Abstract
Background
Diabetes Mellitus is a common chronic metabolic disease in the world population. There is evidences on the anti-hyperglycemic effects of different parts of fennel; however, the reports about antidiabetic activity of fennel leaves are not enough. In this experiment, effects of fennel leaf aqueous extract on biochemical alterations and the histopathology of the pancreas in alloxan induced diabetic rats were studied.
Methods
Fifty adult male rats were divided into five groups: the non-diabetic and the diabetic control groups, and three diabetic groups treated with different doses of fennel leaf extract (50, 100 and 200 mg/kg/day). Blood glucose, body weight, serum insulin and C-peptide levels were determined. The pancreas histology was evaluated by preparation of paraffin sections. They were stained using hematoxylin and eosin stain. Morphometrically, the mean number and the area of the islets of Langerhans were measured.
Results
Fennel leaf extract in different doses caused a reduction in blood glucose, and an increase in body weight, serum insulin and C-peptide. In diabetic treated rats, fennel leaf extract significantly increased the number and area of the Islets of Langerhans.
Conclusions
Our results indicated the anti-hyperglycemic effects of fennel leaf extract and morphologic improvement of the pancreatic islets of Langerhans in alloxan induced diabetic rats.
Keyword: Fennel leaf, Alloxan, Diabetes mellitus, Islets of Langerhans
Introduction
Diabetes mellitus is a common chronic human disorder and is a globally important cause of morbidity and mortality [1]. Diabetes is estimated to cause one in nine deaths among adults aged 20–79 years[2] and its global prevalence is estimated at 529 million people living with diabetes worldwide [3]. Chronic hyperglycemia in diabetes mellitus leads to the development of micro- and macro vascular diseases which cause irreversible damage [4].The damage is primarily due to high oxidative stress, an increase in ROS levels and the activation of inflammatory pathways [5].
For a long time, medicinal plants have been used for the prevention and treatment of diseases. In most developing countries, plant drugs are the primary choice for health care needs [6]. The role of traditional medicine in the treatment of diabetes is attractive in scientific research [7–9]. Phenolic acids, flavonoids, and terpenoids are major antidiabetic components of traditional medicinal plants [10]. The hypoglycemic activity of these components is attributed to stimulating the secretion of insulin by the islets of Langerhans in the pancreas, the reduction of insulin resistance, the antioxidant activity, and the inhibition of α-glucosidase and α-amylase [9].
Fennel is an important medicinal and aromatic plant that is widely used in traditional medicine. Although it is observed that fennel is endemic to the Mediterranean, in other world regions such as Iran this plant wildly grows and is cultivated [11]. The essential oil extracted from fennel exhibited antibacterial, antifungal, antithrombotic, and anti-hirsutism activities [12, 13]. It has long been used for treating respiratory and gastrointestinal disorders [12]. In addition, hypoglycemic and antioxidant activities of the fennel have been reported [13, 14]. The leaves of fennel have been shown to contain a number of flavonoids, fixed oil, protein, and organic acids, which are responsible for antioxidant, and antidiabetic effects [9, 15]. A few reports have shown the effects of fennel leaf on blood glucose levels in streptozotocin induced diabetic rats [14]. In spite of all that, there are little evidence about biochemical and histopathological effects of fennel leaf in diabetes.
The aim of the present study is to evaluate the effects of aqueous extract of fennel leaves on blood glucose level and histopathology of pancreas in alloxan induced diabetic rats.
Material and methods
Extraction method
The fennel was purchased from a local herbal market in Kashan,Iran and approved by Kashan Botanical Garden (H.N: H.K.B.G.1065 GPS:33.96983629910757,51.477861799921016). After identifying the plant, its leaves was separated and dried in the shadow. Then, the ground fennel leaf was extracted using soxhlet extractor (aqueous). After evaporation and reduction of the solvent by a rotary evaporator, it was dried in an oven and refrigerated at 4ºC until use. A total of 100 g of fennel leaves were used for extraction, yielding 8.75 g of extract. All these processes were done in the Kashan Essence Research Center.
Animals and study design
Fifty Sprague–Dawley male rats weighing 150–200 g were obtained from the laboratory animal house, Kashan University of Medical Sciences (KAUMS), Iran. Rats were maintained at 21–24 °C with a 40–60% relative humidity and 12 h light/dark cycle intervals with standard rat feed and water ad libitum. The ethical committee of KAUMS approved the experiment (ethical code: 1911).Animals were divided into five groups, 10 rats in each group. Groups I and II included non-diabetic and diabetic control rats respectively. Groups IIIa, IIIb, and IIIc were three groups for diabetes treatment. Diabetes was induced by a single intraperitoneal injection of alloxan at the dose of 170 mg/kg body weight. Alloxan solutions were freshly prepared and made by alloxan monohydrate (Sigma) dissolved in acetate buffer (pH 4.5). The rats fasted for 12 h before and after the injection. Six days following alloxan injection, blood glucose was measured. Rats with blood glucose levels 220–250 mg/dL were determined as diabetic animals [16]. Groups IIIa, IIIb, and IIIc consisted of diabetic rats that were treated with three different doses of fennel leaf aqueous extract orally (50, 100, and 200 mg/kg body weight respectively).
Measurements of blood glucose and body weight
On days 0, 7, 14, 21, and 30 from the beginning of the experiment, body weight and blood glucose were recorded in each group. For the measurement of blood glucose, after overnight fasting, a sample of tail blood was drawn by introducing the needle into the distal end of the tail and blood glucose was measured with a blood glucose monitoring device[17].
Measurements of blood insulin and C-peptide concentrations
After 30 days from the experiment, the animals from each group was anesthetized with ether, and blood samples were drawn from the heart. After centrifugation of the samples, serum was separated from the blood cells and maintained at −80 °C. Insulin(μIU/mL) and C-peptide(pg/ml) concentrations were measured in serum using the kit (Pars Azmoon Co, Tehran, Iran).
Histopathological study
By the end of the experiment and after collection of blood samples from the heart, rats were sacrificed. The whole pancreas was removed, fixed in 10% buffered formalin, processed, and embedded into paraffin blocks. Paraffin Sects. 6 μm thick were stained with Hematoxylin and Eosin (H&E). The slides were studied under the light microscope (Nikon, Germany). By a camera connected to the microscope, the × 100, × 200 and × 400 images of the sections were prepared. For each Sect. 5 fields were selected and studied using a light microscope (Nikon, Germany). [18].Morphometrically analysis for the islets of Langerhans was done using imaging software using the DS camera Control Unit DS-L2. The mean number and areas of the islets and the mean number of cells in each islet are estimated.
Statistical analysis
Data were analyzed and presented as means ± SD. Differences between data were analyzed using one-way ANOVA followed by Tukey’s post hoc test. P < 0.05 was considered significant.
Results
Effect of fennel leaf aqueous extract on blood glucose concentration
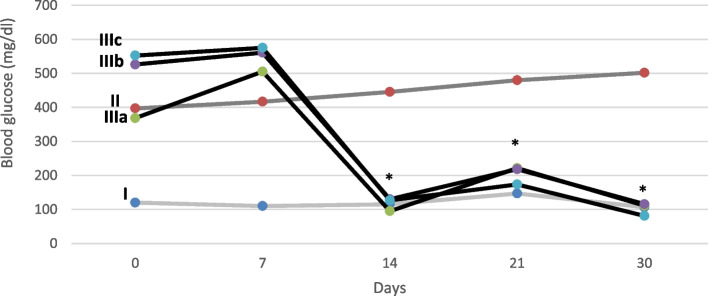
Blood glucose concentration increased significantly (P < 0.05) following alloxan injection compared with the control group during the experiment. The rats in groups IIIa, IIIb and IIIc that received doses of 50, 100 and 200 mg/kg fennel, respectively, showed a significant decrease (P < 0.01) compared with diabetic group II on days 14, 21, and 30 (Table 1; Fig. 1).
Table 1.
Blood glucose concentration in different groups of the experiment
| Experimental groups |
Period of treatment | ||||
|---|---|---|---|---|---|
| 0d | 7d | 14d | 21d | 30d | |
|
Group I (Control) |
120 ± 3.07 | 110 ± 1.65 | 115 ± 1.84 | 146.84 ± 4.42 | 106.65 ± 1.65 |
|
Group II (Diabetic) |
397 ± 41.9 | 416.75 ± 50.04 | 445.5 ± 36.36 | 479.83 ± 59.23 | 501.83 ± 63.9 |
|
Group IIIa (Fennel 50 mg/kg BW) |
368.43 ± 56.35 | 505.29 ± 64.16 | 95.14 ± 12.79* | 221.57 ± 53.97* | 111.14 ± 18.9* |
|
GroupIIIb (Fennel 100 mg/kg BW) |
526 ± 68.3 | 561 ± 18.68 | 130 ± 32.04* | 218.14 ± 67.41* | 115.57 ± 28.51* |
|
GroupIIIc (Fennel 200 mg/kg BW) |
552.5 ± 6.4 | 575.17 ± 31.26 | 126.73 ± 14* | 173.5 ± 10.7* | 81.17 ± 29.9* |
*P < 0.01
Fig. 1.
Blood glucose level on different days in five groups of the experiment. *P < 0.01
Effect of fennel leaf aqueous extract on the body weight
After 30 days from the beginning of the experiment, rats in the diabetic group significantly lost their initial body weight (P < 0.05) as compared with the normal control group (Table 2; Figure 2).
Table 2.
Percentage of body weight changes in different groups of the experiment
| Experimental groups |
Period of treatment | |||||
|---|---|---|---|---|---|---|
| 0d | 6d | 12d | 18d | 24d | 30d | |
|
Group I (Control) |
0 | 0.81 ± 1.3 | 4.55 ± 2.4 | 6.26 ± 2.80 | 3.33 ± 3.096 | 5.04 ± 2.38 |
|
Group II (Diabetic) |
0 | −5.937 ± 0.44 | −8.75 ± 0.83 | −12.86 ± 0.85 | −16.15 ± 0.99 | −17.20 ± 0.83* |
|
Group IIIa (Fennel 50 mg/kg BW) |
0 | 1.86 ± 0.53 | 6.04 ± 0.55 | 5.013 ± 0.77 | 5.08 ± 0.77 | 4.50 ± 0.55** |
|
Group IIIb (Fennel 100 mg/kg BW) |
0 | 7.57 ± 0.78 | 7.24 ± 0.59 | 5.20 ± 0.3 | −0.59 ± 0.5 | 3.55 ± 0.59** |
|
Group IIIc (Fennel 200 mg/kg BW) |
0 | −3.56 ± 0.31 | −0.16 ± 0.59 | −6.56 ± 0.77 | −4.66 ± 0.79 | −7.59 ± 0.59 ** |
*P < 0.05, ** P < 0.01
Fig. 2.
Percentage of body weight changes in different groups of the experiment. *P < 0.05, ** P < 0.01
Following the administration of fennel leaf aqueous extract at 50 mg/kg and 100 mg/kg for 30 days increased their initial body weight to the same level as the normal control group (P < 0.01). After 30 days of treatment with 200 mg/kg of fennel leaf aqueous extract, body weight was significantly greater than that in the diabetic group (P < 0.01) and less than in IIIa, IIIb groups and I (P < 0.05).
Effect of fennel leaf aqueous extract on serum insulin concentration
The mean serum insulin level in the diabetic group decreased significantly (P < 0.05), as compared with the control animals. The mean serum insulin concentration of the diabetic rats that fennel leaf aqueous extract increased as compared with the diabetic animal rats 30th-day of experimental period. The serum insulin concentration of the diabetic rats that received fennel leaf aqueous extract at doses of 50, 100 and 200 mg/kg body weight was significantly higher than in the diabetic rats (P < 0.01) (Fig. 3).
Fig. 3.
Mean serum insulin levels in different groups on the 30th day of experimental period. I. Control nondiabetic group; II. Diabetic group; IIIa. Fennel leaf aqueous extract 50 mg/kg BW; IIIb. Fennel leaf aqueous extract 100 mg/kg BW; IIIc. Fennel leaf aqueous extract 200 mg/kg BW.. * P < 0.05 significantly different from Group I; **P < 0.01 significantly different from Group II
Effect of fennel leaf aqueous extract on blood C-peptide concentration
The mean blood C-peptide level in diabetic group decreased significantly (P < 0.001), compared with the non-diabetic control animals. The mean blood C-peptide concentration of the diabetic rats that received fennel leaf aqueous extract increased compared with the diabetic animals on 30thday of experimental period. The mean blood C-peptide concentration of the diabetic rats that received fennel leaf aqueous extract at doses of 50, 100 and 200 mg/kg body weight was significantly higher than that in the diabetic rats (P < 0.01) (Fig. 4).
Fig. 4.
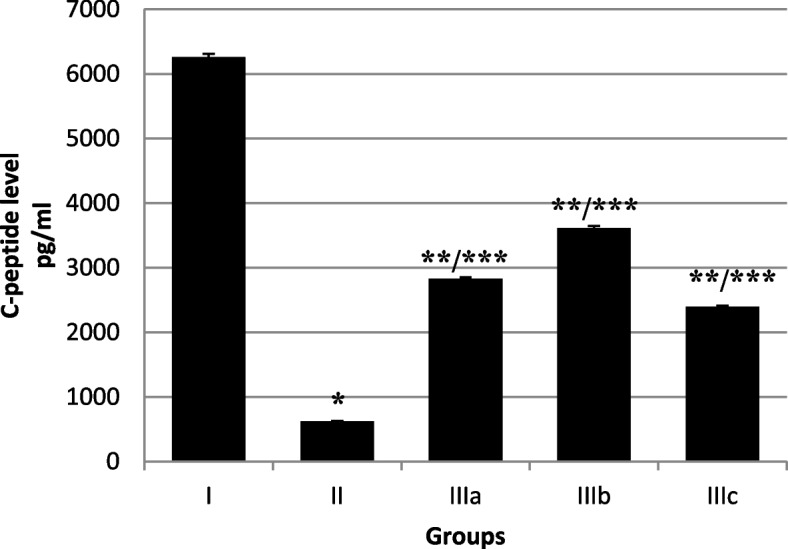
Mean blood C-peptide levels in different groups on the 30th day of experimental period. I. Control non-diabetic group; II. Diabetic group; IIIa. Fennel leaf aqueous extract 50 mg/kg BW; IIIb. Fennel leaf aqueous extract 100 mg/kg BW; IIIc. Fennel leaf aqueous extract 200 mg/kg BW. *P < 0.001, **P < 0.01 significantly different from Group I; ***P < 0.01significantly different from Group II
Histopathology of pancreatic islets
The normal histology of the islets of Langerhans from the control group was shown in Fig. 5A. In the diabetic group, pancreatic islets were atrophied and detracted. Alloxan led to necrotic changes of the islets, especially in the center of the islets. The size and number of islets in the diabetic group obviously reduced (Fig. 5B).
Fig. 5.
Sections of the pancreas stained by H&E. A,a. Section from a control normal rat (group I) showing a normal Islet (arrow); B,b. Section from the pancreas of a diabetic rat (group II) showing an atrophic appearance with irregular outlining of the islet (arrow); C,c; D,d; E,e. Sections from the pancreas of experimental groups IIIa, IIIb and IIIc presenting very similar morphology to the control normal group I with normal Islets (arrows)
Fennel leaf aqueous led to a protective effect in diabetic rats. In experimental groups, the pancreatic tissue retained its normal morphology (Figs. 5 and 6).
Fig. 6.
(A) Mean number of the islets of Langerhans in different groups at 30th-day of experimental period. *P < 0.05, significantly different from Group I; P < 0.05, significantly different from GroupII, (B). Mean cell number of the islets of Langerhans in different groups at 30th-day of experimental period. * P < 0.01, significantly different from Group I; ** P < 0.01, significantly different from GroupII. (C) Mean area of the islets of Langerhans in different groups at 30th-day of experimental period. * P < 0.01, significantly different from Group I; ** P < 0.01, significantly different from GroupII, I. Control nondiabetic group; II. Diabetic group; IIIa. Fennel leaf aqueous extract 50 mg/kg BW; IIIb. Fennel leaf aqueous extract 100 mg/kg BW; IIIc. Fennel leaf aqueous extract 200 mg/kg BW
Discussion
Present study showed fennel leaves aqueous extract can reduce blood glucose levels and intensity of weight loss, and increased serum insulin and C-peptide concentrations significantly; meanwhile morphological investigations showed that fennel leaf aqueous extract could induce significant improvements in morphology of pancreatic islets.
Diabetes mellitus is a common endocrine disorder characterized by hyperglycemia and the pathological changes in the pancreatic islets of Langerhans [19]. Most synthetic drugs have short- and long-term side effects, therefore herbal medicine is becoming increasingly interesting in the treatment of many disorders [20]. The WHO has urged that medicinal herbs be subsequently considered in the treatment of diabetes mellitus [21].
In the present study, biochemical and histopathological effects of fennel leaf aqueous extract were investigated on rats with alloxan-induced diabetes. Alloxan has been widely employed to induce type-1 diabetes in animal models. Alloxan is accessible, inexpensive and one of the most common agent for creation of type- 1 diabetes [22]. Injection of alloxan to the rats led to a reduction in insulin and then elevated serum glucose levels and weight loss.
Fennel is an aromatic plant with yellow flowers, feathery leaves and pharmacological properties. Although the impact of fennel essential oil on the hyperglycemia and pathological abnormalities in experimental diabetes was evaluated in many studies [13, 23], the experimental studies related to the leaf aqueous extract in diabetes are not well-documented. In traditional medicine, fennel tree leaves were recommended for the treatment of mouth ulcer, liver pain, and kidney ailments and diabetes mellitus [24].
The present biochemical findings supported the results of the previous studies, which reported that administration of fennel can lead to a decline of glucose levels, and the severity of weight loss compared to diabetic control group [23, 25].Topical and oral administration of fennel essential oil has shown to reduce glycemic levels [23]. Significant effects of fennel seeds extract on blood glucose were demonstrated [26].El-Ouadyet al described the leaves aqueous extract of fennel has a significant hypoglycemic effect in streptozotocin-induced diabetic rats [14]. The present study aligns with the findings of El-Ouady et al. [14], which showed that fennel leaf extract can reduce blood glucose levels. The main differences between our experiment and theirs include the diabetic model employed, variations in body weight, the geographical region where the fennel was cultivated, the dosage of fennel leaf extract used, the duration of extract administration, and the biochemical parameters measured, such as insulin and C-peptide, as well as histological evaluations. In our experiment, treatment with different doses (50,100 and 200 mg/kg body weight) of fennel leaf extract significantly decreased blood glucose.
Present study found that while 200 mg/kg of fennel leaf extract significantly reduced blood glucose levels, it had minimal impact on body weight. Some evidence supports that higher doses of fennel leaf extract may influence body weight changes, as fennel has been shown to decrease food efficiency [27] and manage appetite [28]. However, other studies indicate that fennel does not affect body weight [14, 29] and [30]. Therefore, it is possible that higher doses of the extract could prevent weight gain.
The identified ingredients of fennel leaf such asphenolic acids (caffeic acid, quinic acid, and chlorogenic acid) have been exhibited to reduce blood glucose and anti-diabetic activity [9]. Caffeic acid reduces blood glucose and reveals a protective effect against diabetes [31]; quinic acid increases mitochondrial Ca2+ and stimulate insulin secretion in pancreatic beta‐cells [32]; chlorogenic acid has a protective effect on pancreatic beta‐cells and blood glucose control [33]. It seems different reasons are responsible for reducing blood-glucose such as: raise the insulin sensitivity of the receptors on cells, changes in energy metabolism and increases in insulin secretion from remaining pancreatic cells, increasing absorption of dietary carbohydrates in alimentary tract or facilitation of glucose consumption by peripheral tissues [34].
Our histological findings showed that fennel leaf extract reduced islets of Langerhans damage in diabetic rats. In diabetic animals, pancreatic β-cells were destroyed and their number declined. This causes a reduction in serum insulin concentration. In our experiment, we considered the effect of fennel leaf aqueous extract on the histopathology of pancreas and the investigation of islets of Langerhans in rats with alloxan-induced diabetes. Our findings showed fennel leaf aqueous extract preserved the size and number of the islets following induction of diabetes. It seems fennel leaf extract can protect the islets against alloxan toxicity. Our results confirm the report of protective effects fennel essential oil on pancreatic islets [25]. The oxidative stress due to hyperglycemia led to the histopathological changes in pancreas gland [35, 36]. Diabetes elevates free radical generation and accordingly decrease antioxidant levels [35]. It seems anti-oxidative effects of fennel improves the changes and carried out production of insulin as a result of pancreatic exocrine and endocrine regeneration [25]. It can be explained that phenolic compounds of fennel underlie the anti-oxidative effects and the regenerative capability of fennel [37].
Our research suggests that regardless of insulin levels, higher amounts of fennel leaf extract do not lead to increased C-peptide levels compared to lower amounts. This result could be associated with nephropathy observed in diabetic animals. While insulin is primarily processed by the liver, C-peptide is mainly metabolized by the kidneys [38]. Because of the potential for diabetes-related neuropathy or the effects of a high dose of fennel leaf extract, there may be an imbalance between insulin and C-peptide ratios. More research is necessary to verify this hypothesis.
Conclusion
We conclude that the administration of fennel leaf aqueous extract improved the hyperglycemia and histopathological changes of the pancreatic islets of Langerhans. The present findings may support the therapeutic effects of fennel leaf extract as complementary treatments in diabetes.
Acknowledgements
Not applicable
Authors’ contributions
MAT and MN contributed to the conception and design of the project and wrote the draft of the manuscript. MA, ZV, SSA and MJN performed the experiments, analyzed and interpreted the data and edited the manuscript. All authors read and approved the final manuscript.
Funding
This work was financially supported by Research Deputy of Kashan University of Medical Sciences (KAUMS, No. 91042) and Anatomical Sciences Research Center of KAUMS.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Kashan University of Medical Sciences (ethical code: 1911).
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Detaille D, Guigas B, Chauvin C, Batandier C, Fontaine E, Wiernsperger N, et al. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes. 2005;54:2179–87. [DOI] [PubMed] [Google Scholar]
- 2.Saeedi P, Salpea P, Karuranga S, Petersohn I, Malanda B, Gregg EW, et al. Mortality attributable to diabetes in 20–79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2020;162: 108086. [DOI] [PubMed] [Google Scholar]
- 3.Collaborators GBDD. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402:203–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miklosz A, Chabowski A. Efficacy of adipose-derived mesenchymal stem cell therapy in the treatment of chronic micro- and macrovascular complications of diabetes. Diabetes Obes Metab. 2024;26:793–808. [DOI] [PubMed] [Google Scholar]
- 5.Wronka M, Krzeminska J, Mlynarska E, Rysz J, Franczyk B. The Influence of Lifestyle and Treatment on Oxidative Stress and Inflammation in Diabetes. Int J Mol Sci. 2022;23:15743 [DOI] [PMC free article] [PubMed]
- 6.Sofowora A, Ogunbodede E, Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr J Tradit Complement Altern Med. 2013;10:210–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sayah K, Marmouzi I, Naceiri Mrabti H, Cherrah Y, Faouzi ME. Antioxidant Activity and Inhibitory Potential of Cistus salviifolius (L.) and Cistus monspeliensis (L.) Aerial Parts Extracts against Key Enzymes Linked to Hyperglycemia. Biomed Res Int. 2017;2017:2789482. [DOI] [PMC free article] [PubMed]
- 8.El Omari N, Sayah K, Fettach S, El Blidi O, Bouyahya A, Faouzi MEA, et al. Evaluation of In Vitro Antioxidant and Antidiabetic Activities of Aristolochia longa Extracts. Evid Based Complement Alternat Med. 2019;2019:7384735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sayah K, El Omari N, Kharbach M, Bouyahya A, Kamal R, Marmouzi I, et al. Comparative Study of Leaf and Rootstock Aqueous Extracts of Foeniculum vulgare on Chemical Profile and In Vitro Antioxidant and Antihyperglycemic Activities. Adv Pharmacol Pharm Sci. 2020;2020:8852570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamba SS, Buch KY, Lewis H, Lamba J. Phytochemicals as potential hypoglycemic agents. Stud Nat Prod Chem. 2000;21:457–96. [Google Scholar]
- 11.Rahimi R, Ardekani MR. Medicinal properties of Foeniculum vulgare Mill. in traditional Iranian medicine and modern phytotherapy. Chin J Integr Med. 2013;19:73–9. [DOI] [PubMed]
- 12.Badgujar SB, Patel VV, Bandivdekar AH. Foeniculum vulgare Mill: a review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed Res Int. 2014;2014: 842674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdelbaky AS, Mohamed A, Abd El-Mageed TA, Rady MM, Alshehri F, El-Saadony MT, et al. Bio-organic fertilizers promote yield, chemical composition, and antioxidant and antimicrobial activities of essential oil in fennel (Foeniculum vulgare) seeds. Sci Rep. 2023;13:13935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Ouady F, Lahrach N, Ajebli M, Haidani AE, Eddouks M. Antihyperglycemic Effect of the Aqueous Extract of Foeniculum vulgare in Normal and Streptozotocin-induced Diabetic Rats. Cardiovasc Hematol Disord Drug Targets. 2020;20:54–63. [DOI] [PubMed] [Google Scholar]
- 15.Picon PD, Picon RV, Costa AF, Sander GB, Amaral KM, Aboy AL, et al. Randomized clinical trial of a phytotherapic compound containing Pimpinella anisum, Foeniculum vulgare, Sambucus nigra, and Cassia augustifolia for chronic constipation. BMC Complement Altern Med. 2010;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rehman HU, Ullah K, Rasool A, Manzoor R, Yuan Y, Tareen AM, et al. Comparative impact of streptozotocin on altering normal glucose homeostasis in diabetic rats compared to normoglycemic rats. Sci Rep. 2023;13:7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. 2010;1:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atlasi MA, Naderian H, Noureddini M, Fakharian E, Azami A. Morphology of Rat Hippocampal CA1 neurons following modified two and four-vessels global ischemia models. Archives of trauma research. 2013;2:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American DA. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rizwan K, Khan SA, Ahmad I, Rasool N, Ibrahim M, Zubair M, et al. A Comprehensive Review on Chemical and Pharmacological Potential of Viola betonicifolia: A Plant with Multiple Benefits. Molecules. 2019;24:3138 [DOI] [PMC free article] [PubMed]
- 21.Kifle ZD, Bayleyegn B, Yimer Tadesse T, Woldeyohanins AE. Prevalence and associated factors of herbal medicine use among adult diabetes mellitus patients at government hospital, Ethiopia: An institutional-based cross-sectional study. Metabol Open. 2021;11: 100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noureddini M, Rezaee-Joshogani F. The comparative effects of aqueous extract of walnut (Juglans regia) leaf and glibenclamide on serum glucose levels of alloxan-induced diabetic rats. Zahedan Journal of Research in Medical Sciences. 2013;15:9-14
- 23.Stefanescu R, Osz BE, Pintea A, Laczko-Zold E, Tero-Vescan A, Vari CE, et al. Fennel Essential Oil as a Complementary Therapy in the Management of Diabetes. Pharmaceutics. 2023;15:2657 [DOI] [PMC free article] [PubMed]
- 24.Shubham PS. A Comprehensive Review on Pharmacological Activity of Foeniculumvulgare. Glob J Pharmaceu Sci; 7:555703. 10.19080/GJPPS.2019.07.
- 25.N AE-S, N E-L, G E-S, WahbyM S, M K, F M, et al. Antidiabetic Activities of FennelMill. Essential Oil in Streptozotocin-Induced Diabetic Rats. Maced J Med Sci. 2011;4:139–46. doi.10.3889/MJMS.1957-5773.2011.0173.
- 26.Samadi-Noshahr Z, Hadjzadeh MA, Moradi-Marjaneh R, Khajavi-Rad A. The hepatoprotective effects of fennel seeds extract and trans-Anethole in streptozotocin-induced liver injury in rats. Food Sci Nutr. 2021;9:1121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hur MH, Kim C, Kim CH, Ahn HC, Ahn HY. The effects of inhalation of essential oils on the body weight, food efficiency rate and serum leptin of growing SD rats. J Korean Acad Nurs. 2006;36:236–43. [DOI] [PubMed] [Google Scholar]
- 28.Bae J, Kim J, Choue R, Lim H. Fennel (foeniculum vulgare) and fenugreek (trigonella foenum-graecum) tea drinking suppresses subjective short-term appetite in overweight women. Clinical nutrition research. 2015;4:168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saghafi N, Ghazanfarpour M, Khadivzadeh T, Babakhanian M, Afiat M. The effect of Foeniculum vulgare (fennel) on body composition in postmenopausal women with excess weight: a double-blind randomized placebo-controlled trial. J Menopausal Med. 2017;23:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zakernezhad F, Barati M, Sanadgol N, Movahhedi M, Majd A, Golab F. The association between fennel extract, serum lipid profile, and leptin receptor expression. Basic and Clinical Neuroscience. 2021;12:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oršolić N, Sirovina D, Odeh D, Gajski G, Balta V, Šver L, et al. Efficacy of Caffeic Acid on Diabetes and Its Complications in the Mouse. Molecules. 2021;26:3262 [DOI] [PMC free article] [PubMed]
- 32.Heikkilä E, Hermant A, Thevenet J, Bermont F, Kulkarni SS, Ratajczak J, et al. The plant product quinic acid activates Ca(2+) -dependent mitochondrial function and promotes insulin secretion from pancreatic beta cells. Br J Pharmacol. 2019;176:3250–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ihara Y, Asahara SI, Inoue H, Seike M, Ando M, Kabutoya H, et al. Chlorogenic Acid and Caffeine in Coffee Restore Insulin Signaling in Pancreatic Beta Cells. Kobe J Med Sci. 2023;69:E1-e8. [PMC free article] [PubMed] [Google Scholar]
- 34.Mohamed NA, Nassier OA. The Antihyperglycaemic Effect of the Aqueous Extract of Origanium Vulgare Leaves in Streptozotocin-Induced Diabetic Rats \\ Jordan Journal of Biological Sciences .- 2013, Vol. 6, No. 1, Pp. 31–38: Hashemite University; 2013.
- 35.Gonzalez P, Lozano P, Ros G, Solano F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int J Mol Sci. 2023;24:9352 [DOI] [PMC free article] [PubMed]
- 36.Alsharif KF, Hamad AA, Alblihd MA, Ali FAZ, Mohammed SA, Theyab A, et al. Melatonin downregulates the increased hepatic alpha-fetoprotein expression and restores pancreatic beta cells in a streptozotocin-induced diabetic rat model: a clinical, biochemical, immunohistochemical, and descriptive histopathological study. Front Vet Sci. 2023;10:1214533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barros L, Heleno SA, Carvalho AM, Ferreira IC. Systematic evaluation of the antioxidant potential of different parts of Foeniculumvulgare Mill. from Portugal. Food Chem Toxicol. 2009;47:2458–64. [DOI] [PubMed]
- 38.Lebowitz MR, Blumenthal SA. The molar ratio of insulin to C-peptide: an aid to the diagnosis of hypoglycemia due to surreptitious (or inadvertent) insulin administration. Arch Intern Med. 1993;153:650–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.