Abstract
Background
Chronic lymphocytic leukemia (CLL) is considered the one of most prevalent hematological diseases in the Western world, with an incidence of 4.2/100 000/year that increases to more than 30/100 000/year at an age of greater than 80 years. The Bruton tyrosine kinase inhibitor ibrutinib has been considered the treatment of choice in treatment naïve and relapsed/refractory settings (R/R). Venetoclax, along with navitoclax, are the selected BCL2 inhibitors in first and second-line settings for chronic lymphocytic leukemia. A degree of acquired resistance for this agents has been observed in clinical settings, and is also determined by scientific rationale.
Methods
A PubMed literature search and Google Scholar search were conducted using the terms “chronic lymphocytic leukemia” AND “novel therapies”, “BTK degraders”, and “acquired resistance”, and “bispecific antibodies”, and “chimeric antigen T cell therapy.” “ recent phase III trials” and “CLL” AND “MURANO trial” AND “BRUIN trial” AND “CLL14 trial” AND “TRANSCEND trial” AND “updates.”
Results
Acquired resistance has been extensively documented in treatment of CLL, mainly due to the mutation Gly101Val that leads to displacement of pro-apoptotic proteins. Newer agents identified include pirtobrutinib and nemtabrutinib, non-covalent, reversible BTK inhibitors, the anti-CD20 monoclonal antibodies employing CD20 target antigen mechanisms ofatumumab, obinutuzumab, lisocabtagene maraleucel, a CD19 chimeric antigen T cell receptor therapy, teclistamab, a BsAb that targets the B cell maturation antigen or BCMA and Siglec-6 monoclonal antibodies.
Conclusion
CLL has demonstrated acquired resistance to BTK inhibitors and BCL2 inhibitors, necessitating the development and evaluating of treatment options beyond their use. Cancer immunotherapies such as bispecific antibodies and chimeric T cell therapies present with viable therapies for CLL. Novel agents have also been developed that enhance the cytotoxic effect of T cells. Future studies may focus on the developing treatments that overcome the acquired resistance that results when treatment with standard of care targeted therapies ibrutinib and venetoclax.
Graphical Abstract
Keywords: Chronic lymphocytic leukemia, BTK degraders, Resistance, Monoclonal antibodies, Bispecific antibodies, Chimeric antigen T cell therapy, Novel therapies
Introduction
The incidence of chronic lymphocytic leukemia is approximately 4.2/100,000 in the Western world and is more prevalent in the elderly [1]. Chemotherapy is considered standard of care, however targeted therapies have entered into first line settings [2]. Chronic lymphocytic leukemia (CLL) overexpresses the B-cell lymphoma or BCL protein that leads to dysregulation of apoptosis and subsequent abnormal cellular survival. CLL cells maintain their viability and survival through signaling from the tumor microenvironment and the B-cell receptor.
Small molecule inhibitors examples being Bruton tyrosine kinase inhibitor ibrutinib and B-cell lymphoma-2 inhibitor venetoclax were developed to target the BCR signaling pathway and the cell death machinery that is aberrantly regulated in CLL. The treatment of CLL is challenging since leukemic cells are resistant to treatment. Age, comorbidities and the presence of the p53 mutation determine treatment, which consists of a wait or watch approach in the asymptomatic early stage. CLL patients respond to chemo-immunotherapy and novel targeted therapies such as BTKs such as ibrutinib and inhibitors of Bcl-2 patients for symptomatic and advanced patients [3]. (Fig. 1)
Fig. 1.
Current treatment agents for chronic lymphocytic leukemia: Imatinib (BTK inhibitor), rituximab (CD20 monoclonal antibody), venetoclax (BCL2 inhibitor, and teclistimab (bispecific antibody for the BCMA antigen)
The Bruton tyrosine kinase inhibitor ibrutinib has been considered the treatment of choice in treatment naive and relapsed/refractory settings (R/R). Venetoclax, along with navitoclax, are the selected BCL2 inhibitors in first and second-line settings for chronic lymphocytic leukemia. A degree of acquired resistance for these agents has been observed in the clinical settings, and as also determined by scientific rationale. Venetoclax’ clinical success has been shown by robust minimal residual rates (MRD). Both have clinical utility as monotherapies, however navitoclax is associated with on-target platelet BCL-XL inhibition leading to thrombocytopenia, limiting its use [3].
Bennett et al. conducted a review of mechanisms of resistance for venetoclax, which can be used in combination with anti-CD20 antibodies such as rituximab and BCL2 inhibitors ibrutinib for treatment of CLL. Baseline BCL2i resistance in CLL patients results from tumor heterogeneity and the relative expression of BCL2 in relation to other BCL2 family member proteins. BCL2 family member proteins include both pro-apoptotic BAX and BAK and anti-apoptotic BCL2 and BCL-XL. When BCL2 is overexpressed in CLL, the accumulation of the protein tips in favor of the anti-apoptotic proteins [3].
In the phase III, multicenter, randomized open-label GLOW trial, fixed duration ibrutinib-venetoclax was evaluated after a 4-year follow-up,. At a median of 46 months, PFS remained superior for the ibrutinib-venetoclax group (HR 0.214 [95% CI 0.138–0.334]. 42-month progression-free survival rates were 74.6% (95% CI 65.0–82.0) for ibrutinib–venetoclax and 24.8% (16.5–34.1) for chlorambucil–Obinutuzumab [4].
Resistance to drug inhibitors
Resistance to BCL2 inhibitors
Acquired resistance to BCL2 and BTK inhibitors is a common feature of treated CLL patients. The mutation Gly101Val is found in clones of CLL and is responsible for BCL2 inhibitor resistance because the overcrowding of the protein’s alpha helix residues that results in anti-apoptosis [3]. As result of the Gly101Val residue mutation, survival of the BCL2 protein function occurs as result of displaced pro-apoptotic proteins from BCL2, and is predictive of the of lack of inhibition of BCL2 inhibitors such as venetoclax, and thus leading to the “selective advantage for the mutated clone.” [3] Resistance in non-mutated Gly101Val cells in the context of BCL-XL overexpression is also observed, as well as dynamic upregulation of BCL-XL in CLL lines which exhibit resistance to venetoclax. D103Y, A113G, R129L, and V156D residue mutations have also been implicated in BCL2 resistance and have led to disease progression in patients on the inhibitor, with their contribution to resistance still unclear [5]. In other studies, it was found that A113G had a variant allelic frequency of 31.7% and F104L had a VAF of 29.2%. The mutation L119V had a low VAF of 0.2%. A novel in-frame insertion of 12 nucleotides resulting in Arg107_Art110dup was seen in patients with CLL-type progression (VAF, 0.4% and 5%). It was also suggested that a copy-number alteration was seen in a patient with Richter transformation, since a rare allele A61T was present at 50% prior to venetoclax administration and decreased to 27% at relapse. In one study, using targeted gene sequencing, Less is known about secondary resistance to venetoclax as a result of Richter transformation however BTK mutations were observed at a frequency of 66% at time of disease progression. Among patients with Richter transformation, SF3B1 mutations were more frequent than BTK mutations [6, 7].
Recent clinical trials such as MURANO and CLL14 have shown that fixed duration therapy can be effective in CLL that do not select for BCL2 resistance mechanisms; however in the event of progression, venetoclax retreatment efficacy is relatively unknown. MURANO evaluated a small patient population that was retreated with venetoclax-rituximab combination when compared with monotherapy and the ORR was 55% [3]. Patients in the MURANO trial showed a 2-year PFS of 84.9% in the venetoclax-rituximab arm and 36.3% in the monotherapy arm. (HR, 0.17; 95% [CI], 0.11 to 0.25). This benefit was seen across all clinical and biologic subgroups, including the subgroup of patients with chromosome 17p deletion, mutation associated with CLL [8]. In the CLL14 trial, PFS was 76.2 versus 36.4 month for the venetoclax-obitunuzumab arm, when compared to 36.4 months for the chlorambucil-obinutuzumab arm (HR 0.40; 95% CI, 0.31–0.52) showed an improved profile [3, 9].
Resistance to BTK inhibitors
Scanland et al. describe the secondary mutations within drug targets that underlie mechanisms of resistance. Mutations in the BH3-binding domain of BCL-2, of which G101V is the most frequent are reported to lead to venetoclax resistance, and is overcome by substituting a glutamate residue with alanine [3]. The mutation of the C481 binding side in BTK in which cysteine is mutated to serine is a common mutation underlying BTK resistance. The most common resistance mechanisms to first-in-class BTK were detected in 19 patients in a study of 29 patients with BTK-resistant CLL (65.6%), with 23 having progressive disease [10].
The mutation of the C481 binding side in BTK in which cysteine is mutated to serine is a common mutation underlying BTK resistance. The most common resistance mechanisms to first-in-class BTK were detected in 19 patients in a study of 29 patients with BTK-resistant CLL (65.6%), with 23 having progressive disease [3].
Bennett et al. also describe clonal complexity at disease relapse that heightens resistance through multiple mechanisms, which is evident in single-cell sequencing studies. Resistance to targeted therapies such as BTK inhibitors (as well as BCL2 inhibitors) is accompanied by multiple resistance mechanisms through convergent clonal evolution [3].
A study of patient samples collected in 4 different ibrutinib studies showed that 85% of the patients that relapsed on treatment had acquired mutations in BTK or PLCG2 [3]. Novel BCL-2 mutations also occur in ibrutinib patients, and many of them have the BTK/PLCG2 mutation, which suggested that the commonly occurring G101B point mutation in BCL-2 is less prevalent in BTK inhibitors [2, 3]. Even further, screening for drug sensitivity of tumor cells reveals that in primary CLL cells collected pre and post treatment of ibrutinib, an “ibrutinib-induced pharmacologically exploitable vulnerabilities to proteasome, PLK1, and mTOR inhibitors were discovered.” [3].
Mato et al. conducted a real world observational study of a de-identified oncology database and evaluated patients receiving covalent BTKi, BCL2 inhibitors, rituximab and chemotherapy and discontinuation across these approved multiple lines of therapy. They found that CLL was not adequately managed and frequently led to progression and adverse events leading to discontinuation of treatment. 9578 patients were identified in the database and of those 4983 received at least one cBTKi, 581 received both a cBTKi and BCL2i, and 218 received cBTKi, BCL2i and rituximab and chemotherapy across multiple lines of therapy [11].
Their extensive study showed in each of the subgroups (cBTKi, (n = 3577); cBTKi + BCL2i (n = 403), cBTKi + BCL2i + rituximab + chemotherapy (n = 218)), patients received treatments in first line settings, treatment discontinuation frequently resulted from death and adverse events. Among patients in the first subgroup, second line therapy included rituximab with or without chemotherapy (n = 183) or venetoclax (n = 173). All patients in this subgroup who discontinued cBTKi therapy regardless of receiving subsequent therapy, the time of discontinuation of treatment was a median of 9.5 months (95% C.I. 8.8–10.4).
The study authors concluded that for CLL patients, treatment options become exhausted due to decreasing outcomes and progressively worse outcomes as result of this lack of sufficient treatment options [11].
Novel agents in pre-clinical and clinical use for CLL
A number of novel targetable pathways have been described for CLL, which address an unmet clinical need. Alternative inhibitors of BTK, BTK degraders, novel BH3-mimetics, therapeutic antibodies towards tumor antigens and immune cell approaches such as bispecific antibodies and cellular therapies are being developed and studied. These agents are now undergoing evaluation for patients with intolerance and/or resistance to BTK inhibitors and BCL2 inhibitors. Resistance to BTK inhibition in treatment discontinuations is observed to be due to mutation in the C481 binding site.
BTK degraders
BTK protein degraders are emerging therapies with potential clinical utility for patients treated with BTK1 and BCL2i and exhibit resistance and relapsed/recurrence. Preliminary data from clinical studies of investigational BTK degraders NX-2127, BGB-16,673, and NX- 5948 showed efficacy in R/R CLL [2].
Non-covalent BTK1 inhibitors
Pirtobrutinib is a non-covalent, reversible inhibitor with a high degree of selectivity.
for BTK that overcomes C481 resistance. In a heavily pretreated population, ORR was 68%.
in the phase 1/2 BRUIN trial, leading to recent FDA approval for cBTKi and BCL2i-treated patients. In the BRUIN trial, the median line of prior therapy was 3, and 100 patients had received a BCL2i, the ORR for pirtobrutinib was 73.3% (95% CI, 67.3 to 78.7), and the percentage was 82.2% (95% CI, 76.8 to 86.7) when partial response with lymphocytosis was included. mPFS was 19.6 months (95% CI, 16.9 to 22.1) [12]. Updated follow-up data revealed an ORR Of 82% with a median PFS of 19.4 months after a median of 27.5 months of follow-up. Numerically inferior responses were observed for heavily pre-treated BCL2i-exposed patients versus BCT2i-naive. Safety data demonstrated a well-tolerated agent, with low rates of Grade 3 adverse events and treatment related discontinuation (2.5%) [2]. Pirtobrutinib was evaluated in an indirect comparison with the combination of venetoclax and rituximab in a recent matching adjusted trial for covalent BTK inhibitor-treated patients. PFS and OS results were not significant since no difference was observed between the two therapies, even though higher ORR and fewer grade ≥ 3 AEs were observed with pirtobrutinib. Pirtobrutinib is administered and approved for post cBTKi and BCL2 treatment and has a favorable safety profile. Overall, pirtobrutinib is an effective agent approved for use after cBTKi and BCL2i exposure and appears well tolerated with low incidence of TEAE. In the updated phase III BRUIN-CLL-321, PFS was significantly improved for pirtobrutinib compared to investigator’s choice of therapy. (HR, 0.58; 95%CI 0.38–0.89. Updated analysis with a median follow-up of 11.6 months, IRC assessed PFS HR was 0.55 (95%CI, 0.38–0.78). INV-assessed PFS also showed benefit, with an HR of 0.42 (95%CI, 0.29–0.62) [13] (Sharman Blood) [3]. Nemtabrutinib, another non-covalent BTKi, has similar efficacy to pirtobrutinib, but was found to be less well-tolerated, as shown in the phase II BELLWAVE-001 in R/R CLL [2]. In this dose-expansion study in R/R CLL patients with 4 lines of therapy, ORR was 53%. Updated results will be provided by the phase III BELLWAVE-008, Bellwave-010 and Bellwave-011 trials [14].
Immunotherapies for CLL
Treatment options for CLL have evolved over time; however, standard chemotherapy approaches such as alkylating agents and purine analogues have not shown real improvement in OS of CLL patients. The relative success of CD20 monoclonal antibody rituximab to chemotherapy has been demonstrated in improved OR and OS rates. The origin of immunological approaches for hematologic disease was demonstrated through allogeneic HSCT, is associated with the graft versus leukemia effect, and involves a process by which donor transferred T cells kill leukemia cells. However, due to more options being available, HSCT is only reserved for high-risk young patients with del(17p) or TP53 alterations. Due to disappointing results in clinical trials, immune checkpoint inhibitors, once thought to hold promise, are not considered options for CLL patients. In this context, cellular approaches such as bispecific antibodies, CAR-modified T cells and NK cells are under development [1, 15, 16].
Cell based immunotherapy is based on the proof of concept of re-engineering patient immune cells and genetically modifying them to kill T cells. (Fig. 2)
Fig. 2.
The CAR T cell construct and tumor cell synapse. The T cell signaling domain of the CAR construct engineered on the T cell membrane leads to the release of cytokines IL-2 and TNF-alpha and cytotoxic granzyme and perforin resulting in apoptosis of the tumor cell
Monoclonal antibodies
Monoclonal antibodies are the first antibody-based immunotherapy to have beneficial outcomes in CLL. Employing mechanisms of cellular cytotoxicity, both antibody-based and complement-dependent, and direct apoptotic effects [1]. Mabs exploit the fact that CD20 is expressed on B cells’ surface and serves as a target antigen in CLL, and has shown success. The mAb rituximab in combination with CT has induced remission along with improved OS. Next generation mAb also employing CD20 target antigen mechanisms are ofatumumab (Phase I-II dose escalating trial: ORR of 48% (13 of 27 patients. with no CR; mPFS was 106 days. Grade 3 or more adverse events included infection, thrombocytopenia and neutropenia) [17].
Obinutuzumab was evaluated in the phase II GAUGIN phase II study in R/R CLL with relapsed/refractory CLL; dosing was 1000 mg on day 1, 8, 15, 22 and then every 3 weeks for a total of 10 infusions. Four grade 3–4 neutropenias were reported. Level of tumor burden was associated with response rate, and suggested greater efficacy when combined with chemoimmunotherapy. In a phase III trial, the ORR led to more complete remissions and improved PFS with the addition of an anti-CD20 mAb (obinutuzumab or rituximab) when compared to CLB monotherapy [18]. Obinutuzumab was also superior to rituximab showing statistically significant and clinically important improvement in PFS (26.7 months versus 11.1 months) and a trend to an OS advantage (p = .08)], with the benefit seen across all patients except those with del(17p). With a lengthier follow-up PFS still remained longer for the obinutuzumab-treated patients (29.2 months) than for the rituximab-treated patients (15.4 months) (p < .001) [19]. (.
In a trial evaluating ublituximab, patients received ibrutinib for a median of 21 months (range 7–67) prior to study enrollment. Fourteen patients (52%) have achieved U-MRD per protocol whereas 78% had at least one U-MRD evaluation. Seventeen patients (63%) have entered TFO after a median of 6.4 months on triplet therapy. Progression-free survival at 12 months was estimated at 95%. Grade ≥ 3 adverse events were hypertension (7%) and diarrhea (4%), and increased ALT/AST (4%) [20]. Ublituximab has shown clinical efficacy in “phase 2 and/or phase 3 clinical trials when used in combination with conventional chemotherapy, Bruton tyrosine kinase (BTK) inhibitors, PI3K inhibitors or venetoclax .” [1] CD20 antigen loss has been observed and may be responsible for their limitations.
Other mAbs in clinical trials as of 2022 are omalizumab in combination with chemotherapy; the combination of tafasitamab with lenalidomide and tafasitamab combined with ibrutinib or venetoclax were administered to CLL patients who were refractory to BTK inhibitors. Tafasitamab plus idelalisib showed in 24 patients that were enrolled (cohort A: n = 11, median time on study, 7.4 months; cohort B: n = 13, median time on study, 15.6 months) TEAEs of anemia and neutropenia, with an ORR of 90.9%. U-MRD in peripheral blood was achieved in 2/8 patients, with the results suggesting this anti-CD19 antibody-based combinations may be important in the treatment of patients with R/R CLL [21] .
Other immunotherapy options for CLL are agents that target antigens for CD37. Otlertuzumab is a chimeric protein created from the fusion of SMP-016 and anti-CD37 protein and displayed full binding activity in truncated form. In a phase 1 trial, it was shown to be well tolerated in treatment-naive and pre-treatment settings. In combination with bendamustine, it also appears to be well tolerated in a phase 1 trial in both treatment-naïve and pre-treated CLL patients; furthermore, otlertuzumab combined with bendamustine was also shown to be efficacious in R/R CLL in a phase 2 trial. Otlertuzumab is a humanized anti-CD37 protein therapeutic that triggers direct caspase-independent apoptosis of malignant B cells and induces antibody-dependent cell-mediated cytotoxicity. In one trial, it was shown that. Thirty-two patients were treated with otlertuzumab and bendamustine and 33 with bendamustine alone showed a ORR of 69% versus 39% in the otlertuzumab and bendamustine arm versus bendamustine alone arm (P = 0·025). mPFS was 15.9 months versus 10.2 months in the otlertuzumab and bendamustine arm versus the monotherapy arm, respectively (P = 0·0192). However, there was a “higher incidence of pyrexia (34% vs. 12%) and neutropenia (59% vs. 39%) with the combination but this did not result in a higher incidence of severe (grade 3/4) infections (13% vs. 27%). This combination significantly increased the response rate and prolonged the PFS over single agent bendamustine in patients with relapsed or refractory CLL [1, 22].
Other anti-CD37 agents are CI 83682f6 that shows efficacy in CLL patients with del (17p) and p53 mutations and is now being evaluated in a phase I trial in combination with ibrutinib in R/R CLL patients [1]. The Fc-engineered DuoHexaBody-CD37 is a anti-CD37 targeted agent which displayed cytotoxic activity in vivo in cell lines and patient-derived xenografts from CLL and is now undergoing clinical evaluation. Another target being explored is the ROR1, or receptor tyrosine kinase-like orphan receptor, that has selective expression only on leukemic cells, now undergoing analysis in a phase1/2 trial displaying tolerability and efficacy. Cirmtuzumab is an example of a ROR1-directed mAb that is being studied with ibrutinib in clinical trials, and is also discussed below. Zilovertamab vedotin (VLS-101) is another mAb in the ROR1 target antigen class that shows potent anti-tumor activity in xenograft models of CLL that have transformed into Richter syndrome, and is being studied in a phase I trial for safety and efficacy in CLL. BAFF, or the B-cell activating factor and its receptor BAFF-R has shown to lead to resistance in CLL due to anti-apoptotic signals, and thus anti-BAFF Ab such as belimumab is being evaluated in combination with ibrutinib and venetoclax in CLL. Another such antibody, ianalumab has shown superior clinical activity to CD20-directed mAbs in preclinical models, particularly in combination with ibrutinib, offering the possibility to discontinue ibrutinib and substitute ianalumab.
Another emerging novel antibody for R/R CLL is the Mab cirmtuzumab which targets ROR1 that is not expressed by normal B-cells, and best responses have been elicited in a small number of patients with stable disease. Patients resistant to BTK1 have responses to anti-BAFF antibody Ianalumab as shown through undetectable MR. Following incomplete response to or resistance mutation to BTKi, anti-BAFF antibodies (Ianalumab). Cirmtuzumab was evaluated in a phase 1 study for CLL, that is self-renewing neoplastic B cells with high expression of ROR1 (95%) that is associated with poor prognosis. 26 patients with progressive R/R CLL showed inhibited ROR1 signalling and CLL stemness gene expression through transcriptome analysis. Cirmtuzumab was found to be safe and effective along with having no dose-limiting toxicity [23]. However, another novel monotherapy, MCL1 AZD5991, when evaluated in a phase 1b/2 study of R/R hematological malignancies (including five patients with CLL) closed “prematurely due to a high incidence of laboratory troponin elevation, with concurrently low response rates.” [2].
Chimeric antigen T cell receptor (CAR) therapies
CAR T cells (Fig. 2) are constructs that combine features and components of both T cells and antibodies that have the CD19 marker and mediate T cell anti-tumor killing. Of the four generation CAR constructs, the 2nd generation construct has been approved for clinical use. A CAR construct (Fig. 2) has an antigen binding domain which includes a single chain variable fragment derived from an immunoglobulin directed against a TAA, and a intracellular domain and a costimulatory domain (intracellular signaling domains of a costimulatory molecule CD28 or 41BB). The latter allows the CAR construct to mediate full activation of T cells in the absence of antigen presenting cells. This is their main advantage: to recognize tumor antigens in a HLA-independent manner, thus eliciting an immune response in immune excluded tumors [1]. Figure 2 describes the mechanism of action of a CAR T cell construct on a cytotoxic T cell interacting with a tumor associated antigen leading to tumor cell apoptosis.
The first use of autologous CAR T cell therapy in CLL was reported in 2011. Two R/R CLL patients and these patients were reinfused with 1.5 × 10^5 autologous CAR cells that had 1000X expansion when compared to initial engraftment. Patients achieved complete response (CR) after treatment, and remission has been sustained for more than ten years after therapy and that CAR T cells are still detectable in blood. These long-persisting CAR T cells are CD4 + cytotoxic T cells. Over 100 CLL patients have been treated with anti-CD19 CAR T cells, most have been refractory to standard conventional treatment, however it has been shown that chemotherapy-induced depletion of lymphocytes prior to CART infusion improved efficacy of CAR T cells, which is proposed to reduced tumor mass and the number of immunosuppressive regulatory T cells [24].
The phase 1/2 TRANSCEND CLL 004 study evaluating lisocabtagene maraleucel, a CD19 chimeric antigen T cell receptor therapy, had a cohort of 23 patients with a median of 4 prior lines of therapy who achieved 75% and 65% undetectable MRD state in blood and bone marrow respectively. Rate of CR or remission was found to be statistically significant at 18% in primary efficacy analysis (n = 9; 95% CI 9–32; p = 0·0006). Grade 3 cytokine release syndrome was reported in ten or 9% out of 11 patients with no grade 3 or 4 events reported. Grade 3 neurological events were seen in 21 or 18% of patients with no grade 5 events. 51 deaths were reported; 43 occurred after liso-cel infusion and 5 were a result of TEAEs that occurred with 90 days after infusion [25]. However, despite these advances, challenges remain, since relapse due to resistance to CD19 CAR T cells due to CD19 antigen loss. Since patients are usually heavily pretreated an thus eliciting a low number of quality T cells, manufacturing and expanding autologous CART cells are challenging. Since resistance can occur as a result of loss of target CD19 antigen due to mutations in the CD19 coding gene and alternative splicing of CD19 mRNA in these settings, new constructs are being studied and developed that target al.ternative antigens other than CD19 (CD20, CD22, ROR-1, Siglec-6) [26–30].
When compared to acute lymphoblastic leukemia and B cell lymphoma, hematologic malignancies that have CAR T cell therapies approved for use for these cancers, CLL is considered relatively more resistant to CAR T cell therapy. (average CR 30%, ranging 0–67%) [5]. Many reasons have been posited accounting for this phenomena. Defective immunological synapse formation between CD4 + and CD8 + T cells and antigen presenting cells due to the higher expression of B7- and TNF-receptor families of transmembrane molecules that serve as inhibitors. The inhibitory characteristics of the immunosuppressive tumor microenvironment are also a factor considering the presence of tumor associated macrophages, myeloid derived stem cells, cancer associated fibroblasts, and M2 macrophages, which create an immunosuppressive environment in the context of T cell exhaustion [27–30].
Ibrutinib was shown to facilitate CAR T cell therapy administration and when dosed in 5 cycles leads to decreased T cell exhaustion, the drug led to the reestablishment of T cell function, and better ex vivo expansion and in vivo functionality of CAR T cells as result of partial loss of PD-1 expression. Fewer CRS and neurological side effects, common toxicities associated with CAR T cell therapy were also observed. Another factor influencing outcomes in CLL patients is the dosage of CAR T cells. One recent study has shown that a higher dose of anti-CD19 CAR T cells (5.0 × 10^8 vs. 5.0 × 10^7) produces higher rates of objective response (55% vs. 31% respectively ) and complete response (36% vs. 8% respectively) Another promising approach is the use of allogeneic T cells from a universal donor with to overcome the limitations of patient derived CAR T cells and its associated complex manufacturing processes, thus broadening CART therapy, However administration of allogeneic T cells from a universal donor can cause serious graft versus host disease (GvHD). This data would suggest that autologous CAR T cell therapy can be an effective therapy for CLL due to its favorable safety profile and positive objective response and complete response rates however they are associated with challenges due to a complex manufacturing process [27–30].
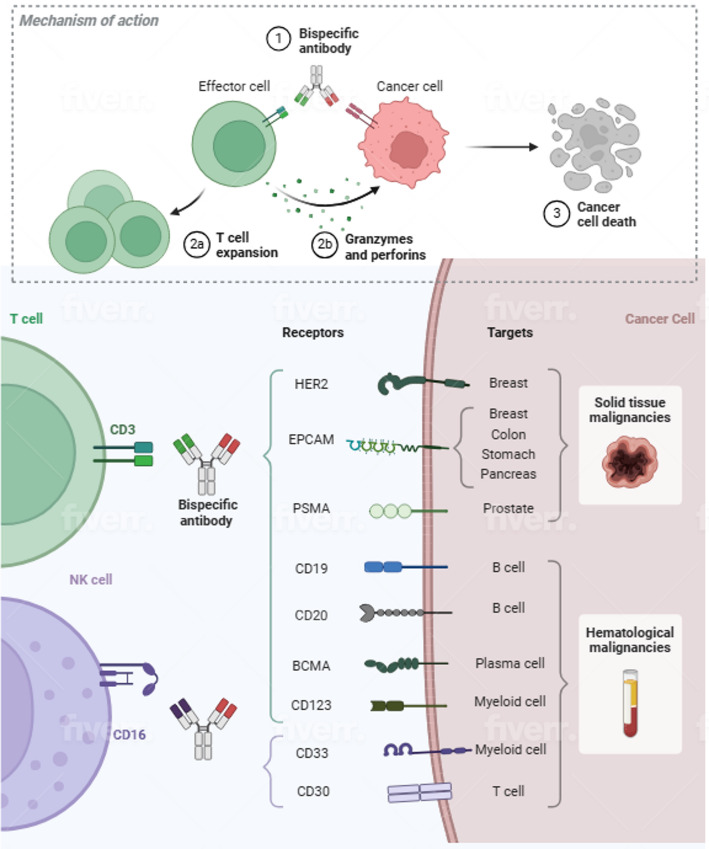
Bispecific antibodies
Bispecific antibodies (Fig. 3) have demonstrated extraordinary efficacy in ALL and NHL are also in development for CLL. In 2013, it was shown that blinatumomab induced T cell killing of CLL cells in vitro. Blinatumumab is a BsAb that is classified as a bispecific T cell engager (BiTE), and consists of a fusion of the scFV anti-CD3 single chain variable fragment with an anti-CD19 scFV linked by a short peptide. Wong et al. showed in a 2013 in vitro set of experiments that blinatumomab overcomes T-cell dysfunction in CLL. Blinatumumab was tested in PBMCs form 28 patients, both treatment naïve and previously treated, and led to secretions of cytokine and granzyme B, and T cell activation and proliferation, inducing tumor cell death by immunological synapse between T cells and tumor cells. Blinatumumab treated cultures induced increased absolute numbers of CD4 + T cells and CD8 + T cells (P = .01), which led to cytotoxicity against leukemic cells, and did not differ between treatment naïve and previously treated patients. Blinatumumab induced death of CLL cells by apoptosis which was tested by PMBC cultures treated with the agent since CLL cells readily undergo apoptosis in culture. T cell proliferation was also observed to be enhanced in that CD4 + and CD8 + T cell numbers in blinatumomab treated cultures were expanded and as shown by increase in the proliferation marker Ki67 in T cells. Blinatumomab treated cells showed a similar effect on percentage of effector memory CD4 + T cells by day 7 compared to untreated controls. In addition, cytokines IL-4, IL-5, and IL-10 were observed in blinatumomab treated cultures. T cells releasing cytotoxic granules and TNF-alpha and Interferon-gamma were increased in number after incubation with blinatumomab. In short, the in vitro experimental study showed activating effects of blinatumomab on T cells, although rapid killing was not observed [31].
Fig. 3.
Bispecific antibodies active for chronic lymphocytic leukemia. Bispecific antibodies are approved for hematologic malignancies with targets for B cells (blinatumomab) and myeloid cells (teclistamab). CLL cells have CD19, CD20 and BCMA antigens targeting by bispecific antibody agents. The bispecific antibody construct is also shown with an anti-CD-3 single chain variable fragment that engages the T-cell through an anti-CD19 scFV linked by a short peptide
In 2018, a study reported a novel CD3/CD20 bispecific antibody with a single-chain Fv-Fc format (CD19/CD3-scFv-Fc) that was developed and had a longer half-life (5 h) than blinatumomab (2.1 h) thus avoiding its rigorous continuous intravenous dosing for efficacy. CD19/CD3-scFv-Fc was engineered by combining human-anti-human CD19 scFv with humanized mouse anti-human CD3 scFv and a human Ig G1 Fc domain to enhance bsAb stability. This drug “demonstrated potent activity against CLL cells” in ibrutinib treated patients. In vitro experiments showed that the novel Ab induced > 90% killing of CLL cells and induced rapid killing of CLL cells that was greater than ibrutinib-treated patients over treatment naïve patients, serving as evidence that the Ab can effective in ibrutinib resistant disease. In addition, the BsAb had a good pharmacokinetic profile and led to immunological synapse formation between CLL cells and autologous T cells. Immune synapse formation was analyzed in cultured PBMC cells after exposure to the antibody and synapse formation between CLL cells and 16.3% (95% CI, 12.3–20.3) of CD8 T cells and 13.5% (95% CI 7.8–19.2) of CD4 T cells took place. When analyzed by flow cytometry, treatment effects after 7 and 12 days of PBMC culture time dependent killing of CLL cells were observed along with T cell expansion. After 12 days, median CLL cell specific killing was 91.6% with CD19/CD3-scFv-Fc and 90.4% with blinatumomab. Compared with controls, the novel agent increased CD8 T cell counts by 13.7-fold on day 7 (P = .002) and 56.4 on day 12 (P < .0001). CD4 T cell counts also increased in with median increase of 29.0-fold on day 7 and 51.8 fold on day 12. T cell proliferation, activation and granzyme B expression were also measured. After 5 and 7 days, CD4 and CD8 T cell proliferation was observed as occurring in cultures treated with the experimental antibody. Granzyme B increase was also observed in treatment naïve PBMCs after incubation with the Ab in both CD8 and CD4 T cells after 7 days with CD19/CD3-scFv-Fc incubation, compared with control with a median of 99.0% of CD8 T cells and 88.4% CD4 T cells that were positive for granzyme B. In vivo activity CLL killing as a result of the activity of the novel BsAb was also investigated in a patient-derived xenograft model. In a cohort of 51 engrafted mice from 4 different patients, 1 injection of reduced leukemic cell counts by > 90% compared to control, and 2 injections eliminated > 99% of CLL cells in blood and > 98% cells in spleen. Similarly, the agent demonstrated potent activity in ibrutinib treated CLL cells (median specific killing of 36.2% vs. 27.5% after 3 days and 94.3% vs21.6% after 7 days, respectively). CLL cells derived from four patients progressing with BTK mutations 34 to 49 months after starting ibrutinib showed that CD19/CD3-scFv-Fc induced time and antibody-dependent in vitro killing eliminating > 90% cells after 7 days. Compared to control, one dose reduced circulating CLLs by 93%. One dose of the construct CD19/CD3-scFv-Fc had an anti-leukemic effect of greater than 93% (range 93.2–99.6, P < .0001). In mice that were engrafted with CLL cells from 3 of 4 patients, 2 doses demonstrated activity in CLL cells (96.9% reduction). A fourth patient’s engrafted tumor had a 73.2% reduction in CLL cells. (P < .0001). As the study authors conclude, “[t]aken together, these data demonstrate the ability of CD19/CD3-scFv-Fc to eliminate ibrutinib-resistant CLL cells in vitro and in vivo.”. All of these results taken together illustrate the promise and potential of this novel antibody [32].
Teclistamab, a BsAb that targets the B cell maturation antigen, or BCMA, was found to have efficacy in multiple myeloma [10], and more recently in chronic lymphocytlc leukemia, since CLL cells have the BCMA, which is enhanced by inhibition of gamma-secretase. The expression of BMCA tumor antigen can target leukemic cells, allowing for effective targeting using teclistamab. Autologous CLL-derived T cells induce lysis of CLL in the presence of BCMA3xCD3. PBMCs of CLL patients were treated with 100 ng/mL teclistamab or control BsAbs for 96 h with or without presence of γ-secretase inhibition. After 48 h, a coculture of CLL in presence of teclistamab, induced cell death average lysis in CLL cells of 12.9% and 16.4% in both T-cell donors. The level of CLL lysis increased to 14.9% and 21.6% on average for both T-cell donors and was 25.8% and 27.4% after treatment of γ-secretase inhibitor, thus the study authors concluded that the inhibition of γ-secretase resulted in a modest increase in lysis, however this was considered nonsignificant due to variation among T cell donors. The lysis was a result of activity of CD8 + T cells rather than CD4 + T cells. While BCMAxCD3 levels are low, they are sufficient to target CLL cells. This study shows that BCMA as a potential target in CLL for T-cell redirection using the BCMAxCD3 [33].
Epcoritamab is a newer investigational agent with the CD3 and CD20 epitopes with potent activation and cytotoxic activity in patients with R/R CLL and was well tolerated.
Common toxicities associated with bispecific antibodies include cytokine release syndrome and neurotoxicities. CRS presents with flu, fever and chills and can occur as early as the first three days. More serious cardiovascular events such as hypotension and tachycardia can also occur. CRS is generally managed with tocilizumab and corticosteroids. Symptoms for neurotoxicities are usually personality changes, tremors and confusion and are reversible with supportive therapy [34]
Other novel agents
Vg9Vd2-T cells were reported in a recent research study by Weerdt et al. to have immunotherapeutic potential with the ability to lyse leukemic cells through the construction of a novel bispecific antibody. This type of T-cell engaging therapy takes advantage of the expression of CD1d on a majority of leukemic cells. Due to their strong “immunotherapeutic potential” as result of being able to trigger the overproduction of phosphoantigens on CLL cells, this bispecific single domain antibody boosts Vg9Vd2-T T cell responses through their specific activation by this antibody, that improves both the efficacy and side effect profile of autologous T cell based therapy. In terms of the experimental therapy designed to evaluate this response, CD1d being expressed in a majority of CLL patients led to induced activation of Vg9Vd2-T T cells, and thus lysing of autologous leukemic cells based on VHH single domain antibodies. When the bispecific VHH antibody is bound, the Vg9Vd2-T cell receptor retains its responsiveness to phosphoantigen, thus demonstrating “the immunotherapeutic potential of this novel CD1d-specific Vg9Vd2-T cell engager in CLL” [35].
Cyr et al. identified a Siglec-6, or sialic acid-binding immunoglobulin like lectin 6, novel target in a report in Journal for Immunotherapy of Cancer, that is absent on healthy cells. Siglec-6 monoclonal antibodies bind to the N-terminal lectin domain with high affinity and have high therapeutic potential. JML, a CLL binding Ab was identified as a target of Siglec-6, which was constructed from a T cell recruiting bispecific antibody with CD3 engineered into a single chain variable fragment Fc and a DART-Fc construct. In vivo it was found that RC-1 and RC-2 Siglec 6 mAbs bind with high affinity and specificity to JML-1; both mAbs were effective in activating T cells killing Siglec 6 target cells. According to report,” [t]he RC-1 clone in the DART–Fc format was the most potent T-biAb tested and was the only anti-Siglec- 6 T-biAb that eliminated Siglec-6 + primary CLL cells via autologous T cells at pathological T-to- CLL cell ratios.” This targeted immunotherapy was considered a pivotal discovery since the authors concluded that it revealed a high potency and specificity for eradicating Siglec 6 + CLL cells and sparing healthy B cells that also had Siglec-6. Another benefit is that diminished suppression of humoral immunity was also observed, a common adverse event associated with other mAbs such as rituximab. Cyr et al. conclude that “[o]ur data corroborate reports that T-biAb efficacy is dependent on synapse geometry and reveal that synapse architecture can be tuned via antibody engineering. Our fully human anti-Siglec- 6 antibodies and T-biAbs have potential for cancer immunotherapy.” [36].
Discussion
There has been robust research leading to increased treatment options for CLL including second-generation agents and cancer immunotherapies. As discussed, novel therapies are also emerging for overcoming the attendant concerns associated with chemotherapy, allogeneic hematopoietic stem cell transplantation, rituximab, BTK and BCL2 inhibitor targeted therapies and blinatumomab. Therapies have been designed to enhance cytotoxic T cell response in the microenvironment of leukemic cells, such Siglec-6 and Vg9Vd2-T cells. While other hematologic malignancies may have more solid therapeutic options, CLL has remains more difficult to treat with relatively higher rates of relapse/recurrence. Bispecific antibodies developed and shown to have efficacy in multiple myeloma such as teclistamab are now shown to be viable treatment options for CLL. Rituximab, the anti-CD20 monoclonal antibody and a mainstay for treatment in CLL, also has limitations that may be overcome by bispecific antibodies and CAR T cell therapies, such lisocabtagene maraleucel, a CD19 CAR T cell therapy.
Characterizing the tumor microenvironment may lead to increasing therapies for CLL. Recent studies have shown that CAR T cells may be combined with natural killer cells for killing of tumor cells in hematologic malignancies. Solid tumors such as melanoma and non-small cell lung cancer have been characterized as immune deserts and immune infiltrating which is relevant for immune checkpoint blockade and resistance however these therapies have found limited use in hematologic malignancies such as CLL.
Precision medicine is also entering the discussions surrounding CLL, particularly in the use of adaptive clinical trials to stratify CLL patients to account for tumor heterogeneity. TP53 mutations usually co-occurring the deletion 17p at the 17p13 locus and can be missense, frameshift or splicing mutations that abolish the tumor suppressive function of p53. The mutation is prognostic since it associated with poor survival and worse OS. IGHV mutations also occur and arise through somatic hypermutation, a “physiological process to generate Ig diversity during normal B-cell maturation which may significant since it is thought that CLL may originate from B cell cells at different stages of maturation.” Emerging prognostic biomarkers include NOTCH1 and ATM [37]. Scanland et al. write that predictive biomarkers for CLL are being identified that lead to improved clinical decision making. Machine learning algorithms are analyzing large data that have been collected, characterizing “genomics guided medicine” that has made “remarkable advances in the literature.” In the PreVent-ACaLL study, an algorithm trained on 4149 patients from the Danish National CLL registry (CLL-TIM) was evaluated for predicting infection risk and providing clinical decision support in newly diagnosed patients with high risk of infection when treated with venetoclax plus acalabrutinib. The development of similar models was suggested towards the goals of treatment guidance for high-risk patients and to prevent resistance. The monitoring of clinical response in real time when resistance is observed was a significant implication of the study [8].
Conclusion
Emerging new classes of small molecule drugs and cancer immunotherapies such as bispecific antibodies and CAR T cell therapies are being developed and evaluated for CLL, a hematologic malignancy that may be considered resistant to treat and high prevalence and mortality rates in the population, particularly in elderly populations who have co-morbidities. Future studies should focus on agents with novel mechanisms that lead to pro-apoptosis of leukemic cells and possibly the development of precision medicine clinical trials designed to stratify patients and prognostic and predictive biomarkers for improved clinical decision making.
Acknowledgements
P.H. wishes to thank Springer Nature Designing Team for the Graphical Abstract in this manuscript.
Author contributions
P.H. designed, wrote, edited and reviewed the manuscript.
Funding
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
P.H. owns stock in Abbvie. Abbvie manufactures venetoclax and ibrutinib, drugs extensively referenced in this review. The presentation of data and discussion of these therapies were not influenced by this competing interest. The paper highlights drug resistance and presents alternatives to venetoclax and ibrutinib as evidence of impartiality towards these therapeutics.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Perutelli F, Jones R, Griggio V, Vitale C, Coscia M. Immunotherapeutic strategies in chronic lymphocytic leukemia: advances and challenges. Front Onc. 2022;12. [DOI] [PMC free article] [PubMed]
- 2.Bennett R, Seymour JF. Update on the management of relapsed/refractory chronic lymphocytic leukemia. Blood Can J. 2024;14:33. [Google Scholar]
- 3.Bennett R, Thompson E, Tam C. SOHO state of the Art updates and next questions. Mechanisms of resistance to BCL2 inhibitor therapy in chronic lymphocytic leukemia and potential future therapeutic directions. Chronic Lymph Myeloma Leuk. 2022;11:795–804. [DOI] [PubMed] [Google Scholar]
- 4.Niemann CU, Munir T, Moreno C, Owen C, Follows GA, et al. Fixed-duration ibrutinib-venetoclax versus chlorambucil-obinutuzumab in previously untreated chronic lymphocytic leukemia (GLOW): 4-year follow-up from a multicentre, open-label, randomized, phase 3 trial. Lancet Oncol. 2023;24(12):1423–33. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Li S, Wang Q, Feng Y, Xing H, et al. Sonrotoclax overcomes BCL2 G101V mutation-induced Venetolax resistance in preclinical models of hematologic malignancy. Blood. 2024;143(18):1825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucas F, Larkin K, Gregory T, Orwick S, Doong T-J et al. Novel BCL2 mutations in venetoclax-resistant CLL patients with BTK/PLCG mutations. Blood. 2020;135(24). [DOI] [PMC free article] [PubMed]
- 7.Kanagal-Shamanna R, Jain P, Patel KP, Routbort M, Bueso-Ramos C et al. Targeted multigene deep sequencing of Bruton tyrosine kinase inhibitor-resistant chronic lymphocytic leukemia with disease progression and Richter transformation. Cancer. 2019;125(4). [DOI] [PubMed]
- 8.Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D’Rozario J et al. Venetoclax-Rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12). [DOI] [PubMed]
- 9.Al-Sawaf O, Robrecht S, Zhang C, Oliverieri S, Chang M et al. Venetoclax-obinutuzumab for previously treated chronic lymphocytic leukemia: 6-year resutsl fo the randomized phase 3 CLL14 study. Blood. 2024;144(18). [DOI] [PMC free article] [PubMed]
- 10.Skanland SS, Mato AR. Overcoming resistance to targeted therapies in chronic lymphocytic leukemia. Blood Adv. 2021. [DOI] [PMC free article] [PubMed]
- 11.Mato AR, Hess LM, Chen Y, Abada PB, Konig H, et al. Outcomes for patients with chronic lymphocytic leukemia (CLL) previously treated with both a covalent BTK and BCL 2 inhibitor in the united states: A real world database study. Chronic Lymph Myeloma Leuk. 2022;23:57–67. [DOI] [PubMed] [Google Scholar]
- 12.Mato A, Woyach J, Brown JR, Chia P, Patel K et al. Pirtobrutinib after a covalent BTK inhibitor in chronic lymphocytic leukemia. NEJM. 2023;389.
- 13.Sharman JP, Munir T, Grosicki S, Roeker L, Burke JM et al. BRUIN CLL-321: randomized phase III trial of pirtobrutinib versus idelalisib plus rituximab (IdelaR) or bendamustine plus rituximab (BR) in BTK inhibitor pretreated chronic lymphocytic leukemia/small lymphocytic lymphoma. Blood. 2024;Abstract 642.
- 14.Tadmor T, Eyre TA, Benjamini O, Chaudrhy, Shen J, Leng S et al. May. BELLWAVE-011: Phase 3 randomized trial of nemtabrutinib versus ibrutinib or acalabrutinib in untreated chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. Meeting Abstract. 2024.
- 15.Roeker LE, Dreger P, Brown JR, Lahoud OB, Eyre TA, Brander DM, et al. Allogeneic stem cell transplantation for chronic lymphocytic leukemia in the era of novel agents. Blood Adv. 2020;4(16):3977–89. 10.1182/bloodadvances.2020001956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu-Monette ZY, Zhou J, Young KH. PD-1 expression and clinical PD-1 Blockade in B-Cell lymphomas. Blood. 2018;131(1):68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.1, Sandhu S, Mulligan SP. Ofatumumab and its role as immunotherapy in chronic lymphocytic leukemia. Hematologica. 2015;100(4). [DOI] [PMC free article] [PubMed]
- 18.Salles GA, Morschhauser F, Solal-Celigny P, Thieblemont C, Lamy T et al. Obinutuzumab (GA101) in patients with relapsed/refractory indolent non-Hodgkin lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31(23). [DOI] [PubMed]
- 19.Owen CJ, Stewart DJ. Obinutuzumab for the treatment of patients with previously untreated chronic lymphocytic leukemia: overview and perspective. Ther Adv Hematol. 2015;4. [DOI] [PMC free article] [PubMed]
- 20.Roeker LE, Leslie LA, Soumerai JD, Falco V, Panton G. A phase 2 study evaluating the addition of ublituximab and umbralisib (U2) to ibrutinib in patients with chronic lymphocytic leukemia (CLL): a minimal residual disease (MRD)-driven, time-limited approach. Blood. 2021;138(Supplement 1).
- 21.Staber PB, Jurczak W, Greil R, Vucinic V, Middeke JM. Tafasitamab combined with idelalisib or venetoclax in patients with CLL previously treated with a BTK inhibitor. Leuk Lymphoma. 2021;62(14). [DOI] [PubMed]
- 22.Robak T, Hellman A, Kloczko J, Loscertales J, Lech-Maranda E et al. Randomized phase 2 study of Otlertuzumab and Bendamustine versus Bendamustine in patients with relapsed chronic lymphocytic leukaemia. B J Haem. 2016. [DOI] [PMC free article] [PubMed]
- 23.Choi MY, Widhopf GF, Ghia Em, Kidwell RL, Kamrui Hasan M et al. Phase I trial: Cirmtuzumab inhibits ROR1 signaling and stemness signatures in patients with chronic lymphocytic leukemia. 2018;22(6). [DOI] [PMC free article] [PubMed]
- 24.Todorovic Z, Todorovic D, Markovic V, Ladjevac N, Zdravkovic N. CAR T cell therapy for chronic lymphocytic leukemia: successes and shortcomings. Curr Oncol. 2022;29:3647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddiqi T, Maloney DG, Kenderian SS, Brander DM, Dorrittie K et al. Lisocabtagene Maraleucel in chronic lymphocytic leukaemia and small lymphocytic lymphoma (TRANSCEND CLL 004): a multicentre, open-label, single-arm, phase 1–2 study. Lancet. 2023;402. [DOI] [PMC free article] [PubMed]
- 26.Fujiwara H. Adoptive immunotherapy for hematological malignancies using T cells gene-modified to express tumor antigenspecific receptors. Pharmaceuticals. 2014;7:1049–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancikova V, Smida M. Current state of CAR T-Cell therapy in chronic lymphocytic leukemia. Int J Mol Sci. 2021;22:5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porter DL, Levine BL, Kalos M, Carroll RG, Binder GK, Teachey D, Samanta M, Lakhal M, Gloss B. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brentjens RJ, Rivière I, Park JH, Wang X, Wang Y, Purdon TJ, Hsu M, Devlin SM, Halton E, Lamanna N, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Can I, Cox MJ, Siegler EL, Sakemura R, Kenderian SS. Challenges of chimeric antigen receptor T-cell therapy in chronic lymphocytic leukemia: lessons learned. Exp Hematol. 2022;108:1–7. [DOI] [PubMed] [Google Scholar]
- 31.Wong R, Pepper C, Brennan P, Nagorsen D, Man S, Fegan C. Blinatumomab induces autologous T-cell killing of chronic lymphocytic leukemia cells. Haematologica. 2013;98(2). [DOI] [PMC free article] [PubMed]
- 32.Robinson HR, Qi J, Cook EM, Nichols C, Dadashian EL, et al. A CD19/CD3 bispecific antibody for effective immunotherapy of chronic lymphocytic leukemia in the ibrutinib era. Blood. 2018;132(5):521–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martens AWJ, Rietveld JM, Boer R, Peters FS, Ngo A, Lotte WHG, et al. Redirecting T-cell activity with Anti-BCMA/Anti-CD3 bispecific antibodies in chronic lymphocytic leukemia and other B-cell lymphomas. Cancer Res Commun. 2022;2(5):330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hays P. Clinical development and therapeutic applications of bispecific antibodies for hematologic malignancies. Cancer Treat Res. 2022;183:287–315. [DOI] [PubMed] [Google Scholar]
- 35.Weerdt I, Lameris R, Rubin JM, Boer R, Kloosterman J, et al. A bispecific Single-Domain antibody boosts autologous V(delta9V(gamma)-T cell responses toward a CD1d in chronic lymphocytic leukemia. Clin Canc Res. 2021;27:1744–55. [DOI] [PubMed] [Google Scholar]
- 36.Cyr MG, Mhibik M, Qi J, Peng H, Chang J et al. Patient-derived Siglec-6-targeting antibodies engineered for T-cell recruitment have a potential therapeutic utility in chronic lymphocytic leukemia. J Imm Can. 2022;10. [DOI] [PMC free article] [PubMed]
- 37.Lee J, Wang L. Prognostic and predictive biomarkers in chronic lymphocytic leukemia. J Mol Diagn. 2020;22(9). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.