Abstract
Background
Due to their contribution to global warming, methane emissions from ruminants have been the subject of considerable scientific interest. It has been proposed that such emissions might be reduced using genetic selection; proposed phenotypes differ in the measurement methods used (direct or predicted methane emissions) and in the unit under consideration (g/d, g/kg of milk, g/kg of intake, residual methane emissions). Identifying the quantitative trait loci (QTLs) and candidate genes responsible for genetic variation in methane emissions allows a better understanding of the underlying genetic architecture of these phenotypes. Therefore, the aim of this study was to identify the genomic regions associated with six methane traits predicted from milk mid-infrared (MIR) spectra (0.33 ≤ R2 ≤ 0.88) in French Holstein dairy cows using genome-wide association studies at the whole-genome-sequence level.
Results
Six methane emission traits—in g/d, in g/kg of fat- and protein-corrected milk, and in g/kg of dry matter intake—were predicted from milk MIR spectra routinely collected by French milk recording companies. A genome-wide association study of the predicted methane emissions of 40,609 primiparous Holstein cows was conducted using imputed whole-genome-sequence data. This analysis revealed 57 genomic regions of interest; between 1 and 8 QTLs were identified on each of the autosomes except 4, 12, 21, 24 and 26. We identified multiple genomic regions that were shared by two or more predicted methane traits, illustrating their common genetic basis. Functional annotation revealed potential candidate genes, in particular FASN, DGAT1, ACSS2, and KCNIP4, which could be involved in biological pathways possibly related to methane production.
Conclusions
The methane traits studied here, which were predicted from milk MIR spectra, appear to be highly polygenic. Several genomic regions associated with these traits contain candidate genes previously associated with milk traits. Functional annotation and comparisons with studies using direct methane measurements support some potential candidate genes involved in biological pathways related to methane production. However, the overlap with genes influencing milk traits highlights the challenge of distinguishing whether these regions genuinely influence methane emissions or reflect the use of milk MIR spectra to predict the phenotypes.
Background
The contribution of the agricultural sector, particularly cattle breeding, to climate change has been extensively highlighted in recent years. Due to the enteric fermentation in their digestive process, cattle are the primary source of agricultural methane (CH4) emissions [1, 2]. Various strategies have been identified to mitigate these emissions, including modifications in feeding strategies, feeding additives, herd management, and genetic selection [3, 4]. Among these proposals, though, only genetic selection offers a cumulative and permanent solution for reducing CH4 emissions.
Methane production in ruminants results from the activity of microorganisms within the rumen. The fermentation of ingested feed by rumen bacteria produces hydrogen, which is primarily converted into CH₄ by archaea through methanogenesis [4]. Numerous studies have analyzed the genetic determinism of CH4 emissions in cattle [5–9], but the ways in which this process is influenced by host genetics are still unclear and require further research.
One effective approach for investigating the genetic basis of CH4 emissions is the use of Genome-Wide Association Studies (GWAS). GWAS identifies genomic markers associated with a phenotype of interest, and significant markers located in the same region collectively indicate Quantitative Trait Loci (QTLs). Within QTL regions, it is possible to identify candidate genes that can improve our understanding of the biological mechanisms underlying CH4 emissions. By combining information on both genetic markers and functional annotation, likely causal variants can be proposed. This biological information can be used to design more precise and effective breeding strategies, ultimately contributing to a more sustainable agricultural sector.
Previous GWASs performed on direct CH4 measurements identified several genomic regions that were associated with CH4 emissions [10–14]. However, the use of direct CH4 phenotypes, which are recorded with low-to-medium throughput methods such as Sniffer, GreenFeed, the SF6 tracer gas method, and respiration chambers, limits the number of animals that can be included in the analyses and therefore both the detection power and resolution of the results. Some studies have used CH4 predicted from feed intake phenotypes [15–17], though these phenotypes are similarly challenging to record at large scale. However, the development of equations to predict CH4 emissions from milk mid-infrared (MIR) spectra [18–21] (0.38 ≤ R2 ≤ 0.88) has made possible the collection of much larger datasets, enabling more accurate genetic and genomic analyses.
The present study had two primary objectives. Using a large database of French Holstein primiparous cows and milk MIR spectra, which are routinely collected by French milk recording companies, we aimed to (1) detect QTLs associated with CH4 emissions predicted from milk MIR spectra, and (2) identify positional candidate genes within those QTLs.
Materials and methods
Animals and spectra data
The initial dataset consisted of 1,221,705 milk MIR spectra collected between 70 and 200 days in milk (DIM) from 359,856 primiparous Holstein cows. The following selection criteria were applied. Only lactations from the A-type recording protocol with samples from both morning and evening milkings were kept. The selected lactations started between 2015 and 2021, and each lactation had at least two records. The CH4 phenotypes were predicted according to Fresco et al. [20], Vanlierde et al. [19], and Chilliard et al. [18] and are described in the next paragraph. All other information was extracted from the bovine national database (INRAE, CTIG Jouy-en-Josas, France).
Methane phenotypes
Methane traits were predicted from milk MIR spectra that were standardized according to Grelet et al. [22]. Different prediction equations were applied to the milk MIR spectra to obtain six distinct CH4-related traits:
MeP_FA (in g/d), obtained from the equation (R2 = 0.88) developed by Chilliard et al. [18] based on fatty acid composition predicted from milk MIR spectra and CH4 measurements with the SF6 tracer gas method in a 4 × 4 Latin square experiment.
MeP_RC (in g/d), obtained from the equation (R2 = 0,60) of Vanlierde et al. [19], which was developed using milk MIR spectra and CH4 measurements with respiration chambers and the SF6 tracer gas method for 299 cows from 7 breeds.
MeP_direct (in g/d, R2 = 0.38), MeY (in g/kg of dry matter intake (DMI), R2 = 0.33), and MeI (g/kg of fat- and protein-corrected milk (FPCM), R2 = 0.47), obtained from the equations developed by Fresco et al. [20] from milk MIR spectra and GreenFeed (C-Lock Inc., Rapid City, SD, USA) measurements for 235 cows from 3 breeds, of which only 76 cows from 1 breed were used to calibrate the equation predicting MeY.
MeP_indirect (in g/d, R2 = 0.38), obtained by multiplying the predicted MeI by the observed FPCM associated with the spectra.
Only test-day data between 70 and 200 days in milk were considered, according to Fresco et al. [20]; outside this interval, the validity of the equations predicting MeP_direct, MeI, and MeY was not guaranteed. Due to contractual restrictions, milk MIR spectra were not directly available to us. Instead, predictions were generated by ESTEL (Nancy, France), the IT company in charge of routinely performing all milk MIR spectra predictions for French milk recording companies.
Adjustment of phenotypes
For each trait, outlier values—defined as those falling outside three standard deviations around the mean—were excluded from the dataset. Univariate repeatability models were applied to estimate environmental effects and then to adjust the phenotypes (i.e. each prediction from the milk MIR spectra), using Genekit software [23]. The pedigree was traced back four generations and contained 903,383 animals. The linear model was the following:
| 1 |
where y is the vector of CH4 predictions; β is the vector including the fixed effects of herd x test-day [168,362 classes], year x month of calving [84 classes], age at calving [17 classes], and the covariate days in milk modeled by third-order Legendre polynomials; X is the corresponding incidence matrix; ) is the vector of breeding values, with A the pedigree relationship matrix among individuals and the additive genetic variance; ) is the vector of permanent environmental effects, with I the identity matrix and the permanent environmental variance; Z and W are the incidence matrices; and ) is the vector of residual effects, with the residual variance. Variance components were estimated from a subset of 579,753 records from 156,381 cows, already described in Fresco et al. [9]. Genetic covariance between the six CH4 traits were estimated using bivariate analyses with Eq. (1). These components were estimated using the AI-REML algorithm, as implemented in Wombat software [24]. The corrected phenotypes were then averaged for each individual for use in the GWAS analyses.
50 K genotypes and imputation to whole-genome sequence
Among the cows with phenotypes, 40,609 had genotypes for the 50 K chip and were subsequently used in GWAS analyses. The 50 K genotypes were imputed to whole-genome sequences (WGS) in two steps, similar to the procedure presented by Tribout et al. [25] and Sanchez et al. [26]. Briefly, we first imputed from 50 K to high density (HD) within breed using FImpute software [27], using 776 major French Holstein bulls genotyped with the HD Illumina chip as a reference, and then from HD to WGS with Minimac software [28], using the WGS variants of a multi-breed Bos taurus bull population from Run9 of the 1000 Bull Genomes Project [29]. This panel included 3414 bulls from 45 breeds, including 1414 Holsteins. After quality control (MAF > 0.01, Minimac imputation R2 > 0.20), 13,366,479 variants were retained for GWAS analyses of the 29 autosomes.
GWAS analyses
Due to computational challenges arising from the large size of the genomic matrix, we performed the GWAS analyses by randomly splitting the population into two equal sub-populations. Single-trait association analyses were then performed across all 13,307,594 variants (MAF > 0.01) for each of the six predicted CH4 traits. All association analyses were performed using the mlma option of GCTA software (version 1.26), which applies a mixed linear model that includes the variant to be tested [30]:
| 2 |
where is the vector of averaged corrected phenotypes for each of the six CH4 traits; is the overall mean; is the additive fixed effect of the variant to be tested for association; is the vector of imputed alternate allele dosages, coded from 0 to 2; ~ (0,) is the vector of random polygenic effects, with the genomic relationship matrix calculated using the 50 K genotypes and the polygenic variance, estimated based on the null model () and then fixed while testing for the association between each variant and the trait; and ~ (0,) is the vector of random residual effects, with the identity matrix and the residual variance. Z was the incidence matrix.
We combined the two GWAS results by performing a meta-analysis (meta-GWAS) using METAL software [31] with the STDERR option, which applies the fixed effects approach. We estimated the inflation factor for each trait (1.10–1.25) and corrected the p-values accordingly. To account for multiple testing, we applied a highly conservative approach, the Bonferroni correction that considered 13,307,594 independent tests in the meta-GWAS. The 5% genome-wide threshold of significance therefore corresponded to a nominal P-value of 3.75 × 10–9 (-log10(P) = 8.4) per test. We determined QTL intervals based on a linkage disequilibrium analysis using the correlations between imputed dosages. We applied the iterative procedure described in Sanchez et al. [32]. In short, this procedure selects the variant with the highest -log10(P) per Bos taurus autosome (BTA), estimates its linkage disequilibrium (LD) with nearby variants within a user-defined window, and defines the QTL confidence interval based on high-LD variants. A new test statistic evaluates whether other variants' effects are explained by LD with the top variant. Variants meeting this criterion and identified in the QTL confidence interval are removed before the next iteration.
For our study, we used the following parameters in the iterative procedure: a minimal linkage disequilibrium of 0.45, a minimal MAF of 0.01, a minimal imputation R2 of 0.2, and a window size set to 4 Mbp. When we observed that the TOP1 variant was in high LD with variants at the edge of the window and that another QTL was detected just outside of the bounds of the window, we performed a second LD analysis using the same iterative procedure with the same parameters on a larger window for the concerned BTAs to determine whether we really had two neighboring QTLs or whether it was a unique QTL with LD at a greater distance. We widened the window to 8 Mbp (BTA1, 3, and 14 for MeP_RC; BTA14 for MeP_direct; BTA14 and 20 for MeI; BTA19 for MeP_FA) or 18 Mbp (BTA14 for MeY and BTA6 for MeP_RC). For each trait, the proportion of genetic variance explained by each QTL () was estimated using , with and the frequency and the estimated allelic substitution effect, respectively, of the variant with the most significant effect (ms) in the QTL.
Annotation of candidate genes
Genomic regions were defined as regions containing one QTL or multiple QTLs whose confidence intervals overlapped. Within these regions, the variant with the most significant effect, i.e., ranked first in the peak, was referred to as the TOP1. The top ten most significant variants within each peak were referred to as the TOP10. QTLs were annotated using the Ensembl gene set integrating the ARS-UCD1.3 bovine reference genome [33]. Positional candidate genes were defined as those containing at least one variant from the TOP10. To further characterize these variants, the Ensembl Variant Effect Predictor (VEP, [34]) was used to provide detailed functional annotations.
Results
Estimation of genetic parameters using a repeatability model
Descriptive statistics for the six CH4 traits during the first lactation, measured between 70 and 200 days in milk, are the same as in Fresco et al. [9] and are presented in Table 1. For the four CH4 traits measured in g/d, the average emissions varied from 367 to 448 g/d, while mean MeI was 14.0 g/kg of FPCM and mean MeY was 17.8 g/kg of DMI. The genetic, permanent environmental, and residual variances for these traits are detailed in Table 2. Heritability estimates varied from 0.23 to 0.42 depending on the CH4 trait (Table 2).
Table 1.
Descriptive statistics of the methane traits predicted from milk MIR spectra, used for estimation of variance components
| Trait | Mean | Standard deviation | Minimum | Maximum |
|---|---|---|---|---|
| MeP_direct (g/d) | 420 | 41.2 | 227 | 572 |
| MeI (g/kg of FPCM) | 14.0 | 1.82 | 7.4 | 19.7 |
| MeY (g/kg of DMI) | 17.8 | 1.40 | 13.4 | 22.9 |
| MeP_indirect (g/d) | 367 | 80.2 | 95 | 630 |
| MeP_FA (g/d) | 448 | 43.61 | 295 | 570 |
| MeP_RC (g/d) | 427 | 41.76 | 292 | 577 |
Table 2.
Variance and heritability estimates for the six methane traits predicted from milk MIR spectra
| Trait | Genetic variance | Permanent environment variance | Residual variance | Heritability |
|---|---|---|---|---|
| MeP_direct (g/d) | 291 | 139 | 310 | 0.39 (0.006) |
| MeI (g/kg of FPCM) | 0.36 | 0.14 | 0.56 | 0.34 (0.006) |
| MeY (g/kg of DMI) | 0.24 | 0.68 | 0.31 | 0.35 (0.006) |
| MeP_indirect (g/d) | 690 | 1204 | 1050 | 0.23 (0.007) |
| MeP_FA (g/d) | 274 | 160 | 459 | 0.31 (0.006) |
| MeP_RC (g/d) | 364 | 133 | 368 | 0.42 (0.006) |
Genome-wide association studies for the six predicted CH4 traits
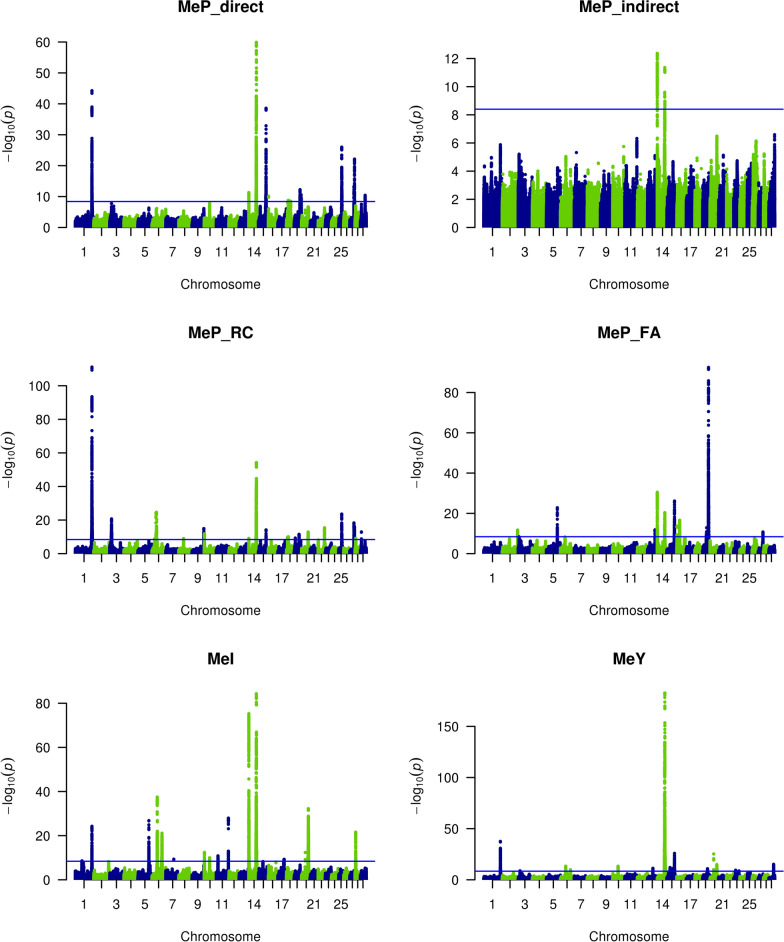
The results of the meta-GWAS combining the single-trait GWAS analyses of the two randomly divided groups of individuals are presented in Fig. 1. The number of variants with a genome-wide significant effect (− log10(P) ≥ 8.4) varied from 157 to 9758 depending on the CH4 trait in question (Table 3). There were between 1 and 3422 significant variants per QTL, with an average of 313 variants (standard deviation = 618). Notably, 86.5% (90 of 104) and 92.0% (897 of 975) of the TOP1 and TOP10 variants were imputed, respectively. Only 4.9% of the TOP1 and 5.1% of the TOP10 variants presented a MAF between 0.01 and 0.02. The maximum -log10(P) value observed was 181, for a variant on BTA14 associated with MeY.
Fig. 1.
-log10(P) values plotted against the position of variants on Bos taurus autosomes for the meta-analyses of methane traits predicted from milk mid-infrared spectra. MeP_direct = prediction of methane production in g/d [20], MeI = prediction of methane intensity in g/kg of fat- and protein-corrected milk [20], MeY = prediction of methane yield in g/kg of dry matter intake [20], MeP_indirect = predicted MeI multiplied by the observed fat- and protein-corrected milk [20], MeP_FA = prediction of methane production in g/d [18], and MeP_RC = prediction of methane production in g/d [19]
Table 3.
Number of significant variants, number of QTLs, and percentage of genetic variance explained
| Trait | Number of significant variants | Number of QTLs | SE of QTL effect | % of genetic variance explained by the QTL | ||
|---|---|---|---|---|---|---|
| Minimum | Maximum | Total | ||||
| MeP_direct (g/d) | 4649 | 17 | 0.01–0.74 | 0.12 | 4.0 | 12.0 |
| MeI (g/kg of FPCM) | 9758 | 25 | 0.01–0.07 | 0.16 | 7.1 | 24.7 |
| MeY (g/kg of DMI) | 7922 | 17 | 0.01–0.02 | 0.14 | 12.5 | 20.7 |
| MeP_indirect (g/d) | 157 | 2 | 0.45–0.57 | 0.63 | 0.9 | 1.5 |
| MeP_FA (g/d) | 2911 | 15 | 0.15–0.42 | 0.12 | 3.1 | 11.7 |
| MeP_RC (g/d) | 7186 | 28 | 0.15–0.90 | 0.14 | 6.4 | 23.0 |
We detected 2 to 28 QTLs per CH4 trait, for a total of 104 QTLs across all six CH4 traits (Table 4a, b, c, d, and e). These QTLs were distributed across 24 of the 29 autosomes, with 1 to 15 QTLs per BTA. The confidence intervals of the QTLs varied from 2 to 11,980,490 bp, and 3 QTL were defined by a single variant. When neighboring QTLs had overlapping confidence intervals, we combined them into genomic regions; this resulted in the identification of 57 distinct genomic regions, each containing between 1 and 8 QTLs. Of these, 22 genomic regions contained at least 2 QTLs affecting one or multiple CH4 traits. Four regions were of particular interest: one located on BTA14 at ~ 64 Mbp (region 28), which was associated with all six CH4 traits; one located on BTA14 at ~ 0.5 Mbp (region 26), which was associated with five of the six CH4 traits; and two regions located on BTA1 at ~ 142 Mbp (region 2) and BTA15 at ~ 65 Mbp (region 32), which were associated with four of the six CH4 traits.
Table 4.
QTLs identified for predicted methane traits
| Region | Trait | BTA | QTL | Confidence interval of the QTL | Variant with the most significant effect (all had an imputation R2 of 1) | Name of genes TOP10 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Start (bp) | End (bp) | N variants | N genes | Position (bp) | -log10P | b | SE | Name of gene TOP1 | |||||
| 1 | MeI | 1 | 1 | 57,130,469 | 57,632,402 | 9 | 4 | 57,631,653 | 8.5 | 0.06 | 0.01 | ENSBTAG00000057099 | CD200, ENSBTAG00000060686, ENSBTAG00000057099, ENSBTAG00000001235 |
| 2 | MeP_RC | 1 | 5 | 139,923,727 | 146,447,185 | 2355 | 47 | 142,814,697 | 111.2 | − 5.80 | 0.22 | SLC37A1 | SLC37A1 |
| 2 | MeP_direct | 1 | 4 | 141,872,915 | 144,306,817 | 1183 | 13 | 142,814,697 | 44.1 | − 3.04 | 0.18 | SLC37A1 | SLC37A1 |
| 2 | MeY | 1 | 3 | 141,915,045 | 144,306,817 | 1177 | 12 | 142,806,542 | 37.5 | − 0.08 | 0.01 | SLC37A1 | SLC37A1 |
| 2 | MeI | 1 | 2 | 142,591,987 | 142,595,307 | 7 | 0 | 142,594,565 | 13.2 | 0.06 | 0.01 | ||
| 2 | MeI | 1 | 6 | 142,604,730 | 142,991,203 | 615 | 6 | 142,879,906 | 24.0 | 0.09 | 0.01 | SLC37A1 | |
| 3 | MeP_FA | 2 | 7 | 131,103,444 | 131,359,941 | 354 | 7 | 131,245,133 | 11.7 | − 2.44 | 0.30 | ENSBTAG00000068711 | |
| 4 | MeP_RC | 3 | 10 | 13,177,910 | 17,220,237 | 65 | 5 | 17,171,600 | 12.2 | − 4.91 | 0.59 | ENSBTAG00000065185 | |
| 4 | MeP_RC | 3 | 8 | 13,672,338 | 17,166,665 | 149 | 28 | 15,470,670 | 20.8 | 3.28 | 0.30 | EFNA1 | SLC50A1, EFNA1, ENSBTAG00000061595 |
| 4 | MeY | 3 | 9 | 15,504,899 | 15,602,932 | 40 | 7 | 15,555,371 | 8.8 | 0.06 | 0.01 | DCST1 | ADAM15, DCST1, DCST2, ZBTB7B |
| 5 | MeI | 5 | 11 | 92,843,362 | 94,221,469 | 16 | 1 | 93,375,037 | 10.0 | 0.09 | 0.01 | EPS8 | |
| 5 | MeI | 5 | 12 | 93,391,179 | 93,749,199 | 205 | 2 | 93,516,066 | 26.6 | − 0.10 | 0.01 | MGST1 | MGST1 |
| 5 | MeP_FA | 5 | 13 | 93,512,771 | 93,751,239 | 124 | 2 | 93,516,319 | 22.7 | − 2.42 | 0.21 | MGST1 | MGST1 |
| 6 | MeP_RC | 6 | 14 | 23,596,912 | 23,604,273 | 3 | 1 | 23,597,653 | 8.9 | 5.49 | 0.78 | PPP3CA | PP3CA |
| 7 | MeP_RC | 6 | 15 | 33,528,107 | 35,173,221 | 31 | 3 | 34,288,754 | 24.1 | − 9.36 | 0.78 | CCSER1 | CCSER1, ENSBTAG00000065318, ENSBTAG00000065966 |
| 8 | MeP_RC | 6 | 16 | 35,571,947 | 36,587,776 | 5 | 3 | 35,594,567 | 11.7 | − 4.82 | 0.59 | ENSBTAG00000062289 | GPRIN3, ENSBTAG00000062289, ABCG2 |
| 9 | MeP_RC | 6 | 17 | 36,955,669 | 37,003,208 | 17 | 2 | 36,997,477 | 11.6 | 4.40 | 0.54 | ENSBTAG00000065096 | |
| 10 | MeP_RC | 6 | 18 | 38,148,560 | 38,485,730 | 34 | 0 | 38,454,876 | 16.5 | − 4.28 | 0.44 | ||
| 11 | MeP_RC | 6 | 19 | 38,485,998 | 40,748,806 | 7 | 1 | 38,494,188 | 24.8 | 10.00 | 0.83 | KCNIP4 | |
| 12 | MeI | 6 | 20 | 44,250,735 | 44,262,727 | 3 | 0 | 44,262,048 | 8.6 | − 0.05 | 0.01 | ||
| 13 | MeI | 6 | 21 | 44,283,395 | 44,308,333 | 8 | 0 | 44,299,242 | 14.7 | − 0.06 | 0.07 | ||
| 14 | MeI | 6 | 22 | 44,329,162 | 46,642,860 | 856 | 19 | 44,747,933 | 37.1 | 0.11 | 0.01 | ENSBTAG00000064660 | |
| 14 | MeY | 6 | 23 | 44,674,698 | 45,967,046 | 48 | 3 | 45,060,087 | 13.3 | 0.06 | 0.01 | ENSBTAG00000064660 | ENSBTAG00000064660 |
| 15 | MeI | 6 | 24 | 82,582,052 | 82,605,868 | 18 | 0 | 82,593,766 | 8.5 | 0.05 | 0.01 | ||
| 16 | MeI | 6 | 25 | 83,640,723 | 85,702,894 | 369 | 17 | 85,503,742 | 20.0 | − 0.10 | 0.01 | ENSBTAG00000060764 | ENSBTAG00000060764, CSN1S2 |
| 16 | MeY | 6 | 28 | 85,424,759 | 86,448,746 | 68 | 7 | 86,221,365 | 9.6 | 0.07 | 0.01 | GRSF1 | CSN1S1, GRSF1 |
| 16 | MeI | 6 | 26 | 85,703,928 | 85,703,928 | 1 | 0 | 85,703,928 | 9.0 | − 0.06 | 0.01 | ||
| 16 | MeI | 6 | 27 | 85,736,321 | 86,158,733 | 35 | 4 | 85,736,621 | 10.8 | − 0.10 | 0.01 | CABS1 | CABS1 |
| 17 | MeI | 7 | 29 | 68,579,274 | 68,592,765 | 58 | 2 | 68,579,369 | 9.3 | 0.09 | 0.01 | ENSBTAG00000057983 | ENSBTAG00000057983, ENSBTAG00000059254 |
| 18 | MeP_RC | 8 | 30 | 41,366,883 | 42,068,650 | 11 | 0 | 41,366,883 | 9.1 | 1.94 | 0.27 | ||
| 19 | MeP_RC | 9 | 31 | 100,858,075 | 102,458,517 | 450 | 13 | 101,345,396 | 15.0 | − 2.11 | 0.23 | ENSBTAG00000059544 | ENSBTAG00000059544 |
| 20 | MeI | 10 | 32 | 1,341,660 | 2,214,558 | 119 | 2 | 2,187,345 | 12.3 | 0.04 | 0.01 | APC, ENSBTAG00000058588 | |
| 20 | MeP_RC | 10 | 33 | 2,137,692 | 2,214,558 | 116 | 0 | 2,191,110 | 11.9 | 1.22 | 0.15 | ||
| 21 | MeY | 10 | 34 | 46,048,106 | 46,548,638 | 438 | 7 | 46,051,704 | 13.2 | − 0.08 | 0.01 | DAPK2 | DAPK2, ENSBTAG00000060901, USP3 |
| 21 | MeI | 10 | 35 | 46,088,320 | 46,548,638 | 172 | 5 | 46,541,616 | 9.9 | − 0.06 | 0.01 | ENSBTAG00000057172 | USP3, ENSBTAG00000057172 |
| 22 | MeI | 11 | 36 | 14,229,710 | 14,642,556 | 128 | 2 | 14,229,992 | 10.6 | − 0.05 | 0.01 | MEMO1 | |
| 23 | MeI | 11 | 37 | 103,226,637 | 103,286,957 | 374 | 4 | 103,254,495 | 27.7 | − 0.12 | 0.01 | ENSBTAG00000014678 | |
| 24 | MeY | 13 | 38 | 45,882,199 | 47,070,574 | 194 | 6 | 46,366,318 | 11.3 | 0.05 | 0.01 | GTPBP4 | |
| 25 | MeP_FA | 13 | 39 | 64,053,550 | 64,262,227 | 52 | 3 | 64,147,879 | 11.7 | − 1.86 | 0.23 | NCOA6 | NCOA6, ACSS2, GSS |
| 26 | MeI | 14 | 46 | 180,920 | 3,104,982 | 220 | 21 | 1,704,582 | 26.7 | − 0.09 | 0.01 | PSCA | PSCA, ENSBTAG00000026340, ABDRB1, TSNARE1 |
| 26 | MeI | 14 | 44 | 233,681 | 993,201 | 522 | 37 | 608,230 | 73.7 | − 0.26 | 0.01 | DGAT1 | CPSF1, ENSBTAG00000000857, SCRT1, DGAT1 |
| 26 | MeP_FA | 14 | 41 | 264,797 | 993,201 | 324 | 33 | 550,784 | 30.3 | − 4.57 | 0.35 | CPSF1 | CPSF1, ADCK5, DGAT1 |
| 26 | MeP_direct | 14 | 43 | 384,242 | 901,826 | 111 | 17 | 568,472 | 11.2 | 3.05 | 0.37 | ADCK5 | ADCK5, DGAT1, HSF1 |
| 26 | MeP_indirect | 14 | 42 | 384,242 | 707,908 | 128 | 16 | 565,009 | 12.5 | − 4.11 | 0.45 | ADCK5 | ADCK5, DGAT1, HSF1 |
| 26 | MeP_RC | 14 | 40 | 497,507 | 955,774 | 73 | 11 | 500,872 | 9.2 | − 3.24 | 0.45 | TONSL | TONSL, CPSF1, ENSBTAG00000000857, SCRT1, DGAT1, HSF1 |
| 27 | MeP_FA | 14 | 45 | 995,464 | 1,915,755 | 136 | 12 | 1,028,657 | 13.5 | − 1.70 | 0.20 | IQANK1 | IQANK1, FAM83H, ZC3H3, ADGRB1 |
| 28 | MeY | 14 | 50 | 59,747,181 | 71,727,670 | 3422 | 59 | 64,752,983 | 181.3 | 0.42 | 0.01 | VPS13B | VPS13B, ENSBTAG00000055757 |
| 28 | MeI | 14 | 47 | 61,219,666 | 68,426,997 | 2910 | 48 | 64,454,721 | 83.2 | 0.23 | 0.01 | VPS13B | VPS13B, ENSBTAG00000055757 |
| 28 | MeP_direct | 14 | 51 | 61,457,257 | 65,916,772 | 1760 | 31 | 64,779,893 | 59.7 | − 8.29 | 0.42 | VPS13B | VPS13B, ENSBTAG00000055757 |
| 28 | MeP_RC | 14 | 52 | 61,527,782 | 65,872,284 | 1411 | 31 | 64,926,343 | 54.5 | − 13.86 | 0.76 | VPS13B | VPS13B, ENSBTAG00000055757 |
| 28 | MeP_FA | 14 | 49 | 63,173,429 | 65,641,447 | 320 | 22 | 64,672,856 | 20.1 | − 4.45 | 0.42 | VPS13B | VPS13B, ENSBTAG00000055757 |
| 28 | MeP_indirect | 14 | 48 | 64,043,186 | 65,402,152 | 29 | 5 | 64,672,856 | 11.3 | 4.96 | 0.57 | VPS13B | VPS13B, ENSBTAG00000055757 |
| 29 | MeY | 15 | 53 | 27,315,640 | 28,691,118 | 212 | 17 | 27,646,671 | 11.4 | 0.05 | 0.01 | SIK3 | ENSBTAG00000067623, SIK3 |
| 30 | MeY | 15 | 54 | 53,137,808 | 53,767,920 | 373 | 8 | 53,359,144 | 11.1 | 0.07 | 0.01 | PAAF1 | MRPL48, COA4, PAAF1, DNAJB13 |
| 31 | MeP_direct | 15 | 55 | 63,019,643 | 63,055,774 | 71 | 1 | 63,055,774 | 9.9 | 1.02 | 0.13 | ENSBTAG00000052796 | ENSBTAG00000052796 |
| 32 | MeP_direct | 15 | 61 | 63,736,668 | 65,705,004 | 252 | 10 | 65,333,701 | 38.5 | − 2.46 | 0.16 | EHF | |
| 32 | MeP_FA | 15 | 58 | 63,804,912 | 65,346,043 | 200 | 5 | 65,312,559 | 26.0 | 1.89 | 0.15 | EHF | |
| 32 | MeY | 15 | 56 | 64,794,022 | 66,090,611 | 67 | 3 | 64,794,049 | 11.9 | 0.04 | 0.01 | ABTB2 | ABTB2 |
| 32 | MeY | 15 | 59 | 64,918,334 | 65,678,067 | 251 | 9 | 65,323,234 | 25.9 | − 0.05 | 0.01 | ENSBTAG00000059416, ENSBTAG00000063234, EHF | |
| 32 | MeP_RC | 15 | 57 | 65,227,767 | 65,239,009 | 3 | 0 | 65,239,009 | 8.6 | 1.23 | 0.18 | ||
| 32 | MeP_RC | 15 | 60 | 65,242,698 | 65,338,417 | 32 | 0 | 65,324,226 | 14.3 | 1.68 | 0.19 | ||
| 32 | MeP_FA | 15 | 62 | 65,355,672 | 65,515,606 | 13 | 3 | 65,355,672 | 9.1 | − 2.01 | 0.29 | PDHX | |
| 32 | MeP_direct | 15 | 63 | 66,074,954 | 66,096,784 | 12 | 0 | 66,089,302 | 10.6 | − 1.17 | 0.15 | ||
| 33 | MeP_FA | 16 | 65 | 1,756,944 | 1,907,186 | 19 | 1 | 1,786,309 | 13.3 | − 2.87 | 0.33 | SOX13 | |
| 33 | MeP_direct | 16 | 64 | 1,784,784 | 1,791,556 | 8 | 0 | 1,786,309 | 10.1 | 2.56 | 0.33 | ||
| 34 | MeP_FA | 16 | 66 | 24,251,952 | 25,510,748 | 148 | 2 | 24,280,507 | 16.5 | − 1.60 | 0.17 | MTARC1 | MTARC1 |
| 35 | MeI | 17 | 67 | 51,586,460 | 51,586,460 | 1 | 0 | 51,586,460 | 8.5 | 0.04 | 0.01 | ||
| 36 | MeI | 17 | 68 | 53,714,812 | 54,541,221 | 51 | 3 | 53,794,372 | 9.2 | 0.08 | 0.01 | KDM2B, RNF34 | |
| 37 | MeP_RC | 18 | 69 | 8,826,358 | 10,690,398 | 10 | 2 | 8,828,778 | 9.6 | 6.42 | 0.88 | ENSBTAG00000069108 | ENSBTAG00000069108, ENSBTAG00000057902 |
| 38 | MeP_RC | 18 | 71 | 15,689,862 | 16,420,403 | 8 | 2 | 16,155,339 | 10.1 | 6.80 | 0.90 | ITFG1, PHKB | |
| 38 | MeP_direct | 18 | 70 | 16,152,862 | 16,155,339 | 2 | 0 | 16,155,339 | 8.8 | 5.32 | 0.74 | ||
| 39 | MeP_RC | 19 | 72 | 10,283,388 | 10,294,221 | 5 | 0 | 10,294,221 | 9.3 | 1.17 | 0.16 | ||
| 40 | MeP_FA | 19 | 75 | 32,335,650 | 34,947,083 | 4 | 2 | 34,947,083 | 8.6 | 1.57 | 0.23 | PLD6 | COPS3, PLD6 |
| 40 | MeP_FA | 19 | 73 | 33,521,281 | 33,536,010 | 30 | 1 | 33,527,729 | 8.7 | − 2.25 | 0.33 | PECC1 | PECC1 |
| 40 | MeP_FA | 19 | 74 | 33,666,818 | 34,856,338 | 28 | 12 | 34,729,508 | 12.7 | 2.17 | 0.26 | RAI1 | ENSBTAG00000063426, EPN2, ENSBTAG00000066713, RAI1 |
| 41 | MeY | 19 | 76 | 42,238,476 | 42,626,158 | 688 | 14 | 42,265,893 | 10.9 | 0.07 | 0.01 | RAB5C | RAB5C, STAT3 |
| 41 | MeP_RC | 19 | 77 | 42,238,476 | 42,626,158 | 689 | 14 | 42,281,725 | 11.6 | − 2.81 | 0.35 | RAB5C, ENSBTAG00000065500 | |
| 42 | MeP_FA | 19 | 80 | 47,997,400 | 54,407,498 | 1102 | 58 | 50,775,172 | 91.5 | − 4.29 | 0.18 | CCDC57, FASN | |
| 42 | MeP_direct | 19 | 78 | 49,996,881 | 50,198,903 | 17 | 2 | 50,198,788 | 10.0 | 1.73 | 0.23 | UTS2R | |
| 42 | MeP_direct | 19 | 79 | 50,207,005 | 50,780,248 | 97 | 4 | 50,721,397 | 12.2 | − 1.66 | 0.19 | CCDC57 | CCDC57, FASN |
| 43 | MeP_direct | 19 | 81 | 60,404,660 | 61,078,226 | 264 | 2 | 60,441,152 | 9.7 | − 1.26 | 0.17 | ENSBTAG00000059677, ENSBTAG00000063788 | |
| 44 | MeY | 20 | 82 | 31,462,828 | 32,320,669 | 13 | 3 | 31,888,449 | 25.2 | 0.13 | 0.01 | GHR | GHR, ENSBTAG00000067324, ENSBTAG00000059721 |
| 44 | MeI | 20 | 83 | 32,018,037 | 32,103,408 | 3 | 2 | 32,018,037 | 9.1 | − 0.09 | 0.01 | GHR | GHR, ENSBTAG00000067324 |
| 45 | MeI | 20 | 85 | 55,290,311 | 60,131,394 | 2933 | 16 | 58,195,251 | 31.9 | 0.12 | 0.01 | ANKH | |
| 45 | MeP_RC | 20 | 84 | 55,514,835 | 57,379,190 | 770 | 10 | 56,489,500 | 13.0 | 2.86 | 0.33 | MYO10 | MYO10, RETREG1 |
| 45 | MeY | 20 | 86 | 58,191,503 | 59,878,202 | 830 | 3 | 58,377,254 | 14.6 | 0.06 | 0.01 | ANKH | ANKH, DNAH5 |
| 46 | MeP_RC | 22 | 87 | 53,951,400 | 54,565,136 | 138 | 3 | 54,475,210 | 14.2 | − 3.07 | 0.34 | ATP2B2 | ATP2B2 |
| 47 | MeP_RC | 22 | 88 | 54,611,746 | 54,677,719 | 42 | 1 | 54,629,523 | 15.5 | 1.44 | 0.15 | ATP2B2 | ATP2B2 |
| 48 | MeY | 23 | 89 | 16,740,006 | 17,219,907 | 2 | 1 | 16,740,006 | 9.4 | 0.05 | 0.01 | PTK7 | PTK7 |
| 49 | MeY | 23 | 90 | 45,223,069 | 45,229,851 | 6 | 1 | 45,223,636 | 8.6 | 0.04 | 0.01 | ELOVL2 | ELOCL2 |
| 50 | MeP_direct | 25 | 91 | 25,899,872 | 25,899,872 | 1 | 0 | 25,899,872 | 8.9 | 1.18 | 0.16 | ||
| 51 | MeP_direct | 25 | 93 | 25,904,431 | 27,723,625 | 621 | 50 | 26,061,709 | 26.0 | − 2.29 | 0.18 | IL27 | ENSBTAG00000064180, ENSBTAG00000050361, IL27 |
| 51 | MeP_RC | 25 | 92 | 25,906,818 | 27,473,500 | 495 | 45 | 26,048,468 | 23.7 | − 2.60 | 0.22 | CLN3 | CLN3 |
| 52 | MeP_RC | 27 | 95 | 36,353,153 | 36,617,333 | 109 | 10 | 36,522,458 | 18.4 | 2.42 | 0.23 | GPAT4 | |
| 52 | MeP_direct | 27 | 94 | 36,378,091 | 36,558,128 | 32 | 4 | 36,522,458 | 13.2 | 1.69 | 0.19 | GINS4, ENSBTAG00000058864, GPAT4, NKX6-3 | |
| 53 | MeP_direct | 27 | 96 | 40,911,676 | 41,646,056 | 158 | 2 | 41,391,206 | 22.1 | − 1.92 | 0.16 | ENSBTAG00000056465 | ENSBTAG00000056465 |
| 53 | MeP_RC | 27 | 97 | 41,375,472 | 41,627,439 | 141 | 2 | 41,391,206 | 16.1 | − 1.94 | 0.20 | ENSBTAG00000056465 | ENSBTAG00000056465 |
| 53 | MeP_FA | 27 | 98 | 41,379,852 | 41,601,820 | 57 | 2 | 41,392,181 | 10.7 | 1.25 | 0.16 | ENSBTAG00000056465 | ENSBTAG00000056465 |
| 54 | MeP_direct | 27 | 99 | 41,737,999 | 41,971,860 | 5 | 3 | 41,971,860 | 8.9 | − 1.68 | 0.23 | ENSBTAG00000064242 | THRB, ENSBTAG00000065779, ENSBTAG00000064242 |
| 55 | MeI | 28 | 101 | 6,192,309 | 7,165,451 | 125 | 2 | 6,458,572 | 21.4 | 0.06 | 0.01 | KCNK1 | KCNK1 |
| 55 | MeP_RC | 28 | 100 | 6,457,299 | 6,529,103 | 8 | 1 | 6,457,425 | 9.2 | 3.33 | 0.46 | KCNK1 | KCNK1 |
| 56 | MeP_RC | 29 | 102 | 9,486,040 | 9,545,883 | 9 | 1 | 9,502,594 | 13.0 | 1.76 | 0.20 | PICALM | |
| 57 | MeY | 29 | 104 | 40,666,856 | 42,245,387 | 93 | 12 | 41,325,720 | 15.2 | 0.17 | 0.02 | SLC22A8, ENSBTAG00000037570 | |
| 57 | MeP_direct | 29 | 103 | 41,149,123 | 42,009,447 | 55 | 7 | 41,319,432 | 10.4 | − 3.83 | 0.49 | SLC22A8 | SLC22A6, SLC22A8 |
The genotyped TOP1 variants are indicated in italics
The CH4 traits with the highest number of genomic regions in common were MeP_direct and MeP_RC (8 genomic regions), followed by MeP_RC and MeY (7), MeI and MeY (7), MeP_direct and MeP_FA (6), and MeP_RC and MeI (6) (Table 5).
Table 5.
Genetic correlations and number of genomic regions shared between pairs of predicted methane traits
| Trait | MeP_direct | MeI | MeY | MeP_indirect | MeP_FA | MeP_RC |
|---|---|---|---|---|---|---|
| MeP_direct (g/j) | 0.25 | 0.81 | 0.36 | 0.21 | 0.62 | |
| MeI (g/kg of FPCM) | 3 | 0.38 | 0.52 | − 0.15 | 0.18 | |
| MeY (g/kg of DMI) | 4 | 7 | 0.36 | 0.13 | 0.50 | |
| MeP_indirect (g/d) | 2 | 2 | 1 | 0.03 | 0.20 | |
| MeP_FA (g/d) | 6 | 3 | 2 | 2 | 0.24 | |
| MeP_RC (g/d) | 8 | 6 | 7 | 2 | 4 |
The percentage of genetic variance explained for each trait varied from 1.5% to 24.7% (Table 3). The variants explaining the highest percentage of genetic variance were found in QTL 51 (4.0%) for MeP_direct; QTL 80 (3.1%) for MeP_FA; QTL 52 (6.4%) for MeP_RC; QTL 42 (0.9%) for MeP_indirect; QTL 44 (7.1%) for MeI; and QTL 50 (12.5%) for MeY. Overall, 30 QTLs contained a TOP1 variant with a -log10(P) value exceeding 20 that we defined as highly significant (Table 4). These included 6 highly significant QTLs for MeP_RC, 5 for MeP_FA, 5 for MeP_direct, none for MeP_indirect, 10 for MeI, and 4 for MeY. Several of these highly significant QTLs were located in the same genomic regions: region 2 on BTA1 at ~ 142 Mbp for MeP_RC, MeP_direct, MeI, and MeY; region 26 on BTA14 at ~ 0.5 Mbp for MeP_FA and MeI; region 32 on BTA15 at ~ 65 Mbp for MeP_direct, MeP_FA, and MeY; and region 51 on BTA25 at ~ 26 Mbp for MeP_RC and MeP_direct.
Functional annotation of GWAS peaks
The total number of genes within the confidence intervals of at least one QTL was 937, with an average of 9 genes per QTL and a range from 0 to 59; in 16 QTLs, no genes were highlighted. Positional candidate genes were defined as those containing at least one of the TOP10 variants for each QTL. We identified 35, 35, 26, 28, 37, and 5 positional candidate genes for MeI, MeY, MeP_direct, MeP_FA, MeP_RC, and MeP_indirect, respectively.
The genomic region associated with all six CH4 traits contained the VPS13B gene, while the genomic regions associated with four or five of the CH4 traits contained the DGAT1, SLC37A1, and EHF/PDHX genes. The QTLs explaining the highest percentage of genetic variance were located in the genomic regions containing the VPS13B, DGAT1, and FASN and CCDC57 genes.
The TOP10 variants for each QTL were annotated using VEP, and some had multiple annotations (Table 6). Between 0% (MeP_indirect) and 18.4% (MeI) of the TOP10 variants were located in intergenic regions, while 47.3% (MeP_direct) to 72.1% (MeP_indirect) were located in intronic regions. Among the TOP10 variants located in coding regions, 1.2% to 2.5% were synonymous variants, and between 0.6% and 5.5% corresponded to missense mutations. Among these missense mutations, some were classified as deleterious based on their SIFT score: two transcripts of the same variant of the VPS13B gene for MeI, MeY, MeP_RC, and MeP_direct; two transcripts of two variants of the gene ENSBTAG00000050361 for MeP_direct; and one transcript of a variant of the PSCA gene for MeI. Additionally, we identified a frameshift variant in the PLD6 gene within QTL 75 on BTA19 at ~ 34 Mbp for MeP_FA.
Table 6.
Functional annotation of the 10 most significant variants of each QTL
| Trait | MeP_direct (g/d) | MeI (g/kg of FPCM) | MeY (g/kg of DMI) | MeP_indirect (g/d) | MeP_FA (g/d) | MeP_RC (g/d) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Variants | 134 | 194 | 142 | 18 | 134 | 226 | ||||||
| Annotations | 383 | 441 | 394 | 68 | 422 | 626 | ||||||
| Intergenic | 45 | 11.7 | 81 | 18.4 | 21 | 5.3 | 27 | 6.4 | 81 | 13.0 | ||
| Intron | 181 | 47.3 | 233 | 52.8 | 282 | 71.6 | 62 | 72.1 | 240 | 56.9 | 414 | 66.2 |
| Downstream_gene | 78 | 20.4 | 53 | 12.0 | 24 | 6.1 | 16 | 18.6 | 58 | 13.8 | 46 | 7.4 |
| Upstream_gene | 42 | 11.0 | 30 | 6.8 | 27 | 6.8 | 6 | 6.9 | 57 | 13.5 | 39 | 6.2 |
| 5_prime_UTR | 11 | 2.5 | 14 | 3.5 | 11 | 1.7 | ||||||
| 3_prime_UTR | 3 | 0.8 | 6 | 1.4 | 3 | 0.8 | 14 | 3.3 | 7 | 1.1 | ||
| Regulatory region | 4 | 1.0 | 5 | 1.1 | 7 | 1.8 | 7 | 1.7 | 9 | 1.4 | ||
| Non_coding_transcript_exon | 1 | 0.2 | 1 | 0.2 | 3 | 0.5 | ||||||
| Synonymous | 8 | 2.1 | 11 | 2.5 | 9 | 2.3 | 1 | 1.2 | 8 | 1.9 | 12 | 1.9 |
| Missense | 21 | 5.5 | 11 | 2.5 | 5 | 1.3 | 9 | 2.1 | 4 | 0.6 | ||
| Frameshift | 1 | 0.2 | ||||||||||
Discussion
To the best of our knowledge, this study is the first GWAS of imputed whole-genome sequences to use such a large population of dairy cattle to investigate CH4 emissions predicted from routinely collected milk MIR spectra.
Genomic regions shared between CH4 traits
We found many genomic regions in common among the six CH4 traits. Out of the 57 genomic regions identified in our study, 21 were shared by at least two CH4 traits, corresponding to 67 QTLs of the 104 identified. The CH4 traits that shared the highest number of regions (8) were MeP_RC and MeP_direct, which is consistent with their methodological similarity: they both represent CH4 emissions in g/d and their prediction equations were calibrated using the same methodology [19, 20]. The number of shared genomic regions was not related to the genetic correlations between the CH4 traits. Indeed, MeP_RC and MeP_direct [genetic correlation of 0.62 (Table 5)] shared 8 genomic regions, while MeP_direct and MeY [genetic correlation of 0.81 (Table 5)] shared 4, and MeP_FA and MeP_direct [genetic correlation of 0.21 (Table 5)] shared 6. Interestingly, the results obtained for MeP_indirect were notably different from those of the other CH4 traits. In this study, MeP_indirect was associated with only two QTLs; these included the genes VPS13B and DGAT1 which were linked with multiple CH4 traits in this study.
In contrast to our results, which revealed multiple genomic regions in common among the CH4 traits investigated, most previously published GWAS analyses of multiple CH4 traits (expressed in different units or predicted using different equations) have reported only a few shared genomic regions; such studies have examined MeP and MeY predicted from milk MIR spectra [35] as well as direct measurements of MeP, MeI, and MeY [10, 11].
Genomic regions shared with different previously published GWAS studies of CH4 traits
The genetic architecture of CH4 emissions has previously been studied using direct CH4 measurements [10–13, 35] or CH4 predicted from traits related to feed intake [15–17], the concentration of volatile fatty acids in the rumen [14], milk fat composition [35], and milk MIR spectra [35] in both dairy and beef breeds. Given the diversity of these approaches and the scarcity of shared regions among previous studies, we anticipated that there would be little overlap between the genomic regions highlighted in this study and those reported in the literature. Indeed, only three of the genomic regions identified here were among those described in the literature. Genomic region 42 on BTA19 at ~ 50 Mbp, which in the present study was associated with MeP_direct and MeP_FA and for which FASN and CCDC57 were the most probable candidate genes, was also highlighted in the studies of van Engelen [35] and Manzanilla-Pech et al. [11], which examined CH4 emissions in g/kg of DMI and g/d, respectively. Genomic region 26 on BTA14 at ~ 0.5 Mbp, which contained the DGAT1 gene, was associated with all predicted CH4 traits except MeY in our study; similarly, this region was reported to be associated with CH4 emissions in both g/d and g/kg of DMI by van Engelen [35]. Finally, genomic region 28 on BTA14 at ~ 64 Mbp was associated with all six predicted CH4 traits analyzed here, with VPS13B being the most likely candidate gene; this region was also identified in the study of Calderón-Chagoya et al. [13], which used CH4 emissions expressed in mg/L.
The small number of genomic regions shared among studies might be the result of the different methods used for measuring CH4 emissions, which are known to have correlations of less than one [36, 37]. Another explanation could be that previous GWAS analyses were based on smaller sample sizes (ranging from 150 to 1962 animals), which limited their detection power. Most of these studies used 50 K genotypes, whereas sequence data, like those used in our study, increase the resolution of QTL detection [38]. Additionally, differences in breed composition among studies (Holstein cows [10–12, 14, 15], beef breeds [10, 16, 17], combination of purebred, crossbred, and Zebu cattle [13]) likely affect the results as well, as different QTLs may segregate in different breeds. This hypothesis is supported by the results of Manzanilla-Pech et al. [10], who highlighted that different genes are involved in the expression of CH4 traits in Angus and Holstein breeds.
CH4 traits are associated with genes related to milk production and composition
Among the positional candidate genes identified in our study, many have been previously confirmed to be associated with milk traits [25, 39], traits predicted from milk MIR spectra [26, 40], and specific wavelengths of milk MIR spectra [41]. Among these, we notably found VPS13B, SLC37A1, DGAT1, FASN, CCDC57, ANKH, PICALM, MTARC1, GPAT4, DNAJB13, KCNK1, MGST1, USP3, GHR, and THRB genes. These genes were frequently present in the genomic regions containing the most significant variants (-log10(P) ≥ 20), the region that explained the highest percentage of genetic variance for the six CH4 traits, or those that were shared by multiple CH4 traits. For instance, genomic region 28 on BTA14 at ~ 64 Mbp, which was shared by all CH4 traits, included the candidate gene VPS13B; genomic region 26 on BTA14 at ~ 0.5 Mbp, which was associated with five CH4 traits, included the candidate gene DGAT1; and genomic region 2 on BTA1 at ~ 142 Mbp, which was associated with four CH4 traits, included the candidate gene SLC37A1. Additionally, TOP10 variants from the VPS13B gene were missense mutations classified as deleterious by the SIFT score for the traits MeI, MeY, MeP_RC, and MeP_direct.
Independent studies using CH4 emissions collected with Sniffers corroborate two of the genes identified in our genomic regions. Manzanilla-Pech et al. [11] identified a SNP significantly associated with MeP within our genomic region 42 (BTA 19 ~ 50 Mbp) for which FASN is the most probable candidate gene, and both Pszczola et al. [12] and Calderón-Chagoya et al. [13] identified SNPs associated with MeP less than 1 Mbp before the region. Calderón-Chagoya et al. [13] also identified a SNP within the genomic region 28 (BTA 14 ~ 64 Mbp) for which the candidate gene is VPS13B. Additionally, using CH4 emissions predicted from milk FA or milk MIR spectra, van Engelen [35] found MeP and MeY to be associated with the DGAT1 gene.
Of these genes, FASN and DGAT1 could be potential functional candidate genes because they are involved in biological relationships underlying the prediction of CH4 from milk MIR spectra. The volatile fatty acids (VFA) produced by the rumen bacteria during fermentation are correlated with hydrogen production, and thus CH4 production. VFAs are absorbed by the ruminal epithelium and transferred to the mammary gland, where acetate and butyrate are used as substrates for the de novo synthesis of short-chain fatty acids (SCFA) in milk [42, 43]. The FASN gene encodes the fatty acid synthase protein, which is involved in the production of SCFAs in the mammary gland from acetyl-CoA and butyryl-CoA derived from acetate and butyrate [44]. The DGAT1 gene encodes the diacylglycerol O-acyltransferase 1 protein which produces triacylglycerol from glycerol and SCFAs [44, 45]. Additionally, here we identified an interesting QTL on BTA13, associated with MeP_FA, in which the candidate gene was ACSS2, whose protein is involved in the production of acetyl-CoA from acetate in the mammary gland [44].
Candidate genes for CH4 emissions related to rumen microbiota
The rumen microbiota is known to differ between low and high CH4-emitters [46, 47] and to be influenced by host genetics [46, 48, 49]. Although one study found that the effect of host genetics on CH4 emissions was almost independent of the effect of host genetics on the microbiota [50], others have proposed developing genetic selection on rumen microbiota to reduce CH4 emissions [51–53]. Multiple studies have been conducted on the relationships between a host’s genes and its microbiota, including GWASs on beef cattle [48, 54], dairy cattle [46, 55], and sheep [56], but there has been little overlap in the QTLs identified in different studies. We looked for overlapping genomic regions between our GWAS analyses of CH4 predicted from milk MIR spectra and the GWAS analyses in the literature to identify possible candidate genes based on biological interpretation. In particular, some of the loci highlighted by Wang et al. [56] are located in the genomic regions identified in our study, which include the candidate genes EPS8, PPP3CA, CCSER1, KCNIP4, NCOA6, ABTB2, ITFG1, PTK7, and KCNK1.
KCNIP4 may be of particular interest as it was a candidate gene in a highly significant QTL for MeP_RC. Moreover, the corresponding genomic region was also found in another study to be associated with members of the bacterial genera Prevotella, Ruminococcus, and Eubacterium [56]. These bacteria can influence VFA and hydrogen production in the rumen, in the case of Prevotella by producing propionate––an alternative to hydrogen production [57]––when digesting starch [58, 59], or, in the case of Ruminococcus and Eubacterium, by producing acetate and butyrate—and thus hydrogen—when digesting cellulose [58, 59]. Through their influence on VFA and hydrogen production, these bacteria may be able to influence CH4 production. Interestingly, they have been found to be overrepresented in the rumen of either high-emitting animals (Ruminococcus and Eubacterium [51, 60, 61]) or low-emitting animals (Prevotella [47, 60, 61]). Other candidate genes in our study were also identified in regions associated with these bacterial genera, such as the gene CCSER1 (Prevotella) and the genes EPS8, PPP3CA, NCOA6, and ABTB2 (Eubacterium) [56].
Quality of the data
In this study, we conducted GWAS using WGS data from a population of over 40,000 cows, which provided high-resolution detection power for detecting QTLs. It is particularly noteworthy that most TOP10 variants we identified were imputed ones (92.0%), which highlights the potential of imputed WGS data for identifying new candidate variants for causal mutations. Additionally, relatively few of these TOP10 variants were annotated as intergenic variants (from 0 to 18.4%), meaning that we mostly identified either the genes or their known regulatory regions. Our results confirm the utility of using WGS data for QTL detection, although in this case our efforts were also supported by the precision of the imputation (average R2 of 0.84 for TOP10 variants), which was permitted by the large reference population of 1414 Holstein bulls in Run9 of the 1000 Bull Genomes Project [29].
Limitations
The phenotypes used in this study are CH4 emissions predicted from milk MIR spectra, which reflect milk composition. The first consequence is that their genetic parameters may be biased compared with those of directly measured traits. Although the heritability values estimated in this study for predicted MeI and MeY are consistent with those estimated from direct measurements (0.30 and 0.33 for MeI [8, 62], 0.38 for MeY [8]), our heritability estimates for predicted MeP (0.23–0.42) are generally higher than the ones reported in the literature (0.12–0.36 [8, 11, 62–65]). This can be partly explained by the inherent heritability of milk MIR spectra. Several studies have estimated the heritability of different wavelengths in the regions of milk MIR spectra used for prediction [66–68], reaching 0.42, 0.27, and 0.30, respectively. Therefore, the traits predicted from milk MIR spectra are heritable because they are a linear combination of heritable wavelength absorbances.
The second consequence is that it was not surprising to find QTLs associated with genes that are already known to be associated with either milk composition or milk MIR spectra. This makes it difficult to verify if these QTLs are truly associated with CH4 production or only with milk production and composition. There can be confounding effects due to the genetic correlations between the predicted CH4 traits and the milk traits. We estimated genetic correlations between the six predicted CH₄ traits and milk traits using bivariate analyses with Eq. (1), that suggest moderate confounding effects with milk production and composition. Except for MeP_indirect, genetic correlations with milk yield ranged from − 0.25 to 0.23, with protein yield from − 0.18 to 0.04, and with fat yield from − 0.39 to 0.42. MeP_indirect showed stronger genetic correlations with these traits, of 0.84, 0.76, and 0.47, respectively, but presented only 2 QTLs, which contain the DGAT1 and the VPS13B genes. The genetic correlations with the fat yield raises concerns about the causality of the association between the predicted CH4 traits and the identified genes. Further research is required to validate or invalidate these potential candidate genes. However, this would necessitate large populations with direct CH4 measurements to prevent the confounding effects of milk production and composition traits that are inherent to prediction from milk MIR spectra.
Conclusions
By studying CH4 emissions predicted from milk MIR spectra, we were able to obtain a large amount of data, which, when combined with imputed WGS data in GWAS analyses, gave us a high detection power and a high resolution of the results. For the six CH4 traits examined here, we were able to identify a total of more than 100 QTLs; of these, several were shared among multiple traits, thus highlighting their common genetic basis. We identified three potential candidate genes that are involved in the production of milk FAs from VFAs (FASN on BTA19, DGAT1 on BTA14, and ACSS2 on BTA13) along with one gene that is associated with bacterial genera (Prevotella, Ruminococcus, and Eubacterium) with known effects on VFA and hydrogen production (KCNIP4 on BTA6). Further research is necessary to determine if these genes are associated with predicted CH4 emissions or if the association is inherent to the nature of the prediction from milk MIR spectra.
Acknowledgements
The authors thank the French milk recording compagnies for providing milk MIR spectra, and Jean-Pierre Allard and Guillaume Courtois (ESTEL, France) for calculating the CH4 predictions. SF acknowledges Sébastien Taussat (Eliance, France) and Louise Brulin (GD Biotech—Gènes Diffusion, France) for methodological support and help in interpreting the results.
Author contributions
PM, DB, and SeF conceived the study. SF performed the statistical analyses and drafted the manuscript. SF, MPS, DB, SeF, and PM participated in interpreting and discussing the results. MPS, DB, SeF, and PM revised and proofread the manuscript. All authors read and approved the final manuscript.
Funding
Data were collected as part of the METHABREED project funded by APIS-GENE (Paris, France). Solène Fresco is the recipient of a CIFRE PhD grant from Eliance, with the financial support of the Association Nationale de la Recherche et de la Technologie (grant number CIFRE n°2021/1241) and APIS-GENE (grant number AP-2021-045).
Availability of data and materials
Raw genotype and phenotype data belong to French farmers and have commercial value. Restrictions apply to their availability, and they are not publicly available. The authors can be contacted for a reasonable request.
Declarations
Ethics approval and consent to participate
This study was based on data routinely collected on dairy farms during milk recording. We did not perform any experiment on animals; therefore, no ethical approval was required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gerber PJ, Steinfield H, Henderson B, Mottet A, Opio C, Dijkman J, et al. Tackling climate change through livestock: a global assessment of emissions and mitigation opportunities. Rome: Food and Agriculture Organization of the United Nations (FAO); 2013. [Google Scholar]
- 2.Nabuurs G-J, Mrabet R, Abu Hatab A, Bustamante M, Clark H, Havlik P, et al. Agriculture, Forestry and Other Land Uses (AFOLU). In: Shukla PR, Skea J, Slade R, Al Khourdajie A, van Diemen R, McCollum D (eds). IPCC, 2022: Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge and New York: Cambridge University Press; 2022, p. 747–860.
- 3.Knapp JR, Laur GL, Vadas PA, Weiss WP, Tricarico JM. Invited review: Enteric methane in dairy cattle production: quantifying the opportunities and impact of reducing emissions. J Dairy Sci. 2014;97:3231–61. [DOI] [PubMed] [Google Scholar]
- 4.Beauchemin KA, Ungerfeld EM, Abdalla AL, Alvarez C, Arndt C, Becquet P, et al. Invited review: current enteric methane mitigation options. J Dairy Sci. 2022;105:9297–326. [DOI] [PubMed] [Google Scholar]
- 5.Kandel PB, Vanrobays M-L, Vanlierde A, Dehareng F, Froidmont E, Gengler N, et al. Genetic parameters of mid-infrared methane predictions and their relationships with milk production traits in Holstein cattle. J Dairy Sci. 2017;100:5578–91. [DOI] [PubMed] [Google Scholar]
- 6.Pszczola M, Rzewuska K, Mucha S, Strabel T. Heritability of methane emissions from dairy cows over a lactation measured on commercial farms. J Anim Sci. 2017;95:4813–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lassen J, Difford GF. Review: genetic and genomic selection as a methane mitigation strategy in dairy cattle. Animal. 2020;14:s473–83. [DOI] [PubMed] [Google Scholar]
- 8.Manzanilla-Pech CIV, Løvendahl P, Mansan Gordo D, Difford GF, Pryce JE, Schenkel F, et al. Breeding for reduced methane emission and feed-efficient Holstein cows: an international response. J Dairy Sci. 2021;104:8983–9001. [DOI] [PubMed] [Google Scholar]
- 9.Fresco S, Boichard D, Fritz S, Martin P. Genetic parameters for methane production, intensity, and yield predicted from milk mid-infrared spectra throughout lactation in Holstein dairy cows. J Dairy Sci. 2024;107:11311–23. [DOI] [PubMed] [Google Scholar]
- 10.Manzanilla-Pech CIV, De Haas Y, Hayes BJ, Veerkamp RF, Khansefid M, Donoghue KA, et al. Genomewide association study of methane emissions in Angus beef cattle with validation in dairy cattle. J Anim Sci. 2016;94:4151–66. [DOI] [PubMed] [Google Scholar]
- 11.Manzanilla-Pech CIV, Difford GF, Sahana G, Romé H, Løvendahl P, Lassen J. Genome-wide association study for methane emission traits in Danish Holstein cattle. J Dairy Sci. 2022;105:1357–68. [DOI] [PubMed] [Google Scholar]
- 12.Pszczola M, Strabel T, Mucha S, Sell-Kubiak E. Genome-wide association identifies methane production level relation to genetic control of digestive tract development in dairy cows. Sci Rep. 2018;8:15164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calderón-Chagoya R, Hernandez-Medrano JH, Ruiz-López FJ, Garcia-Ruiz A, Vega-Murillo VE, Montano-Bermudez M, et al. Genome-wide association studies for methane production in dairy cattle. Genes. 2019;10:995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarghale AJ, Shahrebabak MM, Shahrebabak HM, Javaremi AN, Saatchi M, Khansefid M, et al. Genome-wide association studies for methane emission and ruminal volatile fatty acids using Holstein cattle sequence data. BMC Genet. 2020;21:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Haas Y, Windig JJ, Calus MPL, Dijkstra J, de Haan M, Bannink A, et al. Genetic parameters for predicted methane production and potential for reducing enteric emissions through genomic selection. J Dairy Sci. 2011;94:6122–34. [DOI] [PubMed] [Google Scholar]
- 16.Uemoto Y, Takeda M, Ogino A, Kurogi K, Ogawa S, Satoh M, et al. Genetic and genomic analyses for predicted methane-related traits in Japanese Black steers. Anim Sci J. 2020;91: e13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lakamp AD, Ahlberg CM, Allwardt K, Broocks A, Bruno K, McPhillips L, et al. Variance component estimation and genome-wide association of predicted methane production in crossbred beef steers. J Anim Sci. 2023. 10.1093/jas/skad179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chilliard Y, Martin C, Rouel J, Doreau M. Milk fatty acids in dairy cows fed whole crude linseed, extruded linseed, or linseed oil, and their relationship with methane output. J Dairy Sci. 2009;92:5199–211. [DOI] [PubMed] [Google Scholar]
- 19.Vanlierde A, Dehareng F, Gengler N, Froidmont E, McParland S, Kreuzer M, et al. Improving robustness and accuracy of predicted daily methane emissions of dairy cows using milk mid-infrared spectra. J Sci Food Agric. 2021;101:3394–403. [DOI] [PubMed] [Google Scholar]
- 20.Fresco S, Vanlierde A, Boichard D, Lefebvre R, Gaborit M, Bore R, et al. Combining short-term breath measurements to develop methane prediction equations from cow milk mid-infrared spectra. Animal. 2024;18:101200. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira HR, Sweett H, Narayana S, Fleming A, Shadpour S, Malchiodi F, et al. Symposium review: development of genomic evaluation for methane efficiency in Canadian Holsteins. JDS Commun. 2024;5:756–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grelet C, Pierna JAF, Dardenne P, Soyeurt H, Vanlierde A, Colinet F, et al. Standardization of milk mid-infrared spectrometers for the transfer and use of multiple models. J Dairy Sci. 2017;100:7910–21. [DOI] [PubMed] [Google Scholar]
- 23.Ducrocq V. Genekit, BLUP software 1998. version October 30, 2017. INRA SGQA, Jouy-en-Josas, France.
- 24.Meyer K. WOMBAT—A tool for mixed model analyses in quantitative genetics by restricted maximum likelihood (REML). J Zhejiang Univ Sci B. 2007;8:815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tribout T, Croiseau P, Lefebvre R, Barbat A, Boussaha M, Fritz S, et al. Confirmed effects of candidate variants for milk production, udder health, and udder morphology in dairy cattle. Genet Sel Evol. 2020;52:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez M-P, Rocha D, Charles M, Boussaha M, Hozé C, Brochard M, et al. Sequence-based GWAS and post-GWAS analyses reveal a key role of SLC37A1, ANKH, and regulatory regions on bovine milk mineral content. Sci Rep. 2021;11:7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sargolzaei M, Chesnais JP, Schenkel FS. A new approach for efficient genotype imputation using information from relatives. BMC Genom. 2014;15:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet. 2012;44:955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daetwyler HD, Capitan A, Pausch H, Stothard P, van Binsbergen R, Brøndum RF, et al. Whole-genome sequencing of 234 bulls facilitates mapping of monogenic and complex traits in cattle. Nat Genet. 2014;46:858–65. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez M-P, Escouflaire C, Baur A, Bottin F, Hozé C, Boussaha M, et al. X-linked genes influence various complex traits in dairy cattle. BMC Genom. 2023;24:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrison PW, Amode MR, Austine-Orimoloye O, Azov AG, Barba M, Barnes I, et al. Ensembl 2024. Nucleic Acids Res. 2024;52:D891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GRS, Thormann A, et al. The ensembl variant effect predictor. Genome Biol. 2016;17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Engelen S. The genetic background of methane emission by dairy cows. Wageningen: Wageningen University; 2018. [Google Scholar]
- 36.Hristov AN, Kebreab E, Niu M, Oh J, Bannink A, Bayat AR, et al. Symposium review: uncertainties in enteric methane inventories, measurement techniques, and prediction models. J Dairy Sci. 2018;101:6655–74. [DOI] [PubMed] [Google Scholar]
- 37.Garnsworthy PC, Difford GF, Bell MJ, Bayat AR, Huhtanen P, Kuhla B, et al. Comparison of methods to measure methane for use in genetic evaluation of dairy cattle. Animals. 2019;9:837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez M-P, Govignon-Gion A, Croiseau P, Fritz S, Hozé C, Miranda G, et al. Within-breed and multi-breed GWAS on imputed whole-genome sequence variants reveal candidate mutations affecting milk protein composition in dairy cattle. Genet Sel Evol. 2017;49:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Littlejohn MD, Tiplady K, Fink TA, Lehnert K, Lopdell T, Johnson T, et al. Sequence-based association analysis reveals an MGST1 eQTL with pleiotropic effects on bovine milk composition. Sci Rep. 2016;6:25376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez MP, Wolf V, El Jabri M, Beuvier E, Rolet-Répécaud O, Gaüzère Y, et al. Short communication: confirmation of candidate causative variants on milk composition and cheesemaking properties in Montbéliarde cows. J Dairy Sci. 2018;101:10076–81. [DOI] [PubMed] [Google Scholar]
- 41.Tiplady KM, Lopdell TJ, Reynolds E, Sherlock RG, Keehan M, Johnson TJJ, et al. Sequence-based genome-wide association study of individual milk mid-infrared wavenumbers in mixed-breed dairy cattle. Genet Sel Evol. 2021;53:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindmark MH. Fatty acids in bovine milk fat. Food Nutr Res. 2008;52:1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harvatine KJ, Boisclair YR, Bauman DE. Recent advances in the regulation of milk fat synthesis. Animal. 2009;3:40–54. [DOI] [PubMed] [Google Scholar]
- 44.Bionaz M, Loor JJ. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genom. 2008;9:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jensen RG. The composition of bovine milk lipids: January 1995 to December 2000. J Dairy Sci. 2002;85:295–350. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Q, Difford G, Sahana G, Løvendahl P, Lassen J, Lund MS, et al. Bayesian modeling reveals host genetics associated with rumen microbiota jointly influence methane emission in dairy cows. ISME J. 2020;14:2019–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shinkai T, Takizawa S, Fujimori M, Mitsumori M. Invited Review—The role of rumen microbiota in enteric methane mitigation for sustainable ruminant production. Anim Biosci. 2024;37:360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li F, Li C, Chen Y, Liu J, Zhang C, Irving B, et al. Host genetics influence the rumen microbiota and heritable rumen microbial features associate with feed efficiency in cattle. Microbiome. 2019;7:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abbas W, Howard JT, Paz HA, Hales KE, Wells JE, Kuehn LA, et al. Influence of host genetics in shaping the rumen bacterial community in beef cattle. Sci Rep. 2020;10:15101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Difford GF, Plichta DR, Løvendahl P, Lassen J, Noel SJ, Højberg O, et al. Host genetics and the rumen microbiome jointly associate with methane emissions in dairy cows. PLOS Genet. 2018;14: e1007580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallace RJ, Sasson G, Garnsworthy PC, Tapio I, Gregson E, Bani P, et al. A heritable subset of the core rumen microbiome dictates dairy cow productivity and emissions. Sci Adv. 2019;5:eaav8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martínez-Álvaro M, Mattock J, Auffret M, Weng Z, Duthie C-A, Dewhurst RJ, et al. Microbiome-driven breeding strategy potentially improves beef fatty acid profile benefiting human health and reduces methane emissions. Microbiome. 2022;10:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.González-Recio O, Martínez-Álvaro M, Tiezzi F, Saborío-Montero A, Maltecca C, Roehe R. Invited review: Novel methods and perspectives for modulating the rumen microbiome through selective breeding as a means to improve complex traits: implications for methane emissions in cattle. Livest Sci. 2023;269:105171. [Google Scholar]
- 54.Fan P, Nelson CD, Driver JD, Elzo MA, Peñagaricano F, Jeong KC. Host genetics exerts lifelong effects upon hindgut microbiota and its association with bovine growth and immunity. ISME J. 2021;15:2306–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalez-Recio O, Scrobota N, López-Paredes J, Saborío-Montero A, Fernández A, López de Maturana E, et al. Review: diving into the cow hologenome to reduce methane emissions and increase sustainability. Animal. 2023;17:100780. [DOI] [PubMed] [Google Scholar]
- 56.Wang W, Zhang Y, Zhang X, Li C, Yuan L, Zhang D, et al. Heritability and recursive influence of host genetics on the rumen microbiota drive body weight variance in male Hu sheep lambs. Microbiome. 2023;11:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Janssen PH. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim Feed Sci Technol. 2010;160:1–22. [Google Scholar]
- 58.Hobson PN, Stewart CS, editors. The rumen microbial ecosystem. Dordrecht: Springer, Netherlands; 1997. [Google Scholar]
- 59.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–31. [DOI] [PubMed] [Google Scholar]
- 60.Saborío-Montero A, Gutiérrez-Rivas M, García-Rodríguez A, Atxaerandio R, Goiri I, López de Maturana E, et al. Structural equation models to disentangle the biological relationship between microbiota and complex traits: methane production in dairy cattle as a case of study. J Anim Breed Genet. 2020;137:36–48. [DOI] [PubMed] [Google Scholar]
- 61.Smith PE, Kelly AK, Kenny DA, Waters SM. Differences in the composition of the rumen microbiota of finishing beef cattle divergently Ranked for residual methane emissions. Front Microbiol. 2022;13:855565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richardson CM, Nguyen TTT, Abdelsayed M, Moate PJ, Williams SRO, Chud TCS, et al. Genetic parameters for methane emission traits in Australian dairy cows. J Dairy Sci. 2021;104:539–49. [DOI] [PubMed] [Google Scholar]
- 63.Lassen J, Løvendahl P. Heritability estimates for enteric methane emissions from Holstein cattle measured using noninvasive methods. J Dairy Sci. 2016;99:1959–67. [DOI] [PubMed] [Google Scholar]
- 64.van Breukelen AE, Aldridge MN, Veerkamp RF, Koning L, Sebek LB, de Haas Y. Heritability and genetic correlations between enteric methane production and concentration recorded by GreenFeed and sniffers on dairy cows. J Dairy Sci. 2023;106:4121–32. [DOI] [PubMed] [Google Scholar]
- 65.Lopes LSF, Schenkel FS, Houlahan K, Rochus CM, Oliveira GA, Oliveira HR, et al. Estimates of genetic parameters for rumination time, feed efficiency, and methane production traits in first lactation Holstein cows. J Dairy Sci. 2024;107:4704–13. [DOI] [PubMed] [Google Scholar]
- 66.Soyeurt H, Misztal I, Gengler N. Genetic variability of milk components based on mid-infrared spectral data. J Dairy Sci. 2010;93:1722–8. [DOI] [PubMed] [Google Scholar]
- 67.Bittante G, Cecchinato A. Genetic analysis of the Fourier-transform infrared spectra of bovine milk with emphasis on individual wavelengths related to specific chemical bonds. J Dairy Sci. 2013;96:5991–6006. [DOI] [PubMed] [Google Scholar]
- 68.Zaalberg RM, Shetty N, Janss L, Buitenhuis AJ. Genetic analysis of Fourier transform infrared milk spectra in Danish Holstein and Danish Jersey. J Dairy Sci. 2019;102:503–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw genotype and phenotype data belong to French farmers and have commercial value. Restrictions apply to their availability, and they are not publicly available. The authors can be contacted for a reasonable request.