Abstract
Background
Digital health technology adoption has accelerated in respiratory care, particularly since the COVID-19 pandemic, supporting various applications from self-management to telerehabilitation. While these technologies have transformed health care delivery, their impact on the patient-provider relationship in specialist respiratory care remains poorly understood.
Objective
This study aims to systematically review the literature on the impact of digital health technology on the patient-provider relationship in respiratory secondary care settings and to understand the factors that enhance or diminish this relationship.
Methods
In December 2023, we conducted a systematic review following Cochrane methodology, searching MEDLINE, Embase, CINAHL, Cochrane databases, and PsycINFO. We included qualitative, quantitative, and mixed methods studies examining digital health interventions in respiratory secondary care. Trained volunteers from the European Respiratory Society CONNECT Clinical Research Collaboration performed screening and data extraction. We conducted a qualitative meta-synthesis of findings, followed by an abductive quantitative data analysis. A total of 3 stakeholder workshops were held to interpret findings collaboratively with patients and health care professionals.
Results
From 15,779 papers screened, 97 met the inclusion criteria (55 qualitative/mixed-methods studies, 42 quantitative studies). Studies covered various respiratory conditions, including COPD (32%), asthma (26%), and COVID-19 (13%). Four main themes emerged: trust (foundational to the relationship), adoption factors (including clinical context and implementation drivers), confidence in technology (based on functionality and the evidence base), and connection (encompassing communication and a caring presence). Digital health technology can either enhance or diminish trust between patients and clinicians, with patients' perceptions of the motivations behind its implementation being crucial. While technology facilitated access and communication, remote consultations risked depersonalisation, particularly when not balanced with in-person interactions. Self-monitoring and access to information empowered patients and promoted more equitable patient-provider relationships.
Conclusions
Digital health technology can either strengthen or weaken patient-provider relationships in respiratory care, with effects impacted by adoption factors, confidence in technology, connection, and patient empowerment. Maintaining trust in the era of digital care requires transparent implementation of motivations, consideration of individual circumstances, and reliable technology that supports rather than replaces the therapeutic relationship.
Trial Registration
PROSPERO CRD42024493664; https://www.crd.york.ac.uk/PROSPERO/view/CRD42024493664
Keywords: telemedicine, digital health, patient-provider relationship, respiratory care, systematic review, trust
Introduction
Digital Health Technologies and Their Benefits
Digital technologies encompass a broad range of tools, including telemedicine, electronic health records, mobile health apps, wearable devices, and artificial intelligence (AI)–assisted diagnostics [1]. These technologies have significantly transformed health care delivery by improving accessibility, efficiency, and continuity of care. The World Health Organization (WHO) has highlighted digital health as a strategy to achieve universal health care coverage, particularly by facilitating remote care and enhancing support for vulnerable populations [2,3]. Digital health tools also allow for remote monitoring of disease progression, support for assisted living, and implementation of cloud-based health care systems.
Acceleration of Digital Health Adoption During the COVID-19 Pandemic
The adoption of digital health technologies accelerated dramatically during the COVID-19 pandemic as health care systems adapted to the need for remote care. The crisis led to a surge in teleconsultations, virtual wards, home treatments, and digital self-management tools, many of which have persisted beyond the pandemic [4]. Both patients and health care providers embraced these changes, recognizing their potential to enhance access to care while reducing unnecessary hospital visits. However, the rapid shift to digital health care also raised concerns about digital equity, access disparities, and the potential depersonalization of care.
Relevance for Patients With Respiratory Disease
Patients with respiratory diseases were among the groups most affected by the digital transformation of health care [5]. Conditions such as chronic obstructive pulmonary disease (COPD), asthma, and cystic fibrosis require ongoing monitoring and frequent interactions with health care providers. Given the complexity of managing respiratory diseases, the shift to digital care also raised concerns regarding patient engagement, adherence to treatment, and the impact on the patient-provider relationship, particularly in the secondary care setting, where interactions are typically episodic rather than continuous.
Impact of Digital Health on the Patient-Provider Relationship
Predating widespread adoption of digital health, Ridd et al [6] in primary care conceptualized the patient-provider relationship as being built on continuity, interpersonal communication, and trust. Their framework emphasized that long-term patient-provider interactions foster deeper connections, leading to better health outcomes.
In the digital era, the dynamics of these relationships have shifted. In a review of reviews, Ramachandran et al [7] found that eHealth technologies have a mixed impact on relationships and trust in primary care patient-provider interactions, depending on patient perceptions, provider communication skills, technology design, and organizational factors. They concluded that training providers in technology-specific communication skills and ensuring that eHealth implementations were equitable and considerate of diverse patient needs can foster trust and maintain strong relationships. Similarly, a scoping review that explored the theoretical perspectives underpinning research on the physician-patient relationship in digital health practice emphasized the need for ethical considerations in medical practice to ensure that technology enhances, rather than hinders, the quality of care [8].
These reviews have focused on primary care, where patients benefit from long-term relationships with a single local provider. In contrast, in secondary care, interactions may be with multiple specialists sometimes at a substantial distance, typically short-term rather than continuous. This was a key theme raised by patients and professionals within the Institute for Healthcare Improvement-funded Rapid and Secure AI-enhanced Diagnosis, Precision Medicine and Patient Empowerment Centered Decision Support System for Coronavirus Pandemics (DRAGON) project, which focused on care during the COVID-19 pandemic. We, therefore, aimed to systematically review the literature on the impact of digital health technology on the patient-provider relationship and to understand the factors that enhance or diminish it in respiratory secondary care settings.
Methods
Overview
Our systematic review is registered with the International Prospective Register of Systematic Reviews (PROSPERO; CRD42024493664) and follows the Cochrane methodology [9] and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. There were no deviations from the registered protocol. We used Covidence software to manage the screening and data extraction process [10].
Search Strategy and Selection Criteria
The search strategy had three components: digital health care, respiratory conditions (including COVID-19), and patient-provider relationships. Detailed methods are outlined in Multimedia Appendix 1. We applied English-language search filters but no study design or date limits. Searches were conducted in December 2023 in Ovid MEDLINE, Embase, and CINAHL via ESBCO, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, and PsycINFO. Duplicates were detected and removed using Covidence software.
Eligibility Criteria
Our inclusion and exclusion criteria are presented in Textbox 1.
Inclusion and exclusion criteria.
Inclusion criteria
Population: respiratory disease patients, both adult and pediatric.
Interventions: eHealth technology, mobile health technology, or artificial intelligence.
Comparator: standard care (if appropriate: eg, in controlled intervention studies)
Outcome: the quality of the patient-provider relationship changes in the patient-provider relationship.
Study design: qualitative, mixed-methods studies, randomized controlled trials, cross-sectional
Setting: secondary care settings only. This includes hospital or clinic-based medical specialist care and can consist of urgent and emergency care, or planned elective care.
Exclusion criteria
Systematic reviews, abstracts, conference papers, protocols or commentaries, and studies in primary care settings.
Screening and Data Extraction
Following a training program, 30 volunteer clinicians and researchers from the European Respiratory Society CONNECT Clinical Research Collaboration [11] contributed to the screening of titles and abstracts. The volunteers came from various health care professions, including doctors, nurses, physiotherapists, clinicians, academic researchers, and basic scientists. The training program consisted of multiple stages. For screening titles and abstracts, reviewers attended a session where they received detailed screening guidelines, including clear criteria for “yes,” “no,” and “maybe” decisions. Articles meeting all 3 key criteria (digital health, patient-provider relationship, and respiratory disease) were marked as “yes.” Articles covering none or only one of these areas were marked as “no.” Any articles where reviewers were uncertain or partially met criteria were marked as “maybe.” Following this initial training, reviewers completed a practical exercise screening 100 test articles to gain hands-on experience with the criteria. After 2 weeks of independent screening practice, a second session was held to address questions that had emerged during the preliminary screening exercise and clarify any areas of uncertainty. All articles marked as “maybe” were subsequently reviewed by 2 senior reviewers to ensure quality and consistency. All authors and DRAGON or CONNECT group members performed the title and abstract stage. A total of 6 reviewers (MS, DD, HP, PP, AK, and KH) led by MS and DD resolved conflicts and oversaw quality [12]. Following a similar multistage training program, MS, DD, PP, HP, KH, OM, AA, CT, AG, KBS, and UC conducted the full-text screening with MS and DD, resolving conflicts and overseeing quality.
Quality Assessment
A total of 2 reviewers assessed the methodological quality of the studies using the relevant Critical Appraisal Skills Programme (CASP) checklist [8]. We selected this tool because it includes an assessment of the paper’s relevance to the review aim (“Will the results help our review?”). We anticipated that the patient-provider relationship was unlikely to be a primary objective in most studies, so using the CASP tool enabled us to identify and emphasize the findings from studies whose aims aligned most closely with our research question. CASP does not generate a total score. We classified the studies as those that explored the patient-provider relationship: (1) as the primary aim, (2) as a secondary objective when the authors had explicitly defined it as such in the methods section, or (3) as a coincidental outcome when it was identified in the results but not prespecified in the methods.
Data Analysis
Qualitative Meta-Synthesis
We initially applied the framework from Ridd et al [6], which defines knowledge, trust, loyalty, and regard as constructs of the depth of a patient-provider relationship within the long-term context of primary care relationships. However, because it did not reflect all aspects of digital health care as described in our included papers, MS and DD undertook a thematic meta-synthesis. The data were coded into subthemes and overarching themes using Quirkos software (version 2.3).
Abductive Analysis for Quantitative and Mixed Methods Findings
Categories emerging from the qualitative meta-synthesis provided a framework to organize the quantitative data. A total of 2 researchers (MS and DD) followed an abduction process [13] to consider narratives around elements of the patient-provider relationship affected (positively or negatively) by digital health care. We integrated objective measures (quantitative surface) and latent phenomena to understand these elements.
Stakeholder Engagement Workshops to Interpret Findings
Our work followed the consultation and application approaches to collaborative analysis defined by Jennings et al [14]. Aligned with the methodology of collaborative analysis [15] we presented our preliminary analysis in 3 workshops with patients, patient representatives, and health care professionals. Following an initial analysis within the research team, we carried out the workshops (one for health care professionals and managers, one for patients and patient representatives, and a final workshop for all stakeholder groups. Participants provided verbal and written feedback using Groupmap [16] and engaged in consensus activities, such as using WordCloud functions [17] to inform the interpretation of the findings. Interactive techniques (Wordclouds, GroupMap, and Chat) facilitated engagement in consensus activities, which we used to inform further analysis and interpretation of the findings.
The final analysis stage involved fully integrating the quantitative and qualitative datasets. The research team’s iterative discussions achieved this by aligning the 2 datasets and comparing what is familiar with what is unfamiliar to generate robust explanations [13].
Results
Overview
Following deduplication, we screened titles and abstracts of 15,779 papers; 545 were retrieved for full-text review. We included studies that reported using digital health technologies, including diagnosis, consultations, treatment, and monitoring. We included all studies that comprised respiratory disease patient groups of any age, gender, and disease severity. We excluded studies that did not examine respiratory conditions in secondary care settings or report on the patient-provider relationship.
The primary reason for exclusion at the title and abstract screening stage was the lack of reported primary outcome of interest and the relationship between patient and provider, resulting in 15,234 excluded studies. In the second screening stage of full texts, the main reasons for exclusion were insufficient reporting on the patient-provider aspect (316 full texts excluded), patients with no respiratory disease (45 studies), wrong study design (50 studies in total), and no technology (28 studies). In total, we identified 97 studies that met our inclusion criteria and had sufficiently reported on the aspects of patient-provider relationships (see the PRISMA flow diagram in Figure 1 and checklist in Multimedia Appendix 2).
Figure 1.
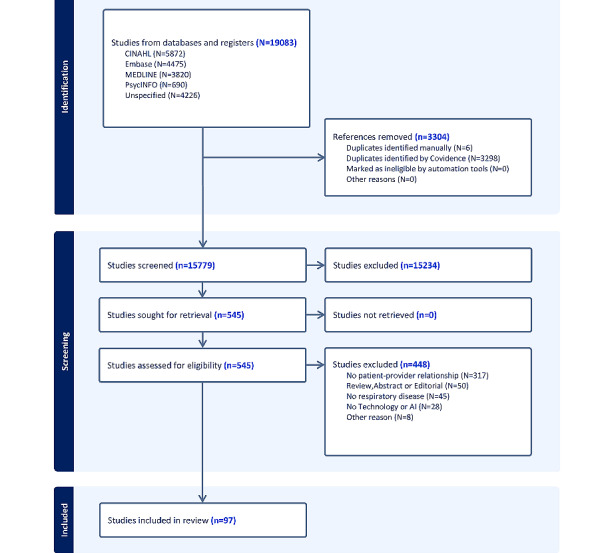
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram.
Characteristics of the Studies Included
Tables 1 (for qualitative studies, 56/97, 58%) and 2 (for quantitative studies, 41/97, 42%) present the included studies.
Table 1.
Included qualitative and mixed methods studies.
| Study characteristics | Theme | |||||||||
| Study | Applicability | Country | System type | Disease | COVID-19 era | Adoption factors | Confidence in technology | Connection | Patient empowerment | |
| Mantzounarisa [18] | Low | Greece | Telehealth (teleconsultation) | Asthma | Pre | Ctxtb | —c | Communication | Self-efficacy | |
| Hibbert et al [19] | Moderate | United Kingdom | Telehealth (telemonitoring) | COPDd | Pre | — | TPe | Depersonalization | — | |
| van Baar et al [20] | Moderate | United Kingdom | Telehealth (teleconsultation) | Asthma | Pre | — | — | Communication, Depersonalization | — | |
| Whitten and Mickus [21] | Moderate | United States | Telehealth (teleconsultation) | COPD | Pre | — | — | Communication | — | |
| Cornwall et al [22] | Moderate | United Kingdom | Communication | Lung cancer | Pre | PoMf | — | Communication | — | |
| Mair et al [23] | Moderate | United Kingdom | Telehealth (telemonitoring) | COPD | Pre | Ctxt, PoM | TP, SoEg | CPh, Depersonalization | — | |
| Hoffman et al [24] | Low | Kenya | Telehealth (telemonitoring) | Tuberculosis | Pre | — | — | Communication | — | |
| Shany et al [25] | Low | Australia | Telehealth (telemonitoring) | COPD | Pre | — | TP | — | Self-efficacy | |
| Cox et al [26] | Low | Australia | Telehealth (telemonitoring) | Lung cancer | Pre | Ctxt | IAi | Depersonalization | — | |
| Fairbrother et al [27] | High | United Kingdom | Telehealth (telemonitoring) | COPD | Pre | — | TP | Communication, CP, CoCj | — | |
| Kim et al [28] | Low | South Korea | Telehealth (telemonitoring) | COPD | Pre | — | — | Communication, CoC | — | |
| Dinesen et al [29] | Moderate | Denmark | Telehealth (telerehabilitation) | COPD | Pre | Ctxt |
|
CP | Self-efficacy | |
| Huniche et al [30] | Moderate | Denmark | Telehealth (self-monitoring) | COPD | Pre | — | — | CP | Self-efficacy | |
| Damhus et al [31] | Moderate | Denmark | Telehealth (telemonitoring) | COPD | Pre | — | — | CP | Self-efficacy | |
| Brown-Johnson et al [32] | Low | United States | Education | Lung cancer | Pre | — | — | Communication | Self-efficacy | |
| Kenealy et al [33] | Moderate | New Zealand | Telehealth (telemonitoring) | COPD | Pre | — | — | Communication, CP | Self-efficacy | |
| Maguire et al [34] | Low | United Kingdom | Telehealth (telemonitoring) | Lung cancer | Pre | — | IA | Communication, CP | Self-efficacy, SDMk | |
| Roberts et al [35] | Low | United States | Telehealth (teleconsultation) | Asthma | Pre | PoM | — | Communication | Self-efficacy | |
| Dichmann Sorknaes [36] | Low | Denmark | Telehealth (teleconsultation) | COPD | Pre | — | IA | Communication, CP | — | |
| Daftary et al [37] | Moderate | Ethiopia | Communication | Tuberculosis | Pre | Ctxt | TP, SoE | — | — | |
| Hirsch-Moverman et al [38] | Moderate | Lesotho | Communication | Tuberculosis | Pre | PoM | — | Communication, CoC | — | |
| Kopanitsa [39] | Moderate | Russia | Communication | Tuberculosis | Pre | PoM, DoIl | SoE | Communication | Self-efficacy | |
| Nhavoto et al [40] | Moderate | Mozambique | Communication | Tuberculosis | Pre | Ctxt | — | Communication | Self-efficacy | |
| Nissen and Lindhardt [41] | Moderate | Denmark | Telehealth (teleconsultation) | COPD | Pre | PoM | TP | Communication, CP, CoC | Self-efficacy | |
| Damhus et al [31] | Moderate | Denmark | Telehealth (telerehabilitation) | COPD | Pre | PoM | TP, IA, SoE | Communication | — | |
| Liacos [42] et al | Low | Australia | Telehealth (telerehabilitation) | Mixed | Pre | — | — | Communication, CP | — | |
| Hamilton et al [43] | Moderate | United States | Decision support | Lung cancer | Pre | PoM | — | Communication | SDM | |
| Patel et al [44] | High | United States | EHRm | Asthma | Pre | — | — | Communication | — | |
| Rudin et al [45] | Moderate | United States | Telehealth (telemonitoring) | Asthma | Pre | — | — | CP | Self-efficacy | |
| Boström et al [46] | High | Sweden | Telehealth (teleconsultation) | COPD | Pre | — | — | Communication, CP | Self-efficacy | |
| Drabble et al [47] | Moderate | United Kingdom | Telehealth (telemonitoring) | CFn | Pre | — | — | CP | Self-efficacy | |
| Kuntz et al [48] | Low | United States | Telehealth (teleconsultation) | COVID-19 | Post | Ctxt, PoM | — | Communication, CP | — | |
| Lewis et al [49] | Low | United Kingdom | Telehealth (teleconsultation) | COVID-19 | Post | Ctxt | — | — | — | |
| Maguire et al [50] | Moderate | United Kingdom | Telehealth (telemonitoring) | Lung cancer | Pre | CC | — | Communication, CP | — | |
| Van Cittersa et al [51] | Moderate | United States | Telehealth (telemonitoring) | CF | Pre | PoM | — | C P | Self-efficacy, SDM | |
| van Lieshout et al [52] | Moderate | Canada | Telehealth (telemonitoring) | COPD | Pre | Ctxt, PoM, DoI | TP, SoE | Communication, CoC | Self-efficacy | |
| Yamada et al [53] | Low | Canada | Decision support | Asthma | Pre | Ctxt, PoM | — | — | Self-efficacy, SDM | |
| Bains et ala [54] | Low | United States | Telehealth (teleconsultation) | COVID-19 | Post | — | — | Communication, CP | — | |
| Jácome et al [55] | Low | Portugal | Telehealth (teleconsultation) | Asthma | Pre | — | — | Communication, CoC | Self-efficacy | |
| Kennedy et al [56] | High | United States | Telehealth (teleconsultation) | Mixed | Post | Ctxt | TP | Communication, Depersonalization | — | |
| Legler et al [57] | Moderate | United States | Telehealth (teleconsultation) | COVID-19 | Post | PoM | — | Communication, CP, Depersonalization | — | |
| Sekandi et al [58] | Moderate | Uganda | Telehealth (telemonitoring) | Tuberculosis | Pre | Ctxt, PoM | IA | Communication, CP, CoC | — | |
| Thomas et al [59] | Moderate | India | Telehealth (telemonitoring) | Tuberculosis | Pre | PoM | — | CP | — | |
| Wu et al [60] | Low | United Kingdom | Telehealth (teleconsultation) | COPD | Post | Ctxt, PoM | — | — | Self-efficacy, SDM | |
| Gillett and Hope-Gill [61] | Moderate | United Kingdom | Telehealth (teleconsultation) | ILDo | Post | PoM, CC | IA | Depersonalization | — | |
| Haynesa et al [62] | Moderate | United States | Telehealth (teleconsultation) | Asthma | Post | Ctxt, PoM | — | Communication, Depersonalization | — | |
| Jiang et al [63] | Moderate | China | Telehealth (telerehabilitation) | COPD | Post | Ctxt, PoM | IA, SoE | Depersonalization | Self-efficacy | |
| Makhecha et al [64] | Low | United Kingdom | Telehealth (teleconsultation) | Mixed | Post | — | — | Communication, Depersonalization | — | |
| Misplon et al [65] | Low | Belgium | Telehealth (telemonitoring) | Lung Cancer | Post | — | — | Communication | — | |
| Nayyar et al [66] | Moderate | Canada | Telehealth (teleconsultation) | Mixed | Post | Ctxt, PoM | — | Depersonalization | — | |
| Sandau et al [67] | Low | Denmark | Medical device | COPD | Post | PoM | TP | CP, Depersonalization | — | |
| Cox et al [68] | Low | New Zealand | Telehealth (telerehabilitation) | Mixed | Pre | Ctxt | TP | Depersonalization | Self-efficacy, SDM | |
| Hattingh et al [69] | Moderate | Australia | Telehealth (teleconsultation) | COVID-19 | Post | Ctxt |
|
Communication, CoC |
|
|
| Kazmerski et al [70] | Moderate | United States | Telehealth (teleconsultation) | CF | Post | — | IA | — | Self-efficacy, SDM | |
| Robinson et al [71] | Low | United Kingdom | Diagnostics | COPD | Post | — | TP, IA, SoE | CP, CoC | Self-efficacy | |
| Watanabe et al [72] | Moderate | United States | Telehealth (self-monitoring) | CF | Pre | — | — | Communication | Self-efficacy, SDM | |
aStudies that included children.
bCtxt: context.
cNot applicable.
dCOPD: chronic obstructive pulmonary disease.
eTP: technology performance.
fPoM: perception of motivations.
gSoE: strength of evidence.
hCP: caring presence.
iIA: interaction anxiety.
jCoC: continuity of care.
kSDM: shared decision-making.
lDoI: drivers of implementation.
mEHR: electronic health record.
nCF: cystic fibrosis.
oILD: interstitial lung disease.
Table 2.
Included quantitative studies.
| Study characteristics | Theme | |||||||||
| Study | Applicability | Country | System type | Disease | COVID-19 era | Adoption factors | Confidence in technology | Connection | Patient empowerment | |
| Pacht et al [73] | Moderate | United States | Telehealth (teleconsultation) | Mix of respiratory diseases | Pre | —a | — | Communication | Self-efficacy | |
| Stepnowsky et al [74] | Moderate | United States | Telehealth (telemonitoring) | Sleep apnea | Pre | — | — | Communication, Depersonalization, CoCb | — | |
| Varkey et al [75] | Moderate | United States | Telehealth (telemonitoring) | Mix of respiratory diseases | Pre | PoMc | TPd, SoEe | Communication | — | |
| Agha et al [76] | Moderate | United States | Telehealth (teleconsultation) | Mix of respiratory diseases | Pre | — | — | Communication, Depersonalization | — | |
| Zamith et al [77] | Low | Portugal | Telehealth (Teleconsultation) | Mix of respiratory diseases | Pre | CoC, PoM | — | CPf | — | |
| Byczkowsi et alg [78] | Low | United States | Telehealth (Telemonitoring) | CFh | Pre | — | IAi | Communication, Depersonalization | — | |
| Nield and Hoo [79] | Low | United States | Telehealth (Teleconsultation) | COPDj | Pre | — | — | CP | Self-efficacy | |
| Chih et al [80] | Low | United States | Telehealth (Teleconsultation) | Lung cancer | Pre | — | — | Communication, CP | Self-efficacy | |
| Pinnock et al [81] | Low | United Kingdom | Telehealth (Telemonitoring) | COPD | Pre | — | — | CoC | Self-efficacy | |
| Cingi et al [82] | Low | Turkey | Telehealth (Teleconsultation) | Asthma | Pre | — | TP, SoE | Communication, CP | — | |
| Apter et al [83] | Low | United States | Telehealth (Teleconsultation) | Asthma | Pre | PoM, CoC | — | Communication, CP | — | |
| Wiechag et al [84] | Low | United States | Telehealth (teleconsultation) | Asthma | Pre | PoM | — | Communication, CP | Self-efficacy | |
| Fadaizadeh et al [85] | Moderate | Iran | Telehealth (Telemonitoring) | Asthma | Pre | — | TP, IA | Communication, CP | — | |
| Rosenberger et al [86] | Low | United States | Telehealth (Telemonitoring) | Mix | Pre | DoIk | TP | Communication, CP | — | |
| Poureslami [87] | Low | Canada | Telehealth (Teleconsultation) | Asthma | Pre | — | IA | Depersonalization | Self-efficacy | |
| Sleathg et al [88] | High | United States | Telehealth (teleconsultation) | Asthma | Pre | DoI | — | Communication, CP | Self-efficacy | |
| Morita et al [89] | Low | Canada | Telehealth (telemonitoring) | Asthma | Pre | — | — | Communication, Depersonalization | Self-efficacy, | |
| Sonneyg et al [90] | Low | United States | Telehealth (telemonitoring) | Asthma | Pre | — | TP | Communication | Self-efficacy, SDMl | |
| Apter et al [91] | Low | United States | Communication | Asthma | Pre | — | IA | Communication, CP | Self-efficacy | |
| Gaynor et al [92] | Low | United States | Telehealth (teleconsultation) | Asthma | Pre | DoI | — | Communication | Self-efficacy, SDM | |
| Kneuertz et al [93] | Low | United States | Telehealth (teleconsultation) | Lung cancer | Pre | — | — | CoC, CP | Self-efficacy | |
| Koff et al [94] | Low | United States | Telehealth (telemonitoring) | COPD | Pre | — | — | — | Self-efficacy, SDM | |
| Opipari-Arrigang et al [95] | Moderate | United States | Communication | CF | Pre | — | — | Communication | Self-efficacy, SDM | |
| Perlman et al [96] | Low | United States | Telehealth (teleconsultation) | COVID-19 | Post | PoM | — | Communication | Self-efficacy, SDM | |
| Al-Sharif [97] | Low | Saudi Arabia | Telehealth (telemonitoring) | COPD | Post | — | IA, SoE | Communication, Depersonalization | — | |
| Davisg [98] | Low | United States | Telehealth (teleconsultation) | Mix of respiratory diseases | Post | PoM | IA, SoE | Depersonalization | — | |
| Gashu et al [99] | Moderate | Ethiopia | Telehealth (teleconsultation) | Tuberculosis | Post | — | — | Communication, CP | Self-efficacy | |
| Hendra et al [100] | Moderate | United States | Telehealth (teleconsultation) | CF | Pre | CoC | IA | — | Self-efficacy | |
| Meshkov et al [101] | Low | Russia | Communication | Tuberculosis | Pre | — | — | Communication | — | |
| Kowatschg et al [102] | Low | Switzerland | Telehealth (teleconsultation) | Asthma | Pre | PoM | — | Communication, Depersonalization | — | |
| Mustafa et al [103] | Low | United States | Telehealth (teleconsultation) | Asthma | Post | Ctxtm | — | Depersonalization | — | |
| Sousa [104] | Moderate | Portugal | Telehealth (teleconsultation) | Asthma | Post | Ctxt | — | Communication, Depersonalization | — | |
| Benson et al [105] | Low | United States | Telehealth (teleconsultation) | Lung cancer | Pre | CoC | IA | Depersonalization | — | |
| Bukstein et al [106] | Low | United States | Telehealth (teleconsultation) | Mix of respiratory diseases | Post | — | SoE, IA | Depersonalization | — | |
| Nelson et al [107] | Low | United States | Telehealth (teleconsultation) | Mixed | Post | Ctxt | — | Communication, Depersonalization | — | |
| Shinoda et al [108] | Moderate | Japan | Telehealth (telemonitoring) | Mix of respiratory diseases | Post | PoM | — | Depersonalization | Self-efficacy | |
| Chun et al [109] | Low | United States | Telehealth (telemonitoring) | Sleep apnea | Pre | — | TP, SoE | Communication, CoC | — | |
| Cokerg et al [110] | Moderate | United States | Telehealth (teleconsultation) | Asthma | Post | — | — | Communication | Self-efficacy, SDM | |
| Lawrenceg et al [111] | Low | Australia | Telehealth (teleconsultation) | COVID-19 | Post | PoM | — | Communication, CoC | — | |
| Varghese et al [112] | High | India | Telehealth (teleconsultation) | COVID-19 | Post | — | — | Communication | — | |
| Zhuge et al [113] | Moderate | China | Diagnostics | COVID-19 | Post | Ctxt, PoM | — | Communication | — | |
aNot applicable.
bCoC: continuity of care.
cPoM: perception of motivations.
dTP: technology performance.
eSoE: strength of evidence.
fCP: caring presence.
gStudies which included children.
hCF: Cystic fibrosis.
iIA: interaction anxiety.
jCOPD: chronic obstructive pulmonary disease.
kDoI: drivers of implementation.
lSDM: shared decision making.
mCtxt: context.
A third of the studies (29/97, 30%) were carried out after the onset of the pandemic. The studies included a broad range of respiratory conditions: COPD (23/97, 24%), asthma (22/97, 23%), lung cancer (10/97, 10%), COVID-19 (9/97, 9%), tuberculosis (9/97, 9%), cystic fibrosis (7/97, 7%), sleep apnea (2/97, 2%), and interstitial lung disease (1/97, 1%) studies. The remaining 14 out of 97 (14%) studies included multiple respiratory conditions (including COVID-19). The primary aim of almost all the studies was testing a device. Only 6 out of 97 (6%) studies had a primary aim directly related to the patient-provider relationship; in 8 out of 97 (8%) studies, the relationship was an explicit secondary objective. Most studies (83/97, 86%) coincidentally reported an outcome relevant to the patient-provider relationship.
Themes from the Integrative Analysis
Initial coding resulted in 12 subthemes synthesized into four main themes: adoption factors, confidence in technology, connection, and empowerment (Figure 2). The figure illustrates how each of the four main themes (each described below) influences the trust within the patient-provider relationship. The quantitative data collected corroborated our 4 main themes. Detailed examples of supporting quantitative evidence for each theme and subtheme are presented in Multimedia Appendix 3, strengthening our thematic framework through methodological triangulation.
Figure 2.
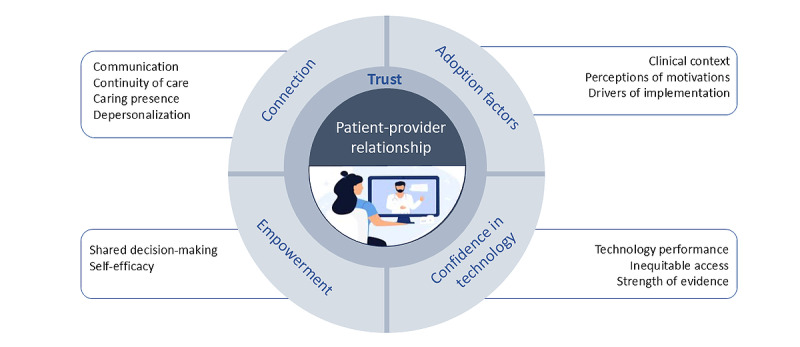
Themes and subthemes.
Figure 2 should be interpreted from the outer elements inwards, illustrating how each layer influences the next: digital respiratory care affects intermediate themes (eg, continuity of care), which influence core themes (eg, connection), ultimately impacting trust within the patient-provider relationship at the center.
We describe the four themes below using data extracts to clarify and confirm their meanings. Each theme was reasonably equally weighted in frequency of occurrence.
Trust
Trust emerged as the foundational element in the patient-provider relationship. Trust was defined as “the patient’s confidence that the physician will do what is best for the patient” [6]. Digital care could impact the other four themes (adoption factors, confidence in technology, connection, and empowerment), enhancing or diminishing trust.
Adoption Factors
The clinical context significantly influenced the adoption [61]. In the context of a pandemic, where visiting hospitals was unsafe, remote care was more acceptable [114].
I think it’s (telephone consulting) the norm because of the circumstances…nobody wants to get COVID [61].
telephone consultations during the pandemic
The severity of the patient’s condition also affected their confidence in using digital health technologies. Those with milder conditions were more willing to use them compared to those with severe or complex conditions, who may prefer in-person consultations [106], for instance, older patients with respiratory diseases during the pandemic).
Patients’ perceptions of the drivers of implementation and the motivation behind using digital health technologies influenced trust. Policy, organizations, and clinicians could drive technology implementation. Or it could be a complex sequence of these as policy pushed organizations to implement change, which then pushed professionals to use technology [115]. Patients could also advocate for using digital health technologies [71].
Patients’ trust was enhanced when digital health care was implemented to increase patient safety and convenience and improve outcomes [100], and it was diminished if technology was used primarily to save time and resources [116].
Confidence in Technology
Trust in technology was intertwined with trust in the health care providers who recommended the technology [117], shaping the overall connection and trust built within the relationship [118]. Patients’ trust in clinicians’ professional judgment was interrelated with how trustworthy patients perceived the technology [40].
Confidence in technology is the belief that you can use technology with ease and competence and that it is reliable. Digital health technology’s performance affects confidence in its functionality. Technology that is not intuitive or functions poorly leads to a loss of confidence, negatively impacting the patient-provider relationship.
Due to technical difficulties, data went missing….There was some loss of trust in the system, and some patients had more outpatient visits and hospital visits [33].
Kenealy et al, 2015
There was a risk of increasing inequities as socioeconomic and demographic factors affected confidence and trust in the technology. For some populations, such as older adults [37], or socioeconomically deprived, accessing health care professionals through technology was challenging.
So we speed ahead with the technology. We think this is all great, but many people do not have access to the right environment. How do you have a confidential conversation with somebody living in a one-bedroom apartment with four people? [66]
Nayyar et al, 2022
For health care providers, the strength of the evidence supporting its use and reliability in clinical practice affected confidence in technology. Clinicians reported instances where technology suggested additional treatment was needed despite their clinical judgment indicating otherwise. In addition, providers were concerned that some patients placed excessive trust in health technology that could prove unreliable [43].
Connection
On a practical level, technology improved patient and professional access and communication:
If I need anything, I have just to phone up…it is a good service [27].
COPD telemonitoring, prepandemic
Continuity of care using telehealth technologies enhanced connection and trust [41] and improved treatment adherence [40]. Clinicians valued continuity of care, observing that patients were more trusting when the same provider consistently performed telemonitoring [45].
I think it is probably best if [telemonitoring is done by] people that are dealing with the patients every single day and have that bit of a relationship with them ... people are more trusting, and it is something more personable for the patient [27].
COPD telemonitoring, prepandemic
Technology can reassure patients that someone is “watching over them” and create a feeling of a “caring presence” compensating for depersonalization due to a lack of physical presence [71].
Grateful that someone kept an eagle eye on my son as it was a very stressful time given his other health issues. Stress levels were significantly reduced by being in his home environment and knowing [the doctor] was a video call away, and he was being monitored remotely. [71]
Physical activity intervention for COPD
However, both patients and providers reported depersonalization due to a lack of in-person interactions, and both expressed a preference for a combination of in-person and remote consultations: In-person consultations could form a stronger bond and strengthen the relationship.
A physical meeting creates more opportunities to instil security and peace. It has an inherent energy, creating a connection, where patients and relatives can ask questions and be recognised. [113]
COPD management in the Swedish population
A study by Jiang et al [63] further emphasized the sense of depersonalization that may occur due to the physical distance during remote consultations.
It is not just the exchange of disease information ... eye contact and a gesture from a doctor or a nurse will give the patient much psychological comfort, which is very difficult to achieve on a mobile phone. [63]
COPD management study for older patients
Both clinicians and patients found remote interactions with a new provider difficult and highlighted that it was difficult to establish trust and rapport with a new provider using telemedicine [62].
Empowerment
Empowerment is important for a balanced patient-provider relationship. Empowered patients have more control and are often involved in decisions about their care, such as how and where services are delivered. Technology helped patients be better informed about their condition [65] and increased their self-efficacy and confidence through home-collected data [33]. This enabled active participation in self-management, enhanced self-efficacy, supported shared decision-making, and facilitated a more equal patient-provider relationship.
Patients feel empowered when contacting the clinician because they have vital readings and their sense of health to explain how they feel and get support. [29]
COPD self-monitoring, prepandemic
Stakeholder Workshops and Interpretation of Findings
Our work followed the consultation and application approaches to collaborative analysis [14]. Following data analysis, and once we had preliminary findings, we carried out stakeholder engagement sessions, where we presented initial findings from the review to stakeholders for feedback. The sessions were conducted remotely and were interactive. All participants were asked to provide verbal and written feedback using Groupmap, WordCloud, and group chat.
The discussion among clinicians and patients at the stakeholder workshops resonated with our findings. The word associations related to digital health technology collected in WordCloud at the start of the session generally reflected positive features of technology identified in our analysis (accessibility, convenience, modernization, and education). At the end of the meeting, after considerable discussion, views were more nuanced, and some of the associations were neutral or more negative (accessibility, followed by disparities, frustrating, potential, complex, and challenge). These views increased confidence in our conclusions as the very positive interpretations from some papers were tempered by the more cautious considerations highlighted in other studies.
Discussion
Principal Findings
We have synthesized the findings of 97 papers on the impact of digital health technology on the patient-professional relationship in specialist practice. Both qualitative and quantitative evidence were used to identify the themes and subthemes, with the qualitative findings revealing underlying nuances and contextual factors. This approach enabled us to quantify key patterns in technology adoption, confidence, connection, and empowerment, while simultaneously understanding the mechanisms through which these factors influenced the patient-provider relationship. Trust was the foundational theme, and digital health technology could either enhance or reduce trust between patients and clinicians. Patients’ perception of why digital health technology was implemented (eg, whether it was used to benefit patient care or used to save time and resources) was a significant factor. The technology could connect patients and clinicians by facilitating access, communication, and continuity of care, but remote consultations risk depersonalization, especially if they are not balanced with in-person interactions. Self-monitoring and access to information can empower patients and promote a more equal patient-provider relationship. Combining qualitative and quantitative measures has provided a fuller picture of patient-provider interactions, as each method offers unique insights into different aspects of the relationship. Qualitative insights into how technology empowers patients to seek information and engage in health care decisions are supported by quantitative data showing improved service engagement and compliance. Integrating qualitative and quantitative data has enriched our understanding of patient-provider relationships by linking communication styles and patient engagement with measurable outcomes. These findings complement each other and can lead to more effective healthcare strategies and improved patient care.
The Pivotal Role of Trust
Trust in the patient-professional relationship was intertwined with trust in technology. While Ridd et al [6] identified trust as a critical factor in determining the depth of the patient and professional relationship in their 2009 primary care study, digital health care technology has introduced a third actor, which can positively or negatively influence relationship formation, development, and maintenance.
Patients’ trust in technology was fundamentally linked to their trust in clinicians. We found that patients trusted technology when they trusted their clinicians to provide the best care and achieve desired outcomes, reflecting broader evidence that patients build trust through receiving quality care in well-functioning health systems [119]. When technology or systems fail, patients’ distrust extends to their providers. This has important clinical implications, as a lack of trust between patients and clinicians is associated with reduced treatment adherence and care-seeking behaviors [37].
In contrast, clinicians approached technology trust differently, primarily based on evidence. They expressed concern that patients might over-rely on technology rather than trusting clinical judgment, highlighting a fundamental tension in how different stakeholders view the role of technology in care delivery.
The Value of Hybrid Delivery
Building on the foundation of trust, our findings emphasize the importance of balancing digital and in-person care. Previous research has shown that psychological and emotional bonds are strengthened through in-person interactions, where nonverbal cues play a crucial role [120]. Our review confirms that in-person and remote consultations can best promote trust and maintain therapeutic relationships. Hybrid arrangements that offer flexibility and choice in consultation mode can help prevent depersonalization while retaining the benefits of digital care [121]. In addition, multiple recent studies have recommended that for effective implementation and to ensure high-quality telehealth care, it is essential to support implementation at each stage of the process. Organizations must provide guidelines and support for health care professionals, anticipating both technological and human challenges and their corresponding solutions [122]. Based on our findings and those from the literature, we recommend providing education and training to support individuals with access to technology effectively, as well as advocating for those who do not have access to these technologies. This could prevent disadvantaged groups from being excluded further. Although we found no trial evidence for specific interventions, it is widely recommended that training not only focus on professional development [123] but also supports to benefit from digital health care [124].
Implementation of Technology
Perceived threats to patient and health care professional relationships have been identified as one of the main barriers to implementing technology [125]. It is thus disappointing that only 11 out of 97 (12%) of our included papers focused explicitly on this crucial issue. Research is needed to ensure that the future application of digital technology aligns with patients’ wishes, and their ability to cope, and build confidence and trust in health technologies [126]. Keywords and phrases include hybrid systems, flexibility, and recognizing individual preferences.
Recognizing that the perceived motivation behind using health technology influences trust in the provider and the relationship [49] enables those driving the implementation of technology to shape perceptions. For instance, Adjekum et al [126] responded to the observation that users trusted public institutions and universities more than profit-making entities.
Multiple contextual factors may influence whether and how digital health care is adopted, as well as its impact on the patient-professional relationship. Time and resource constraints within health care systems already negatively impact patient-professional relationships [127]. Improved communication and efficiency benefits of technology may mitigate these effects—or, conversely, exacerbate them if digital health care places additional demands on services or raises unrealistic expectations. Patient demographics such as gender and age [128] may affect preferences for delivery of care, so the introduction of digital health care will have a different impact on the underlying patient and professional relationship; disease type and severity will further affect the potential of technology to a patient’s ability to interact and engage with health care professionals.
Strengths and Limitations
A key strength of our review was the robust methodology. We followed established systematic review procedures, including duplicate screening and data extraction, supported by a comprehensive search strategy developed in collaboration with a medical librarian. Our novel “crowdsourcing” approach, involving trained volunteers, enabled the timely completion of tasks while maintaining quality through extensive training, careful oversight, and verification of all data extraction by senior reviewers (MS and DD). Another significant strength was the involvement of stakeholders from the CONNECT and DRAGON projects in the design and interpretation of findings. The stakeholder workshop enabled us to “sense-check” our analysis, helping us refine the synthesis and interpretation, as reflected in the schema, which underwent significant revision following the workshop. Using the CASP checklist is a strength because it focuses on the applicability of studies to answer our research question, highlighting that only a minority of the studies had an explicit aim related to exploring the patient-professional relationship. CASP requests an assessment of “validity” but does not provide a summary score for the risk of bias. Whilst this is a limitation of this tool, we were not planning to assess effect size given the highly heterogeneous contexts and secondary outcome measures.
However, several limitations should be noted. We may have missed relevant papers despite screening over 15,000 titles and abstracts. A fundamental limitation was that most included studies were designed to test the efficacy and safety of technology rather than exploring the patient-professional relationship. Few papers explicitly addressed our aim as their primary objective, with most reporting incidental findings about the patient-provider relationship; however, the findings were notably consistent across studies. Our workshop was likely to have attracted participants interested in digital technology and comfortable with the online format. Exploring the perspectives of individuals who are less confident with digital tools is a research priority.
Implications
Recommendations for maintaining a positive patient-provider relationship using digital health technology in respiratory health care are summarized in Textbox 2.
Recommendations for maintaining a positive patient-provider relationship using digital health technology.
Recommendations
Be transparent about implementation motivations.
Ensure technology performance is reliable.
Account for individual circumstances and access.
Offer hybrid care combining remote and in-person consultations.
Consider establishing initial relationships in person.
Maintain continuity of care with the same health care provider.
Offer hybrid care combining remote and in-person consultations.
Support access to self-monitoring tools and health information.
Rationale
Trust was enhanced when digital health care was used to increase patient safety and convenience but diminished if technology was perceived as primarily cost-saving.
Poor functionality led to loss of confidence and negatively impacted patient-provider relationships, with evidence of missing data from monitoring devices leading to loss of trust.
Socioeconomic factors affected confidence and trust in technology, with some populations (older adults and socioeconomically deprived) finding access challenging.
Both patients and providers reported depersonalization with remote-only care.
Both clinicians and patients found remote interactions with new providers difficult and highlighted challenges in establishing trust and rapport.
Patients were more trusting when the same provider consistently performed telemonitoring.
Both patients and providers reported depersonalization with remote-only care.
Technology helped patients be better informed about their condition and increased their self-efficacy through home-collected data, enabling active participation in self-management.
Conclusion
Digital health technology impacts trust in respiratory care through four key mechanisms: adoption factors, confidence in technology, connection, and patient empowerment. Our findings emphasize that successful integration of digital health technology in respiratory care requires transparent implementation motivations, consideration of individual patient circumstances, and maintenance of human connection—all underpinned by reliable technology that supports rather than replaces the therapeutic relationship.
Acknowledgments
We thank all patients and health care providers who participated in the meetings to discuss the systematic review’s findings. We also thank all the members of the DRAGON/CONNECT group consisting of Animesh Ray, Angelos Vontetsianos, Ayşe Önal Aral, Carmine Ruggiero, Christina Anagnostopoulou, Christiana Lekka, Dimitrios Megaritis, Efthymia Papadopoulou, Enya Daynes, Kathleen Condon, Larry Ellee, Nyanti Malek Chaabouni, Miguel Barbosa, Milan Mohammad, Nikolaos Chynkiamis, Roisin Cahalan, Sara Souto Miranda, Shailesh Kolekar, Tonje Reier-Nilsen, Vishakha Kapadia, and Wieke Bouwes. DRAGON received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU; grant agreement 101005122). The JU receives support from the European Union’s Horizon 2020 research and innovation program and EFPIA.
Abbreviations
- AI
artificial intelligence
- CASP
Critical Appraisal Skills Programme
- DRAGON
The Rapid and Secure AI-enhanced Diagnosis, Precision Medicine and Patient Empowerment Centered Decision Support System for Coronavirus Pandemics
- COPD
chronic obstructive pulmonary disease
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
International Prospective Register of Systematic Reviews
- WHO
World Health Organization
Search strategy.
PRISMA checklist.
Quantitative findings.
Footnotes
Authors' Contributions: HP, DD, PP, KH, AA, and MS were responsible for conceptualization and methodology of the research. AG, KBS, UC, OM, CCT, and AG joined at the investigation stages. MS, HP, PP, AA, KH, and DD carried out the formal analysis. MS, DD, and HP wrote the original draft.
Conflicts of Interest: Author HP received a Teva Pharmaceuticals Speaker fee for a sponsored symposium and a Sandoz Pharmaceuticals Speaker fee for a sponsored symposium. All other authors listed no conflicts of interest.
References
- 1.Pinnock H, Poberezhets V, Drummond D. Digital Respiratory Healthcare. Lausanne: European Respiratory Society; 2023. Introduction; p. 8. [Google Scholar]
- 2.Future of digital health systems: report on the WHO symposium on the future of digital health systems in the European region. World Health Organization. 2019. [2019-02-06]. https://iris.who.int/handle/10665/329032 .
- 3.Global diffusion of eHealth: making universal health coverage achievable: report of the third global survey on eHealth. World Health Organization. 2016. [2025-04-09]. https://www.who.int/publications/i/item/9789241511780 .
- 4.Pérez Sust P, Solans O, Fajardo JC, Medina Peralta M, Rodenas P, Gabaldà J, Garcia Eroles L, Comella A, Velasco Muñoz C, Sallent Ribes J, Roma Monfa R, Piera-Jimenez J. Turning the crisis into an opportunity: digital health strategies deployed during the COVID-19 outbreak. JMIR Public Health Surveill. 2020;6(2):e19106. doi: 10.2196/19106. https://publichealth.jmir.org/2020/2/e19106/ v6i2e19106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Digital Respiratory Healthcare. 2023. [2025-04-04]. https://www.ersbookshop.com/products/digital-respiratory-healthcare?srsltid=AfmBOopFT_ubXJnwyKQ0pMHIsA_5l8pgykWU5-99XH0lYG_y5_fAfnPb .
- 6.Ridd M, Shaw A, Lewis G, Salisbury C. The patient-doctor relationship: a synthesis of the qualitative literature on patients' perspectives. Br J Gen Pract. 2009;59(561):e116–e133. doi: 10.3399/bjgp09X420248. https://bjgp.org/lookup/pmidlookup?view=long&pmid=19341547 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramachandran M, Brinton C, Wiljer D, Upshur R, Gray CS. The impact of eHealth on relationships and trust in primary care: a review of reviews. BMC Prim Care. 2023;24(1):228. doi: 10.1186/s12875-023-02176-5. https://europepmc.org/abstract/MED/37919688 .10.1186/s12875-023-02176-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nassehi D, Gripsrud BH, Ramvi E. Theoretical perspectives underpinning research on the physician-patient relationship in a digital health practice: scoping review. Interact J Med Res. 2024;13:e47280. doi: 10.2196/47280. https://www.i-jmr.org/2024//e47280/ v13i1e47280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins J, Thomas J. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Training. 2019. [2024-08-22]. https://training.cochrane.org/handbook .
- 10.Branca Vergano L, Monesi M, Vicenti G, Bizzoca D, Solarino G, Moretti B. Posterior approaches in malleolar fracture: when, why and how. J Biol Regul Homeost Agents. 2020;34(3 Suppl. 2):89–95.14 [PubMed] [Google Scholar]
- 11.van Boven JFM, Drummond D, Chan AHY, Hew M, Hui CY, Adejumo I, Cano I, Hansen K, Poberezhets V, Costello RW, Pinnock H. ERS "CONNECT" Clinical research collaboration - moving multiple digital innovations towards connected respiratory care: addressing the over-arching challenges of whole systems implementation. Eur Respir J. 2023;62(5):2301680. doi: 10.1183/13993003.01680-2023.62/5/2301680 [DOI] [PubMed] [Google Scholar]
- 12.Blog post: crowdsourcing as a way to embed society’s norms and values in digital health innovation. Dragon. [2025-04-04]. https://europeanlung.org/dragon/blog-post-crowdsourcing-as-a-way-to-embed-societys-norms-and-values-in-digital-health-innovation/
- 13.Lipscomb M. Abductive reasoning and qualitative research. Nurs Philos. 2012;13(4):244–256. doi: 10.1111/j.1466-769x.2011.00532.x. [DOI] [PubMed] [Google Scholar]
- 14.Jennings H, Slade M, Bates P, Munday E, Toney R. Best practice framework for Patient and Public Involvement (PPI) in collaborative data analysis of qualitative mental health research: methodology development and refinement. BMC Psychiatry. 2018;18(1):213. doi: 10.1186/s12888-018-1794-8. https://bmcpsychiatry.biomedcentral.com/articles/10.1186/s12888-018-1794-8 .10.1186/s12888-018-1794-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards KAR, Hemphill MA. A practical guide to collaborative qualitative data analysis. J Teach Phys Educ. 2018;37(2):225–231. doi: 10.1123/jtpe.2017-0084. [DOI] [Google Scholar]
- 16.GroupMap. [2025-04-04]. https://www.groupmap.com/
- 17.Fellows I. Wordcloud: Word Clouds, R Package version. 2012. [2025-04-04]. https://cran.r-project.org/web/packages/wordcloud/wordcloud.pdf .
- 18.Mantzouranis EC. User friendliness aspects of home care telematics. Methods Inf Med. 2018;41(05):370–375. doi: 10.1055/s-0038-1634454. [DOI] [PubMed] [Google Scholar]
- 19.Hibbert D, Mair FS, May CR, Boland A, O'Connor J, Capewell S, Angus RM. Health professionals' responses to the introduction of a home telehealth service. J Telemed Telecare. 2004;10(4):226–230. doi: 10.1258/1357633041424386. [DOI] [PubMed] [Google Scholar]
- 20.van Baar JD, Joosten H, Car J, Freeman GK, Partridge MR, van Weel C, Sheikh A. Understanding reasons for asthma outpatient (non)-attendance and exploring the role of telephone and e-consulting in facilitating access to care: exploratory qualitative study. Qual Saf Health Care. 2006;15(3):191–195. doi: 10.1136/qshc.2004.013342. https://europepmc.org/abstract/MED/16751469 .15/3/191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitten P, Mickus M. Home telecare for COPD/CHF patients: outcomes and perceptions. J Telemed Telecare. 2007;13(2):69–73. doi: 10.1258/135763307780096249. [DOI] [PubMed] [Google Scholar]
- 22.Cornwall A, Moore S, Plant H. Embracing technology: patients', family members' and nurse specialists' experience of communicating using e-mail. Eur J Oncol Nurs. 2008;12(3):198–208. doi: 10.1016/j.ejon.2007.09.008.S1462-3889(07)00131-7 [DOI] [PubMed] [Google Scholar]
- 23.Mair FS, Hiscock J, Beaton SC. Understanding factors that inhibit or promote the utilization of telecare in chronic lung disease. Chronic Illn. 2008;4(2):110–117. doi: 10.1177/1742395308092482.4/2/110 [DOI] [PubMed] [Google Scholar]
- 24.Hoffman JA, Cunningham JR, Suleh AJ, Sundsmo A, Dekker D, Vago F, Munly K, Igonya EK, Hunt-Glassman J. Mobile direct observation treatment for tuberculosis patients: a technical feasibility pilot using mobile phones in Nairobi, Kenya. Am J Prev Med. 2010;39(1):78–80. doi: 10.1016/j.amepre.2010.02.018.S0749-3797(10)00259-X [DOI] [PubMed] [Google Scholar]
- 25.Shany T, Hession M, Pryce D, Galang R, Roberts M, Lovell N, Basilakis J. Home telecare study for patients with chronic lung disease in the sydney west area health service. Stud Health Technol Inform. 2010;161:139–148. [PubMed] [Google Scholar]
- 26.Cox A, Illsley M, Knibb W, Lucas C, O'Driscoll M, Potter C, Flowerday A, Faithfull S. The acceptability of e-technology to monitor and assess patient symptoms following palliative radiotherapy for lung cancer. Palliat Med. 2011;25(7):675–681. doi: 10.1177/0269216311399489.0269216311399489 [DOI] [PubMed] [Google Scholar]
- 27.Fairbrother P, Pinnock H, Hanley J, McCloughan L, Sheikh A, Pagliari C, McKinstry B, TELESCOT programme team Continuity, but at what cost? The impact of telemonitoring COPD on continuities of care: a qualitative study. Prim Care Respir J. 2012;21(3):322–328. doi: 10.4104/pcrj.2012.00068. https://doi.org/10.4104/pcrj.2012.00068 .pcrj-2012-02-0028-R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Kim S, Kim HC, Kim KH, Yang SC, Lee CT, Kong HJ, Lee K. Effects of consumer-centered u-health service for the knowledge, skill, and attitude of the patients with chronic obstructive pulmonary disease. Comput Inform Nurs. 2012;30(12):661–671. doi: 10.1097/NXN.0b013e318261c1c.00024665-201212000-00007 [DOI] [PubMed] [Google Scholar]
- 29.Dinesen B, Huniche L, Toft E. Attitudes of COPD patients towards tele-rehabilitation: a cross-sector case study. Int J Environ Res Public Health. 2013;10(11):6184–6198. doi: 10.3390/ijerph10116184. https://www.mdpi.com/resolver?pii=ijerph10116184 .ijerph10116184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huniche L, Dinesen B, Nielsen C, Grann O, Toft E. Patients' use of self-monitored readings for managing everyday life with COPD: a qualitative study. Telemed J E Health. 2013;19(5):396–402. doi: 10.1089/tmj.2012.0135. [DOI] [PubMed] [Google Scholar]
- 31.Damhus CS, Emme C, Hansen H. Barriers and enablers of COPD telerehabilitation – a frontline staff perspective. Int J Chron Obstruct Pulmon Dis. 2018;13:2473–2482. doi: 10.2147/copd.s167501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown-Johnson CG, Berrean B, Cataldo JK. Development and usability evaluation of the mHealth tool for lung cancer (mHealth TLC): a virtual world health game for lung cancer patients. Patient Educ Couns. 2015;98(4):506–511. doi: 10.1016/j.pec.2014.12.006. https://europepmc.org/abstract/MED/25620075 .S0738-3991(14)00528-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kenealy TW, Parsons MJG, Rouse APB, Doughty RN, Sheridan NF, Hindmarsh JKH, Masson SC, Rea HH. Telecare for diabetes, CHF or COPD: effect on quality of life, hospital use and costs. a randomised controlled trial and qualitative evaluation. PLoS One. 2015;10(3):e0116188. doi: 10.1371/journal.pone.0116188. https://dx.plos.org/10.1371/journal.pone.0116188 .PONE-D-14-16859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maguire R, Ream E, Richardson A, Connaghan J, Johnston B, Kotronoulas G, Pedersen V, McPhelim J, Pattison N, Smith A, Webster L, Taylor A, Kearney N. Development of a novel remote patient monitoring system: the advanced symptom management system for radiotherapy to improve the symptom experience of patients with lung cancer receiving radiotherapy. Cancer Nurs. 2015;38(2):E37–E47. doi: 10.1097/NCC.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 35.Roberts NJ, Ghiassi R, Partridge MR. Health literacy in COPD. Int J Chron Obstruct Pulmon Dis. 2008;3(4):499–507. doi: 10.2147/copd.s1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dichmann Sorknaes Anne. Nurses' and Patients' experiences of tele-consultations. Stud Health Technol Inform. 2016;225:885–886. [PubMed] [Google Scholar]
- 37.Daftary A, Hirsch-Moverman Y, Kassie GM, Melaku Z, Gadisa T, Saito S, Howard AA. A qualitative evaluation of the acceptability of an interactive voice response system to enhance adherence to isoniazid preventive therapy among people living with HIV in ethiopia. AIDS Behav. 2017;21(11):3057–3067. doi: 10.1007/s10461-016-1432-8. https://europepmc.org/abstract/MED/27221743 .10.1007/s10461-016-1432-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirsch-Moverman Y, Daftary A, Yuengling KA, Saito S, Ntoane M, Frederix K, Maama LB, Howard AA. Using mHealth for HIV/TB treatment support in lesotho: enhancing patient-provider communication in the START study. J Acquir Immune Defic Syndr. 2017;74(Suppl 1):S37–S43. doi: 10.1097/QAI.0000000000001202. https://europepmc.org/abstract/MED/27930610 .00126334-201701011-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kopanitsa G. A qualitative study of the barriers and opportunities for adoption of web-portals for doctors and patients in Russia. J Med Syst. 2017;41(4):62. doi: 10.1007/s10916-017-0713-8.10.1007/s10916-017-0713-8 [DOI] [PubMed] [Google Scholar]
- 40.Nhavoto JA, Grönlund Å, Klein GO. Mobile health treatment support intervention for HIV and tuberculosis in Mozambique: perspectives of patients and healthcare workers. PLoS One. 2017;12(4):e0176051. doi: 10.1371/journal.pone.0176051. https://dx.plos.org/10.1371/journal.pone.0176051 .PONE-D-16-39057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nissen L, Lindhardt T. A qualitative study of COPD-patients' experience of a telemedicine intervention. Int J Med Inform. 2017;107:11–17. doi: 10.1016/j.ijmedinf.2017.08.004.S1386-5056(17)30203-4 [DOI] [PubMed] [Google Scholar]
- 42.Liacos A, Burge AT, Cox NS, Holland AE. Promoting physical activity using the internet: Is it feasible and acceptable for patients with chronic obstructive pulmonary disease and bronchiectasis? J Aging Phys Act. 2018;26(3):372–381. doi: 10.1123/japa.2017-0123. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton JG, Genoff Garzon M, Westerman JS, Shuk E, Hay JL, Walters C, Elkin E, Bertelsen C, Cho J, Daly B, Gucalp A, Seidman AD, Zauderer MG, Epstein AS, Kris MG. “A Tool, Not a Crutch”: Patient perspectives about IBM watson for oncology trained by memorial sloan kettering. J Oncol Pract. 2019;15(4):e277–e288. doi: 10.1200/jop.18.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel MR, Smith A, Leo H, Hao W, Zheng K. Improving patient-provider communication and therapeutic practice through better integration of electronic health records in the exam room: a pilot study. Health Educ Behav. 2019;46(3):484–493. doi: 10.1177/1090198118796879. https://europepmc.org/abstract/MED/30196720 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudin RS, Fanta CH, Qureshi N, Duffy E, Edelen MO, Dalal AK, Bates DW. A clinically integrated mHealth app and practice model for collecting patient-reported outcomes between visits for asthma patients: implementation and feasibility. Appl Clin Inform. 2019;10(5):783–793. doi: 10.1055/s-0039-1697597. https://europepmc.org/abstract/MED/31618782 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boström E, Ali L, Fors A, Ekman I, Andersson AE. Registered nurses' experiences of communication with patients when practising person-centred care over the phone: a qualitative interview study. BMC Nurs. 2020;19(1):54. doi: 10.1186/s12912-020-00448-4. https://bmcnurs.biomedcentral.com/articles/10.1186/s12912-020-00448-4 .448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drabble SJ, O'Cathain A, Scott AJ, Arden MA, Keating S, Hutchings M, Maguire C, Wildman M. Mechanisms of action of a web-based intervention with health professional support to increase adherence to nebulizer treatments in adults with cystic fibrosis: qualitative interview study. J Med Internet Res. 2020;22(10):e16782. doi: 10.2196/16782. https://www.jmir.org/2020/10/e16782/ v22i10e16782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuntz JG, Kavalieratos D, Esper GJ, Ogbu N, Mitchell J, Ellis CM, Quest T. Feasibility and acceptability of inpatient palliative care e-family meetings during COVID-19 pandemic. J Pain Symptom Manage. 2020;60(3):e28–e32. doi: 10.1016/j.jpainsymman.2020.06.001. https://linkinghub.elsevier.com/retrieve/pii/S0885-3924(20)30433-4 .S0885-3924(20)30433-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis S, Blundell A, Conway J, Meddick E. A virtual respiratory clinic to support patients with Covid-19 after discharge. Nursing Times. 2020. [2020-07-27]. https://tinyurl.com/4jrutp8h .
- 50.Maguire R, Connaghan J, Arber A, Klepacz N, Blyth KG, McPhelim J, Murray P, Rupani H, Chauhan A, Williams P, McNaughton L, Woods K, Moylan A. Advanced symptom management system for patients with malignant pleural mesothelioma (ASyMSmeso): mixed methods study. J Med Internet Res. 2020;22(11):e19180. doi: 10.2196/19180. https://www.jmir.org/2020/11/e19180/ v22i11e19180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Citters AD, Gifford AH, Brady C, Dunitz JM, Elmhirst M, Flath J, Laguna TA, Moore B, Prickett ML, Riordan M, Savant AP, Gore W, Jian S, Soper M, Marshall BC, Nelson EC, Sabadosa KA. Formative evaluation of a dashboard to support coproduction of healthcare services in cystic fibrosis. J Cyst Fibros. 2020;19(5):768–776. doi: 10.1016/j.jcf.2020.03.009. https://linkinghub.elsevier.com/retrieve/pii/S1569-1993(20)30080-1 .S1569-1993(20)30080-1 [DOI] [PubMed] [Google Scholar]
- 52.van Lieshout F, Yang R, Stamenova V, Agarwal P, Cornejo Palma D, Sidhu A, Engel K, Erwood A, Bhatia RS, Bhattacharyya O, Shaw J. Evaluating the implementation of a remote-monitoring program for chronic obstructive pulmonary disease: qualitative methods from a service design perspective. J Med Internet Res. 2020;22(10):e18148. doi: 10.2196/18148. https://www.jmir.org/2020/10/e18148/ v22i10e18148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamada J, Kouri A, Simard S, Segovia SA, Gupta S. Barriers and enablers to using a patient-facing electronic questionnaire: a qualitative theoretical domains framework analysis. J Med Internet Res. 2020;22(10):e19474. doi: 10.2196/19474. https://www.jmir.org/2020/10/e19474/ v22i10e19474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bains J, Greenwald PW, Mulcare MR, Leyden D, Kim J, Shemesh AJ, Bodnar D, Farmer B, Steel P, Tanouye R, Kim JW, Lame M, Sharma R. Utilizing telemedicine in a novel approach to COVID-19 management and patient experience in the emergency department. Telemed J E Health. 2021;27(3):254–260. doi: 10.1089/tmj.2020.0162. [DOI] [PubMed] [Google Scholar]
- 55.Jácome C, Almeida R, Pereira AM, Amaral R, Mendes S, Alves-Correia M, Vidal C, López Freire S, Méndez Brea P, Araújo L, Couto M, Antolín-Amérigo D, de la Hoz Caballer B, Barra Castro A, Gonzalez-De-Olano D, Todo Bom A, Azevedo J, Leiria Pinto P, Pinto N, Castro Neves A, Palhinha A, Todo Bom F, Costa A, Chaves Loureiro C, Maia Santos L, Arrobas A, Valério M, Cardoso J, Emiliano M, Gerardo R, Cidrais Rodrigues JC, Oliveira G, Carvalho J, Mendes A, Lozoya C, Santos N, Menezes F, Gomes R, Câmara R, Rodrigues Alves R, Moreira AS, Bordalo D, Alves C, Ferreira JA, Lopes C, Silva D, Vasconcelos MJ, Teixeira MF, Ferreira-Magalhães M, Taborda-Barata L, Cálix MJ, Alves A, Almeida Fonseca J. Feasibility and acceptability of an asthma app to monitor medication adherence: mixed methods study. JMIR Mhealth Uhealth. 2021;9(5):e26442. doi: 10.2196/26442. https://mhealth.jmir.org/2021/5/e26442/ v9i5e26442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kennedy NR, Steinberg A, Arnold RM, Doshi AA, White DB, DeLair W, Nigra K, Elmer J. Perspectives on telephone and video communication in the intensive care unit during COVID-19. Annals ATS. 2021;18(5):838–847. doi: 10.1513/annalsats.202006-729oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Legler S, Diehl M, Hilliard B, Olson A, Markowitz R, Tignanelli C, Melton GB, Broccard A, Kirsch J, Usher M. Evaluation of an intrahospital telemedicine program for patients admitted with COVID-19: mixed methods study. J Med Internet Res. 2021;23(4):e25987. doi: 10.2196/25987. https://www.jmir.org/2021/4/e25987/ v23i4e25987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sekandi JN, Kasiita V, Onuoha NA, Zalwango S, Nakkonde D, Kaawa-Mafigiri D, Turinawe J, Kakaire R, Davis-Olwell P, Atuyambe L, Buregyeya E. Stakeholders' perceptions of benefits of and barriers to using video-observed treatment for monitoring patients with tuberculosis in uganda: exploratory qualitative study. JMIR Mhealth Uhealth. 2021;9(10):e27131. doi: 10.2196/27131. https://mhealth.jmir.org/2021/10/e27131/ v9i10e27131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas BE, Kumar JV, Periyasamy M, Khandewale AS, Hephzibah Mercy J, Raj EM, Kokila S, Walgude AS, Gaurkhede GR, Kumbhar JD, Ovung S, Paul M, Rajkumar BS, Subbaraman R. Acceptability of the medication event reminder monitor for promoting adherence to multidrug-resistant tuberculosis therapy in two Indian cities: qualitative study of patients and health care providers. J Med Internet Res. 2021;23(6):e23294. doi: 10.2196/23294. https://www.jmir.org/2021/6/e23294/ v23i6e23294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu F, Burt J, Chowdhury T, Fitzpatrick R, Martin G, van der Scheer JW, Hurst JR. Specialty COPD care during COVID-19: patient and clinician perspectives on remote delivery. BMJ Open Respir Res. 2021;8(1):e000817. doi: 10.1136/bmjresp-2020-000817. https://bmjopenrespres.bmj.com/lookup/pmidlookup?view=long&pmid=33414261 .8/1/e000817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gillett M, Hope-Gill B. Telephone clinics during the Covid-19 pandemic-the experiences of interstitial lung disease patients and their carers. J Patient Exp. 2022;9:23743735221133638. doi: 10.1177/23743735221133638. https://journals.sagepub.com/doi/10.1177/23743735221133638?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed .10.1177_23743735221133638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haynes SC, Kamerman-Kretzmer R, Khan SS, Crossen S, Lieng MK, Marcin JP, Kenyon NJ, Kim CH. Telemedicine use for pediatric asthma care: a mixed methods study. J Asthma. 2022;59(12):2431–2440. doi: 10.1080/02770903.2021.2019265. [DOI] [PubMed] [Google Scholar]
- 63.Jiang Y, Sun P, Chen Z, Guo J, Wang S, Liu F, Li J. Patients' and healthcare providers' perceptions and experiences of telehealth use and online health information use in chronic disease management for older patients with chronic obstructive pulmonary disease: a qualitative study. BMC Geriatr. 2022;22(1):9. doi: 10.1186/s12877-021-02702-z. https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-021-02702-z .10.1186/s12877-021-02702-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Makhecha S, Eftychiou L, Tsang V, Vas V, Christiansen N, Crook J, Bentley S. Patient and family perceptions of the provision of medicines as part of virtual outpatient consultations for children during COVID-19 pandemic. BMJ Open Qual. 2022;11(4):e001916. doi: 10.1136/bmjoq-2022-001916. https://bmjopenquality.bmj.com/lookup/pmidlookup?view=long&pmid=36572442 .bmjoq-2022-001916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Misplon S, Marneffe W, Himpe U, Hellings J, Demedts I. Evaluation of the implementation of value-based healthcare with a weekly digital follow-up of lung cancer patients in clinical practice. Eur J Cancer Care (Engl) 2022;31(6):e13653. doi: 10.1111/ecc.13653. [DOI] [PubMed] [Google Scholar]
- 66.Nayyar D, Pendrith C, Kishimoto V, Chu C, Fujioka J, Rios P, Sacha Bhatia R, Lyons OD, Harvey P, O'Brien T, Martin D, Agarwal P, Mukerji G. Quality of virtual care for ambulatory care sensitive conditions: patient and provider experiences. Int J Med Inform. 2022;165:104812. doi: 10.1016/j.ijmedinf.2022.104812. https://europepmc.org/abstract/MED/35691260 .S1386-5056(22)00126-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sandau C, Poulsen I, Nørholm V, Hansen EF, Ringbaek TJ, Suppli Ulrik C, Bove DG. Patients' perspective on automated oxygen administration during hospitalization for acute exacerbation of chronic obstructive pulmonary disease: a qualitative study nested in a randomized controlled trial. COPD. 2022;19(1):345–352. doi: 10.1080/15412555.2022.2141620. https://www.tandfonline.com/doi/10.1080/15412555.2022.2141620?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PubMed] [Google Scholar]
- 68.Cox NS, Lee JYT, McDonald CF, Mahal A, Alison JA, Wootton R, Hill CJ, Zanaboni P, O'Halloran P, Bondarenko J, Macdonald H, Barker K, Crute H, Mellerick C, Wageck B, Boursinos H, Lahham A, Nichols A, Czupryn P, Corbett M, Handley E, Burge AT, Holland AE. Perceived autonomy support in telerehabilitation by people with chronic respiratory disease: a mixed methods study. Chest. 2023;163(6):1410–1424. doi: 10.1016/j.chest.2022.12.023.S0012-3692(22)04344-6 [DOI] [PubMed] [Google Scholar]
- 69.Hattingh HL, Edmunds C, Gillespie BM. Medication management of COVID-19 patients during transition to virtual models of care: a qualitative study. J Pharm Policy Pract. 2023;16(1):127. doi: 10.1186/s40545-023-00633-1. https://www.tandfonline.com/doi/10.1186/s40545-023-00633-1?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed .10.1186/s40545-023-00633-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kazmerski TM, Stransky OM, Wright CE, Albanowski M, Pilewski JM, Talabi MB, Callegari LS, Chang JC, Abebe KZ, Miller E, Deal A, O'Leary R, Borrero S. Feasibility testing of a web-based reproductive decision support tool for cystic fibrosis. J Cyst Fibros. 2024;23(3):404–411. doi: 10.1016/j.jcf.2023.10.003.S1569-1993(23)00924-4 [DOI] [PubMed] [Google Scholar]
- 71.Robinson SA, Shimada SL, Sliwinski SK, Wiener RS, Moy ML. Stakeholder perceptions of a web-based physical activity intervention for COPD: A mixed-methods study. J Clin Med. 2023;12(19):6296. doi: 10.3390/jcm12196296. https://www.mdpi.com/resolver?pii=jcm12196296 .jcm12196296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watanabe AH, Willis C, Ragsdale R, Biskupiak J, Moore K, Brixner D, Young D. Patient perspectives on the use of digital technology to help manage cystic fibrosis. Pulm Med. 2023;2023:5082499. doi: 10.1155/2023/5082499. https://doi.org/10.1155/2023/5082499 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pacht ER, Turner JW, Gailiun M, Violi LA, Ralston D, Mekhjian HS, St John RC. Effectiveness of telemedicine in the outpatient pulmonary clinic. Telemed J. 1998;4(4):287–292. doi: 10.1089/tmj.1.1998.4.287. [DOI] [PubMed] [Google Scholar]
- 74.Stepnowsky CJ, Palau JJ, Marler MR, Gifford AL. Pilot randomized trial of the effect of wireless telemonitoring on compliance and treatment efficacy in obstructive sleep apnea. J Med Internet Res. 2007;9(2):e14. doi: 10.2196/jmir.9.2.e14. https://www.jmir.org/2007/2/e14/ v9i2e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Varkey P, Schumacher K, Swanton C, Timm B, Hagen PT. Telemedicine in the work site: a study of feasibility, and patient and provider satisfaction. J Telemed Telecare. 2008;14(6):322–325. doi: 10.1258/jtt.2008.080512.14/6/322 [DOI] [PubMed] [Google Scholar]
- 76.Agha Z, Roter DL, Schapira RM. An evaluation of patient-physician communication style during telemedicine consultations. J Med Internet Res. 2009;11(3):e36. doi: 10.2196/jmir.1193. https://www.jmir.org/2009/3/e36/ v11i3e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zamith M, Cardoso T, Matias I, Marques Gomes MJ. Home telemonitoring of severe chronic respiratory insufficient and asthmatic patients. Rev Port Pneumol. 2009;15(3):385–417. https://linkinghub.elsevier.com/retrieve/pii/S0873-2159(15)30142-2 .S0873-2159(15)30142-2 [PubMed] [Google Scholar]
- 78.Byczkowski TL, Munafo JK, Britto MT. Variation in use of internet-based patient portals by parents of children with chronic disease. Arch Pediatr Adolesc Med. 2011;165(5):405–411. doi: 10.1001/archpediatrics.2011.55.165/5/405 [DOI] [PubMed] [Google Scholar]
- 79.Nield M, Hoo GWS. Real-time telehealth for COPD self-management using Skype™. COPD. 2012;9(6):611–619. doi: 10.3109/15412555.2012.708067. https://www.tandfonline.com/doi/10.3109/15412555.2012.708067?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PubMed] [Google Scholar]
- 80.Chih M, DuBenske LL, Hawkins RP, Brown RL, Dinauer SK, Cleary JF, Gustafson DH. Communicating advanced cancer patients' symptoms via the Internet: a pooled analysis of two randomized trials examining caregiver preparedness, physical burden, and negative mood. Palliat Med. 2013;27(6):533–543. doi: 10.1177/0269216312457213. https://europepmc.org/abstract/MED/22988042 .0269216312457213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pinnock H, Hanley J, McCloughan L, Todd A, Krishan A, Lewis S, Stoddart A, van der Pol M, MacNee W, Sheikh A, Pagliari C, McKinstry B. Effectiveness of telemonitoring integrated into existing clinical services on hospital admission for exacerbation of chronic obstructive pulmonary disease: researcher blind, multicentre, randomised controlled trial. BMJ. 2013;347:f6070. doi: 10.1136/bmj.f6070. https://www.bmj.com/lookup/pmidlookup?view=long&pmid=24136634 .bmj.f6070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cingi C, Yorgancioglu A, Cingi CC, Oguzulgen K, Muluk NB, Ulusoy S, Orhon N, Yumru C, Gokdag D, Karakaya G, Çelebi Ş, Çobanoglu HB, Unlu H, Aksoy MA. The "physician on call patient engagement trial" (POPET): measuring the impact of a mobile patient engagement application on health outcomes and quality of life in allergic rhinitis and asthma patients. Int Forum Allergy Rhinol. 2015;5(6):487–497. doi: 10.1002/alr.21468. [DOI] [PubMed] [Google Scholar]
- 83.Apter AJ, Bryant-Stephens T, Morales KH, Wan F, Hardy S, Reed-Wells S, Dominguez M, Gonzalez R, Mak N, Nardi A, Park H, Howell JT, Localio R. Using IT to improve access, communication, and asthma in African American and Hispanic/Latino adults: Rationale, design, and methods of a randomized controlled trial. Contemp Clin Trials. 2015;44:119–128. doi: 10.1016/j.cct.2015.08.001.S1551-7144(15)30059-8 [DOI] [PubMed] [Google Scholar]
- 84.Wiecha JM, Adams WG, Rybin D, Rizzodepaoli M, Keller J, Clay JM. Evaluation of a web-based asthma self-management system: a randomised controlled pilot trial. BMC Pulm Med. 2015;15(1):17. doi: 10.1186/s12890-015-0007-1. https://bmcpulmmed.biomedcentral.com/articles/10.1186/s12890-015-0007-1 .10.1186/s12890-015-0007-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fadaizadeh L, Najafizadeh K, Shajareh E, Shafaghi S, Hosseini M, Heydari G. Home spirometry: assessment of patient compliance and satisfaction and its impact on early diagnosis of pulmonary symptoms in post-lung transplantation patients. J Telemed Telecare. 2015;22(2):127–131. doi: 10.1177/1357633x15587435. [DOI] [PubMed] [Google Scholar]
- 86.Rosenberger EM, DeVito Dabbs AJ, DiMartini AF, Landsittel DP, Pilewski JM, Dew MA. Long-term follow-up of a randomized controlled trial evaluating a mobile health intervention for self-management in lung transplant recipients. Am J Transplant. 2017;17(5):1286–1293. doi: 10.1111/ajt.14062. https://linkinghub.elsevier.com/retrieve/pii/S1600-6135(22)24979-X .S1600-6135(22)24979-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Poureslami I, Shum J, Lester RT, Tavakoli H, Dorscheid DR, FitzGerald JM. A pilot randomized controlled trial on the impact of text messaging check-ins and a web-based asthma action plan versus a written action plan on asthma exacerbations. J Asthma. 2018;56(8):1–13. doi: 10.1080/02770903.2018.1500583. [DOI] [PubMed] [Google Scholar]
- 88.Sleath B, Carpenter DM, Davis SA, Watson CH, Lee C, Loughlin CE, Garcia N, Reuland DS, Tudor G. Improving youth question-asking and provider education during pediatric asthma visits. Patient Educ Couns. 2018;101(6):1051–1057. doi: 10.1016/j.pec.2018.01.013. https://europepmc.org/abstract/MED/29402570 .S0738-3991(18)30035-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morita PP, Yeung MS, Ferrone M, Taite AK, Madeley C, Stevens Lavigne A, To T, Lougheed MD, Gupta S, Day AG, Cafazzo JA, Licskai C. A patient-centered mobile health system that supports asthma self-management (breathe): design, development, and utilization. JMIR Mhealth Uhealth. 2019;7(1):e10956. doi: 10.2196/10956. https://mhealth.jmir.org/2019/1/e10956/ v7i1e10956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sonney J, Duffy M, Hoogerheyde LX, Langhauser E, Teska D. Applying human-centered design to the development of an asthma essentials kit for school-aged children and their parents. J Pediatr Health Care. 2019;33(2):169–177. doi: 10.1016/j.pedhc.2018.07.008. https://europepmc.org/abstract/MED/30228032 .S0891-5245(18)30346-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Apter AJ, Bryant-Stephens T, Perez L, Morales KH, Howell JT, Mullen AN, Han X, Canales M, Rogers M, Klusaritz H, Localio AR. Patient portal usage and outcomes among adult patients with uncontrolled asthma. J Allergy Clin Immunol Pract. 2020;8(3):965–970. doi: 10.1016/j.jaip.2019.09.034. https://europepmc.org/abstract/MED/31622684 .S2213-2198(19)30862-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gaynor M, Schneider D, Seltzer M, Crannage E, Barron ML, Waterman J, Oberle A. A user-centered, learning asthma smartphone application for patients and providers. Learn Health Syst. 2020;4(3):e10217. doi: 10.1002/lrh2.10217. https://europepmc.org/abstract/MED/32685685 .LRH210217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kneuertz PJ, Jagadesh N, Perkins A, Fitzgerald M, Moffatt-Bruce SD, Merritt RE, D'Souza DM. Improving patient engagement, adherence, and satisfaction in lung cancer surgery with implementation of a mobile device platform for patient reported outcomes. J Thorac Dis. 2020;12(11):6883–6891. doi: 10.21037/jtd.2020.01.23. https://europepmc.org/abstract/MED/33282391 .jtd-12-11-6883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koff PB, Min S, Freitag TJ, Diaz DLP, James SS, Voelkel NF, Linderman DJ, Diaz del Valle F, Zakrajsek JK, Albert RK, Bull TM, Beck A, Stelzner TJ, Ritzwoller DP, Kveton CM, Carwin S, Ghosh M, Keith RL, Westfall JM, Vandivier RW. Impact of proactive integrated care on chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2021;8(1):100–116. doi: 10.15326/JCOPDF.2020.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Opipari-Arrigan L, Dykes DMH, Saeed SA, Thakkar S, Burns L, Chini BA, McPhail GL, Eslick I, Margolis PA, Kaplan HC. Technology-enabled health care collaboration in pediatric chronic illness: Pre-post interventional study for feasibility, acceptability, and clinical impact of an electronic health record-linked platform for patient-clinician partnership. JMIR Mhealth Uhealth. 2020;8(11):e11968. doi: 10.2196/11968. https://mhealth.jmir.org/2020/11/e11968/ v8i11e11968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perlman A, Vodonos Zilberg A, Bak P, Dreyfuss M, Leventer-Roberts M, Vurembrand Y, Jeffries HE, Fisher E, Steuerman Y, Namir Y, Goldschmidt Y, Souroujon D. Characteristics and symptoms of app users seeking COVID-19-related digital health information and remote services: retrospective cohort study. J Med Internet Res. 2020;22(10):e23197. doi: 10.2196/23197. https://www.jmir.org/2020/10/e23197/ v22i10e23197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alsharif Abdullah H. Cross sctional E-health evaluation study for telemedicine and m-health approaches in monitoring COVID-19 patients with chronic obstructive pulmonary disease (COPD) Int J Environ Res Public Health. 2021 Aug 12;18(16) doi: 10.3390/ijerph18168513. https://www.mdpi.com/resolver?pii=ijerph18168513 .ijerph18168513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Davis J, Gordon R, Hammond A, Perkins R, Flanagan F, Rabinowitz E, Simoneau T, Sawicki GS. Rapid implementation of telehealth services in a pediatric pulmonary clinic during COVID-19. Pediatrics. 2021;148(1):e2020030494. doi: 10.1542/peds.2020-030494.peds.2020-030494 [DOI] [PubMed] [Google Scholar]
- 99.Gashu KD, Gelaye KA, Lester R, Tilahun B. Effect of a phone reminder system on patient-centered tuberculosis treatment adherence among adults in Northwest Ethiopia: a randomised controlled trial. BMJ Health Care Inform. 2021;28(1):e100268. doi: 10.1136/bmjhci-2020-100268. https://informatics.bmj.com/lookup/pmidlookup?view=long&pmid=34172505 .bmjhci-2020-100268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hendra K, Neemuchwala F, Chan M, Ly NP, Gibb ER. Patient and provider experience with cystic fibrosis telemedicine clinic. Front Pediatr. 2021;9:784692. doi: 10.3389/fped.2021.784692. https://europepmc.org/abstract/MED/34900879 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meshkov I, Petrenko T, Keiser O, Estill J, Revyakina O, Felker I, Raviglione MC, Krasnov V, Schwartz Y. Variations in tuberculosis prevalence, Russian Federation: a multivariate approach. Bull World Health Organ. 2019 Nov 01;97(11):737–745A. doi: 10.2471/BLT.19.229997. https://europepmc.org/abstract/MED/31673189 .BLT.19.229997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kowatsch T, Schachner T, Harperink S, Barata F, Dittler U, Xiao G, Stanger C, V Wangenheim F, Fleisch E, Oswald H, Möller A. Conversational agents as mediating social actors in chronic disease management involving health care professionals, patients, and family members: multisite single-arm feasibility study. J Med Internet Res. 2021;23(2):e25060. doi: 10.2196/25060. https://www.jmir.org/2021/2/e25060/ v23i2e25060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mustafa SS, Yang L, Mortezavi M, Vadamalai K, Ramsey A. Patient satisfaction with telemedicine encounters in an allergy and immunology practice during the coronavirus disease 2019 pandemic. Ann Allergy Asthma Immunol. 2020 Oct;125(4):478–479. doi: 10.1016/j.anai.2020.06.027. https://europepmc.org/abstract/MED/32585178 .S1081-1206(20)30435-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sabina Sousa C, Trigueiro Barbosa M, Aguiar R, Benito-Garcia F, Morais-Almeida M. What do asthmatic patients think about telemedicine visits? Eur Ann Allergy Clin Immunol. 2021 May;53(3):138–142. doi: 10.23822/EurAnnACI.1764-1489.182. http://www.eurannallergyimm.com/cont/journals-articles/961/volume-what-asthmatic-patients-think-about.asp . [DOI] [PubMed] [Google Scholar]
- 105.Benson J, Bhandari P, Lui N, Berry M, Liou DZ, Shrager J, Ayers K, Backhus LM. Use of a personalized multimedia education platform improves preoperative teaching for lung cancer patients. Semin Thorac Cardiovasc Surg. 2022;34(1):363–372. doi: 10.1053/j.semtcvs.2021.03.003.S1043-0679(21)00115-5 [DOI] [PubMed] [Google Scholar]
- 106.Bukstein DA, Eghrari-Sabet J, Hart M, Hill T, Parikh P, Winders TA. COVID-19 pandemic impact on telehealth use and perceptions for atopic and respiratory disease: Survey results. Allergy Asthma Proc. 2022;43(3):194–201. doi: 10.2500/aap.2022.43.220019. https://europepmc.org/abstract/MED/35524354 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nelson Sarah E, Steuernagle Jon, Rotello Leo, Nyquist Paul, Suarez Jose I, Ziai Wendy. COVID-19 and telehealth in the intensive care unit setting: a survey. BMC Health Serv Res. 2022 Jun 20;22(1):797. doi: 10.1186/s12913-022-08197-7. https://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-022-08197-7 .10.1186/s12913-022-08197-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shinoda M, Hataji O, Miura M, Kinoshita M, Mizoo A, Tobino K, Soutome T, Nishi T, Ishii T, Miller BE, Tal-Singer R, Tomlinson R, Matsuki T, Jones PW, Shibata Y. A telemedicine approach for monitoring COPD: a prospective feasibility and acceptability cohort study. Int J Chron Obstruct Pulmon Dis. 2022;17(101273481):2931–2944. doi: 10.2147/copd.s375049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chun VS, Whooley MA, Williams K, Zhang N, Zeidler MR, Atwood CW, Folmer RL, Totten AM, Smith CJ, Boudreau EA, Reichert JM, Sarmiento KF. Veterans health administration TeleSleep enterprise-wide initiative 2017-2020: bringing sleep care to our nation's veterans. J Clin Sleep Med. 2023;19(5):913–923. doi: 10.5664/jcsm.10488. https://europepmc.org/abstract/MED/36708262 .jcsm.10488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Coker TR, Mitchell SJ, Lowry SJ, Klein EJ, Stout JW, Brown JC, Liljenquist KS, Wingfield E, Horn IB. Text2Breathe: Text-message intervention for parent communication and pediatric asthma. Acad Pediatr. 2023;23(1):123–129. doi: 10.1016/j.acap.2022.05.004. https://europepmc.org/abstract/MED/35577281 .S1876-2859(22)00242-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lawrence J, Truong D, Dao A, Bryant PA. Virtual hospital-level care-feasibility, acceptability, safety and impact of a pilot Hospital-In-The-Home model for COVID-19 infection. Front Digit Health. 2023;5:1068444. doi: 10.3389/fdgth.2023.1068444. https://europepmc.org/abstract/MED/37090066 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Varghese MP, Selwyn T, Nair S, Samuel S, Chacko B, Pichamuthu K. Assessment of family satisfaction with remote communication for critically Ill COVID-19 patients: an observational cohort study. Indian J Crit Care Med. 2023;27(8):537–544. doi: 10.5005/jp-journals-10071-24504. https://europepmc.org/abstract/MED/37636852 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chang E, Penfold RB, Berkman ND. Patient characteristics and telemedicine use in the US, 2022. JAMA Netw Open. 2024 Mar 04;7(3):e243354. doi: 10.1001/jamanetworkopen.2024.3354. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2024.3354 .2816685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pinnock H, Murphie P, Vogiatzis I, Poberezhets V. Telemedicine and virtual respiratory care in the era of COVID-19. ERJ Open Res. 2022;8(3):00111-2022. doi: 10.1183/23120541.00111-2022. https://europepmc.org/abstract/MED/35891622 .00111-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Connell A, Black G, Montgomery H, Martin P, Nightingale C, King D, Karthikesalingam A, Hughes C, Back T, Ayoub K, Suleyman M, Jones G, Cross J, Stanley S, Emerson M, Merrick C, Rees G, Laing C, Raine R. Implementation of a digitally enabled care pathway (Part 2): qualitative analysis of experiences of health care professionals. J Med Internet Res. 2019;21(7):e13143. doi: 10.2196/13143. https://www.jmir.org/2019/7/e13143/ v21i7e13143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paloumpi E, Ozieranski P, Watson MC, Jones MD. Pharmacy users' perceptions, awareness and future expectations of community pharmacy in England: a focus group study. Int J Pharm Pract. 2024;32(1):39–45. doi: 10.1093/ijpp/riad082. https://strathprints.strath.ac.uk/87386/ 7427626 [DOI] [PubMed] [Google Scholar]
- 117.Walker RC, Tong A, Howard K, Palmer SC. Patient expectations and experiences of remote monitoring for chronic diseases: systematic review and thematic synthesis of qualitative studies. Int J Med Inform. 2019;124:78–85. doi: 10.1016/j.ijmedinf.2019.01.013. https://linkinghub.elsevier.com/retrieve/pii/S1386-5056(18)30982-1 .S1386-5056(18)30982-1 [DOI] [PubMed] [Google Scholar]
- 118.Montague E. Patient source of learning about health technologies and ratings of trust in technologies used in their care. Ergonomics. 2010;53(11):1302–1310. doi: 10.1080/00140139.2010.520746. https://europepmc.org/abstract/MED/20967654 .928457063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sutherland BL, Pecanac K, LaBorde TM, Bartels CM, Brennan MB. Good working relationships: how healthcare system proximity influences trust between healthcare workers. J Interprof Care. 2022;36(3):331–339. doi: 10.1080/13561820.2021.1920897. https://www.tandfonline.com/doi/abs/10.1080/13561820.2021.1920897?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Antonacci G, Benevento E, Bonavitacola S, Cannavacciuolo L, Foglia E, Fusi G, Garagiola E, Ponsiglione C, Stefanini A. Healthcare professional and manager perceptions on drivers, benefits, and challenges of telemedicine: results from a cross-sectional survey in the Italian NHS. BMC Health Serv Res. 2023 Oct 18;23(1):1115. doi: 10.1186/s12913-023-10100-x. https://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-023-10100-x .10.1186/s12913-023-10100-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wilson S, Tolley C, Mc Ardle R, Beswick E, Slight SP. Key Considerations When Developing and Implementing Digital Technology for Early Detection of Dementia-Causing Diseases Among Health Care Professionals: Qualitative Study. J Med Internet Res. 2023 Aug 22;25:e46711. doi: 10.2196/46711. https://www.jmir.org/2023//e46711/ v25i1e46711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Breton M, Sullivan EE, Deville-Stoetzel N, McKinstry D, DePuccio M, Sriharan A, Deslauriers V, Dong A, McAlearney AS. Telehealth challenges during COVID-19 as reported by primary healthcare physicians in Quebec and Massachusetts. BMC Fam Pract. 2021 Sep 26;22(1):192. doi: 10.1186/s12875-021-01543-4. https://bmcfampract.biomedcentral.com/articles/10.1186/s12875-021-01543-4 .10.1186/s12875-021-01543-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee G, Caton E, Ding A. Evaluating digital competencies for pharmacists. Res Social Adm Pharm. 2023 May;19(5):753–757. doi: 10.1016/j.sapharm.2023.01.012. https://linkinghub.elsevier.com/retrieve/pii/S1551-7411(23)00032-3 .S1551-7411(23)00032-3 [DOI] [PubMed] [Google Scholar]
- 124.Bagot KL, Bladin CF, Vu M, Bernard S, Smith K, Hocking G, Coupland T, Hutton D, Badcock D, Budge M, Nadurata V, Pearce W, Hall H, Kelly B, Spencer A, Chapman P, Oqueli E, Sahathevan R, Kraemer T, Hair C, Dion S, McGuinness C, Cadilhac DA. Factors influencing the successful implementation of a novel digital health application to streamline multidisciplinary communication across multiple organisations for emergency care. Evaluation Clinical Practice. 2023 Sep 18;30(2):184–198. doi: 10.1111/jep.13923. [DOI] [PubMed] [Google Scholar]
- 125.Jones WD, Rodts MF, Merz J. Influencing Discharge Efficiency: Addressing Interdisciplinary Communication, Transportation, and COVID-19 as Barriers. Prof Case Manag. 2022;27(4):169–180. doi: 10.1097/NCM.0000000000000549.01269241-202207000-00002 [DOI] [PubMed] [Google Scholar]
- 126.Adjekum A, Blasimme A, Vayena E. Elements of Trust in Digital Health Systems: Scoping Review. J Med Internet Res. 2018 Dec 13;20(12):e11254. doi: 10.2196/11254. https://www.jmir.org/2018/12/e11254/ v20i12e11254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Scott V, Mathews V, Gilson L. Constraints to implementing an equity-promoting staff allocation policy: understanding mid-level managers' and nurses' perspectives affecting implementation in South Africa. Health Policy Plan. 2012 Mar;27(2):138–46. doi: 10.1093/heapol/czr020.czr020 [DOI] [PubMed] [Google Scholar]
- 128.Alkureishi MA, Choo Z, Rahman A, Ho K, Benning-Shorb J, Lenti G, Velázquez Sánchez Itzel, Zhu M, Shah SD, Lee WW. Digitally Disconnected: Qualitative Study of Patient Perspectives on the Digital Divide and Potential Solutions. JMIR Hum Factors. 2021 Dec 15;8(4):e33364. doi: 10.2196/33364. https://humanfactors.jmir.org/2021/4/e33364/ v8i4e33364 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy.
PRISMA checklist.
Quantitative findings.


