Abstract
The disease caused by the duck Tembusu virus (DTMUV) is one of the most prevalent arthropod-borne viral diseases in poultry. DTMUV is classified into three distinct clusters based on significant genetic divergence: Cluster 1, Cluster 2 (subdivided into 2.1 and 2.2), and Cluster 3. The virulence of DTMUV in ducks is potentially associated with the virus genotype. The evaluation of different clusters of DTMUV is based predominantly on the characterization of infected duck hosts, and limited attention has been paid to understanding viral virulence toward the infected mosquito vectors. In this study, we explore the infectivity patterns of DTMUV Cluster 1 (DTMUV 1) and Cluster 2.1 (DTMUV 2.1) in the primary mosquito vector, Culex tritaeniorhynchus. Our objective was to explore the relationship between the mosquito vector and DTMUV genotype, intending to determine whether the mosquito vector alters viral biology, thereby influencing the consequential infectivity characteristics in the host cells. We found that variation in viral nonstructural protein-5 (an RNA-dependent RNA polymerase) may influence the antigenicity process in Cx. tritaeniorhynchus. Our results revealed DTMUV1 underwent higher replication than DTMUV2.1 in mosquito salivary glands and saliva. Furthermore, DTMUV1 derived from mosquito saliva produced larger plaque sizes in baby hamster kidney-21 (BHK-21) cells than DTMUV2.1 derived from mosquito saliva. Interestingly, DTMUV2.1 was more efficient than DTMUV1 in inducing the production of mRNAs for macroglobulin complement-related factor, thioester-containing protein, and antimicrobial peptides (cecropin family) within the mosquito salivary gland. Our findings collectively suggest that Cx. tritaeniorhynchus can influence an environment conducive to modifying the amino acid composition of DTMUV1 and DTMUV2.1 in a manner that may affect the innate immune response, consequently augmenting viral virulence.
Keywords: Culex tritaeniorhynchus, Duck Tembusu virus, NS5, BHK-21 cell, Innate immune response
Graphical abstract
Highlights
-
•
DTMUV1 and DTMUV2.1 differ in infectivity in Culex tritaeniorhynchus mosquitoes.
-
•
Nonstructural protein-5 variations may influence viral replication and immune response.
-
•
The connection between NS5 mutations and antigenicity has not been directly validated.
-
•
DTMUV1 triggers a weaker mosquito immune response than DTMUV2.1.
-
•
Reduced immune response may contribute to the higher replication and transmission of DTMUV1.
1. Introduction
Tembusu virus (TMUV) is a positive-strand RNA virus with an approximately 11 kb genome and a virion size of 30–60 nm in diameter. It is also known as duck egg drop syndrome virus, which belongs to the Ntaya virus group in the genus Orthoflavivirus within the family Flaviviridae (Yun et al., 2012). The viral RNA genome consists of a single open reading frame (ORF) bordered by 5′ and 3′ terminal untranslated regions. The ORF is translated into three structural: envelope (E), membrane precursor (prM), and capsid (C), and seven nonstructural: NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 proteins (Hamel et al., 2021). Among the members of the Orrthoflavivirus, these nonstructural proteins actively inhibit vital signaling pathways essential for mounting an effective immune response, including pathways responsible for virus recognition and interferon activation (Chen et al., 2017). Particularly, NS5 is the largest and most conserved flaviviral protein, which is comprised of an RNA-dependent RNA polymerase (RdRP) domain (Davidson, 2009), which mediates the replication of the viral RNA genome within the replication complex (Klema et al., 2015) and plays an important role in inhibiting the host's antiviral agents (Leite et al., 2022; Pathania et al., 2022).
TMUV was initially identified from the mosquito Culex tritaeniorhynchus in Malaysia in 1955 (Platt et al., 1975). Variants of the original strain have been identified and reported as the causative agents of newly emerging duck egg-drop syndrome and avian outbreaks; hence, the virus was subsequently named duck Tembusu virus (DTMUV) or Baiyangdian virus (BYDV) (Cao et al., 2011; Homonnay et al., 2014). DTMUV has been detected through various surveys across Southeast and East Asia, in countries such as Malaysia, Thailand, China, and Taiwan (Platt et al., 1975; Pandey et al., 1999; Homonnay et al., 2014; Thontiravong et al., 2015; Peng et al., 2020; Sanisuriwong et al., 2020). This virus has been isolated from various avian species, including ducks, chickens, geese, pigeons, and sparrows, as well as from Culex mosquitoes (Pandey et al., 1999; Homonnay et al., 2014; Thontiravong et al., 2015; Peng et al., 2020; Sanisuriwong et al., 2020). In general, ducks are the primary host for DTMUV, while mosquitoes in the genus Culex are the vector, and thus play a significant role in transmitting the virus through mosquito bites (Hamel et al., 2021). Despite the widespread detection of DTMUV across various Culex species in different countries, only Cx. tritaeniorhynchus and Cx. quinquefasciatus have been confirmed to transmit the virus to ducks under natural conditions (Guo et al., 2020).
The bite of an infected mosquito is a mode of transmission for DTMUV; however, ducks can also be infected by the virus through direct contact with infected animals via airborne transmission (Li et al., 2015). Airborne transmission not only accelerates the spread of the virus but also enables its persistence without the presence of the vector, such as during overwintering. This unique mode of arbovirus transmission should be studied systematically and requires thorough evaluation because it could potentially accelerate viral dissemination in various regions with or without arthropod vectors. Currently, DTMUV is classified into three distinct clusters: Cluster 1, Cluster 2 (subdivided into 2.1 and 2.2), and Cluster 3, based on significant genetic divergence among these three clusters (Ninvilai et al., 2019; Cheng et al., 2024). These DTMUV clusters may have originated from MM1775, a Tembusu virus derived from Cx. tritaeniorhynchus in Malaysia (Ninvilai et al., 2019). Interestingly, the virulence and pathogenicity of DTMUV in the ducks are potentially associated with the viral genotype (Meng et al., 2022; Yu et al., 2022). DTMUV genotypes were considered the principal factors that influence its virulence and pathogenicity during infection by direct contact transmission in ducks (Ninvilai et al., 2020; Tunterak et al., 2023). The pathogenesis of different clusters of DTMUV has generally been evaluated based on ducks; nonetheless, the relationships between viral genotypes, infectivity, and antigenicity within mosquito vectors have not been adequately explored.
Several investigations in mammals and poultries suggest that orthoflaviviruses trigger a strong innate immune response that is initiated by the recognition of specific viral components and stimulates the production of a diverse array of complementary antiviral molecules (Takeuchi and Akira, 2009; Morrison et al., 2012; Chen et al., 2013; Lazear and Diamond, 2015; Lee et al., 2022). Orthoflaviviruses possess an inducible gene expression system capable of modulating broad-spectrum antiviral activity against viruses present within mosquitoes (Weng et al., 2022). Mosquitoes lack an immunoglobulin-based adaptive immune system; therefore, innate immune mechanisms assume a principal means of both detecting and combating microbial infections, which hinge upon RNA interference and complement activation (Wang et al., 2006; Sikkeland et al., 2007; Arjona et al., 2011). The complement activation in insects involves thioester-containing proteins (iTEPs) thought to play a pivotal role in complement-like system functions as an innate immunity and antiviral mechanism. Macroglobulin complement-related factor (MCR), which is a subfamily of iTEPs, consists of a set of proteins that usually remains in an inactive form until prompted to potent activation by the formation of antigen-antibody or immune complexes (Blandin et al., 2004; Sikkeland et al., 2007).
Recent studies have reported that TEPs and MCR have a potential role in combating orthoflavivirus infection in various mosquito species. For instance, there were previous studies that revealed the invasion of Aedes aegypti and Aedes albopictus by orthoflaviviruses, such as yellow fever, dengue, and duck Tembusu virus, triggers the activation of MCR, which is responsible for regulating the induction of antimicrobial peptides involved in the elimination of the orthoflaviviruses within the mosquitoes (Xiao et al., 2014; Sri-in et al., 2022, 2023). Cheng et al. (2011) elucidated a role for Ae. aegypti thioester-containing proteins (AaeTEPs) and found that transfection of AaeTEP1 into Ae. aegypti significantly reduced dengue virus infection. Furthermore, Anopheles gambiae thioester-containing protein-1 (AgTEP1) plays a pivotal role in the mosquito immune response to malaria by binding to Plasmodium ookinetes in the basal lamina of the midgut epithelium and directing them for lysis (Blandin et al., 2004; Baxter et al., 2007). While the innate immune response to pathogens in Aedes and Anopheles mosquitoes has been studied extensively, our understanding of this phenomenon in Culex mosquitoes remains largely unexplored.
We hypothesized that the response of each genotype of DTMUV to the innate immune mechanisms within the mosquito vectors varies, consequently influencing the infection characteristics and virulence of the virus. To test this hypothesis, we explored the infectivity characteristics of DTMUV1 and DTMUV2.1 in the primary vector, Cx. tritaeniorhynchus. Given the scarcity of available information and the inherent challenges associated with studying DTMUV within mosquito vectors, we also used cell lines as diagnostic tools for our experiments. In this study, we extensively investigated both in vivo and in vitro, and the development of such diagnostic tools will facilitate the investigation of DTMUV infections and contribute to a deeper understanding of their transmission and characterization.
2. Materials and methods
2.1. Mosquitoes
The mosquitoes used in this study were derived from the Loei strain of Cx. tritaeniorhynchus maintained in the laboratory at the Parasitology Unit, Department of Veterinary Pathology, Faculty of Veterinary Science, Chulalongkorn University, Bangkok, Thailand. Only female mosquitoes at 3–5 days post-eclosion were used in this study. Mosquitoes were maintained as previously described (Meuti et al., 2023). Briefly, mosquitoes were provided with a 10% sucrose solution and maintained at 24–27 °C with a 12:12-h light: dark photoperiod. Mosquitoes were kept in the same cage and fed 5–10 days after adult emergence from established colonies. Female mosquitoes were provided with an artificial blood meal consisting of washed sheep blood (generated from Faculty of Veterinary Science, Chulalongkorn University, Bangkok, Thailand) mixed with a 1% antibiotic-antimycotic solution (Sigma-Aldrich) and a 2% 0.1 M adenosine 5′-triphosphate disodium salt hydrate (ATP) solution (Sigma-Aldrich). ATP helps maintain the freshness of blood and stimulates the mosquitoes to feed until fully satiated. An artificial membrane made from stretched Parafilm (Bemis Company, Inc., Neenah, Wisconsin, USA) was used for blood-feeding of female mosquitoes. Before blood-feeding, sugar sources were removed from the cages for one to two days. Wet sponges were provided as a source of water for the mosquitoes.
2.2. Virus and cell culture
DTMUV Cluster 1 and Cluster 2.1 were represented by strains DK/TH/CU-DTMUV2007 (DTMUV1) and DK/TH/CU-1 (DTMUV2.1), and were kindly provided by Dr Aunyaratana Thontiravong, Department of Veterinary Microbiology, Faculty of Veterinary Science, Chulalongkorn University. As previously described, these virus clusters are predominant in Thailand and were isolated from sick broiler ducks in a disease outbreak area (Ninvilai et al., 2019). DTMUV1 and DTMUV2.1 were propagated in 9-day-old embryonated duck eggs, harvested at 5 days post-infection, and stored at −80 °C (Thontiravong et al., 2015).
BHK-21 cells were cultured in Modified Eagle’s Medium (Opti-MEM®, Gibco, Thermo Fisher Scientific, Grand Island, New York, USA) containing 4% heat-inactivated fetal bovine serum (Gibco), 3% sodium bicarbonate (NaHCO3) (Gibco), 1% L-glutamine (Gibco), and 0.1% antibiotic-antimycotic (Sigma-Aldrich). To prepare DTMUV1 and DTMUV2.1 for infecting female mosquitoes, the viruses were first infected BHK-21 cells and grown at 37 °C with 5% CO2 for 5 days. The virus titer was determined, and approximately 103 TCID50/ml of both DTMUV1 and DTMUV 2.1 were used to infect the mosquitoes.
2.3. Virus infection
2.3.1. Intrathoracic injection
The viruses were administered via intrathoracic injection into female mosquitoes that were immobilized with CO2. Mosquitoes were injected with 207 nl of DTMUV (0.207 TCID50 in 207 nl) into their thorax using a Nanoinject II Injector (Drummond Scientific Company, Broomall, Pennsylvania, USA). The glass needles were generously given by Dr Theerawat Tharasanit, Department of Obstetrics, Gynecology and Reproduction, Faculty of Veterinary Science, Chulalongkorn University. The mosquitoes were then transferred into cylindrical containers fitted with a nylon mesh. The mosquito salivary gland, saliva, and body were collected 14 days after the virus injection, and these were analyzed for the expression level of DTMUV.
2.3.2. Oral infection
Female mosquitoes were transferred into a paper cylinder covered with a nylon mesh and starved for one to two days by sugar deprivation. They were then allowed to feed on DTMUV-infected blood following a simplified method for blood-feeding as previously described (Sri-in et al., 2020). Briefly, 500 μl of washed sheep blood containing 1% antibiotic-antimycotic (Sigma-Aldrich) and 2% 0.1 M ATP solution (Sigma-Aldrich) was gently mixed with 500 μl DTMUV (2.5 × 102 TCID50/ml). The artificial blood meal was wrapped in a stretched Parafilm (Bemis Company, Inc., Neenah, Wisconsin, USA) against human skin and then placed on a heating block set at 37 °C. The mosquitoes were allowed to take the meal for 24 h, during which the infected blood was changed every 4 h. Mosquitoes that had taken a full meal were transferred into a new paper cylinder for further examination.
2.4. RNA extraction, complementary DNA synthesis, and PCR
The mosquito body, salivary gland, and saliva samples were kept in a microcentrifuge tube containing 0.1 ml of Trizol Reagent (Invitrogen, Thermo Fisher Scientific, Carlsbad, California, USA) and stored at −80 °C until further use. The samples were homogenized and ground finely using a rotor-stator homogenizer at room temperature. The material was centrifuged at 3000× g at 4 °C for 5 min to produce a 10% suspension. Total RNA was extracted using Trizol Reagent (Invitrogen) following the manufacturer’s protocol, and diluted in UltraPure™ DNase/RNase-free distilled water Reagent (Invitrogen). The RNA concentration was measured using a spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific, Waltham, MA, USA). Complementary DNA (cDNA) was synthesized using the ImProm-II™ Reverse Transcription System (Promega, Madison, Wisconsin, USA), 250 ng of total RNA, and Random Hexamers primer (Applied Biosystems, Thermo Fisher Scientific, Foster City, California, USA). The resulting cDNA was stored at −20 °C and served as PCR template using the KOD One™ PCR Master Mix Blue kit (Toyobo Co., Ltd., Osaka, Japan). The PCR targeted a sequence of 1018 base pairs (bp) that correspond to a relatively conserved region on the NS5 region of viruses belonging to the genus Orthoflavivirus (Daidoji et al., 2021). The primers used were the FW primer (5′-AGN RCY ATC TGG TAY ATG TGG YTN GG-3′) and the RV primer (5′-BHA GCA TGT CBT CHG TBG TCA TCC A-3′) (Daidoji et al., 2021). The cycling conditions were as follows: initial denaturation at 94 °C for 2 min, followed by 45 cycles of denaturation at 98 °C for 10 s, annealing at 60 °C for 30 s, and extension at 68 °C for 15 or 30 s, and a final extension at 68 °C for 2 min. Positive PCR products of the expected size were cut from the agarose gel and purified using the GenepHlow™ Gel/PCR cleanup kit (Geneaid Biotech Ltd., New Taipei City, Taiwan) following the manufacturer’s protocols.
2.5. Double-stranded RNA synthesis
Double-stranded RNA (dsRNA) was synthesized as previously described (Sri-in et al., 2023). Briefly, target gene fragments containing the T7 polymerase promoter (5′-TAA TAC GAC TCA CTA TA GGG-3′) were amplified using Platinum® Taq DNA Polymerase (Invitrogen). The primers used for dsRNA are listed in Table 1. For the control group, dsRNA specific to the E. coli LacZ operon was synthesized. Amplified fragments were cloned into pCR2.1 TOPO vectors (Invitrogen) and transformed into E. coli HIT-DH5α cells. Positive colonies were purified using a FavorPrep™ Plasmid DNA Extraction Mini Kit (Favorgen Biotech Corporation, Pingtung City, Taiwan) and sequenced to confirm in-frame cDNA generation, and plasmids were digested and purified using a FavorPrep™ Gel/PCR Purification Kit (Favorgen Biotech Corporation). The purified PCR product served as a template for dsRNA synthesis using the T7-Scribe™ Transcription Kit (Epicentre Technologies, Madison, Wisconsin, USA). The in vitro transcription reaction proceeded at 37 °C for 4–12 h. Purified dsRNA was dissolved in DEPC-H2O to a final concentration of 5 μg/μl.
Table 1.
List of specific primers used for dsRNA in this study.
| Primer name | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| dsTEP3 | TAATACGACTCACTATAGGGACCAACAGGCCTCCAACGGA | TAATACGACTCACTATAGGGGTGCAACGTAATCCACTCC |
| dsMCR | TAATACGACTCACTATAGGGATGTCTCGGCTACGGTTCCG | TAATACGACTCACTATAGGGCACCATCGTCCTGAACGAAA |
| dsCEC A | TAATACGACTCACTATAGGGTCGTCCTGCTGGCAGCACTG | TAATACGACTCACTATAGGGAAGTCCTACTTTCCAAGTGC |
| dsCEC B | TAATACGACTCACTATAGGGTGGCCATCTTCCTGGTCCTC | TAATACGACTCACTATAGGGAAAGCTCATTATCCCAAAGC |
| dsCEC C | TAATACGACTCACTATAGGGTTGTCCTGTTGGCCGCGCTGG | TAATACGACTCACTATAGGGCTATTTTGGTCCAAGTGCATTA |
2.6. Sequence and phylogenetic analyses
The PCR products were sequenced by a commercial service (U2Bio, Bangkok, Thailand). The resulting DNA sequences were compared to those in the GenBank database using the NCBI nucleotide Basic Local Alignment Search Tool (BLASTn). Nucleotide sequences were aligned using the ClustalW multiple alignments tool (Thompson et al., 1994) and compared to reference sequences that were selected from previous studies obtained from the databases of the NCBI (http://www.ncbi.nlm.nih.gov/) and VectorBase (https://www.vectorbase.org/). Phylogenetic analysis was performed using the optimal model of nucleotide substitution as determined by the find best DNA/Protein model, and the phylogenetic trees were constructed with 1000 bootstrap replicates using the MEGA X software (Kumar et al., 2018). Multiple protein sequences were aligned using Clustal Omega (https://www.ebi.ac.uk/jdispatcher/msa/clustalo), and the aligned sequences were shaded using the Boxshade program (https://github.com/mdbaron42/pyBoxshade/releases).
2.7. Quantitative real-time PCR analysis
Quantitative real-time PCR (qRT-PCR) was performed using both TaqMan probe and SYBR Green dye binding systems. The TaqMan probe system used the TaqMan™ Fast Advanced Master Mix (Applied Biosystems) and a MicroAmp Fast Optical 96-well reaction plate with the Step One Plus™ Real-Time PCR System (Applied Biosystems). To measure the amount of DTMUV loads in the samples, we established a standard curve using a recombinant plasmid containing DTMUV. The 115 bp long target sequence corresponded to a relatively conserved region of DTMUV. The TaqMan probe (EP) consisted of a reporter dye Fluorescein amidites (FAM) located at the 5′-end, and the quencher (TAMRA) located at the 3′-end of the probe with the sequence (FAM-AGT TCC CAT ATC CAT GTC-TAMRA). The primers used were the EF (forward, 5′-TGT CTT ATG CAG GTA CCG ATG-3′) and ER (reverse, 5′-CGT ATG GGT TGA CTG TTA TCA-3′) primers (Yan et al., 2011).
The SYBR Green dye binding system measures signals of target genes using the Step One Plus™ Real-Time PCR System (Applied Biosystems). The quantification results were normalized relative to the ribosomal protein S7 of Culex species as an internal control. The specific primers used in this study are listed in Table 2. The signal strength was calculated from the cycle threshold values of the target gene and determined using the 2−ΔΔCt method (the relative mRNA expression level). qRT-PCR results are based on data from three independent experiments for each day post-infection.
Table 2.
List of specific primers used in qRT-PCR experiments.
| Primer name | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| TEP3 | CAACAAGGACGTTACCGTTG | GAGACAACCAACTTTGCCCG |
| MCR | GGCTTTGGAATGGCTGAGCG | TTGTTCACGATGTTCTGATA |
| CEC A | AACTTCAACAAGCTTTTCGTCA | CTTGAATCCAGTTACGACTGG |
| CEC B | ACTCTTCCTGCTGCTGGTGG | GCTTTGAATCCGGCCACTAC |
| CEC C | CTTCAACAAACTGTTTGCAA | ATTAATTCCAGCAACCACAG |
| CEC N | CTTTAACAAACTGTTTTTGATT | CGAGGGCCTGCACTCCTGC |
| DEF A | ATGGTGTCCTTACCGAGGAGAC | TCTTGCCGTTGCAGTATCCTC |
| DEF C | CAGGAGTCCGCTGACCAAGTT | ACAGTGCGCCGCGCAGGCACT |
| ATT | AGAATGAAACTGGAGCACGGG | TTGGTTGTAGCTGGCTGAGC |
| GAM | TGTCTATGCGAAAACCTGCTC | GTAATGTAACGATCCTGGAC |
| Ribosomal Protein S7 | GTTCGCAGCTGATCAAGGTC | CCCACTTCTCCATCTCGCAT |
2.8. Median tissue culture infectious dose (TCID50) assay
The experimental mosquitoes were permissive to oral infection by either DTMUV1 or DTMUV2.1. Fourteen days after infection, a total of 180 mosquitoes were fed with the artificial feeding solution to collect DTMUV-infected saliva. The feeding solution containing infectious saliva was transferred to microcentrifuge tubes with 0.22 μm centrifuge tube filters and centrifuged at 12,000× g for 30 min at 4 °C. The filtered saliva samples were adjusted to an equivalent protein concentration at 1 mg/ml, and serial 10-fold dilutions were prepared using Opti-MEM medium (Opti-MEM®, Gibco). Confluent BHK-21 cell monolayers in 96-well plates (Corning Incorporated, Corning, New York, USA) were washed with 1× PBS, inoculated with the dilutions (100 μl/well), and incubated at 37 °C and 5% CO2. After 5 days of incubation, the inoculated cells were removed and fixed with 100 μl of 3.7% formaldehyde for 30 min at room temperature. After the fixation, the residual formaldehyde was washed out using 0.5% phosphate-buffered saline with Tween detergent (PBST). The cells were immune-stained with the primary antibody against flavivirus (50 μl/well of polyclonal Rabbit IgG) (PA5-32200, Invitrogen, Thermo Fisher Scientific, Waltham, Massachusetts, USA) containing 1% bovine serum albumin (BSA) (Gibco) in 0.5% PBST (dilution, 1:400) (Tween™ 20, Thermo Scientific Chemicals, Thermo Fisher Scientific) for 2 h at room temperature. The residual antibody was washed out with 0.5% PBST, and then the cells were stained with the secondary antibody (50 μl/well of HRP-conjugated polyclonal mouse anti-rabbit IgG) (Merck KGaA, Darmstadt, Germany) containing 1% BSA in 0.5% PBST (dilution, 1:300) for 1 h at room temperature. The secondary antibody was removed with the residual washed out with 0.5% PBST. The cells were developed with 50 μl/well of AEC (HRP substrate, Advansta Inc., San Jose, California, USA) for 30 min at room temperature. The cells were washed with tap water and dried for 1 h at room temperature. The virus titer was calculated and expressed as TCID50/ml to quantify virus infectivity.
2.9. Plaque assay
Saliva of infectious Cx. tritaeniorhynchus was collected as described above, and the saliva concentration was adjusted to an equivalent protein concentration of 1 mg/ml. These were then diluted serially 10-fold in OPTI-MEM medium (Opti-MEM®, Gibco). BHK-21 cells were seeded in 24-well plates and incubated at 37 °C and 5% CO2 overnight or until a monolayer had formed. The monolayer cells in each well were exposed to 200 μl of the different dilutions of DTMUV-infected culture fluid for 24 h at 37 °C and 5% CO2. DTMUV-infected culture fluid was then removed, and 500 μl of 1% methylcellulose (Sigma-Aldrich) was added to each well and then incubated for 7 days at 37 °C and 5% CO2. The cells were then fixed with 3.7% formaldehyde solution (Sigma-Aldrich) at room temperature for 1 h. Methylcellulose overlays were then removed from the fixed cells, which were then washed with 1× PBS. The cells were then stained with 1% crystal violet (Sigma-Aldrich) diluted in ethanol for 1 h. Excess stain was discarded, and the cells were washed with water and then dried for 24 h. The plaque sizes were measured under the stereomicroscope using ImageJ software as previously described (Cheang et al., 2021).
2.10. Statistical analysis
The statistical analyses were performed using GraphPad Prism 9.4.1 (GraphPad Software, Boston, Massachusetts, USA). Means of different groups were compared and evaluated with unpaired t-tests and two-way ANOVA. P-value < 0.05 was regarded to be statistically significant.
3. Results
3.1. Phylogenetic analysis and pairwise sequence alignments of DTMUVs
DK/TH/CU-1 and DK/TH/CU-DTMUV2007 represented DTMUV strains and clusters, which were isolated from infected mosquitoes (PP507050-52) or infected BHK21 cells (PP507053-55) (Table 3). The NS5 coding sequence in this study is 840 nucleotides long, and it consists of a partial gene that encodes an NS5 of 280 amino acids. The phylogenetic relationship among clusters of DTMUV was evaluated by comparing the 26 nucleotide sequences of NS5. Phylogenetic analysis indicated that DTMUVs form three lineages designated Clusters 1, 2, and 3. Particularly Cluster 2 is subdivided into two lineages designated clusters 2.1 and 2.2. Although our representative DTMUVs belong to the same cluster, the sequences within lineages varied depending on the originate sources of the virus (Fig. 1). Even though a case of DTMUV Cluster 3 has been reported two times in Thailand (Ninvilai et al., 2020; Hamel et al., 2023), no full-length sequence data for Cluster 3 from Thailand is currently available in GenBank.
Table 3.
Identities and affiliations of DTMUV isolated in this study based on amino acid sequence analysis using the protein Basic Local Alignment Search Tool (BLAST).
| GenBank ID | Source of virus isolation | BLAST result |
|||
|---|---|---|---|---|---|
| Closest sequence | Identity (%) | DTMUV strain | Cluster | ||
| PP507050 | Cx. tritaeniorhynchus (saliva-derived) | QBA86037 | 99.29 | DK/TH/CU-1 | 2.1 |
| PP507051 | Cx. tritaeniorhynchus (saliva-derived) | AVM38076 | 98.92 | DK/TH/CU-DTMUV2007 | 1 |
| PP507052 | Cx. tritaeniorhynchus (saliva-derived) | AVM38076 | 98.92 | DK/TH/CU-DTMUV2007 | 1 |
| PP507053 | BHK-21 (cell culture-derived) | QBA86037 | 100 | DK/TH/CU-1 | 2.1 |
| PP507054 | BHK-21 (cell culture-derived) | AVM38076 | 100 | DK/TH/CU-DTMUV2007 | 1 |
| PP507055 | BHK-21 (cell culture-derived) | AVM38076 | 100 | DK/TH/CU-DTMUV2007 | 1 |
Fig. 1.
Phylogeny of DTMUV based on the partial NS5 gene. The tree with the highest log likelihood is shown. Evolutionary analyses were conducted in MEGA X and phylogenies were inferred using the Maximum Likelihood method and the Tamura-Nei model. The newly generated sequences are indicated in red.
3.2. Infecting mosquitoes alters amino acid compositions of DTMUV clusters 1 and 2.1
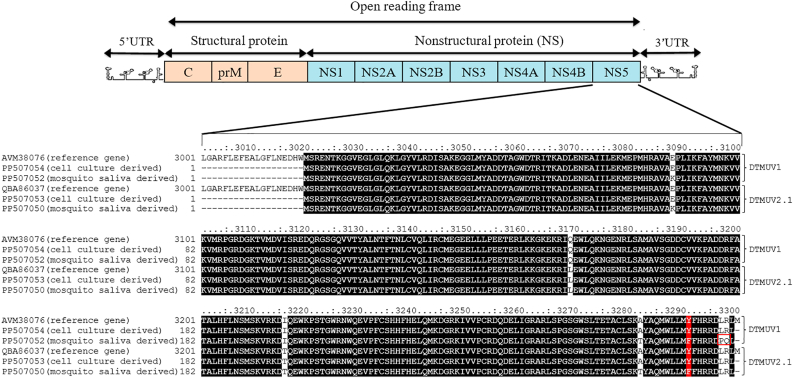
We examined the amino acid sequences of DTMUV1 and DTMUV2.1, including the capsid, envelope, PrM, and NS1-5 proteins, from the original virus and the saliva of infected mosquitoes (Supplementary Fig. S1). We observed that the amino acid sequence of the virus derived from mosquito saliva changed only in the NS5 region. Besides, the amino acid sequences of the NS5 protein in each cluster of DTMUV1 and DTMUV2.1 differ from the consensus at two sites: 3169 and 3216 (Fig. 2). We hypothesized that the variation in the composition of NS5, which possesses RdRP activity, between the two clusters of DTMUV may serve as a factor in the varying responses of the mosquito innate immune system to DTMUVs. The amino acid sequences of DTMUV1 and DTMUV2.1 derived from BHK-21 cell culture (PP507054 and PP507053, respectively) were identical to those of reference genes (AVM38076 and QBA86037, respectively), while those derived from mosquito saliva (PP507052 and PP507050, respectively) differed at 3–5 specific sites within the NS5 protein. The multiple sequence alignment in Fig. 2 shows that amino acid residues between positions 3020 and 3300 of both DTMUV1 and DTMUV2.1, derived from mosquito saliva, are largely identical (highlighted in black). The exceptions are site 3291, which is similar to the consensus (highlighted in red), and sites 3088 and 3282 (not highlighted), which differ from the consensus. It is noteworthy that sites 3297 and 3298 within the DTMUV1 derived from mosquito saliva (PP507052) are distinct from the corresponding sites in other sequences (Fig. 2).
Fig. 2.
Multiple sequence alignment of clusters 1 and 2.1 of DTMUV (DTMUV1 and DTMUV2.1). Members of DTMUV1 include AVM38076 (reference gene), PP507054 (derived from cell culture), and PP507052 (derived from the saliva of Cx. tritaeniorhynchus. Members of DTMUV2.1 include QBA86037 (reference gene), PP507053 (derived from cell culture), and PP507050 (derived from the saliva of Cx. tritaeniorhynchus). Black highlighting indicates identical residues; red indicates similar to consensus; no highlighting indicates different from consensus.
3.3. Characteristics of DTMUV clusters 1 and 2.1 infections in Culex tritaeniorhynchus
We hypothesized that different innate immune responses to infections by DTMUV1 and DTMUV2.1 may affect the replication of viral RNA within the mosquito vector. We measured viral loads in the infected mosquito bodies, salivary glands, and saliva at 3-, 7-, and 14-days post-infection (dpi) by qRT-PCR analysis using DTMUV-specific primers, and the results are presented as the mean ± standard error (SEM) of the number of DTMUV genome copies per 250 ng of RNA from three independent experiments (a total of 180 samples) (Fig. 3A–C). After 14 days of infecting mosquitoes with identical titers of DTMUV1 and DTMUV2.1, we observed that both the salivary glands and saliva infected with DTMUV1 had significantly higher viral loads than those infected with DTMUV2.1 (Fig. 3B and C); however, we observed no notable difference between the viral loads of the bodies. These exhibited DTMUV1-infected mosquitoes, along with a higher growth rate and greater infectivity within both salivary glands and saliva, compared to those infected with DTMUV2.1.
Fig. 3.
Infections of DTMUV clusters 1 and 2.1 in Cx. tritaeniorhynchus. DTMUV1 and DTMUV2.1 were detected by qRT-PCR in the mosquito body (A), salivary gland (B), and saliva (C). Total RNA levels are expressed as log10 DTMUV genome copy number per 250 ng. Each bar represents an average of 30 samples, totaling 180 samples per experiment. These results are from three experiments, and the data are presented as the mean ± standard error (SEM). Statistically significant differences between clusters 1 and 2.1 were analyzed using unpaired t-tests. Asterisks (∗) indicate statistically significant differences: ∗∗∗∗P < 0.0001.
3.4. Effects of DTMUV clusters 1 and 2.1 derived from Culex tritaeniorhynchus saliva on BHK-21 cell cultures
DTMUV1 isolated from infected Cx. tritaeniorhynchus saliva caused the largest plaque sizes on BHK-21 cells (approximately 1 mm in diameter); meanwhile, the sizes of plaques caused by DTMUV2.1 isolated from infected saliva were less than 100 μm, and thus they were difficult to count and measure (Fig. 4A). The plaques sizes on BHK-21 cells infected with saliva-derived from DTMUV1-infected mosquitoes were significantly larger than the corresponding saliva from DTMUV2.1-infected mosquitoes (Fig. 4B). Notably, the median tissue culture infectious dose (TCID50) analysis of virus titers between saliva from DTMUV1- and DTMUV2.1-infected mosquitoes at 14 dpi revealed significantly higher viral titer in DTMUV1-infected mosquitoes compared to that infected by an equivalent titer of DTMUV2.1 (Fig. 4C). Interestingly, we observed that both DTMUV1 and DTMUV2.1 derived from infected mosquito saliva produce larger plaque sizes on BHK-21 cells compared to those infected with DTMUV derived from cell culture (Supplementary Fig. S2). Our results demonstrate that saliva from different clusters of DTMUV-infected Cx. tritaeniorhynchus influences virulence and transmission differently.
Fig. 4.
Effects of DTMUV clusters 1 and 2.1 derived from Cx. tritaeniorhynchus saliva on BHK-21 cell cultures. A Different DTMUV clusters were analyzed by plaque and immunostaining assay including DTMUV1 from Cx. tritaeniorhynchus saliva-derived, DTMUV2.1 from Cx. tritaeniorhynchus saliva-derived, and Uninfected BHK-21 cells treated as control. B Variation in plaque-size of DTMUV1 and DTMUV2.1 derived from infected saliva. Compare the plaque size between clusters 1 and 2.1. C Concentrations of DTMUV1 and DTMUV2.1 from infected mosquito saliva were titrated using TCID50 analysis. Each bar includes saliva from 90 samples, totaling 180 samples in this experiment. The results were performed in triplicate experiments, and the data are presented as the mean ± standard error (SEM). Statistically significant differences between groups were analyzed using an unpaired t-test. Asterisks (∗) indicate statistically significant differences: ∗P < 0.05, ∗∗∗P < 0.001.
3.5. Innate immune response to DTMUV clusters 1 and 2.1 in Culex tritaeniorhynchus
Here, we have focused on thioester-containing protein-3 (TEP3) and the macroglobulin complement-related factor (MCR) of Cx. tritaeniorhynchus to observe whether they are crucial factors in each cluster of DTMUV infection in Cx. tritaeniorhynchus. TEP and MCR are known to influence the antimicrobial peptides (AMPs) that are antiviral factors and restrict orthoflavivirus infection in mosquitoes. We hypothesized that each cluster of DTMUV stimulates a different innate immune response during infection. Furthermore, we speculated that the composition variance in NS5 between DTMUV1 and DTMUV2.1 could potentially associate with specific types of innate immune responses and antiviral agents within the mosquito vector. First, we infected female mosquitoes with DTMUV cluster 1 or cluster 2.1, then we measured the expression of thioester-containing protein-3 (TEP3) and MCR within the salivary glands of Cx. tritaeniorhynchus that may, in turn, influence the virus infection and transmission. The experiment revealed that the gene expression levels of TEP3 and MCR in DTMUV 2.1-infected salivary glands were significantly higher than those in DTMUV1-infected salivary glands (Fig. 5A and B). Furthermore, we measured the expression levels of the following eight AMPs: four cecropin (CEC) genes CEC A, CEC B, CEC C, and CEC N; two defensin (DEF) genes DEF A and DEF C; and one gene each of attacin (ATT) and gambicin (GAM), in response to infection of each cluster of DTMUV in Cx. tritaeniorhynchus salivary glands. We found that the mRNA expression levels of CEC A, CEC B, and CEC C in DTMUV2.1-infected salivary glands were significantly higher than those in DTMUV1-infected salivary glands (Fig. 5C). Therefore, we selected CEC A, CEC B, and CEC C for further study.
Fig. 5.
Innate immune response to DTMUV clusters 1 and 2.1 in Cx tritaeniorhynchus. Transcription levels of thioester-containing protein-3 of Culex tritaeniorhynchus (TEP3) (A), macroglobulin complement-related factor of Cx. tritaeniorhynchus (MCR) (B), and antimicrobial peptides (AMPs) within the salivary glands of Cx. tritaeniorhynchus infected with DTMUV1 and DTMUV2.1 (C). The results are from an experiment with three replications, and the data are presented as the mean ± standard error (SEM). Differences between groups were analyzed using unpaired t-tests. Asterisks (∗) indicate statistically significant differences: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗∗P < 0.0001.
3.6. Macroglobulin complement-related factor and antimicrobial peptides directly influence DTMUV infection in Culex tritaeniorhynchus
We investigated whether TEP3, MCR, and AMPs (CEC A, CEC B, and CEC C) directly interacted with DTMUV infection by silencing their encoding genes in Cx. tritaeniorhynchus using dsRNA. Gene expression of TEP3, MCR, and individual AMPs was significantly decreased after dsRNA treatment (Supplementary Fig. S3). Compared to LacZ-treated control mosquitoes, DTMUV 1 and 2.1 replication tended to increase after silencing these innate immune factors. Particularly, a significant increase in viral load was observed in the salivary glands after individual silencing of MCR or simultaneous silencing of CEC A, CEC B, and CEC C, compared to LacZ-treated control mosquitoes (Fig. 6A–C). However, the individual silence of TEP3, CEC A, CEC B, or CEC C did not significantly affect DTMUV replication (Fig. 6A–C). Interestingly, we found that CEC A, CEC B, and CEC C were silenced in mosquitoes treated with dsMCR, similar to the effect observed with dsCECA + B + C treatment (Fig. 6B–D).
Fig. 6.
Effect of innate immune on DTMUV1 and DTMUV2.1 within the salivary glands of Cx. tritaeniorhynchus.A-B Transcription levels of DTMUV1 (A) and CEC A, B, and C (B) of the DTMUV1- infected mosquitoes. C-D Transcription levels of DTMUV2.1 (C) and CEC A, B, and C (D) of the DTMUV2.1-infected mosquitoes. The data are presented as the mean ± standard error (SEM). Differences between groups were analyzed using unpaired t-tests. Asterisks (∗) indicate statistically significant differences: ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001. Abbreviation: ns, non-significant.
4. Discussion
The phylogenetic tree constructed using NS5 nucleotide sequences demonstrated that the clustering of DTMUVs was not correlated with host species. Our analysis suggested that hosts within each lineage cluster were not limited to a single species. This indicated potential for cross-species transmission of DTMUVs, particularly from mosquitoes to various avian species. This observation aligns with previous research demonstrating the lack of a species barrier for TMUV among avian species, supported by evidence of direct contact transmission and phylogenetic analysis (Liu et al., 2024). Interestingly, the phylogenetic tree showed that DK/TH/CU-DTMUV2007 infecting Cx. tritaeniorhynchus saliva grouped into Cluster 1, while DK/TH/CU-1 grouped into Cluster 2.1. This finding underscores the importance of considering the potential impact of these DTMUV clusters on the mosquito vector.
Our study revealed the NS5 of DTMUV1 and DTMUV2.1 differ at amino acid positions spanning from 3020 to 3300. Furthermore, these amino acid changes also occur after mosquito infection. Orthoflavivirus NS5 is an RNA-dependent RNA polymerase that functions to catalyze the replication of the viral RNA within the replication complex (Klema et al., 2015; Hamel et al., 2021; Goh et al., 2024). Additionally, it is a crucial target for the development of innate immune and antiviral agents (Klema et al., 2015; Hamel et al., 2021; Goh et al., 2024). It plays a key role in effectively managing various RNA viruses such as those causing dengue, influenza, hepatitis, bovine viral diarrhea, etc. (Loddo et al., 2018; Pathania et al., 2022). In all viral regions examined, only the NS5 region of DTMUV1 and DTMUV2.1 in Cx. tritaeniorhynchus saliva undergoes a change in the amino acid composition at various sites. Our findings indicate that there are structural differences in NS5 among the genotypes of DTMUVs after infection in Cx. tritaeniorhynchus. Nonetheless, the molecular mechanisms underlying the differences in viral replication and pathogenicity between Cluster 1 and Cluster 2.1 DTMUVs within mosquito vectors remain unclear and require further investigation. At least our results collectively unveil evidence that NS5 may play a significant role in RNA-based DTMUV replication in Cx. tritaeniorhynchus.
The variation in pathogenicity between Cluster 1 and Cluster 2.1 DTMUVs may result from functional differences in the viral genome or proteins (Tunterak et al., 2023). Previous studies have demonstrated that even minor genomic variations in the NS5 gene of DTMUV can significantly influence viral pathogenicity and disease severity (Ninvilai et al., 2018; Sun et al., 2021). Furthermore, Domingo and Perales (2018) reported that the high mutation rates of RNA viruses are attributable to the inherent proneness of the error of NS5 (viral RdRP). A virus population is composed of a multitude of varying genomes known as quasispecies. Genetic mutations that alter the composition of quasispecies significantly impact viral replication, pathogenesis, and plaque morphology (Kato et al., 2017; Mandary et al., 2019). For instance, the plaques of DENV2 derived from infected mosquito saliva were significantly larger than those produced by culture-derived DENV2 (Cheang et al., 2021). Our observations revealed that both DTMUV1 and DTMUV2.1 derived from mosquito saliva retained their virulence and produced larger plaques compared to those originating from cell culture. This probably suggests that the process of DTMUV infection within the mosquito vector may alter the viral amino acid composition, thereby modifying its characteristics. Our findings underscore that virus replication and infection within the mosquito vector possibly enhance the virus’s virulence.
The present study is supposed to be a hypothesis-generating investigation for future research. Although we propose that amino acid differences in the NS5 region may contribute to the enhanced replication of DTMUV1, this interpretation remains speculative. Without information on full-genome sequencing, it is unclear whether the observed NS5 variations are primary drivers of these differences or merely coincide with other genomic alterations. There is currently no direct or indirect evidence to confirm that NS5 variations alone are responsible for the phenotypic differences observed. Additional studies are required to clarify the functional validation, such as site-directed mutagenesis or reverse genetics. Furthermore, although the larger plaque size of DTMUV1 in BHK-21 cells indicates enhanced cell-to-cell spread in vitro, it does not necessarily reflect increased transmissibility in vivo. Many factors can influence plaque size and cytopathogenic effect (CPE), including cell type and culture conditions. Peng et al. (2020) reported that TMUV-TP1906 infecting the DF-1 chicken fibroblast cell line produced a CPE, while no apparent CPE was observed in Vero- and C6/36 cells. Matrosovich et al. (2006) demonstrated that plaques formed under Avicel-containing media were significantly larger than those formed under agar or methylcellulose overlay media. Therefore, this finding alone does not fully account for the multifactorial nature of mosquito-to-host transmission dynamics. Therefore, researchers should consider these factors to enable more precise investigations in future studies.
Furthermore, our findings suggest that DTMUV1 has greater potential to be transmitted by Cx. tritaeniorhynchus than DTMUV2.1. In contrast, previous findings by Tunterak et al. (2023) indicated that DTMUV1 is less pathogenic and virulent than cluster DTMUV2.1 in 4-week-old ducks. However, their study introduced the virus to ducks via the intradermal route rather than through mosquito transmission. Compared to DTMUV2.1 (DK/TH/CU-1), DTMUV1 (DK/TH/CU-DTMUV2007) has a closer genetic relationship to the original Tembusu virus strains (MM1775 and Sitiawan2000), which were found in mosquitoes (Ninvilai et al., 2019). This may suggest that mosquitoes are the primary hosts of DTMUV1 and the main route of DTMUV1 transmission is through mosquito vectors, which is different from the main route of DTMUV2.1. Our observations imply that the virulence of each DTMUV cluster varies depending on the host of infection. This variation may potentially determine the specific responses of the innate immune system and antiviral agents in mosquito vectors. We compared the immune response and infectivity pattern of DTMUV1 in Cx. tritaeniorhynchus to those of DTMUV2.1. Mosquitoes individually infected with DTMUV1 demonstrated significantly lower efficiency in inducing innate immune products (MCR, TEP, and AMPs) compared to those infected with DTMUV2.1. This diminished immune response may contribute to the higher pathogenicity of DTMUV1 in mosquitoes relative to DTMUV2.1.
Interestingly, three AMPs (CECA, CECB, and CECC) were significantly decreased when MCR was silenced within Cx. tritaeniorhynchus; we hence speculate that these AMPs activities were likely associated with MCR. Corresponding to previous studies that revealed MCR of Ae. aegypti and Ae. albopictus plays a pivotal role in innate immunity by regulating the induction of antimicrobial peptides (Xiao et al., 2014; Sri-in et al., 2023). Our finding emphasized a similar role played by the MCR in Cx. tritaeniorhynchus which exerts antiviral activity against DTMUV infections in mosquito vectors. Our study underscores the significant potential of Cx. tritaeniorhynchus to DTMUV infection and offers valuable information on the innate immune responses within the mosquito vectors. However, we did not thoroughly investigate the downstream signaling pathways, so we cannot determine the specific mechanisms or processes influencing this phenomenon. The underlying mechanism by which DTMUV2.1, rather than DTMUV1, elicits a stronger innate immune response remains unclear. While we hypothesize that amino acid variations in the NS5 region may contribute to the differential immune responses observed between the two strains, this interpretation remains speculative and warrants further investigation. Further studies are required to gain deeper insights into these antiviral activities, including peptide-based treatments, overexpression assays, recognition of pathogen-associated molecular patterns (PAMPs), and viral immune evasion strategies.
5. Conclusions
To the best of our knowledge, this is the first study to compare the clustering of DTMUV in mosquitoes in terms of virulence and transmissibility. Our study revealed that the NS5 proteins of DTMUV1 and DTMUV2.1, derived from mosquito saliva, differ at specific amino acid positions; however, no differences were found in other regions. Furthermore, the RdRp structure is located within the residues of the NS5 region. Therefore, we focused on the NS5 gene to observe the differences in DTMUV infection among clusters within Cx. tritaeniorhynchus. In summary, our findings indicate that the antigenicity and infectivity of each DTMUV genotype are related to the innate immune response within Cx. tritaeniorhynchus. Specifically, the mosquito mounts a stronger innate immune response following infection with DTMUV2.1 compared to DTMUV1. Our data collectively suggests that DTMUV1 from infected Cx. tritaeniorhynchus is more infective and virulent than the corresponding DTMUV2.1. The results of this study underscore the significant potential of mosquito vectors to enhance the severity of viral infections and transmissions. This study highlights the susceptibility of Cx. tritaeniorhynchus to DTMUV infection, which has varying effects across different clusters. We sincerely hope that our study may provide valuable insights into the innate immune responses that may be critical for further impairing the virus within mosquito vectors.
CRediT authorship contribution statement
Chalida Sri-in: Methodology, Investigation, Writing – original draft. Duangduean Prakairungnamthip: Investigation, Writing – review & editing. Kanana Rungprasert: Investigation, Writing – review & editing. Aunyaratana Thontiravong: Methodology, Writing – review & editing. Lyric C. Bartholomay: Methodology, Writing – review & editing. Sonthaya Tiawsirisup: Supervision, Funding acquisition, Methodology, Writing – review & editing.
Ethical approval
The research plan for animal use was approved by the Institutional Animal Care and Use Committee at Chulalongkorn University (Bangkok, Thailand) under approval ID no. IACUC2331078. All procedures are described in the standard operating procedure of the institute. The person involved in animal work has completed animal care training. Furthermore, the protocols used in this study were approved by the Institutional Biosafety Committee under approval ID no. IBC2331026.
Funding
This work was supported by the Thailand Science Research and Innovation Fund and Chulalongkorn University (FOODFF67310023), the Chulalongkorn University Center of Excellence (CE68_053_3100_001), the Chulalongkorn University Second Century Fund, and the National Research Council of Thailand (NRCT) under the NRCT Senior Scholar Grant 2022 awarded to R. Thanawongnuwech (N42A650553).
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Dr Theerawat Tharasanit, Department of Obstetrics, Gynecology and Reproduction, Faculty of Veterinary Science, Chulalongkorn University, for generously providing glass needles.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crpvbd.2025.100274.
Appendix B. Supplementary data
The following is the Supplementary data to this article:
Data availability
The data supporting the conclusions of this article are included within the article and its supplementary files. Raw data generated in the study are available from the corresponding author upon request. The newly generated DNA sequences were submitted to the GenBank database under the accession numbers PP507050-PP507055.
References
- Arjona A., Wang P.H., Montgomery R.R., Fikrig E. Innate immune control of West Nile virus infection. Cell. Microbiol. 2011;13:1648–1658. doi: 10.1111/j.1462-5822.2011.01649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter R.H.G., Chang C.-I., Chelliah Y., Blandin S., Levashina E.A., Deisenhofer J. Structural basis for conserved complement factor-like function in the antimalarial protein TEP1. Proc. Natl. Acad. Sci. USA. 2007;104:11615–11620. doi: 10.1073/pnas.0704967104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandin S., Shiao S.H., Moita L.F., Janse C.J., Waters A.P., Kafatos F.C., Levashina E.A. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell. 2004;116:661–670. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- Cao Z., Zhang C., Liu Y., Ye W., Han J., Ma G., et al. Tembusu virus in ducks, China. Emerg. Infect. Dis. 2011;17:1873–1875. doi: 10.3201/eid1710.101890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheang K.W., Chen W.Y., Wu-Hsieh B.A., Shiao S.H. Infecting mosquitoes alters DENV-2 characteristics and enhances hemorrhage-induction potential in Stat1-/- mice. PLoS Negl. Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Cheng A., Wang M. Innate sensing of viruses by pattern recognition receptors in birds. Vet. Res. 2013;44:82. doi: 10.1186/1297-9716-44-82. http://www.veterinaryresearch.org/content/44/1/82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Wu Z., Wang M., Cheng A. Innate immune evasion mediated by Flaviviridae non-structural proteins. Viruses. 2017;9:291. doi: 10.3390/v9100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G., Liu L., Wang P., Zhang Y., Zhao Y.O., Colpitts T.M., et al. An in vivo transfection approach elucidates a role for Aedes aegypti thioester-containing proteins in flaviviral infection. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Wang R., Wu Q., Chen J., Wang A., Wu Z., et al. Advancements in research on duck Tembusu virus infections. Viruses. 2024;16:811. doi: 10.3390/v16050811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daidoji T., Morales Vargas R.E., Hagiwara K., Arai Y., Watanabe Y., Nishioka K., et al. Development of genus-specific universal primers for the detection of flaviviruses. Virol. J. 2021;18:187. doi: 10.1186/s12985-021-01646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A.D. New insights into flavivirus nonstructural protein 5. Adv. Virus Res. 2009;74:41–101. doi: 10.1016/S0065-3527(09)74002-3. [DOI] [PubMed] [Google Scholar]
- Domingo E., Perales C. Quasispecies and virus. Eur. Biophys. J. 2018;47:443–457. doi: 10.1007/s00249-018-1282-6. [DOI] [PubMed] [Google Scholar]
- Goh J.Z.H., De Hayr L., Khromykh A.A., Slonchak A. The Flavivirus non-structural Protein 5 (NS5): Structure, functions, and targeting for development of vaccines and therapeutics. Vaccines. 2024;12:865. doi: 10.3390/vaccines12080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Jiang T., Jiang Y., Zhao T., Li C., Dong Y., Xing D., Qin C. Potential vector competence of mosquitoes to transmit Baiyangdian virus, a new Tembusu-related virus in China. Vector Borne Zoonotic Dis. 2020;20:541–546. doi: 10.1089/vbz.2019.2523. [DOI] [PubMed] [Google Scholar]
- Hamel R., Phanitchat T., Wichit S., Morales Vargas R.E., Jaroenpool J., Diagne C.T., et al. New insights into the biology of the emerging Tembusu virus. Pathogens. 2021;10:1010. doi: 10.3390/pathogens10081010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel R., Vargas R.E.M., Rajonhson D.M., Yamanaka A., Jaroenpool J., Wichit S., et al. Identification of the Tembusu virus in mosquitoes in northern Thailand. Viruses. 2023;15:1447. doi: 10.3390/v15071447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homonnay Z.G., Kovács E.W., Bányai K., Albert M., Fehér E., Mató T., et al. Tembusu-like flavivirus (Perak virus) as the cause of neurological disease outbreaks in young Pekin ducks. Avian Pathol. 2014;43:552–560. doi: 10.1080/03079457.2014.973832. [DOI] [PubMed] [Google Scholar]
- Kato F., Tajima S., Nakayama E., Kawai Y., Taniguchi S., Shibasaki K. Characterization of large and small-plaque variants in the Zika virus clinical isolate ZIKV/Hu/S36/Chiba/2016. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-16475-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klema V.J., Padmanabhan R., Choi K.H. Flaviviral replication complex: Coordination between RNA synthesis and 5’-RNA capping. Viruses. 2015;7:4640–4656. doi: 10.3390/v7082837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear H.M., Diamond M.S. New insights into innate immune restriction of West Nile virus infection. Curr. Opin. Virol. 2015;11:1–6. doi: 10.1016/j.coviro.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.F., Voon G.Z., Lim H.X., Chua M.L., Poh C.L. Innate and adaptive immune evasion by dengue virus. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.1004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite D.R.B., Mantovani K.M., Cordeiro S.P., Maia F.B., Betim F.C.M., de Bona Sartor E., et al. RNA-dependent RNA polymerase (RdRp) natural antiviral inhibitors: A review. Med. Chem. Res. 2022;31:2089–2102. doi: 10.1007/s00044-022-02963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Shi Y., Liu Q., Wang Y., Li G., Teng Q., et al. Airborne transmission of a novel Tembusu virus in ducks. J. Clin. Microbiol. 2015;53:2734–2736. doi: 10.1128/JCM.00770-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Chen Y.Y., Twu N.C., Wu M.C., Fang Z.S., Dubruel A., et al. A novel goose-origin Tembusu virus exhibits pathogenicity in day-old chicks with evidence of direct contact transmission. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2023.103332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loddo R., Francesconi V., Laurini E., Boccardo S., Aulic S., Fermeglia M., et al. 9-aminoacridine-based agents impair the bovine viral diarrhea virus (BVDV) replication targeting the RNA-dependent RNA polymerase (RdRp) Bioorg. Med. Chem. 2018;26:855–868. doi: 10.1016/j.bmc.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Mandary M.B., Masomian M., Poh C.L. Impact of RNA virus evolution on quasispecies formation and virulence. Int. J. Mol. Sci. 2019;20:4657. doi: 10.3390/ijms20184657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrosovich M., Matrosovich T., Garten W., Klenk H.D. New low-viscosity overlay medium for viral plaque assays. Virol. J. 2006;3:63. doi: 10.1186/1743-422X-3-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng R., Yang B., Feng C., Huang J., Wang X., Zhang D. The difference in CD4+ T cell immunity between high- and low-virulence Tembusu viruses is mainly related to residues 151 and 304 in the envelope protein. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.890263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuti M.E., Siperstein A., Wolkoff M. Rearing and maintaining a Culex colony in the laboratory. Cold Spring Harb. Protoc. 2023;2023 doi: 10.1101/pdb.prot108080. [DOI] [PubMed] [Google Scholar]
- Morrison J., Aguirre S., Fernandez-Sesma A. Innate immunity evasion by Dengue virus. Viruses. 2012;4:397–413. doi: 10.3390/v4030397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninvilai P., Limcharoen B., Tunterak W., Prakairungnamthip D., Oraveerakul K., Banlunara W., Thontiravong A. Pathogenesis of Thai duck Tembusu virus in cherry valley ducks: The effect of age on susceptibility to infection. Vet. Microbiol. 2020;243 doi: 10.1016/j.vetmic.2020.108636. [DOI] [PubMed] [Google Scholar]
- Ninvilai P., Nonthabenjawan N., Limcharoen B., Tunterak W., Oraveerakul K., Banlunara W., et al. The presence of duck Tembusu virus in Thailand since 2007: A retrospective study. Transbound. Emerg. Dis. 2018;65:1208–1216. doi: 10.1111/tbed.12859. [DOI] [PubMed] [Google Scholar]
- Ninvilai P., Tunterak W., Oraveerakul K., Amonsin A., Thontiravong A. Genetic characterization of duck Tembusu virus in Thailand, 2015–2017: Identification of a novel cluster. Transbound. Emerg. Dis. 2019;66:1982–1992. doi: 10.1111/tbed.13230. [DOI] [PubMed] [Google Scholar]
- Pandey B.D., Karabatsos N., Cropp B., Tagaki M., Tsuda Y., Ichinose A., Igarashi A. Identification of a flavivirus isolated from mosquitos in Chiang Mai, Thailand. Southeast Asian J. Trop. Med. Public Health. 1999;30:161–165. [PubMed] [Google Scholar]
- Pathania S., Rawal R.K., Singh P.K. RdRp (RNA-dependent RNA polymerase): A key target providing anti-virals for the management of various viral diseases. J. Mol. Struct. 2022;1250 doi: 10.1016/j.molstruc.2021.131756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S.H., Su C.L., Chang M.C., Hu H.C., Yang S.L., Shu P.Y. Genome analysis of a novel Tembusu virus in Taiwan. Viruses. 2020;12:567. doi: 10.3390/v12050567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt G.S., Way H.J., Bowen E.T., Simpson D.I., Hill M.N., Kamath S., et al. Arbovirus infections in Sarawak, October 1968 - February 1970, Tembusu and Sindbis virus isolations from mosquitoes. Ann. Trop. Med. Parasitol. 1975;69:65–71. doi: 10.1080/00034983.1975.11686984. [DOI] [PubMed] [Google Scholar]
- Sanisuriwong J., Yurayart N., Thontiravong A., Tiawsirisup S. Duck Tembusu virus detection and characterization from mosquitoes in duck farms, Thailand. Transbound. Emerg. Dis. 2020;67:1082–1088. doi: 10.1111/tbed.13474. [DOI] [PubMed] [Google Scholar]
- Sikkeland L.I.B., Thorgersen E.B., Haug T., Mollnes T.E. Complement activation and cytokine response by BioProtein, a bacterial single cell protein. Clin. Exp. Immunol. 2007;148:146–152. doi: 10.1111/j.1365-2249.2007.03339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sri-in C., Thontiravong A., Bartholomay L.C., Tiawsirisup S. Effects of Aedes aegypti salivary protein on duck Tembusu virus replication and transmission in salivary glands. Acta Trop. 2022;228 doi: 10.1016/j.actatropica.2022.106310. [DOI] [PubMed] [Google Scholar]
- Sri-in C., Thontiravong A., Bartholomay L.C., Wechtaisong W., Thongmeesee K., Riana E., Tiawsirisup S. 34-kDa salivary protein enhances duck Tembusu virus infectivity in the salivary glands of Aedes albopictus by modulating the innate immune response. Sci. Rep. 2023;13:9098. doi: 10.1038/s41598-023-35914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sri-in C., Weng S.C., Shiao S.H., Tu W.C. A simplified method for blood-feeding, oral infection, and saliva collection of the Dengue vector mosquitoes. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Sun M., Zhang L., Yu Z., Li J., Xie W., Su J. Amino acid substitutions in NS5 contribute differentially to Tembusu virus attenuation in ducklings and cell cultures. Viruses. 2021;13:921. doi: 10.3390/v13050921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. Innate immunity to virus infection. Immunol. Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. Clustal W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thontiravong A., Ninvilai P., Tunterak W., Nonthabenjawan N., Chaiyavong S., Angkabkingkaew K., et al. Tembusu-related flavivirus in ducks, Thailand. Emerg. Infect. Dis. 2015;21:2164–2167. doi: 10.3201/eid2112.150600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunterak W., Rungprasert K., Wannaratana S., Yurayart N., Prakairungnamthip D., Ninvilai P., et al. Pathogenesis of cluster 1 duck Tembusu virus in ducks reveals the impact of viral genotype on pathogenicity and disease severity. Transbound. Emerg. Dis. 2023;2023 doi: 10.1155/2023/9239953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.H., Aliyari R., Li W.X., Li H.W., Kim K., Carthew R., et al. RNA interference directs innate immunity against viruses in adult Drosophila. Science. 2006;312:452–454. doi: 10.1126/science.1125694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng S.C., Zhou Y.X., Shiao S.H. A flavivirus-inducible gene expression system that modulates broad-spectrum antiviral activity against dengue and Zika viruses. Insect Biochem. Mol. Biol. 2022;142 doi: 10.1016/j.ibmb.2022.103723. [DOI] [PubMed] [Google Scholar]
- Xiao X., Liu Y., Zhang X., Wang J., Li Z., Pang X., Wang P. Complement-related proteins control the flavivirus infection of Aedes aegypti by inducing antimicrobial peptides. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Yan P., Zhou J., Teng Q., Li Z. Establishing a TaqMan-based real-time PCR assay for the rapid detection and quantification of the newly emerged duck Tembusu virus. Virol. J. 2011;8:464. doi: 10.1186/1743-422X-8-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Ren H., Sun M., Xie W., Sun S., Liang N., et al. Tembusu virus infection in laying chickens: Evidence for a distinct genetic cluster with significant antigenic variation. Transbound. Emerg. Dis. 2022;69:e1130–e1141. doi: 10.1111/tbed.14402. [DOI] [PubMed] [Google Scholar]
- Yun T., Ye W., Ni Z., Zhang D., Zhang C. Identification and molecular characterization of a novel flavivirus isolated from Pekin ducklings in China. Vet. Microbiol. 2012;157:311–319. doi: 10.1016/j.vetmic.2012.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the conclusions of this article are included within the article and its supplementary files. Raw data generated in the study are available from the corresponding author upon request. The newly generated DNA sequences were submitted to the GenBank database under the accession numbers PP507050-PP507055.