Abstract
Innate, cellular and humoral immunity in acute HIV infection (AHI) play crucial roles in dictating immunological and pathological outcomes. However, understanding immune cell dynamics in different compartments during AHI is limited. In this study, we characterized immune cells from matched blood and lymph node samples in the RV254 Thai AHI cohort, the RV304 Thai chronic HIV infection cohort, and HIV-negative individuals, using flow cytometry. Our results showed a significant loss of the CD4:CD8 ratio in both PBMCs and LNMCs during AHI. Similarly, we observed increased immune activation of CD4+ and CD8+ T cells in both blood and lymph node compartments during AHI. In contrast, we found no increase in T follicular helper cells (Tfh) and a lack of association between circulating Tfh and lymph node Tfh during AHI. Furthermore, early B cell depletion, particularly in resting memory B cells, was observed in blood but not in lymph nodes during AHI. Additionally, during AHI, plasmacytoid dendritic cell activation was increased in lymph nodes but not in blood. These findings suggest that certain immune cell phenotypes and dynamics are unique in lymph nodes compared to blood during AHI. Understanding the immune cell alteration in these compartments during AHI may help define the mechanisms leading to lack of immune control in natural HIV infection.
Keywords: Acute HIV infection (AHI), Immune cell subsets, Blood, Lymph nodes
1. Introduction
The initial host-pathogen interactions after HIV acquisition are critically important for determining disease progression and outcome even in the presence of antiretroviral therapy (ART). HIV-induced damage appears very early after infection including early depletion of CD4 T cells in mucosal tissues that are not recovered after initiation of ART and early seeding of the HIV reservoir in lymphoid tissues.1, 2, 3, 4, 5, 6 Acute HIV infection (AHI) can be divided into 5 stages based on HIV test results: Stage 1 (S1) is when HIV RNA can initially be detected by PCR; S2 is marked by detection of p24 antigen; S3 corresponds with peak viremia and the detection of anti-HIV antibodies by ELISA; and S4 and S5 encompass the decline in viremia to set point and increasing detection of anti-HIV antibodies by Western blot.7,8 Through twice weekly monitoring of individuals living in Thailand and East Africa who were at high risk for HIV infection, it was shown that peak viremia and viral-load set point were reached approximately 13 and 31 days after first detection of virus, respectively.9 Further analysis of viral sequences from these individuals suggested that HIV infection occurred about 1 week prior to detectable viremia.10 This provides a short window in which to identify individuals in AHI for immunological studies into the earliest immune responses after HIV infection. Critical immunological events occur during the first few weeks of infection in blood and tissues but have not be fully characterized due to the difficulty to enroll participants at these early stages of infection. HIV can be detected in peripheral lymph nodes in S1 and reaches peak levels as early as S2 of AHI.3 Data available in these early stages of infection have been mainly generated in blood due to limited access to tissue samples, as lymph node biopsies are invasive and optional for participants during AHI, albeit lymphoid tissues are a main site of HIV replication and reservoir seeding and the main tissue where immune responses are primed. In many instances, observations in blood are being used as surrogate for immune processes in lymphoid tissues without validation that this extrapolation could be made. Therefore, it is of utmost importance to characterize immune responses during AHI in blood and tissues.
Plasmacytoid dendritic cells (pDCs) are important innate immune mediators that provide a rapid and robust initial type I interferon response after viral infection.11 Loss of both pDCs and myeloid dendritic cells (mDCs) in the blood has been noted in people living with HIV, with their decline correlating with loss of CD4+ T cells after peak viremia.12, 13, 14 Studies of SIV infection in nonhuman primates showed that a similar decrease in pDC frequency in the blood was associated with trafficking to secondary lymphoid organs.15, 16, 17 This trafficking to lymphoid organs is important because, beyond a role in inhibiting viral replication,18 type I interferons and pDCs have been shown to play an important role in T cell priming in cooperation with myeloid dendritic cells (mDCs).19, 20, 21
In acute viral infections, T cells are primed by antigen presenting mDCs in the T cell zones of the lymph node resulting in their proliferation and differentiation into effector cells. A significant increase in activated and proliferating CD8+ T cells in the blood starting in S3 of AHI has been associated with the subsequent decrease in plasma viral load.22, 23, 24, 25 However, this response is not sufficient to completely control the virus in most individuals and prolonged antigen exposure leads to CD8+ T cell dysfunction.26 While CD8+ T cell frequencies increase after peak viremia, CD4+ T cell frequencies decline.9 In particular, it has been shown that significant depletion of gut mucosal CD4+ T cells occurs in S3 of AHI, particularly those with a T helper 17 (Th17) phenotype.1,27 In contrast, untreated HIV infection has been associated with expansion of T follicular helper cells (Tfh) in hyperblastic germinal centers in the lymph nodes.28,29 While the main role of Tfh is to provide B cell help in the germinal center, analysis of circulating Tfh (cTfh) in the peripheral blood has shown Tfh dysfunction and reduced capacity to provide B cell help in S3 of AHI.30, 31, 32
Perturbations in the B cell compartment have long been associated with HIV infection. An early study found a decreased frequency of resting B cells in the blood of seropositive individuals with a concomitant increase in activated and immature B cells.33 More recent work has shown that frequencies of resting memory B cells (CD27+CD21+) declined in the blood within 7 days of detection of plasma viral load in regularly monitored women at high risk for HIV infection.34 This was associated with increased frequencies of activated (CD27+CD21–) and tissue-like (CD27–CD21–) memory B cells, the latter of which include exhausted B cells with disease progression.34,35
To date, much of our knowledge about immune responses in AHI has been discovered through analysis of cellular dynamics in the peripheral blood collected later in AHI, after peak viremia and the onset of clinical symptoms, but activation and differentiation of adaptive immune responses occurs in the tissues. Therefore, the current study aimed to characterize changes in immune cell subsets in peripheral blood mononuclear cells (PBMCs) and lymph node mononuclear cells (LNMCs) to determine if similar changes are seen in both of these compartments. Matching peripheral blood and inguinal lymph node biopsies were collected at the time of diagnosis during AHI from participants in the RV254/SEARCH010 Thai cohort36 and cellular frequencies and phenotypes were measured by flow cytometry. This information could be essential for understanding the development, maintenance and dysfunction of the earliest humoral and cellular immune responses to HIV infection.
2. Materials and methods
2.1. Study participants
Matching PBMCs and LNMCs were analyzed from a total of 68 men who have sex with men (MSM) enrolled in the RV254/SEARCH010 (ClinicalTrials.gov NCT00796146) or RV304/SEARCH013 (ClinicalTrials.gov NCT01397669) cohorts in Bangkok, Thailand. The RV254/SEARCH010 study enrolls participants who are diagnosed in the earliest stages of acute HIV infection (AHI) and immediately initiate antiretroviral therapy (ART) (NCT00796263).1 Participants initiate ART within 3 days of enrollment or diagnosis of AHI. The lymph node procedure did not delay the initiation of ART and was performed on average 2 days after enrollment. The stage of AHI at diagnosis was classified using HIV nucleic acid test (NAT), 3rd generation enzyme immunoassay (3rd Gen EIA), 4th Gen EIA, and Western blot (WB) as previously described7,8: Stage 1 (S1) - NAT+, 4th Gen EIA-, 3rd Gen EIA-; S2 - NAT+, 4th Gen EIA+, 3rd Gen EIA-; S3 - NAT+, 4th Gen EIA+, 3rd Gen+, WB-; S4 - NAT+, 4th Gen EIA+, 3rd Gen EIA+, WB indeterminate; and S5 - NAT+, 4th Gen EIA+, 3rd Gen EIA+, WB+. Matching PBMCs and LNMCs were available from 44 participants in AHI prior to ART initiation (Table 1). The RV304/SEARCH013 study enrolls individuals with chronic HIV infection (CHI) and those who are HIV-negative (HIV−). Matching PBMCs and LNMCs were obtained from 8 participants with untreated CHI. Samples from 16 HIV− participants were included for comparison.
Table 1.
Clinical characterization of people in acute (RV254, AHI) or chronic (RV304B, CHI) HIV infection and people without HIV (RV304A, HIV−) (n = 68).
| Study Cohorts | HIV infection status | Number of participants | Median age (years) | Median CD4+ T cells (cells/cu.mm.) | Median plasma HIV-RNA (copies/ml) |
|---|---|---|---|---|---|
| RV304A | HIV- | 16 | 31 (19–40) | – | – |
| RV254 | AHI Stage1 (S1) | 11 | 26 (20–54) | 654 (307-1302) | 8,970 (789-68,393) |
| RV254 | AHI Stage2 (S2) | 12 | 25 (18–44) | 290 (158–773) | 848,856 (133,705–17,867,500) |
| RV254 | AHI Stage3 (S3) | 13 | 24 (21–60) | 278 (170–794) | 1,456,069 (24,663–31,650,200) |
| RV254 | AHI Stage4/5 (S4/5) | 8 | 30 (20–40) | 477 (233–625) | 684,789 (100,793–6,936,786) |
| RV304B | CHI | 8 | 26 (20–35) | 338 (149–616) | 45,485 (12,200–621,473) |
2.2. Ethics statement
Informed consent was obtained from all participants prior to inclusion in the studies. All studies were approved by the Chulalongkorn University (RV254/SEARCH010; COA No. 835/2008, IRB No. 220/51 and RV304/SEARCH013; COA No. 229/2010, IRB No. 062/53) and Walter Reed Army Institute of Research institutional review boards (RV254/SEARCH010; WRAIR No. 1494, OHRO Log No. A-14430 and RV304/SEARCH013; WRAIR No. 1751, HRPO Log No. A-16391).
2.3. Sample preparation
PBMCs were isolated from peripheral blood by density gradient separation using Ficoll Pacque PLUS (Cytiva, Marlborough, MA). After PBMC separation, cells were washed, counted, and then cryopreserved in fetal bovine serum supplemented with 10 % dimethyl sulfoxide. LNMCs were isolated from inguinal lymph node as previously described.3 We have previously reported that lymph node biopsies are safe and well tolerated in AHI individuals37. After removal of the surrounding fatty tissue, LNs were minced and passed through a 70 μm nylon cell strainer. LNMCs were washed, counted, and then cryopreserved in fetal bovine serum supplemented with 10 % dimethyl sulfoxide.
2.4. Antibodies and reagent for flow cytometry
AlexaFluor 647-labeled anti-CXCR5 (clone RF8B2), AF700-labeled anti-CD3 (clone UCHT1), allophycocyanin H7-labeled anti-CD45RA (clone HI100), BUV395-labeled anti-CD40 (clone 5C3), BUV395-labeled anti-Ki-67 (clone B56), BUV496-labeled anti-CD4 (clone SK3), BUV737- labeled anti-CD69 (clone FN50), BV421-labeled anti-CD27 (clone M-T271), BV510-labeled anti-CD3 (clone UCHT1), FITC-labeled anti-BCl2, were obtained from BD Biosciences. AF700-labeled anti-CD45 (clone 2D1), APC-labeled anti-IgG (clone M1310G05), BV510-labeled anti-CD19 (clone SJ25C1), BV510-labeled anti-CD56 (clone 5.1H11), BV605-labeled anti-CD86 (clone BU63), BV650-labeled anti-CD20 (clone 2H7), BV711-labeled anti-HLA-DR (clone L243), BV785-labeled anti-CD8 (clone RPA-T8), BV785-labeled anti-CD14 (clone M5E2), BV785-labeled anti-CD19 (clone HIB19), Pacific Blue labeled anti-CD11c (clone 3.9), Pacific Blue-labeled anti-Perforin (clone dG9), PE-labeled anti-CD303 (clone 201A), PE-Cy7-labeled anti-PD1 (clone EH12.2H7), PE/Dazzle594-labeled anti-CD1c (clone L161), PE/Dazzle594-labeled anti-CD10 (clone HI10a), PerCP Cy5.5-labeled anti-CD21 (clone Bu32), were obtained from BioLegend. LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (LIVE/DEAD) was from Thermo Fisher Scientific (Molecular Probes). The list of antibodies is shown in the Supplementary Table 1.
2.5. Phenotype staining and analysis by flow cytometry
Frozen PBMC and LNMCs matching were thawed and stained for innate cells, CD8+ T cells, CD4+ T cells, and B cells panels (Supplementary Table 2). For the innate cells and B panel, one million cells in each panel were washed with phosphate-buffered saline containing 2 % fetal bovine serum (2% FBS/PBS; FACS buffer) and first stained with amine-reactive viability dye (Live/Dead Aqua; Life Technologies) for 10 min at RT. The cells were stained for 20 min at 4 °C with the surface markers in the dark. After washed with FACS buffer, cells were fixed with 2 % paraformaldehyde (PFA) and analyzed by flow cytometry (LSRII, BD biosciences). For the CD4+ and CD8+ T cells panels (Supplementary Table 2), one million cells were washed with FACS buffer and first stained with Live/Dead Aqua for 10 min at RT. Then, cells were stained with the other cell surface markers for 20 min at 4 °C followed by 2 times wash with FACS buffer. The cells were fixed/permeabilized in FoxP3 permeabilization buffer (Life Technologies) and stained for Bcl-2, Ki-67 and Perforin, for 30 min at room temperature (RT). After being washed with FACS buffer, cells were fixed with 2% PFA and analyzed by flow cytometry (LSRII, BD biosciences). All data were analyzed using FlowJo (Ashland, OR).
2.6. Statistics
All statistical analyses were conducted using GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA). Differences in population frequencies between HIV-positive (HIV+) groups and HIV-negative (HIV−) controls were measured using the Kruskal-Wallis test with Dunn's multiple comparison test. Differences in cellular frequencies between PBMCs and LNMCs were measured with the Wilcoxon matched-pairs test. Correlations were analyzed with the nonparametric Spearman test. Significance was defined as p < 0.05 for two-sided testing.
3. Results
3.1. Demographics and clinical data from the participants analyzed
Sixty-eight men of similar age living with HIV were enrolled, the majority of whom were found to have acquired the CRF01_AE subtype (Table 1). Highest CD4+ T cell counts were found in AHI S1 (654 cells/cu.mm., range 307–1302 cells/cu.mm.) and peak plasma HIV-RNA at AHI S3 (1,456,069 copies/ml, range 24,633–31,650,200 copies/ml). The LNMC counts and inguinal lymph node size were not different compared to HIV-negative individuals (Supp Fig. 1A and 1B). LNMC numbers recovered from half a lymph node ranged from 5.75x106 to 102.5x106 cells (median 30.1x106 cells) for participants living with HIV and from 2.5x106 to 86x106 cells (median 22.5x106 cells) for HIV− individuals. The lymph node size ranged from 4 to 45 mm (median 13 mm) for participants living with HIV and from 6 to 45 mm (median 10.25 mm) for HIV− individuals. There was a significant correlation between the LNMC counts and lymph node sizes (r = 0.45, p = 0.0023) (Supp Fig. 1C). These results indicate that the LNMC recovery was dependent on lymph node size rather than AHI stage in our study.
3.2. Loss of CD4+ T cells and CD4:CD8 ratio in both PBMCs and LNMCs during AHI
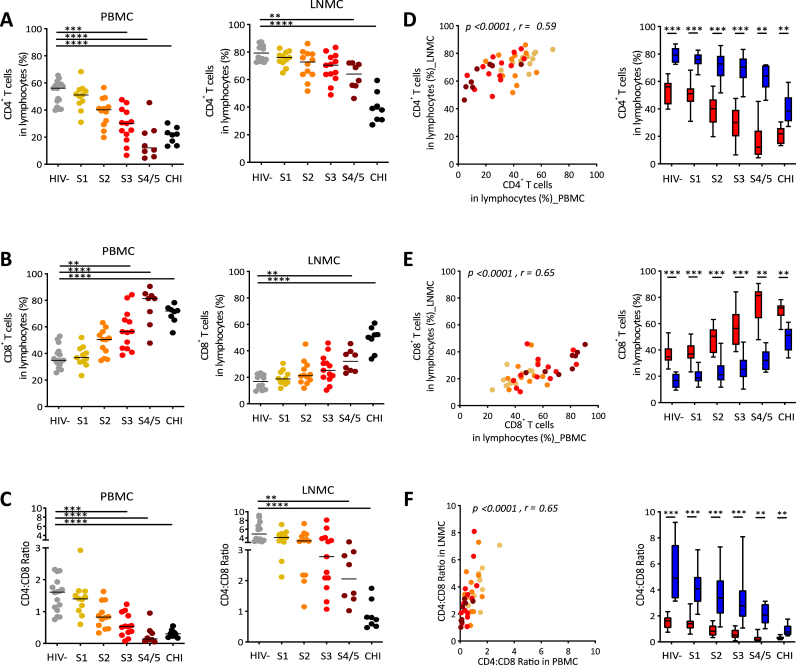
We investigated changes in CD4+ and CD8+ T cells within the PBMCs and LNMCs (Fig. 1 and Supp Fig. 3). We found that the CD4+ T cell frequencies were decreased in both PBMCs and LNMCs during AHI and untreated CHI compared to HIV− individuals, with the decrease reaching significance earlier in the PBMCs (S3, p < 0.001) than in LNMCs (S4/5, p < 0.01) (Fig. 1A). In contrast, the CD8+ T cell frequencies were increased in both PBMCs and LNMCs during AHI and untreated CHI compared to HIV− individuals (Fig. 1B). In addition, the CD4:CD8 ratio was decreased in both PBMCs and LNMCs during AHI and untreated CHI compared to HIV− individuals, with the decrease reaching significance earlier in the PBMCs (S3, p < 0.001) than in LNMCs (S4/5, p < 0.01) (Fig. 1C).
Fig. 1.
Decreased CD4+ T cells, CD4:CD8 ratio and increased CD8+ T cell frequencies in both blood and lymph nodes occurred within weeks of HIV infection. (A) Frequencies of CD4+ T cells in blood and LNMC, (B) Frequencies of CD8+ T cells in blood and LNMC, (C) CD4:CD8 ratio in blood and LNMC, (D) Correlation and comparison of CD4+ T cell frequencies between blood and LNMC, (E) Correlation and comparison of CD8+ T cell frequencies between blood and LNMC, and (F) Correlation and comparison of CD4:CD8 ratio between blood and LNMC from AHI (S1, S2, S3, S4/5) and CHI participants compared to HIV negative (HIV-) participants. Symbols represent data from individual participants. Box and whiskers graphs, red bar as blood and blue bar as LNMC. Statistical analyses were performed using Kruskal-Wallis test, Wilcoxon matched-pairs test and Spearman correlation test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
There was a strong correlation between PBMCs and LNMCs during AHI in frequencies of CD4+ T cells (r = 0.59, p < 0.0001), frequencies of CD8+ T cells (r = 0.65, p < 0.0001) and CD4:CD8 ratio (r = 0.65, p < 0.0001) (Fig. 1D, 1E, 1F). The CD4:CD8 ratio in LNMCs was significantly higher than in PBMCs in all stages of AHI and untreated CHI due to the higher frequencies of CD4+ T cells and the lower frequencies of CD8+ T cell in LNMCs compared to PBMCs (p < 0.001) (Fig. 1D, 1E, 1F). These results indicate that CD4+ T cells depletion and the increase of CD8+ T cells in lymph node was delayed compared to blood (Fig. 1A, 1B, 1C).
3.3. Increased proliferation of CD4+, CD8+ T cells and perforin in CD8+ T cells in both PBMCs and LNMCs during AHI
To look at CD4+ and CD8+ T cell proliferation ex vivo, we measured the frequency of Ki-67+Bcl-2lo cells (Fig. 2 and Supp Fig. 3).22,38, 39, 40, 41 We observed that the frequency of proliferating CD4+ T cells started to increase in S3 and was significantly higher in S4/5 of AHI compared to HIV− individuals in both PBMCs and LNMCs reaching similar levels as in untreated CHI (p < 0.0001) (Fig. 2A). Though the frequency of proliferation of CD4+ T cells in LNMCs showed similar patterns as in PBMCs, it was significantly lower than in PBMCs (Fig. 2B). In addition, we found the frequency of proliferating CD8+ T cells was significantly higher in S3 and S4/5 in both PBMCs and LNMCs in AHI compared to HIV− individuals (p < 0.001) (Fig. 2C). However, CD8+ T cell proliferation was evident earlier (S2) in PBMCs compared to LNMCs (Fig. 2C and 2D). Although CD8+ T cell proliferation was lower in untreated CHI than in the late stages of AHI, it was still increased relative to HIV− individuals in both PBMCs and LNMCs (p < 0.01) (Fig. 2C).
Fig. 2.
Increased proliferation of CD4+, CD8+ T cells and cytotoxicity of CD8+ T cells in blood and LNMC with HIV infection. (A) Frequencies of proliferating (Ki67+BCl2lo) cells in memory CD4+ T cells in blood and LNMC, (B) Correlation and comparison of proliferating (Ki67+BCl2lo) cells in memory CD4+ T cell frequencies between blood and LNMC, (C) Frequencies of Ki67+BCl2lo in memory CD8+ T cells in blood and LNMC, (D) Correlation and comparison of proliferating (Ki67+BCl2lo) cells in memory CD8+ T cell frequencies between blood and LNMC, (E) Frequencies of perforin expression in memory CD8+ T cells in blood and LNMC, and (F) Correlation and comparison of perforin expression in memory CD8+ T cell frequencies between blood and LNMC from AHI (S1, S2, S3, S4/5) and CHI participants compared to HIV negative (HIV-). Symbols represent data from individual participants. Box and whiskers graphs, red bar as blood and blue bar as LNMC. Statistical analyses were performed using Kruskal-Wallis test, Wilcoxon matched-pairs test and Spearman correlation test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
To determine the differentiation and functionality of CD8+ T cells in the blood and lymph nodes during AHI and untreated CHI, we measured the expression of the cytolytic molecule perforin (Fig. 2E and Supp Fig. 3). We found that the frequency of CD8+ T cell expressing perforin was significantly higher in S3 and S4/5 in both PBMCs and LNMCs in AHI compared to HIV− individuals (p < 0.01) (Fig. 2E). Though increased expression of perforin was seen earlier in LNMCs (S2), overall there were fewer CD8+ T cells expressing perforin in LNMCs compared to PBMCs (Fig. 2F). The frequency of perforin expressing CD8+ T cells was similar in S4/5 and untreated CHI participants in PBMCs but lower in CHI compared to AHI S4/5 in LNMCs (Fig. 2E).
There was a strong correlation between PBMCs and LNMCs in proliferation of CD4+ T cells (r = 0.79, p < 0.0001), proliferation of CD8+ T cells (r = 0.92, p < 0.0001) and perforin expressing CD8+ T cells (r = 0.69, p < 0.0001) suggesting active recirculation of these cells between blood and lymph nodes (Fig. 2B, 2D, 2F).
3.4. Lack of Tfh differentiation as well as B cells and resting memory (RM) B cell loss in LNMCs during AHI
One important CD4+ T cell population in lymph nodes is the T follicular helper (Tfh) cell population which provides necessary B cell help and is defined by CXCR5+PD1hi.42,43 In the periphery, it has been shown that the CD4+ T cells with a CXCR5+PD-1+ phenotype, known as circulating Tfh (cTfh), can also provide B cell help in in vitro co-culture systems.30,44 We analyzed changes in the frequency of cTfh in PBMCs and Tfh in LNMCs (LN Tfh) (Fig. 3A and Supp Fig. 3). We found that there was no change in the frequency of cTfh or LN Tfh during AHI compared to HIV− individuals, though a few participants had elevated LN Tfh at S2, as we previously reported (Fig. 3A).45 The frequency of cTfh and LN Tfh cells were significantly higher in untreated CHI compared to HIV− individuals (p < 0.05; p < 0.0001 respectively) (Fig. 3A), as has been previously reported.46,47 Notably, the frequency of cTfh in PBMCs was higher than the frequency of Tfh in LNMCs for most participants, except for people in CHI who had expanded Tfh population in LN (p < 0.01) (Fig. 3B). Interestingly, there was no correlation between the frequency of cTfh in PBMCs and LN Tfh in LNMCs (Fig. 3B) suggesting that cTfh frequencies in PBMCs cannot be used as a correlate of Tfh frequencies in the lymph nodes.
Fig. 3.
Stable Tfh but loss of B cells and resting memory (RM) B cells in the blood but not in the lymph node during AHI. (A) Frequencies of cTfh in blood and LN Tfh in LNMC, (B) Correaltion and comparison of cTfh in blood and LN Tfh in LNMC frequencies between blood and LNMC, (C) Frequencies of B cells in blood and LNMC, (D) Correlation and comparison of B cells frequencies between blood and LNMC, (E) Frequencies of RM B cells (CD21+CD27+IgG+CD10-CD20+CD19+) in blood and LNMC, and (F) Correlation and comparison of RM B cell frequencies between blood and LNMC from AHI (S1, S2, S3, S4/5) and CHI participants compared to HIV negative (HIVof B cells and resting memory (RM) B cells in the blood but not in the lymph node during AHI.). Symbols represent data from individual participants. Box and whiskers graphs, red bar as blood and blue bar as LNMC. Statistical analyses were performed using Kruskal-Wallis test, Wilcoxon matched-pairs test and Spearman correlation test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗ p < 0.001, ∗∗∗∗p < 0.0001.(A) Frequencies of cTfh in blood and LN Tfh in LNMC, (B) Correaltion and comparison of cTfh in blood and LN Tfh in LNMC frequencies between blood and LNMC, (C) Frequencies of B cells in blood and LNMC, (D) Correlation and comparison of B cells frequencies between blood and LNMC, (E) Frequencies of RM B cells (CD21+CD27+IgG+CD10-CD20+CD19+) in blood and LNMC, and (F) Correlation and comparison of RM B cell frequencies between blood and LNMC from AHI (S1, S2, S3, S4/5) and CHI participants compared to HIV negative (HIV-). Symbols represent data from individual participants. Box and whiskers graphs, red bar as blood and blue bar as LNMC. Statistical analyses were performed using Kruskal-Wallis test, Wilcoxon matched-pairs test and Spearman correlation test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗ p < 0.001, ∗∗∗∗p < 0.0001.
To determine how well changes in B cell populations in the blood represent B cell changes in lymph nodes, we analyzed B cells in PBMCs and LNMCs (Fig. 3C and Supp Fig. 4). During AHI, we found that the frequency of B cells was significantly decreased in PBMCs (p < 0.05 for S1, S3, and S4/5) but not in LNMCs during AHI and untreated CHI compared to HIV− individuals (Fig. 3C). These data suggest that the depletion of B cells that has been reported in PBMCs during AHI48 does not extend to LNMCs, with no correlation the frequencies of B cells in PBMCs and LNMCs (r = 0.25, p = 0.0962) (Fig. 3D). As expected, B cells composed a greater frequency of lymphocytes in LNMCs than in the PBMCs during AHI, untreated CHI and HIV− individuals (Fig. 3D).
Loss of resting memory (RM) B cells (CD21+CD27+IgG+CD10−CD20+CD19+) in the blood is a hallmark of HIV infection.48,49 We found that the frequency of RM cells within IgG+ B cells was significantly decreased in PBMCs (p < 0.01 for S1, S2 and S3) but not LNMCs during AHI and untreated CHI (p < 0.001) compared to HIV− individuals (Fig. 3E). In addition, there was no correlation between PBMCs and LNMCs in frequencies of RM B cells (r = 0.02, p = 0.8735) (Fig. 3F). Similarly to the pattern observed with B cell frequencies, there was a higher frequency of RM B cell in LNMCs than in PBMCs (Fig. 3F). We looked at other B cell subsets and found very few differences (Supp Fig. 2). These data indicate that loss of B cell frequencies especially RM B cell frequencies are found only in the blood in early AHI. Together these data indicate that the depletion of B cells that occurs in the blood is not representative of what is occurring in the lymph nodes and that there are few changes in total lymph node B cell subsets in the lymph node during AHI.
3.5. Increased activation of pDCs earlier in lymph nodes than blood during AHI
We measured the frequencies of plasmacytoid dendritic cells (pDC) and their expression of activation markers (Fig. 4 and Supp Fig. 5). We found that there were no significant differences in the frequency of pDCs in PBMCs or LNMCs during AHI and untreated CHI individuals (Fig. 4A). There was a weak correlation between the frequencies of pDCs in the PBMCs and LNMCs at AHI (r = 0.44, p = 0.0052) (Fig. 4B). In addition, we found the pDC frequencies were higher in the lymph node than in the blood during AHI, untreated CHI and HIV− individuals (p < 0.05) (Fig. 4B).
Fig. 4.
Increased activation of pDCs in the lymph node compartment but not in blood in AHI. (A) Frequencies of pDC in CD45+Ln- cells in blood and LNMC, (B) Correlation and comparison of pDC frequency in AHI between blood and LNMC, (C) Frequencies of the activation markers CD40+ cells in pDC in blood and LNMC, (D) Frequencies of the activation markers CD69+ cells in pDC in blood and LNMC, and (E) Frequencies of the activation markers CD86+ cells in pDC in blood and LNMC from AHI (S1, S2, S3, S4/5) and CHI participants compared to HIV negative (HIV-). Symbols represent data from individual participants. Box and whiskers graphs, red bar as blood and blue bar as LNMC. Statistical analyses were performed using Kruskal-Wallis test, Wilcoxon matched-pairs test and Spearman correlation test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
We examined the frequency of pDCs expressing the activation/maturation markers CD40, CD69 and CD86. We found that the frequency of pDCs expressing activation markers increased in LNMCs at S2 in AHI compared to HIV− individuals (p < 0.001 for CD40, CD69, and CD86), whereas only expression of CD40 on pDCs was significantly elevated in PMBCs and not until S3 of AHI (p < 0.001) (Fig. 4C, 4D, 4E). These data indicate that although there were no changes in plasmacytoid dendritic cell frequencies in the lymph node during AHI, pDC activation was earlier and stronger in LNMCs than in PBMCs in AHI.
4. Discussion
This is the first study that provides a comprehensive comparison of immune cell subsets in human blood and lymph node matching from the highest number of people living with HIV (N = 52). Our study characterized CD4+ T cells, CD8+ T cells, T follicular helper cells, B cells and pDCs in both blood and lymph node compartments in AHI and untreated CHI compared to HIV negative individuals. First, our study showed that CD4+ T cell depletion, CD8+ T cell expansion and reduction of CD4:CD8 ratio was prominent earlier in the blood than in the lymph nodes in AHI. The loss of CD4+ T cells, reduction in the CD4:CD8 ratio, and increase of CD8+ T cells are hallmark features of AHI and reflect the profound impact of the virus on the immune system. In addition, these major factors serve as predictors of HIV disease progression.50, 51, 52, 53 These findings were supported by our data and underscore the systemic nature of immune dysregulation during AHI. This depletion reflects the direct cytopathic effects of HIV on CD4+ T cells, as well as the virus-induced immune activation and apoptosis of both infected and uninfected bystander cells.54 Notably, the earlier and more extensive CD4 T cell depletion in PBMCs compared to LNMCs may be due to tissue trafficking, including an influx of CD4 T cells into the lymph nodes during early infection. Concomitant with CD4+ T cell loss, the increase in CD8+ T cells is indicative of ongoing immune activation and the host's attempt to control viral replication. The expansion of CD8+ T cells in both PBMCs and LNMCs highlights their recruitment to sites of viral replication and immune activation in lymphoid tissues.
During AHI, many cells of the immune system show signs of extensive activation and a progressive loss of resting subsets.54 We demonstrated the dynamics of CD4+ and CD8+ T cell proliferation by measuring the Ki-67+Bcl-2lo cells in AHI and untreated CHI individuals. Interestingly, we observed differential patterns of CD4+ T cell proliferation between PBMCs and LNMCs. While both compartments exhibited similar trends, the frequency of CD4+ T cell proliferation was significantly lower in LNMCs compared to PBMCs. In addition, the frequencies of CD4+ T cell proliferation in untreated CHI reached levels comparable to AHI S4/5, suggesting a progressive immune dysregulation that parallels disease progression. We report here, similar to our previous work in blood, that expansion and differentiation of activated CD8+ T cells (defined by Ki-67+BCl-2lo and perforin+ expression within CD45RA−CD8+ T cells) was not significant until AHI S3 corresponding to peak viremia.22 This phenomenon suggests an active expansion of HIV-specific CD8+ T cell responses triggered by viral antigen stimulation, a hallmark of early immune activation in HIV infection.55, 56, 57, 58, 59 Interestingly, our study extends these findings by demonstrating that CD8+ T cell proliferation is detected later in LNMCs compared to PBMCs.
We previously showed that higher levels of proliferating CD8+ T cells in AHI was associated with a reduced seeding of the HIV reservoir after ART initiation.22 Other studies highlighted the importance of an enhanced cytolytic activity of CD8+ T cells to eliminate HIV-infected cells during the early stages of infection.60,61 However, it has been suggested that CD8+ T cells in lymph nodes express perforin during AHI when activated, though a lower frequency of cells were perforin+ than in PBMCs.62 Our study found that the cytolytic molecule perforin increased along with the CD8+ T cells activation at AHI S3 and S4/5 in both PBMCs and LNMCs in AHI suggesting that CD8+ T cells in lymph nodes express perforin during AHI when activated. Further studies would be needed to measure their cytolytic activity against HIV-infected CD4+ T cells.
Our study into the dynamics of T follicular helper (Tfh) cells and B cell populations in both blood and lymph nodes provides valuable data on the complex interplay between these immune cell subsets during AHI and untreated CHI. In our study, we extended this understanding to assess the frequencies of cTfh in the blood, often considered as surrogate for LN Tfh in the lymph node. We found no significant changes in the frequencies of cTfh or LN Tfh cells during AHI compared to HIV− individuals, suggesting a lack of in vivo expansion during AHI. In contrast, the frequencies of both cTfh and LN Tfh in untreated CHI were significantly higher than HIV− individuals, consistent with previous reports.46,47 We found no correlation between the frequencies of cTfh and LN Tfh, suggesting that cTfh in the blood are not representative of the Tfh in the lymph nodes. Then, we aimed to understand changes in B cell frequencies in the blood and LNMCs during AHI and untreated CHI. Our study found that B cell frequencies decreased significantly in PBMCs but remained unchanged in LNMCs. This suggests that B cell depletion happens in the blood during AHI with no correlation between both of compartments. Similarly, in IgG+ B cells, we also found the loss of resting memory (RM) B cell frequencies in the blood but not in the lymph node, in accordance with other studies.48,49 This B cell depletion happens only in the blood may suggest that B cells are trafficking to lymph nodes or other tissue sites during AHI. These data suggest that B cells in the blood are not a good surrogate for B cell dynamics in lymph nodes.
The innate cells particularly plasmacytoid dendritic cells (pDC) are a critical component of immune dysfunction in HIV-1 infection.63, 64, 65 A number of studies have found a transient pDC depletion in the peripheral blood after peak viremia and pDC numbers were found to correlate with CD4 numbers and were inversely correlated with HIV-RNA levels.12, 13, 14,66,67 Previous studies have reported that HIV infection induces upregulation of activation markers (CD40, HLA-DR) and maturation markers (CD80, CD86) on pDC, particularly in later stages of infection.63,64,68,69 In our study, no difference in the frequency of pDCs were observed in PBMCs or LNMCs during AHI. However, we measured increased expression of activation markers on pDCs in AHI. This activation was detected earlier in LN than in the blood.
Our findings have clinical relevance, particularly in demonstrating the need to examine lymph node tissue responses, as blood alone may not fully capture the underlying immunological dynamics. In particular, our study demonstrated depletion of total and resting memory B cells in PBMCs was not observed in LNMCs, suggesting that the decline in PBMCs is not systemic and may be due to cellular trafficking. In addition, pDC activation was significantly elevated in S2 of AHI in LNMCs but not PBMCs, indicating that there is an early innate response in the lymph nodes that cannot be measured in peripheral blood. The activation of pDCs and production of cytokines, especially type I interferon, within the lymph nodes during AHI could affect T cell activation and differentiation. Type I interferons play an important role in CD8+ T cell priming,70,71 and we observed that CD8+ T cell proliferation increased significantly in S3 of AHI. It has also been shown that the timing of type I interferon exposure affects the differentiation of CD4+ T cells into a Th1 or Tfh phenotype,72 which may contribute to the earlier activation of non-Tfh cells in the LNs during AHI.73 While no expansion of total Tfh was observed in PBMCs or LNMCs during AHI, the expansion of Tfh cells was observed in both compartments in CHI and may reflect ongoing antigenic stimulation and prolonged immune activation. This temporal difference underscores the potential benefit of early ART initiation, which may limit prolonged antigenic stimulation and prevent the hyperproliferation of Tfh cells, germinal center disruption, dysregulated B cell help, and HIV reservoir maintenance in chronic infection.46,47 Future longitudinal studies should help determine the extent to which early ART prevents larger immune perturbations observed in participants with CHI. Moreover, using immunohistochemistry (IHC) on lymph node tissue will be highly informative for evaluating the spatial analysis of cellular localization, activation, and interaction, thus providing a more comprehensive understanding of the immune dynamics during early AHI.
Overall, our study provides insights into immune cell subsets over the course of AHI in PBMCs and LNMCs. However, there are some limitations to this study. This study was based solely on dissociated lymph node tissue. Additional immunohistochemistry studies should be conducted on tissue blocks which were not available for our study. Furthermore, we relied on inguinal lymph nodes and immune cell phenotypes may be different in other lymphoid tissues. Our cohort was male, results could defer for female. Finally, the lack of longitudinal sampling after ART initiation limits our ability to evaluate the immune perturbation and restoration in both PBMCs and LNMCs during viral suppression.
5. Conclusions
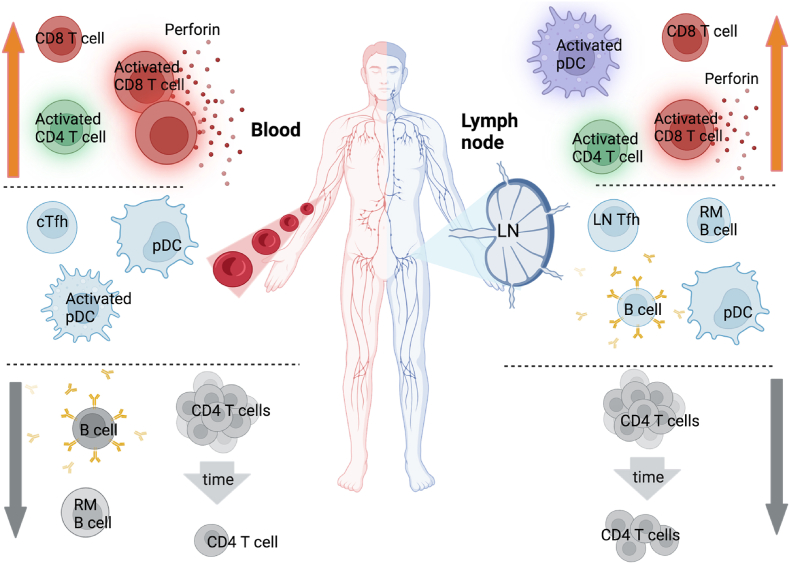
In conclusion, our study reveals significant immune alterations during acute HIV infection (AHI), including depletion of CD4+ T cells and a reduced CD4:CD8 ratio observed in both blood and lymph nodes, indicative of systemic immune impairment. Furthermore, the characterization of CD8+ T cells in blood suggests potential reflection of responses in lymph node, particularly in terms of activation and perforin expression. The lack of CD4+ T follicular helper (Tfh) cell differentiation in both blood (cTfh) and lymph node (LN Tfh) underscores compromised B cell help during AHI. Interestingly, early loss of B cells, particularly RM B cells, is evident in the blood but not significantly different in the lymph node, suggesting compartmentalized effects on B cell populations. Moreover, increased pDC activation is observed in LNMCs but not in PBMCs during AHI, indicate distinct immune activation profiles between these two compartments. These findings are summarized in Fig. 5 and emphasize the complex interplay between systemic and localized immune responses during early AHI, highlighting the need for comprehensive understanding to inform effective therapeutic strategies.
Fig. 5.
Immune alterations during the course acute HIV infection in blood and lymph nodes. Decrease in immune cell frequencies are represented by gray arrows. No change of immune cell frequencies are represented in the middle. Increase in immune cell frequencies are represented by orange arrows.
CRediT authorship contribution statement
Supranee Buranapraditkun: Writing – original draft, Methodology, Formal analysis. Julie L. Mitchell: Writing – original draft, Methodology, Formal analysis. Hiroshi Takata: Writing – review & editing, Methodology. Eugene Kroon: Writing – review & editing, Investigation. Suteeraporn Pinyakorn: Investigation. Nicha Tulmethakaan: Investigation. Sopark Manasnayakorn: Investigation. Suthat Chottanapund: Investigation. Pattarawat Thantiworasit: Investigation. Peeriya Prueksakaew: Investigation. Nisakorn Ratnaratorn: Investigation. Khunthalee Benjapornpong: Investigation. Bessara Nuntapinit: Investigation. Praphan Phanuphak: Investigation. Carlo P. Sacdalan: Writing – review & editing, Investigation. Nittaya Phanuphak: Writing – review & editing, Resources. Kiat Ruxrungtham: Resources. Jintanat Ananworanich: Conceptualization. Sandhya Vasan: Writing – review & editing, Resources. Lydie Trautmann: Writing – original draft, Supervision, Conceptualization.
Informed consent Statement
Informed consent was obtained from all subjects involved in the study.
Institutional review board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Review Committee of the Chulalongkorn University (Bangkok, Thailand) and Walter Reed Army Institute of Research (WRAIR; Silver Spring, MD, USA).
Disclaimer
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. The investigators have adhered to the policies for protection of human participants as prescribed in AR-70-25.
Funding
This study was supported by the following sources: NIH grants R01AI108433, R01MH106466 and UM1AI64560, a cooperative agreement (W81XWH-07-2-0067, W81XWH-11-2-0174, W81XWH-18-2-0040) between the Henry M. Jackson Foundation for the Advancement of Military Medicine Inc. and the U.S. Department of Defense, and, in part, by the Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (AAI20052001). Antiretroviral therapy for RV254/SEARCH 010 participants was supported by the Thai Government Pharmaceutical Organization, Gilead Sciences, Merck and ViiV Healthcare.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank the RV254/SEARCH010 and RV304/SEARCH013 participants as well as the study group members who committed so much of their time for this study. Fig. 5 was created with BioRender.com.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jve.2025.100598.
Contributor Information
Supranee Buranapraditkun, Email: supranee.b@chula.ac.th.
Julie L. Mitchell, Email: mijulie@ohsu.edu.
Hiroshi Takata, Email: takatah@ohsu.edu.
Eugene Kroon, Email: eugene@searchthailand.org.
Suteeraporn Pinyakorn, Email: spinyakorn@global-id.org.
Nicha Tulmethakaan, Email: nicha.t@searchthailand.org.
Sopark Manasnayakorn, Email: sopark.m@chula.ac.th.
Suthat Chottanapund, Email: suthat.c@searchthailand.org.
Pattarawat Thantiworasit, Email: pattarawatth6@gmail.com.
Peeriya Prueksakaew, Email: peeriya.p@searchthailand.org.
Nisakorn Ratnaratorn, Email: nisakorn.r@searchthailand.org.
Khunthalee Benjapornpong, Email: khunthalee.b@gmail.com.
Bessara Nuntapinit, Email: bessaran@hiv-th.org.
Praphan Phanuphak, Email: praphan.p@ihri.org.
Carlo P. Sacdalan, Email: carlo.s@searchthailand.org.
Nittaya Phanuphak, Email: nittaya.p@ihri.org.
Kiat Ruxrungtham, Email: rkiat@chula.ac.th.
Jintanat Ananworanich, Email: jintanat@usthai.us.
Sandhya Vasan, Email: svasan@global-id.org.
Lydie Trautmann, Email: ltrautmann@global-id.org.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Ananworanich J., Schuetz A., Vandergeeten C., et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fletcher C.V., Kroon E., Schacker T., et al. Persistent HIV transcription and variable antiretroviral drug penetration in lymph nodes during plasma viral suppression. Aids. 2022;36(7):985–990. doi: 10.1097/QAD.0000000000003201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leyre L., Kroon E., Vandergeeten C., et al. Abundant HIV-infected cells in blood and tissues are rapidly cleared upon ART initiation during acute HIV infection. Sci Transl Med. 2020;12(533) doi: 10.1126/scitranslmed.aav3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shacklett B.L. Mucosal immunity in acute HIV: a review of recent work. Curr Opin HIV AIDS. 2025;20(3):193–198. doi: 10.1097/COH.0000000000000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tincati C., Bono V., Cannizzo E.S., et al. Primary HIV infection features colonic damage and neutrophil inflammation yet containment of microbial translocation. Aids. 2024;38(5):623–632. doi: 10.1097/QAD.0000000000003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paquin-Proulx D., Lal K.G., Phuang-Ngern Y., et al. Preferential and persistent impact of acute HIV-1 infection on CD4(+) iNKT cells in colonic mucosa. Proc. Natl. Acad. Sci. U.S.A. 2021;118(46) doi: 10.1073/pnas.2104721118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiebig E.W., Wright D.J., Rawal B.D., et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17(13):1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 8.Ananworanich J., Fletcher J.L., Pinyakorn S., et al. A novel acute HIV infection staging system based on 4th generation immunoassay. Retrovirology. 2013;10:56. doi: 10.1186/1742-4690-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robb M.L., Eller L.A., Kibuuka H., et al. Prospective study of acute HIV-1 infection in adults in East Africa and Thailand. N Engl J Med. 2016;374(22):2120–2130. doi: 10.1056/NEJMoa1508952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rolland M., Tovanabutra S., Dearlove B., et al. Molecular dating and viral load growth rates suggested that the eclipse phase lasted about a week in HIV-1 infected adults in East Africa and Thailand. PLoS Pathog. 2020;16(2) doi: 10.1371/journal.ppat.1008179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerkmann M., Rothenfusser S., Hornung V., et al. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J Immunol. 2003;170(9):4465–4474. doi: 10.4049/jimmunol.170.9.4465. [DOI] [PubMed] [Google Scholar]
- 12.Pacanowski J., Kahi S., Baillet M., et al. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood. 2001;98(10):3016–3021. doi: 10.1182/blood.v98.10.3016. [DOI] [PubMed] [Google Scholar]
- 13.Killian M.S., Fujimura S.H., Hecht F.M., Levy J.A. Similar changes in plasmacytoid dendritic cell and CD4 T-cell counts during primary HIV-1 infection and treatment. AIDS. 2006;20(9):1247–1252. doi: 10.1097/01.aids.0000232231.34253.bd. [DOI] [PubMed] [Google Scholar]
- 14.Sabado R.L., O'Brien M., Subedi A., et al. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. 2010;116(19):3839–3852. doi: 10.1182/blood-2010-03-273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruel T., Dupuy S., Demoulins T., et al. Plasmacytoid dendritic cell dynamics tune interferon-alfa production in SIV-infected cynomolgus macaques. PLoS Pathog. 2014;10(1) doi: 10.1371/journal.ppat.1003915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown K.N., Wijewardana V., Liu X., Barratt-Boyes S.M. Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection. PLoS Pathog. 2009;5(5) doi: 10.1371/journal.ppat.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malleret B., Maneglier B., Karlsson I., et al. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood. 2008;112(12):4598–4608. doi: 10.1182/blood-2008-06-162651. [DOI] [PubMed] [Google Scholar]
- 18.Isaacs A., Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147(927):258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- 19.Cervantes-Barragan L., Lewis K.L., Firner S., et al. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proc Natl Acad Sci USA. 2012;109(8):3012–3017. doi: 10.1073/pnas.1117359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brewitz A., Eickhoff S., Dahling S., et al. CD8(+) T cells Orchestrate pDC-XCR1(+) dendritic cell spatial and functional cooperativity to optimize priming. Immunity. 2017;46(2):205–219. doi: 10.1016/j.immuni.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers G.L., Shirley J.L., Zolotukhin I., et al. Plasmacytoid and conventional dendritic cells cooperate in crosspriming AAV capsid-specific CD8(+) T cells. Blood. 2017;129(24):3184–3195. doi: 10.1182/blood-2016-11-751040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takata H., Buranapraditkun S., Kessing C., et al. Delayed differentiation of potent effector CD8(+) T cells reducing viremia and reservoir seeding in acute HIV infection. Sci Transl Med. 2017;9(377) doi: 10.1126/scitranslmed.aag1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koup R.A., Safrit J.T., Cao Y., et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68(7):4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borrow P., Lewicki H., Hahn B.H., Shaw G.M., Oldstone M.B. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68(9):6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freel S.A., Picking R.A., Ferrari G., et al. Initial HIV-1 antigen-specific CD8+ T cells in acute HIV-1 infection inhibit transmitted/founder virus replication. J Virol. 2012;86(12):6835–6846. doi: 10.1128/JVI.00437-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takata H., Kakazu J.C., Mitchell J.L., et al. Long-term antiretroviral therapy initiated in acute HIV infection prevents residual dysfunction of HIV-specific CD8+ T cells. EBioMedicine. 2022;84 doi: 10.1016/j.ebiom.2022.104253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuetz A., Deleage C., Sereti I., et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014;10(12) doi: 10.1371/journal.ppat.1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahlobo B., Laher F., Smidt W., et al. The impact of HIV infection on the frequencies, function, spatial localization and heterogeneity of T follicular regulatory cells (TFRs) within human lymph nodes. BMC Immunol. 2022;23(1):34. doi: 10.1186/s12865-022-00508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O Onabajo O., J Mattapallil J. Expansion or depletion of T follicular helper cells during HIV infection: consequences for B cell responses. Curr HIV Res. 2013;11(8):595–600. doi: 10.2174/1570162x12666140225153552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muir R., Metcalf T., Tardif V., et al. Altered memory circulating T follicular helper-B cell interaction in early acute HIV infection. PLoS Pathog. 2016;12(7) doi: 10.1371/journal.ppat.1005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Locci M., Havenar-Daughton C., Landais E., et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39(4):758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitt N., Bentebibel S.E., Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014;35(9):436–442. doi: 10.1016/j.it.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Maza O., Crabb E., Mitsuyasu R.T., Fahey J.L., Giorgi J.V. Infection with the human immunodeficiency virus (HIV) is associated with an in vivo increase in B lymphocyte activation and immaturity. J Immunol. 1987;138(11):3720–3724. [PubMed] [Google Scholar]
- 34.Mabuka J.M., Dugast A.S., Muema D.M., et al. Plasma CXCL13 but not B cell frequencies in acute HIV infection predicts emergence of cross-neutralizing antibodies. Front Immunol. 2017;8:1104. doi: 10.3389/fimmu.2017.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moir S., Ho J., Malaspina A., et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205(8):1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ananworanich J., Sacdalan C.P., Pinyakorn S., et al. Virological and immunological characteristics of HIV-infected individuals at the earliest stage of infection. Journal of Virus Eradication. 2016;2(1):43–48. doi: 10.1016/S2055-6640(20)30688-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chintanaphol M., Sacdalan C., Chottanapund S., et al. Brief report: safety and tolerability of inguinal lymph node biopsy in individuals with acute HIV infection in Thailand. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2018;79(2):244–248. doi: 10.1097/QAI.0000000000001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akondy R.S., Johnson P.L., Nakaya H.I., et al. Initial viral load determines the magnitude of the human CD8 T cell response to yellow fever vaccination. Proc Natl Acad Sci U S A. 2015;112(10):3050–3055. doi: 10.1073/pnas.1500475112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ndhlovu Z.M., Kamya P., Mewalal N., et al. Magnitude and kinetics of CD8+ T cell activation during hyperacute HIV infection impact viral set point. Immunity. 2015;43(3):591–604. doi: 10.1016/j.immuni.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murali-Krishna K., Altman J.D., Suresh M., et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8(2):177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 41.Miller J.D., van der Most R.G., Akondy R.S., et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28(5):710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 42.Shi J., Hou S., Fang Q., Liu X., Liu X., Qi H. PD-1 controls follicular T helper cell positioning and function. Immunity. 2018;49(2):264–274. doi: 10.1016/j.immuni.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onabajo O.O., George J., Lewis M.G., Mattapallil J.J. Rhesus macaque lymph node PD-1(hi)CD4+ T cells express high levels of CXCR5 and IL-21 and display a CCR7(lo)ICOS+Bcl6+ T-follicular helper (Tfh) cell phenotype. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0059758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buranapraditkun S., Pissani F., Teigler J.E., et al. Preservation of peripheral T follicular helper cell function in HIV controllers. J Virol. 2017;91(14) doi: 10.1128/JVI.00497-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gantner P., Buranapraditkun S., Pagliuzza A., et al. HIV rapidly targets a diverse pool of CD4+ T cells to establish productive and latent infections. Immunity. 2023;56(3):653–668. doi: 10.1016/j.immuni.2023.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindqvist M., Van Lunzen J., Soghoian D.Z., et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. The Journal of clinical investigation. 2012;122(9):3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perreau M., Savoye A.-L., De Crignis E., et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013;210(1):143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moir S., Buckner C.M., Ho J., et al. B cells in early and chronic HIV infection: evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood. 2010;116(25):5571–5579. doi: 10.1182/blood-2010-05-285528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moir S., Fauci A.S. B-cell responses to HIV infection. Immunol Rev. 2017;275(1):33–48. doi: 10.1111/imr.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu W., Mehraj V., Vyboh K., Cao W., Li T., Routy J.P. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. J Int AIDS Soc. 2015;18 doi: 10.7448/IAS.18.1.20052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoenigl M., Chaillon A., Little S.J. CD4/CD8 cell ratio in acute HIV infection and the impact of early antiretroviral therapy. Clin Infect Dis. 2016;63(3):425–426. doi: 10.1093/cid/ciw293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robb M.L., Ananworanich J. Lessons from acute HIV infection. Curr Opin HIV AIDS. 2016;11(6):555. doi: 10.1097/COH.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mudd J.C., Lederman M.M. CD8 T cell persistence in treated HIV infection. Curr Opin HIV AIDS. 2014;9(5):500–505. doi: 10.1097/COH.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Douek D.C., Roederer M., Koup R.A. Emerging concepts in the immunopathogenesis of AIDS. Annu Rev Med. 2009;60:471–484. doi: 10.1146/annurev.med.60.041807.123549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Day C.L., Kaufmann D.E., Kiepiela P., et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 56.Goonetilleke N., Liu M.K., Salazar-Gonzalez J.F., et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med. 2009;206(6):1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Streeck H., Li B., Poon A.F., et al. Immune-driven recombination and loss of control after HIV superinfection. J Exp Med. 2008;205(8):1789–1796. doi: 10.1084/jem.20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brenchley J.M., Schacker T.W., Ruff L.E., et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200(6):749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lichterfeld M., Xu G.Y., Cohen D., et al. HIV-1 Nef is preferentially recognized by CD8 T cells in primary HIV-1 infection despite a relatively high degree of genetic diversity. Aids. 2004;18(10):1383–1392. doi: 10.1097/01.aids.0000131329.51633.a3. [DOI] [PubMed] [Google Scholar]
- 60.Sáez-Cirión A., Lacabaratz C., Lambotte O., et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci. 2007;104(16):6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hersperger AR, Pereyra F, Nason M, Demers K, Sheth P, Shin LY, et al. Perforin Expression Directly Ex Vivo by HIV-specific CD8 T-Cells Is. [DOI] [PMC free article] [PubMed]
- 62.Reuter M.A., Estrada P.M.D.R., Buggert M., et al. HIV-specific CD8+ T cells exhibit reduced and differentially regulated cytolytic activity in lymphoid tissue. Cell Rep. 2017;21(12):3458–3470. doi: 10.1016/j.celrep.2017.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fitzgerald-Bocarsly P., Jacobs E.S. Plasmacytoid dendritic cells in HIV infection: striking a delicate balance. J Leukoc Biol. 2010;87(4):609–620. doi: 10.1189/jlb.0909635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herbeuval J.P., Smith N., Theze J. Characteristics of plasmacytoid dendritic cell and CD4+ T cell in HIV elite controllers. Clin Dev Immunol. 2012;2012 doi: 10.1155/2012/869505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Brien M., Manches O., Bhardwaj N. Plasmacytoid dendritic cells in HIV infection. Adv Exp Med Biol. 2013;762:71–107. doi: 10.1007/978-1-4614-4433-6_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soumelis V., Scott I., Gheyas F., et al. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98(4):906–912. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- 67.Donaghy H., Pozniak A., Gazzard B., et al. Loss of blood CD11c(+) myeloid and CD11c(-) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98(8):2574–2576. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 68.Donhauser N., Pritschet K., Helm M., et al. Chronic immune activation in HIV-1 infection contributes to reduced interferon alpha production via enhanced CD40:CD40 ligand interaction. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mitchell J.L., Takata H., Muir R., Colby D.J., Kroon E., Crowell T.A., et al. Plasmacytoid dendritic cells sense HIV replication before detectable viremia following treatment interruption. J Clin Investig. 2020;130(6):2845–2858. doi: 10.1172/JCI130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Le Bon A., Tough D.F. Type I interferon as a stimulus for cross-priming. Cytokine Growth Factor Rev. 2008;19(1):33–40. doi: 10.1016/j.cytogfr.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 71.Cabral-Piccin M.P., Papagno L., Lahaye X., et al. Primary role of type I interferons for the induction of functionally optimal antigen-specific CD8(+) T cells in HIV infection. EBioMedicine. 2023;91 doi: 10.1016/j.ebiom.2023.104557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Giovanni M., Cutillo V., Giladi A., et al. Spatiotemporal regulation of type I interferon expression determines the antiviral polarization of CD4(+) T cells. Nat Immunol. 2020;21(3):321–330. doi: 10.1038/s41590-020-0596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mitchell J.L., Buranapraditkun S., Gantner P., et al. Activation of CXCR3(+) Tfh cells and B cells in lymph nodes during acute HIV-1 infection correlates with HIV-specific antibody development. J Virol. 2025;99(3) doi: 10.1128/jvi.01532-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.