ABSTRACT
Objective
To determine if targeting higher levels of pH‐controlled permissive hypercapnia beyond postnatal day 7–14 reduces mechanical ventilation duration in preterm infants.
Methods
Single‐center randomized clinical trial with a 1:1 parallel allocation including infants from 22−36 weeks' gestation mechanically ventilated for respiratory distress syndrome on postnatal day 7–14. We targeted higher levels of pH‐controlled permissive hypercapnia (60–75 mmHg and pH ≥ 7.20) or lower levels of pH‐controlled permissive hypercapnia (40–55 mmHg and pH ≥ 7.25) for 28 days after randomization. The primary outcome was the number of days alive and ventilator‐free in the 28 days after randomization.
Results
We enrolled 130 infants with a gestational age (mean ± SD) of 24 weeks and 5 days ± 2 weeks and 0 days and birth weight of 657 ± 198 grams from December 2015 to May 2021. Infants randomized to higher levels of pH‐controlled permissive hypercapnia had more alive ventilator‐free days than infants randomized to lower levels of pH‐controlled permissive hypercapnia (11 ± 10 vs. 6 ± 8; p = 0.009). Grade 2–3 bronchopulmonary dysplasia or death before discharge was not significantly lower in the higher carbon dioxide (PCO2) group (30/62 (44%) vs. 45/68 (59%); adjusted odds ratio (aOR) 0.54, 95% confidence intervals (CI) 0.27–1.08; p = 0.08). Grade 2–3 bronchopulmonary dysplasia among survivors at 36 weeks' postmenstrual age did not differ significantly (higher PCO2 19/53 (35%) vs. lower PCO2 28/53 (50%); aOR 0.56, 95% CI 0.27–1.13; p = 0.12).
Conclusions
Targeting higher levels of permissive hypercapnia from postnatal day 7–14 increased the number of days alive and ventilator‐free and may be lung protective compared with targeting lower levels.
Trial Registration
Clinicaltrials.gov (identifier number NCT02799875). The first infant was enrolled in December 2015 and the trial was not registered until June 2016. The authors confirm that there were no changes made to the Institutional Review Board (IRB) approved trial protocol (dated 10/20/2015) or any amendments made after recruitment started, between the date of first enrollment and the date of clinicaltrials.gov registration, or between study commencement and completion. Furthermore, the authors confirm that the data were not unblinded until after the last infant had been enrolled (March 2021) and discharged from the hospital (August 2021). Study Details | Late Permissive Hypercapnia for Intubated and Ventilated Preterm Infants | ClinicalTrials.gov.
Keywords: artificial, bronchopulmonary dysplasia, hypercapnia, infant, newborn, premature, respiration, respiratory distress syndrome
Abbreviations
- aOR
adjusted odds ratio
- BPD
bronchopulmonary dysplasia
- CI
confidence intervals
- CPAP
continuous positive airway pressure
- IRB
Institutional Review Board
- IVH
intraventricular hemorrhage
- MAP
mean airway pressure
- NDI
neurodevelopmental impairment
- NEC
necrotizing enterocolitis
- NRS
noninvasive respiratory support
- PCO2
partial pressure of carbon dioxide
- RDS
respiratory distress syndrome
- SD
standard deviation
- SpO2
oxygen saturations
- TcCO2
transcutaneous carbon dioxide
1. Introduction
Bronchopulmonary dysplasia (BPD) is the most common major morbidity among preterm infants [1]. In spite of the development of lung protective strategies, rates of BPD remain high and are not decreasing [2, 3]. In addition, preterm infants are at higher risk of longer‐term respiratory disease that may have life‐long implications for lung health [4, 5]. Ventilator‐associated lung injury is a key contributor to the development of BPD [6]. Prolonged mechanical ventilation is associated with higher rates of BPD, mortality, and neurodevelopmental impairment [7]. While avoidance of mechanical ventilation is preferred, many preterm infants with respiratory distress syndrome (RDS) and respiratory failure receive prolonged mechanical ventilation for ongoing lung disease or apnea of prematurity [8, 9, 10]. Permissive hypercapnia, accepting a partial pressure of carbon dioxide (PCO2) above 45 mmHg, is commonly used in neonates to reduce the intensity of mechanical ventilation by allowing a reduction in ventilator pressures/volumes or rates and thereby protect the lungs [11, 12].
Previous randomized controlled trials demonstrated that permissive hypercapnia in the first 7–14 days decreases the use of mechanical ventilation among preterm infants with RDS [13, 14, 15, 16, 17, 18]. There are limited data on the use of higher ranges of PCO2 beyond postnatal days 7–14 [19]. The objective of this trial was to test the hypothesis that among preterm infants from 22 to 36 weeks' gestation with RDS who are mechanically ventilated from day 7 to 14 after birth, higher levels of permissive hypercapnia with a PCO2 goal of 60 to 75 mmHg and pH limit of 7.20 or higher, compared with lower levels of permissive hypercapnia with a PCO2 goal of 40‐55 mmHg and pH limit of 7.25 or higher, would increase the number of days infants are alive and off mechanical ventilation in the 28 days after randomization.
2. Materials and Methods
2.1. Trial Design, Setting, and Participants
This single‐center randomized clinical trial was conducted at a tertiary neonatal intensive care unit following Institutional Review Board approval (IRB‐141120007) and registered on Clinicaltrials.gov (identifier number NCT02799875). We included preterm infants from 22 weeks and 0 days to 36 weeks and 6 days of gestational age, who were ventilated for clinical and radiographic RDS on postnatal day 7–14, who were inborn or transferred to our regional neonatal intensive care unit before postnatal day 7, and in whom informed consent had been obtained. There was no minimum duration of ventilation before enrollment and infants could be enrolled if meeting criteria at any time from postnatal day 7–14. We excluded infants with major congenital malformations or neuromuscular conditions affecting respiration, infants with a terminal illness or decision to withdraw or limit care, or if the parents had refused or withdrawn informed consent.
2.2. Recruitment and Randomization
Randomization was stratified by gestational age at birth into three groups; 22–25 weeks' gestation, 26–28 weeks' gestation, and 29–36 weeks' gestation. Block sizes of two to six were computer generated and a folded piece of paper with group assignment was placed between a paper card and carbon paper in sequentially numbered opaque sealed envelopes by the Pediatric Research Office staff at the university. A study team member wrote the date, time, and participant name on the back of the envelope before opening. Eligible infants were randomized to either group with a 1:1 parallel allocation. Multiples were enrolled in the same group if more than one infant was eligible, as the intervention was not masked.
2.3. Interventions
We randomized infants to two different levels of pH‐controlled permissive hypercapnia based on arterial or capillary blood samples that were commonly used in our unit. The pH and PCO2 targets in the higher permissive hypercapnia group were ≥ 7.20 and ≥ 60 to ≤ 75 mmHg respectively. The pH and PCO2 targets in the lower permissive hypercapnia group were ≥ 7.25 and ≥ 40 to ≤ 55 mmHg respectively. Blood gas testing was performed per clinical team and was typically collected daily while on ventilator support. Either arterial or capillary blood gas pH and PCO2 were utilized as most infants did not have arterial access. Transcutaneous carbon dioxide (TcCO2) monitors (SenTec Inc, Fenton, MO) were routinely used among preterm infants on respiratory support. In both groups the TcCO2 target range was adjusted based on the most recent correlation with the blood gas PCO2 value. The TcCO2 upper alarm limit was set to avoid a pH less than the target or to the maximum intended PCO2 for that assigned group. The lower alarm limit was then set 15–20 mmHg less than this upper alarm limit. Infants remained in their assigned target group for 28 days after enrollment. Masking of the intervention was not performed given the multitude of clinically indicated ventilator adjustments based on TcCO2 monitors used in routine care.
Extubation and reintubation criteria for pH and PCO2 differed between groups. Infants in both groups could be extubated when they met all criteria: SpO2 ≥ 88% with FiO2 ≤ 0.50; conventional ventilator rate ≤ 20 breaths per minute; mean airway pressure (MAP) < 8 cmH2O; amplitude < 2 times the MAP if on high frequency oscillator; and hemodynamically stable (clinically acceptable blood pressure and perfusion per clinical team). In addition, infant in the higher group could be extubated if they had a pH ≥ 7.20 and PCO2 ≤ 75 mmHg, while infants in the lower group could be extubated if they had a pH ≥ 7.25 and PCO2 ≤ 55 mmHg. Infants in both groups could be reintubated if they met any of the following criteria: SpO2 ≥ 88% with FiO2 ≤ 0.80 for ≥ 1 h; repetitive apnea requiring bag and mask ventilation > 1 per hour; clinically defined shock; sepsis; required for surgery; or hemodynamically unstable. In addition, infants in the higher group could be reintubated if they had a pH < 7.20 or a PCO2 > 75 mmHg, whereas infants in the lower group could be reintubated if they had a pH < 7.25 or a PCO2 > 55 mmHg.
Infants in both groups were extubated to noninvasive positive pressure ventilation [20]. If reintubation occurred, infants remained in the same group for the 28 day trial period. The clinician could defer extubation within 7 days of a failed extubation attempt in both groups. An algorithm was used by clinicians while infants remained on invasive mechanical ventilation. This algorithm for out of range carbon dioxide levels suggested the type of ventilator change for infants on mechanical ventilation but not its magnitude. Although the algorithm was based primarily on carbon dioxide measurements, clinical assessments including but not limited to, chest wall movements, breath sounds, and cardiovascular function and perfusion were performed simultaneously. To minimize volutrauma, a high rate was favored over high pressures in both groups for PCO2 elimination [21]. If PCO2 still exceeded the target or if there were concerns for gas trapping, peak inspiratory pressure could be increased. Oxygenation was improved primarily by increasing the FiO2 for infants who were hypoxemic with a FiO2 ≤ 0.40. If the FiO2 was between 0.40 and 0.70, and the infant was hypoxemic, the treating clinician could increase the MAP or FiO2. If the FiO2 was ≥ 0.70, oxygenation was improved predominantly by increasing the MAP. All infants enrolled in the study had routine continuous oxygen monitoring and oxygen saturation targets [22]. Our units protocol permits sodium bicarbonate if the serum bicarbonate is less than 15 mEq/L. Base was not given to accommodate higher levels of permissive hypercapnia.
2.4. Measures
The primary outcome was the number of days alive and ventilator‐free in the 28 days after randomization. Ventilator‐free was defined as being off invasive mechanical ventilation. Secondary outcomes included hospital mortality, grade 2–3 BPD [23], use of postnatal steroids for BPD, pulmonary hypertension diagnosed on routine echocardiography at 28 days ± 7 days [24], presence of a hemodynamically significant patent ductus arteriosus [25], weight and head circumference indices during the 28 day intervention period, the number of days on respiratory support in the 28 days after randomization, and severe neurodevelopmental impairment (NDI). Invasive respiratory support was defined as mechanical ventilation (conventional or high frequency) through an endotracheal tube. Noninvasive respiratory support (NRS) was defined as noninvasive positive pressure ventilation, continuous positive airway pressure, or high‐flow nasal cannula. Oxygen therapy was defined as supplemental oxygen via low flow cannula, head box, or incubator. Additional safety outcomes included number of reintubations during the 28 days, stage ≥ 2 necrotizing enterocolitis (NEC), and late intraventricular hemorrhage (IVH) defined as a new‐onset IVH or extension of an existing IVH after study entry [18]. We collected daily data on pH, PCO2, MAP, ventilator rate, and FiO2 during the randomization period. We used the first blood gas result and ventilator data available on each day. Severe NDI among those who attended for 22–26 month follow‐up was defined as either a cognitive composite score or motor composite score < 70 on Bayley Scales of Infant Development, Gross Motor Function Classification System ≥ 3, blindness with or without some functional vision, or hearing impairment requiring amplification.
2.5. Statistical Analysis
A sample size of 130 infants was required to demonstrate a (mean ± standard deviation) 4 ± 7 day increase in the number of alive ventilator‐free days in the 28 days after randomization with a power of 90% and significance level of 0.05 [16]. All analyses were planned a priori and by intention to treat by a statistician masked to group assignment. For testing differences between groups, we used the generalized linear model based on the estimating equations approach to address the correlation among clusters due to multiples. The correlation structure assumed was compound symmetry. For binary outcomes, we used the logit link function and obtained the estimates for the odds ratios, and for continuous outcomes we assumed the normal distribution. For count outcomes (e.g., number of days free of ventilation, etc.), we used the Poisson regression with log link function using the total number of days in the study for the first 28 days as an offset. For comparing the time to death in the 28‐day period, we used the shared frailty proportional hazard model to account for intra‐cluster dependence in the presences of censoring. SAS version 9.4 (Cary, NC) was used for statistical analyses. A p< 0.05 was considered statistically significant.
3. Results
We enrolled 130 preterm infants with 62 randomized to higher levels and 68 randomized to lower levels of permissive hypercapnia (Figure 1). All 130 infants completed the study and were included in the primary analysis. The gestational age of participants was (mean ± standard deviation) 24 weeks 5 days ± 14 days and birth weight was 657 ± 198 grams. Rates of exposure to antenatal corticosteroids, surfactant, and mechanical ventilation before study entry did not differ between groups (Table 1). There was a higher rate of multiple births in the lower permissive hypercapnia group. Baseline pH, PCO2, and ventilator settings did not differ. The daily pH and PCO2 (mean ± SD, 7.31 ± 0.07 and 55 ± 10 mmHg in the higher group vs. 7.32 ± 0.07 and 52 ± 8 mmHg in the lower group) differed significantly between groups after study entry (all p < 0.05; Table S1 and Figure 2). The daily ventilator rate and FiO2 were both decreased in the higher group compared with the lower group (all p < 0.05; Table S1). The daily mean airway pressure did not differ.
Figure 1.
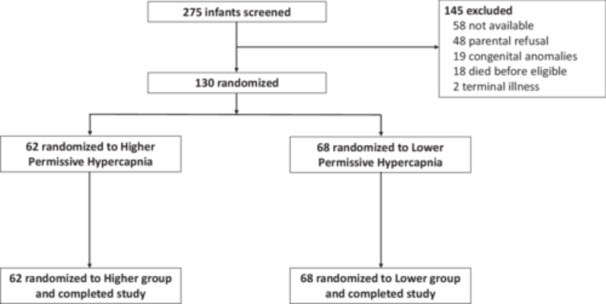
Flow diagram showing the number of infants screened, randomized, and analyzed.
Table 1.
Baseline characteristics and clinical demographics of study participants.
| Higher group (n = 62) | Lower group (n = 68) | |
|---|---|---|
| Gestational age (weeks), mean ± SD | 25 0/7 ± 14/7 | 24 4/7 ± 13/7 |
| Gestational age strata | ||
| 22–25 weeks' gestation, n (%) | 46 (74) | 54 (79) |
| 26–28 weeks' gestation, n (%) | 12 (19) | 11 (16) |
| 29–36 weeks' gestation, n (%) | 4 (6) | 3 (4) |
| Birth weight (grams), mean ± SD | 686 ± 220 | 631 ± 173 |
| Female, n (%) | 31 (50) | 33 (49) |
| Multiple, n (%) | 8 (13) | 24 (35)* |
| Confirmed chorioamnionitis, n (%) | 30 (48) | 42 (62) |
| Cesarean delivery, n (%) | 37 (60) | 34 (50) |
| Any antenatal corticosteroids, n (%) | 55 (89) | 62 (91) |
| Surfactant, n (%) | 62 (100) | 67 (99) |
| Race | ||
| Black, n (%) | 36 (58) | 39 (57) |
| White, n (%) | 23 (37) | 25 (37) |
| Other, n (%) | 3 (5) | 4 (6) |
| PMA at entry (weeks), mean ± SD | 26 2/7 ± 14/7 | 25 5/7 ± 14/7 |
| Days after birth at entry, mean ± SD | 9 ± 2 | 10 ± 3 |
| Days ventilated at entry, mean ± SD | 8 ± 3 | 8 ± 3 |
| Weight at entry (g), mean ± SD | 689 ± 245 | 622 ± 198 |
| Length at entry (cm), mean ± SD | 31.1 ± 3.8 | 30.4 ± 2.8 |
| HC at entry (cm), mean ± SD | 21.9 ± 2.6 | 21.2 ± 2.2 |
| pH at entry, mean ± SD | 7.29 ± 0.08 | 7.30 ± 0.07 |
| PCO2 at entry, mean ± SD | 51 ± 10 | 53 ± 11 |
| Ventilator rate at entry, mean ± SD | 48 ± 28 | 49 ± 30 |
| FiO2 at entry, mean ± SD | 0.45 ± 0.22 | 0.50 ± 0.26 |
| Mean airway pressure at entry, mean ± SD | 8.1 ± 2.3 | 8.2 ± 1.8 |
Abbreviations: FiO2 = fraction of inspired oxygen, HC = head circumference, PCO2 = partial pressure of carbon dioxide, PMA = postmenstrual age.
Significant with p < 0.05.
Figure 2.
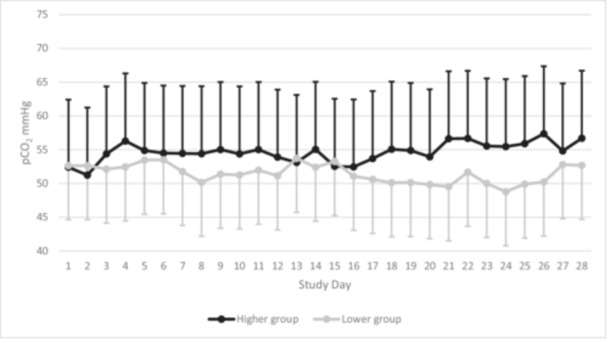
Daily average and standard deviation for carbon dioxide (PCO2) levels on blood gas in the 28 days after study entry by group assignment. The overall PCO2 was 55 ± 10 mmHg in the higher group versus 52 ± 8 mmHg in the lower group.
Infants randomized to the higher permissive hypercapnia group had an increase in the number of days alive and ventilator‐free in the 28 days after randomization compared with the lower permissive hypercapnia group (higher group 11 ± 10 days vs. lower group 6 ± 8 days; p = 0.009) (Table 2). This difference was primarily driven by a decrease in days on invasive ventilation (higher group 14 ± 10 days vs. lower group 19 ± 9 days; p = 0.006) with a corresponding increase in days on NRS among infants in the higher permissive hypercapnia group. There was no difference in the number of days alive during the 28 days (higher group 26 ± 7 days vs. lower group 25 ± 6 days; p = 0.48). The number of days on supplemental oxygen also did not differ.
Table 2.
Primary and secondary outcomes in the 28 days following randomization among infants randomized to higher versus lower levels of permissive hypercapnia.
| Higher (N = 62) | Lower (N = 68) | p value | |
|---|---|---|---|
| Alive ventilator free days, mean ± SD | 11 ± 10 | 6 ± 8 | 0.009* |
| Alive NRS free days, mean ± SD | 3 ± 6 | 2 ± 4 | 0.27 |
| Alive oxygen free days, mean ± SD | 1 ± 2 | 1 ± 3 | 0.79 |
| Days alive, mean ± SD | 26 ± 7 | 25 ± 6 | 0.48 |
| Intubated and ventilated days, mean ± SD | 14 ± 10 | 19 ± 9 | 0.006* |
| NRS days, mean ± SD | 9 ± 9 | 5 ± 6 | 0.005* |
| Oxygen days, mean ± SD | 2 ± 6 | 1 ± 3 | 0.10 |
Abbreviation: NRS = noninvasive respiratory support.
Significant with p < 0.05.
The rate of grade 2–3 BPD or death before discharge was not significantly lower among infants in the higher group compared with the lower group (higher group 27/62 (44%) vs. lower group 40/68 (59%); adjusted odds ratio (aOR) 0.54, 95% confidence intervals (CI) 0.27–1.08; p = 0.08) (Table 3). Grade 2–3 BPD among survivors at 36 weeks' postmenstrual age was not significantly different (higher 19/54 (35%) vs. lower 28/56 (50%); aOR 0.56, 95% CI 0.27–1.13; p = 0.12). The risk of death before discharge did not differ. There was no difference in rates of hemodynamically significant PDA, pulmonary hypertension on postnatal day 28, treatment with postnatal corticosteroids for BPD, proven NEC, late IVH, or discharge beyond 120 days after birth. There were no differences in growth indices between groups (Table S2).
Table 3.
Secondary outcomes among infants randomized to higher versus lower levels of permissive hypercapnia.
| Outcomes | Higher (N = 62) | Lower (N = 68) | Odds ratio (95% CI) | p value |
|---|---|---|---|---|
| Death during 28 day study period, n (%) | 8 (13) | 12 (18) | 0.70 (0.26–1.87) | 0.47 |
| Death before discharge, n (%) | 16 (26) | 20 (29) | 0.84 (0.38–1.87) | 0.68 |
| BPD (grade 2–3) among survivors at 36 weeks' gestation, n (%) | 19/54 (35) | 28/56 (50) | 0.56 (0.27–1.13) | 0.12 |
| BPD (grade 2–3) or death before discharge, n (%) | 27 (44) | 40 (59) | 0.54 (0.27–1.08) | 0.08 |
| Postnatal steroids, n (%) | 30 (48) | 42 (62) | 0.59 (0.29–1.21) | 0.15 |
| Pulmonary hypertension among those alive at Day 28, n (%) | 13/54 (24) | 7/56 (13) | 2.07 (0.74–5.79) | 0.16 |
| Reintubated during study, n (%) | 25 (40) | 31 (46) | 0.75 (0.36–1.53) | 0.42 |
| HS‐PDA, n (%) | 30 (48) | 22 (32) | 1.90 (0.91–4.00) | 0.09 |
| Proven NEC, n (%) | 7 (11) | 16 (24) | 0.49 (0.18–1.33) | 0.16 |
| Late IVH, n (%) | 3 (5) | 5 (7) | 0.64 (0.15–2.81) | 0.56 |
| Discharge home within 120 days, n (%) | 21 (34) | 16 (24) | 1.61 (0.73–3.55) | 0.24 |
| Lost to follow‐up | 12 (19) | 15 (22) | 0.88 (0.45–1.73) | 0·70 |
| Severe neurodevelopmental impairment at 22–26 months, n (%) | 15/33 (46) | 15/33 (46) | 0.96 (0.36–2.59) | 0.94 |
| Severe neurodevelopmental impairment or death at 22–26 months, n (%) | 32/50 (64) | 35/53 (66) | 0.97 (0.73–1.29) | 0.83 |
Abbreviations: BPD = bronchopulmonary dysplasia, HS‐PDA = hemodynamically significant patent ductus arteriosus, IVH = intraventricular hemorrhage, NEC = necrotizing enterocolitis.
The follow‐up rate for formal neurodevelopmental assessment at 22–26 months postmenstrual age was 79.2% and did not differ between groups. There was no difference in rates of severe NDI alone or severe NDI or death between groups (Table 3). Among the 33 infants in both groups who completed Bayley Scales of Infant Development testing the cognitive composite score (mean (SD), 75 (18) in higher group versus 77 (17) in lower group; p = 0.57) and motor composite score (mean (SD), 75 (19) in higher group versus 74 (20) in lower group; p = 0.73) did not differ between groups.
4. Discussion
Targeting higher levels of pH‐controlled permissive hypercapnia among preterm infants intubated for respiratory distress syndrome on postnatal day 7–14 increased the number of days alive and ventilator‐free in the subsequent 28 days compared with targeting lower levels. This was primarily driven by a reduction in the number of days in mechanical ventilation and corresponding increase in the number of days on noninvasive support in the higher PCO2 target group. The reduction in the rate of grade 2–3 BPD or death before discharge among infants in the higher PCO2 target group was not significant, although the current study was not powered for this outcome.
Our study's findings suggest that permissive hypercapnia beyond postnatal day 7–14 may be an important element of strategies to limit exposure to mechanical ventilation in preterm infants, consistent with prior randomized clinical trials of early pH‐controlled permissive hypercapnia [15, 16]. In a pilot study, 49 mechanically ventilated infants preterm infants weighing 601–1250 g were randomized for 96 h to a PCO2 of 45–55 mmHg and a pH ≥ 7.20 compared with a PCO2 of 35‐45 mmHg and a pH ≥ 7.25 [15]. The number of infants ventilated at 96 h was lower in the permissive hypercapnia group (p < 0.005) but major outcomes did not differ. In a randomized controlled trial of 220 extremely low birth weight preterm infants mechanically ventilated before 12 h of life, a PCO2 of > 52 mmHg was compared with a PCO2 < 48 mmHg maintained until postnatal day 10 [16]. Both arms used a pH‐control of > 7.20. There was a reduction noted in the number of infants ventilated at 36 weeks' postmenstrual age, consistent with the current definition of grade 3 BPD, in the higher permissive hypercapnia group although the rate of BPD or death did not differ [23].
In contrast, a randomized trial of early permissive hypercapnia in preterm infants 23–28 weeks' gestation comparing a higher PCO2 range of 55–65 mmHg with a lower PCO2 range of 35–45 mmHg for the first 7 days of life found no difference in the number of days on mechanical ventilation or other outcomes [17]. Both groups targeted a pH ≥ 7.25 which may have limited the ability to target a higher PCO2 as infants may not have developed sufficient metabolic compensation. An additional multicenter randomized trial among 359 preterm infants 23–28 weeks' gestation in the first 7–14 postnatal days, did not find a benefit of higher compared with lower levels of permissive hypercapnia, both in terms of mechanical ventilation or rates of BPD [18]. There was also an increased rate of NEC in the higher group that possibly related to the absence of pH‐control in this study. It is possible that maintaining pH‐control during permissive hypercapnia, as was done in the current study, may reduce complications although the optimal pH target has not been defined.
In studies designed to compare selective surfactant with prophylactic surfactant, pH‐controlled permissive hypercapnia was used alongside early routine CPAP as part of the bundle to reduce mechanical ventilation exposure in the selective surfactant groups [9]. In the SUPPORT trial, 1316 infants with a gestational age of 24 to 27 weeks were randomized to selective or prophylactic surfactant [13]. The selective group used extubation criteria including a PCO2 < 65 mmHg and a pH > 7.20 while the surfactant group had a PCO2 cut off < 50 mmHg and pH > 7.30 until postnatal day 14. The Vermont Oxford Network (VON) trial enrolled 648 infants with a gestational age of 26–29 weeks and compared three approaches to initial respiratory management in the first 7 postnatal days: prophylactic surfactant followed by a period of mechanical ventilation; prophylactic surfactant with rapid extubation to CPAP; or routine CPAP and selective surfactant [14]. The criteria for intubation in the CPAP group included a PCO2 > 65 mmHg. Both these trials individually reported lower rates of mechanical ventilation. Meta‐analyses including these two studies that used routine CPAP and permissive hypercapnia reported a reduction in BPD or death [9]. In the current study there was also a reduction in mechanical ventilation and a nonsignificant reduction in BPD suggesting that permissive hypercapnia may be an important component of strategies to reduce ventilator‐induced lung injury in preterm infants.
4.1. Limitations
This trial was not powered to detect clinically important differences in binary outcomes. Masking was not feasible given the routine use of TcCO2 monitors for ventilator management. Knowledge of group assignment could have resulted in bias related to the frequency or magnitude of weans. Adherence to extubation and reintubation criteria was monitored on a daily basis but we acknowledge that we did not formally measure protocol adherence in either group, which could have been biased by the lack of masking. The use of TcCO2 monitors may have helped maintain PCO2 separation between groups. However, the intended average PCO2 separation between groups was not reached. In some infants this regression to the mean may have occurred as extubated infants were largely in control their own PCO2 levels and/or because of increases in respiratory drive with increasing PCO2 [19]. In other infants the metabolic compensation may not have supported targeting a higher PCO2. This was a single center study in a unit with low rates of prolonged mechanical ventilation and high rates of treatment with noninvasive respiratory support. Infants included in this study may therefore represent a cohort at highest risk of adverse outcomes and the effect of this intervention in other settings is not known. Although our inclusion criteria extended up to 36 weeks' gestation, none of the infants enrolled were 31 weeks' gestation or higher, likely due to the low proportion of infants needing prolonged mechanical ventilation for RDS among infants at higher gestational ages.
5. Conclusion
Higher levels of pH‐controlled permissive hypercapnia among infants who remained mechanically ventilated on postnatal day 7–14 increased the number of alive ventilator‐free days compared with targeting lower levels of permissive hypercapnia. Targeting higher levels of pH‐controlled permissive hypercapnia beyond the first one to 2 weeks after birth may help reduce exposure to mechanical ventilation among preterm infants.
Author Contributions
Colm P. Travers conceptualized and designed the study, coordinated and supervised data collection, recruited infants, drafted the initial manuscript, and critically reviewed and revised the manuscript. Kimberly M. Armstead, Rachel L. Benz, and Deborah Laney designed the data collection instruments, collected data, and critically reviewed and revised the manuscript. Inmaculada Aban prepared the randomization materials, carried out the initial analyses, and critically reviewed and revised the manuscript. Samuel J. Gentle, Vivek V. Shukla, and Aaron J. Yee recruited infants, assisted with data collection, and critically reviewed and revised the manuscript. Namasivayam Ambalavanan and Waldemar A. Carlo conceptualized and designed the study, and critically reviewed and revised the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Conflicts of Interest
Dr. Travers is supported by a grant from Owlet Baby Care Inc. for an investigator‐initiated study (clinicaltrials.gov identifier: NCT05774470). The authors report no other relationships or activities that could appear to have influenced the submitted work. The authors have no conflicts of interest relevant to this article to disclose.
Supporting information
Supporting Material HYFIVE.
Acknowledgments
This study was supported by grants from the National Institute of Health (Travers CP: K23HL157618). The study sponsor(s) had no role in the study design, the collection, analysis, and interpretation of data, the writing of the report, and the decision to submit the manuscript for publication.
Data Availability Statement
Deidentified individual participant data will be made available upon publication through a data use agreement to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal. Proposals should be submitted to cptravers@uabmc.edu.
References
- 1. Bell E. F., Hintz S. R., Hansen N. I., et al., “Mortality, in‐Hospital Morbidity, Care Practices, and 2‐Year Outcomes for Extremely Preterm Infants in the US, 2013–2018,” Journal of the American Medical Association 327, no. 3 (January 2022): 248–263, 10.1001/jama.2021.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Doyle L. W., Carse E., Adams A. M., Ranganathan S., Opie G., and Cheong J. L. Y., “Ventilation in Extremely Preterm Infants and Respiratory Function at 8 Years,” New England Journal of Medicine 377, no. 4 (2017): 329–337, 10.1056/NEJMoa1700827. [DOI] [PubMed] [Google Scholar]
- 3. Horbar J. D., Greenberg L. T., Buzas J. S., Ehret D. E. Y., Soll R. F., and Edwards E. M., “Trends in Mortality and Morbidities for Infants Born 24 to 28 Weeks in the US: 1997–2021,” Pediatrics 153, no. 1 (January 2024): e2023064153, 10.1542/peds.2023-064153. [DOI] [PubMed] [Google Scholar]
- 4. Doyle L. W., Andersson S., Bush A., et al., “Expiratory Airflow in Late Adolescence and Early Adulthood in Individuals Born Very Preterm or With Very Low Birthweight Compared With Controls Born at Term or With Normal Birthweight: A Meta‐Analysis of Individual Participant Data,” Lancet Respiratory Medicine 7, no. 8 (2019): 677–686, 10.1016/S2213-2600(18)30530-7. [DOI] [PubMed] [Google Scholar]
- 5. Islam J. Y., Keller R. L., Aschner J. L., Hartert T. V., and Moore P. E., “Understanding the Short‐ and Long‐Term Respiratory Outcomes of Prematurity and Bronchopulmonary Dysplasia,” American Journal of Respiratory and Critical Care Medicine 192, no. 2 (2015): 134–156, 10.1164/rccm.201412-2142PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McEvoy C. T., Jain L., Schmidt B., Abman S., Bancalari E., and Aschner J. L., “Bronchopulmonary Dysplasia: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases,” supplement, Annals of the American Thoracic Society 11, no. S3 (2014): S146–S153, 10.1513/AnnalsATS.201312-424LD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walsh M. C., Morris B. H., Wrage L. A., et al., “Extremely Low Birthweight Neonates With Protracted Ventilation: Mortality and 18‐Month Neurodevelopmental Outcomes,” Journal of Pediatrics 146, no. 6 (June 2005): 798–804, 10.1016/j.jpeds.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 8. Dargaville P. A., Kamlin C. O. F., Orsini F., et al., “Effect of Minimally Invasive Surfactant Therapy vs Sham Treatment on Death or Bronchopulmonary Dysplasia in Preterm Infants With Respiratory Distress Syndrome: The OPTIMIST—A Randomized Clinical Trial,” Journal of the American Medical Association 326, no. 24 (December 2021): 2478–2487, 10.1001/jama.2021.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rojas‐Reyes M. X., Morley C. J., and Soll R., “Prophylactic Versus Selective Use of Surfactant in Preventing Morbidity and Mortality in Preterm Infants,” Cochrane Database of Systematic Reviews, no. 3 (March 2012): CD000510, 10.1002/14651858.CD000510.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ambalavanan N., Weese‐Mayer D. E., Hibbs A. M., et al., “Cardiorespiratory Monitoring Data to Predict Respiratory Outcomes in Extremely Preterm Infants,” American Journal of Respiratory and Critical Care Medicine 208, no. 1 (July 2023): 79–97, 10.1164/rccm.202210-1971OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Kaam A. H., De Jaegere A. P., and Rimensberger P. C., “Incidence of Hypo‐ and Hyper‐Capnia in a Cross‐Sectional European Cohort of Ventilated Newborn Infants: Table 1,” Archives of Disease in Childhood ‐ Fetal and Neonatal Edition 98, no. 4 (July 2013): F323–F326, 10.1136/archdischild-2012-302649. [DOI] [PubMed] [Google Scholar]
- 12. Thome U. H. and Ambalavanan N., “Permissive Hypercapnia to Decrease Lung Injury in Ventilated Preterm Neonates,” Seminars in Fetal and Neonatal Medicine 14, no. 1 (February 2009): 21–27, 10.1016/j.siny.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 13. Finer N. N., Carlo W. A., Walsh M. C., et al., “For the SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Early CPAP Versus Surfactant in Extremely Preterm Infants.” New England Journal of Medicine (May 2010). 362, 1970–1979. 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dunn M. S., Kaempf J., de Klerk A., et al., “Randomized Trial Comparing 3 Approaches to the Initial Respiratory Management of Preterm Neonates.” Pediatrics (November 2011). 128, e1069–e1076. 10.1542/peds.2010-3848. [DOI] [PubMed] [Google Scholar]
- 15. Mariani G., Cifuentes J., and Carlo W. A., “Randomized Trial of Permissive Hypercapnia in Preterm Infants,” Pediatrics 104, no. 5 Pt 1 (1999): 1082–1088, 10.1542/peds.104.5.1082. [DOI] [PubMed] [Google Scholar]
- 16. Carlo W. A., Stark A. R., Wright L. L., et al., “Minimal Ventilation to Prevent Bronchopulmonary Dysplasia in Extremely‐Low‐Birth‐Weight Infants,” Journal of Pediatrics 141, no. 3 (2002): 370–375, 10.1067/mpd.2002.127507. [DOI] [PubMed] [Google Scholar]
- 17. Thome U. H., Carroll W., Wu T. J., et al., “Outcome of Extremely Preterm Infants Randomized at Birth to Different PaCO2 Targets During the First Seven Days of Life,” Neonatology 90, no. 4 (2006): 218–225, 10.1159/000092723. [DOI] [PubMed] [Google Scholar]
- 18. Thome U. H., Genzel‐Boroviczeny O., Bohnhorst B., et al., “Permissive Hypercapnia in Extremely Low Birthweight Infants (PHELBI): A Randomised Controlled Multicentre Trial,” Lancet Respiratory Medicine 3, no. 7 (2015): 534–543, 10.1016/S2213-2600(15)00204-0. [DOI] [PubMed] [Google Scholar]
- 19. Travers C. P., Carlo W. A., Nakhmani A., et al., “Late Permissive Hypercapnia and Respiratory Stability Among Very Preterm Infants: A Pilot Randomised Trial,” Archives of Disease in Childhood ‐ Fetal and Neonatal Edition 108, no. 5 (September 2023): 530–534, 10.1136/archdischild-2022-325166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lemyre B., Deguise M. O., Benson P., Kirpalani H., De Paoli A. G., and Davis P. G., “Nasal Intermittent Positive Pressure Ventilation (NIPPV) Versus Nasal Continuous Positive Airway Pressure (NCPAP) for Preterm Neonates After Extubation,” Cochrane Database of Systematic Reviews 7, no. 7 (July 2023): CD003212, 10.1002/14651858.CD003212.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Greenough A., Rossor T. E., Sundaresan A., Murthy V., and Milner A. D., “Synchronized Mechanical Ventilation for Respiratory Support in Newborn Infants,” Cochrane Database of Systematic Reviews 9, no. 9 (August 2016): CD000456, 10.1002/14651858.CD000456.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Askie L. M., Darlow B. A., Finer N., et al., “Association Between Oxygen Saturation Targeting and Death or Disability in Extremely Preterm Infants in the Neonatal Oxygenation Prospective Meta‐Analysis Collaboration,” Journal of the American Medical Association 319, no. 21 (June 2018): 2190–2201, 10.1001/jama.2018.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jensen E. A., Dysart K., Gantz M. G., et al., “The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence‐Based Approach,” American Journal of Respiratory and Critical Care Medicine 200, no. 6 (September 2019): 751–759, 10.1164/rccm.201812-2348OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhat R., Salas A. A., Foster C., Carlo W. A., and Ambalavanan N., “Prospective Analysis of Pulmonary Hypertension in Extremely Low Birth Weight Infants,” Pediatrics 129, no. 3 (March 2012): e682–e689, 10.1542/peds.2011-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gentle S. J., Travers C. P., Clark M., Carlo W. A., and Ambalavanan N., “Patent Ductus Arteriosus and Development of Bronchopulmonary Dysplasia‐Associated Pulmonary Hypertension,” American Journal of Respiratory and Critical Care Medicine 207, no. 7 (April 2023): 921–928, 10.1164/rccm.202203-0570OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Material HYFIVE.
Data Availability Statement
Deidentified individual participant data will be made available upon publication through a data use agreement to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal. Proposals should be submitted to cptravers@uabmc.edu.


