Abstract
The plant hormone abscisic acid (ABA) has gained attention for its role in animals and humans, particularly due to its protective effects in various immune and inflammatory disorders. Given its high concentrations in fruits like figs, bilberries and apricots, ABA shows promise as a nutraceutical. However scalability, short half-life and cost limit the use of ABA-enriched fruit extracts and synthetic supplements. In this study, we propose an alternative ABA administration method to overcome these challenges. We genetically engineered a strain of the probiotic Saccharomyces boulardii to produce and deliver ABA directly to the gut of mice. Using the biosynthesis pathway from Botrytis cinerea, four genes (bcaba1-4) were integrated into S. boulardii, enabling ABA production at 30 °C, as previously described in Saccharomyces cerevisiae. Introducing an additional cytochrome P450 reductase gene resulted in a 7-fold increase in ABA titers, surpassing previous ABA-producing S. cerevisiae strains. Supplementation of the ABA-producing S. boulardii in the diet of mice (at a concentration of 5 × 108 CFU/g) led to effective gut colonization but resulted in low serum ABA levels (approximately 1.8 ng/mL). The absence of detectable serum ABA after administration of the ABA-producing probiotic through oral gavage, prompted further investigation to determine the underlying cause. The physiological body temperature (37 °C) was identified as a major bottleneck for ABA production. Modifications to enhance the mevalonate pathway flux improved ABA levels at 37 °C. However, additional modifications are needed to optimize ABA production before testing this probiotic in disease contexts in mice.
Keywords: Saccharomyces boulardii, Abscisic acid, Probiotic engineering, In situ production, Metabolic engineering
Highlights
-
•
Production of phytohormone abscisic acid in probiotic Saccharomyces boulardii.
-
•
Development of method to treat mice with yeast probiotics, inducing minimal stress.
-
•
High Saccharomyces boulardii levels in diet successfully promoted colonization of the mouse gut.
-
•
Physiological body temperature: a critical bottleneck for abscisic acid production.
1. Background
Abscisic acid (ABA) is a well-characterized phytohormone involved in the regulation of key physiological functions, including responses to abiotic stress and plant growth and development (Hey et al., 2010). Although the effects of ABA have been extensively studied within the plant kingdom, its role in animals has only gained significant attention over the past decades (Kim et al., 2020). In animals, the involvement of ABA has been reported in various immune and inflammatory responses (Lievens et al., 2017), and treatments of animals and humans with ABA have demonstrated protective effects against several diseases, such as colitis (Guri et al., 2010a), type 2 diabetes (Ameri et al., 2015; Magnone et al., 2015), atherosclerosis (Guri et al., 2010b) and depression (Qi et al., 2014). Given its high concentrations in fruits like figs, bilberries and apricots (Magnone et al., 2015), ABA presents potential as an interesting nutraceutical substance (Kim et al., 2020). However, challenges such as scalability, short half-life and cost-effectiveness hinder the practical use of ABA-enriched fruit extracts and synthetic ABA supplements (Liang et al., 2011; Siddiqui et al., 2012). In this study, we propose an alternative ABA administration technique that we believe could effectively address these challenges.
Probiotics are defined as “microbial cell preparations or components of microbial cells that have a beneficial effect on the health and well-being of the host” (Salminen et al., 2005). Leveraging decades of advancements in metabolic engineering, next-generation engineered probiotics can offer unique attributes for delivering biomolecules to the gut (Ferreiro et al., 2018; Ozdemir et al., 2018; Pedrolli et al., 2019). Engineered probiotics have traditionally been bacterial, with Escherichia coli Nissle 1917 modified to eliminate pathogens like Pseudomonas aeruginosa (Hwang et al., 2017) or to treat conditions such as hyperammonemia (Kurtz et al., 2019) and cancer (Ho et al., 2018). However, a major disadvantage of bacterial probiotics is their susceptibility to antibiotics and bacteriophages (Sweere et al., 2019). In addition to bacteria, the gastrointestinal tract also contains a diverse fungal population (Wheeler et al., 2016). In this study, we focus on the non-pathogenic yeast Saccharomyces boulardii, a variety of the budding yeast Saccharomyces cerevisiae. S. boulardii is widely recognized as safe and commonly used as a commercial probiotic for treating diarrhea (Czerucka et al., 2007) and various gut-related diseases (Duman et al., 2005; Guslandi et al., 2003; Kotowska et al., 2005; McFarland, 2009, 2010; McFarland et al., 1994, 1995), with its probiotic effects largely attributed to its unusually high acetic acid production (Offei et al., 2019). Through genetic engineering, S. cerevisiae has been modified to produce various plant-based products (Siddiqui et al., 2012), including artemisinic acid, a precursor of the antimalarial drug artemisinin (Paddon and Keasling, 2014; Ro et al., 2006). As well-established protocols developed for S. cerevisiae can be applied to S. boulardii (Ferreira et al., 2018; Jessop-Fabre et al., 2016; Liu et al., 2016), this enables efficient genetic modification. Previous genetic engineering efforts have enabled S. boulardii to secrete lysozyme (Liu et al., 2016) and HIV-1 gag (Palma et al., 2019) in cell culture, the vitamin A precursor β-carotene in the gut of germ-free mice (Durmusoglu et al., 2021), and IL-10 or elevated levels of acetic acid to exert protection in mouse models of inflammatory bowel disease (De Carvalho et al., 2024; Deleu et al., 2024; Michael et al., 2013).
In plants, ABA is synthesized via the degradation of C40 carotenoids produced in plastids (Schwartz et al., 2003). However, plants are not the only organisms capable of producing ABA. ABA production has also been confirmed in phytopathogenic fungi such as Botrytis cinerea (Dörffling et al., 1984), cyanobacteria (Maršálek et al., 1992), the animal parasite Toxoplasma gondii (Nagamune et al., 2008) and mammals, including humans (Bruzzone et al., 2007). Although ABA signaling properties are conserved across different kingdoms of life, biosynthetic pathways vary among organisms. The only well-understood non-plant ABA synthesis pathway has been described in the grey mold B. cinerea, where ABA is synthesized from the C15 molecule farnesyl pyrophosphate (FPP), a product from the mevalonate (MVA) pathway (Hirai et al., 2000; Inomata et al., 2004). Specifically, FPP is cyclized to form the ABA core scaffold and subsequently oxidized by multiple enzymes (Inomata et al., 2004; Takino et al., 2019). In 2006, the gene cluster involved in these subsequent steps was identified to contain two cytochrome P450 monooxygenase (CYP) genes (bcaba1 and bcaba2), a short-chain dehydrogenase/reductase gene (bcaba4), and an alpha-ionylideneethane synthase gene (bcaba3) (Siewers et al., 2004, 2006; Takino et al., 2018). Recently, Otto et al. demonstrated that integrating the ABA biosynthesis gene cluster from B. cinerea (Siewers et al., 2006) into S. cerevisiae (Otto et al., 2019) was sufficient to produce ABA in yeast. This also allowed the authors to elucidate the ABA biosynthesis pathway. Briefly, FPP is cyclized by bcABA3 to form alpha-ionylideneethane, which is then oxidized by bcABA1 to form α-ionylideneacetic acid. This compound is subsequently oxidized by bcABA2 to form 1′,4′-trans-dihydroxy-α-ionylideneacetic acid, which is finally modified by bcABA4 to generate ABA (Otto et al., 2019). Otto et al. did not encounter limitations in precursor supply for ABA production in yeast. Instead, the bottlenecks in this pathway were identified as the activities of CYPs bcABA1 and bcABA2. Further overexpression of the cytochrome P450 reductase (CPR) gene bccpr1, which facilitates electron transfer from NADPH to CYPs (Schenkman and Voznesensky, 1995), increased ABA titers (Otto et al., 2019). The probiotic properties of S. boulardii, combined with the recent report demonstrating that S. cerevisiae can be genetically engineered to produce ABA (Otto et al., 2019), suggest that engineering S. boulardii to produce ABA could offer an efficient strategy for ABA delivery to the gut.
2. Materials and methods
2.1. Microorganisms
NEB® 5-alpha Competent E. coli cells (New England Biolabs) were used for plasmid amplification. The Saccharomyces strains used in this study are listed in detail in Table 1. In short, Enterol (S. boulardii CNCMI-745) was selected because of its widespread use as a probiotic, while SbP was selected because of its high acetic acid production — a trait we hypothesized could enhance in-gut ABA production by improving competition with local microbiota and increasing ABA bioavailability through enhanced trans membrane diffusion at low pH.
Table 1.
Strains used in this study.
| Strain name | Genotype | Source/Reference | |
|---|---|---|---|
| Background strains | SbP | Wild type S. boulardii strains | Lene Jespersen, |
| University of Copenhagen, Denmark (1) | |||
| Enterol (E) | Wild type S. boulardii isolated from Pharmacy product Enterol® | Johan Thevelein, | |
| KU Leuven, Belgium (2) | |||
| Strains with B. cinerea genes | TABA3 | S. cerevisiae CEN.PK113-5D | Verena Siewers, Chalmers University of Technology, Gothenburg, Sweden (3) |
| PPGK1-bcaba1 PPGK1-bcaba2 PTEF1-bcaba3 PTEF1-bcaba4 PTEF1-bccpr1 PTEF1-tHMG1 | |||
| SABA3 | S. cerevisiae CEN.PK113-5D lpp1Δ:loxP dpp1Δ:loxP PERG9Δ:loxP-PHXT1 gdh1Δ:loxP PTEF1-ERG20 PPGK1-GDH2 PTEF1-tHMG1 PPGK1-bcaba1 PPGK1-bcaba2 PTEF1-bcaba3 PTEF1-bcaba4 PTEF1-bccpr1 | Verena Siewers, Chalmers University of Technology, Gothenburg, Sweden (3) | |
| SbP-ABA1 | SbP | This study | |
| PTDH3-bcaba1 PTEF1-bcaba2 PTDH3-bcaba3 PTEF1-bcaba4 | |||
| E-ABA1 | Enterol | This study | |
| PTDH3-bcaba1 PTEF1-bcaba2 PTDH3-bcaba3 PTEF1-bcaba4 | |||
| E-ABA2 | Enterol | This study | |
| PTDH3-bcaba1 PTEF1-bcaba2 PTDH3-bcaba3 PTEF1-bcaba4 | |||
| PTDH3-bcaba1 PTDH3-bcaba3 | |||
| E-ABA3 | Enterol | This study | |
| PTDH3-bcaba1 PTEF1-bcaba2 PTDH3-bcaba3 PTEF1-bcaba4 | |||
| PTEF1-bcaba1 PTEF1-bcaba3 | |||
| E-ABA4 | Enterol | This study | |
| PTDH3-bcaba1 PTEF1-bcaba2 PTDH3-bcaba3 PTEF1-bcaba4 | |||
| PRPL18B-bcaba1 PRPL18B-bcaba3 | |||
| E-ABA5 | Enterol | This study | |
| PTDH3-bcaba1 PTEF1-bcaba2 PTDH3-bcaba3 PTEF1-bcaba4 | |||
| PSAC6-bcaba1 PSAC6-bcaba3 | |||
| E-CABA1 | Enterol | This study | |
| PTDH3-bcaba1 PTEF1-bcaba2 PTDH3-bcaba3 PTEF1-bcaba4 | |||
| PTEF1-bccpr1 | |||
| E-CABA2 | Enterol | This study | |
| PTDH3-bcaba1-bccpr1 PTEF1-bcaba2 PTDH3-bcaba3 PTEF1-bcaba4 | |||
| E-CABA3 | Enterol | This study | |
| PTDH3-bcaba1 PTEF1-bcaba2-bccpr1 PTDH3-bcaba3 PTEF1-bcaba4 | |||
| E-CABA4 | Enterol | This study | |
| PTDH3-bcaba1-bmr PTEF1-bcaba2 PTDH3-bcaba3 PTEF1-bcaba4 | |||
| E-CABA5 | Enterol | This study | |
| PTDH3-bcaba1 PTEF1-bcaba2-bmr PTDH3-bcaba3 PTEF1-bcaba4 | |||
| E-CABA6 | Enterol | This study | |
| PTDH3-bcaba1-bmr PTEF1-bcaba2-bccpr1 PTDH3-bcaba3 PTEF1-bcaba4 | |||
| E-CABA7 | Enterol | This study | |
| PTDH3-bcaba1-bccpr1 PTEF1-bcaba2-bmr PTDH3-bcaba3 PTEF1-bcaba4 | |||
| E-TABA2 | Enterol | This study | |
| PTDH3-bcaba1 PTEF1-bcaba2 PTDH3-bcaba3 PTEF1-bcaba4 | |||
| PTDH3-bcaba1 PTDH3-bcaba3 PTEF1-tHMG1 | |||
| E-TABA3 | Enterol | This study | |
| PTDH3-bcaba1 PTEF1-bcaba2 PTDH3-bcaba3 PTEF1-bcaba4 | |||
| PTEF1-bcaba1 PTEF1-bcaba3 PTEF1-tHMG1 | |||
| E-TABA4 | Enterol | This study | |
| PTDH3-bcaba1 PTEF1-bcaba2 PTDH3-bcaba3 PTEF1-bcaba4 | |||
| PRPL18B-bcaba1 PRPL18B-bcaba3 PTEF1-tHMG1 | |||
| E-TABA5 | Enterol | This study | |
| PTDH3-bcaba1 PTEF1-bcaba2 PTDH3-bcaba3 PTEF1-bcaba4 | |||
| PSAC6-bcaba1 PSAC6-bcaba3 PTEF1-tHMG1 | |||
| E-TCABA2 | Enterol | This study | |
| PTDH3-bcaba1 PTEF1-bcaba2 PTDH3-bcaba3 PTEF1-bcaba4 | |||
| PTDH3-bcaba1 PTDH3-bcaba3 PTEF1-tHMG1 PTEF1-bccpr1 | |||
| E-TCABA3 | Enterol | This study | |
| PTDH3-bcaba1 PTEF1-bcaba2 PTDH3-bcaba3 PTEF1-bcaba4 | |||
| PTEF1-bcaba1 PTEF1-bcaba3 PTEF1-tHMG1 PTEF1-bccpr1 |
2.2. Media and culture conditions
Bacterial (E. coli) cells were propagated in LB medium containing 10 g/L tryptone (Neogen), 5 g/L yeast extract (Neogen), 10 g/L NaCl (ChemLab NV) and 100 mg/L ampicillin (Calbiochem) at 37 °C. For bacterial agar plates, 15 g/L bacto agar (Neogen) was added.
Yeast (Saccharomyces spp) cells were propagated at 30 °C, 33 °C or 37 °C in YPD medium containing 10 g/L yeast extract (Merck), 20 g/L bacteriological peptone (OXOID) and 20 g/L glucose (Merck). Solid nutrient plates for yeast were made with 15 g/L bacto agar (Neogen). Transformed yeast strains were selected on agar plates containing appropriate antibiotics i.e. 200 mg/L geneticin (G418; Gibco) or 100 mg/L nourseothricin (ClonNat).
2.3. Plasmid and strain construction
bcaba1, bcaba2, bcaba3, bcaba4 and bccpr1 from B. cinerea strain ATCC58025 were amplified from plasmids containing codon-optimized sequences for S. cerevisiae generated by Otto et al. (2019). bmr (Sevrioukova et al., 1996) and its natural linker were amplified from BM3 (a kind gift from Dr. Delphine Devriese and Prof. Bart Devreese, Ghent University). tHMG1 (truncated 3-hydroxy-3-methylglutaryl-CoA reductase) was amplified from the genomic DNA of the Enterol S. boulardii strain (Offei et al., 2019). Annotated sequences for each module are available in supplemental data.
Q5 High-Fidelity DNA polymerase (New England Biolabs) was used for PCR of DNA fragments. PCR products were purified using the Wizard® SV Gel and PCR Clean-Up System (Promega). Donor and guide plasmids were generated by Gibson assembly using NEBuilder® HiFi DNA Assembly (New England Biolabs) following the instruction manual provided by the manufacturer. All primers used for plasmid construction are listed in Table S2. Plasmids were amplified in E. coli cultivated at 37 °C in 5 mL LB medium with ampicillin shaking at 200 rpm. The NucleoSpin Plasmid mini kit (Macherey-Nagel) was used for plasmid extraction from E. coli.
Genes were genomically introduced by CRISPR/Cas9 mediated gene integration using the LiAC/SS-DNA/PEG protocol as transformation method (Gietz and Woods, 2006). The Cas9 expression plasmids used in this study was previously described in the report by Cen et al. (2017) and the gRNA plasmids used are described in Table S1. The presence of the introduced genes was verified by colony PCR where DNA was extracted by lysing cells with NaOH (10 mM) for 10 min at 99 °C and PCR was performed using the ALLin™ Red Taq Mastermix (highQu). Next, by sub-culturing 3 times in YPD, the Cas9 and guide plasmids were lost. Plasmid loss was verified by spot assay on YPD supplemented with geneticin or nourseothricin. All constructed strains with information about integration sites and verification primers can be found in Table S1.
2.4. S. boulardii cultivation for ABA measurement
For the comparison of ABA production by the wild-type and engineered S. boulardii strains, cultures were grown at 30 °C, 33 °C or 37 °C, while shaking at 200 rpm. A 3 mL preculture in YPD medium was inoculated by picking one yeast colony from a YPD plate. Three to five biological replicates were prepared for each strain. After approximately 18 h of cultivation, main cultures were inoculated at an OD600 of 0.1–0.2 and grown for 24 h. Main cultures were initially cultured in 50 mL YPD in 250 mL baffled flasks. Subsequently the culture conditions were adapted to allow for evaluations of more clones by down-scaling to 20 mL of YPD in 100 mL baffled flasks, or 3 mL of YPD in test tubes or 24-well deep-well plates as specified in each figure legend. After measuring the OD600, the cells were harvested by centrifugation (3000g, 5 min) and the supernatant was collected for ABA measurement (see below).
2.5. ABA measurement in culture supernatant
ABA measurements were performed using a highly sensitive biosensor, engineered by our lab (Kim et al., 2022). Briefly, this biosensor is a HEK 293T cell line stable transfected with a plasmid containing a PYL1H87P receptor coupled to a VP16 activation domain, the ABA coreceptor ABI1 coupled to a GAL4 binding domain and a plasmid containing a luciferase reporter gene. Presence of ABA brings the VP16 activation domain to the GAL4-dependent promoter, leading to luciferase gene expression. To measure ABA in culture supernatant, reporter cells were seeded at 10.000 cells per well in 96-well plates. One day post seeding, samples were added at a dilution of 1:20 when the medium was refreshed. Positive controls of 8, 4, 2, 1 and 0.5 μM ABA, as well as a negative control were used to create a standard concentration curve. After 24 h, cells were lysed in luciferase lysis buffer (25 mM Tris-phosphate pH7.8, 2 mM DTT, 2 mM CDTA, 10 % glycerol, 1 % Triton X-100). To cell lysates, D-luciferin (E1605, Promega) was added and luminescence signals were measured in triplicate using the Glomax® 96 Microplate Luminometer (Promega).
2.6. Quantitative reverse transcription PCR
For gene expression analysis of aba1-4 at different growth temperatures, yeast cells were grown at 30 °C for approximately 18 h and main cultures were inoculated at an OD600 of 0.1–0.2 and grown for 24 h at either 30 °C or 37 °C. Subsequently, cells were harvested by centrifugation (Eppendorf centrifuge, 4000RPM, 2min) and RNA was extracted from cell pellets using the RiboPure™-Yeast Kit (ThermoFisher Scientific) according to the manufacturer's instructions. RNA concentration and quality were determined with a Nanodrop spectrophotometer (Isogen Life Science). cDNA synthesis was conducted using the SensiFast cDNA Synthesis Kit (GC Biotech) according to the manufacturer's instructions. qPCR was performed on a Real-Time PCR system (Lightcycler 480, Roche) using the SensiFast SYBR No-Rox Kit containing 1x SYBR green (GC Biotech), 0.3 μM primers (Integrated DNA Technologies), 2 ng cDNA and nuclease-free water. mRNA expression was analyzed using the ΔΔCt method, where expression of aba1, aba2, aba3 and aba4 are normalized to expression of the 25S rRNA gene. Primer sequences are provided in Table S3.
2.7. Mice
Male and female wild-type C57BL/6J mice (8–12 weeks old) were obtained from Janvier (France) or bred at Ghent University in specific pathogen–free or germ-free conditions. Mice from Janvier were acclimatized for 2 weeks before initiation of the experiments. The mice were maintained on a 12-h light/dark cycle with free access to water and were fed either a standard chow diet, an ABA-supplemented diet (400 mg/kg, Research diets Inc.) or yeast-supplemented diet (see below), as indicated in the figure legend. All animal procedures were conducted in accordance with the institutional guidelines and approved by the Ethical Committee for Animal Experiments of the VIB site at Ghent University Faculty of Sciences or the Ethical Committee for Animal Experiments of the UZ site at Ghent University Faculty of Medicine and Health Sciences (ECD 21–52).
2.8. Yeast-supplemented mouse diet
Yeast-supplemented diet was prepared by mixing standard powdered mouse food (Sniff Bio Services B.V.) with water and yeast pellets creating a porridge-like formulation. In detail, yeast were precultured in 3 mL YPD at 30 °C. After approximately 18 h of cultivation, main cultures were prepared in 200 mL of YPD and grown for 24 h at 30 °C. The OD600 was measured and an equivalent of 15 × 109 CFU were harvested by centrifugation (3000×g, 5 min). The cell pellets were resuspended in 30 mL of drinking water, forming a yeast suspension. This suspension was mixed with 30 g of powdered mouse diet, resulting in a final concentration of 5 × 108 CFU per gram of food. The mixture was placed in glass jars secured in stainless steel holders within the cages, allowing the mice to consume the diet ad libitum. This process was repeated daily, and fresh yeast-supplemented food was given for 1 week before mice were sacrificed and serum was collected. In these experiments, diet supplemented with ABA-producing yeast was compared to diet-supplemented with wild-type yeast or diet supplemented with water, as indicated in the figure legends.
2.9. Administration of yeast to mice via oral gavage
For experiments in which administration of yeast was not performed through the diet, mice received oral gavage with a PBS solution containing the ABA-producing yeast. Conventional mice were gavaged daily with 5 × 108 CFU in 200 μL of PBS for a period of 1 week, while germ-free mice received a single dose of 5 × 108 CFU.
2.10. ABA measurement in serum
Serum samples were collected at sacrifice, after 1 week for conventional mice and on day 13 for germ-free mice. ABA measurements were performed using the highly sensitive biosensor, engineered by our lab (Kim et al., 2022) as described above. However, samples were added at a dilution of 1:10 when the medium was refreshed and positive controls in a lower range were included to create a standard curve (80, 40, 20, 10, 5, 2.5, 1.25 and 0 nM ABA). The standard curve was spiked with ABA-negative serum to correct for sample type-induced effects.
2.11. CFU count in stool
Fecal samples were collected on days 2, 3 and 4 for conventional mice and on days 3, 7 and 13 for germ-free mice. A piece of stool was collected and weighed to determine fecal mass. Fecal samples were then resuspended in 1 mL PBS per 25 mg feces. Fecal suspensions were diluted 1:100 in 1 mL of PBS and 100 μL of these suspensions were spread on solid YPD media. Plates were incubated at 30 °C for 2 days and colonies were counted manually.
2.12. Statistical analysis
Statistical analyses and data visualization were performed using Prism (Graphpad, La Jolla, CA). Data are presented as mean ± standard error of the mean (SEM). The number of biological replicates is indicated by dots in the figure or denoted as “n” in the legend. Data distribution and variance characteristics were considered for statistical testing. For normally distributed datasets, an ordinary one-way ANOVA with correction for multiple testing or a two-tailed unpaired Student's t-test was used. For non-normally distributed datasets, the Kruskal–Wallis test or Mann–Whitney U test was applied. Statistical significance was defined as p < 0.05, with significance levels indicated as ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001. For mouse experiments, no randomization was done, and the investigator was not blinded to the mouse group allocation. The sample size was determined by power analysis using G∗power software.
3. Results
3.1. Establishment of an ABA-producing S. boulardii strain
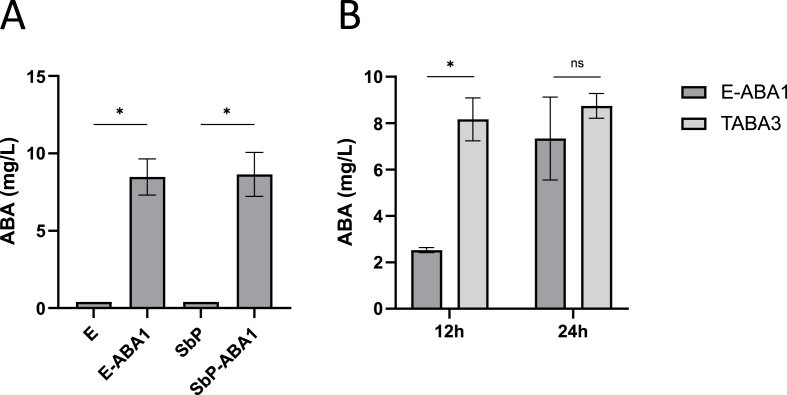
In this study, our objective was to engineer a highly active and fast-growing genetically modified probiotic S. boulardii strain, with minimal modifications while achieving maximal ABA production levels. We selected two specific S. boulardii strains for this purpose: the Enterol (S. bouldardii CNCM I-745) strain, commercialized by Biocodex and commonly used in therapeutic applications, and the high acetic acid-producing SbP strain, known for its potential as a probiotic candidate due to the antimicrobial effects of acetic acid (Offei et al., 2019; Van Der Aa Kühle et al., 2005). Based on the findings of Otto et al., which indicated that the introduction of Botrytis cinerea genes bcaba1, bcaba2, bcaba3 and bcaba4 enables ABA production in S. cerevisiae, we integrated these four genes into specific predefined integration sites (Claes et al., 2020) in the genomes of the S. boulardii strains (Offei et al., 2019) (Table S1). This genetic modification resulted in the creation of the E-ABA1 and SbP-ABA1 strains, respectively (Table 1). Following 24 h of cultivation at 30 °C in 50 mL of yeast extract peptone dextrose (YPD) medium, supernatants of the wild-type strains and the engineered strains were collected. A reporter assay optimized by our group (Kim et al., 2022) was used to measure ABA concentrations in culture supernatants, with (+)-ABA used to generate a standard curve. An ABA production of 8.5 mg/L and 8.6 mg/L was measured in culture supernatant of the E-ABA1 and SbP-ABA1 strains, respectively (Fig. 1A). These measurements confirmed similar levels of ABA production in both the Enterol and SbP strains of S. boulardii expressing bcaba1-4. Notably, a decrease in maximum optical density (OD600) was observed between the ABA-producing strains and the background strains, suggesting a potential trade-off between growth and ABA production (Fig. S1A).
Fig. 1.
ABA production in S. boulardii and S. cerevisiae strains in vitro at 30°C. a ABA titer in supernatant of Enterol and SbP strains containing bcaba1234. Strains were cultivated for 24 h at 30 °C in 50 mL of YPD medium. Average ABA titers were calculated from 5 independent biological replicates. b ABA titer in supernatant of strains E-ABA1 and Table A3. E-ABA1 is based on the genetic background of S. boulardii strain Enterol® and contains bcaba1234, Table A3 (Otto et al., 2019) is based on S. cerevisiae strain CENK.PK113-5D and contains bcaba1234, bccpr1 and tHMG1. Strains were cultivated for 12 h or 24 h at 30 °C in 20 mL of YPD medium. Average ABA titers were calculated from 3 independent biological replicates. Data are represented as mean ± SEM and statistically analyzed using a Student's t-test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Subsequently, we compared ABA production levels in the E-ABA1 strain to those in one of the best ABA-producing S. cerevisiae strains generated by Otto et al. (Table A3)(containing bcaba1-4, bccpr1 and tHMG1) (Otto et al., 2019). Our results show that after 12 h of cultivation at 30 °C, the E-ABA1 strain produced significantly less ABA than the Table A3 strain. However, by 24 h, there was no significant difference in ABA production between the two strains (Fig. 1B). While the Table A3 strain reached its maximum ABA production levels within 12 h, the E-ABA1 strain required 24 h to achieve similar production levels. This suggests that, despite having fewer integrated pathway genes, the E-ABA1 strain was able to produce ABA levels comparable to the Table A3 strain after 24 h.
3.2. Expression of NADPH cytochrome P450s further increases ABA production titers
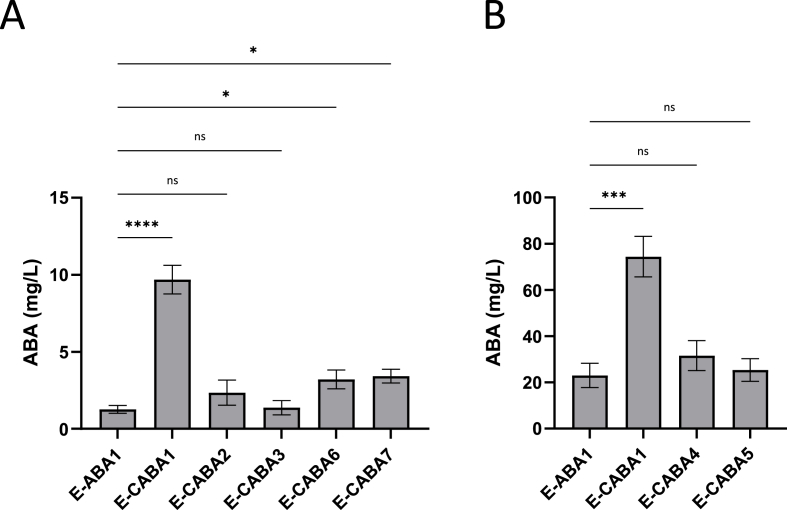
A well-established strategy to enhance ABA production yield involves optimizing the expression of cytochrome P450 monooxygenases (CYPs) (Hausjell et al., 2018; Renault et al., 2014). In S. cerevisiae strains engineered to produce ABA via a heterologous pathway, the CYPs BcABA1 and BcABA2 have been identified as critical bottlenecks. Overexpression of CYPs can increase the demand for NADP+ and thereby potentially causes metabolic imbalances within the organism. These imbalances can be mitigated by co-overexpression of cytochrome P450 reductases (CPRs), which provide the necessary NADP+ equivalents (Hausjell et al., 2018). Otto et al. demonstrated that the CPR from B. cinerea (bccpr1) was more effective in enhancing ABA production in S. cerevisiae than the native cpr gene (Otto et al., 2019). Building on this finding, we evaluated the effect of integrating a copy of bccpr1 into the E-ABA1 genome (Table 1). We constructed the E-CABA1 strain and showed that after 24 h of cultivation at 30 °C in 3 mL of YPD, the inclusion of bccpr1 resulted in a 7-fold increase in ABA titers in the supernatant (1.3 mg/L in E-ABA1; 9.7 mg/L in E-CABA1) (Fig. 2A).
Fig. 2.
ABA production in E-ABA strains containing a copy of a cytochrome P450 reductase gene. (a–b) All strains were based on the E-ABA1 strain. Average ABA titers were calculated from 4 to 6 independent biological replicates. Effect of bccpr1 (E-CABA1), fusion of bccpr1 to bcaba1 (E-CABA2) or bcaba2 (E-CABA3), fusion of bmr to bcaba1 (E-CABA4) or bcaba2 (E-CABA5) and combined fusion of bccpr1 to bcaba1 and bmr to bcaba2 (E-CABA6) or bccpr1 to bcaba2 and bmr to bcaba1 (E-CABA7) on ABA titers. a Strains were cultivated for 24 h at 30 °C in 3 mL of YPD medium in 24-well deep well plates. b Strains were cultivated for 24 h at 30 °C in 3 mL of YPD medium in test tubes. Data are represented as mean ± SEM and statistically analyzed using a one-way ANOVA. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Furthermore, multiple reports have indicated that engineering redox partner fusions can support CYP activity by reducing complexity of the P450 system and improving electron transfer properties, thereby enhancing catalytic performance and redirecting flux towards particular pathway branches (Bakkes et al., 2017; Siddiqui et al., 2012). We hypothesized that fusing bccpr1 to the CYPs bcaba1 and bcaba2 could further increase ABA production. The corresponding strains were named E-CABA2 and E-CABA3, respectively (Table 1). Surprisingly, no significant differences in ABA titers were observed between E-ABA1 and E-CABA2 or E-CABA3 (Fig. 2A). In addition to the bccpr1 fusion, we evaluated an alternative reductase, BMR, from Bacillus megaterium BM3. This reductase is naturally fused to CYP enzymes and its fusion has been shown to dramatically increase CYP activity in other metabolic engineering applications (Galanie et al., 2015; Scheps et al., 2013). Therefore, we hypothesized that BMR could be better suited as a fusion partner. The strains where bmr was fused to either aba1 or aba2 were named E-CABA4 and E-CABA5, respectively (Table 1). Consistent with the results of the bccpr1 fusion, fusing bmr to either aba1 or aba2 did not result in significant changes in ABA expression levels (Fig. 2B). Additionally, we also explored whether the titer could be increased by fusing both cyps to a cpr gene. To this end, aba1 was fused to bmr and aba2 to cpr1, and vice versa, resulting in the strains E-CABA6 and E-CABA7, respectively (Table 1). No big differences in OD600 were observed between E-ABA1 and the strains containing additional cprs (Fig. S1B and C). After 24 h of cultivation at 30 °C in 3 mL of YPD medium, we observed a 2.5-fold increase in ABA production in the strains with a CPR fused to both aba1 and aba2 compared to E-ABA1 (E-ABA1 = 1.3 mg/L; E-CABA6 = 3.2 mg/L; E-CABA7 = 3.4 mg/L) (Fig. 2A). Nonetheless, the increase in ABA production observed for CPR-fusions was still lower than the 7-fold increase observed when cpr1 was integrated separately.
3.3. Analysis of mouse gut yeast colonization and mouse serum ABA levels
To ensure effective ABA delivery to the gut, the engineered yeast strain must be able to produce ABA not only in culture but also within the gut environment. Considering the significant differences between in vitro culture conditions and the gut, we evaluated the strain's ability to colonize the gut and measured serum ABA levels in mice.
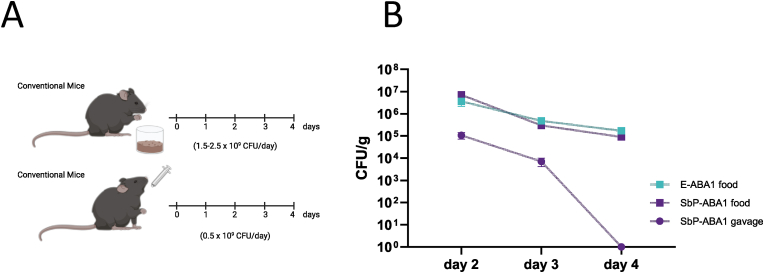
To administer yeast strains to mice, oral gavage is a commonly used method. However, since daily oral gavage is known to induce considerable stress in animals, we developed an optimized, less stressful approach for yeast delivery. In this method, yeast was incorporated into the mice's diet. The yeast-supplemented diet was prepared by mixing standard powdered mouse food with water and yeast pellets to form a porridge-like consistency (Fig. S2A).This mixture was placed into glass jars secured in stainless steel holders to prevent moving or tipping over of the jars (Fig. S2B). The feeding system was then placed into the cages, allowing ad libitum consumption (Fig. S2C). In preliminary tests comparing various yeast concentrations per gram of food, we observed that mice showed no preference for low or high concentrations. As a result, we conducted colonization experiments using the highest concentration, with 5 × 108 colony forming units (CFU) added per gram of food. Since mice typically consume 3–5 g per day, this corresponded to a daily dose of approximately 1.5-2.5 × 109 CFU. In the oral gavage set-up, a lower dose of yeast (5 × 108 CFU) was administered due to limitations in the amount of yeast pellet that could be dissolved within a volume suitable for gavage.
To assess the ability of yeast strains to colonize the gut, mice were administered an engineered yeast for three consecutive days, either through a yeast-supplemented diet with strains E-ABA1 or SbP-ABA1, or through oral gavage with strain SbP-ABA1 (experimental design in Fig. 3A). Yeast presence was monitored in the stool via CFU counting. While high levels of yeast were still detectable in the stool two days after the last feeding with yeast-supplemented diet, yeast was undetectable in the stool of gavaged mice after the same period (Fig. 3B).
Fig. 3.
Determination of CFU count in the feces of conventional mice that received either the yeast supplemented diet or were administered the yeast through oral gavage. a Schematic representation showing treatment of the mice. First, conventional mice were fed a yeast-supplemented diet containing 5 × 108 CFU/g of food. The diet included either E-ABA1 or SbP-ABA1 and was administered for 3 consecutive days (day 0, day 1, day 2) (yellow dots). Second, conventional mice received 3 doses of 5 × 108 CFU of SbP-ABA1 through oral gavage (day 0, day 1, day2) (yellow dots). The time points for collection of feces are depicted by brown dots. b Fecal carriage of yeast in both set-ups. Fecal samples were collected as shown in the timelines, plated on YPD media and kept at 30 °C for 2 days. Data are represented as mean ± SEM (n = 3 mice).
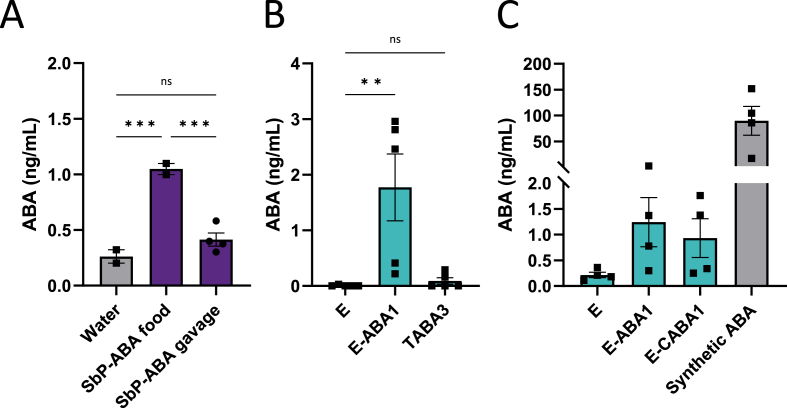
Additionally, we measured ABA levels in the serum of mice following 1 week of treatment with either a yeast-supplemented diet or yeast administered via oral gavage. While oral gavage of the SbP-ABA1 strain did not result in an increase in serum ABA levels (Fig. 4A), mice fed the SbP-ABA1 and E-ABA1 strains exhibited serum ABA levels of 1.0 ng/mL and 1.8 ng/mL, respectively, both of which were significantly higher than the levels observed in mice fed the wild-type Enterol strain or a regular diet (Fig. 4A and B). Interestingly, this increase in serum ABA was not observed in mice treated with the most productive ABA-producing S. cerevisiae strain, Table A3 (Otto et al., 2019) (Fig. 4B). However, it is important to note that the CFU dose administered to these mice was 2-fold lower due to slower growth rate of this strain. Despite the higher ABA production observed in YPD cultures for the E-CABA1 strain compared to E-ABA1 (Fig. 2A), no corresponding increase in serum ABA levels was detected in mice receiving this strain (Fig. 4C). Furthermore, the ABA levels in mice fed E-ABA1 were approximately 70 times lower than those observed in mice treated with 0.3–0.5 mg of ABA via a diet supplemented with 100 mg/kg of synthetic ABA (Fig. 4C).
Fig. 4.
ABA titers in serum of mice fed or gavaged with an ABA-producing yeast for 1 week. a Mice were either fed a yeast-supplemented diet containing ABA-producing strain SbP-ABA1 (5 × 108 CFU/g of food) or received oral gavage with this strain (5 × 108 CFU). b Mice were fed a yeast-supplemented diet containing either wild-type Enterol (5 × 108 CFU/g of food), the ABA-producing Enterol strain E-ABA1 (5 × 108 CFU/g of food) or the ABA-producing S. cerevisiae strain Table A3 (2 × 108 CFU/g of food). c Mice were fed a yeast-supplemented diet containing either wild-type Enterol (5 × 108 CFU/g of food), the ABA-producing Enterol strains E-ABA1 (5 × 108 CFU/g of food) or E-CABA1 (5 × 108 CFU/g of food) or a diet supplemented with synthetic ABA (100 mg/kg of food). Serum was taken at sacrifice after 1 week of treatment. Each dot represents one mouse. Data are represented as mean ± SEM and statistically analyzed using a one-way ANOVA. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Given that ABA was detected in the serum of mice fed the yeast-supplemented diet but not in mice treated via oral gavage, it remained unclear whether the detected ABA was produced by the yeast when in the gut or synthesized by the yeast in the feedings bowl prior to ingestion. To investigate this, we kept feeding bowls containing yeast-supplemented food at room temperature for 0, 8 or 24 h. To measure ABA levels, water was added to the food to create a diluted, homogeneous suspension, and ABA was then quantified in the supernatant. We found that ABA was detectable in feeding bowls stored at room temperature for 24 h (Fig. S3A). This duration corresponds to the time the feeding bowls remained in the cage before being refreshed during the mouse experiment, suggesting that at least some of the ABA detected in serum may have been produced in the feeding bowls rather than within the gut. We hypothesized that a low or absent ABA production within the gut could be a result of genetic variations arising during gastrointestinal transit or competitive interactions with the microbiome. To investigate whether the absence of ABA production was due to genetic alterations in the yeast after passing through the gastrointestinal tract, stool pellets from mice fed the yeast-supplemented diet were plated on agar plates and individual yeast colonies were isolated and cultured in liquid media at 30 °C for 24 h. ABA was detected in the supernatant of these cultures, indicating that the yeast retained its ability to produce ABA after passage through the gut (Fig. S3B). To explore whether competition with the native gut microbiota affected ABA production, germ-free mice were gavaged with the engineered ABA-producing yeast strain E-ABA1 (experimental design in Fig. S4A). The mice received a single dose of E-ABA1 on day 0 and the presence of this yeast was assessed in stool samples on days 3, 7 and 13 post-gavage. ABA levels were measured in serum on day 13 post-gavage. Despite detecting high CFU counts of E-ABA1 in the stool (Fig. S4B), ABA remained undetectable in the serum (data not shown), indicating that microbial competition was not the cause for low ABA levels in serum.
3.4. Identification of physiological body temperature (37 °C) as ABA production bottleneck
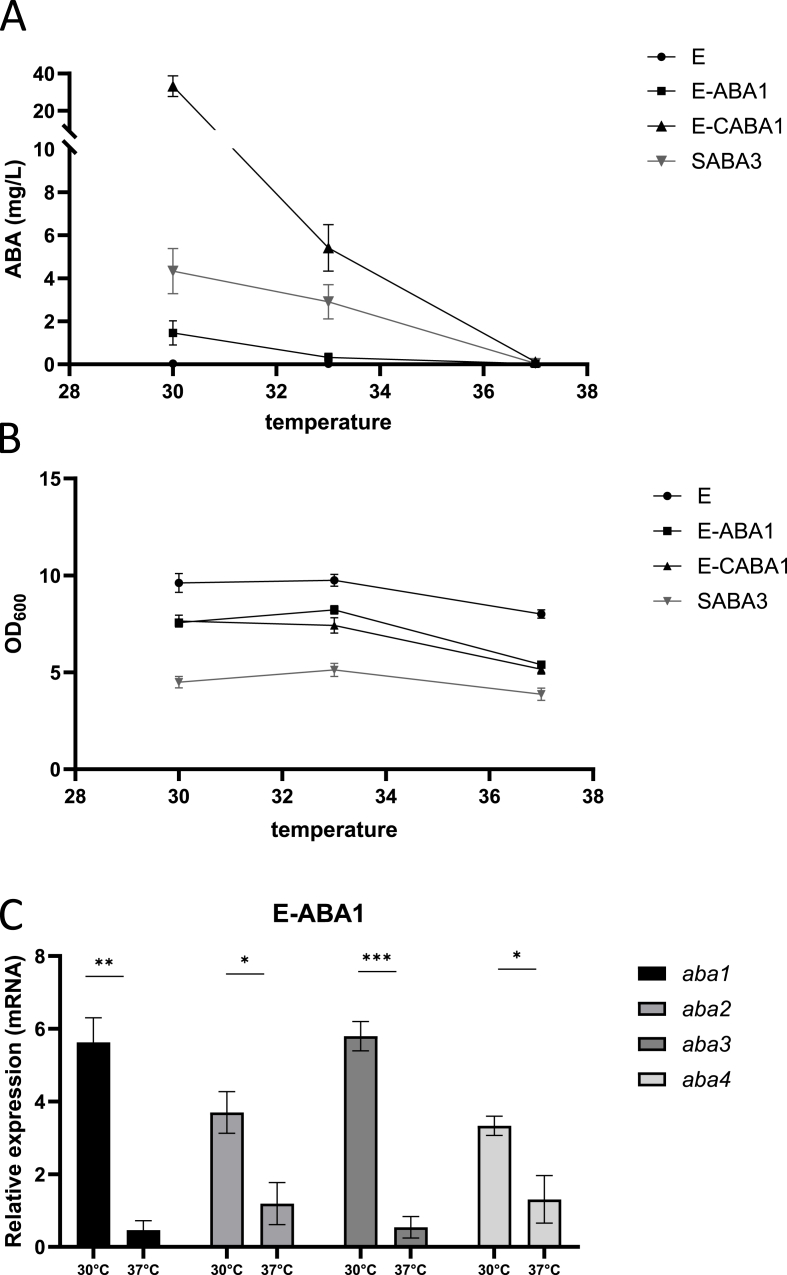
The engineered yeast successfully colonized the gut (Fig. 3B) and remained viable, with stable expression of the ABA biosynthesis pathway following passage through the gastrointestinal tract (Fig. S3B). However, we were unable to detect ABA in serum of mice that received and oral gavage with this yeast (Fig. 4A). To investigate this limitation, the yeast strain's ability to produce ABA at various culturing temperatures was evaluated. Three specific temperatures were selected for this assessment: 30 °C, 33 °C and 37 °C, representing in vitro growth conditions, an intermediate measurement, and the physiological temperature of mice, respectively. ABA production was compared between E-ABA1, E-CABA1 and the SABA3 S. cerevisiae strain of Otto et al. (2019) (Fig. 5A). The SABA3 strain, in addition to the modifications made in the previously described Table A3 strain, was intensively modified to enhance upstream flux through the MVA pathway and to reduce the formation of side products, thereby boosting the flux towards ABA synthesis. All yeasts in this experiment were pre-cultured at 30 °C and subsequently adjusted to an OD600 of 0.2 before being incubated for 24 h at 30 °C, 33 °C or 37 °C. At 30 °C, the SABA3 strain demonstrated higher ABA production than the E-ABA1 strain, whereas the E-CABA1 strain exhibited seven times higher ABA production than the SABA3 strain. At 33 °C, ABA production in the E-ABA1 strain was nearly abolished, and the E-CABA1 strain showed a 6-fold reduction in ABA production. In contrast, ABA levels in the SABA3 strain remained relatively stable under the same conditions, exhibiting only a 1.5-fold decrease. At 37 °C, ABA production was completely abrogated in all strains tested. This reduction or loss of ABA production was not linked to decreased growth, as OD600 values at 33 °C were comparable to those at 30 °C. Although a reduction in OD600 was observed at 37 °C, it was similar to that observed in the wild-type Enterol strain, suggesting that the reduced growth was not attributable to the introduced genetic modifications (Fig. 5B).
Fig. 5.
Effect of temperature on ABA production levels and RNA expression levels of bcaba1234. a ABA titer in supernatant of strains E, E-ABA1, E-CABA1 and SABA3. E-ABA1 and E-CABA1 are based on the genetic background of S. boulardii strain Enterol and contain bcaba1234, and bcaba1234 and bccpr1 respectively. SABA3 (Otto et al., 2019) is based on S. cerevisiae strain CENK.PK113-5D and contains various modifications as shown in Table 1. Strains were cultivated for 24 h in 3 mL of YPD medium in 24-well deep well plates. Average ABA titers were calculated from 3 independent biological replicates and error bars indicate the standard error of the mean. b OD600 of strains analyzed in Fig. 5A c mRNA expression of aba1, aba2, aba3 and aba4 in the pellet of a 24 h culture of E-ABA1 grown at 30 °C or 37 °C in 3 mL of YPD in test tubes. Average expression was calculated from 3 independent biological replicates. Data are represented as mean ± SEM and statistically analyzed using a Student's t-test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
3.5. Effect of promoter strength on ABA production at physiological body temperature
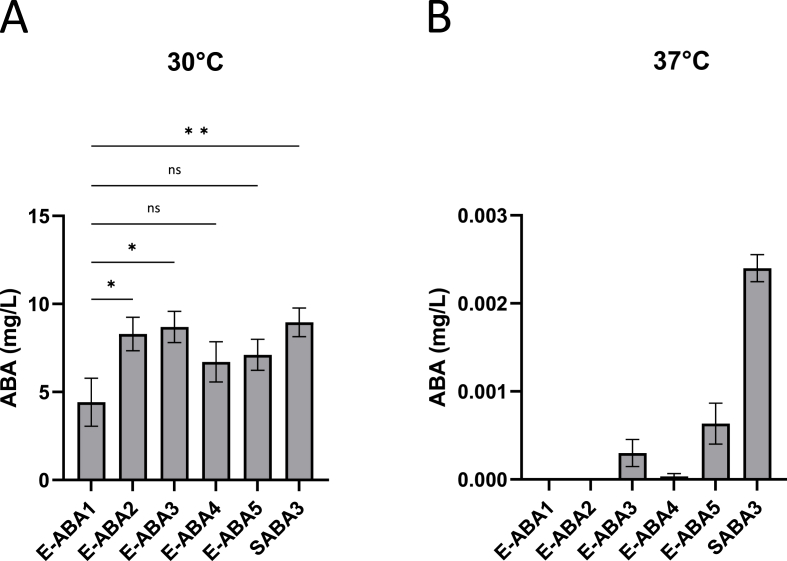
Given the unexpected decrease in ABA production at higher temperatures, qPCR was conducted to compare the expression levels of the different aba genes in the heterologous pathway at both low (30 °C) and high (37 °C) temperatures. This analysis aimed to identify potential bottleneck genes in the pathway. We observed that the expression of all four aba genes was significantly reduced at 37 °C compared to at 30 °C (Fig. 5C). Specifically, the expression levels of aba2 and aba4 decreased by 3-fold and 2.5-fold, respectively, while aba1 and aba3 exhibited a more pronounced decrease of 12-fold and 11-fold, respectively. Notably, aba1 and aba3 were integrated into the genome in a bicistronic manner under the TDH3 promoter, while aba2 and aba4 were expressed in a bicistronic manner under the TEF1 promoter. A similar effect was observed in the Table A3 strain, where genes regulated by the TEF1 promoter (aba3 and aba4) exhibited a 2-fold decrease in expression levels, while those controlled by the PGK1 promoter (aba1 and aba2) showed a more pronounced, 4-fold reduction in expression (Fig. S5). This prompted us to hypothesize that the reduction in ABA production at 37 °C might be a result of decreased promoter activity at higher temperatures. To investigate this, we introduced an additional copy of the aba1-aba3 expression cassette under various promoters with different expression strengths, including TDH3, TEF1, RPL18B and SAC6 (Durmusoglu et al., 2021), resulting in the creation of strains E-ABA2, E-ABA3, E-ABA4 and E-ABA5, respectively (Table 1). At 30 °C, the inclusion of an extra copy of aba1 and aba3 under the control of the TDH3, TEF1 or SAC6 promoter led to increased expression of these genes, whereas the RPL18B promoter did not show the same effect (Fig. S6A and B). At 37 °C, an increase in aba1 and aba3 gene expression was observed with the additional copy under the control of the TEF1, RPL18B or SAC6 promoters, but not under the TDH3 promoter (Fig. S6A and B). Introduction of an additional copy of aba1-3 did not result in changes is OD600 (Fig. S6C
). The integration of an additional copy of these genes significantly increased ABA production titers at 30 °C for the strong promoters TDH3 and TEF1, with a trend towards increased ABA titers for the intermediate and weak promoters RPL18B and SAC6 (Fig. 6A). However, at 37 °C ABA titers remained near zero for all strains, regardless of the promoter used (Fig. 6B). Therefore, decreased promoter activity was not identified as the primary cause of the abrogated ABA production at 37 °C.
Fig. 6.
ABA production in E-ABA strains containing an additional copy of aba1 and aba3 genes expressed under various promoters. All described strains were based on the E-ABA1 strain. Average ABA titers were calculated from 3 independent biological replicates. Effect of an additional copy of aba1 and aba3 expressed under control of the TDH3 (E-ABA2), TEF1 (E-ABA3), RPL18B (E-ABA4) or SAC6 (E-ABA5) promoter on ABA titers. Strains were cultivated for 24 h in 3 mL of YPD medium in 24-well deep well plates. a Cultures were grown at 30 °C. b Cultures were grown at 37 °C. Data are represented as mean ± SEM and statistically analyzed using a one-way ANOVA. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
3.6. Generation of a yeast strain producing low levels of ABA at 37 °C
Recognizing the need to achieve high production titers for the therapeutic application of an ABA-producing probiotic, we explored various strategies to further enhance ABA yields. Given the stability of ABA production at 33 °C compared to 30 °C in the S. cerevisiae strain SABA3, which is heavily boosted in MVA flux, we hypothesized that increasing MVA flux in our Enterol strains could similarly boost ABA production at higher temperatures. To test this hypothesis, we integrated truncated 3-hydroxy-3-methylglutaryl-CoA reductase (tHMG1), coding for a rate-limiting enzyme in the MVA pathway, into the genome. This modification was introduced into the strains with an additional copy of aba1 and aba3, resulting in the creation of strains E− (Table 1). Our results showed that at 30 °C, E-TABA2 and E-TABA3 exhibited 2.5-fold and 5.5-fold higher ABA production comparedTABA2, E-TABA3, E-TABA4 and E-TABA5 to E-TABA4 and E-TABA5, respectively (Fig. 7A). Although ABA levels were lower at 37 °C, E-TABA2 and E-TABA3 still produced twice as much ABA as E-TABA4 and E-TABA5 (Fig. 7B). E-TABA2 and E-TABA3 were identified as the highest-producing strains containing tHMG1, and E-TABA2 in particular demonstrated a statistically significant, 1.5-fold, increase in ABA titers compared to E-ABA2 (Fig. 7C). To further enhance ABA yield, we introduced a copy of cpr1, which encodes a cytochrome P450 reductase and is known from previous experiments to substantially increase ABA titers (Fig. 2A). The resulting strains, named E-TCABA2 and E-TCABA3 (Table 1), showed the highest ABA production levels at 30 °C among all strains tested (25.2 mg/L in E-TCABA2; 28.6 mg/L in E-TACABA3), significantly outperforming SABA3, E-CABA1 and their E-TABA counterparts (1.9 mg/L in SABA3; 17 mg/L in E-CABA1; 4.3 mg/L in E-TABA2, 3.4 mg/L in E-TABA3) (Fig. 7D). At 37 °C, these strains produced less than 0.5 mg/L of ABA, but for the first time, ABA levels showed a more than 10-fold increase compared to background ABA levels measured in wild-type Enterol (0.32 mg/L in E-TCABA2; 0.20 mg/L in E-TCABA3; 0.02 mg/L in E) (Fig. 7E). Notably, E-TCABA2 and E-TCABA3 also produced significantly higher ABA titers than their E-TABA counterparts (0 mg/L in E-TABA2; 0.01 mg/L in E-TABA3), with E-TCABA2 outperforming E-CABA1 (0.15 mg/L in E-CABA1), as was also observed at 30 °C. Consequently, we successfully generated a strain capable of producing low amounts of ABA at 37 °C, with levels equal to or higher than 0.2 mg/L. This represents a significant increase compared to production levels measured in previously generated strains.
Fig. 7.
ABA production in E-ABA strains containing a copy of tHMG1 and/or cpr1. a-c The effect of adding tHMG1 to E-ABA2, E-ABA3, E-ABA4 and E-ABA5 on ABA titers during cultivation at 30 °C (a) or 37 °C (b,c). d-e The effect of adding cpr1 to E-Table A2 and E-Table A3 on ABA titers during cultivation at 30 °C (d) or 37 °C (e). Strains were cultivated for 24 h in 3 mL of YPD medium in 24-well deep well plates. Average ABA titers were calculated from 3 to 5 independent biological replicates. Data are represented as mean ± SEM and statistically analyzed using a one-way ANOVA. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
4. Discussion
ABA is considered an interesting nutraceutical substance that may explain some of the health benefits associated with consumption of ABA-rich foods (Kim et al., 2020). Since challenges such as scalability, short half-life and cost-effectiveness hinder the practical use of ABA-enriched fruit extracts and synthetic ABA supplements (Liang et al., 2011; Siddiqui et al., 2012), we propose an alternative ABA administration technique in this study by engineering an ABA-producing probiotic strain of Saccharomyces boulardii. Consistent with observations made in S. cerevisiae (Otto et al., 2019), Yarrowia lipolytica (Arnesen et al., 2022) and Aspergillus oryzae (Takino et al., 2019), we demonstrated that the integration of the aba1-4 expression cassette from Botrytis cinerea is sufficient to establish ABA production in S. boulardii (Fig. 1A). Additionally, the subsequent incorporation of the cpr1 gene led to a 7-fold increase in ABA production (Fig. 2A), and already surpassed the performance of the highest-producing S. cerevisiae strain previously generated by Otto et al. (2019) (Fig. 5A). To further increase ABA production, we engineered redox partner fusions, which are generally recognized for enhancing CYP activity by improving electron transfer efficiency and catalytic performance (Bakkes et al., 2017; Hausjell et al., 2018; Siddiqui et al., 2012). In our study, fusing cpr1 and bmr to the CYP enzymes aba1 and aba2 resulted in only a 2.5-fold increase in ABA production, which was lower than the 7-fold increase observed when cpr1 was integrated separately (Fig. 2A). Previous studies have indicated that the length of the linker is a critical determinant of enzyme activity (Govindaraj and Poulos, 1996) and that the activity of fusion constructs can be fine-tuned through linker engineering (Bakkes et al., 2017). Therefore, optimizing linker length remains a promising strategy to further enhance ABA production.
To develop a genetically engineered probiotic strain effectively delivering ABA to the gut, the strain should possess the following attributes: tolerance to low pH to ensure survival and genetic stability during gastrointestinal transit, the ability to adhere to the intestinal epithelium for effective colonization, and efficient ABA production at the epithelial cell surface (Ouwehand et al., 1999).
We demonstrated that our ABA-producing S. boulardii remained viable, with stable expression of the ABA biosynthesis pathway following passage through the gastrointestinal tract as colonies recovered from stool samples were able to produce ABA in YPD cultures at 30 °C with titers comparable to those observed in the original cultures prior to ingestion (Fig. S3B).
By feeding mice a yeast-supplemented diet, we showed that our ABA-producing S. boulardii effectively colonized the gut, as evidenced by a high number of CFU detected in the stool two days after the cessation of the yeast-supplemented diet (Fig. 3A and B). In contrast, when mice received an oral gavage with a yeast dose that was three times lower than the amount received through the yeast-supplemented diet, the yeast was cleared from the gut more rapidly, with no detectable CFU two days after the last gavage. These results suggest that enhancing the gut adhesion capacity of S. boulardii could be beneficial. A potential strategy to improve gut adhesion involves genetic engineering to introduce or overexpress specific adhesion-related proteins. One approach could be the integration of the adhesin gene epa1 from Candida glabrata, or the creation of a chimeric protein by combining the endogenous S. boulardii flo1 homolog with the N-terminal carbohydrate-binding domain of Cgepa1 (Frieman et al., 2002). Additionally, other EPA proteins with different glycan-binding specificities could be explored to enhance gut colonization (Zupancic et al., 2008). Although C. glabrata is closely related to Saccharomyces (as Candida is not monophyletic), extensive genetic modifications would be required to confer pathogenic traits to S. boulardii (Roetzer et al., 2011), suggesting that this modification could be safe. However, introducing a pathogenicity-associated factor like EPA1 into a probiotic yeast such as S. boulardii still poses a potential risk that requires careful evaluation before clinical application. If proven successful and safe, this approach could potentially benefit a wide range of engineered probiotics based on S. boulardii.
Although successful gut colonization was achieved in mice fed the yeast-supplemented diet, we observed only minimal levels of ABA in their serum (Fig. 4A–C). Notably, no ABA was detected when the yeast was administered via oral gavage (Fig. 4A). Our findings suggest that the low serum ABA levels in mice fed the yeast-supplemented diet were due to ABA being produced in the feedings bowl at room temperature prior to ingestion, rather than within the gut at 37 °C (Fig. S3A). In vitro experiments confirmed that the physiological body temperature of 37 °C is likely a critical factor limiting ABA production by the engineered S. boulardii within the gut (Fig. 5A).
Recognizing the importance of achieving high ABA production titers for the therapeutic application of an ABA-producing probiotic, we explored several strategies to further enhance ABA yields at 37 °C. Firstly, we considered the possible inactivation of the Botrytis ABA biosynthesis enzymes at temperatures above 30oC. However, our in silico predictions for the thermal stability of BcABA3 and BcABA4 did not suggest that these enzymes are particularly temperature-sensitive (Miotto et al., 2019, 2022). However, due to their large size, BcABA1 and BcABA2 could not be analyzed with the in silico tool. Despite this, 3D modeling of these proteins indicates a complex network of interactions, suggesting no significant issues with their thermal stability. Nevertheless, it could be worthwhile to explore alternative enzymes in the ABA biosynthesis pathway from other phytopathogenic fungi, such as Leptosphaeria maculans (Darma et al., 2019) and Magnaporthe oryzae (Spence et al., 2015). Secondly, the availability of molecular oxygen may also be a potential rate-limiting factor for ABA production in the gut, as two catalytic steps in the ABA biosynthesis pathway require oxidation. While the gut lumen is mostly anaerobic, there is an oxygen gradient, with oxygen levels near the gut wall being similar to those in the bloodstream (Albenberg et al., 2014). Therefore, yeasts that adhere efficiently to the gut epithelium might still access sufficient oxygen for ABA production. Decreased thermotolerance of the cells is also an important factor to consider in bioengineering (Huang et al., 2018). Thermotolerance, a polygenic trait, is crucial for cell survival and growth at high temperatures. Previous studies in S. cerevisiae have shown that increasing the gene copy number of heterologous CYPs and CPRs results in decreased growth rates, increased formation of reactive oxygen species (ROS) (Zhao et al., 2016) and reduced resistance to other cellular stresses such as elevated temperature (Arnesen et al., 2022). This suggests that reduced thermotolerance might be contributing to the poor ABA production observed in our strain at 37 °C. However, our observations indicated that cell survival at 37 °C was not compromised, as measured by acridine orange counting (data not shown). Additionally, although the OD600 of ABA-producing S. boulardii was significantly lower at 37 °C compared to 30 °C after 24 h, this reduction was similar to the decrease observed in wild-type S boulardii under the same conditions (Fig. 5B). Nonetheless, improving thermotolerance might be an interesting strategy for increasing ABA production at 37 °C. One method to enhance thermotolerance is experimental evolution, where yeast are gradually exposed to higher temperatures (Huang et al., 2018). However, this process requires many generations to achieve successful adaptation (Caspeta et al., 2014). Alternatively, thermotolerance can be enhanced more rapidly through whole-genome transformation (Deparis et al., 2021) or through introduction of specific mutations in genes known to be involved in sustaining high thermotolerance. Mutations in the ergosterol biosynthesis pathway (e.g. in the genes erg3, erg4 and erg5) have been shown to improve membrane fluidity, cell viability, cell integrity and overall thermotolerance (Caspeta et al., 2014; Caspeta and Nielsen, 2015; Yang et al., 2023). Furthermore, another promising method to further enhance ABA yield is balancing the heterologous ABA production pathway with the yeast's native metabolism. To maximize ABA production at 37 °C, it is crucial to achieve high expression levels of the aba1-4 genes while maintaining robust expression of the native metabolism. A well-documented and effective strategy for balancing pathways in S. cerevisiae involves modifying promoter strength to coordinate gene expression (He et al., 2023; Xie et al., 2015). Consistent with previous studies showing a strong reduction in promoter activity under stress conditions, including elevated temperature (Xiong et al., 2018), we observed that the expression of the promoter driving aba1 and aba3 was decreased by 12- and 11-fold respectively at 37 °C (Fig. 5C). This reduction may account for the absence of ABA production at 37 °C. In an attempt to counteract this decreased expression, we integrated additional copies of the aba1 and aba3 genes under promoters with varying strength. Interestingly, while TEF1-, RPL18B- and SAC6-driven expression of aba1 and aba3 was higher at 37 °C than at 30 °C, ABA production remained below 0.001 mg/L at 37 °C in these strains (Fig. 6B). Consequently, decreased promoter activity was not identified as the primary cause of the abrogated ABA production at 37 °C. Moreover, balancing the heterologous pathway may also be achieved through redirection of redox cofactors and reduction of the metabolic burden on the cells (Chen et al., 2014). Redox constraints are known to limit the yield of many fermentation processes (Görgens et al., 2001; Moreiradossantos et al., 2003) and rerouting of NADPH-dependent synthetic pathways has been used to enhance heterologous production in S. cerevisiae (Kim et al., 2018; Kwak et al., 2020). However, since Arnesen et al. showed that improving NADPH supply did not significantly increase ABA production in Y. lipolytica, this strategy might not be the most promising for increasing ABA titers in our S. boulardii strain (Arnesen et al., 2022).
Lastly, the ABA levels in the SABA3 strain, which is engineered to enhance the upstream flux through the MVA pathway, remained relatively stable at 33 °C compared to 30 °C, exhibiting only a 1.5-fold decrease. In contrast, the E-CABA1 strain showed a more pronounced reduction, with a 6.1-fold decrease in ABA levels at 33 °C (Fig. 5A). These findings suggested that optimizing the biosynthetic pathway upstream of FPP and minimizing the formation of side products could be a promising strategy to further increase ABA production at 37 °C. In our most modified Enterol strain, containing aba1-4, an additional copy of aba1 and aba3, and a copy of cpr1 and tHMG1, we observed an approximately 15-fold increase in ABA levels at both 30 °C and 37 °C compared to the highest-producing strain generated by Otto et al. (2019). At 37 °C, ABA concentrations reached or exceeded 0.2 mg/L (Fig. 7E).
5. Conclusion
In summary, we report that despite successful colonization and survival in the gut, ABA production by our S. boulardii strain was hindered and the physiological body temperature (37 °C) was identified as a significant bottleneck. Since serum ABA levels remained very low in mice fed a yeast-supplemented diet, further modifications are required before this probiotic can be tested in disease contexts in mice. Suggested modifications include optimizing the upstream biosynthesis pathway and enhancing thermotolerance through random or targeted mutations. Once the issue of in vitro production at 37 °C is resolved, in vivo ABA serum levels can be assessed and possibly further increased through modifications that enhance gut adhesion.
CRediT authorship contribution statement
Femke Van Gaever: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis. Paul Vandecruys: Writing – review & editing, Resources, Methodology. Yasmine Driege: Writing – review & editing, Methodology. Seo Woo Kim: Writing – review & editing, Methodology. Johan M. Thevelein: Writing – review & editing, Resources. Rudi Beyaert: Writing – review & editing, Resources, Funding acquisition. Jens Staal: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Funding acquisition, Data curation, Conceptualization.
Material availability
All plasmids and yeast strains described are available upon request.
Ethics statement
This study was approved by and conducted in accordance with the recommendations of the institutional animal care and use committees at the VIB site of Ghent University Faculty of Science or the UZ site of Ghent University Faculty of Medicine and Health Sciences.
Funding
This work was supported by an FWO PhD Grants (1SE0521N to F.V.G), an FWO Project Grant (G021119N), and Ghent University Project Grants (bof/baf/2y/2024/01/024 and BOF19-GOA).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank all the people of the IRC Animal House Facility, as well as L. Vereecke and his team from the Germ-free Facility at MRB2. We would also like to thank V. Siewers (Chalmers University of Technology, Gothenburg) for her kind supply of bcaba plasmids and ABA-producing S. cerevisiae strains, and D. Devriese from the lab of B. Devreese for their kind supply of bmr DNA (Ugent, Ghent). We would like to thank Charly and Geert from the VIB workshop for their help with the construction of the feeding systems. We would also like to thank all member of the Unit of Molecular Signal Transduction in Inflammation at VIB-UGent for their helpful feedback and suggestions during the preparation of this manuscript.
Handling editor: Mattheos Koffas
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mec.2025.e00263.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- Albenberg L., Esipova T.V., Judge C.P., Bittinger K., Chen J., Laughlin A., Grunberg S., Baldassano R.N., Lewis J.D., Li H., Thom S.R., Bushman F.D., Vinogradov S.A., Wu G.D. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology (New York, N. Y., 1943) 2014;147:1055–1063.e8. doi: 10.1053/j.gastro.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameri P., Bruzzone S., Mannino E., Sociali G., Andraghetti G., Salis A., Ponta M.L., Briatore L., Adami G.F., Ferraiolo A., Venturini P.L., Maggi D., Cordera R., Murialdo G., Zocchi E. Impaired increase of plasma abscisic Acid in response to oral glucose load in type 2 diabetes and in gestational diabetes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0115992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnesen J.A., Jacobsen I.H., Dyekjær J.D., Rago D., Kristensen M., Klitgaard A.K., Randelovic M., Martinez J.L., Borodina I. Production of abscisic acid in the oleaginous yeast Yarrowia lipolytica. FEMS Yeast Res. 2022;22:foac015. doi: 10.1093/femsyr/foac015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkes P.J., Riehm J.L., Sagadin T., Rühlmann A., Schubert P., Biemann S., Girhard M., Hutter M.C., Bernhardt R., Urlacher V.B. Engineering of versatile redox partner fusions that support monooxygenase activity of functionally diverse cytochrome P450s. Sci. Rep. 2017;7:9570. doi: 10.1038/s41598-017-10075-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone S., Moreschi I., Usai C., Guida L., Damonte G., Salis A., Scarfì S., Millo E., De Flora A., Zocchi E. Abscisic acid is an endogenous cytokine in human granulocytes with cyclic ADP-ribose as second messenger. Proc. Natl. Acad. Sci. U. S. A. 2007;104:5759–5764. doi: 10.1073/pnas.0609379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspeta L., Chen Y., Ghiaci P., Feizi A., Buskov S., Hallström B.M., Petranovic D., Nielsen J. Altered sterol composition renders yeast thermotolerant. Sci. Technol. Humanit. 2014;346:75–78. doi: 10.1126/science.1258137. [DOI] [PubMed] [Google Scholar]
- Caspeta L., Nielsen J. Thermotolerant yeast strains adapted by laboratory evolution show trade-off at ancestral temperatures and preadaptation to other stresses. mBio. 2015;6 doi: 10.1128/mBio.00431-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen Y., Timmermans B., Souffriau B., Thevelein J.M., Van Dijck P. Comparison of genome engineering using the CRISPR-Cas9 system in C. glabrata wild-type and lig4 strains. Fungal Genet. Biol. 2017;107:44–50. doi: 10.1016/j.fgb.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Chen X., Li S., Liu L. Engineering redox balance through cofactor systems. Trends Biotechnol. 2014;32:337–343. doi: 10.1016/j.tibtech.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Claes A., Deparis Q., Foulquié-Moreno M.R., Thevelein J.M. Simultaneous secretion of seven lignocellulolytic enzymes by an industrial second-generation yeast strain enables efficient ethanol production from multiple polymeric substrates. Metab. Eng. 2020;59:131–141. doi: 10.1016/j.ymben.2020.02.004. [DOI] [PubMed] [Google Scholar]
- Czerucka D., Piche T., Rampal P. Review article: yeast as probiotics – Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007;26:767–778. doi: 10.1111/j.1365-2036.2007.03442.x. [DOI] [PubMed] [Google Scholar]
- Darma R., Lutz A., Elliott C.E., Idnurm A. Identification of a gene cluster for the synthesis of the plant hormone abscisic acid in the plant pathogen Leptosphaeria maculans. Fungal Genet. Biol. 2019;130:62–71. doi: 10.1016/j.fgb.2019.04.015. [DOI] [PubMed] [Google Scholar]
- De Carvalho B.T., Subotić A., Vandecruys P., Deleu S., Vermeire S., Thevelein J.M. Enhancing probiotic impact: engineering Saccharomyces boulardii for optimal acetic acid production and gastric passage tolerance. Appl. Environ. Microbiol. 2024;90 doi: 10.1128/aem.00325-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleu S., Jacobs I., Vazquez Castellanos J.F., Verstockt S., Trindade De Carvalho B., Subotić A., Verstockt B., Arnauts K., Deprez L., Vissers E., Lenfant M., Vandermeulen G., De Hertogh G., Verbeke K., Matteoli G., Huys G.R.B., Thevelein J.M., Raes J., Vermeire S. Effect of mutant and engineered high-acetate-producing Saccharomyces cerevisiae var. boulardii strains in dextran sodium sulphate-induced colitis. Nutrients. 2024;16:2668. doi: 10.3390/nu16162668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deparis Q., Duitama J., Foulquié-Moreno M.R., Thevelein J.M. Whole-genome transformation promotes tRNA anticodon suppressor mutations under stress. mBio. 2021;12 doi: 10.1128/mBio.03649-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörffling K., Petersen W., Sprecher E., Urbasch I., Hanssen H.-P. Abscisic acid in phytopathogenic fungi of the genera Botrytis, ceratocystis, Fusarium, and rhizoctonia. Z. Naturforsch. C Biosci. 1984;39:683–684. doi: 10.1515/znc-1984-0626. [DOI] [Google Scholar]
- Duman D.G., Bor S., Ozütemiz O., Sahin T., Oğuz D., Iştan F., Vural T., Sandkci M., Işksal F., Simşek I., Soytürk M., Arslan S., Sivri B., Soykan I., Temizkan A., Beşşk F., Kaymakoğlu S., Kalayc C. Efficacy and safety of Saccharomyces boulardii in prevention of antibiotic-associated diarrhoea due to Helicobacterpylori eradication. Eur. J. Gastroenterol. Hepatol. 2005;17:1357–1361. doi: 10.1097/00042737-200512000-00015. [DOI] [PubMed] [Google Scholar]
- Durmusoglu D., Al'Abri I.S., Collins S.P., Cheng J., Eroglu A., Beisel C.L., Crook N. In situ biomanufacturing of small molecules in the mammalian gut by probiotic Saccharomyces boulardii. ACS Synth. Biol. 2021;10:1039–1052. doi: 10.1021/acssynbio.0c00562. [DOI] [PubMed] [Google Scholar]
- Ferreira R., Skrekas C., Nielsen J., David F. Multiplexed CRISPR/Cas9 genome editing and gene regulation using Csy4 in Saccharomyces cerevisiae. ACS Synth. Biol. 2018;7:10–15. doi: 10.1021/acssynbio.7b00259. [DOI] [PubMed] [Google Scholar]
- Ferreiro A., Crook N., Gasparrini A.J., Dantas G. Multiscale evolutionary dynamics of host-associated microbiomes. Cell. 2018;172:1216–1227. doi: 10.1016/j.cell.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieman M.B., McCaffery J.M., Cormack B.P. Modular domain structure in the Candida glabrata adhesin Epa1p, a beta1,6 glucan-cross-linked cell wall protein. Mol. Microbiol. 2002;46:479–492. doi: 10.1046/j.1365-2958.2002.03166.x. [DOI] [PubMed] [Google Scholar]
- Galanie S., Thodey K., Trenchard I.J., Filsinger Interrante M., Smolke C.D. Complete biosynthesis of opioids in yeast. Sci. Technol. Humanit. 2015;349:1095–1100. doi: 10.1126/science.aac9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R.D., Woods R.A. Yeast transformation by the LiAc/SS Carrier DNA/PEG method. Methods Mol. Biol. 2006;313:107–120. doi: 10.1385/1-59259-958-3:107. [DOI] [PubMed] [Google Scholar]
- Görgens J.F., van Zyl W.H., Knoetze J.H., Hahn-Hägerdal B. The metabolic burden of the PGK1 and ADH2 promoter systems for heterologous xylanase production by Saccharomyces cerevisiae in defined medium. Biotechnol. Bioeng. 2001;73:238–245. doi: 10.1002/bit.1056. [DOI] [PubMed] [Google Scholar]
- Govindaraj S., Poulos T.L. Probing the structure of the linker connecting the reductase and heme domains of cytochrome P450BM-3 using site-directed mutagenesis. Protein Sci. 1996;5:1389–1393. doi: 10.1002/pro.5560050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guri A.J., Hontecillas R., Bassaganya-Riera J. Abscisic acid ameliorates experimental IBD by downregulating cellular adhesion molecule expression and suppressing immune cell infiltration. Clin Nutr. 2010;29:824–831. doi: 10.1016/j.clnu.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guri A.J., Misyak S.A., Hontecillas R., Hasty A., Liu D., Si H., Bassaganya-Riera J. Abscisic acid ameliorates atherosclerosis by suppressing macrophage and CD4+ T cell recruitment into the aortic wall. J. Nutr. Biochem. 2010;21:1178–1185. doi: 10.1016/j.jnutbio.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guslandi M., Giollo P., Testoni P.A. A pilot trial of Saccharomyces boulardii in ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 2003;15:697–698. doi: 10.1097/00042737-200306000-00017. [DOI] [PubMed] [Google Scholar]
- Hausjell J., Halbwirth H., Spadiut O. Recombinant production of eukaryotic cytochrome P450s in microbial cell factories. Biosci. Rep. 2018;38 doi: 10.1042/BSR20171290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Zhang Z., Lu W. Natural promoters and promoter engineering strategies for metabolic regulation in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2023;50 doi: 10.1093/jimb/kuac029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hey S.J., Byrne E., Halford N.G. The interface between metabolic and stress signalling. Ann. Bot. 2010;105:197–203. doi: 10.1093/aob/mcp285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai N., Yoshida R., Todoroki Y., Ohigashi H. Biosynthesis of abscisic acid by the non-mevalonate pathway in plants, and by the mevalonate pathway in fungi. Biosci. Biotechnol. Biochem. 2000;64:1448–1458. doi: 10.1271/bbb.64.1448. [DOI] [PubMed] [Google Scholar]
- Ho C.L., Tan H.Q., Chua K.J., Kang A., Lim K.H., Ling K.L., Yew W.S., Lee Y.S., Thiery J.P., Chang M.W. Engineered commensal microbes for diet-mediated colorectal-cancer chemoprevention. Nat. Biomed. Eng. 2018;2:27–37. doi: 10.1038/s41551-017-0181-y. [DOI] [PubMed] [Google Scholar]
- Huang C.-J., Lu M.-Y., Chang Y.-W., Li W.-H. Experimental evolution of yeast for high-temperature tolerance. Mol. Biol. Evol. 2018;35:1823–1839. doi: 10.1093/molbev/msy077. [DOI] [PubMed] [Google Scholar]
- Hwang I.Y., Koh E., Wong A., March J.C., Bentley W.E., Lee Y.S., Chang M.W. Engineered probiotic Escherichia coli can eliminate and prevent Pseudomonas aeruginosa gut infection in animal models. Nat. Commun. 2017;8 doi: 10.1038/ncomms15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata M., Hirai N., Yoshida R., Ohigashi H. The biosynthetic pathway to abscisic acid via ionylideneethane in the fungus Botrytis cinerea. Phytochemistry. 2004;65:2667–2678. doi: 10.1016/j.phytochem.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Jessop-Fabre M.M., Jakočiūnas T., Stovicek V., Dai Z., Jensen M.K., Keasling J.D., Borodina I. EasyClone-MarkerFree: a vector toolkit for marker-less integration of genes into Saccharomyces cerevisiae via CRISPR-Cas9. Biotechnol. J. 2016;11:1110–1117. doi: 10.1002/biot.201600147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-E., Jang I.-S., Sung B.H., Kim S.C., Lee J.Y. Rerouting of NADPH synthetic pathways for increased protopanaxadiol production in Saccharomyces cerevisiae. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-34210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.W., Alci K., Van Gaever F., Driege Y., Bicalho K., Goeminne G., Libert C., Goossens A., Beyaert R., Staal J. Engineering a highly sensitive biosensor for abscisic acid in mammalian cells. FEBS Lett. 2022;596:2576–2590. doi: 10.1002/1873-3468.14431. [DOI] [PubMed] [Google Scholar]
- Kim S.W., Goossens A., Libert C., Van Immerseel F., Staal J., Beyaert R. Phytohormones: multifunctional nutraceuticals against metabolic syndrome and comorbid diseases. Biochem. Pharmacol. 2020;175 doi: 10.1016/j.bcp.2020.113866. [DOI] [PubMed] [Google Scholar]
- Kotowska M., Albrecht P., Szajewska H. Saccharomyces boulardii in the prevention of antibiotic‐associated diarrhoea in children: a randomized double‐blind placebo‐controlled trial. Aliment. Pharmacol. Ther. 2005;21:583–590. doi: 10.1111/j.1365-2036.2005.02356.x. [DOI] [PubMed] [Google Scholar]
- Kurtz C.B., Millet Y.A., Puurunen M.K., Perreault M., Charbonneau M.R., Isabella V.M., Kotula J.W., Antipov E., Dagon Y., Denney W.S., Wagner D.A., West K.A., Degar A.J., Brennan A.M., Miller P.F. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci. Transl. Med. 2019;11:eaau7975. doi: 10.1126/scitranslmed.aau7975. [DOI] [PubMed] [Google Scholar]
- Kwak S., Yun E.J., Lane S., Oh E.J., Kim K.H., Jin Y. Redirection of the glycolytic flux enhances isoprenoid production in Saccharomyces cerevisiae. Biotechnol. J. 2020;15 doi: 10.1002/biot.201900173. [DOI] [PubMed] [Google Scholar]
- Liang F.-S., Ho W.Q., Crabtree G.R. Engineering the ABA plant stress pathway for regulation of induced proximity. Sci. Signal. 2011;4 doi: 10.1126/scisignal.2001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievens L., Pollier J., Goossens A., Beyaert R., Staal J. Abscisic acid as pathogen effector and immune regulator. Front. Plant Sci. 2017;8:587. doi: 10.3389/fpls.2017.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.-J., Kong I.I., Zhang G.-C., Jayakody L.N., Kim H., Xia P.-F., Kwak S., Sung B.H., Sohn J.-H., Walukiewicz H.E., Rao C.V., Jin Y.-S. Metabolic engineering of probiotic Saccharomyces boulardii. Appl. Environ. Microbiol. 2016;82:2280–2287. doi: 10.1128/AEM.00057-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnone M., Ameri P., Salis A., Andraghetti G., Emionite L., Murialdo G., De Flora A., Zocchi E. Microgram amounts of abscisic acid in fruit extracts improve glucose tolerance and reduce insulinemia in rats and in humans. FASEB J. 2015;29:4783–4793. doi: 10.1096/fj.15-277731. [DOI] [PubMed] [Google Scholar]
- Maršálek B., Zahradníčková H., Hronková M. Extracellular abscisic acid produced by cyanobacteria under salt stress. J. Plant Physiol. 1992;139:506–508. doi: 10.1016/S0176-1617(11)80503-1. [DOI] [Google Scholar]
- McFarland L.V. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. WJG. 2010;16:2202. doi: 10.3748/wjg.v16.i18.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland L.V. Evidence-based review of probiotics for antibiotic-associated diarrhea and Clostridium difficile infections. Anaerobe. 2009;15:274–280. doi: 10.1016/j.anaerobe.2009.09.002. [DOI] [PubMed] [Google Scholar]
- McFarland L.V., Surawicz C.M., Greenberg R.N., Elmer G.W., Moyer K.A., Melcher S.A., Bowen K.E., Cox J.L. Prevention of beta-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am. J. Gastroenterol. 1995;90:439–448. [PubMed] [Google Scholar]
- McFarland L.V., Surawicz C.M., Greenberg R.N., Fekety R., Elmer G.W., Moyer K.A., Melcher S.A., Bowen K.E., Cox J.L., Noorani Z. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271:1913–1918. [PubMed] [Google Scholar]
- Michael S., Keubler L.M., Smoczek A., Meier M., Gunzer F., Pöhlmann C., Krause-Buchholz U., Hedrich H.-J., Bleich A. Quantitative phenotyping of inflammatory bowel disease in the IL-10-deficient mouse by use of noninvasive magnetic resonance imaging. Inflamm. Bowel Dis. 2013;19:185–193. doi: 10.1002/ibd.23006. [DOI] [PubMed] [Google Scholar]
- Miotto M., Armaos A., Di Rienzo L., Ruocco G., Milanetti E., Tartaglia G.G. Thermometer: a webserver to predict protein thermal stability. Bioinformatics. 2022;38:2060–2061. doi: 10.1093/bioinformatics/btab868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto M., Olimpieri P.P., Di Rienzo L., Ambrosetti F., Corsi P., Lepore R., Tartaglia G.G., Milanetti E. Insights on protein thermal stability: a graph representation of molecular interactions. Bioinformatics. 2019;35:2569–2577. doi: 10.1093/bioinformatics/bty1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreiradossantos M., Thygesen G., Kotter P., Olsson L., Nielsen J. Aerobic physiology of redox-engineered strains modified in the ammonium assimilation for increased NADPH availability. FEMS Yeast Res. 2003;4:59–68. doi: 10.1016/S1567-1356(03)00155-7. [DOI] [PubMed] [Google Scholar]
- Nagamune K., Hicks L.M., Fux B., Brossier F., Chini E.N., Sibley L.D. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature. 2008;451:207–210. doi: 10.1038/nature06478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offei B., Vandecruys P., De Graeve S., Foulquié-Moreno M.R., Thevelein J.M. Unique genetic basis of the distinct antibiotic potency of high acetic acid production in the probiotic yeast Saccharomyces cerevisiae var. boulardii. Genome Res. 2019;29:1478–1494. doi: 10.1101/gr.243147.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M., Teixeira P.G., Vizcaino M.I., David F., Siewers V. Integration of a multi-step heterologous pathway in Saccharomyces cerevisiae for the production of abscisic acid. Microb. Cell Fact. 2019;18:205. doi: 10.1186/s12934-019-1257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwehand A.C., Kirjavainen P.V., Shortt C., Salminen S. Probiotics: mechanisms and established effects. Int. Dairy J. 1999;9:43–52. doi: 10.1016/S0958-6946(99)00043-6. [DOI] [Google Scholar]
- Ozdemir T., Fedorec A.J.H., Danino T., Barnes C.P. Synthetic biology and engineered live biotherapeutics: toward increasing system complexity. Cell Syst. 2018;7:5–16. doi: 10.1016/j.cels.2018.06.008. [DOI] [PubMed] [Google Scholar]
- Paddon C.J., Keasling J.D. Semi-synthetic artemisinin: a model for the use of synthetic biology in pharmaceutical development. Nat. Rev. Microbiol. 2014;12:355–367. doi: 10.1038/nrmicro3240. [DOI] [PubMed] [Google Scholar]
- Palma M.L., Garcia-Bates T.M., Martins F.S., Douradinha B. Genetically engineered probiotic Saccharomyces cerevisiae strains mature human dendritic cells and stimulate Gag-specific memory CD8+ T cells ex vivo. Appl. Microbiol. Biotechnol. 2019;103:5183–5192. doi: 10.1007/s00253-019-09842-8. [DOI] [PubMed] [Google Scholar]
- Pedrolli D.B., Ribeiro N.V., Squizato P.N., de Jesus V.N., Cozetto D.A. Engineering microbial living therapeutics: the synthetic biology toolbox. Trends Biotechnol. 2019;37:100–115. doi: 10.1016/j.tibtech.2018.09.005. [DOI] [PubMed] [Google Scholar]
- Qi C.-C., Zhang Z., Fang H., Liu J., Zhou N., Ge J.-F., Chen F.-H., Xiang C.-B., Zhou J.-N. Antidepressant effects of abscisic acid mediated by the downregulation of corticotrophin-releasing hormone gene expression in rats. Int. J. Neuropsychopharmacol. 2014;18:pyu006. doi: 10.1093/ijnp/pyu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault H., Bassard J.-E., Hamberger B., Werck-Reichhart D. Cytochrome P450-mediated metabolic engineering: current progress and future challenges. Curr. Opin. Plant Biol. 2014;19:27–34. doi: 10.1016/j.pbi.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Ro D.-K., Paradise E.M., Ouellet M., Fisher K.J., Newman K.L., Ndungu J.M., Ho K.A., Eachus R.A., Ham T.S., Kirby J., Chang M.C.Y., Withers S.T., Shiba Y., Sarpong R., Keasling J.D. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–943. doi: 10.1038/nature04640. [DOI] [PubMed] [Google Scholar]
- Roetzer A., Gabaldón T., Schüller C. From Saccharomyces cerevisiae to Candida glabratain a few easy steps: important adaptations for an opportunistic pathogen. FEMS Microbiol. Lett. 2011;314:1–9. doi: 10.1111/j.1574-6968.2010.02102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen S.J., Gueimonde M., Isolauri E. Probiotics that modify disease risk. J. Nutr. 2005;135:1294–1298. doi: 10.1093/jn/135.5.1294. [DOI] [PubMed] [Google Scholar]
- Schenkman J.B., Voznesensky A.I. In: Molecular Aspects of Oxidative Drug Metabolizing Enzymes. Arinç E., Schenkman J.B., Hodgson E., editors. Springer Berlin Heidelberg; Berlin, Heidelberg: 1995. Interaction between cytochrome P450 and reductase; pp. 47–63. [DOI] [Google Scholar]
- Scheps D., Honda Malca S., Richter S.M., Marisch K., Nestl B.M., Hauer B. Synthesis of ω-hydroxy dodecanoic acid based on an engineered CYP153A fusion construct. Microb. Biotechnol. 2013;6:694–707. doi: 10.1111/1751-7915.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S.H., Qin X., Zeevaart J.A.D. Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiol. 2003;131:1591–1601. doi: 10.1104/pp.102.017921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevrioukova I., Truan G., Peterson J.A. The flavoprotein domain of P450BM-3: expression, purification, and properties of the flavin adenine dinucleotide- and flavin mononucleotide-binding subdomains. Biochemistry. 1996;35:7528–7535. doi: 10.1021/bi960330p. [DOI] [PubMed] [Google Scholar]
- Siddiqui M.S., Thodey K., Trenchard I., Smolke C.D. Advancing secondary metabolite biosynthesis in yeast with synthetic biology tools. FEMS Yeast Res. 2012;12:144–170. doi: 10.1111/j.1567-1364.2011.00774.x. [DOI] [PubMed] [Google Scholar]
- Siewers V., Kokkelink L., Smedsgaard J., Tudzynski P. Identification of an abscisic acid gene cluster in the grey mold Botrytis cinerea. Appl. Environ. Microbiol. 2006;72:4619–4626. doi: 10.1128/AEM.02919-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewers V., Smedsgaard J., Tudzynski P. The P450 monooxygenase BcABA1 is essential for abscisic acid biosynthesis in Botrytis cinerea. Appl. Environ. Microbiol. 2004;70:3868–3876. doi: 10.1128/AEM.70.7.3868-3876.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence C.A., Lakshmanan V., Donofrio N., Bais H.P. Crucial roles of abscisic acid biogenesis in virulence of rice blast fungus Magnaporthe oryzae. Front. Plant Sci. 2015;6:1082. doi: 10.3389/fpls.2015.01082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweere J.M., Van Belleghem J.D., Ishak H., Bach M.S., Popescu M., Sunkari V., Kaber G., Manasherob R., Suh G.A., Cao X., de Vries C.R., Lam D.N., Marshall P.L., Birukova M., Katznelson E., Lazzareschi D.V., Balaji S., Keswani S.G., Hawn T.R., Secor P.R., Bollyky P.L. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Sci. Technol. Humanit. 2019;363 doi: 10.1126/science.aat9691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takino J., Kozaki T., Ozaki T., Liu C., Minami A., Oikawa H. Elucidation of biosynthetic pathway of a plant hormone abscisic acid in phytopathogenic fungi. Biosci. Biotechnol. Biochem. 2019;83:1642–1649. doi: 10.1080/09168451.2019.1618700. [DOI] [PubMed] [Google Scholar]
- Takino J., Kozaki T., Sato Y., Liu C., Ozaki T., Minami A., Oikawa H. Unveiling biosynthesis of the phytohormone abscisic acid in fungi: unprecedented mechanism of core scaffold formation catalyzed by an unusual sesquiterpene synthase. J. Am. Chem. Soc. 2018;140:12392–12395. doi: 10.1021/jacs.8b08925. [DOI] [PubMed] [Google Scholar]
- Van Der Aa Kühle A., Skovgaard K., Jespersen L. In vitro screening of probiotic properties of Saccharomyces cerevisiae var. boulardii and food-borne Saccharomyces cerevisiae strains. Int. J. Food Microbiol. 2005;101:29–39. doi: 10.1016/j.ijfoodmicro.2004.10.039. [DOI] [PubMed] [Google Scholar]
- Wheeler M.L., Limon J.J., Bar A.S., Leal C.A., Gargus M., Tang J., Brown J., Funari V.A., Wang H.L., Crother T.R., Arditi M., Underhill D.M., Iliev I.D. Immunological consequences of intestinal fungal dysbiosis. Cell Host Microbe. 2016;19:865–873. doi: 10.1016/j.chom.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Ye L., Lv X., Xu H., Yu H. Sequential control of biosynthetic pathways for balanced utilization of metabolic intermediates in Saccharomyces cerevisiae. Metab. Eng. 2015;28:8–18. doi: 10.1016/j.ymben.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Xiong L., Zeng Y., Tang R.-Q., Alper H.S., Bai F.-W., Zhao X.-Q. Condition-specific promoter activities in Saccharomyces cerevisiae. Microb. Cell Fact. 2018;17:58. doi: 10.1186/s12934-018-0899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Wu W., Chen J., Jiang S., Zheng Z., Deng Y., Lu J., Wang H., Zhou Y., Geng Y., Wang K. Thermotolerance improvement of engineered Saccharomyces cerevisiae ERG5 Delta ERG4 Delta ERG3 Delta, molecular mechanism, and its application in corn ethanol production. Biotechnol Biofuels Bioprod. 2023;16:66. doi: 10.1186/s13068-023-02312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Bai P., Liu T., Li D., Zhang X., Lu W., Yuan Y. Optimization of a cytochrome P450 oxidation system for enhancing protopanaxadiol production in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2016;113:1787–1795. doi: 10.1002/bit.25934. [DOI] [PubMed] [Google Scholar]
- Zupancic M.L., Frieman M., Smith D., Alvarez R.A., Cummings R.D., Cormack B.P. Glycan microarray analysis of Candida glabrata adhesin ligand specificity. Mol. Microbiol. 2008;68:547–559. doi: 10.1111/j.1365-2958.2008.06184.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.