., Choi W. S.. Phase Transitions via Selective Elemental Vacancy Engineering in Complex Oxide Thin Films. Sci. Rep. 2016;6(1):23649. doi: 10.1038/srep23649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assis M., Pontes Ribeiro R. A., Carvalho M. H., Teixeira M. M., Gobato Y. G., Prando G. A., Mendonça C. R., de Boni L., Aparecido de Oliveira A. J., Bettini J., Andrés J., Longo E.. Unconventional Magnetization Generated from Electron Beam and Femtosecond Irradiation on α-Ag2WO4: A Quantum Chemical Investigation. ACS Omega. 2020;5(17):10052–10067. doi: 10.1021/acsomega.0c00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragelli B. D. L., Assis M., Rodolpho J. M. A., Godoy K. F., Líbero L. O., Anibal F. F., Longo E.. Modulation of Cell Death Mechanisms via α-Ag2WO4Morphology-Dependent Factors. Journal of Photochemistry and Photobiology B: Biology. 2024;257:112947. doi: 10.1016/j.jphotobiol.2024.112947. [DOI] [PubMed] [Google Scholar]
- Sczancoski J. C., Maya-Johson S., Da Silva Pereira W., Longo E., Leite E. R.. Atomic Diffusion Induced by Electron-Beam Irradiation: An in Situ Study of Ag Structures Grown from α-Ag2WO4. Cryst. Growth Des. 2019;19(1):106–115. doi: 10.1021/acs.cgd.8b01076. [DOI] [Google Scholar]
- Liu D., Huang W., Li L., Liu L., Sun X., Liu B., Yang B., Guo C.. Experimental and Theoretical Investigation on Photocatalytic Activities of 1D Ag/Ag2WO4 Nanostructures. Nanotechnology. 2017;28(38):385702. doi: 10.1088/1361-6528/aa7d96. [DOI] [PubMed] [Google Scholar]
- Silva G. S., Gracia L., Fabbro M. T., Serejo dos Santos L. P., Beltrán-Mir H., Cordoncillo E., Longo E., Andrés J.. Theoretical and Experimental Insight on Ag2CrO4Microcrystals: Synthesis, Characterization, and Photoluminescence Properties. Inorg. Chem. 2016;55(17):8961–8970. doi: 10.1021/acs.inorgchem.6b01452. [DOI] [PubMed] [Google Scholar]
- Nobre F. X., Bastos I. S., dos Santos Fontenelle R. O., Júnior E. A. A., Takeno M. L., Manzato L., de Matos J. M. E., Orlandi P. P., de Fátima Souza Mendes J., Brito W. R., da Costa Couceiro P. R.. Antimicrobial Properties of α-Ag2WO4 Rod-like Microcrystals Synthesized by Sonochemistry and Sonochemistry Followed by Hydrothermal Conventional Method. Ultrasonics Sonochemistry. 2019;58:104620. doi: 10.1016/j.ultsonch.2019.104620. [DOI] [PubMed] [Google Scholar]
- Xu D., Cao S., Zhang J., Cheng B., Yu J.. Effects of the Preparation Method on the Structure and the Visible-Light Photocatalytic Activity of Ag2CrO4. Beilstein Journal of Nanotechnology. 2014;5:658–666. doi: 10.3762/bjnano.5.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade Neto N. F., Oliveira P. M., Bomio M. R. D., Motta F. V.. Effect of Temperature on the Morphology and Optical Properties of Ag2WO4 Obtained by the Co-Precipitation Method: Photocatalytic Activity. Ceram. Int. 2019;45(12):15205–15212. doi: 10.1016/j.ceramint.2019.05.006. [DOI] [Google Scholar]
- Andrade Neto N. F., Oliveira M. C., Nascimento J. H. O., Longo E., Ribeiro R. A. P., Bomio M. R. D., Motta F. V.. Effect of the Cross-Section Morphology in the Antimicrobial Properties of α-Ag2WO4 Rods: An Experimental and Theoretical Study. Applied Nano. 2023;4:213–225. doi: 10.3390/applnano4030012. [DOI] [Google Scholar]
- Roca R. A., Sczancoski J. C., Nogueira I. C., Fabbro M. T., Alves H. C., Gracia L., Santos L. P. S., De Sousa C. P., Andrés J., Luz G. E., Longo E., Cavalcante L. S.. Facet-Dependent Photocatalytic and Antibacterial Properties of α-Ag 2 WO 4 Crystals: Combining Experimental Data and Theoretical Insights. Catalysis Science and Technology. 2015;5(8):4091–4107. doi: 10.1039/C5CY00331H. [DOI] [Google Scholar]
- da Silva Sousa G., Nobre F. X., Júnior E. A. A., Sambrano J. R., dos Reis Albuquerque A., dos Santos Bindá R., da Costa Couceiro P. R., Brito W. R., Cavalcante L. S., Santos M. R. D. M. C., de Matos J. M. E.. Hydrothermal Synthesis, Structural Characterization and Photocatalytic Properties of β-Ag2MoO4Microcrystals: Correlation between Experimental and Theoretical Data. Arabian J. Chem. 2020;13(1):2806–2825. doi: 10.1016/j.arabjc.2018.07.011. [DOI] [Google Scholar]
- Wang J., Liu H., Xiong H., Chen Y., Xiao J.. Enhancing Ammonia Sensing Performance of Ag2MoO4-Based Mixed-Potential Sensor by Introducing Ionic and Electronic Conductance. Sens. Actuators, B. 2024;416:135997. doi: 10.1016/j.snb.2024.135997. [DOI] [Google Scholar]
- Laier L. O., Assis M., Foggi C. C., Gouveia A. F., Vergani C. E., Santana L. C. L., Cavalcante L. S., Andrés J., Longo E.. Surface-Dependent Properties of α-Ag2WO4: A Joint Experimental and Theoretical Investigation. Theor. Chem. Acc. 2020;139(7):108. doi: 10.1007/s00214-020-02613-z. [DOI] [Google Scholar]
- Miclau M., Kumar S., Ursu D., Orha C., Miclau N., Rueff J.-M., Malo S., Martin C.. Ag2CrO4 for Photocatalytic Applications: High Performance of Crystals Designed by Autogenous Pressure. Mater. Charact. 2019;158:109970. doi: 10.1016/j.matchar.2019.109970. [DOI] [Google Scholar]
- Warmuth L., Ritschel C., Feldmann C.. Facet-, Composition- And Wavelength-Dependent Photocatalysis of Ag2MoO4. RSC Adv. 2020;10(31):18377–18383. doi: 10.1039/D0RA02953J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo N. G., Gouveia A. F., Roca R. A., Assis M., Gracia L., Andrés J., Leite E. R., Longo E.. Surfactant-Mediated Morphology and Photocatalytic Activity of α-Ag 2 WO 4 Material. J. Phys. Chem. C. 2018;122(15):8667–8679. doi: 10.1021/acs.jpcc.8b01898. [DOI] [Google Scholar]
- Ribeiro L. K., Gouveia A. F., Silva F. D. C. M., Noleto L. F. G., Assis M., Batista A. M., Cavalcante L. S., Guillamón E., Rosa I. L. V, Longo E., Andrés J., Luz Júnior G. E.. Tug-of-War Driven by the Structure of Carboxylic Acids: Tuning the Size, Morphology, and Photocatalytic Activity of α-Ag2WO4. Nanomaterials. 2022;12(19):3316. doi: 10.3390/nano12193316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Fu C., Wang P., Yu H., Yu J.. Hierarchically Porous Metastable β-Ag2WO4 Hollow Nanospheres: Controlled Synthesis and High Photocatalytic Activity. Nanotechnology. 2013;24(16):165602. doi: 10.1088/0957-4484/24/16/165602. [DOI] [PubMed] [Google Scholar]
- Andrade Neto N. F., Silva J. M. P., Tranquilin R. L., Longo E., Bomio M. R. D., Motta F. V.. Stabilization of the γ-Ag2WO4Metastable Pure Phase by Coprecipitation Method Using Polyvinylpyrrolidone as Surfactant: Photocatalytic Property. Ceram. Int. 2020;46(10, Part A):14864–14871. doi: 10.1016/j.ceramint.2020.03.012. [DOI] [Google Scholar]
- Li Z., Chen X., Xue Z.-L.. Microwave-Assisted Hydrothermal Synthesis of Cube-like Ag-Ag2MoO4 with Visible-Light Photocatalytic Activity. Science China Chemistry. 2013;56(4):443–450. doi: 10.1007/s11426-013-4845-5. [DOI] [Google Scholar]
- Wang Z., Dai K., Liang C., Zhang J., Zhu G.. Facile Synthesis of Novel Butterfly-like Ag2MoO4 Nanosheets for Visible-Light Driven Photocatalysis. Mater. Lett. 2017;196:373–376. doi: 10.1016/j.matlet.2017.03.078. [DOI] [Google Scholar]
- Teodoro V., Gouveia A. F., Machado T. R., Trench A. B., Jacomaci N., Assis M., Marques G. E., Teodoro M. D., San-Miguel M. A., Andrés J., Bettini J., Longo E.. Connecting Morphology and Photoluminescence Emissions in β-Ag2MoO4Microcrystals. Ceram. Int. 2022;48(3):3740–3750. doi: 10.1016/j.ceramint.2021.10.156. [DOI] [Google Scholar]
- Assis M., de Foggi C. C., Teodoro V., de Campos da Costa J. P., Silva C. E., Robeldo T., Caperucci P. F., Vergani C. E., Borra R. C., Sorribes I., Gouveia A. F., San-Miguel M. A., Andrés J., Longo E.. Surface-Dependent Photocatalytic and Biological Activities of Ag2CrO4: Integration of Experiment and Simulation. Appl. Surf. Sci. 2021;545:148964. doi: 10.1016/j.apsusc.2021.148964. [DOI] [Google Scholar]
- Oliveira C. A., Volanti D. P., Nogueira A. E., Zamperini C. A., Vergani C. E., Longo E.. Well-Designed β-Ag2MoO4 Crystals with Photocatalytic and Antibacterial Activity. Materials & Design. 2017;115:73–81. doi: 10.1016/j.matdes.2016.11.032. [DOI] [Google Scholar]
- de Foggi C. C., de Oliveira R. C., Fabbro M. T., Vergani C. E., Andres J., Longo E., Machado A. L.. Tuning the Morphological, Optical, and Antimicrobial Properties of α-Ag2WO4Microcrystals Using Different Solvents. Cryst. Growth Des. 2017;17(12):6239–6246. doi: 10.1021/acs.cgd.7b00786. [DOI] [Google Scholar]
- Fabbro M. T., Foggi C. C., Santos L. P. S., Gracia L., Perrin A., Perrin C., Vergani C. E., Machado A. L., Andrés J., Cordoncillo E., Longo E.. Synthesis, Antifungal Evaluation and Optical Properties of Silver Molybdate Microcrystals in Different Solvents: A Combined Experimental and Theoretical Study. Dalton Transactions. 2016;45(26):10736–10743. doi: 10.1039/C6DT00343E. [DOI] [PubMed] [Google Scholar]
- De Foggi C. C., De Oliveira R. C., Assis M., Fabbro M. T., Mastelaro V. R., Vergani C. E., Gracia L., Andrés J., Longo E., Machado A. L.. Unvealing the Role of β-Ag2MoO4Microcrystals to the Improvement of Antibacterial Activity. Materials Science and Engineering: C. 2020;111:110765. doi: 10.1016/j.msec.2020.110765. [DOI] [PubMed] [Google Scholar]
- Cunha F. S., Sczancoski J. C., Nogueira I. C., de Oliveira V. G., Lustosa S. M. C., Longo E., Cavalcante L. S.. Structural{,} Morphological and Optical Investigation of β-Ag2MoO4Microcrystals Obtained with Different Polar Solvents. CrystEngComm. 2015;17(43):8207–8211. doi: 10.1039/C5CE01662B. [DOI] [Google Scholar]
- Chen H., Xu Y.. Photoactivity and Stability of Ag2WO4 for Organic Degradation in Aqueous Suspensions. Appl. Surf. Sci. 2014;319:319–323. doi: 10.1016/j.apsusc.2014.05.115. [DOI] [Google Scholar]
- Della Rocca D. G., Schneider M., Fraga F. C., De Noni Júnior A., Peralta R. A., Rodríguez-Aguado E., Rodríguez-Castellón E., Moreira R. F. P. M.. A Comprehensive Insight into the Parameters That Influence the Synthesis of Ag2MoO4 Semiconductors via Experimental Design. Journal of Materials Science: Materials in Electronics. 2023;34(19):1500. doi: 10.1007/s10854-023-10897-7. [DOI] [Google Scholar]
- Liu Y., Yu H., Cai M., Sun J.. Microwave Hydrothermal Synthesis of Ag2CrO4 Photocatalyst for Fast Degradation of PCP-Na under Visible Light Irradiation. Catal. Commun. 2012;26:63–67. doi: 10.1016/j.catcom.2012.04.017. [DOI] [Google Scholar]
- de Annunzio S. R., Lima Moraes B. de, Assis M., Barbugli P. A., de Oliveira V. H. F. P., Longo E., Vergani C. E.. Antimicrobial Activity and Biocompatibility of Alpha-Silver Tungstate Nanoparticles. Heliyon. 2025;11(4):e42648. doi: 10.1016/j.heliyon.2025.e42648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tello A. C. M., Assis M., Menasce R., Gouveia A. F., Teodoro V., Jacomaci N., Zaghete M. A., Andrés J., Marques G. E., Teodoro M. D., da Silva A. B. F., Bettini J., Longo E.. Microwave-Driven Hexagonal-to-Monoclinic Transition in BiPO4: An In-Depth Experimental Investigation and First-Principles Study. Inorg. Chem. 2020;59(11):7453–7468. doi: 10.1021/acs.inorgchem.0c00181. [DOI] [PubMed] [Google Scholar]
- Patil, S. ; Jagadale, S. . Chapter 3 - Co-Precipitation Methods for the Synthesis of Metal Oxide Nanostructures. In Solution Methods for Metal Oxides Nanostructures; Mane, R. ; Jadhav, V. ; Al-Enizi, A. M. Eds.; Elsevier, 2023; pp 39–60. 10.1016/B978-0-12-824353-4.00016-6. [DOI] [Google Scholar]
- Modeshia D. R., Walton R. I.. Solvothermal Synthesis of Perovskites and Pyrochlores: Crystallisation of Functional Oxides under Mild Conditions. Chem. Soc. Rev. 2010;39(11):4303–4325. doi: 10.1039/b904702f. [DOI] [PubMed] [Google Scholar]
- Demazeau G.. Solvothermal Reactions: An Original Route for the Synthesis of Novel Materials. J. Mater. Sci. 2008;43(7):2104–2114. doi: 10.1007/s10853-007-2024-9. [DOI] [Google Scholar]
- Schütz M. B., Lisong X., Thomas L., Thomas F., Mathur S.. Microwave-Assisted Synthesis of Nanocrystalline Binary and Ternary Metal Oxides. Int. Mater. Rev. 2018;63(6):341–374. doi: 10.1080/09506608.2017.1402158. [DOI] [Google Scholar]
- Palchik O., Zhu J., Gedanken A.. Microwave Assisted Preparation of Binary Oxide Nanoparticles. J. Mater. Chem. 2000;10(5):1251–1254. doi: 10.1039/a908795h. [DOI] [Google Scholar]
- Xu H., Zeiger B. W., Suslick K. S.. Sonochemical Synthesis of Nanomaterials. Chem. Soc. Rev. 2013;42(7):2555–2567. doi: 10.1039/C2CS35282F. [DOI] [PubMed] [Google Scholar]
- Głowniak S., Szczęśniak B., Choma J., Jaroniec M.. Recent Developments in Sonochemical Synthesis of Nanoporous Materials. Molecules. 2023;28:2639. doi: 10.3390/molecules28062639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezk A. R., Ahmed H., Ramesan S., Yeo L. Y.. High Frequency Sonoprocessing: A New Field of Cavitation-Free Acoustic Materials Synthesis, Processing, and Manipulation. Advanced Science. 2021;8(1):2001983. doi: 10.1002/advs.202001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wang S., Wu J., Zhou W.. Recent Progress in Defective TiO2 Photocatalysts for Energy and Environmental Applications. Renewable and Sustainable Energy Reviews. 2022;156:111980. doi: 10.1016/j.rser.2021.111980. [DOI] [Google Scholar]
- Macchi C., Petinardi G. M., Freire L. A., Castro M. S., Aldao C. M., Luiz T. M., Moura F., Simões A. Z., Moreno H., Longo E., Somoza A., Assis M., Ponce M. A.. Tracking of Structural Defects Induced by Eu-Doping in β-Ag2MoO4: Their Influences on Electrical Properties. Dalton Trans. 2024;53(2):525–534. doi: 10.1039/D3DT03385F. [DOI] [PubMed] [Google Scholar]
- Cruz L., Teixeira M. M., Teodoro V., Jacomaci N., Laier L. O., Assis M., Macedo N. G., Tello A. C. M., Silva L. F. da, Marques G. E., Zaghete M. A., Teodoro M. D., Longo E.. Multi-Dimensional Architecture of Ag/α-Ag2WO4 Crystals: Insights into Microstructural, Morphological, and Photoluminescence Properties. CrystEngComm. 2020;22(45):7903–7917. doi: 10.1039/D0CE00876A. [DOI] [Google Scholar]
- Arandiyan H., Mofarah S. S., Sorrell C. C., Doustkhah E., Sajjadi B., Hao D., Wang Y., Sun H., Ni B.-J., Rezaei M., Shao Z., Maschmeyer T.. Defect Engineering of Oxide Perovskites for Catalysis and Energy Storage: Synthesis of Chemistry and Materials Science. Chem. Soc. Rev. 2021;50(18):10116–10211. doi: 10.1039/D0CS00639D. [DOI] [PubMed] [Google Scholar]
- Koczkur K. M., Mourdikoudis S., Polavarapu L., Skrabalak S. E.. Polyvinylpyrrolidone (PVP) in Nanoparticle Synthesis. Dalton Trans. 2015;44(41):17883–17905. doi: 10.1039/C5DT02964C. [DOI] [PubMed] [Google Scholar]
- Ali U., Abd Karim K. J., Abd Karim K., Buang N. A.. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA) Polym. Rev. 2015;55(4):678–705. doi: 10.1080/15583724.2015.1031377. [DOI] [Google Scholar]
- Pereira W. D. S., Ferrer M. M., Botelho G., Gracia L., Nogueira I. C., Pinatti I. M., Rosa I. L. V., La Porta F. D. A., Andrés J., Longo E.. Effects of Chemical Substitution on the Structural and Optical Properties of α-Ag2–2xNixWO4 (0 ≤ x ≤ 0.08) Solid Solutions. Phys. Chem. Chem. Phys. 2016;18(31):21966–21975. doi: 10.1039/C6CP00575F. [DOI] [PubMed] [Google Scholar]
- Penha M. D., Gouveia A. F., Teixeira M. M., de Oliveira R. C., Assis M., Sambrano J. R., Yokaichya F., Santos C. C., Gonçalves R. F., Siu Li M., San-Miguel M. A., Andrés J., Longo E.. Optical Properties, and Photocatalytic Activity of α-Ag2W0.75Mo0.25O4. Mater. Res. Bull. 2020;132:111011. doi: 10.1016/j.materresbull.2020.111011. [DOI] [Google Scholar]
- dos Santos Pereira P. F., De Foggi C. C., Gouveia A. F., Pinatti I. M., Cabral L. A., Guillamon E., Sorribes I., San-Miguel M. A., Vergani C. E., Simões A. Z., da Silva E. Z., Cavalcante L. S., Llusar R., Longo E., Andrés J.. Disclosing the Biocide Activity of α-Ag2–2xCuxWO4 (0 ≤ x ≤ 0.16) Solid Solutions. Int. J. Mol. Sci. 2022;23(18):10589. doi: 10.3390/ijms231810589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira P. F. S., Santos C. C., Gouveia A. F., Ferrer M. M., Pinatti I. M., Botelho G., Sambrano J. R., Rosa I. L. V, Andrés J., Longo E.. α-Ag2–2xZnxWO4 (0 ≤ x ≤ 0.25) Solid Solutions: Structure, Morphology, and Optical Properties. Inorg. Chem. 2017;56(13):7360–7372. doi: 10.1021/acs.inorgchem.7b00201. [DOI] [PubMed] [Google Scholar]
- Patrocinio K. L., Santos J. R., Granone L. I., Ponce M. A., Churio M. S., Ribeiro L. K., Teodoro M. D., Llusar R., Andrés J., Longo E., Assis M.. Tuning the Morphology to Enhance the Catalytic Activity of α-Ag2WO4 through V-Doping. Dalton Transactions. 2023;52(41):14982–14994. doi: 10.1039/D3DT02352D. [DOI] [PubMed] [Google Scholar]
- Gupta S. K., Sudarshan K., Ghosh P. S., Mukherjee S., Kadam R. M.. Doping-Induced Room Temperature Stabilization of Metastable β-Ag2WO4 and Origin of Visible Emission in α- And β-Ag2WO4: Low Temperature Photoluminescence Studies. J. Phys. Chem. C. 2016;120(13):7265–7276. doi: 10.1021/acs.jpcc.6b00078. [DOI] [Google Scholar]
- Pinatti I. M., Fern G. R., Longo E., Ireland T. G., Pereira P. F. S., Rosa I. L. V., Silver J.. Luminescence Properties of α-Ag2WO4 Nanorods Co-Doped with Li+ and Eu3+ Cations and Their Effects on Its Structure. J. Lumin. 2019;206:442–454. doi: 10.1016/j.jlumin.2018.10.104. [DOI] [Google Scholar]
- Pinatti I. M., Pereira P. F. S., de Assis M., Longo E., Rosa I. L. V.. Rare Earth Doped Silver Tungstate for Photoluminescent Applications. J. Alloys Compd. 2019;771:433–447. doi: 10.1016/j.jallcom.2018.08.302. [DOI] [Google Scholar]
- Khairy M., Mohamed M. M., Khalifa M. E., Abdelrahman E. A., Sameeh M.. Surfactant-Assisted Synthesis of α-and β-Ag2WO4Modified with Sulphur, Phosphorous, and Boron and Their Applications in Wastewater Elimination. J. Photochem. Photobiol., A. 2024;450:115458. doi: 10.1016/j.jphotochem.2024.115458. [DOI] [Google Scholar]
- Sudarshan K., Gupta S. K., Sonawane K., Kadam R. M.. Room Temperature Synthesis, Concentration Quenching Study and Defect Formation in β-Ag2MoO4:Dy3+- Photoluminescence and Positron Annihilation Spectroscopy. J. Lumin. 2019;212:293–299. doi: 10.1016/j.jlumin.2019.04.031. [DOI] [Google Scholar]
- Souza J. C., Lemos S. C. S., Assis M., Fernandes C. H. M., Ribeiro L. K., Núñez-de la Rosa Y., Teodoro M. D., Gracia L., Andrés J., Mascaro L. H., Longo E.. Boosted Photocatalytic Activities of Ag2CrO4 through Eu3+-Doping Process. ACS Omega. 2024;9(33):35537–35547. doi: 10.1021/acsomega.4c02683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida P. B., Pinatti I. M., de Oliveira R. C., Teixeira M. M., Santos C. C., Machado T. R., Longo E., Rosa I. L. V.. Structural, Morphological and Photoluminescence Properties of β-Ag2MoO4 Doped with Eu3+ Chemical Papers. 2021;75(5):1869–1882. doi: 10.1007/s11696-020-01489-4. [DOI] [Google Scholar]
- Assis M., Macedo N. G., Machado T. R., Ferrer M. M., Gouveia A. F., Cordoncillo E., Torres-Mendieta R., Beltrán-Mir H., Mínguez-Vega G., Leite E. R., Sambrano J. R., Andrés J., Longo E.. Laser/Electron Irradiation on Indium Phosphide (InP) Semiconductor: Promising Pathways to In Situ Formation of Indium Nanoparticles. Part. Part. Syst. Charact. 2018;35(11):1800237. doi: 10.1002/ppsc.201800237. [DOI] [Google Scholar]
- Assis M., Carvalho De Oliveira M., Machado T. R., Macedo N. G., Costa J. P. C., Gracia L., Andrés J., Longo E.. In Situ Growth of Bi Nanoparticles on NaBiO 3, δ-, and β-Bi 2 O 3 Surfaces: Electron Irradiation and Theoretical Insights. J. Phys. Chem. C. 2019;123(8):5023–5030. doi: 10.1021/acs.jpcc.8b11566. [DOI] [Google Scholar]
- Machado T. R., Macedo N. G., Assis M., Doñate-Buendia C., Mínguez-Vega G., Teixeira M. M., Foggi C. C., Vergani C. E., Beltrán-Mir H., Andrés J., Cordoncillo E., Longo E.. From Complex Inorganic Oxides to Ag–Bi Nanoalloy: Synthesis by Femtosecond Laser Irradiation. ACS Omega. 2018;3(8):9880–9887. doi: 10.1021/acsomega.8b01264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assis M., Cordoncillo E., Torres-Mendieta R., Beltrán-Mir H., Mínguez-Vega G., Gouveia A. F., Leite E., Andrés J., Longo E.. Laser-Induced Formation of Bismuth Nanoparticles. Phys. Chem. Chem. Phys. 2018;20(20):13693–13696. doi: 10.1039/C8CP01225C. [DOI] [PubMed] [Google Scholar]
- Andrés J., Gracia L., Gonzalez-Navarrete P., Longo V. M., Avansi W., Volanti D. P., Ferrer M. M., Lemos P. S., La Porta F. A., Hernandes A. C., Longo E.. Structural and Electronic Analysis of the Atomic Scale Nucleation of Ag on α-Ag2WO4 Induced by Electron Irradiation. Sci. Rep. 2014;4:1–7. doi: 10.1038/srep05391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo V. M., De Foggi C. C., Ferrer M. M., Gouveia A. F., André R. S., Avansi W., Vergani C. E., Machado A. L., Andrés J., Cavalcante L. S., Hernandes A. C., Longo E.. Potentiated Electron Transference in α-Ag2WO4Microcrystals with Ag Nanofilaments as Microbial Agent. J. Phys. Chem. A. 2014;118(31):5769–5778. doi: 10.1021/jp410564p. [DOI] [PubMed] [Google Scholar]
- Alvarez Roca R., Lemos P. S., Andrés J., Longo E.. Formation of Ag Nanoparticles on Metastable β-Ag2WO4Microcrystals Induced by Electron Irradiation. Chem. Phys. Lett. 2016;644:68–72. doi: 10.1016/j.cplett.2015.11.048. [DOI] [Google Scholar]
- Fabbro M. T., Gracia L., Silva G. S., Santos L. P. S., Andrés J., Cordoncillo E., Longo E.. Understanding the Formation and Growth of Ag Nanoparticles on Silver Chromate Induced by Electron Irradiation in Electron Microscope: A Combined Experimental and Theoretical Study. J. Solid State Chem. 2016;239:220–227. doi: 10.1016/j.jssc.2016.03.050. [DOI] [Google Scholar]
- Fabbro M. T., Saliby C., Rios L. R., La Porta F. A., Gracia L., Li M. S., Andrés J., Santos L. P. S., Longo E.. Identifying and Rationalizing the Morphological, Structural, and Optical Properties of β-Ag2MoO4Microcrystals, and the Formation Process of Ag Nanoparticles on Their Surfaces: Combining Experimental Data and First-Principles Calculations. Sci. Technol. Adv. Mater. 2015;16(6):065002. doi: 10.1088/1468-6996/16/6/065002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assis M., Robeldo T., Foggi C. C., Kubo A. M., Mínguez-Vega G., Condoncillo E., Beltran-Mir H., Torres-Mendieta R., Andrés J., Oliva M., Vergani C. E., Barbugli P. A., Camargo E. R., Borra R. C., Longo E.. Ag Nanoparticles/α-Ag2WO4 Composite Formed by Electron Beam and Femtosecond Irradiation as Potent Antifungal and Antitumor Agents. Sci. Rep. 2019;9(1):9927. doi: 10.1038/s41598-019-46159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira M. M., Santos L. C., Tello A. C. M., Almeida P. B., Silva J. S. da, Laier L., Gracia L., Teodoro M. D., Silva L. F. da, Andrés J., Longo E.. α-Ag2WO4 under Microwave, Electron Beam and Femtosecond Laser Irradiations: Unveiling the Relationship between Morphology and Photoluminescence Emissions. J. Alloys Compd. 2022;903:163840. doi: 10.1016/j.jallcom.2022.163840. [DOI] [Google Scholar]
- Macedo N. G., Machado T. R., Roca R. A., Assis M., Foggi C. C., Puerto-Belda V., Mínguez-Vega G., Rodrigues A., San-Miguel M. A., Cordoncillo E., Beltrán-Mir H., Andrés J., Longo E.. Tailoring the Bactericidal Activity of Ag Nanoparticles/α-Ag2WO4 Composite Induced by Electron Beam and Femtosecond Laser Irradiation: Integration of Experiment and Computational Modeling. ACS Appl. Bio Mater. 2019;2(2):824–837. doi: 10.1021/acsabm.8b00673. [DOI] [PubMed] [Google Scholar]
- Lin Z., Li J., Zheng Z., Yan J., Liu P., Wang C., Yang G.. Electronic Reconstruction of α-Ag2WO4 Nanorods for Visible-Light Photocatalysis. ACS Nano. 2015;9(7):7256–7265. doi: 10.1021/acsnano.5b02077. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Pinheiro A., Gouveia A. F., Longo E., Andrés J., San-Miguel M. A.. Mechanistic Insights into Ag Nanoparticle Formation on β-Ag2WO4 Surfaces through Electron Beam Irradiation. ACS Physical Chemistry Au. 2025;5:139. doi: 10.1021/acsphyschemau.4c00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos C. C., de Assis M., Machado T. R., dos Santos Pereira P. F., Minguez-Vega G., Cordoncillo E., Beltran-Mir H., Doñate-Buendía C., Andrés J., Longo E.. Proof-of-Concept Studies Directed toward the Formation of Metallic Ag Nanostructures from Ag3PO4 Induced by Electron Beam and Femtosecond Laser. Particle & Particle Systems Characterization. 2019;36(6):1800533. doi: 10.1002/ppsc.201800533. [DOI] [Google Scholar]
- Rosales-Martínez C., Assis M., Castillo-Blas C., Abánades Lázaro I.. Tuning the Electronic Properties of Zr UiO-66 through Defect-Functionalised Multivariate Modulation. Chem. Commun. 2024;60(63):8280–8283. doi: 10.1039/D4CC02581D. [DOI] [PubMed] [Google Scholar]
- Rosales-Martínez C., López-Alcalá D., Assis M., Castillo-Blas C., Baldoví J. J., Abánades Lázaro I.. Promoting Photoswitching in Mismatching Mixed-Linker Multivariate Zr6MOFs. RSC Adv. 2024;14(51):37984–37992. doi: 10.1039/D4RA07366E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sczancoski J. C., Cavalcante L. S., Badapanda T., Rout S. K., Panigrahi S., Mastelaro V. R., Varela J. A., Li M. S., Longo E.. Structure and Optical Properties of [Ba1–XY2x/3](Zr0.25Ti0.75)O3 Powders. Solid State Sci. 2010;12(7):1160–1167. doi: 10.1016/j.solidstatesciences.2010.04.002. [DOI] [Google Scholar]
- Motta F. V., Marques A. P. A., Li M. S., Abreu M. F. C., Paskocimas C. A., Bomio M. R. D., Souza R. P., Varela J. A., Longo E.. Preparation and Photoluminescence Characteristics of In(OH)3:XTb3+ Obtained by Microwave-Assisted Hydrothermal Method. J. Alloys Compd. 2013;553:338–342. doi: 10.1016/j.jallcom.2012.11.063. [DOI] [Google Scholar]
- Chaves A. C., Lima S. J. G., Araújo R. C. M. U., Maurera M. A. M. A., Longo E., Pizani P. S., Simões L. G. P., Soledade L. E. B., Souza A. G., dos Santos I. M. G.. Photoluminescence in Disordered Zn2TiO4. J. Solid State Chem. 2006;179(4):985–992. doi: 10.1016/j.jssc.2005.12.018. [DOI] [Google Scholar]
- Longo E., Volanti D. P., Longo V. M., Gracia L., Nogueira I. C., Almeida M. A. P., Pinheiro A. N., Ferrer M. M., Cavalcante L. S., Andrés J.. Toward an Understanding of the Growth of Ag Filaments on α-Ag 2WO4 and Their Photoluminescent Properties: A Combined Experimental and Theoretical Study. J. Phys. Chem. C. 2014;118(2):1229–1239. doi: 10.1021/jp408167v. [DOI] [Google Scholar]
- Souza J. C., Ribeiro R. A. P., da Trindade L. G., Oliveira R. C. de, Costa L. D., de Oliveira M. C., de Lazaro S. R., Sambrano J. R., Mendonça C. R., de Boni L., Pontes F. M. L., de Oliveira A. J. A., Leite E. R., Longo E.. Unconventional Disorder by Femtosecond Laser Irradiation in Fe2O3. ACS Omega. 2021;6(42):28049–28062. doi: 10.1021/acsomega.1c04079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna D. N. G., Philip J.. Review on Surface-Characterization Applications of X-Ray Photoelectron Spectroscopy (XPS): Recent Developments and Challenges. Applied Surface Science Advances. 2022;12:100332. doi: 10.1016/j.apsadv.2022.100332. [DOI] [Google Scholar]
- Haneman D.. Review of Electron Paramagnetic Resonance Investigations of Semiconductor Surfaces. Jpn. J. Appl. Phys. 1974;13(S2):371. doi: 10.7567/JJAPS.2S2.371. [DOI] [Google Scholar]
- Amaral D. C., Rocha L. S. R., Granone L. I., Lage M. M., Churio M. S., Sanchez M. D., Longo E., Nascimento H. M. S., Assis M., Moura F., Ponce M. A.. Manganese Defective Clustering: Influence on the Spectroscopic Features of Ceria-Based Nanomaterials. Inorg. Chem. 2023;62(31):12392–12402. doi: 10.1021/acs.inorgchem.3c01597. [DOI] [PubMed] [Google Scholar]
- Watkins, G. D. Chapter 1 EPR and ENDOR Studies of Defects in Semiconductors. In Identification of Defects in Semiconductors; Stavola, M. ; Weber, E. R. ; Ed.; Elsevier, 1998; Vol. 51, pp 1–43. 10.1016/S0080-8784(08)63053-7. [DOI] [Google Scholar]
- Shen Z., Zhao Z., Wen J., Qian J., Peng Z., Fu X.. Role of Oxygen Vacancies in the Electrical Properties of WO3‑x Nano/Microrods with Identical Morphology. J. Nanomater. 2018;2018:7802589. doi: 10.1155/2018/7802589. [DOI] [Google Scholar]
- Nakamura I., Negishi N., Kutsuna S., Ihara T., Sugihara S., Takeuchi K.. Role of Oxygen Vacancy in the Plasma-Treated TiO2 Photocatalyst with Visible Light Activity for NO Removal. J. Mol. Catal. A: Chem. 2000;161:205–212. doi: 10.1016/S1381-1169(00)00362-9. [DOI] [Google Scholar]
- Grafutin V. I., Prokop'ev E. P.. Positron Annihilation Spectroscopy in Materials Structure Studies. Phys.-Usp. 2002;45(1):59–74. doi: 10.1070/PU2002v045n01ABEH000971. [DOI] [Google Scholar]
- Triantou K., Mergia K., Stamatelatos I. E.. Positron Annihilation Lifetime Spectroscopy in the Study of Defects in Materials. HNPS Advances in Nuclear Physics. 2019;24:235–238. doi: 10.12681/hnps.1872. [DOI] [Google Scholar]
- Singh A. N.. Positron Annihilation Spectroscopy in Tomorrow’s Material Defect Studies. Appl. Spectrosc. Rev. 2016;51(5):359–378. doi: 10.1080/05704928.2016.1141290. [DOI] [Google Scholar]
- Onue L. A., Ribeiro L. K., Gonçalves M. O., Longo E., Paiva de Sousa C., Assis M., Cruz S. A.. Unveiling Antimicrobial Properties and Crystallization Induction in PLA Using α-Ag2WO4 Nanoparticles. ACS Applied Polymer Materials. 2024;6(6):3233–3242. doi: 10.1021/acsapm.3c03012. [DOI] [Google Scholar]
- Huang K., Li C., Zheng Y., Wang L., Wang W., Meng X.. Recent Advances on Silver-Based Photocatalysis: Photocorrosion Inhibition, Visible-Light Responsivity Enhancement, and Charges Separation Acceleration. Sep. Purif. Technol. 2022;283:120194. doi: 10.1016/j.seppur.2021.120194. [DOI] [Google Scholar]
- Feng Y., Wu J., Chi Q., Li W., Yu Y., Fei W.. Defects and Aliovalent Doping Engineering in Electroceramics. Chem. Rev. 2020;120(3):1710–1787. doi: 10.1021/acs.chemrev.9b00507. [DOI] [PubMed] [Google Scholar]
- Zhang S., Si Y., Li B., Yang L., Dai W., Luo S.. Atomic-Level and Modulated Interfaces of Photocatalyst Heterostructure Constructed by External Defect-Induced Strategy: A Critical Review. Small. 2021;17(1):2004980. doi: 10.1002/smll.202004980. [DOI] [PubMed] [Google Scholar]
- Zhong K., Sun P., Xu H.. Advances in Defect Engineering of Metal Oxides for Photocatalytic CO2 Reduction. Small. 2024:e2310677. doi: 10.1002/smll.202310677. [DOI] [PubMed] [Google Scholar]
- Wang G., Lv S., Shen Y., Li W., Lin L., Li Z.. Advancements in Heterojunction, Cocatalyst, Defect and Morphology Engineering of Semiconductor Oxide Photocatalysts. Journal of Materiomics. 2024;10(2):315–338. doi: 10.1016/j.jmat.2023.05.014. [DOI] [Google Scholar]
- Lipsky F., Lacerda L. H. da S., Gracia L., Foschiani B. G., Assis M., Oliva M., Longo E., Andrés J., San-Miguel M. A.. A Tale of Reactive Oxygen Species on the Ag3PO4(110) Surface. J. Phys. Chem. C. 2023;127(48):23235–23245. doi: 10.1021/acs.jpcc.3c06321. [DOI] [Google Scholar]