Abstract
This study investigates the effects of 3-indoleacrylic acid (IAA), a bacterial metabolite of tryptophan, on LPS-induced liver injury in laying hens. The aim is to explore the potential hepatoprotective mechanisms of IAA, particularly through the modulation of inflammatory and antioxidant pathways. A total of 48, 24-week-old No. 6 Jingfen laying hens were randomly divided into four groups of 12 hens each: the CON group (basal diet), IAA group (basal diet + 150 mg/kg IAA), LPS group (basal diet), and IAA+LPS group (basal diet + 150 mg/kg IAA). The feeding period lasted 8 weeks, and at the end of the 8th week, the laying hens in the LPS and IAA+LPS groups were intraperitoneally injected with 0.5 mg/kg LPS. The four groups were uniformly slaughtered and sampled 12 h after LPS injection. The expression of pro-inflammatory cytokines (IL-1β, TNF-α, IL-6) was significantly alleviated (P < 0.05), and antioxidant enzymes (T-SOD, CAT, GSH-PX) were significantly elevated (P < 0.05) in the serum and liver of the IAA+LPS group. Histological analysis revealed reduced hepatocyte necrosis and inflammation in the IAA-treated groups (P < 0.05). Additionally, the activation of MAPK and Toll-like receptor (TLR) signaling pathways was modulated through transcript analyses, thereby reducing inflammation and oxidative stress. In summary, IAA effectively mitigates liver inflammation and oxidative stress through the regulation of MAPK and TLR signaling pathways, providing a promising strategy for improving liver health in hens. This study represents the first report on the application of IAA in poultry, demonstrating its potential as a novel therapeutic agent for alleviating liver injury.
Keywords: 3-Indoleacrylic acid, Liver inflammatory damage, Oxidative stress, MAPK signaling pathway, Lay hens
Introduction
Similar to mammals, the liver in birds performs a wide range of metabolic and homeostatic functions. It is regarded as a biochemical factory responsible for most synthesis, metabolism, excretion, and detoxification processes. The liver is crucial for digestion and metabolism, overseeing the production, storage, and release of lipids, carbohydrates, and proteins (Hamid et al., 2019; Zaefarian et al., 2019). Efficient liver function ensures optimal synthesis of proteins, lipids, and carbohydrates, which are essential for the formation of egg components such as albumen, yolk, and shell. According to statistics, approximately 30 % to 40 % of diseases in layer chicken farming are closely related to abnormal liver function (Hu et al., 2022). Thus, liver health directly impacts egg production, growth, and the overall well-being of the birds. However, liver health is often compromised due to various factors, such as overnutrition (high-energy diets), nutritional deficiencies (e.g., lack of choline, vitamin E, or selenium), bacterial infections (e.g., Escherichia coli), leading to reduced egg production, poor egg quality, decreased feed efficiency, oxidative stress, liver damage, and increased mortality rates (Emami et al., 2020; Fouad and El-Senousey, 2014; Geng et al., 2018; Zhang et al., 2025; Zhou et al., 2025). The liver synthesizes a diverse array of proteins, including blood proteins, enzymes, hormones, clotting factors, and immune-related molecules. Studies have shown that after liver cell damage, inflammatory factors such as TNF-α and IL-1β are released. This inflammatory response further activates immune cells and hepatic stellate cells, leading to additional liver cell damage and death (Kubes and Mehal, 2012; Xu et al., 2019). Therefore, protecting liver health is of great importance for maintaining layer production. Previous studies have shown that tryptophan deficiency affects intestinal immunity in mice and leads to an imbalance in the intestinal microbiota (Gao et al., 2018; Hashimoto et al., 2012; Taleb, 2019). Some studies have also demonstrated that tryptophan is transported to intestinal epithelial cells via transporters and then reaches the liver through enterohepatic circulation, thus influencing liver function and metabolism (Shao et al., 2012; Tsuji et al., 2023). Therefore, it is widely believed that tryptophan and its metabolites play crucial roles in organ function and metabolism.
Tryptophan (Trp), an essential amino acid for protein synthesis, also serves as a precursor for key metabolites that support the metabolism and physiological functions of laying hens. It plays a crucial role in hormone regulation, immune organ development, and improving egg production and quality, especially under controlled or stressed conditions (Zhong et al., 2024). Tryptophan is metabolized by intestinal bacteria, producing a variety of indole compounds (Agus et al., 2018; Gasaly et al., 2021). Microbiota-derived metabolites are essential intermediaries in the interactions between the host and microorganisms, playing significant roles in disease development. Tryptophan undergoes multiple metabolic pathways, resulting in several indole-derived metabolites, including indole, tryptamine, indole ethanol, indole-3-propionic acid (IPA), indole-3-acetic acid, indole-3-lactic acid, skatole, indole aldehyde, and 3-indoleacrylic acid (IAA) (Min et al., 2024). In recent years, the potential applications of 3-indoleacrylic acid in animal health and disease prevention have garnered significant attention (Amarasiri et al., 2025). 3-indoleacrylic acid has been reported to promote intestinal epithelial barrier function and suppress inflammatory responses, and increasing the production of IAA by restoring tryptophan metabolism in the intestine could have a therapeutic effect in IBD patients by enhancing anti-inflammatory responses (Wlodarska et al., 2017). Recent studies have demonstrated that indole metabolites are effective in providing hepatic protection. Notably, emerging research has linked IAA to pro-inflammatory pathways and reactive oxygen species (ROS) production (Effiong et al., 2022). For instance, a multi-omics investigation revealed that Porphyromonas gingivalis-mediated gut-kidney axis dysfunction involves IAA-driven ROS generation and renal inflammatory responses (Dong et al., 2025). Additionally, gut microbiome-derived IAA has been shown to enhance ferroptosis resistance in colorectal cancer by inducing oxidative stress and inflammatory signaling (Zhang et al., 2024a). These findings highlight the potential of IAA as a key mediator of inflammatory and redox imbalance pathways, motivating further investigation into its role in inflammatory diseases. However, despite its growing relevance in other fields, research on IAA in the context of laying hens remains limited and warrants further exploration.
This study aimed to investigate the impact of IAA on mitigating LPS-induced liver damage and systemic inflammation in laying hens, exploring its protective effects. We performed transcriptome sequencing, serum and liver biochemical analysis, and histological examination of liver tissue from IAA-treated hens to assess its impact on host metabolism and anti-inflammatory functions in the liver. The findings enhance our understanding of IAA's protective effects on liver health.
Materials and methods
Ethics statement
All animal experiments were conducted with approval from the Laboratory Animal Welfare and Ethical Review Committee of China Agricultural University, Beijing, China, under permit number AW21213202-4.
Chemicals
The 3-indoleacrylic acid (cis-IAA, purity≥98 %, Cat: 1204-06-4) product was provided by Shanghai Aladdin Biochemical Technology Co., Ltd. The LPS (Escherichia coli O55:B5) was provided by Sigma-Aldrich Life Science & Technology Co., Ltd.
Experimental design and diets
Forty-eight 24-week-old Jingfen No.6 laying hens were randomly divided into 4 groups with 6 replicates per group and 2 hens per replicate. They were CON group (basal diet), IAA group (basal diet+150 mg/kg IAA), LPS group (basal diet), IAA+LPS group (basal diet+150 mg/kg IAA). The feeding period lasted for 8 weeks, and the experimental chickens were housed in a controlled condition with ad libitum, 25±2°C, 50 ± 10 % humidity, and 16 h light/8 h dark with a dark cycle respectively in Zhuozhou breeding base of China Agricultural University (Zhuozhou, China). The basal diet was formulated to meet or slightly exceed Chinese Feeding Standard of Chicken (NY/T 33-2004) and National Research Council (NRC, 1994) requirements (Table S3). At the end of the feeding period of the eighth week, the LPS group and IAA+LPS group were challenged with LPS at a dose of 0.5 mg/kg body weight. The LPS dose (0.5 mg/kg BW) was selected based on established protocols for inducing acute inflammation in laying hens. All laying hens were slaughtered after 12 h challenge, and the serum and liver tissues were collected and stored at -80°C for further analysis.
Serum and liver biochemistry analysis
At the end of the experiment, 12 hens from each treatment group were sacrificed and consequently the blood samples from the jugular vein were collected into 10 mL tubes, left to stand for 2–3 h, and then centrifuged at 3000 r/min for 15 min to collect the serum, which was stored at -80 °C. The concentrations of inflammatory cytokines in the serum and liver, including interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-a (TNF-α) were measured using ELISA kits, following the protocols provided by the Nanjing Jiancheng Biology Engineering Institute (Nanjing, China). Additionally, the levels of oxidative stress markers in the serum and liver, such as catalase (CAT), total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-PX), total antioxidant capacity (T-AOC) and malondialdehyde (MDA), were also quantified using the commercial assay kits and same method. Finally, the contents of alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) in the serum were measured and automated biochemical analyzer.
Hematoxylin-eosin staining
Liver tissues from hens were collected, fixed in 4 % paraformaldehyde, and embedded in paraffin. Sections of 5 mm thickness were prepared and stained with Hematoxylin and eosin following the manufacturer's protocol. The stained sections were then imaged and analyzed using Case Viewer 2.4 software (3DHISTECH Ltd., Budapest, Hungary).
Transmission electron microscopy
Liver tissues are collected and cut into 1 mm³ pieces, then fixed in 2.5 % glutaraldehyde for at least 6 h, followed by storage at 4 °C. The samples are washed with 0.1 M phosphate-buffered saline (PBS) four times, each for 10 min, and then dehydrated using graded ethanol solutions (50 %, 70 %, and 90 %) for 15 min each, followed by 90 % acetone for 20 min. The tissues are then infiltrated with a mixture of acetone and resin (1:1, 1:2, 1:3) and finally embedded in resin overnight at room temperature. The embedded samples are polymerized at 60 °C for 48 h. Ultra-thin sections (50–70 nm) are cut using an ultramicrotome and stained with heavy metals (uranyl acetate and lead citrate) to enhance contrast. The sections are observed under a transmission electron microscope, and images are captured using the microscope's imaging system. The images are analyzed using specialized software (Case Viewer 2.4) to evaluate the ultrastructural changes in liver cells (Wuhan Servicebio Technology Co., Ltd., China).
Immunofluorescence analysis
Liver samples are collected and fixed in 4 % formaldehyde in PBS for at least 24 h at 4 °C, then embedded in paraffin and sectioned into 5 µm thick slices. Sections are deparaffinized and rehydrated through graded ethanol solutions, followed by optional antigen retrieval using EDTA buffer (pH 9.0) for 15 min. After permeabilization with 1 % Triton X-100 in PBS for 15 min, sections are blocked with 10 % normal serum in PBS for 1 h. The primary antibody against TGR5 (GPBAR1) is applied and incubated at room temperature for 2 h or at 4 °C overnight. Sections are then washed with PBS and incubated with a fluorophore-conjugated secondary antibody for 1 h in the dark. After final washing, sections are mounted with a coverslip using Fluoromount-G or Fluoro-Gel and observed under a fluorescence microscope (Wuhan Servicebio Technology Co., Ltd., China).
TUNEL analysis
Liver tissue samples are collected and fixed in 4 % paraformaldehyde for at least 24 h at 4 °C, then embedded in paraffin and sectioned into 5 µm thick slices. Sections are deparaffinized and rehydrated through graded ethanol solutions, followed by antigen retrieval using EDTA buffer (pH 9.0) in a pressure cooker for 15 min. After blocking with 10 % BSA in PBS for 1 h, the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) reaction mixture is applied and incubated at 37 °C for 60 min. Sections are washed with PBS, optionally counterstained with DAPI, and mounted with antifade medium. Fluorescence microscopy is used to capture images, and TUNEL-positive cells are quantified to determine the percentage of apoptotic cells (Wuhan Servicebio Technology Co., Ltd., China).
Transcriptome analysis
Extraction of total RNA from liver samples and determination of the concentration and purity of the extracted RNA was performed using Nanodrop 2000. RNA-Seq was performed by Shanghai Majorbio Technology Co., Ltd. The Illumina Novaseq 6000 platform was used to construct RNA libraries and generate reads of 300 bp long paired-end (Illumina, San Diego, CA, USA). The read number of each gene was transformed into FPKM (fragments per kilobase of exon model per million mapped reads). After quality control of the raw data, the clean data were compared with the house reference genome using TopHat software to obtain mapped data, which were used for transcript assembly and expression calculation. The expression levels of genes and transcripts were quantified using RSEM (http://deweylab.github.io/RSEM/). After obtaining read counts of genes, differentially expressed genes (DEGs) were identified using the DESeq2 (V1.24.0) package68, and the identification criteria was P adjust <0.05 and |log2FC|≥ 1. Gene Ontology (GO) enrichment analysis was performed to categorize DEGs into biological processes, molecular functions, and cellular components using tools like Cluster Profiler. KEGG pathway enrichment was conducted to identify significantly altered pathways among DEGs. This helps in understanding the biological processes and signaling pathways affected in the liver.
RNA extraction and real-time quantitative PCR
Total RNA samples were obtained from the livers using the FastPure cell/Tissue Total RNA Isolation Kit V2 (Vazyme Biotech Co., Ltd., Nanjing, China) and were then reverse-transcribed into cDNA using the HiScript II O RT SuperMix for qPCR Kit (R223-01Vazyme Biotech Co, Ltd.) with the gDNA wiper Kit (Takara, Beijing, China), in accordance with the manufacturer's instructions. The mRNA expression levels of the antioxidant genes and inflammation genes (IL-1β,TNF-α,IL-6,T-SOD,CAT,GSH-PX) in the livers were evaluated. The mRNA expression was assessed using a commercially available kit (Takara). The qPCR protocol comprised denaturation at 95°C for 1 min, followed by 40 cycles of 15 s at 95 °C and 15 s at 60 °C. The house keeping gene β-actin was used as the internal reference gene. The primer sequences are shown in Table S1.
Statistical analysis
All data are presented as mean ± standard deviation. Differences between the two groups were evaluated using a two-tailed unpaired Student’s t-test. Multiple comparisons were performed using Duncan's method. Data visualization and statistical analyses were performed using GraphPad Prism 9.5. *P < 0.05, **P < 0.01, *** P < 0.001.
Results
Suppression of 3-indoleacrylic acid on LPS-induced inflammation and oxidative stress in the serum of laying hens
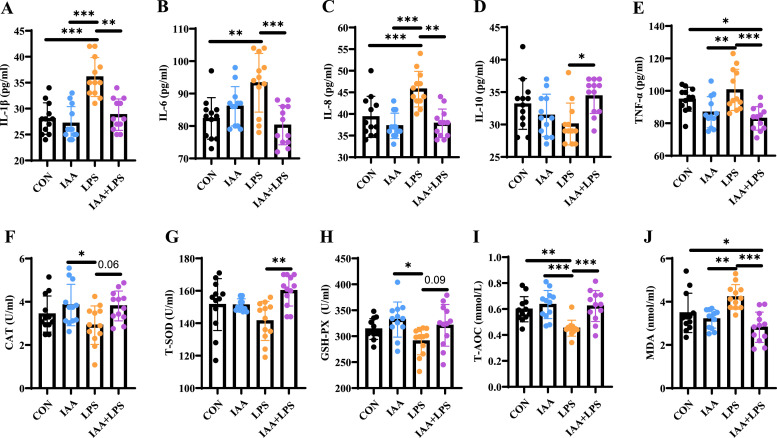
To determine the effects of IAA on host inflammation induced by LPS, the levels of inflammatory cytokines in the serum were detected. The pro-inflammatory cytokines IL-1β, IL-6, IL-8, and TNF-α were significantly increased in the serum of the LPS group (P < 0.05) compared to the CON group. However, IAA administration reduced the levels of pro-inflammatory cytokines compared with those of the LPS group (Fig. 1 A–C, E). In contrast, anti-inflammatory cytokines IL-10 levels were elevated in the IAA+LPS group, indicating an improved inflammatory state (Fig. 1 D). Consistently, the antioxidant markers CAT, T-SOD, GSH-PX, and T-AOC were significantly increased in the serum of the IAA+LPS group after LPS challenge (Fig. 1 F–I). Moreover, the level of MDA in the IAA+LPS hens were notably inhibited (P < 0.05) compared to the LPS group (Fig. 1 J). These results suggested that IAA contributes to alleviating the host inflammatory damage and oxidative stress induced by LPS.
Fig. 1.
Effects of 3-indoleacrylic acid on inflammatory cytokines and oxidative stress in a lipopolysaccharide-induced liver injury model in laying hens. Serum biochemical indices. (A) IL-1β (B) IL-6 (C) IL-8 (D) IL-10 (E) TNF-α (F) CAT (G) T-SOD (H) GSH-PX (I) T-AOC (J) MDA. All data are presented as mean ± standard deviation. *P < 0.05, **P < 0.01, *** P < 0.001.
Suppression of 3-indoleacrylic acid on LPS-induced inflammation and oxidative stress in the liver of laying hens
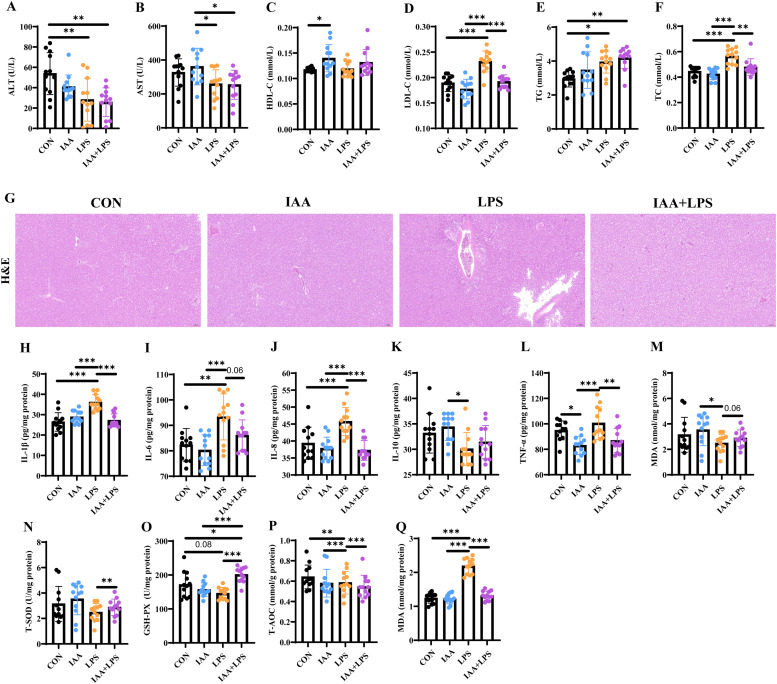
The results of serum biochemical analysis showed that compared with the CON group, LPS treatment significantly increased the levels of TG, TC, and LDL-C in serum (P < 0.05), while IAA pre-treatment significantly decreased these levels after LPS treatment (P < 0.05) (Fig. 2 D–F). However, the concentrations of ALT and AST were both significantly lower (P < 0.05) in LPS group (Fig. 2A, B), which was inconsistently with previous results. LPS and IAA treatment had no significant effect on serum HDL-C levels (P > 0.05) (Fig. 2 C). As shown in Fig. 2 G, H&E analysis showed that more fat vacuoles and lipid droplets appeared in the LPS treatment group than in the other groups indicating that inflammation in the liver was induced by LPS, while this phenomenon was alleviated in the IAA treatment group. Correspondingly, the levels of IL-1β, IL-6, IL-8, TNF-α and MDA in the liver were significantly higher (P < 0.05) in the LPS group than CON group (Fig. 2H–J, L, Q). However, these increases were significantly reduced (P < 0.05) in the liver under IAA administration. Moreover, the levels of IL-10, CAT, T-SOD, GSH-PX, and T-AOC was significantly increased in the liver (Fig. 2L, M–P). These results indicated that LPS could induce oxidative stress in the liver, and the addition of IAA could alleviate this injury.
Fig. 2.
Effects of 3-indoleacrylic acid on liver morphology and inflammation and antioxidant indexes in a lipopolysaccharide-induced liver injury model in laying hens. (A) ALT in serum. (B) AST in serum (C) HDL-C in serum (D) LDL-C in serum (E) TG in serum (F) TC in serum (G) Pathological histological sections stained with Hematoxylin and Eosin (HE) of the liver. (H) IL-1β in liver (I) IL-6 in liver (J) IL-8 in liver (K) IL-10 in liver (L) TNF-α in liver (M) CAT in liver (N) T-SOD in liver (O) GSH-PX in liver (P) T-AOC in liver (Q) MDA in liver. All data are presented as mean ± standard deviation. *P < 0.05, **P < 0.01, *** P < 0.001.
Liver transcriptome expression profiles induced by 3-indoleacrylic acid treatment in laying hens
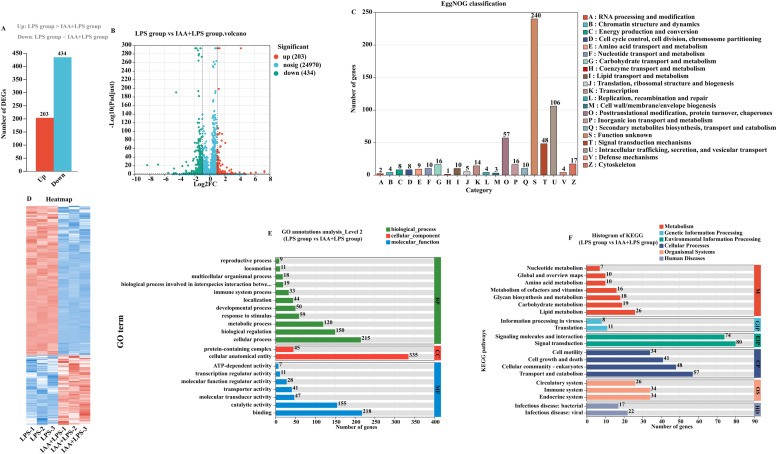
To characterize the protective immune mechanism of IAA, RNA-seq was conducted on the liver tissue of hens. Compared with the LPS group, a total of 637 differentially expressed genes were obtained in the IAA+LPS group, of which 203 differentially expressed genes were significantly upregulated and 434 differentially expressed genes were significantly downregulated (Fig. 3 A, B). To further elucidate the functions of the differentially expressed genes, we annotated the DEGs in liver tissues by EggNOG analysis, GO annotations analysis and KEGG analysis. Comparison with the EggNOG database showed that the function of EggNOG-enriched genes in the IAA+LPS group was mainly involved in Posttranslational modification, protein turnover, chaperones, Amino acid transport and metabolism, Secondary metabolites biosynthesis, transport and catabolism, and Signal transduction mechanisms (Fig. 3C). The GO analysis of DEGs primarily focused on cellular component and biological process, including cellular anatomical entity and protein-containing complex, binding, catalytic activity, molecular transducer activity, and transporter activity (Fig. 3E). KEGG results revealed that DEGs were mainly related to Signal transduction, Signaling molecules and interaction, Transport and catabolism and Lipid metabolism (Fig. 3F). Specific genes associated with lipid pathways were extracted from the DEG analysis. Four upregulated DEGs (FABP6, PLD5, ATP5L and PLA2G3) and six downregulated DEGs (PLA2G4A, TMEM68, SLC16A6, ACSL4, OXCT1 and ACSS1A) were found to be related to lipid metabolism (Table.S2).
Fig. 3.
Transcriptomic analysis of differentially expressed genes and functional enrichment based on the comparison between the LPS and IAA+LPS groups (the IAA+LPS group as the test group and the LPS group as the control). (A) Number of differentially expressed genes (DEGs). (B) Volcano plot analysis of DEGs between LPS and IAA+LPS groups. (C) EggNOG classification of DEGs. (D) Heatmap of DEGs. (E) GO classification enrichment analysis of DEGs. (F) KEGG pathway enrichment analysis of DEGs.
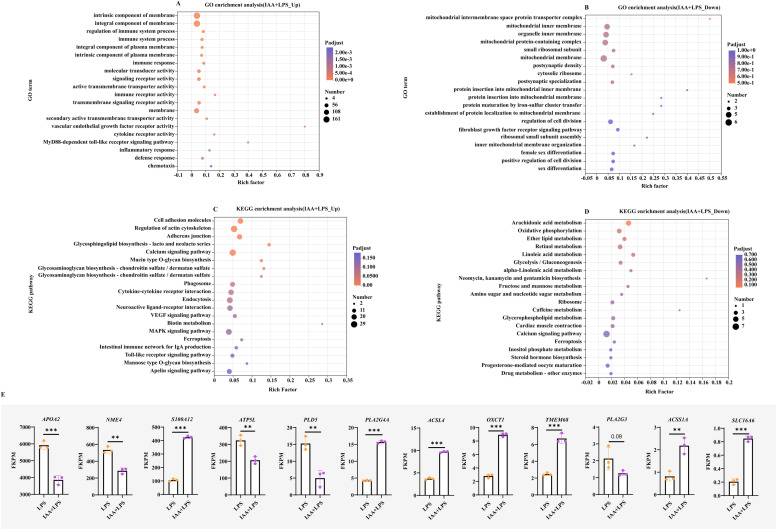
Next, in this study, pathway enrichment analysis was performed using both GO and KEGG. As shown in Fig. 4A, the GO enrichment results indicated that the downregulated DEGs were associated with intrinsic component of the membrane, the integral component of the membrane, the regulation of immune system process, immune system process, and signaling receptor activity (Fig. 4A). The functions of upregulated DEGs genes were mainly concentrated in the mitochondrial intermembrane spatial protein transporter complex, the mitochondrial inner membrane and organelle inner membrane, and the mitochondrial protein-containing complex (Fig. 4B). The KEGG enrichment results showed that the downregulated DEGs were mainly involved in cell adhesion molecules, actin cytoskeleton regulation, VEGF signaling pathway, calcium signaling pathway, Toll-like receptor signaling pathway and MAPK signaling pathway (Fig. 4C). The upregulated DEGs were mainly enriched in arachidonic acid metabolism, oxidative phosphorylation, and ether lipid metabolism (Fig. 4D). In addition, we focused on specific genes in these signaling pathways. Notably, the PLA2G4A gene was enriched in VEGF and AMPK signaling pathways. The levels of gene APOA2, NME4, ATP5L, PLD5 and PLA2G3 were significantly lower in the IAA+LPS group than those in LPS. In contrast, the levels of gene S100A12, PLA2G4A, ACSL4, OXCT1, TMEM68, ACSS1A, and SLC16A6 were significantly higher in the IAA+LPS group than those in LPS (Fig. 4E).
Fig. 4.
Transcriptomic analysis reveals the regulatory mechanisms based on the comparison between the LPS and IAA+LPS groups (the IAA+LPS group as the test group and the LPS group as the control). (A) GO enrichment analysis of genes downregulated by IAA. (B) GO enrichment analysis of genes upregulated by IAA. (C) KEGG pathway enrichment analysis of genes downregulated by IAA. (D) KEGG pathway enrichment analysis of genes upregulated by IAA. (E) Expression levels of DEGs between LPS and IAA groups. All data are presented as mean ± standard deviation. *P < 0.05, **P < 0.01, *** P < 0.001.
Suppression of 3-indoleacrylic acid on LPS-induced mitochondrial changes and apoptosis
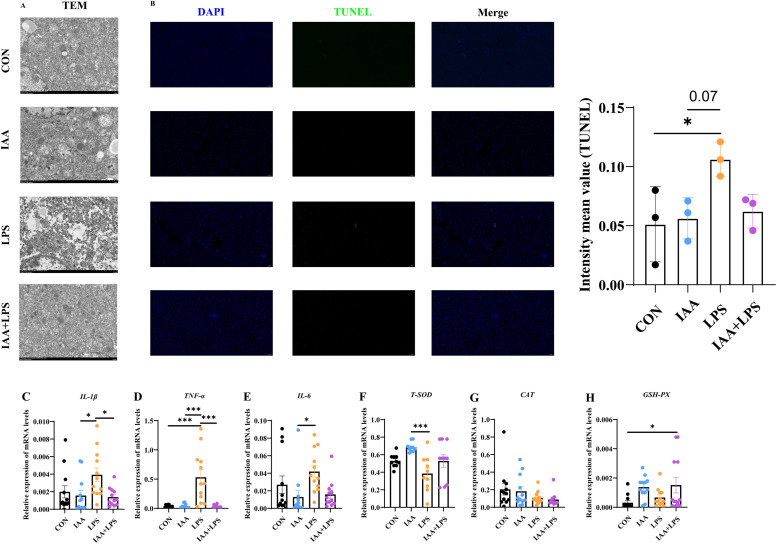
In this study, the effects of LPS treatment on mitochondrial morphology in liver tissue were investigated using transmission electron microscopy (Gultemirian et al.). It was found that in the control group, mitochondria were normal and well-arranged, with distinct membranes and normal vacuoles. In contrast, after LPS treatment, the cristae membranes of mitochondria in hepatocytes were reduced and disordered, with increased cristae density. The inner and outer membranes of mitochondria were disrupted, and most mitochondria exhibited condensation. Additionally, larger vacuoles were observed (Fig. 5A). This study demonstrated that LPS can induce significant morphological changes in the mitochondria of liver cells. The TUNEL analysis was employed to assess the occurrence of apoptosis in liver cells among the four treatment groups. In comparison to the control group, the LPS group exhibited a significant increase in TUNEL-positive cells within liver tissue, while the IAA+LPS group demonstrated a notable reduction compared to the LPS group. No significant difference was observed between the IAA group and IAA+LPS group (Fig. 5B). These findings suggested that administration of LPS at this dosage can induce hepatocyte apoptosis, whereas IAA effectively mitigates LPS-induced hepatocyte apoptosis in laying hens.
Fig. 5.
TEM and TUNEL analysis of liver and Relative mRNA expression of genes in laying hens. (A) Transmission electron microscopy (Gultemirian et al.) analysis of liver tissue. (B) TUNEL staining analysis of liver tissue. Relative mRNA expression levels of (C) IL-1β, (D) TNF-α, (E) IL-6, (F) T-SOD, (G) CAT, (H) GSH-PX in liver. All data are presented as mean ± standard deviation. *P < 0.05, **P < 0.01, *** P < 0.001.
Suppression of 3-indoleacrylic acid on LPS-induced changes in the expression of inflammatory cytokines and antioxidant enzymes
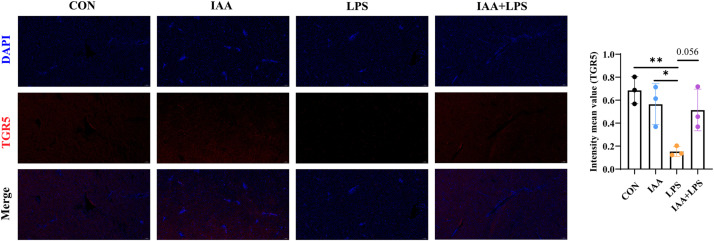
Next, RNA expression levels in liver were further determined by RT-qPCR analysis. The gene expression of IL-1β, TNF-α, and IL-6 were significantly upregulated (P < 0.05) in the LPS group compared with CON group (Fig. 5C–E). Additionally, the expression of T-SOD was significantly decreased (P < 0.05) due to LPS challenge, while IAA supplementation decreased the expression of the inflammatory genes and improved antioxidative ability in LPS-challenged laying hens (Fig. 5F, H). However, no significant (P > 0.05) changes were observed in the gene expression of CAT between the LPS and IAA + LPS groups (Fig. 5G). These results indicated that IAA supplementation reduced inflammatory and increased antioxidant-related gene expression in LPS-challenged laying hens. As shown in Fig. 6, the immunofluorescence intensity of TGR5 in the LPS group was significantly lower than that in the other groups, while the immunofluorescence intensity of TGR5 was significantly increased in the IAA treatment group (P < 0.05).
Fig. 6.
Immunofluorescence analysis of TGR5 protein expression in liver tissue. All data are presented as mean ± standard deviation. *P < 0.05, **P < 0.01.
Discussion
The liver is a crucial metabolic organ in the body, and disorders in hepatic lipid metabolism, hepatic inflammatory responses, and liver oxidative stress are closely linked to the onset and progression of liver damage (Scott and Billiar, 2008; Shao et al., 2012; Tsuji et al., 2023). Endotoxins, particularly lipopolysaccharide (LPS), are significant contributors to liver damage, with LPS playing a critical role in this process (Bender et al., 2023). As a result, LPS is commonly used in experimental studies of bacterial infections in livestock and poultry (French et al., 2020; González-Fernández et al., 2021; Liu et al., 2025). However, due to the implementation of policies that ban or restrict antibiotic use, research into alternative strategies has intensified. Tryptophan and its derivatives have been shown to regulate lipid metabolism in the liver (Smith et al., 2016). Elevated levels of indoles in the intestine can inhibit lipogenesis, suppress appetite, and reduce fat absorption and lipid accumulation in the liver (Macia et al., 2015). Despite these findings, research on IAA remains limited. The results of this study demonstrate that IAA can mitigate LPS-induced hepatic injury by modulating the MAPK and Toll-like receptor signaling pathways. Specifically, IAA effectively suppresses proinflammatory and oxidative markers while enhancing the expression of TGR5 in the liver.
Hepatocellular damage and subsequent disruption of liver function are typically accompanied by an elevation in serum levels of certain enzymes. ALT and AST, key transaminases synthesized by the liver, are released into the bloodstream when liver cells undergo necrosis or increased membrane permeability due to hepatic injury (Abo Ghanima et al., 2023; Ma et al., 2022; Rehman et al., 2018). Thus, ALT and AST levels serve as reliable biomarkers for assessing liver damage. However, in this study, we found that the levels of ALT and AST in the LPS group were not significantly increased, which is inconsistent with previous studies. This lack of increase may be due to factors such as the modeling method, dosage, type of liver injury, or individual differences among experimental animals. Although ALT and AST are commonly used biomarkers, studies suggest that relying on a single marker may not fully reflect the complexity of liver damage. Therefore, combining other markers, such as bile acids and γ-glutamyl transferase, may provide a more accurate assessment of liver injury. Previous studies have shown that LPS stimulates immune cells to produce inflammatory factors like IL-1β, IL-6, and TNF-α, which lead to excessive immune activation and inflammatory responses (Peng and Jiang, 2014). These pro-inflammatory factors interact with proteins in inflammatory signaling pathways, modulating downstream transcription and causing a sustained inflammatory response, cytokine dysregulation, immune deficiency, and impaired liver function (Takaki et al., 2014; Yuan et al., 2012; Zhong et al., 2016). In our study, LPS significantly increased the levels of IL-1β, IL-6, IL-8, and TNF-α in both serum and liver, consistent with previous research (Peng and Jiang, 2014). IAA pretreatment significantly reduced these inflammatory factors compared to the LPS group, suggesting that IAA has an anti-inflammatory role in LPS-induced liver injury. Moreover, gene expression analysis in liver tissue revealed that LPS significantly upregulated IL-1β, IL-6, and TNF-α, further confirming our results.
Oxidative stress is a key marker of liver damage, contributing to both chronic liver diseases, such as non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (Yamada et al., 2019), as well as acute liver injury (Saeidnia and Abdollahi, 2013). Additionally, oxidative stress is closely linked to inflammatory factors. MDA, a byproduct of lipid peroxidation, accumulates excessively, inducing hepatocyte damage and necrosis, which triggers the release of inflammatory factors that further exacerbate oxidative stress and worsen liver damage. Conversely, antioxidants like CAT, GSH-PX, and SOD alleviate this damage by scavenging ROS and neutralizing free radicals, thus protecting tissues from oxidative injury (Shan and Miao, 2022; Yang et al., 2025; Yu et al., 2022). In our study, intraperitoneal injection of LPS significantly elevated MDA levels, a marker of lipid peroxidation, indicating that LPS induced severe liver damage via lipid peroxidation. At the same time, we observed a substantial decrease in GSH-PX, T-SOD, CAT, and T-AOC levels, consistent with previous research (Jiang et al., 2019). Notably, IAA treatment mitigated these effects, suggesting that its hepatoprotective action is linked to its antioxidant properties and ability to quench ROS-induced inflammatory responses. Moreover, gene expression analysis revealed that IAA significantly upregulated SOD expression in the liver, supporting our findings. Apoptosis is a critical pathway in LPS-induced liver injury, driven by inflammation and oxidative stress. TUNEL staining showed a significant increase in apoptosis signals (marked by green fluorescence) in the LPS group, indicating that both inflammation and oxidative stress contribute to hepatocyte apoptosis. This finding aligns with previous studies showing that LPS induces apoptosis through caspase activation and the mitochondrial pathway (Chen and Nuñez, 2010; Koyama and Brenner, 2017). However, IAA treatment significantly reduced apoptosis signals, suggesting its protective role in preventing LPS-induced hepatocyte apoptosis. This protective effect is likely due to IAA's combined anti-inflammatory and antioxidant properties, which reduce cellular stress and mitigate apoptosis.
Transmission electron microscopy (TEM) is a powerful tool for visualizing ultrastructural changes in hepatocytes, providing detailed insights into cellular and subcellular alterations associated with liver injury (Tasci et al., 2008; Gultemirian et al., 2014). In this study, TEM was used to examine the effects of LPS-induced liver injury and the potential protective role of IAA treatment on hepatocyte morphology. TEM analysis revealed significant ultrastructural alterations in hepatocytes following LPS administration, including mitochondrial damage, endoplasmic reticulum (ER) stress, lysosomal alterations, and cytoskeletal disorganization (Chen et al., 2024; Wang, 2015). In contrast, IAA treatment significantly mitigated these changes, suggesting its protective role against liver injury. The findings highlight the therapeutic value of IAA in preserving mitochondrial function, reducing ER stress, enhancing lysosomal function, and stabilizing the cytoskeleton, thus protecting hepatocytes from LPS-induced damage. The observed ultrastructural changes provide a comprehensive understanding of IAA's hepatoprotective mechanisms. Future studies should investigate the molecular pathways involved in IAA's effects, such as modulation of mitochondrial dynamics, ER stress pathways, and autophagy. Additionally, exploring the therapeutic potential of IAA in other liver injury models, including those induced by toxins, metabolic disorders, or viral infections, is warranted. In conclusion, this study demonstrates that IAA can significantly alleviate LPS-induced liver injury by preserving hepatocyte ultrastructure and function, underscoring its potential as a novel therapeutic agent for liver diseasess.
This study utilized transcriptomic analysis to investigate the molecular mechanisms underlying LPS-induced liver injury and the potential protective effects of IAA. The results highlight the involvement of the MAPK and Toll-like receptor (TLR) signaling pathways in liver damage and the therapeutic potential of IAA in modulating these pathways. The MAPK pathway, a key regulator of stress responses such as inflammation and oxidative stress, showed significant enrichment of differentially expressed genes (DEGs) in the LPS-treated group. This pathway activates transcription factors like NF-κB and AP-1, which drive the expression of pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) (Yu et al., 2023), indicating a robust inflammatory response. Toll-like receptors (TLRs) play a crucial role in initiating immune responses by recognizing pathogen-associated molecular patterns (PAMPs) like LPS (Kiziltas, 2016). We observed significant downregulation of genes in the TLR pathway in the LPS group, suggesting a dysregulated immune response that may contribute to liver injury. IAA treatment modulated both the MAPK and TLR pathways, attenuating the upregulation of pro-inflammatory genes and restoring the expression of downregulated genes, indicating its anti-inflammatory and hepatoprotective effects. Specifically, IAA reduced the expression of genes involved in NF-κB and AP-1 activation, dampening the inflammatory response and restoring immune homeostasis. These findings suggest that IAA has therapeutic potential for mitigating LPS-induced liver injury and other inflammatory liver diseases (Zhang et al., 2024b). Overall, this study underscores the importance of the MAPK and TLR pathways in LPS-induced liver injury and highlights IAA as a potential therapeutic agent. Future studies should further explore the specific molecular mechanisms by which IAA modulates these pathways and its broader applications in liver disease treatment.
TGR5 (G protein-coupled bile acid receptor 1) plays a key role in maintaining liver homeostasis and regulating metabolic processes. Activation of TGR5 has anti-inflammatory and anti-fibrotic effects and promotes bile acid signaling, which is essential for liver health (Kawai and Akira, 2010). In this study, we examined the impact of LPS-induced liver injury and IAA treatment on TGR5 expression in the liver. LPS treatment significantly reduced TGR5 expression, suggesting that inflammation and oxidative stress impair bile acid signaling, contributing to liver dysfunction. Previous studies have shown that TGR5 activation is crucial for liver homeostasis, and its downregulation increases susceptibility to liver injury (Bertolini et al., 2022; Guo et al., 2016). Therefore, the observed reduction in TGR5 expression in the LPS group likely exacerbates liver damage. In contrast, IAA treatment significantly increased TGR5 expression, indicating that IAA may enhance bile acid signaling and mitigate LPS-induced liver injury. The upregulation of TGR5 by IAA suggests that it may exert hepatoprotective effects by improving bile acid metabolism and enhancing liver resilience against inflammation and oxidative stress (Holter et al., 2020). These findings highlight the potential of IAA as a therapeutic agent for liver injury, suggesting the need for further studies on its mechanisms and clinical applications.
Conclusion
This study is the first to report the application of 3-indoleacrylic acid (IAA), a bacterial metabolite of tryptophan, in laying hens. Our findings show that IAA, at a dosage of 150 mg/kg, effectively alleviates liver injury by modulating key signaling pathways and enhancing antioxidant defenses. Specifically, IAA mitigates liver inflammation by regulating the MAPK and Toll-like receptor (TLR) pathways, reducing pro-inflammatory cytokine expression and boosting antioxidant enzyme activity. This study underscores the therapeutic potential of IAA in managing liver inflammation and oxidative stress, offering a novel strategy to improve liver health and productivity in laying hens.
Disclosures
The authors have no conflicts of interest to declare regarding this research.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
CRediT authorship contribution statement
Qiyue Zhang: Data curation, Methodology, Software, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Yue Wang: Data curation, Methodology, Software, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Yanwei Wang: Data curation, Methodology, Validation, Writing – review & editing. Jielu Yuan: Data curation, Methodology, Validation, Writing – review & editing. Yaru Wang: Data curation, Methodology, Validation, Writing – review & editing. Yongqi Zeng: Data curation, Methodology, Validation, Writing – review & editing. Haihua Zhang: Investigation, Validation, Writing – review & editing. Hui Yang: Investigation, Validation, Writing – review & editing. Qiugang Ma: Writing – review & editing. Donghui Shi: Formal analysis, Investigation, Methodology, Resources, Supervision, Writing – review & editing. Shimeng Huang: Formal analysis, Investigation, Methodology, Resources, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by the National Science Foundation of China (Grant No. 32472952) and the National Key Research and Development Program of China (Grant No. 2023YFD1301000). It was also funded by the Modern Agricultural Industrial Technology System in Hebei Province (Grant No. HBCT2023210202) and the Special Fund for the China Agricultural Research System Program (Grant No. CARS-40-K08). Additionally, support was provided by the Fujian Provincial Modern Agricultural Industry Technology System - Poultry Industry Technology System Project (2025).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2025.105307.
Contributor Information
Donghui Shi, Email: shidonghui@jzmu.edu.cn.
Shimeng Huang, Email: shimengh@cau.edu.cn.
Appendix. Supplementary materials
References
- Abo Ghanima M.M., Abd El-Hack M.E., Al-Otaibi A.M., Nasr S., Almohmadi N.H., Taha A.E., Jaremko M., El-Kasrawy N.I. Growth performance, liver and kidney functions, blood hormonal profile, and economic efficiency of broilers fed different levels of threonine supplementation during feed restriction. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agus A., Planchais J., Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host. Microbe. 2018;23:716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Amarasiri R., Hyun J., Lee S.W., Kim J.I., Lee H.G., Ryu B., Jeon Y.J. Therapeutic potential of tryptophan metabolite indoleacrylic acid in inflammatory bowel disease: From cellular mechanisms to zebrafish stress-like behavior. Int. Immunopharmacol. 2025;149 doi: 10.1016/j.intimp.2025.114207. [DOI] [PubMed] [Google Scholar]
- Bender M.J., McPherson A.C., Phelps C.M., Pandey S.P., Laughlin C.R., Shapira J.H., Medina Sanchez L., Rana M., Richie T.G., Mims T.S., et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell. 2023;186:1846–1862.e1826. doi: 10.1016/j.cell.2023.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini A., Fiorotto R., Strazzabosco M. Bile acids and their receptors: modulators and therapeutic targets in liver inflammation. Semin. Immunopathol. 2022;44:547–564. doi: 10.1007/s00281-022-00935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.Y., Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Yao L., Yuan M., Wang Z., Zhang Q., Jiang Y., Li L. Mitochondrial dysfunction: A promising therapeutic target for liver diseases. Genes Dis. 2024;11 doi: 10.1016/j.gendis.2023.101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Ji Z., Sun J., Hu J., Jiang Q., Wei W. Multi-omics investigation of Porphyromonas gingivalis exacerbating acute kidney injury through the gut-kidney axis. mSystems. 2025;10 doi: 10.1128/msystems.01136-24. e01136-01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effiong K., Hu J., Xu C., Zhang Y., Yu S., Tang T., Huang Y., Lu Y., Li W., Zeng J., et al. 3-Indoleacrylic acid from canola straw as a promising antialgal agent - Inhibition effect and mechanism on bloom-forming Prorocentrum donghaiense. Mar. Pollut. Bull. 2022;178 doi: 10.1016/j.marpolbul.2022.113657. [DOI] [PubMed] [Google Scholar]
- Emami N.K., Jung U., Voy B., Dridi S. Radical response: effects of heat stress-induced oxidative stress on lipid metabolism in the avian liver. Antioxidants. 2020:10. doi: 10.3390/antiox10010035. . (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad A.M., El-Senousey H.K. Nutritional factors affecting abdominal fat deposition in poultry: a review. Asian Australas. J. Anim. Sci. 2014;27:1057–1068. doi: 10.5713/ajas.2013.13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French C.E., Sales M.A., Rochell S.J., Rodriguez A., Erf G.F. Local and systemic inflammatory responses to lipopolysaccharide in broilers: new insights using a two-window approach. Poult. Sci. 2020;99:6593–6605. doi: 10.1016/j.psj.2020.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Xu K., Liu H., Liu G., Bai M., Peng C., Li T., Yin Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell Infect. Microbiol. 2018;8:13. doi: 10.3389/fcimb.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasaly N., de Vos P., Hermoso M.A. Impact of bacterial metabolites on gut barrier function and host immunity: a focus on bacterial metabolism and its relevance for intestinal inflammation. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.658354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y., Ma Q., Wang Z., Guo Y. Dietary vitamin D(3) supplementation protects laying hens against lipopolysaccharide-induced immunological stress. Nutr. Metab. 2018;15:58. doi: 10.1186/s12986-018-0293-8. (Lond) [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Fernández C., Basauri A., Fallanza M., Bringas E., Oostenbrink C., Ortiz I. Fighting against bacterial lipopolysaccharide-caused infections through molecular dynamics simulations: a review. J. Chem. Inf. Model. 2021;61:4839–4851. doi: 10.1021/acs.jcim.1c00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gultemirian M.L., Corti H.R., Chaia A.P., Apella M.C. Fermentation in vitro of a mixture of dietary fibers and cane molasses by the cecal microbiota: Application on mineral absorption through the laying hen's colonic epithelium. Anim. Feed. Sci. Technol. 2014;191:76–82. [Google Scholar]
- Guo C., Chen W.D., Wang Y.D. TGR5, not only a metabolic regulator. Front. Physiol. 2016;7:646. doi: 10.3389/fphys.2016.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid H., Zhang J.Y., Li W.X., Liu C., Li M.L., Zhao L.H., Ji C., Ma Q.G. Interactions between the cecal microbiota and non-alcoholic steatohepatitis using laying hens as the model. Poult. Sci. 2019;98:2509–2521. doi: 10.3382/ps/pey596. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Perlot T., Rehman A., Trichereau J., Ishiguro H., Paolino M., Sigl V., Hanada T., Hanada R., Lipinski S., et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holter M.M., Chirikjian M.K., Govani V.N., Cummings B.P. TGR5 signaling in hepatic metabolic health. Nutrients. 2020;12 doi: 10.3390/nu12092598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G.L., Xiong J., Liu Y., Yang H.J., Hu L.L., Chen P., Wang X., Liao S., Lv T., Liu C.J., et al. Effects of lecithin supplementation in feed of different fat levels on serum indexes and liver health of laying hens. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.892585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Qi L., Lv Z., Jin S., Wei X., Shi F. Dietary stevioside supplementation alleviates lipopolysaccharide-induced intestinal mucosal damage through anti-inflammatory and antioxidant effects in broiler chickens. Antioxidants. 2019:8. doi: 10.3390/antiox8120575. . (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kiziltas S. Toll-like receptors in pathophysiology of liver diseases. World J. Hepatol. 2016;8:1354–1369. doi: 10.4254/wjh.v8.i32.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y., Brenner D.A. Liver inflammation and fibrosis. J. Clin. Invest. 2017;127:55–64. doi: 10.1172/JCI88881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P., Mehal W.Z. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Liu S., Liang W., Wu J., Bao E., Tang S. Alleviation of lipopolysaccharide-induced heart inflammation in poultry treated with carnosic acid via the NF-κB and MAPK pathways. J. Anim. Sci. 2025;103 doi: 10.1093/jas/skae373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B., Xing T., Li J., Zhang L., Jiang Y., Gao F. Chronic heat stress causes liver damage via endoplasmic reticulum stress-induced apoptosis in broilers. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia L., Tan J., Vieira A.T., Leach K., Stanley D., Luong S., Maruya M., Ian McKenzie C., Hijikata A., Wong C., et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- Min B.H., Devi S., Kwon G.H., Gupta H., Jeong J.J., Sharma S.P., Won S.M., Oh K.K., Yoon S.J., Park H.J., et al. Gut microbiota-derived indole compounds attenuate metabolic dysfunction-associated steatotic liver disease by improving fat metabolism and inflammation. Gut Microbes. 2024;16 doi: 10.1080/19490976.2024.2307568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Jiang Y. Protective effects of Lactobacillus plantarum NDC 75017 against lipopolysaccharide-induced liver injury in mice. Inflammation. 2014;37:1599–1607. doi: 10.1007/s10753-014-9886-1. [DOI] [PubMed] [Google Scholar]
- Rehman Z.U., Meng C., Sun Y., Safdar A., Pasha R.H., Munir M., Ding C. Oxidative Stress in Poultry: Lessons from the Viral Infections. Oxid. Med. Cell Longev. 2018;2018 doi: 10.1155/2018/5123147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeidnia S., Abdollahi M. Toxicological and pharmacological concerns on oxidative stress and related diseases. Toxicol. Appl. Pharmacol. 2013;273:442–455. doi: 10.1016/j.taap.2013.09.031. [DOI] [PubMed] [Google Scholar]
- Scott M.J., Billiar T.R. Beta2-integrin-induced p38 MAPK activation is a key mediator in the CD14/TLR4/MD2-dependent uptake of lipopolysaccharide by hepatocytes. J. Biol. Chem. 2008;283:29433–29446. doi: 10.1074/jbc.M803905200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan C., Miao F. Immunomodulatory and antioxidant effects of hydroxytyrosol in cyclophosphamide-induced immunosuppressed broilers. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao B., Munford R.S., Kitchens R., Varley A.W. Hepatic uptake and deacylation of the LPS in bloodborne LPS-lipoprotein complexes. Innate Immun. 2012;18:825–833. doi: 10.1177/1753425912442431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B.K., Marcinko K., Desjardins E.M., Lally J.S., Ford R.J., Steinberg G.R. Treatment of nonalcoholic fatty liver disease: role of AMPK. Am. J. Physiol. Endocrinol. Metab. 2016;311:E730–e740. doi: 10.1152/ajpendo.00225.2016. [DOI] [PubMed] [Google Scholar]
- Takaki A., Kawai D., Yamamoto K. Molecular mechanisms and new treatment strategies for non-alcoholic steatohepatitis (NASH) Int. J. Mol. Sci. 2014;15:7352–7379. doi: 10.3390/ijms15057352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleb S. Tryptophan dietary impacts gut barrier and metabolic diseases. Front. Immunol. 2019;10:2113. doi: 10.3389/fimmu.2019.02113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasci I., Mas N., Mas M.R., Tuncer M., Comert B. Ultrastructural changes in hepatocytes after taurine treatment in CCl4 induced liver injury. World J. Gastroenterol. 2008;14:4897–4902. doi: 10.3748/wjg.14.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji A., Ikeda Y., Yoshikawa S., Taniguchi K., Sawamura H., Morikawa S., Nakashima M., Asai T., Matsuda S. The tryptophan and kynurenine pathway involved in the development of immune-related diseases. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms24065742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. Autophagy and apoptosis in liver injury. Cell Cycle. 2015;14:1631–1642. doi: 10.1080/15384101.2015.1038685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarska M., Luo C., Kolde R., d'Hennezel E., Annand J.W., Heim C.E., Krastel P., Schmitt E.K., Omar A.S., Creasey E.A., et al. Indoleacrylic acid produced by commensal peptostreptococcus species suppresses inflammation. Cell Host Microbe. 2017;22:25–37. doi: 10.1016/j.chom.2017.06.007. e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu E., Zhang L., Yang H., Shen L., Feng Y., Ren M., Xiao Y. Transcriptome profiling of the liver among the prenatal and postnatal stages in chickens. Poult. Sci. 2019;98:7030–7040. doi: 10.3382/ps/pez434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Hino S., Iijima H., Genda T., Aoki R., Nagata R., Han K.H., Hirota M., Kinashi Y., Oguchi H., et al. Mucin O-glycans facilitate symbiosynthesis to maintain gut immune homeostasis. EBioMedicine. 2019;48:513–525. doi: 10.1016/j.ebiom.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Shao Y., Yang J., Xing X., Yang H., Wang Z. Betaine enhances hepatic antioxidant activity and thymus-associated immunity in lipopolysaccharide-challenged goslings. BMC Vet. Res. 2025;21:77. doi: 10.1186/s12917-025-04527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., Zhang Y., Wang T., Guo J., Kong C., Chen Z., Ma X., Qiu T. MAPK signaling pathways in hepatic ischemia/reperfusion injury. J. Inflamm. Res. 2023;16:1405–1418. doi: 10.2147/JIR.S396604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Li Q., Zeng X., Xu Y., Jin K., Liu J., Cao G. Effects of probiotics on the growth performance, antioxidant functions, immune responses, and caecal microbiota of broilers challenged by lipopolysaccharide. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.846649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Li L., Zheng W., Wan J., Ge P., Li H., Zhang L. Antidiabetic drug metformin alleviates endotoxin-induced fulminant liver injury in mice. Int. Immunopharmacol. 2012;12:682–688. doi: 10.1016/j.intimp.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Zaefarian F., Abdollahi M.R., Cowieson A., Ravindran V. Avian liver: the forgotten organ. Animals. 2019;9 doi: 10.3390/ani9020063. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Kang R., Tang D. Gut microbiome mediates ferroptosis resistance for colorectal cancer development. Cancer Res. 2024;84:796–797. doi: 10.1158/0008-5472.CAN-24-0275. [DOI] [PubMed] [Google Scholar]
- Zhang S., You M., Shen Y., Zhao X., He X., Liu J., Ma N. Improving fatty liver hemorrhagic syndrome in laying hens through gut microbiota and oxylipin metabolism by bacteroides fragilis: a potential involvement of arachidonic acid. Anim. Nutr. 2025;20:182–199. doi: 10.1016/j.aninu.2024.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li S., Fan X., Wu Y. Pretreatment with indole-3-propionic acid attenuates lipopolysaccharide-induced cardiac dysfunction and inflammation through the AhR/NF-κB/NLRP3 pathway. J. Inflamm. Res. 2024;17:5293–5309. doi: 10.2147/JIR.S466777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S., Sun Y.Q., Huo J.X., Xu W.Y., Yang Y.N., Yang J.B., Wu W.J., Liu Y.X., Wu C.M., Li Y.G. The gut microbiota-aromatic hydrocarbon receptor (AhR) axis mediates the anticolitic effect of polyphenol-rich extracts from Sanghuangporus. Imeta. 2024;3:e180. doi: 10.1002/imt2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W., Qian K., Xiong J., Ma K., Wang A., Zou Y. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-κB related signaling. Biomed. Pharmacother. 2016;83:302–313. doi: 10.1016/j.biopha.2016.06.036. [DOI] [PubMed] [Google Scholar]
- Zhou M., Lv J., Chen X., Shi Y., Chao G., Zhang S. From gut to liver: exploring the crosstalk between gut-liver axis and oxidative stress in metabolic dysfunction-associated steatotic liver disease. Ann. Hepatol. 2025;30 doi: 10.1016/j.aohep.2025.101777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.