Abstract
Recent studies have revealed that cardiac tissue regeneration is promoted by administering an initial dose of exogenous lactate and locally maintaining an abundant concentration of this compound for a prolonged period (i.e., around 10–14 days) through sustained release. The aim of this study is to develop a scaffold based on poly(lactic acid) (PLA) for achieving a sustained daily release of lactate from the first day to the end of the recommended period. First, a five-layered electroresponsive scaffold has been engineered using three PLA layers (first, third, and fifth), each composed of electrospun microfibers (MFs), separated by spin coated lactate (second) and poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) (fourth) intermediate layers. The hydrophobicity of the outer PLA layers (first and fifth) has been used to maintain the release of lactate from the intermediate second layer over 3 days, while the conducting fourth PEDOT:PSS layer has ensured a complete lactate release by electrostimulation. After that, in a second step, the same scaffold has been re-engineered to maintain the sustained release not only for a short period (3 days) but also for a prolonged period (>10 days). For this purpose, the PLA MFs of the intermediate third layer have been substituted by plasma-treated proteinase K-containing PLA MFs, obtained by electrospinning a PLA:enzyme mixture. The activity of the enzyme, which decomposes the ester bonds of PLA, combined with the effect of the plasma on the PLA structure, results in a prolonged sustained release that, in addition, can be modulated.
Keywords: poly(lactic acid), electrospinning, electroresponsive scaffolds, enzymatic degradation, conducting polymer, cardiac tissue regeneration


Introduction
Electrospun microfibers (MFs) are attracting great interest for their potential applications in biomedicine, such as tissue engineering scaffolds, wound healing, antibactericidal patches, and drug release systems. − This success is due, on one hand, to the simplicity and versatility of the electrospinning technology, which produces long-lasting continuous fibers with controlled thickness, morphology, and mechanical properties, and, on the other hand, to the huge number of polymers that can be processed using this fabrication method, including natural, synthetic, and blended polymers.
Drug release and tissue regeneration are biomedical applications that are often interconnected. For example, cell differentiation and proliferation can be helped, and even promoted, through the controlled release of substances. − This is the case of heart tissue, for which Engel and co-workers found that cardiomyocyte proliferation was enhanced by administering an initial dose of exogenous lactate and locally maintaining an abundant concentration of this compound for a prolonged period (i.e., at least 6 mM per day during 10–14 days in human induced pluripotent stem cells (hiPSC)) through sustained release. Specifically, using in vitro mouse neonatal and human pluripotent stem-cell-derived cardiomyocytes, it was observed that exogenous lactate increased cardiomyocyte cell cycle activity, expressed markers of stemness, and modulated the production of inflammatory cytokines. These results were consistent with a previous study dealing with the regeneration of the mammalian heart, where it was observed that dedifferentiated cardiomyocytes that appear de novo from adult cardiomyocytes switched to a glycolytic metabolism and, therefore, depended more on lactate than mature cardiomyocytes. Since injecting exogenous lactate daily for a period of 10–14 days is a very invasive approach, it would be desirable to engineer a platform capable of controlled release of lactate Ideally, such a platform should provide a sustained lactate concentration of around 6 mM for the period necessary to promote cardiac tissue regeneration.
As cardiovascular diseases stand as the leading cause of mortality on a global scale, with an alarming 45% of all deaths in Europe attributed to them, the development of new bioplatforms to achieve a controlled release of lactate has captured our interest lately. In a recent study, we electrospun feeding solutions containing poly(lactic acid) (PLA) and proteinase K, which is a serine protease that exhibits broad cleavage activity, to induce cardiac tissue regeneration. A plasma treatment was applied to the resulting PLA(K) MFs to induce faster PLA degradation. This approach was successful in achieving a sustained and abundant release of exogenous lactate, even though the release did not start before 2–3 days, the time necessary for the enzyme to develop its activity. In order to achieve a sustained lactate delivery during the first 2–3 days, both lactate and PLA(K) MFs were incorporated into an alginate (Alg) hydrogel matrix using an in situ approach. While the sustained and prolonged lactate release from PLA(K) MFs was not affected by the hydrogel, the release of the lactate directly loaded in the Alg hydrogel was very quick, reaching 100% after only a few hours. This ultrafast and uncontrolled release was attributed to the similar environmental conditions of lactate between the hydrophilic environment provided by the Alg hydrogel and the physiological release medium favoring its diffusion. Consequently, sustained lactate delivery for the first 2–3 days became a challenging task.
Although some other lactate delivery systems have been reported for different biomedical applications, they do not fulfill the requirements for cardiac tissue regeneration (i.e., sustained and abundant release over 10–14 days). For example, no controlled lactate release was achieved using silica nanoparticles as carriers, while the delivery from poly(d,l-lactide-co-glycolide) (PLGA) microspheres and multilayered PLA-based films was too slow and/or scarce. Regarding multilayered flexible and self-supported PLA films, which were formed by two spin-coated PLA layers separated by an electropolymerized poly(3,4-ethylenedioxythiophene) (PEDOT) layer, different strategies were considered to load and release lactate. Among them, the one based on the utilization of the outer PLA layers during the spin-coating process for loading lactate provided the best results. Nevertheless, the lactate released using such an approach, even applying electrostimulation, was still insufficient and not enough sustained with respect to the requirements proposed by Engel and co-workers, which was attributed to the compactness of PLA films. On the other hand, lactate-loaded mucoadhesive polyelectrolyte chitosan hydrogels only released lactate when the ionic strength of the environment changed.
In this work, we reformulate our previous approach, which was based on the loading of lactate and plasma-treated PLA(K) MFs into Alg hydrogels, eliminating the hydrophilic environment provided by the hydrogel, creating a multilayered electroresponsive PLA-based scaffold, and applying electrical stimulation to regulate the release of lactate. The capacity to respond against electrical stimuli has been achieved by incorporating a layer of poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS), which is an intrinsically conductive mixture of two ionomers. − While PEDOT is a biocompatible conjugated polymer with impressive electrical conductivity and mechanical flexibility, PSS is a polymer surfactant that acts as a dopant agent and helps disperse and stabilize PEDOT in water. More specifically, in the first part of this study, we focused on the construction and characterization of a five-layered scaffold specifically designed to release lactate in a controlled manner over 72 h. The sustained delivery of lactate over such a short period was achieved without degrading the PLA MFs. In fact, the release during such 72 h came from a lactate layer that was explicitly included in the engineered scaffold and responded to the application of external electrical stimuli. In the second part, the release of lactate beyond 10 days was achieved by the effect of enzyme proteinase K in the intermediate PLA MF layer.
Methods
Materials
PLA 2002D, a product of Natureworks, was kindly supplied by Nupik International (Polinyà, Spain). PLA has a D content of 4.25%, a residual monomer content of 0.3%, a density of 1.24 g/cm, a glass transition temperature (T g) of 58 °C, a melting temperature (T m) of 153 °C, a number-average molecular weight (M n) of 98,100 g/mol, and a polydispersity index (PDI) of 1.9. Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) 1.3 wt % dispersion in water, tris(hydroxymethyl)aminomethane (Tris), sodium l-lactate (≥99.0%), and proteinase K were acquired from Sigma-Aldrich. The solvents, chloroform (≥99.8%), acetone (≥99.5%), and ethanol absolute (≥99.5%), were used as received from Panreac Applichem.
Electrospinning
Electrospun mats of MFs were obtained at room temperature using parameters previously optimized for PLA (i.e., polymer concentration, solvent, flow rate, distance to the needle, and voltage). In brief, the feeding solution was obtained by dissolving PLA in a 2:1 chloroform/acetone mixture to produce a 16.6 wt % polymer solution that was stirred at a constant speed of 1200 rpm for 4 h at a temperature of 25 °C. Electrospinning was carried out in an E-Fiber EF100 electrospinning/electrospraying equipment (SKE, Italy). The feeding solution was loaded in a syringe with a stainless steel needle (inner diameter of 0.84 mm). The flow rate and the needle tip-collector distance were 4.5 mL/h and 28 cm, respectively. A voltage of 15 kV was applied to produce randomly oriented MFs, which were collected on an aluminum foil. PLA MFs loaded with proteinase K, hereafter named PLA(K), were prepared using the same protocol but adding 42 mg of enzyme per 1 g of PLA to the feeding solution.
Spin-Coating
After performing assays with different concentrations of lactate, the choice of the conditions used to spin-coat the lactate layer was carried out by adjusting a final lactate release of around 10 mM per day. More specifically, the lactate layer was prepared using an l-lactate solution (15% w/v) in a 9:1 of H2O:ethanol mixture. 100 μL of such solution (i.e., 20 mM) were deposited onto a 1 × 1 cm2 PLA MF layer and spin-coated using a WS-400BZ-6NPP/A1/AR1 spin-coater (Laurell Technologies Corporation) at a speed of 1600 rpm for 1 min. The resulting two-layered system was removed from the spin-coater with the help of tweezers and taken to the electrospinning equipment to deposit the second PLA MF layer.
PEDOT:PSS was spin-coated by depositing 200 μL of the PEDOT:PSS 1.3 wt % dispersion onto the three-layered system previously prepared. The spin-coating was performed by using the operational conditions described above for the lactate layer. Also, the resulting four-layered system was removed from the spin-coater with the help of tweezers and taken again to the electrospinning to deposit the fifth PLA MF layer.
Plasma Treatment
The 3-layered system formed by two PLA MFs mats separated by a spin-coated lactate layer was treated with plasma using a Diener Electronics Zepto low-pressure plasma device. Samples were introduced inside a 2.6 dm3 chamber. The chamber was purged under a vacuum and filled with 0.3 mbar of oxygen gas using a flux of ∼20 sccm. Then, low-pressure plasma was induced using a 13.56 MHz electric discharge with a maximum power of 100 W. The samples were treated under low-pressure plasma for 20 s.
Characterization
Scanning electron microscopy (SEM) studies were performed using a focus ion beam Zeiss Neon 40 instrument (Carl Zeiss, Germany). Samples with an area of 1 × 1 cm2 were mounted on a double-sided adhesive carbon disc and sputter-coated with a thin layer of carbon to prevent sample charging problems. All micrographs were recorded at an accelerating voltage of 5 kV. The diameter of the PLA MFs was measured with ImageJ software.
FTIR spectra were recorded using a Jasco FTIR 4700 spectrometer equipped with an attenuated total reflection accessory (top plate) with a diamond crystal (Specac model MKII Golden Gate heated single reflection diamond ATR). Each spectrum was obtained using an average of 64 scans in the range of 600–4000 cm–1 with a resolution of 4 cm–1.
Raman analyses were performed using an inVia Qontor confocal Raman microscope (Renishaw) equipped with a Renishaw Centrus 2957T2 detector and both 785 and 532 nm lasers. The 785 nm laser was selected unless explicitly specified. In order to obtain representative data, 32 single-point spectra were averaged. Micro-Raman mappings were obtained by collecting 3078 spectra organized in grids of 57 × 54 points separated 1 μm. The 532 nm laser with an exposure time of 0.2 s was used. Finally, a principal component analysis algorithm was applied to generate the maps.
Water contact angle (WCA) measurements were carried out with the sessile drop method at room temperature. Images of milli-Q water drops (2.6 μL) were recorded with a DSA25S (Krüss GmbH) after stabilization (10 s) and analyzed using Advance (Krüss) software. For each sample, the average WCA value and the corresponding standard deviation were derived from at least 10 independent measures at least.
Electrochemical characterization was carried out by cyclic voltammetry (CV) using a VIONIC potentiostat and Intello software. Experiments were conducted in a three-electrode cell using directly the corresponding MFs mesh (of 1.0 × 1.0 cm2) as the working electrode and a platinum wire as the counter electrode. The reference electrode was a Ag|AgCl electrode containing a KCl-saturated aqueous solution (E 0 = 0.222 V at 25 °C). All electrochemical assays were performed using a 0.07 M Tris aqueous solution (pH = 7.6) at room temperature. The initial and final potentials were −1.0 V, while the reversal potential was +1.0 V. The scan rate was 100 mV/s.
Mechanical properties of two-layered and five-layered systems were evaluated with a Zwick Z2.5/TN1S testing machine with integrated testing software (testXpert, Zwick). The deformation rate for stress–strain assays was 10 mm/min. Samples with surface area of 0.5× 3 cm2 and thickness of 0.9 ± 0.2 mm and 1.6 ± 0.2 mm were cut for the two- and five-layered scaffolds, respectively. Assays were performed considering eight independent samples for each system.
Theoretical Calculations
The strength of PEDOT···PLA interaction was examined using density functional theory (DFT) calculations, which were performed using the Gaussian 09 computer package. The geometries of the different investigated model complexes were fully optimized with the B3LYP − functional combined with the 6–311++G(d,p) basis set. Geometry optimizations were performed in an aqueous environment, which was described through a simple self consistent reaction field (SCRF) method. More specifically, the polarizable continuum model , (PCM) was used in the framework of the B3LYP/6–31++G(d,p) level. No symmetry constraints were used in the geometry optimizations.
Release of Lactate
Lactate release from the engineered five-layered scaffolds was evaluated in Tris buffer solution (pH 7.3) at 37 °C, without and with electrical stimulation. For the release without external stimulation (i.e., diffusional release), each membrane was introduced in the medium, and no voltage was applied in the whole assay. On the other hand, the applied signal to the lactate-loaded system during electrostimulation consisted of a fixed voltage of – 0.5 V along the multilayered system for 30 min, which was applied after a certain silent time of 5 min, 1 h, 2 h, 6 h, 24 h, and 48 h. Thus, the period of time with electrical stimulation took 30 min × 6 = 3 h from a total of 72 h. All experiments were carried out at least three times.
Lactate was quantified at different time intervals (5 min, 10 min, 15 min, 30 min, 1 h, 2 h, 6 h, 1 day, and, after that, daily for 11 days) by UV–vis spectroscopy using a commercial kit from Nzytech (l-lactic acid kit) and a volume of 20 μL. The medium was changed every 24 h. The kit applies an enzymatic reaction for the oxidation of l-lactate to pyruvate by lactate dehydrogenase (17 U/mL) in the presence of nicotinamide adenine dinucleotide (2.95 mg/mL) (NAD), which is reduced from NAD+ to NADH. The amount of NADH, which is proportional to the amount of L-lactate in the release medium was spectrophotometrically measured (λ = 340 nm) after 20 min (end of the enzymatic reaction) with a microplate reader. The concentration of lactate was obtained from a previously performed calibration plot.
Cell Viability Assays
Cell Culture
Cellular assays were performed using the C2C12 cell line (mouse myoblasts), which were cultured in Dulbecco’s modified Eagle medium (DMEM) high glucose, supplemented with 16% fetal bovine serum (FBS) and 1% antibiotic solution (penicillin 100 units/mL and streptomycin 100 μg/mL). Cultures were maintained in a humidified incubator with an atmosphere of 5% CO2 and 95% O2 at 37 °C. Culture media were changed every 2 days. Cell passaging was performed in a 1:4 split ratio. When the cells reached 80–90% confluence, they were detached using 1.5 mL of trypsin (0.25% trypsin/EDTA) for 3 min at 37 °C. Finally, cells were resuspended in 5 mL of fresh medium and their concentration was determined using an automatic cell counter (Countess 3, ThermoFisher).
MTT Assays
The tested systems were placed in 24-well plates and sterilized using UV irradiation for 30 min on each side in a laminar flow hood. Then, the samples were incubated for 24 h and 3 days at 37 °C in DMEM medium, supplemented with FBS and antibiotic solution, to extract leachable compounds from the samples to the medium (leachable medium).
For cell viability assays, cells were seeded at a density of 5 × 104 cells/mL in 96-well plates for 24 h in a humidified incubator with an atmosphere of 5% CO2 and 95% O2 at 37 °C. The cells were further cultured for 24 h in the leachable medium from the samples at varying concentrations. Cellular viability was evaluated by the colorimetric MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] assay. Specifically, after discarding the leachable medium, 200 μL of MTT solution (5 mg/mL in PBS) was added to each well in the 96-well plates and incubated for 3 h at 37 °C. To dissolve formazan crystals, 200 μL of DMSO was added to each well after MTT solution removal. Finally, the absorbance was measured in a microplate reader (SpectraMax Mini, Molecular Devices LLC) at 540 mm. The viability results correspond to the average of three independent replicas (n = 3) for each system. Results were normalized to the control for relative percentages. Statistical analyses were performed with a confidence level of 95% (p < 0.05) using Student’s t test.
Confocal Microscopy
The tested systems were placed in 24-well plates and sterilized using UV irradiation for 30 min on each side in a laminar flow hood. Controls were simultaneously performed by culturing C2C12 cells directly on the surfaces of tissue culture wells. For adhesion and proliferation assays, 5 × 104 cells/mL were seeded on the surface of each well, and direct contact with the sample was promoted by incubation inside the plate wells for 1 and 3 days. After the incubation period, cells were fixed using 2.5% glutaraldehyde solution in PBS, and stained with Alexa Fluor 488 phalloidin for visualizing the actin cytoskeleton and with Hoechst dye to visualize the nuclei. Samples were protected from light and kept at 4 °C before imaging, which was performed using a 10× objective of an Axio Observer 7 confocal laser microscope (Carl ZEISS LSM 800, Germany). Image processing was completed with ZEN software (ZEISS, Germany) and ImageJ software (Wayne Rasband, NIH, USA).
Results and Discussion
Construction of the Scaffold
Initially, we focused on the construction of a scaffold for the short-term sustained release of lactate (i.e., during the first 72 h) since, in previous works, , a prolonged and sustained lactate release, which started at 72 h, was achieved using PLA(K) MFs. A scheme of the designed scaffold, which consisted of a five-layered system, is sketched in Figure a. The first layer was made up of a PLA MF mat by electrospinning a 2:1 chloroform:acetone PLA solution (16.6 wt %) onto the collector (Figure b). Such a PLA mat was used as a support for a lactate layer, which was deposited by spin coating a 9:1 water:ethanol lactate solution (15% w/v) (Figure c). Thus, the hydrophilic lactate was in contact with hydrophobic PLA MFs (PLA/Lact), which acts as a barrier preventing the immediate diffusion of lactate toward the physiological environment. With the same aim, the lactate layer was coated with another electrospun PLA MF mat using the same operational parameters as before (Figure b). Then, the resulting three-layered system (PLA/Lact/PLA) was subjected to a short plasma treatment (Figure d) to adjust the lactate release during the first 72 h by slightly reducing the hydrophobicity of PLA MFs. It should be noted that PLA can be electrically activated by applying plasma, although the operational conditions (i.e., gas pressure, power, and application time) and the type of samples (i.e., robust samples obtained by 3D printing) are very different from those used in this work. , Thus, the plasma conditions used in this work only seek to slightly functionalize the surface to reduce the intrinsic hydrophobicity of PLA.
1.

(a) Sketch of the PLA/Lact/PLA/PEDOT/PLA five-layered scaffold. (b) Electrospinning of PLA MF layers. (c) Spin-coating of the lactate layer. (d) Plasma treatment applied to the PLA/Lact/PLA system. (e) Spin-coating of the PEDOT:PSS layer.
On the plasma-treated PLA/Lact/PLA surface, a fourth layer was incorporated by spin-coating 200 μL of the 1.3 wt % PEDOT:PSS aqueous dispersion (Figure e). PEDOT:PSS is an electroresponsive and biocompatible material able to modify its volume when activated by applying an external voltage. − Therefore, through electrostimulation, some control on the release of lactate is expected in the four-layered system (PLA/Lact/PLA/PEDOT). Finally, the PEDOT:PSS layer, which could be dispersed in a physiological medium, was protected by another hydrophobic PLA MFs mat, thus resulting in the final five-layered scaffold (PLA/Lact/PLA/PEDOT/PLA). It is worth noting that no enzyme was loaded into the system at this stage, as the first objective of this study was to achieve a controlled release of lactate in the short term (during the first 72 h).
Morphological and Chemical Characterization
Figure a displays digital photographs of the electrospun PLA MFs mat (a1), the PLA/Lact (a2), plasma-treated PLA/Lact/PLA (a3), and the complete PLA/Lact/PLA/PEDOT/PLA scaffold (a4). While the incorporation of lactate converts the white color of PLA MFs into a pale orange color, the dark blue color of PEDOT:PSS dominates the aspect of the whole scaffold. SEM micrographs of the first PLA layer, as well as the diameter distribution graphic, are displayed in Figure b. MFs with a well-defined cylindrical morphology, smooth surface, and average diameter of 3.3 ± 0.9 μm are randomly oriented. Despite the success of its incorporation into the scaffold, as it is proved by FTIR and Raman (see below), lactate was only intuited by SEM. Thus, the only difference between PLA and PLA/Lact systems was the appearance of micrometric protuberances scattered at the surface of the MFs, which increased their roughness (Figure c). This fact suggests that lactate organizes in aggregates adhering to the surface of PLA MFs.
2.
(a) Digital photographs of PLA MFs (a1), PLA/Lact (a2), plasma-treated PLA/Lact/PLA (a3), and PLA/Lact/PLA/PEDOT/PLA (a4) scaffolds. (b–e) SEM micrographs of the (b) PLA MFs mat (the diameter distribution graphic is included), (c) PLA/Lact, (d) plasma-treated PLA/Lact/PLA, and (e) PLA/Lact/PLA/PEDOT:PSS four-layered system. The red boxes in (c) and the blue ellipses in (e) show protuberances associated with lactate aggregates and interfiber PEDOT:PSS aggregates, respectively.
In the PLA/Lact/PLA three-layered system, the morphology of PLA MFs changed after the application of the low-pressure O2 plasma. Thus, the smooth surface of PLA MFs became rough, showing a combination of both small cavities and flat protrusions on account of superficial delamination (Figure d). The average diameter of MFs after such a physical change was 3.2 ± 1.1 μm, which was very similar to that obtained for the untreated ones. Amazingly, spin-coated PEDOT:PSS did not form a homogeneous layer itself. Instead, PEDOT:PSS was infiltrated into the plasma-treated PLA/Lact/PLA three-layered system forming a homogeneous distribution of conducting nano- and microaggregates (Figure e) that in many cases were percolated (as proved below by the increment in the electrochemical response), connecting the MFs. Finally, the last PLA layer was formed by smooth fibers practically identical to those displayed in Figure b.
The fact that the spin-coated PEDOT:PSS did not form a homogeneous layer but nano- and microaggregates homogeneously distributed suggests that PLA···PEDOT interactions are probably much less favored than PEDOT···PEDOT interactions. In order to corroborate such a hypothesis, DFT calculations were performed on eight model systems that were constructed by assembling PLA and PEDOT chains constituted by 4 (4-PLA) and 8 (8-PEDOT) repeat units, respectively (Figure a). Such amounts of repeat units were chosen not only to facilitate comparison with the interaction between PEDOT and other compounds, such as alginate and PSS, which were calculated in previous work (see below) but also because the interaction between PEDOT···PLA is too weak when modeled using a smaller number of repeat units. While 4-PLA was in the neutral state, 8-PEDOT was in the oxidized state and exhibited a half-positive charge per repeat unit, according to previous measurements. Before the assemblies were constructed, the geometries of the two model molecules were optimized. While 8-PEDOT formed the expected anti-planar conformations because of the π-conjugation, the oxygen electron-donating effect, and the attractive S···O intramolecular noncovalent interactions, the lowest energy conformation of 4-PLA was a helical structure close to the 107 helix, which is typically found in the α form of PLA. Thus, for the 4-PLA, the average values of the two flexible dihedral angles (i.e., those adjacent to the dihedral of the ester bond) were −72 and 165° (Figure a), while the dihedrals reported for the 107 helix of the most stable α form of PLA were −65 and 149°.
3.
(a) Minimum energy conformations found for 4-PLA and 8-PEDOT model molecules. (b) Two lowest energy assemblies, which are isoenergetic, found for the 4-PLA···8-PEDOT complexes.
The eight model systems constructed by assembling the conformations of the lowest energy found for 8-PEDOT and 4-PLA (Figure a) were optimized. Figure b displays the two optimized complexes of the lowest energy, which were almost isoenergetic (i.e., ΔE < 0.2 kcal/mol). The two molecules interact through weak unspecific van der Waals interactions, as 4-PLA is not able to form specific hydrogen bonds. The binding energy (ΔE b) between the two model molecules, which was determined considering the basis set superposition error, was −2.2 kcal/mol for both complexes. This strength is much weaker than the ones predicted for complexes involving molecules different from 4-PLA. For example, the ΔE b was determined for the interaction of 8-PEDOT with model molecules containing four alginate repeat units (4-Alg) was −55 kcal/mol. The strength of the interactions decreased to ΔE b = −36 kcal/mol when 4-PEDOT was complexed with four repeat units of poly(styrenesulfonate) (4-PSS), while it increased to ΔE b = −90 kcal/mol when 4-PEDOT interacted with three repeat units of cellulose (3-CEL). The interaction of PEDOT with Alg, PSS, and CEL was through strong unspecific electrostatic forces (all cases), accompanied by specific hydrogen bonds (ALG and CEL) or π–π stacking interactions (PSS). In the case of PEDOT···PEDOT interactions, sophisticated theoretical calculations using correlated methods to properly represent π–π stacking indicated that the ΔE b of two π-staked thiophene rings is around −1.7 kcal/mol, this value increasing to −5.8 kcal/mol when the number of π-staked thiophene rings was four.
Overall, the theoretical calculations confirmed our hypothesis about the weakness of PLA···PEDOT interactions, which explains the singular structure found for the PEDOT layer. Thus, in order to minimize PEDOT···PLA interactions, the spin-coated PEDOT chains distributed in infiltrated domains, which was favored by PEDOT···PSS and PEDOT···PEDOT interactions rather than forming a thin and homogeneous 2D layer.
The FTIR spectra of PLA MFs, a lactate solution, and PEDOT:PSS film are displayed in Figure a. PLA shows characteristic peaks at 2997 and 2942 cm–1 (asymmetric and symmetric C–H stretching of CH3, respectively), 1747 cm–1 (CO stretching), 1451 and 1361 cm–1 (−CH3 asymmetric and symmetric bending, respectively), 1181 cm–1 (C–O stretching), 1079 cm–1 (−C–O–C stretching in esters), and 866 and 751 cm–1 (C–C stretching of amorphous and crystalline phases, respectively). , Many such bands are also detected for the lactate solution, even though with some shifts. However, the most important difference with respect to PLA is the hydroxyl vibrations at the 3400–3100 cm–1 region and the shifted CO stretching, −CH3 asymmetric stretching, and C–O–C stretching to 1595, 1457, and 1033 cm–1, respectively. , It is worth noting that the FTIR spectrum of PLA/Lact contains fingerprints of PLA and lactate. Regarding the latter, the broad band centered at around ∼3250 cm–1 (marked by the ellipsoid in Figure a) and the sharp peak at 1595 cm–1 can be clearly identified in the two-layered system.
4.

(a) FTIR and (b) Raman spectra recorded for PLA MFs, a lactate solution, a PEDOT:PSS film, the PLA/Lact two-layered system, and the complete PLA/Lact/PLA/PEDOT/PLA scaffold. Transmittance and Raman intensities are given in arbitrary units.
The FTIR spectrum of PEDOT:PSS shows the characteristic vibrations of the polymer backbone, including the CC stretching in the thiophene ring at 1567 cm–1, the vibrations of the fused dioxane ring at 1265 and 1124 cm–1, the stretch of the C–S bond in the thiophene ring at 858 cm–1. Moreover, the bands at 1164 and 1057 cm–1 have been attributed to the S–O and S–phenyl vibrations of PSS chains. However, the FTIR spectrum of the PLA/Lact/PLA/PEDOT/PLA scaffold, which is clearly dominated by PLA, does not exhibit the characteristic fingerprints of PEDOT:PSS. The similarity between the spectra of PLA MFs and the final PLA/Lact/PLA/PEDOT/PLA scaffold is attributed not only to the abundance of PLA MFs (i.e., three of five layers) compared to PEDOT:PSS but also to the overlapping of some PEDOT:PSS peaks by those of PLA.
Figure b compares the Raman spectra obtained for PLA, lactate, PEDOT:PSS, PLA/Lact, and PLA/Lact/PLA/PEDOT/PLA. The spectrum of the PLA mat shows bands at 3004–2886 cm–1 (C–H stretching modes of CH3), 1770 cm–1 (CO groups), 1455 and 1301 cm–1 (−CH3 asymmetric and symmetric bending, respectively), 1134 cm–1 (CH3 asymmetric groups), 1094 cm–1 (symmetric C–O–C), 1041 cm–1 (C–CH3 stretching), and 871 cm–1 (C–COO vibration). All of these bands were also detected for the lactate solution and the PLA/Lact bilayer. In addition, the successful incorporation of lactate can be corroborated by the vibrations at the region comprised between 2889 and 2914 cm–1 (marked by the ellipsoid in Figure b). On the other hand, the Raman spectrum of PLA/Lact/PLA/PEDOT/PLA is clearly dominated by the fingerprints of PEDOT:PSS (Figure b), which was attributed to the increment in the intensity of PEDOT bands ascribed to the resonance Raman effect (i.e., the laser energy coincides with the frequency of the electronic transition of PEDOT). , More specifically, the peaks ascribed to PEDOT are asymmetric and symmetric Cα=Cβ stretching (1490 and 1424 cm–1, respectively), Cα–Cα′ inter-ring stretching vibrations (1367 and 1259 cm–1), oxyethylene ring deformation (988 cm–1), and symmetric C–S–C (701 cm–1). ,
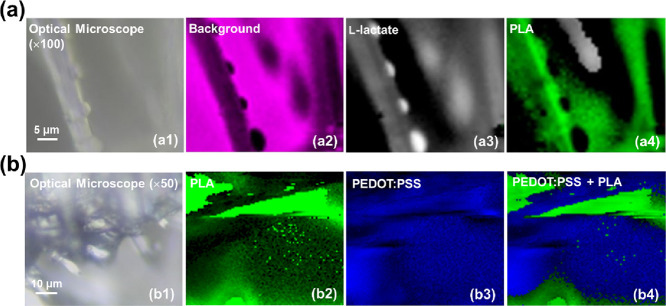
Although the combination of FTIR and Raman spectroscopies allowed us to confirm the successful incorporation of the lactate and PEDOT:PSS into the multilayered scaffold, additional assays were conducted to ascertain the distribution of such compounds when added onto the PLA MFs mats. Micro-Raman mapping images, which were collected using the 532 nm laser, of PLA/Lact and PLA/PEDOT systems, are displayed in Figure a,b, respectively. More specifically, the optical micrograph shown in Figure a1 allows the identification of a nMF from the PLA/Lact bilayer, while Figure a2 stands out the contour of such microfiber once the background is highlighted in pink color. Figure a3,a4 displays the intensity of the l-lactate (white) and PLA (green) marker bands (2900 and 2943 cm–1, respectively), which is evidence that lactate organizes into small particles that decorate the surface of the PLA MFs, in agreement with SEM results (Figure c). Similarly, the PLA/PEDOT system shown in the optical micrograph (Figure b1) is formed by PLA MFs (green; 2943 cm–1) (Figure b2) and PEDOT (blue; 1424 cm–1) (Figure b3). The combination of both micro-Raman mapping images (Figure b4) demonstrates that PEDOT is infiltrated between the PLA fibers.
5.
Optical micrographs (a1 and b1) and micro-Raman mapping images (a2–a4, b2–b4) of PLA/Lact and PLA/PEDOT, respectively. A typical background map (corresponding to the pink area) is displayed in a2 for PLA/Lact, while b4 combines the Raman mapping images collected for PLA and PEDOT.
Properties of the Scaffold
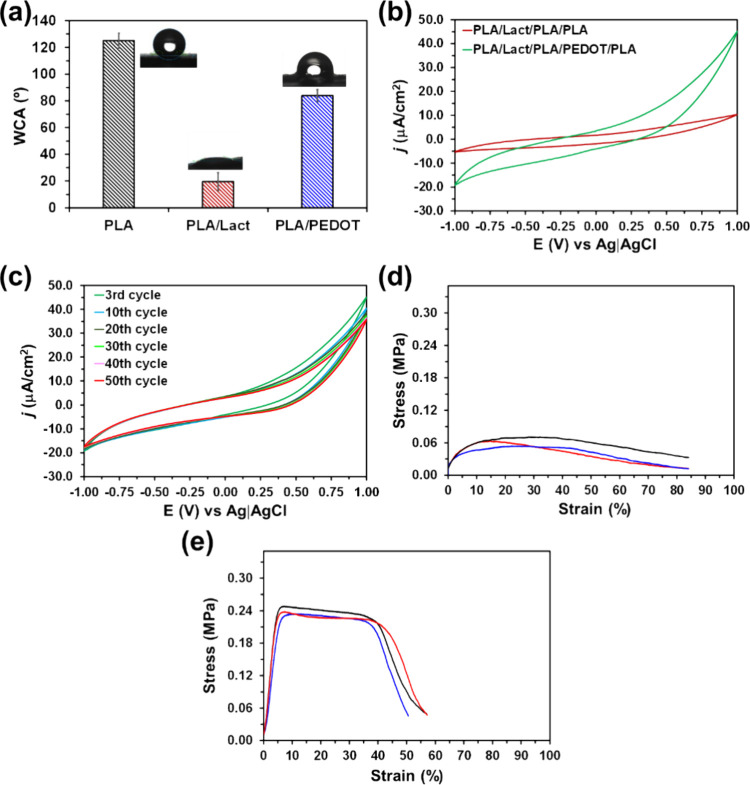
The WCA values obtained for PLA, PLA/Lact, and PLA/PEDOT systems, which are compared in Figure a, show that hydrophobic PLA MFs (125° ± 6°) transform into hydrophilic upon the incorporation of PEDOT:PSS (84° ± 5°) and, especially, lactate (<20°). This change in the surface wettability corroborates that the PLA/Lact/PLA/PEDOT/PLA scaffold contains regions with very different surface properties (i.e., hydrophilic and hydrophobic regions). This heterogeneity is expected to be an advantage in extending the release of added lactate over a larger period of time than the one achieved using only hydrophilic uniform environments, such as the one provided by the Alg hydrogels. Thus, heterogeneity is expected to be a rate-limiting factor in the diffusion of lactate, hindering its immediate release.
6.
(a) Average values of the water contact angle (WCA) for PLA, PLA/Lact, and PLA/PEDOT (standard deviations are indicated). (b) Control cyclic voltammograms recorded for PLA/Lact/PLA/PLA and PLA/Lact/PLA/PEDOT/PLA. (c) Cyclic voltammograms recorded for PLA/Lact/PLA/PEDOT/PLA after 3, 10, 20, 30, 40, and 50 redox cycles. The scan rate used in (b) and (c) was 100 mV/s. (d, e) Stress–strain curves recorded for (d) PLA/Lact and (e) PLA/Lact/PLA/PEDOT/PLA (eight independent samples for each one).
Figure b compares the cyclic voltammograms recorded for PLA/Lact/PLA/PLA and PLA/Lact/PLA/PEDOT/PLA differing only in the presence of PEDOT. Since the areas of the cathodic and anodic scans indicate the electrochemical activity of the scaffold, it can be concluded that the PLA/Lact/PLA/PLA system shows lower electrochemical activity than the scaffold with PEDOT. Thus, the infiltration of PEDOT:PSS between PLA fibers results in a significant increment of the voltammetric charge. Furthermore, the electrochemical response of PLA/Lact/PLA/PEDOT/PLA was maintained with a number of consecutive oxidation and reduction cycles, as shown in Figure c for 50 redox cycles, evidencing the electrochemical stability of the system. Overall, these features indicate that the PEDOT:PSS-containing five-layered scaffold is electroresponsive, which is expected to contribute to the control of lactate release through electrostimulation.
Typical stress–strain curves of PLA/Lact and PLA/Lact/PLA/PEDOT/PLA, which contain one and three layers of PLA MFs, respectively, are plotted in Figure d,e. The mechanical properties extracted from such curves are summarized in Table . It is worth noting that Lact was the only nonpolymeric layer, and, therefore, the comparison between the mechanical properties of PLA and PLA/Lact was considered interesting. No significant difference was found between such one- and two-layered systems (Table ), which was consistent with the organization of Lact not as a continuous layer but as aggregates that adhered to the surface of PLA MFs (Figures c and a3). On the other hand, as it was expected, the strain–stress curves were strongly affected by the number of assembled PLA layers. Thus, the average elastic modulus and maximum strength increased by around 5–6 times when the number of PLA layers grew from one to three, which indicates that the assembly of MF layers results in stronger and more rigid scaffolds. In addition, the elongation at break is around two times smaller for the complete scaffold than for the two-layered system, which is also consistent with the enhanced rigidity associated with the assembly of three PLA MF layers.
1. Mechanical Properties Obtained for PLA/Lact and PLA/Lact/PLA/PEDOT/Lact by Averaging the Results Derived from Strain–Stress Curves of Eight Independent Samples (Standard Deviations Are Indicated).
| PLA | PLA/Lact | PLA/Lact/PLA/PEDOT/PLA | |
|---|---|---|---|
| Young's modulus (MPa) | 0.64 ± 0.17 | 0.75 ± 0.19 | 4.20 ± 0.66 |
| maximum strength (MPa) | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.24 ± 0.01 |
| elongation at break (%) | 78 ± 6 | 83 ± 11 | 44 ± 15 |
Lactate Release
Lactate release from PLA/Lact/PLA/PEDOT/PLA scaffolds by electrostimulation was quantified by UV–vis spectroscopy using the l-lactic acid kit, as described in the Methods section. The calibration plot, which represents the variation of the absorbance against the concentration of lactate, is displayed in Figure a. In addition, control and blank release assays were conducted using PLA/Lact/PLA/PEDOT/PLA and PLA/PLA/PEDOT/PLA scaffolds, respectively, without applying any voltage. The diffusional lactate release (control) was studied in the former assay using five-layered scaffolds identical to those employed for electrostimulation, while for the blank assay, four-layered scaffolds were constructed using the same procedure but without the lactate layer.
7.

(a) Calibration curve used to quantify the amount of lactate released. (b) Cumulative lactate release profiles from PLA/Lact/PLA/PEDOT/PLA scaffolds without and with electrical stimulation (ST). Electrical stimuli consisted of the application of a voltage of −0.5 V for a time interval of 30 min. (c) Strategically chosen times (5 min, 1h, 2 h, 6 h, 24 h, and48 h) at which the electrostimulation protocol was applied. Standard deviations are displayed in parts (a) and (b).
As expected, no lactate was released from the PLA/PLA/PEDOT/PLA scaffolds (not shown) since no lactate was loaded into them, and the time period considered for the release test (72 h) was too short to reflect the degradation of PLA MFs. Figure b shows the cumulative release profiles determined for PLA/Lact/PLA/PEDOT/PLA without and with electrical stimulation. In the absence of electrical stimuli, PLA/Lact/PLA/PEDOT/PLA shows a burst release, which is characterized by the release of a large amount of lactate immediately upon placement of the scaffold in the release medium. After only 5 min, the released lactate was 65 ± 13%, stabilizing after 30 min only with a release of 80 ± 10%. The lactate delivered during the next 71.5 h was very small (∼5%). Overall, the maximum diffusional release after 3 days was 85 ± 1%.
The effect of the electrostimulation on the lactate release was first explored considering different voltages and times, which allowed us to optimize the protocol (results not shown). The application of a constant voltage of −0.5 V during cycles of 30 min was selected as the most reliable, even though such electrostimulation cycles were not applied at regular time intervals but, after a trial and error process to be as close as possible to complete release, at strategically chosen times (5 min, 1 h, 2 h, 6 h, 24 h, and 48 h) (Figure c). As can be seen, a total of six intervals was chosen, which represents a total electrostimulation time of 3 h from a total release time of 72 h.
Although the shape of the electrostimulated release profile was apparently similar to the nonelectrostimulated (diffusional) profile (Figure b), some subtle differences appeared. At short times (<1 h), a burst effect was observed with and without electrostimulation; however, the amount of released lactate was slightly lower with electrostimulation than the one reached without any stimulus (i.e., 74 ± 11% vs 80 ± 10% after 30 min). This feature suggests that initially, the applied negative voltage could affect the transport of lactate molecules, slightly delaying the diffusion of the lactate across the scaffold boundaries. Nevertheless, the statistical analysis of variance using the data obtained during the first 6 h indicated that the difference between the electrostimulated and nonelectrostimulated release profiles was not significant enough at such a time period to clearly identify the effect of applied voltage. On the contrary, when the variance was analyzed using the release profiles comprised between 24 and 72 h, statistically significant differences appeared among them. Thus, after 72 h, the amount of lactate released by electrostimulation increased to 99 ± 3%, evidencing that practically all the loaded lactate was supplied along such 3 days. This was attributed to very subtle structural variations induced by the electrostimulation cycles, especially by the first four ones that were applied at the following times: 5 min, 1 h, 2 h, and 6 h. Thus, such small and visually imperceptible structural changes act as diffusion paths. This result represents a significant advantage since this platform was designed to ensure the sustained release of lactate in the short term (i.e., the first 3 days) before the proteinase K enzyme loaded in PLA-based scaffolds acted.
Prototyping the Scaffold for Sustained Release in Both the Short and Long Terms
Once the complete release of the lactate incorporated in the second layer of the PLA/Lact/PLA/PEDOT/PLA scaffold was achieved within a time period of 72 h, the scaffold was re-engineered to obtain a sustained lactate release over a whole period of around 1–2 weeks. For this purpose, the intermediate (third) layer of PLA MFs was substituted by a layer of electrospun PLA MFs loaded with proteinase K, PLA(K), and further plasma treated. The aim, based on previous studies, is that the cleavage activity of proteinase K ensures the abundant and sustained delivery of lactate over a relatively prolonged period, , and once the intermediate Lact layer is completely released along the first 3 days, the rest of the layers used for the new scaffold, hereafter named PLA/Lact/PLA(K)/PEDOT/PLA, were fabricated by using the same procedure described above for PLA/Lact/PLA/PEDOT/PLA.
Figure displays details of the surface morphology of PLA(K) MFs from the PLA/Lact/PLA(K) system before and after plasma treatment. First, it is important to highlight that the smooth PLA MFs surface previously observed (Figure b) changed when proteinase K was introduced into the MFs. Thus, PLA(K) MFs exhibited abundant nanometric craters (pores) homogeneously distributed at the surface (Figure a), which were ascribed to the phase separation between the organic solvent from the PLA solution and the water from the enzyme solution. Furthermore, the average diameter of the MFs increased up to 4.7 ± 1.8 μm. The effect of the plasma treatment, which produced superficial delamination in PLA MFs (Figure d), was much more pronounced in PLA(K) (Figure b). Thus, plasma-treated PLA(K) MFs showed not only delamination but also grooves and large cavities at the surface. These morphological changes, which were attributed to oxidation, breakage, and detachment of PLA chains, mainly around the above-mentioned nanometric craters, were found to accelerate considerably the cleavage activity of the enzyme by degrading PLA into lactate.
8.
Representative SEM micrographs of PLA(K) (a) before and (b) after plasma treatment.
The release of lactate from PLA/Lact/PLA(K)/PEDOT/PLA was investigated considering four different conditions: (1) room temperature without electrical stimulation (25 °C, without ST); (2) room temperature with electrical stimulation (25 °C, with ST); (3) physiological temperature without electrical stimulation (37 °C, without ST); and (4) physiological temperature with electrical stimulation (37 °C, with ST). We followed the same electrical stimulation protocol as before: a voltage of – 0.5 V was applied for 30 min after certain times covering the first 72 h (Figure c). The accumulated release profiles obtained for the four previous conditions are represented in Figure a, while the daily average amount of released lactate for the same conditions is represented in Figure b.
9.

(a) Accumulated and (b) daily release profiles obtained at 25 (room temperature) and 37 °C (physiological temperature), with and without electrical stimulation. (c) Daily average amount of released lactate considering 10.5 day assays.
Comparison of the diffusional release profiles obtained at 25 and 37 °C without stimulation indicates that the temperature especially affects the release of lactate from the intermediate PLA(K) layer (Figure a,b). Thus, after 3 days, the lactates released at 25 and 37 °C were 18.3 ± 2.0 and 21.8 ± 2.5 mM, respectively, both values being identified with the amount of lactate loaded in the Lact layer (20 mM). Conversely, the effect of the temperature on the activity of the enzyme (from day 3) was much higher as the difference between the profiles at room and physiological temperatures increased with time. The increment in the activity of the enzyme at 37 °C considerably affected the daily average amount of released lactate, which increased from 3.4 ± 3.0 mM at 25 °C to 10.7 ± 2.6 mM at 37 °C (Figure c).
A comparison of the release profiles recorded at room temperature without and with stimulation reveals that the applied external voltage plays a key role in the lactate delivery, not only for the first 3 h but also during the 10.5 studied days. Thus, the accumulated lactates released in the first 72 h at 25 °C without and with electrical stimulation were 18.2 ± 2.0 and 32.6 ± 2.7 mM, increasing after 10.5 days to 37.8 ± 7.7 and 111.8 ± 9.9 mM, respectively (Figure a). These values clearly indicate that the applied electrical voltage affected the activity of proteinase K, accelerating the lactate release from the intermediate PLA(K) layer. Accordingly, after 3 days, the lactate released with stimulation exceeded by ∼65% the amount of lactate loaded in the Lact layer (20 mM). Furthermore, after 10.5 days, the amount of lactate released with stimulation was almost three times that delivered without it (Figure a,b). This affected the daily average amounts of released lactate obtained at 25 °C, which were 3.4 ± 3.0 and 10.2 ± 2.6 mM without and with electrical stimulation, respectively (Figure c).
A similar behavior, but much less pronounced in the long term than at 25 °C, was observed when the two release profiles recorded at physiological temperature were compared (Figure a,b). In this case, the accumulated lactate released in the first 72 h at 37 °C without and with electrical stimulation was 21.8 ± 2.5 and 35.7 ± 3.0 mM, respectively, which represent a global increment of 3–4 mM with respect to 25 °C. The value obtained at 37 °C with stimulation was almost twice the amount of lactate loaded in the Lact layer (20 mM), which was consistent with our previous observation at 25 °C and corroborated the stimulating effect of the applied electrical voltage on the activity of the proteinase K. After 10.5 days, the accumulated release without and with electrical stimulation was of 117.4 ± 9.5 and 131.3 ± 12.0 mM, respectively. Indeed, the daily average amount of released lactate increased by ∼20% (from 10.7 ± 2.6 to 12.8 ± 2.2 mM) when the stimulation was applied at physiological temperature, while such an increment was of ∼200% (from 3.4 ± 3.0 to 10.2 ± 2.6 mM) at room temperature (Figure c). This was attributed to the effect of the temperature on the activity of the enzyme discussed above.
Considering that the daily dose of lactate to enhance cardiomyocyte proliferation is at least 6 mM per day for 10–14 days, the release profiles displayed in Figure indicate that the proposed PLA/Lact/PLA(K)/PEDOT/PLA platform can be used to achieve such doses. Specifically, the activity of the proteinase K enzyme is enhanced by the effect of both temperature and electrical stimulation. The latter affects not only the long-term release (10.5 days) but also the short-term release (≤3 days), which is probably ascribed to the impact of the voltage on the structure of the PLA from the intermediate PLA(K) layer. Thus, the structure of the PLA(K) layer, which is already porous and, in addition, is structurally affected by the plasma treatment (Figure ), is probably further altered by the effect of the electric potential, facilitating the access of the enzyme to the PLA chains. On the other hand, the synergy between the effect of the physiological temperature, which directly speeds the enzymatic reaction without denaturing the enzyme structure, and the effect of the voltage is relatively low, as is deduced from the comparison between the recorded profiles.
Cell Viability
Cell viability MTT assays were performed to examine the toxicity of compounds leached along 3 days from PLA MFs (blank), PLA/Lact/PLA(K) after plasma treatment, and the complete PLA/Lact/PLA(K)/PEDOT/PLA platform, while tissue culture wells were used as control. For this purpose, the C2C12 cell line, which is an immortalized mouse myoblast cell line often used in muscle and cardiac cell research, , was used. Cell viabilities after 1 and 3 days, considering different dilutions of the leached media, are displayed in Figure a,b. As can be seen, no cytotoxic effect was observed, even at the lowest dilution factor, independently of the incubation days. Indeed, no significant between the different studied platforms and the control was observed. Such nontoxicity of the leached compounds was corroborated by confocal microscopy. Figure c shows that for the three studied systems, not only the amount of cells but also the morphology of the cells was very similar to that of the control when no dilution was considered (dilution factor 1).
10.
Cell viability results after (a) 1 and (b) 3 days in a leachable medium from PLA MFs, PLA/Lact/PLA(K) after plasma treatment, and the complete PLA/Lact/PLA(K)/PEDOT/PLA platform. (c) Confocal microscopy images obtained after 1 and 3 days in the leachable medium using a dilution factor of 1.
Conclusions
Because of its biodegradability and biocompatibility, PLA is widely used in biomedical applications, making it an ideal candidate as a lactate supplier for cardiac tissue regeneration.
A novel electroresponsive multilayered scaffold has been successfully designed and fabricated for controlled lactate release combining hydrophobic PLA MFs, lactate, plasma-treated PLA(K) MFs, and PEDOT:PSS. The lactate layer was loaded between hydrophobic PLA MFs, allowing a sustained release over 3 days upon electrostimulation. On the other hand, the combination of PLA MFs and plasma-treated PLA(K) MFs ensured a sustained release of lactate over a prolonged period (>10 days). The largest daily amount of released lactate was obtained by electrostimulation at physiological temperature (∼13 mM/day), even though a sustained daily release of 10–11 mM can be achieved directly at such temperature without stimuli. On the other hand, the nontoxicity of the leached compounds was proved using C2C12 myoblast cells.
Overall, the obtained results demonstrate that a sustained release of different ranges of lactate can be achieved by combining different PLA layers with different functionalities. Lactate must be properly controlled at the heart tissue, in terms of not only concentration but in duration. The scaffold developed in this work has two levels of release: one related to diffusion and the other to the proteinase activity. Both can be tuned with physiological temperature and electric stimulation, resulting in a tailored scaffold for different applications.
Acknowledgments
This publication is part of the I+D+i projects PID2021-125767OB-I00, PLEC2022-009279, PID2021-128412OB-I00, PDC2022-133755-I00, and CEX2023-001300-M funded by MCIN/AEI/10.13039/501100011033 and, as appropriate, by “ERDF A way of making Europe” and European Union Next Generation EU (REGOMUC and BIOMATCH projects). The authors thank the Agència de Gestió d’Ajuts Universitaris i de Recerca (2021 SGR 00387) for financial support. P.A.H.-G. thanks CONACYT (México) for the financial support through a postgraduate scholarship (836603 CVU 347614). S.C. thanks the Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR; Generalitat de Catalunya) for a FI Fellowship. E.E. received funding from the CERCA program by the Generalitat de Catalunya and the ″Centro de Excelencia Severo Ochoa″ (grant CEX2023-001282-S, funded by MICIU/AEI/10.13039/501100011033). M.M.P.-M. thanks the Ministerio de Ciencia, Innovación y Universidades for the Junior Beatriz Galindo Award (BG20/00216). J.G.-T. acknowledges the Serra Hunter program of the Generalitat de Catalunya. Support for the research of E.E. and C.A. was also received through the prize “ICREA Academia” for excellence in research funded by the Generalitat de Catalunya.
The authors declare no competing financial interest.
References
- Dai Y., Lu T., Li L., Zhang F., Xu H., Li H., Wang W., Shao M., Lyu F.. Electrospun Composite PLLA-PPSB Nanofiber Nerve Conduits for Peripheral Nerve Defects Repair and Regeneration. Adv. Healthcare Mater. 2024;13:e2303539. doi: 10.1002/adhm.202303539. [DOI] [PubMed] [Google Scholar]
- Randhawa A., Dutta S. D., Ganguly K., Patil T. V., Lim K. T.. Manufacturing 3D Biomimetic Tissue: A Strategy Involving the Integration of Electrospun Nanofibers with a 3D-Printed Framework for Enhanced Tissue Regeneration. Small. 2024;20:e2309269. doi: 10.1002/smll.202309269. [DOI] [PubMed] [Google Scholar]
- dos Santos F. V., Siqueira R. L., de Morais Ramos L., Yoshioka S. A., Branciforti M. C., Correa D. S.. Silk Fibroin-Derived Electrospun Materials for Biomedical Applications: A Review. Int. J. Biol. Macromol. 2024;254:127641. doi: 10.1016/j.ijbiomac.2023.127641. [DOI] [PubMed] [Google Scholar]
- CeCe R., Jining L., Islam M., Korvink J. G., Sharma B.. An Overview of the Electrospinning of Polymeric Nanofibers for Biomedical Applications Related to Drug Delivery. Adv. Eng. Mater. 2024;26:2301297. doi: 10.1002/adem.202301297. [DOI] [Google Scholar]
- Mannai F., Elhleli H., Feriani A., Otsuka I., Belgacem M. N., Moussaoui Y.. Electrospun Cactus Mucilage/Poly(vinyl alcohol) Nanofibers as a Novel Wall Material for Dill Seed Essential Oil (Anethum graveolens L.) Encapsulation: Release and Antibacterial Activities. ACS Appl. Mater. Interfaces. 2023;15:58815–58827. doi: 10.1021/acsami.3c13289. [DOI] [PubMed] [Google Scholar]
- Gürtler A. L., Rades T., Heinz A.. Electrospun Fibers for the Treatment of Skin Diseases. J. Controlled Release. 2023;363:621–640. doi: 10.1016/j.jconrel.2023.10.009. [DOI] [PubMed] [Google Scholar]
- Kaniuk Ł., Stachewicz U.. Development and Advantages of Biodegradable PHA Polymers Based on Electrospun PHBV Fibers for Tissue Engineering and Other Biomedical Applications. ACS Biomater Sci. Eng. 2021;7:5339–5362. doi: 10.1021/acsbiomaterials.1c00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasmal P., Datta P.. Tranexamic Acid-Loaded Chitosan Electrospun Nanofibers as Drug Delivery System for Hemorrhage Control Applications. J. Drug Delivery Sci. Technol. 2019;52:559–567. doi: 10.1016/j.jddst.2019.05.018. [DOI] [Google Scholar]
- Ghasemiyeh P., Mohammadi-Samani S., Nokhodchi A.. An Overview of Nanofibers and Microfibers for Improved Oral Delivery of Medicines: Challenges and Advances. J. Drug Delivery Sci. Technol. 2024;91:105235. doi: 10.1016/j.jddst.2023.105235. [DOI] [Google Scholar]
- Bishnoi A., Tiwari R. K., Chanda S., Ajmal G., Bonde G. V.. Elecrospun Nanofibers: The Versatile Platform as a Drug Delivery System in Healthcare. J. Drug Delivery Sci. Technol. 2023;90:105127. doi: 10.1016/j.jddst.2023.105127. [DOI] [Google Scholar]
- Rambhia K. J., Ma P. X.. Controlled Drug Release for Tissue Engineering. J. Controlled Release. 2015;219:119–128. doi: 10.1016/j.jconrel.2015.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah E., Wu Z., Thakur S. S., O’Carroll S. J., Svirskis D.. Externally Triggered Release of Growth Factors - A Tissue Regeneration Approach. J. Controlled Release. 2021;332:74–95. doi: 10.1016/j.jconrel.2021.02.015. [DOI] [PubMed] [Google Scholar]
- Li L., Hao R., Qin J., Song J., Chen X., Rao F., Zhai J., Zhao Y., Zhang L., Xue J.. Electrospun Fibers Control Drug Delivery for Tissue Regeneration and Cancer Therapy. Adv. Fiber Mater. 2022;4:1375–1413. doi: 10.1007/s42765-022-00198-9. [DOI] [Google Scholar]
- Hu B., Gao M., Boakye-Yiadom K. O., Ho W., Yu W., Xu X., Zhang X.-Q.. An Intrinsically Bioactive Hydrogel with On-Demand Drug Release Behaviors for Diabetic Wound Healing. Bioact. Mater. 2021;6:4592–4606. doi: 10.1016/j.bioactmat.2021.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Wang L., Luan X., Pang Y., Zhang K., Cheng Y., Ji Z., Pang J.. Adhesive Injectable Cellulose-Based Hydrogels with Rapid Self-Healing and Sustained Drug Release Capability for Promoting Wound Healing. Carbohydr. Polym. 2023;320:121235. doi: 10.1016/j.carbpol.2023.121235. [DOI] [PubMed] [Google Scholar]
- Ordoño J., Perez-Amodio S., Ball K., Aguirre A., Engel E.. The Generation of a Lactate-Rich Environment Stimulates Cell Cycle Progression and Modulates Gene Expression on Neonatal and hiPSC-Derived Cardiomyocytes. Biomater. Adv. 2022;139:213035. doi: 10.1016/j.bioadv.2022.213035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A., Montserrat N., Zacchigna S., Nivet E., Hishida T., Krause M. N., Kurian L., Ocampo A., Vazquez-Ferrer E., Rodriguez-Esteban C., Kumar S., Moresco J. J., Yates J. R., Campistol J. M., Sancho-Martinez I., Giacca M., Izpisua Belmonte J. C.. In Vivo Activation of a Conserved MicroRNA Program Induces Mammalian Heart Regeneration. Cell Stem Cell. 2014;15:589–604. doi: 10.1016/j.stem.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Lancet Regional Health – Europe. Navigating Disparities in Cardiovascular Disease Outcomes Across Europe: A Call to Action. Lancet Reg. Health – Eur. 2023;33:100746. doi: 10.1016/j.lanepe.2023.100746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macor L.-P., Colombi S., Tamarit J. L., Engel E., Pérez-Madrigal M. M., García-Torres J., Alemán C.. Immediate-Sustained Lactate Release Using Alginate Hydrogel Assembled to Proteinase K/Polymer Electrospun Fibers. Int. J. Biol. Macromol. 2023;238:124117. doi: 10.1016/j.ijbiomac.2023.124117. [DOI] [PubMed] [Google Scholar]
- Colombi S., Macor L. P., Ortiz-Membrado L., Pérez-Amodio S., Jiménez-Piqué E., Engel E., Pérez-Madrigal M. M., García-Torres J., Alemán C.. Enzymatic Degradation of Polylactic Acid Fibers Supported on a Hydrogel for Sustained Release of Lactate. ACS Appl. Bio Mater. 2023;6:3889–3901. doi: 10.1021/acsabm.3c00546. [DOI] [PubMed] [Google Scholar]
- Chavarria V., Ortiz-Islas E., Salazr A., Pérez de la Cruz V., Espinosa-Bonilla A., Figueroa R., Ortíz-Plata A., Sotelo J., Sánchez-García F. J., Pinea B.. Lactate-Loaded Nanoparticles Induce Glioma Cytotoxicity and Increase the Survival of Rats Bearing Malignant Glioma Brain Tumor. Pharmaceutics. 2022;14:327. doi: 10.3390/pharmaceutics14020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trindade A. R., Kiyohara P. K., de Araujo P. S., Bueno da Costa M. H.. PLGA Microspheres Containing Bee Venom Proteins for Preventive Immunotherapy. Int. J. Pharm. 2012;423:124–133. doi: 10.1016/j.ijpharm.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Puiggalí-Jou A., Ordoño J., del Valle L. J., Pérez-Amodio S., Engel E., Alemán C.. Tuning Multilayered Polymeric Self-Standing Films for Controlled Release of L-Lactate by Electrical Stimulation. J. Controlled Release. 2021;330:669–683. doi: 10.1016/j.jconrel.2020.12.049. [DOI] [PubMed] [Google Scholar]
- Bonferoni M. C., Giunchedi P., Scalia S., Rossi S., Sandri G., Caramella C.. Chitosan Gels for the Vaginal Delivery of Lactic Acid: Relevance of Formulation Parameters to Mucoadhesion and Release Mechanisms. AAPS PharmSciTech. 2006;7:E141–E147. doi: 10.1208/pt0704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Yang L., Liu N.. Recent Progress on Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) Bioelectrodes. Small Sci. 2023;3:2300008. doi: 10.1002/smsc.202300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Zhang L., Guan X., Cheng H., Liu X., Yu S., Wei J., Ouyang J.. Biocompatible Conductive Polymers with High Conductivity and High Stretchability. ACS Appl. Mater. Interfaces. 2019;11:26185–26193. doi: 10.1021/acsami.9b07325. [DOI] [PubMed] [Google Scholar]
- Wang Z., Liu R.. PEDOT:PSS-Based Electrochromic Materials for Flexible and Stretchable Devices. Mater. Today Electron. 2023;4:100036. doi: 10.1016/j.mtelec.2023.100036. [DOI] [Google Scholar]
- Frisch, M. J. ; Trucks, G. W. ; Schlegel, H. B. ; Scuseria, G. E. ; Robb, M. A. ; Cheeseman, J. R. ; Scalmani, G. ; Barone, V. ; Petersson, G. A. ; Nakatsuji, H. ; Li, X. ; Caricato, M. ; Marenich, A. V. ; Bloino, J. ; Janesko, B. G. ; Gomperts, R. ; Mennucci, B. ; Hratchian, H. P. ; Ortiz, J. V. ; Izmaylov, A. F. ; Sonnenberg, J. L. ; Williams-Young, D. ; Ding, F. ; Lipparini, F. ; Egidi, F. ; Goings, J. ; Peng, B. ; Petrone, A. ; Henderson, T. ; Ranasinghe, D. ; Zakrzewski, V. G. ; Gao, J. ; Rega, N. ; Zheng, G. ; Liang, W. ; Hada, M. ; Ehara, M. ; Toyota, K. ; Fukuda, R. ; Hasegawa, J. ; Ishida, M. ; Nakajima, T. ; Honda, Y. ; Kitao, O. ; Nakai, H. ; Vreven, T. ; Throssell, K. ; Montgomery, J. A., Jr. ; Peralta, J. E. ; Ogliaro, F. ; Bearpark, M. J. ; Heyd, J. J. ; Brothers, E. N. ; Kudin, K. N. ; Staroverov, V. N. ; Keith, T. A. ; Kobayashi, R. ; Normand, J. ; Raghavachari, K. ; Rendell, A. P. ; Burant, J. C. ; Iyengar, S. S. ; Tomasi, J. ; Cossi, M. ; Millam, J. M. ; Klene, M. ; Adamo, C. ; Cammi, R. ; Ochterski, J. W. ; Martin, R. L. ; Morokuma, K. ; Farkas, O. ; Foresman, J. B. ; Fox, D. J. . Gaussian 16, Revision C.01, Gaussian, Inc.: Wallingford CT, 2016. [Google Scholar]
- Becke A. D.. Thermochemistry Density-Functional III. The Role of Exact Exchange. J. Chem. Phys. 1993;98:5648–5652. doi: 10.1063/1.464913. [DOI] [Google Scholar]
- Lee C., Yang W., Parr R. G.. Development of the Colle-Salvetti Correlation-Energy Formula Into a Functional of the Electron Density. Phys. Rev. B. 1988;37:785–789. doi: 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- Vosko S. H., Wilk L., Nusair M.. Accurate Spin-Dependent Electron Liquid Correlation Energies for Local Spin Density Calculations: A Critical Analysis. Can. J. Phys. 1980;58:1200–1211. doi: 10.1139/p80-159. [DOI] [Google Scholar]
- Miertus S., Scrocco E., Tomasi J.. Electrostatic Interaction of a Solute with a Continuum. a Direct Utilization of Ab Initio Molecular Potentials for the Prevision of Solvent Effects. Chem. Phys. 1981;55:117–129. doi: 10.1016/0301-0104(81)85090-2. [DOI] [Google Scholar]
- Miertus S., Tomasi J.. Approximate Evaluations of the Electrostatic Free Energy and Internal Energy Changes in Solution Processes. Chem. Phys. 1982;65:239–245. doi: 10.1016/0301-0104(82)85072-6. [DOI] [Google Scholar]
- Fontana-Escartín A., Lanzalaco S., Bertran O., Alemán C.. Electrochemical Multi-Sensors Obtained by Applying an Electric Discharge Treatment to 3D-Printed Poly(lactic acid) Appl. Surf. Sci. 2022;597:153623. doi: 10.1016/j.apsusc.2022.153623. [DOI] [Google Scholar]
- Fontana-Escartín A., Lanzalaco S., Pérez-Madrigal M. M., Bertran O., Alemán C.. Electrochemical Activation for Sensing of Three-Dimensional-Printed Poly(lactic acid) Using Low-Pressure Plasma. Plasma Processes Polym. 2022;19:e2200101. doi: 10.1002/ppap.202200101. [DOI] [Google Scholar]
- Alhashmi Alamer F., Althagafy K., Alsalmi O., Aldeih A., Alotaiby H., Althebaiti M., Alghamdi H., Alotibi N., Saeedi A., Zabarmawi Y., Hawsawi M., Alnefaie M. A.. Review on PEDOT:PSS-Based Conductive Fabric. ACS Omega. 2022;7:35371–35386. doi: 10.1021/acsomega.2c01834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W., Gwon G., Ha J. H., Kim D., Eom K. J., Park J. H., Kang S. J., Kwak B., Hong J. I., Lee S., Hyun D. C., Lee S.. Enhancing the Conductivity of PEDOT:PSS Films for Biomedical Applications Via Hydrothermal Treatment. Biosens Bioelectron. 2021;171:112717. doi: 10.1016/j.bios.2020.112717. [DOI] [PubMed] [Google Scholar]
- Lehane R. A., Gamero-Quijano A., Malijauskaite S., Holzinger A., Conroy M., Laffir F., Kumar A., Bangert U., McGourty K., Scanlon M. D.. Electrosynthesis of Biocompatible Free-Standing PEDOT Thin Films at a Polarized Liquid|Liquid Interface. J. Am. Chem. Soc. 2022;144:4853–4862. doi: 10.1021/jacs.1c12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina B. G., Cuesta S., Besharatloo H., Roa J. J., Armelin E., Alemán C.. Free-Standing Faradaic Motors Based on Biocompatible Nanoperforated Poly(lactic acid) Layers and Electropolymerized Poly(3,4-ethylenedioxythiophene) ACS Appl. Mater. Interfaces. 2019;11:29427–29435. doi: 10.1021/acsami.9b08678. [DOI] [PubMed] [Google Scholar]
- Ocampo C., Oliver R., Armelin E., Alemán C., Estrany F.. Electrochemical Synthesis of Poly(3,4-ethylenedioxythiophene) on Steel Electrodes: Properties and Characterization. J. Polym. Res. 2006;13:193–200. doi: 10.1007/s10965-005-9025-7. [DOI] [Google Scholar]
- Poater J., Casanovas J., Solà M., Alemán C.. Examining the Planarity of Poly(3,4-ethylenedioxythiophene): Consideration of Self-Rigidification, Electronic, and Geometric Effects. J. Phys. Chem. A. 2010;114:1023–1028. doi: 10.1021/jp908764s. [DOI] [PubMed] [Google Scholar]
- De Santis P., Kovacs A. J.. Molecular Conformation of Poly(S-lactic acid) Biopolymers. 1968;6:299–306. doi: 10.1002/bip.1968.360060305. [DOI] [PubMed] [Google Scholar]
- Babeli I., Ruano G., Casanovas J., Ginebra M. P., García-Torres J., Alemán C.. Conductive, Self-Healable and Reusable Poly(3,4-ethylenedioxythiophene)-Based Hydrogels for Highly Sensitive Pressure Arrays. J. Mater. Chem. A. 2020;8:8654–8667. doi: 10.1039/D0TC01947J. [DOI] [Google Scholar]
- Saborío M. G., Svelic P., Casanovas J., Ruano G., Pérez-Madrigal M. M., Franco L., Torras J., Estrany F., Alemán C.. Hydrogels for Flexible and Compressible Free Standing Cellulose Supercapacitors. Eur. Polym. J. 2019;118:347–357. doi: 10.1016/j.eurpolymj.2019.06.011. [DOI] [Google Scholar]
- Rodríguez-Ropero F., Casanovas J., Alemán C.. Ab Initio Calculations on π-Stacked Thiophene Dimer, Trimer, and Tetramer: Structure, Interaction Energy, Cooperative Effects, and Intermolecular Electronic Parameters. J. Comput. Chem. 2008;29:69–78. doi: 10.1002/jcc.20763. [DOI] [PubMed] [Google Scholar]
- Su L., Zou J., Dong S., Hao N., Xu H.. Influence of Different β-Nucleation Agents on Poly(L-lactic acid): Structure, Morphology, and Dynamic Mechanical Behavior. RSC Adv. 2017;7:55364–55370. doi: 10.1039/C7RA10550A. [DOI] [Google Scholar]
- Liu Y., Jiang S., Yan W., He M., Qin J., Qin S., Yu J.. Crystallization Morphology Regulation on Enhancing Heat Resistance of Polylactic Acid. Polymers. 2020;12:1563. doi: 10.3390/polym12071563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayemitte P. E., Gerliani N., Raymond P., Aïder M.. Study of the Electro-Activation Process of Calcium Lactate, Calcium Ascorbate Solutions, and Their Equimolar Mixture: Assessment of Their Physicochemical Properties. ACS Omega. 2021;6:8531–8547. doi: 10.1021/acsomega.1c00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua S., Ma H., Li X., Yang H., Wang A.. pH-Sensitive Sodium Alginate/Poly(vinyl alcohol) Hydrogel Beads Prepared by Combined Ca2+ Crosslinking and Freeze-Thawing Cycles for Controlled Release of Diclofenac Sodium. Int. J. Biol. Macromol. 2010;46:517–523. doi: 10.1016/j.ijbiomac.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Yuniarto K., Purwanto Y. A., Purwanto S., Welt B. A., Purwadaria H. K., Sunarti T. C.. Infrared and Raman Studies on Polylactide Acid and Polyethylene Glycol-400 Blend. AIP Conf. Proc. 2016;1725:020101. doi: 10.1063/1.4945555. [DOI] [Google Scholar]
- Shakoor A., Rizvi T. Z.. Raman Spectroscopy of Conducting Poly(methylmethacrylate)/Polyaniline-Dodecylbenzenesulfonate Blends. J. Raman Spectrosc. 2010;41:237–240. doi: 10.1002/jrs.2414. [DOI] [Google Scholar]
- Salvatierra R. V., Moura L. G., Oliveira M. M., Pimienta M. A., Zarbin A. J. G.. Resonant Raman Spectroscopy and Spectroelectrochemistry Characterization of Carbon Nanotubes/Polyaniline Thin Film Obtained Through Interfacial Polymerization. J. Raman Spectrosc. 2012;43:1094–1100. doi: 10.1002/jrs.3144. [DOI] [Google Scholar]
- Saborio M. G., Bertran O., Lanzalaco S., Häring M., Franco L., Puiggalí J., Díaz D. D., Estrany F., Alemán C.. Isomeric Cationic Ionenes as n-Dopant Agents of Poly(3,4-ethylenedioxythiophene) for In Situ Gelation. Soft Matter. 2018;14:6374–6385. doi: 10.1039/C8SM00969D. [DOI] [PubMed] [Google Scholar]
- Alessandri I., Torricelli F., Cerea B., Speziani M., Romele P., Kovacs-Vajna Z. M., Vassalini I.. Why PEDOT:PSS Should not Be Used for Raman Sensing of Redox States (and How It Could Be) ACS Appl. Mater. Interfaces. 2022;14:56363–56373. doi: 10.1021/acsami.2c17147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebedin E., Mille M., Speiser M., Zarrabi T., Sandtner W., Latzenhofer B., Todt H., Hilber K.. C2C12 Skeletal Muscle Cells Adopt Cardiac-Like Sodium Current Properties in a Cardiac Cell Environment. AJP-Heart Circ. 2007;292:H439–H450. doi: 10.1152/ajpheart.00119.2006. [DOI] [PubMed] [Google Scholar]
- Koh G. Y., Klug M. G., Soonpaa M. N., Field L. J.. Differentiation and Long-Term Survival of C2C12 Myoblast Grafts in Heart. J. Clin. Invest. 1993;92:1548–1554. doi: 10.1172/JCI116734. [DOI] [PMC free article] [PubMed] [Google Scholar]