Abstract
This paper describes the public repository that houses multimodal data collected as part of Aging Brain Cohort study being conducted at the University of South Carolina (ABC@UofSC). Ultimately, the ABC@UofSC Repository will contain diverse data from cross sectional (N = 800, age = 20–80) and longitudinal (N = 200, age = 60–80, interesting interval = 4 years) samples of healthy South Carolinians which include socio demographic data, raw and preprocessed functional (resting-state and task based fMRI, ASL) and structural (T1, T2 FLAIR, DWI, SWI) MRI data, raw and preprocessed resting-state EEG, comprehensive blood work, measures of physical and sensory function, genetic data derived from whole blood and buccal swabs, and results from a unique constellation of social, emotional, cognitive and language measures. Currently, data has been collected from 65 participants (ages 60–80) in the longitudinal arm of the project. The ABC@UofSC Repository is unique in its broad range of measures, choice of state-of-the art MRI sequences, inclusion of complex language discourse measures, and demographic diversity. Data from the ABC@UofSC Repository are easily accessible upon request (abc.sc.edu), and our publicly available statistics and visualization tools provide collaborating researchers with the ability to identify associations between brain structure and function in relation to genetic variation and behavioral measures across the adult life-span with unprecedented ease and rapidity.
Keywords: Data repository, Neuroimaging, Multimodal, Healthy aging, Older adults, Lifestyle, Cognition, Language
Highlights
-
•
The Aging Brain Repository contains multimodal data from a large, ongoing study on healthy aging.
-
•
This publicly accessible database contains a unique constellation of biological and behavioral measures.
-
•
The database contains particularly rich data on human language perception and production.
-
•
This repository will illuminate relationships between genetics, brain structure/function, lifestyle and age.
Glossary of terms
- ABC@UofSC
Aging Brain Cohort Study at the University of South Carolina
- AAL
automated anatomical labelling
- ABC
Activities-specific Balance Confidence
- AFNI
Analysis of Functional NeuroImages
- AHRQ
Adult Reading History Questionnaire
- AICHA
atlas of intrinsic connectivity of homotopic areas
- AP
absolute power
- AR
activation ratio
- ASL
arterial spin labeled
- BASIL
Bayesian Inference for Arterial Spin Labelling
- BET
brain extraction tool
- BP
blood pressure
- BRFSS
Behavioral Risk Factor Surveillance System
- CAT
Computational Anatomy Toolbox
- CBC
complete blood count
- CBF
cerebral blood flow
- CHAT
Codes for Human Analysis of Transcripts
- CIU
content information units
- CLAN
Computer Language ANalysis
- CMP
comprehensive metabolic panel
- CSV
cerebrospinal volume
- CTOPP
Comprehensive Test of Phonological Processing
- DNA
deoxyribonucleic acid
- DWI
diffusion weighted imaging
- EEG
electroencephalogram
- EPI
echo-planar imaging
- eTIV
estimated total intracranial volume
- FA
fractional anisotropy
- FFT
fast fourier transform
- FLAIR
fluid-attenuated inversion recovery
- FLEX
Foundations of Lipids and EXercise
- FMR1
Fragile X mental retardation 1
- fMRI
functional magnetic resonance imaging
- FOV
field of view
- FWHM
full width at half maximum
- GM
gray matter
- hA1c
hemoglobin a1C
- HAPPE
Harvard Automated Preprocessing Pipeline for Electroencephalography
- HIPAA
Health Insurance Portability and Accountability Act
- ICA
independent components analysis
- IPAQ
International Physical Activity Questionnaire
- MCBI
McCausland Center for Brain Imaging
- MD
mean diffusivity
- MGH
Massachusetts General Hospital
- MNI
Montreal Neurological Institute
- MoCA
MOntreal Cognitive Assessment
- mPCR
methylated polymerase chain reaction
- MPRAGE
magnetisation prepared gradient echo
- MRI
magnetic resonance imaging
- NHIS
National Health Interview Survey
- NIH
National Institutes of Health
- PASE
Physical Activity Scale for the Elderly
- PCASL
pseudo-continuous arterial spin labelling
- PCR
polymerase chain reaction
- PE-GRE
phase-encoded gradient echo
- PLD
post-label delay
- PROMIS
Patient-Reported Outcomes Measurement Information System
- PSD
power spectral density
- PSQI
Pittsburgh Sleep Quality Index
- QOLI
Quality of Life Inventory
- RAND-SF
Research and Development Short Form
- REDCap
Research Electronic Data Capture
- ROI
region of interest
- SAS
Statistical Analysis System
- SC
South Carolina
- SL
slice number
- SNP
single-nucleotide polymorphism
- SPM
Statistical Parametric Mapping
- SPSS
Statistical Package for the Social Sciences
- SSQ
Speech Spatial Qualities
- ST
slice thickness
- SWI
susceptibility-weighted imaging
- TE
echo time
- TR
repetition time
- TSH
thyroid-stimulating hormone
- UCR-BGC
University of California Riverside - Brain Game Center
- UofSC
University of South Carolina
- VBM
voxel based morphometry
- Vol
number of volumes
- VS
voxel size
- WM
white matter
- WMH
white matter hyperintensity
- XML
eXtensible Markup Language
1. Introduction
The Aging Brain Cohort at the University of South Carolina (ABC@UofSC) repository is a multimodal database derived from a planned, large-scale longitudinal and cross-sectional study of aging. Emerging evidence suggests the aging brain is shaped by both genetic and environmental factors (Barter and Foster, 2018; Cammarata et al., 2019; Cornwell and Waite, 2009; Coyle and Dugan, 2012; Finch and Ruvkun, 2001; Hueluer and Rebok, 2019; Kowald and Kirkwood, 2016; Onaolapo et al., 2019; Phillips, 2017; Pruchno et al., 2018; Rodríguez-Rodero et al., 2011; Schoentgen et al., 2020; Steptoe and Zaninotto, 2020) but little is known about the interaction between these factors and their influence on brain development and cognitive functioning. Decline in brain health underlies age-related cognitive decline (Fletcher et al., 2018), including memory loss (Pelletier et al., 2017; Small, 2001), reduced motor control (Seidler et al., 2010; Ward and Frackowiak, 2003), impairments in executive function (McDonald et al., 2018) and poor attention (Milham et al., 2002). Although some progress has been made towards understanding the relationship between cognitive decline and brain health, many of the important questions remain unanswered. This not only has important implications for understanding normal aging but is also vital for interpreting data from neurologically compromised populations, such as stroke or Alzheimer’s disease patients. Dementia and mild cognitive impairment are both common, but even those who do not experience these conditions may experience steady, and often subtle, cognitive decline associated with aging (Salthouse, 2019). These normal cognitive changes are important to understand because, first, they can affect an older adult’s day-to-day function and, second, they can help distinguish normal from disease states (Harada et al., 2013). A key aim of the ABC@UofSC project is to improve our understanding of healthy aging by identifying associations between brain structure and function in relation to genetic variation and behavioral/lifestyle measures across the adult life-span. The ABC@UofSC database currently contains data for 65 participants, but the plan is to grow it to a sample size of 800.
Several features of the ABC@UofSC repository make it unique. First, the breadth of measurements taken in this study rivals or surpasses other similar multimodal databases (Bookheimer et al., 2019; Razlighi et al., 2017; Schoffelen et al., 2019; Taylor et al., 2017). Following completion of multiple hours of survey-based physical and mental health measures, diet measures and other questionnaires, participants in the ABC@UofSC study must complete two days of extensive on-site neurocognitive and health testing, which includes collection of structural (T1-weighted, T2-weighted FLAIR, diffusion weighted imaging [DWI], susceptibility weighted imaging [SWI]) and functional (resting-state and task-based functional magnetic resonance imaging [fMRI] and aerial spin labeled [ASL] images) data using best-of-breed sequences, electroencephalographic (EEG) data including eyes-open and eyes-closed resting-state sequences, measurements of physiological variables related to blood pressure, hearing, vision, smell and somatosensation, genetic testing (blood and buccal swabs) comprehensive blood panels, and a complex and utterly unique battery of cognitive tests some of which are well known (NIH Toolbox, MoCA, etc) and some of which are in-house measures developed by our team. The second aspect of this study that makes it unique is its focus on, and measurement depth within, the field of human language processing. Because our core group has significant expertise in these domains, our cognitive testing battery was designed to include an abundance of speech and language tests, including sentence repetition, multiple discourse tasks, etc., which are not typically found in large longitudinal studies of aging (Razlighi et al., 2017; Taylor et al., 2017) but may provide insights into age related challenges in communication and their causes. Third, our neuroimaging data are processed using best-of-breed methods encapsulated and presented in a simple to distribute format that, along with our custom analysis software, allows researchers to examine the relationship between brain data and behavioral data with unprecedented ease and rapidity. Finally, this study is one-of-a-kind in that it is conducted in the state of South Carolina and is designed to assess a representative sample of that state’s unique population. Stroke rates within South Carolina are among the highest in the country (Howard and Howard, 2020), and cases of dementia, already startlingly high (Koller and Bynum, 2015), are projected to top 120,000 by 2025 (AARC, 2020). Based on these statistics, the state of South Carolina has decided that better understanding the process of healthy aging is particularly vital. Additionally, because the state of South Carolina is highly economically and racially diverse, the ABC@UofSC repository will allow researchers to explore the relationship between these variables and healthy aging with high fidelity.
Data for the repository will be collected in three stages over a project span of five years. In the first stage of data collection (year one), we recruit healthy participants in the age range of 60–80 (currently 65 have completed; target N = 200). These participants are part of our longitudinal dataset and will be tested at two time points separated by four years. Prior to enrollment, all participants are screened for eligibility using an electronic survey hosted by our university website (abc.sc.edu) which gathers data on age, gender, race, and socioeconomic status. Once eligibility is established, participants complete questionnaires designed to measure health history, diet, quality of life, sleep, physical function, speech and hearing abilities, reading history, access to healthcare, social interactions, and family engagement. Additional behavioral testing, collection of biological specimens used for genetic analysis (blood and saliva), and measurements of brain structure and function (using EEG and MRI) are accomplished via in-person sessions conducted at the on-campus ABC@UofSC testing suite, and at McCausland Center for Brain Imaging (MCBI) at Prisma Health heart hospital. In the second stage of the study, which will be conducted in years 2–4, we will collect a cross-sectional dataset including approximately 600 participants between the ages of 20 and 60. Testing for these participants will be identical to testing conducted for participants in the first stage of data collection. Finally, in the third stage of the project (year five) we will conduct follow-up evaluations of participants that were evaluated in stage one. This subset of participants will go through the same initial evaluation they completed during the first year of the project. The full dataset will yield a cross-sectional database of adults 20–80 years old (N = 600) as well as a more restricted longitudinal database containing pre- and post-evaluation (4 year inter-evaluation interval, N = 200) of adults between the ages of 60 and 80. Both datasets will be interrogated for behavioral and brain changes related to normal aging.
2. Recruitment
Recruitment goals were established to reflect the diversity, with regards to age, gender, race and socioeconomic status, of South Carolina’s (SC’s) elderly population. Eligible participants, as determined by the initial eligibility survey described above (viewable at https://is.gd/abceligibility), are stratified based on these criteria. We used the Hollingshead Index (Hollingshead and Others, 1975), a multifactor measurement tool, to assign participants to low, middle and high income categories, and participants were recruited using an adaptive strategic plan to meet our stratified sampling goals. A dedicated recruiter created and distributed study materials. Flyers and brochures were distributed to local churches, senior citizen centers, wellness and community centers, community events, health fairs and libraries to reach adults aged 60–80 in the first stage of the study. Promotional items such as t-shirts, chip clips, and pens were distributed during events to prospective participants. Recruitment materials provided on our website were designed to give potential participants easy access to contact information, clear information regarding the study, a virtual tour of study activities, and a link to our eligibility survey. In addition to typical flyer locations, we aimed to target a more diverse group by distributing recruitment materials at local barber/beauty shops, laundromats, thrift stores, and food banks. A press release was also developed and distributed to mainstream as well as minority media outlets. In addition to recruitment materials, social media accounts were established on Facebook, Instagram and Twitter to aid in recruitment (and retention) of participants by connecting with the community, brain health and health research communities as well as fellow researchers.
Overall, we tailored our strategies for recruitment with age groups and cultural sensitivity in mind. Initially, recruitment focused on the first stage of the study. Strategies to promote and maintain a positive relationship between participants and the study team are important in longitudinal studies (Lyons et al., 2004). We also recognize that retention of participants over time is a challenge in longitudinal studies (Lyons et al., 2004; Polit and Beck, 2008). Useful strategies to promote a positive relationship included sending newsletters via email to participants, sending email updates, maintaining consistency of contact person, and expressing appreciation for participants’ time and effort. Useful strategies for maintaining contact with participants included obtaining contact information at every data collection point, as well as maintaining birthdates and preferred contact information and chart numbers in our repository database (Hanna et al., 2014; Lyons et al., 2004; Polit and Beck, 2008). Social media platforms were also used to maintain social contact with participants, provide updates on study progress, introduce team members to the community, and share introduce content to promote engagement. The recruitment plan is likely to shift towards online media as we move into the second, cross-sectional arm of the study.
3. Database general overview
Data collected from the current study are stored primarily (with the exception of large MRI and EEG related datasets) in the ABC@UofSC Repository. This ABC@UofSC Repository leverages the Research Electronic Data Capture (REDCap) system, a HIPAA-compliant, highly secure web-based application developed by Vanderbilt University and designed to capture and store data from large studies (Harris et al., 2019). REDCap is sponsored through Health Sciences of South Carolina, and it allows for flexibility in the design of studies, including longitudinal design and multiple-assessment studies. REDCap allows for easy data entry either by uploading a pre-formatted Excel document or by direct entry of data into the online interface. Furthermore, it provides automated export procedures for seamless data downloads to Excel and common statistical packages (SPSS, SAS, Stata, R), as well as a built-in project calendar, a scheduling module, ad hoc reporting tools, and advanced features, such as branching logic, file uploading, and calculated fields. Data stored in the REDCap database include basic demographic data (Table 1), information regarding personal medical history (Table 2), as well as various raw/computed scores from initial and in person surveys (Table 3 and Table 4 respectively). Summary statistics (mean, standard-deviation and range) from various physical, cognitive, emotional and language tests can be found in Table 5. Results from blood tests, FMR1 analysis of buccal swabs, and select preprocessed/computed and de-identified data derived from in-house analysis of MRI and EEG data (discussed below) are also available. Raw brain imaging data (MRI and EEG) are stored on a separate, secure UofSC server.
Table 1.
Demographic information for the current ABC@UofSC study sample.
| Demographic Information (N = 65) | N | Percent | |
|---|---|---|---|
| Age | 60–65 | 21/65 | 32% |
| 66–70 | 24/65 | 37% | |
| 71–75 | 11/65 | 17% | |
| 76–80 | 9/65 | 2% | |
| Sex | Female | 43/65 | 66% |
| Male | 22/65 | 34% | |
| Race | White | 46/65 | 71% |
| Black | 19/65 | 29% | |
| Other | 0/65 | 0% | |
| Education | Primary School | 0/65 | 0% |
| High School | 15/65 | 23% | |
| College | 32/65 | 49% | |
| Graduate School | 8/65 | 12% | |
| Post-Graduate | 10/65 | 16% | |
Table 2.
Frequency of self-reported medical conditions in the current ABC@UofSC sample.
| Condition | Frequency | Percent |
|---|---|---|
| high cholesterol | 34/65 | 52.30% |
| joint pain | 28/65 | 43.10% |
| high blood pressure | 27/65 | 41.50% |
| allergies | 26/65 | 40.00% |
| arthritis | 25/65 | 38.50% |
| GERD/Reflux | 19/65 | 29.20% |
| obesity | 14/65 | 21.50% |
| cancer | 12/65 | 18.50% |
| type-2 diabetes | 11/65 | 16.90% |
| pneumonia | 11/65 | 16.90% |
| asthma | 10/65 | 15.40% |
| irregular heartbeat | 10/65 | 15.40% |
| murmur | 10/65 | 15.40% |
| depression | 9/65 | 13.80% |
| anemia | 8/65 | 12.30% |
| migraine | 7/65 | 10.80% |
| glaucoma | 6/65 | 9.20% |
| hypothyroidism | 6/65 | 9.20% |
| blood transfusions | 5/65 | 7.70% |
| kidney stone | 5/65 | 7.70% |
| peptic ulcer disease | 4/65 | 6.20% |
| osteoporosis | 4/65 | 6.20% |
∗Fewer than 5% of participants reported COPD/Emphysema, blocked blood vessels, lymphoma, gastrointestinal disease, hepatitis, hyperlipid, thyroid disease, kidney disease, dyslexia, myocardial infarction, congestive heart failure, connective tissue disease, solid tumor, hyperthyroidism, heart attack, lung disease, rheumatoid arthritis, attention deficit disorder. ∗No participants reported peripheral vascular disease, stroke, transient ischaemic event, dementia, leukemia, liver disease, hemiplegia, chronic kidney disease, AIDS, HIV, cirrhosis, colitis, gestational diabetes, seizure, multiple sclerosis, rheumatic arthritis, speech language impairment or learning disability. GERD = Gastrointestinal Reflux Disease; ADD = attention deficit disorder; HIV = human immunodeficiency virus; AIDS = acquired immunodeficiency syndrome: COPD = chronic obstructive pulmonary disease.
Table 3.
Selected physical, cognitive, emotional and language variables from the current ABC@UofSC sample (N = 65).
| Physical Measures | Mean | STD | Range |
|---|---|---|---|
| PASE (total score) | 145.61 | 88.84 | 27–571 |
| NIH Toolbox | |||
| Grip Strength Dominant (percentile)∗ | 98.61 | 10.13 | 82–129 |
| Grip Strength Non-Dominant (percentile)∗ | 98.2 | 10.18 | 78–125 |
| 4-Meter Walk Time (seconds) | 3.72 | 0.81 | 1.68–6.38 |
| Pegboard Dominant∗ | 95.57 | 11.29 | 59–113 |
| Pegboard Non-Dominant∗ | 99.94 | 6.66 | 83–114 |
|
Cognitive Measures | |||
| MoCA | 25.1 | 3.35 | 15–30 |
| NIH Toolbox | |||
| NIH Toolbox-Flanker∗ | 90.99 | 7.19 | 69–105 |
| Flanker Task Mean RT (sec) | 1.08 | 0.31 | 0.66–1.94 |
| Picture Sequence Memory Test∗ | 93.15 | 11.52 | 76–124 |
| List-Sorting Memory Test∗ | 96.24 | 10.58 | 74–120 |
| Pattern Comparison Test∗ | 84.03 | 14.64 | 57–113 |
| Emotional Measures | |||
| QOLI (total score) | 3.4 | 1.2 | 0.31–5.37 |
| Satisfaction with Life Scale (total score) | 27.22 | 4.3 | 15–35 |
| NIH Toolbox | |||
| Emotional Support (raw score) | 35.02 | 4.83 | 22–40 |
| Perceived Stress (raw score) | 21.22 | 5.52 | 13–43 |
| LanguageMeasures | |||
| Discourse | 3.4 | 1.2 | 0.31–5.37 |
| Cat Rescue Narrative Duration (seconds) | 47.46 | 25.33 | 15–144 |
| Cat Rescue Words/Minute | 146.21 | 27.02 | 84.6–202.5 |
| Cookie Theft Narrative Duration (seconds) | 53.35 | 42.77 | 14–244 |
| Cookie Theft Words/Minute | 129.09 | 30.61 | 57.55–215 |
| Self-Paced Reading Task (% accuracy) | 83 | 7.94 | 62.5–97.9 |
| Sentence Repetition Task total score | 281.83 | 14.89 | 230–297 |
PASE = Physical Activity Scale for the Elderly; MoCA = Montreal Cognitive Assessment; NIH = National Institutes of Health; QOLI = Quality of Life Index; STD = Standard Deviation; RT = Reaction Time; ∗uncorrected standard score.
Table 4.
List of questionnaires completed by participants in the ABC@UofSC study prior to in-person testing.
| Questionnaire/Survey | What it measures |
|---|---|
| Short-Form Health Survey (RAND-SF 36) (Hays, 1994) | Provides an indication of perceived change in health by measuring physical functioning, bodily pain, role limitations due to physical health problems and personal or emotional problems, emotional well-being, social functioning, energy/fatigue, and general health perceptions. |
| BRFSS 2009 Section 11 Tobacco use (Rolle-Lake and Robbins, 2020) | Collects uniform, state-specific data on preventive health practices and risk behaviors that are linked to chronic diseases, injuries, and preventable infectious diseases that affect the adult population. Factors assessed by the BRFSS include tobacco use. |
| BRFSS 2009 Section 15 Alcohol Consumption (Rolle-Lake and Robbins, 2020) | Assesses levels of binge drinking by sex, age group, race/ethnicity, education level, income level, and disability status at the individual level, as well as geographic disparities in binge drinking at the state level. |
| NHIS Dietary Screener (National Cancer Institute (NCI), 2019) | Assesses the health and nutritional status of adults of children in the U.S. It collects detailed information about food, nutrients, and supplement intake and other dietary information. |
| Food Security Supplement-CPS-FSS (United States Department of Agriculture (USDA), 2019) | The questions detail food security, food expenditures, and use of food and nutrition assistance programs to determine what is needed for the population. It is a source of statistics on food insecurities. |
| Lifetime Discrimination Subscale of the Perceived (Williams et al., 1997) | Measures how often people feel that others treat them badly or unfairly on the basis of race, ethnicity, gender, age, religion, physical appearance, sexual orientation, or other characteristics. The scale covers discrimination in different areas of life, including at school, at work, and in one’s neighborhood. |
| Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989) | Measures the quality and patterns of sleep in the older adult. It differentiates “poor” from “good” sleep by measuring seven domains: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction over the last month. |
| Physical Activity Scale for the Elderly (PASE) (Washburn et al., 1993) | Measures the level of self-reported physical activity in individuals 65 and over. The PASE score combines information on leisure, household and occupational activity. |
| IPAQ Short Form (self-administered) (Craig et al., 2017) | Self-reported intensity of physical activity and sitting time that individuals do as a part of their daily lives. It is considered to estimate total physical activity in MET-min/week and time spent sitting. |
| PROMIS 57 Profile V20 (Cella et al., 2010) | Collection of short forms containing a fixed number of items from seven PROMIS domains (Depression, Anxiety, Physical Function, Pain Interference, Fatigue, Sleep Disturbance, and Ability to Participate in Social Roles and Activities). They assess all domains over the past seven days except for Physical Function which has no timeframe specified. |
| Speech, Spatial, and Qualities of Hearing Scale (SSQ12) (Noble et al., 2013) | Measures a range of hearing disabilities across several domains. Particular attention is given to hearing speech in a variety of competing contexts, and to the directional, distance and movement components of spatial hearing. |
| Adult Reading History Question-Revised (AHRQ) (Lefly and Pennington, 2000) | Self-report screening tool designed to measure risk of reading disability (i.e. dyslexia) in adults. The AHRQ asks adults about their own reading history and current reading habits in order to estimate the risk that they may have a reading disability. |
| Satisfaction with Life Scale (Diener et al., 1985) | A 5-item scale designed to measure global cognitive judgments of one’s life satisfaction (not a measure of either positive or negative affect). |
| Pearlin Mastery Scale (Pearlin and Schooler, 1978) | Measures the extent to which an individual regards their life chances as being under their personal control rather than fatalistically ruled. |
| Social Relationships Survey (Burt, 1984) | Name generator is used to collect information regarding anyone an individual has had personal contact or communication with over the past 7 and 30 days, excluding professional interactions and their role-relation. The degree of participation in social clubs, organizations, and other types of events is also collected. |
| Collective Efficacy Scale (Sampson et al., 1997) | Two-part scale that measures how well communities work together to make things happen. The informal social control section assesses how likely neighbors are to intervene when there is trouble, and the social cohesion and trust section assesses how likely neighbors are to support each other in times of need. |
| PROMIS SF V20 Emotional Support 8a (Hays et al., 2018) | The PROMIS Emotional Support item bank (currently adults only) assesses perceived feelings of being cared for and valued as a person; having confident relationships. |
| PROMIS SF V20 Instrumental Support (Hays et al., 2018) | Assesses self-reported perceived availability of assistance with material, cognitive or task performance. The instrumental support short forms are universal rather than disease-specific. |
| PROMIS SF V20 Ability to Participate Social Roles and Activities 8a (Hays et al., 2018) | Assesses the perceived ability to perform one’s usual social roles and activities. Items are worded negatively in terms of perceived limitations, but responses are reverse-coded so that higher scores represent fewer limitations (better abilities). |
| PROMIS SF V20 Social Isolation 8a (Hays et al., 2018) | Assesses perceptions of being avoided, excluded, detached, disconnected from, or unknown by, others. The item bank does not use a time frame (e.g. over the past seven days) when assessing social isolation. |
| Quality of Life Inventory (QOLI) (Frisch, 1994) | Provides a score that indicates a person’s overall satisfaction with life based on how well their needs, goals, and wishes are being met in important areas of life. |
Table 5.
List of tests conducted during in-person testing for the ABC@UofSC study.
| Task | Description | What it measures |
|---|---|---|
| Small et al. Sentence Repetition (Small et al., 2000) | Participants repeat sentences that are presented in a fixed, pseudo-random order at maximum volume via an application on a laptop. The sentence task includes five sentences each of six various sentence types (Active, Passive, Object-Subject, Object-Object, Subject-Subject, and Subject-Object) for a total of 30 sentences. | Overall measure of sentence-level language skills. Sentence repetition has been consistently found to have strong classification accuracy for identifying adults as well as children with developmental language disorder (cf., Poll, Betz and Miller, 2010). |
| Comprehensive Test of Phonological Processing, 2nd Edition (CTOPP 2)-nonword repetition subtest (Wagner et al., 2013) | Participants repeat made-up words that are presented via audio recording in a fixed order. | Assesses phonological processing skills. Proposed clinical marker of developmental language disorders and dyslexia. |
| Self-paced reading task (Just et al., 1982) | Participants are presented with sentences in 4 separate parts advancing to each part of the sentence at their own rate. The participant answers a Yes/No question following each sentence with a keyboard response. | Provides data on the length of time in seconds to read each sentence part as well as comprehension accuracy for the following sentence types: Active and Subject-Relative Active, Passive, Subject-Relative, and Object-Relative. |
| Montreal Cognitive Assessment (MoCA) Version 8.1 English (Nasreddine et al., 2005) | Screening tool for individuals with mild cognitive dysfunction. The test addresses 8 domains of cognitive functioning and provides a total score and memory index score. | Assesses visuospatial/executive functioning, naming, immediate/delayed recall, attention, language, abstraction, and orientation. |
| Boston Diagnostic Aphasia Examination- Cookie Theft picture description (Goodglass et al., 2001) | The “Cookie Theft” picture is presented and participants are instructed to, “Tell me everything you see going on in this picture.” This task is recorded for later transcription and analysis. | Discourse analysis of an elicited language sample of a picture description task. |
| “Cat Rescue” discourse measure from the AphasiaBank Non-Aphasia Control protocol (Nicholas and Brookshire, 1993) | The “Cat Rescue” picture is presented and participants are instructed to look at everything that’s happening and then tell the researcher a story about what they see, i.e. "Tell me a story with a beginning, a middle, and an end.” This task is recorded for later transcription and analysis. | Discourse analysis of an elicited language sample with a prompt to tell a story based on the picture provided. |
| Peer Conflict Resolution (PCR) discourse measure (Nippold et al., 2007) | A short story that illustrates a potential conflict scenario in which either male or female characters are presented. Participants are instructed to retell the story in their own words and then answer a set of questions based on what they thought about the issues and how they were handled. | Discourse analysis of an elicited expository language sample. The Nippold et al. (2007) found that an expository discourse sample contained more use of complex syntactic structures than conversational or narrative samples, with typical language and/or with language impairment. |
| Vowel Production Task (Boersma, 2001) | Participants are asked to read aloud monosyllabic American-English words. Six American-English vowels are presented embedded in/hVC/context (/i ϵ æ ɑ ʌ u/: ‘heat, head, hat, hot, hub, and hoot’). Stimuli are randomly presented in written form on a computer screen, with 15 presentations per stimulus type. Participant responses are analyzed using Praat software (Boersma, 2001). | We will derive the following measures: (1) formant centralisation ratio, a measure of individual vowel space, and (2) formant dispersion, a measure of articulatory stability. |
| NIH Toolbox- Flanker Inhibitory Control and Attention Test 12 + (attention & executive functioning) (Gershon et al., 2013) | Participant’s are presented with rows of arrows pointing either left or right via the NIH Toolbox application on an iPad. They are instructed to choose the button that represents the direction the middle arrow is pointing relative to a fixed location (i.e. ‘home base’). | The allocation of one’s limited capacities to deal with an abundance of environmental stimulation. |
| NIH Toolbox-Picture Sequence Memory Test 8 + (episodic memory) (Gershon et al., 2013) | Participants are asked to reproduce the sequence of pictures that is shown on the screen via the NIH Toolbox application on an iPad. | Cognitive processes involved in the acquisition, storage and retrieval of new information. |
| NIH Toolbox-List Sorting Working Memory Test 7 + (working memory) (Gershon et al., 2013) | Pictures of different foods and animals are displayed with both an accompanying audio recording and written text that name the item via the NIH Toolbox application on an iPad. The participant is asked to say the items back to the examiner in size order from smallest to largest. | Ability to store information until the amount of information to be stored exceeds one’s capacity to hold that information. |
| NIH Toolbox- Oral Reading Recognition Test 3 + (language) (Gershon et al., 2013) | Adaptive assessment where participants pronounce a word presented on the screen via the NIH Toolbox application on an iPad. | Reading decoding skill which is considered one of the crystallized abilities; those abilities are generally more dependent upon past learning experiences and consistent across the adult life span. |
| NIH Toolbox-Pattern Comparison Processing Speed 7 + (processing speed) (Gershon et al., 2013) | Requires participants to discern whether two side-by-side pictures are the same or not by choosing a yes or no button on the iPad screen always starting from a fixed location ("home base"). | Measures the amount of information that can be processed within a certain unit of time. Items are simple so as to purely measure processing speed. |
| Auditory Verbal Learning Test 8 + subdomain (immediate recall) (Gershon et al., 2013) | Participants are presented with a set of 15 unrelated words and are instructed to recall as many as they can for a total of three separate trials. | Measures immediate recall. Unrelated words presented via audio recording and participant recalls as many as possible. |
| NIH Toolbox- Oral Symbol Digit Test subdomain (processing speed) (Gershon et al., 2013) | Participants are presented with a key and rows of symbols. Following practice, participants are instructed to speak the number for each symbol, progressing row by row for a duration of 120 s. | Measures attention switching ability and processing speed. |
| Audiometric thresholds | Thresholds are captured by a trained speech language pathologist using an Amplivox 170 audiometer set to automatic testing mode (familiarization = ON, set at 2/3 correct responses). | Air conduction measurements. Responses are measured to the following frequencies in both the left and right ears: 250, 500, 1000, 2000, 3000, 4000, 6000, and 8000. |
| NIH Toolbox Words in Noise 6 + (Gershon et al., 2013) | A recorded voice tells the participant to listen to and repeat words via the NIH Toolbox application on an iPad. Background noise gets louder, reducing the signal-to-noise ratio. | Measures a person’s ability to recognize single words amid varying levels of background noise, measuring difficulty a person might have hearing in a noisy environment. |
| University of California- Riverside Brain Game Center UCR-BGC: Spatial Release App (Gallun et al., 2013) | Listening scenarios are presented (Single Talker, Progressive, and Separated) via the UCR-BGC Spatial Release application on an iPad. For each task, participants are instructed to listen to and select the color and number they hear from the target call sign. | Measures auditory thresholds for speech alone, speech in the presence of other talkers and the exert to which spatial auditory cues can rescue speech comprehensibility in the presence of other talkers. Studies have shown that it can dissociate effects of age from those of hearing loss. |
| Standing Balance (Gershon et al., 2013) | Participants progress through five different poses while wearing a gait belt with an iPod attached to detect any sway (each pose is held for 50 s). | This task is designed to assess postural sway and to determine participants’ vestibulo-spinal function. |
| Grip Strength (Gershon et al., 2013) | Participants complete a practice and test trial using a dynamometer to measure the grip strength of both hands in lbs. | The capacity of a muscle to produce the tension and power necessary for maintaining posture, initiating movement, or controlling movement during conditions of loading the musculoskeletal system. |
| 4-Meter Walk Gait Speed (Gershon et al., 2013) | Participants are instructed to walk a 4 m path at their normal walking pace for a practice trial and two timed trials to record the time in seconds. | Measures locomotion. |
| 9-Hole Pegboard Dexterity (Gershon et al., 2013) | Participant’s are instructed to place 9 pegs in a pegboard (placed front and center) one at a time and then remove all 9 pegs one at a time. The task is timed using the NIH Toolbox application on an iPad. | One’s ability to coordinate the fingers and manipulate objects in a timely manner. |
| Odor Identification (Gershon et al., 2013) | Participants are presented with scratch and sniff cards in a fixed order and instructed to identify which of four pictures on the screen matches the odor they have just smelled. | The ability to detect odors, to recognize and discriminate odor qualities, and to identify the sources of odors in our world. |
| Visual Acuity (Gershon et al., 2013) | Participants are seated 3 m from the screen and letters are displayed one at a time to measure distance vision. | As the participant successfully identifies letters of a given size, smaller ones appear, until the software ascertains the smallest size the participant can successfully identify. |
| NIH Emotion Battery 18 + (Gershon et al., 2013) | Participants select the answers that best represent their thoughts, feelings and behaviors. This test is self-administered utilizing the NIH Toolbox app on an iPad. | The surveys measure constructs such as, positive affect, general life satisfaction, emotional support, friendship, loneliness, perceived rejections, perceived hostility, self-efficacy, sadness, perceived stress, fear, anger, instrumental support, and meaning and purpose. |
| Activities-specific Balance Confidence (ABC) Scale (Powell and Myers, 1995) | Participants indicate their level of confidence in doing a specified activity without losing their balance or becoming unsteady from choosing one of the percentage points on the 11-point scale from 0% to 100%. | Provides a measure of balance confidence that can provide insight into individuals they may be at a higher risk of falling. |
3.1. Eligibility survey and remote testing questionnaires
The initial eligibility survey, as well as various remotely-administered questionnaires and assessments (Table 4) are administered to participants in paper, or online using REDCap’s virtual testing functionality, prior to the first in-person appointment. In order to officially enroll in the ABC@UofSC study, participants first fill out a paper version (5/65 current participants) or online version (60/65 current participants) of the ABC@UofSC eligibility survey. The eligibility survey is designed to gather basic demographic data which is used to fill cells within the stratification table we use to match our study’s distribution of age, gender and race to that of the state of South Carolina. Additionally, participants fill out a shortened MRI screening form to ensure they will be able to safely participate in the neuroimaging arm of the study.
Once eligibility is confirmed and participants are officially enrolled in the study, they are either sent a paper packet containing the ABC@UofSC surveys and questionnaires, or they are sent a link which allows them to access online versions of these same surveys and questionnaires. Data collected from pen and paper surveys was mailed back and manually entered into the REDCap database. Data collected electronically are automatically entered into our deidentified REDCap database with minimal staff intervention. Results from behavioral measures taken during the first day of in-person testing are manually entered into the ABC@UofSC Repository by study staff with the exception of the NIH toolbox (Table 5). In the case of the NIH toolbox, data is sent to our secure ABC@UofSC server and reshaped into user-specified REDCap variables using custom scripts available online (Joseph and Newman-Norlund, 2020) and subsequently automatically uploaded into the ABC@UofSC Repository. The ABC@UofSC Repository houses various instruments which are administered, including the initial eligibility screening forms, the ABC@UofSC questionnaire database, a database of behavioral measures, behavioral data and a protected database linking participant IDs to contact information. The eligibility screening forms and the initial ABC questionnaires are primarily sent to participants via an individualized REDCap link to allow the data to automatically be stored in our database. The behavioral data stored in our database includes, but is not limited to, demographic information, photo/video release authorization, speech/language and cognitive measures, auditory function, physiological measures, EEG data, and MRI summary. In addition, a procedural checklist is utilized to ensure key components of the study are completed and for future use as a reference when contacting participants who are eligible to return for the second stage of our longitudinal study.
4. Cognitive, behavioral and language measures
Each participant enrolled in the study was administered the same comprehensive battery of behavioral and language assessments. We chose a wide range of measures to provide us an overview of each participant’s cognition, language, hearing, motor and sensation (Table 2, Table 3). Our assessment battery consists of both standardized and informal measures as well as battery tests from the NIH Toolbox (Institute and National Cancer Institute, 2020), which is a comprehensive set of neuro-behavioral measurements. Increasing age is associated with a decrease in the speed with which cognitive processes can be executed, leading to impairments in various cognitive functions, including working memory, free recall, and verbal fluency, although to different extents depending on task complexity and domain (Drag and Bieliauskas, 2010). The cognitive tasks of the NIH Toolbox (Weintraub et al., 2013) help assess various cognitive domains including attention, processing speed, memory, executive functioning and language. An important aspect of the ABC@UofSC Repository is that it contains many complex language measures, including discourse measures which capture interactions among diverse cognitive processes such as semantic storage and retrieval, executive functions, and working memory. Taking multiple measures into account more closely approximates language production in everyday contexts than do standardized tests (Mueller et al., 2018).
Because normal aging is often accompanied by hearing loss (Humes et al., 2012; Lin et al., 2011), we included a series of hearing assessments in this project. Specifically, we assess standard audiometric thresholds, spatial release from speech masking and a words in noise task. It is not only important to evaluate a participant’s hearing sensitivity for calibrated pure tones but also to look further into a common complaint of many aging adults, which is difficulty hearing in noisy environments. Spoken language processing in noisy environments, a hallmark of the human brain, is subject to age-related decline, even when peripheral hearing might be intact (Wong et al., 2009).
Beyond measures of audition, we also target other modalities of sensation including the odor identification and visual acuity tests from the NIH Toolbox Sensation Battery. There is ample evidence for aging related changes in sensory function, particularly for olfaction and vision which are also associated with emergent cognitive impairment as well as systematic within-person change in cognitive function (MacDonald et al., 2018; Wong et al., 2009).
Aging additionally affects sensorimotor control and functioning. Age-related decline in fine motor control, gait and balance affect the ability of older adults to perform activities of daily living and maintain their independence (MacDonald et al., 2018; Seidler et al., 2010; Wong et al., 2009). We collect data on usual gait speed with a distance of 4 meters, standing balance, fine motor control and grip strength utilizing the subtests from the NIH Toolbox Motor Battery. There has been research recommending grip strength as a useful indicator for overall health (Forrest et al., 2018) and proposing grip strength as a biomarker for aging (Bohannon, 2019; Forrest et al., 2018; Sayer and Kirkwood, 2015).
One of the unique features of the ABC@UofSC Repository is that it includes rich discourse measures not typically found in similar large-scale databases. Participants in the ABC study perform three separate discourse tasks: Cat Rescue (Nicholas and Brookshire, 1993), Cookie Theft (Borod et al., 1980), and Peer Conflict Resolution (Nippold et al., 2007). The Cat Rescue and Cookie Theft tasks involve participant-generated language production in response to the presentation of a visual image, with the former involving creation of a narrative with a beginning middle and end, and the latter involving generation of a description of the scene. The Peer Conflict Resolution task involves analysis of a textually described conflict between two individuals and involves social communication and critical thinking abilities and is a good measure of expository discourse skills. For each of these tasks, trained study staff generate transcripts, separate utterances into communication units and code the transcripts for specific linguistic variables using the CHAT transcription format for automatic analyses by the CLAN program (available at https://childes.talkbank.org/). The CLAN program is then used to generate a number of descriptive measures that can be used in subsequent data analysis including duration of utterance, mean lexical units per utterance, type-token ratio, number of content words per minute, verbs per utterance, percentage word errors, total utterance errors, propositional density, total fillers, total false starts, total retracings, total repetitions, total pauses (2–5 s) and total pauses greater than 5 s. Additionally, trained staff complete a Correct Information Units (CIU) analysis for each discourse measure by duplicating the coded CHAT file already created, identifying the correct information units following guidelines proposed by Nicholas and Brookshire and then completing an automatic analysis using the CLAN program (Nicholas and Brookshire, 1993). The CLAN analysis provides the total words and total CIU, and study staff use this to compute the additional variables of percent CIU and CIU per minute. Full transcripts for each task, as well as each of these calculated variables are available in the ABC@UofSC Repository.
In general, behavioral measures were administered on paper, laptop (MacBook) or on an iPad. As a part of our battery of assessments we utilize subtests from the NIH Toolbox Cognition, Motor and Sensation batteries. The researchers administering the cognition battery have C-level qualifications and obtained a cognitive unlock code from NIH staff prior to beginning the study. Several language measures including sentence repetition, nonword repetition and discourse measures are recorded via an application on the laptop that is automatically uploaded to a HIPAA compliant server for later transcription and scoring. Considerations were taken into planning the assessment day to provide ample breaks and spread the measures that require increased cognitive load to reduce negative effects of fatigue on performance.
5. Physiological, blood and buccal swab measures
5.1. Blood pressure data
Blood pressure and pulse wave analysis were obtained by a trained operator using the SphygmoCor XCEL PWA system. All blood pressure measurements are taken following a 5 min rest period in a supine position. The pulse wave analysis is obtained using a standard brachial cuff placed on the arm by a trained study member. The analysis captures the brachial and aortic systolic and diastolic pressures as well as the aortic waveform. The SphygmoCoris programmed to take three separate blood pressure measurements, always disregarding the initial and averaging the final two. The device then captures the brachial waveform and analyzes it to provide a measure of central blood pressure. The brachial and aortic systolic and diastolic blood pressure values, along with aortic augmentation pressure and heart rate, are stored in the ABC@UofSC Repository.
5.2. Blood data
Each participant provides 25 ml (5 × 5 cc vials) of blood from a non-fasting blood draw collected midway through the first day of in-person testing (just prior to lunch). The blood is collected by a trained phlebotomist with UofSC Foundations of Lipids and Exercise (FLEX) laboratory using universal precautions (i.e., treating all blood as if it was infectious) and following the University of South Carolina’s bloodborne pathogen guidelines for the handling and storage of blood, including an approved exposure control plan. Two vials of the blood are sent to a commercial blood analysis company, LabCorp in Columbia, South Carolina, for analysis which included hemoglobin A1c, Vitamin B12, thyroid-stimulating hormone (TSH), complete blood count (CBC) with differential/platelet, and a complete metabolic panel. All blood measures are available on request. One aliquot of whole blood is stored at −20 °C until shipped in batches to AKESogen where for DNA extraction. AKESOgen will run Illumina’s Infinium Global Screening Array-24 v.3.0 on the samples. This array provides data on a subset of 654,027 single nucleotide polymorphisms (SNPs) (Infinium Global Screening Array-24 v3.0 BeadChip, 2020). This analysis will be completed in batches of 100, and the first batch has yet to ship. All remaining blood is centrifuged at 1300 g for 10 min and the plasma fraction collected and aliquoted into 1.5 mL cryotubes and stored at −80 °C. These blood plasma samples are banked for future, as yet undetermined analyses.
5.3. Buccal swab data
A buccal swab is collected for each participant for purposes of FMR1 genotyping and to evaluate possible influence on cognitive performance and impaired cognition with aging. Prior to collection, participants were instructed to not eat, drink, smoke, or chew gum for 30 min. Collection consists of rolling a small brush along the inside of the participants cheek approximately 30 times and then dipping it into a vial of liquid for the same number of times. Buccal swab collection is completed on both cheeks to ensure an adequate sample of outer cheek cells for analysis. The vials are labeled with the participant’s study identifier and sent for analysis in batches of at least 60 vials (results from the initial 65 participants are currently available) to Rush University Medical Center. FMR1 repeat-primed PCR is performed using a recently developed, highly sensitive method utilizing a commercially available kit (Asuragen, Inc., Austin TX) which detects all FMR1 expansions and allows accurate quantification of allele-specific CGG repeat length (Chen et al., 2010; Filipovic-Sadic et al., 2010) including identification of AGG interspersions. For FMR1 methylation PCR (mPCR) an mPCR kit (Chen et al., 2011) (Asuragen, Inc., Austin TX) is used to determine activation rations (AR) for the normal allele (Hadd et al., 2016).
6. MRI brain measures
A major advantage of the ABC@UofSC Repository is the unprecedented ease with which researchers can examine the relationship between brain data from multiple modalities including fMRI (both task-based and resting-state), DWI, ASL, structural T1 and T2-weighted FLAIR images, and SWI, and other raw and derived scores collected as part of the study. As described below, neuroimaging data collected as part of the ABC@UofSC study goes through our custom processing pipeline which outputs a single de-identified MATLAB (.mat) file (one file per participant) containing preprocessed MRI data which can readily be associated with other database variables using MATLAB (2017) and our free NiiStat (Rorden et al., n.d.) software. For example, testing the relationship between resting-state functional connectivity and blood pressure is as simple as creating a one-column Excel file which stores the blood pressure of each participant, placing it in the same directory as the study’s MATLAB (.mat) files, and running the NiiStat graphical user interface. Details regarding MRI data collection and preprocessing are described below and represented graphically in Fig. 1. A complete description of NiiStat, including tutorials for running analyses, can be found online (Rorden et al., n.d.) (Fig. 2).
Fig. 1.
After completing an initial eligibility survey, participants are officially enrolled in the ABC@UofSC study and complete multiple online questionnaires delivered through REDCap (Harris et al., 2019) prior to scheduling their 2-day sequence of in-person testing. The first day of in-person testing involves collection of behavioral data, physiological data, blood, saliva, and EEG data and the second day involves collection of MRI data at the McCausland Center for Neuroimaging located in Columbia, SC. As indicated in the diagram, the ABC@UofSC project collects a wide range of data (demographic, physiologic, cognitive, audition, vision, smell, motor function, blood/genetics, neuroimaging) the goal of which is to improve understanding of healthy aging. 1Cognitive measures from the National Institutes of Health (NIH) Toolbox included the Flanker Inhibitory Control and Attention Test 12+ (home base), Picture Sequence Memory Test 8+, List Sorting Working Memory Test 7+, Oral Reading Recognition Test 3+, Pattern Comparison Processing Speed 7+, Auditory Verbal Learning Test 8+, Oral Symbol Digit Test-subdomain. 2Discourse tasks include ‘Cat Rescue’, ‘Cookie Theft’ and ‘Peer Conflict Resolution’ tasks. 3Blood panel including CBC with differential, CMP, hemoglobin A1c, Vitamin B12 and TSH. ∗These measures are components of the NIH Toolbox Test Battery. CBC = complete blood panel, CMP = comprehensive metabolic panel, TSH = thyroid stimulating hormone, BP = blood pressure, EEG = electroencephalogram, MoCA = Montreal Cognitive Assessment, SWI = susceptibility weighted image, DTI = diffusion tensor image, DWI-FA = diffusion weighted image – functional anisotropy, DWI-MD = diffusion weighted image – mean diffusivity, CBF = cerebral blood flow derived from arterial spin labeled (ASL) imaging, fMRI = functional magnetic resonance imaging.
Fig. 2.
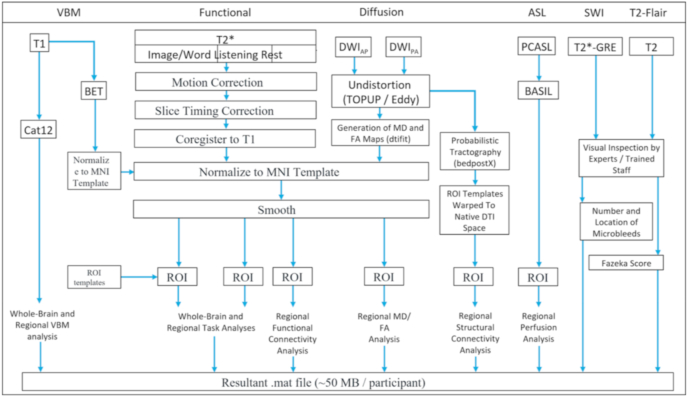
Participants in the ABC@UofSC Repository are scanned once (cross-sectional sample, ages 20–80) or twice (longitudinal sample, ages 60–80, separated by 4 years). Generally speaking, gray and white matter volumes were computed from the T1-structural image using Cat12 (CAT12, n.d.). Functional images, both task-based and resting-state, are put through our lab’s custom preprocessing pipeline, nii_preprocess (Rorden et al., 2020), which is scripted in MATLAB, 2017 and leverages multiple public image processing tools, prior to calculation whole-brain (voxelwise) and region of interest-based values for connectivity and specific task-based contrasts respectively. Diffusion weighted data is processed in native space using FSL’s TOPUP and Eddy, dtifit and bedpostX in order to generate mean diffusivity and functional anisotropy maps as well as ROI-based connectivity values (Smith et al., 2004). Arterial spin labeled images are processed using the FSL group’s Bayesian Inference for Arterial Spin Labelling (BASIL) tool (Chappell et al., 2008) tool, and the susceptibility weighted images and T2-Flair images are manually inspected by trained staff for microbleeds and white-matter hyperintensities. See text for full details regarding sequence parameters and data processing/preprocessing procedures. BET = brain extraction tool, VBM = voxel based morphometry, MNI = Montreal Neurological Institute, ROI = region of interest, DWIAP = diffusion weighted imaging collected anterior to posterior, DWIPA = diffusion weighted imaging collected posterior to anterior direction, PCASL = pulsed continuous arterial spin labelling, GRE = gradient recalled echo, BASIL = Bayesian Inference for Arterial Spin Labeling, CAT12 = Computational Anatomy Toolbox 12.
6.1. MRI data collection
MRI data were collected at the McCausland Center for Brain Imaging (MCBI) located at Prisma Health Hospital, Columbia SC using a Siemens 3 T Prisma Fit scanner equipped with a 20 channel head coil. Foam cushions were used to stabilize participants’ heads and minimize unwanted movement during scanning. For the task based fMRI, stimuli were displayed back-projected onto an MRI compatible screen placed at the back of the scanner and visible through the bore via an adjustable mirror attached directly to the head coil. Auditory stimuli were presented through Serene Sound MRI compatible headphone system (Resonance Technology, Inc., www.mrivideo.com), and manual responses, when required, were made using the MRI compatible Celeritas Fiber Optic Response System (Psychology Software Tools, www.pstet.com) secured to the participant’s right hand.
Structural (T1-weighted, T2-weighted FLAIR, DWI, SWI) and functional (ASL, resting-state fMRI and task-based fMRI) data were collected from participants over the course of a 1 h and 15 min scanning session. Scanning parameters for all acquired scans are summarized in Table 6. Resting-state scanning was collected with eyes-closed and lasted 12 min and 3 s.
Table 6.
Scanning parameters for ABC@UofSC MRI sequences.
| Functional Scans | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scan Type | Seq. | TR | TE | FA | FOV | VS | Vol | SL | ST | Gap | Order | Taska |
| Resting-state | EPI | 1650 | 35 | 72 | 216 × 216 × 120 | 2.4 × 2.4 × 2.0 | 427 | 50 | 2 | 20 | Interleaved, Ascending | Rest with eyes closed |
| Auditory Listening | EPI | 953 | 26.2 | 44 | 215 × 215 × 135 | 3.0 × 3.0 × 3.0 | 452 | 45 | 3 | 0 | Interleaved, Ascending | Listen to passages; Foreign/English. |
| Word/Picture Viewing | EPI | 953 | 26.2 | 44 | 215 × 215 × 135 | 3.0 × 3.0 × 3.0 | 452 | 45 | 3 | 0 | Interleaved, Ascending | Rate familiarity of words and pictures. |
| Structural Scans | |||||||
|---|---|---|---|---|---|---|---|
| Scan Type | Seq. | TR | TE | FA | FOV | VS | Other |
| T1-weighted | MGH multiecho MPRAGE | 2530 | {1.44,2.9,4.36,5.82,7.28} | 8 | 256 × 256 × 192 | 1 × 1 × 1 | GRAPPA = 2, TI = 1100 ms |
| T2-weighted | Siemens T2 Flair | 5000 | 387 | Var. | 230 × 230 × 173 | 0.9 × 0.9 × 0.9 | GRAPPA = 2, TI = 1800 ms |
| Diffusion Weighted (DWI) | Siemens DWI | 4500 | 103 | 90 | 220 × 220 × 128 | 2.2 × 2.2 × 2.2 | 137 dir., Monopolar, slices = 58, averages = 1, Diffusion Values = 2 {0 s/mm2, 2000 s/mm2) |
| DWI phase reversed (DWI rev) | Siemens DWI | 4500 | 103 | 90 | 220 × 220 × 128 | 2.2 × 2.2 × 2.2 | ∗Identical to above, but acquired P ≪ A |
| Arterial Spin Labeled (ASL) | Oxford 6-PLD PCASL | 5000 | 14 | 90 | 208 × 218 × 167 | 3.4 × 3.4 × 4.5 | |
| Resting-state fMRI Scan | EPI | 1650 | 35 | 72 | 215 × 215 × 128 | 2.4 × 2.4 × 2.0 | 427 vol, Ascending Interleaved, Eyes Closed |
Notes: TR = repetition time (ms); TE = echo time (ms); TI = inversion time (ms); ST = Slice Thickness (mm); FOV = field of view (mm); Seq = Sequence Name; FA = Flip Angle (Degrees); VS = Voxel Size (mm); Vols = Number of Volumes; SL = Slices (N); EPI = T2∗-weighted gradient echo echo planar image; PE-GRE = phase-encoded gradient echo;PCASL = pseudo-continuous arterial spin labelling; NIH = National Institutes of Health; MGH = Massachusetts General Hospital; MPRAGE = magnetisation prepared gradient echo. aTask: see text for details.
Auditory Listening Task: This task was 8 min long. It consisted of a 6 min passage spoken in English, chosen at random from a bank of 46 passages (23 different passages read by a female and a male speaker). The passages were chosen to vary along a number of dimensions, including overall difficulty/grade level, affect, syntactic complexity, genre, reference (repeated names/pronouns), use of nonliteral language, and voice. Participants were instructed to listen to each passage attentively, and were informed that they will be asked some comprehension questions afterwards. After the task, four questions about the passage were asked. Each question had three choices, selected with a button press. Before and after the English passage, a 50 s foreign language (one Hindi and one Thai) passage was played, with gender of the speaker the same as that of the English passage. The passages were separated by 8 s of rest.
Word/picture Familiarity Task: During this task, which was also 8 min long, participants viewed 95 stimuli that were words, nonwords, or pictures. These were chosen at random from a set of 1524 words, 110 nonwords, and 180 pictures. There were 77 words, 8 nonwords, and 10 pictures presented to each participant. Each item was displayed for 2 s, with a variable inter-trial interval of 2.5, 3, or 3.5 s. The stimuli varied on a number of dimensions, in order to facilitate examination of a variety of research questions. These included transitive/intransitive verbs, event/object nouns, famous/non-famous persons and places, several types of abstract concepts, and variation in frequency, concreteness/imageability, spelling-sound consistency, emotional valence/arousal, and for nonwords, bigram frequency and orthographic neighborhood size. Pictures were indoor and outdoor scenes, emotional faces, several categories of living and nonliving things, and nonsense objects. Participants responded to each stimulus by categorizing it as either ‘highly familiar’, ‘somewhat familiar’, or ‘unfamiliar’ using the index, middle, and ring fingers, respectively, on their right hand. This task was chosen to encourage deep semantic processing and encourage attention to each item, while being neutral with respect to various categories and dimensions of stimuli. Complete details on auditory and picture/word stimuli, as well as the complete bank of word and picture stimuli are publicly accessible online (Riccardi, 2020).
While individual raw MRI images of any modality can be requested using our ABC@UofSC Data Request Form, in an effort to enhance the rate of scientific discovery, and with the full realization that processing of MRI images from varying modalities takes considerable technical expertise/investment not present at all inquiring institutions, we also make available the output of our fully vetted in-house multimodal MRI processing stream as a single, deidentified MATLAB (.mat) file for each participant. Importantly, this processing stream has been used as the basis of analysis in multiple published papers by our group (William; W.; Fridriksson et al., 2015; Hickok et al., 2018; Hillis et al., 2018; Karnath et al., 2018; Matchin et al., 2020; Matchin et al., 2020; Yourganov et al., 2018). Each participant’s MATLAB (.mat) file contains normalized statistical maps representing the final stages of processing for each modality. The file also contains results from specific contrasts calculated using the task-based fMRI data (contrast images and statistical T-maps). Specifically, we provide the contrasts for four basic conditions versus rest: words, pictures, English passages, and foreign language passages. These statistical maps were created using the Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996). The standardized preprocessing pipeline followed AFNI’s most recent recommendations (Taylor et al., 2018). The pipeline involved despiking the data by fitting a smooth curve to each voxel time series and registration of functional images to the anatomy (Saad et al., 2009). Functional images were co-registered (Cox and Jesmanowicz, 1999) and projected into standard stereotaxic space (Evans et al., 1993) using nonlinear warping through the @SSwarper command, with the MNI152_2009_template_SSW volume as the target anatomical. The normalized images were smoothed with an isotropic 7-mm FWHM Gaussian kernel and converted to percent signal change. Using 3dREMLfit, the resulting preprocessed fMRI time series were analyzed on a subject-by-subject basis using an event-related approach for words and pictures and a block design for the English and foreign language passages. For words and pictures, voxel-wise multiple linear regression was used with regressors for both conditions convolved with a canonical hemodynamic response function. For English and foreign language passages, the regressors for both conditions were convolved with a boxcar hemodynamic response function matching the length of the corresponding passage. Several noise covariates of no interest were included in the model: six motion parameters and their derivatives, signal extracted from cerebrospinal fluid, and signal associated with response button presses for the word and picture viewing task. The AFNI scripts used for preprocessing and data analysis are available online (Riccardi, 2020). Preprocessed resting-state fMRI data are provided in the form of full connectivity matrices calculated using regions of interest delineated by 6 popular brain parcellation maps: AICHA (Joliot et al., 2015), Brodmann (2007), AAL (Tzourio-Mazoyer et al., 2002), JHU (Oishi et al., 2009), Catani (Catani and Thiebaut de Schotten, 2008), and FOX (Fox, 1995).
6.2. MRI data analysis
All of our MRI data were processed using the publicly available nii_preprocess pipeline, which has been used in numerous prior publications (Rorden et al., 2020). Briefly, this fully automatic pipeline uses a MATLAB wrapper to leverage what we consider to be ‘best-of-breed’ processing tools for each imaging modality, and generates a single MATLAB (.mat) file (~50 mb) per participant which contains the main structural image (T1) as well as modality-specific calculated measures of interest described below. In order to keep file sizes reasonable, data for most calculated measures of interest are provided as within-ROI mean values based on commonly used neuroanatomical atlases cited above. The processing stages for MRI data are summarized in Fig. 1. Voxel based morphometry (VBM) is accomplished using the SPM12 (Penny et al., 2011) program run in MATLAB (and the Computational Anatomy Toolbox (CAT12) (CAT12, n.d.), using default settings. In addition to computing normalized smoothed modulated gray and white matter maps which can be used to compute voxelwise statistics, we also provide the following numeric values for each participant: estimated total intracranial volume [eTIV], gray matter volume [GM], cerebrospinal fluid volume [CSF], white matter volume [WM], and volume of white matter hyperintensities [WMH]. The XML files containing these details, along with ROI-based gray matter volume results computed for the AAL atlas are also available upon request. Cerebral blood flow (CBF) data (perfusion weighted images and calibrated perfusion weighted images) are computed using FSL′s Bayesian Inference for Arterial Spin Labelling (BASIL) tool (Chappell et al., 2008; Smith et al., 2004) which was developed to specifically support processing of the 6-PLD PCASL sequences developed by Oxford and used in the current study. We used BASIL’s recommended (default) preprocessing settings as described (in great detail) in the ASL ‘white paper’ (Alsop et al., 2015). The DWI branch of the pipeline generates mean diffusivity (MD) and functional anisotropy (FA) maps in standard space using FSL’s dtifit, as calculates probabilistic tractography using FSL’s bedpostX (Smith et al., 2004). The fMRI stream has separate branches for processing resting-state fMRI, and two task-based fMRI runs, with each task running through an identical preprocessing stream that includes motion correction, slice timing correction, coregistration to the T1 image, normalization to standard space, and spatial smoothing. Voxelwise and region of interest based statistical maps for key contrasts (along with documentation describing the value of each contrast) are provided in the MATLAB (.mat) file for each participant. Voxelwise and region of interest based resting-state connectivity maps are also created for each participant and included in the final MATLAB (.mat) file. SWI images are rated by a team trained to identify/quantify microbleeds, and the T2-flair images are rated by the same team for both periventricular as well as white matter hyperintensities according to the Fazekas scale (Fazekas et al., 1987). Finally, each participant’s MATLAB (.mat) file contains a copy of their deidentified (defaced) high resolution T1-structural image.
7. EEG brain measures
7.1. Resting-state EEG data acquisition
EEG data werecollected from all participants following lunch on the first day of in-person testing. EEG signals were recorded from 128 sites on fifty-four participants’ scalp using the Brain Vision actiCAP slim (actiCAP slim/actiCAP snap, 2020) electrode layout with 500 Hz sampling frequency while participants were sitting quietly in a room using the ActiCHAMP EEG plus system (actiCHamp Plus, 2020). Electrode set up and high viscosity gel (10% salt) application took approximately 30 min and voltages were referenced online to a fronto-central reference electrode (Fz) and impedances were maintained below 10 kΩ. For each participant, approximately 6 min of resting-state EEG activity (3 min eyes-closed and 3 min eyes-open) was collected. Currently, resting-state EEG data has been subjected to power-spectrum analysis (described below). In the future, resting-state inter-electrode connectivity will be calculated and made available for exploration.
7.2. EEG preprocessing
Artifact removal and data preprocessing was done using the Harvard Automated Processing Pipeline for EEG (HAPPE) (Gabard-Durnam et al., 2018). This pipeline integrates MATLAB functions with open source toolboxes including EEGLAB and the HAPPE processing pipeline (Gabard-Durnam et al., 2018) (Delorme and Makeig, 2004). First, raw resting-state EEG data were converted to MATLAB files using the EEGLAB toolbox and locations of the electrodes used to record EEG signals were imported into MATLAB. All the signals were passed through a 1 Hz high-pass filter and a subset of electrodes (54 out of 128) were selected to be processed in HAPPE given the length and sampling rate of the recorded signals. Electrodes selection was based on two goals: covering the entire scalp, and optimizing the distance between electrodes to avoid redundant signals. Subsequently, electrical line noise was removed using the Cleanline program and channels with high impedances or displacement during recording were labeled to be excluded from the further preprocessing steps. Next, wavelet-enhanced independent components analysis (ICA) followed by ICA with automated component rejection were implemented. This step allowed us to first remove some of the most severe artifacts, then perform ICA decomposition, and finally remove additional artifacts. The resulting data was then divided into non-overlapping 2000 ms (ms) segments and artifact-contaminated segments were eliminated. After having cleaned the data of all electrodes, data from channels previously labeled as “bad channels” were replaced with data interpolated from nearby electrodes to preserve all the selected channels and maximize artifact removal at the same time. Finally, voltages were re-referenced offline to the average reference.
7.3. Spectral analysis
Three minutes of resting-state EEG activity with eyes open, as well as 3 min of resting-state EEG activity with eyes closed, were collected and preprocessed for each participant. For the eyes-open condition, an average of 80.55 epochs (min = 69, max = 86) were analyzed per participant (N = 54) after the post-segmentation rejection step. For the eyes-closed condition, an average of 81.09 epochs (min = 68, max = 88) were analyzed per participant (N = 43, this data was not collected from the first 10 participants, and one participant was excluded due to poor data quality). Spectral analysis was performed using Welch’s method as implemented in the pop_spectopo function of EEGLAB package. This is an approach which involves sectioning the signal of each electrode and calculating the fast fourier transform (FFT) for each section and power spectral density (PSD) is then computed as an average of FFTs over all sections for each electrode. As EEG signals were high-pass filtered as part of preprocessing, they had a frequency somewhere within the 1 Hz–250 Hz range. The frequency content of the signals was then divided into five frequency bands (Delta [1–4 Hz], Theta [4–8], Alpha [8–13], Beta [13–30] and Gamma [30–45]). Finally, in order to calculate the mean absolute power over each frequency band for each individual, we took the power spectra from the first boundary to the second boundary of each frequency band and those values were averaged. Data from the eyes-open (5∗54∗54, frequency-bands by electrodes by participants) and eyes-closed conditions (5∗54∗43, frequency-bands by electrodes by participants) are available in table format and represent the average power per frequency band per participant and values for each electrode are provided as power spectral density (PSD) in dB, and as absolute power (AP) in squared microvolts (μv2).
8. Data access
Data in the ABC@UofSC Repository is currently stored in a REDCap database maintained by Healthsciences South Carolina and operated out of the University of South Carolina. Neuroimaging data (MRI, EEG), which, due to their large size, are not suited to REDCap, are stored on a secure UofSC server. Researchers interested in requesting data from the ABC@UofSC Repository are required to complete the electronic ABC Data Request Form (Newman-Norlund et al., 2020). Researchers are asked to include a summary of their project, a brief description of their proposed research question or hypothesis and details regarding the subset (where applicable) of the ABC@UofSC Repository population they wish to include in their request. A list of all measures are provided to researchers to allow them to request the data relevant to their proposed project(s). While a number of calculated/derived data are provided, researchers may also request data in its raw form (unprocessed survey and questionnaire responses, raw reaction time data, transcripts of discourse, etc.). Specifically for MRI and EEG analysis, access to raw, unprocessed data, may be necessary for certain types of analyses. Prior to receiving data, researchers are required to sign a Data Usage Agreement. A list of researchers who have requested access to date from the ABC@UofSC Repository, as well as their goals and results (once available) are available on the ABC@UofSC Repository website (ABC Data Requests, 2019).
9. Summary
The ABC@UofSC Repository houses a unique, muti-modal, cross-sectional lifespan database designed to allow researchers to ask questions about healthy aging in the state of South Carolina. The database contains raw and preprocessed neuroimaging data (functional, resting-state and structural MRI, as well as resting-state EEG), physiological data (pulse, blood pressure), blood data (CBC with differential, CMP, hA1c, B12, Folate), FMR1 data from buccal swabs, calculated scores from various well-known and commonly used tests of cognition along with less frequently encountered measures of language use and processing ability, as well as health-related self-report measures, and demographic data. This unique dataset will allow researchers to probe the relationship between healthy aging and brain/genetic/behavioral factors in ways that no other database in the world can.
Conflicts of interest
Roger Newman-Norlund, Sarah E. Newman-Norlund, Sara Sayers, Samaneh Nemati, Nick Riccardi, Chris Rorden and Julius Fridriksson report no conflicts of interest.
Funding source
University of South Carolina Excellence Initiative, Aging Brain Cohort (ABC) Project.
Acknowledgements
This study was funded by the University of South Carolina through the Excellence Initiative (awarded to PI: Julius Fridriksson). Multiple collaborators helped us put together our comprehensive battery of assessments and questionnaires: Dirk den Ouden, William Matchin, Alexandra Basilakos, Lorelei Phillip, Leigh Ann Spell, Brielle Stark, Jessica Klusek, Jill Stewart, Stacy Fritz, Rutvik Desai, Roozbeh Behroozmand, Suzanne Adlof, Dan Fogerty, Svetlana Shinkareva, Scott Decker, Souvik Sen, Robert Davis Moore, Troy Herter, Leonardo Bonilha, Spencer Moore, Daniela Friedman, Anthony Alberg, Alexander McLain, and Feifei Xiao. Special thanks to Mark Sarzynski and Charles S. Schwartz with the Foundations of Lipids and Exercise (FLEX) Laboratory at UofSC for their time and efforts with the blood draw collections for our study. We would like to thank the rest of our Aging Brain Cohort team at UofSC including our recruitment specialist, Briana Davis, and PhD student, Hunter Steiner, as well as our team of graduate and undergraduate students for their dedication to the success of the study.
References
- AARC Aging South Carolina. 2020. https://aging.sc.gov/programs-initiatives/alzheimers-resource-coordination-center-arcc [WWW Document] accessed 8.13.20.
- ABC Data Requests 2019. https://abc.sc.edu/abc-repository-data-requests/ [WWW Document] accessed 2.19.21.
- actiCAP slim/actiCAP snap 2020. https://brainvision.com/products/acticap-slim-acticap-snap/ [WWW Document] accessed 2.19.21.
- actiCHamp Plus BrainProducts. 2020. https://brainvision.com/products/actichamp-plus/ [WWW Document] accessed 2.19.21.
- Alsop D.C., Detre J.A., Golay X., Günther M., Hendrikse J., Hernandez-Garcia L., Lu H., MacIntosh B.J., Parkes L.M., Smits M., vanOsch M.J.P., Wang D.J.J., Wong E.C., Zaharchuk G. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 2015;73:102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barter J.D., Foster T.C. Aging in the brain: new roles of epigenetics in cognitive decline. Neuroscientist. 2018;24:516–525. doi: 10.1177/1073858418780971. [DOI] [PubMed] [Google Scholar]
- Bohannon R.W. Grip Strength: an indispensable biomarker for older adults. Clin. Interv. Aging. 2019;14:1681–1691. doi: 10.2147/CIA.S194543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S.Y., Salat D.H., Terpstra M., Ances B.M., Barch D.M., Buckner R.L., Burgess G.C., Curtiss S.W., Diaz-Santos M., Elam J.S., Fischl B., Greve D.N., Hagy H.A., Harms M.P., Hatch O.M., Hedden T., Hodge C., Japardi K.C., Kuhn T.P., Ly T.K., Smith S.M., Somerville L.H., Uğurbil K., van der Kouwe A., Van Essen D., Woods R.P., Yacoub E. The lifespan human connectome project in aging: an overview. Neuroimage. 2019;185:335–348. doi: 10.1016/j.neuroimage.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borod J.C., Goodglass H., Kaplan E. Normative data on the boston diagnostic aphasia examination, parietal lobe battery, and the boston naming Test. J. Clin. Neuropsychol. 1980;2:209–215. [Google Scholar]
- Brodmann K. Springer Science & Business Media; 2007. Brodmann’s: Localisation in the Cerebral Cortex. [Google Scholar]
- Burt R.S. Network items and the general social survey. Soc. Network. 1984;6:293–339. [Google Scholar]
- Buysse D.J., Reynolds C.F., 3rd, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr. Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cammarata G., Duro G., Chiara T.D., Curto A.L., Taverna S., Candore G. Circulating miRNAs in successful and unsuccessful aging. A mini-review. Curr. Pharmaceut. Des. 2019;25:4150–4153. doi: 10.2174/1381612825666191119091644. [DOI] [PubMed] [Google Scholar]
- CAT12 [WWW Document], n.d. URL http://www.neuro.uni-jena.de/cat/(accessed 9.30.20).
- Catani M., Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Cella D., Riley W., Stone A., Rothrock N., Reeve B., Yount S., Amtmann D., Bode R., Buysse D., Choi S., Others The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J. Clin. Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell M.A., Groves A.R., Whitcher B. Variational Bayesian inference for a nonlinear forward model. IEEE Trans. Signal Process. 2008;57:223–236. [Google Scholar]
- Chen L., Hadd A.G., Sah S., Houghton J.F., Filipovic-Sadic S., Zhang W., Hagerman P.J., Tassone F., Latham G.J. High-resolution methylation polymerase chain reaction for fragile X analysis: evidence for novel FMR1 methylation patterns undetected in Southern blot analyses. Genet. Med. 2011;13:528–538. doi: 10.1097/GIM.0b013e31820a780f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Hadd A., Sah S., Filipovic-Sadic S., Krosting J., Sekinger E., Pan R., Hagerman P.J., Stenzel T.T., Tassone F., Latham G.J. An information-rich CGG repeat primed PCR that detects the full range of fragile X expanded alleles and minimizes the need for southern blot analysis. J. Mol. Diagn. 2010;12:589–600. doi: 10.2353/jmoldx.2010.090227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell E.Y., Waite L.J. Social disconnectedness, perceived isolation, and health among older adults. J. Health Soc. Behav. 2009;50:31–48. doi: 10.1177/002214650905000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox R.W., Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn. Reson. Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Coyle C.E., Dugan E. Social isolation, loneliness and health among older adults. J. Aging Health. 2012;24:1346–1363. doi: 10.1177/0898264312460275. [DOI] [PubMed] [Google Scholar]
- Craig C., Marshall A., Sjostrom M., Bauman A., Lee P., Macfarlane D., Lam T., Stewart S. 2017. International Physical Activity Questionnaire-Short Form. [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Diener E., Emmons R.A., Larsen R.J., Griffin S. The satisfaction with life scale. J. Pers. Assess. 1985;49:71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- Drag L.L., Bieliauskas L.A. Contemporary review 2009: cognitive aging. J. Geriatr. Psychiatr. Neurol. 2010;23:75–93. doi: 10.1177/0891988709358590. [DOI] [PubMed] [Google Scholar]
- Evans A.C., Collins D.L., Mills S.R., Brown E.D., Kelly R.L., Peters T.M. vol. 3. 1993. 3D statistical neuroanatomical models from 305 MRI volumes; pp. 1813–1817. (1993 IEEE Conference Record Nuclear Science Symposium and Medical Imaging Conference). [Google Scholar]
- Fazekas F., Chawluk J.B., Alavi A., Hurtig H.I., Zimmerman R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am. J. Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- Filipovic-Sadic S., Sah S., Chen L., Krosting J., Sekinger E., Zhang W., Hagerman P.J., Stenzel T.T., Hadd A.G., Latham G.J., Tassone F. A novel FMR1 PCR method for the routine detection of low abundance expanded alleles and full mutations in fragile X syndrome. Clin. Chem. 2010;56:399–408. doi: 10.1373/clinchem.2009.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch C.E., Ruvkun G. The genetics of aging. Annu. Rev. Genom. Hum. Genet. 2001;2:435–462. doi: 10.1146/annurev.genom.2.1.435. [DOI] [PubMed] [Google Scholar]
- Fletcher E., Gavett B., Harvey D., Farias S.T., Olichney J., Beckett L., DeCarli C., Mungas D. Brain volume change and cognitive trajectories in aging. Neuropsychology. 2018;32:436–449. doi: 10.1037/neu0000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest K.Y.Z., Williams A.M., Leeds M.J., Robare J.F., Bechard T.J. Patterns and correlates of grip strength in older Americans. Curr. Aging Sci. 2018;11:63–70. doi: 10.2174/1874609810666171116164000. [DOI] [PubMed] [Google Scholar]
- Fox P.T. Spatial normalization origins: objectives, applications, and alternatives. Hum. Brain Mapp. 1995;3:161–164. [Google Scholar]
- Fridriksson J., Basilakos A., Hickok G., Bonilha L., Rorden C. Speech entrainment compensates for Broca’s area damage. Cortex. 2015;69:68–75. doi: 10.1016/j.cortex.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch M.B. Pearson; 1994. QOLI: Quality of Life Inventory : Manual and Treatment Guide. [Google Scholar]
- Gabard-Durnam Laurel, Leal Adriana, Wilkinson Carol, Levin April. The Harvard Automated Processing Pipeline for Electroencephalography (HAPPE): Standardized Processing Software for Developmental and High-Artifact Data. Frontiers in Neuroscience. 2018;12:97. doi: 10.3389/fnins.2018.00097. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Mendez Leal A.S., Wilkinson C.L., Levin A.R. The harvard automated processing pipeline for Electroencephalography (HAPPE): standardized processing software for developmental and high-artifact data. Front. Neurosci. 2018;12:97. doi: 10.3389/fnins.2018.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallun F.J., Diedesch A.C., Kampel S.D., Jakien K.M. Independent impacts of age and hearing loss on spatial release in a complex auditory environment. Front. Neurosci. 2013 doi: 10.3389/fnins.2013.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon R.C., Wagster M.V., Hendrie H.C., Fox N.A., Cook K.F., Nowinski C.J. NIH Toolbox for assessment of neurological and behavioral function. Neurology. 2013;80:S2–S6. doi: 10.1212/WNL.0b013e3182872e5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H., Kaplan E., Barresi B. third ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2001. BDAE-3: Boston Diagnostic Aphasia Examination- [Google Scholar]
- Hadd A.G., Filipovic-Sadic S., Zhou L., Williams A., Latham G.J., Berry-Kravis E., Hall D.A. A methylation PCR method determines FMR1 activation ratios and differentiates premutation allele mosaicism in carrier siblings. Clin. Epigenet. 2016;8:130. doi: 10.1186/s13148-016-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna K.M., Scott L.L., Schmidt K.K. Retention strategies in longitudinal studies with emerging adults. Clin. Nurse Spec. 2014 doi: 10.1097/nur.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada C.N., Natelson Love M.C., Triebel K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013 doi: 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O’Neal L., McLeod L., Delacqua G., Delacqua F., Kirby J., Duda S.N., REDCap Consortium The REDCap consortium: building an international community of software platform partners. J. Biomed. Inf. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays R.D. 1994. The Medical Outcomes Study (MOS) Measures of Patient Adherence. [Google Scholar]
- Hays R.D., Spritzer K.L., Schalet B.D., Cella D. PROMIS®-29 v2.0 profile physical and mental health summary scores. Qual. Life Res. 2018;27:1885–1891. doi: 10.1007/s11136-018-1842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G., Rogalsky C., Matchin W., Basilakos A., Cai J., Pillay S., Ferrill M., Mickelsen S., Anderson S.W., Love T., Binder J., Fridriksson J. Neural networks supporting audiovisual integration for speech: a large-scale lesion study. Cortex. 2018;103:360–371. doi: 10.1016/j.cortex.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis A.E., Beh Y.Y., Sebastian R., Breining B., Tippett D.C., Wright A., Saxena S., Rorden C., Bonilha L., Basilakos A., Yourganov G., Fridriksson J. Predicting recovery in acute poststroke aphasia. Ann. Neurol. 2018;83:612–622. doi: 10.1002/ana.25184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A.B., Others . 1975. Four Factor Index of Social Status. [Google Scholar]
- Howard G., Howard V.J. Twenty years of progress toward understanding the stroke belt. Stroke. 2020;51:742–750. doi: 10.1161/STROKEAHA.119.024155. [DOI] [PubMed] [Google Scholar]
- Hueluer G., Rebok G.W. The role of work and retirement in cognitive and brain aging. Innovation in Aging. 2019;3 S24–S24. [Google Scholar]
- Humes L.E., Dubno J.R., Gordon-Salant S., Lister J.J., Cacace A.T., Cruickshanks K.J., Gates G.A., Wilson R.H., Wingfield A. Central presbycusis: a review and evaluation of the evidence. J. Am. Acad. Audiol. 2012;23:635–666. doi: 10.3766/jaaa.23.8.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infinium Global Screening Array-24 v3.0 BeadChip Illumina. 2020. https://www.illumina.com/content/dam/illumina-marketing/documents/products/datasheets/infinium-global-screening-array-data-sheet-370-2016-016.pdf [WWW Document] accessed 1.24.21.
- Institute, N.C., National Cancer Institute . Definitions; 2020. NIH Toolbox. [DOI] [Google Scholar]
- Joliot M., Jobard G., Naveau M., Delcroix N., Petit L., Zago L., Crivello F., Mellet E., Mazoyer B., Tzourio-Mazoyer N. AICHA: an atlas of intrinsic connectivity of homotopic areas. J. Neurosci. Methods. 2015;254:46–59. doi: 10.1016/j.jneumeth.2015.07.013. [DOI] [PubMed] [Google Scholar]
- Joseph R., Newman-Norlund R.D. rogiedodgie/ABC_NIH_TOOLBOX_REDCAP_PROCESSING: nii_preprocess_August_2019_2. 2020. [DOI]
- Just M.A., Carpenter P.A., Woolley J.D. Paradigms and processes in reading comprehension. J. Exp. Psychol. Gen. 1982;111:228–238. doi: 10.1037//0096-3445.111.2.228. [DOI] [PubMed] [Google Scholar]
- Karnath H., Sperber C., Rorden C. Mapping human brain lesions and their functional consequences. Neuroimage. 2018;165:180–189. doi: 10.1016/j.neuroimage.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller D., Bynum J.P.W. Dementia in the USA: state variation in prevalence. J. Public Health. 2015;37:597–604. doi: 10.1093/pubmed/fdu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowald A., Kirkwood T.B.L. Can aging be programmed? A critical literature review. Aging Cell. 2016;15:986–998. doi: 10.1111/acel.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefly D.L., Pennington B.F. Reliability and validity of the adult reading history questionnaire. J. Learn. Disabil. 2000;33:286–296. doi: 10.1177/002221940003300306. [DOI] [PubMed] [Google Scholar]
- Lin F.R., Thorpe R., Gordon-Salant S., Ferrucci L. Hearing loss prevalence and Risk factors among older adults in the United States. The Journals of Gerontology Series A: Biol. Med. Sci. 2011 doi: 10.1093/gerona/glr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons K.S., Carter J.H., Carter E.H., Rush K.N., Stewart B.J., Archbold P.G. Locating and retaining research participants for follow-up studies. Res. Nurs. Health. 2004;27:63–68. doi: 10.1002/nur.20001. [DOI] [PubMed] [Google Scholar]
- MacDonald S.W.S., Keller C.J.C., Brewster P.W.H., Dixon R.A. Contrasting olfaction, vision, and audition as predictors of cognitive change and impairment in non-demented older adults. Neuropsychology. 2018;32:450–460. doi: 10.1037/neu0000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchin W., Basilakos A., den Ouden D.-B., Stark B.C., Hickok G., Fridriksson J. Neuroanatomical dissociations of syntax and semantics revealed by lesion-symptom mapping. 2020. [DOI]
- Matchin W., Basilakos A., Stark B.C. Agrammatism and paragrammatism: a cortical double dissociation revealed by lesion-symptom mapping. Neurobiology of Language. 2020 doi: 10.1162/nol_a_00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATLAB . The Mathworks Inc.; Natick, Massachusetts: 2017. Version 9.3.0.713579 (R2017b) [Google Scholar]
- McDonald A.P., D’Arcy R.C.N., Song X. Functional MRI on executive functioning in aging and dementia: a scoping review of cognitive tasks. Aging Med (Milton) 2018;1:209–219. doi: 10.1002/agm2.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham M.P., Erickson K.I., Banich M.T., Kramer A.F., Webb A., Wszalek T., Cohen N.J. Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain Cognit. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Mueller K.D., Hermann B., Mecollari J., Turkstra L.S. Connected speech and language in mild cognitive impairment and Alzheimer’s disease: a review of picture description tasks. J. Clin. Exp. Neuropsychol. 2018;40:917–939. doi: 10.1080/13803395.2018.1446513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Cummings J.L., Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute (NCI) Dietary screener questionnaire in the NHANES 2009-2010. 2019. http://epi.grants.cancer.gov/nhanes/dietscreen/ [WWW Document]
- Newman-Norlund S., Newman-Norlund R., Sayers S. ABC RedCap data request form. 2020. https://redcap.healthsciencessc.org/surveys/?s=943KWC8LNH [WWW Document]. URL. accessed 2.1.21.
- Nicholas L.E., Brookshire R.H. A system for quantifying the informativeness and efficiency of the connected speech of adults with aphasia. J. Speech Hear. Res. 1993;36:338–350. doi: 10.1044/jshr.3602.338. [DOI] [PubMed] [Google Scholar]
- Nippold M.A., Mansfield T.C., Billow J.L. Peer conflict explanations in children, adolescents, and adults: examining the development of complex syntax. Am. J. Speech Lang. Pathol. 2007;16:179–188. doi: 10.1044/1058-0360(2007/022). [DOI] [PubMed] [Google Scholar]
- Noble W., Jensen N.S., Naylor G., Bhullar N., Akeroyd M.A. A short form of the Speech, Spatial and Qualities of Hearing scale suitable for clinical use: the SSQ12. Int. J. Audiol. 2013;52:409–412. doi: 10.3109/14992027.2013.781278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K., Faria A., Jiang H., Li X., Akhter K., Zhang J., Hsu J.T., Miller M.I., van Zijl P.C.M., Albert M., Lyketsos C.G., Woods R., Toga A.W., Pike G.B., Rosa-Neto P., Evans A., Mazziotta J., Mori S. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer’s disease participants. Neuroimage. 2009;46:486–499. doi: 10.1016/j.neuroimage.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaolapo A.Y., Obelawo A.Y., Onaolapo O.J. Brain ageing, cognition and diet: a review of the emerging roles of food-based nootropics in mitigating age-related memory decline. Curr. Aging Sci. 2019;12:2–14. doi: 10.2174/1874609812666190311160754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin L.I., Schooler C. The structure of coping. J. Health Soc. Behav. 1978;19:2–21. [PubMed] [Google Scholar]
- Pelletier A., Bernard C., Dilharreguy B., Helmer C., Le Goff M., Chanraud S., Dartigues J.-F., Allard M., Amieva H., Catheline G. Patterns of brain atrophy associated with episodic memory and semantic fluency decline in aging. Aging. 2017;9:741–752. doi: 10.18632/aging.101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny W.D., Friston K.J., Ashburner J.T., Kiebel S.J., Nichols T.E. Elsevier; 2011. Statistical Parametric Mapping: the Analysis of Functional Brain Images. [Google Scholar]
- Phillips C. Lifestyle modulators of neuroplasticity: how physical activity, mental engagement, and diet promote cognitive health during aging. Neural Plast. 2017:3589271. doi: 10.1155/2017/3589271. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polit D.F., Beck C.T. Lippincott Williams & Wilkins; 2008. Nursing Research: Generating and Assessing Evidence for Nursing Practice. [Google Scholar]
- Powell L.E., Myers A.M. The activities-specific balance confidence (ABC) scale. J. Gerontol. A Biol. Sci. Med. Sci. 1995:M28–M34. doi: 10.1093/gerona/50a.1.m28. 50A. [DOI] [PubMed] [Google Scholar]
- Pruchno R., Heid A.R., Wilson-Genderson M. Successful aging, social support, and ownership of a companion animal. Anthrozoös. 2018;31:23–39. [Google Scholar]
- Razlighi Q.R., Habeck C., Barulli D., Stern Y. Cognitive neuroscience neuroimaging repository for the adult lifespan. Neuroimage. 2017;144:294–298. doi: 10.1016/j.neuroimage.2015.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi N. 2020. Aging Brain Cohort (ABC): Functional Neuroimaging Stimuli and Data-Processing Pipeline. [DOI] [Google Scholar]
- Rodríguez-Rodero S., Fernández-Morera J.L., Menéndez-Torre E., Calvanese V., Fernández A.F., Fraga M.F. Aging genetics and aging. Aging Dis. 2011;2:186. [PMC free article] [PubMed] [Google Scholar]
- Rolle-Lake L., Robbins E. StatPearls. StatPearls Publishing; Treasure Island (FL: 2020. Behavioral Risk factor surveillance system (BRFSS) [PubMed] [Google Scholar]
- Rorden C., McKinnon E., Hanayik T., Yourganov G., Newman-Norlund R.D., Reddy D.P. rogiedodgie/nii_preprocess: zenodo DOI release. 2020. [DOI]
- Rorden, C.R., Yourganov, G., Newman-Norlund, R.D., Joseph, R., n.d. NiiStat [WWW Document]. URL https://www.nitrc.org/projects/niistat/(accessed 2.19.21)..
- Saad Z.S., Glen D.R., Chen G., Beauchamp M.S., Desai R., Cox R.W. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage. 2009;44:839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T.A. Trajectories of normal cognitive aging. Psychol. Aging. 2019;34:17–24. doi: 10.1037/pag0000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson R.J., Raudenbush S.W., Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997;277:918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- Sayer A.A., Kirkwood T.B.L. Grip strength and mortality: a biomarker of ageing? Lancet. 2015 doi: 10.1016/s0140-6736(14)62349-7. [DOI] [PubMed] [Google Scholar]
- Schoentgen B., Gagliardi G., Défontaines B. Environmental and cognitive enrichment in childhood as protective factors in the adult and aging brain. Front. Psychol. 2020;11:1814. doi: 10.3389/fpsyg.2020.01814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelen J.-M., Oostenveld R., Lam N.H.L., Uddén J., Hultén A., Hagoort P. Publisher Correction: a 204-subject multimodal neuroimaging dataset to study language processing. Sci Data. 2019;6:38. doi: 10.1038/s41597-019-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R.D., Bernard J.A., Burutolu T.B., Fling B.W., Gordon M.T., Gwin J.T., Kwak Y., Lipps D.B. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010;34:721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J.A., Kemper S., Lyons K. Sentence repetition and processing resources in Alzheimer’s disease. Brain Lang. 2000;75:232–258. doi: 10.1006/brln.2000.2355. [DOI] [PubMed] [Google Scholar]
- Small S.A. Age-related memory decline: current concepts and future directions. Arch. Neurol. 2001;58:360–364. doi: 10.1001/archneur.58.3.360. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Steptoe A., Zaninotto P. Lower socioeconomic status and the acceleration of aging: an outcome-wide analysis. Proc. Natl. Acad. Sci. U.S.A. 2020;117:14911–14917. doi: 10.1073/pnas.1915741117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.R., Williams N., Cusack R., Auer T., Shafto M.A., Dixon M., Tyler L.K., Henson R.N., Others The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) data repository: structural and functional MRI, MEG, and cognitive data from a cross-sectional adult lifespan sample. Neuroimage. 2017;144:262–269. doi: 10.1016/j.neuroimage.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P.A., Chen G., Glen D.R., Rajendra J.K. FMRI processing with AFNI: some comments and corrections on “exploring the impact of analysis software on task fMRI results. bioRxiv. 2018 [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture (USDA) Current population survey food security supplement 2018. 2019. https://www.ers.usda.gov/data-products/food-security-in-the-united-states/food-security-in-the-united-states/#Current%20Population%20Survey%20(CPS) [WWW Document] accessed 2.19.21.
- Wagner R.K., Torgesen J.K., Rashotte C.A., Pearson N.A. 2013. CTOPP-2: Comprehensive Test of Phonological Processing. Pro-ed. [Google Scholar]
- Ward N.S., Frackowiak R.S.J. Age-related changes in the neural correlates of motor performance. Brain. 2003;126:873–888. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn R.A., Smith K.W., Jette A.M., Janney C.A. The physical activity scale for the elderly (PASE): development and evaluation. J. Clin. Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- Williams D.R., Yan Yu, Jackson J.S., Anderson N.B. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J. Health Psychol. 1997;2:335–351. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- Wong P.C.M., Jin J.X., Gunasekera G.M., Abel R., Lee E.R., Dhar S. Aging and cortical mechanisms of speech perception in noise. Neuropsychologia. 2009;47:693–703. doi: 10.1016/j.neuropsychologia.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourganov G., Fridriksson J., Rorden C. Estimating the statistical significance of spatial maps for multivariate lesion-symptom analysis. Cortex. 2018;108:276–278. doi: 10.1016/j.cortex.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]