Abstract
Background:
Secondhand smoke exposure (SHSe) is a public health threat for people with cystic fibrosis (CF) and other lung diseases. Primary smoking reduces CFTR channel function, the causative defect in CF. We reported that SHSe worsens respiratory and nutritional outcomes in CF by disrupting immune responses and metabolic signaling. Recently, electronic cigarette (e-cigs) usage by caregivers and peers has increased rapidly, causing new secondhand e-cig vape exposures. Primary vaping is associated with immunologic deficits in healthy people, but it is unknown if e-cigs similarly impacts CF immune function or how it differs from SHSe.
Methods:
Human CF and non-CF blood monocyte derived macrophages (MDMs) and bronchial epithelial cells (HBECs) were exposed to flavored and unflavored e-cigs. The effect of e-cigs on CFTR expression and function, bacterial killing, cytokine signaling, lipid mediators, and metabolism was measured during treatment with CFTR modulators.
Results:
E-cigs decreased CFTR expression and function in CF and non-CF MDMs and negated CFTR functional restoration by elexacaftor/tezacaftor/ivacaftor (ETI). E-cigs also negated the restoration of anti-inflammatory PGD2 expression in CF MDMs treated with ETI compared to controls. Flavored but not unflavored e-cigs increased pro-inflammatory cytokine expression in CF MDMs and e-cigs promoted glycolytic metabolism. E-cigs did not impact bacterial killing. Overall, HBECs were less impacted by e-cigs compared to MDMs.
Conclusion:
E-cigs reduced macrophage CFTR expression and hindered functional CFTR restoration by CFTR modulators, promoting a glycolytic, pro-inflammatory state. E-cigs are an emerging public health threat that may limit the efficacy of CFTR modulators in people with CF.
Keywords: cystic fibrosis, inflammation, proteomics, modulators
Graphical Abstract

Introduction
Electronic-cigarette (e-cig) usage has increased dramatically in the United States. As a result, children have increased exposure to the aerosols (vape) created from e-cigs by personal use, their caregivers, or friends. Primary combustible cigarette smoke exposure decreases CF transmembrane conductance regulator (CFTR) expression in airway epithelial cells and macrophages, and e-cig vapors also may reduce epithelial CFTR function.(1–6) However, another study suggests e-cig induced changes in epithelial CFTR currents may be milder compared to combustible cigarettes.(7) Our prior studies demonstrated that secondhand smoke exposure (SHSe) from combustible cigarettes is associated with worsened early CF outcomes including decreased lung function, increased bacterial infections and oxidative stress, altered metabolism, disruptions in gut microbiota, and abnormal monocyte-derived macrophage (MDM) function.(8–12) In contrast, limited studies exist on the impact of e-cigarette exposure (e-cig) in CF. CFTR gene variants also result in abnormal macrophage and epithelial cell function with an associated decrease in bacterial clearance and increased inflammation that is independent of environmental exposures.(13, 14) How emerging exposures such as e-cigs influence already altered immunologic function and inflammation in CF remain to be determined. The increased usage of CFTR modulator therapies at younger ages has also increased the clinical significance of secondhand environmental exposures upon CFTR restoration. Therefore, we sought to determine if e-cigs impact the immune (macrophages) and airway epithelial cells that are responsible for protection against environmental exposures. We utilized primary cell exposure to smoked/vaped extracts, as this represents the aqueous constituents of direct or passively inhaled exposures. We hypothesized that e-cigs would decrease CFTR function resulting in impairment in phagocytic function and heightened inflammatory responses.
Methods
Cell isolation:
MDMs were isolated as previously described(15) as approved by the Institutional Review Boards (STUDY00004965, IRB16–01020). In brief, blood was obtained during routine clinical labs and monocytes were isolated from whole blood using Lymphocyte Separation Medium. Monocytes were cultured in RPMI plus 20% human AB serum and differentiated for 5–7 days at 37°C into MDMs in Teflon wells. MDMs were plated in monolayers with fresh RPMI and 10% AB serum and rested prior to experimentation. Human bronchial epithelial cells (HBECs, CF- homozygous F058del, from Nationwide Epithelial Core) were isolated as previously described.(16) Cells were isolated from human bronchial brushings and detached from the brush by agitation and cultured on irradiated fibroblast feeder layers in medium as published.(17) Passage 1 epithelial cells at 80% confluence were recovered by double trypsinization and re-plated on collagen-coated transwells in proliferation medium.(17) At confluence, the cultures were transitioned to Pneumacult-ALI medium and differentiated for 21 days prior to experimentation with differentiation assessed as reported.(18)
E-cig and cigarette exposure extract preparation:
Flavored or unflavored e-cig extract was prepared using a commercial 3rd generation device at 60 watts. E-cigarette solutions contained a standard concentration of 18 mg nicotine with 50% vegetable glycerin and 50% propylene glycol. E-cig extract was collected by 3-second puffs every minute for 60 minutes into 1X PBS. Extract was then filter sterilized and diluted to the desired concentration prior to experimentation. Cigarette extracts were prepared as previously described.(19)
Apoptosis/TEER:
Cell toxicity was assayed as previously described(15) using the apoptosis detection kit (Biolegend, 640932). In brief, MDMs were plated in 12 well plates at 1 × 106/ml prior to exposure to increasing concentrations of e-cig or cigarette extracts daily for 3 days. Elexacaftor/tezacaftor/ivacaftor (ETI, Vertex) was added at 3 μM for each component. Cells were detached for labeling by APC Annexin V and propidium iodide for 15 min at room temperature in the dark, and then analyzed for the percentage of apoptotic cells using a BD Accuri C6 plus flow cytometer and analyzed via BD flow jo software version 10.8.2. TEER was measured as previously described using a voltohmmeter (EVOM2, World Precision Instrument).(20)
CFTR expression:
Intracellular staining of CFTR was performed as previously described.(13, 14) Individual wells were treated with 1% e-cig extract or 0.25 mg/mL Nicotine (Sigma, N3876) with or without ETI at 3μM for each component. The wells were treated with e-cig extract, nicotine, and ETI every 24 hours for 3 days. Post 3-day treatment and exposure, cells were harvested from the wells using accutase solution (Fisher, 25058C1). MDMs (1.0×106/per condition) were incubated in fixation buffer (Invitrogen, 00822249) for 20 min at room temperature and then treated with permeabilization buffer (Invitrogen, 00833356) for 15 min after 2 washes with cold PBS. Cells were centrifuged at 390×g for 5 min at 4°C and resuspended in 500 μL blocking buffer containing 5% goat serum (Gibco, 16210072) in permeabilization buffer for 20 min at RT. Before flow cytometry, MDMs were incubated in 100 μL permeabilization buffer with CFTR antibodies at a dilution of 1:100 (CFTR-596 from CFTR Antibody Distribution Program) for 30 min at RT in the dark, and then stained with 100 μL permeabilization buffer with Alexa Fluor488 goat anti-mouse antibody at a dilution of 1:300 (Invitrogen, A31620A) for 20 min at RT. The stained cells were acquired using BD Accuri Flow Cytometer. CFTR expression by immunoblotting was performed as previously published.(21) In brief, HBECs were lysed in Tris lysis buffer. Thirty μg of total proteins were separated with SDS-PAGE and CFTR (antibody 769 obtained from UNC distribution program) and GAPDH (Santa Cruz Biotechnology, Dallas, TX) proteins were probed followed by HRP-conjugated secondary antibody (Pierce, Rockford, IL).
CFTR function:
CFTR function was assayed via CFTR-mediated halide efflux assay as previously described.(14, 15) Briefly, 1 × 106 MDMs were seeded on 96-well plates and rested for 4 days prior to treatments. Cells were washed with efflux solution and loaded with MQAE for 5 min at 37°C in a CO2 incubator and the maximum fluorescence was recorded. The basal fluorescence was measured after exposure to warmed NaI buffer for 15 min at 37°C in the dark. The minimum fluorescence was obtained by exposure to quenching solution for 30 min at 37°C. Chloride efflux was induced by treatment with 20 μM forskolin plus 100 μM IBMX or 5 μM CFTR inhibitor (CFTRinh172) at 37°C and fluorescence measured every 5 min for 30 min.
Bacterial killing:
Bacteria strains isolated from CF sputum, including a gentamicin-sensitive strain of B. cenocepacia (B.c), methicillin-resistant Staphylococcus aureus (MRSA) isolate, and a multi-drug resistant Pseudomonas aeruginosa isolate, were grown for 24h in LB media prior to use. A colony forming unit (CFU) assay was performed as previously described (22). Briefly, 0.8 ×106 MDMs were cultured on 24-well plates and were treated with ETI (3 μM, ivacaftor one-time dosing prior to experimentation, repeated elexacaftor/tezacaftor dosing over 48h prior to experimentation) before adding bacteria. MDMs were infected with MRSA (5 MOI) B.c (10 MOI) or P. aeruginosa (10 MOI) for 2 hour at 37°C. Extracellular bacteria were killed by gentamicin (50 μg/ml, Gibco, 15750060) for 40 min at 37°C. MDMs were replaced with fresh media with AB serum and incubated overnight. MDMs were lysed in PBS 0.1% Triton X-100 (Acros Organics, 9002093–1) and bacteria load was quantified by plating serial dilutions on LB agar plates and analyzed for CFUs.
Lipidomics:
Targeted eicosanoid lipidomics was performed on extracellular supernatants from MDMs or HBECs during exposure to flavored and unflavored e-cigs, nicotine, ETI, or combinations. Lipidomics was performed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) using Multiple Reaction Monitoring (MRM) and stable isotope dilution for quantitation as published.(19, 23) Specifically, each sample was spiked with a deuterated eicosanoid internal standard solution then extracted with organic solvents (CHCl3:MeOH). Samples were injected into an Applied Biosystems 4000 QTrap LC/MS/MS equipped with a Shimadzu HPLC. Individual calibration curves were generated for each class of analytes and sample concentrations were calculated using isotope dilution corrections.
Cytokine expression:
Extracellular supernatants from MDMs or HBECs during exposure to flavored and unflavored e-cigs, nicotine, ETI, or combinations were analyzed for 44 cytokines via the Human XL cytokine performance assay on the Luminex platform according to the manufacturer’s instructions (LKTM014). 50μl of biofluids at 2-fold dilution were analyzed in a 96 well plate, with 38 cytokines detected from MDMs and 34 from HBECs.
Seahorse metabolism:
The Seahorse XF HS Mini Analyzer was used to determine the oxygen consumption rate (OCR), extracellular acidification rate (ECAR), and proton efflux rate (PER). MDMs were seeded on XF miniplates at a density of 4.5 × 105 cell/well, with two control wells filled with RPMI. The cell seeding plate was rested for one hour at RT, and incubated overnight at 37° C. Additionally, biosensors were prepared, and cartridges hydrated per manufacturer protocols and incubated without CO2 at 37°C overnight. The next day, XF assay media was created by mixing 9.7 mL XF RPMI warmed to 37°C and 100 μL each of XF Glucose, XF Pyruvate, and XF L-Glutamine. From each well on the cell culture plate, 60 μL of fluid was discarded. The seeded cells in the plate were washed with XF Assay Media and incubated with 200 μL XF Assay Media per well in a non-CO2 incubator for an hour. The sensors were hydrated with XF Calibrant and incubated in a non-CO2 incubator for one hour. Diluted injection solutions of Oligomycin and Rotenone + Antimycin A were loaded into their corresponding ports per manufacturer protocol. Immediately before the experiment, XF Assay Media in each well was discarded and 160 μL of XF Assay Media was added prior to loading the plates into the machine. For experimental groups representing acute e-cig exposure, e-cig extract was added to each well on the cell culture plate. Similarly, for the experimental groups representing acute e-cig exposure with ETI treatment, solution was added to each well on the cell culture plate. The assay was then run following manufacturer’s directions.
Statistical Analysis:
For each hypothesis, post-hoc Dunnett’s F multiple comparison test following a one-way Analysis of Variance (ANOVA) was used for pairwise comparisons to control for the overall Type I error rate. All comparisons were made in reference to a non-CF NT group, or a CF NT group if the experiment did not include a non-CF NT group. Comparisons are listed in the individual figure legends. Bioinformatics was performed with Reactome pathway analysis with Voronoi visualization (https://reactome.org/) and hierarchical clustering using the average linkage method (Morpheus software, Broad Institute).
Results
E-cigs alter cellular stability
We first determined if repeated, intermittent, secondhand exposure altered the integrity and viability of MDMs and well differentiated HBECs. Over 3–5 days, we found that both CF and non-CF MDMs tolerated repeated low doses of cigarette extracts up to 5% with no change in apoptosis markers, like prior acute exposures(19) (Fig. 1A). Similarly, both CF and non-CF MDMs tolerated repeated low doses of e-cig extracts up to 5%, although apoptosis increased significantly at 5% (Fig. 1B). CF HBECs also demonstrated a significant decrease in TEER with repeated apical e-cig exposure (Fig. 1C), indicative of increased susceptibility to damage from e-cigs. In contrast, TEER for non-CF epithelia remained relatively stable (Fig. 1C). Therefore, for subsequent experiments we used 1% extracts, which were well tolerated and allowed cellular stability during extended culture.
Figure 1: E-cigs alter cellular stability.
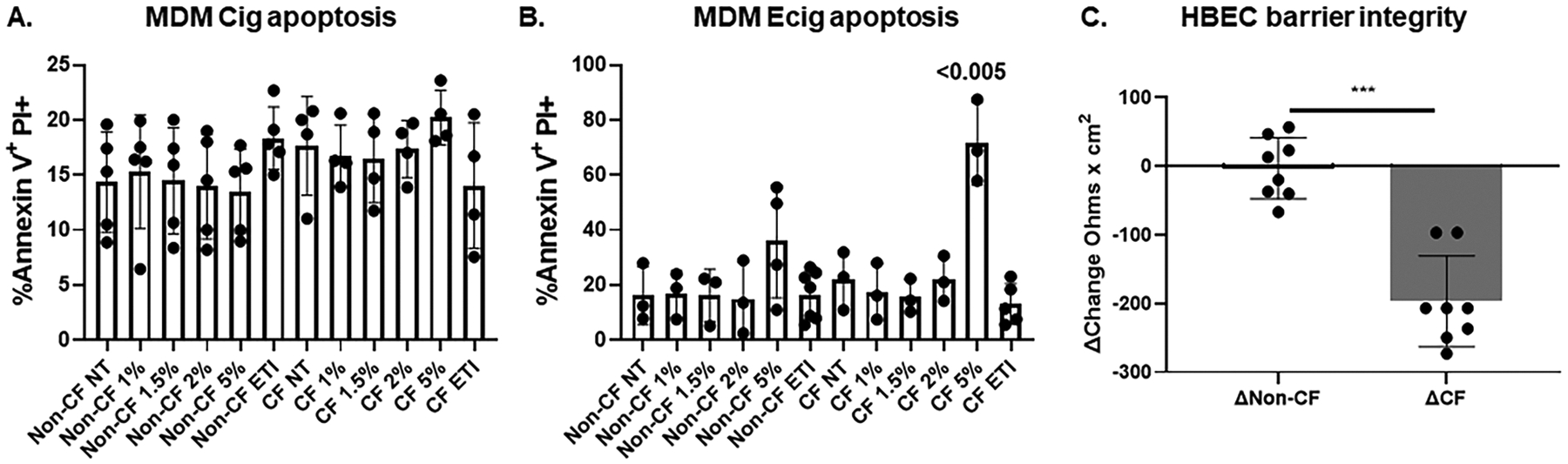
A) Apoptosis was measured via flow cytometry detection of Annexin V+ PI+ non-CF and CF human MDMs exposed to vehicle (NT) or increasing concentrations of cigarette extracts or ETI, n=4–5. B) Apoptosis was measured via flow cytometry detection of Annexin V+ PI+ non-CF and CF human MDMs exposed to increasing concentrations of e-cig extracts or ETI, n=3–5. C) Change in trans-epithelial electrical resistance (TEER) measurements over 5 days (Ohms × cm2) in CF and non-CF well-differentiated HBECs during 5-day intermittent apical exposures to 1% unflavored and flavored e-cigs, n=8 biologic replicates. “*” = p value <0.05, “**” = p value < 0.01, “***” = p value< 0.001. Statistics via one-way ANOVA for 1A, 1B with post-hoc Dunnett’s F multiple comparison test and unpaired t-test for 1C.
E-cigs decrease MDM CFTR expression and function
Next, we examined the impact of one-time and repeated 1% e-cig exposure on CFTR expression and function in macrophages and epithelial cells compared to treatment with CFTR modulators. Treatment of MDMs with ETI or tezacaftor/ivacaftor (TI) significantly improved CFTR expression (Fig. 2A) and function (Fig. 2C). In contrast, acute, one-time e-cig exposure reduced CF MDM CFTR expression (Fig. 2A) and function (Fig. 2C). Further, e-cigs partially negated ETI or TI mediated rescue of CFTR expression and function (Fig. 2A, 2C). While nicotine alone did not reduce CFTR expression or alter ETI effects, it did reduce both CFTR function and ETI functional rescue, similar to e-cigs (Fig. 2A, 2C). During repeated exposure, CF MDMs also demonstrated increased CFTR expression (Fig. 2B) and function (Fig. 2D) after ETI treatment as well as decreased expression and function after e-cigs. Similar to acute exposures, nicotine only reduced CFTR function (Fig. 2D). However, e-cigs negated CFTR restoration by ETI, while nicotine did not (Fig. 2B, 2D). As a control, we found that repeated cigarette exposure also partially negated ETI functional CFTR restoration in CF MDMs (Fig. 2D). In contrast to MDM findings, CFTR expression in HBECs was unaffected by repeated e-cigs, including flavored e-cigs (Fig. 2E). These findings suggest cell-specific impacts of e-cigs on CFTR and raise concern regarding the efficacy of CFTR modulator restoration of innate immune function in children with e-cigs.
Figure 2: E-cigs decrease MDM CFTR function and expression.

CFTR expression was measured in CF and non-CF MDMs via flow cytometry with or without exposure to vehicle (NT) or A) acute (1-time) or B) repeated (3-day) ETI, 1% e-cig, nicotine alone (nico), or combinations, n=3–6. CFTR function measured via halide efflux in non-CF and CF MDMs with or without C) acute or D) repeated exposures to ETI, 1% e-cig, nicotine, or combinations. Repeated exposure also with 1% cigarettes (cig). n=3–6 for A-D, “*” = p value <0.05, “**” = p value < 0.01, “***” = p value< 0.001 via one-way ANOVA with Dunnett’s F multiple comparison test for CF compared to non-CF NT control. Additional within exposure one-way ANOVA with Tukey multiple comparison test denoted with bracketed individual comparisons of significance for A-D. E) CFTR expression measured via Western blot in CF and non-CF HBECs with repeated e-cig exposure, representative blots shown with GAPDH as loading control. HBECs additionally exposed to cinnamon-flavored e-cig extracts.
E-cigs and bacterial killing
Prior studies demonstrated that secondhand cigarette exposure reduces macrophage-mediated bacterial killing.(19, 24–26) Therefore, we tested the impact of e-cigs upon CF MDM-mediated killing of CF clinical isolates. Consistent with prior studies, we found a significant increase in MRSA bacterial load in CF MDMs with combustible SHSe, but not e-cigs (Fig. 3A). Additionally, there were no significant differences in bacterial load for P. aeruginosa, or B.cenocepacia during SHSe or e-cigs (Fig. 3A–C), although P. aeruginosa bacterial loads for CF and non-CF were similar at baseline. There were no differences in bacterial load for any pathogen during SHSe or e-cigs combined with ETI treatment (Fig. 3A–C), although ETI reduced CF B. cenocepacia burden as anticipated.
Figure 3: E-cigs and bacterial killing.

A) MRSA, B) Pseudomonas aeruginosa (Pa), and C) Burkholderia cenocepacia (Bc) bacterial killing via CFU assay in response to repeated e-cig or cigarettes (cig) exposure, treatment with ETI, or combinations. N=3–6, p value via one-way ANOVA with Dunnett’s F multiple comparison test, “*” = p value <0.05, “***” = p value <0.01.
E-cigs alter immunometabolism
Our prior work showed that SHSe alters anti-inflammatory PGD2 and other arachidonic acid metabolite expression in CF MDMs.(19) Therefore, we examined a targeted panel of pro- and anti-inflammatory lipid metabolites in CF and non-CF MDMs and HBECs in response to e-cigs. We found that e-cigs negated ETI-mediated increases in PGD2 expression in CF MDMs (Fig. 4A). However, both e-cigs and ETI treatment had limited impacts on other lipid metabolites examined (Fig. 4A). In contrast, e-cigs did not alter PGD2 expression in CF HBECs, but it did alter other lipid metabolites such as 2,3-dinor-8-iso-PGF2α and 15-deoxy-D12,14 PGJ2. (Fig. 4B). Because of the observed alterations in other lipids besides PGD2 we also tested the impact of flavored e-cig extracts. We did not find any significant differences between flavored and unflavored e-cigs in HBECs (Fig. 4B).
Figure 4: E-cigs and immunometabolites.

A) MDM production of eicosanoid metabolites in response to ecigs, ETI, nicotine, or combinations. B) HBEC production of eicosanoid metabolites in response to flavored or non-flavored ecigs, ETI, nicotine, or combinations. N=3–4, significance via one-way ANOVA with Dunnett’s F multiple comparison test.
We then tested whether e-cigs alter pathways of inflammation that may be downstream of CFTR signaling. CF MDMs and HBECs were exposed to unflavored e-cigs alone or in combination with ETI and analyzed for cytokine production via a 44-cytokine panel Luminex assay. We also tested exposure to cinnamon-flavored e-cigs, as flavorings can be associated with additive inflammatory properties. At baseline, CF MDMs had an increased expression of inflammatory cytokines (Fig. 5A). Exposure to flavored e-cigs was associated with further upregulation of many cytokines in CF MDMs including GRO-α (CXCL1), GRO-β (CXCL2), IL-2, IL-6, IL-10, IL-33, and IP-10 (Fig. 5A). While unflavored e-cigs had minimal effects on non-CF MDMs, flavored e-cigs were also associated with an upregulation of inflammatory cytokines in non-CF MDMs (Fig. 5A). ETI was associated with a general down-regulation of all cytokines in CF MDMs, with mild loss of downregulation during combined ETI treatment plus e-cigs (Fig. 5A). We then examined all cytokine changes for biologically relevant pathways affected during e-cig exposure in CF MDMs compared to no exposure. Reactome pathway analysis of affected cytokines demonstrated alterations in multiple areas including neutrophil degranulation (n=10 reactions, p value 0.0044), PI3K/AKT signaling (n=8, p value 7.37e-04), MAPK1/MAPK3 signaling (n=10, p value 0.006), the senescence-associated secretory phenotype (SASP, n=5, p value 0.002), and GPCR ligand binding (n=11, p value 0.014) in association with e-cigs. A Voronoi visualization of impacted pathways is depicted in Figure 5C.
Figure 5: E-cigs and cytokine pathways.
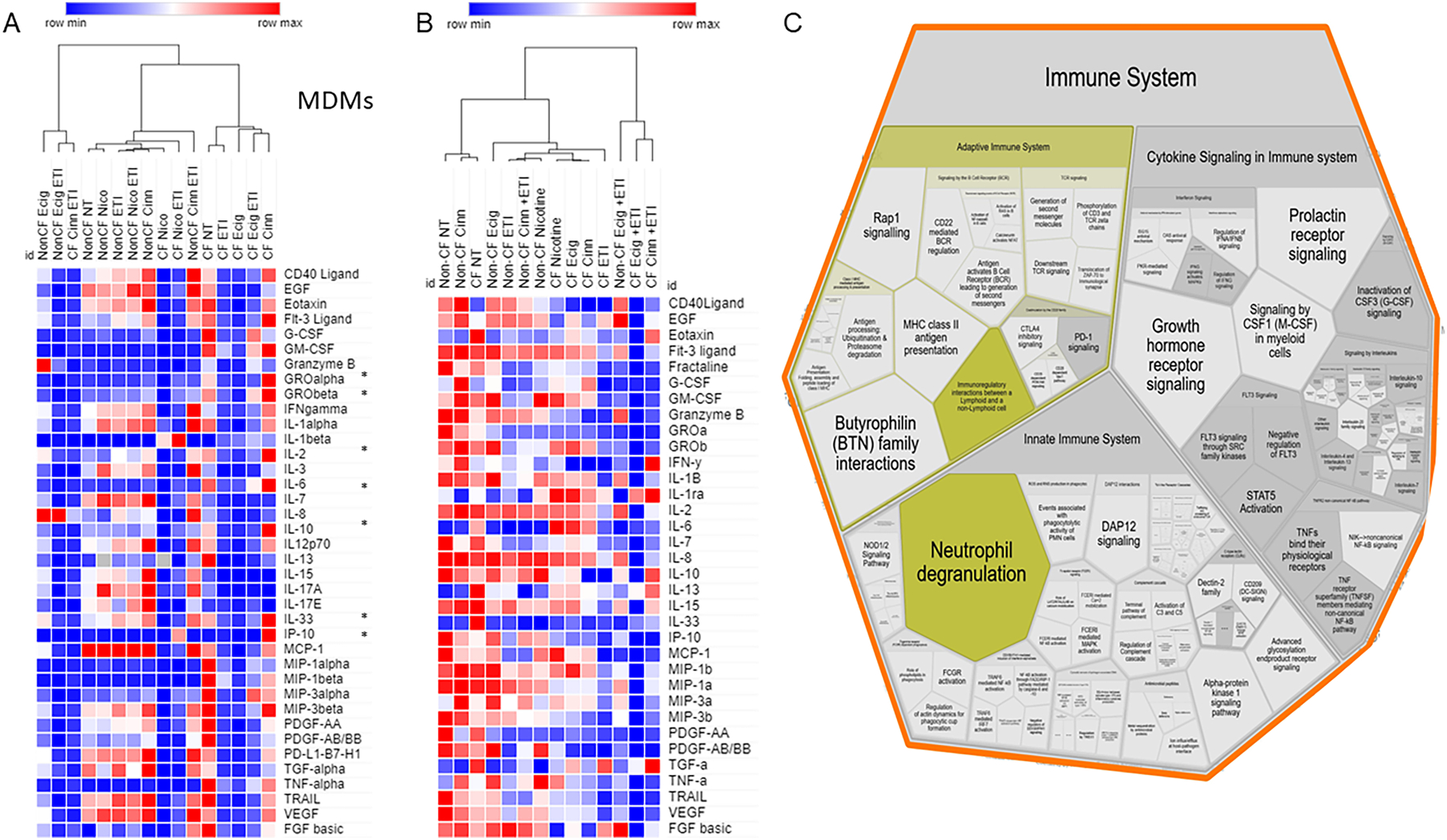
Luminex cytokine results for 44 cytokines in A) CF and non-CF MDMs (38 detected) and B) HBECs (34 detected) during exposure to flavored and unflavored e-cigs, nicotine, ETI or combinations. Columns represent summed exposures (n=4) grouped by hierarchical clustering. A color-coded heatmap is displayed for expression values (red=high, blue=low). “*” indicates cytokines altered by flavored e-cigs. C) Voronoi pathway visualization from Reactome analysis of e-cig exposure in CF MDMs from 5A. The size of the component indicates a higher amount of related cytokine pathways, with the most significantly upregulated pathways in yellow.
E-cig exposure had modest changes in cytokine expression in exposed HBECs, but it did not alter changes induced by ETI treatment, which generally suppressed cytokine function in CF but not non-CF. (Fig. 5B). Changes induced by flavored e-cigs in HBECs were also less pronounced compared to MDMs. Together, these findings suggest that HBECs are less susceptible to e-cigs compared to MDMs.
Last, we examined measures of immunometabolism via seahorse assays in MDMs in response to e-cigs or ETI. Figure 6A demonstrates the overall dynamics of measures of basal and non-mitochondrial respiration and proton leak during the seahorse assays. We found that maximal macrophage basal respiration was slightly lower in CF MDMs compared to non-CF (first 15 minutes), with blunting of basal respiration in both CF and non-CF MDMs in response to e-cigs and rescue by ETI (Figs. 6B–C). E-cig exposure was also associated with increased glycolytic metabolism in both CF and non-CF MDMs (Fig. 6D), with again no impact by ETI treatment. Extracellular acidification rates (ECAR) were also blunted in CF MDMs at baseline (Fig. 6E). E-cigs decreased ECAR over time in non-CF MDMs only (Fig. 6F), due to the already blunted baseline of CF MDMs (Fig. 6E). These findings support disease-agnostic alterations in macrophage immunometabolism in response to e-cigs.
Figure 6: E-cigs and immunometabolism.

CF and non-CF MDM metabolism during e-cig exposure alone or in combination with ETI. Outputs measured were oxygen consumption rate (OCR), proton efflux rate (PER) and extracellular acidification rate (ECAR). Mean results for n=3 over 60 minutes. A) Schematic of seahorse OCR. B) CF and C) Non-CF MDM OCR basally, in response to e-cigs, ETI, or combinations. D) Percent glycolytic and oxidative phosphorylation (OXPHOR) rates in CF and non-CF MDMs in response to e-cigs, ETI, or combinations. ECAR in E) CF and F) Non-CF MDMs in response to e-cigs, ETI, or combinations. “*” = p value < 0.05, one-way ANOVA with Dunnett’s F multiple comparison test.
Discussion
With the advent of electronic nicotine delivery systems, alternatives to cigarettes have become increasingly common,(27) even among populations with existing pulmonary health conditions or their caregivers.(28, 29) Recent literature has suggested that e-cig exposure is not as carcinogenic as combustible cigarettes, though there is still a high risk of developing obstructive airway diseases or acute lung injury.(30) However, the long-term cancer risk associated with e-cig use remains uncertain.(31) While the negative impacts of SHSe on health outcomes in people with CF have been well studied,(8, 19, 32, 33) not enough research has been conducted to understand the effects of e-cigs on people with CF. In the present study, we identified specific cellular impacts of e-cigs on CF macrophages and to a lesser extent, epithelial cells, including impaired restoration of CFTR function during treatment with CFTR modulators. These findings have important implications for continued monitoring and health policy for people with CF.
Prior to our study, combustible cigarette smoke was demonstrated to decrease CFTR function in HBECs.(34) When HBECs are exposed to cigarette smoke, CFTR channels are internalized, leading to a decrease in active CFTR in acute and chronic settings of exposure.(35–38) It is suggested that SHSe causes an increase in calcium levels, causing CFTR to aggregate in the interior of the cell to allow the cell to function in highly toxic environments, like those experienced in chronic SHSe.(36) Similar to prior cigarette studies, we found that e-cig exposure alters CFTR expression and function in human macrophages and importantly, partially negates the benefits of CFTR modulators. However, these findings were not recapitulated in HBECs and were only partially dependent on nicotine, suggesting other shared mechanisms between e-cig and cigarette exposures. Acrolein has been proposed as one such shared mechanism.(3) It is also possible that HBEC CFTR function may still be impacted in some fashion by e-cigs even though expression was not changed. Many other aspects of e-cig exposure on CFTR trafficking and function are less known. Due to their mechanism of delivery, e-cigs and vape can be flavored with different chemical agents. Some, like the chocolate flavoring 2,5-dimethylpyrazine or vanilla flavoring vanillin, may cause chronic activation of epithelial CFTR channels, which could have negative effects on airway surface function or potentially, immune system responsiveness.(39) Other studies have suggested that electronic nicotine delivery systems do not lead to a significant decrease in airway epithelial CFTR function.(7) We found that e-cig flavors differentially impacted inflammatory cytokine production in both MDMs and HBECs, suggesting product specific impacts on inflammatory outcomes. It is also possible that circulatory and airway structural cells experience different levels of e-cig exposure, which may account for findings in this study. Although our overall results are consistent with a less significant impact of e-cig exposure on epithelial cell CFTR, other harmful effects such as barrier disruption were present, emphasizing the importance of protection against e-cig exposure for people with CF, regardless of the cell type being impacted.
In addition to the above-described defects, we also found that e-cigs metabolically re-programmed CF macrophages, inducing a glycolytic, pro-inflammatory state that was not reversed by CFTR modulators. Similar findings have been found in non-CF mouse studies,(40) with signaling impacted by both flavored and unflavored exposures. Cigarette extracts also similarly impact alveolar macrophages.(4) In mouse precision cut lung slices or HBECs, the impact of e-cigs on glycolysis and mitochondrial metabolism was dose-dependent, with abrogation of responses at higher exposures.(41, 42) However, alveolar macrophages from individuals with e-cig, or vaping, product use-associated lung injury (EVALI) also show skewing towards a pro-inflammatory phenotype.(43) While macrophages can utilize glycolysis acutely to combat bacterial infections, prolonged glycolytic states can cause heightened inflammation and eventual exhaustion.(44) Macrophage metabolites are also critically involved in cellular epigenetic modifications.(45) Overall, these studies suggest cell-specific impacts of e-cig exposure upon immunometabolism.
If primary or secondary tobacco, or vaping exposure are present for people with CF, cessation measures are the first recommended step. When cessation or avoidance is not possible, therapeutic rescue may be necessary. CFTR potentiators like ivacaftor, icenticaftor, or GLPG2196 have shown potential in recovering CFTR function in cigarette-smoke exposed individuals.(46–48) Other agents that activate CFTR via cAMP signaling have also shown benefit.(48) However, the beneficial impact of CFTR modulators or other therapies for e-cig exposed lungs are less well known. Conversely, our findings suggest that the negative impact of e-cig exposure on CFTR modulator treated immune cells in people with CF could lead to heterogeneous immune responses. Our recent work also demonstrated that the level of CFTR restoration in human macrophages correlates with clinical outcomes.(13) Tobacco exposure has been shown in epidemiologic studies to nullify the beneficial effects of CFTR modulators on lung function.(49) Whether e-cig exposure impacts clinical outcomes in CF through immune-mediated or other mechanisms remains to be studied. Therefore, we recommend careful screening and counseling regarding all forms of tobacco/vape exposure in people with CF. Further, these and other studies can be used to advocate for stricter public health policy to prevent e-cig exposure in children with chronic diseases like CF.
While advances in e-cig toxicity awareness and prevention advocacy have been made among youth,(50) continued exposures are still common, despite encouraging reports of overall decreased primary usage by high schoolers.(51) Importantly, rates among middle school youth are not decreasing at the same pace as older youth.(51) Further, underage youth frequently access products online, including through social media, which circumvents many legal policies to curb access.(52) Continued evolution of vaping devices and products also shift youth usage preferences and create new users.(53) New FDA regulations on e-cig flavoring agents would potentially impact youth usage, however the FDA has also authorized menthol flavored e-cigarettes, which still put youth at risk, especially among minoritized populations.(54) Emerging evidence supports this unintended consequence of patchy flavor restrictions, with increased usage amongst youth.(55) All told, until e-cigarette access is completely eliminated for youth, both primary and secondary users will continue to experience negative side effects, especially amongst children with chronic lung diseases like CF.
In summary, e-cig exposure reduced macrophage CFTR expression and hindered functional CFTR restoration by CFTR modulators, promoting a glycolytic, pro-inflammatory state in macrophages. E-cig exposure is an emerging public health threat that may limit the therapeutic efficacy of CFTR modulators in people with CF.
Acknowledgements:
Thank you to our research donors and caregivers for their time and effort. Thank you to Lynette Rogers for assistance with lipidomics performance. The Cure Cystic Fibrosis Columbus Research Development Program (C3RDP) Epithelial Cell Core (ECC) at Nationwide Children’s Hospital and The Ohio State University provided the primary human bronchial epithelial cells used in this work. C3RDP is supported by a Research Development Program Grant (MCCOY17R2) from the Cystic Fibrosis Foundation. MDMs were supplied by the C3 Immune Core as well as the Children’s Healthcare of Atlanta and Emory University Pediatric CF Discovery Core and Immunophenotyping Core. This study was supported by the Cystic Fibrosis Foundation (KOPP20P0-CI) and the NIH (R01HL158747, R01HL148171). Graphical abstract produced via Biorender.com.
Footnotes
Conflict of interest: The authors declare no relevant conflicts of interest.
Literature Cited
- 1.Rasmussen LW, Stanford D, LaFontaine J, Allen AD, and Raju SV. Nicotine aerosols diminish airway CFTR function and mucociliary clearance. Am J Physiol Lung Cell Mol Physiol 324: L557–L570, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim MD, Chung S, Dennis JS, Yoshida M, Aguiar C, Aller SP, Mendes ES, Schmid A, Sabater J, Baumlin N, and Salathe M. Vegetable glycerin e-cigarette aerosols cause airway inflammation and ion channel dysfunction. Front Pharmacol 13: 1012723, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin VY, Fain MD, Jackson PL, Berryhill TF, Wilson LS, Mazur M, Barnes SJ, Blalock JE, Raju SV, and Rowe SM. Vaporized E-Cigarette Liquids Induce Ion Transport Dysfunction in Airway Epithelia. Am J Respir Cell Mol Biol 61: 162–173, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aridgides DS, Mellinger DL, Armstrong DA, Hazlett HF, Dessaint JA, Hampton TH, Atkins GT, Carroll JL, and Ashare A. Functional and metabolic impairment in cigarette smoke-exposed macrophages is tied to oxidative stress. Sci Rep 9: 9624, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyrrell J, Ghosh A, Manzo ND, Randell SH, and Tarran R. Evaluation of chronic cigarette smoke exposure in human bronchial epithelial cultures. J Appl Toxicol 43: 862–873, 2023. [DOI] [PubMed] [Google Scholar]
- 6.Raju SV, Jackson PL, Courville CA, McNicholas CM, Sloane PA, Sabbatini G, Tidwell S, Tang LP, Liu B, Fortenberry JA, Jones CW, Boydston JA, Clancy JP, Bowen LE, Accurso FJ, Blalock JE, Dransfield MT, and Rowe SM. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med 188: 1321–1330, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rayner RE, Makena P, Prasad GL, and Cormet-Boyaka E. Cigarette and ENDS preparations differentially regulate ion channels and mucociliary clearance in primary normal human bronchial 3D cultures. Am J Physiol Lung Cell Mol Physiol 317: L295–L302, 2019. [DOI] [PubMed] [Google Scholar]
- 8.Kopp BT, Ortega-Garcia JA, Sadreameli SC, Wellmerling J, Cormet-Boyaka E, Thompson R, McGrath-Morrow S, and Groner JA. The Impact of Secondhand Smoke Exposure on Children with Cystic Fibrosis: A Review. International journal of environmental research and public health 13: 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopp BT, Sarzynski L, Khalfoun S, Hayes D Jr., Thompson R, Nicholson L, Long F, Castile R, and Groner J. Detrimental effects of secondhand smoke exposure on infants with cystic fibrosis. Pediatr Pulmonol 50: 25–34, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Kopp BT, Thompson R, Kim J, Konstan R, Diaz A, Smith B, Shrestha C, Rogers LK, Hayes D Jr., Tumin D, Woodley FW, Ramilo O, Sanders DB, Groner JA, and Mejias A. Secondhand smoke alters arachidonic acid metabolism and inflammation in infants and children with cystic fibrosis. Thorax 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wisniewski BL, Shrestha CL, Zhang S, Thompson R, Gross M, Groner JA, Uppal K, Ramilo O, Mejias A, and Kopp BT. Metabolomics profiling of tobacco exposure in children with cystic fibrosis. J Cyst Fibros 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loman BR, Shrestha CL, Thompson R, Groner JA, Mejias A, Ruoff KL, O’Toole GA, Bailey MT, and Kopp BT. Age and environmental exposures influence the fecal bacteriome of young children with cystic fibrosis. Pediatr Pulmonol 55: 1661–1670, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Shrestha CL, Robledo-Avila F, Jaganathan D, Wisniewski BL, Brown N, Pham H, Carey K, Amer AO, Hall-Stoodley L, McCoy KS, Bai S, Partida-Sanchez S, and Kopp BT. Cystic fibrosis macrophage function and clinical outcomes after elexacaftor/tezacaftor/ivacaftor. Eur Respir J 61: 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Shrestha CL, Wisniewski BL, Pham H, Hou X, Li W, Dong Y, and Kopp BT. Consequences of CRISPR-Cas9-Mediated CFTR Knockout in Human Macrophages. Front Immunol 11: 1871, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S, Shrestha CL, Robledo-Avila F, Jaganathan D, Wisniewski BL, Brown N, Pham H, Carey K, Amer AO, Hall-Stoodley L, McCoy KS, Bai S, Partida-Sanchez S, and Kopp BT. Cystic fibrosis macrophage function and clinical outcomes after elexacaftor/tezacaftor/ivacaftor. Eur Respir J 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Assani K, Shrestha CL, Rinehardt H, Zhang S, Robledo-Avila F, Wellmerling J, Partida-Sanchez S, Cormet-Boyaka E, Reynolds SD, Schlesinger LS, and Kopp BT. AR-13 reduces antibiotic-resistant bacterial burden in cystic fibrosis phagocytes and improves cystic fibrosis transmembrane conductance regulator function. J Cyst Fibros 18: 622–629, 2019. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds SD, Rios C, Wesolowska-Andersen A, Zhuang Y, Pinter M, Happoldt C, Hill CL, Lallier SW, Cosgrove GP, Solomon GM, Nichols DP, and Seibold MA. Airway Progenitor Clone Formation is Enhanced by Y-27632-dependent Changes in the Transcriptome. Am J Respir Cell Mol Biol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds SD, Rios C, Wesolowska-Andersen A, Zhuang Y, Pinter M, Happoldt C, Hill CL, Lallier SW, Cosgrove GP, Solomon GM, Nichols DP, and Seibold MA. Airway Progenitor Clone Formation Is Enhanced by Y-27632-Dependent Changes in the Transcriptome. Am J Respir Cell Mol Biol 55: 323–336, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopp BT, Thompson R, Kim J, Konstan R, Diaz A, Smith B, Shrestha C, Rogers LK, Hayes D Jr., Tumin D, Woodley FW, Ramilo O, Sanders DB, Groner JA, and Mejias A. Secondhand smoke alters arachidonic acid metabolism and inflammation in infants and children with cystic fibrosis. Thorax 74: 237–246, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rayner RE, Wellmerling J, Osman W, Honesty S, Alfaro M, Peeples ME, and Cormet-Boyaka E. In vitro 3D culture lung model from expanded primary cystic fibrosis human airway cells. J Cyst Fibros 19: 752–761, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wellmerling J, Rayner RE, Chang SW, Kairis EL, Kim SH, Sharma A, Boyaka PN, and Cormet-Boyaka E. Targeting the EGFR-ERK axis using the compatible solute ectoine to stabilize CFTR mutant F508del. FASEB J 36: e22270, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang S, Shrestha CL, and Kopp BT. Cystic fibrosis transmembrane conductance regulator (CFTR) modulators have differential effects on cystic fibrosis macrophage function. Sci Rep 8: 17066, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez JT, Rogers LK, Kellogg C, Iazbik MC, Couto CG, Pressler BM, Hoepf TM, and Radin MJ. Plasma Vasoprotective Eicosanoid Concentrations in Healthy Greyhounds and Non-Greyhound Dogs. J Vet Intern Med 30: 583–590, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pehote G, Bodas M, Brucia K, and Vij N. Cigarette Smoke Exposure Inhibits Bacterial Killing via TFEB-Mediated Autophagy Impairment and Resulting Phagocytosis Defect. Mediators Inflamm 2017: 3028082, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulkarni R, Caskey J, Singh SK, Paudel S, Baral P, Schexnayder M, Kim J, Kim N, Kosmider B, Ratner AJ, and Jeyaseelan S. Cigarette Smoke Extract-Exposed Methicillin-Resistant Staphylococcus aureus Regulates Leukocyte Function for Pulmonary Persistence. Am J Respir Cell Mol Biol 55: 586–601, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ni I, Ji C, and Vij N. Second-hand cigarette smoke impairs bacterial phagocytosis in macrophages by modulating CFTR dependent lipid-rafts. PLoS One 10: e0121200, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowler RP, Hansel NN, Jacobson S, Graham Barr R, Make BJ, Han MK, O’Neal WK, Oelsner EC, Casaburi R, Barjaktarevic I, Cooper C, Foreman M, Wise RA, DeMeo DL, Silverman EK, Bailey W, Harrington KF, Woodruff PG, Drummond MB, for C, and Investigators S. Electronic Cigarette Use in US Adults at Risk for or with COPD: Analysis from Two Observational Cohorts. J Gen Intern Med 32: 1315–1322, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clapp PW, and Jaspers I. Electronic Cigarettes: Their Constituents and Potential Links to Asthma. Curr Allergy Asthma Rep 17: 79, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaziri S, McGarry ME, Huang CY, Cuneo AA, Willen SM, Iwanaga K, Neemuchwala F, Gibb ER, Chan M, and Ly NP. Time to be blunt: Substance use in cystic fibrosis. Pediatr Pulmonol 59: 1015–1027, 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esteban-Lopez M, Perry MD, Garbinski LD, Manevski M, Andre M, Ceyhan Y, Caobi A, Paul P, Lau LS, Ramelow J, Owens F, Souchak J, Ales E, and El-Hage N. Health effects and known pathology associated with the use of E-cigarettes. Toxicol Rep 9: 1357–1368, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahu R, Shah K, Malviya R, Paliwal D, Sagar S, Singh S, Prajapati BG, and Bhattacharya S. E-Cigarettes and Associated Health Risks: An Update on Cancer Potential. Adv Respir Med 91: 516–531, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collaco JM, Vanscoy L, Bremer L, McDougal K, Blackman SM, Bowers A, Naughton K, Jennings J, Ellen J, and Cutting GR. Interactions between secondhand smoke and genes that affect cystic fibrosis lung disease. JAMA 299: 417–424, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubin BK. Exposure of children with cystic fibrosis to environmental tobacco smoke. N Engl J Med 323: 782–788, 1990. [DOI] [PubMed] [Google Scholar]
- 34.Sloane PA, Shastry S, Wilhelm A, Courville C, Tang LP, Backer K, Levin E, Raju SV, Li Y, Mazur M, Byan-Parker S, Grizzle W, Sorscher EJ, Dransfield MT, and Rowe SM. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS One 7: e39809, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cantin AM. Cystic Fibrosis Transmembrane Conductance Regulator. Implications in Cystic Fibrosis and Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc 13 Suppl 2: S150–155, 2016. [DOI] [PubMed] [Google Scholar]
- 36.Rasmussen JE, Sheridan JT, Polk W, Davies CM, and Tarran R. Cigarette smoke-induced Ca2+ release leads to cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction. J Biol Chem 289: 7671–7681, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, Zeman KL, Worthington EN, Gentzsch M, Kreda SM, Cholon D, Bennett WD, Riordan JR, Boucher RC, and Tarran R. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J 26: 533–545, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bodas M, Min T, and Vij N. Critical role of CFTR-dependent lipid rafts in cigarette smoke-induced lung epithelial injury. Am J Physiol Lung Cell Mol Physiol 300: L811–820, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherwood CL, and Boitano S. Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine. Respir Res 17: 57, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muthumalage T, and Rahman I. Pulmonary immune response regulation, genotoxicity, and metabolic reprogramming by menthol- and tobacco-flavored e-cigarette exposures in mice. Toxicol Sci 193: 146–165, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herbert J, Kelty JS, Laskin JD, Laskin DL, and Gow AJ. Menthol flavoring in e-cigarette condensate causes pulmonary dysfunction and cytotoxicity in precision cut lung slices. Am J Physiol Lung Cell Mol Physiol 324: L345–L357, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clapp PW, Lavrich KS, van Heusden CA, Lazarowski ER, Carson JL, and Jaspers I. Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am J Physiol Lung Cell Mol Physiol 316: L470–L486, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warren KJ, Beck EM, Callahan SJ, Helms MN, Middleton E, Maddock S, Carr JR, Harris D, Blagev DP, Lanspa MJ, Brown SM, and Paine R 3rd. Alveolar macrophages from EVALI patients and e-cigarette users: a story of shifting phenotype. Respir Res 24: 162, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogger PP, and Byrne AJ. Macrophage metabolic reprogramming during chronic lung disease. Mucosal Immunol 14: 282–295, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soto-Heredero G, Gomez de Las Heras MM, Gabande-Rodriguez E, Oller J, and Mittelbrunn M. Glycolysis - a key player in the inflammatory response. FEBS J 287: 3350–3369, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez FJ, Criner GJ, Gessner C, Jandl M, Scherbovsky F, Shinkai M, Siler TM, Vogelmeier CF, Voves R, Wedzicha JA, Bartels C, Bottoli I, Byiers S, Cardenas P, Eckert JH, Gutzwiller FS, Knorr B, Kothari M, Parlikar R, Tanase AM, and Franssen FME. Icenticaftor, a CFTR Potentiator, in COPD: A Multicenter, Parallel-Group, Double-Blind Clinical Trial. Am J Respir Crit Care Med 208: 417–427, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raju SV, Lin VY, Liu L, McNicholas CM, Karki S, Sloane PA, Tang L, Jackson PL, Wang W, Wilson L, Macon KJ, Mazur M, Kappes JC, DeLucas LJ, Barnes S, Kirk K, Tearney GJ, and Rowe SM. The Cystic Fibrosis Transmembrane Conductance Regulator Potentiator Ivacaftor Augments Mucociliary Clearance Abrogating Cystic Fibrosis Transmembrane Conductance Regulator Inhibition by Cigarette Smoke. Am J Respir Cell Mol Biol 56: 99–108, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaza N, Lin VY, Stanford D, Hussain SS, Falk Libby E, Kim H, Borgonovi M, Conrath K, Mutyam V, Byzek SA, Tang LP, Trombley JE, Rasmussen L, Schoeb T, Leung HM, Tearney GJ, Raju SV, and Rowe SM. Evaluation of a novel CFTR potentiator in COPD ferrets with acquired CFTR dysfunction. Eur Respir J 60: 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baker E, Harris WT, Rowe SM, Rutland SB, and Oates GR. Tobacco smoke exposure limits the therapeutic benefit of tezacaftor/ivacaftor in pediatric patients with cystic fibrosis. J Cyst Fibros 20: 612–617, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller CC, Xiao M, Lay DJ, Miley SN, Vanderford NL, and Ickes MJ. The Impact of a Virtual Tobacco Prevention and Advocacy Training Among Youth in Appalachian Kentucky Communities. Tob Use Insights 16: 1179173X221150747, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jamal A, Park-Lee E, Birdsey J, West A, Cornelius M, Cooper MR, Cowan H, Wang J, Sawdey MD, Cullen KA, and Navon L. Tobacco Product Use Among Middle and High School Students - National Youth Tobacco Survey, United States, 2024. MMWR Morb Mortal Wkly Rep 73: 917–924, 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaiha SM, Lempert LK, Lin C, and Halpern-Felsher B. E-cigarette access and age verification among adolescents, young adults, and adults. Addict Behav 161: 108193, 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hammond D, Reid JL, Burkhalter R, and East K. Use of disposable e-cigarettes among youth who vape in Canada, England and the United States: Repeat cross-sectional surveys, 2017–2023. Addiction 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubin R Controversial FDA Decision Authorizes Menthol-Flavored E-Cigarettes Despite Risks to Youth. JAMA 332: 692–696, 2024. [DOI] [PubMed] [Google Scholar]
- 55.Borowiecki M, Kim Y, and Emery S. A Patchy Prohibition: Product and Flavor Substitution After the Food and Drug Administration’s Prioritized Enforcement Policy on Flavored E-cigarettes. Nicotine Tob Res 26: 527–535, 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]


