Abstract
Hepatic glucolipotoxicity, characterized by the synergistic detrimental effects of elevated glucose levels combined with excessive lipid accumulation in hepatocytes, plays a central role in the pathogenesis of various metabolic liver diseases. Despite recent advancements, the precise mechanisms underlying this process remain unclear. Employing cultured AML12 and HepG2 cells exposed to excess palmitate, with and without high glucose, as an in vitro model, we aimed to elucidate the cellular and molecular mechanisms underlying hepatic glucolipotoxicity. Our data showed that palmitate exposure induced the integrated stress response (ISR) in hepatocytes, evidenced by increased eIF2α phosphorylation (serine 51) and upregulated ATF4 expression. Moreover, we identified mTORC1 as a novel upstream kinase responsible for palmitate-triggered ISR induction. Furthermore, we showed that either mTORC1 inhibitors, ISRIB (an ISR inhibitor), or ATF4 knockdown abolished palmitate-induced cell death, indicating that the mTORC1-eIF2α-ATF4 pathway activation plays a mechanistic role in mediating palmitate-induced hepatocyte cell death. Our continuous investigations revealed that GPAT4-mediated metabolic flux of palmitate into the glycerolipid synthesis pathway is required for palmitate-induced mTORC1 activation and subsequent ISR induction. Specifically, we uncovered that saturated phosphatidic acid production contributes to palmitate-triggered mTORC1 activation. Our study provides the first evidence that high glucose enhances palmitate-induced activation of the mTORC1-eIF2α-ATF4 pathway, thereby exacerbating palmitate-induced hepatotoxicity. This effect is mediated by the increased availability of glycerol-3-phosphate, a substrate essential for phosphatidic acid synthesis. In conclusion, our study highlights that the activation of the mTORC1-eIF2α-ATF4 pathway, driven by saturated phosphatidic acid overproduction, plays a mechanistic role in hepatic glucolipotoxicity.
Keywords: Palmitate, phosphatidic acid, mTORC1, ISR, glucolipotoxicity
Graphical Abstract

Introduction
Lipotoxicity refers to the detrimental effects resulting from the excessive accumulation of free fatty acids (FFAs), particularly saturated fatty acids (SFAs), and their toxic metabolites in non-adipose tissues such as the liver, pancreas, and skeletal muscle(1). The concurrent presence of elevated glucose levels, as seen in hyperglycemia, has been shown to exacerbate lipotoxicity through synergistic mechanisms, giving rise to a pathological state known as glucolipotoxicity, a concept first introduced by Prentki and Corkey(2). Glucolipotoxicity underscores the compounding adverse effects of lipid and glucose toxicity, which together play a pivotal role in the development and progression of metabolic disorders, including metabolic dysfunction-associated fatty liver disease (MAFLD), the hepatic manifestation of metabolic syndrome(3).
The MAFLD encompasses a range of conditions characterized by excessive lipid accumulation (hepatic steatosis) that can progress to hepatocyte injury, inflammation, and cirrhosis(4). Palmitate, a 16-carbon SFA, is the predominant SFA found in human circulation and has consistently been observed at elevated levels in MAFLD both in experimental models(5) and clinical settings(6). It has frequently served as a standard experimental model for inducing lipotoxicity in various cell types, including hepatocytes(7, 8). Despite substantial advancements in recent decades, the precise mechanisms underlying glucolipotoxicity remain incompletely understood. Therefore, elucidating the molecular mechanisms by which hepatocytes respond to the combined elevation of saturated fatty acids and glucose is essential for developing targeted therapeutic strategies for the treatment of MAFLD.
The integrated stress response (ISR) is a complex cellular signaling pathway that is activated in response to various stressors such as nutrient deprivation, viral infection, oxidative stress, and accumulation of unfolded proteins in the endoplasmic reticulum (ER)(9). ISR is initiated by the phosphorylation of eukaryotic translation initiation factor 2 alpha (eIF2α), which is regulated by one of four well-established upstream kinases: protein kinase R (PKR), PKR-like ER kinase (PERK), heme-regulated inhibitor kinase (HRI), and general control nonderepressible 2 (GCN2)(10). While the phosphorylation of eIF2α results in the inhibition of general protein synthesis, it preferentially initiates the translation of a subset of mRNAs, specifically activating transcription factor 4 (ATF4), a key transcription factor that regulates the expression of genes involved in cellular stress responses(11). Phosphorylation of eIF2α at serine 51 and the subsequent induction of ATF4 translation are hallmark features of ISR activation(12).
The mammalian target of rapamycin complex 1 (mTORC1) is a well-established regulator of cell growth and metabolism in response to nutrient availability, growth factors, and cellular energy status(13). mTORC1 activation is orchestrated by a complex signaling network that integrates multiple inputs, primarily growth factors such as insulin and IGF-1, and amino acids, particularly leucine(14). Once activated, mTORC1 phosphorylates downstream targets such as S6K (which phosphorylates protein S6) and 4E-BP1, thereby regulating critical cellular processes, including protein synthesis, metabolism, and cell growth(7). Intriguingly, dysregulated mTORC1 activity has also been documented to contribute to cell death under certain conditions, such as nutrient deprivation or prolonged stress(15). Several groups, including ours, have independently reported that mTORC1 activation contributes to palmitate-induced lipotoxicity in different cell types, including hepatocytes(1, 7, 16). Specifically, we documented that nicotinamide N-methyltransferase upregulation is a downstream event in response to palmitate-induced mTORC1-ATF4 pathway activation, which leads to hepatocyte cell death(17). These previous studies suggest that mTORC1 not only acts as a sensor for cellular lipid overload but might also be an integral part of the ISR.
Palmitate undergoes diverse metabolic processes inside the cell after being activated to palmitoyl-CoA by fatty acyl-CoA synthase(18). In mitochondria, it undergoes β-oxidation, a pathway that breaks down palmitoyl-CoA into acetyl-CoA for energy production. Palmitate can also be used to synthesize complex lipids such as glycerolipid, in which glycerol-3-phosphate acyltransferase (GPAT) catalyzes the initial reaction between glycerol-3-phosphate and palmitoyl-CoA(1). Additionally, palmitate is a precursor for de novo biosynthesis of ceramide, a bioactive lipid involved in various signaling pathways. Moreover, palmitate can undergo desaturation catalyzed by stearoyl-CoA desaturase-1 (SCD-1), forming monounsaturated fatty acids(19). These metabolic processes collectively regulate the cellular energy balance, lipid homeostasis, and signaling cascades.
In this study, we investigated the cellular and molecular mechanisms driving glucolipotoxicity using cultured hepatocytes. Our findings reveal that the accumulation of saturated phosphatidic acids, which arise from the metabolism of both glucose and saturated fatty acids, plays a mechanistic role in the pathogenesis of hepatic glucolipotoxicity through activating mTORC1 and subsequent induction of the ISR.
Materials and methods
Reagents
Chemicals, including palmitate (St. Louis, USA), bovine serum albumin (BSA, A7030), myriocin (M1177), and dimethyl sulfoxide DMSO (D2650) were purchased from Sigma-Aldrich. The other chemicals used in this study were purchased as follows: ISRIB (MCE, HY-12495), GSK-2606414 (MCE, S7307), GCN2 inhibitor (MCE, HY-100877), PKR inhibitor (EMD Millipore Corp., 527450), Etomoxir (MCE, HY-50202A), Torin1 (APExBIO Technology LLC, A8312), and Rapamycin (APExBIO Technology LLC, 53123–88-9). The palmitate-albumin complex was prepared using a saponification method as described by Goldstein et al.(20), employing palmitate (C16:0) to form a complex with bovine serum albumin (BSA), which is essentially free of fatty acids. The specific procedure was performed as described in Dou et al(21).
Cell culture
AML12 cells (purchased from ATCC) were cultured in Dulbecco’s modified Eagle’s medium (DMEM)-F12 supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin, 0.1 mg/mL streptomycin, 40 ng/mL dexamethasone, and ITS supplement containing 5 mg/L insulin, 5 mg/L transferrin, and 5 μg/L selenium. HepG2 cells (purchased from ATCC) were maintained in DMEM supplemented with 10% FBS. Both cell lines were grown in 75 cm2 flasks at 37°C in a humidified atmosphere with 5% CO2. The cells were allowed to reach 80% confluence before being subjected to various experimental interventions.
RNA interference
Cultured cells were transfected with ATF4 siRNA (Santa Cruz Biotechnology, sc-35113), Agpat1 siRNA (Invitrogen, ASO2NJUL), Agpat3 siRNA (Invitrogen, ASO2NJUM), Gpam siRNA (Santa Cruz Biotechnology, sc-145677), Gpat4 siRNA (Santa Cruz Biotechnology, sc-75691), Gpd1 siRNA (Santa Cruz Biotechnology, sc-145683), Hri siRNA (Santa Cruz Biotechnology, sc-39053), Lpin1 siRNA (Invitrogen, AM16708), and Lpin2 siRNA (Invitrogen, ASO2MY54) using Lipofectamine 2000 according to the manufacturer’s instructions. Cells in the control group were transfected with a scrambled siRNA (Santa Cruz Biotechnology, sc-37007).
Intracellular TAG content
Cells were seeded at a density of 1×10⁵ cells/mL in 24-well plates and cultured overnight. Hepatocytes in the wells were washed twice with phosphate-buffered saline (PBS). Lipids were extracted using a 500 μL mixture of hexane:isopropanol (3:2). After two hours of shaking at room temperature, 20 μL of the supernatant was transferred to a new tube and centrifuged at 10,000 rpm for 5 min. The solvent was evaporated by using a SpeedVac concentrator. TAG levels were measured by adding TAG assay reagent (Triglyceride assay kit, MedTest Dx, Canton) and incubating at 37°C for 6 min, with vortexing every 3 min. TAG levels were normalized to the cellular protein concentration under the same conditions to minimize variability.
LDH release assay
The cells were seeded at a density of 1×10⁵ cells/mL in 24-well plates and incubated overnight. After the designated treatments, the culture supernatant was collected and analyzed using the CyQUANT LDH Cytotoxicity Assay Kit (Thermo Scientific Inc., C20301). Absorbance readings at 490 and 680 nm were obtained using a SPECTRAmax 340PC microplate spectrophotometer (Molecular Devices Corp., Sunnyvale, CA). Relative lactate dehydrogenase (LDH) release levels were quantified and expressed as fold-change compared to the untreated control group.
Mice administrations
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Illinois Chicago (Chicago, IL, USA) and adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All mice were housed in a controlled environment with a temperature range of 18–23°C, humidity levels between 40–60%, and a 12 h light/dark cycle. Ten-week-old male C57BL/6 mice were administered rapamycin intraperitoneally at 5 mg/kg body weight per day for 9 consecutive days. Rapamycin was prepared as a vehicle solution consisting of 2% DMSO, 5% Tween 80, and 30% PEG300 in ddH2O. Control mice were administered an equivalent volume of the vehicle solution.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted using TRIzol® reagent (Invitrogen, Carlsbad, CA, USA) and subsequently reverse-transcribed into cDNA using reverse transcription kits (Promega, Madison, WI, USA), following the manufacturer’s instructions. The synthesized cDNAs were then amplified in 384-well PCR plates using SYBR Green/ROX qPCR Master Mix (2X) on an Applied Biosystems 7300 Real-Time PCR System (Thermo Fisher Scientific). Gene expression levels were normalized to 18s rRNA and calculated using the 2−△△Ct method. All primers were synthesized by Integrated DNA Technologies (IDT) with the following sequences:
Mouse Atf4 forward, ATGGCGCTCTTCACGAAATC;
Mouse Atf4 reverse, ACTGGTCGAAGGGGTCATCAA;
Mouse Agpat1 forward, TAAGATGGCCTTCTACAACGGC;
Mouse Agpat1 reverse, CCATACAGGTATTTGACGTGGAG;
Mouse Agpat3 forward, CTGCTTGCCTACCTGAAGACC;
Mouse Agpat3 reverse, GATACGGCGGTATAGGTGCTT;
Mouse Hri forward, TGCCAGACTTTTATCAAGATGGG;
Mouse Hri reverse, GTAGGGCTTACCTGACGGAC;
Mouse lipin1 forward, CATGCTTCGGAAAGTCCTTCA;
Mouse lipin1 reverse, GGTTATTCTTTGGCGTCAACCT;
Mouse lipin2 forward, GAAGTGGCGGCTCTCTATTTC;
Mouse lipin2 reverse, AGAGGGTTACATCAGGCAAGT;
Mouse Gpd1 forward, ATGGCTGGCAAGAAAGTCTG;
Mouse Gpd1 reverse, CGTGCTGAGTGTTGATGATCT;
Mouse Gpat1 forward, ACAGTTGGCACAATAGACGTTT;
Mouse Gpat1 reverse, CCTTCCATTTCAGTGTTGCAGA;
Mouse Gpat4 forward, AGCTTGATTGTCAACCTCCTG;
Mouse Gpat4 reverse, CCGTTGGTGTAGGGCTTGT;
Mouse 18s forward, TAACCCGTTGAACCCCATT;
Mouse 18s reverse, CCATCCAATCGGTAGTAGCG.
Western blot analysis
Total protein from AML12 hepatocytes and liver tissues was obtained using a lysis buffer from Thermo Scientific Inc. (Rockford, USA). The samples were chilled on ice with frequent vortex for 15 minutes and centrifuged for 10 minutes at 12,000g. Protein concentrations of samples were determined using the Enhanced BCA Protein Assay Kit from Thermo Scientific Inc. (Rockford, IL, USA) according to the manufacturer’s instructions. Equal amounts of protein (30 μg) were loaded onto 10% or 8% SDS-PAGE, depending on the molecular weight of the desired proteins, and transferred to a nitrocellulose transfer membrane (Pall Corporation, New Port Richey, FL). Following the transfer, membranes were cut based on the molecular weight of target proteins and blocked in 5% (wt./vol.) non-fat dry milk in PBS-0.1% Tween 20 and probed with specific antibodies. The following primary antibodies were used: p-S6 (1:1000, CST, 4858S), S6 (1:1000, CST, 2217S), p-eIF2α (1:1000, ABclonal, AP0692), eIF2α (1:1000, ABclonal, A21221), ATF4 (1:1000, ABclonal, A0201), GPD1 (1:1000, ABclonal, A5715), GPAT4 (1:1000, ABclonal, A20804), LPIN2 (1:1000, ABclonal, A15762), and anti-β-actin (1:1000, CST, 4970 L). β-actin served as an internal control. IRDye secondary antibodies (anti-rabbit and anti-mouse, LI-COR, NE, USA) were used for signal detection. The specificity of the primary antibodies was validated based on manufacturer-provided data, expected molecular weights observed in our Western blot analyses, and normalization using β-actin as an internal loading control. Antibodies from Cell Signaling Technology (CST) and ABclonal were selected due to their extensive validation in prior studies. The observed bands consistently corresponded to the predicted molecular weights of the target proteins, with minimal evidence of non-specific binding. Immunoreactive bands corresponding to the predicted molecular weights were visualized using the LI-COR Odyssey CLx System (Lincoln, NE, USA).
Statistical analysis
All data were analyzed using GraphPad Prism 9 and are presented as the mean ± standard deviation (SD). A two-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test was performed for comparisons among multiple groups. All experiments were repeated at least three times independently, and each dot represented an independent biological replicate. Statistical significance was defined as P < 0.05.
Results
mTORC1 activation contributes to palmitate-induced lipotoxicity in hepatocytes.
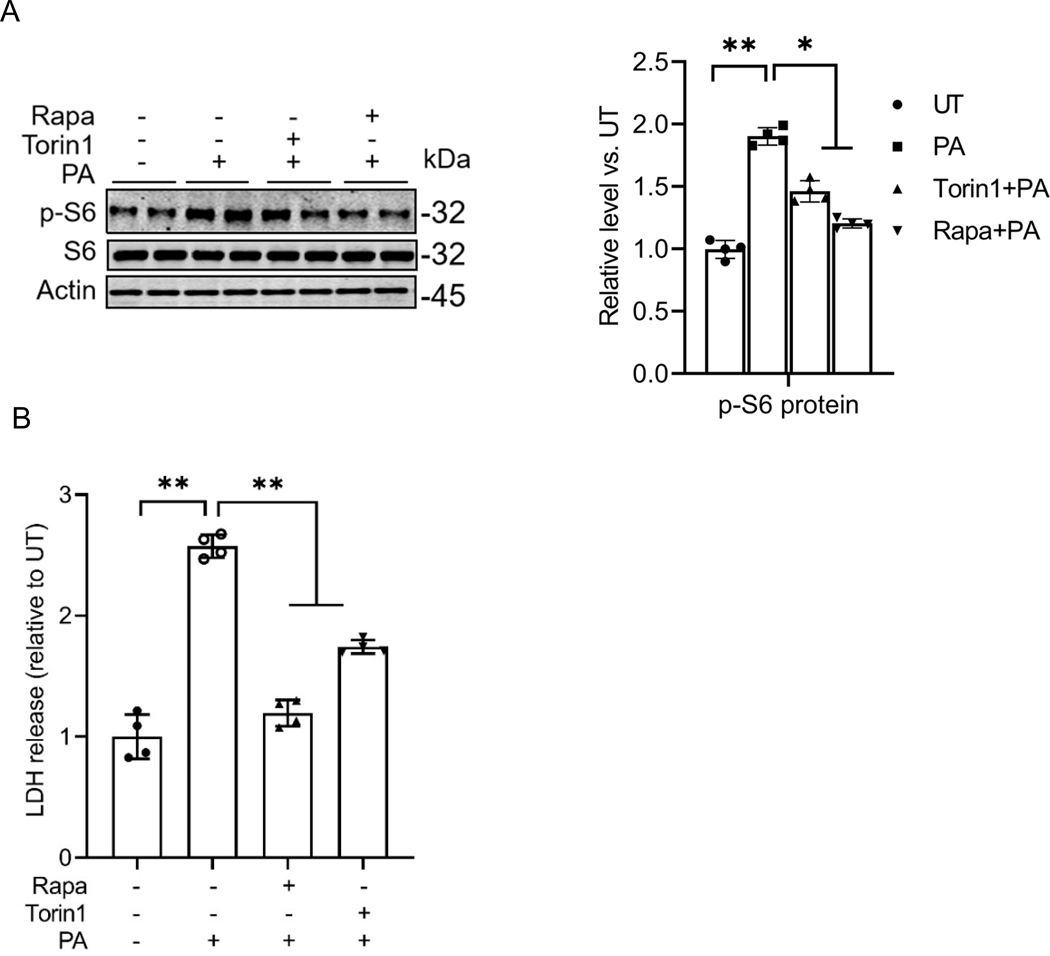
In this study, we employed cultured AML12 cells exposed to palmitate, a 16-carbon saturated fatty acid, as an in vitro model to investigate lipotoxicity. To evaluate the effects of palmitate on mTORC1 activation, AML12 cells were treated with palmitate (0.4 mM) for 16 hours, either with or without a 2-hour pretreatment of mTORC1 inhibitors (Torin1 or rapamycin). The activation status of mTORC1 was assessed by detecting the abundance of phosphorylated S6 protein (p-S6), a well-established downstream target of mTORC1, via Western blot analysis. The level of p-S6 was used as a marker to indicate the activation status of mTORC1. As shown in Fig. 1A, palmitate exposure led to a significant increase in the abundance of phosphorylated S6 protein (p-S6) compared to control cells treated with an equivalent amount of albumin, indicating enhanced mTORC1 activation. Pretreatment with either of the mTORC1 inhibitors, Torin1 or rapamycin, abolished the palmitate-induced activation of mTORC1. Notably, as shown in Fig. 1B, both mTORC1 inhibitors also mitigated palmitate-induced hepatocyte cell death, suggesting that the activation of mTORC1 plays a mechanistic role in mediating palmitate-induced lipotoxicity in hepatocytes.
Fig. 1. mTORC1 activation contributes to palmitate-induced lipotoxicity in hepatocytes.
(A) AML12 cells were pretreated with mTORC1 inhibitors (rapamycin, 0.05 mM and torin1, 0.25 μM) for 2 h before palmitate addition (0.4 mM). Protein abundance of phosphorylated and total S6 was detected by Western blot. (B) LDH release. *p < 0.05 and **p < 0.01 indicate statistically significant differences.
Palmitate induces the integrated stress response (ISR) in hepatocytes.
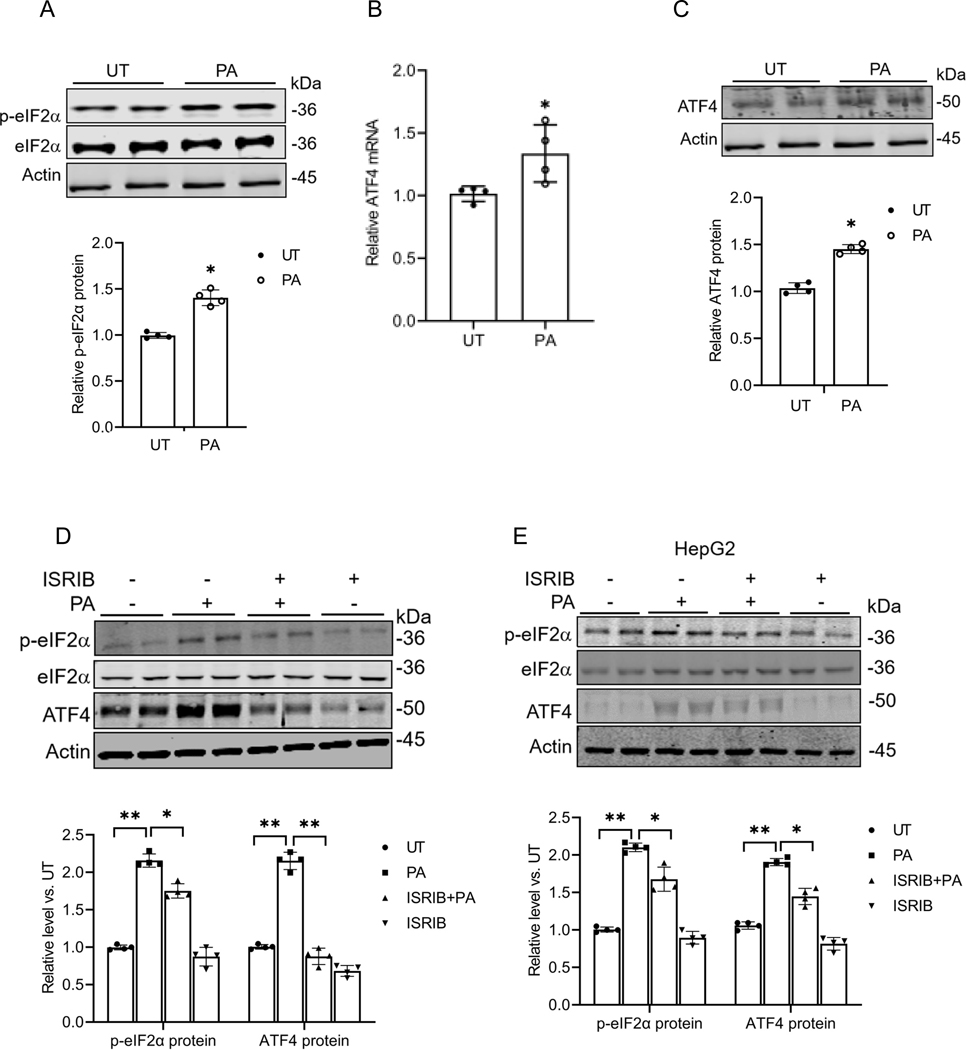
The ISR is a cellular signaling pathway that coordinates various cellular stress responses. The key event in the ISR is the phosphorylation of eIF2α at Serine 51. This leads to global translational repression and selective translational activation of ATF4. Our previous finding that the exposure of hepatocytes to palmitate upregulated ATF4 expression(22) prompted us to explore whether palmitate acts as an ISR inducer. In this study, AML12 cells were primarily used to test our hypothesis, and the key findings were further validated in HepG2 cells. As shown in Fig. 2A, palmitate exposure of AML12 cells led to an increased protein abundance of phosphorylated eIF2α at Serine 51, concurrent with upregulated ATF4 expression at both mRNA and protein levels (Figs. 2B & C). Pretreatment with ISRIB, an ISR inhibitor that prevents eIF2α phosphorylation at Serine 51, abolished palmitate-induced ATF4 upregulation (Fig. 2D). A similar effect of palmitate on eIF2α (serine 51) phosphorylation and ATF4 expressions was observed in HepG2 cells (Fig. 2E). Collectively, these results indicate that palmitate triggers ISR in hepatocytes.
Fig. 2. Palmitate activates the ISR (integrated stress response) in hepatocytes.
(A) Western blot analysis of phosphorylated and total eIF2α expression with/without palmitate (0.4mM) exposure in AML12 cells. (B) qRT-PCR assay of ATF4 gene expression with/without palmitate exposure in AML12 cells. (C) Western blot detection of ATF4 protein abundance with/without palmitate exposure in AML12 cells. (D, E) AML12 and HepG2 cells were pretreated with ISRIB (10 μM), an inhibitor of the integrated stress response (ISR), for 2 h before palmitate exposure (0.4 mM). The hepatic eIF2α phosphorylation and ATF4 expressions were detected by Western blot. *p < 0.05 and **p < 0.01 indicate statistically significant differences. Note: The same internal control (actin) band was utilized for Figures A & C since all protein bands originated from the same membrane.
The ISR induction contributes to palmitate-induced hepatotoxicity.
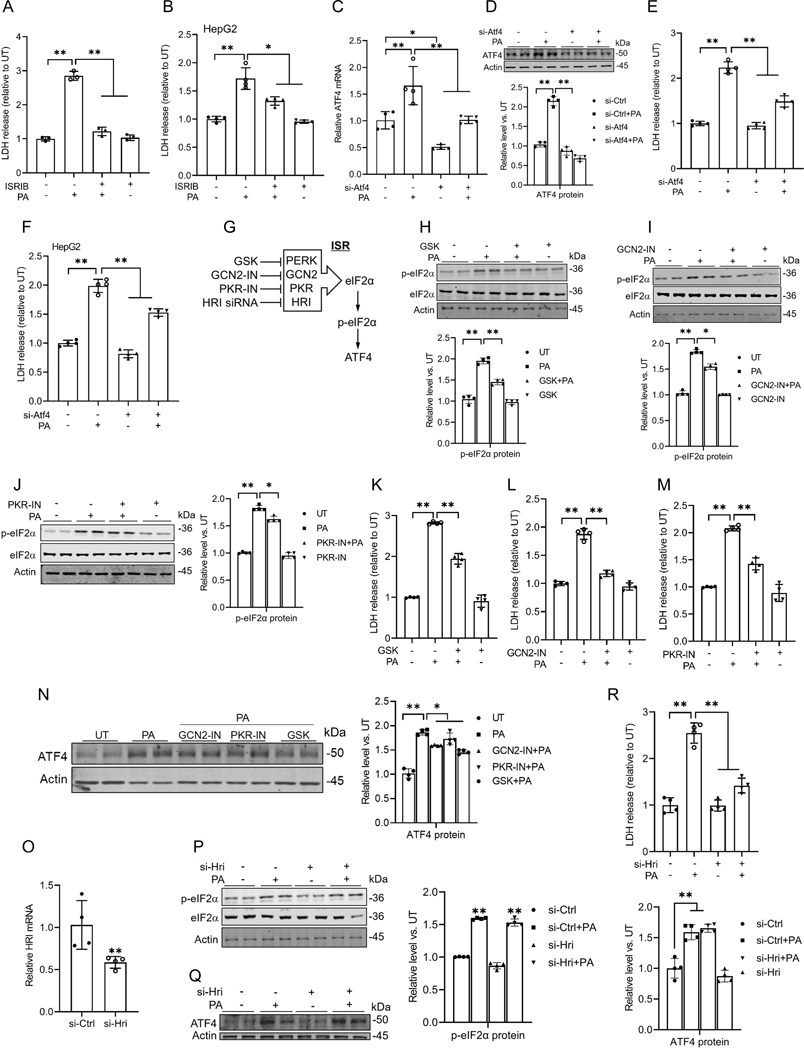
To determine the critical role of ISR activation in palmitate-induced hepatotoxicity, we first treated AML12 and HepG2 cells with ISRIB, an ISR inhibitor, for 2 hours before the palmitate challenge. Cell death was measured after 16 hours using an LDH release assay kit. ISR inhibition almost completely prevented palmitate-induced cell death in hepatocytes (Figs. 3A & B). Next, to assess the role of ATF4 upregulation in palmitate-induced cell death, we genetically knocked down ATF4 in AML12 cells using Atf4-targeted siRNA, followed by exposure to palmitate for 16 hours. As shown in Figs. 3C-E, genetic knockdown of ATF4 attenuated palmitate-induced cell death in AML12 cells. A similar protective effect of Atf4 siRNA knockdown was observed in HepG2 cells (Fig. 3F). It has been well-documented that during ISR, eIF2α is phosphorylated by one of four well-established upstream kinases, including protein kinase R (PKR), PKR-like ER kinase (PERK), heme-regulated inhibitor kinase (HRI), and general control nonderepressible 2 (GCN2) in response to different upstream stimuli(23). To further confirm the role of ISR induction in palmitate-induced lipotoxicity, we examined the effects of inhibiting each individual upstream kinase on palmitate-induced cell death. Our data demonstrated that inhibiting these four kinases provides varying degrees of protection against cell death induced by palmitate (Figs. 3G-R). Specifically, inhibition of PERK and GCN2 significantly attenuated the induction of p-eIF2α and the subsequent upregulation of ATF4 (Figs. 3H, I, and N), whereas inhibition of PKR and HRI exhibited notably weaker effects (Figs. 3J, O-Q). Intriguingly, despite their differential impacts on p-eIF2α and ATF4 levels, inhibition of each of these four kinases provided varying degrees of protection against palmitate-induced cell death (Figs. 3K-M, R). This discrepancy suggests that, beyond their roles in ISR activation, these kinases, particularly HRI, may promote palmitate-induced cell death through additional mechanisms. Nevertheless, our findings collectively demonstrate that ISR activation plays a mechanistic role in palmitate-induced lipotoxicity.
Fig. 3. The ISR induction contributes to palmitate-induced hepatotoxicity.
(A, B) The cell death of AML12 and HepG2 cells was determined 16 h later by LDH release measurement. ISRIB treatment rescued the hepatocyte death induced by palmitate. (C, D) AML12 cells were transfected with either scramble siRNA or Atf4 siRNA (si-Atf4) for 24 h before palmitate exposure (0.4 mM) for 16 h. qRT-PCR and Western blot analysis of ATF4 expression. (E, F) ATF4 gene knockdown alleviated palmitate-induced cell death in AML12 and HepG2 cells, determined by LDH release. (G) The schematic illustration of four canonical upstream kinases. (H-M) AML12 cells were pretreated with GSK-2606414 (PERK inhibitor, 10 μM), GCN2 inhibitor (GCN2-IN, 10 μM), or PKR inhibitor (PKR-IN, 10 μM) for 2 h before palmitate addition (0.4 mM). Protein abundances of phosphorylated and total eIF2α were detected by Western blot, and cell death was determined 16 h later by LDH release. (N) Protein abundances of ATF4 were detected by Western blot. (O) The transfection efficiency of HRI siRNA in AML12 cells was assayed by qRT-PCR. (P-R) AML12 cells were transfected with either scramble siRNAor HRI siRNA (si-Hri) for 24 h before palmitate exposure (0.4 mM) for 16 h. The eIF2α phosphorylation and ATF4 expressions were detected by Western blot, and cell death was determined by LDH release. *p < 0.05 and **p < 0.01 indicate statistically significant differences.
mTORC1 is an upstream signal contributing to palmitate-triggered ISR activation.
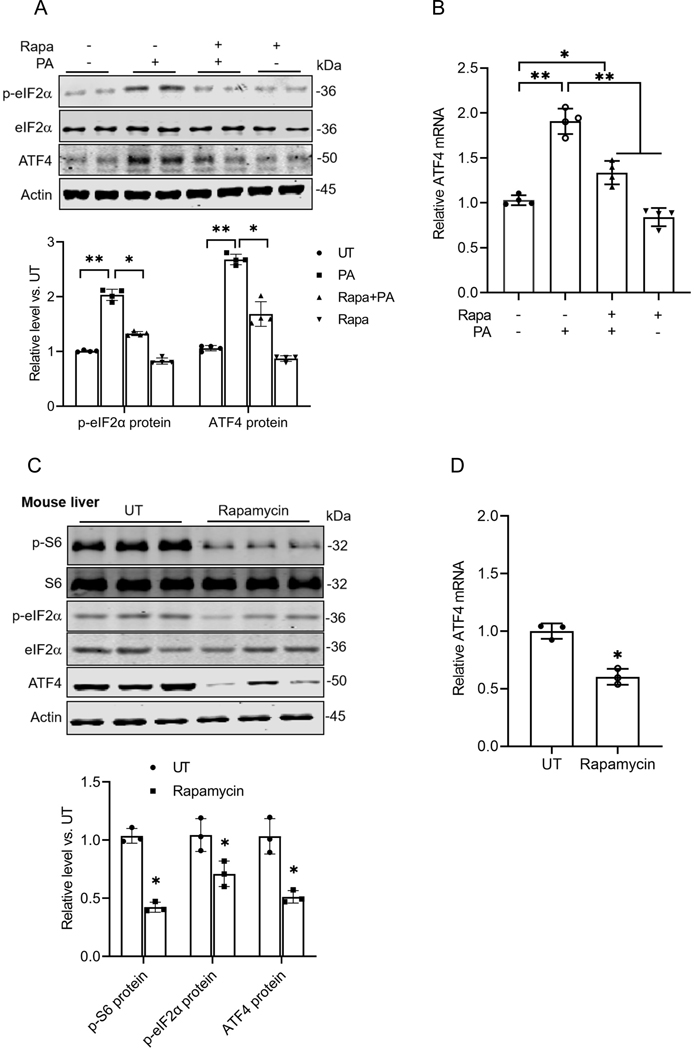
Based on our observations that inhibition of both mTORC1 and the ISR protects against palmitate-induced lipotoxicity, we postulated that these two pathways are closely interconnected. To investigate whether mTORC1 activation acts as an upstream regulator of ISR induction in response to palmitate exposure, AML12 cells were pretreated with rapamycin, a mTORC1 inhibitor, for 2 hours prior to a 16-hour exposure to palmitate. As shown in Figs. 4A & B, mTORC1 inhibition almost eliminated the palmitate-induced increase in eIF2α phosphorylation and significantly blunted ATF4 upregulation. The notion was further supported by the subsequent animal studies in which male C57BL/6 mice were administered rapamycin (5 mg/kg BW/day, ip) for 9 days. As shown in Figs. 4C & D, rapamycin administration substantially reduced eIF2 phosphorylation and ATF4 expression in the mouse liver. Taken together, these observations indicate that mTORC1 functions as an upstream kinase mediating ISR initiation following palmitate exposure in hepatocytes.
Fig. 4. mTORC1 is an upstream signal contributing to palmitate-triggered ISR activation.
(A, B) AML12 cells were pretreated with rapamycin for 2 h before palmitate addition (0.4 mM). Protein abundances of phosphorylated and total eIF2α, as well as ATF4, were detected using Western blot, while gene expression was assessed through qRT-PCR. (C) Hepatic protein abundances of phosphorylated and total S6, phosphorylated and total eIF2α, and ATF4 were detected by Western blot in mice administrated with/without rapamycin (n=3/group). (D) qRT-PCR analysis of hepatic ATF4 expression in mice administrated with/without rapamycin (n=3/group). Rapa: rapamycin. *p < 0.05 and **p < 0.01 indicate statistically significant differences.
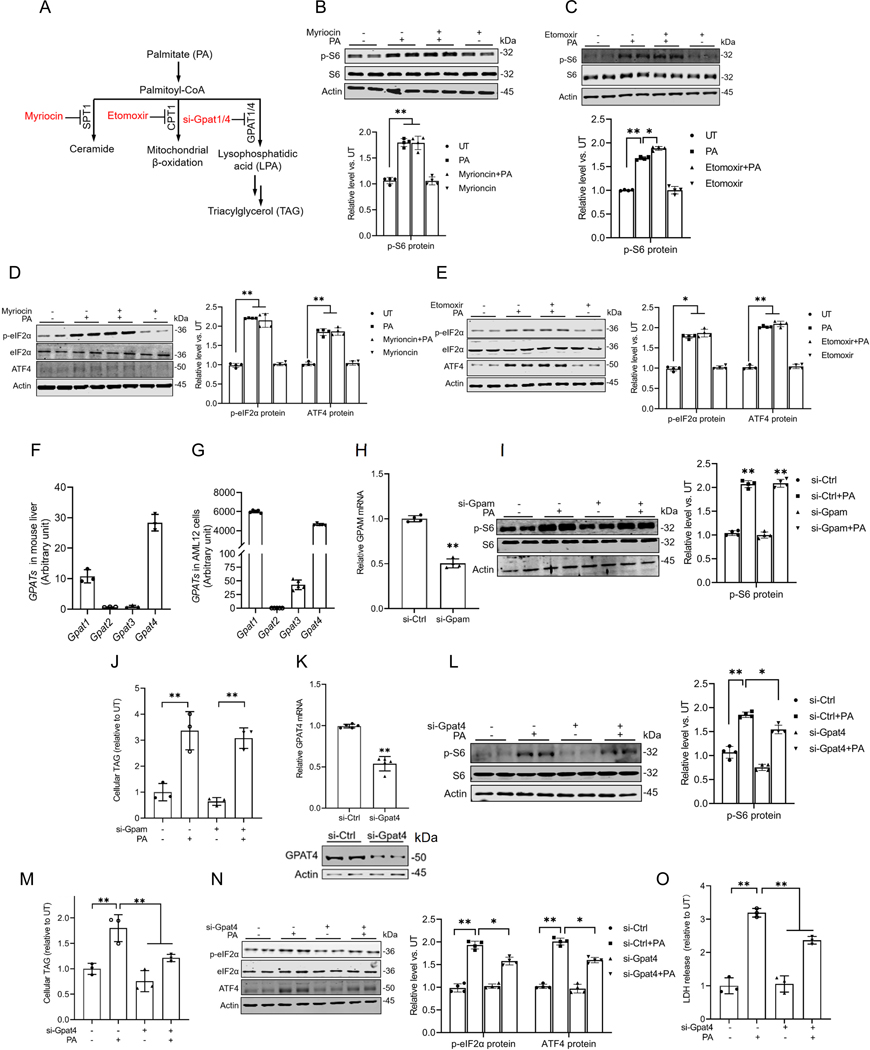
Metabolic flow into the glycerolipid synthesis pathway contributes to palmitate-triggered mTORC1 activation.
We previously reported that both the conversion of palmitate to palmitoyl-CoA and the preservation of its saturation status are required to induce mTORC1 activation in hepatocytes(7). Upon conversion to palmitoyl-CoA, palmitate can participate in three major metabolic pathways, including ceramide biosynthesis, mitochondrial β-oxidation, and the biosynthesis of lysophosphatidic acid (LPA), which subsequently contributes to the production of other glycerolipids (Fig. 5A). To identify the specific metabolic pathway contributing to palmitate-induced mTORC1 activation, we systematically inhibited the rate-limiting enzymes of these pathways using either pharmacological inhibitors or genetic approaches prior to exposing the cells to palmitate. As shown in Figs. 5B & C, the inhibition of neither SPT1, the rate-limiting enzyme in ceramide biosynthesis, nor CPT1, a key enzyme in fatty acid β-oxidation, impacted palmitate-induced mTORC1 activation. Similarly, neither intervention affected the induction of the ISR (Figs. 5D & E), excluding the involvement of these two pathways in palmitate-triggered mTORC1 activation. The metabolic flux of palmitate into the glycerolipid biosynthesis pathway is initiated by the formation of LPA from fatty acyl-CoA and glycerol-3-phosphate, which is mediated by glycerol-3-phosphate acyltransferase (GPAT). The mammalian cells possess four distinct isoforms of GPAT, GPAT1–4. Notably, our RNA-sequencing analysis of both mouse liver (Fig. 5F) and AML12 cells (Fig. 5G) confirmed that the mouse hepatocytes predominantly express two isoforms, GPAT1/GPAM and GPAT4. To determine the role of saturated LPA synthesis from palmitate acyl-CoA in mTORC1 activation upon palmitate stress, we conducted gene knockdown experiments targeting Gpam and Gpat4. Knockdown of Gpam had no significant effect on palmitate-induced mTORC1 activation (Figs. 5H & I) or intracellular TAG levels (Fig. 5J). In contrast, siRNA-mediated knockdown of Gpat4 attenuated mTORC1 activation in response to palmitate exposure (Figs. 5K & L), which was accompanied by a significant reduction in palmitate-induced intracellular TAG accumulation (Fig. 5M). These findings suggest that metabolic flow into the glycerolipid synthesis pathway contributes to palmitate-triggered mTORC1 activation. Consistently, Gpat4 knockdown blunted palmitate-induced ISR activation, as evidenced by a reduction in eIF2α phosphorylation and ATF4 upregulation (Fig. 5N). This was accompanied by reduced palmitate-induced LDH release (Fig. 5O), indicative of decreased cellular damage.
Fig. 5. Metabolic flow into the glycerolipid synthesis pathway contributes to palmitate-triggered mTORC1 activation.
(A) Schematic illustration of major metabolic pathways of palmitate. (B-E) AML12 cells were pretreated with either myriocin (100 μM), the SPT1 inhibitor, or etomoxir (20 μM), the CPT1 inhibitor, for 2 h before palmitate addition (0.4 mM). The protein abundances of phosphorylated and total S6/eIF2α, and ATF4 were detected by Western blot. (F) The gene expression of 4 isoforms of GPATs in mouse liver. (G) The expression of 4 isoforms of GPATs in AML12 cells. AML12 cells were transfected overnight with Gpam siRNA, prior to palmitate exposure for 16 h. (H) The transfection efficiency of Gpam siRNA in AML12 cells was assayed by qRT-PCR. (I) The protein abundances of phosphorylated and total S6 were detected by Western blot. (J) Intracellular TG content. AML12 cells were transfected overnight with Gpat4 siRNA, prior to palmitate exposure for 16 h. (K) The transfection efficiency of Gpat4 siRNA in AML12 cells was assayed by qRT-PCR and Western blot. (L) The protein abundances of phosphorylated and total S6 were detected by Western blot. (M) Intracellular TG content. (N) The protein abundances of phosphorylated and total eIF2α and ATF4 were detected by Western blot. (O) The cell death was determined by LDH release measurement. *p < 0.05 and **p < 0.01 indicate statistically significant differences.
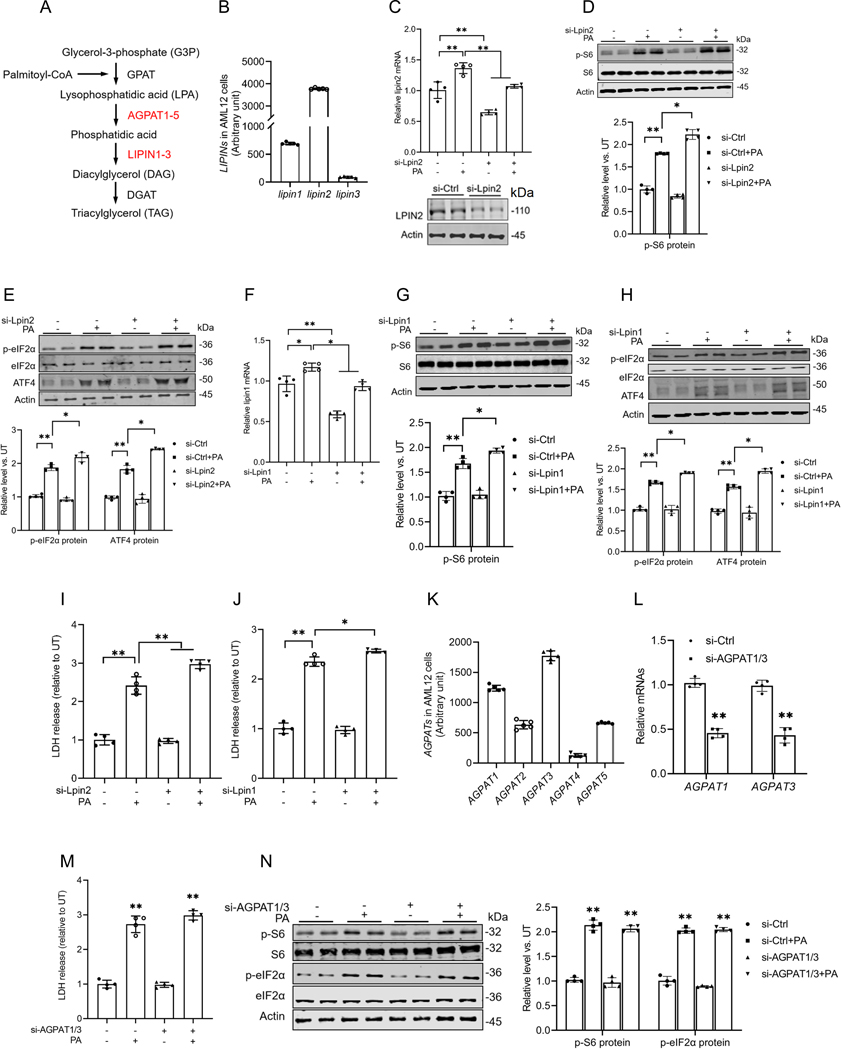
Saturated phosphatidic acids contribute to palmitate-triggered mTORC1 activation, ISR induction, and hepatocyte cell death.
In the glycerolipid biosynthesis pathway, palmitoyl-CoA is initially converted to LPA, which is subsequently transformed into phosphatidic acid. Phosphatidic acid is then converted into diacylglycerol (DAG) by the enzyme LIPIN (Fig. 6A). This LIPIN family consists of three isoforms, LIPIN1–3, with Lipin1 and Lipin2 being the predominant isoforms in AML12 cells, as confirmed by our RNA sequencing analysis (Fig. 6B). DAG is a well-documented lipotoxic mediator implicated in cellular dysfunction and damage across various cell types(24, 25). To determine whether saturated phosphatidic acid formation or the subsequent generation of DAG contributes to palmitate-induced mTORC1 activation, ISR induction, and cell death, we transfected AML12 cells with either Lpin1 or Lpin2 siRNAs overnight before being exposed to palmitate, respectively. As shown in Figs. 6D-J, either Lpin1 or Lpin2 gene knockdown exacerbated the palmitate-induced increase in the protein abundance of p-S6, phosphorylation of eIF2α (p-eIF2α), and ATF4, as well as LDH release. Although direct measurement of lysophosphatidic acid and phosphatidic acid would undoubtedly further enhance our mechanistic understanding and represents a limitation of the current study, our targeted gene knockdown experiments provide compelling evidence that the intracellular accumulation of saturated phosphatidic acids mechanistically contributes to palmitate-induced mTORC1 activation, subsequent ISR induction, and eventual cell death.
Fig. 6. Saturated phosphatidic acid accumulation contributes to palmitate-triggered ISR induction and lipotoxicity in hepatocytes.
(A) Schematic illustration of the metabolic flux of palmitate into the glycerolipid biosynthesis pathway. (B) The gene expression of three isoforms of LIPIN in AML12 cells. (C-E) AML12 cells were transfected overnight with Lpin2 siRNA, prior to palmitate exposure for 16 h. The expression of lipin2 in AML12 cells was tested by qRT-PCR and Western blot. Protein abundances of phosphorylated and total S6, phosphorylated and total eIF2α, and ATF4 were detected by Western blot. (F-H) AML12 cells were transfected overnight with Lpin1 siRNA, prior to palmitate exposure for 16 h. The expression of lipin1 in AML12 cells was tested by qRT-PCR. Protein abundances of phosphorylated and total S6, phosphorylated and total eIF2α, and ATF4 were detected by Western blot. (I, J) AML12 cells were transfected overnight with Lpin1 and Lpin2 siRNAs, prior to palmitate exposure for 16 h. The cell death was determined by LDH, respectively. (K) The gene expression of 5 isoforms of AGPATs in AML12 cells. (L, M) AML12 cells were transfected overnight with AGPAT1/3 siRNAs, prior to palmitate exposure for 16 h exposure. The expressions of AGPAT1 and AGPAT3 in AML12 cells were measured by qRT-PCR and the cell death was determined by LDH release measurement. (N) Protein abundances of phosphorylated and total S6, and phosphorylated and total eIF2α were detected by Western blot. *p < 0.05 and **p < 0.01 indicate statistically significant differences.
The enzyme 1-acylglycerol-3-phosphate O-acyltransferase (AGPAT) converts LPA into phosphatidic acid (Fig. 6A). The AGPAT family consists of five isoforms, APGAT1–5, with Agpat1 and Agpat3 being the predominant isoforms present in AML12 cells, as confirmed by our RNA sequencing analysis (Fig. 6K). To differentiate the effects of phosphatidic acid and LPA during these processes, we further knocked down AGPAT by overnight transfection of AML12 cells with siRNAs targeting Agpat1 or Agpat3 (Fig. 6L) before exposing them to palmitate. As shown in Figs. 6M & N, Agpat1/3 gene knockdown failed to alter the palmitate-induced cell death, mTORC1 activation, and ISR induction, implying that saturated LPA and phosphatidic acid may equally contribute to palmitate-induced mTORC1 activation.
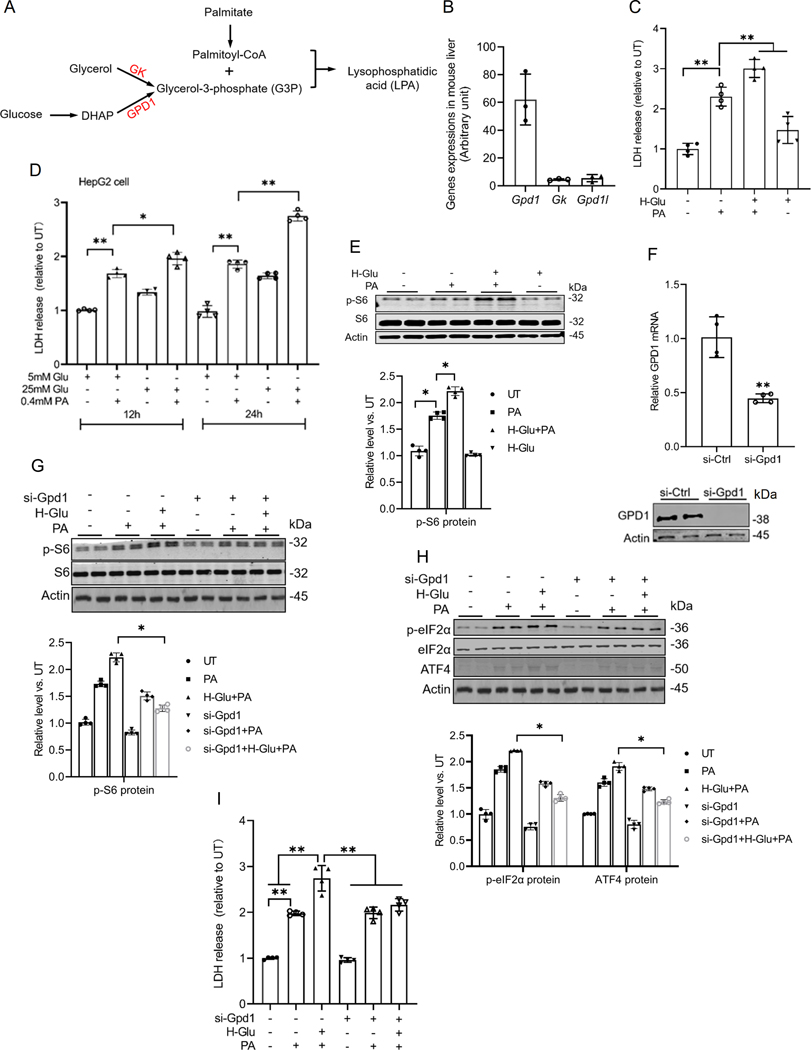
Glucose exacerbates palmitate-induced hepatotoxicity by promoting the generation of glycerol-3-phosphate, a co-substrate for phosphatic acid biosynthesis.
Glucolipotoxicity refers to the synergistic detrimental effects of prolonged exposure to elevated glucose and free fatty acids (particularly saturated fatty acids) on cellular function and viability(26, 27). Although glucolipotoxicity has been well-established to implicate the pathogenesis of various metabolic diseases(27–30), the exact mechanism remains elusive. Phosphatidic acid formation from palmitoyl-CoA requires glycerol-3-phosphate (G3P) as a co-substrate (Fig. 7A). In the liver, G3P is primarily produced by two enzymes, glycerol kinase (GK), which converts glycerol to G3P, and glycerol-3-phosphate dehydrogenase-1 (GPD1), which generates G3P from dihydroxyacetone phosphate (DHAP), a product of glycolysis (Fig. 7A). Based on this, we propose that glucose-derived G3P may amplify palmitate-induced mTORC1 activation, ISR induction, and cell death by acting as a precursor for phosphatidic acid biosynthesis, thereby contributing to glucolipotoxicity. From our RNA sequencing data analysis, we found that the expression of Gpd1 in the mouse liver was significantly higher than that of GK and glycerol-3-phosphate dehydrogenase 1-like (Gpd1l), another isoform of GPD1 (Fig. 7B). To test our hypothesis, we investigated the effects of varying glucose concentrations on palmitate-induced lipotoxicity in both AML12 and HepG2 cells. As shown in Figs. 7C & D, high-glucose media exacerbated palmitate-induced hepatotoxicity. Consistently, high-glucose conditions also enhanced palmitate-induced mTORC1 activation (Fig. 7E). Notably, genetic knockdown of Gpd1 via siRNA transfection (Fig. 7F) effectively attenuated mTORC1 activation induced by the combination of high glucose and palmitate (Fig. 7G), suppressed ISR induction (Fig. 7H), and reduced cell death (Fig. 7I). These results underscore the critical role of G3P formation from glucose in mediating glucolipotoxicity.
Fig. 7. Glucose enhances palmitate-induced lipotoxicity by promoting the generation of glycerol-3-phosphate, a co-substrate for LPA biosynthesis.
(A) Schematic illustration of the formation of LPA. (B) The expression of Gpd1, Gk, and Gpd1l in mouse liver. AML12 cells were pretreated with high glucose (H-Glu, 25 mM) for 2 h before palmitate addition (0.4 mM). (C) Cell death was determined by LDH release measurement. (D) LDH release in HepG2 cells under different glucose and palmitic acid (PA) conditions. Cells were treated with 5 mM or 25 mM glucose (Glu) in the presence or absence of 0.4 mM PA for 12 h and 24 h. (E) AML12 cells were transfected overnight with Gpd1 siRNA followed by glucose (25 mM) pretreatment for 2 h, prior to a 16 h palmitate exposure. Protein abundance of phosphorylated and total S6 was detected by Western blot. (F) The transfection efficiency of Gpd1 siRNA in AML12 cells was assayed by qRT-PCR and Western blot. (G-I) The protein abundances of phosphorylated and total S6, phosphorylated and total eIF2α, and ATF4 were detected by Western blot, and the cell death was determined by LDH. *p < 0.05 and **p < 0.01 indicate statistically significant differences. Note: The same internal control (actin) band was utilized for Figures G & H since all protein bands originated from the same membrane.
Discussions
This study demonstrated that palmitate exposure induces eIF2α phosphorylation at serine 51 and upregulates ATF4 in hepatocytes, both hallmark features of integrated stress response (ISR) activation. Notably, our findings revealed that inhibiting eIF2α phosphorylation or knocking down ATF4 using siRNA protected hepatocytes from palmitate-induced cell death, highlighting a mechanistic role for ISR activation in palmitate-induced hepatotoxicity. Moreover, our study showed that palmitate activates mTORC1 in hepatocytes, and this activation is required for both eIF2α phosphorylation and ATF4 upregulation upon palmitate exposure, indicating that mTORC1 is a novel upstream kinase responsible for palmitate-induced ISR activation in hepatocytes. Further mechanistic investigations revealed that the formation of saturated phosphatidic acid is essential for activating the mTORC1-eIF2α-ATF4 pathway in response to palmitate exposure. Importantly, our data uncovered that glucose exacerbated palmitate-induced lipotoxicity, a phenomenon termed glucolipotoxicity, by supplying glycerol-3-phosphate (G3P) to promote phosphatidic acid biosynthesis and subsequent mTORC1 activation and ISR induction. Collectively, our findings suggest that mTORC1 activation and subsequent ISR induction by cellular saturated phosphatidic acids represents an important mechanism driving glucolipotoxicity in hepatocytes.
The eIF2α-ATF4 pathway is the central component of the ISR(11). In this study, we provide evidence that ISR induction plays a mechanistic role in palmitate-induced hepatotoxicity and identified mTORC1 as an upstream kinase contributing to palmitate-triggered eIF2α phosphorylation. mTORC1 activation has been documented to contribute to palmitate-induced lipotoxicity in various cell types, including hepatocytes(7, 16). In our previous study, we documented that the activation of the mTORC1-ATF4 pathway plays a mechanistic role in palmitate-induced hepatotoxicity by upregulating nicotinamide N-methyltransferase in hepatocytes(17). Moreover, mTORC1-mediated ATF4 activation has been reported by Torrence et al. to promote protein and glutathione synthesis downstream of growth signals(31). There is also indirect evidence supporting that mTORC1 may act as an upstream kinase of eIF2α, contributing to ISR(32). In this study, we directly tested this hypothesis, and our findings provide clear evidence that mTORC1 plays a key role in palmitate-induced ISR activation. First, we observed that inhibiting mTORC1 completely blocked palmitate-triggered eIF2α phosphorylation at serine 51. Second, both mTORC1 inhibitors and ISRIB, an inhibitor of eIF2α phosphorylation, abolished palmitate-induced ATF4 upregulation. These results confirm that mTORC1 is a novel upstream kinase driving palmitate-induced ISR activation.
In our previous studies, we documented that the intracellular metabolism of palmitate, specifically its conversion to palmitoyl-CoA and the preservation of its saturation state, is essential for mTORC1 activation(7). In the present study, we aimed to gain deeper insights into the mechanisms by which palmitate activates mTORC1 in hepatocytes by assessing the involvement of key intracellular metabolic pathways associated with palmitate metabolism downstream of palmitoyl-CoA, including ceramide biosynthesis, mitochondrial β-oxidation, and glycerolipid synthesis. Our findings confirmed that, while neither de novo ceramide biosynthesis nor mitochondrial β-oxidation contributes to palmitate-induced mTORC1 activation, the metabolic flux of palmitoyl-CoA into the glycerolipid synthesis pathway is required in this process. Palmitoyl-CoA enters the glycerolipid synthesis pathway by reacting with G3P to form lysophosphatidic acids (LPAs), which are catalyzed by glycerol-3-phosphate acyltransferase (GPAT)(33). Among the four isoforms of GPAT, GPAT4 is one of the two GPATs, along with GPAT1 (GPAM), which is abundantly expressed in hepatocytes and responsible for LPA formation. Our data revealed that the genetic knockdown of GPAT4, but not GPAT1, diminished the activation of the mTORC1-eIF2α-ATF4 pathway upon palmitate exposure, concurrent with a reduction in cellular TAG content, suggesting that GPAT4-mediated flux of palmitate into the glycerolipid biosynthesis pathway contributes to its mTORC1-activating effect. This observation aligns with findings from previous studies conducted in other cell types. For instance, GPAT4-generated saturated LPAs induce lipotoxicity in smooth muscle cells via a mechanism involving autophagy inhibition resulting from the formation of abnormal omegasomes(34). In MEFs and HEK293 cells, phospholipase D-derived phosphatidic acid is required for both amino acid and growth factor input to mTORC1 via promoting mTORC1 lysosomal translocation(35).
In the glycerolipid biosynthetic pathway, LPAs are subsequently converted to phosphatidic acids. Lipins are a family of phosphatidic acid phosphatase, catalyzing the removal of phosphate groups from phosphatidic acid to generate diacylglycerol(36). There are three known lipins in mammals (lipin-1, −2, and −3), with lipin-2 being the predominant isoform in the liver. In the present study, we found that in contrast to the effect of GPAT4 knockdown, which prevented palmitate-triggered mTORC1 activation, lipin2 knockdown exacerbated the activation of the mTORC1-eIF2α-ATF4 pathway and increased cell death upon palmitate exposure, suggesting that the formation of phosphatidic acid is responsible for palmitate-induced mTORC1 activation and subsequent cytotoxicity in hepatocytes.
Although it has been documented that glucose exacerbates palmitate-induced lipotoxicity, a phenomenon known as glucolipotoxicity(37), its exact mechanism remains unclear. Given that the formation of phosphatic acids from palmitoyl-CoA requires G3P as a co-substrate, we hypothesized that glucose may exacerbate palmitate-induced hepatotoxicity through supplying G3P through the glycolytic intermediate dihydroxyacetone phosphate (DHAP), which can be converted into G3P by a reaction catalyzed by GPD1. Although hepatocytes have been well known to express GK, which can directly convert glycerol to G3P, intriguingly, our RNA sequencing data indicated that the expression of GPD1 in mouse liver was much higher than that of GK. Importantly, our findings revealed that genetic knockdown of GPD1 mitigated the exacerbating effects of high glucose on palmitate-induced activation of the mTORC1-eIF2α-ATF4 pathway and hepatocyte cell death. These results collectively suggest that G3P, as a substrate for the metabolic flux of palmitate into the glycerolipid synthesis pathway, plays a critical role in driving glucolipotoxicity.
In conclusion, our study establishes that ISR induction is a key mechanistic factor in palmitate-induced hepatic lipotoxicity and identifies mTORC1 as a novel upstream kinase responsible for palmitate-induced ISR activation. Importantly, our study is the first to demonstrate that the intracellular formation of saturated phosphatidic acids mediates palmitate-induced and glucose-amplified activation of the mTORC1-eIF2α-ATF4 signaling pathway, thereby contributing to glucolipotoxicity. The findings in this study provide new mechanistic insights and identify potential therapeutic targets for the treatment of metabolic liver diseases associated with hepatic glucolipotoxicity.
New and Noteworthy:
ISR activation contributes to palmitate-induced lipotoxicity in hepatocytes.
mTORC1 acts as an upstream kinase essential for palmitate-mediated ISR activation and hepatocyte death.
The formation of saturated phosphatidic acid mechanistically regulates hepatic mTORC1 activation induced by palmitate.
Glucose-enhanced generation of saturated phosphatidic acid amplifies palmitate-induced hepatotoxicity, contributing to glucolipotoxicity.
Acknowledgements
We thank all authors and funders who contributed to this article.
Funding
This work was partly funded by the US NIH Grant NIAAA R01AA026603 (to Z. S.).
Abbreviations
- ATF4
activating transcription factor 4
- EIF2α
eukaryotic translation initiation factor 2 alpha
- G3P
glycerol-3-phosphate
- GPAT
glycerol-3-phosphate acyltransferase
- GPD1
glycerol-3-phosphate dehydrogenase-1
- LPA
lysophosphatidic acid
- MAFLD
metabolic dysfunction-associated fatty liver disease
- mTORC1
mammalian target of rapamycin complex 1
- ISR
integrated stress response
- PBS
phosphate-buffered saline
- SFA
saturated fatty acid
- SCD1
stearoyl-CoA desaturase-1
- TAG
triacylglycerol
Footnotes
Declarations
Ethics approval and consent to participate
All animal experiments were conducted with the approval of the Institutional Animal Care and Use Committee at the University of Illinois at Chicago on June 11, 2022 (approval number: ACC-22–052), and in accordance with the rules of Basel Declaration and “Guide for The Care and Use of Laboratory Animals” 8th Edition.
Consent for publication
All authors in this article have consented to publication.
Competing interests
The authors declare no competing interests.
Availability of data and materials
All data and materials are available.
References:
- 1.Lipke K, Kubis-Kubiak A, and Piwowar A. Molecular Mechanism of Lipotoxicity as an Interesting Aspect in the Development of Pathological States-Current View of Knowledge. Cells 11: 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prentki M, Segall L, Roche E, Thumelin S, Brun T, McGarry JD, Corkey BE, and Assimacopoulos-Jeannet F. [Gluco-lipotoxicity and gene expression in the pancreatic beta cell]. Journ Annu Diabetol Hotel Dieu 17–27, 1998. [PubMed] [Google Scholar]
- 3.Unger RH, Clark GO, Scherer PE, and Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta 1801: 209–214, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Kuchay MS, Choudhary NS, and Mishra SK. Pathophysiological mechanisms underlying MAFLD. Diabetes Metab Syndr 14: 1875–1887, 2020. [DOI] [PubMed] [Google Scholar]
- 5.Lee JY, Cho HK, and Kwon YH. Palmitate induces insulin resistance without significant intracellular triglyceride accumulation in HepG2 cells. Metabolism 59: 927–934, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Rosqvist F, Kullberg J, Stahlman M, Cedernaes J, Heurling K, Johansson HE, Iggman D, Wilking H, Larsson A, Eriksson O, Johansson L, Straniero S, Rudling M, Antoni G, Lubberink M, Orho-Melander M, Boren J, Ahlstrom H, and Riserus U. Overeating Saturated Fat Promotes Fatty Liver and Ceramides Compared With Polyunsaturated Fat: A Randomized Trial. J Clin Endocrinol Metab 104: 6207–6219, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Chen Y, Song Q, Griffiths A, and Song Z. mTORC1-IRE1alpha pathway activation contributes to palmitate-elicited triglyceride secretion and cell death in hepatocytes. Exp Biol Med (Maywood) 245: 1268–1279, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen C, Ma W, Ding L, Li S, Dou X, and Song Z. The TLR4-IRE1alpha pathway activation contributes to palmitate-elicited lipotoxicity in hepatocytes. J Cell Mol Med 22: 3572–3581, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa-Mattioli M, and Walter P. The integrated stress response: From mechanism to disease. Science 368: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bond S, Lopez-Lloreda C, Gannon PJ, Akay-Espinoza C, and Jordan-Sciutto KL. The Integrated Stress Response and Phosphorylated Eukaryotic Initiation Factor 2alpha in Neurodegeneration. J Neuropathol Exp Neurol 79: 123–143, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wek RC. Role of eIF2alpha Kinases in Translational Control and Adaptation to Cellular Stress. Cold Spring Harb Perspect Biol 10: 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neill G, and Masson GR. A stay of execution: ATF4 regulation and potential outcomes for the integrated stress response. Front Mol Neurosci 16: 1112253, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saxton RA, and Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell 168: 960–976, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sengupta S, Peterson TR, and Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40: 310–322, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu J, Wang H, and Jiang X. mTORC1 beyond anabolic metabolism: Regulation of cell death. J Cell Biol 221: 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Griffiths A, Wang J, Zhang T, Song Q, and Song Z. Inositol-requiring enzyme 1alpha links palmitate-induced mTOR activation and lipotoxicity in hepatocytes. Am J Physiol Cell Physiol 319: C1130–C1140, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths A, Wang J, Song Q, Iyamu ID, Liu L, Park J, Jiang Y, Huang R, and Song Z. Nicotinamide N-methyltransferase upregulation via the mTORC1-ATF4 pathway activation contributes to palmitate-induced lipotoxicity in hepatocytes. Am J Physiol Cell Physiol 321: C585–C595, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandel NS. Lipid Metabolism. Cold Spring Harb Perspect Biol 13: 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tracey TJ, Steyn FJ, Wolvetang EJ, and Ngo ST. Neuronal Lipid Metabolism: Multiple Pathways Driving Functional Outcomes in Health and Disease. Front Mol Neurosci 11: 10, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein JL, Basu SK, and Brown MS. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol 98: 241–260, 1983. [DOI] [PubMed] [Google Scholar]
- 21.Dou X, Wang Z, Yao T, and Song Z. Cysteine aggravates palmitate-induced cell death in hepatocytes. Life Sci 89: 878–885, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffiths A, Wang J, Song Q, Lee SM, Cordoba-Chacon J, and Song ZY. ATF4-mediated CD36 upregulation contributes to palmitate-induced lipotoxicity in hepatocytes. Am J Physiol-Gastr L 324: G341–G353, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donnelly N, Gorman AM, Gupta S, and Samali A. The eIF2alpha kinases: their structures and functions. Cell Mol Life Sci 70: 3493–3511, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rockenfeller P, Smolnig M, Diessl J, Bashir M, Schmiedhofer V, Knittelfelder O, Ring J, Franz J, Foessl I, Khan MJ, Rost R, Graier WF, Kroemer G, Zimmermann A, Carmona-Gutierrez D, Eisenberg T, Buttner S, Sigrist SJ, Kuhnlein RP, Kohlwein SD, Gourlay CW, and Madeo F. Diacylglycerol triggers Rim101 pathway-dependent necrosis in yeast: a model for lipotoxicity. Cell Death Differ 25: 767–783, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yazici D, Demir SC, and Sezer H. Insulin Resistance, Obesity, and Lipotoxicity. Adv Exp Med Biol 1460: 391–430, 2024. [DOI] [PubMed] [Google Scholar]
- 26.Poitout V, and Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 29: 351–366, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunes A, Schmitt C, Bilodeau L, Huet C, Belblidia A, Baldwin C, Giard JM, Biertho L, Lafortune A, Couture CY, Cheung A, Nguyen BN, Galun E, Bemeur C, Bilodeau M, Laplante M, Tang A, Faraj M, and Estall JL. IL-6 Trans-Signaling Is Increased in Diabetes, Impacted by Glucolipotoxicity, and Associated With Liver Stiffness and Fibrosis in Fatty Liver Disease. Diabetes 72: 1820–1834, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu T, Shao Y, Li X, Wu T, Yu L, Liang J, Zhang Y, Wang J, Sun T, Zhu Y, Chang X, Wang S, Chen F, and Han X. NR3C1/Glucocorticoid receptor activation promotes pancreatic beta-cell autophagy overload in response to glucolipotoxicity. Autophagy 19: 2538–2557, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bar-Tana J mTORC1 syndrome (TorS): unified paradigm for diabetes/metabolic syndrome. Trends Endocrinol Metab 34: 135–145, 2023. [DOI] [PubMed] [Google Scholar]
- 30.Guo J, Li C, Yang C, Li B, Wei J, Lin Y, Ye P, Hu G, and Li J. Liraglutide reduces hepatic glucolipotoxicity‑induced liver cell apoptosis through NRF2 signaling in Zucker diabetic fatty rats. Mol Med Rep 17: 8316–8324, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torrence ME, MacArthur MR, Hosios AM, Valvezan AJ, Asara JM, Mitchell JR, and Manning BD. The mTORC1-mediated activation of ATF4 promotes protein and glutathione synthesis downstream of growth signals. Elife 10: 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Rodriguez A, Mayoral R, Agra N, Valdecantos MP, Pardo V, Miquilena-Colina ME, Vargas-Castrillon J, Lo Iacono O, Corazzari M, Fimia GM, Piacentini M, Muntane J, Bosca L, Garcia-Monzon C, Martin-Sanz P, and Valverde AM. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis 5: e1179, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wendel AA, Lewin TM, and Coleman RA. Glycerol-3-phosphate acyltransferases: rate limiting enzymes of triacylglycerol biosynthesis. Biochim Biophys Acta 1791: 501–506, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiozaki Y, Miyazaki-Anzai S, Okamura K, Keenan AL, Masuda M, and Miyazaki M. GPAT4-Generated Saturated LPAs Induce Lipotoxicity through Inhibition of Autophagy by Abnormal Formation of Omegasomes. iScience 23: 101105, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frias MA, Mukhopadhyay S, Lehman E, Walasek A, Utter M, Menon D, and Foster DA. Phosphatidic acid drives mTORC1 lysosomal translocation in the absence of amino acids. J Biol Chem 295: 263–274, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang P, and Reue K. Lipin proteins and glycerolipid metabolism: Roles at the ER membrane and beyond. Biochim Biophys Acta Biomembr 1859: 1583–1595, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachar E, Ariav Y, Ketzinel-Gilad M, Cerasi E, Kaiser N, and Leibowitz G. Glucose amplifies fatty acid-induced endoplasmic reticulum stress in pancreatic beta-cells via activation of mTORC1. PLoS One 4: e4954, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are available.