Abstract
Background
High-throughput sequencing technologies play an increasingly active role in the surveillance of major global health challenges, such as the emergence of antimicrobial resistance. The post-weaning period is of critical importance for the swine industry and antimicrobials are still required when infection occurs during this period. Here, two sequencing approaches, shotgun metagenomics and metatranscriptomics, have been applied to decipher the effect of different treatments used in post-weaning diarrhea on the transcriptome and resistome of pig gut microbiome. With this objective, a metagenome-assembled genome (MAG) catalogue was generated to use as a reference database for transcript mapping obtained from a total of 140 pig fecal samples in a cross-sectional and longitudinal design to study differential gene expression. The different treatments included antimicrobials trimethoprim/sulfamethoxazole, colistin, gentamicin, and amoxicillin, and an oral commercial vaccine, a control with water acidification, and an untreated control. For metatranscriptomics, fecal samples from pigs were selected before weaning, three days and four weeks post-treatment.
Results
The final non-redundant MAGs collection comprised a total of 1396 genomes obtained from single assemblies and co-assemblies per treatment group and sampling time from the metagenomics data. Analysis of antimicrobial resistance genes (ARGs) at this assembly level considerably reduced the total number of ARGs identified in comparison to those found at the reads level. Besides, from the metatranscriptomics data, half of those ARGs were detected transcriptionally active in all treatment groups. Differential gene expression between sampling times after treatment found major number of differential expressed genes (DEGs) against the group treated continuously with amoxicillin, with DEGs being correlated with antimicrobial resistance. Moreover, at three days post-treatment, a high number of significantly downregulated genes was detected in the group treated with gentamicin. At this sampling time, this group showed an altered expression of ribosomal-related genes, demonstrating the rapid effect of gentamicin to inhibit bacterial protein synthesis.
Conclusions
Different antimicrobial treatments can impact differently the transcriptome and resistome of microbial communities, highlighting the relevance of novel sequencing approaches to monitor the resistome and contribute to a more efficient antimicrobial stewardship.
Supplementary Information
The online version contains supplementary material available at 10.1186/s42523-025-00418-8.
Keywords: Metatranscriptomics, Metagenome-assembled genomes, Post-weaning, Swine, Antimicrobial resistance
Background
For decades, antimicrobials have been widely implemented in veterinary medicine as therapeutics, but also at low dosages to prevent bacterial infections, taking advantage of their growth promoting effect [1, 2]. However, the emergence of antimicrobial resistance (AMR) worldwide poses a great concern that must be urgently addressed [3–5]. In the European Union, the use of antibiotics as growth promoters in animal feed was banned in 2006. More recently, EU Regulation 2019/6 prohibited the routine or preventive use of antimicrobials in animal husbandry, as well as the supplementation of feed with high doses of zinc oxide, which has also been demonstrated to enhance the emergence of resistant bacteria [6–8]. Notwithstanding, antimicrobials are still essential when a bacterial infection occurs, and their effectiveness should be preserved from a One Health perspective.
The role of the gut microbiome in overall health and growth of piglets has been widely described in recent years, especially at early life [9–11]. Shifts in microbial diversity and composition, influenced by factors like weaning, are known to be correlated with diarrhoea susceptibility, lower feed intake, and colonization of pathogens, leading to disease [12–15]. At this phase, post-weaning diarrhoea (PWD) is a frequent and multifactorial condition associated with a loss of productivity and increased mortality, leading to major financial losses and increased use of antimicrobials [16, 17]. In the current context of antimicrobial reduction, many alternative strategies have been implemented to improve piglets gut health during post-weaning. These strategies include preventive measures such as controlling the housing environment, revising vaccine programmes, formulation of new diets, and even the inclusion of essential oils, prebiotics, probiotics, or antimicrobial peptides [16, 18–20]. Besides, other measures focusing on the rapid monitoring of the resistome within the pig gut microbiome could also have an impact reducing the use of non-effective antimicrobials and protecting health systems.
Nowadays, the advances in high-throughput sequencing technologies have revolutionized the way to analyse microbial communities in a specific environment. Shotgun metagenomics allows to recover microbial genomes, determine their taxonomic composition, and predict their potential functionality, while metatranscriptomics enables the identification and quantification of genes that are being expressed within the community at a particular moment [21, 22]. In the past years, many studies have focused on understanding the pig gut microbiome and resistome using shotgun metagenomics, but not that many have implemented metatranscriptomics to gain insight into the microbiome functionalities [23–26]. Notwithstanding, both shotgun sequencing techniques have their own intrinsic limitations, such as the presence of eukaryotic DNA or ribosomal RNA in the samples adding complexity to the taxonomic classification, or the challenge to link the identified resistance genes to the host bacterial species and/or their genetic context to predict their mobilizable potential.
In our previous study [23], we determined the longitudinal and cross-sectional effect of different PWD treatments in the bacterial communities and the resistome from pig faecal samples using shotgun metagenomics alone. The study included seven groups of piglets, four treated with antimicrobials (gentamicin, colistin, amoxicillin, and trimethoprim/sulfamethoxazole), one treated with an oral attenuated vaccine, and two control groups, one with the addition of acidifiers in the drinking water, and the last one untreated. The analysis of microbial diversity indexes showed that a continuous and prolonged amoxicillin treatment significantly decreased diversity four weeks after treatment. On the other hand, the different treatments contributed differently to the emergence of resistance, observing that gentamicin and amoxicillin treatments significantly contributed to the increase in abundance of antimicrobial resistance genes (ARGs) [23]. In the present study, shotgun metagenomics data from the previous study have been implemented to create a collection of metagenome-assembled genomes (MAGs) representing the microbial communities existing in the pigs’ gut microbiome. These MAGs have been further applied as a gene reference catalogue for the mapping of the metatranscriptomics data from the same experimental design with the objective to determine the effect of the PWD treatments on the transcriptome of pig gut microbiomes. Furthermore, this multi-omics approach allowed for the identification and quantification of the expressed ARGs in this cross-sectional and longitudinal study.
Methods
Experimental design
The experimental design was performed as previously described in Guitart-Matas et al., 2024 [23], including a total of 210 piglets divided into seven different treatment groups. The farm of origin, located in Catalonia (Spain), was selected for previous records of PWD outbreaks. After weaning, one of the groups remained at the farm of origin (GG), following the routine amoxicillin treatment performed at the farm at the time of study, whereas the other groups were transferred to an experimental farm from the Institut de Recerca i Tecnologia Agroalimentàries (IRTA), and subjected to six different treatments: trimethoprim/sulfamethoxazole (G1), colistin (G2), commercial oral E. coli vaccine (G3), gentamicin (G4), untreated control with water acidification (G5), and untreated control (G6). The selected antibiotic treatments were known to be commonly used in the treatment of PWD in pigs and were selected based on previous epidemiological data from the farm [23, 27]. All antibiotics were applied orally in water for five days when individual animals showed signs of PWD, eleven days after arrival at the experimental farm. Dosages and concentrations were determined by the summary of product characteristics (SmPC) of Methoxasol for the trimethoprim/sulfamethoxazole treatment (25 mg/kg/day, Genera Inc.), Apsasol for the colistin treatment (1.5 × 105 international units (IU)/kg/day, Andrés Pintaluba, S.A.), and Gentavet for the gentamicin treatment (2 × 103 IU/kg/day, Fatro S.p.A.). The commercial vaccine (Coliprotec F4/F18, Elanco GmbH) was applied orally in a single dose the day of arrival, and for the untreated control with water acidification, drinking water was acidified with phosphoric acid 75% from the arrival until the end of the study.
The experimental farm was previously decontaminated, cleaned, and disinfected, and biosecurity measures were applied to prevent cross-contamination between the different treatment groups. Clinical signs were monitored daily, piglets’ weight was measured at the beginning and at the end of the experiment, and faecal samples were collected on four different sampling times and frozen instantly in dry ice: at the farm of origin the day before departure to the experimental farm and before treatment (ST1), three days post-treatment (ST2), two weeks (ST3), and four weeks post-treatment (ST4). Faecal samples were also collected from animals that remained at the farm of origin treated with amoxicillin at the same sampling times (Fig. 1).
Fig. 1.
Experimental design describing the seven different treatment groups (G1–G6, GG) sampled at four sampling times (ST1–ST4). Metagenomics sequencing was performed from DNA extracted from the faeces of ten animals per group at all sampling times, while metatranscriptomics sequencing was performed from RNA extracted from the faeces of seven animals per group at three sampling times
Ethical statement
Animals in the experimental farm were exposed to the same conditions as in the conventional farm and were allocated following legislation in animal welfare. Antimicrobial treatments followed the Summary of Product Characteristics (SmPC), and no disease was induced. The Ethics Committee for Animal Experimentation (CEEA) guidelines reviewed and authorized the procedures of this study with the ID number CEEA103/2018.
Shotgun metagenomics and metatranscriptomics sequencing
For shotgun metagenomics sequencing, total DNA from faecal samples of ten animals per group at all sampling times (n = 280) was extracted using the QIAamp® PowerFecal® Pro DNA Kit (Qiagen) according to the manufacturer’s instructions. For metatranscriptomics, total RNA was extracted using the NucleoSpin™ RNA Stool (Macherey–Nagel™) from seven animals per treatment group at ST1, ST2, and ST4 (n = 147). Sample selection for metatranscriptomics corresponded to a pre-weaning pre-treatment sampling (ST1), and a short-term (ST2) and long-term (ST4) effect of the treatments. DNA and RNA were quantified using the Qubit dsDNA Broad Range and RNA High-Sensitivity assay kits (Thermo Fisher Scientific), respectively. All extracted nucleic acids were paired-end sequenced (2 × 150 bp) for shotgun metagenomics and shotgun metatranscriptomics on an Illumina NovaSeq 6000 platform at 10 and 12 Gb sequencing depth, respectively (Novogene Bioinformatics Technology and Centro Nacional de Análisis Genómico (CNAG-CRG), respectively). For detailed information about animals selected for metagenomics see BioProject PRJNA1010706 and for metatranscriptomics metadata see Additional File: Table S1.
Metagenome-assembled genomes as a reference database
Metagenomic trimmed and host decontaminated reads were obtained as described in Guitart-Matas et al., 2024 [23]. From paired reads, single sample assemblies (SA) were performed with MetaSPAdes assembler v3.15.5 [28] and co-assemblies per treatment group and sampling time were generated with Megahit v1.0.2 [29] with a minimum contig length of 1000 nt and the “–presets meta-large” option for large and complex metagenomes. From both SA and co-assemblies, bins were generated with the binning module of the MetaWRAP software v1.3 [30]. For SA, the binning tools implemented were CONCOCT, MaxBin2, and MetaBAT2 [31–33]. However, for co-assemblies, only MaxBin2 and MetaBAT2 were used due to the vast computational requirements of CONCOCT with large files. Then, all bins obtained from SA per treatment group and sampling time were refined together with the two sets of bins obtained from co-assemblies (MaxBin2 and MetaBAT2) using the refinement module of MetaWRAP to obtain a final set of refined bins, referred as single- and co-assemblies (SCOA) bins set of MAGs per treatment group and sampling time. Parameters for the refinement module were set at a minimum completion of 70% (-c 70) and a maximum contamination of 10% (- × 10). Pairwise comparison analyses between treatment groups and sampling times of SA and SCOA were performed in R version 4.3.2 and corrected for multiple comparisons using the Benjamini–Hochberg method [34].
Subsequently, all MAGs generated per treatment group and sampling time were integrated to create a reference database. The de-replication tool dRep v3.4.2 was implemented to remove repeated genomes and select the most representative ones among similar MAGs of the integrated set [35]. Completeness and contamination of each individual MAG was assessed with the “lineage_wf” workflow of CheckM v1.2.0 [36], and coverage to calculate the mapping rate of metagenomic reads against the MAGs was measured with CoverM v0.7.0 with default relative abundance method [37]. Phylogenetic tree was generated with PhyloPhlAn v3.1.68 with the phylophlan database and the diversity parameter set at “high” [38]. Final maximum likelihood tree was constructed using RAxML v8.2.12 with the PROTCATLG model for amino acid substitution, and edited with iTOL v6.9 online tool [39, 40]. Taxonomy annotation of final MAGs was improved with GTDB-Tk v2.0.1 (Genome Taxonomy DataBase Toolkit) using the database release 207, and functional annotation was performed with DRAM v1.5.0 with the following databases: Uniref90, PFAM-A, KOfam, and dbCAN-V10 (all downloaded in June 2023) [41, 42]. The R package ‘distillR’ was used to transform raw DRAM annotations of each MAG into multiple Genome-Inferred Functional Traits (GIFTs) [43, 44]. Finally, ARGs from final MAGs were also estimated with Resfinder v4.3.0 [45].
Metatranscriptomics analysis against the reference database
Metatranscriptomic raw reads were filtered with the KneadData software, that included a trimming step with Trimmomatic v0.39.2 and a host decontamination step with Bowtie2 v2.4.4. The same features specified for the metagenomics data were also used for the metatranscriptomic reads [46, 47]. Additionally, ribosomal RNA (rRNA) was removed from metatranscriptomic reads with SortMeRNA v4.3.6 with the Rfam and Silva databases (silva-bac-16 s-id90, silva-arc-16 s-id95, silva-bac-23 s-id98, silva-arc-23 s-id95, silva-euk-18 s-id95, silva-euk-28 s-id98), and the parameters: “–out2 –paired_out –fastx” [48–50].
For the quantification of transcript abundances, the cleansed metatranscriptomic reads were pseudoaligned with the open reading frames (ORFs) encoded by the MAGs using the Kallisto software v0.50.1 [51]. The resultant count abundance table including all samples was analysed for differential expression with DESeq2 Bioconductor package in R 4.3.2 using the median of ratios as normalization method [52]. The final model included a variable for the treatment group and sampling time, and the sow ID variable to control for batch differences. In a first step, principal component analysis (PCA) was performed to study clustering among samples and remove outliers. Three outliers were removed at this step from the groups treated with colistin (G2, n = 1) and gentamicin (G4, n = 1) before weaning (ST1) and the group treated with amoxicillin (GG, n = 1) three days post-treatment (ST2) for being positioned between clusters, and another outlier was excluded from the gentamicin-treated group (G4, n = 1) before weaning (ST1) for falling outside the corresponding sampling time cluster (ST4). Secondly, a differential gene expression analysis between treatment groups at the different sampling times, after a pre-filtering step to remove genes with less than 10 counts, was performed. Comparison results were filtered at a 1% significance level after correction for multiple testing (P-adj < 0.01) and were represented as heatmaps with the ‘ggplot2’ R package [53].
Expressed resistome
ARGs were determined from two different analyses. On the one hand, differential expressed genes (DEG) associated with resistance were filtered from the comparison result tables from DESeq2. The terms used for filtering were the following: “resistan”, “antibiotic”, “antimicrobial”, “colistin”, “cillin”, “efflux”, “pump”, “drug”, “multi”, “lactam”, “cyclin”, “macrolid”, “midazol”, “penem”, “cepha”, “quinolon”, “mycin”, “polypeptide”, “sulfonamid”, and “phenicol”. On the other hand, resistance profiles were independently determined with Resfinder v4.2.5 [45] from quality trimmed transcriptomic reads, performing the steps for normalization and quantification calculated in transcripts per million (TPM) as described in Guitart-Matas et al., 2024 [23]. Resistance gene normalized expression counts per antibiotic class were compared between treatment groups and sampling times also using the nonparametric Wilcoxon pairwise test with the Benjamini–Hochberg method for P-value correction. A multivariate comparison between ARGs abundance estimated by metagenomics and metatranscriptomics data of common samples from both datasets was assessed by performing a Procrustes analysis using the ‘vegan’ R package [54].
Results
Metagenome-assembled genome (MAG) collection from pig fecal samples
The collection of MAGs from pig faecal samples generated in this study was constructed from a total of 280 single assemblies and 28 co-assemblies, one per treatment group (n = 7) and sampling time (n = 4). From the single assemblies (SA), a total of 10,531 high-quality bins were obtained after the refinement module, with a completeness mean of 87.75% (SD = 8.48%) and a contamination mean of 1.66% (SD = 1.75%). The mean size of the bins was 2.10 Mbp, with a maximum bin size of 6.27 Mbp and a minimum of 594,565 bp. Pairwise comparisons of mean completeness and mean contamination did not show significant differences between treatment groups (Fig. 2A).
Fig. 2.
A. Percentage of completeness and contamination means per treatment group and sampling time for both single assembly (SA) bins and final refined bins from single assemblies and co-assemblies (SCOA). B. Taxonomic classification at the phylum level of SCOA per treatment group and sampling times. C. Percentage of completeness and contamination means of the final bins included in the metagenome-assembled genome (MAG) collection refined from 280 pig faecal samples. D. Taxonomic classification at the phylum level of the final bins included in the MAG collection. E. Phylogenetic reconstruction of the collection of MAGs. Different colours (same as panel D) indicate different phyla and layers indicate phyla, presence (purple) or absence (white) of resistance genes identified in each MAG, completeness (grey gradient scale), contamination (red gradient scale), and genome size (grey bars). For Bacillota, s.s. are sensu stricto. G1: trimethoprim/sulfamethoxazole, G2: colistin, G3: oral vaccination, G4: gentamicin, G5: untreated control with water acidification, G6: untreated control, GG: amoxicillin (farm of origin). ST1: one day before weaning, ST2: three days post-treatment, ST3: two weeks post-treatment, ST4: four weeks post-treatment
SA bins per treatment group and sampling times were then refined together with bins generated using MaxBin2 and MetaBAT2 from co-assemblies (SCOA). After this step, the number of SCOA bins increased up to a total of 17,698 high-quality bins, with a mean number of bins per condition of 632.07 bins (SD = 138.08 bins). The highest number of bins was found in the group treated with trimethoprim/sulfamethoxazole two weeks post-treatment (G1_ST3, n = 837) and the minimum in the colistin-treated group at four weeks post-treatment (G2_ST4, n = 365). The completeness mean and the contamination mean of the SCOA bins increased slightly compared with the SA bins to 90.07% (SD = 1.81%) and 2.04% (SD = 0.31), respectively (Fig. 2A). The mean size of the bins was similar to the SA bins with 2.15 Mbp, but the range was less extensive with a maximum bin size of 2.48 Mbp and a minimum of 1.93 Mbp. Pairwise comparisons showed similar results as for SA bins.
The total number of bins obtained per treatment group and sampling time showed common longitudinal variations in all groups, except for the control group with water acidification (G5). As depicted in Fig. 2B, a significantly lower number of SCOA bins was observed before weaning (ST1) and four weeks post-treatment (ST4) compared with three days (ST2, P = 0.001 and 0.003, respectively) and two weeks post-treatment (ST3, P = 0.001 and 0.001, respectively) in all groups except G5. The taxonomic classification of SCOA bins represented in Fig. 2B showed that Bacillota and Bacteroidota were the most abundant phyla in all groups at all sampling times. Moreover, a large proportion of non-identified bacteria was found in all conditions, with a mean percentage of non-classified bacteria of 15.84% (SD = 2.60%) of the SCOA bins per treatment group and sampling time. No major changes were observed for SA bins (Additional File: Figure S1 A).
After de-replication of the 17,698 bins, the MAGs collection contained a total of 1,396 bins with a completeness mean of 89.63% (SD = 8.23%) and a contamination mean of 1.50% (SD = 1.77%) (Fig. 2C). Of these, a total of 806 (57.74%) were defined as high-quality bins according to the Minimum Information about a Metagenome-Assembled Genome (MIMAG) standards (> 90% completeness, < 5% contamination) [55]. The mean size of the collection bins was 2.16 Mbp with a mean number of scaffolds of 254.34. The mean percentage of mapped metagenomic reads against the complete MAG collection was 70.29% (SD = 6.17%) of the total reads (Additional File: Figure S1B). Taxonomically, the collection of MAGs had a higher representation of non-classified bacteria after de-replication compared with the SA and SCOA bins. Bacillota was still the most representative phylum, followed by Bacteroidota, Actinomycetota, and Spirochaetota (Fig. 2D).
The final constructed phylogeny of the complete collection of MAGs from pig fecal samples is represented in Fig. 2E. The resultant phylogenetic tree clustered the collection of MAGs by phylum, except for the Bacillota sublineages A and sensu stricto (s.s.) that separated from Bacillota sublineages B (“Desulfotomaculota”) and C (“Selenobacteria”) among other phyla. Functional annotation enabled studying the metabolic potential of the gut microbiome included in the collection of MAGs. Most represented functions involved biosynthesis of different compounds, such as nucleic acids, amino acids, short-chain fatty acids, and vitamins, although metabolic pathways related with sugar, amino acid, and antibiotic degradation were also identified. A total of 1,024 MAGs (73.35%) were identified with at least one functional trait related with antibiotic degradation. Functions related with cellular structure were mainly identified among Bacteroidota, while spore formation was more predominant among Bacillota (Additional File: Figure S2).
The analysis of ARGs at the bin level identified 72 MAGs (5.16%) with resistance genes. A total of 84 different ARGs were detected belonging to the aminoglycoside, beta-lactam, phenicol, trimethoprim, macrolide, quinolone, sulphonamide, tetracycline, and glycopeptide antibiotic classes. The MAGs with the highest number of encoding ARGs belonged to the species Campylobacter coli and Prevotella sp900771975 (n = 8), followed by Collinsella sp002391315 (n = 6), and Clostridioides mangenotii, JG1575 sp 012511315, Parasutterella excrementihominis, and Ruminococcus gnavus (n = 5). Moreover, seven MAGs were identified with ARGs conferring resistance to three or more antibiotic classes, showing multi-drug resistance at the genomic level. Specific genes per antibiotic class identified in these MAGs are summarised in Table 1. Besides, most of the genes identified among the MAGs conferred resistance to tetracyclines (n = 31), macrolides (n = 29), aminoglycosides (n = 29), and beta-lactams (n = 27). For detailed information about all ARGs detected with Resfinder, including their phenotype, mechanism of resistance, predicted genetic context, and taxonomic rank of the MAGs where they were identified see Additional File: Table S2.
Table 1.
ARGs identified in the seven MAGs showing multi-drug resistance at the genomic level
| Phylum | Species | Total Classes | Aminoglycoside | Beta-lactam | Phenicol | Trimethoprim | Macrolide | Sulphonamide | Tetracycline |
|---|---|---|---|---|---|---|---|---|---|
| Actinomycetota | Collinsella sp | 5 |
aadA2 aph(6)-Id |
floR | lnu(F) | sul1 | tet(L) | ||
| Bacteroidota | Bacteroides fragilis_A | 4 | ant(6)-Ia | cfiA4 | erm(G) | tet(X) | |||
| Bacteroidota | Prevotella sp900771975 | 4 | ant(6)-Ia | cfr(C) |
erm(F) lnu(C) mef(A) |
tet(40) tet(Q) tet(W) |
|||
| Pseudomonadota | Basfia_A porcinus | 3 | aadA14 | blaROB-1 | tet(B) | ||||
| Bacillota_A | Clostridioides_A mangenotii_A | 3 | ant(9)-Ia |
lnu(B) lsa(E) |
tet(M) tetB(P) |
||||
| Bacillota_A | JG1575 sp012511315 | 3 |
aadA14 aph(2'')-Ic |
dfrA20 |
mph(E) msr(E) |
||||
| Bacillota | Enterococcus_B faecium | 3 | aac(6')-Ii | msr(C) | tet(L) |
The taxonomic classification of each metagenome-assembled genome (MAG) is shown at the phylum and species level and antimicrobial resistance genes (ARGs) identified with Resfinder are classified and counted per antibiotic class
Metatranscriptomics sequencing and differential gene expression analysis
After excluding low-quality sequences, trimming off adapters, host decontamination, and removal of rRNA from shotgun metatranscriptomic reads of the 140 faecal samples, a median per sample of 1.04 million paired reads (Q1 = 0.70M, Q3 = 1.34M) was obtained. After trimming, host contamination accounted for 1.21% (SD = 3.55%), while removal of rRNA reduced the total number of reads by 96.40% (SD = 1.15%). From the remaining reads, the mean percentage of pseudoaligned reads against the MAGs functional database was 40.38% (SD = 6.26%). See Additional File: Table S1 for detailed information per sample.
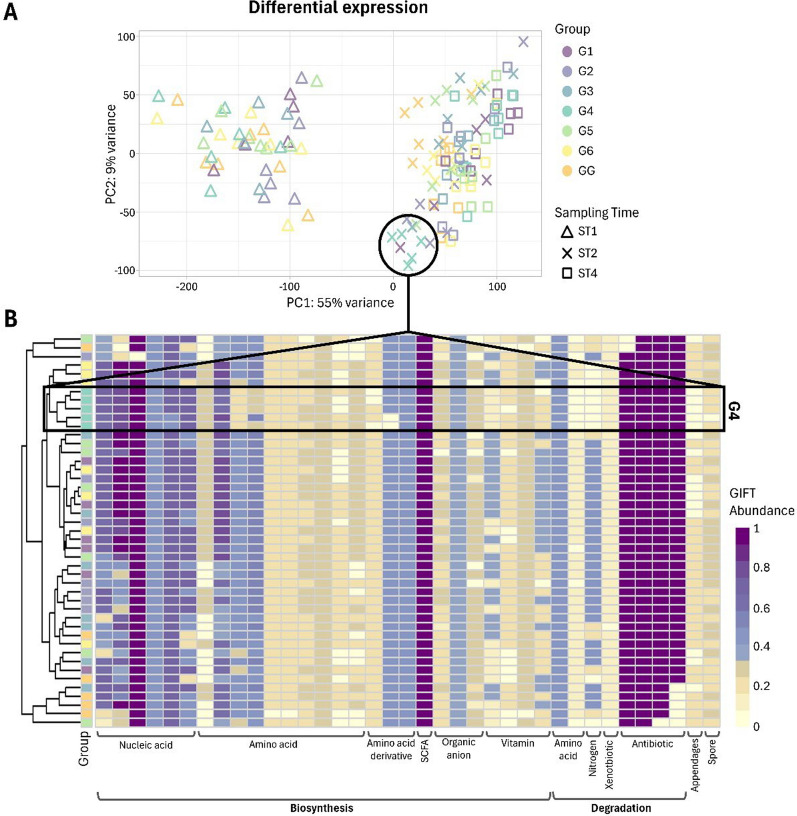
Principal component analysis (PCA) of expression profiles from metatranscriptomics data clustered samples per sampling time with 55% of the total variance explained by the first principal component. Samples collected before weaning (ST1) clustered together and separated from the samples collected after treatment (ST2 and ST4) (Fig. 3A). Differential expression analysis with DESeq2 comparing treatment groups within sampling times (Additional File: Table S3 A) identified major number of DEGs against the gentamicin-treated group (G4) at three days post-treatment (ST2). Individual samples from this condition (G4, ST2) were also observed to cluster together in the PCA (Fig. 3A). A total of 5,960 genes were found differentially expressed (3,819 downregulated and 2141 upregulated) in the gentamicin-treated group (G4), detecting main differences compared with the vaccinated group for downregulated genes (G3; 931 genes), and with the group treated with amoxicillin for upregulated genes (GG; 1,314 genes) (P-adj < 0.01). DEGs against the gentamicin-treated group (G4) at a 1% confidence level (P-adj < 0.01) were further investigated (Additional File: Table S3 A). Among them, a mean of 25.68% (SD = 3.53%) of the genes encoded hypothetical proteins. For the remaining DEGs, a relevant proportion of the genes appeared to be related to ribosomal proteins; 15.57% (SD = 1.87%) downregulated and 7.73% (SD = 2.39%) upregulated genes. Within the downregulated genes, most of them encoded ribosomal proteins of the small (30S) and large (50S) subunits. In contrast, several genes encoding ribosomal-associated proteins were found to be significantly upregulated, such as the ABC-F type ribosomal protection protein Msr(D), the small ribosomal subunit biogenesis GTPase RsgA, and the ribosome hibernation promotion factor (Additional File: Table S3; ST2). The taxonomic phyla associated to these DEGs belonged to Bacillota, Bacteroidota, Actinomycetota, Campylobacterota, Verrucomicrobiota, and Pseudomonadota from the Bacteria kingdom, and to Methanobacteriota from the Archaea kingdom. At the species level, main ribosomal DEGs were found in Clostridium sp000435835, Terrisporobacter sp022782605, and Paratractidigestivibacter faecalis. Distillation of the functional annotations of the DEGs identified against the gentamicin-treated group (G4) at three days post-treatment (ST2) showed that functions related with amino acid and vitamin biosynthesis were underrepresented in comparison to the other treatment groups. Also, functions related to nitrogen compound degradation and spores were less represented in all samples of this treatment group (Fig. 3B).
Fig. 3.
A. Principal component analysis (PCA) of metatranscriptomic expression profiles from different treatment groups and sampling times. B. Functional traits of the differential expressed genes (DEGs) against the gentamicin-treated group (G4) at three days post-treatment (ST2) at a 1% significance level (P-adj < 0.01). G1: trimethoprim/sulfamethoxazole, G2: colistin, G3: oral vaccination, G4: gentamicin, G5: untreated control with water acidification, G6: untreated control, GG: amoxicillin (farm of origin). ST1: one day before weaning, ST2: three days post-treatment, ST4: four weeks post-treatment
To be noted, filtered results of DEGs associated with resistance specific terms were compared between treatment groups within each sampling time. Before weaning (ST1), no significant differences between treatment groups were observed (P-adj > 0.01 and |log2-fold change (FC)|< 2). However, several differences were observed at three days post-treatment (ST2) between groups. Among the DEGs identified, the gene encoding the mupirocin-resistant isoleucine—tRNA ligase MupA was found to be significantly downregulated in the group treated with gentamicin (G4) in comparison to the colistin-treated group (G2, FC−1 = 61.39, P-adj = 0.008), the vaccinated group (G3, FC−1 = 146.02, P-adj = 0.009), and the group treated with amoxicillin (GG, FC−1 = 33.13, P-adj = 0.007). Several other genes encoding resistance-related proteins were also significantly upregulated in the gentamicin-treated group (G4) compared with the group treated with amoxicillin (GG): tetracycline resistance ribosomal protection protein Tet(44) (FC = 134.36, P-adj = 0.001), mupirocin-resistant isoleucine—tRNA ligase MupB (FC = 1,097.50, P-adj = 0.000), ferrous-iron efflux pump FieF (FC = 1,640.59, P-adj = 0.001), multidrug export protein MepA (FC = 357.05, P-adj = 0.003), penicillin-binding protein A (FC = 955.43, P-adj = 0.007), and phosphoribosylaminoimidazole-succinocarboxamide synthase (FC = 257.78, P-adj = 0.008). Finally, the gene encoding the multiple stress resistance protein BhsA was upregulated in the group treated with amoxicillin (GG) compared with the groups treated with the following antibiotics: trimethoprim/sulfamethoxazole (G1, FC = 1,031.12, P-adj = 0.005), colistin (G2, FC = 634.73, P-adj = 0.004), and gentamicin (G4, FC = 617.37, P-adj = 0.004).
Besides, comparisons of DEGs between sampling times within the different treatment groups (Additional File: Table S3B) identified main differences before weaning (ST1) and sampling times post-treatment (ST2 and ST4). Main differences were detected for the untreated control group (G6) with 10,912 upregulated genes at four weeks post-treatment (ST4) compared to before weaning (ST1). Filtered results of DEGs associated with resistance specific terms identified downregulated genes at three days post-treatment (ST2) compared to before weaning (ST1) encoding multidrug efflux pumps (AcrABF, TtgA, EmrAB, BepE, AaeB), multiple antibiotic resistance proteins (MarAR, MdtC), and penicillin-binding proteins (1B, 1 A, LpoAB) for all treatment groups. Also, the gene encoding the multiple stress resistance protein BhsA was downregulated in all groups, although it was not significant for the group that remained at the farm of origin treated with amoxicillin (GG). Other genes were upregulated at three days post-treatment (ST2): the multidrug export protein MepA was upregulated in all groups except the group treated with amoxicillin (GG), and the mupirocin-resistant isoleucine-tRNA ligase MupA was upregulated in all treatment groups except the groups treated with trimethoprim/sulfamethoxazole (G1) and gentamicin (G4) (Fig. 4A). The taxonomic species harbouring most of these resistance-associated DEGs were Escherichia coli and Parabacteroides distasonis, followed by Butyricimonas virosa and Absicoccus porci. Between post-treatment sampling times, most differences were observed for the group that remained at the farm of origin treated with amoxicillin (GG) with 2,564 upregulated genes at four weeks post-treatment (ST4) compared with three days post-treatment (ST2) (Additional File: Table S3B). Analysis of the DEGs among this group between sampling times identified an upregulation of antimicrobial-related genes at four weeks post-treatment (ST4). Most of these genes encoded efflux pumps and multidrug proteins, such as MepA, MdtE, MdtK and NorM. Also, specific genes conferring resistance to colistin, tetracycline, linearmycin, macrolide, penicillin, and streptomycin were identified (Fig. 4B). The taxonomic species harbouring most of these DEGs in the group treated with amoxicillin (GG) were Prevotella sp945863825, Absicoccus porci, and Parabacteroides distasonis.
Fig. 4.
A. Significant differentially expressed genes (P-adj < 0.01) associated with resistance specific terms at three days post-treatment (ST2) in comparison to before weaning (ST1) for all treatment groups. G1: trimethoprim/sulfamethoxazole, G2: colistin, G3: oral vaccination, G4: gentamicin, G5: untreated control with water acidification, G6: untreated control, GG: amoxicillin (farm of origin). B. Significant differentially expressed genes (P-adj < 0.01) associated with resistance specific terms between sampling times for the group that remained at the farm of origin treated with amoxicillin (GG). ST1: one day before weaning, ST2: three days post-treatment, ST4: four weeks post-treatment
Expression profile of ARGs between treatment groups and sampling times
Resistance profiles were determined using Resfinder. This software identified a total of 116 different ARGs, conferring resistance to nine different antibiotic classes. The mean expression of ARGs per sample was 18.18 (SD = 7.19), with those conferring resistance to aminoglycosides (n = 27), macrolides (n = 25), and tetracyclines (n = 23) being the most predominant. A total of 244,934 reads covered the sequence regions of the ARGs identified among samples. After normalization, a total of 196,090 TPMs were calculated, detecting major expression levels of ARGs conferring resistance to macrolides (n = 89,081.67 TPMs), tetracyclines (n = 70,396.36 TPMs), and aminoglycosides (n = 27,209.18 TPMs), followed by sulphonamides (n = 13,201.16 TPMs) and beta-lactams (n = 10,501.91 TPMs) (Additional File: Table S4).
Pairwise comparisons of ARG expression levels between treatment groups and sampling times are depicted in Fig. 5. Comparisons between treatment groups did not identify significant differences of ARG expression levels before weaning (ST1) and three days post-treatment (ST2) for any antibiotic class. However, significant differences were found between treatment groups four weeks post-treatment (ST4) for aminoglycosides, beta-lactams, and tetracyclines. A significant increased expression of ARGs conferring resistance to aminoglycosides was observed for the group that remained at the farm of origin treated with amoxicillin (GG) in comparison to the group treated with trimethoprim/sulfamethoxazole (G1, P-adj = 0.028), the vaccinated group (G3, P-adj = 0.033), and the control group with water acidification (G5, P-adj = 0.028). Specific ARGs identified in the GG group four weeks post-treatment (ST4) conferring resistance to aminoglycosides were ant(6)-Ia, ant(6)-Ib, and aph(3’)-III (considering ARGs found in three or more samples). Also, an increase in ARGs expression levels conferring resistance to beta-lactams was observed in the GG group compared to the vaccinated group (G3, P-adj = 0.049) and the gentamicin-treated group (G4, P-adj = 0.049). The most prevalent ARGs were cfxA and cfxA6. For ARGs conferring resistance to tetracyclines, a significant increased expression was observed in the untreated control group (G6) in comparison with the group treated with trimethoprim/sulfamethoxazole (G1, P-adj = 0.025) at four weeks post-treatment (ST4). The most predominant ARGs in this group (G6) at four weeks post-treatment were tet(40), tet(L), tet(O/W), tet(Q), tet(W), and tet(W/32/O).
Fig. 5.
Expression levels of ARGs to the different antibiotic classes represented in a logarithmic scale of transcripts per million (TPM) of transcriptomic reads by treatment group and sampling times. Only ARGs found in at least 2% of the samples are represented. G1: trimethoprim/sulfamethoxazole, G2: colistin, G3: oral vaccination, G4: gentamicin, G5: untreated control with water acidification, G6: untreated control, GG: amoxicillin (farm of origin). ST1: one day before weaning, ST2: three days post-treatment, ST4: four weeks post-treatment
Besides, when studying ARGs expression longitudinally, a significant decrease for ARGs conferring resistance to macrolides and tetracyclines was observed at three days (ST2) and four weeks (ST4) post-treatment in all treatment groups (P-value < 0.05, Fig. 5). The ARGs for macrolides that showed major decreased expression were erm(F), erm(N), mef(C), mph(A), mph(B), and mph(G). In the case of ARGs conferring resistance to tetracycline, main differences were observed for tet(A), tet(Q), and tet(X).
Correlation of ARGs abundances from metagenomic and metatranscriptomic reads
To assess the similarities in global profiles of the ARGs estimated by shotgun metagenomics [23] and metatranscriptomics, a combination of univariate and Procrustes analysis was implemented. A total of 115 ARGs were common between both datasets, suggesting that in our experimental conditions 50.22% of the ARGs from the total ARGs identified in the metagenomics analysis (n = 229) were being expressed. All the ARGs detected from the metatranscriptomic reads were also detected from the metagenomic reads, except the beta-lactam blaTEM-213 resistant gene. An overall multivariate correlation of 0.79 was observed between metagenomics and metatranscriptomics data from ninety-six common samples (Additional File: Figure S3 A).
Furthermore, we also evaluated the individual correlation patterns of specific ARGs between the metagenomic and metatranscriptomic datasets, and observed 23 genes (20%) with a positive correlation (> 0.6), with seven genes having a strong positive correlation (> 0.8): aph(3’)-Ib with a correlation of 0.92, nimD and sul2 with 0.87 correlation, mph(A) with 0.85, and cepA−44, mef(C), and mph(G) with a correlation of 0.82. A second Procrustes analysis to compare global similarity patterns between datasets was repeated using only the common genes between the metagenomic and metatranscriptomic datasets (n = 115 ARGs), and a higher correlation similarity was observed (R = 0.85) (Additional File: Figure S3B).
Discussion
Antimicrobial resistance is one of the major challenges threatening global health [56, 57]. Understanding how common antimicrobial treatments affect the emergence and selection of ARGs is of great importance in a One Health context, since ARGs can disseminate among different environments [58–60]. Traditionally, methods for the identification of ARGs have relied on culture-based techniques that limit the analysis to the estimated 1% of culturable bacteria under laboratory conditions [61–63]. In the omics era, novel sequencing technologies have revolutionized how microbial communities and their associated resistome can be investigated. The two shotgun sequencing technologies implemented in this study allowed the generation of a metagenome catalogue, and to comprehensively reveal the effect of multiple different PWD treatments on the transcriptome and resistome of the pig gut microbiome longitudinally. Furthermore, the non-redundant MAGs catalogue generated herein will potentially contribute as a valuable resource to better characterize and further study the pig gut microbiome and resistome.
Despite bioinformatics software to analyse metagenomics and metatranscriptomics data are continuously emerging, the strategy performed here appears to be a robust approach that, to our knowledge, has not previously been performed from pigs treated with different antibiotics in similar experimental settings, reducing the possible bias introduced by the farm environment, different diets, and other management practices that can affect the microbial composition of the gut microbiota. First, it took advantage of the genetic content available from metagenomics sequencing to generate a highly refined non-redundant MAGs catalogue obtained from assemblies of individual samples and co-assemblies per treatment group and sampling time. Refinement of both assembly sets improved bins completeness and length, while increasing the number of final bins per condition, and subsequently, of the catalogue. Second, the metatranscriptomics data generated from the same experimental design was mapped against the MAGs catalogue to decipher which populations and genes found in the same microbiome were transcriptionally active, reducing the bias of mapping against an integrated database. Moreover, the taxonomic diversity and total bins abundance identified for the SCOA and SA bins per treatment group and sampling time resembled to that observed in previous studies of our group from the metagenomic reads [23]. Microbial diversity variations represented by the Shannon indexes in previous work showed an increase in diversity three days and two weeks post-treatment that decreased to initial amounts at four weeks post-treatment [23], the same pattern observed for bins abundance herein at the different sampling times. Treatment groups with reduced microbial diversity measured from bins abundance at four weeks post-treatment were the colistin-treated group, the untreated control, and the group that remained at the farm of origin continuously treated with amoxicillin. Besides, the treatment group with increased number of bins by the end of the experiment was the vaccinated group, followed by the control group with water acidification, as observed by the Shannon indexes at four weeks post-treatment [23]. These results suggest that microbial diversity could also be estimated and compared from total MAGs abundance when comparing different conditions from the same experimental setting.
The prevalence of unclassified bacteria to any taxonomic level among the bins generated per treatment group and sampling time, as well as for the final non-redundant MAGs catalogue, highlights the importance of investigating new methods for the identification of these unknown bacteria to unravel their characteristics and functionalities in the pig gut microbiome. Furthermore, it is interesting to note that the number of ARGs identified at the bin level is substantially reduced in comparison to the total ARGs identified at the reads level. The fact that detection of ARGs decreased after downstream assembly steps, including binning, suggests that much information may be lost during these steps [64]. These observations raise questions concerning the capacity of the bins to detect ARGs that represent the whole resistome, and the possibility of deciphering the potential reservoirs of ARGs within the pig gut microbiome [65, 66]. Considering our results, Campylobacter coli and Prevotella sp900771975 (recently renamed Segatella) were found as the major reservoirs of ARGs in the gut microbiome of pigs.
The identification of ARGs from the metatranscriptomics data provided further insights into the resistance genes that were actively expressed in the pig gut microbiome. To our knowledge, no previous data was available on the extent to which ARGs are expressed in the gut of pigs, especially under antimicrobial selective pressures. Despite the high amount of ARGs identified from the metagenomics data [23], 50% of them were transcriptionally active, as previously found in other studies [22, 24]. A diverse range of ARGs were expressed before treatment and in all treatments, including the untreated control group. As previously reported [23], the decrease of some ARGs after weaning may be correlated with the microbial diversity shifts observed after weaning carrying those specific ARGs. Perhaps, these microorganisms harbouring ARGs were transmitted from the sows to the piglets after the antibiotic pressure applied for years at the farm. Future studies including samples from the sows or the environment at birth would allow to investigate into this hypothetical correlation. However, most of these microorganisms switched after weaning, resulting in a reduced number of ARGs in the gut of the piglets. In this study, the most expressed ARG classes regarding diversity and abundance conferred resistance to macrolides, tetracyclines, and aminoglycosides, in concordance with other studies performed in pigs [67, 68]. However, transcript abundances showed great variability and were not very consistent between treatment groups.
Parallelly, differences in gene expression were mainly observed when comparing sampling times after weaning, either at three days or four weeks post-treatment, and the sampling time before weaning, for all treatment groups. Major differences were observed for the untreated control group, with more than 10,000 upregulated genes four weeks post-treatment. This observation could suggest that the different treatments implemented here, even the addition of acidifiers in the water, could potentially select for specific microorganisms reducing the total number of functions differentially expressed after weaning. Moreover, nonspecific genome-inferred functional trait was distinctly identified in the untreated control group in comparison to the other treatment groups when contrasting these two sampling times. Besides, comparisons between sampling times after weaning observed major differences for the group that remained at the farm of origin continuously treated with amoxicillin. This group showed the highest number of upregulated genes at four weeks post-treatment in comparison to three days post-treatment, with a substantial abundance of resistance correlated genes, such as efflux pumps and multidrug resistance proteins. This effect on the metatranscriptome, together with previous findings showing a disruption of the microbial communities at this sampling time [23], can again be correlated with the longer prescription period of the treatment, most probably as a result of an adaptation to the continuous antibiotic pressure [69, 70]. Another relevant observation of this study was the effect of the gentamicin treatment on the transcriptome compared with the rest of the treatments. Interestingly, this group had significantly altered the expression of ribosomal-related genes showing an effect at the transcriptome level already three days after treatment. These results reflect the rapid effect of gentamicin against bacteria, which acts by binding the 30S ribosomal subunit to inhibit bacterial protein synthesis [71, 72]. However, when the antibiotic treatment was withdrawn, these changes in the transcriptome were restored by the end of the experiment.
The overall high amount of ARGs found in the intestinal tract of animals and humans is known to contribute to the spread of resistance worldwide [65, 73]. Noteworthy, some ARGs identified in this study, aph(3’)-III, erm(B), lnu(C), and tet(40), were found to be actively expressed continuously in all or nearly all the samples collected. Although antimicrobial resistance genes were expressed even in the absence of selective pressure, we provided evidence of the significant impact that some commonly used antimicrobials have on the selection of resistance. For instance, the continuous use of amoxicillin not only increased expression levels for beta-lactam resistance genes, but also for aminoglycosides. In contrast, gentamicin had a rapid and reversible effect on the microbial transcriptome. The usage of shotgun sequencing technologies must be considered to further investigate the prevalence of antimicrobial resistance within microbiomes to decipher potential drivers and reservoirs. The findings of this study underscore the importance of characterizing the microbiome and resistome at different levels to implement effective antimicrobial stewardship practices on farm.
Conclusions
By combining shotgun metagenomics and metatranscriptomics, this study has generated a metagenome assembled catalogue of the pig gut microbiome to study differential gene expression in a cross-sectional and longitudinal experimental design. The main findings showed that half of the antimicrobial resistance genes present in the metagenomics data were transcriptionally active. Gentamicin and amoxicillin treatments had major effects on the microbial transcriptome compared to the rest of the treatments. These results also evidence that the application of high-throughput sequencing technologies offers a new paradigm for the surveillance of antimicrobial resistance worldwide.
Supplementary Information
Supplementary Material 1: Table S1. General information of selected animals of the study for metatranscriptomics sequencing, including host and rRNA contamination, mapping rate, and NCBI SRA Accession numbers from BioProject PRJNA1010706. Weights are expressed in Kg. ADWG: Average Daily Weight Gain (information not available for the GG group). G1: trimethoprim/sulfamethoxazole, G2: colistin, G3: oral vaccinated, G4: gentamicin, G5: untreated control with water acidification, G6: untreated control, GG: amoxicillin (farm of origin). ST1: one day before weaning, ST2: three days after treatment, ST4: three weeks after treatment. Table S2. Antimicrobial resistance genes (ARGs) detected with Resfinder in the collection of 1,396 metagenome-assembled genomes (MAGs) from pig faecal samples, including their taxonomic rank and gene metadata obtained from Resfinder and CARD databases. Table S3. A. Number of differential expressed genes between treatment groups at the three different sampling times at a 1% significance level. B. Number of differential expressed genes between sampling times within each treatment group at a 1% significance level. Blue bars indicate downregulated genes, while purple bars are upregulated genes. G1: trimethoprim/sulfamethoxazole, G2: colistin, G3: oral vaccinated, G4: gentamicin, G5: untreated control with water acidification, G6: untreated control, GG: amoxicillin (farm of origin). ST1: one day before weaning, ST2: three days after treatment, ST4: three weeks after treatment. Table S4. Sum of antimicrobial resistance genes (ARGs) abundance per antibiotic class from normalized data of resistance genes identified by Resfinder. G1: trimethoprim/sulfamethoxazole, G2: colistin, G3: oral vaccinated, G4: gentamicin, G5: untreated control with water acidification, G6: untreated control, GG: amoxicillin (farm of origin). ST1: one day before weaning, ST2: three days post-treatment, ST4: four weeks post-treatment.
Supplementary Material 2: Figure S1. A. Taxonomic classification at the phylum level of refined bins from single assemblies per treatment group and sampling time. B. Percentage of mapped metagenomic reads against the collection of metagenome-assembled genomes per treatment group and sampling time. G1: trimethoprim/sulfamethoxazole, G2: colistin, G3: oral vaccination, G4: gentamicin, G5: untreated control with water acidification, G6: untreated control, GG: amoxicillin. ST1: one day before weaning, ST2: three days post-treatment, ST3: two weeks post-treatment, ST4: four weeks post-treatment.
Supplementary Material 3: Figure S2. Heatmap showing the abundance of the different genome-inferred functional traits represented in each metagenome-assembled genome of the collection. Abundance for each genome-inferred functional trait is represented in a gradient scale, excluding those represented in less than 5% of the genomes.
Supplementary Material 4: Figure S3. A. Procrustes analysis comparing all antimicrobial resistance gene abundances from metagenomic and metatranscriptomic reads. B. Procrustes analysis comparing only common antimicrobial genes between metagenomic and metatranscriptomic datasets.
Acknowledgements
We thank the personnel from the IRTA experimental farm and Noemí Giler-Baquerizo for her technical support. The Saga HPC Sigma2—the National Infrastructure for High Performance Computing and Data Storage in Norway are also acknowledged for providing computational resources that have contributed to some of the meta-omics analyses described in this study.
Author contributions
L.M.G. designed the experiment and obtained the funding. L.M.G., L.F., and J.G.M. performed the experiments and Y.R.C., A.V.P.L., and J.G.M. conducted the bioinformatic and statistical analyses. M.B., L.M.G., J.G.M., A.V.P.L., P.B.P., T.R.H., and Y.R.C. discussed and interpreted the results. J.G.M. wrote the first draft of the manuscript, and all authors reviewed the manuscript.
Funding
This study was funded by the I + D + I National Program RTI2018-095586-B-C22 and the CERCA program. J.G.M. is a PhD student from the Autonomous University of Barcelona, Biotechnology Program, with an IRTA fellowship from the strategic initiative on antimicrobial reduction in animal production and performed part of the analysis at the Norwegian University of Life Sciences (NMBU) with an EMBO Scientific Exchange Grant (10385). Y.R.C. is recipient of a Ramon y Cajal postdoctoral fellowship (RYC2019-027244-I) from the Spanish Ministry of Science and Innovation.
Availability of data and materials
Metagenomics and metatranscriptomics raw data have been submitted to the NCBI’s sequence read archive with the BioProject accession number PRJNA1010706.
Declarations
Ethics approval and consent to participate
Animals on the experimental farm were exposed to the same conditions as on the conventional farm and were allocated following legislation in animal welfare. Antimicrobial treatments followed the summary of product characteristics (SmPC) of the products, and no disease was induced. The Ethics Committee for Animal Experimentation (CEEA) guidelines reviewed and authorized the procedures of this study with the ID number CEEA103/2018.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuliaxis Ramayo-Caldas, Email: yuliaxis.ramayo@irta.cat.
Lourdes Migura-Garcia, Email: lourdes.migura@irta.cat.
References
- 1.Kirchhelle C. Pharming animals: a global history of antibiotics in food production (1935–2017). Palgrave Commun. 2018. 10.1057/s41599-018-0152-2. [Google Scholar]
- 2.Cromwell GL. Why and how antibiotics are used in swine production. Anim Biotechnol. 2006;13(1):7–27. 10.1081/abio-120005767. [DOI] [PubMed] [Google Scholar]
- 3.Allel K, Day L, Hamilton A, Lin L, Furuya-Kanamori L, Moore CE, et al. Global antimicrobial-resistance drivers: an ecological country-level study at the human–animal interface. Lancet Planet Health. 2023;7(4):e291–303. 10.1016/s2542-5196(23)00026-8. [DOI] [PubMed] [Google Scholar]
- 4.Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet. 2022;399(10325):629–55. 10.1016/s0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh TR, Gales AC, Laxminarayan R, Dodd PC. Antimicrobial resistance: addressing a global threat to humanity. PLoS Med. 2023;20(7): e1004264. 10.1371/journal.pmed.1004264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regulation 1831/2003/EC on additives for use in animal nutrition, replacing Directive 70/524/EEC on additives in feeding-stuffs. Official Journal of the European Union. 2003.
- 7.Regulation (EU) 2019 of the European Parliament and of the Council of 11 December 2018 on veterinary medicinal products and repealing Directive 2001/82/EC. Official Journal of the European Union. 2018.
- 8.Bednorz C, Oelgeschläger K, Kinnemann B, Hartmann S, Neumann K, Pieper R, et al. The broader context of antibiotic resistance: Zinc feed supplementation of piglets increases the proportion of multi-resistant Escherichia coli in vivo. Int J Med Microbiol. 2013;303(6–7):396–403. 10.1016/j.ijmm.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Oh JK, Chae JP, Pajarillo EAB, Kim SH, Kwak MJ, Eun JS, et al. Association between the body weight of growing pigs and the functional capacity of their gut microbiota. Anim Sci J. 2020. 10.1111/asj.13418. [DOI] [PubMed] [Google Scholar]
- 10.Guevarra RB, Hong SH, Cho JH, Kim B-R, Shin J, Lee JH, et al. The dynamics of the piglet gut microbiome during the weaning transition in association with health and nutrition. J Anim Sci Biotechnol. 2018. 10.1186/s40104-018-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frese SA, Parker K, Calvert CC, Mills DA. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome. 2015. 10.1186/s40168-015-0091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergamaschi M, Tiezzi F, Howard J, Huang YJ, Gray KA, Schillebeeckx C, et al. Gut microbiome composition differences among breeds impact feed efficiency in swine. Microbiome. 2020. 10.1186/s40168-020-00888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang H, Fang S, Yang H, Chen C. Identification of the relationship between the gut microbiome and feed efficiency in a commercial pig cohort. J Anim Sci. 2021. 10.1093/jas/skab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Guo Y, Wen Z, Jiang X, Ma X, Han X. Weaning stress perturbs gut microbiome and its metabolic profile in piglets. Sci Rep. 2018. 10.1038/s41598-018-33649-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loh G, Dou S, Gadonna-Widehem P, Rome V, Hamoudi D, Rhazi L, et al. Characterisation of early-life fecal microbiota in susceptible and healthy pigs to post-weaning diarrhoea. PLoS ONE. 2017;12(1): e0169851. 10.1371/journal.pone.0169851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhouma M, Fairbrother JM, Beaudry F, Letellier A. Post weaning diarrhea in pigs: risk factors and non-colistin-based control strategies. Acta Vet Scand. 2017. 10.1186/s13028-017-0299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lallès J-P, Bosi P, Smidt H, Stokes CR. Weaning—a challenge to gut physiologists. Livest Sci. 2007;108(1–3):82–93. 10.1016/j.livsci.2007.01.091. [Google Scholar]
- 18.He L, Zhao X, Li J, Yang C. Post-weaning diarrhea and use of feedstuffs in pigs. Anim Front. 2022;12(6):41–52. 10.1093/af/vfac079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canibe N, Højberg O, Kongsted H, Vodolazska D, Lauridsen C, Nielsen TS, et al. Review on preventive measures to reduce post-weaning diarrhoea in piglets. Animals. 2022;12(19):2585. 10.3390/ani12192585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suiryanrayna MVAN, Ramana JV. A review of the effects of dietary organic acids fed to swine. J Anim Sci Biotechnol. 2015. 10.1186/s40104-015-0042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcelino VR, Wille M, Hurt AC, González-Acuña D, Klaassen M, Schlub TE, et al. Meta-transcriptomics reveals a diverse antibiotic resistance gene pool in avian microbiomes. BMC Biol. 2019. 10.1186/s12915-019-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Hu Y, Gao GF. Combining metagenomics and metatranscriptomics to study human, animal and environmental resistomes. Med Microecol. 2020;3: 100014. 10.1016/j.medmic.2020.100014. [Google Scholar]
- 23.Guitart-Matas J, Ballester M, Fraile L, Darwich L, Giler-Baquerizo N, Tarres J, et al. Gut microbiome and resistome characterization of pigs treated with commonly used post-weaning diarrhea treatments. Anim Microbiome. 2024. 10.1186/s42523-024-00307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munk P, Knudsen BE, Lukjancenko O, Duarte ASR, Van Gompel L, Luiken REC, et al. Abundance and diversity of the faecal resistome in slaughter pigs and broilers in nine European countries. Nat Microbiol. 2018;3(8):898–908. 10.1038/s41564-018-0192-9. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Guo Q, Li Y-Y, Song T-Y, Ge J-Q. Metagenomic analysis fecal microbiota of dysentery-like diarrhoea in a pig farm using next-generation sequencing. Front Vet Sci. 2023. 10.3389/fvets.2023.1257573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaio D, DeMaere MZ, Anantanawat K, Eamens GJ, Liu M, Zingali T, et al. A large-scale metagenomic survey dataset of the post-weaning piglet gut lumen. GigaScience. 2021. 10.1093/gigascience/giab039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vilaró A, Novell E, Enrique-Tarancon V, Balielles J, Migura-García L, Fraile L. Antimicrobial susceptibility testing of porcine bacterial pathogens: investigating the prospect of testing a representative drug for each antimicrobial family. Antibiotics. 2022;11(5):638. 10.3390/antibiotics11050638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nurk S, Meleshko D, Korobeynikov A, Pevzner PA. metaSPAdes: a new versatile metagenomic assembler. Genome Res. 2017;27(5):824–34. 10.1101/gr.213959.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li D, Liu C-M, Luo R, Sadakane K, Lam T-W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics. 2015;31(10):1674–6. 10.1093/bioinformatics/btv033. [DOI] [PubMed] [Google Scholar]
- 30.Uritskiy GV, DiRuggiero J, Taylor J. MetaWRAP—a flexible pipeline for genome-resolved metagenomic data analysis. Microbiome. 2018. 10.1186/s40168-018-0541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alneberg J, Bjarnason BS, de Bruijn I, Schirmer M, Quick J, Ijaz UZ, et al. Binning metagenomic contigs by coverage and composition. Nat Methods. 2014;11(11):1144–6. 10.1038/nmeth.3103. [DOI] [PubMed] [Google Scholar]
- 32.Wu Y-W, Simmons BA, Singer SW. MaxBin 2.0: an automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics. 2016;32(4):605–7. 10.1093/bioinformatics/btv638. [DOI] [PubMed] [Google Scholar]
- 33.Kang DD, Li F, Kirton E, Thomas A, Egan R, An H, et al. MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ. 2019;7: e7359. 10.7717/peerj.7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Team RC: R: A language and environment for statistical computing. [Internet]. In.; 2022.
- 35.Olm MR, Brown CT, Brooks B, Banfield JF. dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. ISME J. 2017;11(12):2864–8. 10.1038/ismej.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25(7):1043–55. 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodcroft B: CoverM. In.; 2019.
- 38.Asnicar F, Thomas AM, Beghini F, Mengoni C, Manara S, Manghi P, et al. Precise phylogenetic analysis of microbial isolates and genomes from metagenomes using PhyloPhlAn 3.0. Nat Commun. 2020. 10.1038/s41467-020-16366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–3. 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49(W1):W293–6. 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaumeil P-A, Mussig AJ, Hugenholtz P, Parks DH, Hancock J. GTDB-Tk: a toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics. 2020;36(6):1925–7. 10.1093/bioinformatics/btz848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaffer M, Borton MA, McGivern BB, Zayed AA, La Rosa SL, Solden LM, et al. DRAM for distilling microbial metabolism to automate the curation of microbiome function. Nucleic Acids Res. 2020;48(16):8883–900. 10.1093/nar/gkaa621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanehisa M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.anttonalberdi: distillR: R package for distilling functional annotations of bacterial genomes and metagenomes. In: Github; 2022.
- 45.Bortolaia V, Kaas RS, Ruppe E, Roberts MC, Schwarz S, Cattoir V, et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J Antimicrob Chemother. 2020;75(12):3491–500. 10.1093/jac/dkaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20. 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–9. 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kopylova E, Noé L, Touzet H. SortMeRNA: fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics. 2012;28(24):3211–7. 10.1093/bioinformatics/bts611. [DOI] [PubMed] [Google Scholar]
- 49.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2012;41(D1):D590–6. 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griffiths-Jones S. Rfam: an RNA family database. Nucleic Acids Res. 2003;31(1):439–41. 10.1093/nar/gkg006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34(5):525–7. 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 52.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014. 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wickham H. ggplot2: elegant graphics for data analysis. 2nd ed. Springer International Publishing; 2016. [Google Scholar]
- 54.Oksanen J, Simpson GL, Blanchet FG, Kindt R, Legendre P, Minchin PR, et al. vegan: Community Ecology Package. 2001; 10.32614/CRAN.package.vegan.
- 55.Bowers RM, Kyrpides NC, Stepanauskas R, Harmon-Smith M, Doud D, Reddy TBK, et al. Minimum information about a single amplified genome (MISAG) and a metagenome-assembled genome (MIMAG) of bacteria and archaea. Nat Biotechnol. 2017;35(8):725–31. 10.1038/nbt.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.EclinicalMedicine. Antimicrobial resistance: a top ten global public health threat. eClinicalMedicine. 2021;41:101221. 10.1016/j.eclinm.2021.101221. [DOI] [PMC free article] [PubMed]
- 57.Coque TM, Cantón R, Pérez-Cobas AE, Fernández-de-Bobadilla MD, Baquero F. Antimicrobial resistance in the global health network: known unknowns and challenges for efficient responses in the 21st century. Microorganisms. 2023;11(4):1050. 10.3390/microorganisms11041050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu Y, Yang X, Li J, Lv N, Liu F, Wu J, et al. The bacterial mobile resistome transfer network connecting the animal and human microbiomes. Appl Environ Microbiol. 2016;82(22):6672–81. 10.1128/aem.01802-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forsberg KJ, Reyes A, Wang B, Selleck EM, Sommer MOA, Dantas G. The shared antibiotic resistome of soil bacteria and human pathogens. Science. 2012;337(6098):1107–11. 10.1126/science.1220761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lawther K, Santos FG, Oyama LB, Rubino F, Morrison S, Creevey CJ, et al. Resistome analysis of global livestock and soil microbiomes. Front Microbiol. 2022. 10.3389/fmicb.2022.897905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rappé MS, Giovannoni SJ. The uncultured microbial majority. Annu Rev Microbiol. 2003;57(1):369–94. 10.1146/annurev.micro.57.030502.090759. [DOI] [PubMed] [Google Scholar]
- 62.Hugenholtz P. Exploring prokaryotic diversity in the genomic era. Genome Biol. 2002;3(2):reviews0003.1. 10.1186/gb-2002-3-2-reviews0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hofer U. The majority is uncultured. Nat Rev Microbiol. 2018;16(12):716–7. 10.1038/s41579-018-0097-x. [DOI] [PubMed] [Google Scholar]
- 64.Lapidus AL, Korobeynikov AI. Metagenomic data assembly—the way of decoding unknown microorganisms. Front Microbiol. 2021. 10.3389/fmicb.2021.613791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anthony WE, Burnham C-AD, Dantas G, Kwon JH. The gut microbiome as a reservoir for antimicrobial resistance. J Infect Dis. 2021;223(Supplement_3):S209–13. 10.1093/infdis/jiaa4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diebold PJ, Rhee MW, Shi Q, Trung NV, Umrani F, Ahmed S, et al. Clinically relevant antibiotic resistance genes are linked to a limited set of taxa within gut microbiome worldwide. Nat Commun. 2023. 10.1038/s41467-023-42998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Versluis D, D’Andrea MM, Ramiro Garcia J, Leimena MM, Hugenholtz F, Zhang J, et al. Mining microbial metatranscriptomes for expression of antibiotic resistance genes under natural conditions. Sci Rep. 2015. 10.1038/srep11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Hu Y, Liu F, Cao J, Lv N, Zhu B, et al. Integrated metagenomic and metatranscriptomic profiling reveals differentially expressed resistomes in human, chicken, and pig gut microbiomes. Environ Int. 2020;138: 105649. 10.1016/j.envint.2020.105649. [DOI] [PubMed] [Google Scholar]
- 69.Oz T, Guvenek A, Yildiz S, Karaboga E, Tamer YT, Mumcuyan N, et al. Strength of selection pressure is an important parameter contributing to the complexity of antibiotic resistance evolution. Mol Biol Evol. 2014;31(9):2387–401. 10.1093/molbev/msu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hughes D, Andersson DI. Evolutionary consequences of drug resistance: shared principles across diverse targets and organisms. Nat Rev Genet. 2015;16(8):459–71. 10.1038/nrg3922. [DOI] [PubMed] [Google Scholar]
- 71.Yoshizawa S. Structural origins of gentamicin antibiotic action. EMBO J. 1998;17(22):6437–48. 10.1093/emboj/17.22.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kotra LP, Haddad J, Mobashery S. Aminoglycosides: perspectives on mechanisms of action and resistance and strategies to counter resistance. Antimicrob Agents Chemother. 2000;44(12):3249–56. 10.1128/aac.44.12.3249-3256.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bengtsson-Palme J, Angelin M, Huss M, Kjellqvist S, Kristiansson E, Palmgren H, et al. The human gut microbiome as a transporter of antibiotic resistance genes between continents. Antimicrob Agents Chemother. 2015;59(10):6551–60. 10.1128/aac.00933-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Table S1. General information of selected animals of the study for metatranscriptomics sequencing, including host and rRNA contamination, mapping rate, and NCBI SRA Accession numbers from BioProject PRJNA1010706. Weights are expressed in Kg. ADWG: Average Daily Weight Gain (information not available for the GG group). G1: trimethoprim/sulfamethoxazole, G2: colistin, G3: oral vaccinated, G4: gentamicin, G5: untreated control with water acidification, G6: untreated control, GG: amoxicillin (farm of origin). ST1: one day before weaning, ST2: three days after treatment, ST4: three weeks after treatment. Table S2. Antimicrobial resistance genes (ARGs) detected with Resfinder in the collection of 1,396 metagenome-assembled genomes (MAGs) from pig faecal samples, including their taxonomic rank and gene metadata obtained from Resfinder and CARD databases. Table S3. A. Number of differential expressed genes between treatment groups at the three different sampling times at a 1% significance level. B. Number of differential expressed genes between sampling times within each treatment group at a 1% significance level. Blue bars indicate downregulated genes, while purple bars are upregulated genes. G1: trimethoprim/sulfamethoxazole, G2: colistin, G3: oral vaccinated, G4: gentamicin, G5: untreated control with water acidification, G6: untreated control, GG: amoxicillin (farm of origin). ST1: one day before weaning, ST2: three days after treatment, ST4: three weeks after treatment. Table S4. Sum of antimicrobial resistance genes (ARGs) abundance per antibiotic class from normalized data of resistance genes identified by Resfinder. G1: trimethoprim/sulfamethoxazole, G2: colistin, G3: oral vaccinated, G4: gentamicin, G5: untreated control with water acidification, G6: untreated control, GG: amoxicillin (farm of origin). ST1: one day before weaning, ST2: three days post-treatment, ST4: four weeks post-treatment.
Supplementary Material 2: Figure S1. A. Taxonomic classification at the phylum level of refined bins from single assemblies per treatment group and sampling time. B. Percentage of mapped metagenomic reads against the collection of metagenome-assembled genomes per treatment group and sampling time. G1: trimethoprim/sulfamethoxazole, G2: colistin, G3: oral vaccination, G4: gentamicin, G5: untreated control with water acidification, G6: untreated control, GG: amoxicillin. ST1: one day before weaning, ST2: three days post-treatment, ST3: two weeks post-treatment, ST4: four weeks post-treatment.
Supplementary Material 3: Figure S2. Heatmap showing the abundance of the different genome-inferred functional traits represented in each metagenome-assembled genome of the collection. Abundance for each genome-inferred functional trait is represented in a gradient scale, excluding those represented in less than 5% of the genomes.
Supplementary Material 4: Figure S3. A. Procrustes analysis comparing all antimicrobial resistance gene abundances from metagenomic and metatranscriptomic reads. B. Procrustes analysis comparing only common antimicrobial genes between metagenomic and metatranscriptomic datasets.
Data Availability Statement
Metagenomics and metatranscriptomics raw data have been submitted to the NCBI’s sequence read archive with the BioProject accession number PRJNA1010706.