Abstract
The clinical development of novel 5-HT2C receptor (5-HT2CR) therapies has been limited due to concerns over lack of selectivity and potential for off-target effects. Here, we report that the introduction of a secondary amide substituent into a 6,5,7-tricyclic benzodiazepine scaffold provided compounds with unprecedented selectivity for the 5-HT2CR in both functional and binding assays. An early lead compound, 7b, had an in vivo half-life that was shorter than desired, which was improved by a targeted reduction in renal clearance. This provided the clinical candidate compound (+)-19m (later named bexicaserin), which displayed excellent oral bioavailability, good central nervous system partitioning, and decreased renal clearance in vivo. (+)-19m was also a potent inhibitor of acute refeeding in the fasted rat, suggesting on-target effects. (+)-19m demonstrated excellent selectivity for 5‑HT2CR over 5-HT2AR and 5-HT2BR and maximal activity exceeding that induced by the endogenous ligand 5-HT. (+)-19m has since progressed into clinical development.
Introduction
The 5-HT receptors, also known as serotonin receptors, are a large family of G-protein coupled receptors consisting of several subtypes with distinct expression patterns, biological roles, and therapeutic potentials. , The 5-HT2C receptor (5-HT2CR) is an important mediator of diverse biological functions, including food intake and body weight regulation, mood/affect modulation, motor behavior, and endocrine secretion. ,− As such, 5‑HT2CR modulators are promising potential targets for several disorders with unmet medical need, including obesity, substance abuse disorders, anxiety, depression, stress incontinence, schizophrenia, and seizure disorders. ,,−
Several lines of evidence suggest that 5-HT2CR agonists may be effective in the treatment of seizure disorders, including developmental and epileptic encephalopathies (e.g., Dravet syndrome, Lennox-Gastaut syndrome [LGS]), which are often resistant to current antiseizure therapy. − 5-HT2C knockout mice display spontaneous tonic-clonic seizures and reduced seizure threshold in response to activating stimuli, suggesting a role for 5-HT2C in seizure onset. , In humans, the nonselective 5-HT2R agonist fenfluramine (and its metabolite norfenfluramine) reduces seizure frequency in patients with seizure disorders , and is US Food and Drug Administration (FDA) approved for the treatment of seizures associated with Dravet syndrome and LGS. − Finally, limited clinical evidence suggests that the selective 5-HT2C agonist lorcaserin may reduce seizure frequency in patients with Dravet syndrome, LGS, and other severe childhood-onset epilepsy disorders. ,,
Despite the promising evidence suggesting a role for 5-HT2CR agonists in seizure disorders, clinical development of 5-HT2CR therapies has been significantly hindered due to safety concerns; specifically, the undesirable off-target effects arising from nonselective compounds. Nonselective 5-HT2 receptor agonists, including fenfluramine and dexfenfluramine, were widely prescribed anorectic agents in the 1990s, particularly when used in combination with phentermine (i.e., Fen-Phen). However, retrospective safety analyses of these patients showed a strong association with cardiac valvulopathy as well as a connection to pulmonary arterial hypertension (PAH), resulting in their withdrawal from the market in the USA in 1997. The induction of both valve disease and PAH have since been linked to the off-target activation of the 5-HT2BR. , Although less well studied until more recently, 5-HT2AR agonists are also associated with potentially negative effects, including serotonergic hallucinations, in both preclinical species and human patients. ,,
As a result of these safety issues, the major challenge for new compounds in the field has been to significantly improve receptor selectivity, particularly with respect to 5-HT2BR. This approach eventually resulted in the discovery and FDA approval of lorcaserin, a more selective 5-HT2CR agonist (compound 1, Belviq, Figure ), for use in weight management; however, lorcaserin was later withdrawn from the market for reasons unrelated to receptor selectivity. − Thus, there remains a need to identify new 5-HT2CR agonist compounds with clean safety profiles and high selectivity for 5-HT2CR to avoid the risk of cardiovascular side effects associated with 5-HT2BR agonism. We herein report that the incorporation of a secondary amide substituent into a previously identified 6,5,7-tricyclic benzodiazepine scaffold resulted in several compounds with unprecedented levels of 5-HT2CR selectivity, one of which is in clinical development with recently initiated Phase 3 studies for the treatment of seizures associated with developmental and epileptic encephalopathies.
1.
Structures of selective 5-HT2CR agonists with their literature binding-affinity data (Figure -) and numbering of carbon atoms (Figure and ).
Results and Discussion
We have recently reported on several previously underexplored 5-HT2R scaffolds where we measured activity and selectivity in both functional and binding assays. In that work, we identified a lead 6,5,7-tricyclic scaffold and demonstrated that small alkyl substituents at the 7-position of the scaffold (on the 5-membered partially saturated ring) and simple alkyl or halo-substituents at the 8-position (on the aromatic ring) were well tolerated and could provide compounds with good potency and functional selectivity. Of note, some earlier highly advanced 5‑HT2CR agonist compounds exhibited clear binding to 5-HT2AR and 5-HT2BR but did not appear to produce an agonist effect on the Gq pathway in vitro. , However, lingering concerns remained that compounds retaining significant receptor binding affinity may still exhibit agonism via alternative second messenger pathways not captured with a Gq functional assay. Thus, we placed a major focus on obtaining high binding selectivity as well as high Gq functional selectivity as one of our optimization criteria.
Several examples from the earlier series, such as compound 2 (Figure ), had good pharmacokinetic (PK) properties in the rat, including high oral bioavailability and good central nervous system (CNS) partitioning, and as a result in a simple pharmacodynamic assay, were able to decrease acute food intake in the rat after oral administration. With this encouraging data in hand, we next moved on to the investigation of more hydrophilic substituents, to try and reduce the overall lipophilicity of the series, with a focus on the 8-position, which had proven amenable to manipulation. To maintain rapid access to new analogues, we prepared derivatives only from commercially available building blocks to keep the synthesis to 4–5 steps. This one cluster of derivatives available to us was a series of amides that was readily accessed via an indoline ester building block. Such compounds turned out to have highly desirable properties.
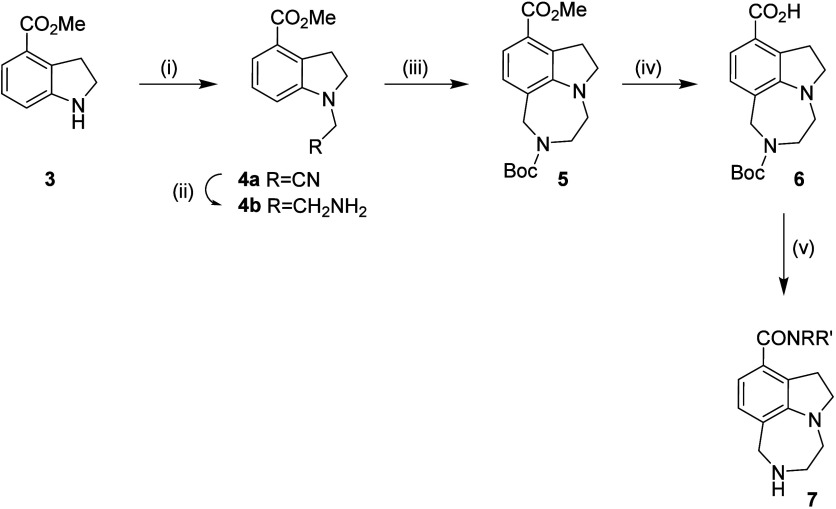
The first target compounds 7, were prepared as outlined in Scheme . Methyl indoline-4-carboxylate (3) is commercially available in gram quantities, or if needed on a larger scale, can be cleanly prepared from the corresponding indole by reduction with triethyl silane. Condensation of 3 with hydroxyacetonitrile, followed by reduction of the nitrile group, provided the primary amine 4. A Pictet-Spengler ring closure of 4 with formaldehyde provided the tricyclic core, which was then Boc-protected for ease of purification (for both this intermediate and the final compounds) to give 5. Hydrolysis of the ester provided the key acid intermediate 6, which was used to prepare a library of amide derivatives by hexafluorophosphate azabenzotriazole tetra‑methyl uronium (HATU) coupling to the appropriate amine and finally Boc-deprotection to provide the test compounds 7.
1. Preparation of the First Amide Library for the 8-Carboxamide Derivatives 7 .
a Reagents and Conditions: (i) a) HOCH2CN, water, 110 °C; (ii) Raney Ni, 7 M NH3 in MeOH, H2, 50 atm; (iii) a) formaldehyde, MeOH, TFA, 110 °C; b) Boc2O; (iv) NaOH, MeOH, water; (v) a) HATU, RR’NH, Et3N, DMF; b) HCl, dioxan.
As shown in Table , the incorporation of an amide group at the 8-position of the 6,5,7 diazepine ring system completely abolished all functional agonist activity at 5-HT2AR and 5‑HT2BR, and secondary amides showed good 5-HT2CR agonist activity. The primary amide analogue 7a was much less active than the secondary amides, whereas the dimethyl amide 7c was devoid of significant 5-HT2CR agonist activity.
1. Agonist Functional Activity and Binding Affinity for the 8-Carboxamide Derivatives Compound 7 and Compound 12.

[125I]-DOI ligand. [125I]-DOI: [125I]-2,5-dimethoxy-4-iodoamphetamine. EC50: half maximal effective concentration. E max: maximum effect. Ki: inhibition constant. n.d.: not determined.
Several additional tertiary amide analogues, including those prepared from cyclic amines, were also inactive (data not shown). In contrast, the monomethyl amide 7b was a moderately potent 5-HT2CR agonist with at least 200-fold selectivity over 5-HT2AR and 5-HT2BR in inositol triphosphate (IP3) functional assays. At that time, 7b was the most selective compound that we had prepared in these tricyclic series. Whereas others have seen levels of functional selectivity that approach this level in vitro, − compound 7b also showed selectivity close to 200-fold in the [125I]-2,5-dimethoxy-4-iodoamphetamine (125I-DOI) binding assay. This level of receptor binding selectivity for 5-HT2CR agonists over each of the other two members of the family was, to the best of our knowledge, unprecedented.
Extending the length of the primary amine up to four carbon atoms did not greatly affect the binding or functional activity, but the introduction of branching in the chain was detrimental, even in the cases where a smaller cyclopropyl group (7h, 7g) was included. The incorporation of heteroatoms into this straight chain substituent provided a modest improvement in agonist potency while maintaining outstanding functional and binding selectivity, rising to >500-fold in binding for 7i and 7j. There was little difference between oxygen or sulfur in filling this role, but replacement of the thioether with a sulfone abolished 5-HT2CR activity. Although further elongation of the chain did incrementally improve 5-HT2CR binding and functional potency (7l and 7m), this increase in lipophilicity also led to increased binding affinity for both 5-HT2AR and 5-HT2BR, resulting in an overall decrease in binding selectivity. Branching was tolerated beyond the oxygen atom in the chain (7n), whereas branching earlier in the chain (7q and 7r) resulted in a significant loss of receptor potency and selectivity. The use of a phenyl ether at the end of this amide substituent (7p) had a similar effect, although binding selectivity close to 100-fold was maintained.
It is worth noting that several compounds in this series (and in later series), including 7b, showed consistently higher intrinsic efficacy than serotonin in our 5-HT2CR functional assay system, with some having close to 120% of serotonin efficacy.
Having previously expressed our preference for the 6,5,7-ring system described herein (based on its desirable potency and PK properties), we prepared some examples from the 6,6,7-ring system that contained the secondary amide substituent in the appropriate position to confirm the superiority of our chosen ring system. The compounds were made using a similar synthetic strategy as with the 6,5,7 series (Scheme ). Methyl 1,2,3,4-tetrahydroquinoline-5-carboxylate 8 was heated with neat 2-bromoethanamine hydrobromide to provide the amino ethyl intermediate 9. Pictet-Spengler cyclization and Boc protection provided the cyclized ester product 10, which was hydrolyzed to provide the carboxylic acid 11, the common precursor to the target compounds. Amide formation and Boc deprotection completed the sequence. In this series, the amide substituent had a similar effect in providing the outstanding 5‑HT2CR agonist selectivity already observed. However, 12a–c all showed significantly weaker 5-HT2CR activity than the corresponding 6,5,7 series analogues with the same amide substituents (Table ). As a result, these compounds were not pursued further although they did serve to further emphasize the importance of the secondary amide substituent for selectivity.
2. Preparation of 6,6,7-Analogues .
a Reagents and Conditions: (i) a) Bromoethanamine (neat), 122 °C; (ii) a) formaldehyde, MeOH, TFA, 110 °C; b) Boc2O; (iii) NaOH, MeOH, water; (iv) a) HATU, RR’NH, Et3N, DMF; b) HCl, dioxan.
In an attempt to understand the remarkable selectivity afforded by incorporation of a secondary amide at the 8-position of the 6,5,7 series, we retrospectively performed a docking analysis of several members of the series using the crystal structure of the 5-HT2CR with bound ergotamine as a model. As shown in Figure , the major binding interaction with the receptor appears to be still driven by the ionic interaction between the basic nitrogen and the transmembrane domain 3 aspartate group D134 (Ballesteros-Weinstein position 3.32). In every simulation, compound 7i (>700-fold selectivity in functional assays; > 800-fold selectivity in binding assays) is situated somewhat lower in the pocket than we expected, likely because of the presence of extra pocket volume left by removal of the much larger ergotamine hydrophobic core. The observed selectivity of the 8-substituted 6,5,7 series is, we believe, likely driven by two interactions where the 5-HT2CR differs substantially from the 5-HT2AR and 5-HT2BR receptors. First in TM2 at position A113 (Ballesteros-Weinstein position 2.64) of 5-HT2CR, which is a threonine in 5-HT2AR and 5-HT2BR and may therefore inhibit ligand binding, and second the polar functionality of the amide group may not allow positive interactions in the 5-HT2AR and 5-HT2BR with the poorly conserved extracellular loop 2, which seems to form the cap of the receptor-binding pocket.
2.
Overlay of 5-HT2CR receptor (green) and the 5-HT2BR receptor (cyan) with compound 7i docked into the binding pocket. Transmembrane helices, loops, and residue interactions are labeled with the 5-HT2CR residue positions. PDB ID: 6BQG.
All the 6,5,7 series compounds tested were highly stable in liver microsomes from mouse, rat, and human. Thus, we began to screen selected examples in our in vivo pharmacodynamic (PD) model, an acute refeeding assay in the fasted rat at a screening dose of 10 mg/kg administered orally (PO). Institutional approval for in vivo experiments was provided by Arena Pharmaceuticals Institutional Animal Care and Use Committee. Although we were not focused on developing any potential candidate for obesity or metabolic indications, our indications of interest (e.g., childhood seizure and psychiatric disorders) would, we presumed, require a similar profile in terms of oral bioavailability and CNS partitioning. Therefore, the acute refeeding assay, when used as a PD screen, would allow us to identify compounds for multiple indications that had adequate exposure after oral administration and were CNS penetrant. In this assay, active compounds would be expected to produce a significant decrease in food intake over 1–2 h and may also induce other well-characterized 5-HT2CR-mediated behaviors (such as penile grooming and decreased locomotor activity) in the rat, providing some confidence that any observed hypophagic effect were on target. ,
We compared the activity of 7b in this model with 7i and 7l, two analogues of comparable or better receptor agonist activity, following an oral dose of 10 mg/kg. Somewhat surprisingly, only 7b showed a good effect at this screening dose (we later determined an ED50 of 6.4 mg/kg PO for this compound, calculated from 4 active doses), whereas the two compounds with the longer side chains were essentially inactive. To investigate this difference further, we tested 7i at a higher oral dose (20 mg/kg) where we saw a modest effect (25% inhibition at 1 h), and at 10 mg/kg subcutaneous (SC), which had the same very weak effect as 10 mg/kg PO (7% inhibition). With this scaffold having previously shown good oral exposure, and with the failure to see an effect even after SC dosing, we hypothesized that this compound was most likely not accessing the target receptors in the brain. We therefore followed up with a CNS partitioning study and noted that the brain concentration and brain-to-plasma ratio of 7i was much lower than that for 7b, which had a good in vivo effect at 10 mg/kg (Table ). In addition, 7b had a very high free fraction in both rat plasma (fraction unbound [fu] 87%) and rat brain (fu 75%), whereas 7i was significantly more highly protein bound. Taken together, we believe these data can account for the difference in the acute in vivo activity between the two compounds.
2. In Vivo PD and PK Data in the Rat for the 8‑Carboxamide Derivatives 7 .
| Abbreviated
CNS PK Study (10 mg/kg PO) Tissue Concentrations
at 1 h |
Cell
Permeability P-gp Expressing LLCPK2 Cells |
||||
|---|---|---|---|---|---|
| Compound | Inhibition of Food Intake at 1 h (%) with 10 mg/kg po | Plasma (ng/mL) | Brain (ng/g) | A-B (×10–6 cm s–1) | Efflux Ratio |
| 7b | 87 | 1340 | 973 | 9.83 | 1.21 |
| 7i | 9 | 2040 | 295 | 6.05 | 2.76 |
| 7j | n.d. | n.d. | n.d. | 7.3 | 3.5 |
| 7l | 26 | n.d. | n.d. | 3.7 | 5.7 |
| 7o | n.d. | n.d. | n.d. | 12.1 | 3.5 |
| 7s | n.d. | n.d. | n.d. | 21.9 | 1.19 |
Mean data for n = 6 (food intake) or n = 3 (PK study) animals per time point. CNS: central nervous system; n.d.: not determined. PD: pharmacodynamic. P-gp: p-glycoprotein. PK: pharmacokinetic. PO: oral administration.
In search of an explanation for the much lower brain-to-plasma ratio for 7i, we next looked at the permeability of selected potent analogues in P-gp–expressing LLCPK2 cells (Table ). In this in vitro test, we observed that although the apical to basal permeability of all the analogues was in the moderate-to-high range, compounds with elongated side-chains that included ether groups had a significantly increased efflux ratio, suggesting that such compounds may be actively transported from the brain in vivo. A similar tendency for an increase in efflux resulting from an increase in size of 5-HT2C agonist substituents was previously observed in a structurally unrelated series.
Although several compounds of this type appeared more potent than 7b at 5-HT2CR, because of their efflux properties, we viewed it as unlikely that they would be more potent in vivo. However, we did note that a compound that included the incorporation of a halogen substituent, 7s, retained good receptor activity and potency, was highly permeable, and had a very low efflux ratio. We did not consider 7s as a viable compound to investigate further due to its potential for covalent binding, so we instead prepared and tested the fluorinated analogues 7t-7v; however, these compounds were significantly less potent agonists of 5-HT2CR and were not pursued further at this stage.
In a broader examination of the pharmacological profile of 7b, we demonstrated that the essentially maximal hypophagic effect at 10 mg/kg po after 1 h was blocked by preadministration of the selective and brain penetrant 5-HT2CR antagonist SB242084 (1 mg/kg PO) helping to further increase confidence that the effect was on target.
More extensive profiling of 7b showed this compound to be highly selective against all other receptors tested (<25% binding at 10 mM against an 87 target Eurofins safety screen panel) and, as might be predicted from the low lipophilicity (cLogP = 0.62) and moderate basicity (measured pK a = 7.93), there was no significant inhibition of human ether-a-go-go-related gene (hERG) activity up to 30 mM in a patch clamp assay. In addition, there was no effect in a screening Ames test and no inhibition of the six most highly expressed CYP enzymes (IC50 > 30 mM vs CYP3A4, 2D6, 2C8, 2C9, 2C19, and 1A2). The compound had good stability in hepatocytes and microsomes and had excellent oral bioavailability across species (Table ) but with a somewhat short intravenous (iv) half-life. The good CNS partitioning properties observed in the rat were also confirmed with a second PK study in cynomolgus monkeys measuring compound concentration in both plasma and cerebrospinal fluid (CSF) after oral administration. The CSF/plasma ratio in this study based on the area under curve (AUC) measurements was calculated to be around 0.7.
3. IV and PO PK Parameters for 7b in Multiple Species .
| IV Parameters
(2 mg/kg dose) | |||||
|---|---|---|---|---|---|
| Species | t1/2 (h) | AUC0‑inf (μg·h/mL) | Cl (L/h/kg) | Vss (L/kg) | ClR (L/h/kg) |
| Mouse | 0.41 | 0.34 | 5.94 | 2.44 | n.d. |
| Rat | 0.56 ± 0.05 | 0.77 ± 0.13 | 2.66 ± 0.47 | 1.89 ± 0.34 | 1.44 ± 0.50 |
| Dog | 1.38 ± 0.48 | 1.83 ± 0.08 | 1.10 ± 0.05 | 1.41 ± 0.18 | 0.07 ± 0.08 |
| Cyno | 1.49 ± 0.18 | 2.0 ± 0.18 | 1.0 ± 0.1 | 1.80 ± 0.35 | 0.27 ± 0.19 |
| PO Parameters
(5 mg/kg dose) | |||||
|---|---|---|---|---|---|
| Species | t1/2 (h) | tmax (h) | Cmax (μg/mL) | AUC0‑inf (μg·h/mL) | %F |
| Mouse | 0.6 | 0.25 | 0.94 | 0.72 | 85.6 |
| Rat | 1.51 ± 0.66 | 0.58 ± 0.38 | 0.57 ± 0.08 | 2.02 ± 0.13 | 105 ± 7 |
| Dog | 1.90 ± 0.43 | 0.92 ± 0.95 | 1.82 ± 0.60 | 4.13 ± 1.27 | 90.2 ± 27.8 |
| Cyno | 2.07 ± 0.97 | 2.33 ± 1.53 | 0.70 ± 0.33 | 3.58 ± 1.18 | 71.2 ± 23.4 |
%F: % oral bioavailability. AUC0‑inf: area under the curve extrapolated to infinity. C max: maximum concentration. Cyno: cynomolgus monkey. IV: intravenous. n.d.: not determined. PO: oral administration. t 1/2: half-life. T max: time to maximum concentration.
However, allometric scaling of the PK data to predict a half-life for dosing in humans provided an estimate of 3 h, which we regarded as too short to meet the target product profile (TPP) of once- or twice-daily dosing in patients. As we had previously observed with our 6,5,7 core scaffold, renal clearance was responsible for a significant proportion of the total clearance for compound 7b, particularly in the rat and the cynomolgus monkey. Therefore, we aimed to prolong compound 7b’s half-life by modestly increasing the lipophilicity of this highly water-soluble core (water solubility of 7b, free base = 91 mg/mL) while maintaining receptor activity, which might in turn increase plasma protein-binding and reduce renal clearance. We had already shown that increasing lipophilicity by including larger groups on the carboxamide function may lead to compounds with poorer selectivity profiles and limited in vivo activity, as a result of their increased tendency for efflux. We therefore turned our attention to the addition of substituents to the 7-position, which we had previously shown could also tolerate small alkyl groups, to increase lipophilicity. Such compounds were first synthesized as racemic mixtures as shown in Scheme . Acylation (R=CF3, R=CH3, R=CHF2) or formylation (R=H) of methylindole-4-carboxylate provided the intermediates 14a–d. The intermediates 14a and 14b could be reduced in one pot with trimethylsilane to provide the 3-alkyl indolines 15a and 15b. In the case of 14c and 14d, the reduction to the indoline was more efficient on a small scale when completed in two steps. From the indoline intermediates 15a–d, the route to provide the final compounds followed the same strategy as for 7. In this sequence, however, alkylation of 15a–d with 2-bromoethylamine to provide 16a–d directly was used, as this avoided the high-pressure reduction of the nitrile used in the earlier sequence. Pictet-Spengler cyclization, Boc protection, ester hydrolysis and amide formation followed by a final Boc deprotection provided the racemic target compounds (±)-19 (Table ).
3. Preparation of 7-Substituted 8-Carboxamide Analogues .
a Reagents and Conditions: (i) R=CF3: TFAA, 40 °C; R=CH3: Ac2O, 40 °C; R=CHF2: difluoroacetic anhydride, 40 °C; R=H: oxalyl chloride; (ii) For R=CF3, CHF2: Et3SiH, rt For R=Me, NaBH4, BF3.OEt2, For R=H: a) 4-methylbenzene sulfonohydrazide, p-toluenesulfonic acid, sulfolane, 100 °C, 1 h b) NaHCNBH3 rt c) Et3SiH, rt; (iii) bromoethanamine hydrobromide (neat), 120 °C; (iv) a) formaldehyde, MeOH, TFA, 110 °C; b) Boc2O; (v) NaOH, MeOH, water; (vi) a) HATU, RR’NH, Et3N, DMF; b) HCl, dioxan.
4. In Vitro Receptor Functional and Binding Data and Cell Permeability Measurements for Racemic 7-Substituted 8‑Carboxamide Analogues 19.

[125I]-DOI ligand.
In P-gp expressing LLCPK2 cells.
[125I]-DOI: [125I]-2,5-dimethoxy-4-iodoamphetamine. EC50: half maximal effective concentration. E max: maximum effect. Ki: inhibition constant. P-gp: p-glycoprotein. n.d.: not determined.
Screening of these compounds as their racemates showed that the combination of a secondary amide in the 8-position and a small alkyl group in the 7-position was very well tolerated, providing potent 5-HT2CR agonists with very good selectivity, particularly in functional assays where nonmeasurable agonism for either the 5-HT2CR or 5-HT2BR was observed for any of these compounds in the IP3 assay (Table footnote). With the trifluoroethyl group at the 7-position, an increase in the size of the 8-amide substituent marginally improved the 5-HT2CR agonist effect and decreased binding selectivity, particularly for 5-HT2CR over 5-HT2AR. In addition, we again observed an increase in the efflux ratio for such compounds, with a magnitude of effect similar to that we had observed in the absence of the 7-substituent in the first amide series. Interestingly, incorporating halogen substituents by mono- and difluorination of the ethyl amide substituent ((±)-19e and (±)-19f) partially restored the loss of selectivity, and these two compounds were significantly more potent than the analogues without a 7-substituent. However, trifluorination in the ethyl amide substituent resulted in a 10-fold reduction in 5-HT2CR agonist effect compared with difluorination. In addition, with the smaller size of these substituents, there was a trend toward a lower efflux ratio.
Still trying to balance the potency, selectivity, and efflux ratio, we looked at additional substituents at the 7-position of the core scaffold. In this position, the difluoroethyl analogue (±)-19h was comparable to (±)-19a in terms of 5-HT2CR potency but showed poorer selectivity against 5-HT2BR and a higher efflux ratio. The 7-ethyl analogue (±)-19i was a somewhat weaker 5‑HT2CR agonist and, although it had excellent binding selectivity, its efflux ratio was not improved compared with (±)-19a. In this case, the insertion of mono- or difluoroethyl groups into the 8‑amide had a detrimental effect on binding selectivity against 5‑HT2AR and did not improve the efflux ratio. Reducing the size of the 7-substituent further to a methyl group resulted in a significant loss in 5-HT2CR activity for the 8-methyl amide (±)-19l. The difluoroethylamide (±)-19m however had a good 5-HT2CR agonist effect and was exquisitely selective in both the functional and binding assays. Moreover, this compound had the lowest efflux ratio among any of the 7-substituted 8-amide analogues prepared.
From these studies, we selected five compounds to be separated into their constituent enantiomers. Based on our previous experience in the absence of an 8-amide substituent, we expected that only one 7-position isomer would show any 5‑HT2CR agonist activity, but we also wanted to ensure that resolution would not have an adverse effect on selectivity or efflux ratio. Hence, in addition to the most promising analogues, our selections included additional compounds with a range of selectivity and efflux ratios for the racemic compounds. These compounds were initially separated by either chiral high-performance liquid chromatography (HPLC) or supercritical fluid chromatography (SFC; of either the free amines or the Boc-protected analogues) to provide the single enantiomers in >99% enantiomeric excess (ee). The enantiomers were screened as described above and the data are shown in Table .
5. In Vitro Receptor Functional and Binding Data and Cell Permeability and In Vivo Data for Chiral 7-Substituted 8‑Carboxamide Analogues 19.
| 5-HT2CR |
5-HT2BR |
5-HT2AR |
Cell
Permeability
|
Inhibition
of Food Intake at 1 h (%) |
Abbreviated
CNS PK Study (10 mg/kg PO) Tissue Concentrations
at 1 h |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | EC50, nM (E max, %, n) | Ki, nM (n) | Ki, nM (n) | Ki, nM (n) | A-B (×10–6 cm s–1) | Efflux ratio | 10 mg/kg PO screen | ED50 (mg/kg po) | Plasma (ng/mL) | Brain (ng/g) |
| (+)-19a | 11.5 (106, 25) | 19.2 (18) | 4240 (12) | 1270 (17) | 14.85 | 2.40 | 83 (41) | 5.2 | 1660 | 440 |
| (−)-19a | >25,000 (n.m., 6) | 10,000 (2) | >50,000 (2) | >50,000 (2) | n.d. | n.d. | 0 | n.d. | n.d. | n.d. |
| (+)-19c | 22.5 (107, 3) | 33.4 (3) | >50,000 (3) | 7270 (3) | n.d. | n.d. | 53 | n.d. | n.d. | n.d. |
| (+)-19i | 52 (105, 20) | 38 (13) | >50,000 (113) | 9250 (12) | 7.73 | 3.79 | 63 | 6.4 | 1010 | 222 |
| (−)-19i | >25,000 (n.m., 9) | 5570 (4) | >50,000 (4) | >50,000 (4) | n.d. | n.d. | 0 | n.d. | n.d. | n.d. |
| (+)-19k | 8 (112, 12) | 17.6 (15) | >50,000 (14) | 23,800 (12) | 12.8 | 2.16 | 27 | >5 | 866 | 454 |
| (+)-19m | 42 (112, 23) | 39 (27) | >50,000 (23) | >50,000 (26) | 8.9 | 1.9 | 81 | 2.8 | 960 | 832 |
[125I]-DOI ligand.
In P-gp-expressing LLCPK2 cells.
Mean of two determinations.
5 mg/kg dose. [125I]-DOI: [125I]-2,5-dimethoxy-4-iodoamphetamine. CNS: central nervous system. ED50: effective dose for 50% of the population. n.d.: not determined. n.m.: not measurable. PK: pharmacokinetic. PO: oral administration.
We once again confirmed that only one isomer was responsible for all the 5-HT2CR activity and showed with several analogues that the (−)-enantiomer in each case had essentially no 5-HT2CR agonist effect. We also tested the inactive isomers of two compounds ((−)-19a and (−)19i) in our in vivo feeding assay in a blinded fashion alongside the corresponding (+)-enantiomers. As expected, these compounds had no measurable in vivo effect (Table ). The active (+)-enantiomers retained all the binding and functional selectivity properties of the racemates, and in the cases where the racemate and (+)-enantiomer were both tested, there was no meaningful difference in the permeability or efflux ratio. Several of the active enantiomers were then screened in the in vivo refeeding assay in the rat and showed varying degrees of inhibition of food intake at 1 h that were broadly reflective of their receptor activity and brain concentration (Table ). In turn, the brain concentration or brain/plasma ratio appeared most dependent on the efflux ratio for each compound. For example, despite its modest brain/plasma ratio, the brain concentration of (+)-19a was sufficient for it to be among the most potent compounds we had seen in the in vivo screening assay. An ED50 determination showed this compound to be only around 2-fold less potent in vivo than lorcaserin, (ED50 for lorcaserin = 2.6 mg/kg at 1 h in this model, calculated from 4 active doses) which was used as a positive control. (+)-19k, although a potent 5-HT2CR agonist with a reasonable efflux ratio and a comparable dose-equivalent brain exposure to (+)-19a, was marginally less potent in vivo. (+)-19m, the compound with the lowest efflux ratio and highest brain/plasma ratio – and as a result, the compound with the highest brain concentration – was shown to be the most potent compound in vivo with an ED50 of 2.8 mg/kg PO, an effect comparable to lorcaserin under the same conditions. At the time we noted that the CSF/plasma ratio was also very high (∼0.4) indicating to us the likelihood of a high free concentration in brain. We were later able to show that this figure is comparable to the Kpuu (0.66) for this compound when we determined the rat brain protein-binding (53% binding).
As a result of these clear advantages over other compounds in the series, we examined (+)-19m further to see if there was an improvement in the in vivo PK profile over 7b. Our hypothesis was that an increase in lipophilicity would decrease renal clearance, and that the increase in cLogP from 0.62 for 7b to 1.48 for (+)-19m did produce a modest increase in plasma protein binding from 13% in the rat and 17% in human for 7b to 34% (in both human and rat) for (+)-19m (Table ). However, this still represents very low protein binding. The IV PK parameters across multiple species showed that this modest increase in lipophilicity did indeed provide a significant decrease in renal clearance, particularly in higher species. However, as the total clearance rates were similar, the clear improvement in IV half-life in the rat and cynomolgus monkey may therefore be better attributed to a greater volume of distribution in these species. (+)-19m also had a much greater overall exposure (AUC) after oral administration in higher species. With all the data taken together, allometric scaling for this compound predicted a half-life of approximately 7 h in human, which was much more in keeping with the TPP requirement for once- or twice-daily dosing, and so (+)-19m was investigated further.
6. Comparison of Pharmacokinetic Parameters for (+)-19m in Multiple Species.
| IV Parameters
(2 mg/kg dose) | |||||
|---|---|---|---|---|---|
| Species | t1/2 (h) | AUC0‑inf (μg·h/mL) | Cl (L/h/kg) | Vss (L/kg) | ClR (L/h/kg) |
| Mouse | 0.66 ± 0.47 | 0.318 ± 0.15 | 4.13 ± 1.21 | 2.98 ± 1.74 | n.d. |
| Rat | 1.6 ± 0.24 | 0.577 ± 0.079 | 3.51 ± 0.5 | 3.69 ± 0.19 | 0.579 ± 0.056 |
| Dog | 1.3 ± 0.063 | 1.62 ± 0.147 | 1.24 ± 0.11 | 1.85 ± 0.18 | 0.0002 ± 0.00001 |
| Cyno | 6.8 ± 2.61 | 2.97 ± 0.48 | 0.69 ± 0.12 | 4.36 ± 0.6 | 0.0018 ± 0.006 |
| PO Parameters
(5 mg/kg dose) | |||||
|---|---|---|---|---|---|
| Species | t1/2 (h) | tmax (h) | Cmax (μg/mL) | AUC0‑inf (μg·h/mL) | %F |
| Mouse | 1.5 ± 1.13 | 0.25 ± 0.087 | 0.82 ± 0.19 | 0.91 ± 0.092 | ∼100 |
| Rat | 2.5 ± 1.0 | 0.5 ± 0.43 | 0.45 ± 0.12 | 1.28 ± 0.14 | 89 |
| Dog | 4.0 ± 1.78 | 0.58 ± 0.38 | 1.59 ± 1.02 | 3.92 ± 2.92 | 97 |
| Cyno | 5.52 ± 0.95 | 3.0 ± 0.00 | 0.43 ± 0.059 | 4.30 ± 0.2 | 58 |
The mouse (2 mg/kg) and rat (10 mg/kg) PO doses were dose adjusted to 5 mg/kg assuming dose-linearity. %F: % oral bioavailability. AUC0‑inf: area under the curve extrapolated to infinity. Cl: Total clearance. CIR: renal clearance. C max: maximum concentration. Cyno: cynomolgus monkey. iv: intravenous. po: oral administration. t 1/2: half-life. T max: time to maximum concentration. Vss: volume of distribution.
Similar to previous compounds in these series, (+)-19m showed no significant inhibition of any of the major CYP enzymes (IC50 > 30 mM for CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP3A4) and no time-dependent inhibition was observed. In addition, no measurable inhibition of the hERG channel was observed in a patch clamp assay (IC50 > 30 mM) and the compound was clean in an Ames test. In addition to the 5-HT2C family, (+)-19m demonstrated outstanding selectivity against all other targets tested (<25% binding at 10 μM against a > 140 target Eurofins safety screen panel, see Supporting Information). Finally, we identified a crystalline, nonhygroscopic HCl salt with a 1:1 stoichiometry that appeared suitable for early development. (+)-19m. HCl had high aqueous solubility (>386 mg/mL, self-pH = 2.5), showed <0.1% weight gain at 90% relative humidity and 25 °C in a dynamic moisture sorption test, and decomposed at >200 °C without melting during 10 °C/min differential scanning calorimetry and thermogravimetric analysis. An X-ray crystal structure determination of (+)-19m proved our early hypothesis, based on the known stereochemistry of vabicaserin, that the chirality of the methyl group in the molecule was most likely to be of the R-configuration (see Supporting Information). Based on the exceptional selectivity, excellent in vivo potency, and adequate projected human PK profile, (+)-19m (later renamed LP-352 or bexicaserin) was selected for further development.
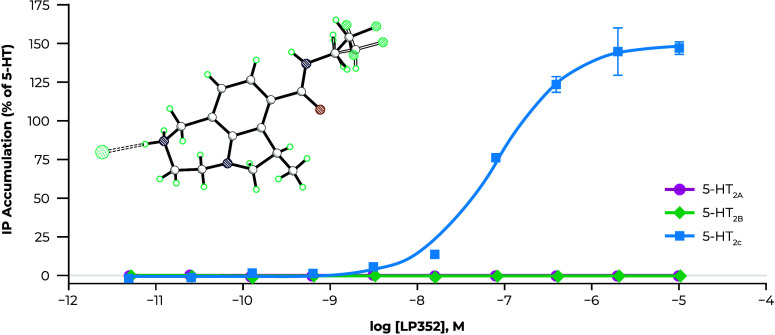
In 5-HT2CR IP accumulation assays in cloned cell lines with no receptor reserve, (+)-19m routinely demonstrated a modestly greater maximal efficacy than that induced by the endogenous ligand 5-HT (serotonin) consistent with classification as a superagonist at 5-HT2CR. − This was confirmed using dynamic mass redistribution (DMR) assays that can capture additional second messenger pathways in HEK293 cells expressing human 5-HT2CR, where the maximal cellular response of (+)-19m was greater than both the 5-HT2CR partial agonist lorcaserin and the endogenous ligand 5-HT (Figure A). Similar results were observed using primary rat choroid plexus cells, which express endogenous 5-HT2C, where (+)-19m displayed superagonist activity as measured by both IP accumulation and DMR assays (Figure B). Having observed this phenomenon in both cloned cell lines and primary cells, we believe that this observed superagonism is a real effect; however, it remains unknown whether such superagonism translates into any additional in vivo or clinical effect.
3.
(A) Dynamic mass redistribution assay in human 5-HT2C–expressing HEK293 cells and (B) inositol phosphate (IP) accumulation and dynamic mass redistribution (DMR) assays in primary rat choroid plexus cells.
Conclusions
In summary, a lead optimization campaign around a series of compounds wherein a secondary amide substituent was introduced into our previously described scaffold, provided compounds with unprecedented selectivity for the 5-HT2CR in both functional assays and, uniquely, in binding assays. Based on allometric scaling, it was predicted that an early lead compound 7b, was likely to have too short a half-life in human to meet our target profile. Further lead optimization targeted a reduction in renal clearance and provided (+)-19m that had a good balance of properties, including excellent oral bioavailability and good CNS partitioning. In our in vivo PD assay, this compound was a potent inhibitor of food intake in the rat and overall, showed a good developability profile. As a result, (+)-19m (bexicaserin) was selected for preclinical development and has progressed into clinical trials. A variety of preclinical models of seizure activity (e.g., zebrafish with scn1lab–/– mutations, zebrafish larvae treated with ethyl ketopentenoate to reduce GABA, zebrafish larvae treated with kainic acid to activate glutamate receptors, and mice administered the GABA-A antagonist pentylenetetrazol) generated data showing the potential of bexicaserin to reduce seizure activity stemming from a wide variety of underlying causes: genetic mutations in neuronal sodium channels, reduced GABAergic signaling, and excessive glutamatergic excitation. Following favorable safety and efficacy results from a Phase 2 study, the clinical development of bexicaserin has progressed with initiation of a global Phase 3 study program. Breakthrough Therapy Designation also was granted from the United States Food and Drug Administration for bexicaserin the treatment of seizures associated with developmental and epileptic encephalopathies in patients 2 years of age and older.
Experimental Section
All compounds were >95% pure by HPLC and/or LC/MS.
Chemistry
Proton nuclear magnetic resonance (1H NMR) spectra were recorded on a Bruker Avance III-400 equipped with a 5 mm BBFO probe. Chemical shifts are given in parts per million (ppm) with the residual solvent signal used as reference. NMR abbreviations are used as follows: s = singlet, d = doublet, dd = doublet of doublets, t = triplet, q = quartet, m = multiplet, bs = broad singlet, sxt = sextet. Microwave irradiations were carried out using a Smith Synthesizer or an Emrys Optimizer (Biotage). Liquid chromatography mass spectrometry (LC-MS) specifications: HPLC- Agilent 1200; pumps: G1312A; DAD:G1315B; Autosampler: G1367B; Mass spectrometer-Agilent G1956A; ionization source: electrospray ionization; Drying Gas Flow:10 L/min; Nebulizer Pressure: 40 psig; Drying Gas Temperature: 350 °C; Capillary Voltage: 2500 V) Software: Agilent Chemstation Rev.B.04.03.
Thin-layer chromatography (TLC) was performed on silica gel 60 F254 (Merck), preparatory thin-layer chromatography (prep TLC) was performed on PK6F silica gel 60 Å 1 mm plates (Whatman) and column chromatography was carried out on a silica gel column using Kieselgel 60, 0.063–0.200 mm (Merck). Evaporation was done under reduced pressure on a Büchi rotary evaporator. Celite 545 was used for filtration of palladium.
Preparation of Methyl 1-(Cyanomethyl)indoline-4-carboxylate (4a)
2-hydroxyacetonitrile (3.073 g, 29.63 mmol) was added to a mixture of methyl indoline-4-carboxylate (3, 5.0 g, 28.22 mmol) in H2O (2.5 mL). The reaction was heated at 110 °C for 15 h then cooled and the mixture filtered and the resultant solid was collected and recrystallized from MeOH (12 mL) to give the title compound (5.6 g). LC-MS m/z = 217.2 [M + H]+; 1H NMR (400 MHz, CDCl3) δ 3.37–3.45 (m, 2H), 3.46–3.54 (m, 2H), 3.89 (s, 3H), 4.11 (s, 2H), 6.71 (d, J = 7.6 Hz, 1H), 7.22 (t, J = 7.9 Hz, 1H), 7.47 (dd, J 1= 8.0 Hz, J 2 = 0.9 Hz, 1H).
Preparation of Methyl 1-(2-Aminoethyl)indoline-4-carboxylate (4b)
Raney-Nickel 2800 (2.606 g, 44.40 mmol) was added to a Parr reaction vessel. The catalyst was washed with methanol multiple times before the addition of ammonia in methanol (7 M, 95.13 mL, 665.9 mmol). 4a (4.8 g, 22.20 mmol) was added and the reaction was shaken under H2 (50 atm) for 20 h at 23 °C. The mixture was then filtered and the filtrate was concentrated to give the title compound (3.9 g). LC-MS m/z = 221.4 [M + H]+.
Preparation of Methyl 1,2,3,4,6,7-Hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxylate (5)
Trifluoroacetic acid (TFA; 10.57 mL, 138.0 mmol) was added to a solution of 4b (3.8 g, 17.25 mmol) and formaldehyde (1.658 g, 55.21 mmol) in MeOH (250 mL). The reaction was stirred at 80 °C for 1 h and the mixture cooled and concentrated. The residue was purified by HPLC to give the secondary amine as its TFA salt (3.93 g). LC-MS m/z = 233.4 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 3.25–3.30 (m, 2H), 3.34–3.40 (m, 2H), 3.49–3.56 (m, 4H), 3.88 (s, 3H), 4.32 (s, 2H), 7.12 (d, J = 8.1 Hz, 1H), 7.40 (d, J = 8.0 Hz, 1H). To a solution of this TFA salt (3.95 g, 11.41 mmol) and triethylamine (6.359 mL, 45.62 mmol) in CH2Cl2 (55 mL), was added a solution of di-tert-butyl dicarbonate (2.489 g, 11.41 mmol) in CH2Cl2 (55 mL). The reaction was stirred at 23 °C for 2 h. The mixture was washed with H2O, the organic portion was concentrated and the residue was purified by column chromatography to give the title compound (3.7 g). LC-MS m/z = 333.6 [M + H]+.
Preparation of 2-(tert-Butoxycarbonyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxylic acid (6)
Five M sodium hydroxide (3.389 mL, 16.95 mmol) was added to a solution of 5 (2.8165 g, 8.473 mmol) in MeOH (12.5 mL). The reaction was stirred at 65 °C for 3 h. The mixture was concentrated. Four M hydrogen chloride (4.025 mL, 16.10 mmol) was added to the residue. The reaction was stirred for 10 min. The mixture was concentrated to give the title compound (2.89 g). LC-MS m/z = 319.2 [M + H]+.
Preparation of 1,2,3,4,6,7-Hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7a)
To a solution of 6 (40 mg, 0.095 mmol), ammonia (0.136 mL, 0.955 mmol), and triethylamine (53.24 μL, 0.382 mmol) in N,N-dimethylformamide (DMF; 0.8 mL), was added HATU (54.46 mg, 0.143 mmol) and the reaction mixture was stirred at room temperature for 15 h. The mixture was concentrated and the residue purified by HPLC to give tert-butyl 8-carbamoyl-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1- hi]indole-2(1H)-carboxylate (Boc-7a) as a white solid. This product was then dissolved in a 1.25 M methanolic solution of HCl (2 mL). The mixture was stirred at rt for 24 h then concentrated, and the residue was purified by HPLC to give the title compound as a trifluoroacetate salt (11 mg, 53%) LC-MS m/z = 218.4 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 3.22–3.29 (m, 4H), 3.49–3.53 (m, 4H), 4.31 (s, 2H), 7.11 (m, 2H).
Preparation of N-Methyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7b)
HATU (0.204 g, 0.537 mmol) was added to a solution of 6 (150 mg, 0.358 mmol), 33% methanamine in ethanol (EtOH; 0.374 mL, 3.581 mmol), and triethylamine (0.150 mL, 1.074 mmol) in DMF (3 mL). The reaction was stirred at 23 °C for 15 h. The mixture was concentrated and purified by HPLC to give tert-butyl 8-(methylcarbamoyl)-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2(1H)-carboxylate (Boc-7b) as a white solid. The solid was then dissolved in 1.25 M methanolic solution of HCl (2 mL). The reaction was stirred at 50 °C for 2 h then the mixture was cooled and concentrated to give the title compound (78 mg). LC-MS m/z = 232.2 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 2.88 (s, 3H), 3.22–3.29 (m, 4H), 3.47–3.55 (m, 4H), 4.31 (s, 2H), 7.02 (d, J = 7.9 Hz, 1H), 7.11 (d, J = 7.9 Hz, 1H).
N,N-Dimethyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7c)
HATU (54.46 mg, 0.143 mmol) was added to a solution of 6 (40 mg, 0.095 mmol), dimethylamine (0.477 mL, 0.955 mmol), and triethylamine (39.93 μL, 0.286 mmol) in DMF (0.8 mL). The reaction was stirred at 23 °C for 15 h. The mixture was concentrated and purified by HPLC to give tert-butyl 8-(dimethylcarbamoyl)-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2(1H)-carboxylate (Boc-7c) as a white solid. This solid was then dissolved in a 1.25 M methanolic solution of HCl (2 mL) and the mixture was stirred at rt for 24 h. The mixture was then concentrated and the residue purified by HPLC to give the title compound as its trifluoroacetate salt. LC-MS m/z = 232.2 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 2.92 (s, 3H) 2.97 (t, J = 8 Hz, 2H), 3.09 (s, 3H) 3.25–3.27 (m, 2H), 3.47–3.53 (m, 4H), 4.29 (s, 2H), 6.73 (d, J = 7.9 Hz, 1H), 7.10 (d, J = 7.9 Hz, 1H).
Preparation of N-Ethyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7d)
From 6 and ethanamine, the title compound was obtained as a white solid using a similar method to the one described in 7b. LC-MS m/z = 246.2 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 1.21 (d, J = 7.2 Hz, 3H), 3.26 (t, J = 8.5 Hz, 2H), 3.29–3.34 (m, 2H), 3.38 (q, J = 7.2 Hz, 2H), 3.50–3.59 (m, 4H), 4.33 (s, 2H), 7.06 (d, J = 7.9 Hz, 1H), 7.14 (d, J = 7.9 Hz, 1H).
Preparation of N-Butyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7e)
From 6 and butan-1-amine, the title compound was obtained as a white solid using a similar method to the one described for 7b. LC-MS m/z = 274.2 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 0.97 (t, J = 7.3 Hz, 3H), 1.36–1.47 (m, 2H), 1.54–1.64 (m, 2H), 3.24 (t, J = 8.5 Hz, 2H), 3.27–3.32 (m, 2H), 3.34 (t, J = 7.1 Hz, 2H), 3.48–3.58 (m, 4H), 4.32 (s, 2H), 7.03 (d, J = 7.9 Hz, 1H), 7.12 (d, J = 7.9 Hz, 1H).
Preparation of N-Isopropyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7f)
From 6 and isopropylamine, the title compound was obtained as a white solid using a similar method to the one described for 7b. LC-MS m/z = 260.2 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 1.23 (d, J = 6.6 Hz, 6H), 3.23 (t, J = 8.4 Hz, 2H), 3.27–3.30 (m, 2H), 3.49–3.58 (m, 4H), 4.10–4.22 (m, 1H), 4.32 (s, 2H), 7.03 (d, J = 7.9 Hz, 1H), 7.13 (d, J = 7.9 Hz, 1H).
Preparation of N-Cyclopropyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7g)
From 6 and cyclopropanamine, the title compound was obtained as a white solid using a similar method to the one described for 7b. LC-MS m/z = 260.2 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 0.58–0.64 (m, 2H), 0.77–0.83 (m, 2H), 2.78–2.86 (m, 1H), 3.21–3.28 (m, 2H), 3.28–3.32 (m, 2H), 3.49–3.58 (m, 4H), 4.32 (s, 2H), 7.03 (d, J = 7.9 Hz, 1H), 7.12 (d, J = 7.9 Hz, 1H).
Preparation of N-(Cyclopropylmethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7h)
From 6 and cyclopropylmethanamine, the title compound was obtained as a white solid using a similar method to the one described in 7b. LC-MS m/z = 272.4 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 0.21–0.35 (m, 2H), 0.45–0.60 (m, 2H), 1.01–1.15 (m, 1H), 3.19–3.34 (m, 6H), 3.50–3.60 (m, 4H), 4.33 (s, 2H), 7.07 (d, J = 7.9 Hz, 1H), 7.14 (d, J = 7.9 Hz, 1H).
Preparation of N-(2-Methoxyethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7i)
HATU (0.430 g, 1.131 mmol) was added to a solution of 6 (300 mg, 0.754 mmol), 2-methoxyethanamine (67.95 mg, 0.905 mmol), and triethylamine (0.315 mL, 2.262 mmol) in DMF (6 mL). The reaction was stirred at 23 °C for 15 h and concentrated then purified by HPLC to give tert-butyl 8-((2-methoxyethyl)carbamoyl)-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2(1H)-carboxylate (Boc-7i) as a white solid. The solid was then dissolved in HCl (1.25 M in methanol) and the reaction was stirred at rt for 24 h. The mixture was concentrated to give the title compound (107 mg). LC-MS m/z = 276.2 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 3.20–3.29 (m, 4H), 3.38 (s, 3H), 3.47–3.58 (m, 8H), 4.31 (s, 2H), 7.03 (d, J = 8.1 Hz, 1H), 7.11 (d, J = 8.0 Hz, 1H).
Preparation of N-(2-(Methylthio)ethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7j)
From 6 and 2-(methylthio)ethanamine, the title compound was obtained as a white solid using a similar method to the one described for 7b. LC-MS m/z = 292.2 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 2.14 (s, 3H), 2.72 (t, J = 6.9 Hz, 2H), 3.02 (s, 2H), 3.24–3.37 (m, 4H), 3.52–3.62 (m, 4H), 4.34 (s, 2H), 7.09 (d, J = 7.9 Hz, 1H), 7.15 (d, J = 8.0 Hz, 1H).
Preparation of N-(2-(Methylsulfonyl)ethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7k)
To a solution of 6 (14 mg, 35.18 μmol), 2-(methylsulfonyl)ethanamine hydrochloride (6.458 mg, 40.46 μmol), and triethylamine (14.71 μL, 0.106 mmol) in MeCN (0.5 mL) was added HATU (20.06 mg, 52.77 μmol). The reaction mixture was stirred at 23 °C for 15 h the concentrated and the residue purified by HPLC to give tert-butyl 8-((2-(methylsulfonyl)ethyl)carbamoyl)-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2(1H)-carboxylate (Boc-7k) as a white solid. This solid was then dissolved in 1.25 M methanol solution of HCl (2 mL). The reaction was stirred at 55 °C for 3 h and then the mixture was concentrated to give the title compound as its hydrochloride salt (9 mg, 64%). LC-MS m/z = 324.2 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 3.20 (s, 3H), 3.31–3.40 (m, 6H), 3.48–3.62 (m, 4H), 3.75–3.84 (m, 2H), 4.32 (s, 2H), 7.10–7.20 (m, 2H).
Preparation of N-(2-Ethoxyethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7l)
HATU (21.78 mg, 57.29 μmol) was added to a solution of 6 (16 mg, 38.19 μmol), 2-ethoxyethanamine (3.745 mg, 42.01 μmol), and triethylamine (15.97 μL, 0.115 mmol) in DMF (0.5 mL). The reaction was stirred at 23 °C for 15 h. The mixture was concentrated and purified by HPLC to give tert-butyl 8-((2-ethoxyethyl)carbamoyl)-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2(1H)-carboxylate as a white solid. The solid was then dissolved in 1.25 M methanol solution of HCl (2 mL). The reaction was stirred at rt for 24 h. The mixture was concentrated to give the title compound as its hydrochloride salt (10.2 mg). LC-MS m/z = 290.0 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 1.20 (t, J = 7.1 Hz, 3H), 3.21–3.30 (m, 4H), 3.48–3.62 (m, 10H), 4.32 (s, 2H), 7.05 (d, J = 7.9 Hz, 1H), 7.11 (d, J = 8.0 Hz, 1H).
Preparation of N-(2-Propoxyethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7m)
From 6 and 2-propoxyethanamine, the title compound was obtained as a white solid using a similar method to the one described for 7b. LC-MS m/z = 304.4 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 0.93 (t, J = 7.3 Hz, 3H), 1.54–1.64 (m, 2H), 3.25 (t, J = 8.5 Hz, 2H), 3.27–3.31 (m, 2H), 3.45 (t, J = 6.5 Hz, 2H), 3.49–3.57 (m, 6H), 3.57–3.62 (m, 2H), 4.33 (s, 2H), 7.06 (d, J = 7.9 Hz, 1H), 7.13 (d, J = 7.9 Hz, 1H).
Preparation of N-(2-Isopropoxyethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7n)
From 6 and 2-isopropoxyethanamine, the title compound was obtained as a white solid using a similar method to the one described for 7b. LC-MS m/z = 304.4 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 1.16 (d, J = 6.2 Hz, 6H), 3.24–3.37 (m, 4H), 3.50 (t, J = 5.5 Hz, 2H), 3.53–3.70 (m, 7H), 4.35 (s, 2H), 7.10 (d, J = 7.9 Hz, 1H), 7.16 (d, J = 7.9 Hz, 1H).
Preparation of N-(2-(Ethylthio)ethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7o)
From 6 and 2-(ethylthio)ethanamine, the title compound was obtained as a white solid using a similar method to the one described for 7b. LC-MS m/z = 306.4 [M + H]+.
Preparation of N-(2-Phenoxyethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7p)
From 6 and 2-phenoxyethanamine, the title compound was obtained as a white solid using a similar method to the one described for 7b. LC-MS m/z = 338.4 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 3.23 (t, J = 8.5 Hz, 2H), 3.26–3.30 (m, 2H), 3.46–3.57 (m, 4H), 3.74 (t, J = 5.5 Hz, 2H), 4.15 (t, J = 5.6 Hz, 2H), 4.31 (s, 2H), 6.89–6.98 (m, 3H), 7.04 (d, J = 7.9 Hz, 1H), 7.12 (d, J = 7.9 Hz, 1H), 7.22–7.30 (m, 2H).
Preparation of N-(1-Methoxypropan-2-yl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7q)
From 6 and 1-methoxypropan-2-amine, the title compound was obtained as a white solid using a similar method to the one described for 7b. LC-MS m/z = 290.0 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 1.22 (d, J = 6.8 Hz, 3H), 3.19–3.28 (m, 2H), 3.28–3.34 (m, 2H), 3.37 (s, 3H), 3.38–3.49 (m, 2H), 3.50–3.58 (m, 4H), 4.22–4.30 (m, 1H), 4.33 (s, 2H), 7.05 (d, J = 7.9 Hz, 1H), 7.14 (d, J = 7.9 Hz, 1H).
Preparation of N-(2-Methoxypropyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7r)
From 6 and 2-methoxypropan-1-amine, the title compound was obtained as a white solid using a similar method to the one described for 7b. LC-MS m/z = 290.4 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 1.18 (d, J = 6.2 Hz, 3H), 3.26 (t, J = 8.4 Hz, 3H), 3.31–3.35 (m, 2H), 3.33–3.47 (m, 2H), 3.38 (s, 3H), 3.52–3.60 (m, 5H), 4.34 (s, 2H), 7.08 (d, J = 7.9 Hz, 1H), 7.15 (d, J = 7.9 Hz, 1H).
Preparation of N-(2-Chloroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7s)
From 6 and 2-chloroethanamine, the title compound was obtained as a white solid using a similar method to the one described for 7b. LC-MS m/z = 280.2 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 3.23–3.33 (m, 4H), 3.49–3.57 (m, 4H), 3.63–3.75 (m, 4H), 4.33 (s, 2H), 7.07 (d, J = 7.9 Hz, 1H), 7.14 (d, J = 7.9 Hz, 1H).
Preparation of N-(2-Fluoroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7t)
From 6 and 2-fluoroethanamine, the title compound was obtained as a white solid using a similar method to the one described for 7b. LC-MS m/z = 264.2 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 3.28 (t, J = 8.5 Hz, 2H), 3.32–3.37 (m, 2H), 3.52–3.59 (m, 4H), 3.61 (t, J = 5.1 Hz, 1H), 3.68 (t, J = 5.1 Hz, 1H), 4.35 (s, 2H), 4.49 (t, J = 5.1 Hz, 1H), 4.61 (t, J = 5.1 Hz, 1H), 7.11 (d, J = 7.9 Hz, 1H), 7.17 (d, J = 7.9 Hz, 1H).
Preparation of N-(2,2-Difluoroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7u)
HATU (28.66 mg, 75.38 μmol) was added to a solution of 6 (20 mg, 50.26 μmol), 2,2-difluoroethanamine (4.481 mg, 55.28 μmol), and triethylamine (21.01 μL, 0.151 mmol) in MeCN (0.5 mL). The reaction was stirred at 23 °C for 15 h. The mixture concentrated and the residue purified by HPLC to give tert-butyl 8-((2,2difluoroethyl)carbamoyl)-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2(1H)-carboxylate (Boc-7u) as a white solid. This solid was then dissolved in 1.25 M methanol solution of HCl (2 mL) and the mixture was stirred at 55 °C for 2 h. The mixture was cooled and concentrated to give the title compound as its hydrochloride salt (7.8 mg, 44%). LC-MS m/z = 282.4 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 3.08–3.13 (m, 2H), 3.20 (t, J = 8.5 Hz, 2H), 3.44 (t, J = 8.5 Hz, 2H), 3.65–3.80 (m, 4H), 4.43 (s, 2H), 5.98 (tt, J 1 = 56.2 Hz, J 2 = 4 Hz, 1H), 7.01 (m, 2H).
Preparation of N-(2,2,2-Trifluoroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (7v)
HATU (21.78 mg, 57.29 μmol) was added to a solution of 6 (16 mg, 38.19 μmol), 2,2,2-trifluoroethanamine (4.162 mg, 42.01 μmol), and triethylamine (15.97 μL, 0.115 mmol) in DMF (0.5 mL) was added. The reaction was stirred at 23 °C for 15 h. The mixture was concentrated and purified by HPLC to give tert-butyl 8-((2,2,2-trifluoroethyl)carbamoyl)-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2(1H)-carboxylate (Boc-7v) as a white solid. This solid was then dissolved in 1.25 M methanol solution of HCl (2 mL). The mixture was stirred at rt for 24 h and then concentrated to give the title compound as its hydrochloride salt (14.2 mg, 64%). LC-MS m/z = 300.4 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 3.23–3.27 (m, 4H), 3.50–3.55 (m, 4H), 4.05 (AB m, J = 8 Hz, 2H), 4.32 (s, 2H), 7.06 (d, J = 7 Hz, 1H), 7.14 (d, J = 7 Hz, 1H).
Preparation of Methyl 1-(2-aminoethyl)-1,2,3,4-tetrahydroquinoline-5-carboxylate (9)
A mixture of methyl 1,2,3,4-tetrahydroquinoline-5-carboxylate (8, 118 mg, 0.617 mmol) and 2-bromoethanamine hydrobromide (139 mg, 0.678 mmol) were combined and heated at 122 °C. After 16 h, the mixture was cooled and the product purified by HPLC (CH3CN/H2O + 0.1% TFA 5–100% gradient). Fractions containing product were concentrated and dried under high vacuum to give the title compound as a trifluoroacetate salt (84.5 mg, 0.243 mmol, 39.3%). LC-MS m/z = 235.0 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 1.90–1.97 (m, 2H), 2.98 (t, J = 7.5 Hz, 2H), 3.15 (t, J = 7.5 Hz, 2H), 3.57 (t, J = 7.5 Hz, 2H), 3.83 (s, 3H), 6.84–6.87 (dd, J 1 = 7.5 Hz, J 2 = 1.5 Hz, 1H), 7.06–7.13 (m, 2H).
Preparation of 2-(tert-Butyl) 9-methyl 3,4,7,8-tetrahydro-6H-[1,4]diazepino[6,7,1-ij]quinoline-2,9(1H)-dicarboxylate (10)
A mixture of 9 (TFA salt, 83.5 mg, 0.240 mmol), 37% formaldehyde (26.8 μL, 0.360 mmol), and TFA (27.7 μL, 0.362 mmol) in 2 mL MeOH was stirred at 80 °C (oil bath temperature). After 1 h, further 37% formaldehyde (0.027 mL) was added and stirring continued for 50 h at 80 °C. The mixture was then concentrated and the residue purified by column chromatography (eluant AcOEt + 5% 2 M NH3 in MeOH) to give the title compound (44.7 mg, 0.181 mmol, 76%). LC-MS m/z = 247.0 [M + H]+; 1H NMR (400 MHz, CDCl3) δ 1.81–1.86 (m, 2H), 3.02–3.08 (m, 6H), 3.21–3.24 (m, 2H), 3.80 (s, 3H), 3.90 (s, 2H), 6.99 (d, J 1 = 7.5 Hz, 1H), 7.32 (d, J 1 = 7.5 Hz, 1H).
Preparation of 2-(tert-Butoxycarbonyl)-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinoline-9-carboxylic acid (11)
A solution of 10 (50.6 mg, 0.146 mmol, 88.6%) in tetrahydrofuran (THF)/MeOH/H2O (1.5 mL, 3:1:1 v/v/v) was treated with lithium hydroxide hydrate (19.1 mg, 0.455 mmol). The mixture was stirred at rt for 72 h, then diluted with water, acidified by the addition of 2 M HCl, and extracted with CH2Cl2. The combined extracts were dried over MgSO4, filtered, and concentrated to give the title compound (46.1 mg, 0.139 mmol, 96.3%) that was used in the next step without additional purification. LC-MS m/z = 333.0 [M + H]+; 1H NMR (400 MHz, CDCl3) δ 1.42 (s, 9H), 1.81–1.87 (m, 2H), 3.08–3.12 (m, 2H), 3.13–3.15 (m, 2H), 3.22–3.24 (m, 2H), 3.64 (m, 2H), 4.36–4.42 (m, 2H), 7.10 (br. d, J = 7.5 Hz, 1H), 7.53 (d, J = 7.5 Hz, 1H).
Preparation of N-Methyl-1,2,3,4,7,8-hexahydro-6H-[1,4]diazepino[6,7,1-ij]quinoline-9-carboxamide (12a)
HATU (32 mg, 84.16 μmol) was added to a solution of 11 (19.4 mg, 58.36 μmol) in DMF (1 mL), followed by 2 M methanamine in THF (146 μL, 0.292 mmol). The mixture was stirred at rt for 1 h, then diluted with water and extracted with CH2Cl2. The combined organic phases were dried over MgSO4, filtered, and concentrated. The residue was the purified by column chromatography (eluting with a hexane/ethyl acetate [EtOAc] gradient) to give tert-butyl 9-(methylcarbamoyl)-3,4,7,8-tetrahydro-1H-[1,4]diazepino[6,7,1-ij]quinoline-2(6H)-carboxylate (Boc-12a, 18.5 mg, 53.56 μmol, 91.8%). To a solution of this intermediate in CH2Cl2 (0.5 mL), was added TFA (89 μL, 1.162 mmol). After stirring at rt for 4 h, the solution was diluted with CH2Cl2 and extracted with 1 M NaOH. The aqueous phase was back-extracted several times with CH2Cl2. The combined organic phases were dried over MgSO4, filtered, and concentrated to provide the title compound (9.2 mg, 37.50 μmol, 70.0%). LC-MS m/z = 246.2 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 1.84–1.88 (m, 2H), 2.82 (t, J = 7.5 Hz, 2H), 2.87 (s, 3H), 3.28–3.44 (m, 6H), 4.2 (s, 2H), 6.88 (d, J = 7.5 Hz, 1H), 7.16 (d, J = 7.5 Hz, 1H).
Preparation of N-(2-Methoxyethyl)-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinoline-9-carboxamide (12b)
To a solution of 11 (11.7 mg, 35.20 μmol) and HATU (21 mg, 55.23 μmol) in 1 mL DMF, was added 2-methoxyethanamine (15.3 μL, 0.178 mmol). After stirring at rt for 1 h, the mixture was concentrated and residue was purified by column chromatography (SiO2, hexane/EtOAc gradient) to give tert-butyl 9-((2-methoxyethyl)carbamoyl)-3,4,7,8-tetrahydro-1H-[1,4]diazepino[6,7,1-ij]quinoline-2(6H)-carboxylate (Boc-12b, 9.2 mg, 23.62 μmol, 67.1%). This product was taken up in dichloromethane (DCM; 1 mL) and treated with 4 M hydrogen chloride in dioxane (1 mL, 4 mmol) with stirring overnight. The mixture was concentrated and dried under high vacuum to provide the title compound as its hydrochloride salt (8.5 mg, 23.23 μmol, 99.4%). LC-MS m/z = 290.0 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 1.84–1.88 (m, 2H), 2.84 (t, J = 7.5 Hz, 2H), 3.30–3.37 (m, 4H), 3.50–3.60 (m, 5H) 3.65–3.80 (m, 5H), 4.25 (s, 2H), 6.91 (d, J = 7.5 Hz, 1H), 7.20 (d, J = 7.5 Hz, 1H).
Preparation of N-(2-Ethoxyethyl)-2,3,4,6,7,8-hexahydro-1H-[1,4]diazepino[6,7,1-ij]quinoline-9-carboxamide (12c)
To a solution of 11 (11.7 mg, 35.20 μmol) and HATU (21 mg, 55.23 μmol) in DMF (1 mL) was added 2-ethoxyethanamine (18 μL, 0.176 mmol). After stirring at rt for 1 h, mixture was concentrated and residue was purified by column chromatography (SiO2, hexane/AcOEt gradient) to give tert-butyl 9-((2-ethoxyethyl)carbamoyl)-3,4,7,8-tetrahydro-1H-[1,4]diazepino[6,7,1-ij]quinoline-2(6H)-carboxylate (Boc-12c, 10.1 mg, 25.03 μmol, 71.1%). This product was taken up in DCM (1 mL) and treated with 4 M hydrogen chloride in dioxane (1 mL, 4 mmol) with stirring overnight. The mixture was concentrated and dried under high vacuum to provide the title compound as its hydrochloride salt (9.4 mg, 24.73 μmol, 99.8%). LC-MS m/z = 290.0 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 1.20 (t, J = 7.5 Hz, 3H) 1.84–1.88 (m, 2H), 2.84 (t, J = 7.5 Hz, 2H), 3.30–3.37 (m, 4H), 3.50–3.80 (m, 8H) 4.25 (s, 2H), 6.91 (d, J = 7.5 Hz, 1H), 7.20 (d, J = 7.5 Hz, 1H).
Preparation of Methyl 3-(2,2,2-trifluoroacetyl)indole-4-carboxylate (14a)
TFAA (0.317 mL, 2.283 mmol) at rt was added to a solution of methyl 1H-indole-4-carboxylate (13, 0.40 g, 2.283 mmol) in DMF (15 mL). The reaction was stirred at 40 °C for 2 h. The mixture was then cooled to rt and poured into sodium bicarbonate solution (100 mL). The precipitate was filtered. The filtrate was extracted with EtOAc. The organic extract was dried over Na2SO4 and concentrated. The residue was purified by column chromatography to give the title compound (220 mg). LC-MS m/z = 272.2 [M + H]+; 1H NMR (400 MHz, CD3Cl3) δ 3.99 (s, 3H), 7.37 (t, J = 7.9 Hz, 1H), 7.51–7.57 (m, 2H), 8.00–8.05 (m, 1H), 9.50 (bs, 1H).
Preparation of Methyl 3-(2,2,2-trifluoroethyl)indoline-4-carboxylate (15a)
To a solution of methyl 3-(2,2,2-trifluoroacetyl)-1H-indole-4-carboxylate (0.10 g, 0.369 mmol) in TFA (1.412 mL, 18.44 mmol) in an ice-bath was added Triethylsilane (0.589 mL, 3.687 mmol) dropwise under N2. The reaction was stirred at 23 °C for 15 h. The mixture was poured into saturated NaHCO3 solution and extracted with EtOAc. The combined organics were concentrated and the residue was purified by silica gel column chromatography to give the title compound (40 mg). LC-MS m/z = 260.0 [M + H]+; 1H NMR (400 MHz, CD3Cl3) δ 2.31–2.44 (m, 1H), 3.56–3.65 (m, 2H), 3.91 (s, 3H), 4.03–4.12 (m, 1H), 6.82 (d, J = 7.8 Hz, 1H), 7.14 (t, J = 7.8 Hz, 1H), 7.37–7.44 (m, 1H).
Preparation of Methyl 1-(2-aminoethyl)-3-(2,2,2-trifluoroethyl)indoline-4-carboxylate (16a)
A mixture of methyl 3-(2,2,2-trifluoroethyl)indoline-4-carboxylate (0.040 g, 0.154 mmol) and 2-bromoethanamine hydrobromide (34.78 mg, 0.170 mmol) was heated at 122 °C for 15 h. The mixture was dissolved in 2 M HCl and purified by HPLC to give the title compound as its TFA salt (20 mg). LC-MS m/z = 303.0 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 2.34–2.64 (m, 2H), 3.10–3.30 (m, 4H), 3.58–3.72 (m, 2H), 3.89 (s, 3H), 4.03–4.12 (m, 1H), 6.88 (d, J = 7.9 Hz, 1H), 7.25 (t, J = 7.9 Hz, 1H), 7.40 (d, J = 7.9 Hz, 1H).
Preparation of 2-tert-Butyl 8-methyl 7-(2,2,2-trifluoroethyl)-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2,8(1H)-dicarboxylate (17a)
TFA (11.04 μL, 0.144 mmol) was added to a solution of 16a (60 mg, 0.144 mmol) and formaldehyde (4.327 mg, 0.144 mmol) in MeOH (4 mL). The reaction was stirred at 80 °C for 1 h. The mixture was concentrated and the residue was purified by HPLC to give methyl 7-(2,2,2-trifluoroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxylate (60 mg). LC-MS m/z = 315.2 [M + H]+. To a solution of this product in TFA (60 mg, 0.140 mmol) and triethylamine (78.10 μL, 0.560 mmol) in CH2Cl2 (1.2 mL), a solution of ditert-butyl dicarbonate (30.57 mg, 0.140 mmol) in CH2Cl2 (1.2 mL) was added. The reaction was stirred at 23 °C for 2 h. The mixture was extracted with 1 M NaOH. The organic extract was concentrated. The residue was purified by column chromatography to give the title compound (44 mg). LC-MS m/z = 415.6 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 1.32–1.47 (m, 9H), 2.24–2.40 (m, 2H), 2.81–3.00 (m, 1H), 3.12–3.27 (m, 2H), 3.35–3.48 (m, 1H), 3.58–3.65 (m, 1H), 3.90 (s, 3H), 3.97–4.13 (m, 3H), 4.63–4.93 (m, 1H), 6.95–7.16 (m, 1H), 7.42 (d, J = 7.9 Hz, 1H).
Preparation of 2-(tert-Butoxycarbonyl)-7-(2,2,2-trifluoroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxylic acid (18a)
5 M sodium hydroxide (40.35 μL, 0.202 mmol) was added to a solution of 17a (0.044 g, 0.106 mmol) in MeOH (0.2 mL). The reaction was stirred at 65 °C for 3 h. The mixture was then concentrated to give the title compound that was used in the next step without purification. LC-MS m/z = 401.4 [M + H]+.
N-Methyl-7-(2,2,2-trifluoroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (19a)
33% methanamine in EtOH (62.68 μL, 0.599 mmol) was added to a solution of 18a (0.030 g, 59.94 μmol), HATU (34.19 mg, 89.91 μmol), and triethylamine (25.06 μL, 0.180 mmol) in MeCN (0.6 mL). The reaction was stirred at 23 °C for 3 h. The mixture was then concentrated and the residue was purified by HPLC to give tert-butyl 8-(methylcarbamoyl)-7-(2,2,2-trifluoroethyl)-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2(1H)-carboxylate (Boc-19a) as a white solid. The solid was then dissolved in 1.25 M methanol solution of HCl (2 mL) and the mixture stirred at 55 °C for 5 h. The mixture was then concentrated and lyophilized to give the title compound as its hydrochloride salt (18.5 mg, 80%). LC-MS m/z = 314.2 [M + H]+; 1H NMR (400 MHz, CD3OD) δ ppm 2.25–2.40 (m, 1H), 2.49–2.61 (m, 1H), 2.89 (s, 3H), 3.15–3.20 (m, 1H), 3.39–3.50 (m, 3H), 3.60–3.66 (m, 3H), 4.01–4.11 (m, 1H), 4.22 (d, J = 15.0 Hz, 1H), 4.47 (d, J = 15.0 Hz, 1H), 7.05 (d, J = 7.9 Hz, 1H), 7.19 (d, J = 7.9 Hz, 1H).
Preparation of N-Ethyl-7-(2,2,2-trifluoroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (19b)
HATU (8.426 mg, 22.16 μmol) was added to a solution of 18a (9.5 mg, 14.77 μmol), ethanamine in methanol (73.87 μL, 0.148 mmol), and triethylamine (6.178 μL, 44.32 μmol) in MeCN (0.25 mL)The reaction was stirred at 23 °C for 15 h. The mixture was concentrated and purified by HPLC to give tert-butyl 8-(ethylcarbamoyl)-7-(2,2,2-trifluoroethyl)-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2(1H)-carboxylate (Boc-19b) as a white solid. The solid was then dissolved in 1.25 M methanol solution of HCl (12 mL) and the reaction was stirred at rt for 24 h. The mixture was then concentrated and lyophilized to give the title compound (3.1 mg, 52%). LC-MS m/z = 328.4 [M + H]+; 1H NMR (400 MHz, CD3OD) δ ppm 1.21 (t, J = 8 Hz, 3H), 2.25–2.35 (m, 1H), 2.44–2.60 (m, 1H), 3.15–3.20 (m, 1H), 3.39–3.50 (m, 6H), 3.60–3.66 (m, 2H), 4.01–4.11 (m, 1H), 4.22 (d, J = 15.0 Hz, 1H), 4.47 (d, J = 15.0 Hz, 1H), 7.04 (d, J = 7.9 Hz, 1H), 7.19 (d, J = 7.9 Hz, 1H).
Preparation of N-Propyl-7-(2,2,2-trifluoroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (19c)
HATU (6.209 mg, 16.33 μmol) was added to a solution of 18a (7 mg, 10.89 μmol), propan-1-amine (1.074 μL, 13.06 μmol), and triethylamine (4.552 μL, 32.66 μmol) in MeCN (0.2 mL) . The reaction was stirred at 23 °C for 15 h. The mixture concentrated and purified by HPLC to give tert-butyl 8-(propylcarbamoyl)-7-(2,2,2-trifluoroethyl)-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2(1H)-carboxylate (Boc-19c) as a white solid. The solid was then dissolved in 1.25 M methanol solution of HCl (12 mL). The reaction was stirred at 55 °C for 1 h and then concentrated to give the title compound (3.3 mg, 73%). LC-MS m/z = 342.4 [M + H]+; 1H NMR (400 MHz, CD3OD) δ ppm 0.97 (t, J = 8 Hz, 3H), 1.62 (q, J = 8 Hz, 2H), 2.28–2.39 (m, 1H), 2.46–2.60 (m, 1H), 3.15–3.20 (m, 1H), 3.22–3.50 (m, 5H), 3.60–3.66 (m, 2H), 4.01–4.11 (m, 1H), 4.22 (d, J = 15.0 Hz, 1H), 4.47 (d, J = 15.0 Hz, 1H), 7.04 (d, J = 7.9 Hz, 1H), 7.19 (d, J = 7.9 Hz, 1H).
Preparation of N-(2-Methoxyethyl)-7-(2,2,2-trifluoroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (19d)
19d was prepared from 18a (7 mg, 10.89 μmol) and 2-methoxyethanamine (1.125 μL, 13.06 μmol), using the method described above for 19c. Yield 3.2 mg, 62%. LC-MS m/z = 358.4 [M + H]+; 1H NMR (400 MHz, CD3OD) δ ppm 2.28–2.39 (m, 1H), 2.46–2.60 (m, 1H), 3.15–3.20 (m, 1H), 3.28–3.65 (m, 13H), 4.01–4.11 (m, 1H), 4.22 (d, J = 15.0 Hz, 1H), 4.47 (d, J = 15.0 Hz, 1H), 7.04 (d, J = 7.9 Hz, 1H), 7.19 (d, J = 7.9 Hz, 1H).
Preparation of N-(2-Fluoroethyl)-7-(2,2,2-trifluoroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (19e)
19e was prepared from 18a (10 mg, 19.98 μmol) and 2-fluoroethanamine (2.103 μL, 23.98 μmol), using the method described above for 19b. Yield 5.2 mg, 62%. LC-MS m/z = 358.4 [M + H]+; 1H NMR (400 MHz, CD3OD) δ ppm 2.28–2.39 (m, 1H), 2.46–2.60 (m, 1H), 3.15–3.20 (m, 1H), 3.40–3.52 (m, 3H), 3.60–3.75 (m, 5H), 4.01–4.11 (m, 1H), 4.22 (d, J = 15.0 Hz, 1H), 4.47 (d, J = 15.0 Hz, 1H), 4.53 (dt, J 1 = 56.0, J 1 = 3.9 Hz, 2H), 7.08 (d, J = 7.9 Hz, 1H), 7.19 (d, J = 7.9 Hz, 1H).
Preparation of N-(2,2-Difluoroethyl)-7-(2,2,2-trifluoroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (19f)
HATU (11.40 mg, 29.97 μmol) was added to a solution of 2-(tert-butoxycarbonyl)-7-(2,2,2-trifluoroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxylic acid (18a, 10 mg, 19.98 μmol), 2,2-difluoroethanamine (2.703 μL, 23.98 μmol), and triethylamine (8.355 μL, 59.94 μmol) in MeCN (0.2 mL). The reaction was stirred at 23 °C for 15 h. The mixture was purified by HPLC to give tert-butyl 8-((2,2-difluoroethyl)carbamoyl)-7-(2,2,2-trifluoroethyl)-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2(1H)-carboxylate as a white solid. LC-MS m/z = 464.2 [M + H]+. The solid was then dissolved in 1.25 M methanol solution of HCl (2 mL). The reaction was stirred at rt for 24 h. The mixture was concentrated to give the title compound. LC-MS m/z = 364.4 [M + H]+; 1H NMR (400 MHz, CD3OD) δ ppm 2.25–2.43 (m, 1H), 2.47–2.62 (m, 1H), 3.12–3.22 (m, 1H), 3.38–3.52 (m, 3H), 3.58–3.79 (m, 4H), 4.01–4.11 (m, 1H), 4.23 (d, J = 15.0 Hz, 1H), 4.48 (d, J = 15.0 Hz, 1H), 5.83–6.16 (m, 1H), 7.09 (d, J = 7.9 Hz, 1H), 7.21 (d, J = 7.9 Hz, 1H).
Preparation of (+)-N-(2,2-difluoroethyl)-7-(2,2,2-trifluoroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide and (−)-N-(2,2-difluoroethyl)-7-(2,2,2-trifluoroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide.
The (+)- and (−)-enantiomers of N-(2,2-difluoroethyl)-7-(2,2,2-trifluoroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide were obtained from the racemate 19f by separation using supercritical fluid chromatography.
Preparation of N,7-Bis(2,2,2-trifluoroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (19g)
19g was prepared from 18a (10 mg, 19.98 μmol) and 2,2,2-trifluoroethanamine (3.303 μL, 23.98 μmol), using the method described above for 19b. Yield 2.4 mg, 26%. LC-MS m/z = 381.8 [M + H]+; 1H NMR (400 MHz, CD3OD) δ ppm 2.28–2.39 (m, 1H), 2.46–2.60 (m, 1H), 3.15–3.20 (m, 1H), 3.40–3.52 (m, 4H), 3.60–3.72 (m, 2H), 3.95–4.11 (m, 3H), 4.24 (d, J = 15.0 Hz, 1H), 4.47 (d, J = 15.0 Hz, 1H), 7.09 (d, J = 7.9 Hz, 1H), 7.22 (d, J = 7.9 Hz, 1H).
Preparation of Methyl 3-(2,2-difluoroacetyl)-1H-indole-4-carboxylate (14b)
1 M solution of diethylaluminum chloride in hexane (4.281 mL, 4.281 mmol) at ice–water bath under N2 was added to a solution of methyl 1H-indole-4-carboxylate (0.50 g, 2.854 mmol) in CH2Cl2 (10 mL). After 30 min, 2,2-difluoroacetic anhydride (0.994 g, 5.708 mmol) was added dropwise. The reaction mixture was stirred at ice–water bath for 2 h. Water was added, then sat. NaHCO3 was added to adjust the pH to 7. The aqueous mixture was extracted three times with with DCM. The combined organics were concentrated and the resulting residue purified by silica gel column chromatography to give the title compound (67 mg, 9.3%). LC-MS m/z = 254.2 [M + H]+.
Preparation of Methyl 3-(2,2-difluoroethyl)indoline-4-carboxylate (15b)
Triethylsilane (0.169 mL, 1.058 mmol) dropwise under N2 was added to an ice-cooled solution of 14b (0.067 g, 0.265 mmol) in TFA (1.013 mL, 13.23 mmol) was added. The reaction was then stirred at 23 °C for 15 h. The resulting mixture was poured into 2 M Na2CO3 solution and extracted twice with EtOAc. The combined organics were then concentrated and the residue purified by silica gel column chromatography (0–50%EtOAc/Hex, product elutes around 30%) to give the title compound (26 mg. 41%). LC-MS m/z = 242.4 [M + H]+
Preparation of Methyl 1-(2-aminoethyl)-3-(2,2-difluoroethyl)indoline-4-carboxylate (16b)
A mixture of 15b (26 mg, 0.108 mmol) and 2-bromoethanamine hydrobromide (23.19 mg, 0.113 mmol) was heated at 122 °C for 3 h. The mixture was dissolved in 2 M HCl and purified by HPLC to give the title compound as its TFA salt (13.2 mg, 40%). LC-MS m/z = 285.4 [M + H]+
Preparation of Methyl 7-(2,2-difluoroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxylate (17b)
TFA (7.613 μL, 99.42 μmol) was added to a solution of 16b (13.2 mg, 33.14 μmol) and formaldehyde (2.756 μL, 99.42 μmol) in MeOH (1 mL). The reaction was stirred at 80 °C for 1 h. The mixture was concentrated. The residue was purified by HPLC to give the title compound as its TFA salt (6.0 mg, 44%). LC-MS m/z = 297.4 [M + H]+. 1H NMR (400 MHz, CD3OD) δ ppm 3.12–3.22 (m, 1H), 3.24–3.34 (m, 1H), 3.38–3.52 (m, 3H), 3.58–3.70 (m, 3H), 3.90 (s, 3H), 4.01–4.11 (m, 1H), 4.18 (d, J = 15.0 Hz, 1H), 4.52 (d, J = 15.0 Hz, 1H), 6.05 (tt, J 1 = 56.0, J 2 = 3.9 Hz, 1H), 7.19 (d, J = 8 Hz, 1H), 7.47 (d, J = 8 Hz, 1H). To this product in CH2Cl2 (0.1 mL) and triethylamine (8.152 μL, 58.49 μmol) was added a solution of ditert-butyl dicarbonate (3.191 mg, 14.62 μmol) in CH2Cl2 (0.1 mL). The reaction was stirred at 23 °C for 2 h and the mixture was extracted with 1 M NaOH. The organic portion was concentrated and the residue purified by column chromatography (0–50%EtOAc/Hex, eluted 35%) to give the title compound (3.5 mg, 60%). LC-MS m/z = 397.0 [M + H]+.
Preparation of 2-(tert-Butoxycarbonyl)-7-(2,2-difluoroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxylic acid (18b)
5 M sodium hydroxide (6.614 μL, 33.07 μmol) was added to a solution of 17b (6.9 mg, 17.41 μmol) in MeOH (0.05 mL). The reaction was stirred at 65 °C for 3 h. The mixture was then concentrated to provide the sodium salt of the title compound that was used in the next step without purification. LC-MS m/z = 383.0 [M + H]+.
Preparation of 7-(2,2-Difluoroethyl)-N-methyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (19h)
HATU (10.55 mg, 27.75 μmol) was added to a mixture of 18b (Na+ salt, 7.50 mg, 18.50 μmol), 33% methanamine in EtOH (19.35 μL, 0.185 mmol), and triethylamine (7.736 μL, 55.50 μmol) in MeCN (0.3 mL) was added. The reaction was stirred at 23 °C for 15 h and then concentrated. The residue was purified by HPLC to give tert-butyl 7-(2,2-difluoroethyl)-8-(methylcarbamoyl)-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2(1H)-carboxylate (Boc-19h) LC-MS m/z = 396.2 [M + H]+; 1H NMR (400 MHz, CD3OD) δ ppm 1.36 (s, 5.5 H), 1.43 (s, 4.5 H), 1.90–2.08 (m, 2H), 2.87 (s, 3H), 2.95–3.02 (m, 1H), 3.12–3.22 (m, 1H), 3.35 (d, J= 8 Hz, 1H), 3.45 (d, J= 8 Hz, 1H), 3.52–3.60 (m, 1H), 3.80–3.90 (m, 2H), 4.20–4.24 (m, 1H), 4.58–4.65 (m, 1H), 5.96 (tt, J 1 = 56.0, J 2 = 3.9 Hz, 1H), 6.93 (d, J = 8 Hz, 1H), 7.00 (d, J = 8 Hz, 1H). This solid was then dissolved in 1.25 M methanol solution of HCl (2 mL). The reaction was stirred at 55 °C for 1 h. The mixture was concentrated to give the title compound as its hydrochloride salt (2.6 mg, 38%). LC-MS m/z = 296.2 [M + H]+; 1H NMR (400 MHz, CD3OD) δ ppm 1.95–2.20 (m, 2H), 2.89 (s, 3H), 2.95–3.02 (m, 1H), 3.38–3.49 (m, 3H), 3.54–3.62 (m, 2H), 3.90–4.00 (m, 1H), 4.20 (d, J= 8 Hz, 1H), 4.45 (d, J= 8 Hz, 1H), 5.99 (tt, J 1 = 56.0, J 2 = 3.9 Hz, 1H), 7.03 (d, J = 8 Hz, 1H), 7.16 (d, J = 8 Hz, 1H).
Preparation of Methyl 3-acetyl-1H-indole-4-carboxylate (14c)
To a solution of methyl 1H-indole-4-carboxylate (1 g, 5.708 mmol) in DCM (20 mL) was added 1 M solution of diethylaluminum chloride (8.562 mL, 8.562 mmol) in an ice–water bath under nitrogen. After 30 min, acetyl chloride (0.812 mL, 11.42 mmol) was added dropwise. The reaction was stirred for 2 h in an ice–water bath. The reaction was quenched with water, then saturated NaHCO3 solution was added to adjust the pH to 7. The mixture was extracted with EtOAc. The combined organic fractions were concentrated. The residue was purified by silica gel column chromatography to give the title compound (1.18 g) as an off-white solid. LC-MS m/z = 218.2 [M + H]+; 1H NMR (400 MHz, CDCl3) δ 2.46 (s, 3H), 3.99 (s, 3H), 7.28 (dd, J = 8.2 and 7.4 Hz, 1H), 7.43 (dd, J = 7.4 and 1.0 Hz, 1H), 7.46 (dd, J = 8.2 and 1.0 Hz, 1H), 7.77 (d, J = 3.1 Hz, 1H), 9.14 (bs, 1H).
Preparation of Methyl 3-ethylindoline-4-carboxylate (15c)
Sodium borohydride (0.139 g, 3.683 mmol) under nitrogen in an ice–water bath was added to a solution of 14c (400 mg, 1.841 mmol) in anhydrous THF (10 mL). Boron trifluoride diethyl etherate (0.700 mL, 5.524 mmol) was then added dropwise. The reaction was stirred for 2 h while warmed to rt. The reaction was poured into a mixture of ice–water and 5% aqueous NaHCO3 and extracted with EtOAc. The combined organic fractions were concentrated. The residue was purified by silica gel column chromatography to give methyl 3-ethyl-1H-indole-4-carboxylate (320 mg) as an oil. LC-MS m/z = 204.4 [M + H]+; 1H NMR (400 MHz, CDCl3) δ 1.25 (t, J = 7.4 Hz, 3H), 2.87–2.94 (m, 2H), 3.97 (s, 3H), 7.11–7.14 (m, 1H), 7.19 (t, J = 7.8 Hz, 1H), 7.51 (dd, J = 8.1 and 1.0 Hz, 1H), 7.60 (dd, J = 7.4 and 1.0 Hz, 1H), 8.16 (bs, 1H). To a solution of this product (320 mg, 1.575 mmol) in TFA (10 mL) in an ice–water bath, 1 M solution of borane THF complex (2.677 mL, 2.677 mmol) was added dropwise under nitrogen. The reaction was stirred for 30 min. A 20% NaOH solution was added to neutralize the mixture. The mixture was then extracted with EtOAc. The combined organic fractions were concentrated. The residue was purified by HPLC. The combined fractions were neutralized with saturated NaHCO3, partially concentrated, and extracted with EtOAc. The combined organic fractions were dried over anhydrous Na2SO4, filtered, then concentrated to give the title compound (270 mg) as a yellow oil. LC-MS m/z = 206.2 [M + H]+; 1H NMR (400 MHz, CDCl3) δ 0.96 (t, J = 7.4 Hz, 3H), 1.50–1.70 (m, 2H), 3.42 (dd, J = 8.7 and 1.7 Hz, 1H), 3.60 (t, J = 8.6 Hz, 1H), 3.68–3.75 (m, 1H), 3.88 (s, 3H), 6.77 (dd, J = 7.8 and 1.0 Hz, 1H), 7.07 (t, J = 7.8 Hz, 1H), 7.33 (dd, J = 7.8 and 1.0 Hz, 1H).
Preparation of Methyl 1-(2-aminoethyl)-3-ethylindoline-4-carboxylate (16c)
15c (205 mg, 0.999 mmol) and 2-bromoethanamine hydrobromide (0.246 g, 1.199 mmol) were heated at 120 °C for 15 h. The mixture was dissolved in methanol and purified by HPLC to give the title compound as a TFA salt (148 mg). LC-MS m/z = 249.4 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 1.00 (t, J = 7.4 Hz, 3H), 1.53–1.68 (m, 2H), 3.14–3.29 (m, 4H), 3.52 (dd, J = 8.8 and 1.8 Hz, 1H), 3.57–3.65 (m, 1H), 3.65–3.73 (m, 1H), 3.88 (s, 3H), 6.81 (d, J = 7.5 Hz, 1H), 7.17 (t, J = 7.8 Hz, 1H), 7.31 (dd, J = 7.9 and 0.9 Hz, 1H).
Preparation of 2-tert-Butyl 8-methyl 7-ethyl-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2,8(1H)-dicarboxylate (17c)
TFA (0.148 mL, 1.932 mmol) was added to a solution of 16c (140 mg, 0.386 mmol) and 37% formaldehyde in water (86.30 μL, 1.159 mmol) in methanol (5 mL). The reaction was stirred at 80 °C for 2 h. The mixture was concentrated and the residue was dissolved in EtOAc. The organic solution was washed with saturated NaHCO3, water and brine, dried with Na2SO4, filtered, then concentrated to give crude methyl 7-ethyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxylate. The crude product obtained above was dissolved in DCM (5 mL). Triethylamine (81.43 μL, 0.580 mmol) was added, followed by ditert-butyl dicarbonate (0.110 g, 0.502 mmol). The reaction was stirred at rt overnight. The mixture was concentrated. The residue was purified by silica gel column chromatography to give the title compound (92 mg) as an oil. LC-MS m/z = 361.2 [M + H]+; 1H NMR (400 MHz, CDCl3) δ 0.95 (t, J = 7.4 Hz, 3H), 1.35–1.45 (br, 9H), 1.40–1.50 (m, 1H), 1.54–1.64 (m, 1H), 2.80–2.98 (m, 1H), 3.10–3.18 (m,1H), 3.24 (t, J = 8.9 Hz, 1H), 3.34–3.45 (m, 2H), 3.68–3.76 (m, 1H), 3.87 (s, 3H), 3.97–4.08 (m, 2H), 4.62–4.88 (m, 1H), 6.91–7.06 (m, 1H), 7.36 (d, J = 8.0 Hz, 1H).
Preparation of 2-(tert-Butoxycarbonyl)-7-ethyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxylic acid (18c)
1 M solution of lithium hydroxide in water (1.531 mL, 1.531 mmol) was added to a solution of 17c (92 mg, 0.255 mmol) in dioxane (5 mL). The reaction was stirred at 90 °C overnight. A 5% citric acid solution was added to adjust the pH of the mixture to 3–4. The mixture was then extracted with EtOAc. The combined organic fractions were concentrated to give the title compound (90 mg) as gum. LC-MS m/z = 347.2 [M + H]+.
Preparation of 7-Ethyl-N-methyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (19i)
HATU (41.16 mg, 0.108 mmol) was added to a stirred solution of 18c (25 mg, 72.17 μmol) and triethylamine (30.18 μL, 0.216 mmol) in DMF (2 mL). After 10 min, 2 M methanamine in THF (0.361 mL, 0.722 mmol) was added. The reaction mixture was stirred at rt overnight, then purified by HPLC (10–70% CH3CN/H2O with 0.1% TFA over 30 min). The combined fractions were lyophilized to give tert-butyl 7-ethyl-8-(methylcarbamoyl)-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2(1H)-carboxylate (Boc-19i), which was then dissolved in methanol (1 mL), then 4 M HCl in dioxane (1 mL) was added. The reaction mixture was stirred at rt for 2 h and concentrated, and the residue was purified by preparative HPLC (5–60% CH3CN/H2O with 0.1% TFA over 30 min). The combined fractions were lyophilized to give the title compound as its trifluoroacetate salt (15 mg, 56%). LC-MS m/z = 260.2 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 0.92 (t, J = 7.4 Hz, 3H), 1.40–1.50 (m, 1H), 1.54–1.62 (m, 1H), 2.87 (s, 3H), 3.10–3.16 (m, 1H), 3.32–3.45 (m, 4H), 3.55–3.62 (m, 1H), 3.68–3.74 (m, 1H), 4.18 (d, J = 15.2 Hz, 1H), 4.39 (d, J = 15.2 Hz, 1H), 6.95 (d, J = 8.0 Hz, 1H), 7.10 (d, J = 8.0 Hz, 1H).
Preparation of 7-Ethyl-N-(2-fluoroethyl)-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (19j)
2-fluoroethanamine (12.29 mg, 0.195 mmol) was added to the solution of 18c (45 mg, 0.130 mmol), HATU (74.04 mg, 0.195 mmol), and triethylamine (36.21 μL, 0.260 mmol) in DMF (2 mL). The reaction mixture was stirred at rt overnight. The crude product was purified by preparative HPLC (15–85% CH3CN/H2O with 0.1% TFA over 30 min). The combined fractions were lyophilized to give tert-butyl 7-ethyl-8-((2-fluoroethyl)carbamoyl)-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2(1H)-carboxylate (Boc-19j). This product was dissolved in dioxane (0.5 mL) and 4 M HCl in dioxane (0.5 mL) was added. The reaction mixture was stirred at rt for 5 h, then concentrated. The residue was purified by preparative HPLC (5–60% CH3CN/H2O with 0.1% TFA over 30 min). The combined fractions were lyophilized to give the title compound as its trifluoroacetate salt (35 mg, 66%). LC-MS m/z = 292.2 [M + H]+; 1H NMR (400 MHz, CD3OD) δ 0.90 (t, J = 7.4 Hz, 3H), 1.40–1.50 (m, 1H), 1.54–1.64 (m, 1H), 3.10–3.18 (m, 1H), 3.32–3.45 (m, 4H), 3.55–3.72 (m, 4H), 4.18 (d, J = 15.2 Hz, 1H), 4.39 (d, J = 15.2 Hz, 1H), 4.55 (td, J 1 = 56 Hz, J 1 = 4 Hz, 2H), 6.98 (d, J = 8.0 Hz, 1H), 7.12 (d, J = 8.0 Hz, 1H).
Preparation of N-(2,2-Difluoroethyl)-7-ethyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (19k)
2,2-difluoroethanamine (17.55 mg, 0.216 mmol) was added to the solution of 18c (50 mg, 0.144 mmol), HATU (82.27 mg, 0.216 mmol), and triethylamine (40.23 μL, 0.289 mmol) in DMF (2 mL). The reaction mixture was stirred at room temperature overnight. The crude was purified by semi preparative HPLC (15–85% CH3CN/H2O with 0.1% TFA over 30 min). The combined fractions were lyophilized to give tert-butyl 8-((2,2-difluoroethyl)carbamoyl)-7-ethyl-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2(1H)-carboxylate, which was dissolved in dioxane (0.5 mL), and 4 M HCl in dioxane (0.5 mL) was added. The reaction mixture was stirred at room temperature for 4 h and concentrated. The residue was purified by semi preparative HPLC (5–60% CH3CN/H2O with 0.1% TFA over 30 min). The combined fractions were lyophilized to give the title compound as the TFA salt (40 mg). LC-MS m/z = 310.6 [M + H]+. 1H NMR (400 MHz, CD3OD) δ 0.89 (t, J = 7.4 Hz, 3H), 1.40–1.52 (m, 1H), 1.54–1.65 (m, 1H), 3.09–3.18 (m, 1H), 3.32–3.50 (m, 4H), 3.54–3.72 (m, 4H), 4.18 (d, J = 4.9 Hz, 1H), 4.40 (d, J = 4.9 Hz, 1H), 6.00 (tt, J 1 = 56.0, J 1 = 3.9 Hz, 1H), 6.98 (d, J = 7.8 Hz, 1H), 7.12 (d, J = 7.9 Hz, 1H).
Preparation of (+)-N-(2,2-Difluoroethyl)-7-ethyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide ((+)-19k) and (−)-N-(2,2-Difluoroethyl)-7-ethyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide ((−)-19k)
Enantiomers of N-(2,2-difluoroethyl)-7-ethyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide were obtained by chiral HPLC separation using following conditions. Column: Chiralpak IC column 250 × 20 mm (L x I.D.); flow: 10 mL/min; eluent: 15% EtOH/hexanes with 0.1% Et3N; detector: UV 254 nm; retention time: first eluting enantiomer = 18.3 min, second eluting enantiomer = 20.5 min. After separation, both enantiomers were further purified by semi preparative HPLC (5–50% CH3CN/H2O with 0.1% TFA over 30 min). The combined fractions were lyophilized to give the title compounds as their TFA salts.
Preparation of Methyl 3-formyl-1H-indole-4-carboxylate (14d)
A 2 M solution of oxalyl dichloride in DCM (1.712 mL, 3.425 mmol) was added to DCM (15 mL) cooled down in an ice–water bath. N,N-dimethylformamide (0.250 g, 3.425 mmol) was added dropwise under nitrogen. The reaction mixture was stirred at 0 °C for 30 min, then methyl 1H-indole-4-carboxylate (0.5 g, 2.854 mmol) in DCM (10 mL) was added. The reaction mixture was warmed to room temperature and stirred for 1 h. The solvent was removed. THF (15 mL) and 20% aqueous ammonium acetate were added. The reaction mixture was stirred under reflux (∼70 °C) for 30 min. The reaction mixture was then extracted with EtOAc. The combined organics (organic phases) were concentrated; the residue was purified by silica gel column chromatography with 90% EtOAc/hexanes to give the title compound (551 mg, 95.0%) as a white solid. LC-MS m/z = 204.2 [M + H]+; 1H NMR (400 MHz, CDCl3) δ ppm 4.00 (s, 3H), 7.34 (t, J = 7.8 Hz, 1H), 7.63 (dd, J = 8.0 and 1.0 Hz, 1H), 7.87 (dd, J = 7.5 and 1.0 Hz, 1H), 8.10 (d, J = 3.2 Hz, 1H), 9.08 (br s, 1H), 10.53 (s, 1H).
Preparation of Methyl 3-methyl-1H-indole-4-carboxylate
4-methylbenzenesulfonohydrazide (0.657 g, 3.525 mmol) followed by p-toluenesulfonic acid monohydrate (77.37 mg, 0.407 mmol) and tetramethylene sulfone (sulfolane, 8 mL) was added to a stirred solution of methyl 3-formyl-1H-indole-4-carboxylate (14d, 551 mg, 2.712 mmol) in DMF (8 mL). The reaction mixture was stirred at 100 °C for 1 h and cooled to room temperature. Sodium cyanoborohydride (0.682 g, 10.85 mmol) was added portionwise. The mixture was then stirred at 100 °C for 2 h. The reaction mixture was cooled, diluted with water, and extracted with 50% EtOAc in hexanes. The organic extracts were concentrated and the residue was purified by silica gel column chromatography with 20% EtOAc/hexanes to give the title compound (355 mg, 69.2%) as off-white solid. LC-MS m/z = 190.4 [M + H]+; 1H NMR (400 MHz, CDCl3) δ ppm 2.41 (d, J = 1.0 Hz, 3H), 3.96 (s, 3H), 7.07–7.10 (m, 1H), 7.18 (t, J = 7.8 Hz, 1H), 7.50 (dd, J = 8.0 and 1.0 Hz, 1H), 7.64 (dd, J = 7.5 and 1.0 Hz, 1H), 8.12 (bs, 1H).
Preparation of Methyl 3-methylindoline-4-carboxylate (15d)
To a solution of methyl 3-methyl-1H-indole-4-carboxylate (1.253 g, 6.622 mmol) in TFA (trifluoroacetic acid) (4.06 mL) in an ice–water bath was added triethylsilane (4.231 mL, 26.49 mmol) dropwise under N2. The reaction mixture was warmed to room temperature and stirred overnight. The mixture was concentrated and added water. After adjusting the pH to 8 with saturated aqueous NaHCO3 solution, the mixture was extracted with EtOAc. The combined organics were concentrated. The residue was purified by silica gel column chromatography with 25% EtOAc/hexanes (column prewashed with 0.1% Et3N/hexanes) to give the title compound (1.013 g, 80.0%) as an orange-red oil. LC-MS m/z = 192.2 [M + H]+; 1H NMR (400 MHz, CDCl3) δ ppm 1.25 (d, J = 6.9 Hz, 3H), 3.25 (dd, J = 8.6 and 1.7 Hz, 1H), 3.68 (t, J = 8.5 Hz, 1H), 3.83–3.92 (m, 1H), 3.90 (s, 3H), 6.78 (dd, J = 7.8 and 1.0 Hz, 1H), 7.07 (t, J = 7.8 Hz, 1H), 7.34 (dd, J = 7.8 and 1.0 Hz, 1H).
Preparation of 2-tert-Butyl 8-methyl 7-methyl-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2,8(1H)-dicarboxylate (17d)
A mixture of methyl 3-methylindoline-4-carboxylate (15d, 1.013 g, 5.297 mmol) and 2-bromoethanamine hydrobromide (1.302 g, 6.357 mmol) was heated at 115 °C overnight. The residue was dissolved in methanol and purified by preparative HPLC (5–60% CH3CN/H2O with 0.1% TFA over 30 min). The combined fractions were then concentrated to give methyl 1-(2-aminoethyl)-3-methylindoline-4-carboxylate (16d). LC-MS m/z = 235.4 [M + H]+; 1H NMR (400 MHz, CDCl3) δ ppm 1.24 (d, J = 7.0 Hz, 3H), 2.92–3.00 (m, 3H), 3.25 (dd, J = 8.5 and 1.7 Hz, 1H), 3.30–3.40 (m, 2H), 3.80–3.90 (m, 1H), 3.88 (s, 3H), 6.64 (d, J = 7.7 Hz, 1H), 7.11 (t, J = 7.8 Hz, 1H), 7.27 (dd, J = 7.9 and 0.8 Hz, 1H). This product was dissolved in methanol (10 mL) and 37% formaldehyde in water (1.183 mL, 15.89 mmol) was added, followed by TFA (1.217 mL, 15.89 mmol). The reaction mixture was heated at 80 °C for 1 h and then concentrated. The residue was dissolved in THF (8 mL), and saturated aqueous NaHCO3 (8 mL) solution and ditert-butyl dicarbonate (0.776 mL, 5.297 mmol) added. The reaction mixture was stirred at room temperature overnight, diluted with water, and extracted with EtOAc. The combined organics were concentrated. The residue was purified by silica gel column chromatography with 25% EtOAc/hexanes to give the title compound (1.212 g, 66.0%) as a colorless oil. LC-MS m/z = 347.2 [M + H]+; 1H NMR (400 MHz, CDCl3) δ ppm 1.20 (d, J = 6.9 Hz, 3H), 1.35–1.45 (br, 9H), 2.80–2.95 (m, 1H), 3.08–3.18 (m,1H), 3.24–3.35 (m, 2H), 3.35–3.45 (m, 1H), 3.85–3.95 (m, 1H), 3.86 (s, 3H), 3.97–4.08 (m, 2H), 4.62–4.88 (m, 1H), 6.91–7.06 (m, 1H), 7.36 (d, J = 8.0 Hz, 1H).
Preparation of 2-(tert-Butoxycarbonyl)-7-methyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxylic acid (18d)
To a solution of 2-tert-butyl 8-methyl 7-methyl-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2,8(1H)-dicarboxylate (17d, 1.212 g, 3.499 mmol) in dioxane (10 mL) was added a 1 M solution of lithium hydroxide in water (13.99 mL, 13.99 mmol). The reaction mixture was stirred at 80 °C for 2 h. Organic solvent was evaporated. The residue was diluted with water, and the pH adjusted to 3–4 with aqueous 5% citric acid. The off-white precipitate was collected and dried to give the title compound (1.116 g, 96.0%) as an off-white solid, which was used in the next step without further purification. LC-MS m/z = 333.4 [M + H]+.
Preparation of N,7-Dimethyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (19l)
HATU (37.75 mg, 99.28 μmol) was added to a stirred solution of 18d (22 mg, 66.19 μmol) and triethylamine (27.68 μL, 0.199 mmol) in DMF (2 mL). After 10 min, 2 M methanamine in THF (0.331 mL, 0.662 mmol) was added. The reaction mixture was stirred at rt overnight, then concentrated and purified by HPLC (10–70% CH3CN/H2O with 0.1% TFA over 30 min). The combined fractions were lyophilized to give tert-butyl 7-methyl-8-(methylcarbamoyl)-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2(1H)-carboxylate (Boc-19l). This product was taken up in methanol (0.5 mL) and 4 M hydrogen chloride in dioxane (0.5 mL, 2.000 mmol) added. The reaction mixture was stirred at rt for 3 h, then concentrated, and the residue was lyophilized to give N,7-dimethyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide as its hydrochloride salt (14 mg, 73%). LC-MS m/z = 246.2 [M + H]+; 1H NMR (400 MHz, CD3OD) δ ppm 1.21 (d, J = 7 Hz, 3H), 3.15–3.22 (m, 1H), 3.26–3.42 (m, 2H), 3.45–3.85 (m, 6H), 4.23 (d, J = 14.9 Hz, 1H), 4.42 (d, J = 14.9 Hz, 1H), 6.99 (d, J = 7.8 Hz, 1H), 7.14 (d, J = 7.9 Hz, 1H).
Preparation of N-(2,2-Difluoroethyl)-7-methyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide (19m)
To the solution of 2-(tert-butoxycarbonyl)-7-methyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxylic acid (18d, R = H, 25 mg, 75.21 μmol), HATU (42.87 mg, 0.113 mmol), and triethylamine (20.97 μL, 0.150 mmol) in DMF (2 mL) was added 2,2-difluoroethanamine (9.146 mg, 0.113 mmol). The reaction was stirred at room temperature overnight. The mixture was purified by semipreparative HPLC (15–85% CH3CN/H2O with 0.1% TFA over 30 min). The combined fractions were lyophilized to give tert-butyl 8-((2,2-difluoroethyl)carbamoyl)-7-methyl-3,4,6,7-tetrahydro-[1,4]diazepino[6,7,1-hi]indole-2(1H)-carboxylate (Boc-19m), which was dissolved in dioxane (0.5 mL). A solution of 4 M HCl in dioxane (0.5 mL) was added. The reaction mixture was stirred at room temperature for 4 h and concentrated. The residue was purified by semipreparative HPLC (5–60% CH3CN/H2O with 0.1% TFA over 30 min). The combined fractions were lyophilized to give the title compound as its TFA salt (17 mg, 55.2%). LC-MS m/z = 296.2 [M + H]+; 1H NMR (400 MHz, CD3OD) δ ppm 1.18 (d, J = 6.9 Hz, 3H), 3.10–3.20 (m, 1H), 3.26–3.40 (m, 2H), 3.40–3.64 (m, 3H), 3.65–3.85 (m, 3H), 4.21 (d, J = 14.9 Hz, 1H), 4.40 (d, J = 14.9 Hz, 1H), 6.00 (tt, J = 56.0 and 3.9 Hz, 1H), 6.99 (d, J = 7.8 Hz, 1H), 7.12 (d, J = 7.9 Hz, 1H).
Preparation of (+)-N-(2,2-Difluoroethyl)-7-methyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide ((+)-19m) and (−)-N-(2,2-Difluoroethyl)-7-methyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide ((−)-19m)
The enantiomers of N-(2,2-difluoroethyl)-7-methyl-1,2,3,4,6,7-hexahydro-[1,4]diazepino[6,7,1-hi]indole-8-carboxamide were obtained by chiral HPLC separation using the following conditions. Column: Chiralpak IC column 250 × 20 mm (L x I.D.); flow: 12 mL/min; eluent: 12% EtOH/8% mTBE/80% hexanes with 0.1% Et3N; detector: UV 254 nm; retention time: first eluting enantiomer = 22.0 min, second eluting enantiomer = 23.5 min. After separation, both enantiomers were further purified by semi preparative HPLC (5–60% CH3CN/H2O with 0.1% TFA over 30 min). The combined fractions were lyophilized to give the title compounds as their TFA salts.
Radioligand Binding Assays
Binding assays were performed following standard procedures with [125I]-DOI as radioligand. Competition binding experiments used 5-HT2-receptor–expressing HEK293 cell membranes (5–25 mg/well membrane protein) and radioligand at final assay concentrations of 0.4–0.6 nM. Assays consisted of addition of 95 μL of assay buffer (20 mM HEPES, pH 7.4, 10 mM MgCl2), 50 μL of membranes, 50 μL of radioligand stock, and 5 μL of test compound diluted in assay buffer to 96-well microtiter plates, which were then incubated for 1 h at room temperature. Assay incubations were terminated by rapid filtration through PerkinElmer GF/C plates (PerkinElmer Life and Analytical Sciences, Boston, MA) followed by a 3x wash with ice-cold assay buffer. Plates were dried at 45 °C and BetaScint scintillation cocktail (25 mL/well) was added prior to counting on a Packard TopCount scintillation counter.
IP Accumulation Assays
5-HT2-receptor–expressing HEK293 cells were added to sterile poly-d-lysine-coated 96-well microtiter plates (20–35,000 cells/well) and labeled with 0.6 mCi/well [3H]inositol in myoinositol-free Dulbecco’s modified Eagle’s medium for 18 h. Unincorporated [3H]inositol was removed by aspiration and media replaced with fresh myoinositol-free Dulbecco’s modified Eagle’s medium containing pargyline (10 mM) and lithium chloride (10 mM). Test compound incubations were conducted at 37 °C and assays terminated by lysing cells with ice cold 0.1 M formic acid followed by freezing at – 80 °C. After thawing, total [3H]inositol phosphates were resolved from [3H]inositol using AG1-X8 ion-exchange resin (Biorad), and [3H]inositol phosphates were measured by scintillation counting using a PerkinElmer TopCount scintillation counter. All potency determinations were performed at a minimum total of 8 or 10 different concentrations and triplicate determinations were made at each test concentration.
Measurement of Food Intake in the Rat
Male Sprague–Dawley rats (Harlan, San Diego, CA) weighing 250–350g were triple-housed within a holding room controlled for humidity, temperature, and light (lights off 1800h–0600h) and received food and water ad libitum unless stated otherwise. Eighteen h prior to food intake testing (1600h), food was removed from home cages. On the next day, animals were weighed and placed into cages with grid floors at 1000h and allowed to acclimate to these cages for a 90 min period with free access to water and no food. At 1130h, rats (8 animals per group) were injected with the required dose of compound via oral gavage. Thirty min after injection (1200h), animals were allowed free access to food. Food was then weighed at 30 min after food exposure (1 h after drug administration). Upon test conclusion, rats were returned to home cages with ad-libitum access to food and water. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.
Molecular Modeling
Docking simulations were performed using Fred (v4.0; Openeye Scientific). Receptor preparation of the HTR2C structure 6BQG was performed using Receptor (4.0; Openeye Scientific). Three-dimensional conformation generation and searching was performed using Omega2 (v4.1; Openeye Scientific), and 200 unique conformations for each structure were generated. Docked structure comparisons and visualizations were performed in PyMol.
Supplementary Material
Acknowledgments
This study was sponsored by Arena Pharmaceuticals, Inc. (San Diego, CA, USA). Medical writing assistance was provided by ApotheCom (San Diego, CA, USA) and funded by Longboard Pharmaceuticals.
Glossary
Abbreviations
- %F
% oral bioavailability
- 125I-DOI
[125I]-2,5-dimethoxy-4-iodoamphetamine
- 1H NMR
proton nuclear magnetic resonance
- 5-HT
serotonin
- 5-HT2CR
5-HT2C receptor
- AUC
area under the curve
- Cl
clearance
- ClR
renal clearance
- C max
maximum concentration
- CNS
central nervous system
- CSF
cerebrospinal fluid
- DCM
dichloromethane
- DMF
dimethylformamide
- DMR
dynamic mass redistribution
- DSC
differential scanning calorimetry
- EC50
half maximal effective concentration
- ED50
effective dose for 50% of maximal in vivo response
- ee
enantiomeric excess
- E max
maximum effect
- ESI
electrospray ionization
- FDA
US Food and Drug Administration
- fu
fraction unbound
- hERG
human Ether-a-go-go-Related Gene
- HPLC
high-performance liquid chromatography
- IP3
inositol triphosphate
- IV
intravenous
- Ki
inhibition constant
- LC-MS
liquid chromatography–mass spectrometry
- LGS
Lennox-Gastaut syndrome
- n.d.
not determined
- PAH
pulmonary arterial hypertension
- PD
pharmacodynamics
- PK
pharmacokinetics
- PO
oral administration
- ppm
parts per million
- SC
subcutaneous
- SFC
supercritical fluid chromatography
- t 1/2
half-time
- TFA
trifluoroacetic acid
- THF
tetrahydrofuran
- TLC
thin-layer chromatography
- t max
time to maximum concentration
- TPP
target product profile
- Vss
volume of distribution.
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jmedchem.4c02923.
Blocking effect of the 5-HT2CR antagonist (SB242084) on acute (1h) food intake reduction with 7b and the structurally distinct 5-HT2CR agonist AR630; Spectroscopic and LC-MS data for key late stage compounds; Off-target (CEREP) panel data for AR503352 ((+)-19m, LP-352, bexicaserin); Crystal Structure and stereochemistry determination of (+)-19m; View of LP-352 HCl salt from the crystal structure showing the atom numbering scheme (PDF)
All authors contributed to the data curation, analysis, validation, and writing of the manuscript.
No external funding was received.
The authors declare the following competing financial interest(s): Albert Ren, Xiuwen Zhu, Juerg Lehmann, Michelle Kasem, Thomas O. Schrader, Kevin T. Whelan, and Michael E. Morgan are employees and/or hold stock options in Arena Pharmaceuticals. Huong Dang, John Frazer, David J. Unett, Andrew J. Grottick, Carleton R. Sage, and Graeme Semple are employees and/or hold stock options in Eurofins Beacon Discovery. Nuggehally R. Srinivas, Minh Le, and Dewey McLin are employees and/or hold stock options in Longboard Pharmaceuticals.
References
- Wold E. A., Wild C. T., Cunningham K. A., Zhou J.. Targeting the 5-HT2C receptor in biological context and the current state of 5-HT2C receptor ligand development. Curr. Top Med. Chem. 2019;19(16):1381–1398. doi: 10.2174/1568026619666190709101449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagraoui A., Thibaut F., Skiba M., Thuillez C., Bourin M.. 5-HT2C receptors in psychiatric disorders: A review. Prog. Neuropsychopharmacol Biol. Psychiatry. 2016;66:120–135. doi: 10.1016/j.pnpbp.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Cortes-Altamirano J. L., Olmos-Hernandez A., Jaime H. B., Carrillo-Mora P., Bandala C., Reyes-Long S., Alfaro-Rodríguez A.. Review: 5-HT1, 5-HT2, 5-HT3 and 5-HT7 receptors and their role in the modulation of pain response in the central nervous system. Curr. Neuropharmacol. 2018;16(2):210–221. doi: 10.2174/1570159X15666170911121027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao T., He J., Cui Z., Wang R., Bao K., Huang Y., Wang R., Liu T.. Central 5-HTR2C in the control of metabolic homeostasis. Front Endocrinol (Lausanne) 2021;12:694204. doi: 10.3389/fendo.2021.694204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins G. A., Fletcher P. J.. Therapeutic potential of 5-HT2C receptor agonists for addictive disorders. ACS Chem. Neurosci. 2015;6(7):1071–1088. doi: 10.1021/acschemneuro.5b00025. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D. O., Ambrogini P., Chruścicka B., Lindskog M., Crespo-Ramirez M., Hernández-Mondragón J. C., Perez de la Mora M., Schellekens H., Fuxe K.. The role of central serotonin neurons and 5-HT heteroreceptor complexes in the pathophysiology of depression: A historical perspective and future prospects. Int. J. Mol. Sci. 2021;22(4):1927. doi: 10.3390/ijms22041927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J. H., Zhao Y., Rosenzweig-Lipson S., Popp D., Williams J. B., Giller E., Detke M. J., Kane J. M.. A 6-week randomized, double-blind, placebo-controlled, comparator referenced trial of vabicaserin in acute schizophrenia. J. Psychiatr Res. 2014;53:14–22. doi: 10.1016/j.jpsychires.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Schoonjans A. S., Lagae L., Ceulemans B.. Low-dose fenfluramine in the treatment of neurologic disorders: experience in Dravet syndrome. Ther Adv. Neurol Disord. 2015;8(6):328–338. doi: 10.1177/1756285615607726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolete P., Knupp K., Karlovich M., DeCarlo E., Bluvstein J., Conway E., Friedman D., Dugan P., Devinsky O.. Lorcaserin therapy for severe epilepsy of childhood onset: A case series. Neurology. 2018;91(18):837–839. doi: 10.1212/WNL.0000000000006432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giovanni G., De Deurwaerdère P.. New therapeutic opportunities for 5-HT2C receptor ligands in neuropsychiatric disorders. Pharmacol Ther. 2016;157:125–162. doi: 10.1016/j.pharmthera.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Mbaki Y., Gardiner J., McMurray G., Ramage A. G.. 5-HT 2A receptor activation of the external urethral sphincter and 5-HT 2C receptor inhibition of micturition: a study based on pharmacokinetics in the anaesthetized female rat. Eur. J. Pharmacol. 2012;682(1–3):142–152. doi: 10.1016/j.ejphar.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Ouchi M., Kitta T., Kanno Y., Higuchi M., Togo M., Moriya K., Shinohara N.. Effect of a 5-HT2c receptor agonist on urethral closure mechanisms in female rats. Neurourol Urodyn. 2018;37(8):2382–2388. doi: 10.1002/nau.23586. [DOI] [PubMed] [Google Scholar]
- Ishigami T., Ueshima K., Ukai M., Asai N., Takamatsu H., Yokono M., Takeda M., Masuda N.. Effect of ASP2205 fumarate, a novel 5-HT(2C) receptor agonist, on urethral closure function in rats. J. Pharmacol Sci. 2019;139(4):333–339. doi: 10.1016/j.jphs.2019.02.004. [DOI] [PubMed] [Google Scholar]
- Tecott L. H., Sun L. M., Akana S. F., Strack A. M., Lowenstein D. H., Dallman M. F., Julius D.. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374(6522):542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Applegate C. D., Tecott L. H.. Global increases in seizure susceptibility in mice lacking 5-HT2C receptors: a behavioral analysis. Exp. Neurol. 1998;154(2):522–530. doi: 10.1006/exnr.1998.6901. [DOI] [PubMed] [Google Scholar]
- Ceulemans B., Boel M., Leyssens K., Van Rossem C., Neels P., Jorens P. G., Lagae L.. Successful use of fenfluramine as an add-on treatment for Dravet syndrome. Epilepsia. 2012;53(7):1131–1139. doi: 10.1111/j.1528-1167.2012.03495.x. [DOI] [PubMed] [Google Scholar]
- Knupp K. G., Scheffer I. E., Ceulemans B., Sullivan J. E., Nickels K. C., Lagae L., Guerrini R., Zuberi S. M., Nabbout R., Riney K.. et al. Efficacy and safety of fenfluramine for the treatment of seizures associated with lennox-gastaut syndrome: A randomized clinical trial. JAMA Neurol. 2022;79(6):554–564. doi: 10.1001/jamaneurol.2022.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon K., Sheckley H., Anderson C. L., Liu Z., Carney P. R.. A review of fenfluramine for the treatment of Dravet syndrome patients. Curr. Res. Pharmacol Drug Discov. 2022;3:100078. doi: 10.1016/j.crphar.2021.100078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabbout R., Mistry A., Zuberi S., Villeneuve N., Gil-Nagel A., Sanchez-Carpintero R., Stephani U., Laux L., Wirrell E., Knupp K.. et al. Fenfluramine for treatment-resistant seizures in patients with dravet syndrome receiving stiripentol-inclusive regimens: A randomized clinical trial. JAMA Neurology. 2020;77(3):300–308. doi: 10.1001/jamaneurol.2019.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagae L., Sullivan J., Knupp K., Laux L., Polster T., Nikanorova M., Devinsky O., Cross J. H., Guerrini R., Talwar D.. et al. Fenfluramine hydrochloride for the treatment of seizures in Dravet syndrome: a randomised, double-blind, placebo-controlled trial. Lancet. 2019;394(10216):2243–2254. doi: 10.1016/S0140-6736(19)32500-0. [DOI] [PubMed] [Google Scholar]
- UCB . FINTEPLA® (fenfluramine) oral solution; 40, Smyrna, GA, 2023. https://www.ucb-usa.com/fintepla-prescribing-information.pdf.
- Bialer M., Perucca E.. Lorcaserin for dravet syndrome: A potential advance over fenfluramine? CNS Drugs. 2022;36(2):113–122. doi: 10.1007/s40263-022-00896-3. [DOI] [PubMed] [Google Scholar]
- Silenieks L. B., Carroll N. K., Van Niekerk A., Van Niekerk E., Taylor C., Upton N., Higgins G. A.. Evaluation of selective 5-HT(2C) agonists in acute seizure models. ACS Chem. Neurosci. 2019;10(7):3284–3295. doi: 10.1021/acschemneuro.8b00739. [DOI] [PubMed] [Google Scholar]
- Connolly H. M., Crary J. L., McGoon M. D., Hensrud D. D., Edwards B. S., Edwards W. D., Schaff H. V.. Valvular heart disease associated with fenfluramine-phentermine. N Engl J. Med. 1997;337(9):581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- Rothman R. B., Baumann M. H., Savage J. E., Rauser L., McBride A., Hufeisen S. J., Roth B. L.. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102(23):2836–2841. doi: 10.1161/01.CIR.102.23.2836. [DOI] [PubMed] [Google Scholar]
- Huang X. P., Setola V., Yadav P. N., Allen J. A., Rogan S. C., Hanson B. J., Revankar C., Robers M., Doucette C., Roth B. L.. Parallel functional activity profiling reveals valvulopathogens are potent 5-hydroxytryptamine(2B) receptor agonists: implications for drug safety assessment. Mol. Pharmacol. 2009;76(4):710–722. doi: 10.1124/mol.109.058057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt A. L.. Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav Brain Res. 2015;277:99–120. doi: 10.1016/j.bbr.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Giménez J. F., González-Maeso J.. Hallucinogens and serotonin 5-HT(2A) receptor-mediated signaling pathways. Curr. Top Behav Neurosci. 2017;36:45–73. doi: 10.1007/7854_2017_478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. M., Smith J. M., Tsai J. H., Schultz J. A., Gilson C. A., Estrada S. A., Chen R. R., Park D. M., Prieto E. B., Gallardo C. S.. et al. Discovery and structure-activity relationship of (1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-benzazepine (Lorcaserin), a selective serotonin 5-HT2C receptor agonist for the treatment of obesity. J. Med. Chem. 2008;51(2):305–313. doi: 10.1021/jm0709034. [DOI] [PubMed] [Google Scholar]
- Thomsen W. J., Grottick A. J., Menzaghi F., Reyes-Saldana H., Espitia S., Yuskin D., Whelan K., Martin M., Morgan M., Chen W.. et al. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J. Pharmacol Exp Ther. 2008;325(2):577–587. doi: 10.1124/jpet.107.133348. [DOI] [PubMed] [Google Scholar]
- Smith S. R., Weissman N. J., Anderson C. M., Sanchez M., Chuang E., Stubbe S., Bays H., Shanahan W. R.. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Eng. J. Med. 2010;363(3):245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- Ren A., Zhu X., Feichtinger K., Lehman J., Kasem M., Schrader T. O., Wong A., Dang H., Le M., Frazer J.. et al. Discovery of a lead series of potent benzodiazepine 5-HT(2C) receptor agonists with high selectivity in functional and binding assays. Bioorg. Med. Chem. Lett. 2020;30(5):126929. doi: 10.1016/j.bmcl.2019.126929. [DOI] [PubMed] [Google Scholar]
- Storer R. I., Brennan P. E., Brown A. D., Bungay P. J., Conlon K. M., Corbett M. S., DePianta R. P., Fish P. V., Heifetz A., Ho D. K.. et al. Multiparameter optimization in CNS drug discovery: design of pyrimido[4,5-d]azepines as potent 5-hydroxytryptamine 2C (5-HT2C) receptor agonists with exquisite functional selectivity over 5-HT2A and 5-HT2B receptors. J. Med. Chem. 2014;57(12):5258–5269. doi: 10.1021/jm5003292. [DOI] [PubMed] [Google Scholar]
- Dunlop J., Watts S. W., Barrett J. E., Coupet J., Harrison B., Mazandarani H., Nawoschik S., Pangalos M. N., Ramamoorthy S., Schechter L.. et al. Characterization of vabicaserin (SCA-136), a selective 5-hydroxytryptamine 2C receptor agonist. J. Pharmacol Exp Ther. 2011;337(3):673–680. doi: 10.1124/jpet.111.179572. [DOI] [PubMed] [Google Scholar]
- Rouquet G., Moore D. E., Spain M., Allwood D. M., Battilocchio C., Blakemore D. C., Fish P. V., Jenkinson S., Jessiman A. S., Ley S. V.. et al. Design, synthesis, and evaluation of tetrasubstituted pyridines as potent 5-HT2C receptor agonists. ACS Med. Chem. Let. 2015;6(3):329–333. doi: 10.1021/ml500507v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kim Y. J., Londhe A. M., Pae A. N., Choo H., Kim H. J., Min S. J.. Synthesis and biological evaluation of disubstituted pyrimidines as selective 5-HT(2C) agonists. Molecules. 2019;24(18):3234. doi: 10.3390/molecules24183234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., McCorvy J. D., Giguere P. M., Zhu H., Kenakin T., Roth B. L., Kozikowski A. P.. Design and discovery of functionally selective serotonin 2C (5-HT2C) receptor agonists. J. Med. Chem. 2016;59(21):9866–9880. doi: 10.1021/acs.jmedchem.6b01194. [DOI] [PubMed] [Google Scholar]
- Zhang G., McCorvy J. D., Shen S., Cheng J., Roth B. L., Kozikowski A. P.. Design of fluorinated cyclopropane derivatives of 2-phenylcyclopropylmethylamine leading to identification of a selective serotonin 2C (5-HT(2C)) receptor agonist without 5-HT(2B) agonism. Eur. J. Med. Chem. 2019;182:111626. doi: 10.1016/j.ejmech.2019.111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., McCorvy J. D., Harpsøe K., Lansu K., Yuan S., Popov P., Qu L., Pu M., Che T., Nikolajsen L. F.. et al. 5-HT(2C) receptor structures reveal the structural basis of GPCR polypharmacology. Cell. 2018;172(4):719–730. doi: 10.1016/j.cell.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. R., Bös M., Jenck F., Moreau J., Mutel V., Sleight A. J., Wichmann J., Andrews J. S., Berendsen H. H., Broekkamp C. L.. et al. 5-HT2C receptor agonists: pharmacological characteristics and therapeutic potential. J. Pharmacol Exp Ther. 1998;286(2):913–924. doi: 10.1016/S0022-3565(24)37669-4. [DOI] [PubMed] [Google Scholar]
- Millan M. J., Peglion J. L., Lavielle G., Perrin-Monneyron S.. 5-HT2C receptors mediate penile erections in rats: actions of novel and selective agonists and antagonists. Eur. J. Pharmacol. 1997;325(1):9–12. doi: 10.1016/S0014-2999(97)89962-1. [DOI] [PubMed] [Google Scholar]
- Kennett G. A., Wood M. D., Bright F., Trail B., Riley G., Holland V., Avenell K. Y., Stean T., Upton N., Bromidge S.. et al. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology. 1997;36(4–5):609–620. doi: 10.1016/S0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- Ballard, P. ; Brassil, P. ; Bui, K. H. ; Dolgos, H. ; Petersson, C. ; Tunek, A. ; Webborn, P. J. H. . Metabolism and pharmacokinetic optimization strategies in drug discovery. In Drug Discovery and Development, 2013; p 135 10.1016/B978-0-7020-4299-7.00010-X. [DOI] [Google Scholar]
- Schrage R., De Min A., Hochheiser K., Kostenis E., Mohr K.. Superagonism at G protein-coupled receptors and beyond. Br. J. Pharmacol. 2016;173(20):3018–3027. doi: 10.1111/bph.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrage R., Seemann W. K., Klöckner J., Dallanoce C., Racké K., Kostenis E., De Amici M., Holzgrabe U., Mohr K.. Agonists with supraphysiological efficacy at the muscarinic M2 ACh receptor. Br. J. Pharmacol. 2013;169(2):357–370. doi: 10.1111/bph.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead C. J., Christopoulos A.. Supra-physiological efficacy at GPCRs: superstition or super agonists? Br. J. Pharmacol. 2013;169(2):353–356. doi: 10.1111/bph.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alix K., Khatri S., Mosier P. D., Casterlow S., Yan D., Nyce H. L., White M. M., Schulte M. K., Dukat M.. Superagonist, full agonist, partial agonist, and antagonist actions of arylguanidines at 5-hydroxytryptamine-3 (5-HT3) subunit A receptors. ACS Chem. Neurosci. 2016;7(11):1565–1574. doi: 10.1021/acschemneuro.6b00196. [DOI] [PubMed] [Google Scholar]
- Danks, A. M. ; Peters, M. ; Kaye, R. . LP352, a 5-HT2C Superagonist, Has Broad Antileptic Activity in Preclinical Seizure Models. In American Epilepsy Society (AES) Annual Meeting December 1–5, 2023; Orlando, FL, 2023. [Google Scholar]
- Kaye, R. ; Orevillo, C. ; Dlugos, D. J. ; Scheffer, I. E. . Efficacy and safety of bexicaserin (LP352) in adolescents and adult participants with developmental and epileptic encephalopathies: Results of the phase 1B/2A pacific study. In 76th Annual Meeting of the American Academy of Neurology, April 13–18, 2024, Denver, CO, 2024. [Google Scholar]
- Longboard Pharmaceuticals . Longboard Pharmaceuticals initiates Phase 3 DEEp SEA study evaluating bexicaserin in Dravet Syndrome. Receives breakthrough therapy designation for bexicaserin (LP352). Longboard Pharmaceuticals, Inc.: La Jolla, CA, 2024. [Google Scholar]
- Longboard Pharmaceuticals . Longboard Pharmaceuticals receives breakthrough therapy designation for bexicaserin (LP352). Longboard Pharmaceuticals, Inc.; La Jolla, CA, 2024. [Google Scholar]
- Schrodinger. PyMOL by Schrödinger. Schrodinger, Inc., 2023. https://pymol.org/2/ (accessed 2023). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.