Abstract
Many studies have recognized that a woman’s reproductive history influences the survival status of her fetus and the newborn. In the esteemed literature of demography, abundant evidence acknowledges the linkage between maternal exposure to offspring and their associated adult outcomes and the other way around. This study examines the link between maternal risk factors at birth and long-term outcomes for daughters in India. Using national health survey data, it focuses on three maternal risks: young age, high parity, and short birth intervals. Applying regression analysis to cohort data, the study finds these early-life disadvantages are associated with daughters experiencing stunted growth, undernutrition, child mortality, and low birth weight, as well as limited education and employment. Conversely, daughters of educated mothers have better outcomes, highlighting the importance of maternal education. The pseudo-cohort approach provides valuable longitudinal insights from cross-sectional surveys. The study underscores the need for policies promoting healthy reproductive practices and education access to improve long-term outcomes for women in India.
Keywords: Maternal risk, Pseudo cohort, Beta regression, Child development
Introduction
The demographic conditions into which a child is born play a significant role in shaping the sequence of events throughout her life [11], [38]. Studies have established that disadvantages in one’s early life cycle significantly influence the future trajectories of life course events [10], [34]. The disadvantages are not only transferred in one’s life from one event to another but also from one generation to another generation as well. Such vulnerabilities are cyclical, intergenerationally transferring from generation to generation [17, 26]. The intergenerational penetration of vulnerabilities mostly occurs at critical demographic events such as the birth of a child. Evidently, it has been argued that maternal demographic and reproductive health history determine the health and well-being of the newborn [14],[45], [39]. It is also suggested that the maternal risk factors associated with a child at the time of birth impact her biological, demographic, and cognitive development. Illustratively, a child born to either a younger woman (< 18 years) or an older one (> 35 years) or born at four or higher parity (4 +) or shortly after the previous birth (birth interval < 18 months) are considered as ‘Maternal risk factors’ or ‘risky birth’ in demographic literature [41]. A multitude of studies has advocated that births in these settings are likely to experience severe demographic and health problems. These include nutritional deficiency, recurrent morbidities, and depreciated cognitive development, including infant and child mortality in many cases [38], [5] [15, 24]. In demographic literature, a female born in risky conditions is termed “female with maternal risk factors” [23]. It is noteworthy to mention that the association between maternal risk factors, the survival of the newborn, and the growth of offspring are quite complicated. The empirical derivatives linking a mother’s and child’s health reveal that maternal and infant health are inseparable. In this regard, the investment in reproductive and child health care has an important place in subscribing to the right to health under the Millennium Development Goals (MDGs) and Sustainable Development Goals (SDGs) [42] [40]).
Underlined as a strong maternal risk factor, pregnancy in the teenage period places adverse pressure on both the mother and her fetus due to women’s physiological and structural factors [45] [44]. Teenage mothers are more susceptible to health risks such as anaemia and nutritional deficiencies, which in turn elevate the risk of associated neonatal, perinatal, and maternal mortality [38], [9]. Among teenage mothers, besides demographic adversities, risky birth behaviour may bring complications in deliveries, such as pre-term labour, premature births, and low birth weight. Teenage mothers remain exposed to the risk of anaemia and postpartum haemorrhage [27, 32]. Besides young women, older women are also associated with pregnancy complications such as lower conception chances, congenital problems, and miscarriages. Studies have shown that children born to mothers under 19 years old are more likely to have low birth weight, be born early, and be stunted at 2 years old, even when other factors are considered [50]. Similar findings in Nigeria and Kenya indicate that adolescent mothers face higher risks of anemia, lack of prenatal care, and having low birth weight babies compared to adult women [33]. On average, babies born to adolescent mothers exhibited lower birth weights, shorter lengths, and reduced mid-upper arm circumference [13, 51].
Frequent pregnancy cycles are strongly associated with higher neonatal and infant mortality, forming a vicious circle of nutritional deficiencies leading to mother depletion syndrome [45], [46] [49]). An insufficient nutritious supply will cause a situation of biological competition in which the welfare of both the mother and the fetus is at risk, which significantly contributes to impaired pregnancy and offspring outcomes [5, 37]. Healthy timing and spacing between births are globally acknowledged as essential family planning instruments to improve maternal and child health [23]. The inverted U-shaped relation between birth spacing and newborn survival has been known for decades. A Meta-analysis performed by Rutstein [35] demonstrated the effect of birth spacing on pregnancy outcomes. The author proposed that both long (longer than 59 months) and short (shorter than 18 months) birth intervals were seen as contributing to adverse pregnancy outcomes. It also leads to a higher incidence of morbidity in the gestational period. These findings also complemented studies performed in developing and developed countries, which argue that these adverse results persist even in mothers from educated, affluent, and resourceful settings [29], [36]. Sibling hypotheses can explain the relationship between short birth intervals and child development. Some researchers have suggested that closely spaced children compete with limited household resources and maternal attention. Such children are prone to cross-infection [4]. Additionally, births at a higher parity disinvest various social and health advantages, specifically when the birth is female. Not surprisingly, girls who are adequately nurtured in their teen years are more likely to have more physically balanced lives [6]. Maternal factors are not the sole determinants of a child’s development,a mother’s socioeconomic conditions also play a crucial role. Anyamele et al. [3] found a significant association between a mother’s education, living conditions such as her residential area and wealth status, and infant and child mortality.
The reproductive profile of a mother’s risky birth behaviour in demographic research is full of observational studies such as cross-sectional, cohort, or case–control studies. Granular and reliable data on demographic markers such as maternal factors at birth, perinatal conditions, and physical and mental growth of the child are rarely available in developing countries [1, 15]. Due to the inadequate vital registration system and imperfect routine information, national surveys have become a significant source of population-based evidence in developing countries. This study builds on previous research by [34], which links early maternal risk factors with a daughter’s physical and cognitive development and reproductive health outcomes. As future child-bearers, daughters play a critical role in shaping the health and well-being of subsequent generations. A supportive prenatal environment significantly impacts their growth, development, and future reproductive health. This research seeks to provide a nuanced and comprehensive analysis of the unique experiences of daughters in order to better understand the specific gendered dynamics at play. In the absence of nationally representative panel data, this paper applies a pseudo-cohort approach to examine how early maternal risks at birth affect a daughter’s adulthood outcomes. By simulating a longitudinal analysis using cross-sectional data, this study examines the influence of maternal risk factors on the reproductive and socioeconomic outcomes of the next generation in low-income settings such as India [30]. This study aims to inform policy interventions that promote long-term health and socioeconomic stability by identifying key maternal risk factors that affect daughters.
Data and methodology
Data source
Due to a lack of nationally representative panel data in many developing countries, researchers heavily rely on cross-sectional surveys conducted through the Demographic and Health Survey (DHS). The Indian DHS, named as National Family Health Survey (NFHS), conducted by IIPS, Mumbai, with the funding of ORC Macro and the Bill and Melinda Gates Foundation, is the only authentic source that endows a wide range of information on birth histories, maternal and child health conditions at the national and sub-national levels. The uniform sampling across the survey rounds provides immense help in the construction of cohorts. To date, five rounds of NFHS have been completed, starting from 1992 to 2020. This gathered information from women and men of reproductive age groups 15–49 years and 15–54 years, respectively. The survey provides estimates of fertility, mortality, maternal and child health care services, and reproductive health facilities at the national and sub-national levels. A detailed description of the study design, sampling procedure, frame, and non-response rate is published in the round of specific reports (IIPS and ICF, 1995, [18–22]. The present study draws data from all five rounds of the survey, utilizing information on women classified within the maternal risk and outcome categories. This data offers fresh insights into the impact of early maternal risk exposures on daughters’ well-being, supporting the application of a panel data model for deeper analysis.
Construction of cohort
Deaton [7] first introduced the concept of panel techniques on repeated cross-sectional data for life cycle analysis. After that, pseudo-panel data became a popular statistical technique for making a panel without longitudinal data. This methodology became widely acceptable to various branches of study, including behavioural economics and demography [2, 8, 25, 34]. Unlike other dimensions, intergenerational studies need data for longer durations, and multi-round cross-sectional surveys fulfil this requirement more often than panel data. This benefit makes repeated surveys more efficacious than panel observations. Talking about the Indian setting, the frequency of exploitation of multiple rounds of data for panel insights remained low. Unlike panel data, the principle of a pseudo-panel is to follow a group of individuals called a cohort rather than individuals over time [16]. Researchers have propounded a set of rules for the construction of pseudo cohorts with its limitations [16, 31, 43]. The present study attempts to utilize this methodology of cohort construction in Indian settings to capture the intergenerational linkages between the two life courses in two different settings.
The first criterion for constructing a cohort is stability, which means each observable characteristic should not vary with reference to time. This criterion becomes a basis for the partition of the population, such that each study unit falls into precisely one cohort. Now, investigating the second condition for cohort studies, one must address the measurement and sampling error inherent in data; this error can be reduced by including cohorts of large sizes in the study. The empirical study done by [43] proposes that sample size in a cohort of around 100 or more is enough to reduce a significant amount of bias and imprecision of the estimators. However, forming large cohorts may cause variability loss, which reduces the precision of the estimators. So, the third criterion is to increase the accuracy of measures without losing the variability present in data.
In the present work, the “year of daughter’s birth” is selected as the key base for making cohorts as it fulfils many of the above requirements. Obviously, it is a perfect example of a stable selection criterion, and it is readily available in the survey data. Furthermore, large samples of females in all four rounds of NFHS enabled us to divide the base from year of birth and to month of birth due to an adequate number of females in each cohort. Following the [43] recommendations, we restricted the size to a minimum of 100 females. For making the cohort, we grouped women born in the same month of a year and followed them in subsequent surveys; for example, women born in January 1980 were clubbed together to make a generation, which was then followed across time in the following surveys. The study utilized birth histories recorded in the NFHS (named as a base survey) for computing the maternal risk factors present at a daughter’s birth and then linked them to the next survey (named outcome survey) for measuring the development of those children as adults.
Cohort measures
Unlike traditional panel data, the principle of the pseudo panel follows a generation or a group of women with the same birth year rather than following an individual over a period of time. Thus, the cohort becomes the actual unit of analysis instead of the female. In this process, the cohort level proportions, or averages, are computed for each study variable, and values thus obtained are replaced by their corresponding means against each birth cohort. The measurement of early birth exposures with reference to a daughter’s birth is aggregated and linked with their counterparts from subsequent survey rounds. In other words, measured nutritional status (height and BMI), socioeconomic development (education and work status), and reproductive health of females are aggregated by the month of birth for a given year. The estimated cohort level means, or proportions are then aligned with the maternal risk factors for the cohort of daughters born in the same month (year).
After applying the minimum threshold of 100 females per cohort, the analysis utilized 718 cohorts drawn from all five rounds of the National Family Health Survey. Given that reproductive outcomes such as child mortality and low birth weight are experienced by women who have ever given birth, the cohort-level measures for these outcomes were aggregated at the maternal level. Similarly, for the analysis of low birth weight, the survey only recorded the mother’s self-reported birth weights for children born in the five years preceding the survey, further restricting the cohort sample to only those mothers who had given birth within that timeframe. By adhering to these sample restrictions, the final analytical sample consisted of 588 cohorts of mothers who had ever given birth and 569 cohorts of daughters with available birth size information.
Methodology
Figure 1 presents the study’s analytic framework, where early exposure conditions at birth, combined with maternal background characteristics, are hypothesized to impact adult reproductive and socioeconomic outcomes, analyzed using cohorts as the units of analysis.
Fig. 1.
Schematic presentation of the study framework
We reviewed the literature to identify disadvantages mothers face at a daughter’s birth, considering them as risk factors for the child’s growth [23],[34]. Three key adversities were identified: young maternal age (daughters born to mothers under 18), short birth interval (daughters born within 18 months of a previous sibling), and higher parity (daughters born at or after a mother’s fourth birth). Adult development outcomes were measured for female respondents aged 15–49 years, focusing on physical growth through short stature (< 145 cm) and underweight (BMI < 18.5 kg/m2), reflecting poor nutritional status and potential negative impacts on childbearing [47]. Reproductive outcomes of interest included experiences of child mortality under five and self-reported births of “very small” or “small” infants (weight < 2500 g). Lastly, we assessed socioeconomic well-being through educational status (average schooling years) and current employment status, indicators that indirectly reflect cognitive development and productive work capacity, based on available socioeconomic data from the DHS.
Besides these outcome variables, the analysis in the study is controlled by socioeconomic variables such as maternal education defined by the proportion of daughters born to mothers with any schooling, dwelling setting, having resided in urban areas, religion, and caste of mother coded as muslim vs. others and schedule caste & scheduled tribe (SC/ST)1 vs. other and economic status of the family (poor vs. non-poor). For analysis, the national level data was further divided into two groups: Empowered action group (EAG)2 (consisting of 9 states: Bihar, Chhattisgarh, Jharkhand, Madhya Pradesh, Orissa, Rajasthan, Uttaranchal, and Uttar Pradesh) and Non-EAG states. The birth year of a daughter and her age at the outcome survey is also used in the analysis as control variables. A daughter’s birth year and age of daughter measured in outcome surveys, are treated as control variables in the analysis.
Out of all six dependent variables, the education of the daughter is taken as the mean year of schooling and means for each cohort have been estimated; the remaining five outcomes are cohort proportions. So, the multivariate linear regression model is applied to check the association between explanatory variables and the mean years of schooling. However, a linear model is not suitable for cohort proportions due to its bounded nature (0, 1). This assumption, however, is not always valid, but in the current scenario, outcome proportions of all cohorts are bounded within the interval (0, 1). This study applies a beta regression model to assess the relationship between the independent variables and the outcome variable presented in cohort proportions [12]. The functional form of the beta model is given below:
| 1 |
Here, Yc is the proportion of an outcome in cohort C, xi and βi (i = 1, 2, 3... n) are the covariates and coefficient to be estimated from the model.
Let = µc, where µc is the mean of dependent variable Y conditional on covariates X for a cohort C. Equation 1 is rewritten as,
| 2 |
where µc is computed by the next equation.
| 3 |
The model uses the quasi-maximum likelihood estimation method to estimate consistent, efficient, and asymptomatically normal statistics. To facilitate the interpretation of models, we conducted a post-estimation simulation exercise to compute the risk-eliminated cohort proportions for each outcome. Furthermore, the analysis includes a univariate presentation of trends before and after eliminating risky maternal factors. All analyses were performed using Stata version 17.
Results
Descriptive measures
Before delving into the detailed analysis of models on how early risk factors affect the development of daughters, we must describe their behaviour according to the variables involved in the present study. Table 1 shows the summary statistics such as mean, standard deviation (SD), and range (represented as minimum and maximum values of variable proportions) of all covariates and outcome variables involved in the study. Converting the means to percentage terms, we found that, on average, 13 percent of daughters were born to younger mothers (age < 18 years). The average cohort percentage of daughters born within 18 months of their preceding siblings is 12 percent. Further, in the last risk factor, we found that out of 100 births, on average, 29 daughters were born on four or higher parity, which is the highest among all three risk factors.
Table 1.
Summary statistics of cohort proportions on maternal risk factors, adult reproductive, socioeconomic and development outcomes for India
| Explanatory variables | Mean | SD | Min | Max |
|---|---|---|---|---|
| Maternal risk factor present at the time of daughter’s birth | ||||
| Young mother | 0.127 | 0.038 | 0.044 | 0.307 |
| Short birth interval | 0.118 | 0.025 | 0.040 | 0.197 |
| Higher parity | 0.289 | 0.075 | 0.019 | 0.564 |
| Covariates present at daughter’s birth | ||||
| Mother’s education | 0.380 | 0.067 | 0.154 | 0.557 |
| Mother’s residence | 0.311 | 0.049 | 0.019 | 0.564 |
| Mother’s caste (SC/ST vs. others) | 0.231 | 0.034 | 0.011 | 0.631 |
| Mother’s religion (Muslim vs. others) | 0.101 | 0.024 | 0.007 | 0.324 |
| Mother’s wealth (poor vs. others) | 0.137 | 0.042 | 0.013 | 0.571 |
| Region (EAG vs Non EAG) | 0.156 | 0.032 | 0.017 | 0.432 |
| Year of daughter’s birth | 1982.29 | 8.94 | 1964 | 2005 |
| Age of adult daughters | 25.22 | 5.41 | 15.50 | 35.50 |
| Development measures | ||||
| Short stature | 0.125 | 0.018 | 0.058 | 0.170 |
| Underweight | 0.263 | 0.078 | 0.122 | 0.495 |
| Reproductive measures | ||||
| Experiencing child death | 0.138 | 0.052 | 0.048 | 0.300 |
| Low birth weight infant | 0.190 | 0.057 | 0.083 | 0.389 |
| Socioeconomic measures | ||||
| Educational status | 2.993 | 0.712 | 1.475 | 4.559 |
| Working status | 0.195 | 0.119 | 0.014 | 0.423 |
Moving towards the outcome variables of interest, we observed that adult females experience 14 percent of child deaths below the age of five by taking the average of all 588 cohort proportions. Around 30 percent of mothers reported losing a child under age five in a cohort, which is the highest child mortality recorded in the sample. Based on the proportion of mothers who gave birth five years prior to the survey, the average number of infants reported as small or very small is nearly 19 percent. A female’s nutritional status indicates that around 13 to 26 percent of adult daughters are undernourished with height less than 145 cm and BMI below 18.5 kg/m2, respectively. Furthermore, looking at the last two columns of the table, we noted that in these health outcomes, short stature/stunted daughters are limited from 6 to 17 percent and 12 to 50 percent in the samples. Measuring the socioeconomic variables, we found that the average years of a female’s schooling has emerged as three years, while around 20 percent of females are employed in the sample.
From the tabulation of covariates selected in the study, it is observed that almost 31 percent of daughters are born to mothers who live in urban areas of the country, while the average of cohort percentage of daughters born to mothers with any schooling is 38 percent. The findings highlighted that the average cohort percentage of daughters born in EAG states siblings is 16 percent, ranging from 11 to 43 percent. With regard to the religion and caste-related variation, we found that 57 percent of daughters were born to a Muslim woman, the highest birth recorded in the sample. The survey collects information only for women aged 15 to 49 years, so all the cohort proportions calculated from outcome surveys are based on daughters older than 15 years, whereas by summing all round surveys, our sample allows for measuring the outcomes of adults up to age 35.5 years only. The cohort based on the daughter’s birth started in the year 1964, which is 28 years before the first NFHS-1, and continued till 2005. After aggregating all of these, we arrived at a cohort with a mean age of 25.5 years.
Trends in birth year
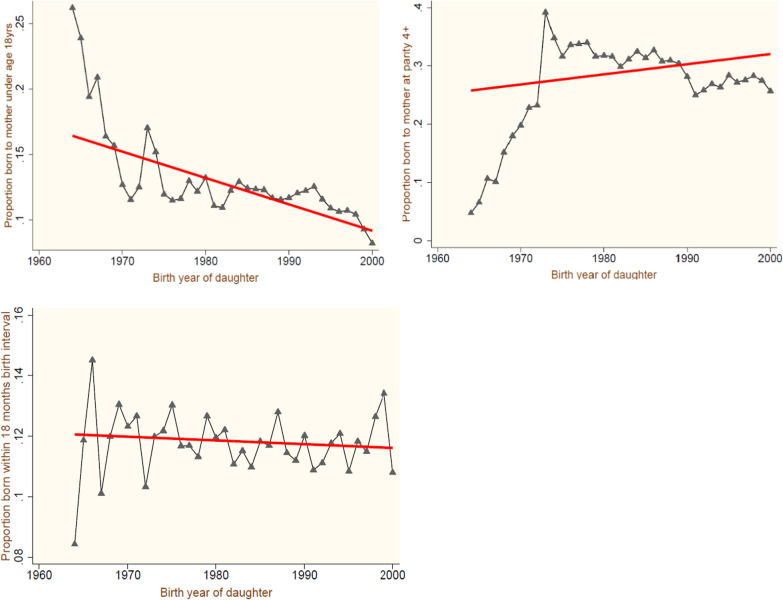
Table 1 only provides the overall view of the data. To visualize the trend of cohort proportions of all six outcomes, three maternal risk factors, and the remaining control variables, we plotted the proportion averages for the birth years of daughters. The trend line shown in Fig. 2 offered some improvements in two risk exposures, i.e., in young mothers and in those who had short birth intervals. The plots suggest that the number of births to mothers of age < 18 years and to those who have shorter gaps between successive births, becomes less as the daughters are born at a later age, but the cohort averages of daughters born at four or above parity shows an opposite trend in that as the year of birth increases the chances of births occurring at a higher parity increase. Now examining the rate of change among the first two causes, we found a more rapid decline for those at a younger maternal age with a minimum average cohort proportion recorded at less than 0.1 compared to the short birth interval with a range of cohort averages almost zero.
Fig. 2.
Average Proportions of the cohort for maternal risk factors by birth year of daughters
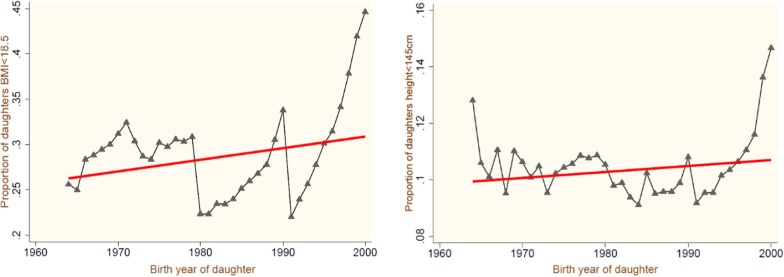
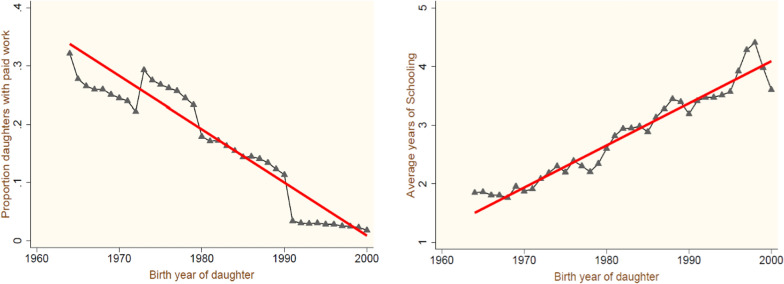
Figure 3 helps to observe the pattern of two selected reproductive outcomes of a daughter’s development by birth years. Both trend lines suggest some advancement in the outcomes experienced by mothers over a period. In contrast to the nutritional outcomes measured in terms of height and body mass index, the reports show a hike in the cohort proportions, though the pace of growth is lower for short-stature cohort proportions compared to underweight females. The cohort level averages and trend lines are shown in Fig. 4. The remaining two socioeconomic cohort-level outcomes are also plotted, and the trend lines with year-wise averages are presented in Fig. 5, where clear progress is observed in the average years of a female’s education. In contrast, the proportion of females engaged in the paid workforce has decreased over time. However, the irregular trends (i.e., the increasing proportions of higher parity births, short and undernourished development of females, and lower engagement in paid work industries) reflect a larger share of cohorts from relatively socio-economically backward states compared to southern and northern affluent states.
Fig. 3.
Average Proportions of the cohort for reproductive health outcomes by birth year of daughters
Fig. 4.
Average Proportions of the cohort for development outcomes by birth year of daughters
Fig. 5.
Average Proportions of the cohort for socioeconomic outcomes by birth year of daughters
Regression results
To test the hypothesis that maternal risk factors influence the growth of daughters, the Beta regression model has been applied. Multivariate linear regression has been used to assess maternal risk factors’ effect on academic achievement. The computed odds ratios for the rest of the dependent variables with 95 percent confidence interval and significance levels are presented in Table 2. This study discusses the model results primarily using relative risk rather than coefficients, except for educational outcomes, which are directly interpretable. From equation one, unlike the conventional logit link model, the risk is the ratio of two proportions rather than two probabilities. So, the calculated ratios give the proportional change of study variables. The impact of three selected risky maternal factors in the sample is seen in the first three rows, where each is statistically significant with one or more of the outcomes.
Table 2.
Results of beta and linear regression models estimation on cohort proportions for reproductive and socioeconomic outcomes in India
| Covariates/ Relative risk |
Short Stature | Under-weight | Experiencing Child death | Low Birth Weight Infant | Educational Status | Working Status |
|---|---|---|---|---|---|---|
| Young mother | 1.222 | 2.291* | 1.188* | 3.097* | − 2.141* | 0.022* |
| [0.52, 1.26] | [1.50, 3.48] | [1.10, 1.73] | [1.68, 3.09] | [− 2.73, − 1.43] | [0.007, 0.064] | |
| Short birth interval | 1.785* | 1.165 | 1.147* | 0.546 | − 1.126* | 0.498 |
| [1.01, 3.22] | [1.05, 2.05] | [1.09, 1.95] | [0.23, 1.24] | [− 2.01, − 0.18] | [0.96, 2.56] | |
| Higher parity | 1.334* | 1.588* | 1.140 | 4.254* | − 0.346* | 0.114* |
| [1.02, 1.52] | [1.29, 1.95] | [0.90, 1.19] | [3.18, 5.67] | [− 0.66, − 0.025] | [0.069, 0.187] | |
|
Mother’s education (Literate vs illiterate) |
0.957 | 0.604* | 0.758 | 6.201* | 1.623* | 52.691* |
| [0.62, 1.45] | [0.40, 0.90] | [0.51, 1.10] | [3.52, 10.91] | [0.97, 2.27] | [17.07, 62.64] | |
|
Mother’s residence (Urban vs Rural) |
0.977 | 1.208 | 0.818 | 0.145* | 1.147* | 0.021* |
| [0.64, 1.47] | [0.81, 1.79] | [0.81, 1.83] | [0.08, 0.26] | [0.50, 1.78] | [0.006, 0.066] | |
|
Mother’s Caste (SC/ST vs others) |
1.310* | 1.090 | 1.230* | 1.300* | − 2.480* | 0.050* |
| [1.12,1.53] | [0.78,1.52] | [1.03,1.47] | [1.15,1.46] | [− 3.77, − 1.63] | [0.01,0.49] | |
|
Mother’s Religion (Muslim vs others) |
1.22 | 1.420* | 1.63* | 1.240* | − 1.58* | 0.490* |
| [0.95,1.55] | [1.10,1.82] | [1.06,2.51] | [1.10,1.39] | [− 2.26, − 1.10] | [0.44,0.55] | |
|
Mother’s wealth (Poor vs others) |
1.39* | 1.030 | 0.96 | 1.350* | 0.390* | 0.950 |
| [1.17,1.65] | [0.75,1.41] | [0.68,1.36] | [1.13,1.60] | [0.16,0.93] | [0.86,1.06] | |
|
Region (EAG vs non-EAG) |
1.01* | 1.040 | 1.71* | 1.010* | 0.740 | 0.630* |
| [1.01,1.02] | [0.79,1.37] | [1.37,2.12] | [1.00,1.02] | [− 0.50,1.08] | [0.49,0.80] | |
| Year of daughter’s birth | 0.999 | 0.975* | 0.955* | 0.970* | 0.076* | 0.897* |
| [0.99, 1.01] | [0.97, 0.98] | [0.95, 0.96] | [0.96, 0.97] | [0.071, 0.80] | [0.88, 0.90] | |
| Age of adult daughters | 0.99* | 0.922* | 1.010* | 0.936* | 0.014* | 0.932* |
| [0.98, 0.99] | [0.91, 0.92] | [1.00, 1.01] | [0.93, 0.94] | [0.008, 0.019] | [0.92, 0.94] |
95% confidence interval given in parenthesis, * p < 0.05
In Table 2, the first two columns support the hypothesized associations between cohort proportions of risk factors and the cohort experiencing poor physical growth outcomes. The results for young mothers are positive and not significantly associated with both outcomes. A one-point increase in the proportion of daughters born to younger mothers suggests a rise in the risk of having short height by 22 percent, while the risk of the proportion of daughters of low BMI in the cohort is doubled. These figures conclude that a cohort with a high proportion of daughters born to young mothers appears to have a higher proportion of adults of short stature and low BMI. Similarly, the influences of the remaining two early risk exposures, i.e., birth at higher parity and short birth interval, come out statistically significant with both adverse health outcomes. The relative risks for higher parity and short birth intervals are increased by 33 and 79 percent, respectively, for short stature indicators. The risks of low BMI show an increment of 57 and 17 percent after having one-point change in respective maternal factors. These findings suggest that cohorts with a high proportion of births occurring at short birth intervals and higher parity (parity > = 4) are significantly associated with the proportions of undernourished daughters in a cohort.
The strength of the association of maternal education exhibits a significantly negative association with some of these selected outcomes. Among nutritional outcomes, it is negatively related to a daughter’s lower BMI, as the cohort proportions of daughters born to educated mothers increase by one unit, and the risk of having low BMI decreases by 40 percent. The second control variable, the proportion of girls born to mothers living in urban areas, fails to significantly impact nutritional outcomes measured in outcome surveys.
Talking about the relationship between risky birth conditions and daughters’ reproductive outcomes (the regression results are shown in columns 4 and 5 of Table 1.2). We found that only two of them are substantially and positively correlated with the proportion of mothers who experienced the death of a child. In the sample, the risk of child loss rose by 19 and 15 percent when the cohort proportion of a daughter was born to younger mothers, while those born at a short interval after a sibling increased by one point. This strong association is suggestive of the impact of early birth conditions on a daughter’s adverse reproductive outcomes. Except for short birth intervals listed in maternal risk factors, an increase in the proportion of births occurring to mothers below 18 years or at four or higher parity is significantly associated with a higher proportion of adult daughters experiencing low birth weight. The relative risk in Table 2 shows that the risk of having a small baby to adult daughters is 3.1 for younger mothers and 4.3 for high parity births.
A mother’s education and residence are taken as the control variables in the study, offering significant results for low-birth infants and becoming inconsistent for females experiencing child loss, as seen in columns 4 and 5 of Table 2. An increase in the proportion of daughters born to educated mothers significantly increases the risk of adults reporting low-weight infants; with a relative risk of 6.2, there is some significant impact on women experiencing low birth weight. On the other hand, a higher proportion of births occurring to women living in urban areas significantly decreases the proportion of daughters producing small babies as adults. Mother’s socioeconomic background also affects the growth of the daughter- daughters born to poor mothers had 35 percent higher risk of experiencing low birth weight and 39 percent higher risk of having short stature. Daughters born to SC/ST mothers experience higher child loss than daughters born to mothers from other castes. Combining these results, we conclude that these control variables – a mother’s schooling, economic status, religion, caste, and residence- have a mixed effect on her daughter’s reproductive outcomes. Daughters from EAG states have a 71 percent higher risk of experiencing child loss as an adult than those from non-EAG states.
The strength of the association of early risk exposure at a daughter’s birth with adult socioeconomic measures is mixed. Cohorts with a high proportion of daughters born to younger mothers and at higher parity are associated with a lower proportion of daughters with paid work status (see column 7 of Table 2). The relative risk of a daughter’s working status calculated from the model was statistically significant for younger mothers (0.02) and high parity births (0.11). Daughters born within 18 months are also negatively linked with economic consequences; a one-point increase in the proportion of this risk factor provides a reduction of 50 percent in the working ratios of daughters, but the association is not significant.
Moving toward the linear regression results in column 6 of Table 2, we found that the coefficient for hazardous factors present at birth is sizable, negative, and significant for a daughter’s education. A one-unit change in cohort proportions born to young mothers, at higher parity and shortly after their sibling is associated with 2.11, 0.35, and 1.11 fewer years of schooling, respectively. The positive relationship between cohort proportion born to educated mothers and a daughter’s academic status in adulthood suggests an intergenerational transfer of school education privilege by adding 1.62 more years of education in their lives. Not only the schooling but also the residence of mothers affects the average years of a daughter’s schooling. The regression coefficient is 1.47 years of schooling. Daughters born to affluent mothers have higher average mean years of schooling.
The strength of the association of the remaining two control variables with adult development outcomes is mixed, and the results are given in the last two rows of Table 2. The age of the daughter’s cohort in the outcome survey is statistically and negatively associated with the physical well-being of daughters. However, the difference offered by this variable on the proportion of undernourished daughters is minimal. Except for child loss, an increase in the age of daughters recorded at outcome surveys is negatively associated with all selected development measures. Combine these outcomes. This study concludes that the age at the time of the survey captures some improvement in the physical and reproductive well-being of adult daughters. Similar results are obtained for the year of birth variables with a similar magnitude. The regression coefficient for age variables with proportions of daughters having paid work status is also significant and in the same direction, which shows that younger women have higher involvement in the workforce.
Trends in eliminated proportions
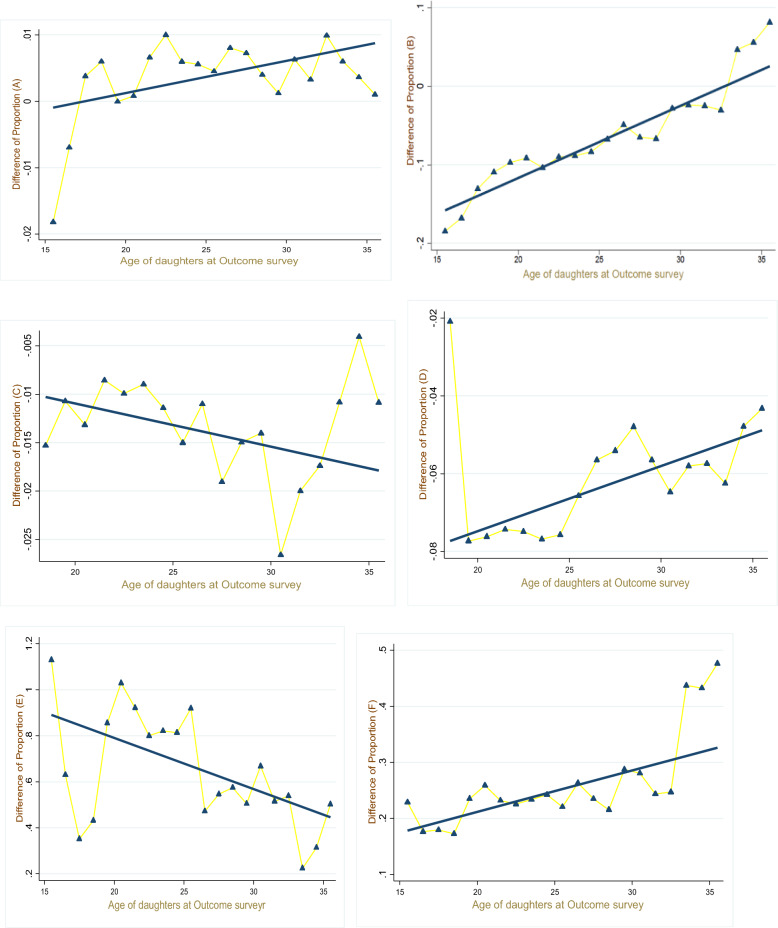
After eliminating the three risk factors, we compute the summarized view (combining all cohorts) of changes computed in all outcome variables. Also, in addition to the association of risk factors, all six outcome proportions are also influenced by the age of daughters noted from the outcome surveys (see Table 2). Combining these two concerns of the study, we plotted the difference between estimated (simulated after eliminating all risk factors) and observed cohort proportions over age and the trends of each outcome variable, as given in Fig. 6.
Fig. 6.
Average Proportion (eliminated–observed) by the age of daughter at outcome survey, A: Difference in proportion of adults with height < 145 cm, B: Difference in proportion of adults with BMI < 18.5, C Difference in proportion of adult losing child under age 5, D: Difference in proportion of adult having small baby, E: Difference in proportion of adult with working status, F: Difference in proportion of adult’s average years of schooling
Cohort proportions related to a daughter’s nutritional status, as shown in Fig. 6, indicate that younger women experience more benefit from non-exposure to maternal risk factors for both short-stature and undernourished outcomes compared to older women. By investigating the trend lines, we found that as the age of females increases, the difference between estimated and observed proportions also increases, but the range of this difference is shifted from a negative to a positive scale, indicating that for older women, simulated values are larger than observed values. Comparing these two trends, we found that the gain obtained by removing the risk factors from a daughter’s birth is larger for BMI < 18.5 kg/m2 compared to short-stature outcomes.
To study the child loss experience, the difference is plotted against ages, and the result is shown in Fig. 6. Moving from larger to smaller differences, the trend offers a higher gain from non-exposure to hazardous conditions present at birth to younger women, compared to older cohorts, while for the proportion of infants born small, the estimated benefits are greater for older daughters.
The socioeconomic gains with respect to age are presented in Fig. 6—the benefits of work status and educational status offer different trends. Older females are estimated to drive more considerable benefits compared to younger women, which leads to the conclusion that after eliminating the risk factors, estimated proportions of working females as compared to observed values increase with age. In contrast, the trend of benefit for average years of schooling is larger among younger daughters.
Looking at the last two figures, we conclude that removing maternal risk factors from the sample not only gives advantages on overall averages but also shows a positive impact at cohort-level proportions. The negative difference between the eliminated and estimated proportions projected on the vertical axis of the graph also expresses the gains that we calculated after eliminating the conditional factors; for example, the difference for child loss and small-size births is negative, which indicates that simulated proportions are lower than observed proportions. Similar results are found for socioeconomic outcomes with the opposite signs of range. The results for nutritional outcomes are not shown to be significantly associated with early risk exposures; estimated proportions are higher than the observed ones.
Conclusion, policy implication, and recommendation
This study provides a detailed examination of how maternal risk factors, specifically young maternal age, short birth intervals, and high parity, affect the long-term physical, reproductive, and socioeconomic outcomes of daughters in India. The pseudo-cohort analysis has been extensively utilized in economics, particularly for investigating skill formation and labor market dynamics [30]. Yet, its application within public health research remains relatively limited. Notably, Li Q et al. (2016) employed this methodological approach to assess the influence of maternal risk factors on child development across a combination of African and Asian nations. Building upon this foundation, the present study applies this robust analytical framework to conduct a comprehensive examination within the Indian context, which is a crucial setting given India’s remarkable advancement in reproductive health outcomes over recent decades. Through the pseudo-cohort approach, using NFHS cross-sectional data across multiple survey rounds, this research simulates longitudinal insights into life course dynamics, which are traditionally accessible only through panel data. The results indicate that early maternal risk factors at birth significantly correlate with increased risk of undernutrition, stunted growth, child mortality, and low birth weight in daughters, suggesting a notable intergenerational transmission of health and socioeconomic disadvantage.
The findings emphasize the role of maternal age and parity as influential factors in shaping a daughter’s growth and reproductive outcomes. Daughters born to mothers under 18 years of age are notably more likely to experience adverse health outcomes, as are those born at higher parity or within 18 months of a previous sibling. These outcomes align with existing literature that associates maternal risk factors with poorer child health, particularly in resource-limited settings [44, 48]. The results underscore that daughters born to educated mothers experience fewer adverse outcomes, highlighting the importance of maternal education as a protective factor [28]. This connection suggests that maternal education may buffer against some of the detrimental impacts of early maternal risk factors, potentially by enabling better health practices and increased awareness of family planning and child health.
A significant observation from this study is the socioeconomic influence of maternal background on daughters’ well-being. Daughters born in urban areas or mothers from non-poor households showed relatively better physical and socioeconomic outcomes [28]. This affirms that broader socio-environmental factors play an essential role in mitigating the negative impacts of maternal risk factors on the next generation. Moreover, while the adverse effects of maternal age and high parity are consistent across settings, regional differences, particularly between EAG (Empowered Action Group) and non-EAG states, suggest that targeted interventions in high-need regions could improve outcomes for the most vulnerable populations.
Methodologically, this study offers a novel application of the pseudo-cohort approach in demographic research within India, where comprehensive longitudinal data are scarce. By leveraging repeated cross-sectional data from the NFHS, this study bridges a crucial gap in understanding life course dynamics in low-resource settings. The consistency in results between the pseudo-cohort analysis and traditional longitudinal insights validates the utility of this approach, especially when panel data are unavailable. The use of cohort averages to model proportions of affected daughters effectively reduces recall bias and enhances the robustness of the findings.
Besides interesting findings, this study also has certain limitations. Some of these are produced due to the formation of the cohort approach by using cross-sectional surveys. For example, in the truncation bias, female respondents have an age limit of 15 to 49 years; thus, women with completed cohorts are truncated from the analysis. Also, a short interval between two consecutive surveys restricts the age of cohorts and provides less scope to follow them over the reproductive life span. The current study includes the cohort mean age as a control variable to address the potential bias. This limitation can be mitigated over time with the increasing number of national representative surveys with the same sampling design. The second possible limitation of using population surveys is differentials of mortality and migration, which can be addressed by weighing the data using survival probability distributions [7]. However, cohort averages curtailed the chance of survival bias. In addition to these, the potential bias from in and out-migration in many countries is likely to be small, and due to lack of data, our sample cannot be adjusted for this bias. This study utilized the socioeconomic conditions at the time of a daughter’s birth as a proxy to control the model. However, these socioeconomic factors may evolve as the daughter transitions into adulthood, representing a limitation. This assumption fails to fully capture the dynamic nature of socioeconomic influences across different life stages. While the pseudo-cohort approach enables analysis without longitudinal data, it remains a significant drawback compared to true longitudinal studies, which can better account for these temporal changes. The last one is the retrospective recall bias present in measuring some of the information. We believe that this bias is random and somewhat mitigated with the use of cohort averages.
Improving maternal and child well-being in India necessitates targeted policies addressing the intergenerational impacts of maternal risk factors, including young maternal age, short birth intervals, and high fertility. Policy priority must be given to maternal education, as educated mothers demonstrate better health and socioeconomic outcomes for their daughters, breaking cycles of disadvantage. Crucial family planning initiatives promoting healthy birth intervals are essential for reducing undernutrition and child mortality. Expanding these services through community health workers, especially in rural and underprivileged areas, can bolster awareness and access. Vulnerable populations, such as daughters from poorer households, marginalized castes, and EAG states facing pronounced socioeconomic challenges, warrant special focus. Tailored interventions offering nutritional support, quality healthcare, and educational resources could help bridge these gaps. Comprehensive sexual education and adolescent health services can also delay early pregnancies, fostering improved outcomes for young mothers and their children. By integrating healthcare, education, and economic support policies, India can cultivate an environment where maternal and child health improvements are sustainable across generations, empowering both mothers and daughters with opportunities for healthier, more economically secure lives.
Acknowledgements
We would like to thank The Demographic and Health Surveys (DHS) Program for providing access to the data. We would like to thank the Asian Development Bank Institute for supporting this project. The views expressed here are those of the authors and do not necessarily reflect the views of the authors’ institutions, and the usual disclaimer applies.
Author contributions
AS: Led conceptualization, data cleaning, analysis, write-up, editing, and structuring. DRB: Contributed to the conceptualization, analysis, write-up, editing, and structuring. KKS: Contributed to the conceptualization, write-up, editing, and structuring.
Funding
This study was supported by the Asian Development Bank Institute (ADBI), Tokyo.
Availability of data and materials
Data is publicly available from The Demographic and Health Surveys (DHS) Program upon request, and the third party is not allowed to share it. https://dhsprogram.com/data/ We would be happy to share the data and related material upon request.
Declarations
Ethics approval and consent to participate
Procedures and questionnaires for standard DHS surveys have been reviewed and approved by the ICF Institutional Review Board (IRB). Additionally, country-specific DHS survey protocols are reviewed by the ICF IRB and typically by an IRB in the host country. ICF IRB ensures that the survey complies with the U.S. Department of Health and Human Services regulations for the protection of human subjects (45 CFR 46), while the host country IRB ensures that the survey complies with the laws and norms of the nation.
Consent for publication
The Demographic and Health Surveys (DHS) Program obtained consent for publication at the time of the survey.
Competing interests
The authors declare no competing interests.
Footnotes
Scheduled Castes (SC) and Scheduled Tribes (ST) are historically marginalized groups recognized by the Constitution of India. These groups have faced social exclusion, economic disadvantage, and discrimination over centuries.
The EAG states account for a significant portion of India's population and face challenges such as high fertility rates, lower literacy, and higher infant and maternal mortality rates. Recognizing these disparities, the Government of India and the National Health Mission (NHM) initiated targeted programs to improve health infrastructure, family planning, and socioeconomic outcomes in these states.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.AbouZahr C. Global burden of maternal death and disability. Br Med Bull. 2003;67(1):1–11. [DOI] [PubMed] [Google Scholar]
- 2.Afsa C, Buffeteau S. Female labor force participation in France: a cohort analysis. Institut National de la Statistique et des Etudes Economiques. 2005;(2005–02).
- 3.Anyamele OD, Ukawuilulu JO, Akanegbu BN. The role of wealth and Mother’s education in infant and child mortality in 26 sub-Saharan African countries: evidence from pooled demographic and health survey (DHS) data 2003–2011 and African development indicators (ADI), 2012. Soc Indic Res. 2017;130:1125–46. [Google Scholar]
- 4.Boerma JT, Bicego GT. Preceding birth intervals and child survival: searching for pathways of influence. Stud Fam Plann. 1992;23(4):243–56. [PubMed] [Google Scholar]
- 5.Irene C, Arianna L. The importance of maternal nutrition for health. J Pediat Neonatal Individ Med. 2015. 10.7363/040220. [Google Scholar]
- 6.Chan KA, Tsoulis MW, Sloboda DM. Early-life nutritional effects on the female reproductive system. J Endocrinol. 2015;224(2):R45-62. [DOI] [PubMed] [Google Scholar]
- 7.Deaton A. Panel data from time series of cross-sections. J Econom. 1985;30(1–2):109–26. [Google Scholar]
- 8.Duhautois R. Le ralentissement de l’in vestissementest plutôt le fait des petites enterprises tertiaires. Economie et Statistique. 2001;341–342:47–66. [Google Scholar]
- 9.Duncan GJ, Lee KT, Rosales-Rueda M, Kalil A. Maternal age and child development. Demography. 2018;55(6):2229–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elder GH Jr, Johnson MK, Crosnoe R. The emergence and development of life course theory. In: Handbooks of Sociology and Social Research. Springer US; 2003:3–19.
- 11.Ferraro KF, Schafer MH, Wilkinson LR. Childhood disadvantage and health problems in middle and later life: Early imprints on physical health?: Early imprints on physical health? Am Sociol Rev. 2016;81(1):107–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari S, Cribari-Neto F. Beta regression for modelling rates and proportions. J Appl Stat. 2004;31(7):799–815. [Google Scholar]
- 13.Gebreegziabher E, Bountogo M, Sié A, Zakane A, Compaoré G, Ouedraogo T, Lebas E, et al. Influence of maternal age on birth and infant outcomes at 6 months: a cohort study with quantitative bias analysis. Int J Epidemiol. 2023;52(2):414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbs CM, Wendt A, Peters S, Hogue CJ. The impact of early age at first childbirth on maternal and infant health: Impact of early age at childbirth on maternal and infant health. Paediatr Perinat Epidemiol. 2012;26(Suppl 1):259–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham WJ. Now or never: the case for measuring maternal mortality. Lancet. 2002;359(9307):701–4. [DOI] [PubMed] [Google Scholar]
- 16.Guillerm Marine. Pseudo‑panel methods and an example of application to Household Wealth data. Economie et Statistique / Economics and Statistics N° 2017; 491–492.
- 17.Hyppönen E, Power C. An intergenerational study of birthweight: investigating the birth order effect. BJOG. 2004;111(4):377–9. [DOI] [PubMed] [Google Scholar]
- 18.International Institute for Population Sciences - IIPS/India and ORC Macro. 2000. India National Family Health Survey (NFHS-2) 1998–99. Mumbai, India: IIPS and ORC Macro.
- 19.International Institute for Population Sciences - IIPS/India and Macro International. 2007. India National Family Health Survey (NFHS-3) 2005–06. Mumbai, India: IIPS and Macro International.
- 20.International Institute for Population Sciences - IIPS/India and ICF. 2017. India National Family Health Survey NFHS-4 2015–16. Mumbai, India: IIPS and ICF.
- 21.International Institute for Population Sciences (IIPS) and Macro International. 2017. National Family Health Survey (NFHS-4), 2015–16: India: Volume 1. Mumbai: IIPS.
- 22.International Institute for Population Sciences (IIPS) and Macro International. 2022. National Family Health Survey (NFHS-5), 2019–20: India: Volume 1. Mumbai: IIPS.
- 23.Khan ME, Phillip Sebastian M, Sharma U, Idnani R, Kumari K, Maheshwari B. Promoting Healthy Timing and Spacing of Births in India through a Community-Based Approach. FRONTIER, Population Council; 2008.
- 24.Kim YN, Choi DW, Kim DS, Park EC, Kwon JY. Maternal age and risk of early neonatal mortality: a national cohort study. Sci Rep. 2021;11(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lelièvre M, Sautory O, Pujol J. Niveau de vie par âge et génération entre. Published online 2010:23–35.
- 26.Magnus P, Bakketeig LS, Skjaerven R. Correlations of birth weight and gestational age across generations. Ann Hum Biol. 1993;20(3):231–8. [DOI] [PubMed] [Google Scholar]
- 27.Malviya MK, Bhardwaj VK, Chansoria M, Khare S. Anthropometric profile and perinatal outcome of babies born to young women (< 18 years). Indian Pediatr. 2003;40(10):971–6. [PubMed] [Google Scholar]
- 28.Mani A, Mullainathan S, Shafir E, Zhao J. Poverty impedes cognitive function. Science. 2013;341(6149):976–80. [DOI] [PubMed] [Google Scholar]
- 29.Marquis GS, Penny ME, Zimmer JP, Díaz JM, Marín RM. An overlap of breastfeeding during late pregnancy is associated with subsequent changes in colostrum composition and morbidity rates among Peruvian infants and their mothers. J Nutr. 2003;133(8):2585–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIntosh S. Using Pseudo Cohorts to Track Changes in the Qualifications of National Populations. Center for Economic Performance, London School of Economics, Department for Education and Skills; 2005.
- 31.Moffitt R. Identification and estimation of dynamic models with a time series of repeated crosssections. J Economet. 1993;59:99–123. [Google Scholar]
- 32.Nadarajah S, Leong NK. Adolescent pregnancies managed at KK Hospital. Singapore Med J. 2000;41(1):29–31. [PubMed] [Google Scholar]
- 33.Nguyen PH, Scott S, Neupane S, Tran LM, Menon P. Social, biological, and programmatic factors linking adolescent pregnancy and early childhood undernutrition: a path analysis of India’s 2016 National Family and Health Survey. The Lancet Child & Adolescent Health. 2019;3(7):463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Tsui AO. Maternal risk exposure and adult daughters’ health, schooling and employment a constructed cohort analysis of 50 developing countries. Demography. 2016;53(3):835–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutstein S. Effects of preceding birth intervals on neonatal, infant and under-five years’ mortality and nutritional status in developing countries: Evidence from the Demographic and Health Surveys. Int J Gynecol Obstet. 2005;89(Suppl. 1):7–24. [DOI] [PubMed] [Google Scholar]
- 36.Rutstein SO. Further evidence of the effects of preceding birth intervals on neonatal, infant, and under-five-years mortality and nutritional status in developing countries: Evidence from the demographic health surveys.” DHS Working Paper. 2008;(41). [DOI] [PubMed]
- 37.Sayour N. The impact of maternal care on child development: Evidence from sibling spillover effects of a parental leave expansion. Labour Econ. 2019;58:167–86. [Google Scholar]
- 38.Sloboda DM, Hickey M, Hart R. Reproduction in females: the role of the early life environment. Hum Reprod Update. 2011;17(2):210–27. [DOI] [PubMed] [Google Scholar]
- 39.Suárez-Idueta L, Bedford H, Ohuma EO, Cortina-Borja M. Maternal risk factors for small-for-gestational-age newborns in mexico: analysis of a nationwide representative Cohort. Front Public Health. 2021;9:707078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Starbird E, Norton M, Marcus R. Investing in family planning: Key to achieving the sustainable development goals. Glob Health Sci Pract. 2016;4(2):191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stover J, Ross J. Changes in the distribution of high-risk births associated with changes in contraceptive prevalence. BMC Public Health. 2013;13 Suppl 3(Suppl 3):S4. [DOI] [PMC free article] [PubMed]
- 42.Unicef, Fund UNC. The state of the world’s children 2009: maternal and newborn health. 2008;9.
- 43.Verbeek M, Nijman T. Can cohort data be treated as genuine panel data? In: Panel Data Analysis. Physica-Verlag HD; 1992:9–23.
- 44.Wemakor A, Garti H, Azongo T, Garti H, Atosona A. Young maternal age is a risk factor for child undernutrition in Tamale Metropolis. Ghana BMC Res Notes. 2018;11(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winikoff B, Castle MA. The maternal depletion syndrome: Clinical diagnosis or eco-demographic condition? Biolog Sci. 1987;5:163–70. [Google Scholar]
- 46.Winkvist A, Rasmussen KM, Habicht JP. A new definition of maternal depletion syndrome. Am J Public Health. 1992;82(5):691–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization (WHO). A WHO collaborative study of maternal anthropometry and pregnancy outcomes. International Journal of Gynecology and Obstetrics. 1997; 57:1–15. [PubMed]
- 48.Yount KM, Zureick-Brown S, Halim N, Lavilla K. Fertility decline, girls’ well-being, and gender gaps in children’s well-being in poor countries. Demography. 2014;51(2):535–61. [DOI] [PubMed] [Google Scholar]
- 49.Ytterberg K, Jacobsson Bo, Flatley C, Juodakis J, Nilsson S, Solé-Navais P. Exploring the association of parity and its interaction with history of pre-term delivery on gestational duration. Ann Epidemiol. 2023;87:60–8. [DOI] [PubMed] [Google Scholar]
- 50.Yu SH, Mason J, Crum J, Cappa C, Hotchkiss DR. Differential effects of young maternal age on child growth. Glob Health Action. 2016;9(1):31171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu WH, Yan HX. Maternal age, early childhood temperament and youth outcomes. Demography. 2022;59(6):2215–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is publicly available from The Demographic and Health Surveys (DHS) Program upon request, and the third party is not allowed to share it. https://dhsprogram.com/data/ We would be happy to share the data and related material upon request.